
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
STATE OF INDIANA
Family and Social Services Administration
Office of Medicaid Policy and Planning
INDIANA MEDICAID MEDICAL ASSISTANCE PROGRAM
MEDICAL POLICY MANUAL
TABLE OF CONTENTS
Page
I
. PREFACE 6
II. INDIANA HEALTH COVERAGE PROGRAMS OVERSIGHT 7-24
AND DELIVERY SYSTEMS
III. OTHER STATE PROGRAMS 25
I
V. SERVICES, LIMITATIONS AND EXCLUSIONS 26-29
V. PRIOR AUTHORIZATION 30
VI. MEDICAL POLICIES BY TOPIC
Abortion 31-33
Anesthesia Services 34-44
Bariatric Surgery and Revisions 45-53
Cardiac Rehabilitation 54-60
Case Management—Pregnant Women 61-65
Chiropractic Services
66-78
Clinic Services—FQHC and Rural Health Clinic Services 79-83
Clinical Trials 84-97
Collagen Implants for Stress Urinary Incontinence 98-100
Consultations—Second Opinions
101-106
Dental Services
107-122
Dermatology
123-125
Diabetes Self-Management Training 126-128
01/31/2007 Index
Medical Policy Manual
1

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Diagnostic Studies 129-130
Emergency Medicine—Cardiopulmonary Resuscitation (CPR)
131-132
Emergency Medicine—Emergency Room
133-136
Emergency Medicine—Emergency Services 137-144
EPSDT—HealthWatch 145-156
Evaluation and Management Services 157-162
Family Planning
163-169
Gastroenterology
170-173
Genetic Testing—BRCA1 and BRCA2 for Breast and Ovarian Cancer 174-178
Gynecology Services 179-187
HIV Care Coordination 188-196
Home Health Services 197-226
Hospice 227-243
Hospital Inpatient 244-254
Hospital Inpatient—Readmissions/General/Same Provider 255-256
Hospital Outpatient
257-263
Hyperbaric Oxygen 264-267
Immunizations and Vaccines 268-277
Intermediate Care Facilities for the Mentally Retarded
278-287
Laboratory Services
288-294
Laboratory Services—Group A Beta Hemolytic Streptococcal
Pharyngitis Tests 295-296
Laboratory Services—HER 2/neu Gene Detection Test 297-299
01/31/2007 Index
Medical Policy Manual
2

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Laboratory Services—Human Immunodeficiency Virus
(HIV) Testing 300-302
Laboratory Services—Sweat Chloride Test
303-304
Laboratory Services—Salivary Estriol
305-306
Locum Tenens & Substitute Physician Policy 307-311
Long-Term Acute Care Hospitals 312-318
Medical Supplies and Durable Medical Equipment (DME) Overview 319-332
Medical Supplies and Equipment —Automatic External Defibrillators
333-337
Medical Supplies and Equipment—Beds
338-344
Medical Supplies and Equipment—Gloves 345-348
Medical Supplies and Equipment—Implantable Infusion Pumps 349-354
Medical Supplies and Equipment—Incontinence Supplies 355-358
Medical Supplies and Equipment—Monitoring Devices 359-362
Medical Supplies and Equipment—Negative Pressure Wound Therapy 363-366
Medical Supplies and Equipment—Non-Invasive Respiratory Assist
Devices 367-374
Medical Supplies and Equipment—Patient-Activated Event Recorder—
Implantable Loop Recorder (ILR) 375-379
Medical Supplies and Equipment—Phrenic Nerve Stimulator 380-383
Medical Supplies and Equipment—Power Wheelchairs 384-391
Medical Supplies and Equipment—Programmable Hearing Aids
392-395
Medical Supplies and Equipment—Prothrombin Time
396-399
Medical Supplies and Equipment—Standers
400-406
Medical Supplies and Equipment—Standing Wheelchair
407-408
Medical Supplies and Equipment—ThAIRapy Vest 409-411
01/31/2007 Index
Medical Policy Manual
3

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Medical Supplies and Equipment – Vagus Nerve Stimulator for Epilepsy
412-419
Medical Supplies and Equipment—Ventricular Assist Device
(VAD) 420-431
Medical Supplies and Equipment—Wheelchair Accessories
432-448
Mental Health/Behavioral Health—Inpatient Services 449-459
Mental Health/Behavioral Health—Outpatient Services 460-470
Nursing Facilities 471-480
Nursing Services 481-490
Obstetric Care 491-513
Oncology 514-517
Oncology—Breast and Cervical Cancer 518-520
Ophthalmologic Services 521-534
Osteogenic Bone Growth Stimulator 535-539
Out-of-State Services 540-544
Pharmacy 545-566
Pharmacy—Botulinum Toxin Type A (BOTOX)
567-572
Pharmacy—Synagis
® and Respigam® 573-576
Plasmapheresis
577-579
Podiatry
580-586
Radioimmunotherapy
587-595
Radiology
596-607
Radiology-PET Scans
608-614
Screening Services—Newborn Screening 615-618
01/31/2007 Index
Medical Policy Manual
4

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Index
Medical Policy Manual
5
Smoking Cessation 619-622
Speech and Hearing
623-634
Substance Abuse
635-639
Surgery—Multiple Procedures/Same Operative Session 640-641
Surgery—Office Visits 642-643
Surgery—Plastic Reconstructive Surgery–Genitourinary Breast 644-649
Surgery—Plastic Reconstructive Surgery–Facial and Maxillofacial
650-671
Surgery—Plastic Reconstructive Surgery–Panniculectomy
672-675
Surgery—Removal of Implants 676-678
Surgery—Services Requiring Prior Authorization 679-680
Surgery—Surgeon and Assistant Surgeon, Same Provider 681-682
Surgery— Surgery and Anesthesia by the Same Provider 683-684
Surgery—Surgical Services 685-692
Surgery—Suture of Wounds 693-696
Surgery—Transplants 697-729
Therapy Services
730-740
Transportation Services 741-765
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
I. PREFACE
Health Care Excel, Incorporated, is a private, not-for-profit corporation
established for the purpose of providing clinically-based, objective, and
independent monitoring of the quality, appropriateness, and medical necessity of
health care services. Health Care Excel, in its role as the Indiana Health Coverage
Programs’ (IHCP) Medical Policy and Review Services contractor, is responsible
for the Medical Policy (MP), Prior Authorization (PA), and Surveillance and
Utilization Review (SUR) business functions. The Medical Policy Manual has
been developed to ensure the success of the IHCP. This manual will be used as a
reference handbook for the Family and Social Services Administration’s (FSSA)
Office of Medicaid Policy and Planning (OMPP), Health Care Excel (HCE), and
other State contractors and partners.
Within the Medical Policy and Review Services contract, the formulation of, and
support for, IHCP medical policies will involve an array of individuals and a
complex set of tasks for each policy. The management of medical policy must
involve the careful consideration of the stakeholders–the State, the practitioner
and provider community, and the IHCP member community. It must be
collaborative in nature to promote a positive, effective, and responsive approach
to customer service. By its unique nature, medical policy must strengthen the
foundation of the IHCP, irrespective of the governing agency or health care
delivery system.
This manual addresses the policies of the IHCP. The information regarding prior
authorization, payment methodology, and maximum fees may vary for providers
rendering services to members enrolled in the risk-based managed care (RBMC)
delivery system. Detailed descriptions of all IHCP covered services, as well as
exclusions and limitations, are also included. The objective is to take a proactive
approach in the development of new policy and the review of existing policies to
ensure the manual is reflective of the IHCP. The manual will serve as a living
document, providing flexibility to accommodate change, and promote ease of use.
01/31/2007 Narrative 6
Medical Policy Manual
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
II. INDIANA HEALTH COVERAGE PROGRAMS OVERSIGHT
AND DELIVERY SYSTEMS
A. Overview of the Indiana Family and Social Services Administration
The Indiana Family and Social Services Administration (FSSA) is the
umbrella agency responsible for administering Indiana’s public assistance
programs. FSSA includes the offices and divisions listed below.
♦ Office of Medicaid Policy and Planning (OMPP)
♦ Division of Disability and Rehabilitative Services (DDARS)
♦ Division of Family Resources (DFR)
♦ Division of Mental Health and Addiction (DMHA)
♦ Division of Aging (IDA)
The Director of Medicaid and Health Policy is responsible for OMPP.
Other agencies that administer programs that impact IHCP include the
Division of Disability and Rehabilitative Services, Division of Family
Resources, Division of Mental Health and Addiction, and Division of
Aging. These agencies are described below.
♦ Division of Disability and Rehabilitative Services (DADRS)
manages aging and in-home services, guardianship, and adult
protective services, and determines medical eligibility for the
Supplemental Security Income (SSI) and Social Security Disability
(SSD) programs for the Federal government. It provides case
management services for persons with developmental disabilities
including supervision services for four developmental centers for
clients with disabilities, operation of several State institutions,
vocational rehabilitation case management services, independent
living services for the deaf and hard of hearing, and services for the
blind and visually impaired.
♦ Division of Family Resources (DFR), through its county offices, is
responsible for determining eligibility for IHCP services. Following
the eligibility determination, county offices enroll individuals meeting
eligibility standards and maintain eligibility files using the Indiana
Client Eligibility System (ICES) for the IHCP member population.
01/31/2007 Narrative 7
Medical Policy Manual
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
♦ Division of Mental Health and Addiction (DMHA) ensures the
availability of accessible, acceptable, and effective mental health and
substance abuse related disorder services for Hoosiers. The division is
responsible for providing funding support for mental health and
addictions services to target populations with financial need through a
network of managed care providers, certifying all community mental
health centers and managed care providers (licensing inpatient
psychiatric hospitals and operating State behavioral health hospitals),
and administering Federal funds earmarked for substance abuse
prevention projects.
♦ Division of Aging (IDA) provides in-home and community based
services to older adults and people of all ages with disabilities.
Services focus on areas such as prevention, early intervention,
protection, and advocacy. The IDA collaborates with communities
and local organizations to provide appropriate services to individuals
and their families to ensure community resources are accessible.
In-home services provide assistance to enable independent living,
private homes and community living settings. These services include
attendant care, homemaker, home health services and supplies, respite
care, home delivered meals, adult day care, transportation, CHOICE
(Community and Home Options to Institutional Care for the Elderly
and Disabled), and other appropriate services.
Community based services provide a variety of services. These
services include Adult Guardianship, Title V Senior Employment, Pre-
Admission Screening Annual Resident Review, Indiana Pre-Admission
Screening, Assistance to Residents in County Homes, Room and Board
Assistance, USDA Meals Reimbursement, Title III/VII of the Older
Americans Act, Long Term Care Ombudsman, Money Management
Program, and the Developmental Disabilities Waiver Ombudsman.
Community based services are also accessible to family members of
older and/or disabled people to increase community outreach and
continuity of services.
Adult protective services (APS) address and investigate reports of
abuse, neglect, and exploitation of adults. The state of Indiana
coordinates with Indiana's prosecuting attorneys, law enforcement,
and the Family and Social Services Administration to ensure the safety
of adults in need. Multiple services are available through APS which
is dependent upon the level-of-need of the individual.
01/31/2007 Narrative 8
Medical Policy Manual
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
B. Indiana Health Coverage Programs Eligibility
In 2004, the IHCP provided medical assistance to approximately 760,000
eligible members. This estimate includes the categorically needy
population, as well as, those individuals eligible for, or receiving,
federally-aided financial assistance or deemed categorically needy, and
eligible for services under Federally-authorized waiver programs.
Persons in the categorical groups listed below are eligible for the IHCP,
subject to income and asset criteria.
♦ Aged, blind, and disabled people
♦ Families receiving assistance through the Temporary Assistance
for Needy Families (TANF) program
♦ Children under nineteen years of age with family incomes at or
below a designated percent of the Federal poverty level
♦ In addition, limited IHCP benefits are available to certain
population groups, as listed below.
♦ Qualified Medicare Beneficiaries (QMB)
♦ Pregnant women whose family income exceeds TANF program
limits, but is at or below a designated percent of the Federal
poverty level
♦ Qualified Disabled Working Individuals (QDWI) who lost
Medicare Part A due to employment status
♦ Specified Low Income Medicare Beneficiaries (SLIMB)
♦ Undocumented or unqualified aliens
C. Medicaid Waiver Programs
1. Overview of CMS’ Medicaid Waivers
States may apply to the Centers for Medicare and Medicaid Services
(CMS) for waivers of certain Federal regulations. There are three
major types of waivers; 1115, 1915(b), and 1915(c). Of these, Indiana
has no 1115 waivers, but does have one 1915(b) waiver, and several
waivers under 1915(c). States have the flexibility to design each
waiver program and select the mix of waiver services to best meet the
needs of the population they wish to serve. Waiver services may be
provided statewide or may be limited to specific geographic
01/31/2007 Narrative 9
Medical Policy Manual
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
subdivisions. The following Waiver services are provided statewide in
Indiana.
♦ CMS’ Home and Community-Based Waivers
♦ CMS’ Freedom of Choice Waivers
♦ CMS’ Research and Demonstration Waivers
2. Indiana Waiver Program Overview
The eight home-and community-based waivers currently offered
through the Indiana Medicaid program are listed below.
♦ Aged and Disabled
♦ Assisted Living
♦ Autism
♦ Traumatic Brain Injury
♦ Developmental Disability
♦ Medically Fragile Children
♦ Support Services
♦ Severely Emotionally Disturbed (SED) Children
FSSA’s DDARS administers the Home and Community Based
Services (HCBS) waiver program with assistance from, and oversight
by, OMPP. The HCBS waivers provide services to eligible recipients
to address special health care needs for persons who would be
institutionalized in the absence of community-based services.
The IHCP Medical Policy Manual will no longer contain policies
specific to Medicaid Waiver Programs. Clarification of policies for
Medicaid Waiver Programs can be found by contacting the Medicaid
Waiver Unit by phone at 1-800-545-7763, or in writing at the
following.
Medicaid Waiver Unit
Bureau of Aging and In-Home Services
Division of Disability, Rehabilitative Services
402 West Washington Street, W-454
Post Office Box 7083, MS-21
Indianapolis, IN 46207-7083
01/31/2007 Narrative 10
Medical Policy Manual
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
D. Traditional Medicaid
1. Overview
The Traditional Medicaid program provides coverage for health care
services rendered to the following eligibility groups.
♦ Wards and foster children who do not voluntarily enroll in a
managed care program
♦ Persons in nursing homes and other institutions, such as ICF/MR
facilities
♦ Undocumented aliens
♦ Waiver or hospice services
♦ Spenddown recipients
Eligible members receive health care services from enrolled IHCP
providers. Providers bill services rendered to members enrolled in
Traditional Medicaid subject to fee-for-service (FFS) directly to the
IHCP claims processing contractor, EDS. Providers are required to sign
a Medicaid Provider Agreement.
2. Delivery System
Traditional Medicaid is part of the FFS delivery system and
includes four benefit packages.
♦ Standard Plan–Members enrolled in the Traditional Medicaid are
eligible for full coverage.
♦ Spenddown–Some members with income in excess of the
Traditional Medicaid threshold can be enrolled under the
spenddown provision. These members are enrolled in Traditional
Medicaid with a spenddown. Spenddown is similar to a deductible
in that members must incur medical expenses in the amount of their
excess income each month before becoming eligible for Traditional
Medicaid. It is the member’s responsibility to provide verification
of incurred medical expenses to the county Division of Family
Resources (DFR) office. When spenddown is met, the member
becomes eligible for the remainder of the month. Members eligible
for assistance under the spenddown provision are listed below.
o Aged 65 and over
o Blind
01/31/2007 Narrative 11
Medical Policy Manual
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
o Disabled
o QMB-Also in combination with another aid category
o Those who spenddown their income to the correct
percentage of the federal poverty level in any given month
according to Hayes versus Paine
♦ Waiver–Waiver programs cover a variety of Home and
Community-Based Services (HCBS) not otherwise reimbursed by
the IHCP. Waiver programs are available to those IHCP-eligible
members who require the level-of-care (LOC) provided in a nursing
facility, hospital, or intermediate care facility for the mentally
retarded (ICF/MR), but choose to remain in the home. Eligibility
for all waiver programs requires the following.
o The member must meet IHCP guidelines
o The member would require institutionalization in the
absence of the waiver or other home-based services
o The total IHCP cost of serving the member on the waiver
(waiver cost plus other IHCP services) cannot exceed the
total cost to IHCP for serving the member in an appropriate
institutional setting
o Providers must verify member eligibility and if a member is
enrolled in managed care, the member needs to be
disenrolled from managed care to participate in the HCBS
Waiver Programs
♦ Qualified Medicare Beneficiary (QMB)–Federal law requires that
state Medicaid programs pay Medicare premiums, coinsurance, and
deductibles for certain elderly and disabled people. QMBs must
meet the following eligibility criteria to receive assistance with
Medicare-related costs.
o Entitled to Medicare
o Low income
o Age 65 years or older, or younger than 65 years old and
entitled to Medicare
o Few personal resources
QMB coverage falls into the following three categories.
o QMB-Only coverage: the member’s benefits are limited to
payment of the member’s Medicare premiums as well as
01/31/2007 Narrative 12
Medical Policy Manual
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
deductibles and coinsurance for Medicare-covered services
only
o QMB-Also coverage without spenddown: the member’s
benefits include payment of the member’s Medicare
premiums, deductibles, and coinsurance on Medicare-
covered services in addition to Traditional Medicaid
benefits throughout each month of eligibility
o QMB-Also coverage with spenddown: the member’s
benefits include payment of the member’s Medicare
premiums, deductibles, and coinsurance on Medicare-
covered services in addition to Traditional Medicaid
benefits beginning with the date on which the monthly
spenddown is met and continuing through the end of the
month
Claims processing and payment for these three types of QMBs
differ as follows.
o All QMBs–Medicaid pays the Medicare Part B premiums as
well as Medicare deductibles and coinsurance on Medicare-
covered services for which the Medicare payment amount is
less than the Medicaid allowed reimbursement amount. The
member is never responsible for the amount disallowed
(paid at zero) when Medicare paid more than the Medicaid
allowed amount for the service.
o QMB-Only–IHCP pays for only those services covered by
Medicare. For these member’s claims, the IHCP pays the
member’s Medicare deductible and coinsurance on
Medicare-covered services only. Claims for services not
covered by Medicare are denied as Medicaid non-covered
services. The member must make payment in full for
medical supplies, equipment, and other services not offered
by Medicare, such as routine physicals, dental care, hearing
aids, and eyeglasses.
o QMB-Also Without Spenddown–Medicaid claims for
services not covered by Medicare must be submitted as
regular Medicaid claims and not as crossover claims. These
QMB-Also members are enrolled in the Medicaid Select
program and their care is managed by a primary medical
provider (PMP).
o QMB-Also coverage with spenddown-In addition to
coverage for Medicare-related costs, QMBs who are also
eligible for another Medicaid aid category under the
spenddown provision have Traditional Medicaid benefits for
a portion of the months in which they meet their
01/31/2007 Narrative 13
Medical Policy Manual
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
spenddown. When spenddown is met for the month, the
member becomes eligible for the full array of services
covered by the Traditional Medicaid program. However, as
with the QMB-Only, the member must pay for services not
covered by Medicare if the Medicare non-covered service is
provided prior to the date when spenddown is met.
IHCP eligibility verification systems are designed to inform a provider of
a member’s Traditional Medicaid/QMB dual eligibility status when
spenddown has not been met for the month. If the QMB member is only
eligible for the coinsurance and deductible for Medicare covered services,
Medicare does not cover the service and Traditional Medicaid does not
cover the service.
Members enrolled in Traditional Medicaid are not assigned to a PMP and
certification codes are not required. However, prior authorization is
required for services as designated by IAC 405-5.
E. Hoosier Healthwise
1. Overview
Hoosier Healthwise is Indiana’s Medicaid Managed Care program
administered by OMPP and the Office of the Children’s Health
Insurance Program (CHIP). The State of Indiana requested approval
of this program through a waiver under the authority of Section
1915(b)(1) of the Social Security Act. The objective of the waiver
program is to reduce costs, prevent unnecessary utilization, reduce
inappropriate utilization, and assure adequate access to primary care
by Medicaid members.
Hoosier Healthwise provides coverage for parents and children who
receive Temporary Assistance for Needy Families (TANF) and for
low-income pregnant women and children. This program
encompasses the following four member eligibility packages.
♦ Package A–Standard Plan
♦ Package B–Pregnancy Coverage Only
♦ Package C–Children’s Health Insurance Plan (CHIP)
♦ Package E–Emergency Services Only (in FFS only)
OMPP began phasing in Hoosier Healthwise for TANF members and
low-income pregnant women and children in selected counties in July
1994. The program became statewide on July 1, 1996.
01/31/2007 Narrative 14
Medical Policy Manual
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Narrative 15
Medical Policy Manual
All Hoosier Healthwise participating primary medical providers
(PMP) in Indiana must enroll with an MCO in the RBMC delivery
system.
The goals of Hoosier Healthwise are listed below.
♦ To ensure access to primary and preventive care services
♦ To improve access to all necessary health care
services
♦ To encourage quality, continuity, and appropriateness of medical
care
♦ To provide medical coverage in a cost-effective manner
2. Delivery System
The Office of Medicaid Policy and Planning (OMPP) implemented a
statewide Hoosier Healthwise mandatory risk-based managed care
(RBMC) enrollment for all Indiana counties in 2005. This transitioned
PrimeStep Hoosier Healthwise managed care members from Primary
Care Case Management (PCCM) into local managed care
organizations (MCOs) in the RBMC delivery system. OMPP
submitted a request for federal approval for modification of Indiana's
1915(b) waiver to the Centers for Medicare and Medicaid Services
(CMS). The State anticipated that these counties will be approved for
mandatory MCO enrollment as well. This mandatory transition was
expected to be completed November 2005.
Under RBMC, OMPP contracts with Managed Care Organizations
(MCOs). MCOs are paid a capitated rate per month, per enrolled
Medicaid member by OMPP. Members in RBMC must obtain most
services from the network of the MCO in which they are enrolled.
The RBMC delivery system is a fully capitated prepayment plan in
which the MCOs are at risk to arrange for or administer the provision
of a comprehensive set of covered services to Hoosier Healthwise
members. The MCO accepts a per-member-per-month fee to provide
an agreed upon bundle of services, including high-cost services such
as inpatient hospitalization. Hoosier Healthwise member enrollees
enter the RBMC system by choosing as their PMP a primary care
physician who has contracted with an MCO.
The MCO must purchase reinsurance from a commercial reinsurer and
must establish reinsurance agreements meeting the requirements
stipulated by OMPP. The attachment point must be equal to or less
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
than $125,000. The MCO electing to establish commercial
reinsurance agreements with an attachment point greater than
$125,000 must receive approval from OMPP before changing the
attachment point. The MCO must receive reinsurance coverage of at
least $2,000,000 per member per year.
3. Primary Medical Providers
A basic and pervasive tenet of Hoosier Healthwise is that eligible
members are allowed to select their PMP. Physicians enrolled in
Hoosier Healthwise as PMPs provide preventive and primary care
through an ongoing patient/physician relationship, as well as
authorization and referral for most specialty services. The PMP or a
designee must be available 24 hours a day, seven days a week. The
PMP assists the member in gaining access to the health care system
and monitors, on an ongoing basis, the member's condition, health care
needs, and service delivery. The PMP is responsible for locating,
coordinating, and monitoring all primary care and other medical and
rehabilitation services on behalf of members enrolled in Hoosier
Healthwise.
The Hoosier Healthwise program encourages eligible Medicaid
members to select a PMP. However, if a member in the mandatory
program fails to make a PMP selection within 30 days of being
determined eligible for Medicaid (or re-determined eligible), a PMP is
assigned to the member through an auto-assignment process.
The intent of Hoosier Healthwise is to enhance existing provider-
patient relationships, or to establish a relationship when none exists.
Members enrolled in Hoosier Healthwise are restricted to services
included under Hoosier Healthwise either from the chosen or assigned
PMP or from another qualified provider to whom the member was
referred by the PMP. The member's health care will be managed by the
PMP. However, the member is allowed self-referral for the following
services.
♦ Chiropractic services
♦ Dental services
♦ Diabetes self management services
♦ Emergency services
♦ Family planning services
♦ HIV/AIDS targeted case management
♦ Vision services (except eye care surgical services)
01/31/2007 Narrative
Medical Policy Manual
16
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
♦ Podiatry services
A PMP must be a physician qualified in General Practice, Family
Practice, General Pediatrics, General Internal Medicine, or
Obstetrics/Gynecology (OB/GYN). Primary care physicians in any
setting are eligible to be PMPs and may serve as the PMP for any
member within their normal scope of practice. Physicians who enroll
in Hoosier Healthwise agree to be listed as a PMP in the listing of
approved practitioners and agree to accept a panel.
The PMP is responsible for providing or authorizing most primary and
preventive care services. PMP services include, but are not limited to:
physician services; hospital inpatient and outpatient services; and some
ancillary services such as laboratory and radiology;
orthotic/prosthetics; audiology; durable medical equipment and
supplies; home health services; and Early and Periodic Screening,
Diagnosis, and Treatment (EPSDT). PMPs who authorize another
provider to render services must document the referral in the patient's
medical record.
4. Process for Primary Medical Provider Enrollment
A physician interested in becoming a PMP is referred to one of the
participating Hoosier Healthwise MCOs and is required to sign a
contract with one MCO to participate in the RBMC delivery system. A
PMP can only contract with one MCO, however, specialists and other
providers may participate in more than one MCO. This does not
prohibit the PMP from rendering fee-for-service treatment for non-
Hoosier Healthwise members or from serving as a PMP in Medicaid
Select.
5. Process for Member Enrollment
In the DFR county offices, applicants for medical assistance receive a
brief presentation on Medicaid managed care, how to select a PMP,
and a description of the RBMC Hoosier Healthwise program from a
Benefit Advocate (BA). Benefit Advocates are employed by
AmeriChoice through a contract with OMPP. Videotapes and
brochures are available to describe the managed care programs.
Information provided includes a toll-free number to call for further
assistance. The member also is given: (1) a list of qualified PMPs
serving the member's geographic area; (2) a form for choice of a PMP
in RBMC; and (3) brochures for any risk-based managed care MCOs
in their geographic region. Qualified PMPs include only those PMPs
who are currently accepting new enrollees into the RBMC Hoosier
Healthwise program.
01/31/2007 Narrative
Medical Policy Manual
17
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Newly eligible members and members whose eligibility has been re-
determined have up to 30 days to choose a PMP. The member must
indicate a choice of a PMP by either mailing in the form or
telephoning the program. The member is then enrolled with the
selected PMP if he or she is accepting new members.
Qualified PMPs are free to encourage patients to choose them as a care
manager. When a provider is not on the list of qualified PMPs, the
member may encourage the provider to enroll or contact the program
to see if the provider qualifies. OMPP and its contractors market
enrollment to non-participating providers. If the member does not
choose a PMP within thirty (30) days, Hoosier Healthwise assigns the
member to a qualified and suitable PMP via an auto-assignment
process.
The member and the PMP are informed by mail of the member's
enrollment. The PMP may refuse the assignment if he or she does not
feel medically qualified to accept the case or if no further assignments
are being accepted. With the exception of newborns, enrollment in
managed care is not retroactive, so services rendered before the
effective date of Hoosier Healthwise enrollment are not subject to the
waiver's referral requirements.
F. Medicaid Select
1. Overview
Beginning January 1, 2003, IHCP implemented a new aged, blind, and
disabled (ABD) managed care program.
OMPP began phasing in Medicaid Select for ABD members in January
2003. The program became statewide in 2004. The goals of Medicaid
Select are listed below.
♦ To ensure access to primary and preventive care services
♦ To improve access to all necessary health care
services
♦ To encourage quality, continuity, and appropriateness of medical
care
♦ To provide medical coverage in a cost-effective manner
01/31/2007 Narrative
Medical Policy Manual
18
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
2. Delivery System
Medicaid Select operates as a PCCM System The PMP is responsible
for providing or authorizing most primary and preventive care
services. PMP services include, but are not limited to: physician
services; hospital inpatient and outpatient services; and some ancillary
services such as laboratory and radiology; orthotic/prosthetics;
audiology; durable medical equipment and supplies; home health
services; and Early and Periodic Screening, Diagnosis, and Treatment
(EPSDT). PMPs who authorize another provider to render services
must document the referral in the patient's medical record.
Members will continue to have the same ID number and will use the
same Hoosier Health Card. PMPs will be required to provide a
referral by phone or in writing, which will include the Provider ID
number and a special two digit certification code that will allow the
rendering provider to bill and receive reimbursement.
There are no certification code requirements for Medicare crossover
claims in Medicaid Select. However, for services that Medicare does
not cover but Medicaid does, a certification code is required.
Some services are self-referral and will not require PMP authorization.
These services include the following.
♦ Chiropractic services
♦ Dental services
♦ Diabetes self management services
♦ Emergency services
♦ Family planning services
♦ HIV/AIDS targeted case management
♦ Vision services (except eye care surgical services)
♦ Podiatry services
In addition to an administrative fee payment of $4.00 per member/per
month, PMP’s will be reimbursed, as usual, based on the fee-for-
service schedule. Claims will be submitted to the state’s fiscal agent,
EDS, for processing and payment.
3. Primary Medical Providers
Medicaid Select has five standard PMP categories: Family
Practitioner, General Practitioner, Internist, Pediatrician and
01/31/2007 Narrative
Medical Policy Manual
19
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Obstetrician / Gynecologist. In addition, any physician specialist, such
as a Cardiologist, Psychiatrist, or Urologist, may serve as a PMP.
Medicaid Select members are encouraged to select a PMP. Members
will have up to 60 days to select a PMP before they are auto-assigned
to the five traditional PMP types or to a non-traditional PMP type
(specialist), if they have previously been linked to a non-traditional
PMP on a self-selection basis. However, members are able to change
their PMP if they are auto-assigned or choose to see a different doctor
than originally selected.
4. Process for Primary Medical Provider Enrollment
Providers contact a Provider Services Representative at AmeriChoice
to receive information regarding enrollment in Medicaid Select. To
serve as a PMP, providers must be enrolled as an IHCP provider and
are required to sign a Medicaid Provider Agreement Addendum to
provide services to Medicaid Select members.
The five standard PMP categories are allowed member panels of 50 to
1000. All specialist provider types are allowed panels of 1-1000. A
specialist may enroll as a PMP to see only one or two existing patients.
The Medicaid Select panel is maintained separately and cannot be
combined with the Hoosier Healthwise panel.
5. Process for Member Enrollment
Medicaid Select includes all Medicaid recipients in the following aid
categories.
♦ Children receiving adoptive services
♦ Aged recipients
♦ Blind recipients
♦ Physically and mentally disabled recipients
♦ Medicare and Medicaid dual eligible recipients
♦ Individuals receiving room and board assistance
The following individuals are excluded from enrollment in Medicaid
Select.
♦ Breast and cervical cancer group
♦ Wards of the court and foster children
♦ Persons in nursing homes
01/31/2007 Narrative
Medical Policy Manual
20
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
♦ Intermediate Care Facilities for the Mentally Retarded and state-
operated facilities
♦ Waiver recipients
♦ Hospice service recipients
♦ Individuals for whom Medicaid pays only the Medicare premiums
♦ Spenddown recipients
6. Managed Care Administrative Contracts
OMPP has contracted many of the administrative activities essential
for managed care, such as development of a management information
system, claims processing, and member and provider enrollment. In
addition to the contracts with risk-based managed care providers, there
are (in 2005) three major administrative contracts: AmeriChoice,
primarily for member education and enrollment; Electronic Data
Systems (EDS) as a part of a larger contract to act as the State's fiscal
agent and pharmacy benefits manager; and HCE, which serves as the
Medical Policy and Review Services contractor.
AmeriChoice has the following specific responsibilities.
♦ Member education and enrollment facilitation
♦ Member hotline development and management
♦ Member outreach and follow-up
♦ Deployment of benefits advocates and related database
development and management
♦ Administrative plan development and execution
♦ Medicaid Select Provider recruitment and orientation
♦ Quality assurance/quality control execution, including member and
provider surveys
♦ Submission of program monitoring reports
EDS responsibilities in relation to managed care are stated below.
♦ Development and administration of an information system for
managed care as a component of IndianaAIM
♦ PMP enrollment
♦ Fee-for-service claims processing
01/31/2007 Narrative
Medical Policy Manual
21
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
♦ Monthly administrative payments to PMPs
participating in Medicaid Select
♦ Monthly capitation payments to MCOs participating in the RBMC
program
♦ Processing encounter claims from MCOs (MCOs are paid a
capitated rate but are expected to provide encounter-based data in
the form of shadow claims)
HCE responsibilities in relation to Hoosier Healthwise are listed
below.
♦ Assessing and coordinating existing medical policy
♦ Evaluating input from providers, members, and operational
activities and determining if there are policy implications,
including consideration of who is impacted, what policy needs to
be addressed, why the policy implication is important, when a
policy should be changed or added, where the impact will be felt
the most, and how to best address identified issues
♦ Coordinating the resolution of issues that bridge operations and
policy areas
♦ Recommending new medical policy to address emerging issues
♦ Determining and analyzing indicators to evaluate utilization of
services, access, preventive care, quality of care, and disease
management
♦ Prior authorization
♦ Surveillance and Utilization Review
G. Indiana Health Coverage Programs Provider Participation
Within the restrictions of federal and state law, OMPP (through its
contractors) enrolls various types of medical practitioners, providers of
institutional care, other agencies, and managed care organizations to
provide services to IHCP members.
A provider is said to be participating in the IHCP during a particular time
period if the provider bills for services rendered to IHCP members during
that time. The number and participation rate of enrolled IHCP providers
fluctuates. The number of participating providers can decrease when
some providers cease to treat IHCP members. Increases in the number of
participating providers can be seen when a new service is added or
previous restrictions are lifted. In 2004, there were approximately 46,000
providers approved to participate in the IHCP. The IHCP places a high
01/31/2007 Narrative
Medical Policy Manual
22
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
priority on provider participation so that IHCP members may have
appropriate access to care and choice of providers.
H. Indiana Health Coverage Programs Provider Payment Methodologies
Within the limitations prescribed by federal laws and regulations, the State
has established various methods of determining provider reimbursement
levels for the provision of IHCP covered services. These methods are
described briefly below. It should be noted that, with the exception of
copayment requirements, federal law requires providers to accept IHCP
payment in full, thereby prohibiting providers from billing members for
additional payment. In general, federal regulations require that
reimbursement rates be sufficient to ensure that the availability of health
care services to IHCP members is no less than that for the general
population. The payment methodologies currently utilized by the IHCP
are as follows.
♦ Diagnosis-Related Grouping (DRG) is used for pricing hospital
inpatient claims. If the inpatient claim can be grouped into one of the
established DRG numbers, a DRG number is assigned by IndianaAIM,
the claims processing system used by the IHCP. The principal
diagnosis, surgical procedure code, patient discharge status, and
member's age and sex are used to group the claim into one of the
established DRG numbers. Claims that do not group into an
established DRG number will be paid based on diagnoses, surgical
procedures, and revenue codes reported on the claim. Burn treatment,
rehabilitation, and psychiatric inpatient claims are paid according to
the level-of-care.
♦ Facility-specific per diem rates are used for nursing homes,
Intermediate Care Facilities for Mentally Retarded (ICF/MR),
Community Residential Facilities for the Developmentally Disabled
(CRF/DD), and Psychiatric Residential Treatment Facilities (PRTF).
♦ A case mix reimbursement methodology was implemented, effective
October 1, 1998, for IHCP nursing facilities that provided both skilled
and intermediate care to residents. Under this methodology, payment
is based upon one Medicaid rate, determined each quarter, for all
Medicaid residents in a Medicaid-certified and duly licensed nursing
facility.
♦ The Resource Based Relative Value Scale (RBRVS) system is used to
reimburse most services provided by physicians and other
practitioners.
01/31/2007 Narrative
Medical Policy Manual
23
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
♦ The lowest of the state maximum allowable charge (SMAC), amount
billed, federal upper limit or estimated acquisition cost (EAC) (for
legend drugs), plus a dispensing fee where applicable, is used for
pharmacy claims.
♦ Deductible and coinsurance amounts are paid by Medicaid for
Medicare crossover claims, so long as the Medicaid payment does not
exceed the Medicaid allowable for the service.
♦ Dental, Current Procedural Terminology (CPT), and Health Care
Common Procedure Coding System (HCPCS) codes not subject to
RBRVS are priced based on the lower of a maximum fee or the
submitted charge.
♦ Payment for hospital outpatient services depends on the revenue codes
billed. Surgery revenue codes are priced using Ambulatory Surgical
Center (ASC) rates on file. Treatment room revenue codes are priced
using a flat rate on file. Laboratory revenue codes are priced using the
laboratory pricing methodology described below. Radiology revenue
codes are priced based on the lower of a maximum fee or the
submitted charge. All other revenue codes are priced using a flat rate
on file.
♦ Laboratory fees are calculated at the Medicare-allowed amount based
upon the HCPCS procedure codes with a pricing indicator for
laboratory.
♦ In the RBMC delivery system, capitation fees are paid to contracted
managed care organizations (MCO).
♦ In the PCCM delivery system, an administrative fee of four dollars
($4.00) per member per month is paid to the Primary Medical Provider
(PMP). In addition, the PMP is paid according to the fee-for-service
schedule for services provided.
♦ Home health services are paid according to overhead and wage rates.
♦ Hospice services are reimbursed according to one of four all-inclusive
per diem rates, based on levels of service.
01/31/2007 Narrative
Medical Policy Manual
24
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
III. OTHER STATE PROGRAMS
The State of Indiana also funds various other medical assistance programs for its
population. Other State-funded programs are listed below.
♦ The 590 Program provides coverage for certain health care services
provided off-site to members who are residents of state-owned facilities.
These facilities operate under the direction of the Family FSSA, DMHA,
and the Indiana State Department of Health (ISDH).
♦ Aid to Residents in County Homes (ARCH) provides case review services
to certain residents of county nursing homes.
01/31/2007 Narrative
Medical Policy Manual
25
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
IV. SERVICES, LIMITATIONS, AND EXCLUSIONS
A. Services
Covered services, prior approval requirements, and limitations of coverage
for the Indiana Medicaid program are set out in the Indiana Administrative
Code (IAC) at 405 IAC 5.
B. Limitations
Medicaid limits the provision of certain covered services. 405 IAC 5
specifies the limited services and the conditions of the limitations.
Certain covered services as specified in 405 IAC 5 are available only with
prior authorization. The provider must submit a properly completed
Medicaid prior review and authorization request and receive written notice
indicating the approval for provision of such service prior to providing any
Medicaid service that requires prior authorization except as provided in
405 IAC 5-3-2, which allows for specific providers to request prior
authorization by telephone for specific services.
Any non emergent Medicaid service requiring prior authorization, which
is provided without first receiving prior authorization, shall not be
reimbursed by Medicaid. Services provided out-of-state with exceptions,
require prior authorization. Any authorization of a service by the
contractor is limited to authorization for payment of Medicaid allowable
charges and is not an authorization of the provider’s estimated fees.
Requests for prior authorization are reviewed for appropriate completion
of the request form, the medical and social information provided on the
request form or documentation accompanying the request form, the
criteria set out in 405 IAC 5 for the service requested, and the medical
reasonableness and necessity of the requested service based upon current
professional standards commonly held to be applicable to the case. Refer
to Section V, page 30, for additional information about the prior
authorization function.
Certain Medicaid members have restricted utilization of their Medicaid
cards when it has been determined that services must be controlled. A
provider other than the one to whom the member is restricted may provide
treatment to the member with a referral from the authorized provider, or
without a referral form if the diagnosis is an emergency diagnosis.
01/31/2007 Narrative
Medical Policy Manual
26
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
All Indiana Medicaid providers are subject to ongoing surveillance and
utilization review (SUR) activities. The SUR responsibilities have been
contracted to HCE. Based on paid claim information, statistical profiles
are established on provider peer and class groups to monitor the delivery
and receipt of medical services to identify misutilization and aberrant
practices by analyzing and comparing providers to their peer groups.
Based on the results of the off-site review and/or medical record review
the specific aberrant practice and billing patterns are identified and
prepayment review criteria are developed, unique to each provider. The
established criteria describe specifically what documentation and/or
practice is expected, and what procedure will be followed for each of the
review measures.
The provider will be notified and the prepayment reviewer will initiate the
appropriate system file changes to ensure that the provider’s claims that
meet the prepayment review criteria suspend for manual adjudication. A
minimum of three months of documentation submitted with the dates of
service during the prepayment review period must be reviewed to
determine compliance with the IAC and review criteria before the
prepayment review status can be terminated.
C. Exclusions
The following services are not covered by Medicaid.
1. Services that are not medically reasonable or necessary according to
current professional standards commonly held to be applicable to the
case.
2. Services provided outside the scope of a provider's license,
registration, certification, or other authority to practice under state or
federal law.
3. Experimental drugs, treatments, or procedures, and all related services.
4. Any new product, service, or technology not specifically covered in
IAC. The product, service, or technology will remain a noncovered
product, service, or technology until such time as the OMPP
authorizes the coverage of the product, service, or technology. This
does not apply to legend drugs.
5. Personal comfort or convenience items, including, but not limited to,
television, radio, or telephone rental.
6. Services for the remediation of learning disabilities.
7. Treatments or therapies of an educational nature.
01/31/2007 Narrative
Medical Policy Manual
27
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
8. Experimental radiological or surgical or other modalities and
procedures, including, but not limited to, the following items.
♦ Acupuncture
♦ Biofeedback therapy
♦ Carbon dioxide five percent inhalator therapy for inner ear disease
♦ Hyperthermia
♦ Hypnotherapy
9. Hair transplants
10. Fallopian tuboplasty (reanastomosis of the fallopian tubes) for
infertility or vasovasostomy (reanastomosis of the vas deferens). This
procedure is covered only in conjunction with disease
11. Augmentation mammoplasty for cosmetic purposes
12. Dermabrasion surgery for acne pitting or marsupialization
13. Rhinoplasty or bridge repair of the nose in the absence of a significant
obstructive breathing problem
14. Otoplasty for protruding ears unless one of the following applies to the
case.
♦ Multifaceted craniofacial abnormalities due to congenital
malformation or maldevelopment, for example, Pierre Robin
Syndrome
♦ A member has pending or actual employment where protruding ears
would interfere with the wearing of required protective devices
15. Scar removals or tattoo removals by excision or abrasion
16. Ear lobe reconstruction
17. Removal of keloids complicating pierced ears unless one of the
following is present
♦ Keloids are larger than three centimeters
♦ Obstruction of the ear canal is 50% or more
18. Rhytidectomy
19. Penile implants
20. Perineoplasty for sexual dysfunction
21. Reconstructive or plastic surgery unless deformity is related to disease
or trauma
22. Sliding mandibular osteotomies unless related to prognathism or
micrognathism
01/31/2007 Narrative
Medical Policy Manual
28
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
23. Blepharoplasties when not related to a significant obstructive vision
problem
24. Radial keratotomy
25. Miscellaneous procedures or modalities, including, but not limited to
the following items
♦ Autopsy
♦ Cryosurgery for chloasma
♦ Conray dye injection supervision
♦ Day care or partial day care or partial hospitalization except when
provided pursuant to 405 IAC 5
♦ Formalized and predesigned rehabilitation programs, including, but
not limited to, the following programs
o Pulmonary
o Cardiovascular (Cardiac Rehabilitation Phase 3 is non-covered.)
o Work-hardening or strengthening
♦ Telephone transmitter used for transtelephonic monitor
♦ Telephone, or any other means of communication, consultation from
one doctor to another
♦ Artificial insemination
26. Cognitive rehabilitation, except for treatment of traumatic brain injury
27. Ear piercing
28. Cybex evaluation or testing or treatment
29. High colonic irrigation
30. Services that are not prior authorized under the level-of-care
methodology as required by 405 IAC 5-19
31. Amphetamines when prescribed for weight control or treatment of
obesity
32. Under federal law, drug efficacy study implementation drugs not
covered by Medicaid
33. All anorexics, except amphetamines, both legend and nonaligned
34. Physician samples
01/31/2007 Narrative
Medical Policy Manual
29
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Narrative
Medical Policy Manual
30
V. PRIOR AUTHORIZATION
The Indiana Medicaid Program allows reimbursement for those services which
are outlined in the Covered Services and Limitation and Medical Policy Rule 405
IAC 5. The IAC contains the rules and regulations that govern IHCP, and serves
as a comprehensive reference to covered services and prior authorization (PA)
procedures and parameters. It is the responsibility of each IHCP provider to read
the portions of the IAC that apply to his/her area of service. Specific prior
authorization criteria should be obtained from 405 IAC 5.
The Health Care Excel Prior Authorization department reviews all requests for
prior authorization for traditional Medicaid, the 590 Program, and the Hoosier
Healthwise and Medicaid Select PCCM programs on an individual case-by-case
basis. The risk-based managed care companies, or their designees, review
requests for prior authorization for members enrolled in the Hoosier Healthwise
Risk-Based Managed Care program. The decision to authorize, modify, or deny a
given request is based upon medical reasonableness and necessity and other
criteria set forth in the IAC. Prior Authorization decisions may also be based on
OMPP approved internal criteria, in addition to the IAC prior authorization
guidelines.
The primary objective of prior authorization review is to allow payment only for
those treatments and/or services that are medically necessary, appropriate, cost-
effective, and to reduce over-utilization and/or abuse of certain services.

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: ABORTION
DESCRIPTION:
Surgical abortion is the induced termination of pregnancy before the fetus has developed
to a viable state. This does not include a spontaneous abortion, missed abortion,
incomplete abortion, or medical interventions required in the case of ectopic pregnancy.
MEDICAL TOPICS CROSS-REFERENCES:
Gynecology – Laminaria
Gynecology – Pelvic Exam under Anesthesia
RULES, CITATIONS, AND SOURCES:
42 CFR 50 Subpart C Abortions and Related Medical Services in Federally Assisted
Programs
42 CFR 441.200 Services: Requirements and Limits Applicable to Specific Services
Abortions
405 IAC 5-28-7 Medical and Surgical Services--Abortion
405 IAC 5-27-6 Radiology Services-- Sonography
Indiana State Department of Public Welfare Medical Policy Manual 1991
Indiana Medical Assistance Program Provider Manual 1994
Indiana Health Coverage Programs Provider Manual 1999
01/31/2007 Abortion
Medical Policy Manual
31

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Initial Policy Issue Effective
Date
Implementation
Date
Retroactive
Date
405 IAC 1-7-14
Repealed
8/24/97
Surgical
Services
01/01/1992
405 IAC 1-7-
19(e) Repealed
8/24/97
Sonography 01/01/1992
Revisions:
42 CFR 441.200 Abortions 10/01/1997
405 IAC 5-28-7 Abortion 08/24/1997
405 IAC 5-27-6 Sonography 08/24/1997
APPLICABLE INDIANAAIM EDITS AND AUDITS:
4012-Abortion Diagnosis/Procedure Indicated
4022-Abortion Diagnosis/Procedure Indicated
COVERAGE CRITERIA:
Medicaid reimbursement is available for abortions only if performed to preserve the life of
the pregnant woman or in other circumstances when the abortion is required to be covered
by Medicaid under federal law subject to limitations and restrictions set out in 42 CFR
Subpart C Sec.50.301, 50.302, 50.303, 50.304, 50.306, 42 CFR 441.200 Sec 441.200,
441.201, 441.202, 441.203, 441.206, 441.207, 441.208, 405 IAC 5-28-7 and 405. All
appropriate documentation must be attached to the claim and to claims for directly related
services before reimbursement shall be made.
01/31/2007 Abortion
Medical Policy Manual
32

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Abortion
Medical Policy Manual
33
ABORTION FACT SHEET
ADDENDUM A
Note: This addendum contains provider notifications that have been published since the
review of the Abortion Fact Sheet. The information in this addendum will be
incorporated into the fact sheet at the next review.
Provider Notification: BT200511 Publication Date: 06/01/2005
Subject: Abortion Diagnosis/Procedure Claim Notes
Date Added to Manual: 07/29/2005
Text of Publication
In the claim note, the IHCP accepts indication of medical documentation that supports the
need to save the mother’s life or a police report that indicates rape or incest.

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: ANESTHESIA SERVICES
DESCRIPTION
Stedman’s Medical Dictionary defines anesthesia as the loss of sensation resulting from
pharmacologic depression of nerve function or from Neurologic dysfunction.
Additionally, anesthesia is a broad term for anesthesiology as a clinical specialty.
Anesthesia services may include but are not limited to general, regional, supplementation
of local anesthesia, or other supportive services in order to give a patient anesthesia care
deemed optimal by the anesthesiologist to reduce or mitigate pain during any procedure.
The services include the usual preoperative and postoperative visits, anesthesia care
during the procedure, the administration of fluids and/or blood and the usual monitoring
services (for example, ECG, temperature, blood pressure, oximetry, capnography, and
mass spectrometry.) Other monitoring services (for example, intra-arterial, central
venous, and Swan-Ganz) are not included.
SUMMARY OF CURRENT POLICY
Indiana Health Coverage Programs (IHCP) reimbursement is available for covered
anesthesia services subject to the limitations and restrictions set out in the Indiana
Administrative Code (IAC). Providers who are eligible for reimbursement include
licensed anesthesiologists, certified registered nurse anesthetists (CRNA), and licensed
anesthesiologist assistants. Anesthesia services associated with canceled surgeries are
not reimbursed.
The IHCP provides separate reimbursement for the following types of anesthesia, when
provided by a physician other than the operating surgeon.
Epidural
Field Block
Inhalation
Intravenous
Nerve Block
Regional
Spinal
01/31/2007 Anesthesia Services
Medical Policy Manual
34
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Health Insurance Portability and Accountability Act (HIPAA) requirements mandated the
adoption of standards for anesthesia CPT codes. Providers submitting claims for
anesthesia services must use anesthesia CPT codes 00100 through 01999, effective
October 16, 2003. Anesthesia charges must be submitted using the anesthesia CPT code
that corresponds to the surgical procedure performed. General regional or epidural
anesthesia administered by the same provider who performs the surgical or obstetrical
delivery procedure is not reimbursable as it is included in the surgical delivery fee.
COVERAGE CRITERIA (Including Billing Requirements)
Anesthesia services are reimbursed according to a statewide fee schedule calculated on
the total base units, time units, add-on units, and additional units for specific physical
modifiers (as applicable), multiplied by the anesthesia conversion factor established by
the Office of Medicaid Policy and Planning (OMPP). Providers submitting anesthesia
services must use the anesthesia CPT codes 00100 through 01999. Anesthesia charges
must be submitted using the anesthesia CPT code that corresponds to the surgical
procedure performed.
Time
Time starts when the anesthesiologist or CRNA begins preparing the patient for the
procedure in the operating room or other appropriate area. It is not appropriate to start
counting time when the preoperative examination occurs. The preoperative exam is
reimbursed via the base units. Time ends when the anesthesiologist or CRNA releases
the patient to the postoperative unit and is no longer in constant attendance.
Base relative value units (RVU’s) are loaded in IndianaAIM. However, the actual time of
the procedure in minutes, is indicated in Locator 24G of the CMS-1500 claim form or the
837P electronic transaction. IndianaAIM calculates the time units. One unit is allowed
for each 15-minute period or fraction thereof.
Base Units
Base unit values have been assigned to each CPT code that would normally include
anesthesia. Providers should not report the base units on claims. The IndianaAIM claims
processing system automatically determines the base units for the procedure code as
submitted on the CMS-1500 claim form or the 837P electronic transaction. The system
converts each 15 minute block of time to one unit.
For the following procedure codes, IndianaAIM calculates one time unit or each fifteen
minute block of time billed in the first hour of service, and for subsequent hours of
service, calculates one unit of service for every sixty minute block of time or portion
thereof billed.
01960 – Anesthesia for vaginal delivery only
01967 – Neuraxial labor analgesia for anesthesia for planned vaginal delivery
(this includes any repeat subarachnoid needle placement and drug
01/31/2007 Anesthesia Services
Medical Policy Manual
35

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
injection and/or any necessary replacement of an epidural catheter during
labor)
Additional Units
The IndianaAIM claims processing system recognizes the following circumstances and
calculates any additional units appropriate for reimbursement.
Patient age – IndianaAIM applies additional units to the base units for
members under one year of age or over seventy years of age
Procedure code 99140 – Use this code on a separate line item of the claim to
indicate that the anesthesia provided was complicated by emergency
conditions
Physical status – Providers must use the appropriate modifier to denote any
patient conditions that may warrant payment of additional units these are
listed in Table 1, below
Table 1 – Physical Status Modifiers for Anesthesia
Modifier Description
Additional
Units
Allowed
P1 A normal healthy patient for an elective operation 0 units
P2 A patient with mild systemic disease 0 units
P3 A patient with severe systemic disease that limits activity but is
not incapacitating
1.0 units
P4 A patient with a severe system disease that is a constant threat
to life
2.0 units
P5 A moribund patient who is not expected to survive without the
operation
3.0
P6 A declared brain-dead patient whose organs are being removed
for donor purposes
0 units
Anesthesiologists performing the following procedures must bill with the AA modifier.
These procedures must be billed in units, instead of minutes.
36555 – Insertion of non-tunneled centrally inserted central venous catheter;
under 5 years of age
36556 – Insertion of non-tunneled centrally inserted central venous catheter; age 5
years or older
36568 – Insertion of peripherally inserted central venous catheter (PICC), without
subcutaneous port or pump; under 5 years of age
36569 – Insertion of peripherally inserted central venous catheter (PICC), without
subcutaneous port or pump; age 5 years or older
36580 – Replacement, complete, of a non-tunneled centrally inserted central
venous catheter, without subcutaneous port or pump, through same
venous access
01/31/2007 Anesthesia Services
Medical Policy Manual
36

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
36584 – Replacement, complete, of a peripherally inserted central venous catheter
(PICC), without subcutaneous port or pump through same venous access
36620 – Arterial catheterization or cannulation for sampling, monitoring or
transfusion (separate procedure); percutaneous
36625 – Arterial catheterization or cannulation for sampling, monitoring or
transfusion (separate procedure); cutdown
93503 – Insertion and placement of flow directed catheter (for example, Swan
-Ganz) for monitoring purposes
99116 – Anesthesia complicated by utilization of total body hypothermia
99183 – Physician attendance and supervision of hyperbaric oxygen therapy, per
session
99185 – Hypothermia, regional
Medical Direction and CRNA
The IHCP reimburses 30 percent of an anesthesia CPT procedure code’s allowed amount
when an anesthesiologist submits for medical direction of qualified anesthetists.
Anesthesia details submitted by a CRNA are reimbursed at 60 percent of the procedure
code’s allowed amount.
CRNAs must use anesthesia CPT codes (00100-01999) and bill with the appropriate
medical direction and/or physical status modifiers. CRNA providers use the same
physical status modifiers that apply to anesthesiologists, shown in Table 1 on the
preceding page. Table 2 – Anesthesia Modifiers lists the only modifiers used to identify
services provided by CRNAs.
Table 2 – Anesthesia Modifiers
Modifier Description
QS Monitored anesthesia care service
QX Certified Registered Nursing Anesthetist (CRNA) service, with medical
direction by a physician
QZ CRNA without medical direction by a physician
Reimbursement is available for medical direction of a procedure involving an anesthetist
only when the direction is by an anesthesiologist, and only when the anesthesiologist
medically directs two, three, or four concurrent procedures involving qualified
anesthetists. An anesthesiologist billing for medical direction uses modifier QK, Medical
direction of two, three, or four concurrent anesthesia procedures involving qualified
individuals. An anesthesiologist involved in medically directing more than one and up to
four procedures may not be personally performing procedures at the same time. Criteria
for medical direction include the following.
Ensure that only qualified people administer anesthesia
Monitor anesthesia at frequent intervals
Participate in the most demanding portions of the procedures including induction
and emergence, if applicable
Perform the preoperative evaluation
01/31/2007 Anesthesia Services
Medical Policy Manual
37
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Perform the postoperative evaluation
Prescribe anesthesia plan
Remain immediately available and not perform other services
The IHCP does not allow medical supervision by an anesthesiologist for more than four
concurrent procedures. Therefore, reimbursement is not allowed for services billed with
the AD modifier.
Regional Anesthesia
Regional anesthesia or nerve blocks involve blocking nerve impulses with a local
anesthetic, steroid, narcotic, or other agent. It is administered by a physician and requires
special techniques and attention, especially during the initial phase of instituting the
block. Nerve blocks performed as a surgical procedure for the treatment of a condition
such as chronic pain are billed with the appropriate nerve block code, quantity of one,
with no anesthesia modifier.
When billing regional anesthesia as the anesthesia type for a given surgical procedure
that is performed by a qualified anesthesia professional, regional anesthesia is billed and
paid in the same manner as a general anesthetic, using base units plus time. (See the
section entitled “Coverage Criteria”.) The bilateral procedure code modifier 50 is not
used in conjunction with anesthesia modifiers.
Monitored Anesthesia
Monitored anesthesia care (MAC) involves the intraoperative monitoring of patient vital
signs in anticipation of the need for administration of general anesthesia or the
development of adverse physiological patient reaction to the surgical procedure or
anesthesia. MAC also includes the performance of a preanesthestic examination and
evaluation, prescription of the anesthesia care required, administration of any necessary
oral or parenteral medications (such as Atropine, Demerol, or Valium), and the provision
of indicated postoperative anesthesia care.
The IHCP allows payment for medically reasonable and necessary MAC services on the
same basis as other anesthesia services. The QS modifier must be added to the
appropriate CPT code in addition to other applicable modifiers to identify the services as
monitored anesthesia care.
Postoperative Pain Management Services
The IHCP reimburses for postoperative epidural catheter management services using
CPT procedure code 01996. Procedure code 01996 is not separately paid on the same
day the epidural is placed. Rather, this code is billed on subsequent days when the
epidural is actually being managed. This code is used for daily management of patients
receiving continuous epidural, subdural, or subarachnoid analgesia, and is limited to one
unit of service for each day of management. Procedure code 01996 is only reimbursable
during active administration of the drug. When monitored by an anesthesia provider, no
modifier is appended. Claims submitted with anesthesia procedure codes and the
postoperative pain management codes listed in Table 3 – CPT Codes for Postoperative
01/31/2007 Anesthesia Services
Medical Policy Manual
38

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Pain Management, in Table 3, billed on the same day of surgery, must be used in
conjunction with modifier 59, Distinct procedural service, and is subject to post payment
review.
TABLE 3 – CPT Codes for Postoperative Pain Management
CPT
CODE
DESCRIPTION
62310
Injection, single (not via indwelling catheter), not including neurolytic
substances, with or without contrast (for either localization or epidurography),
of diagnostic or therapeutic substances(s) (including anesthetic,
antispasmodic, opiod, steroid, other solution), epidural or subarachnoid;
cervical or thoracic.
62311
Injection, single (not via indwelling catheter), not including neurolytic
substances, with or without contrast (for either localization or epidurography),
of diagnostic or therapeutic substances(s) (including anesthetic,
antispasmodic, opiod, steroid, other solution), epidural or subarachnoid;
lumbar, sacral (caudal)
62318
Injection, including catheter placement, continuous infusion or intermittent
bolus, not including neurolytic substances, with or without contrast (for either
localization or epidurography), of diagnostic or therapeutic substances(s)
(including anesthetic, antispasmodic, opiod, steroid, or solution), epidural or
subarachnoid; cervical or thoracic
62319
Injection, including catheter placement, continuous infusion or intermittent
bolus, not including neurolytic substances, with or without contrast (for either
localization or epidurography), of diagnostic or therapeutic substances(s)
(including anesthetic, antispasmodic, opiod, steroid, or solution), epidural or
subarachnoid; lumbar, sacral (caudal)
64412
Injection, anesthetic agent; spinal accessory nerve
64413
Injection, anesthetic agent; cervical plexus
64415
Injection, anesthetic agent; brachial plexus, single
64416
Injection, anesthetic agent; brachial plexus, continuous infusion by catheter
(including catheter placement) including daily management for anesthetic
agent administration
64417
Injection, anesthetic agent; axillary nerve
64420
Injection, anesthetic agent; intercostal nerve, single
64421
Injection, anesthetic agent; intercostal nerves, multiple, regional block
64445
Injection, anesthetic agent; sciatic nerve, single
64446
I
njection, anesthetic agent; sciatic nerve, continuous infusion by catheter
(including catheter placement) including daily management for anesthetic
agent administration
64447
Injection, anesthetic agent; femoral nerve, single
64448
Injection, anesthetic agent; femoral nerve, continuous infusion by catheter
(including catheter placement) including daily management for anesthetic
agent administration
64449
Injection, anesthetic agent; lumbar plexus, posterior approach, continuous
infusion by catheter (including catheter placement) including daily
01/31/2007 Anesthesia Services
Medical Policy Manual
39

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
TABLE 3 – CPT Codes for Postoperative Pain Management
CPT DESCRIPTION
CODE
management for anesthetic agent administration
64450
Injection, anesthetic agent; other peripheral nerve or branch
ANESTHESIA FOR DENTAL SERVICES
IHCP reimbursement for general anesthesia, billed using code D9220 for the first 30
minutes and D9221 for each additional 15 minutes, is only covered in a dentist’s office
for individuals under the age of 21 years of age. General anesthesia is covered for adults
only if the procedure is performed in a hospital (inpatient or outpatient) or an ambulatory
surgical center. Nitrous oxide analgesia is covered only for members twenty years of age
and younger.
Reimbursement for IV sedation in a dental office for IHCP members of all ages when
provided for oral surgery services only. Documentation in the member’s record must
include specific reasons why such services are needed and is subject to post payment
review.
Documentation for general anesthesia (adults or children) should include why the
individual could not receive necessary dental services unless general anesthesia was
administered. These records must be retained in the member’s file for at least three
years.
Adult dental patients who may qualify for hospital or surgical center general anesthesia
include, but are not limited to adults with the following medical conditions.
Mental incapacitation such that the member’s ability to cooperate with
procedures is impaired, including mental retardation and organic brain disease
Severe physical disorders affecting the tongue or jaw movements
Seizure disorders
Significant psychiatric disorders resulting in impairment of the recipient’s
ability to cooperate with procedures
Previously demonstrated idiosyncratic or severe reactions to IV sedation
medication
A dental cap of $600 applies to ICHP dental services provided in a dental office. Table 4
– CDT Codes Included in the $600 Dental Cap, on the following page, describes
anesthesia codes that are included in the dental cap for IHCP members. Providers can
bill their usual and customary charge to the member for any services provided after the
cap has been exhausted. However, if a service is partially paid by the IHCP because of
the cap limit, the member can only be billed for the difference between what the IHCP
would have reimbursed to the provider and what the IHCP actually paid. The following
guidelines must be met for the IHCP provider to hold a member responsible for payment.
01/31/2007 Anesthesia Services
Medical Policy Manual
40

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
The service rendered by the provider must be an IHCP non-covered service
The member has exceeded program limitations for a particular service
The member failed to inform the provider of eligibility. The provider must
maintain documentation to show that the provider billed the IHCP member of
that the provider requested the information within one year of the filing limit
The member must understand before receiving the service that the service is
not covered by the IHCP, and that the member is responsible for the charges
associated with the service.
The provider must maintain documentation that the member voluntarily chose
to receive the service knowing that the IHCP would not cover the service.
Table 4 – CDT Codes Included in the $600 Dental Cap
HCPCS Code Description
D9220 Deep sedation/general anesthesia – first 30 minutes
D9221 Deep sedation/general anesthesia – each additional 15 minutes
D9230 Analgesia, anxiolysis, inhalation of nitrous oxide
D9248 Non-intravenous conscious sedation
D9610 Therapeutic drug injection, by report
Monitored sedation for children (Dental procedures)
IHCP reimbursement for monitored sedation, provided in a dentist’s office, is available
for members under the age of 21. Monitored sedation is the administration of
subcutaneous, intramuscular, intravenous or oral sedation, in combination with
monitoring the patient’s vital signs. This service should be billed utilizing procedure
code D9248, Non-intravenous conscious sedation.
ANESTHESIA SERVICES FOR OBSTETRICAL SERVICES
General, regional, or epidural anesthesia administered by the same provider who
performs the surgical or obstetrical delivery procedure is not reimbursable as it is
included in the surgical delivery fee.
Providers billing anesthesia services for vaginal or cesarean deliveries must use the
appropriate anesthesia CPT codes as listed in Table 5 – Anesthesia CPT Procedure
Codes for Vaginal or Cesarean Delivery. This method of billing is the same as for any
other surgery, and should be used for obstetrical anesthesia, regardless of the type of
anesthesia provided, e.g. general or regional, including epidural anesthesia.
Table 5 – Anesthesia CPT Procedure Codes for Vaginal or Cesarean Delivery
CPT Procedure
Code
Description
01960 Anesthesia for vaginal delivery only
01961 Anesthesia for cesarean delivery only
01962 Anesthesia for urgent hysterectomy following delivery
01/31/2007 Anesthesia Services
Medical Policy Manual
41

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 5 – Anesthesia CPT Procedure Codes for Vaginal or Cesarean Delivery
CPT Procedure
Description
Code
01963
Anesthesia for cesarean hysterectomy without any labor
analgesia/anesthesia care
01964 Anesthesia for abortion procedures
01967
Neuraxial labor analgesia/anesthesia for planned vaginal delivery
(this includes any repeat subarachnoid needle placement and drug
injection and/or any necessary replacement of an epidural
catheter during labor)
*10968
Anesthesia for cesarean delivery following neuraxial labor
analgesia/anesthesia (list separately in addition to code for
primary procedure performed)
*01969
Anesthesia for cesarean hysterectomy following neuraxial labor
analgesia/anesthesia (list separately in addition to code for
primary procedure performed)
* Add-on codes that must be used in conjunction with 01967
When an anesthesiologist starts an epidural anesthesia for labor, and it becomes
necessary to switch to a general anesthetic for a delivery, the total times are combined
and billed for the procedure performed, such as vaginal delivery or cesarean section (C-
section).
When a provider, other than the surgeon or obstetrician, is billing for epidural anesthesia,
that provider is reimbursed in the same manner as is reimbursed for general anesthesia.
MANAGED CARE
The same policies and procedures pertaining to anesthesia services for traditional
Medicaid apply to the PrimeStep program. Anesthesiology services do not require the
PMP certification code for payment. Risk Based Managed Care (RBMC) may have
other, specific standards and practices. For RBMC specific policies, please consult the
appropriate managed care organization.
RELATED MEDICAL TOPICS
Consultations – Second Opinion
Dental Services
Obstetric Care
Surgery – Multiple Procedures/Same Operative Session
Surgery – Removal of Implants
Surgery – Services Requiring Prior Authorization
Surgery – Surgeon and Assistant Surgeon, Same Provider
Surgery – Surgical Services
01/31/2007 Anesthesia Services
Medical Policy Manual
42

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Surgery – Suture of Wounds
RULES, CITATIONS, AND SOURCES
405 IAC 5-10 - Anesthesia Services
405 IAC 5-14-11 - Analgesia (Dental)
405 IAC 5-14-15 - General Anesthesia and Intravenous Sedation (Dental)
405 IAC 5-25-1 - Physician Services
405 IAC 5-28-1(h) – Medical and Surgical Services (Reimbursement Limitations)
Hoosier Healthwise Manual for Primary Medical Providers and Office Staff
January 2003
Indiana Health Coverage Programs Provider Manual
1999
July 2004, Version 5.0
Indiana Health Coverage Programs Provider Banner
BR199947 – General Anesthesia – Dental Services
BR200350 – Modifiers for Medical Direction and CRNA Billing Requirements
Indiana Health Coverage Programs Provider Bulletin
BT200324 – Changes to the $600 Dental Cap
BT200353 – HIPAA requirements
BT200401 – 2004 Annual HCPCS Update
Origination Date: 12/31/2000
Revisions and Reviews Reason Date
Indiana State Dept. Of
Public Welfare Medical
Policy Manual 1991
Anesthesia Services Global Fee billing
07/01/1991
470 IAC 5-9-27 Transferred Anesthesia Services 07/01/1991
405 IAC 1-7-26 Repealed
8/24/97
Anesthesia Services
01/01/1992
405 IAC 1-7-26 (1) Anesthesia Reimbursement 12/01/1993
Indiana Medicaid Update
Bulletin 95-21, 05/02/95
Anesthesia Services
02/25/1995
Indiana Medicaid Update
Bulletin 96-26,
Anesthesia Services
07/29/1996
405 IAC 5-25-1 Physician Services 08/24/1997
405 IAC 5-10 Anesthesia Services 08/24/1997
405 IAC 5-14-11 and 405
IAC 5-14-15
Analgesia for Dental Procedures;
General Anesthesia and Intravenous
Sedation for Dental Procedures 08/24/1997
Indiana Medicaid Update
Bulletin 8-03
Anesthesia Services
01/06/1998
405 IAC 5-28-1(h) Medical and Surgical Services,
Reimbursement limitations 07/27/2001
Revision - Updates HIPAA requirements, Dental Services, 04/29/2005
01/31/2007 Anesthesia Services
Medical Policy Manual
43

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Anesthesia Services
Medical Policy Manual
44
APPLICABLE INDIANAAIM EDITS AND AUDITS
6002 – Any Two Anesthesiology Provider Same Procedure Requires Review
6096 – The CPT/HCPCS Code Billed Is Not Payable According to the PPS
Reimbursement Methodology
6156 – Procedure 99140 Must Be Billed With Anesthesia Code
6652 – Multiple Surgeries Must Be Billed on Same Claim
6666 – Anesthesia Services Not Allowed by Provider Billing for Surgery

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: BARIATRIC SURGERY AND REVISIONS
DESCRIPTION
Morbid obesity is defined as a body mass index
1
(BMI) of at least 35 kilograms per meter
squared, with co-morbidity or co-existing medical conditions, such as hypertension,
cardiopulmonary conditions, sleep apnea, or diabetes; or a BMI of at least 40 kilograms
per meter squared without a co-morbidity.
This document is intended to serve as a general summary of the IHCP policies regarding
this service. More specific information may be found in the IHCP provider manual,
program notices, the Indiana Administrative Code (IAC) or other sources as appropriate.
See the “Rules, Citations, and Sources” section of this document for further information.
I. BARIATRIC SURGERY
Bariatric surgery is a procedure of last resort, used to treat morbid obesity, when other
methods of weight management have failed. The term “bariatric surgery” is a collective
term, used to refer to procedures that involve restricting the stomach size with or without
a bypass of the stomach to alter the digestive system. The primary goal of bariatric
surgery is to achieve weight loss through restriction of the ability to eat, restriction of the
body’s ability to absorb nutrients and calories, or a combination of both. These surgeries
are categorized as “restrictive” or “malabsorptive” dependent upon the procedure
utilized.
Restrictive Operations
Restrictive operations limit food intake but do not interfere with the normal digestive
process. This type of surgery restricts the patient’s ability to eat large quantities of food
at one sitting.
♦ Adjustable gastric banding – a hollow band made of special material is placed around
the stomach near its upper end, creating a small pouch and a narrow passage into the
larger remainder of the stomach. The band is then inflated with a saline solution and
can be tightened or loosened over time to change the size of the passage by increasing
or decreasing the amount of saline solution.
♦ Vertical banded gastroplasty (VBG)
– VBG has been the most common restrictive
operation for weight control. Staples are used to create a small stomach pouch. A
1
Body mass index is equal to weight in kilograms divided by height in meters squared.
01/31/2007 Bariatric Surgery and Revisions
Medical Policy Manual
45

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
band of non-absorbable material is placed to limit the emptying of food from that
pouch.
Malabsorptive Operations
Malabsorptive operations are the most common gastrointestinal surgeries for weight
reduction. These operations restrict the amount of food a patient is able to eat, as well as
limit the ability to absorb calories and nutrients.
♦ Roux-en Y gastric bypass (RGB)
– RGB is the most common and successful
malabsorptive surgery. A small stomach pouch is created to restrict food intake. A
Y-shaped section of the small intestine is attached to the pouch to allow food to
bypass the lower stomach. This bypass reduces the amount of calories and nutrients
the body absorbs.
♦ Biliopancreatic diversion (BPD)
– BPD is a more complicated operation that involves
removal of a portion of the stomach. The small pouch that remains is connected
directly to the final segment of the small intestine. This procedure is used less
frequently because of the higher risk for nutritional deficiencies.
COVERAGE CRITERIA – BARIATRIC SURGERY
Bariatric surgery is recognized as medically necessary when used for the treatment of
morbid obesity. All types of bariatric surgery require prior authorization (PA) and are
subject to the following conditions.
1. Scope and duration of failed weight loss therapy must meet the following
criteria.
a. Morbid obesity has persisted for at least five years duration, and
b. Physician-supervised non-surgical medical treatment
2
has bee
unsuccessful for at least 18 consec
n
utive months.
Or
Member has successfully achieved weight loss after participating in
physician-supervised non-surgical medical treatment but has been
unsuccessful at maintaining weight loss for two years [ > 3 kg (6.6 lb.)
weight gain].
Successful weight loss therapy is defined as the ability to reduce body weight by
approximately 10% from baseline in a period of eight months. Unsuccessful weight loss
maintenance is defined as a weight regain of > 3 kg (6.6 lb.) in two years and the inability
to maintain a sustained reduction in waist circumference of at least four centimeters.
2. Age and maturity requirements must include both of the following.
a. Member must be between 21 and 65 years of age.
2
Includes a diet to help create a 500 to 1,000 kcal/day deficit; an increase in physical activity; and
strategies to change eating and physical activity behaviors.
01/31/2007 Bariatric Surgery and Revisions
Medical Policy Manual
46

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
b. Member must be physically mature as shown by sexual maturity and the
closure of growth plates.
Documentation in the member’s medical record must be maintained to substantiate the
following.
1. A psychological or psychiatric evaluation by a Health Service Provider in
Psychology (HSPP) or a psychiatrist is required prior to surgery. Members with
the following contraindications will not be candidates for bariatric surgery.
a. Active abuse of alcohol, illicit and/or social drugs and other chemicals,
and/or tobacco use during the six months prior to the request.
b. DSM IV criteria for Bulimia or Binge Eating Disorder (BED).
c. Other eating patterns that are deemed likely to interfere with post-surgical
safety and success.
d. Active psychosis.
e. Uncontrolled depression.
f. Borderline personality disorder.
g. Other complex psychiatric problems that might interfere with a successful
weight-loss outcome.
2. Member is able to understand, tolerate, and comply with all phases of care and is
committed to long-term follow-up requirements.
3. Member is abstinent from alcohol use, illicit drug use, and tobacco use; member
has a negative urine drug screen.
4. Member’s treatment plan includes pre-operative and post-operative dietary
evaluations.
5. Member has received a thorough explanation of the risks, benefits, and complications
of the procedure.
6. Member’s post-operative expectations have been addressed prior to the bariatric
surgery.
7. Member has agreed in writing to participate in all pre-operative and post-
operative evaluations and sessions considered essential to his or her having a
successful outcome to the bariatric surgery.
NONCOVERED SERVICES
The IHCP does not provide reimbursement for the following.
1. Procedures that are considered investigational and/or not meeting safety and/or
efficacy standards will not be covered.
2. Panniculectomy following gastric bypass procedures performed for cosmetic
reasons, even if performed incidentally to a ventral herniorrhaphy, is a non-
covered service.
3. The IHCP will not provide reimbursement for bariatric surgeries reported with
miscellaneous CPT procedure codes. Refer to the billing guidelines in this
document for further information.
01/31/2007 Bariatric Surgery and Revisions
Medical Policy Manual
47
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
PRIOR AUTHORIZATION – BARIATRIC SURGERY
All bariatric surgeries, as described in Table 1 in the “Billing Requirements – Bariatric
Surgery” section of this document, require PA. Surgical procedures performed to correct
or revise the initial surgical procedure require PA and are described in the “Surgical
Revisions” section of this document.
HCPCS code S2083, Adjustment of gastric band diameter via subcutaneous port by
injection or aspiration of saline, does not require PA. This procedure is considered a
routine, frequently performed, office procedure that involves accessing a subcutaneous
port, using a needle and syringe, and injecting or aspirating saline and is not a surgical
procedure. Injection or aspiration of saline results in an increase or decrease in the
diameter of the gastric band. The adjustment of the gastric band diameter is based on the
patient’s symptomatology and weight loss, as determined by the surgeon.
Documentation of an attempt to follow a physician-supervised, non-surgical medical
treatment for a minimum of 18 consecutive months must be presented with the request
for PA. Documentation of unsuccessful weight loss or unsuccessful weight maintenance
after successful weight loss must be submitted.
The physician requesting PA is responsible for referral of the member to a psychiatrist or
an HSPP at any time before or during the non-surgical treatment. The consultation would
include an assessment for any psychosocial needs with recommendation for treatment, if
necessary.
Additionally, the request for PA for bariatric surgery must be accompanied by the
following documentation requirements.
1. A signed statement from the member acknowledging an understanding of pre- and
post-operative expectations.
2. Documentation by the primary care physician of the results of the physician-
supervised non-surgical weight loss program for at least 18 consecutive months,
including unsuccessful weight loss or maintenance after successful weight loss.
3. Documentation by a psychiatrist or psychologist licensed as an HSPP that reflects
a psychiatric evaluation for possible behavioral health conditions that are
contraindications to the surgery.
4. Consultation reports from other practitioners who have seen the member for
evaluation (eg. anesthesiologist, pulmonologist, cardiologist, etc).
II. SURGICAL REVISIONS
COVERAGE CRITERIA – REVISIONS
Members may require subsequent surgery due to a complication during the perioperative
period or a revision to correct a technical failure post-operatively. Re-operation to repair
01/31/2007 Bariatric Surgery and Revisions
Medical Policy Manual
48
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
a complication or to correct a technical failure requires PA. Examples of perioperative
complications of surgery include, but are not limited to, the following.
1. Gastrointestinal leakage.
2. Stomal stenosis.
3. Anastomatic leakage.
4. Abscess.
Post-operative technical failures of the primary operation include, but are not limited to,
the following.
1. Staple line disruption - documented by x-ray or endoscopy.
2. Gastrogastric fistula with weight gain.
3. Expanded outlet - documented by gastroscopy.
4. Enlarged anastomosis -- documented by gastroscopy.
5. Intolerance to solid food after a band procedure.
6. Intractable reflux after a band procedure.
7. Weight loss as a result of anastomotic stenosis.
8. Stomal ulceration.
PRIOR AUTHORIZATION – REVISIONS
PA is required for re-operation to repair a complication or to correct a technical failure.
PA for revision or conversion to Roux-en-Y shall include a medical review of
documentation. Documentation of medical necessity must include the reason for the
failure and the date of the original surgery. Examples of peri-operative complications
and technical failures are provided in the coverage criteria for surgical revisions.
PA for revision of bariatric surgery due to reasons other than technical failure or due to
noncompliant behavior of the member requires six months of documentation in the
medical record to include the following.
1. Member participation in all pre-operative and post-operative evaluations and
sessions included in the treatment plan.
2. Consultations with the bariatric dietician with documentation in the medical
record of member’s compliance with the post operative dietary treatment plan.
3. When failure is at least in part due to noncompliant behavior of the member,
evaluation by a psychiatrist or psychologist licensed as an HSPP that reflects the
absence of behavioral health contraindications to a successful outcome to revision
of the bariatric surgery.
01/31/2007 Bariatric Surgery and Revisions
Medical Policy Manual
49

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MANAGED CARE
Members enrolled in Primary Care Case Management (PrimeStep) are subject to the
same benefit limits, restrictions, and medical necessity criteria as those individuals
enrolled in traditional Medicaid. Providers should contact the individual managed care
organizations to determine any additional criteria or requirements for bariatric surgery.
Senate Enrolled Act 360 from the 2005 session of the Indiana General Assembly outlines
the following criteria for Risk Based Managed Care (RBMC).
• Morbid obesity is defined as a body mass index* of at least 35 kilograms per
meter squared, with co-morbidity or coexisting medical conditions such as
hypertension, cardiopulmonary conditions, sleep apnea, or diabetes; or a body
mass index of at least 40 kilograms per meter squared without co-morbidity.
*Body mass index is equal to weight in kilograms divided by height in meters
squared.
• Physician-supervised non-surgical medical treatment has to have been
unsuccessful for at least 18 consecutive months.
• Members less than 21 years of age must have documentation in the medical
record by two physicians who have determined that bariatric surgery is
necessary to save the life of the member, or restore the member's ability to
maintain a major life activity defined as self-care, receptive and expressive
language, learning, mobility, self-direction, capacity for independent living or
economic self-sufficiency.
BILLING REQUIREMENTS
The IHCP will provide reimbursement for the bariatric procedures described in Table 1.
Providers must report ICD-9-CM diagnosis code 278.01, Morbid obesity with the most
specific procedure code available that represents the procedure performed.
Table 1 – Bariatric Surgery Codes
Code Description
43644
Laparoscopy, surgical, gastric restrictive procedure; with gastric bypass and
Roux-en-Y gastroenterostomy (roux limb 150 cm or less)
43645
Laparoscopy, surgical, gastric restrictive procedure; with gastric bypass and
small intestine reconstruction to limit absorption
43842
Gastric restrictive procedure, without gastric bypass, for morbid obesity;
vertical-banded gastroplasty
43843
Gastric restrictive procedure, without gastric bypass, for morbid obesity; other
than vertical-banded gastroplasty
01/31/2007 Bariatric Surgery and Revisions
Medical Policy Manual
50

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 1 – Bariatric Surgery Codes
Code Description
43845
Gastric restrictive procedure with partial gastrectomy, pylorus preserving
duodenoileostomy and ileoileostomy (50 to 100 cm common channel) to limit
absorption (biliopancreatic diversion from duodenal switch)
43846
Gastric restrictive procedure, with gastric bypass for morbid obesity; with short
limb (150 cm or less) Roux-en-Y gastroenterostomy
43847
Gastric restrictive procedure, with gastric bypass for morbid obesity; with small
intestine reconstruction to limit absorption
43770
Laparoscopy, surgical; gastric restrictive procedure; placement of adjustable
gastric band (gastric band and subcutaneous port)
43771
Laparoscopy, surgical; gastric restrictive procedure; revision of adjustable gastric
band component only
43772
Laparoscopy, surgical; gastric restrictive procedure; removal of adjustable
gastric band component only
43773
Laparoscopy, surgical; gastric restrictive procedure; removal and replacement of
adjustable gastric band component only
43774
Laparoscopy, surgical; gastric restrictive procedure; removal of adjustable
gastric band and subcutaneous port components
43848
Revision, open, of gastric restrictive procedure for morbid obesity, other than
adjustable gastric band (separate procedure)
43886
Gastric restrictive procedure, open; revision of subcutaneous port component
only
43887
Gastric restrictive procedure, open; removal of subcutaneous port component
only
43888
Gastric restrictive procedure, open; removal and replacement of subcutaneous
port component only
43999 Unlisted procedure, stomach
The IHCP does not provide reimbursement for HCPCS code S2083, Adjustment of
gastric band diameter via subcutaneous port by injection or aspiration of saline, during
the 90-day global period for CPT code 43770, 43773, 43771, 43886, or 43888.
01/31/2007 Bariatric Surgery and Revisions
Medical Policy Manual
51

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
RELATED MEDICAL TOPICS
Anesthesia Services
Consultations – Second Opinion
Diagnostic Studies
Gastroenterology
Radiology
RULES, CITATIONS, AND SOURCES
Indiana Health Coverage Programs Provider Manual
405 IAC 5-3-13 Services Requiring PA
405 IAC 5-29 Non-Covered Services
Indiana Health Coverage Programs Bulletin BT200410
Indiana Health Coverage Programs Bulletin BT200430
Medical Policy Administrative Report May 2004
Senate Enrollment Act 360
Origination Date 10/01/2000
REVIEW AND REVISIONS REASON DATE
Review Add new codes 02/01/2005
Revision
Senate Enrollment Act 360 and
addition of PA criteria for
bariatric surgery revisions
10/31/2005
01/31/2007 Bariatric Surgery and Revisions
Medical Policy Manual
52

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Bariatric Surgery and Revisions
Medical Policy Manual
53
APPLICABLE INDIANAAIM EDITS AND AUDITS
3000 – Units Exceed PA Master
3001 – Date(s) of Service Not on PA Database
3010 – Out-of-State Provider Requires Prior Authorization
3011 – Out-of-State Provider Requires Prior Authorization-Rendering Provider
6002 – Any Two Anesthesiology Providers Same Procedure Requires Review
6003 – Manual Pricing for Split Care Billing
6022 – Components Not Payable When Global Paid-Digestive Services
6023 – Global Payable at a Reduced Fee When Components Paid-Digestive Services
6034 – Global Surgery Payable at Reduced Amount When Components of Surgical Care
Paid
6035 – Components of Surgical Care Not Payable when Global Surgery Paid
6037 – One Assistant Surgeon Allowed for Select Surgeries
6040 – Co-Surgeon Paid at Reduced Amount when Assistant Surgeon Paid
6096 – CPT/HCPCS Code Billed is Not Payable According to the PPS Reimbursement
Methodology
6652 – Multiple Surgeries Must be Billed On Same Claim
6661 – Duramorph Cannot Be Billed on Same Day as Surgery
6666 – Anesthesia Services Not Allowed by Providers Billing for Surgery

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: CARDIAC REHABILITATION
DESCRIPTION:
Progressive exercise programs have demonstrated benefit in the management and
rehabilitation of individuals with cardiac disease, especially following certain cardiac
events. Cardiac rehabilitation programs are typically divided into three stages. The
initial stage (Phase I) involves the most intensive supervision and occurs in an inpatient
setting. A Phase I program is typically initiated during the acute convalescent period
following a cardiac event. Phase II begins with an overall treatment plan including a
physician’s prescription for progressive exercise based on the individual’s clinical status
and physical capacity. Phase II programs incorporate close monitoring and
individualized progressive increases in the intensity of physical activity, as well as
lifestyle changes such as dietary modifications and smoking cessation. Phase II exercise
programs for cardiac patients may be conducted in specialized, freestanding, cardiac
rehabilitation clinics, as well as in outpatient hospital departments. Phase III is an
ongoing maintenance period, consisting of continued lifestyle changes and aerobic
exercise. All phases of cardiac rehabilitation programs include individualized exercises
and behavior change therapy with the intention of returning the patient to an active life
with minimized symptoms.
This document is intended to serve as a general summary of the Indiana Health Coverage
Programs’ (IHCP) policies regarding this service. More specific information may be
found in the IHCP Provider Manual, program notices, the Indiana Administrative Code
(IAC) or other sources as appropriate. See the “Rules, Citations, and Sources” section of
this document for further information.
COVERAGE CRITERIA
IHCP mirrors Medicare guidelines and regulations for the Cardiac Rehabilitation Phase I
and Phase II services. The cardiac rehabilitation program is to include a medical
evaluation, and a program to modify cardiac risk factors, such as nutritional counseling,
prescribed exercise, education, and behavioral counseling. Phase I is included in the
inpatient DRG; therefore, IHCP does not provide separate reimbursement. IHCP does
not provide reimbursement for Phase III programs.
Reimbursement is available for Phase II cardiac rehabilitation programs when considered
medically reasonable and necessary. Members must be referred by the physician, and
have at least a moderate level of risk stratification.
01/31/2007 Cardiac Rehabilitation
Medical Policy Manual
54
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
The member must have either of the following criteria.
1. Stable angina pectoris (ICD-9-CM diagnosis codes 413.0 or 413.9) with reduced
activity tolerance substantially altering lifestyle. Stable angina is defined as
exertional chest pains with a constant threshold, predictable symptoms, and the
ability to adjust one’s activity and medications in order to avoid symptoms.
Members who qualify for a Phase II cardiac rehabilitation program are expected
to have a functional classification of Class II or Class III on the Canadian
Cardiovascular Society Functional Classification, as follows.
• Class I
Ordinary physical activity, such as walking and climbing stairs, does not
cause angina. Angina may occur with strenuous, rapid, or prolonged
exertion at work or recreation
• Class II
Slight limitation of ordinary activity including, walking or climbing stairs
rapidly walking uphill, walking or stair climbing after meals, in cold, in
wind, or when under emotional stress, or only during the few hours after
awakening. Walking more than two blocks on a level surface and
climbing more than one flight of ordinary stairs at a normal pace and
under normal conditions
• Class III
Marked limitation of ordinary physical activity. Walking one to two
blocks on a level surface and climbing more than one flight in normal
conditions
• Class IV
Inability to carry on any physical activity without discomfort; anginal
syndrome may be present at rest
2. Have had one of the following preceding the initiation of the Phase II program.
• Coronary artery bypass surgery
• Acute myocardial infarction (within the previous twelve months)
• Old myocardial infarction (that is less than 52 weeks from the date of the
infarction)
• Heart valve repair/replacement
• Percutaneous transluminal coronary angioplasty (PTCA) or coronary stenting
• Heart or heart-lung transplant
Cardiac rehabilitation programs may be provided either by the outpatient department of a
hospital or in a freestanding cardiac rehabilitation facility. Cardiac rehabilitation services
must be conducted only when a physician is on the premise and is available to perform
medical duties at all times the facility is open. The IHCP member must be under the care
of a hospital or clinic physician. The facility must have available for immediate use all
01/31/2007 Cardiac Rehabilitation
Medical Policy Manual
55
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
the necessary cardiopulmonary emergency diagnostic and therapeutic life saving
equipment accepted by the medical community as medically necessary, such as oxygen,
cardiopulmonary resuscitation equipment, and defibrillator.
Services provided in connection with a cardiac rehabilitation exercise program may be
considered reasonable and necessary for up to 36 sessions, usually a maximum of three
sessions a week, in a single 12- week period. Coverage for continued participation in
cardiac exercise programs beyond 12 weeks are approved on a case-by-case basis with
exit criteria taken into consideration.
Documentation in the medical record must show the medical necessity for unusual
frequency or duration of Phase II. Documentation must be specific in terms of exit
criteria and/or setbacks that changes the exercise prescription. Claims for Phase II
accompanied documentation indicating the member has not reached an exit level within
12 weeks are considered on a case-by-case basis. Reimbursement is not available for
Phase II exceeding a maximum of 24 weeks.
A member may be progressed to the maintenance (Phase III) program when the following
criteria are met.
1. The member has achieved a stable level of exercise tolerance without ischemia or
dysrhythmia as evidenced by electrocardiogram (ECG)
2. Symptoms of angina or dyspnea are stable at the member’s maximum exercise
level
3. The member’s resting blood pressure and heart rate are within normal limits, or
are stable on optimal medical therapy
4. The stress test is not positive during exercise. (A positive test in this context
means an ECG with a junctional depression of greater than or equal to 2 mm
associated with slowly rising, horizontal, or down-sloping ST segment)
A routine cardiac rehabilitation visit must include at least one of the following services.
1. Continuous ECG telemetric monitoring during exercise
2. ECG rhythm strip with interpretation and physician’s revision of exercise
prescription
3. Physician evaluation to assess the member’s performance, adjust medication, or
other treatment changes. This physician evaluation is considered an element of
the cardiac rehabilitation visit, and is not a separate reimbursement. Additional
physician reimbursement requires documentation of a separately identifiable
evaluation and management (E/M) service
Other cardiac rehabilitation services may include, but are not limited to, the following.
1. New patient comprehensive evaluation, including history, physical, and
preparation of initial exercise prescription. One comprehensive evaluation is
allowed and separately payable at the beginning of the program, if not
01/31/2007 Cardiac Rehabilitation
Medical Policy Manual
56

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
already performed by the member’s attending physician, or if the evaluation
performed by the member’s attending physician is not acceptable to the
program’s director. An assessment performed by a nurse or other personnel
does not meet this requirement.
2. ECG stress test (treadmill or bicycle ergometer) with physician monitoring
and report. One is allowed at the beginning of the program and one after 3
months (usually at the completion of the program). Pharmacologic stress
testing may be indicated in certain circumstances and would be allowed only
on a case-by-case basis with appropriate documentation of medical necessity.
PRIOR AUTHORIZATION
Prior authorization is not required for Cardiac Rehabilitation services.
BILLING GUIDELINES
Phase II Cardiac Rehabilitation services are to be reported with the appropriate CPT
procedure code as noted in Table 1 and an appropriate ICD-9-CM Diagnosis code as
described in Table 2. According to the ICD-9-CM coding narratives, cardiac
rehabilitation that begins within eight weeks of the date of the infarction should be coded
as 410.00 - 410.92. Cardiac rehabilitation beginning eight weeks or greater from the date
of the infarction (but less than 52 weeks) should be coded as 412 or 414.8.
Table 1–CPT Procedure Codes for Phase II Cardiac Rehabilitation
Code Description
93797 Physician services for outpatient cardiac rehabilitation; without continuous
ECG monitoring (per session)
93798 Physician services for outpatient cardiac rehabilitation; with continuous ECG
monitoring (per session)
Table 2–ICD-9-CM Diagnosis Codes for Phase II Cardiac Rehabilitation
Code Description
410.00 Acute myocardial infarction of anterolateral wall; episode of care unspecified
410.01 Acute myocardial infarction of anterolateral wall; initial episode of care
410.02 Acute myocardial infarction of anterolateral wall; subsequent episode of care
410.10 Acute myocardial infarction of other anterior wall; episode of care unspecified
410.11 Acute myocardial infarction of other anterior wall; initial episode of care
410.12 Acute myocardial infarction of other anterior wall; subsequent episode of care
410.20 Acute myocardial infarction of inferolateral wall; episode of care unspecified
410.21 Acute myocardial infarction of inferolateral wall; initial episode of care
410.22 Acute myocardial infarction of inferolateral wall; subsequent episode of care
410.30 Acute myocardial infarction of inferoposterior wall; episode of care
unspecified
01/31/2007 Cardiac Rehabilitation
Medical Policy Manual
57

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 2–ICD-9-CM Diagnosis Codes for Phase II Cardiac Rehabilitation
Code Description
410.31 Acute myocardial infarction of inferoposterior wall initial episode of care
410.32 Acute myocardial infarction of inferoposterior wall subsequent episode of care
410.40 Acute myocardial infarction of other inferior wall; episode of care unspecified
410.41 Acute myocardial infarction of other inferior wall; initial episode of care
410.42 Acute myocardial infarction of other inferior wall; subsequent episode of care
410.50 Acute myocardial infarction of other lateral wall; episode of care unspecified
410.51 Acute myocardial infarction of other lateral wall; initial episode of care
410.52 Acute myocardial infarction of other lateral wall; subsequent episode of care
410.60 Acute myocardial infarction true posterior wall infarction; episode of care
unspecified
410.61 Acute myocardial infarction true posterior wall infarction; initial episode of
care
410.62 Acute myocardial infarction true posterior wall infarction; subsequent episode
of care
410.70 Acute myocardial infarction subendocardial infarction; episode of care
unspecified
410.71 Acute myocardial infarction subendocardial infarction; initial episode of care
410.72 Acute myocardial infarction subendocardial infarction; subsequent episode of
care
410.80 Acute myocardial infarction of other specified sites; episode of care
unspecified
410.81 Acute myocardial infarction of other specified sites; initial episode of care
410.82 Acute myocardial infarction of other specified sites; subsequent episode of care
410.90 Acute myocardial infarction unspecified site; episode of care unspecified
410.91 Acute myocardial infarction unspecified site; initial episode of care
410.92 Acute myocardial infarction unspecified site; subsequent episode of care
412 Old myocardial infarction
413.0 Angina decubitus*
413.9 Other and unspecified angina pectoris
414.8 Other specified forms of chronic ishemic heart disease
V15.1 Surgery to heart and great vessels
V42.1 Organ or tissue replaced by transplant; heart
V42.2 Organ or tissue replaced by transplant; heart valve
V43.3 Organ or tissue replaced by other means; heart valve
V45.09 Cardiac device in situ; other specified cardiac device
V45.81 Postsurgical status, aortocoronary bypass
V45.82 Percutaneous transluminal coronary angioplasty status
*Includes nocturnal angina
It is the responsibility of the provider to code to the highest level specified in the ICD-9-
CM (e.g., to the fourth or fifth digit). The correct use of an ICD-9-CM code listed above
does not assure coverage of a service. The service must be reasonable and necessary in
the specific case and must meet the criteria specified in this policy.
01/31/2007 Cardiac Rehabilitation
Medical Policy Manual
58

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
The appropriate revenue code for cardiac rehabilitation services is 943. All charges
associated with the elements of a cardiac rehabilitation service as noted previously in this
document, including telemetry and supplies for telemetry, are to be included in this
charge. Separate reimbursement for charges for telemetry, electrodes, etc. are not
provided. One unit equals one cardiac rehabilitation visit. The number of units must be
shown on the UB-92 in field 46. A stress test may be billed using revenue code 482. The
date of onset or surgery must be indicated on the UB-92 in fields 32-35 with occurrence
code 11. The date of the first cardiac rehabilitation session must be indicated in fields
32-35 with occurrence code 46. The total number of cardiac rehabilitation visits from the
start of care, including the current claim, must be entered on the UB-92 in fields 39-41
with value code 53.
Reasons for Denial
The IHCP will deny reimbursement for, but not limited to, the following reasons.
Although members may meet a provider’s protocol for cardiac rehabilitation services,
they must also meet the IHCP coverage criteria for medical necessity.
1. Lack of documentation of a covered diagnosis
2. Lack of documentation of the elements of a cardiac rehabilitation visit
3. Duration beyond 12 weeks without documentation showing medical
necessity as indicated above
4. Services determined to be not reasonable and necessary as stated previously
in this document
Documentation Requirements
The diagnosis of stable angina should be substantiated with a physician history and
physical, a hospital discharge summary, or a physician statement to confirm the
diagnosis. The member’s medical record must contain documentation that fully supports
the medical necessity for cardiac rehabilitation, as it is covered by IHCP. This
documentation includes, but is not limited to, medical records confirming the diagnosis,
evidence of the elements of a cardiac rehabilitation session (e.g., telemetry monitoring
strips), and medical reason for extension of the 12-week limit.
MANAGED CARE
For members enrolled in Risk Based Managed Care (RBMC), providers must contact the
member’s managed care organization (MCO) for more specific guidelines. Refer to the
Provider Manual, Chapter 1, for detailed information about the fee-for-service (FFS),
Primary Care Case Management (PCCM), and Risk Based Managed Care (RBMC)
delivery systems.
IHCP members enrolled in Medicaid Select PCCM receive the same benefit coverage,
and are subject to the same limitations as traditional Medicaid FFS. Refer to the
Medicaid Select Manual for Primary Care Providers and Office Staff for further
information.
01/31/2007 Cardiac Rehabilitation
Medical Policy Manual
59

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Cardiac Rehabilitation
Medical Policy Manual
60
RELATED MEDICAL TOPICS
Medical/Surgical
Hospital Inpatient
Hospital Outpatient
RULES, CITATIONS, AND SOURCES:
405 IAC 5-2-17 – Medically reasonable and necessary service
Indiana Health Coverage Program Provider Manual, Version 5.1
Origination Date: 12/31/2002
Review and
Revision
Reason Date
405-IAC-5-2-17 Medically reasonable and necessary service 07/25/1997
Review Scheduled 01/31/2007
APPLICABLE INDIANAAIM EDITS AND AUDITS:
6096 – The CPT/HCPCS Code Billed is Not Payable According to the PPS
Reimbursement Methodology
6652 – Multiple Surgeries Must be Billed On the Same Claim
6768 – Services Not Covered for Telemedicine

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: CASE MANAGEMENT—PREGNANT WOMEN
DESCRIPTION
Case management (care coordination) for pregnant women is an active, ongoing process
of assisting a member to identify, access, and utilize community resources. Care
coordinators perform prenatal risk assessments to identify Indiana Health Coverage
Programs (IHCP) benefit eligible women whose pregnancies are at risk of preterm birth,
low birth weight, or poor pregnancy outcomes. Case managers coordinate the services to
meet member needs by locating community resources, making appointments for services,
and following up to verify or reschedule appointments. Pregnant women, identified by
the Prenatal Risk Assessment Form as high-risk members due to medical or psychosocial
conditions, may also receive pregnancy care coordination services through the IHCP.
The Prenatal Risk Assessment Form can be located on the IHCP website at
www.indianamedicaid.com, under forms.
This document is intended to serve as a general summary of the Indiana Health Coverage
Programs’ (IHCP) policies regarding this service. More specific information may be
found in the IHCP Provider Manual, program notices, the Indiana Administrative Code
(IAC) or other sources as appropriate. See the “Rules, Citations and Sources” section of
this document for further information.
COVERAGE CRITERIA
IHCP reimbursement is available for care coordination services provided to eligible
pregnant women by any of the following providers.
• A physician licensed by the state
• A registered nurse licensed by the state
• A social worker with a baccalaureate or master’s degree from a school accredited
by the Council on Social Work Education
• A social worker certified by the state
• A dietician registered with the Commission on Dietetic Registration of the
American Dietetic Association and with certified training in pregnancy case
management to combat poor pregnancy outcomes
• A community health worker may perform all care coordination services except
the home encounter portion of the initial assessment and the postpartum or
newborn assessment
01/31/2007 Case Management – Pregnant Women
Medical Policy Manual
61
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
A care coordinator must be certified by and have successfully completed perinatal care
coordination training from a program approved by the Office of Medicaid Policy and
Planning (OMPP). Care coordination includes the following services.
• Case finding
• Risk assessment
• Plan-of-care development
• Coordination
• Referral
• Linkage
• Follow-up monitoring
All eligible pregnant women may receive initial assessment services; however, only those
deemed at risk, according to a prenatal risk assessment form approved by OMPP, are
eligible for additional services. Reassessment and postpartum assessment are not covered
services, when an initial assessment determines a pregnancy is not at risk. However,
services may be covered later in the pregnancy if risk factors from the prenatal risk
assessment form, which were not evident or present during the initial assessment, are
discovered.
Care coordination services are exempt from prior authorization (PA) requirements,
except for members with Primary Care Case Management (PCCM). PCCM pregnancy
care coordination services require authorization by the member’s Primary Medical
Provider (PMP).
Pregnancy care coordination services are designed to prevent preterm births or poor
pregnancy outcomes by facilitating the linking of the pregnant women to all necessary
services, including medical, health promotion, and social services. The following
services, available on a trimester basis, may be provided by physicians, registered nurses,
social workers, and registered dieticians with certified training in pregnancy case
management to combat poor pregnancy outcomes.
• Home visits, including the initial and postpartum home visit
• Referral to social service agencies
• Follow-up activities to ensure services were received
Intensive intervention by the pregnancy care coordinators must occur if the initial
prenatal risk assessment indicates a high-risk condition that could potentially result in a
poor pregnancy outcome. The pregnancy care coordinator should conduct up to two
additional prenatal reassessments and a postpartum assessment, as well as the required
home visit following the delivery to gather vital information about the pregnancy
outcome and assessment of the newborn care needs. The postpartum visit is the only
service that requires a completed copy of the Care Coordination Outcome Report to be
attached to the claim.
01/31/2007 Case Management – Pregnant Women
Medical Policy Manual
62

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Effective for claims with dates of service of November 20, 2001 and later; the IHCP
requires the use of the new Prenatal Care Coordination Outcome Report Form. The new
form permits accumulation of statistical data regarding care coordination services in
Indiana. In the past, the Care Coordination Outcome Report was completed by the care
coordinator and submitted with the postpartum visit billing claim sent to EDS. Prenatal
care coordinators no longer send claims or outcome reports to EDS unless a client is in
Medicaid Select or not enrolled in a Managed Care Organization (MCO). In addition,
Risk-Based Managed Care (RBMC) is mandatory in all counties. The assessment forms
for RBMC members are sent to the MCO in which the member is enrolled. To reduce
reports received by the MCOs the data found on the Care Coordination Outcome Report
has been incorporated into the Combined Initial and Reassessment Prenatal Care
Coordination Assessment Form (CIRPNCCAF) and the Postpartum Assessment Form
(PPAF). All providers are required to complete the CIRPNCCAF and the PPAF. These
forms can be located on the IHCP website at www.indianamedicaid.com
, under forms.
A member may not appear to be high-risk by traditional assessment methods.
Physicians/practitioners may acquire additional information regarding the risk of preterm
delivery by performing the salivary estriol test, which would allow them to take steps to
treat the member. Please refer to the Medical Policy (MP) Fact Sheet regarding
Laboratory Services-Salivary Estriol Test for Preterm Labor Risk Assessment for
additional information.
PRIOR AUTHORIZATION
PA is not required for care coordination services. However, PCCM members require
authorization from the member’s PMP.
MANAGED CARE
For members enrolled in RBMC, providers must contact the member’s MCO for more
specific guidelines. Refer to the Provider Manual, Chapter 1, for detailed information
about the fee-for-service (FFS), PCCM, and RBMC delivery systems.
IHCP members enrolled in Medicaid Select PCCM receive the same benefit coverage,
and are subject to the same limitations as traditional Medicaid FFS. Refer to the
Medicaid Select Manual for Primary Medical Providers and Office Staff for further
information.
BILLING REQUIREMENTS
Services that will be reimbursed by the IHCP include a prenatal risk assessment, one
initial assessment and follow-up, one reassessment and follow-up per trimester (occurring
01/31/2007 Case Management – Pregnant Women
Medical Policy Manual
63

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
after the initial assessment), and one home visit postpartum assessment. The following
codes are used to report these services.
• Healthcare Common Procedure Coding System (HCPCS) codes that are used to
report prenatal care coordination for the initial assessment and re-assessments are
as follows.
• H1000, Prenatal care, at-risk assessment-initial
• H1004, Prenatal care, at-risk enhanced service; antepartum management-
reassessment
• Current Procedural Terminology (CPT) code 99501, Home visit for postnatal
assessment and follow-up care, is used to report the postpartum visit and is the
only service that requires a copy of the Care Coordination Outcome Report form
be completed by the care coordinator and submitted with the claim when billing
for the visit.
RELATED MEDICAL TOPICS
Family Planning
Gynecology−Pelvic Exam under Anesthesia
Laboratory Services-Salivary Estriol Test for Preterm Labor Risk Assessment
Obstetric Care
Surgery−Surgical Services
Transportation Services
RULES, CITATIONS, AND SOURCES
405 IAC 5-11, Case Management Services for Pregnant Women
Indiana Health Coverage Programs Provider Manual 2006
Indiana Health Coverage Programs Provider Bulletin
BT200014-Salivary Estriol Test For Preterm Labor Risk Assessment
Indiana Health Coverage Programs Provider Bulletin
BT200353-HIPAA-Mandated Elimination of Local Codes and Local Code
Modifiers
Indiana Health Coverage Programs Provider Bulletin
BT200604-Assessment Forms and Outcome Report as a Result of State-Wide
Hoosier Healthwise Mandatory MCO Transition
Indiana Health Coverage Programs Provider Banner
BR200207
Indiana Health Coverage Programs Provider Banner
BR200214
Indiana Health Coverage Programs Provider Banner
BR200316
01/31/2007 Case Management – Pregnant Women
Medical Policy Manual
64

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Case Management – Pregnant Women
Medical Policy Manual
65
Indiana Health Coverage Programs Provider Banner
BR200317
Indiana Health Coverage Programs Provider Newsletter
NL200508
Origination Date: 07/01/1991
Revisions and
Review
Reason Date
470 IAC 5-9-25.1
Transferred
Case Management Services For Pregnant
Women
07/01/1991
405 IAC 1-7-24
Repealed 08/24/1997
Care Coordination Services For Pregnant
Women
01/01/1992
405 IAC 5-11 Case Management-Pregnant Women 08/24/1997
405 IAC 5-11-1
Amended
Case Management-Pregnant Women 10/27/1999
405 IAC 5-11-7
Amended
Case Management-Pregnant Women 10/27/1999
Review Scheduled 10/31/2006
APPLICABLE INDIANAAIM EDITS AND AUDITS
6050-Care Coordinator-Reassessment
6051-Care Coordinator-Initial Assessment
6052-Care Coordination Post-Partum Assessment/Outcome
6096-The CPT/HCPCS Code Billed Is Not Payable
6652-Multiple Surgeries Must Be Billed On Same Claim
6768-Services Not Covered For Telemedicine
6800-Care Coordination-Transportation For Home Visits
6801-Care Coordination-Transportation For Home Visits
6802-Care Coordination-Transportation (Postpartum)
6916-Global-Home Uterine Monitoring
6917-Components-Home Uterine Monitoring

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: CHIROPRACTIC SERVICES
DESCRIPTION
Chiropractic services are defined in Indiana Code (IC) 25-10 as the diagnosis and
analysis of any interference with normal nerve transmission and expression, the
procedure preparatory to and complementary to the correction thereof by an adjustment
of the articulations of the vertebral column, its immediate articulation, and includes other
incidental means of adjustments of the spinal column and the practice of drugless
therapeutics.
COVERAGE CRITERIA
Indiana Health Coverage Programs (IHCP) reimbursement is available for covered
services provided by a licensed chiropractor subject to restrictions and limitations set out
in the Indiana Code (IC) 25-10-1-1, 5.5, 13, 14, and the Indiana Administrative Code
(IAC) 405 IAC 5-12 and 407 IAC 3-12. Reimbursement is not available for any
chiropractic services provided outside the scope of IC 25-10-1 and 846 IAC 1-1, or for
any chiropractic service for which federal financial participation is not available.
Effective July 1, 2003, reimbursement is limited to a total of fifty visits and spinal
manipulations or physical medicine treatments per member per rolling twelve month
period. As part of this limitation, reimbursement will be made for no more than five
office visits out of the total fifty visits allowed per member per rolling twelve month
period. New patient office visits are reimbursed once per lifetime, per recipient, per
provider, or provider of the same specialty and in the same practice within a three year
time period. Reimbursement is not available for the following types of extended or
comprehensive office visits.
• New patient detailed
• New patient comprehensive
• Established patient detailed
• Established patient comprehensive
Reimbursement is not available for durable medical equipment (DME) provided by
chiropractors. Additionally, electromyogram (EMG) testing is no longer a covered IHCP
service for chiropractors.
01/31/2007 Chiropractic Services
Medical Policy Manual
66
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Reimbursement is limited to one set of full spinal x-rays per year. Chiropractors must
provide the actual x-ray films previously taken at no cost to IHCP members when
requested. The IHCP will not reimburse for additional x-rays necessitated by the failure
of a practitioner to forward x-rays or related documentation to a chiropractic provider
when requested. Chiropractors are entitled to receive x-rays from other providers at no
charge to the member upon the member’s written request to the other providers and upon
reasonable notice.
Claim payment is limited for chiropractic practitioners (specialty 150) to the Current
Procedural Terminology (CPT) procedure codes listed in tables one through four and the
International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-
CM) diagnosis codes listed in the tables five and six included in this document.
Prior Authorization
Manual muscle testing services require prior authorization according to 405 IAC 5-12-5.
Package C chiropractic services are restricted to five visits and fourteen procedures per
rolling 12 month period. Additional procedures that are medically necessary may be
reimbursed subject to prior authorization according to 407 IAC 3-12-2. There is a 50
treatment limit per rolling 12 month period, which includes no more than five office
visits.
Managed Care
Chiropractic services are self-referral for Risk Based Managed Care (RBMC). Self-
referral services do not require Primary Medical Provider (PMP) authorization.
Benefits under Hoosier Healthwise Package A - Standard Plan - have not been modified.
Reimbursement is limited to a total of 50 visits, which includes no more than five office
visits per recipient per rolling 12 month period.
Benefits under the Hoosier Healthwise Package B - Pregnancy Coverage Only - have not
been modified. Coverage is limited to services related to pregnancy, conditions that may
complicate the pregnancy, or urgent care services. Three principal diagnosis codes were
covered specifically for Package B members effective July 1, 2003 and are listed in table
five in this document. Refer to the Obstetric Care Fact Sheet for more information
regarding billing chiropractic services for Package B members.
Benefits under the Hoosier Healthwise Package C - Children’s Health Insurance
Program - have not been modified. Package C chiropractic services are restricted to five
visits and fourteen procedures per rolling 12 month period. Additional procedures that
are medically necessary may be reimbursed subject to prior authorization. There is a 50
01/31/2007 Chiropractic Services
Medical Policy Manual
67

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
rreatment limit per rolling 12 month period, which includes no more than five office
visits.
Tables one through four identify the procedure codes that can be billed to the IHCP by
chiropractors.
Table 1–Covered IHCP Chiropractic Codes for Office Visits
CPT
Code
Description
99201
Office or other outpatient visit for evaluation and management of new patient,
problems are self-limited or minor
99202
Office or other outpatient visit for evaluation and management of new patient,
presenting problems are of low to moderate severity
99203
Office or other outpatient visit for evaluation and management of new patient,
presenting problems are of moderate severity
99211
Office or other outpatient visit for the evaluation and management of an
established patient, presenting problem(s) are minimal
99212
Office or other outpatient visit for the evaluation and management of an
established patient, presenting problem(s) are self-limited or minor
99213
Office or other outpatient visit for the evaluation and management of an
established patient, presenting problem(s) are low to moderate severity
Table 2–Covered IHCP Chiropractic Codes for Manipulative Treatment
CPT
Code
Description
98940 Chiropractic manipulative treatment (CMT); spinal, one to two regions
98941 Chiropractic manipulative treatment (CMT); spinal, three to four regions
98942 Chiropractic manipulative treatment (CMT); spinal, five regions
98943
Chiropractic manipulative treatment (CMT); extraspinal, one or more regions
Chiropractors may perform laboratory tests, which fall within their scope of
practice for the State of Indiana (IC 25-10-1 and Title 846, which include blood
analysis and urinalysis).
01/31/2007 Chiropractic Services
Medical Policy Manual
68

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 3–Covered IHCP Chiropractic Codes for Radiology
CPT
Code
Description
72010 Radiologic examination, spine, entire, survey study, anteroposterior and lateral
72020 Radiologic examination, spine, single view, specify level
72040 Radiologic examination, spine, cervical; two or three views
72050 Radiologic examination, spine, cervical; minimum of four views
72052 Complete, including oblique and flexion and /or extension studies
72069 Radiologic examination, spine, thoracolumbar, standing (scoliosis)
72070 Radiologic examination, spine, thoracic, two views
72072 Radiologic examination, spine, thoracic, three views
72074 Radiologic examination, spine; thoracic, minimum of four views
72080 Radiologic examination, spine; thoracolumbar, two views
72090
Radiologic examination, spine; scoliosis study, including supine and erect
studies
72100 Radiologic examination, spine; lumbosacral, two or three views
72110 Radiologic examination, spine; lumbosacral, minimum of four views
72114
Radiologic examination, spine; lumbosacral, complete, including bending
views
72120
Radiologic examination, spine, lumbosacral, bending views only, minimum of
four views
72170 Radiologic examination, pelvis; one or two views
72190 Radiologic examination, pelvis; complete, minimum of three views
72200 Radiologic examination, sacroiliac joints; less than three views
72202 Radiologic examination, sacroiliac joints; three or more views
72220 Radiologic examination, sacrum and coccyx, minimum of two views
73000 Radiologic examination; clavicle, complete
73010 Radiologic examination; scapula, complete
73020 Radiologic examination, shoulder; one view
73030 Radiologic examination, shoulder; complete, minimum of two views
73050
Radiologic examination; acromioclavicular joints, bilateral, with or without
weighted distraction
73060 Radiologic examination; humerus, minimum of two views
73070 Radiologic examination, elbow; two views
73080 Radiologic examination, elbow; complete, minimum of three views
73090 Radiologic examination; forearm, two views
73100 Radiologic examination, wrist; two views
73110
Radiologic examination, wrist; complete, minimum of three views
73120 Radiologic examination, hand; two views
73130
Radiologic examination, hand; minimum of three views
01/31/2007 Chiropractic Services
Medical Policy Manual
69
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 3–Covered IHCP Chiropractic Codes for Radiology
CPT
Code
Description
73140
Radiologic examination, finger (s), minimum of two views
73500
Radiologic examination, hip, unilateral; one view
73510
Radiologic examination, hip, unilateral; complete, minimum of two views
73520
Radiologic examination, hips, bilateral, minimum of two views of each
hip, including anteroposterior view of pelvis
73550
Radiologic examination, femur, two views
73560
Radiologic examination, knee; one or two views
73562
Radiologic examination, knee; three views
73564
Radiologic examination, knee; complete, four or more views
73565 Radiologic examination, knee; both knees, standing, anteroposterior
73590
Radiologic examination; tibia and fibula, two views
73600
Radiologic examination, ankle; two views
73610
Radiologic examination, ankle; complete, minimum of three views
73620
Radiologic examination, foot; two views
73630
Radiologic examination, foot; complete, minimum of three views
73650
Radiologic examination; calcaneus, minimum of two views
73660
Radiologic examination; toe(s), minimum of two views
Table 4–Covered IHCP Chiropractic Codes for Medicine Services
CPT
Code
Description
95831
Muscle testing, manual (separate procedure) with report; extremity (excluding
hand) or trunk
95832
Muscle testing, manual (separate procedure) with report; hand, with or without
comparison with normal side
97010 Application of a modality to one or more areas; hot or cold packs
97012 Application of a modality to one or more areas; traction, mechanical
97014
Application of a modality to one or more areas; electrical stimulation
(unattended)
97016 Application of a modality to one or more areas; vasopneumatic devices
97018 Application of a modality to one or more areas; paraffin bath
97020
Application of a modality to one or more areas; microwave
97022 Application of a modality to one or more areas; whirlpool
97024 Application of a modality to one or more areas; diathermy
97026 Application of a modality to one or more areas; infrared
97028 Application of a modality to one or more areas; ultraviolet
97032
Application of modality to one or more areas; electrical stimulation (manual),
each 15 minutes
97033
Application of modality to one or more areas; iontophoresis, each 15 minutes
01/31/2007 Chiropractic Services
Medical Policy Manual
70

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 4–Covered IHCP Chiropractic Codes for Medicine Services
CPT
Code
Description
97034
Application of modality to one or more areas; contrast baths, each 15
Minutes
97035
Application of modality to one or more areas; ultrasound, each 15
Minutes
97036
Application of modality to one or more areas; Hubbard tank, each 15
Minutes
97039
Unlisted modality (specify type and time if constant attendance)
97110
Therapeutic procedure, one or more areas, each 15 minutes; therapeutic
Exercises to develop strength and endurance, range of motion and flexibility.
97112
Therapeutic procedure, one or more areas, each 15 minutes; neuromuscular
reeducation of movement, balance, coordination, kinesthetic sense, posture,
and/or proprioception for sitting and/or standing activities
97113
Therapeutic procedure, one or more areas, each 15 minutes; aquatic therapy
with therapeutic exercises
97116
Therapeutic procedure, one or more areas, each 15 minutes; gait training
(includes stair climbing)
97124
Therapeutic procedure, one or more areas, each 15 minutes; massage,
including effleurage, petrissage and/or tapotement (stroking, compression,
percussion)
97139
Therapeutic procedure, one or more areas, each 15 minutes; unlisted therapeutic
procedure (specify)
97140
Manual therapy techniques (e.g. mobilization/manipulation, manual
lymphatic drainage, manual traction), one or more regions, each 15
minutes
Tables five and six identify diagnosis codes appropriate for chiropractic services billed to
the IHCP. Table five lists principal diagnosis codes and table six lists secondary
diagnosis codes.
01/31/2007 Chiropractic Services
Medical Policy Manual
71

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
739.3
Nonallopathic lesions, not elsewhere classified – Lumbar region
739.4 Nonallopathic lesions, not elsewhere classified – Sacral region
739.5 Nonallopathic lesions, not elsewhere classified – Pelvic region
739.6 Nonallopathic lesions, not elsewhere classified – Lower extremities
739.7 Nonallopathic lesions, not elsewhere classified – Upper extremities
739.8 Nonallopathic lesions, not elsewhere classified – Rib cage
Table 5– Diagnosis Codes for Chiropractic Services, Principal ICD-9-CM Codes
Principal ICD-9-CM Codes
Dia
g
nosis
Codes
Description
646.93* Unspecified complication of pregnancy, antepartum condition or complication
648.73*
Bone and joint disorders of the back, pelvis, and lower limbs, antepartum
condition or complication
648.93*
Other current conditions classifiable elsewhere, antepartum condition or
complication
739.0 Nonallopathic lesions, not elsewhere classified – Head region
739.1 Nonallopathic lesions, not elsewhere classified – Cervical region
739.2 Nonallopathic lesions, not elsewhere classified – Thoracic region
*Limited to medically necessary services rendered to Package B members
effective July 1, 2003.
Table 6–Diagnosis Codes for Chiropractic Services, Secondary ICD-9-CM Codes
Secondary ICD-9-CM Codes
Dia
g
nosis
Codes
Description
307.81 Tension headache
333.83 Spasmodic torticollis
346.00 Classical migraine without mention of intractable migraine
346.01 Classical migraine with intractable migraine, so stated
346.10 Common migraine without mention of intractable migraine
346.11 Common migraine with intractable migraine, so stated
346.20 Variants of migraine without mention of intractable migraine
346.21 Variants of migraine with intractable migraine, so stated
346.80 Other forms of migraine without mention of intractable migraine
346.81 Other forms of migraine with intractable migraine, so stated
346.90 Migraine, unspecified, without mention of intractable migraine
346.91 Migraine, unspecified, with intractable migraine, so stated
353.0 Brachial plexus lesions
01/31/2007 Chiropractic Services
Medical Policy Manual
72

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 6–Diagnosis Codes for Chiropractic Services, Secondary ICD-9-CM Codes
Secondary ICD-9-CM Codes
Dia
g
nosis
Codes
Description
353.1 Lumbosacral plexus lesions
353.2 Cervical root lesions, not elsewhere classified
353.3 Thoracic root lesions, not elsewhere classified
353.4 Lumbosacral root lesions, not elsewhere classified
353.8 Other nerve root and plexus disorders
353.9 Unspecified nerve root and plexus disorder
354.4 Causalgia of upper limb
354.8 Other mononeuritis of upper limb
354.9 Mononeuritis of upper limb, unspecified
719.40 Pain in joint, site unspecified
719.48 Pain in joint, other specified sites
719.49 Pain in joint, multiple sites
720.0 Ankylosing spondylitis
720.1 Spinal enthesopathy
721.0 Cervical spondylosis without myelopathy
721.2 Thoracic spondylosis without myelopathy
721.3 Lumbosacral spondylosis without myelopathy
721.6 Anklyosing vertebral hyperostosis
721.7 Traumatic spondylopathy
721.90 Spondylosis of unspecified site without mention of myelopathy
722.0 Displacement of cervical intervertebral disc without myelopathy
722.10 Lumbar intervertebral disc without myelopathy
722.11 Thoracic intervertebral disc without myelopathy
722.2 Displacement of intervertebral disc, site unspecified, without myelopathy
722.30 Schmorl’s nodes, unspecified region
722.31 Schmorl’s nodes, thoracic region
722.32 Schmorl’s nodes, lumbar region
722.4 Degeneration of cervical intervertebral disc
722.51 Degeneration of thoracic or thoracolumbar intervertebral disc
722.52 Degeneration of lumbar or lumbosacral intervertebral disc
722.6
Degeneration of intervertebral disc, site unspecified
722.80
Postlaminectomy syndrome, unspecified region
722.81
Postlaminectomy syndrome, cervical region
722.82
Postlaminectomy syndrome, thoracic region
722.83
Postlaminectomy syndrome, lumbar region
722.90
Other and unspecified disc disorder, unspecified region
01/31/2007 Chiropractic Services
Medical Policy Manual
73

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 6–Diagnosis Codes for Chiropractic Services, Secondary ICD-9-CM Codes
Secondary ICD-9-CM Codes
Dia
g
nosis
Codes
Description
722.91
Other and unspecified disc disorder, cervical region
722.92
Other and unspecified disc disorder, thoracic region
722.93
Other and unspecified disc disorder, lumbar region
723.0
Spinal stenosis in cervical region
723.1
Cervicalgia
723.2
Cervicocranial syndrome
723.3
Cervicobrachial syndrome (diffuse)
723.4
Brachial neuritis or radiculitis NOS
723.5
Torticollis, unspecified
723.8
Other syndromes affecting cervical region
723.9
Unspecified musculoskeletal disorders and symptoms referable to neck
724.00
Spinal stenosis, unspecified region
724.01 Spinal stenosis, thoracic region
724.02 Spinal stenosis, lumbar region
724.09 Spinal stenosis, other
724.1 Pain in thoracic spine
724.2 Lumbago
724.3 Sciatica
724.4 Thoracic or lumbosacral neuritis or radiculitis, unspecified
724.5 Backache, unspecified
724.6 Disorders of sacrum
724.70 Unspecified disorder of coccyx
724.79 Disorders of coccyx, other
724.8 Other symptoms referable to back
724.9 Other unspecified back disorders
728.71 Plantar fascial fibromatosis
728.85 Spasm of muscle
729.1 Myalgia and myositis, unspecified
729.4 Fasciitis, unspecified
732.0 Juvenile osteochondrosis of spine
737.0
Adolescent postural kyphosis
737.10
Kyphosis (acquired) (postural)
737.12
Kyphosis, postlaminectomy
737.19
Kyphosis, other
737.20
Lordosis (acquired) (postural)
737.21
Lordosis postlaminectomy
737.22
Other postsurgical lordosis
737.29
Lordosis, other
737.30
Scoliosis [and kyphoscoliosis], idiopathic
01/31/2007 Chiropractic Services
Medical Policy Manual
74

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 6–Diagnosis Codes for Chiropractic Services, Secondary ICD-9-CM Codes
Secondary ICD-9-CM Codes
Dia
g
nosis
Codes
Description
737.31
Resolving infantile idiopathic scoliosis
737.32
Progressive infantile idiopathic scoliosis
737.34
Thoracongenic scoliosis
737.39
Kyphoscoliosis and scoliosis – other
737.40
Curvature of spine, unspecified
737.41
Curvature of spine associated with other conditions, kyphosis
737.42
Curvature of spine associated with other conditions, lordosis
737.43
Curvature of spine associated with other conditions, scoliosis
737.8
Other curvatures of spine
737.9
Unspecified curvature of spine
738.4
Acquired spondylolisthesis
754.1
Certain congenital musculoskeletal deformities of sternocleidomastoid muscle
754.2
Certain congenital musculoskeletal deformities of spine
756.11
Spondylolysis, lumbrosacral region
756.12
Spondylolisthesis
784.0
Headache
839.00
Cervical vertebra, closed – cervical vertebra, unspecified
839.01
Cervical vertebra, closed – first cervical vertebra
839.02
Cervical vertebra, closed – second cervical vertebra
839.03
Cervical vertebra, closed – third cervical vertebra
839.04
Cervical vertebra, closed – fourth cervical vertebra
839.05
Cervical vertebra, closed – fifth cervical vertebra
839.06
Cervical vertebra, closed – sixth cervical vertebra
839.07
Cervical vertebra, closed – seventh cervical vertebra
839.08
Cervical vertebra n, closed – multiple cervical vertebra
839.20
Lumbar vertebra, closed
839.21
Thoracic vertebra, closed
846.0
Sprains and strains of sacroiliac region, lumbosacral (joint) (ligament)
846.1
Sprains and strains of sacroiliac region, sacroiliac ligament
846.2
Sprains and strains of sacroiliac region, sacrospinatus (ligament)
846.3
Sprains and strains of sacroiliac region, sacrotuberous (ligament)
846.8
Sprains and strains of sacroiliac region, other specified sites of sacroiliac
region
846.9
Sprains and strains of sacroiliac region, unspecified site of sacroiliac region
847.0
Sprains and strains of other and unspecified parts of back, neck
847.1
Sprains and strains of other and unspecified parts of back, thoracic
847.2
Sprains and strains of other and unspecified parts of back, lumbar
847.3
Sprains and strains of other and unspecified parts of back, sacrum
847.4
Sprains and strains of other and unspecified parts of back, coccyx
847.9
Sprains and strains of other and unspecified parts of back, unspecified site of
back
907.3
Late effect of injury to nerve root(s), spinal plexus(es), and other nerves of
trunk
01/31/2007 Chiropractic Services
Medical Policy Manual
75

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 6–Diagnosis Codes for Chiropractic Services, Secondary ICD-9-CM Codes
Secondary ICD-9-CM Codes
Dia
g
nosis
Codes
Description
953.0
Injury to nerve roots and spinal plexus, cervical root
953.1
Injury to nerve roots and spinal plexus, dorsal root
953.2
Injury to nerve roots and spinal plexus, lumbar root
953.3
Injury to nerve roots and spinal plexus, sacral root
953.4
Injury to nerve roots and spinal plexus, brachial plexus
953.5
Injury to nerve roots and spinal plexus, lumbrosacral plexus
956.0
Injury to peripheral nerve(s) of pelvic girdle and lower limb, sciatic nerve
956.1
Injury to peripheral nerve(s) of pelvic girdle and lower limb, femoral nerve
956.2
Injury to peripheral nerve(s) of pelvic girdle and lower limb, posterior tibial
nerve
956.3
Injury to peripheral nerve(s) of pelvic girdle and lower limb, peroneal nerve
956.4
Injury to peripheral nerve(s) of pelvic girdle and lower limb, cutaneous
sensory nerve, lower limb
956.5
Injury to peripheral nerve(s) of pelvic girdle and lower limb, other specified
nerve(s) of pelvic girdle and lower limb
956.8
Injury to peripheral nerve(s) of pelvic girdle and lower limb, multiple nerves
of pelvic girdle and lower limb
956.9
Injury to peripheral nerve(s) of pelvic girdle and lower limb, unspecified nerve
of pelvic girdle and lower limb
RELATED MEDICAL TOPICS
Consultation – Second Opinions
Diagnostic Studies
Physical Rehabilitation Services
Medical Supplies and Equipment
Out-of-State Services
Package B
Obstetric Care
RULES, CITATIONS, AND SOURCES
IC 25-10-Chiropractors
405 IAC 5-12-Chiropractic Services
407 IAC 3-12-Chiropractic Services
846 IAC-Chiropractic Examiners
Indiana Health Coverage Programs Provider Bulletins
BT200323
BT200329
Indiana Health Coverage Programs Provider Newsletter
NL2004099
State Medicaid Plan - Chiropractors’ Services
01/31/2007 Chiropractic Services
Medical Policy Manual
76

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Origination Date: 12/31/2000
Revisions and
Reviews
Reasons Date
405 IAC 5-12-1 Chiropractic Services 08/24/1997
405 IAC 5-5 Out-of-State services 08/24/1997
405 IAC 5-12-2 Office Visits
08/24/1997
modified
05/01/2003
405 IAC 5-12-3 Chiropractic X-ray Services
08/24/1997
modified
05/01/2003
405 IAC 5-12- 4 Laboratory Services
08/24/1997
modified
05/01/2003
405 IAC 5-12-6
Repealed 5/1/2003
Electromyography Services 08/24/1997
405 IAC 5-12-7 Durable Medical Equipment
08/24/1997
modified
05/01/2003
Indiana Health
Coverage
Provider Bulletins
BT200323 and
BT200329
Changes in Chiropractic Services
Chiropractic Service Limitations
07/01/2003
Indiana Health
Coverage Provider
Newsletter
NL2004099
Addition of Pregnancy Diagnosis
Codes for Package B Members
04/30/2005
01/31/2007 Chiropractic Services
Medical Policy Manual
77
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Chiropractic Services
Medical Policy Manual
78
APPLICABLE INDIANAAIM EDITS AND AUDITS
4070 - Select Radiological Procedures Billed by a Chiropractor (inactive)
4071 - Lab Services Billed by Chiropractor Require Review (inactive)
6098 - Chiropractic Services Limited to Procedure and Diagnosis Codes Identified
6100 - Max of 50 Chiropractic Therapeutic Physical Medicine Treatments
6101 - Chiropractic Restrictive Office Visits Codes (NP)
6102 - Chiropractic Office Visits Limited to 5 Per Year
6103 - Component Spinal X-Rays vs Full Spine X-ray
6104 - DME Rental from Chiropractor of More Than 1 Month
6105 - One Full Spine X-Ray Per Year for Chiropractor
6106 - Component Spinal X-Rays Greater Than $95.00/Year
6107 - Full Spinal X-Ray Payable at Reduced Amount When Component Previously
Paid
6108 - Global Payable at a Reduced Fee When Components Paid
6111 - Chiropractic Office Visits Limited to Five per Year
6112 - Maximum of 14 Chiropractic Therapeutic Physical Medicine Treatments Per
Calendar Year
6122 - Chiropractic Therapeutic Physical Medicine Treatments 15 Through 50 Require
Prior Authorization

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: CLINIC SERVICES—FQHC AND RURAL HEALTH
CLINIC SERVICES
DESCRIPTION
Federally Qualified Health Clinics (FQHC) and Rural Health Centers (RHC) are facilities
for physical examination and treatment of ambulatory patients who are not hospitalized
where preliminary diagnosis is made and treatment is provided. Often, a RHC may
provide services to a medically underserved area.
This document is intended to serve as a general summary of the Indiana Health Coverage
Programs’ (IHCP) policies regarding this service. More specific information may be
found in the IHCP Provider Manual, program notices, the Indiana Administrative Code
(IAC) or other sources as appropriate. See the “Rules, Citations, and Sources” section of
this document for further information.
COVERAGE CRITERIA
Reimbursement is available for IHCP members seeking medical care in rural health
clinics and other qualified health clinics. The provider may be a physician, nurse
practitioner, physician assistant, clinical psychologist, or clinical social worker. A valid
encounter visit is described as a face-to-face encounter between a clinic patient and a
provider, as noted above.
IHCP reimbursement is available for services and supplies incidental to such services,
which would otherwise be covered if furnished by a physician or an incident to a
physician’s services. Services to a home bound individual are only available in the case
of those FQHCs located in an area with a shortage of home health agencies as determined
by Medicaid. Any other ambulatory service included in the IHCP state plan is considered
a covered FQHC service if the FQHC offers such a service. FQHC services are defined
the same as services provided by RHCs.
FQHCs receive funds through the Public Health Services and receive FQHC status from
the Centers for Medicare and Medicaid Services (CMS). To enroll as an FQHC with the
IHCP, the CMS letter granting the FQHC status must be forwarded to EDS Provider
Enrollment with a completed application. RHCs receive their Medicare designation
01/31/2007 Clinic Services – FQHC and Rural Health Clinic Services
79
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
through the CMS and must contact the Indiana State Department of Health (ISDH) to
receive RHC status as an IHCP provider.
IHCP reimbursement is limited to one encounter per IHCP member, per provider, per day
unless the diagnosis differs. Should a member visit an office twice on the same day with
a different diagnosis, the second claim can be submitted. This policy does not allow a
provider to bill multiple claims for one visit with multiple diagnoses by separating the
diagnoses on different claims. Documentation includes, but is not limited to, the
following.
• Visits performed at separate times of the day that indicate the times and
reasons for each visit on the face of the claim or on a claim attachment
• Visits provided by different providers on the same day that indicate the type
of provider that rendered each visit and denote which practitioner treated
which diagnosis
• Documentation in the medical record supports the medical reason for an
additional visit and includes presenting symptoms or reason for the visit, onset
of symptoms and treatment rendered
• Documentation that the diagnosis for each encounter is different
IHCP reimbursement is also available for services and supplies incidental to such
services as would otherwise be covered if furnished by a physician or as an incident to a
physician’s services. Services such as drawing blood, collecting urine specimens,
performing laboratory tests, taking x-rays, filling and dispensing prescriptions, or
providing optician services do not constitute encounters. These services can be included
in the encounter reimbursement when performed in conjunction with the office visit to a
valid provider. These services are not reimbursable through claim submission if
performed without a face-to-face visit to a valid provider.
FQHCs and RHCs can provide preventive services and encounters, care coordination,
and Health Watch services. Refer to the IHCP Provider Manual or the Health Watch
Early and Periodic Screening, Diagnostic & Treatment Program supplemental provider
manual for further information about those services.
The valid FQHC/RHC encounter code list is reviewed annually and is published on the
Myers and Stauffer [the rate setting contractor for the Office of Medicaid Policy and
Planning (OMPP)] Website at http://www.mslcindy.com
.
PRIOR AUTHORIZATION
FQHCs and RHCs are subject to the same prior authorization (PA) requirements for
IHCP services as Traditional Medicaid. The provider may contact the PA department for
specific information regarding requirements and guidelines. Additionally, further
information can be located in the IHCP Provider Manual, Chapter 6, Prior Authorization.
01/31/2007 Clinic Services – FQHC and Rural Health Clinic Services
80
Medical Policy Manual
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MANAGED CARE
An FQHC/RHC can participate with a managed care organization (MCO). The MCO
provider contract must specify the contractual arrangements to ensure that the FQHC or
RHC is reimbursed for services.
Claims for members in a Risk-Based Managed Care (RBMC) plan should continue to be
billed in the manner applicable to the specific MCO. The T1015 encounter code should
not be included on these claims. All MCO claims will be reconciled to the provider
specific Prospective Payment System (PPS) rate on a quarterly basis by the rate setting
contractor and settlements will be made at that time. These reconciliations will continue
until such time that the MCOs adapt the systems to the PPS methodology.
Claims submitted for members currently in the Medicaid Select Managed Care will
continue to include all Primary Medical Provider (PMP) information on the CMS-1500
claim form and the 837P electronic transaction. PMP information is required on the
claim in the following fields: 17, PMP name; 17a, PMP’s nine-digit IHCP provider
number; and 19, the PMP’s two-digit certification code.
BILLING REQUIREMENTS
All FQHC and RHC facilities are required to submit claims using the appropriate HCPCS
code and HCPCS code T1015, Clinic visit/encounter, all inclusive. HCPCS code T1015
is reimbursed at a facility-specific PPS rate determined by the rate-setting contractor for
the specific FQHC/RHC provider enrollment file.
For claims submitted with a Place of Service 50, Federally Qualified Health Center, 72,
Rural Health Clinic; 11, Office; 12, Home; 31, Skilled Nursing Facility; or 32, Nursing
Facility, providers must use both the T1015 encounter code and the appropriate CPT or
HCPCS procedure code. If the claim contains both T1015 and one of the allowable
procedure codes from a list of valid CPT/HCPCS codes approved by OMPP, the CPT or
HCPCS code will correctly deny. The T1015 encounter code will be reimbursed
according to the usual and customary charge as established in the provider’s file.
If the CPT or HCPCS code billed is not on the list of allowable procedure codes from the
encounter criteria for place of service 50, 72, 11, 12, 31, or 32 the claim will deny.
Services provided at these place of service locations that are not valid encounters with the
appropriate provider, such as injections performed by a nurse without a corresponding
visit to satisfy the valid encounter definition, should instead be reflected in the facility’s
end of year cost report.
Claims submitted with a Place of Service 20-26, described in Table 1 on the next page,
will reimburse each line item detail at the current rate for that CPT/HCPCS code. It is
not necessary to include the T1015 encounter code on claims with Place of Service 20-
26. These services are not considered FQHC/RHC services by a valid provider.
01/31/2007 Clinic Services – FQHC and Rural Health Clinic Services
81
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 1 – Place of Service Codes for Other Than
RHC/FQHC Setting
Code Description
20 Urgent Care Center
21 Inpatient Hospital
22 Outpatient Hospital
23 Emergency Room – Hospital
24 Ambulatory Surgical Center
25 Birthing Center
26 Military Treatment Facility
Only one valid encounter per IHCP member, per provider, per day is allowed unless the
diagnosis code differs. Valid encounters with differing diagnosis codes for a member
that exceeds the allowed one encounter per day can be submitted to the IHCP for manual
processing.
All Third Party (TPL), patient liability, and co-payments will continue to apply as
appropriate. Previous TPL payments and spenddown will be applied to the total amount
due. All Medicare crossover claims are excluded from the PPS logic as well as the
crossover reimbursement methodology, and will continue to pay co-insurance and
deductible amounts.
DENTAL CLAIMS
Dental claims for FQHC/RHC are to be billed on a dental claim form using Current
Dental Terminology (CDT) codes. The T1015 encounter code should not be included on
the dental claim form. Dental claims will be reconciled to the provider-specific PPS rate.
Reconciliation of claims will occur until a national dental code is established to act as an
all inclusive code on the dental claim form.
RELATED MEDICAL TOPICS
Evaluation and Management Services
Family Planning
Nursing Services
RULES, CITATIONS, AND SOURCES
405 IAC 5-16-5 Rural health clinics and federally qualified health clinics; reimbursement
405 IAC 5-16-6 Free-standing clinics and surgical centers; limitations
Indiana State Department of Public Welfare Medical Policy Manual 1991
Indiana Health Coverage Programs Provider Bulletins
BT200318, Change in Method of Filing Claims
01/31/2007 Clinic Services – FQHC and Rural Health Clinic Services
82
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Clinic Services – FQHC and Rural Health Clinic Services
Medical Policy Manual
83
BT200357, Update of FQHC/RCH Valid Encounters
BT200340, Hoosier Healthwise Mandatory MCO Transition
Indiana Health Coverage Programs Provider Manual
Version 5.1, March, 2005
Origination date: 12/31/2000
Revisions and Reviews Reason Date
Indiana State Department of
Public Welfare Medical Policy
Manual 1991
Rural Health Clinics 07/1/1991
470 IAC 5-8-11
Transferred
Rural Clinics, FQHC 07/1/1991
405 IAC 1-6-11
Repealed 8/24/97
Home Health Agency, Clinic, FQHC,
and Laboratory Services
01/1/1992
405 IAC 5-16-5 RHC and FQHC; reimbursement 08/24/1997
405 IAC 5-16-6
Free-standing clinics and surgical
centers; limitations
08/24/1997
Review Scheduled 10/31/2006
APPLICABLE INDIANAAIM EDITS AND AUDITS:
4124 – FQHC and RHC Services Must be Billed According to the PPS reimbursement
methodology
6096 – The CPT/HCPCS code billed is not a valid encounter

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: CLINICAL TRIALS
DESCRIPTION
On September 19, 2000, Medicare established guidelines to cover the routine costs of
approved clinical trials, as well as reasonable and necessary items and services used to
diagnose and treat complications arising from beneficiaries’ participation in clinical
trials. In 2000, the Office of Medicaid Policy and Planning (OMPP) began following
Medicare’s guidelines to reimburse for routine costs and complications of clinical trials.
In 2003 the IHCP 1) developed a definition of experimental and investigational services;
2) defined investigational and experimental procedures, and routine costs in the context
of clinical trials; and 3) adopted IHCP policy for coverage of clinical trials based on
Medicare policy.
SUMMARY OF CURRENT POLICY
The IHCP covers the routine costs of approved clinical trials as well as reasonable and
necessary items and services used to prevent complications and to diagnose and treat
complications arising from participation in all clinical trials. Routine costs include items
and services that would otherwise be covered by the IHCP if they were not provided in a
clinical trial. The investigational item(s) or service(s) of the clinical trial are experimental
or investigational, are not considered routine costs involved in the trial and are not
covered by the IHCP.
RELATED MEDICAL TOPICS
Experimental services
Investigational services
Investigational new drug
Investigational device exemption
Wegener’s Granulomatosis
Crohn’s Disease
01/31/2007 Clinical Trials
84
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
RULES, CITATIONS, SOURCES
§1862 (a)(1)(E) of the Social Security Act
Coverage Issues Manual 30-1, Clinical Trials
IC 25-22.5-1-2.1
Anthem Blue Cross Blue Shield of Kentucky – Definition of
Experimental/Investigational Services
Washington State Administrative Code WAC 284-44-043 (2) – Disclosure Requirements
for Experimental or Investigational Services
Blue Cross of Oregon – Definition of Experimental or Investigational Services
21 CFR312 – Investigational New Drug Application
21 CFR812 – Investigational Device Exemption,
21 CFR 405.201 and 405.203 - Class I-III devices
HCFA Fact Sheet – Medicare Coverage Routine Costs of Beneficiaries in Clinical Trials
ORIGINATION, REVISION, AND REVIEWS
Origination Date: 10/31/04
Revisions and Reviews
Reason Date
Medical Policy Administrative
Report – Clinical Trials
Clinical Trials Policy – Approved by OMPP 10/29/04
APPLICABLE AIM EDITS AND AUDITS
None
01/31/2007 Clinical Trials
85
Medical Policy Manual
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
EXPERIMENTAL AND INVESTIGATIONAL SERVICES
The terms “experimental” and “investigational” are used interchangeably, are described
under the same definition, and have the same coverage guidelines. Medicare defines an
experimental item or service as for “research use only” or for “investigational use only.”
Section 1862(a)(1) of the Social Security Act states experimental services are denied for
coverage as not reasonable and necessary because they are not proven safe and effective.
DEFINITION OF EXPERIMENTAL AND/ OR INVESTIGATIONAL SERVICES
Experimental or Investigational Services: Procedures, treatments, supplies, devices,
equipment, drugs, biologicals, hospitalizations, or medical services (hereinafter called
services) which are:
1. Not of proven benefit for the particular diagnosis or treatment of the member’s
particular condition. (This would include services currently covered by the IHCP for
specific diagnoses, but that are being tested for use outside the scope of coverage);
2. Not yet approved by the U.S. Food and Drug Administration for other than
experimental, investigational, or clinical trials testing;
3. Provided or performed in special settings for research purposes or under a controlled
environment or clinical protocol or subject of a Phase I, II, or III clinical trial;
4. Not generally recognized in peer-reviewed medical literature as having a definitive
positive effect on health outcomes (i.e., the service’s medical benefits do not
outweigh any harmful effects); or
5. Not recognized by a federal government agency or a national professional medical
society as effective or appropriate for the particular diagnosis or treatment of the
member’s particular condition.
CLINICAL TRIALS - DEFINED
A clinical trial is a research study in human volunteers to answer specific health
questions. Clinical trials are performed to find new ways of using known treatments and
to determine whether new drugs, devices, and procedures are both safe and effective for
general use. Carefully conducted clinical trials are the fastest and safest way to
determine what new treatments will be effective in improving the health of the public.
Clinical trials are conducted in four phases.
• Phase I – Researchers test a new drug, treatment, or device in a small group for the
first time to evaluate safety, determine dosage range, and identify side effects.
01/31/2007 Clinical Trials
86
Medical Policy Manual
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• Phase II – Expands the study to a larger group of people to further evaluate safety
and effectiveness.
• Phase III – A still larger group is studied to confirm effectiveness, monitor side
effects, and compare to commonly used treatments.
• Phase IV – Studies performed after drug, treatment, or device is marketed. Study is
continued to collect information about the effect of the drug, treatment, or device in
various populations and to identify any side effects associated with long-term use.
All clinical trials must have peer-reviewed, written protocol with clear definitions and
statements that describe the study objectives and trial criteria. The trial criteria should
include specifications of study size, study duration, statistical requirements and
endpoints, patient eligibility criteria, diagnostic protocol, and treatment protocol. Every
clinical trial in the U.S. must be approved and monitored by a federally registered
Institutional Review Board (IRB). The IRB is an independent committee of physicians,
statisticians, community advocates, and others that ensures a clinical trial is ethical and
that the rights of study participants are protected.
A clinical trial is sponsored or funded by an organization or individual that initiates, but
does not actually conduct, the investigation. Sponsors can include physicians, medical
institutions, foundations, pharmaceutical companies, and federal agencies. Some of the
foremost federal agencies that sponsor clinical trials are the National Institute of Health
(NIH), the Department of Defense (DOD), the Department of Veteran Affairs (VA), the
U.S. Food and Drug Administration (FDA), and the Centers for Disease Control (CDC).
EXPERIMENTAL AND INVESTIGATIONAL PRODUCTS AND PROCEDURES
RELATED TO CLINICAL TRIALS
Drugs and Devices
In order for a drug or device to be tested in a clinical trial, it must be approved by the
FDA and/or an IRB. Most drugs must have an approved Investigational New Drug
(IND) Application to qualify for clinical trials (see 21 CFR 312.2 for exemptions).
Medical devices must have an approved Investigational Device Exemption (IDE) to
qualify for clinical trials. If the device poses a significant risk, meaning that it presents a
potential for serious risk to the health, safety, or welfare of a clinical trial subject, it must
be approved by the FDA before studies can begin. If the device does not pose a
significant risk, the study can be approved by an IRB. Table 1 defines IND, IDE, and
related terms.
Procedures
Procedures are not regulated by the FDA or other guiding entities. In order for a
procedure to be tested in a clinical trial, an organization must conclude that the procedure
is appropriate for further evaluation in a clinical trial and agree to sponsor the research.
The principal investigator must write protocol for the trial and submit it to an IRB for
approval and monitoring during the trial.
01/31/2007 Clinical Trials
87
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 1 – Clinical Trial Terms
TERM SOURCE DEFINITION
Experimental
drug
Clinicaltrials.go
v
A drug that is not FDA licensed for use in humans, or as a
treatment for a particular condition.
Investigation
al New Drug
(IND)
21 CFR 312.3 IND means a new drug or biological drug that is used in a
clinical investigation. The term also includes a biological
product that is used in vitro for diagnostic purposes.
IND
application
21 CFR 312.1 An investigational new drug for which an IND application is in
effect in accordance with this part is exempt from the
premarketing approval requirements that are otherwise
applicable and may be shipped lawfully for the purpose of
conducting clinical investigations of that drug.
IND
exemption
21 CFR 312.2 Certain drugs can be lawfully marketed or studied in the U.S.
without having to be approved by the FDA through an IND
application. Some examples include drugs that are not
intended to be reported to the FDA with a new indication for
use, the drug is already marketed in the US and the
investigation is not going to significantly change the
advertising of the drug, etc. Refer to the CFR for further
details.
Investigation
al Device
21 CFR 812.3
(g)
Investigational device means a device, including a transitional
device, which is the object of an investigation.
Investigation
al Device
Exemption
21 CFR 812.1 An approved investigational device exemption permits a
device that otherwise would be required to comply with a
performance standard or have premarket approval to be
shipped lawfully for the purpose of conducting investigations
of that device.
Experimental
/
Investigation
al (Category
A) Devices
42 CFR
405.201
The FDA assigns devices with an FDA-approved IDE to one
of two categories: A&B.
Category A includes innovative devices believed to be in Class
III for which “absolute risk” of the device type has not been
established (that is initial questions of safety and effectiveness
have not been resolved and the FDA is unsure whether the device
type can be safe and effective)
Non-
experimental
/
Investigation
al (Category
B) Devices
42 CFR
405.201
The FDA assigns devices with an FDA-approved IDE to one
of two categories: A&B.
Category B includes devices believed to be in Class I or Class
II, or a device believed to be in Class III for which the
incremental risk is the primary risk in question (that is,
underlying questions of safety and effectiveness of the device
type have been resolved), or it is known that the device type
can be safe and effective because, for example, other
manufacturers have obtained FDA approval for that device
type.
Class I 42 CFR Devices for which general controls of the Food, Drug, and
01/31/2007 Clinical Trials
88
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Clinical Trials
89
Table 1 – Clinical Trial Terms
TERM SOURCE DEFINITION
405.201 Cosmetics Act, such as adherence to good manufacturing
practice regulations, are sufficient to provide a reasonable
assurance to safety and effectiveness.
Class II 42 CFR
405.201
Devices that, in addition to general controls, require special
controls, such as performance standards or postmarket
surveillance, to provide a reasonable assurance of safety and
effectiveness.
Class III 42 CFR
405.201
Devices that cannot be classified into Class I or Class II
because insufficient information exists to determine that either
special or general controls would provide reasonable assurance
of safety and effectiveness. Class III devices require
premarket approval.
(NOTE: Categories assigned to devices by the FDA prior to clinical trials are subject to
change during and after clinical trials. The Centers for Medicare and Medicaid Services
(CMS) uses the categorization of the device as a factor in making Medicare coverage
decisions.)
CLINICAL TRIAL COVERAGE
Routine Costs - Defined
This section provides definitions and examples of routine costs and investigational costs.
General case examples of routine costs in national clinical trials are presented. Two
specific case examples of clinical trials in which IHCP participation was requested are
also outlined to illustrate proposed IHCP coverage of routine costs.
The IHCP will pay most of a member’s costs in a clinical trial once the clinical trial is
considered an approved clinical trial. Specifically, the IHCP will cover the routine costs
involved in the clinical trial that are provided in either the experimental or the control
arms of a clinical trial. The routine items must not be items that are otherwise excluded
from coverage by the program. There are five types of covered routine costs associated
with a clinical trial.
1. Items and services that would otherwise be covered by the program if they were not
provided in the context of a clinical trial. For example:
• Nursing/staffing fees
• Patient monitoring and evaluation
• DME equipment
• IVs/catheters/line placement
2. Items or services required for the administration and provision of the investigational
item or service, up to but not including the actual cost of the investigational item or
service. For example:
• Administration fee for investigational chemotherapeutic agent
Medical Policy Manual
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• Equipment and ancillary staffing for the implantation of investigational device
• Provision of a nebulizer to administer an investigational drug
• Room and board as part of a hospital stay required as part of the clinical trial
3. Items or services required for the clinically appropriate monitoring of the effects of
the investigational item or service. For example:
• ECGs, EEGs,
• Monitoring blood pressures
4. Items and services required for the prevention of complications. For example:
• Costs of anti-nausea drug for investigational chemotherapeutic agent
5. Items or services needed for reasonable and necessary care arising from the provision
of an investigational item or service – in particular, for the diagnosis or treatment of
complications. For example:
• Treatment of pneumonia caused by an investigational lung procedure
The IHCP also adopted a Medicare policy on coverage of routine costs for items and
services that are the subject of a clinical trial, but are already covered items or services
under policy. The IHCP will extend coverage to routine care provided in clinical trials,
but not withdraw IHCP coverage for items and services that are currently covered outside
the context of a clinical trial as a result of, national coverage policies, statutes, rules, etc.
The policy also does not expand coverage beyond what would ordinarily be covered
outside a clinical trial. Therefore, if a clinical trial is investigating an item or service that
is covered outside the context of a clinical trial, the IHCP would continue to cover the
investigational item or service in accordance with existing coverage rules and regulations.
The IHCP policy on clinical trials will not render these investigational items or services
noncovered.
Investigational Costs
Items that are not considered routine costs and are not covered by the IHCP include the
following.
1. The investigational item(s) or service(s).
2. Items and services provided solely to satisfy data collection and analysis needs that
are not used in the direct clinical management of the patient. For example:
• Monthly CT scans for a condition usually requiring only a single scan.
• Weekly blood draws not needed to monitor side effects.
• Quarterly PAP smears for a condition requiring yearly PAP smears.
3. Items and services customarily provided by the research sponsors free of charge for
any enrollee in the trial. For example:
• Peak flow meters will not be reimbursed in a respiratory trial if the sponsor
provides them free of charge to the enrollees.
01/31/2007 Clinical Trials
90
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Clinical Trials Case Examples
On the following pages are several case examples of clinical trials. Cases 1 and 2
illustrate clinical trial policy applied to current national clinical trials. Case 3 applies the
policy to an actual clinical trial that the IHCP reimbursed in 2003 for a member with
Crohn’s disease. Case 4 illustrates application of the policy to a request for clinical trial
coverage received from Northwestern Hospital on behalf of a member with Wegener’s
Granulomatosis. This trial has not been reimbursed by the IHCP because the trial is not
funded and the member has not been able to raise the funds necessary to cover the
investigational costs.
Case 1
Title Diagnostic Study of CT Scans in Women at High Risk for Lung Cancer
Description
To determine the ability of CT to detect early lung abnormalities in women at
high risk for lung cancer, and determine the number of abnormal findings
detected by CT that develop into lung cancer in these patients.
Outline of the
Clinical Trial
1. Questionnaire including medical history and demographics.
2. CT scans without contrast for all patients.
a. Patients with normal results receive additional CT every 12 months.
b. Patients with abnormal results receive diagnostic CT scan with contrast.
3. Patients with indeterminate nodules have repeat scans at 6 months and 1
year.
4. Patients with abnormalities suspicious for malignancy undergo
bronchoscopy with biopsy and bronchoaveolar lavage (BAL).
a. If no confirmed malignancy, repeat CT scans at 6 months and 1 year.
b. If confirmed malignancy, refer for definitive treatment.
Coverage
1. Non-routine, non-covered items include the questionnaire, the initial CT
scans, and any additional CT scans not necessary for the direct clinical
management of the patient. These are noncovered costs because they are
items and services provided to satisfy data collection and analysis.
2. If policy allows for a repeat CT scan of an indeterminate finding at 6
months, or a bronchoscopy with biopsy for a suspicious abnormality, these
procedures would be considered routine costs and would be reimbursable.
3. Complications such as reactions to anesthesia during bronchoscopy,
reactions to contrast dye, or development of infection after bronchoscopy
would be considered routine reimbursable costs.
Case 2
Title Combination Chemotherapy in Treating Patients With Advanced Cancer
Description
To determine the effects of the combination of Flavopiridol, Fluorouracil, and
Leucovorin Calcium with and without Irinotecan on the destruction of tumor
cells in patients who have advanced cancer with no known therapies.
Outline of the
Clinical Trial
1. Two patient groups reside in the hospital for 5 days every 3 weeks while
receiving chemotherapy.
2. One group receives the combination chemotherapy only.
3. The second group of patients receives the combination chemotherapy plus
01/31/2007 Clinical Trials
91
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Irinotecan.
4. Tumors will be monitored by CT, MRI, or other appropriate clinical
determinants.
Coverage
1. Noncovered services would include the actual chemotherapeutic drugs
being administered because the trial protocol indicated there are no known
standard therapies for these tumors. The CTs, MRIs, etc. would also be
noncovered for the same reasons.
2. Routine care services that would otherwise be reimbursed by the IHCP if
not included in the context of a clinical trial encompasses nursing services,
room and board, and supplies that would be used to administer the
chemotherapy, (e.g., intravenous pumps and tubing).
3. Services required for the administration of investigational items or services
would also be covered such as administration of the chemotherapeutic
agents.
4. Other routine costs include prevention of complications such as treatment
of nausea and treatment of complications such as an electrolyte imbalance,
or an acute toxicity reaction.
Case 3
Title
Immune Ablation with Chemotherapy followed by Stem Cell Transplant for
Patients with Severe Crohn’s Disease
Description
To determine the safety and efficacy of immune ablation with high-dose
cyclophosphamide and anti-thymocyte globulin followed by autologous
peripheral blood stem cell rescue in patients with severe Crohn’s disease.
Outline of the
Clinical Trial
1. Mobilization of peripheral stem cells with cyclophosphamide IV on the first
day and filgrastim beginning on the third day until adequate blood counts
are reached.
2. Undergo leukapheresis for approximately 10 days until target number of
cells are reached.
3. A central line is placed, and an immune-ablation conditioning period is
initiated on a 5 day countdown with cyclophosphamide IV days 5-2 and
anti-thymocyte globulin IV on days 4-2.
4. Undergo autologous T lymphocyte-depleted peripheral stem cell transplant
after completing routine labs and tests on day 1.
5. Receive filgrastim on day 0.
6. Follow-up visits with serial small intestine absorption assessments at 3, 6,
and 12 months, then annually thereafter.
Coverage
1. Noncovered services include all chemotherapeutic agents because
chemotherapeutic agents are not peer-approved, nationally approved, or
FDA approved drugs for the treatment of Crohn’s disease. The
chemotherapeutic agents include cyclophosphamide, anti-thymocyte, and
filgrastim. The leukapheresis would be noncovered even though it is a
covered code by the IHCP because leukapheresis is not a procedure
normally performed on patients with Crohn’s disease, but it is a procedure
that is part of the investigational component of this trial. The stem cell
01/31/2007 Clinical Trials
92
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Clinical Trials
93
Case 3
Immune Ablation with Chemotherapy followed by Stem Cell Transplant for
Title
Patients with Severe Crohn’s Disease
transplant is also noncovered as an investigational procedure because a
peripheral stem cell transplant for Crohn’s disease is outside the scope of
coverage for stem cell transplant. Serial small intestine absorption
assessments would not be covered because these are services to satisfy data
collection.
2. Routine costs include placement of the central line, administration fees for
all drugs, including the investigational chemotherapeutic agents, and
Methylprednisone, room and board for any necessary hospital stay during
the conditioning phase and the transplant, and the costs of the labs and tests
(that have not been performed in the last 90 days) that are required for the
transplant.
3. Methylprednisone is covered because this is an approved treatment for
Crohn’s disease. The costs of services to prevent side effects, such as the
cost of the MESNA would be covered because MESNA is a drug used to
prevent side effects of Cytoxan.
4. The costs of the labs and tests prior to the transplant are covered if they
have not already been performed within the last 90 days because IHCP
policy states that the policy is to extend coverage to routine care provided in
clinical trials, but not to withdraw coverage for items and services that are
currently covered outside the context of a clinical trial. The IHCP current
policy for stem cell transplant requires that these tests must have been done
within the last 90 days.
5. Routine costs will also include any physician or nursing fees, monitoring
fees, or equipment fees that may arise during the clinical trial that would
normally be considered a covered service if not performed in the context of
a clinical trial. If any additional medications are needed to prevent side
effects or if complications occurred, these would also be covered services.
Case 4
Title
Hematopoietic Stem Cell Therapy for Patients with Systemic Necrotizing
Vasculitis (Wegener’s Granulomatosis)
Description
To determine the safety and efficacy of immune ablation with high-dose
cyclophosophamide and anti-thymocyte globulin followed by autologous
peripheral blood stem cell rescue in patients with Wegener’s Granulomatosis.
Outline of
the Clinical
Trial
1. Mobilization of peripheral stem cells with Cytoxan 2gm/m
2
(cyclophosphamide) and filgrastim 10 mcg/kg until adequate blood counts
are reached.
2. Undergo leukapheresis for approximately 4 days until target number of cells
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Clinical Trials
94
Case 4
Hematopoietic Stem Cell Therapy for Patients with Systemic Necrotizing
Title
Vasculitis (Wegener’s Granulomatosis)
are reached.
3. Rest for approximately 3-4 weeks.
4. A central line is placed, and an ablation conditioning period is completed
with combinations of high-dose Cytoxan 50mg/kg, Rabbit ATG 0.5mg/kg,
Methylprednisone 1gm, and MESNA 50mg/kg per day for 6 days.
5. Undergo stem cell transplant after checking routine labs and tests.
6. Receive filgrastim 5mcg/kg the day after transplant.
7. Inpatient transplant recovery as needed.
Coverage
1. The trial is sponsored by Northwestern Hospital which would not make it an
automatically qualified clinical trial; however, the trial has an approved
investigational new drug application by the FDA which does qualify it as an
approved clinical trial. Therefore, routine costs would be covered.
2. Noncovered services include all chemotherapeutic agents except the Cytoxan
used in the mobilization phase of therapy. The Cytoxan used in the
mobilization phase of therapy is dosed at 2gm/m
2
. Cytoxan is a prescribed
treatment for Wegener’s Granulomatosis at this dose. The remainder of the
chemotherapeutic agents are not peer-approved, nationally approved, or
FDA approved drugs for the treatment of Wegener’s. The chemotherapeutic
agents include Cytoxan 50mg/kg, filgrastim, and Rabbit ATG. The
leukapheresis would be noncovered even though it is a covered code by the
IHCP because leukapheresis is not a procedure normally performed on
patients with Wegener’s Granulomatosis, but it is a procedure that is part of
the investigational component of this trial. The stem cell transplant is also
noncovered as an investigational procedure because a transplant for
Wegener’s is outside the scope of coverage for stem cell transplant.
3. Routine costs include placement of the central line, administration fees for
all drugs, including the investigational chemotherapeutic agents, coverage of
the MESNA and Methylprednisone, room and board for any necessary
hospital stay during the conditioning phase and the transplant, and the costs
of the labs and tests (that have not been performed in the last 90 days) that are
required for the transplant.
4. The cost of the MESNA is covered because MESNA is a drug used to
prevent side effects of Cytoxan. Methylprednisone is covered because this is
an approved treatment for Wegener’s.
5. The costs of the labs and tests prior to the transplant are covered if they have
not already been performed within the last 90 days because IHCP policy
states that the policy is to extend coverage to routine care provided in clinical
trials, but not to withdraw coverage for items and services that are currently
covered outside the context of a clinical trial. The IHCP current policy for
stem cell transplant requires that these tests must have been done within the
last 90 days.
6. Routine costs will also include any physician or nursing fees, monitoring
fees, or equipment fees that may arise during the clinical trial that would
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Clinical Trials
95
Case 4
Hematopoietic Stem Cell Therapy for Patients with Systemic Necrotizing
Title
Vasculitis (Wegener’s Granulomatosis)
normally be considered a covered service if not performed in the context of a
clinical trial. If any additional medications are needed to prevent side effects
or if complications occurred, these would also be covered services.
IHCP CLINICAL TRIALS POLICY
The IHCP covers the routine costs of approved clinical trials as well as reasonable and
necessary items and services used to prevent complications and to diagnose and treat
complications arising from participation in all clinical trials.
Routine costs of a clinical trial include all items and services that are available to IHCP
members (i.e., there exists a benefit category and the item or service is not listed as a
noncovered service in the Indiana Administrative Code) that are provided in either the
experimental or the control arms of the trial.
Routine costs in clinical trials include the following.
• Items and services that would otherwise be covered by the program if they were
not provided in the context of a clinical trial.
• Items or services required for the administration and provision of the
investigational item or service, up to but not including the actual cost of the
investigational item or service (e.g., administration for a noncovered
chemotherapeutic agent).
• Items required for the clinically appropriate monitoring of the effects of the
investigational item or service.
• Items and services required for the prevention of complications.
• Items or services needed for reasonable and necessary care arising from the
provision of an investigational item or service; in particular, for the diagnosis or
treatment of complications.
• Items or services already covered by the IHCP will be considered a routine cost in
accordance with existing coverage rules and regulations even if the item or
service is the investigational item or service. The IHCP policy on clinical trials
will not render these investigational items or services noncovered. However, if
the investigational item or service is currently covered only for certain medical
conditions and is being tested for use outside the scope of coverage, the item or
service will still be considered investigational.
Medical Policy Manual
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Items that are not considered routine costs in a clinical trial and are not covered by the
IHCP include the following.
• The investigational item(s) or service(s).
• Items and services provided solely to satisfy data collection and analysis needs
that are not used in the direct clinical management of the patient (e.g., monthly
CT scans for a condition usually requiring only a single CT scan).
• Items and services customarily provided by the research sponsors free of charge
for any enrollee in the trial.
All investigators requesting IHCP coverage of routine costs for a clinical trial must first
meet the following three requirements.
1. The subject or purpose of the trial must be the evaluation of an item or service that
would be covered under Indiana Administrative Code (IAC) guidelines. The items or
service being investigated must not be a non-covered item or service as listed under
405 IAC 5-10-5, 5-19-18, 5-24-3, 5-29-1, or 5-30-3.
2. If a clinical trial has one objective, it must have a therapeutic intent. If a clinical trial
has multiple objectives, it must have a therapeutic intent as a primary objective. It
must have some ability to improve a subject’s condition such as prolongation of life,
shrinkage of a tumor, or improved quality of life even though cure or dramatic
improvement may not necessarily be affected. The trial cannot be designed
exclusively to test toxicity or disease pathology.
3. Trials of therapeutic intervention only must enroll members with diagnosed disease
rather than healthy members. Trials including diagnostic interventions may enroll
healthy members in order to have a proper control group.
The three requirements above are insufficient by themselves to qualify a clinical trial for
IHCP coverage of routine costs. In order for the IHCP to cover the routine costs involved
in clinical trials, the clinical trial must be deemed “automatically qualified” under
Medicare guidelines. The following clinical trials are deemed to be automatically
qualified as approved clinical trials.
• Trials funded by the NIH, CDC, AHRQ, CMS, DOD, or VA.
• Trials supported by centers or cooperative groups that are funded by the NIH,
CDC, AHRQ, CMS, DOD, or VA including but not limited to the FDA, NHLBI,
National Human Genome Research Institute, NCI, NIDDK, NIMH, and others.
01/31/2007 Clinical Trials
96
Medical Policy Manual
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Clinical Trials
Medical Policy Manual
97
• Trials conducted under an Investigational New Drug (IND) application reviewed
by the FDA.
• Drug trials that are exempt from having an IND under 21 CFR 312.2 (b)(1) will
be deemed automatically qualified.

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: COLLAGEN IMPLANTS FOR STRESS URINARY
INCONTINENCE
DESCRIPTION
Stress urinary incontinence (SUI) can be caused by incompetence of the urethral
sphincter mechanism at the bladder neck. This type of SUI may be caused by scarring
from previous surgery (e.g. prostatectomy), urethral hypermobility, myelomeningocele,
epispadias, trauma, radiation, or sacral cord lesions or by any process that limits the
ability of the proximal sphincter to form an effective watertight seal.
Collagen implants, such as Contigen are used to control urinary incontinence due to SUI
caused by intrinsic sphincter deficiency. The collagen implant is injected endoscopically
into the submucosal tissue of the urethra and/or bladder neck, increasing tissue and
urethral resistance. Collagen injections thicken the urethra sphincter mechanism at the bladder
neck, allowing the proximal urethra to form a watertight seal during bladder filling and to
maintain this seal during physical stress.
Collagen is a natural fibrous protein found in human bone, cartilage, and connective
tissue. The collagen used for treatment of SUI is sterile, biocompatible, biodegradable,
purified bovine dermal collagen. Allergic reactions to collagen are infrequent but can
occur, so patients must be evaluated preoperatively to limit the possibility of adverse
reactions to collagen. Urologists who perform this treatment must complete training
programs specifically related to this treatment.
RELATED MEDICAL TOPICS
Anesthesia Services
Consultations – Second Opinion
Diagnostic Studies
Surgery – Global Billing/Payment Guidelines
Surgery – Services Requiring Prior Authorization
Surgery – Surgical Services
01/31/2007 Collagen Implants for Stress Urinary Incontinence
98
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
RULES, CITATIONS, AND SOURCES
Indiana Medicaid Update Bulletin 95-21
Indiana Health Coverage Programs Provider Manual
ORIGINATION, REVIEWS, AND REVISIONS
Origination date: 7/11/94
Review and Revisions Reason Date
Review and Revision Routine 10/29/04
APPLICABLE INDIANAAIM EDITS AND AUDITS
6061
6706
COVERAGE CRITERIA
Indications for the implants are as follows.
♦ Indicated for the treatment of urinary incontinence due to Intrinsic Sphincter
Deficiency (ISD) – Diagnosis: Code 599.82
♦ Should be considered after a patient with ISD has demonstrated consistent symptoms
and signs of urinary incontinence for at least twelve months.
♦ Since the collagen is potentially immunogenic, the patient must be evaluated by a
skin test prior to treatment.
♦ Providers should be skilled in the endoscopic procedures necessary to carry out the
injection and have credentials to perform these procedures in the respective
institution. Additionally, FDA requirements mandate that the performing physician
must have completed a Contigen Implant Training Program.
♦ Repeat injections of the collagen should be performed no less than seven days after
the initial treatment. Patients who have failed to demonstrate improvement after five
injection sessions are considered treatment failures; further attempts to use collagen
should not be undertaken.
Restrictions and limitations to the use of collagen implants are as follows.
♦ Limit 12 injections per member for same date of service
01/31/2007 Collagen Implants for Stress Urinary Incontinence
99
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Collagen Implants for Stress Urinary Incontinence
Medical Policy Manual
100
♦ Five units (sessions) per member per lifetime
♦ Diagnosis code 599.82 Intrinsic Sphincter Deficiency (ISD) must be included on the
claim
♦ Prior authorization is not required
♦ Contraindications for collagen therapy include:
a. Untreated urinary tract infections
b. Unmanaged detrusor muscle instability
c. Known hypersensitivity to bovine collagen
♦ Place of service: Inpatient/Outpatient/Physician Office
BILLING INFORMATION
Medicaid reimbursement is available for collagen implants used to control urinary
incontinence due to intrinsic sphincter deficiency, demonstrated by consistent symptoms
and signs of urinary incontinence for at least twelve months. Appropriate HCPCS codes
for billing are listed in Table 1 – HCPCS CODES. Prior authorization is not required.
Diagnosis code 599.82, Intrinsic Sphincter Deficiency, must be included with the HCPCS
code in the request for reimbursement.
Table 1 – HCPCS CODES
HCPCS CODE DEFINITION
LIMITATIONS/
RESTRICTIONS
L8603
Collagen implant, urinary tract, per
2.5cc syringe, includes shipping and
necessary supplies
Limit 12 injections per
recipient for each date of
service
51715
Endoscopic injection of implant
material into the submucosal tissues of
the urethra and/or bladder neck
Five units (sessions) per
lifetime.
Q3031
Collagen skin test To be administered and
evaluated over a four week
period.
95028
Intracutaneous (intradermal) tests with
allergenic extracts, delayed type
reaction, including reading, specify
number of tests

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: CONSULTATIONS—SECOND OPINIONS
DESCRIPTION
A consultation is the rendering of a medical opinion by a physician regarding evaluation
or management of a specific condition requested by another physician. It requires the
consulting physician to examine the patient, unless the applicable standard of care does
not require a physical examination.
Second or third opinions (confirmatory consultation) may be requested for members
having a procedure for which a choice of treatment modalities may be applicable, such as
surgical procedures.
This document is intended to serve as a general summary of the Indiana Health Coverage
Programs (IHCP) policies regarding this service. More specific information may be
found in the IHCP provider manual, program notices, the Indiana Administrative Code
(IAC) or other sources as appropriate. See the “Rules, Citations, and Sources” section of
this document for further information.
COVERAGE CRITERIA
The following four subcategories of consultations are covered by the IHCP.
Office or other outpatient consultation
Confirmatory consultation
Initial inpatient consultation
Follow-up inpatient consultation
Reimbursement for an initial consultation is limited to one per consultant, per member,
per inpatient hospital or nursing facility admission. The IHCP will not reimburse
consultation codes when a member is referred for management of a condition or the
consulting physician assumes management of the member’s care.
The medical record must contain written documentation of the request for consultation by
the requesting physician, if a consultation code is billed. This documentation should be
maintained in the member’s medical record by both the physician requesting a
consultation and the physician providing the consultation.
01/31/2007 Consultations – Second Opinions
Medical Policy Manual
101
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Reimbursement is available for consultative pathology and radiology services. These
consultations do not require the consulting physician to examine the member.
Office Consultation (CPT codes 99241 – 99245)
A consulting physician may initiate diagnostic or therapeutic services. An office or other
outpatient consultation must address a specific condition not previously diagnosed or
managed by the consulting physician. If an additional request for an opinion or advice
regarding the same or a new problem is received from the attending physician and
documented in the medical record, the office consultation codes may be used by the
consulting physician again. If the consulting physician initiates a follow-up visit, the
follow-up visit is reported using the appropriate office or other outpatient code for
established patients (CPT codes 99211-99215).
Confirmatory Consultation
A confirmatory consultation must be specifically requested by the member or the IHCP
contractor and is used for second and third opinions or advice only. A confirmatory
consultation to substantiate medical necessity may be required as part of the prior
authorization (PA) process.
Initial Inpatient Consultation (CPT codes 99251 – 99255)
Only one initial consultation may be reported by a consultant per admission. The request
for consultation must be documented. These codes are used to report consultations
provided to hospital inpatients, residents of nursing facilities, or patients in a partial
hospital setting. These codes should be utilized by the consulting physician for the initial
encounter with the patient and then subsequent hospital care codes for additional
encounters thereafter.
Follow-up Inpatient Consultation
A follow-up consultation is a subsequent visit needed to complete the initial consultation
or subsequent consultative visits requested by the attending physician. These
consultative visits include monitoring progress, recommending management
modifications, or advising on a new plan of care in response to changes in the patient’s
status. If the consulting physician has initiated treatment at the initial consultation, and
participates thereafter in the patient’s management, the codes for subsequent hospital care
should be used (CPT codes 99231 – 99233).
Pathology Services
Consultative pathology services are reimbursable if they are requested by the member’s
attending physician in writing and meet the following criteria.
• The consult relates to a test result that lies outside the clinically significant normal
or expected range in view of the condition of the member
• The consultant provides a written narrative report to be included in the member’s
medical record
• Medical judgment is required by the consulting physician.
01/31/2007 Consultations – Second Opinions
Medical Policy Manual
102

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Dental Services
The American Dental Association (ADA) has indicated that a consultation is to be used
as a second opinion. When billing for a dental consultation, providers are to report CDT
code D9310, Consultation (diagnostic service provided by dentist or physician other than
practitioner providing treatment), or one of the oral evaluation codes, not both on the
same date of service, for the same member, by the consulting dentist. Please refer to the
MP Manual fact sheet on Dental Services for further information.
Podiatry Services
A second or third opinion substantiating the medical necessity or approach may be
required for bunionectomy procedures and all surgical procedures involving the foot. A
confirmatory consultation is required regardless of the setting in which the surgery is
performed, including ambulatory surgical centers, hospitals, clinics, or in the office.
Please refer to the MP Manual fact sheet on Podiatry for further information.
Consultation services rendered by a podiatrist in a nursing facility are not covered when
performed on members on a routine basis for screening purposes, except in those cases
where a specific foot ailment is involved. Documentation must be maintained in the
member’s medical record. Please refer to the MP Manual fact sheet on Podiatry Services
for further information.
PRIOR AUTHORIZATION
The CPT codes for evaluation and management (E&M) services that are used to report
second opinion and consultative services and that also require prior authorization after 50
visits per member per provider per rolling calendar year are listed in Table 1 – Services
Requiring PA After 50 Visits per Member per Rolling Calendar Year. The Billing
Requirements section of this fact sheet provides information regarding appropriate use of
the codes listed in Table 1 for reporting second opinion and consultation services
provided to IHCP members.
Table 1 - Services Requiring PA
After 50 Visits per Member per Rolling Calendar Year*
CPT Code Ranges
for E&M Services
Description
99201-99205
Office or other outpatient visit for the evaluation and management
of a new patient
99211-99215
Office or other outpatient visit for the evaluation and management
of an established patient
99241-99245
Office or other outpatient consultation for a new or established
patient
99271-99275 Confirmatory consultation for a new or established patient
*This table contains the E&M codes used for second opinion and consultative services
and is not meant to be inclusive of all E&M codes.
01/31/2007 Consultations – Second Opinions
Medical Policy Manual
103
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MANAGED CARE
The Hoosier Healthwise PrimeStep member’s primary medical provider (PMP) must
make a referral for a second opinion if requested by the member. Hoosier Healthwise
members may choose a qualified Hoosier Healthwise provider from whom they desire to
seek a second opinion. The PMP certification code is required for the consultative
physician services to be reimbursed. Any subsequent treatment by the second opinion
provider, if necessary, requires a separate referral.
Hoosier Healthwise members enrolled in Risk-Based Managed Care (RBMC) are
allowed to receive a second medical opinion and consultative services. Hoosier
Healthwise members that are enrolled in RBMC are the financial responsibility of the
managed care organization (MCO) in which the member is enrolled. The MCO may
have its own requirements for authorization of second opinion and consultative services;
therefore, providers must contact the MCO for further information regarding the referral
process.
BILLING INFORMATION
Using the appropriate modifier, consultations and second opinions may be reimbursed
according to Medicaid guidelines. Documentation may be requested to determine
medical necessity for the claim to be paid. In the claim note information on the
electronic 837 claim, the provider can indicate the medical reason for a second opinion
during the 15 days before or after the billed consultation.
Consultation codes should not be used for the evaluation of a self-referred or non-
physician referred patient. A consultation implies collaboration between a requesting and
a consulting physician. Follow-up visits in the consultant’s office or other outpatient
facility initiated by the consulting physician are reported using office visit codes for
established patients, 99211-99215. If an additional request for an opinion or advice about
a new problem is received from the attending physician and documented in the medical
record, the office consultation codes may be used again.
A physician providing a confirmatory consultation (CPT codes 99271–99275) is expected
to provide an opinion and/or advice only. Any services provided subsequent to the
opinion are coded at the appropriate level of office visit, established patient, or
subsequent hospital care.
The IHCP provides reimbursement to providers billing CPT codes 99251-99255 for
initial inpatient consultations with new or established patients in the inpatient hospital
setting. The IHCP recognizes CPT codes 99261-99263 for follow-up inpatient
consultations.
01/31/2007 Consultations – Second Opinions
Medical Policy Manual
104

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
RELATED MEDICAL TOPICS
Dental Services
Diagnostic Studies
Evaluation and Management Services
Physician Services
Podiatry Services
Surgery – Surgical Services
RULES, CITATIONS, AND SOURCES
405 IAC 5-8 - Consultations and Second Opinions
405-IAC 5-9-1 - Evaluation and Management Services, Limitations
405 IAC 5-18-4 - Nonanatomical laboratory procedures
405 IAC 5-26-2 (4) – Podiatric Services, General restrictions
405 IAC 5-26-10 – Surgical Procedures; Confirmatory Consultations
Hoosier Healthwise Manual for Primary Medical Providers and Office Staff, January,
2003
Indiana Health Coverage Programs Provider Manual
1999
Version 5.1, March 2005
Indiana Health Coverage Programs Provider Bulletins
BT200262 - PrimeStep PMP Certification Code Changes
BT200511 – HIPAA Modifications
Origination Date: 12/31/2000
Revisions and
Review
Reason Date
470 IAC 5-9-8;
5-9-9
Transferred
Consultations; Second Opinions 7/1/91
405 IAC 1-7-8;
1-7-9 Repealed
8/24/97
Consultation And Second Opinions 1/1/92
405 IAC 5-8 Consultations and Second Opinions 8/24/97
Review Scheduled 7/29/05
01/31/2007 Consultations – Second Opinions
Medical Policy Manual
105

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Consultations – Second Opinions
Medical Policy Manual
106
APPLICABLE INDIANAAIM EDITS AND AUDITS:
6006 – Only 1 New Patient Visit Per Three (3) Years
6011 – Therapeutic or Diagnostic Injections
6012 – Medical Services 30 per year
6014 – Global Payable at a Reduced Fee When Component Paid
6019 – Initial/Established Visits Not Payable Same Day of Service
6028 – Initial and Periodic Visits Not Payable Same Day of Service
6041 – E&M Codes Not Reimbursable With Prenatal Codes
6042 – Prenatal Codes Not Reimbursable with E&M Codes
6064 – Components Not Payable When Global Paid Medical System
6069 – Office Visits 50 per Year
6090 – Office Visits Limited to One per Year – Podiatrist
6091 – One Initial Office Visit per Recipient - Podiatrists
6096 – The CPT/HCPCS Code Billed is Not Payable According to the PPS
Reimbursement Methodology
6098 – Chiropractic Services Limited to Procedure and Diagnosis
6099 – Reimbursement is Limited to 50 Chiropractic Services
6101 – Chiropractic Restrictive Office Visit Codes (NP)
6102 – Chiropractic Office Visits Limited to 5 per year
6111 – Chiropractic Office Visits Limited to Five per Year
6122 – Chiropractic Therapeutic Physical Medicine Treatment
6150 – Consultation Billed 15 Days Before or After Another
6151 – Consultation Billed 7 Days Before or After Surgery
6152 – Surgery Payable at Reduced Amount When Consult Paid
6236 – Dental Services Limited $600 for 21 and Over
6238 – Dental Services Limited $600 for 21 and Over
6610 – Routine Vision Exam Limit to 1/12 Months Age 1-18 years
6611 – Routine Vision Exam Limited to 1/24 Months Ages 19-999 years
6637 – Drug Administration is Not Payable on the Same Day of Service
6649 – Surgery Payable at Reduced Amount When Related POS
6652 – Multiple Surgeries Must Be Billed on the Same Claim
6653 – Post Op Care Within 00-90 Days of Surgery
6654 – Pre-Operative Care Within 1 day of Surgery
6655 – Surgery Payable at Reduced Amount When Pre-op Care
6656 – Post-Op Care Within 10 Days of Select Surgery
6657 – Pre-Op Care on Day of Surgery
6658 – Surgery Payable at Reduced Amount When Pre-Op Care
6659 – Surgery Payable at Reduced Amount When Related POS
6660 – Pre/Post-Operative Care Billed with Unlisted Surgery
6903 – PCCM Limits Office Visits Greater Than 30 Visits

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: DENTAL SERVICES
DESCRIPTION:
Dental services include diagnostic, preventive, or corrective procedures provided by or under the
supervision of a dentist in the practice of his or her profession. These include treatment of the
teeth and associated structures of the oral cavity, disease, injury, or impairment that may affect
the oral or general health of the member. Other services include those offered by dental
specialists, such as endodontists, orthodontists and periodontists.
COVERAGE CRITERIA
Procedure Code Multiple Units
The Indiana Health Care Programs (IHCP) accepts multiple units per service line for applicable
dental procedure codes. The dental provider will be able to submit multiple units on one claim
service line. For example, D1110, Prophylaxis, adult, is not appropriate for submitting multiple
service units; however, D4341, Periodontal scaling and root planning, four or more contiguous
teeth or bounded teeth spaces, per quadrant, can be submitted with up to four units on one
service line with the number of quadrants treated on the date of service.
The multiple units must be rendered on the same date of service and a single service line cannot
span more than one date. All procedure code limitations remain in place and services that exceed
these limitations are reduced to the unit limitation allowed for the procedure code. This change is
effective for dates of service November 14, 2003, and after.
Dental Extractions
Effective for dates of service on or after January 1, 2004, the following billing requirements and
reimbursement policies apply to tooth extractions. Only one tooth number is allowed per service
line for dental extractions.
A provider submitting a claim for D7140, Extraction, erupted tooth or exposed root (elevation
and/or forceps removal), must indicate the tooth number for each tooth extracted on a separate
service line in Field 59 on the American Dental Association (ADA) 2000 Dental Claim form.
The IHCP will pay 100 percent of the maximum allowed amount or the billed amount,
whichever is less, for the initial extraction. For multiple extractions within the same quadrant on
the same date of service, the IHCP will pay 90 percent of the maximum allowed amount for
procedure code D7140 or the billed amount, whichever is less.
01/31/2007 Dental Services
Medical Policy Manual
107
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Dental Cap
Effective March 1, 2003, The Office of Medicaid Policy and Planning (OMPP) established a $600
cap for members 21 years of age and older. The affected procedures are limited to a total of $600
of services per member, per calendar year. Dental services included in the dental cap are
considered non-covered when the cap is reached for that calendar year. For years 2004 and
beyond, the calendar year for the dental cap will begin on January 1 and end December 31. If
additional dental services are needed for a member beyond the $600 cap during a specific calendar
year, providers can hold members financially responsible. For a list of codes included in the $600
dental cap, please refer to Table 8.86 in Chapter 8, Section 4 of the IHCP Provider Manual.
The dental cap applies only to the IHCP paid dental services provided in a dental office. Dental
services for root planing and scaling, intravenous sedation provided in conjunction with oral
surgery, and osseous surgery are excluded from the dental cap.
In certain circumstances, providers can bill their usual and customary charge, provided that the cap
has been exhausted. However, if the service is partially paid by the IHCP because of the cap limit,
the member can only be billed for the difference between what the IHCP would have reimbursed to
the provider and what the IHCP actually paid.
Diagnostic and Preventative
IHCP members are eligible for diagnostic and preventive services; however, many of the
services offered to IHCP members are included in the $600 dental cap.
Currently, a member can receive one (1) periodic oral evaluation (D0120) every six (6) months.
Likewise, D0150, Comprehensive oral evaluation, and D0160, detailed and extensive oral
evaluation, are limited to two visits per member per year. Other diagnostic and preventive
services that members are eligible to receive include, D0140, limited oral evaluation-problem
focused and D0170, Re-evaluation-limited, problem focused (established; patient; not post
operative visit).
Prophylaxis is a covered IHCP service, but is limited to the following restrictions. The program
allows for one (1) application of prophylaxis every six months, for non-institutionalized
members twelve (12) months of age up to their twenty first birthday. One (1) unit of prophylaxis
is allowed, every 12 months, for non-institutionalized members that are twenty-one (21) years of
age and older. Institutionalized members may receive up to one unit of prophylaxis every six
months, regardless of age. Members 12 months of age and younger are not eligible for
prophylaxis service.
Prophylaxis and fluoride that is provided on same date of service, should be billed under one
code—D1201, topical application of fluoride. Likewise D1110, prophylaxis-adult, and D1204,
topical application of fluoride (prophylaxis not included), are no longer separately reimbursed.
D1204 is a non-covered procedure code. Providers should be billing this joint service using
either D1201 for children up to the age of 12 and D1205 for children 13 up to age 21, when
fluoride and prophylaxis are provided on the same day.
01/31/2007 Dental Services
Medical Policy Manual
108
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Either full mouth series radiographs or panorex x-rays are limited to one (1) set per member
every three (3) years. Bitewing, intraoral, and extraoral radiographs are limited to one (1) set per
recipient every twelve months. One (1) set of bitewings is defined as a total of four (4) single
films.
Restorative
IHCP reimburses for dentures and partials once every six years, but only with prior authorization
(PA) and if medically necessary. IHCP also reimburses for reline and repairs to dentures and
partials, provided PA is obtained and that the service is for members 21 years of age and older.
Repairs and relines are only approved to extend the useful life of a prosthesis that is at least six
(6) years old. Eight posterior teeth in occlusion, four maxillary and four mandibular teeth in
functional contact with each other, are considered adequate for functional purposes. If a member
is taking parenteral/enteral nutritional supplements, dentures and partials will not be approved
unless the dentist submits a plan of care with the PA request that indicates dentures or partials
are needed to wean the member from the nutritional supplements.
Local codes for complete and partial dentures were eliminated effective January 1, 2004.
Reimbursement rates for dentures are determined by the age of the member. Complete and
partial dentures do not require PA for members under twenty-one (21), while those 21 and older
do require PA. Repairs and relines require PA and are only approved to extend the life of the
prosthesis that is at least six years old.
Providers must submit D7999 to denote supernumerary tooth extractions. A claim attachment
(note of explanation) is required with the ADA Claim Form when billing D7999. The attachment
should indicate the type of extraction performed and whether it is an erupted or an impacted
tooth. An impacted tooth must be documented as to whether it is soft tissue, partially bony,
completely bony, with unusual complications, and so forth.
IHCP provider bulletin BT200433, published December 23, 2004, stated that procedure code
D7283, placement of a device to facilitate eruption of an impacted tooth, was a covered service
effective January 1, 2005. Further review indicates that this procedure is performed as an
orthodontic service. The IHCP covers comprehensive orthodontic services with PA, as outlined
in IHCP provider bulletin BT200230, published June 19, 2002. Procedure code D7283 includes
placement of an orthodontic bracket or band to facilitate eruption of an unerupted tooth after
surgical exposure. Placement of an orthodontic bracket is included in the reimbursement for
comprehensive orthodontic services; therefore, procedure code D7283 is not separately
reimbursed.
Resin crown codes D2336, D2337, D2335, and D2932 are covered by IHCP. IHCP will also pay
for resin fillings, amalgam fillings and steel crowns.
Dentures and the $600 Dental Cap
Dentures are subject to the annual $600 dental cap for members age 21 or older. Services subject
to the dental cap that are provided after the cap has been reached are not covered by the IHCP. If
the member has exceeded the annual $600 dental cap, the provider must inform the member of
01/31/2007 Dental Services
Medical Policy Manual
109
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
the non-covered services as described in the IHCP Provider Manual. If the member has been
informed of the amount of the non-covered charge(s) prior to the service being rendered, the
provider can charge the member the usual and customary fee for the service(s).
If the member exhausted the cap during the course of treatment, the member is responsible only
for the Medicaid allowed amount over the annual $600 dental cap. The following scenario
demonstrates the correct method for charging a member when the dental cap is exhausted during
treatment. The member receives dental services that exhaust $400 of the dental cap. During the
same year, the member requires a complete upper denture, a service that is also included in the
dental cap. The current IHCP maximum reimbursement for a complete upper denture (D5110) is
$391.25. Therefore, the member will exceed the $600 cap and the member will be responsible
for $191.25, if notified of the charges prior to the delivery of the dentures. The provider must
maintain documentation to substantiate member notification of the member’s portion of financial
responsibility prior to providing the service. The IHCP Provider Manual, Chapter 4, Section 5,
describes the policy for charging members for non-covered services.
Example:
$600 cap - $400 cap exhausted = $200 cap remaining
$391.25 maximum fee - $200 cap remaining = $191.25 billable to the member
The service of providing dentures to any patient is not complete until the completed denture has
been delivered to the patient. The date of the provision of the finished product is the date of
service that must be used for claims filing and must be supported by record documentation. The
provider must bill the IHCP according to when the services are rendered. The IHCP requires that
provider records be maintained in accordance with 405 IAC 1-5-1. Per 405 IAC 1-5-1(b)(4), the
medical record must contain the date when the service was rendered. In addition, according to
405 IAC 1-1-4, denial of claim payment can occur if the services claimed are not documented in
accordance with 405 IAC 1-5-1.
If the member is no longer eligible, the former member can be charged for the dentures. The
IHCP policy for charging members for non-covered services does not apply if the member is no
longer eligible; therefore, a non-covered services waiver is not required.
Providers are responsible for verifying member eligibility prior to rendering services. Providers
are urged to advise members that if their eligibility is terminated prior to the dentures being
completed, the cost of the dentures will be the member’s responsibility. If the provider has
verified that the member is no longer eligible, the provider can charge the member according to
the provider’s usual practices for other customers not enrolled in the IHCP.
Members Under Twenty One
Reimbursement is available for treatment of dental caries, extraction of teeth, and space
maintenance in children with deciduous molar teeth. General anesthesia, nitrous oxide analgesia,
and pre-anesthetic medication are also covered under IHCP reimbursement for general
anesthesia provided in the dentist’s office and available only for members under twenty-one (21)
years of age.
01/31/2007 Dental Services
Medical Policy Manual
110
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
IHCP offers reimbursement for monitored sedation ‘provided in the dentist’s office’ for
recipients under the age of twenty-one (21). Monitored sedation is the administration of either
subcutaneous, intramuscular, intravenous or oral sedation, in combination with monitoring of the
patient’s vital signs.
Prophylaxis is a covered IHCP service, but is limited to the following restrictions. The program
allows for one (1) application of prophylaxis every six months, for non-institutionalized
members twelve (12) months of age, up to their twenty first birthday. One (1) unit of prophylaxis
is allowed, every 12 months, for non-institutionalized members that are twenty-one (21) years of
age and older. Institutionalized members may receive up to one unit of prophylaxis every 12
months. Members 12 months of age and younger are not eligible for prophylaxis service.
Prophylaxis and fluoride that is provided on same date of service, should be billed under one
code—D1201, topical application of fluoride. Likewise, D1110, prophylaxis-adult and D1204,
topical application of fluoride (prophylaxis not included) are no longer separately reimbursed.
Providers should be billing this joint service using either D1201 for children up to the age of 12
and D1205 for children 13 up to age 21, when fluoride and prophylaxis are provided on the same
day.
Periodontics
D4341, scaling and root planing is limited to four quadrants per lifetime for members 21 years of
age and older who are not institutionalized. Institutionalized members are restricted to four (4)
quadrants every two years. Providers billing D4341 must attach documentation that demonstrates
that the member has periodontal disease by showing pocket markings, or evidence of attachment
loss, and that the procedure was necessary for the removal of cementum and dentin that is rough,
permeated by calculus, or contaminated with toxins or micro-organisms.
Periodontics surgery is a covered service for cases of drug-induced periodontal hyperplasia; prior
authorization is required. Admission of a member to a hospital for performing any elective
dental service, or any elective dental service performed on an inpatient basis, requires prior
authorization. IHCP providers shall be required, based upon the facts of the case, to obtain a
second or third opinion substantiating the medical necessity or approach for maxillofacial surgery
related to diseases and conditions of the jaws and contiguous structures.
Orthodontics and Oral Surgery
Orthodontic procedures for IHCP are covered only for members younger than 21 years old. The
OMPP requires PA, effective August 5, 2002 for all orthodontic services. Prior authorization
requests must be submitted on the IHCP Medical Prior Authorization Form not the IHCP Prior
Authorization Dental Request Form.
The patient must be diagnosed by a member of a recognized craniofacial anomalies team, such as
a member of the American Cleft Palate-Craniofacial Association. The patient must be treated by
a licensed practitioner, who minimally accepts routine craniofacial patients for orthodontic
services, such as those patients with the cleft lip and palate.
01/31/2007 Dental Services
Medical Policy Manual
111
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
A signed statement from the practitioner, who is a member of a hospital based craniofacial team,
must certify the correct craniofacial diagnosis and malocclusion. The diagnosis must include
information descriptive of facial and soft tissue, skeletal, dental/occlusal, functional, and
applicable medical or other conditions. Documentation for orthodontic services must be
maintained in the patient’s dental or medical record, as required by 405 IAC 1-5-1, Medical
records; contents and retention.
Procedure code D8680, Orthodontic retention-removal of appliances, construction and
placement of retainer(s) is non-covered and is included in the reimbursement for orthodontic
treatment. Procedure codes D8691, Repair of orthodontic appliance, and D8692, Replacement of
lost or broken retainer are also non-covered. These services are included in the reimbursement
for orthodontic treatments and will not be separately reimbursed.
General anesthesia is covered for adults only if the procedure is performed in a hospital (in-
patient or outpatient) or an ambulatory surgical center. Reimbursement for IV sedation and
nitrous oxide analgesia is available to all members, regardless of age.
Mobile Dental Services
Effective July 1, 2002, 828 IAC 4-1-1 requires providers of mobile dental services to be licensed
as a mobile dental facility. Providers of mobile dental services should contact the Health
Professions Bureau at (317) 234-2010 for an application and forward a copy of the mobile dental
license to EDS Provider Enrollment.
Dental Forms and Provider Billing Issues
Dental providers wishing to submit paper claims must do so only on the ADA 2000 approved
claim form. Effective January 1, 2004, dental providers will be using CDT-4 codes exclusively
for billing.
During the week of June 6, 2005, the IHCP identified a high number of claim denials for edit
1008, rendering provider must have an individual number. This error occurs when a provider
submits a billing group number in the detail line. According to IHCP provider bulletin
BT200511, published June 1, 2005, all group providers must use their rendering provider
numbers. To expedite claims, providers should follow these guidelines:
• Group provider using a paper claim – Enter the group number and location codes in
field 44A. Enter the individual rendering numbers in the Administrative column adjacent
to each detail submitted.
• Group provider using Web InterChange – Enter the group number and location code
in the provider number field. Enter the individual rendering number in the rendering
provider field.
• Individual billing provider using a paper claim – Enter the individual billing number
and location code in field 44A. Enter the individual billing number in the Administrative
column adjacent to each detail submitted.
01/31/2007 Dental Services
Medical Policy Manual
112
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• Individual billing provider using Web InterChange – Enter the individual billing
number and location code in the provider number field. Enter the individual billing
number in the rendering provider field.
Dental Rendering Provider Information at the Service Line Level
For all dental claims, dental providers are required to submit rendering provider information at
the service line level when the billing provider is a group. Rendering providers are required to be
associated with the billing provider’s group. The requirement to record the individual dentist
performing the service is an added HIPAA requirement. The IHCP captures the rendering
provider information at the service line level.
The provider must include rendering provider number in the administrative column on the ADA
2000 Dental Claim form. Providers may also submit dental rendering provider information via
Web InterChange. Dental rendering provider information is contained in the 835 transaction.
Identification of Supernumerary Tooth Extractions
If using the claim note segment, the provider should identify the affected tooth by one of the
following:
• Adult – Designate the tooth ID by the appropriate tooth number followed by an A
• Child – Designate the tooth ID by the appropriate tooth letter followed by a 1
RBMC Carve Out Dental Guidelines
Dental services, which are performed by the following dental specialists and billed on the ADA
1999 Version 2000 Dental Claim Form (ADA 2000) or the 837 Health Care Claim: Dental (837D)
electronic transaction, are carved out or excluded from the responsibility of Hoosier Healthwise
Risk-based Managed Care (RBMC). The specialties include:
Endodontists
General Dentistry Practitioners
Oral Surgeons
Orthodontists
Pediatric Dentists
Periodontists
Mobile Dentists
Prosthodontists
Dental Clinic
All dental services billed using CDT-5 procedure codes must be submitted to EDS using either
the ADA 2000 claim form or the 837D transaction.
Dental providers that provide treatment for medical conditions may be billing the E/M codes,
and these services may include treatment of sleep apnea or Temporomandibular joint (TMJ)
syndrome. The scope of practice, defined in IC 25-14-1-23, allows for diagnosis or treatment of
the human oral cavity, teeth, gums, maxillary or mandibular structures.
01/31/2007 Dental Services
Medical Policy Manual
113

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
When dental services will be provided in an inpatient, outpatient hospital setting, or an ASC for
an RBMC member, the dental providers must first contact the member’s managed care
organization (MCO) before rendering services to determine whether prior authorization is
required. When the provider obtains MCO authorization and provides services, the services
must then be billed as follows:
Dental-related facility charges must be billed on a UB-92 claim form. Dental services provided
in an inpatient, outpatient, or ASC setting can be billed with CDT-5 codes on a dental claim
form. These services are carved out of RBMC, and must be billed to EDS using the ADA 2000
claim form or the 837D transaction. All other associated professional services (such as oral
surgery, radiology, and anesthesia) as well as ancillary services related to the dental services
must be billed to the MCO on the CMS-1500 claim form or the 837P transaction, along with
appropriate authorization information.
Package E Dental Provider Notice
Dental providers may have received inappropriate reimbursement for non-emergency services
rendered to Package E members. With the assistance of the Dental Advisory Panel (DAP), the
IHCP created a table of the CDT-5 codes that are allowed for reimbursement of emergency
services provided to Package E members.
CDT-5 Codes Allowed for Package E Members
CDT-5
Code
Description
D0140 Limited oral evaluation– problem focused
D0210 Intraoral – complete series (including bitewings)
D0220 Intraoral– periapical– first film
D0230 Intraoral– periapical– each additional film
D0240 Intraoral– occlusal film
D0270 Bitewing– single film
D0272 Bitewings– two films
D0274 Bitewing– four films
D0330 Panoramic film
D7111 Extraction, coronal remnants– deciduous tooth *
D7140 Extraction, erupted tooth or exposed root
D7210
Surgical removal of erupted tooth requiring elevation of mucoperiosteal flap and
removal of bone and/or section of tooth
D7220 Removal of impacted tooth– soft tissue
D7230 Removal of impacted tooth– partially bony
D7240 Removal of impacted tooth– completely bony
D7241 Removal of impacted tooth– completely bony, with unusual surgical complications
01/31/2007 Dental Services
Medical Policy Manual
114

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
CDT-5 Codes Allowed for Package E Members
CDT-5
Description
Code
D7250 Surgical removal of residual tooth roots (cutting procedure)
D7260 Oroantral fistula closure
D7261 Primary closure of sinus perforation
D7270 Tooth reimplantation and/or stabilization of accidentally evulsed or displaced tooth
D7280 Surgical access of unerupted tooth (impacted tooth not intended for extraction)
D7282 Mobilization of erupted or malpositioned tooth to aid eruption
D7285 Biopsy of oral tissue– hard
D7286 Biopsy of oral tissue– soft
D7288 Brush biopsy– transepithelial sample collection
D7510 Incision and drainage of abscess– intraoral soft tissue
D7511
Incision and drainage of abscess– intraoral soft tissue – complicated (includes drainage
of multiple fascial spaces)
D7520 Incision and drainage of abscess– extraoral soft tissue
D7521
Incision and drainage of abscess– extraoral soft tissue– complicated (includes drainage
of multiple fascial spaces)
D7560 Maxillary sinusotomy for removal of tooth fragment or foreign body
D7610 Maxilla– open reduction (simple fracture)
D7620 Maxilla– closed reduction (simple fracture)
D7630 Mandible– open reduction (simple fracture)
D7640 Mandible– closed reduction (simple fracture)
D7650 Malar and/or zygomatic arch– open reduction (simple fracture)
D7660 Malar and/or zygomatic arch– closed reduction (simple fracture)
D7670 Alveolus– closed reduction, may include stabilization of teeth (simple fracture)
D7671 Alveolus– open reduction, may include stabilization of teeth (simple fracture)
Radiographs must only be billed when the member presents with symptoms that warrant the
diagnostic service. In addition, providers are encouraged to refer to the IHCP Provider Manual
and IHCP provider newsletters NL200410 and NL200504 for related information.
NL200410 reminded dental providers of the importance of eligibility verification prior to
rendering services to IHCP members. It is important to verify eligibility prior to each visit, as
eligibility can change, be terminated, or include service limitations dependent on the program in
which the member is enrolled.
NL200504 reiterated the policy stated in the IHCP Provider Manual about field 53 of the ADA
Dental Claim Form. Field 53 is a required field and must be used to specify if the services
01/31/2007 Dental Services
Medical Policy Manual
115

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
performed were for emergency care. Providers must indicate “Yes” for all emergency care. All
services are subject to post-payment review and documentation must support medical necessity
for the services performed.
MCO Billing Codes
Refer to Table 6.4, Dental Related Anesthesia, Surgery, Radiology, and Laboratory CPT
Codes for a list of CPT codes that may be billed to a member’s managed care organization
(MCO). Dental providers should only bill the codes listed in Table 6.4 when a CDT-5 is not
appropriate.
Table 6.4
Dental Related Anesthesia, Surgery, Radiology, and Laboratory CPT Codes
Code Description
00100– 00352 Anesthesia (Head and Neck)
10021– 11646 Removal of Lesions or Skin Tags
12001– 16036 Wound Repair, Skin Grafts and Flaps, Burns
17000– 17999 Lesions
20150– 20694 TMJ Treatments, Biopsy
20900– 20926 Grafts
20999 Unlisted Procedure, Musculoskeletal System, General
21010– 21499 Musculoskeletal System Repairs
29800– 29804 Arthroscopy, TMJ
40490– 42999 Oral Surgery (above Esophagus)
64716 Neuroplasty and/or Transposition; Cranial Nerve
70100– 70380 Radiology
71010 Radiological Exam, Chest, Single View, Frontal
72020 Radiological Exam, Spine, Single View, Specify Level
72040 Radiological Exam, Spine, Cervical; Two or Three Views
72072 Radiological Exam, Spine, Thoracic, Three Views
72146 MRI, Spinal Canal and Contents, Thoracic
72285
Diskography, Cervical or Thoracic, Radiological Supervision
and Interpretation
76100
Radiological Exam, Single Plane Body Section, other than with
Urography
76536
Ultrasound, Soft Tissues of Head and Neck, B-Scan, and/or
Real Time with Image
80048– 89399 Pathology and Laboratory Codes
Note: If a member is enrolled in the PCCM delivery system, the PMP must authorize services
rendered in an inpatient, outpatient, or ASC setting for providers to receive reimbursement.
01/31/2007 Dental Services
Medical Policy Manual
116

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MANAGED CARE
The MCOs are responsible for determining which services require PA for its members. The
MCOs’ decisions to authorize, modify, or deny a given request is based on medical necessity,
reasonableness, and other criteria. A provider must make requests for reviews and appeals by
contacting the appropriate MCO.
RELATED MEDICAL TOPICS
Anesthesia Services
EPSDT - HealthWatch
Locum Tenens
Radiology
Surgery– Surgery and Anesthesia by the Same Provider
Surgery Surgical Services
RULES, CITATIONS, AND SOURCES
42 CFR 440.100 Dental Services
42 CFR 440.50 Physicians' services and medical and surgical services of a dentist
42 CFR 440.120 Prescribed drugs, dentures, prosthetic devices, and eyeglasses
405 IAC 5-14 Dental Services
405 IAC 5-1-5 Global fee billing; codes
405 IAC 5-2-2 Definitions
405 IAC 5-13-4 Intermediate Care Facilities for the Mentally Retarded
IHCP Provider Bulletin BT200511
IHCP Provider Bulletin BT200433
IHCP Provider Bulletin BT200326
IHCP Provider Bulletin BT200324
IHCP Provider Bulletin BT200321
IHCP Provider Bulletin BT200318
IHCP Provider Bulletin BT200313
IHCP Provider Bulletin BT200311
IHCP Provider Bulletin BT200302
IHCP Provider Bulletin BT200250
IHCP Provider Bulletin BT200230
IHCP Provider Bulletin BT200227
IHCP Provider Bulletin BT200141
IHCP Provider Bulletin BT200142
IHCP Provider Bulletin BT200003
Indiana Medicaid Update Bulletin 98-32
Indiana Medicaid Update Bulletin 98-23
Indiana Medicaid Update Bulletin 98-07
Indiana Medicaid Update Bulletin 98-03
Indiana Medicaid Update Bulletin 95-21
01/31/2007 Dental Services
Medical Policy Manual
117
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
IHCP Provider Banner BR200532
IHCP Provider Banner BR200526
IHCP Provider Banner BR200318
IHCP Provider Banner BR200310
IHCP Provider Banner BR200309
IHCP Provider Banner BR200308
IHCP Provider Banner BR200305
IHCP Provider Banner BR200245
IHCP Provider Banner BR200243
IHCP Provider Banner BR200241
IHCP Provider Banner BR200224
IHCP Provider Banner BR200219
IHCP Provider Banner BR200213
IHCP Provider Banner BR200208
IHCP Provider Banner BR200207
IHCP Provider Banner BR200206
IHCP Provider Banner BR200149
IHCP Provider Banner BR200104
IHCP Provider Banner BR200049
IHCP Provider Banner BR200045
IHCP Provider Banner BR200038
IHCP Provider Banner BR200226
IHCP Provider Banner BR199945
IHCP Provider Banner BR199905
IHCP Provider Banner BR199903
Indiana Medicaid Banner Page 1/19/99
Indiana Medicaid Banner Page 1/12/99
Indiana Medicaid Banner Page 1/5/99
Indiana Medicaid Banner Page 8/11/98
Indiana Medicaid Banner Page 8/4/98
Indiana Medicaid Banner Page 7/28/98
Indiana Health Coverage Programs Provider Manual 2003
HealthWatch Manual
01/31/2007 Dental Services
Medical Policy Manual
118

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 1 Sources For Policy Issues and Updates
Revisions and
Review
Reason Date
Indiana State
Department of Public
Welfare Medical
Policy Manual 1991
Dental; second opinion procedures, dental;
out-of-state medical services; intermediate
care facilities for the mentally retarded;
7/1/91
470 IAC 5-8-8
Transferred
Dental services
7/1/91
405 IAC 1-6-8
Repealed 8/24/97
Coverage of dental services
1/1/92
Indiana Medicaid
Update Bulletin 95-21
(DATED 5/2/95)
IndianaAIM Medicaid Medical Policy
Update- Dental Services Requiring
Anesthesia on an Outpatient Hospital or
Ambulatory Surgical Center (ASC)
2/20/95
Indiana Medicaid
Update Bulletin 94-42
(dated 9/6/94)
Dental Services
10/5/94
405 IAC 5-13-4 Intermediate care facilities for the
mentally retarded
8/24/97
405 IAC 5-14 Dental services policy
8/24/97
405 IAC 5-1-5 Global fee billing; codes
8/24/97
405 IAC 5-2-2 Definitions—ADA
8/24/97
42 CFR 440.100 Dental services
10/1/97
42 CFR 440.50 Physicians' services and medical and
surgical services of a dentist.
10/1/97
42 CFR 440.120 Prescribed drugs, dent-rues, prosthetic
devices, and eyeglasses.
10/1/97
828 IAC 4-1-1 Licensing of Mobile Dental Units
7/1/02
405 IAC 5-14-2
405 IAC 5-14-4
405 IAC 5-14-6
405 IAC 5-14-11
405 IAC 5-14-15
405 IAC 5-14-16
405 IAC 5-14-17
405 IAC 5-14-18
Covered Services
Topical Fluoride
Prophylaxis
Analgesia
Anesthesia
Periodontics; surgical
Oral surgery
Hospital Admission
2/10;03
2/10/03
2/10/03
2/10/03
2/10/03
2/10/03
2/10/03
2/10/03
405 IAC 5-14-1 $600 Dental Cap
3/1/03
Indiana Medicaid
Update Bulletin 94-42
Dental services
10/5/94
01/31/2007 Dental Services
Medical Policy Manual
119

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Revisions and
Reason Date
Review
Indiana Medicaid
Update Bulletin 95-21
IndianaAIM Medicaid medical policy
update—dental services requiring
anesthesia in an outpatient hospital or
ambulatory surgical center (ASC)
2/20/95
Indiana Medicaid
Update Bulletin 98-03
Dental Services
9/1/97
Indiana Medicaid
Update Bulletin 98-07
Dental services and managed care organ-
izations
8/1/98
Indiana Medicaid
Update Bulletin 98-23
Dental changes: Rate Increase
PA Lifted RBMC Changes
5/1/98
9/1/97
8/1/98
Indiana Medicaid
Update Bulletin 98-32
Update on dental procedure codes
6/5/98
IHCP BT199933 Smoking Cessation TX
10/27/99
IHCP BT200003 Coverage of dentures and partials
1/14/00
IHCP BT200141 Dental claim instructions
11/01/01
IHCP BT200142 D7999, D9999
12/17/01
IHCP BT200201 Locum Tenens Policy
1/18/02
01/31/2007 Dental Services
Medical Policy Manual
120

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Revisions:
Revisions and
Review
Reason Date
IHCP BT200227 Dental code changes
7/29/02
IHCP BT200230 Criteria for orthodontic services
8/05/02
IHCP BT200250 End dating and limitations of dental codes 11/01/02
10/15/02
IHCP BT200301 Implementation of the $600 dental cap
1/15/03
IHCP BT200302 Implementation of the $600 dental cap
1/15/03
IHCP BT200310 $600 dental limit for members 21 and
older
3/1/03
IHCP BT200311 Delayed implementation of the $600
dental cap
3/1/03
IHCP BT200313 New 2003 HCPCS Coding Systems.
4/1/01
IHCP BT200318 Change in method of filing RHC and
FQHC claims.
3/07/03
IHCP BT200321 Correct Codes for Billing of IHCP Dental
Services
4/1/01
IHCP BT200324 Changes to the $600 Dental Cap
06/01/03
ICHP BT200326 Change in Dental Coverage
5/31/03
APPLICABLE INDIANAAIM EDITS AND AUDITS
0261, Tooth number missing
0262, Tooth number invalid
0263, One or more of the tooth surface codes billed is invalid
0266, Insufficient number of valid tooth surface codes
3011, Out-of-state provider requires prior authorization- rendering provider
4211, Tooth number/procedure code combination invalid
5010, Exact duplicate- tooth surface
5011, Possible duplicate- tooth surface
6000, Manual pricing required
6032, Extraction/surgical procedures payable at reduced amount when suturing paid
6033, Prophylaxis limited to two treatments every six months- institutionalized
6036, Oral surgery payable at reduced rate when apicoectomy previously paid
6199, Fluoride limited to one treatment every six months
6200, Panoramic/full mouth versus bitewings/periapical
6201, More than one reline/rebase/repair of dentures within three years
6202, Palliative versus other emergency services
6203, Relines and rebases vs. initial denture lower placement
6204, Pulpotomy versus root canal therapy
01/31/2007 Dental Services
Medical Policy Manual
121
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Dental Services
Medical Policy Manual
122
6205, Apicoectomy versus oral surgery
6208, Occlusal films limited to two units
6209, Full mouth or panoramic x-rays limited to once every three years
6210, Prophylaxis limited to one treatment every six months for non-institutionalized age 18
months to 21 years.
6211, Periodic/limited oral evaluation limit one every six months
6212, Fluoride treatments limited to one every six months for members ages 18 months
through 18 years of age
6213, Prosthodonic adjustment
6214, Root canal payable at reduced amount when pulpotomy paid
6216, Lower denture relines limited to one per 36 months
6217, Gingival curettage payable at a reduced amount
6218, One pulp cap per tooth per lifetime
6219, Periodontal scaling and planing- two quadrants per day
6220, Replacement greater than three teeth- denture partial or complete
6221, Periodontal root planing and scaling limited to four treatments every two years
non-instituionalized
6222, Periodontal root planing and scaling limited to four treatments every two years
member regardless of age
6223, Periodontal root planing & scaling non-institutionalized member 21 years or older
limited to 4 treatments per lifetime
6224, One tooth extraction per tooth per lifetime
6225, One sealant per tooth per lifetime/21 years or younger
6226, Comprehensive/extensive oral examination limited to one per lifetime
6227, Emergency services versus palliative treatment
6228, Denture reline versus denture repair
6229, Relines and rebases versus initial denture upper replacement
6232, Prophylaxis- adult will be limited to one treatment every six months for
institutionalized members 13 years old and older
6234, Suturing versus extractions and surgical procedures
6235, Prophylaxis non-institutional 21 years or older limited to 1 (one) per 12 months
6236, Dental services limited to $600 for members 21 years old or older

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: DERMATOLOGY
DESCRIPTION
As defined in Dorland’s Medical Dictionary, Edition 28, 1994, dermatology is the
medical specialty concerned with the diagnosis and treatment of diseases of the skin.
This document is intended to serve as a general summary of the Indiana Health Coverage
Programs’ (IHCP) policies regarding this service. More specific information may be
found in the IHCP Provider Manual, program notices, the Indiana Administrative Code
(IAC) or other sources as appropriate. See the “Rules, Citations, and Sources” section of
this document for further information.
COVERAGE CRITERIA
The IHCP will provide for reimbursement for dermatology services billed with an
appropriate procedure code when medically necessary. Generally, prior authorization
(PA) is not required; however, specific procedure codes may require PA. Documentation
in the medical record must support medical necessity. According to Indiana
Administrative Code (IAC) citation 405 IAC 5-29-1, the following are noncovered
services :
• Dermabrasion surgery for acne pitting or marsupialization
• Scar removals or tattoos removals by excision or abrasion
• Removal of keloids from pierced ears when they are less than 3 centimeters or they
obstruct fifty percent of the ear canal
PRIOR AUTHORIZATION
Generally, prior authorization is not required.
01/31/2007 Dermatology
Medical Policy Manual
123

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MANAGED CARE
For members enrolled in Risk Based Managed Care (RBMC), providers must contact the
member’s managed care organization (MCO) for more specific guidelines. Refer to the
Provider Manual, Chapter 1, for detailed information about the fee-for-service (FFS),
Primary Care Case Management (PCCM), and Risk Based Managed Care (RBMC)
delivery systems.
IHCP members enrolled in Medicaid Select PCCM receive the same benefit coverage,
and are subject to the same limitations as traditional Medicaid FFS. Refer to the
Medicaid Select Manual for Primary Medical Providers and Office Staff for further
information.
BILLING REQUIREMENTS
Providers must bill for dermatology services using the established billing guidelines.
RELATED MEDICAL TOPICS
Consultations - Second Opinion
Evaluation and Management Services
Physician Services
RULES, CITATIONS, AND SOURCES
405 IAC 5-29-1 Noncovered services
Indiana Health Coverage Programs Provider Manual
Version 5.1, March 2005
Origination Date: 12/31/2000
Revisions and
Review
Reason Date
470 IAC 5-9-1
Transferred
Global Billing Fee 07/01/1991
405 IAC 1-7-1
Repealed 8/24/97
Global Fee Billing 01/01/1992
405 IAC 1-6-1;
1-6-9; 1-6-15; 1-
7-15; Repealed
8/24/97
Noncovered Services 01/01/1992
405 IAC 5-1-5 Global Billing Fee 08/24/1997
405 IAC 5-29-1 Noncovered Services 08/24/1997
01/31/2007 Dermatology
Medical Policy Manual
124

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Dermatology
Medical Policy Manual
125
Revisions and
Review
Reason Date
405 IAC 5-29-1
Amended
Noncovered Services 10/27/1999
Review Scheduled 10/31/2006
APPLICABLE INDIANAAIM EDITS AND AUDITS
6649 – Surgery Payable at Reduced Amount When Related POS
6651 – Surgical Cutback Procedure – 50%
6653 – Post Op Care Within 00-90 Days of Surgery
6654 – Pre-Operative Care Within 1 Day of Surgery
6655 – Surgery Payable at Reduced Amount When Pre-Op Care
6656 – Post Op Care Within 10 Days of Select Surgery
6657 – Pre-Operative Care on Day of Surgery
6658 – Surgery Payable at Reduced Amount When Pre-Op Care
6659 – Surgery Payable at Reduced Amount When Related POS
6660 – Pre/Post-Operative Care Billed with Unlisted Surgery

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: DIABETES SELF-MANAGEMENT TRAINING
DESCRIPTION
Indiana Health Coverage Programs’ (IHCP) diabetes self-management training services
are intended to enable or enhance the member’s ability to properly manage their diabetic
condition. The purpose of these services is to optimize personal therapeutic treatment
regimens.
This document is intended to serve as a general summary of the Indiana Health Coverage
Programs’ (IHCP) policies regarding this service. More specific information may be
found in the IHCP Provider Manual, program notices, the Indiana Administrative Code
(IAC) or other sources as appropriate. See the “Rules, Citations, and Sources” section of
this document for further information.
COVERAGE CRITERIA
The IHCP will provide reimbursement for diabetes self-management training services
when medically necessary, ordered in writing by a physician or podiatrist, and provided
by a health care professional licensed under applicable Indiana law. The health care
professional providing the service must have specialized training in the management of
diabetes. Some examples of the training include, but would not be limited to, instruction
regarding the diabetic disease state, nutrition, exercise and activity, medication
counseling, blood glucose self-monitoring training, insulin injection training, foot, skin,
eye, and dental care.
The IAC citation, 405 IAC 5-36 mandates coverage limitations of diabetes self-
management training to a total of sixteen units with each unit equaling 15 minutes. IHCP
reimbursement of diabetes self-management training services is limited to a total of eight
units of G0108, Diabetes outpatient self-management training services, individual, per
30 minutes, and G0109, Diabetes self-management training services, group session (2 or
more), per 30 minutes, for diabetes self-management training services per member, per
rolling calendar year, applicable under any of the following circumstances.
• Receipt of a diagnosis of diabetes
• Receipt of a diagnosis that represents a significant change in the insured’s
symptoms or condition
• Re-education or refresher training
01/31/2007 Diabetes Self-Management Training
Medical Policy Manual
126
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Practitioners, both ordering and rendering, are to maintain sufficient documentation of
the diabetes self-management training services provided to substantiate medical necessity
and the provision of the service itself. Examples of documentation that are to be
maintained by the provider include, but are not limited to, the following.
• Written order(s) for the service
• Date of service
• Amount of time used for the training session
• General content of the training session
• Units of service billed and charge amount
• Pertinent patient history and clinical data
• Practitioner notes from the training sessions
PRIOR AUTHORIZATION
Prior authorization is NOT required for initial units of diabetes self-management training
services that do not exceed the established limits for the service. However, additional
units may be authorized through the standard prior authorization request process.
Documentation must be maintained to provide evidence of medical necessity for
additional units requested.
It is the responsibility of the ordering physician or podiatrist to ensure that initial and all
subsequent orders for diabetes self-management training are fully substantiated by
medical necessity of the service.
MANAGED CARE
For members enrolled in Risk Based Managed Care (RBMC), providers must contact the
member’s managed care organization (MCO) for more specific guidelines. Refer to the
Provider Manual, Chapter 1, for detailed information about the fee-for-service (FFS),
Primary Care Case Management (PCCM), and RBMC delivery systems.
IHCP members enrolled in Medicaid Select PCCM receive the same benefit coverage,
and are subject to the same limitations as traditional Medicaid FFS. Refer to the
Medicaid Select Manual for Primary Medical Providers nd Office Staff for further
information.
BILLING REQUIREMENTS
Providers must bill for diabetes self management training service on the HCFA 1500
claim form or 837P electronic transaction, utilizing HCPCS procedure codes G0108,
Diabetes outpatient self-management training services, individual, per 30 minutes, and
G0109, Diabetes self-management training services, group session (2 or more), per 30
minutes. Fractional units of service can be billed on the CMS 1500 with allowance for
01/31/2007 Diabetes Self-Management Training
Medical Policy Manual
127

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Diabetes Self-Management Training
Medical Policy Manual
128
two decimal places when submitting partial units. The IHCP does not provide
reimbursement for a diabetes self-management training service which is provided to the
general public at no charge, including diabetes self-management training services.
RELATED MEDICAL TOPICS
Endocrinology – Insulin
Nursing Services
Podiatry
RULES, CITATIONS, AND SOURCES
Indiana Senate Enrolled Act No. 184, IC 27-8-14.5, effective January 1, 1998
Indiana Medicaid Update Bulletin 98-05
Indiana Health Coverage Programs Provider Manual
Version 5.1, March 2005
Medicaid Select Manual for Primary Care Providers and Office Staff, 2003
Origination Date: 12/31/2000
Revisions and Reviews Reason Date
Indiana SEA No. 184,
IC 27-8-14.5; Indiana
Medicaid Update
Bulletin 98-05
Diabetes Self-Management Training 01/01/1998
Revision 405 IAC 5-36 Amended 10/27/1999
Review Scheduled 10/31/2006
APPLICABLE INDIANAAIM EDITS AND AUDITS
6096 – The CPT/HCPCS Code Billed is Not Payable
6652 – Multiple Surgeries Must Be Billed on Same Claim
6798 – Services Not Covered for Telemedicine
6920 – Diabetes Management Limited to 8 Units in 12 Months

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: DIAGNOSTIC STUDIES
DESCRIPTION:
Diagnostic Studies are invasive and non-invasive tests completed to determine the
probable cause of the patient’s signs and symptoms. These studies can be done as an
inpatient or outpatient, dependent on the severity of the signs and symptoms and the
treatment they require.
SUMMARY OF CURRENT POLICY:
Medical diagnostic services may not be fragmented and billed separately.
MEDICAL TOPICS CROSS-REFERENCES:
Consultations – Second Opinion
Endocrinology - Insulin
Gastroenterology
Hematology
Mental/Behavioral Health – Inpatient Services
Mental/Behavioral Health – Outpatient Services
Obstetric Care
Oncology
Physician Services – Visits/New patient vs. Established Patient Visits: Office, Home,
Nursing Facility, Emergency Room
Urology
RULES, CITATIONS, AND SOURCES:
405 IAC 5-28-2
Indiana State Department of Public Welfare Medical Policy Manual 1991
Indiana Health Coverage Programs Provider Manual
01/31/2007 Diagnostic Studies
Medical Policy Manual
129

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Diagnostic Studies
Medical Policy Manual
130
Initial Policy Issue
Effective
Date
Implementation
Date
Retroactive
Date
470 IAC 5-9-1
Transferred
Global Fee Billing
7/1/91
405 IAC 1-7-1
Repealed 8/24/97
Coverage of
Diagnostic
Procedures
1/1/92
Revisions:
405 IAC 5-28-1
Diagnostic Studies 8/24/97
405 IAC 5-28-2
Diagnostic Studies 8/24/97
APPLICABLE INDIANAAIM EDITS AND AUDITS:
6001
6011
6014
6024
6025
6026
6064

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: EMERGENCY MEDICINE—CARDIOPULMONARY
RESUSCITATION (CPR)
DESCRIPTION
Cardiopulmonary Resuscitation is the process used to return the patient presenting with
sudden cardiac arrhythmia and/or sudden respiratory failure to normal respiratory and
cardiac function.
SUMMARY OF CURRENT POLICY
Cardiopulmonary Resuscitation is a covered service. It is an all inclusive procedure and
includes central venous pressure catheterization, insertion of arterial lines, endotracheal
intubation, and cardioversion.
MEDICAL TOPICS CROSS-REFERENCESRELATED MEDICAL TOPICS
Emergency Medicine – Emergency Room
Emergency Medicine – Emergency Services
Hospital Outpatient
Physician Services – Visits/New patient vs. Established Patient Visits: Office, Home,
Nursing Facility, Emergency Room
Surgery – Office Visits
RULES, CITATIONS, AND SOURCES
405 IAC 5-10-3 Anesthesia Services
Indiana State Department of Public Welfare Medical Policy Manual 1991
ORIGINATION, REVISIONS, AND REVIEWS
Origination Date: 12/31/00
Revisions and Reviews Reason Date
405 IAC 5-10-3 Anesthesia Services 8/24/97
Reviewed Without Revision Scheduled Review 4/30/04
01/31/2007 Emergency Medicine – Cardiopulmonary Resuscitation (CPR)
Medical Policy Manual
131
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Emergency Medicine – Cardiopulmonary Resuscitation (CPR)
Medical Policy Manual
132
APPLICABLE INDIANAAIM EDITS AND AUDITS
COVERAGE CRITERIA
Cardiopulmonary Resuscitation (CPR)
Cardiopulmonary resuscitation (92950) is an all inclusive procedure. Therefore, separate
charges for central venous pressure catheterization (36488, 36489), insertion of arterial
lines (36620), endotracheal intubation (31500), and cardioversion (92960) will be denied
as included in the charge for CPR. Additional charges for care of the member on the
same day by the same physician (for example, intensive care visits, hospital visits,
emergency room visits) will also be denied as included in the charge for the
cardiopulmonary resuscitation.
1. Payment in full is allowed for a Swan-Ganz in addition to the CPR charge.
2. These guidelines apply when the same physician bills separately for CPR and any of
the above components. Physicians other than the primary physician will be paid for
services they provide during the cardiopulmonary resuscitation.
3. These guidelines apply when the same physician bills separately for CPR and any of
the above components. Physicians other than the primary physician should be paid
for services that they provide during the cardiopulmonary resuscitation.

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: EMERGENCY MEDICINE—EMERGENCY ROOM
DESCRIPTION
Emergency services are services provided in the emergency department of a hospital.
Emergency services include services provided to an Indiana Heath Coverage Programs
(IHCP) member after the sudden onset of a medical condition manifesting itself by acute
symptoms of sufficient severity that the absence of immediate medical attention could
reasonably be expected to result in placing the member's health in serious jeopardy;
serious impairment to bodily functions; or serious dysfunction of any bodily organ or part.
SUMMARY OF CURRENT POLICY
Reimbursement is available for emergency department services provided by IHCP
enrolled providers. Emergency services (as described above) do not require prior
authorization. Emergency services are excluded from co-payment requirements.
However, non-emergency services treated in an emergency department setting are subject
to co-payments. Members on restricted utilization may receive treatment without a
referral from the authorized provider if the diagnosis is an emergency diagnosis.
Members enrolled in the RBMC component of Hoosier Healthwise can seek emergency
care from providers not contracted with the managed care organization without prior
authorization, subject to the prudent layperson standard for emergency medical
conditions. This means that a layperson with an average knowledge of health and
medicine could reasonably expect the absence of immediate medical attention to result in
(a) serious jeopardy to the health of the individual or, in the case of a pregnant women,
the health of the woman or her unborn child; (b) serious impairment to bodily functions,
or (c) serious dysfunction of any bodily organ or part.
The Primary Care Case Management (PCCM) component of the Hoosier Healthwise
managed care program does not require Primary Medical Provider (PMP) authorization
for federally required medical screening examinations performed by a physician in the
emergency department of a hospital. CPT codes reflecting the appropriate level of
screening exam must be billed on a HCFA 1500 form:
99281
Emergency Department Visit for evaluation and management;
presenting problem(s) are self limiting or minor
99282
Emergency Department Visit for evaluation and management;
presenting problem(s) are low to moderate severity
01/31/2007 Emergency Medicine – Emergency Room
Medical Policy Manual
133

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
99283
Emergency Department Visit for evaluation and management;
presenting problem(s) are of moderate severity
99284
Emergency Department Visit for evaluation and management;
presenting problem(s) are high severity; require urgent evaluation
by physician but do not pose immediate threat to life or
physiologic function
99285
Emergency Department Visit for evaluation and management;
presenting problem(s) are high severity and pose immediate threat
to life or physiologic function
Related services that do not have an emergency diagnosis and emergency indicator on the
claim will be denied if the claim does not contain the PMP authorization.
If a physician uses an emergency room as a substitute for his or her office for non-
emergency services, these visits should be billed as office visits and will be reimbursed as
such.
RELATED MEDICAL TOPICS
Emergency Medicine – Cardiopulmonary Resuscitation (CPR)
Emergency Medicine – Emergency Services
Hospital Outpatient
Physician Services – Visits/New patient vs. Established patient visits: Office, Home,
Nursing Facility, Emergency Room
Out-of-State Services
RULES, CITATIONS, AND SOURCES
IC 12-15-15-2.5 Payment for physician services provided in the emergency department of
a hospital
405 IAC 5-2-9 “Emergency service” defined
405 IAC 5-3-12 Prior authorization; exceptions
Indiana Medicaid Update Bulletin 96-36
Indiana Medicaid Update Bulletin 95-51
Indiana Medical Assistance Program Provider Manual 1994
Indiana Health Coverage Programs Provider Manual 1999
Indiana Health Coverage Programs Provider Manual 2003
01/31/2007 Emergency Medicine – Emergency Room
Medical Policy Manual
134

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Origination Date 7/1/91
REVIEW AND REVISIONS REASON DATE
470 IAC 5-9-1 Transferred Global Fee Billing 7/1/91
405 IAC 1-7-1 Repealed 8/24/97 Global Fee Billing 1/1/92
Indiana Medicaid Update Bulletin
95-51
Medical Screening
Exams in the
Emergency Room
1995
Indiana Medicaid Update Bulletin
96-36
COPAYMENTS
FOR
EMERGENCY
ROOM VISITS
1/1/97
IC 12-15-15-2.5
Payment for
Physician Services
Provided in the
Emergency
Department of a
Hospital
5/13/97
405 IAC 5-2-9
"Emergency
Service" Defined
8/24/97
405 IAC 5-3-12
Prior
Authorization;
Exceptions
8/24/97
405 IAC 5-9-2
Evaluation and
Management
Services--
Restrictions
8/24/97
IC 12-15-15-2.5(f)
Payment for
Physician Services
Provided in the
Emergency
Department of a
Hospital
7/1/99
405 IAC 5-3-12
AMENDED
Prior
Authorization;
Exceptions
10/27/99
Review
Scheduled
7/30/04
01/31/2007 Emergency Medicine – Emergency Room
Medical Policy Manual
135

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Emergency Medicine – Emergency Room
Medical Policy Manual
136
APPLICABLE INDIANAAIM EDITS AND AUDITS:
440
6514
Provider Type and Specialty List
PROVIDER TYPE Provider Specialty
31 Physician 315 Emergency Medicine Practitioner

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: EMERGENCY MEDICINE—EMERGENCY SERVICES
DESCRIPTION
Emergency services include unscheduled episodic services provided to IHCP members
who require immediate medical attention.
SUMMARY OF CURRENT POLICY
IHCP reimbursement is available for emergency services provided to IHCP members. As
defined by the prudent layperson provision in the Omnibus Budget Reconciliation Act
(OBRA) of 1986, an emergency may be perceived by the sudden onset of a medical
condition manifesting itself by acute symptoms of such severity that the absence of
immediate medical attention could reasonably be expected to result in (1) placing the
patient’s health in serious jeopardy, (2) serious impairment to bodily functions, and (3)
serious dysfunction of any bodily organ or part.
Hoosier Healthwise Package E members (aliens, non-citizens) receive only emergency
services and the services are reimbursed as fee for service. Package E services must meet
emergency criteria as noted in the paragraph above. In the case of pregnant women eligible
for coverage under Package E, labor and delivery services are also considered emergency
medical conditions.
Providers must denote that a provided service was emergent by indicating such in the
appropriate field on submitted claim forms.
COVERAGE CRITERIA
IHCP provides reimbursement for emergency services; guidelines for these services are
subject to the member’s program enrollment. Hoosier Healthwise Programs A and B
enrollees are required to pay a copayment for non-emergency services provided in the
emergency department. Per federal regulations, IHCP providers may not deny services to
any member due to the member’s inability to pay the copayment on the date of service.
This federal requirement does not apply to a member who is able to pay, nor does a
member’s inability to pay eliminate his or her liability for the copayment. It is the
member’s responsibility to inform the provider that he or she cannot afford to pay the
copayment on the date of service. The provider may bill the member for copayments not
01/31/2007 Emergency Medicine – Emergency Services
Medical Policy Manual
137
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
made on the date of service. The following information discusses emergency services by
program area, as applicable.
Cardiopulmonary Resuscitation
The IHCP reimburses cardiopulmonary resuscitation. Refer to the Medical Policy fact
sheet on Emergency Services – Cardiopulmonary Resuscitation for specific coverage
information.
Emergency Dental Services
The IHCP reimburses medically necessary emergency dental services. Hoosier
Healthwise Package E members are eligible only for services to treat an emergency
medical condition. Preventive treatments such as sealants, prophylaxis, and fluoride
treatments do not meet the definition of an emergency medical condition. Package E
members who seek dental services that are non-emergencies are responsible for payment
of such services.
Field 53 on the ADA dental claim form must be used to specify if the services performed
were for emergency care. Providers must indicate Yes for emergency care rendered to
Package E members. All services are subject to post-payment review, and documentation
must support medical necessity for the services performed.
Palliative treatment of facial pain, such as an abscess incision and drainage is limited to
emergency treatment only. HCPCS code D0140 can be billed for the emergency exam.
If the procedure for the palliative care has a corresponding ADA code, the code for the
procedure is billed rather than the code for palliative care.
Emergency Services Related to Hospice
When an IHCP member elects the IHCP hospice benefit, care for the terminal condition
comes under the supervision of the IHCP hospice provider. The IHCP covers the IHCP
hospice member’s medical care for conditions not related to the terminal illness.
If emergency services are related to the terminal illness and the hospice member has not
revoked the hospice benefit, the hospice provider is responsible for hospice and
transportation charges associated with all emergency services. If the emergency services
are unrelated to the terminal illness, the IHCP covers transportation and facility services
associated with the emergency services according to the member’s program enrollment,
(i.e. Fee-for-Service and Hoosier Healthwise). Refer to the Indiana Health Coverage
Programs Hospice Provider Manual and the Medical Policy fact sheet on Hospice
Services for specific coverage information.
Inpatient Services
Inpatient services for diagnoses reimbursed under the level of care payment methodology
and emergency substance abuse require prior authorization (PA). Emergency inpatient
admissions for these diagnoses must be reported to PA within forty-eight hours of
admission, not including Saturdays, Sundays, or legal holidays in order to receive IHCP
reimbursement.
01/31/2007 Emergency Medicine – Emergency Services
Medical Policy Manual
138
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Pharmacy Services
Pharmacy services are exempt from copayment requirements when emergency services
are provided in a hospital, clinic, office, or other facility equipped to furnish emergency
care.
In accordance with federal law, the IHCP allows for the provision of at least a 72 hour
supply of a prescribed drug in an emergency situation, without otherwise applicable PA
(such as, on a weekend, holiday, and so forth). Pharmacy providers should document the
circumstances that support providing the emergency supply and are subject to post
payment review. Providers should refer to the Medical Policy fact sheet on Pharmacy
Services for specific coverage and billing information.
Emergency Department Physicians
ICHP reimbursement is available to emergency department physicians who render
medically necessary emergency service to IHCP members. PCCM members are not required
to obtain a PMP authorization for the federally required medical screening examinations
performed by a physician in the emergency department of a hospital. One of the CPT
codes listed below, reflecting the appropriate level of screening exam, must be billed on
the CMS 1500 claim form or the 837P transaction.
99281 – Emergency department visit for evaluation and management,
usually, presenting problem(s) are self limiting or minor
99282 – Emergency department visit for evaluation and management,
usually, presenting problem(s) are of low to moderate severity
99283 – Emergency department visit for evaluation and management,
usually, presenting problem(s) are of moderate severity
99284 – Emergency department visit for evaluation and management,
presenting problem(s) of high severity, require urgent
evaluation by the physician, do not pose significant threat to
life/physiologic function
99285 – Emergency department visit for evaluation and management,
presenting problem(s) of high severity, pose immediate
significant threat to life/physiologic function
Psychiatric Services
The physician or HSPP must be available for emergencies and must either see the patient
or review the information obtained by the mid-level practitioner within seven days of the
intake process. IHCP reimbursement is available for emergency mental health admissions
in cases of a sudden onset of a psychiatric condition manifesting in acute symptoms of
such severity that the absence of immediate medical attention could reasonable be
expected to result in danger to the member or to others. Refer to the Medical Policy fact
sheets for Mental and Behavioral Health Services for inpatient hospitalization and
outpatient services for specific coverage information.
Transportation Services
01/31/2007 Emergency Medicine – Emergency Services
Medical Policy Manual
139
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Emergency transportation services are covered by the IHCP. Refer to the Medical Policy
Fact sheet on Transportation Services for specific coverage information.
BILLING REQUIREMENTS
Facility payments for services rendered in the emergency department are the same for
emergent and non-emergent care. Under federal regulations (42 CFR 447.53), emergency
services are excluded from copayment requirements.
Revenue codes 70X, 71X, 72X, and 76X are reimbursed at a flat rate regardless of the
diagnosis code on the claim. For a service to be considered an emergency, an applicable
emergency diagnosis code must be entered as the principal diagnosis in Locator 67 on the
UB-92.
Providers submitting claims for reimbursement of services to Hoosier Healthwise Package E
members must indicate emergency in the proper form locator on the claim form. The
diagnosis codes must include an appropriate code from the comprehensive list of codes
considered as emergencies, located in the Provider Manual, Chapter 8, entitled
“Emergency Department Diagnosis Codes.”
PRIOR AUTHORIZATION
Prior authorization (PA) is not required for emergency services.
Emergency inpatient admissions for diagnoses reimbursed under the level of care
payment methodology and emergency substance abuse inpatient admissions must be
reported to PA within forty-eight (48) hours of admission, not including Saturdays,
Sundays, or legal holidays, in order to receive IHCP reimbursement. IHCP reimbursement is
available for emergency mental health admissions only in cases of a sudden onset of a
psychiatric condition manifesting in acute symptoms of such severity that the absence of
immediate medical attention could reasonably be expected to result in danger to the
member or to others.
Continuation of inpatient treatment and hospitalization, following out-of-state emergency
services is subject to prior authorization requirements. Refer to the Medical Policy fact
sheet for Out-of-State Services for further information.
MANAGED CARE
IHCP members enrolled in Hoosier Healthwise programs are encouraged to take
responsibility for the choices they make in seeking medical care, particularly with regard
to use of the emergency room. Each Hoosier Healthwise member has chosen or been
assigned to a primary medical provider (PMP) who is available 24 hours a day to give
medical advice. Members are educated to contact their PMP prior to seeking treatment in
01/31/2007 Emergency Medicine – Emergency Services
Medical Policy Manual
140

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
the emergency room except in cases of true emergencies. Members are encouraged to
seek emergency room treatment only when it is appropriate. Thus, to the extent
permitted by federal and state regulations, a copayment is charged when there is not a
true emergency and the emergency room setting was chosen without the authorization of
the PMP.
While authorization is not required for emergencies, non-authorized services, such as
facility charges, labs, and x-ray services, rendered to a PCCM member without an
emergency diagnosis may not be covered. These claims may be suspended for review to
determine if the prudent layperson standard (see page 1 of this fact sheet) for an
emergency medical condition has been met. If the review results in the determination
that the prudent layperson standard has not been met, the claim will be denied. If the
prudent layperson standard has been met, then only revenue code 45X will be paid, as
long as the eight digit PMP license number is indicated in Field 83B of the UB-92 claim
form. As noted previously on page 3, a PMP authorization is not required for a federally
required medical screening examination performed by a physician in the emergency
department of a hospital.
For members enrolled in Risk Based Managed Care (RBMC), providers must contact the
member’s MCO for more specific guidelines. Refer to the Provider Manual, Chapter 1,
for detailed information about the fee-for-service (FFS), Primary Care Case Management
(PCCM), and RBMC delivery systems.
RELATED MEDICAL TOPICS
Dental Services
Emergency Medicine – Emergency Room
Emergency Medicine – Cardiopulmonary Resuscitation
Hospice Services
Hospital Outpatient
Mental Health Services – Inpatient
Out-of-State Services
Physician Services – Visits/New patient vs. Established patient visits: Office, Home,
Nursing Facility, Emergency Room
Transportation Services
RULES, CITATIONS, AND SOURCES
42 CFR 447.15 – Member copayment
405 IAC 5-2-9 – “Emergency service” defined
405 IAC 5-3-12 – Prior authorization; exceptions
405 IAC 5-4-2 – Provider agreement requirements for transportation services
405 IAC 5-17-3 – Emergency; weekend inpatient admissions
405 IAC 5-20-6 – Emergency admissions
01/31/2007 Emergency Medicine – Emergency Services
Medical Policy Manual
141

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Indiana Medicaid – Update Bulletins
95-17, 96-36, and 98-04
Indiana State Department of Public Welfare Medical Policy Manual 1991
Indiana Health Coverage Programs Hospice Provider Manual, March 2004, Version 4.0
Indiana Health Coverage Programs Provider Bulletin BT200311 – Package E Dental
Services
Indiana Health Coverage Programs Provider Newsletter NL200410 – Dental Services
Indiana Health Coverage Programs Provider Manuals
1999
July 2004, Version 5.0
ORIGINATION, REVISIONS AND REVIEWS
Origination Date: 12/31/2000
Revisions and Reviews Reason Date
Indiana State Department
of Public Welfare Medical
Policy Manual 1991
Emergency Services 7/1/91
Indiana Medicaid Update
Bulletin 95-17
MCOs’ Coverage Of Emergency Services 3/28/95
Indiana Medicaid Update
Bulletin 96-36
Copayments For Urgent And Nonemergency
Services Provided In An Emergency Room
1/97
405 IAC 5-2-9 "Emergency service" defined 8/24/97
Indiana Medicaid Update
Bulletin 98-04,
Emergency Room Transportation 2/9/98
Indiana Health Coverage
Programs BT 200311
Package E Dental Services 2/7/03
Review Scheduled 4/29/05
APPLICABLE INDIANAAIM EDITS AND AUDITS
440 – Emergency Indicator Invalid
4090 – Drug and Supply Codes are Included in Treatment
4091 – Add-on Service was Billed Without a Treatment Room
6514 – No More than One Emergency Room Visit per Day
01/31/2007 Emergency Medicine – Emergency Services
Medical Policy Manual
142

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
EMERGENCY MEDICINE – EMERGENCY SERVICES
ADDENDUM A
Note: This addendum contains provider notifications that have been published since the
review of the Emergency Medicine – Emergency Services Fact Sheet. The information
in this addendum will be incorporated into the fact sheet at the next review.
Provider Notification: BR200539 Publication Date: 09/27/2005
Subject: Emergency Department Diagnosis Codes
Date Added to Manual: 10/31/2005
Text of Publication
Beginning October 1, 2005 please use the following updated International Classification
of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. The new, revised,
and discontinued codes may be viewed at
http://www.cms.hhs.gov/medlearn/icd9code.asp. To ensure Health Insurance Portability
and Accountability Act (HIPAA) compliance, the 90-day grace period no longer applies
to ICD-9-CM updates. Providers must use the appropriate ICD-9-CM diagnosis and
procedure codes that are valid for the date of service. Codes not valid for the dates of
service will deny. The ICD-9-CM diagnosis and procedure codes are billable and
reimbursable October 1, 2005.
The following new ICD-9-CM diagnosis codes will be added to Table 8.13 – Emergency
Department Diagnosis Codes in the IHCP Provider Manual, Chapter 8, Section 2. These
codes are effective October 1, 2005.
ICD-9-CM Diagnosis Codes Added to Table 8.13 Emergency Department Diagnosis
Codes
276.50 567.38 651.70 770.12 770.86 996.44
276.51 567.39 651.71 770.13 779.84 996.45
276.52 567.81 651.73 770.14 799.01 996.46
567.21 567.82 760.77 770.15 799.02 996.47
567.22 567.89 760.78 770.16 996.40 996.49
567.23 585.6 763.84 770.17 996.41 V46.14
567.29 599.60 770.10 770.18 996.42 V62.84
567.31 599.69 770.11 770.85 996.43
01/31/2007 Emergency Medicine – Emergency Services
Medical Policy Manual
143

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Emergency Medicine – Emergency Services
Medical Policy Manual
144
The following ICD-9-CM diagnosis codes will be removed from Table 8.13 – Emergency
Department Diagnosis Codes in the IHCP Provider Manual, Chapter 8, Section 2
effective October 1, 2005. These codes are no longer valid codes.
ICD-9-CM Diagnosis Codes Removed from Table 8.13 Emergency Department
Diagnosis Codes
276.5 567.2 567.8 599.6 770.1 799.0 996.4
The following new ICD-9-CM diagnosis codes will be added to Table 8.63 – High Risk
Pregnancy – ICD-9-CM Diagnosis Codes in the IHCP Provider Manual, Chapter 8,
Section 3. These codes are effective October 1, 2005.
ICD-9-CM Diagnosis Codes Added to Table 8.63 High Risk Pregnancy – ICD-9-CM
Diagnosis Codes
276.50 291.82 567.38 585.5 V46.13 V85.25 V85.37
276.51 362.07 567.39 585.6 V46.14 V85.30 V85.38
276.52 426.82 567.81 585.9 V62.84 V85.31 V85.39
278.02 567.21 567.89 599.60 V85.0 V85.32 V85.4
287.30 567.22 585.1 599.69 V85.21 V85.33
287.31 567.23 585.2 651.70 V85.22 V85.34
287.33 567.29 585.3 651.71 V85.23 V85.35
287.39 567.31 585.4 651.73 V85.24 V85.36
The following ICD-9-CM diagnosis codes will be removed from Table 8.63 High Risk
Pregnancy – ICD-9-CM Diagnosis Codes in the IHCP Provider Manual, Chapter 8,
Section 3 effective October 1, 2005. These diagnosis codes are no longer valid.
Invalid ICD-9-CM Diagnosis Codes Removed
from Table 8.63 High Risk Pregnancy – ICD-9-CM Diagnosis Codes
287.3 585
The following new ICD-9-CM procedures are not covered by the IHCP. According to the
Indiana Administrative Code (IAC) 405 IAC 5-29-1 (3), experimental treatment or
procedures are not covered by the IHCP.
ICD-9-CM Non-Covered ServicesCode Description
37.41 Implantation of prosthetic cardiac support device around the heart
84.58 Implantation of interspinous process decompression device

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: EPSDT/HEALTHWATCH
DESCRIPTION
The Early and Periodic Screening, Diagnostic, and Treatment (EPSDT) program, referred
to as HealthWatch in Indiana, is a federally mandated preventive health care program
designed to improve the overall health of eligible infants, children and adolescents.
Special emphasis is given to early detection and treatment since these efforts can reduce
the risk of more harmful and costly treatment or hospitalization, when detection is
delayed. IHCP members from birth to age twenty-one (21) may participate in the
program on a voluntary basis. Division of Family and Children staff from each of the 92
counties are responsible for HealthWatch program outreach and for informing IHCP
eligible persons about the availability of HealthWatch services. The Hoosier Healthwise
program plays a significant role in the administration of the EPSDT program. Hoosier
Healthwise enrollees must consult their managed care organization (MCO) for outreach
and information on EPSDT services.
SUMMARY OF CURRENT MEDICAL POLICY
EPSDT program services covered by the IHCP are subject to certain coverage limita-
tions. The purpose of EPSDT is to facilitate the introduction of young IHCP members
into a continuing health care system that will detect abnormalities before such
abnormalities become chronic or debilitating.
An initial screening is performed by the EPSDT screening provider when referred by the
Office of Medicaid Policy and Planning (OMPP) or its designee or upon the initial
request of the member for EPSDT services. The initial screening and subsequent,
periodic screenings must be performed in accordance with the HealthWatch Periodicity
and Screening Schedule (periodicity schedule) shown on Table 1. A comprehensive
health and developmental history includes assessment of both physical and mental health
development and a comprehensive unclothed physical exam. Provisions are outlined for
other services such as nutritional assessment, developmental assessment, appropriate vision
and hearing testing, dental screening, health education, including anticipatory guidance,
and include administration of or referral for any other test, procedure or immunization that
is medically necessary or clinically indicated.
Any treatment found necessary to correct or ameliorate defects and physical and mental
illnesses and conditions as a result of a diagnosis identified during an initial or periodic
screening may be provided subject to any prior authorization requirements for the services.
If a service is not covered by the IHCP, it is still available to EPSDT eligible members,
only if it is necessary to correct or ameliorate defects and physical or mental illnesses and
01/31/2007 EPSDT – Health Watch
Medical Policy Manual
145

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
conditions discovered by the screening services, subject to prior authorization requirements
of 405 IAC 5-4
Table 1: HealthWatch/EPSDT Periodicity and Screening Schedule
RELATED MEDICAL TOPICS
Immunizations
Laboratory Services
Pediatrics
Screening Services – Newborn screening
Speech and Hearing
RULES, CITATIONS, AND SOURCES
405 IAC 5-15 Early and Periodic Screening, Diagnostic, and Treatment Services
405 IAC 5-14-19 Prior authorization for early and periodic screening, diagnostic, and
treatment covered services
405 IAC 5-14-2 Covered services
405 IAC 5-23-3 Covered vision care services
Department of Health and Human Services Health Care Financing Administration
Transmittal No. 12 September 1998
HealthWatch EPSDT Provider Manual, Version 1.0, February 2002
Indiana Health Coverage Programs Provider Manual, July 2004
01/31/2007 EPSDT – Health Watch
Medical Policy Manual
146

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
ORIGINATION, REVISIONS, AND REVIEWS
Origination Date: August 25, 1997
Revisions and Reviews Reason Date
405 IAC 5-2-10 “EPSDT” defined 8/25/97
405 IAC 5-15
Early and Periodic Screening, Diagnostic, and
Treatment Services
8/25/97
405 IAC 5-14-19 Prior Authorization 8/25/97
405 IAC 5-14-2 Covered Services 8/25/97
HealthWatch EPSDT
Provider Manual
Provider Manual 2/2002
BT 200207 New HCPCS Codes 2/15/02
BR200217 RHC and FQHC Coding Change 4/23/02
BT 200353
Elimination of Local Codes
X3067 (Missed Appointment)
8/15/03
IHCP Provider Manual Vaccines for Children 7/04
Review Schedule Routine Review 10/04
APPLICABLE INDIANAAIM EDITS AND AUDITS:
6028 – Initial and periodic visits not payable on same date of service
6702 – Newborn screening limited to one per lifetime
9050 – EPSDT pricing
COVERAGE CRITERIA
Member Requirements
Hoosier Healthwise Primary Care Case Management (PCCM) or risk-based managed
care (RBMC) enrollees receive HealthWatch/EPSDT screening examinations from
physicians enrolled in the IHCP as Hoosier Healthwise primary medical providers
(PMPs). Children who are not enrolled in PCCM or RBMC can receive screenings from
any IHCP provider. EPSDT screenings for children not enrolled in Hoosier Healthwise
are provided at designated federally qualified health centers (FQHC) and rural health
clinics (RHC).
HealthWatch Screening Examinations
The primary goal of the HealthWatch/EPSDT program is to ensure that all IHCP eligible
children receive necessary, age-appropriate, comprehensive, preventive services.
Components of the screening examinations and recommended frequency of the screens
are listed in the periodicity schedule. The periodicity schedule is listed in the Indiana
Administrative Code (IAC) 405 IAC 5-15-8. These screenings or any portion of the
screening is not required when lack of medical necessity is documented by the provider.
01/31/2007 EPSDT – Health Watch
Medical Policy Manual
147
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
However, when a member receives components of an EPSDT screening or is referred
elsewhere for these components, they must be accurately reflected in the member’s
medical record.
Any treatment found necessary as a result of a diagnosis reached during an initial or
periodic screening may be provided subject to any prior authorization requirement for the
specified service. If a service is not covered by the IHCP, it is still available to a
HealthWatch/EPSDT-eligible member if it is necessary to correct or improve a physical
or mental illness or condition discovered during a screening subject to prior authorization
requirements.
HealthWatch/EPSDT screens are reimbursed at a higher rate than other child exams.
Reimbursement for the initial patient exam is limited to the first screen performed by the
screening provider during the member’s lifetime. In order to claim a higher
reimbursement, the following components of the screening must be provided and
documented in the member’s medical record.
• A health and developmental history, including assessment of both physical
and mental health development
• An unclothed physical exam
• A nutritional assessment
• A developmental assessment
• Vision observation at each screening and direct referral to an optometrist or
ophthalmologist starting when objective screening methods indicate a referral
is warranted
• Hearing observation at each screening and objective testing with audiometer
at four years, administered or referred
• Dental observation at each screening. Direct referral to a dentist starting at 24
months old. Dental referrals may be made as early as 12 months old when
indicated.
• Laboratory tests, including blood lead level assessment appropriate for age
and risk factors
• Immunizations administered or referred, if needed at time of the screening
• Health education, including anticipatory guidance
Further details of the components of HealthWatch/EPSDT screenings are available in the
HealthWatch EPSDT Provider Manual.
Billing HealthWatch/EPSDT Screening Examinations
HealthWatch/EPSDT visits are billed on the CMS-1500 claim form or 837P transaction.
HealthWatch/EPSDT claims billed to the IHCP must be coded with the following.
• The appropriate patient examination code (99381-99395, 99391-99395) must
be included on the first detail line of the medical claim form
01/31/2007 EPSDT – Health Watch
Medical Policy Manual
148

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• The preventive health diagnosis code, V20.2 (Routine infant or child health
check), as the primary diagnosis. Physicians are encouraged to include all
applicable diagnosis codes (up to four) and procedure codes on the claim for
each HealthWatch/EPSDT visit
• The appropriate EPSDT reimbursement rate for the initial or established
patient exam billed.
The required examination codes providers are to use vary by the age of the member. The
appropriate examination procedure codes are listed in Table 2 by age.
Table 2: EPSDT Screening Examination Codes
Age Initial Patient Exam Established Patient Exam
Less than one year 99381 99391
One to four years 99382 99392
Five to 11 years 99383 99393
12 to 17 years 99384 99394
18 to 20 years 99385 99395
HealthWatch/EPSDT screening services are not subject to third party liability (TPL).
Providers do not have to submit EPSDT claims for TPL payment prior to submitting the
claim to IHCP
Providers may bill and be reimbursed for services provided in addition to the EPSDT
visit. In this instance, the problem-oriented exam can be billed separately, with the -25
modifier (separately significantly identifiable E/M service). The visit must require
additional moderate level evaluation to qualify as a separate service on the same date.
IHCP reimbursement will be at the lesser of the submitted charge or the maximum fee for
each additional service.
Members who miss HealthWatch/EPSDT appointments or follow-up appointments must
be identified and their names forwarded to the Hoosier Healthwise Helpline or the
Hoosier Healthwise benefit advocate. Claim submission for missed appointments is not
required and reimbursement will not be made for missed appointments. Providers may
not bill IHCP members for missed appointments.
Required EPSDT Referrals
HealthWatch/EPSDT providers are required to make dental, vision, hearing, and lead
screening referrals when screening results indicate a problem. Providers may refer
members for dental services beginning at 24 months old or as early as 12 months, if
indicated in the screening. Vision referrals must be made when objective screening
methods indicate a problem is present. Newborns with hearing deficit identified under
the universal newborn screening program must be referred to the First Steps program.
Older members in need of additional testing should be referred for additional testing and
treatment when screening results indicate a possible hearing deficit. Tables 2, 3, and 4
show the schedules for dental, vision and hearing observation and screenings
01/31/2007 EPSDT – Health Watch
Medical Policy Manual
149

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 3: Periodicity Schedule for HealthWatch/EPSDT
Dental Observation and Screening
Age of Child
Recommended (S) or
Required (R)
Services
12 to 24 months S
Direct referral to a dentist if
necessary
24 months R
Direct referral to a dentist
for examination, preventive
dental care, and anticipatory
guidance
24 months through 20 years R
Regular dental assessments
at intervals defined by the
dentist, approximately
every six months. The
individual member
assessment should include
examination, preventive
dental care, and anticipatory
guidance.
Table 4: Periodicity Schedule for HealthWatch/EPSDT
Vision Observation and Screening
Age of Child
Recommended (S) or
Required (R)
Services
Up to 3 years S
Visual observation with an
external eye examination;
subjective screening by
history. Refer child to an
appropriate specialist if
abnormality is suspected.
3 to 5 years R
Annual objective screening
test by standard testing
method. If warranted, refer
child to an appropriate
specialist.
6,8,14,16,18 years S
Visual observation with an
external eye examination;
subjective screening by
history. Refer child to an
appropriate specialist if
abnormality is suspected.
01/31/2007 EPSDT – Health Watch
Medical Policy Manual
150

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 4, con’t.
Age of Child
Recommended (S) or
Required (R)
Services
10, 12, 18 R
Objective screening test by
a standard testing method.
If warranted, refer child to
an appropriate specialist.
Table 5: Periodicity Schedule for HealthWatch/EPSDT
Hearing Observation and Screening
Age of Child
Recommended (S) or
Required (R)
Services
Newborn S
Newborn hearing screening
via fully automated brain
stem response, if available
Newborn R
All members considered to
be at risk for hearing deficit
should be screened at this
time.
Under 12 months S
Subjective screening, by
history and/or other infant
screening using standard
testing method. Refer those
at risk or suspected of
hearing deficit to a
specialist, if warranted.
12 months through 3 years R
As early as possible,
perform an objective
screening using standard
testing method. Refer those
at risk or suspected of
hearing deficit to a
specialist.
4-5 years R
Audiometric screening with
an audiometer or
audioscope (refer to
audiologist if necessary).
Refer child at risk or
suspected of hearing deficit
to an appropriate specialist.
01/31/2007 EPSDT – Health Watch
Medical Policy Manual
151

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 5, con’t.
Age of Child
Recommended (S) or
Required (R)
Services
4, 8, 14, 16, 20 years S
Subjective screening, by
history and/or other
method; refer child with
suspected hearing deficit to
an appropriate specialist.
10, 12, 18 years R
Objective hearing screening
by a standard testing
method; (hearing tests are
given by Indiana Dept. of
Education in grades 1, 4,7,
and 10 – several schools
also test kindergarten
students). Do not duplicate
school screenings unless a
child is considered at risk
and rescreening is
warranted.
Blood lead screenings must be performed at the nine-month, 12 month, and 24 month
visits. If the member is at high risk for lead exposure, the initial screening should be
done at the six-month visit. The OMPP recommends that blood samples drawn for lead
screening be sent to labs participating in the Indiana Childhood Lead Poisoning
Prevention Program (ICLPPP). Providers that send blood samples to ISDH/ICLPPP
laboratories can use code 36415, collection of venous blood by venipuncture, to indicate
that blood draws were made. Sending blood samples to a participating lab ensures that
the results are recorded in the ILCPPP database. The three ILCPPP laboratories are the
Vanderburgh County Department of Health, Marion County Department of Health, and
the Indiana State Department of Health in Marion County. The ILCPPP provides
medical supplies, mailing containers and postage for providers registered in the program
and tracks blood lead screening results geographically to identify areas at risk. The IHCP
will not reimburse a conveyance fee for sending samples to the lab if the ILCPP postage
paid kit is used. Providers that send blood sample to private labs should use the codes in
Table 2, as appropriate.
Table 6: CPT Codes for Blood Samples Sent to Private Labs
CPT
Code
Definition
36415 Collection of venous blood by venipuncture
99000
Handling and/or conveyance of specimen for transfer from
physician’s office to a laboratory
99001
Handling and/or conveyance of specimen for transfer from the patient in other
than a physician’s office to laboratory (distance may be indicated)
01/31/2007 EPSDT – Health Watch
Medical Policy Manual
152
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Vaccines for Children
The Vaccines for Children (VFC) program is a federally funded program that works to
raise childhood immunization levels in the United States by supplying health care
providers with free vaccines for children 18 years old and younger. To be eligible for the
VCF program, a child must meet one of the following criteria.
• Enrollment in IHCP
• Without health insurance
• Identified by parent or guardian as American Indian or Alaskan native
• Underinsured, for example, the child has health insurance that does not
cover immunizations
The VFC program currently offers free vaccines against the following diseases.
• Diphtheria
• Hemophilus influenza type B (Hib)
• Hepatitis B(HepB)
• Measles, mumps and rubella (MMR)
• Pertussis (Whooping cough)
• Polio
• Tetanus
• Varicella (Chickenpox)
• Pneumoccocal disease
IHCP providers are not required to participate in this program. If a provider chooses not
to participate in this program, they must provide appropriate vaccine referrals, patient
follow-up, and documentation of immunization history. If a Hoosier Healthwise provider
chooses not to participate in the program, they must have a mechanism in place to ensure
that members under their care are immunized.
VFC vaccines provided by fee-for-service providers are reimbursed at the lesser of the
submitted charge or the current VFC administration fee. Reimbursement under the
RBMC delivery system is administered by the managed care organizations.
To bill the IHCP for VFC vaccine administration ICD-9 code V20.2 must be the primary
diagnosis and the appropriate procedure code for the specific vaccine being administered.
Providers are not to bill for more than the VFC vaccine administration time on the date of
service. The IHCP only allows one vaccine administration fee per encounter, regardless
of the number of vaccines given at one time. If the only service provided during the
encounter is vaccine administration, the provider may not bill for an office visit. An
office visit can only be billed if a separate, identifiable service is performed during the
same visit Table 7 shows the procedure codes for vaccines covered in the VFC program.
01/31/2007 EPSDT – Health Watch
Medical Policy Manual
153

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 7: Procedure Codes for VFC Vaccines
Procedure
Code
Vaccine
90645
Hemophilus influenza b vaccine (Hib), HbOC conjugate
(4 dose schedule), for intramuscular use
90647
Hemophilus influenza b vaccine (Hib), PRP-OMP conjugate
(3 dose schedule), for intramuscular use
90648
Hemophilus influenza b vaccine (Hib) PRP-T conjugate
(4 dose schedule), for intramuscular use
90655
Influenza Virus Vaccine, split virus, preservative free, for children 6-35
months dosage, for IM or jet injection use, per 0.25 ml
90656
Influenza Virus Vaccine, split virus, preservative free, for children and
adults three years of age and above, dosage for IM or jet injection use,
per 0.5 ml
90657
Influenza Virus Vaccine, split virus, for children 6-35 months dosage, for
IM or jet injection use, per 0.25 ml
90658
Influenza Virus Vaccine, split virus, for children and adults three years
of age and above, dosage for IM or jet injection use, per 0.5 ml
90660 Influenza virus vaccine, live, for intranasal use
90669
Pneumoccocal Conjugate, polyvalent, for children under 5 years for
intramuscular use
90700 DTaP
90702 DT
90707 MMR
90713 Inactivated Polio Vaccine (e-IPV)
90716 Varicella
90718 Td
90721 DTaP-HIB
90723 DTaP-IPV-HepB, brand name Pedirix
90744 HEP B-Ped
90748 HepB-Hib combination, brand name Comvax
01/31/2007 EPSDT – Health Watch
Medical Policy Manual
154

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
EPSDT-HEALTHWATCH FACT SHEET
ADDENDUM A
Note: This addendum contains provider notifications that have been published since the
review of the EPSDT-Healthwatch Fact Sheet. The information in this addendum will
be incorporated into the fact sheet at the next review.
Provider Notification: NL200506 Publication Date: 06/2005
Subject: Vaccines for Children and Injectables
Date Added to Manual: 07/29/2005
Text of Publication
Provider-Purchased Vaccine
When a provider administers immunizations using the provider’s private stock, refer to
IHCP provider bulletin, BT200151, for use of the administration code 90788, as
appropriate, for the additional $2.75 rate.
Administration Fee
Separate reimbursement is allowed when the administration of the drug is the only
service billed by the practitioner. In addition, if more than one injection is given on the
same date of service and no E/M code is billed, providers may bill a separate
administration fee for each injection using 90788. When billing for privately purchased
vaccine, bill an administration code in addition to the CPT code to obtain reimbursement
for both vaccine and its administration. Do not bill an administration CPT code when
billing for VFC vaccine. VFC vaccines must be billed with the CPT code for the vaccine
and the provider’s charge (not to exceed $8) for VFC vaccine administration. Medicaid
maximum fee information can be found on the www.indianamedicaid.com Web site. Be
aware of the member’s primary medical provider assignment, managed care delivery
system assignment, and third party liability resource(s).
RHCs and FQHCs
Note: RHC- and FQHC-specific encounter rates already include payment for
immunizations.
When submitting RHC and FQHC claims to track encounters (such claims will be
denied), bill no more than the $8 VFC administration fee for use of VFC influenza
vaccine or bill the usual and customary rate for the influenza vaccine CPT® plus the
administration CPT 90782 for use of providerpurchased meningococcal vaccine. All
immunization dollars should be included and totaled on the line specific for
immunizations in cost reports submitted to Myers & Stauffer.
01/31/2007 EPSDT – Health Watch
Medical Policy Manual
155

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 EPSDT – Health Watch
Medical Policy Manual
156
EPSDT-HEALTHWATCH FACT SHEET
ADDENDUM B
Note: This addendum contains provider notifications that have been published since the
review of the EPSDT Healthwatch Fact Sheet. The information in this addendum will be
incorporated into the fact sheet at the next review.
Provider Notification: BR200539 Publication Date: 09/27/2005
Subject: EPSDT Annual Exam or Well-Baby Exam
Date Added to Manual: 10/31/2005
Text of Publication
This notification clarifies the policy and billing requirements regarding problem-oriented
exams rendered on the same date as an EPSDT annual exam or well-baby exam. The
HealthWatch Provider Manual states, “If a patient is evaluated and treated for a problem
during the same visit as an EPSDT annual exam or well child service, the problem-
oriented exam can be billed separately accompanied by the -25 modifier (separate
significantly identifiable E&M service). The problem must require additional moderate
level evaluation to qualify as a separate service on the same date.”
Some have interpreted this statement from the manual to mean that Evaluation and
Management (E&M) codes 99211, 99212, or 99213 could not be reimbursed if provided
on the same date as the EPSDT annual or well baby exam. This is incorrect.
IHCP reimburses for all E&M codes billed by a physician who is providing a problem-
oriented exam on the same date as the EPSDT annual or well-baby exam. This includes
E&M codes 99211 through 99215. These services should be billed with modifier -25 to
identify a separate significantly identifiable E&M service.

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: EVALUATION AND MANAGEMENT SERVICES
DESCRIPTION
Evaluation and management (E&M) services are those that are provided for the
assessment of a member’s health or condition and direction of a member’s health care.
E&M services must include the following three components: (1) obtaining a medical and
social history, (2) a physical examination, and (3) medical decision making.
This document is intended to serve as a general summary of the Indiana Health Coverage
Programs (IHCP) policies regarding this service. More specific information may be
found in the IHCP provider manual, program notices, the Indiana Administrative Code
(IAC) or other appropriate sources. See the “Rules, Citations, and Sources” section of
this document for further information.
COVERAGE CRITERIA
E&M services must be appropriate to the diagnosis and treatment given, and providers
are advised to select the CPT code that most closely describes the services rendered, and
includes the three components listed above. IHCP reimbursement is available for office
visits, limited to a maximum of 50 per year, per member, without prior authorization
(PA). If a physician uses an emergency room as a substitute for the physician’s office for
nonemergency services, such services should be billed as office visits with the site of
service indicated.
New patient office visits are limited to one per member, per provider within the last three
years. A new patient is one who has not received any professional services from a
provider within the same specialty and group practice, within the preceding three years.
If a surgical procedure is performed during the course of an office visit, the surgical fee
includes the medical visit unless the member has never been seen by the provider prior to
the surgical procedure, or the determination to perform surgery is made during the
evaluation of the member. If an evaluation of a separate clinical condition is performed
on the same day as the surgery, both the evaluation and the surgery may be billed
separately.
The IHCP will provide reimbursement for eye examinations that are reported using an
appropriate E&M code. Providers are advised to select the most descriptive code for the
01/31/2007 Evaluation and Management Services
Medical Policy Manual
157

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
service rendered from the E&M codes listed in Table 1. Refer to the Medical Policy fact
sheet for Ophthalmological Services for further information.
Table 1 – Evaluation and Management Services to Report Eye Examinations
CPT Code Description
92002 – 92014 Ophthalmological services: medical examination and evaluation with
initiation of diagnostic treatment program
99201 – 99205 Office or other outpatient visit for the evaluation and management of a
new patient
99211 – 99215 Office or other outpatient visit for the evaluation and management of an
established patient
99241 – 99245 Office consultation for a new or established patient
99251 - 99255 Initial inpatient consultation for a new or established patient
99261 - 99263 Follow-up inpatient consultation for an established patient
99271 - 99275 Confirmatory consultation for a new or established patient
An initial prenatal visit can be reported with the appropriate E&M codes (CPT codes
99201 through 99215), and the appropriate trimester modifier and the expected date of
delivery. Additionally, outpatient office visits rendered to pregnant members, if related
to a concurrent medical condition requiring medical care or consultative referral, are to be
reported with the appropriate E&M codes. Medical problems that complicate labor and
delivery management may require additional resources that are reported utilizing an
appropriate E&M code. Refer to the Medical Policy fact sheet for Obstetrical Services
for further information.
PRIOR AUTHORIZATION
E&M services as described in Table 2, which exceed 50 visits, per rolling calendar year,
per member require prior authorization (PA). Additionally, PA is required for any
physician service that is rendered during an inpatient hospital stay and reimbursed using a
level-of-care methodology, such as psychiatric, rehabilitation, and burn treatment.
Table 2 – Evaluation and Management Services Requiring PA After 50 Visits Per
Member Per Rolling Calendar Year
CPT Code Description
99201 – 99205 Office or other outpatient visit for the evaluation and management of a new patient
99211 – 99215 Office or other outpatient visit for the evaluation and management of an established
patient
99241 – 99245 Office consultation or a new or established patient
99271 - 99275 Confirmatory consultation for a new or established patient
99381 – 99387 Initial comprehensive preventive medicine evaluation and management of an
individual
99391 – 99397 Periodic comprehensive preventive medicine reevaluation and management of an
individual
01/31/2007 Evaluation and Management Services
Medical Policy Manual
158
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MANAGED CARE
For members enrolled in Risk Based Managed Care (RBMC), providers must contact the
member’s managed care organization (MCO) for guidelines about E&M codes specific to
the MCO. Refer to the Provider Manual, Chapter 1, for detailed information about the
fee-for-service (FFS), Primary Care Case Management (PCCM), and Risk Based
Managed Care (RBMC) delivery systems.
IHCP members enrolled in Hoosier Healthwise PrimeStep (PCCM) receive the same
benefit coverage, and are subject to the same limitations as traditional Medicaid FFS.
Refer to the Hoosier Healthwise Manual for Primary Medical Providers and Office Staff
for further information.
Hoosier Healthwise providers must report family planning office visits with the
appropriate E&M codes for the initial and each established patient office or outpatient
visit. Appropriate documentation should be maintained in the member’s medical record
to support the level of coding appropriate to the service rendered.
BILLING REQUIREMENTS
Professional services rendered during the course of a hospitalization must be submitted
on the CMS-1500 or via an 837P electronic transaction. If a physician uses an emergency
department as a substitute for the physician’s office for nonemergency services, these
visits should be billed with a CPT code used for a visit in the office with the site of
service indicated. Providers who perform procedures in an outpatient setting or place of
service 22, 23, or 62, which are normally provided in a physician's office, are subject to a
site of service payment adjustment, which is 80 percent of the practice expense
component of the statewide RBRVS IHCP Fee Schedule. The IHCP does not provide
reimbursement for E&M codes reported with treatment room or emergency room revenue
codes 45X, 51X, 52X, 70X, 71X, 72X, and 76X.
New patient office visits are limited to one visit per member, per provider, within the last
three years. A new patient is one who has not received any professional services from a
provider within the same specialty and group practice, within the preceding three years.
If a surgical procedure is performed during the course of an office visit, the surgical fee
includes the medical visit unless the member has never been seen by the provider prior to
the surgical procedure, or the determination to perform surgery is made during the
evaluation of the member. If an evaluation of a separate clinical condition is performed
on the same day as the surgery, both the evaluation and the surgery may be billed
separately. Providers are advised to report the appropriate E&M code with Modifier 25,
Significant, separately identifiable evaluation and management service by the same
physician on the same day of the procedure or other service, to indicate that there was a
significant, separately identifiable E&M service by the same physician on the same day
of a procedure. Modifier 57, Decision for surgery, is to be reported to indicate that an
01/31/2007 Evaluation and Management Services
Medical Policy Manual
159

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
E&M service resulted in the initial decision to perform surgery. Appropriate documentation must
be included in the medical record to substantiate the need for an office visit code in
addition to the procedure code on the same date of service.
The first prenatal visit can be billed with E&M codes, CPT codes 99201 through 99215,
with the appropriate trimester modifier and the expected date of delivery indicated on the
CMS-1500 or 837P electronic transaction.
RELATED MEDICAL TOPICS
Chiropractic Services
Clinic Services – FQHC and Rural Health Clinic Services
Consultations – Second Opinion
Emergency Medicine – Emergency Services
EPDST
Family Planning
Hospital Inpatient
Hospital Outpatient
Obstetric Services
Ophthamological Services
Podiatry
RULES, CITATIONS, AND SOURCES
405 IAC 5-9-1 - Evaluation and Management Services
Hoosier Healthwise Manual for Primary Medical Providers and Office Staff
January 2003
Hoosier Healthwise EPSDT Provider Manual
Version 1.0, February 2002
Indiana Health Coverage Programs Provider Manual
1999
Version 5.1, March 2005
Indiana Health Coverage Programs Provider Bulletins
BT199916 – Changes in Policy and Billing of Vision Services
BT200136 – Outpatient Hospital and Ambulatory Surgical Centers
01/31/2007 Evaluation and Management Services
Medical Policy Manual
160

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Origination Date: 12/31/2000
Revisions and
Review
Reason Date
Revision
407 IAC 5-9-9 Transferred - Second
Opinions
470 IAC 5-9-10; 5-9-11 Transferred -
Office Visits
7/1/91
Revision
405 IAC 1-7-9 - Second Opinions -
Repealed 8/24/97
405 IAC 5-9 - Evaluation and
Management Services
1/1/92
Review Scheduled Review 10/31/05
APPLICABLE INDIANAAIM EDITS AND AUDITS:
4108 – No ASC on file.
6004 – Established Intermediate Office Visit (One Every 30 Days)
6005 – Established Extended Office Visit (One Every 60 Days)
6006 – Only One New Patient Visit per Three Years
6009 – Medical Versus Medical Overlaps Previous In-Hospital Care
6010 – Excessive Physical Exam/One Physical Exam (Same Provider Within 12 Months)
6012 – Medical Services 30 per Rolling Calendar Year
6013 – Multiple In-Hospital Visits Same Day Same Provider
6014 – Global Payable at a Reduced Fee When Components Paid – Respiratory System
6019 – Initial or Established Visits Not Payable Same Date of Service as W6511 or
W6512
6027 – Select Medical Services Reimbursable Only Once per Day
6028 – Initial and Periodic Visits Not Payable Same DOS (EPSDT)
6041 – E&M Codes Not Reimbursable with Prenatal Codes
6042 – Prenatal Codes Not Reimbursable with E&M Codes
6064 – Components Not Payable When Global Paid – Medical System
6069 – Office Visits 50 per Year
6082 – Nursing Facility Visits Versus DME Service
6090 – Office Visits Limited to One per Year - Podiatrist
6091 – One Initial Office Visit per Member - Podiatrist
6096 – CPT/HCPCS Code Billed is Not Payable According to the PPS Reimbursement
Methodology
6098 – Chiropractic Services Limited to Procedure and Diagnosis Codes Identified
6099 – Reimbursement is Limited to 50 Chiropractic Services
6101 – Chiropractic Restrictive Office Visits Codes (New Patient)
6102 – Chiropractic Office Visit Limited to Five per Year
6111 – Chiropractic Office Visits Limited to Five (5) per Calendar Year
6150 – Consultation Billed 15 Days Before or After Another Consultation
6151 – Consultations Billed Seven Days Before or After Surgery
01/31/2007 Evaluation and Management Services
Medical Policy Manual
161
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Evaluation and Management Services
Medical Policy Manual
162
6152 – Surgery Payable at Reduced Amount When Consultation Paid Days Before or
After Surgery
6211 – Periodic/Limited Oral Eval Limit One Every Six Months
6508 – Same Day Discharge
6610 – Routine Vision Exam Limited to One Exam per Twelve (12) Months for ages 1-
18 years
6611 – Routine Vision Exam Limited to One Exam per Twenty-four (24) Months for
ages 19-999 years
6637 – Drug Administration is Not Payable on the Same DOS
6649 – Surgery Payable at Reduced Rate Amount When Related Postoperative Care Paid
6650 – Lifetime Procedures are Limited to One per Lifetime
6653 – Postoperative Care Within Zero to 90 Days of Surgery
6654 – Preoperative Care Within One Day of Surgery
6655 – Surgery Payable at Reduced Amount When Preoperative Care Paid
6656 – Postoperative Care Within 10 Days of Select Surgery
6657 – Preoperative Care on Day of Surgery
6658 – Surgery Payable at Reduced Amount When Preoperative Care Paid Same Date of
Service
6659 – Surgery Payable at Reduced Amount When Related Postoperative Care Paid
6660 – Preoperative and Postoperative Care Billed With Unlisted Surgeries Requires
Review
6858 – Excessive Nursing Home Visits – More Than One per 27 Days
6903 – PCCM Office Visits Greater than 30 Visits per Member per Year Requires PA
6911 – Therapeutic or Diagnostic Injection

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: FAMILY PLANNING
DESCRIPTION
Family planning services are those services provided to individuals of childbearing age to
temporarily or permanently prevent or delay pregnancy. Such services include the
following.
♦ Diagnosis and treatment of sexually transmitted diseases (STDs), if medically
indicated
♦ Follow up care for complications associated with contraceptive methods issued by the
family planning provider
♦ Health education and counseling necessary to make informed choices and understand
contraceptive methods
♦ Laboratory tests, if medically indicated as part of the decision making process for
choice of contraceptive methods
♦ Limited history and physical examinations
♦ Pregnancy testing and counseling
♦ Provision of contraceptive pills, devices, and supplies
♦ Screening, testing, and counseling of members at risk for HIV and referral and
treatment
♦ Tubal ligations, Essure sterilization procedure
♦ Vasectomies
♦ Abortions, in accordance with 405 IAC 5-28-7 (consult the MP fact sheet entitled
“Abortion.”)
SUMMARY OF CURRENT POLICY
Indiana Health Coverage Programs (IHCP) reimbursement is available for family
planning services rendered by IHCP enrolled providers, including but not limited to
physicians, certified nurse-midwives, family planning clinics, and hospitals. The IHCP
does not place any restrictions on access to family planning services for members of
childbearing age and who desire such services and supplies. Hoosier Healthwise
members may obtain information about accessing family planning services from their
Primary Medical Provider (PMP).
PAP smears are included as a family planning service if performed according to the
United States Preventative Services Task Force Guidelines. The Task Force has
01/31/2007 Family Planning
Medical Policy Manual
163

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
concluded that most cases of cervical cancer occur in women who are not screened
adequately. The guidelines recommend cervical cancer screening every one to three
years, based on the presence of risk factors such as early onset of sexual intercourse and
multiple sexual partners. However, PAP smear annual frequency may be reduced if three
or more annual smears are normal.
IHCP reimbursement for abortions is available only if the procedure is performed to
preserve the life of the pregnant woman or in other circumstances when the abortion is
required to be covered by Medicaid under federal law. The IHCP reimburses for medical
abortion by oral ingestion of medication using the same coverage criteria applicable to
surgical abortions. For more information on abortion guidelines, please consult the MP
fact sheet entitled “Abortion.”
RELATED MEDICAL TOPICS
Abortion
Clinic Services – FQHC and Rural Health Clinic Services
EPDST
Gynecology – Pelvic Exam under Anesthesia
Gynecology – Services Requiring Prior Authorization
Pharmacy
Sterilization
RULES, CITATIONS, AND SOURCES
42 CFR 441.20 Family Planning Services
42 CFR 431.51(b)(2) Free Choice of Providers
IC 12-15-5 Services Provided
IC 16-36-1-3 – Consent to own health care; minors
405 IAC 5-24-7 Pharmacy Services-- Copayment for legend and nonlegend drugs
405 IAC 5-22-3 Nursing and Therapy Services-- Certified nurse midwife services
State Medicaid Plan 08/01/95
Social Security Act, Section 1905 (a)(4)(C)
Indiana Medicaid Update Bulletin 95-17
Indiana Medicaid Update Bulletin 96-38
Indiana Medical Assistance Program Provider Manual 1994
Indiana Health Coverage Programs Provider Manuals 1999, 2002, and 2004
HealthWatch EPSDT Provider Manual, Version 1.0, February 2002
Hoosier Healthwise Manual for Primary Medical Providers and Office Staff, January
2003
Indiana Health Coverage Programs Newsletter NL200403
Indiana Health Coverage Programs Newsletter NL200405 – Essure Sterilization
Indiana Health Coverage Programs Bulletin BT200433
01/31/2007 Family Planning
Medical Policy Manual
164

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Indiana Health Coverage Programs Newsletter NL200412
State of Indiana Request for Proposals (RFP) 4-79 –“Solicitation for Managed Care
Organization Services”
ORIGINATION, REVIEWS, AND REVISIONS
Origination date: 12/31/2000
Review and Revisions Reason Date
Revision –Bulletin 96-38
MCO’s coverage of Family
Planning
7/1/91
Revision - 470 IAC 5-8-19
Transferred
Nursing and Therapy Services 7/1/91
Revision - 405 IAC 1-6-20 Repealed
08/24/97
Nursing and Therapy Services 1/1/92
Revision - 405 IAC 1-6-21.1
Repealed 08/24/97
Family Planning and Pharmacy
Services
11/4/93
Revision - State Medicaid Plan
Family Planning and Copay
exemptions
8/1/95
Revision - 405 IAC 5-24-7
Pharmacy Services-- Copayment
For Legend And Nonlegend
Drugs
8/24/97
42 CFR 441.20 Family Planning Services
405 IAC 5-22-3
Nursing And Therapy Services--
Certified Nurse Midwife Services
8/24/97
IC 12-15-5-1 Services Provided
Public Law 2-1992, SEC. 9;
Amended by Public Law 24-
1997, SEC. 48; Public Law 149-
2001, SEC.1.
405 IAC 1-8-4 Client copayment
12/02/93,
readopted
6/27/01
Review and Revision
Scheduled review, Essure
Sterilization Procedure, Medical
Abortion by Oral Medication
1/31/05
APPLICABLE INDIANAAIM EDITS AND AUDITS
1011 – Member’s PMP Is Missing
6704 – Family Planning/One Within 12 Months
6998 – Sterilization Consent Form Improperly Completed
01/31/2007 Family Planning
Medical Policy Manual
165
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
COVERAGE CRITERIA
Contraception
Norplant and related services are reimbursable once per member, per five years. If
removal and re-implantation at the same or different incision site is performed prior to
five years from the previous implantation, reimbursement is available for the removal
only. Specific information for reimbursement of the Norplant system is available in the
Provider Manual, Chapter 8.
Condoms are considered medically necessary for men and women in the prevention of
pregnancy and to reduce the risk of sexually transmitted disease. Therefore,
reimbursement is available for HCPCS code A4267, Contraceptive supply, condom, male
for both male and female IHCP members.
Sterilization (Essure device)
The IHCP covers the Essure implant device as a sterilization option. Essure is an implant
device providing a non-incision permanent sterilization option. The implant can be
performed by a medical doctor (MD) or a doctor of osteopathy (DO) trained in the
procedure and can be performed in the office, at an outpatient hospital facility, or in an
Ambulatory Surgical Center (ASC).
An outpatient hospital facility or ASC must adhere to the following billing instructions to
receive reimbursement in addition to the outpatient ASC rate. No additional
reimbursement is available if the service is performed in an inpatient setting.
♦ Outpatient hospitals and ASCs must bill for the device using HCPCS code A9900,
Miscellaneous supply, accessory, and or service component of another HCPCS
code on the CMS-1500 claim form under their professional number or durable
medical equipment (DME) number.
♦ Outpatient hospitals and ASCs must submit a cost invoice with the claim to
support the actual cost of the device. Reimbursement will be the lesser of the
following amounts.
- 130 percent of the amount listed on the cost invoice
- The provider’s usual and customary charge
- The Statewide maximum
♦ Providers must submit a valid, signed Sterilization Consent Form with the
claim.
♦ Providers must enter ICD-9-CM V25.2 – Sterilization as the primary
diagnosis on the claim.
♦ Providers must print “Essure Sterilization” in the body of the claim form or on
the accompanying invoice.
The following instructions must be followed when the procedure is performed in the
office.
01/31/2007 Family Planning
Medical Policy Manual
166
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
♦ Providers must submit the professional service on the CMS-1500 claim form
using Current Procedural Terminology (CPT) code 58565, Hysteroscopy,
surgical; with bilateral fallopian tube cannulation to induce occlusion by
placement of permanent implants.
♦ Providers must submit the signed Sterilization Consent Form with the claim.
♦ Providers must bill the device on a separate line item on the claim using HCPCS
code A9900.
♦ Providers must submit a cost invoice with the claim to support the actual cost of
the device. Reimbursement is the lesser of the following amounts.
- 130 percent of the amount listed on the cost invoice
- The provider’s usual and customary charge
- The statewide maximum
Sexually Transmitted Diseases (STDs)
Based on CMS’ policies, initial diagnosis and treatment of STDs, HIV testing and
counseling provided during a family planning encounter are considered part of the
family planning services. Ongoing follow-up of STDs and visits for treatment of chronic
STDs are not part of family planning services. Referral to the primary medical provider
should be made for ongoing treatment and follow up of chronic STDs to maintain
continuity of patient care.
BILLING INFORMATION
Services and supplies not classified as drugs or biologicals must be billed using the CMS
claim form 1500 or 837P. These services should be billed using appropriate CPT or
HCPCS codes and appropriate ICD-9-CM diagnosis codes for the services rendered or
the condition treated.
Physicians and family planning clinics using the appropriate NDCs on the IFSSA Drug
Claim Form can bill for contraceptive pills, devices, and supplies, including Norplant.
For specific information regarding claims filing, refer to the IHCP Provider Manual,
under Family Planning Services.
Family planning services and supplies furnished to members of child-bearing age are
exempt from the pharmacy services co-payment requirement, including oral
contraceptives. Pharmacy services provided to RBMC members follow the specific
guidelines of the appropriate MCO.
Specific billing information for the Essure device is available in the Coverage Criteria
section of this fact sheet.
01/31/2007 Family Planning
Medical Policy Manual
167

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Hoosier Healthwise/Risk Based Managed Care
Family planning services under Federal regulation 42 CFR 431.51(b)(2) require a
freedom of choice of providers and access to family planning services and supplies.
Family planning services are those services provided to individuals of childbearing age to
temporarily or permanently prevent or delay pregnancy including, but not limited to, birth
control pills. Hoosier Healthwise members may not be restricted in choice of a family
planning service provider. The IHCP Provider Manual provides a complete and current
list of family planning services.
The MCOs participating in Hoosier Healthwise must allow their members to obtain birth
control pills on a self-referral basis. OMPP recognizes the need for appropriate
management of prescription medication in the interest of the member’s health; however,
OMPP also recognizes the importance of removing barriers to family planning services.
To reduce potential barriers to obtaining birth control pills, which may include, but may
not be limited to, transportation to pharmacies for periodic refills, the MCOs must, at a
minimum, reimburse for the dispensation of up to a 90 calendar day supply for birth
control pills at one time per member, if prescribed.
For more information on family planning in the Risk Based Managed Care program,
please consult the individual MCO.
Hoosier Healthwise/Primestep
The Hoosier Healthwise Manual for Primary Medical Providers (PMPs) and Office Staff
Section 4.12, Family Planning, page 23, indicates family planning is covered in the
following manner.
Members may seek family planning services from any qualified IHCP enrolled provider
without PMP authorization. However, if a family planning provider diagnoses a
particular condition in a member and subsequently initiates treatment (i.e. sexually-
transmitted diseases) the family planning provider must refer the patient back to the PMP
if treatment continues for more than one month. At that time, the PMP will assume case
management and determine whether or not further treatment is medically necessary. If
additional treatment is required, the PMP may either continue treatment at the PMP site
or authorize the family planning provider to do so.
01/31/2007 Family Planning
Medical Policy Manual
168

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Family Planning
Medical Policy Manual
169
FAMILY PLANNING FACT SHEET
ADDENDUM A
Note: This addendum contains provider notifications that have been published since the
review of the Family Planning Fact Sheet. The information in this addendum will be
incorporated into the fact sheet at the next review.
Provider Notification: BR200539 Publication Date: 09/27/2005
Subject: HCPCS code J7303, Contraceptive supply, hormone
containing vaginal ring, each, and J7305, Contraceptive
supply, hormone containing patch, each
Date Added to Manual: 10/31/2005
Text of Publication
This article advises providers that the Indiana Health Coverage Programs (IHCP) has
approved coverage of Healthcare Common Procedure Coding System (HCPCS) codes
J7303 – Contraceptive supply, hormone containing vaginal ring, each, and J7304 –
Contraceptive supply, hormone containing patch, each, effective October 1, 2005.
Providers must bill J7303 and J7304 instead of a miscellaneous supply code because they
are more specific to the service being supplied. HCPCS code J7303 reimburses a max fee
rate of $41.48 and HCPCS code J7304 reimburses a max fee rate of $14.31. Direct
questions about this article to customer assistance at (317) 655-3240 in the Indianapolis
local area or 1-800-577-1278.

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: GASTROENTEROLOGY
DESCRIPTION
Gastrointestinal disorders are disorders that affect the digestive system including, but not
limited to, the esophagus, stomach, small intestines, and large intestines. Such disorders often
simultaneously affect multiple digestive organs. Symptoms associated with these disorders
include, but are not limited to, abdominal pain, flatulence, bleeding, difficulties swallowing,
poor appetite, nausea, and vomiting.
Diagnostic procedures utilized to determine appropriate treatment methods include endoscopy,
computed tomography (CT)/magnetic resonance imaging (MRI), and manometry. Below are
brief descriptions of these diagnostic procedures.
• Endoscopy
This procedure utilizes a flexible tube to view different internal structures. Evidence of
abnormalities such as inflammation, infection, or tumors may be detectable.
Laparoscopy is an examination of the abdominal cavity with an endoscope. Other uses
for this procedure include obtaining tissue samples and reparative procedures.
• Computed Tomography (CT)/Magnetic Resonance Imaging (MRI)
A magnetic field allows cross-sections of organs to be evaluated. The visual image
contains scans of the underlying organs. These diagnostic procedures determine size and
location of abnormalities in organs such as tumors, changes in the path and size of blood
vessels, and inflammation.
• Manometry
A tube connected to pressure gauges is inserted in the esophagus. This test determines
whether the muscle contractions of the esophagus are adequate for swallowing purposes.
This document is intended to serve as a general summary of the Indiana Health Coverage
Programs (IHCP) policies regarding this service. More specific information may be found in
the IHCP provider manual, program notices, the Indiana Administrative Code (IAC) or other
sources as appropriate. See the “Rules, Citations, and Sources” section of this document for
further information.
COVERAGE CRITERIA
IHCP reimbursement is available for medically necessary gastrointestinal services for the
evaluation, diagnosis, and treatment of such disorders.
01/31/2007 Gastroenterology
Medical Policy Manual
170
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
PRIOR AUTHORIZATION
Services must be medically reasonable and necessary and required for the care and well-being
of the member. Services must be provided in accordance with generally accepted standards of
medical or professional practice. Gastroplasty and gastrointestinal surgeries require prior
authorization (PA). Services rendered without appropriate PA are noncovered. Refer to the
medical topics cross reference section of this fact sheet and the IHCP Provider Manual for
specific procedure requirements and limitations.
The following information describes the coverage for the diagnostic examinations
computerized tomography and upper gastrointestinal studies. Services rendered outside
of these guidelines are noncovered.
Computerized tomography
Diagnostic examinations performed by computerized tomography (CT) scanners
are covered services and do not require PA. These examinations are subject to the
following guidelines.
• The scan should be reasonable and necessary for the individual member.
• The use of a CT scan must be found to be medically appropriate considering
the member’s symptoms and preliminary diagnosis.
• Reimbursement will be made for CT scans performed with equipment
certified by the Food and Drug Administration.
• CT scans for the treatment of cancer, whole abdomen or whole pelvis areas
(greater than 20 cuts), are reimbursable.
Upper gastrointestinal studies
Medicaid reimbursement is available for upper gastrointestinal (GI) studies when
performed for the detection and evaluation of diseases of the esophagus, stomach,
and duodenum.
• An upper GI study is not a covered service for a patient with a history of
duodenal or gastric ulcer disease unless recently symptomatic.
• An upper GI study is not a covered service in the preoperative
cholecystectomy patient unless symptoms indicate an upper GI abnormality
in addition to the cholelithiasism or if the etiology of the abdominal pain is
uncertain.
BILLING REQUIREMENTS
Multiple diagnostic and operative procedures billed on the same day, by the same provider, are
subject to the IHCP multiple surgery and reimbursement guidelines.
MANAGED CARE ORGANIZATIONS
For members enrolled in Risk Based Managed Care (RBMC), providers must contact the
member’s managed care organization (MCO) for more specific guidelines. Refer to the IHCP
01/31/2007 Gastroenterology
Medical Policy Manual
171

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Provider Manual, Chapter 1, for detailed information about the fee-for-service (FFS), Primary
Care Case Management (PCCM), and RBMC delivery systems.
IHCP members enrolled in Medicaid Select receive the same benefit coverage, and are subject
to the same limitations as Traditional Medicaid FFS. Refer to the Medicaid Select Provider
Manual for Primary Medical Providers and Office Staff for further information.
MEDICAL TOPICS
Anesthesia Services
Bariatric Services
Consultations – Second Opinion Services
Diagnostic Services
Emergency and Evaluation Management Services
Home Health Services
Inpatient Hospital Services
Managed Care Organization Services
Nursing Services
Outpatient Hospital Services
Pharmacy Services
Radiology
Surgery Services
Transplant Services
Weight Reduction Services
RULES, CITATIONS, AND SOURCES
Indiana Administrative Code (IAC)
405 IAC 5-3-13 – Services requiring prior authorization
405 IAC 5-1-5 – Global Fee Billing
405 IAC 5-27-3 – Computerized tomography; general
405 IAC 5-27-5 – Upper gastrointestinal studies
405 IAC 5-28-1 – Medical and Surgical Services; Reimbursement limitations
405 IAC 5-28-2 – Medical and Surgical Services; Medical diagnostic procedures
405 IAC 5-29-1 – Services Not Covered by Medicaid; Noncovered services
Indiana Health Coverage Programs Banner (BR)
BR200513 – Gastrointestinal Tract Imaging
Indiana Health Coverage Programs Bulletin (BT)
BT200306 – All Pharmacy Providers and Prescribing Practitioners
Indiana Health Coverage Programs (IHCP) Provider Manual
1994
1999
2005
01/31/2007 Gastroenterology
Medical Policy Manual
172

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Gastroenterology
Medical Policy Manual
173
Origination Date: 12/31/2000
Revisions and
Reviews
Reason Date
Revision
470 IAC 5-9-1 Transferred
Global Fee Billing
07/01/1991
Revision
405 IAC 1-7-1 Repealed 08/24/1991
Global Fee Billing
01/01/1992
Review
405 IAC 1-7-19 Repealed 08/24/1997
Radiology Services-Upper GI studies
01/01/1992
Review
405 IAC 5-27-5 Radiology Services Upper
gastrointestinal studies
08/24/1997
Review
405 IAC 5-29-1 Services Not Covered By
Medicaid—Noncovered Services
08/24/1997
Revision 405 IAC 5-1-5 Global Fee Billing; Codes 08/24/1997
Review
405 IAC 5-29-1Amended Services Not
Covered By Medicaid—Noncovered Services
10/27/1999
Review Scheduled 10/31/2006
APPLICABLE INDIANAAIM EDITS AND AUDITS
6022– Components not billable when global fee paid
6023– Global payable at a reduced fee when components paid

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: GENETIC TESTING – BRCA1 AND BRCA2 FOR
BREAST AND OVARIAN CANCER
DESCRIPTION
Two genes (BRCA1 and BRCA2)
1
are associated with susceptibility to breast and
ovarian cancer. Often, family members of those diagnosed with breast or ovarian cancer
share a gene mutation. Therefore, testing of the diagnosed individuals and family
members can identify those family members at higher risk of developing breast or
ovarian cancer. Presence of a gene mutation does not predict the certainty of cancer, but
indicates the individual is at higher risk for developing breast or ovarian cancer and can
utilize increased surveillance and screening measures, as well as make appropriate
lifestyle changes to decrease the likelihood of developing a malignancy.
This document is intended to serve as a general summary of the Indiana Health Coverage
Programs’ (IHCP) policies regarding this service. More specific information may be
found in the IHCP provider manual, program notices, the Indiana Administrative Code
(IAC) or other sources, as appropriate. See the “Rules, Citations, and Sources” section of
this document for further information.
COVERAGE CRITERIA
The IHCP provides reimbursement for BRCA1 and BRCA2 genetic testing when
determined to be medically necessary for women with a personal history of breast cancer,
developing contralateral disease (disease in the opposite breast), or families with history
of breast and ovarian cancer. IHCP members referred to an oncologist or geneticist for
BRCA1 and BRCA2 testing must have a completed personal and family cancer history
that should include three generations on both maternal and paternal sides of the family in
the member’s medical record to include the following.
a. relatives with breast, ovarian, and other relevant cancers, such as prostate
and colon cancer
b. age at diagnosis in affected family members
c. other significant factors, such as ethnic background
1
The acronym BRCA has been given to the genes that are specific to breast and ovarian cancer.
01/31/2007 Genetic Testing – BRCA1 and BRCA2 for Breast and Ovarian Cancer
Medical Policy Manual
174

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
PRIOR AUTHORIZATION
Prior authorization will be given for BRCA1 and BRCA2 genetic testing, utilizing the
HCPCS codes listed in Table 1, when medically necessary in the following
circumstances.
1. Clinically affected individuals (invasive breast cancer or ovarian cancer at any
age) meeting at least one of the following criteria.
a. One or more first (mother, father, sister, or daughter) or second-degree
(aunt, uncle, grandmother, niece, or granddaughter) relatives with
invasive breast cancer diagnosed before age 50
b. One or more first or second-degree relatives with ovarian cancer
c. One or more first or second-degree relatives with male breast cancer
2. Individuals with a personal history of at least one of the following (no family
history required).
a. Invasive breast cancer before age 50
b. Ovarian cancer at any age
c. Both invasive breast cancer and ovarian cancer at any ages
d. Male breast cancer at any age
3. Individuals with a family member (related by blood) with a known BRCA1 or
BRCA2 mutation.
4. Individuals with Ashkenazi (Eastern European) Jewish ancestry with invasive
breast cancer at any age, or meeting any of the above listed (1-3) criteria.
Table 1–HCPCS Codes for Reporting BRCA Genetic Testing
HCPCS
Code
Description Comments
S3818
Complete gene sequence
analysis; BRCA1 gene
S3819
Complete gene sequence
analysis; BRCA2
S3818 and S3819 are not covered for ICD-9-CM
diagnosis codes in Table 2. (Refer to S3820.)
However, reimbursement may be available for
testing for other types of cancer, such as
pancreatic carcinoma.
S3820
Complete BRCA1 and
BRCA2 gene sequence
analysis for susceptibility to
breast and ovarian cancer
S3820 encompasses the testing for all the
genetic variations involving BRCA1 and
BRCA2. Testing required is more extensive
than required for the specific gene variations
reported by S3822 and S3823.
S3822
Single mutation analysis (in
individual with a known
BRCA1 or BRCA2 mutation
in the family) for
susceptibility to breast and
ovarian cancer
S3822 designates the test required for detection
of BRCA gene mutation for an individual with a
family known to have BRCA1 or BRCA2
mutation.
01/31/2007 Genetic Testing – BRCA1 and BRCA2 for Breast and Ovarian Cancer
Medical Policy Manual
175

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 1–HCPCS Codes for Reporting BRCA Genetic Testing
HCPCS
Description Comments
Code
S3823
Three-mutation BRCA1 and
BRCA2 analysis for
susceptibility to breast and
ovarian cancer in Ashkenazi
individuals
S3823 reports genetic testing for individuals of
Ashkenazi Jewish descent. Since there are
known to be fewer and more specific changes in
this population group, the amount of testing
required is significantly less than the general
population.
Men rarely develop breast cancer and, thus, there may not be an affected first-degree
relative, and the size of the family may not permit analysis of possible autosomal
dominant inheritance.
2
BRCA testing of men with breast cancer is considered medically
necessary for either of the following indications.
1. To assess the man’s risk of recurrent breast cancer; or
2. To assess the breast cancer risk of a female member where the affected male is a
first or second degree blood relative of that member. BRCA1 and BRCA2
testing to assess the risk of breast or prostate cancer in men without breast cancer
is considered not medically necessary.
MANAGED CARE
Hoosier Healthwise members enrolled in risk-based managed care (RBMC) are allowed
to receive genetic testing services for breast and ovarian cancer that are determined to be
medically necessary. Hoosier Healthwise members that are enrolled in RBMC are the
financial responsibility of the managed care organization (MCO) in which the member is
enrolled. The MCO may have its own requirements for authorization of genetic testing
services; therefore, providers must contact the MCO for further information.
BILLING REQUIREMENTS
The IHCP provides reimbursement for BRCA1 and BRCA2 genetic testing billed with
the appropriate HCPCS codes, noted in Table 1, and with the appropriate ICD-9-CM
diagnosis code, reflected in Table 2 on the next page.
HCPCS codes S3820, S3822, and S3823 are limited to once per lifetime. If the member
has S3820 completed, the IHCP will not provide reimbursement for S3822 and S3823.
HCPCS codes S3818 and S3819 will not be covered for the ICD-9-CM diagnosis codes
noted in Table 2. S3818 and S3819 are components of S3820.
2
This is a type of genetic inheritance which can be inherited form a single affected parent. Sons and
daughters have an equal chance of inheriting the gene.
01/31/2007 Genetic Testing – BRCA1 and BRCA2 for Breast and Ovarian Cancer
Medical Policy Manual
176

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 2 – ICD-9-CM Codes Supporting Medical Necessity
Code Description
174.0 Malignant neoplasm of female breast; nipple and areola
174.1 Malignant neoplasm of female breast; central portion
174.2 Malignant neoplasm of female breast; upper-inner quadrant
174.3 Malignant neoplasm of female breast; lower-inner quadrant
174.4 Malignant neoplasm of female breast; upper-outer quadrant
174.5 Malignant neoplasm of female breast; lower-quadrant
174.6 Malignant neoplasm of female breast; axillary tail
174.8 Malignant neoplasm of female breast; other specified sites of breast
174.9 Malignant neoplasm of female breast; unspecified
175.0 Malignant neoplasm of male breast; nipple and areola
175.9 Malignant neoplasm of male breast; other and unspecified sites of male
breast
183.0 Malignant neoplasm of ovary and other uterine adnexa; ovary
183.2 Malignant neoplasm of ovary and other uterine adnexa; fallopian tube
183.3 Malignant neoplasm of ovary and other uterine adnexa; broad ligament
183.4 Malignant neoplasm of ovary and other uterine adnexa; parametrium
183.5 Malignant neoplasm of ovary and other uterine adnexa; round ligament
183.8 Malignant neoplasm of ovary and other uterine adnexa; other specified sites
of uterine adnexa
183.9 Malignant neoplasm of ovary and other uterine adnexa; uterine adnexa,
unspecified
V10.3 Personal history of malignant neoplasm of breast
V10.43 Personal history of malignant neoplasm of ovary
V16.3 Family history of malignant neoplasm of breast
V16.41 Family history of malignant neoplasm of ovary
RELATED MEDICAL TOPICS
Laboratory Services
Oncology – Breast and Ovarian Cancer
RULES, CITATIONS, AND SOURCES
Indiana Administrative Code
405 IAC 5-3-13
Indiana Health Coverage Programs Provider Manual
Version 5.1, March 2005
Indiana Health Coverage Programs Provider Notifications
BT200605 – Billing Requirements and PA Criteria
01/31/2007 Genetic Testing – BRCA1 and BRCA2 for Breast and Ovarian Cancer
Medical Policy Manual
177

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Genetic Testing – BRCA1 and BRCA2 for Breast and Ovarian Cancer
Medical Policy Manual
178
Origination Date: April 28, 2006
Revisions and
Review
Reason Date
APPLICABLE INDINAAIM EDITS AND AUDITS
6177 – S3818, S3819, S3822, S3823 Not Allowed if S3820 Pd
6178 – S3820, S3822, and S3823 Are Limited to Specific Dx
6179 – Genetic Testing Codes not Reimbursed with Specific Dx
6650 – Lifetime Procedures Are Limited to 1 per Lifetime

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: GYNECOLOGY SERVICES
DESCRIPTION
This document is intended to serve as a general summary of the Indiana Health Coverage
Programs’ (IHCP) policies regarding gynecologic services. More specific information
may be found in the IHCP provider manual, program notices, the Indiana Administrative
Code (IAC) or other sources as appropriate. See the “Rules, Citations, and Sources”
section of this document for further information.
As defined by Stedman’s Electronic Medical Dictionary , V.5.0
, gynecology is the medical
specialty concerned with diseases of the female genital tract, as well as endocrinology and
reproductive physiology of the female. Many gynecologic conditions create infertility.
CURRENT COVERAGE
A. Pelvic Exam Under Anesthesia
A pelvic exam performed under anesthesia may be done as part of another gynecological
surgical procedure or as a single procedure. The IHCP provides reimbursement when the
member requires anesthesia/conscious sedation to enable the practitioner to complete
the exam. Based on accompanying documentation, medically necessary care
provided prior to surgery will be reimbursed. If the examination is completed in
adjunct with a surgical procedure, it will be included in the global fee schedule with
the appropriate reduction applied.
Procedures requiring prior authorization (PA) cannot be rendered before acquiring
PA. For such services, reimbursement will be denied if PA is not obtained. Prior
authorization must be requested for emergency procedures within 48 hours of the
admission or on the next business day if on a weekend or holiday.
B. Laminaria
Laminaria is derived from a perennial kelp plant, Laminaria digitata, which is
hydrophilic. When placed in the cervical canal, laminaria gradually absorbs moisture,
swells, and gently dilates the cervix. Laminaria is most frequently used to induce
abortion and can be utilized to assist the dilatation of the cervix when labor is
induced.
The IHCP will provide reimbursement for the laminaria, when it is used for induction
of labor and the delivery is paid. When the HCPCS CPT code 59200, Insertion of
cervical dilator (eg, laminaria, prostaglandin) (separate code) appears with a code
01/31/2007 Gynecology Services
Medical Policy Manual
179
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
indicating an abortion procedure, the claim will be denied and documentation of
medical necessity will be requested.
C. Hysterectomy
Hysterectomy is a covered service only when the member has given her informed
consent. The member must have been informed orally and in writing that this
procedure will render her permanently incapable of reproducing, and has signed a
written acknowledgement of receipt of that information. The IHCP does not provide
reimbursement for a hysterectomy performed solely for the purpose of rendering an
individual permanently incapable of reproducing, whether performed as a primary or
secondary procedure.
The “acknowledgement requirement”, which must be signed by the member or
member’s representative, is NOT required in the following situations.
1. The member is already sterile.
2. A life-threatening emergency situation exists for which the physician determines
prior acknowledgement is not possible.
However, in the situations noted above, the physician performing the hysterectomy
must certify one of the following in writing.
1. The member was already sterile at the time the hysterectomy was performed, and
states the cause of the sterility at the time of the hysterectomy.
2. The hysterectomy was performed under a life-threatening emergency situation
and that prior acknowledgement was not possible. The physician must also
include a description of the nature of the emergency.
Retroactive Eligibility
The IHCP will provide reimbursement for hysterectomies performed during a period
of a member’s retroactive Medicaid eligibility, if the physician, who performed the
hysterectomy, certifies in writing one of the following.
1. The member was informed before the operation that the hysterectomy would
make her permanently incapable of reproducing.
2. The member was already sterile before the hysterectomy.
3. The member requires a hysterectomy because of a life-threatening emergency
situation in which the physician determined that prior acknowledgment is not
possible. The physician who performed the hysterectomy must also include a
description of the nature of the emergency.
Documentation Requirements
No specific format is mandated and examples are provided in the IHCP Provider
Manual including the information necessary to satisfy IHCP documentation and
certification requirements for hysterectomy procedures. The sterilization consent
form cannot be used for hysterectomy procedures under any circumstances.
01/31/2007 Gynecology Services
Medical Policy Manual
180

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
PRIOR AUTHORIZATION
The IHCP advises providers to report the appropriate CPT code that describes the service
provided. Table 1 lists CPT codes used to report services that include total or partial
hysterectomies. This list may not be inclusive and PA requirements are as noted.
Table 1 – CPT Codes for Reporting Total or Partial Hysterectomy
CPT
Code
Description
PA
Requirement
51925 Closure of vesicouterine fistula; with hysterectomy No
58150
Total abdominal hysterectomy (corpus and cervix), with or without
removal of tube(s), with or without removal of ovary(s)
Yes
58152
Total abdominal hysterectomy (corpus and cervix), with or without
removal of tubes(s), with or without removal of ovary(s); with
colpo-urethrocystopexy (eg, Marshall-Marchetti-Krantz type)
Yes
58180
Supracervical abdominal hysterectomy (subtotal hysterectomy),
with or without removal of tube(s), with or without removal of
ovary(s)
Yes
58200
Total abdominal hysterectomy, including partial vaginectomy, with
para-aortic and pelvic lymph node sampling, with or without
removal of tube(s), with or without removal of ovary(s)
No
58210
Radical abdominal hysterectomy, with bilateral total pelvic
lymphadenectomy and para-aortic lymph node sampling (biopsy),
with or without removal of tube(s), with or without removal of
ovary(s)
No
58260 Vaginal hysterectomy, for uterus 250 grams or less Yes
58262
Vaginal hysterectomy for uterus 250 grams or less; with removal of
tubes(s), and/or ovary
Yes
58263
Vaginal hysterectomy for uterus 250 grams or less; with removal of
tubes(s), and/or ovary, with repair of enterocele
Yes
58267
Vaginal hysterectomy, for uterus 250 grams or less; with colpo-
urethrocystopexy (Marshall-Marchetti-Krantz type, Pereyra type)
with or without endoscopic control
Yes
58270
Vaginal hysterectomy, for uterus 250 grams or less; with repair of
enterocele
Yes
58275 Vaginal hysterectomy, with total or partial vaginectomy Yes
58280
Vaginal hysterectomy, with total or partial vaginectomy; with repair
of enterocele
Yes
58285 Vaginal hysterectomy, radical (Schauta type operation) No
58290 Vaginal hysterectomy, for uterus greater than 250 grams Yes
58291
Vaginal hysterectomy, for uterus greater than 250 grams; with
removal of tube(s) and/or ovary(s)
Yes
58292 Vaginal hysterectomy, for uterus greater than 250 grams; with Yes
01/31/2007 Gynecology Services
Medical Policy Manual
181

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 1 – CPT Codes for Reporting Total or Partial Hysterectomy
CPT
Code
PA
Description
Requirement
removal of tube(s) and/or ovary(s), with repair of enterocele
58293
Vaginal hysterectomy, for uterus greater than 250 grams; with colp-
urethrocystopexy (Marshall-Marchetti-Krantz type, Pereyra type)
with or without endoscopic control
Yes
58294
Vaginal hysterectomy, for uterus greater than 250 grams; with repair
of enterocele
Yes
58550
Laparoscopy surgical, with vaginal hysterectomy, for uterus 250
grams or less
Yes
58552
Laparoscopy surgical, with vaginal hysterectomy, for uterus 250
grams or less; with removal of tube(s) and/or ovary(s)
Yes
58553
Laparoscopy, surgical, with vaginal hysterectomy, for uterus greater
than 250 grams
Yes
58554
Laparoscopy, surgical, with vaginal hysterectomy, for uterus greater
than 250 grams; with removal of tube(s) and/or ovary (s)
Yes
58951
Resection of ovarian, tubal or primary peritoneal malignancy with
bilateral salpingo-oophorectomy and omentectomy; with total
abdominal hysterectomy, pelvic and limited para-aortic
lymphadenectomy
No
58953
Bilateral salpingo-oophorectomy with omentectomy, total
abdominal hysterectomy and radical dissection for debulking
No
58954
Bilateral salpingo-oophorectomy with omentectomy, total
abdominal hysterectomy and radical dissection for debulking; with
pelvic lymphadenectomy and limited para-aortic lymphadenectomy
No
58956
Bilateral salpingo-oophorectomy with total omentectomy, total
abdominal hysterectomy for malignancy
No
59525
Subtotal or total hysterectomy after cesarean delivery (List
separately in addition to code for primary procedure)
No
Prior authorization will be granted for members with documentation supporting one of
the following.
1. Non-malignant uterine tumor causing abnormal pressure or bleeding (lasting >
eight days for > two cycles, requiring additional bleeding protection defined as
large clots and gushes, limiting activity).
2. Non-malignant uterine tumor causing one of the following.
• Uterus of 12 week gestational size or larger, with ill defined adnexa (<12
week gestational size, or <8 cm could have vaginal procedure).
• Post-menopausal enlargement (>12 week gestational size necessitates
abdominal procedure).
• Rapid uterine growth over last six months.
• Pressure on adjacent organs.
3. Cervical intraepithelial neoplasia (CIN) III diagnosed by endocervical curettage
uncontrolled by conservative surgery, such as laser excision, loop electrosurgical
01/31/2007 Gynecology Services
Medical Policy Manual
182
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
excision procedure (LEEP), large loop excision of transformation zone (LLETZ),
or loop surgical excision.
4. Fibroids in premenopausal woman with both of the following.
• Uterus >12 weeks size or documentation of need for abdominal, rather than
vaginal, approach.
• And one of the following.
Abnormal bleeding.
Uterus size doubled within one year.
Ureteral compression by ultrasound (US) or intravenous pyelogram
(IVP).
Other symptoms, such as pelvic or abdominal pain or discomfort
without other explanation, urinary frequency or urgency, or
dyspareunia.
5. Fibroids in postmenopausal woman with all of the following.
• Uterus >12 weeks size or documentation of need for abdominal rather than
vaginal approach.
• And one of the following.
Uterus size doubled within any time period.
Ureteral compression by US or IVP.
Other symptoms, such as pelvic or abdominal pain or discomfort
without other explanation, urinary frequency or urgency, or
dyspareunia.
Papanicolaou (PAP) smear within six months.
6. Dysfunctional uterine bleeding with all of the following.
• Premenopausal woman.
• Abnormal bleeding uncontrolled by conservative therapy, such as hormonal
therapy.
• No evidence of cancer demonstrated by hysteroscopy, endometrial biopsy,
Dilation and Curettage (D&C) or transvaginal ultrasound.
• PAP smear within six months.
7. Postmenopausal bleeding with all of the following.
• Abnormal bleeding continued after change in or discontinuation of hormone
replacement therapy.
• No evidence of cancer demonstrated by hysteroscopy, endometrial biopsy,
D&C or transvaginal ultrasound.
• PAP smear within six months.
8. Pelvic inflammatory disease with one of the following.
• Suspected rupture or leakage of pelvic abscess.
• Unsuccessful management with antibiotics for 10-14 days.
• Surgery for residual, inactive but symptomatic, disease if conservative therapy
not possible.
• Chronic Pelvic Inflammatory Disease (PID) with both of the following.
Chronic pelvic pain.
Adhesions, scarring, or hydrosalpinx.
9. Recurrent abnormal uterine bleeding (lasting > eight days for > two cycles,
requiring additional protection defined as large clots and gushes, with limitations
01/31/2007 Gynecology Services
Medical Policy Manual
183
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
of normal activity) and benign endometrial biopsy after failed medication therapy
[excluding members on birth control pills or with an Intrauterine Device (IUD)].
10. Chronic incapacitating pelvic pain, unresponsive to conservative therapy, such as
analgesics, and evidence of normal gastrointestinal/genitourinary (GI/GU)
evaluations.
• A four to six month failed trial of oral contraceptives, diuretics, anti-
inflammatories, or induced amenorrhea.
• Negative examinations of urinary tract (UT), GI tract, and musculoskeletal.
• Psychological and psychosexual counseling reveals no etiology of pain.
11. Post-menopausal bleeding > one year after last menstrual period (LMP), (with
D&C or endometrial biopsy within past six months). Positive cytology of cervix
requires abdominal procedure (cervical intra-epithelial neoplasia including
carcinoma-in-situ).
12. Pre-malignant adenomatous hyperplasia or adenocarcinoma of the endometrium,
confirmed by pathology report.
13. Post-menopausal (> one year) with benign or malignant ovarian tumor and/or
cyst.
14. Abdominal procedure when associated with abdominal procedure for correction
of urinary stress incontinence or vaginal repair of cystocele, rectocele, enterocele,
or uterine prolapse.
15. Uncontrolled postpartum bleeding within six hours of delivery uncontrolled by
drug therapy (e.g., Pitocin, Methergine, or Prostaglandin therapy) or D&C.
16. Endometriosis uncontrolled by hormonal therapy (e.g., depot
medroxyprogesterone, oral contraceptives, GnRH agonist, or danazol), surgical
ablation, or excision.
17. Tubo-ovarian abscess.
18. Urinary incontinence due to fistula into vagina, uterus, or perineum, and fistula
demonstrated by cystoscopy, radiological examination, visual inspection, or
probing.
19. Uterine prolapse, second or third degree and one of the following.
• Pain.
• Pelvic pressure.
• Stress incontinence.
• Ulceration of vaginal mucosa or cervix with bleeding or spotting.
• Vaginal splinting.
BILLING REQUIREMENTS
The appropriate documentation must be attached to the claim form, or sent separately to
the Electronic Claims and Attachments address for claims submitted electronically. All
providers rendering hysterectomy related services (e.g. anesthesiologist, etc.) must attach
an EXACT photocopy of the appropriate sterilization acknowledgement or physician
certification statement(s) to the claim(s). To ensure timely reimbursement, the primary
service provider is advised to forward copies of the sterilization acknowledgement or
physician certification statement(s) to these related service providers.
01/31/2007 Gynecology Services
Medical Policy Manual
184

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MANAGED CARE
For members enrolled in Risk Based Managed Care (RBMC), providers must contact the
member’s managed care organization (MCO) for specific guidelines. Refer to the IHCP
Provider Manual, Chapter 1, for detailed information about fee-for-service (FFS),
Primary Care Case Management (PCCM), and Risk Based Managed Care (RBMC)
delivery systems.
IHCP members enrolled in Hoosier Healthwise PrimeStep (PCCM) receive the same
benefit coverage, and are subject to the same limitations as traditional Medicaid FFS.
Refer to the Hoosier Healthwise Manual for Primary Medical Providers and Office Staff
for further information.
RELATED MEDICAL TOPICS
Abortion
Anesthesia Services
Case Management Services – Pregnant Women
Clinic Services – FQHC and Rural Health Clinic Services
Family Planning
Hospital Inpatient Services
Hospital Outpatient Services
Obstetric Services
Pathology Services
Surgery-Multiple Procedures/Same Operative Session
Surgery-Office Visits
Surgery-Surgeon and Assistant Surgeon, Same Provider
Surgery-Surgery and Anesthesia By the Same Provider
Surgery-Surgical Services
Surgery-Suture of Wounds
RULES, CITATIONS, AND SOURCES
Code of Federal Register 42 CFR 441.255
Indiana Administrative Code
405 IAC 5-17 – Hospital Services
405 IAC 5-28 – Medical and Surgical Services
405 IAC 5-28-7 – Abortion
Indiana State Department of Public Welfare Medical Policy Manual 1991
Indiana Health Coverage Programs Provider Manual
1999
01/31/2007 Gynecology Services
Medical Policy Manual
185

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
March 2005, Version 5:1
Indiana Medical Assistance Program Provider Manual 1994
Indiana Health Coverage Programs Provider Notifications
Banner Page 8/19/97
Origination Date: 12/31/2000
Revisions and
Review
Reason Date
470 IAC 5-9-1 – Global Fee Billing
470 IAC 5-9-3 – Prior Authorization
5-9-9 – Second Opinions
470 IAC 5-9-12 – Medical Services
Surgical Services
470 IAC 5-9-14 – Surgical Services
Specifically Gynecology Transferred
7/1/91
Indiana State Department of Public
Welfare Medical Policy Manual 1991 -
Laminaria or Dilateria
7/1/91
405 IAC 1-7-1 – Global Fee Billing
405 IAC 1-7-3 – Services Requiring
Prior Authorization
405 IAC 1-7-9 – Second Opinions
405 IAC 1-7-12 & 14 – Surgical
Services
1/1/92
405 IAC 5-1-5 – Global Fee Billing
405 IAC 5-3 – Prior Authorization
405 IAC 5-8 – Consultations and
Second Opinions
405 IAC 5-17-2 – Hospital Services-
Prior Authorization
405 IAC 5-17-3 – Hospital Services-
Emergency, Weekend Inpatient
Admissions
405 IAC 5-28 – Medical and Surgical
Services
8/24/97
Revision
Combination of Gynecology –
Hysterectomy, Laminaria, and Pelvic
Under Anesthesia
01/31/06
01/31/2007 Gynecology Services
Medical Policy Manual
186

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Gynecology Services
Medical Policy Manual
187
APPLICABLE INDIANAAIM EDITS AND AUDITS
4012 – Abortion Diagnosis/Procedure Indicated
4073 – Hysterectomy Requires Manual Review
6002 – Any Two Anesthesiology Providers Same Procedure Requires Review
6003 – Manual Pricing for Split Care Billing
6023 – Global Payable at a Reduced Fee When Components Paid – Digestive System
6034 – Global Surgery Payable at Reduced Amount When Components of Surgical Care
Paid
6035 – Components of Surgical Care Not Payable When Global Surgery Paid
6037 – Only One Assistant Surgeon Allowed for Select Surgeries
6039 – Assistant Surgeon Not Payable When Co-Surgeon Paid
6040 – Co-Surgeon Paid at Reduced Amount When Assistant Surgeon
6061 – Components Not Payable When Global Paid – Urinary/Reproductive Systems
6096 – The CPT / HCPCS Code Billed Is Not Payable According to the PPS
Reimbursement Methodology
6649 – Surgery Payable at Reduced Amount When Related Postoperative Care Paid
6650 – Lifetime Procedures Are Limited to One Per Lifetime
6652 – Multiple Surgeries Must be Billed on Same Claim
6653 – Postoperative Care Within Zero to 90 Days of Surgery
6654 – Preoperative Care Within One Day of Surgery
6655 – Surgery Payable at Reduced Amount When Postoperative Care Paid
6656 – Postoperative Care Within 10 Days of Select Surgery
6657 – Preoperative Care on Day of Surgery
6658 – Surgery Payable at Reduced Amount When Preoperative Care Paid Same Date of
Service
6659 – Surgery Payable at Reduced Amount When Related Postoperative Care Paid
6660 – Preoperative and Postoperative Care Billed with Unlisted Surgeries Requires
Review
6706 – Global Payable at a Reduced Fee When Components Paid – Genital
Urinary/Reproductive Systems

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: HIV/AIDS CARE COORDINATION
DESCRIPTION
According to the Centers for Disease Control and Prevention, the Human
Immunodeficiency Virus (HIV) is a viral infection that gradually destroys the immune
system. The compromised immune system leaves the body vulnerable to a variety of
life-threatening illnesses. Common bacteria, yeast, parasites, and viruses, that ordinarily
do not cause serious disease in people with fully functional immune systems, can cause
fatal illnesses in people with HIV. People with severely compromised immune systems
are diagnosed with Acquired Immune Deficiency Syndrome (AIDS). AIDS is the final
and most serious stage of the HIV viral infection.
HIV/AIDS can be transmitted from person to person in several manners. Blood, semen,
vaginal secretions, and breast milk have been the only proven secretions to transmit the
diseases. Most transmissions of HIV/AIDS occur by sexual contact, blood transfusion,
needle sharing, mother to fetus through shared blood circulation, and mother to infant
through breastfeeding. Other transmission methods are rare and include accidental
needle injury, artificial insemination with donated semen, and through a donated organ.
Treatment for HIV/AIDS may delay the progression of diseases for many years and
improve the quality of life. Most treatments are used to strengthen the immune system
and increase the T-cell counts.
This document is intended to serve as a general summary of the Indiana Health Coverage
Programs (IHCP) policies regarding this service. More specific information may be
found in the IHCP provider manual, program notices, the Indiana Administrative Code
(IAC), or other sources as appropriate. See the “Rules, Citations, and Sources” section of
this document for further information.
COVERAGE CRITERIA
HIV/AIDS care coordination is a specialized form of case management for members with
HIV/AIDS diagnoses. Care coordination consists of goal-oriented activities that
facilitate, coordinate, and monitor the full-range of HIV-related health and human
services including, but not limited to, medical, psychological, social, and educational
services. To assure freedom of choice, members sign a Freedom of Choice/Intent to
Participate Form acknowledging an understanding of the services provided and
identifying the chosen care coordination provider.
01/31/2007 HIV Care Coordination
Medical Policy Manual
188
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
PRIOR AUTHORIZATION
Prior authorization is not required for this service. Refer to the following sections
outlining member eligibility, member services, and provider enrollment for other relevant
information.
Member Eligibility
To be eligible for HIV/AIDS care coordination services, members must be a Traditional
Medicaid member, a Hoosier Healthwise member, or a Medicaid Select member with a
documented HIV viral infection/AIDS diagnosis. Medical documentation or verification
of a related medical diagnosis must be maintained in the member’s care coordination file.
The diagnosis may be verified with the following types of documentation.
• Confidential positive HIV test result
• Physician's statement
• Hospital discharge statement or other medical reports which verify the
diagnosis
• Medical prescription for Azidothymidine (AZT), Didanosine (ddl), or
Zalcitabine (ddC)
• Copy of approval for participation in the AIDS Drug Assistance Program
(ADAP) or the Early Intervention Program (EIP)
The provider must verify that the member is eligible for Medicaid services during the
specific month in which services are provided. Failure to do so may result in denial of
payment. The member’s name must appear on the claim form exactly as it appears on the
eligibility file. Eligibility must be checked each time that a service is rendered.
HIV/AIDS Care Coordination Services
HIV/AIDS care coordination providers must maintain written documentation of all
services provided. Documentation is subject to post-payment review by the Office of
Medicaid Policy and Planning (OMPP) or its agent. The following records must be
available in each member’s file and updated annually, if applicable.
• Determination of eligibility
• Documentation of verified diagnosis of HIV/AIDS diagnosis
• Intake form
• Assessment and re-evaluations of the member’s presenting problems,
indications of medical/physical status, indications of psychological status,
quality of social supports, living arrangements, and status on other relevant
factors
• Documentation of service provider referrals
• Progress notes
• Comprehensive plan of care
• Documentation of member and service provider contacts, including face-to-
face contacts
• Signed release of information form
01/31/2007 HIV Care Coordination
Medical Policy Manual
189
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Providers must comply with all regulations delineated by OMPP and the Division of
Disability, Aging, and Rehabilitative Services (DDARS). The following information
outlines these guidelines.
• Providers must permit access and examination of records by persons
authorized by the OMPP, its agent, and Federal personnel. Records must be
maintained for seven years. Record destruction must be done in a confidential
manner such as burning or shredding of documents.
• Providers must obtain input from members on service satisfaction and
summarize this input at least annually. This information must be available for
review.
• Providers are required to provide quarterly reports to the DDARS on the
approved forms.
• Providers must have data collection and analysis capability required to prepare
monthly statistical reports concerning member demographics, including but not
limited to, age, race, gender, risk exposure category, and other relevant data in
a format approved by the DDARS.
• Providers must develop and maintain a current listing of support services in the
community and the established referral process.
• Providers will be required to maintain member files in a secure area to protect
member confidentiality. Information stored on computerized data systems
must have password protection.
• Providers must adopt and adhere to a written policy protecting confidentiality
that states that all information regarding the member is confidential
information.
• Providers may not use any information obtained about the member in any
manner except as necessary for the proper discharge of responsibilities. All
information, personal facts and circumstances concerning the member, must be
treated as privileged communication and may not be divulged without the
written consent of the member or the member’s legal representative.
• Providers will assure that services will be available to all eligible members
regardless of age, race, gender, sexual/affectional orientation or preference,
ethnicity, religion, national origin, or handicap.
• Providers must comply with licensing and accreditation standards as required
by the OMPP or its agent.
• Providers will determine if a member is eligible for receipt of services in
accordance with OMPP and the DDARS established policies and procedures.
• Providers will comply with procedures for administrative review of appeals for
applicants and members.
There are specific services with limitation that must be followed when care coordinators
provide services to eligible members. The following information outlines the HIV/AIDS
care coordinator services provided to IHCP members.
A. Intake and Assessment
The care coordinator completes a Freedom of Choice/Intent to Participate Form
and an Assessment of Care Coordination/Worksheet for Data Entry Form. As
01/31/2007 HIV Care Coordination
Medical Policy Manual
190
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
determined by the Assessment of Intensity of Care Coordination/Worksheet for
Data Entry Form, care coordination services may not exceed a maximum of 128
units (32 hours), per calendar quarter (three month period), per member. This form
should be completed at the beginning of each quarter to determine the maximum
number of hours allowed per member. It is expected that most members will
require between 6.6 and 9 hours per quarter with some using less. Furthermore, it is
anticipated that few members will require more than nine hours per quarter. This
form describes the supporting factors for the number of care coordination hours
used. Supporting factors must be substantiated through self-report, medical records,
caregiver records, other agency reports, and physical observation. Substantiation of
services must be documented in each member’s file. Sections of the assessment
must contain information regarding presenting problems, medical/physical status,
indicators of psychosocial status, indicators of developmental and intellectual
status, living arrangements, and other relevant factors.
Once the maximum number of hours allowed is determined in a given quarter, the
need for additional hours will require the submission of a new and updated
Assessment of Intensity of Care Coordination/Worksheet for Data Entry Form.
B. Plan of Care Development
Based upon the information gathered in the assessment, the care coordinator and the
member develop a comprehensive plan of care. Services are structured and time is
goal oriented. The plan of care must be developed on or before the 30
th
day
following the date of the initial intake. The following factors must be considered in
the development of the plan.
• Prioritizing the needs that were identified in the assessment
• Developing outcome goals that will address the identified needs
• Identifying factors that may impinge upon the implementation of the plan of
care
• Proposing and discussing preliminary strategies for meeting outcome goals and
obtaining signed approval forms from the member or representative
• Specifying the time frame for meeting outcome goals
• Developing evaluation criteria to measure whether outcome goals are met
• Identifying specific services, costs, and sources of payment
• Contracting with the member or representative to apportion the rights and
responsibilities between the member and the care coordinator
• Developing procedures for emergency situations
01/31/2007 HIV Care Coordination
Medical Policy Manual
191
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
C. Implementation
To implement the plan of care, the care coordinator negotiates agreements with
service providers, coordinates delivery of service, and maintains the member’s
record. In addition, the care coordinator acts as a facilitator in resolving access
problems that arise in implementing the plan of care, as well as, advocating for the
development of new services. It is the responsibility of the care coordinator to
ensure services are cost effective and not duplicated.
D. Monitoring
The care coordinator assures implementation of the services identified in the plan of
care by monitoring planned interventions in a timely fashion and obtaining
confirmation of scheduled interventions by the member, the service provider, or
both.
E. Evaluation
The care coordinator periodically engages in the following activities to measure the
quality and effectiveness of the plan of care. The following describes the
evaluation process.
• Analysis of information generated through monitoring the quality and
effectiveness of interventions, appropriateness of goals and strategies, the
member’s or representative’s commitment to participate in the plan of care,
and the member’s or representative's satisfaction with the plan of care
• Comparison of plan of care objectives with actual outcomes of intervention
F. Re-evaluation
The care coordinator schedules plan of care re-evaluations to determine continuing
or changing needs the member may have. Evaluations and re-evaluations require
ongoing contact with the member. The Assessment of Care
Coordination/Worksheet for Data Entry Form should be completed from the date of
the initial intake and the beginning of every quarter, as part of the re-evaluation
process. Where there is a change in a member’s needs, the care coordinator will do
one of the following.
• Revise plan of care outcome goals
• Re-evaluate the priority of member needs
• Revise plan of care strategies
• Realign technical service resources
• Propose and discuss new strategies for meeting outcome goals subject to the
member’s or representative’s approval
• Specify new time frames for meeting outcome goals
• Enter into a new contract with the member or representative to reapportion the
rights and responsibilities of the member and the care coordinator
G. Termination
Upon re-evaluation of the plan of care, the care coordinator and the member or
representative determines whether services should continue. The decision will be
made based on the following information.
01/31/2007 HIV Care Coordination
Medical Policy Manual
192
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• HIV/AIDS care coordination services are still required
• Member or representative elects to continue care coordination services
• Member or representative is upholding the contracted agreement set forth in
the plan of care
• Member or representative is cooperating with agreed upon plan of care
• Member is aggressive or non-cooperative with the care coordinator
HIV/AIDS Care Coordinator Eligibility
In order to receive reimbursement for HIV/AIDS care coordination services, the service
provider must be enrolled as an IHCP provider. In addition, the care coordinator must
meet the following minimum qualifications described below.
• Registered Nurse licensed in Indiana with a minimum of one year case
management experience
• Master of Social Work/Master of Social Sciences or equivalent degree with a
minimum of one year case management experience
• Bachelor of Social Work or equivalent degree with a minimum of one year
case management experience
• Master of Science in Nursing or equivalent master's nursing degree with a
minimum of one year case management experience
• Bachelor of Science in Nursing or equivalent nursing degree with a minimum
of one year case management experience
All HIV/AIDS care coordinators are required to enroll and complete a State-approved
HIV/AIDS specific care coordination training course prior to certification. If training is
not available within six weeks of the provider's letter of intent to enroll, the provider may
request a temporary waiver for this training. If the waiver is granted, the training will
need to be completed within three months of the conditional enrollment. The request to
waive may be made under these circumstances.
• If the provider has at least one year of documented HIV/AIDS care
coordination experience
• If the provider is under the supervision of a currently enrolled HIV/AIDS care
coordinator
Once minimum qualifications are met, the provider may complete enrollment documents
and submit them to the Medicaid Waiver Unit. These documents may be requested from
the following address.
Medicaid Waiver Unit
P.O. Box 7083
Indianapolis, IN 46207-7083
(317) 232-5710
Once the enrollment process is completed, the provider will be notified in writing of the
assigned care coordinator provider number. Additionally, the provider will receive the
appropriate provider manual along with an initial supply of forms. All changes in status,
location, or personnel should also be reported in writing to the Medicaid Waiver Unit.
01/31/2007 HIV Care Coordination
Medical Policy Manual
193
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MANAGED CARE
HIV/AIDS care coordination services are self-referral services under the Hoosier
Healthwise and Medicaid Select managed care programs. Providers serving members in
the risk-based managed care (RBMC) delivery system should contact the appropriate
managed care organization to file claims.
HIV/AIDS care coordination claims are not subject to managed care edits; therefore,
there is no requirement for a PMP certification code or a provider number on the HCFA-
1500 claim form.
BILLING REQUIREMENTS
HIV/AIDS care coordinator, provider specialty 211, is reimbursed for the Healthcare
Common Procedural Coding System (HCPCS) code G9012, Coordinated care fee, risk
adjusted maintenance, other specified care management. Effective October 1, 2004, this
code is the only procedure code that will be reimbursed. Additionally, providers must
use primary diagnosis code 042, Human Immunodeficiency virus {HIV} disease, for
billing purposes. Each unit of the HCPCS code G9012 is equal to 15 minutes of services.
Non-billable Services
Non-billable services include time spent completing or reviewing claim forms for care
coordination, implicit paperwork, and activities not related to a particular member.
Administrative expenses are not billable; however, these expenses are included in the
reimbursement rate. Transporting members and general provider recruitment are not
billable.
Early and Periodic Screening, Diagnosis, and Treatment/HealthWatch Program
Early and Periodic Screening, Diagnosis, and Treatment (EPSDT)/HealthWatch services
may be provided to IHCP members younger than 21 years old. Primary Medical
Providers (PMP) are not required to provide or authorize HIV/AIDS targeted case
management services. Reimbursement for case management services is on a fee-for-
service basis.
Targeted Case Management Services for Pregnant Women
HIV-related targeted case management services for pregnant women are limited to 60
hours per quarter. Coverage is limited to services related to pregnancy, including
prenatal, delivery, and postpartum services, as well as, conditions which may complicate
the pregnancy or urgent care services. The following Current Procedural Terminology
(CPT) codes must be used for HIV testing and screening.
• CPT code 86701, HIV-1
• CPT code 86689, HIV antibody confirmatory test (e.g., Western Blot)
01/31/2007 HIV Care Coordination
Medical Policy Manual
194

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
RELATED MEDICAL TOPICS
Early and Periodic Screening, Diagnosis, and Treatment/HealthWatch Services
Inpatient Hospital Services
Laboratory Services
Managed Care Services
Obstetric Care
Outpatient Hospital Services
Screening Services–Newborn Screening
Targeted Case Management Services for Pregnant Women
Waiver Services
RULES, CITATIONS, AND SOURCES
Indiana Administrative Code (IAC)
405 IAC 5-15 – Early and Periodic Screening, Diagnosis, and Treatment Services
Indiana Code (IC)
IC 16-41-6 – Communicable Disease: Mandatory Testing of Individuals with
Communicable or Dangerous Diseases
Indiana Health Coverage Programs Provider Manual
1999
2005 Version 5.1
Medicaid Provider Manual for Waiver Providers
Indiana Health Coverage Programs Provider Banner (BR)
BR200510 – IHCP Policy for Billing HIV Care Coordination Services
BR200433 – HIV Care Coordinator Provider Code Set
Indiana Health Coverage Programs Provider Bulletin (BT)
BT200526 – Notice of Program Change Due to the New Medicare Prescription
Drug Coverage PDPs must cover all, or substantially all, drugs in the
following six categories
BT200424 – Early and Periodic Screening, Diagnosis, and Treatment/
HealthWatch Services CPT codes HIV Aesting
BT200360 – DRG Relative Weight Table
BT200359 – Antiviral (Anti-Herpetic) Agents, W5A; HMG CoA Reductase
Inhibitors, M4E Valtrex Pravachol
BT200255 – Preferred Drug List–Pravastatin (PravacholÒ)
BT200006 – Package C Claim Submission and Coverage Information
BT199909 – Removal of Services from Prior Authorization
01/31/2007 HIV Care Coordination
Medical Policy Manual
195

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 HIV Care Coordination
Medical Policy Manual
196
Origination Date: 04/13/2006
Revisions and Reviews Reason Date
Indiana Medical
Assistance Program
Provider Manual
HIV/AIDS 1994
IC 16-41-6
Communicable Disease: Mandatory
Testing of Individuals with Communicable
or Dangerous Diseases
1993;1996;
1998
Review Scheduled 10/31/2006
APPLICABLE INDIANAAIM EDITS AND AUDITS
1020 – Rendering Provider Specialty Not Eligible to Render Procedure Code
6051 – Care Coordinator – Initial Assessment
6053 – HIV Case Management
6800 – Care Coordination Transportation for Home Visits (Initial Assessment)
6801 – Care Coordination – Transportation for Home Visits (Reassessment)

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: HOME HEALTH SERVICES
DESCRIPTION
Home health services are available to Indiana Health Care Programs (IHCP) members
medically confined to home, when services are ordered by the member’s physician, and
performed in accordance with the written plan of care. IHCP members who, because of
illness or injury, are unable to leave home without assistance of another person or an
assistive device such as a wheelchair or walker, or for whom leaving the home is contrary
to medical advice are considered to be medically confined to home. Home health services
may be utilized for: care and treatment of acute or chronic conditions, rehabilitation,
education regarding care, coordination of community services, or to avoid prolonged or
repeated hospitalizations and/or higher and more costly levels of care.
SUMMARY OF CURRENT POLICY
IHCP reimbursement is available to home health agencies for skilled nursing care
services provided by a registered nurse (RN) or licensed practical nurse (LPN), home
health aide (HHA) services, physical, occupational, or speech therapy services,
respiratory therapy services, renal dialysis, and tocolytic infusion therapy subject to
limitations in 405 IAC 5-3, 5-16, and 5-22.
All home health services require prior authorization (PA) except those services provided
by a RN, LPN, or HHA ordered in writing by a physician prior to the member’s discharge
from a hospital and that do not exceed 120 hours within 30 days of discharge. Services
may not continue beyond 30 calendar days unless PA is received. Any combination of
therapy services ordered in writing by a physician prior to the member’s hospital
discharge does not require initial PA, but may not continue beyond 30 hours, sessions, or
visits in 30 calendar days unless PA is received.
Home health care is available to eligible IHCP members who are in need of intermittent
or part-time home health services. The type and extent of service required must be
documented in the plan of treatment and included with the PA request. Home health care
services must be rendered as indicated on the plan of treatment. The plan of treatment
must be signed by the attending physician and reviewed every 60 days. The PA request,
the plan of treatment, supporting documentation and hourly determination guidelines will
be when considered when evaluating the number of hours to approve for home health
care services. The hourly determination guidelines are used as an aid, but do not take the
place of clinical judgment when determining appropriate hours of service.
01/31/2007 Home Health Services
Medical Policy Manual
197
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Case management services for pregnancy and HIV/AIDS, and Diabetes Self Management
Training services can be provided in a home setting, but are not included under home
health care services. Each of these services has specific coverage criteria for
reimbursement. See individual policies for details.
DEFINITIONS
Encounter: An encounter occurs when a home health care provider enters a home,
provides services to one or more individuals within that home, and then departs.
Multiple member care situation: A home care situation in which more than one
member is receiving home health services in a single household. When this situation
occurs, care must be coordinated in the most efficient manner. Multiple care member
situations must be reported on each member’s individual PA request. When one member
of a home health agency provides care to multiple members during an encounter, only
one overhead may be billed.
Home Health Care Provider: Registered Nurse (RN), Licensed Practical Nurse (LPN),
Physical Therapist (PT), Occupational Therapist (OT), Speech Therapist (ST), Speech
Language Pathologist (SPL), Respiratory Therapist (RT), or a home health aid (HHA)
RELATED MEDICAL TOPICS
Aged and Disabled –Waiver Services
Autism – Waiver Services
Case Management - Pregnant Women
Case management - HIV/Aids Coordination
Diabetes Self-Management Training
Hospice
Intermediate Care Facilities for the Mentally Retarded
Medical Supplies and Equipment
Medically Fragile Children – Waiver Services
Nursing Services
Physical Rehabilitation Services
01/31/2007 Home Health Services
Medical Policy Manual
198

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
RULES, CITATIONS, AND SOURCES
405 IAC 5-16 Home Health Agency and Clinic Services
405 IAC 5-16-1 Providers eligible for reimbursement
405 IAC 5-16-2 Home health agency services
405 IAC 5-16-3 Home health agency services; limitations
405 IAC 5-3-13 Prior Authorization--Services requiring prior authorization
405 IAC 5-19-6 Durable medical equipment subject to prior authorization
405 IAC 5-19-12 Home hemodialysis equipment
405 IAC 5-22 Nursing and Therapy Services
405 IAC 5-11 Case management Services for Pregnant Women
405 IAC 5-34 Hospice Services
405 IAC 5-36 Diabetes Self Management Training
405 IAC 5-35 Case Management Services for Infants and Toddlers With Disabilities
Indiana Code 16-41-6 Communicable Disease
42 CFR 441.15 Subpart A--General Provisions
405 IAC 5-19-6 Durable medical equipment subject to prior authorization
405 IAC 5-19-12 Home hemodialysis equipment
42 CFR 441.15 Subpart A--General Provisions
42 CFR 440.70 Subpart A--Definitions
Indiana Medicaid Update Bulletin 97-12-- Overhead Component
Indiana Medicaid Update Bulletin 97-14--Home Tocolytic Infusion Therapy Utilizing A
Home Uterine Monitoring Device
Indiana Medicaid Update Bulletin 97-05 --Clarification of Indiana Medicaid
Indiana Medicaid Update Bulletin 97-03--Billing Procedures for Home Infusion/Enteral
State Medicaid Plan 01/01/95--Home Health Services
Indiana medical Assistance Provider Manual 1994
Indiana Health Coverage Programs Manual 1999
Indiana Health Coverage Bulletin BT200353 – HIPPA-Mandated Elimination of Local
Codes and Local Code Modifiers
Indiana Health Coverage Programs Home Health Care Hourly Determination Guidelines
Indiana Health Coverage Banner BR200207
Indiana Health Coverage Bulletin BT200237 – Required Documentation for Prior
Authorization Requests for Home Health Services
Indiana Health Coverage Bulletin BT200349 – Change in Reimbursement Rates for
Home Health Providers
Indiana Health Coverage Banner BR200411
Indiana Health Coverage Provider Manual 2003
01/31/2007 Home Health Services
Medical Policy Manual
199

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
ORIGINATION, REVISION, AND REVIEWS
Origination Date: 12/31/00
Revisions and Reviews Reason Date
Indiana Medicaid Update
Bulletin 97-14
Tocolytic Home Infusion and Uterine
Monitoring Device
5/27/95
Indiana Medicaid Update
Bulletin 97-06
Clarification of Policies 1/1/97
Indiana Medicaid Update
Bulletin 97-03
Home Health Infusion 3/1/97
Indiana Medicaid Update
Bulletin 97-12, 3/31/97
Home Health Services 5/27/97
405 IAC 5-3-13
Prior Authorization-Services Requiring Prior
Authorization
8/24/97
405 IAC 5-16 Home Health Agency and Clinic Services- 8/24/97
405 IAC 5-19-6
Durable Medical Equipment Subject to Prior
Authorization
8/24/97
405 IAC 5-19-12 Home Hemodialysis Equipment 8/24/97
42 CFR 441.15 Subpart A-General Provisions 10/1/97
42 CFR 440.70 Subpart A-Definitions 10/1/97
405 IAC 5-16-2 Amended Home Health Agency and Clinic Services- 9/27/99
405 IAC 5-16-3 Amended Home Health Agency and Clinic Services- 9/27/99
405 IAC 5-19-3.1 Amended Home Health Agency and Clinic Services- 9/27/99
405 IAC 5-16-2 Amended Home Health Agency and Clinic Services- 10/1/99
405 IAC 5-16-3 Amended Home Health Agency and Clinic Services- 10/1/99
Revision
Home Health Care hourly Determination
Guidelines
4/30/04
BT200335
HIPAA-Mandated Elimination of Local Codes
and Love Code Modifiers, Updated
4/30/04
BT200117
PA Requests for Home Health, Quarterly
Update
7/30/04
BR200207 PA Request Criteria, Quarterly Update 7/30/04
BT200237
Required Documentation for Prior
Authorization Requests for Home Health
Services, Quarterly Update
7/30/04
IHCP Provider Manual
2003
Medically Confined to Home, Billing
Requirements, Quarterly Update
7/30/04
01/31/2007 Home Health Services
Medical Policy Manual
200
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
APPLICABLE INDIANAAIM EDITS AND AUDITS:
515
551
552
3016
6073
6260
6261
6752
6753
6916
6917
01/31/2007 Home Health Services
Medical Policy Manual
201
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
COVERAGE CRITERIA
HOME HEALTH CARE
IHCP reimbursement is available to members medically confined to home for
intermittent or part-time home health care services provided by home health care
providers. In order for home health services to be approved, the services must be
medically reasonable and necessary, and home care must be less expensive than
alternative modes of care.
Home health services may consist of the following.
♦ skilled nursing services provided by a registered nurse or licensed practical nurse;
♦ home health aide services;
♦ physical, occupational, and speech therapy services;
♦ respiratory therapy services;
♦ renal dialysis; and
♦ home tocolytic infusion therapy
Home health services require prior authorization (PA), except in the following
circumstances.
♦ Services provided by a registered nurse, licensed practical nurse, or home health aid
which have been ordered in writing by a physician prior to the member’s discharge
from a hospital that do not exceed one hundred twenty (120) hours within thirty (30)
days of discharge do not require PA. These services may not continue beyond thirty
(30) calendar days unless prior authorization is received.
♦ Any combination of therapy services which have been ordered in writing by a physician
prior to the member’s hospital discharge that does not exceed thirty (30) units in thirty
(30) calendar days does not require PA. These services may not continue beyond 30
days following discharge unless prior authorization is received.
♦ Home tocolytic infusion therapy does not require PA, effective April 4, 2002.
The PA request for home health services must contain information required for all prior
authorizations, as specified in 405 IAC 5-3-5, including but not limited to:
♦ the appropriate diagnosis and related information
♦ services or supplies requested with the appropriate codes
♦ name of suggested provider of services and supplies
01/31/2007 Home Health Services
Medical Policy Manual
202
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
♦ description of previous services or supplies
♦ plan of treatment
♦ rehabilitation potential
In addition, the following information must be submitted with the PA request form for
home health services:
♦ An estimate of the costs for the services ordered by the physician and set out in the
written plan of treatment. The cost estimate must be provided with the plan of
treatment and signed by the attending physician. The estimate must reflect the cost of
each service requested, plus the overhead rate for the time period(s) requested, as
reflected on the plan of treatment.
♦ PA requests for home health services should provide documentation of all services
received by the IHCP member. For example, Medicare, CHOICE, IHCP waiver
programs, private insurance, and any other paid caregivers. The number of hours per
day and the number of days per week should be listed for each service.
♦ PA requests for home health services should indicate the number of non-paid
caregivers, (even if there are none) available to provide care for the member,
including consideration of whether the caregiver works outside the home or attends
school outside the home. A copy of the caregiver’s work schedule from the employer
or the class schedule from the school must be submitted with the PA request. The
provider is responsible for coordinating home care services with the caregiver’s work
or school schedule to meet the member’s needs, and should clearly document
caregiver information on the PA request form.
♦ PA requests for home health services should document whether the member works or
attends school outside the home, including what assistance is required.
♦ When there is a multiple member situation and more than one member is receiving
home health services in a single household, care must be coordinated in order to
utilize service in the most efficient manner. Only one overhead component can be
billed per encounter. Agencies are responsible for reporting this aspect of the case,
and should indicate this fact on the PA request submitted for each member of the
household.
A copy of the current plan of treatment, developed by the attending physician,
therapist(s), and agency personnel, and signed by the attending physician, must also be
included with the PA request for home health services. The plan of treatment should
include the date of onset of the medical problem(s) and progress notes regarding the
necessity, effectiveness, and goals of therapy services. The plan of treatment should
detail the types of services and equipment required, frequency of visits, prognosis,
rehabilitation potential, functional limitation, activities permitted, nutritional
requirements, medications and treatments, safety measures to protect against injury, and
any other relevant items.
01/31/2007 Home Health Services
Medical Policy Manual
203
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
NON-COVERED SERVICES
The following services are noncovered home health services, except as specified under
the applicable IHCP Waiver Service programs.
♦ Transportation to and from grocery stores, drug stores, banks, etc.
♦ Homemaker services, including shopping, laundry, cleaning, meal preparation, etc.
♦ Companion or sitter services, including escort services, activity planning, etc.
♦ Chores, including picking up prescriptions, household supplies and/or groceries, etc.
♦ Respite care
HOME HEALTH CARE HOURLY DETERMINATION GUIDELINES
The following are guidelines for determining the appropriate number of hours
reimbursable for general categories of home health care services. These are guidelines
only and do not override medical decisions based on individual case review.
Factors for consideration when determining the hours of service to be approved
include:
♦ Severity of illness and symptoms
♦ Stability of the condition and symptoms
♦ Change in medical condition that affects the type or units of service that can be
authorized
♦ Intensity of care required to meet needs
♦ Complexity of needs
♦ Amount of time required to complete treatment tasks
♦ Treatment plan, including identified goals
♦ History of previous response to care
♦ Whether the member works or attends school outside of the home, including what
assistance is required
♦ Caregivers available to provide care for the member, including the following
considerations:
01/31/2007 Home Health Services
Medical Policy Manual
204
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Number of caregivers available.
Physical limitations of available caregiver(s) that limit that ability of the
caregiver(s) to provide care to the member.
Number of hours requested, compared to availability of caregiver(s) available
time.
If the caregiver has additional child care responsibilities.
If the caregiver works outside the home.
♦ Other home care services currently being utilized including, but not limited to,
Medicare, Medicaid Waiver Programs, CHOICE, vocational rehabilitation, and
private insurance.
12-16 HOURS/DAY HOME HEALTH CARE SERVICES
Members requiring 24-hour monitoring may be authorized for up to 12 hours/day skilled
nursing or home health aide services to prevent deterioration in life sustaining systems.
Examples of these conditions include, but are not limited to:
♦ Severe respiratory conditions resulting from pulmonary disorders such as,
bronchopulmonary dysplasia, severe respiratory complications of cystic fibrosis,
bronchitis, asthma; central nervous system disorders, cardiovascular disorders such
as cardiac anomalies; neuromuscular disorders such as muscular dystrophy, Guillain
Barré syndrome,
♦ Dependency on mechanical ventilator assistance
♦ Tracheostomy
Special situations may occur where home health hours may be approved for up to 16
hours per day of skilled care on an ongoing basis, although each individual situation must
be evaluated with a PA request. These special situations include but are not limited to:
♦ A single caregiver is available who also works full-time (or a significant number of
hours part time) outside the home. This also applies to situations where there may be
two adults present but one is unable to provide any, or a very limited amount, of care
due to physical disability or severe physical limitations. The disabled caregiver’s
physician must substantiate this in writing.
♦ Significant additional childcare responsibilities. Significant is defined as:
a) three or more children under the age of six, or four or more children under the
age of 10,
b) one or more children in the home with special medical care needs requiring
extensive medical and physical care above and beyond the needs of the
average well child. If Medicaid is not providing services to this child at home
also, the child’s physician must provide a statement of the child’s medical
01/31/2007 Home Health Services
Medical Policy Manual
205
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
needs. The same caregiver(s) must be caring for these children as well as the
member for whom the PA request has been submitted.
Special situations may occur where additional home health hours may be authorized on a
short term or temporary basis, that are evaluated individually, on a case-by-case basis.
Examples of these situations are as follows:
♦ Significant deterioration in the condition of the member, particularly if additional
hours will prevent an inpatient or extended inpatient hospital admission.
♦ Major illness or injury of the caregiver with expectation of recovery, including, but
not limited to:
a) illness or injury that requires an inpatient acute care stay;
b) chemotherapy or radiation treatments; or
c) a broken limb, which would impair the caregiver’s ability to lift the member.
♦ Temporary, but significant, change in the home situation, including, but not limited
to:
a) a caregiver’s call to military duty; or
b) temporary unavailability due to employment responsibilities.
(These must be substantiated in writing by the commanding officer, other
military representative, or by the employer.)
♦ Significant permanent change in the home situation, including, but not limited to,
death or divorce with loss of a caregiver. Additional units of service may be
authorized for a short period of time to assist in providing a transition.
8 HOURS /DAY HOME HEALTH CARE SERVICES
Members who require extensive care and daily monitoring of their medical/physical
condition, but do not possess the same degree of potential to deteriorate quickly into life
threatening situations as do members requiring 24-hour monitoring, may receive up to 8
hours of care daily. An additional hour or two may be allowed for transportation to and
from work in situations where the caregiver(s) work full time outside the home.
Examples of these situations/conditions may include, but are not limited to:
♦ Chronic, debilitating conditions such as severe forms of cerebral palsy, muscular
dystrophy, spina bifida, and other congenital anomalies, and quadriplegia.
♦ Conditions that require equipment or treatment needs with potential for serious
complications. For example, central lines, or Hickman catheters.
♦ Frequent treatments such as respiratory therapy required (in the form of updrafts,
CPT, etc.)
♦ Nutrition is provided by hyperalimentation or by gastrostomy tube feedings in
addition to one of the above.
♦ Skilled nursing assistance required to attend school.
♦ The member receives multiple medications that require monitoring for severe side
effects or responses.
01/31/2007 Home Health Services
Medical Policy Manual
206
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Special situations may occur in which additional home health hours may be authorized on
a short term or temporary basis. These will be evaluated individually, on a case-by-case
basis. Examples of these situations are:
♦ Significant deterioration in the condition of the member, particularly if additional
hours will prevent an inpatient or extended inpatient hospital admission.
♦ Major illness or injury of the caregiver with expectation of recovery, including, but
not limited to:
a) illness or injury that requires an inpatient acute care stay;
b) chemotherapy or radiation treatments; or
c) a broken limb, that impairs the caregiver’s ability to lift the member.
♦ Temporary, but significant, change in the home situation, including, but not limited
to:
a) a caregiver’s call to military duty; or
b) temporary unavailability due to employment responsibilities.
(These must be substantiated in writing by the commanding officer, other
military representative, or by the employer.)
♦ Significant permanent change in the home situation, including, but not limited to,
death or divorce with loss of a caregiver. Additional units of service may be
authorized for a short period of time to assist the member with a transition.
3 - 7 HOURS/DAY HOME HEALTH CARE SERVICES
Members without the severity of conditions noted above, who require primarily heavy
physical care, with some skilled nursing monitoring to avoid deterioration, may receive 3
to 7 hours of care per day. These members are generally chronic but stable and may have
conditions such as congenital anomalies, neuromuscular disorders, central nervous
system disorders, or other disorders that severely disrupt the capacity to care for self.
Adults requiring care/assistance must be homebound as certified by the attending primary
physician. However, consideration may be given to paraplegics, quadriplegics, or other
disabled members unable to provide self-care such as bathing or dressing, who are able to
drive mechanically altered vehicles in order to maintain meaningful employment and a
relationship with the community. Such adults may be considered for assistance from a
Home Health Aide for up to 3-4 hours per day. The agency may split the hours between
morning and evening to attend to the bedtime needs of the member. This service is
subject to medical necessity and documentation must demonstrate the need.
BILLING PROCEDURES
♦ The following is the computation of the total reimbursement rate:
♦ The overhead cost rate; plus
♦ The staffing cost rate multiplied by the number of hours spent performing
billable patient care activities.
01/31/2007 Home Health Services
Medical Policy Manual
207
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
♦ Each component of the total home health reimbursement rate is based on statewide
weighted median costs calculated for each component. The statewide weighted
median rate for each component is determined by calculating the per visit or per hour
cost of each component for each home health agency. These costs are ranked from the
highest to the lowest, calculating the cumulative number of Medicaid visits or hours,
and locating the point on the array in which half of the respective Medicaid visits or
hours were provided by agencies with a higher cost and half were provided by
agencies with a lower cost.
♦ The overhead cost rate per visit for each home health provider is based on total patient-
related costs, less the direct staffing and employee benefit costs, less the semi-
variable costs, divided by the total number of home health agency visits during the
Traditional Medicaid reporting period for that provider. The result of this calculation
is the overhead cost per visit for each home health provider that was included in the
statewide overhead array. The semi-variable cost was removed from the overhead
cost rate calculated, and included in the staffing cost rates.
♦ The staffing cost rate per hour for each discipline in the home health agency is based
on the total patient-related direct staffing and employee benefit costs, plus the semi-
variable cost divided by the total number of home health agency hours worked. The
result of this calculation is the staffing cost rate per hour per discipline for each home
health agency.
Occurrence Codes
Providers should use the UB-92 Occurrence Code, Occurrence Date, and Occurrence
Span for Locators 32–36, a–b, on the UB-92 to indicate the appropriate overhead fees.
The following six codes can be used to identify the overhead rate:
♦ Code 61 indicates that one encounter with the member occurred on the date shown.
♦ Code 62 indicates that two encounters occurred on the date shown:
- These may be the same service or a combination of services provided on one day.
- Example: One skilled nurse encounter and one home health aide encounter or two
home-health aides encounters of care.
♦ Code 63 indicates that three encounters occurred on the date shown:
- These may be the same service or a combination of services provided on one day.
- Example: One physical therapy encounter, one skilled nurse encounter, and one
home-health aide encounter.
♦ Code 64 indicates that four encounters occurred on the date shown:
- These may be the same service or a combination of services provided on one day.
- Example: In limited pediatric cases, the need for more than three overhead charges
in one 24-hour period may occur.
01/31/2007 Home Health Services
Medical Policy Manual
208
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
♦ Code 65 indicates that five encounters occurred on the date shown:
- These may be the same service or a combination of services provided on one day.
- Example: In limited pediatric cases, the need for more than four overhead charges
in one 24-hour period may occur.
♦ Code 66 indicates that six encounters occurred on the date shown:
- These may be the same service or a combination of services provided on one day.
- Example: In limited pediatric cases, the need for more than five overhead charges
in one 24-hour period may occur.
All home health visits must be documented on any PA request submitted on behalf of
members. All home health claims utilizing occurrence codes 64, 65, and 66 which
indicate more than three visits a day are subject to post-payment review and will continue
to be monitored closely by the OMPP.
If the dates of service billed are not consecutive, the provider should enter the correct
occurrence code corresponding to each date-of-service billed on the UB-92 in the
Occurrence Code and Occurrence Date fields, Locators 32–35 a–b, on the UB-92. If the
dates of service billed are consecutive, and one encounter was provided per day, then
Occurrence Code 61 and the dates of service being billed are entered in the Occurrence
Span Code field, locator 36 a-b. Occurrence Codes 62 through 66 may not be used in the
Occurrence Span Code field.
Providers that submit more than one UB-92 claim form in a multiple member care
situation should submit only one of the forms with the overhead attached. As long as the
overhead is attached to only one member, it does not matter to which member it is
attached.
Multiple Visit Billing
When multiple visits for the same prior authorized service are made to a member in one
day, providers should bill all visits on the same claim form. Billing these same-day
services on one claim form allows the system to bypass duplicate editing. If these
services are billed on separate claim forms, one or more of the services will be denied as
a duplicate service. It is not appropriate for home health agency (HHA) providers to
rotate personnel in the home merely to increase billing.
Partial Units of Service
Partial units of service must be rounded to the closest whole unit when calculating
reimbursement. A partial unit of service of 30 minutes or more should be rounded up to
the next highest unit. A partial unit of service of 29 minutes or less should be rounded
down to the next lowest unit. One unit of service equals 60 minutes.
♦ Example 1: 85 minutes spent on billable patient care activities is rounded down to one
unit.
♦ Example 2: 95 minutes spent on billable patient care activities is rounded up to two units.
01/31/2007 Home Health Services
Medical Policy Manual
209

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
HOME HEALTH SERVICES GENERAL GUIDELINES
CPT/HCPCS CODES
Local codes effective on or before 12/31/03
CROSSWALKED CODES
EFFECTIVE 1/1/04
Y0601 – Skilled nurse, per hour 99600 TD – Skilled nurse, RN
Y0501 – Home health aide, per hour 99600 – Home health aide
X3069 – Licensed practical nurse, per hour 99600 TE – Skilled nurse, LPN/LVN
Z5016 – Home tocolytic therapy
S9349 – Home infusion therapy, tocolytic
infusion therapy
W6503 – Physical therapy, individual, by the
unit; modalities not requiring use of capital
equipment
G0151 – Services of physical therapist is
home health setting, each 15 minutes
W7402 – Occupational therapy, by the unit,
individual
G0152 – Services of occupational
therapist in home health setting, each 15
minutes
W9083 – Speech therapy, home health
G1053 – Services of speech and language
pathologist in home health setting, each
15 minutes
TOCOLYTIC THERAPY
Local code effective on
or before 12/31/03
Crosswalked Code in
effect1/1/04 – 3/31/04
2004 HCPCS code change
effective 1/1/04
99601 – Home infusion, up to 2
hours Z5017 – Home tocolytic
therapy
99553 – Home infusion for
tocolytic therapy, per visit
99602 – Home infusion, each
additional hour
Indicators for Home Health Services
ONE of the following indicators from each category must be present for a member
to be eligible for home health services.
Category I: Member
♦ The member is at risk of respiratory failure, severe deterioration or hospitalization
without constant monitoring.
♦ The member requires total care 24 hour/day monitoring.
♦ The member desires to stay in the home, rather than a long term care facility.
♦ The medical condition of the member has deteriorated, creating the need for more
intense short-term care. (Physician’s statement required)
♦ The member does not have a primary caregiver or access to other care.
Category II: Caregiver
01/31/2007 Home Health Services
Medical Policy Manual
210
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
♦ Primary caregiver(s) is employed and absent from the home, or is unable to provide
the necessary care.
♦ Primary caregiver(s) has additional child care responsibilities disallowing the time
needed to care for the member. (3 or more under 6 years of age, or 4 or more under
the age of 10 years.)
♦ Primary caregiver(s) also has additional child(ren) with special needs to care for.
(One or more children with special health care needs requiring extensive medical and
physical care.)
♦ Major illness or injury of caregiver(s) with expectation of recovery. (Physician’s
statement required)
♦ Temporary but significant change in the availability of caregiver(s) for example,
military service. (Commanding officer, other military representative, or employer’s
statement required)
♦ Significant permanent change in status of caregiver(s), for example, death or divorce
with loss of one caregiver. (Physician’s statement required)
01/31/2007 Home Health Services
Medical Policy Manual
211

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
HOME HEALTH CARE FOR CENTRAL NERVOUS SYSTEM DISORDERS
CPT/HCPCS CODES
Local codes effective on or before 12/31/03
CROSSWALKED CODES
EFFECTIVE 1/1/04
Y0601 – Skilled nurse, per hour 99600 TD – Skilled nurse, RN
Y0501 – Home health aide, per hour 99600 – Home health aide
X3069 – Licensed practical nurse, per hour 99600 TE – Skilled nurse, LPN/LVN
Z5016 – Home tocolytic therapy
S9349 – Home infusion therapy, tocolytic
infusion therapy
W6503 – Physical therapy, individual, by the
unit; modalities not requiring use of capital
equipment
G0151 – Services of physical therapist is
home health setting, each 15 minutes
W7402 – Occupational therapy, by the unit,
individual
G0152 – Services of occupational
therapist in home health setting, each 15
minutes
W9083 – Speech therapy, home health
G1053 – Services of speech and language
pathologist in home health setting, each
15 minutes
Indicators for CNS Disorders
ONE of the following indicators must be present for a member to receive home
health care for CNS disorders.
♦ Altered level of consciousness
♦ Respiratory distress
♦ Potential for increased intracranial pressure
♦ Body temperature fluctuations (hypothalamus involvement)
♦ Posturing (decerebrate/decorticate)
♦ Seizure activity (current)
♦ Spasticity (severe)
♦ Pain
♦ Impaired motor/sensory function to include
paresis
paralysis
vision impairment
hearing impairment
impaired gag reflex
decreased tactile sensation
♦ Potential for self injury
♦ Need for constant supervision
01/31/2007 Home Health Services
Medical Policy Manual
212

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
ONE of the following services must also be necessary to receive either skilled or non-
skilled nursing care for CNS disorders.
SERVICES REQUIRING SKILLED
CARE
1. Vital signs
2. Ventilator operation/maintenance
3. Central line maintenance/dressings
4. Complex treatment modalities
(sterile dressings, soaks, packing,
etc.)
5. Parenteral/Enteral nutrition
6. Oxygen therapy
7. Respiratory treatments
8. Tracheostomy maintenance/change
9. Suctioning (frequency/secretion
type)
10. Stimulation (verbal/tactile)
11. Tube feedings/maintenance of tube
12. IV medication administration
13. Urinary catheter maintenance/change
14. Exercise (active/passive)
Services Requirin
g
Non-Skilled Care
1. Bathing/linen change/dressing
2. Catheter care
3. Skin care
4. Minor treatment modalities
5. Oral care
6. Stimulation
7. Continue plan of OT/PT
8. Assist with transfers/ambulation
9. Positioning
10. I & O records
11. Assist with oral feedings
12. Splint or brace application
13. Exercise (active or passive)
14. Ensure safety measures (seizure
precautions)
15. Vital signs
01/31/2007 Home Health Services
Medical Policy Manual
213

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
HOME HEALTH CARE FOR GASTROINTESTINAL DISORDERS
CPT/HCPCS CODES
Local codes effective on or before 12/31/03
CROSSWALKED CODES
EFFECTIVE 1/1/04
Y0601 – Skilled nurse, per hour 99600 TD – Skilled nurse, RN
Y0501 – Home health aide, per hour 99600 – Home health aide
X3069 – Licensed practical nurse, per hour 99600 TE – Skilled nurse, LPN/LVN
Z5016 – Home tocolytic therapy
S9349 – Home infusion therapy, tocolytic
infusion therapy
W6503 – Physical therapy, individual, by the
unit; modalities not requiring use of capital
equipment
G0151 – Services of physical therapist is
home health setting, each 15 minutes
W7402 – Occupational therapy, by the unit,
individual
G0152 – Services of occupational
therapist in home health setting, each 15
minutes
W9083 – Speech therapy, home health
G1053 – Services of speech and language
pathologist in home health setting, each
15 minutes
Indicators for Gastrointestinal Disorders
ONE of the following indicators must be present for a member to receive home
health care for gastrointestinal disorders.
♦ Nutritional impairment
malabsorption
mechanical cause
♦ Stomatitis, pharyngitis, esophagitis
♦ Swallowing disorders
♦ Gastric reflux
♦ Vomiting
♦ Anorexia
♦ Pain
♦ Orthostatic B/P
♦ Significant rapid weight loss
♦ Morbid obesity >200% optimal weight
♦ Periorbital/perirectal lesions
♦ Unhealed wound(s)
surgical
fistula, abscess, fissures
♦ Bacterial/parasitic infections
♦ Diarrhea
01/31/2007 Home Health Services
Medical Policy Manual
214

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
♦ Constipation
♦ Subtotal/total gastrectomy
♦ Ostomies
♦ Anemia
♦ Weakness and fatigue
ONE of the following services must also be necessary to receive either skilled or non-
skilled nursing care for gastrointestinal disorders.
Services Requiring Skilled Care
1. Vital signs
2. IV medication administration
3. Parenteral/Enteral nutrition
4. Administration/maintenance
5. Central line maintenance
6. Oral medication administration
7. Gastric tube medication
administration
8. Placement of nasogastric tubes
9. Complex treatment/wound care,
sterile dressings/wound
packing/medicated soaks, etc.
10. Ostomy care/irrigation
11. Oxygen therapy
12. Bowel training
13. Weight
14. I & O
Services Requirin
g
Non-Skilled Care
1. Bathing/linens/dressing
2. Oral care
3. Skin care
4. Feedings (oral)
5. Force fluid
6. Assist with ambulation
7. Exercise active\passive
8. Reinforce teaching of OT/PT/ST
9. I & O
10. Weight
01/31/2007 Home Health Services
Medical Policy Manual
215

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
HOME HEALTH CARE FOR MUSCULOSKELETAL DISORDERS
CPT/HCPCS CODES
Local codes effective on or before 12/31/03
CROSSWALKED CODES
EFFECTIVE 1/1/04
Y0601 – Skilled nurse, per hour 99600 TD – Skilled nurse, RN
Y0501 – Home health aide, per hour 99600 – Home health aide
X3069 – Licensed practical nurse, per hour 99600 TE – Skilled nurse, LPN/LVN
Z5016 – Home tocolytic therapy
S9349 – Home infusion therapy, tocolytic
infusion therapy
W6503 – Physical therapy, individual, by the
unit; modalities not requiring use of capital
equipment
G0151 – Services of physical therapist is
home health setting, each 15 minutes
W7402 – Occupational therapy, by the unit,
individual
G0152 – Services of occupational
therapist in home health setting, each 15
minutes
W9083 – Speech therapy, home health
G1053 – Services of speech and language
pathologist in home health setting, each
15 minutes
Indicators for Musculoskeletal Disorders
ONE of the following indicators must be present for a member to receive home
health care for musculoskeletal disorders.
♦ Pain
♦ Loss of locomotor ability
♦ Decreased muscle strength
♦ Stiffness
♦ Joint pain, swelling, redness, tenderness
♦ Muscle wasting
♦ Paralysis
♦ Post amputation
♦ Multiple fractures
♦ Muscle spasms
♦ Potential for injury to self
01/31/2007 Home Health Services
Medical Policy Manual
216

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
ONE of the following services must also be necessary to receive either skilled or non-
skilled nursing care for musculoskeletal disorders.
SERVICES REQUIRING SKILLED
CARE
1. Assist with prostheses, braces,
splints
2. Treatments requiring sterile
procedures
SERVICES REQUIRING NON-
SKILLED CARE
1. Bathing/linen/dressing
2. Assistance with ADL’s
3. Assistance with
transfers/ambulation
4. Assist with prostheses, braces,
splints
5. Exercise active or passive
6. Position changes
7. Non-invasive treatments, comfort
measures
01/31/2007 Home Health Services
Medical Policy Manual
217
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
HOME HEALTH CARE FOR RESPIRATORY DISORDERS
CPT/HCPCS CODES
Local codes effective on or before 12/31/03
CROSSWALKED CODES
EFFECTIVE 1/1/04
Y0601 – Skilled nurse, per hour 99600 TD – Skilled nurse, RN
Y0501 – Home health aide, per hour 99600 – Home health aide
X3069 – Licensed practical nurse, per hour 99600 TE – Skilled nurse, LPN/LVN
Z5016 – Home tocolytic therapy
S9349 – Home infusion therapy, tocolytic
infusion therapy
W6503 – Physical therapy, individual, by the
unit; modalities not requiring use of capital
equipment
G0151 – Services of physical therapist is
home health setting, each 15 minutes
W7402 – Occupational therapy, by the unit,
individual
G0152 – Services of occupational
therapist in home health setting, each 15
minutes
W9083 – Speech therapy, home health
G1053 – Services of speech and language
pathologist in home health setting, each
15 minutes
Indicators
ONE of the following indicators must be present for a member to receive home
health care for respiratory disorders.
♦ Dyspnea
▪ quality of respiration (shallow, air hunger, etc.)
rate of respiration's
dyspnea at rest
dyspnea with exertion
cyanosis
use of accessory muscles
apnea/bradycardia
♦ Abnormal breath sounds
♦ Splinting respirations
♦ Strenuous coughing
♦ Excessive, tenacious secretions
♦ Ineffective airway clearance
♦ Abnormal ABG’s
♦ Decreased ability to be mobile due to dyspnea
♦ Irritability/depression
♦ Fatigue/weakness
♦ Anxiety
01/31/2007 Home Health Services
Medical Policy Manual
218

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
ONE of the following services must also be necessary to receive either skilled or non-
skilled nursing care for respiratory disorders.
SERVICES REQUIRING SKILLED
CARE
1. Oral medication administration
2. IV medication administration
3. Parenteral/Enteral nutrition
4. Vital signs
5. Ventilator operation/maintenance
6. Tracheostomy maintenance/change
7. Suctioning
8. Complex treatment modalities
(sterile dressing, wound care)
9. Respiratory treatments
SERVICES REQUIRING NON-
SKILLED CARE
1. Assist with bathing, dressing, ADL’s
(total care may be required)
2. Skin care
3. Oral care
4. Force fluids as instructed
5. Assist with ambulation
6. Exercise active/passive
7. Assist with meals (oral feeding)
8. Vital Signs
01/31/2007 Home Health Services
Medical Policy Manual
219

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
HOME TOCOLYTIC INFUSION THERAPY
TOCOLYTIC THERAPY CODE Z5016
Local code effective on or before 12/31/03
Crosswalked code effective 1/1/04
Z5016 – Home tocolytic therapy
S9349 - Home infusion therapy, tocolytic
infusion therapy
TOCOLYTIC THERAPY CODE Z5017
Local code effective on
or before 12/31/03
Crosswalked code
effective 1/1/04 – 3/31/04
2004 code change effective
1/1/04
99601 – Home infusion, up to 2
hours Z5017 – Home tocolytic
therapy
99553 – Home infusion for
tocolytic therapy, per visit
99602 - Home infusion, each
additional hour
Indicators
ALL of the following indicators must be present for a member to receive home
health care for home tocolytic infusion therapy.
♦ The member must be at least 24 to 34 weeks gestation;
♦ The member must be in current preterm labor (preterm labor being defined as greater
than or equal to six contractions per hour);
♦ The member must have a cervical dilation of greater than or equal to 1 cm, or an
effacement of greater than or equal to 75%;
♦ The member must have experienced secondary failure to wean from infused
tocolytics, or have failed oral therapy and requires continued infusion therapy; and
♦ The member must have direct home telephone access to providers.
Agency guidelines for home tocolytic infusion therapy
Home Health care agencies must meet the following minimum guidelines to be
reimbursed for home tocolytic infusion therapy.
♦ Provide home health care to the pregnant member on a 24 hour/day, 7 day/week
basis;
♦ Provide the member with a tocolytic infusion pump and a uterine monitoring device
(including set up and delivery); provide member education regarding the use of the
equipment and be available for trouble shooting for the equipment on a 24 hour/day,
7 day/week basis;
♦ Provide pharmacological consultation regarding the use of tocolytics and
individualized member dosing on a 24 hour/day, 7 day/week basis;
♦ Provide member education regarding uterine contractions and other subtle symptoms
of preterm labor.
01/31/2007 Home Health Services
Medical Policy Manual
220
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
♦ Contact the member’s physician at least weekly for updates on member
condition/compliance.
01/31/2007 Home Health Services
Medical Policy Manual
221

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
HOME HEALTH CARE FOR URINARY/RENAL DISORDERS
CPT/HCPCS CODES
Local codes effective on or before 12/31/03
CROSSWALKED CODES
EFFECTIVE 1/1/04
Y0601 – Skilled nurse, per hour 99600 TD – Skilled nurse, RN
Y0501 – Home health aide, per hour 99600 – Home health aide
X3069 – Licensed practical nurse, per hour 99600 TE – Skilled nurse, LPN/LVN
Z5016 – Home tocolytic therapy
S9349 – Home infusion therapy, tocolytic
infusion therapy
W6503 – Physical therapy, individual, by the
unit; modalities not requiring use of capital
equipment
G0151 – Services of physical therapist is
home health setting, each 15 minutes
W7402 – Occupational therapy, by the unit,
individual
G0152 – Services of occupational
therapist in home health setting, each 15
minutes
W9083 – Speech therapy, home health
G1053 – Services of speech and language
pathologist in home health setting, each
15 minutes
Indicators for Urinary/Renal Disorders
ONE of the following indicators must be present for a member to receive home
health care for urinary/renal disorders.
♦ Anemia
♦ Dyspnea
♦ Increased BUN/creatinine
♦ Decreased mental acuity
♦ Increased B/P
♦ Abnormal electrolytes
♦ Oliguria
♦ Weakness/fatigue
♦ Decreased mobility
♦ Neuropathies
♦ New diagnosis of renal failure
♦ Vascular access
♦ Newly initiated hemodialysis
♦ Recent admission for renal failure
♦ Recent admission for urinary tract surgery
♦ Peritoneal dialysis
♦ Pain
♦ Edema
♦ Potential for self injury
01/31/2007 Home Health Services
Medical Policy Manual
222

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
ONE of the following services must also be necessary to receive either skilled or non-
skilled nursing care for urinary/renal disorders.
SERVICES REQUIRING SKILLED
CARE
1. Complex treatment modalities
sterile dressings
special catheter care (ureteral
catheters, irrigation, etc.)
2. Urinary, suprapubic catheter care
3. I & O
4. Weight
5. Vital signs
SERVICES REQUIRING NON-
SKILLED CARE
1. Assist bathing/linens/dressing
2. Skin care
3. Oral care
4. Assist with exercise and ambulation
5. Reinforce nutritional teaching
6. Weight
7. I & O
8. Vital signs
9. Safety measures
01/31/2007 Home Health Services
Medical Policy Manual
223

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
HOME HEALTH SERVICES
ADDENDUM A
Note: This addendum contains provider notifications that have been published since the
review of the Home Health Services Fact Sheet. The information in this addendum will
be incorporated into the fact sheet at the next review.
Provider Notification: NL200509 Publication Date: 09/2005
Subject: Physician Signature Stamps
Date Added to Manual: 10/31/2005
Text of Publication
Effective January 24, 2004, CMS Transmittal 59 allows for the acceptance of a
physician’s rubber stamp signature for clinical record documentation, provided it is
permitted by Federal, state, and local law, and authorized by the home health agency’s or
hospice agency’s policy. This newsletter article addresses the impact this new policy will
have on the Medicaid prior authorization process for home health and hospice services by
referring providers to the appropriate regulations for Medicaid.
Chapter 6 of the IHCP Provider Manual and state regulations at 405 IAC 5-5-5 specify
that the provider must approve the Indiana Prior Review and Authorization Request form
by personal signature, or providers and their designees may use a signature stamp.
Providers that are agencies, corporations, or business entities may authorize one or more
representatives to sign requests for prior authorization (PA). Providers should note that
this section of the IHCP Provider Manual and state regulation address permissible
signature requirements for the Indiana Prior Review and Authorization Request form, and
must be differentiated from the signature requirements for physician orders and care
plans. Under the above-mentioned regulation, it is permissible for the agency to use a
signature stamp for the Indiana Prior Review and Authorization Request form.
The following state regulations apply to Medicaid prior authorization request for home
health services and can be viewed on the internet at www.accessindiana.com
:
• 405 IAC 5-16-3.1 Home health agency services; limitations: does not address
physician signature stamps for physician orders or written care plans.
• 405 IAC 5-22-2 Nursing services; prior authorization requirements does not address
physician signature stamps for prior authorization of nursing services.
In conclusion, physician signature stamps may be used on the Indiana Prior Review and
Authorization Request form when requesting Medicaid prior authorization for home
health services; however, any physician order or plan of treatment that is attached to the
Indiana Prior Review and Authorization Request form must include an original signature
by the physician.
01/31/2007 Home Health Services
Medical Policy Manual
224

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
State regulations for the Medicaid hospice benefit do not specifically provide for
physician signature stamps. The following regulations do apply to Medicaid prior
authorization request for hospice services with regard to the hospice physician
certification and the hospice plan of care. They can be viewed on the internet at
www.accessindiana.com .
• 405 IAC 5-34-5 Physician certification
• 405 IAC 5-34-7 Plan of care
In order to ensure that the medical director or physician member of the hospice reviewed
the plan of care, an original signature is required.
In conclusion, physician signature stamps may be used on the Indiana Prior Review and
Authorization Request form when requesting Medicaid prior authorization for hospice
services; however, any Medicaid Hospice Physician Certification form or Medicaid
Hospice Plan of Care that is attached to the Indiana Prior Review and Authorization
Request form must include an original signature by the physician.
Furthermore, the IHCP notes that electronic signatures are not acceptable on plans of care
submitted to the HCE Prior Authorization Unit.
Home health and hospice providers should contact the Acute Care Division of the Indiana
State Department of Health at (317) 233-7474 with regard to ISDH home health and
hospice survey rules.
Information To Be Read In Conjunction with Provider Bulletin BT200117 Prior
Authorization Request for Home Health
This information should be read in conjunction with information already published in
BT200117 (April 27, 2001 release date). BT200117 may be viewed on the Indiana
Medicaid Web site at www.indianamedicaid.com .
Providers are informed that there have been no changes to Medicaid state regulations at
405 IAC 5-16-3(d)(2)(G), which requires a home health agency to state the amount of
time required to complete the treatment task on the plan of care. However, the IHCP has
made a change to the directions in BT200117, which specified that the Indiana Prior
Review and Authorization Request form and the signed plan of care must reflect the
specific frequency and duration of care.
This newsletter notes the following change:
• The Indiana Prior Review and Authorization Request form may now reflect the
maximum amount of time it may require for the home health agency to care for the
patient; however, the provider should only bill the IHCP the actual service units
provided on each visit.
ISDH regulations regarding patient care and the medical plan of care that were referenced
in BT200117 have changed. The new home health regulations may be viewed by
01/31/2007 Home Health Services
Medical Policy Manual
225

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Home Health Services
Medical Policy Manual
226
accessing the IAC on the Web site at www.accessindiana.com . The new regulations may
be viewed as follows:
• Encounter defined may be viewed at 410 IAC 17-9-12.
• Frequency of visits defined may be viewed at 410 IAC 17-9-13.
EDS Page 4 of 20 P.O. Box 7263 Indianapolis, IN 46207-7263 For more information
visit www.indianamedicaid.com Indiana Health Coverage Programs Provider Monthly
Newsletter NL200509 September 2005
• Information regarding patient care and the medical plan of care may be viewed at 410
IAC 17-13-1.
It is the responsibility of home health providers to ensure that their plans of care are
compliant with Medicaid regulations and ISDH survey regulations.
Home health providers may direct any questions regarding the ISDH home health survey
process to the ISDH Acute Care Unit at (317) 233-7472. Home health providers may
direct any questions regarding Medicaid home health prior authorization to the HCE Prior
Authorization Unit at (317) 347-4511 or 1-800-457-4518.

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: HOSPICE
DESCRIPTION:
The Indiana Health Coverage Programs (IHCP) hospice benefit program mirrors the
covered services and reimbursement methodology of the Medicare hospice program.
IHCP hospice providers are required to comply with the federal hospice regulations located
in the Code of Federal Regulations (CFR), 42 CFR Part 418 et al implementing the
Balanced Budget Act of 1997. These regulations require hospice providers to list all hospice
covered services in frequency and scope on the hospice plan of care necessary to treat the
terminal illness and related conditions. Additionally, IHCP hospice providers must be
Medicare-certified and licensed as hospice providers by the Indiana State Department of
Health as a condition of provider enrollment.
Hospice providers are health care providers who own or operate hospice
programs/facilities that use interdisciplinary teams directed by licensed physicians.
These programs provide planned and continuous care for hospice program members and
their families. Hospice programs are designed to alleviate the physical, emotional, social,
and spiritual discomforts of a member who is experiencing the last phase of a terminal
illness or disease.
This document is intended to serve as a general summary of the IHCP policies regarding
this service. More specific information may be found in the IHCP provider manual,
program notices, the Indiana Administrative Code (IAC), or other sources as appropriate.
See the “Rules, Citations, and Sources” section of this document for further information.
COVERAGE CRITERIA
The IHCP consults hospice criteria published by the fiscal intermediary for Indiana
Medicare hospice providers, Palmetto Government Benefits Administrators, LLC.
Palmetto has established these guidelines as a matter of protocol for medical criteria.
Providers are to use current professional guidelines, including the Local Medical Review
Policy (LMRP), to determine when members meet medical necessity for hospice
services. Hospice providers are reminded that the IHCP recognizes the LMRP is a
guideline to determine when members are appropriate for hospice and palliative services.
The LMRP is not meant to replace the overall clinical evaluation by the hospice provider,
01/31/2007 Hospice
Medical Policy Manual
227
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
IHCP, or its contractor when evaluating the unique clinical condition of each hospice
member.
Any IHCP member, who is terminally ill and meets medical necessity criteria, may
receive services from an IHCP hospice provider. Additional to service compliance with
42 CFR Part 418 et. al and the Balanced Budget Act of 1997, providers are required to
document in the member’s hospice medical record support for a terminal diagnosis versus
a chronic condition.
PRIOR AUTHORIZATION
Hospice care is dependent upon a physician’s certification endorsing a member's
prognosis of life-expectancy to be six months or less, if the terminal condition runs its
normal course. Hospice services must be reasonable and meet medical necessity for the
palliation and management of the terminal illness. Coverage for hospice care is strongly
dependent upon documentation of the member's condition and the overall decline in the
member's health status as recorded in the physician certification and the hospice plan of
care. Additionally, documentation must include any co-morbidities. Documentation is
utilized in the prior authorization (PA) and review processes to determine medical
necessity. Each case is evaluated on its own merit. The IHCP, Medicare, and its
contractors are not prevented from requesting medical documentation regarding hospice
members at any time during the member’s enrollment the hospice program. This practice
is consistent with the IHCP provider agreement.
All IHCP hospice providers are required to be Medicare-certified and licensed as a
hospice by the Indiana State Department of Health before enrolling as an IHCP hospice
provider. Each hospice agency must ensure that the medical documentation submitted to
Health Care Excel (HCE) meets Medicare conditions of participation. The IHCP requires
hospice providers to request PA for members at the beginning of each hospice benefit
period. PA requests for hospice services may be modified by the HCE PA department.
When entering the third hospice benefit period of 60 continuous days, hospice providers
must submit specific medical documentation that supports the need of continued hospice
care. If the HCE PA department determines that the information is insufficient to process
the request, the PA hospice reviewer will return it for the required documentation. The
following information must be documented and sent to the PA department for initial and
continued hospice care.
• Member has a terminal prognosis, as well as, a physician certification that
meets the Medicare guidelines for participation.
• Clinical evidence must support a terminal diagnosis at the time of the initial
certification and each subsequent certification.
• Documentation must describe why a member’s condition is terminal, not
chronic (Medical history may provide clarification for documentation that
reflects a chronic condition).
01/31/2007 Hospice
Medical Policy Manual
228
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• For each hospice benefit period, the interdisciplinary team must assess the
member's condition and service appropriateness. Documentation must
distinguish between exacerbation and stabilization, as well as, exacerbation and
deterioration.
• Documentation must include the most specific and appropriate terminal
diagnosis from the International Classification of Diseases 9
th
Edition, Clinical
Modification (ICD-9-CM).
• Documentation must specify any necessary medications, treatments, and
services that are considered aggressive treatments.
• Document must detail the member's decline.
• Document must describe how the systems of the body are in a terminal
condition.
Hospice Forms
IHCP hospice providers must complete and submit the appropriate forms for each
member. Required forms are dependent upon the member’s status and requested actions.
Refer to the IHCP Hospice Provider Manual and the Indiana Family and Social Services
Administration web site for detailed information regarding these forms. Below is a brief
description of the hospice forms available to providers.
• Medicaid Hospice Election
Members complete this form to elect the hospice benefit. Additionally, it is
used to select a particular hospice provider. Election of the hospice benefit
requires the member to waive: (a) other forms of health care treatment of the
terminal illness for which hospice care was elected or for treatment of a
condition related to the terminal illness; (b) services provided by another
provider that are equivalent to the care provided by the elected hospice
provider; and (c) hospice services other than those provided by the elected
hospice provider or its contractors.
• Medicaid Physician Certification
Providers complete this form in conjunction with the Hospice Election Form
and the Hospice Plan of Care when requesting the first hospice benefit period.
Assuming the information is sufficient and accurate, an initial benefit period of
90 days is approved. If benefit periods beyond the first 90 days are necessary,
then recertification on the IHCP Physician Certification form and an updated
Hospice Plan of Care is required for hospice authorization of the next benefit
period.
• Medicaid Hospice Plan of Care
Providers complete this form must be completed by an interdisciplinary team
member and who must confer with at least one other member of the
interdisciplinary team. One of the conferees must be a licensed physician or
nurse. All team members must review the plan of care. All of the services
stipulated within the plan of care must be reasonable and necessary for
palliation or management of the terminal illness and related condition. The
01/31/2007 Hospice
Medical Policy Manual
229
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
plan of care must be signed by the hospice medical director and include two
other signatures from any of the disciplines listed on the IHCP Hospice Plan of
Care.
• Hospice Authorization Notice for Dually-Eligible Medicare/Medicaid Nursing
Facility Residents
Providers complete this form for dully-eligible Medicare/Medicaid members.
These members must elect, revoke, and change providers under both the
Medicare and the IHCP programs at the same time. The hospice provider is
required to notify both programs of any changes in the dually-eligible
Medicare/IHCP member’s hospice care status. The IHCP requires that the
hospice provider submit all the required certification forms
• Hospice Provider Change Request Between Indiana Hospice Providers
Providers complete this form when a member, or representative of the
member, is not satisfied with the hospice provider. A member may change
hospice providers once during any benefit period. This change does not
constitute a revocation of services.
• Change in Status of Medicaid Hospice Patient
Providers complete this form when there is an eligibility status change.
• Medicaid Hospice Discharge
Providers complete this form when a hospice provider discharges a member
from future services. Refer to the section, Discharge by a Provider, to
determine appropriate discharge criteria.
• Medicaid Hospice Revocation
Providers complete this form when a member, or representative of the
member, is not satisfied with hospice care and revokes hospice services. This
form includes a signed statement that the individual revokes the election of
IHCP hospice services for the remaining days in the election period. A
member can elect to receive hospice care intermittently, rather than
consecutively, over the three benefit periods and can therefore elect and revoke
hospice coverage an unlimited number of times.
Covered Services
Hospice core services are covered services in the Medicare and IHCP hospice per diem
that must be provided directly to the member by hospice employees. Hospice core
services include nursing services, medical social work services, and counseling services
(including bereavement, dietary, spiritual, and other services). Hospice noncore services
are services in the Medicare or IHCP hospice per diem not identified as hospice core
services. The following list includes hospice services included in the Medicare and
Medicaid hospice per diem:
• Nursing care provided by or under the supervision of a registered nurse
01/31/2007 Hospice
Medical Policy Manual
230
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• Medical social work services provided by a social worker with at least a
bachelor’s degree working under the supervision of a physician
• Physician services provided by the medical director or a physician part of
the interdisciplinary team participating in services as follows.
• General supervising services, participating in the plan of care, conducting
periodic reviews, establishing governing policies, and providing direct-care
to members
• Counseling services provided to the member, member’s family, and other
people caring for the member
• Short-term inpatient care provided on a hospice inpatient unit, participating
hospital, and nursing home setting
• Medical equipment and supplies, including palliative drugs, related to the
palliation and management of the member’s terminal illness
• Home health services furnished by qualified aides
• Homemaker services that assist in providing a safe and healthy environment
• Physical therapy, occupational therapy, and speech-language pathology
services provided for the purpose of symptom control
• Inpatient respite care
• Room and board (dually-eligible hospice members) residing in long-term
care facilities
• Room and board for IHCP-only hospice members who reside in long-term
care facilities
• Any other item or service specified in the member’s plan of care, if the item
or service is a covered service under the Medicare program
Treatment of Nonterminal Conditions
The IHCP covers medical care for conditions unrelated to the terminal illness. The IHCP
expects the hospice provider to actively interface and coordinate these services with other
IHCP providers. Medical care for nonterminal conditions may be met by one of the
following methods.
• Outpatient physician services
• Inpatient and outpatient hospital admissions
• Emergency admissions to a nursing facility from a private home
If the IHCP hospice member requires an inpatient or outpatient hospital admission for
conditions unrelated to the terminal illness, the hospital must bill the IHCP directly for
these services. The hospice provider coordinates the inpatient or outpatient hospital
services. Hospice provider responsibility for the treatment of nonterminal conditions is
case specific. The following guidelines provide clarification for hospice providers
regarding this issue.
• If the hospice member currently does not receive treatment for a nonterminal
condition, the hospice provider is required to locate appropriate IHCP
services for the treatment of a nonterminal condition.
01/31/2007 Hospice
Medical Policy Manual
231
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• To ensure that the hospice member is not billed for these services, the
hospice provider must ensure that the hospital is enrolled as an IHCP hospice
provider.
• The hospice provider must communicate and coordinate with the hospital’s
medical personnel does not compromise the member’s hospice care.
• If the IHCP hospice member is admitted to the hospital from a private home,
the hospice provider must submit to the HCE PA department a Change in
Status of Medicaid Hospice Patient form. This form reflects the hospice
member’s change of care. The same form must be completed once the
hospice member is discharged from the hospital to either another institutional
care setting or to a private home.
The IHCP provider billing for the treatment of the nonterminal illness must obtain PA for
these services. The following services do NOT require PA for the treatment of
nonterminal conditions.
• Pharmacy services not related to the member’s terminal condition
• Dental services
• Vision care services
Discharge by Hospice Provider
Once a hospice provider chooses to admit a member, the provider may not automatically
or routinely discharge the member at its discretion, even if the care is costly or
inconvenient. The election of the hospice benefit is the member’s choice, rather than the
hospice’s choice; therefore, the hospice may not revoke the member’s election.
Additionally, hospice providers may not request or demand hospice revocation. Reasons
a hospice provider may discharge a member from care include the following situations.
• Member dies
• Member’s prognosis is determined to be greater than six months
• Member moves out of the hospice service area
• Member’s safety or hospice staff safety is compromised
When a member moves out of the service area, the hospice provider notifies the fiscal
intermediary of the discharge so that hospice services and billings are terminated as of the
discharge date. In this situation, the member loses the remaining days in the benefit
period; however, there is no increase cost to the member.
For circumstances when a member’s safety is compromised, the hospice must make
every effort to resolve these problems satisfactorily before discharge is considered an
option. All efforts by the hospice to resolve the problem must be documented in detail in
the member’s record. The hospice must notify the fiscal intermediary and the State
Survey Agency (SA) of the circumstances surrounding the impending discharge.
Hospice providers must submit specific forms to facilitate the discharge. For
reimbursement, hospice program guidelines must be followed.
01/31/2007 Hospice
Medical Policy Manual
232
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• Hospice providers may fax the Medicaid Hospice Discharge form to the
HCE PA department, as long as all the hospice benefit periods preceding the
hospice discharge date have been previously authorized.
• For members residing in nursing facilities, hospice providers are encouraged
to provide a copy of the discharge form to the appropriate staff in the
nursing facility to ensure the form is included in the hospice member’s
nursing facility clinical record. These coordination procedures ensure staff is
aware of the exact date that the hospice provider discharged the member.
Additionally, hospice providers must provide a copy of this form to the
nursing facility billing department.
• Hospice providers must bill the IHCP for the hospice per diem for nursing
facility room and board for the hospice discharge date.
• Nursing facilities may resume billing the IHCP directly for nursing facility
care for the date of service following the hospice discharge date once the
hospice provider has provided a copy of the PA form reflecting an updated
hospice discharge claim.
• Hospice providers are reminded that it is a violation of medical records
standard to predate the hospice discharge. The documented discharge date
cannot precede the actual discharge.
Noncompliance
As part of the admissions process, hospice providers should explain to members covered
hospice services, actions that may constitute noncompliance with the hospice care
philosophy, and charges members may be responsible for paying. When a hospice
provider believes a member has reflected significant noncompliance with the hospice
plan of care, documentation standards outlined below must be followed.
• Must have clear written admissions policies
• Must have informed the member of the hospice benefit responsibilities
• Must have thorough documentation of the noncompliance issues
Once the hospice provider follows these guidelines, the SA must be contacted. The SA
contacts the Centers for Medicare and Medicaid Services (CMS) for further discharge
consideration.
Dually-Eligible and Medicaid-Only Hospice Members in Nursing Facilities
The IHCP hospice benefits must comply with the Omnibus Budget Reconciliation Act of
1989 (OBRA 1989). OBRA 1989 requires dually-eligible Medicare/IHCP members to
elect, revoke, or change providers under both the Medicare and the IHCP programs
simultaneously. Hospice providers are required to notify both programs of any changes
in the member’s hospice care status. Additionally, hospice providers are required to
coordinate regularly with nursing facility providers. To ensure that the IHCP member's
enrollment in the IHCP hospice benefit is clear to both hospice and nursing facility staffs,
the hospice provider must furnish the nursing facility staff and the nursing facility’s
billing department with the member’s Medicaid hospice forms.
01/31/2007 Hospice
Medical Policy Manual
233
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Waiver Members
Once hospice service criteria are met, IHCP waiver members are eligible for hospice
services. Waiver members are not required to disenroll from the waiver program;
however, they must be under direct-care of an IHCP hospice provider for those services
that both programs have in common. Member may receive waiver services unrelated and
non-duplicative of the hospice services. Hospice providers must coordinate with
nonhospice providers to ensure overall care is met and the hospice plan of care is not
compromised. The number of hours related to the member’s nonterminal condition is
determined on a case specific basis. Additional waiver services should not be provided
due to the hospice election.
MANAGED CARE ORGANIZATIONS
Members enrolled in an IHCP Managed Care Organization (MCO) must disenroll.
Disenrollment is necessary for hospice authorization to be completed. Members become
eligible for hospice services the day following disenrollment from the MCO.
To facilitate the hospice authorization process, the hospice provider may fax the
Medicaid Hospice Election form to the HCE PA department initiating MCO
disenrollment. The corresponding Medicaid Hospice Physician Certification form and
Medicaid Hospice Plan of Care form must be sent to the HCE PA department within ten
business days. If the hospice provider fails to verify IHCP eligibility or fails to fax the
Medicaid Hospice Election form to the HCE PA department, the hospice provider will
not receive payment for these dates of service the member is MCO member.
REIMBURSEMENT INFORMATION
Hospice providers must provide care based on the medical acuity of the member at one of four
distinct hospice levels of care: routine home care, continuous home care, general inpatient
hospice care, and inpatient hospice respite. Hospice inpatient care must be provided in an
inpatient unit or contracted inpatient facility that meets the parameters at 42 CFR Part 418.100
et al. The following information describes the four levels of service and the two levels of
care available to members. Table 1 outlines the hospice reimbursement methodology.
• Routine home care delivered in a private home
• Continuous home care delivered in a private home
• Routine home care delivered in a nursing facility
• Continuous home care delivered in a nursing facility
• Inpatient respite care (available to private home hospice members only)
• General inpatient hospice care
01/31/2007 Hospice
Medical Policy Manual
234

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 1 Hospice Reimbursement Methodology
Revenue
Code
Revenue Code Descriptions and Explanations
180
Nursing Facility Bed Hold Non-Paid Revenue Code***
The hospice provider should bill the IHCP using this revenue code for leave
days when the nursing facility occupancy is less than 90%. This code
generates an IHCP denial; however, providers may charge members for the
nonreimbursed bed hold days.
183
Nursing facility bed hold for hospice therapeutic leave days***
Therapeutic leave days are reimbursed at 50% of the 95% of the nursing
facility room and board per diem rate. Eighteen therapeutic leave days are
reimbursable per member per calendar year. Hospice providers should not
bill the IHCP using this revenue code when the nursing facility occupancy
rate is below 90%.
185
Nursing facility bed hold for hospitalization for services unrelated to the
terminal illness***
Bed holds for hospitalization for services unrelated to the terminal illness are
reimbursed at 50% of the 95% of the nursing facility room and board per
diem rate. Fifteen days per hospitalization is reimbursable. Hospice
providers should not bill the IHCP using this revenue code when the nursing
facility occupancy rate is below 90%.
651
Routine home care in a private home
The hospice provider is paid at the routine home care rate for each day the
member is at home, under the care of the hospice provider, and not receiving
continuous home care. This rate is paid without regard to the volume or
intensity of routine home care services.
652
Continuous home care in a private home *
Continuous home care per diem rate is calculated into an hourly rate. The
hourly rate is reimbursed to the hospice provider to 24 hours a day. Home
health aides may supplement the nursing care in the total continuous care
hours. All hours must be counted. Documentation must clearly indicate the
nature of the medical crisis, need for skilled intervention, and illustrate hourly
and daily what level of staffing and the services provided.
653
Routine home care in a nursing facility
The hospice provider is paid at the routine home care rate for each day the
member is in a nursing facility, under the care of the hospice provider, and
not receiving continuous home care. The rate is paid without regard to the
volume or intensity of routine home care services. The hospice provider is
paid an additional room and board per diem at 95% of the lowest nursing
facility rate for contracted nursing facility cost.
01/31/2007 Hospice
Medical Policy Manual
235

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Revenue
Revenue Code Descriptions and Explanations
Code
654
Continuous home care in a nursing facility
The continuous home care rate is calculated into an hourly rate. The hourly
rate is reimbursed up to 24 hours a day. Home health aides may supplement
the nursing care in the total continuous care hours. All hours must be
counted. Documentation must clearly indicate the nature of the medical
crisis, need for skilled intervention, and illustrate hourly and daily what level
of staffing and the services provided. The hospice provider is paid an
additional room and board per diem at 95% of the lowest nursing facility rate
for contracted nursing facility cost.
655
Inpatient respite care**
Respite care is an occasional, short-term inpatient care provided to hospice
members to relieve caregivers. Respite care is available to members residing
in private homes. The hospice provider is paid at the inpatient respite care
rate for each day the member resides in an approved inpatient facility and
receives respite care. Payment for respite care is for a maximum of five
consecutive days per stay. Payment for the sixth day and subsequent days is
at the routine home care rate.
656
General inpatient hospice care
The hospice provider is paid at the general inpatient hospice rate for each day
the member resides in an approved inpatient hospice facility and receives
general inpatient hospice care. Inpatient hospice care is for pain control and
acute/chronic symptom management not manageable in other settings.
657
Hospice direct-care physician services
Physician services, hospice provider employee or authorized hospice
provider, are separately reimbursable on a fee-for-service basis. Services are
billed by the hospice provider utilizing the hospice provider number. This
code may be billed on the same day other hospice revenue codes are billed.
659
Room and Board for Dually-eligible Medicare/Medicaid nursing facility
members only (Room and board portion of the hospice per diem rate)
The hospice provider must bill Medicare for the hospice services and
Medicaid for room and board. The hospice provider is paid 95% of the
lowest nursing facility per diem to cover the room and board cost incurred by
the contracted nursing facility. Revenue code 659 may not billed with the
hospice related revenue codes 651, 652, 653, 654, 655, or 656. These codes
are designated for IHCP-only hospice services.
*Continuous home care is provided only during a period of crisis requiring continuous care for acute medical
symptoms, palliation and management treatments. A nurse, registered or licensed practical nurse, must
provide over half the total care. This care need not be continuous and uninterrupted.
**Inpatient facility is defined as a hospital, long-term care facility, or the facility of a hospice provider that
provides care 24 hours a day.
***Please have hospice revenue codes 180, 183, and 185 follow hospice revenue code 659 in the table.
01/31/2007 Hospice
Medical Policy Manual
236
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Reimbursement for Room and Board on Date of Death or Date of Physical
Discharge
The OMPP does not pay the nursing facility per diem or room and board services for the
day a member is discharged from the nursing facility. When a hospice member dies in a
nursing facility, the date of death follows the same reimbursement procedures as the date
of physical discharge from the nursing facility. If a hospice member is admitted and dies
in the nursing facility on the same day, the nursing facility is not paid the room and board
per diem for that day; however, hospice providers may bill the IHCP for the hospice per
diem for either a physical or death discharge. Providers bill revenue code 653 or 654
with occurrence code 51.
Hospice Member Liability Residing in a Nursing Facility
An IHCP member (dually-eligible Medicare/IHCP or IHCP-only) residing in a nursing
facility is responsible for the member's portion of the payment before the IHCP pays the
remaining balance of nursing facility care (i.e., room and board services). Member
liability includes, but is not limited to, personal savings account, Medicare pension funds,
or Social Security funds. Member liability is deducted the first date of service the
member resides and eligible for the IHCP nursing facility LOC.
Hospice providers can obtain a member’s patient liability for a particular month by
contacting EDS Customer Assistance or using one of the eligibility verification system
(EVS). When a provider obtains the patient liability amount, the RA is used to determine
how EDS calculates the paid amount. The following formula is used if the RA does not
match the rates the provider submitted on the claim.
1. Nursing facility case mix rate on file x 95% (.95) = allowed amount on the RA
for room and board
2. (Number of dates of service x allowed amount on RA) minus member liability
= room and board amount
Prior Authorized Physician Services
Additional reimbursement is available for an independent physician's direct-care services
in accordance with the IHCP reimbursement physician service methodology. Hospice
providers may not bill these services under the hospice provider number. Services must
be the physician's professional services. Costs for services related to the terminal
condition, such as laboratory or X-rays, may not be included in this billing. These
services are included in the per diem rates.
Emergency Service Charges
Emergency service charges related to the terminal illness for hospice members are the
responsibly of the hospice provider. These responsibilities include all hospice and
transportation charges associated with emergency. Emergency service charges unrelated
to the terminal illness are separately reimbursable by the IHCP by the appropriate
provider.
01/31/2007 Hospice
Medical Policy Manual
237
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
NURSING FACILITY QUALITY ASSESSMENT
The IHCP began retro rate adjustments to nursing home rates for the nursing facility
quality assessment fee July 2005. The change in nursing facility rates, due to the quality
assessment fee results in retro rate adjustments for room and board to hospice providers
retroactive to July 1, 2003. Hospice and nursing facility providers are reminded that all
coordination and payment arrangements should be reflected in the hospice contract with
the nursing facility. Hospice claims reflecting room and board payments for the dates of
service July 1, 2003 to present are included in the retro rate adjustment. OBRA 1989 and
405 IAC 1-16-4 require the IHCP to reimburse hospice providers nursing facility room
and board payments; Hospice providers reimburse nursing facilities according to their
contract. The IHCP pays the hospice 95% of the nursing home rate on file to the hospice.
INDIANA HEALTH COVERAGE PROGRAMS HOSPICE PROVIDER
MANUAL 4.0
The IHCP Hospice Provider Manual provides a comprehensive, single-source document
outlining policies and procedures associated with the IHCP hospice program. The
manual does not address general aspects of IHCP policy such as IHCP member
eligibility, third party liability, medical policy, PA, utilization review, or inspection of
care. The manual is divided into nine main sections.
• Section 1: Introduction
This section outlines key policies and procedures
• Section 2: Provider Enrollment
This section outlines the conditions for provider participation and enrollment
procedures. Special attention is given to provider certification requirements,
locational service issues, and institutional policies.
• Section 3: Member Eligibility
This section outlines the target group by population category, hospice benefit
periods, and member certification requirements.
• Section 4: Election and Revocation
This section explains election and revocation hospice services, discharging hospice
members, and changing hospice providers.
• Section 5: Hospice Authorization Process
This section defines the procedures and policies that determine the framework of a
member's hospice services. Emphasis is placed on the informational requirements
associated with the hospice benefit periods and the prior authorization process for
services unrelated to the terminal condition.
01/31/2007 Hospice
Medical Policy Manual
238

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• Section 6: Reimbursement
This section describes reimbursement for hospice services detailing billing revenue
codes and level-of-care categories.
• Section 7: IHCP Recoupment
This section describes methods of IHCP recoupment and overpayment.
• Section 8: Most Common Error Codes
This section describes common reimbursement denials, explanation of benefit
codes, and methods to correct errors.
• Section 9: Hospice Contracts with Nursing Facilities
This section explains hospice provider and nursing facilities contracts.
RELATED MEDICAL TOPICS
Emergency Services
Home Health Services
Hospital Inpatient Services
Hospital Outpatient Services
Long-Term Care Services
Managed Care Organization Services
Mental Health Services
Nursing Facilities Services
Out-of-State Servies
Pharmacy Services
Therapy Services
Waiver Services
RULES, CITATIONS, AND SOURCES
Code of Federal Regulations (CFR)
42 CFR 1001.952 – Safe Harbor Regulation
42 CFR 418 – Conditions of Participation for Hospice Care
01/31/2007 Hospice
Medical Policy Manual
239
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Indiana Administrative Code (IAC)
405 IAC 1-16 – Reimbursement for Hospice Services
405 IAC 5-2-10.2 – “Hospice Program” defined
405 IAC 5-5-1 – Out-of-State Services; general
405 IAC 5-34 – Hospice Services
Indiana Code (IC)
IC 4-21.5-3-33 – Adjudicative Proceedings, Records
IC 16-25-3 – Licensure of Hospices
Indiana Health Coverage Programs Provider Manual
1999
Revised 2005, Version 5.0
Indiana Health Coverage Programs Hospice Provider Manual
Revised March 2004, Version 4.0
Indiana Health Coverage Programs Banner Page (BR)
BR200536 – Retro Rate Adjustments under Hospice
BR200513 – Information about Hospice Retro Rate Room and Board Adjustment
Procedures
BR200503 – Change Order to Expedite the Adjustment of Hospice Claims for
Room and Board
BR200502 – Hospice and Nursing Facility Change Order
BR200501 – Hospice and Nursing Facilities Claims Changes
BR200452 – Hospice and Nursing Facilities Update
BR200446 – Hospice for Managed Care Member Update
BR200442 – Hospice Update
BR200413 – Hospice Counties 1-9 EOB code 4014
BR200412 – Hospice Update as in BT200372
BR200411 – Hospice Changes to Authorization Process
BR200410 – Hospice Providers Enrolled in MCO and Hospice Authorization
Update
BR200331 – Hospice Verification Responses
BR200315 – Hospice Update
BR200216 – Hospice Rule Change
BR200043 – Hospice Agency Review Process
BR200025 – Hospice Benefits Clarification
BR199919 – Hospice Claims Mass Adjustments
BR12-29-1998 – Hospice Forms
BR12-15-1998 – Hospice “Private Pay”
BR11-10-1998 – Medicaid Hospice Forms
Indiana Health Coverage Programs Provider Bulletin (BT)
BT200372 – Changes in Hospice Authorization Process
BT200367 – Hospice Rates Effective October 1, 2003 and Hospice Benefits
Updates
BT200365 – Pharmacy Hard Edits and Hospice Review Process
BT200331 – Changes to the Hospice Benefit Rules
BT200259 – Detailed Explanation of Rule Changes and the Impact of the
Changes on Hospice Providers for IHCP Hospice Authorization
01/31/2007 Hospice
Medical Policy Manual
240

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
BT200234 – System Changes Affecting Hospice Revenue Codes 655 and 656
BT200147 – Hospice Rates Effective October 1, 2001
BT200146 – Nursing Facility Bed Hold Days
BT200116 – Nursing Facility Retro Rate Adjustments for Hospice Members
Receiving Hospice Care Prior to July 1, 1999
BT200112 – Revised Indiana Health Coverage Programs Hospice Rates Effective
April 1, 2001
BT200107 – Notification of Systems Issues Regarding Incorrect Payments to
Hospice Providers for Room and Board Payments on Member's
Date of Death
BT200102 – New Hospice Rate Effective October 1, 2000
BT200041 – Incorrect Payment to Hospice Providers for Room and Board
Payments for Member’s Date of Death
BT200030 – Revised Hospice Provider Manual
BT200011 – New Hospice Policy for Nursing Facility Residents
BT200002 – Use of Forms 450B and OMPP 450B SA/DE
BT199940 – New Rates
BT199939 – Billing Procedures, Edit1024
BT199925 – Policies and Procedures for Treatment for Non-Terminal Conditions
BT199924 –Treatment for Non-Terminal Conditions for Hospice Recipients
Admitted to a Nursing Facility After a Hospital Stay.
BT199919 – New Effective Date OMPP Recoupment Based on Noncompliance
with 405 IAC 1-16-4
BT199905 – Exceptional Services Managed Care (Medicaid Hospice)
Information
BT199904 – Election of Hospice Services by Home and Community-Based
Waiver Recipients Information
BT199846 – New Hospice Rates
BT199840 – Updated Form 450B Information
BT199838 – Hospice Authorization and Claim Payment Issues
BT199836 – Contracted Hospice Services in Nursing Facility
BT199830 – Reimbursement and Survey Issues (Hospice Benefits)
BT199817 – Procedures to Request Hospice Forms
BT199802 – Medicaid Hospice Billing Procedures
Indiana Health Coverage Programs Provider Newsletter (NL)
NL200411 – Hospice Services
Omnibus Budget Reconciliation Act of 1989 (OBRA 1989)
Social Security Act, Section 1902(a)(57)
Origination Date: 12/31/2000
Revision
and Review
Reason Date
Revision E97-25 New Hospice Benefit 07/01/97
Revision E98-15 New HCPC Procedure Codes 01/01/98
01/31/2007 Hospice
Medical Policy Manual
241

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Revision
Reason Date
and Review
Revision E98-02 Medicaid Hospice Billing Procedures 01/09/98
Revision
E98-30 Reimbursement and Survey Issues Related to the
Hospice Benefit
09/18/98
Revision E98-17 Procedures to Request Hospice Forms 09/18/98
Revision E98-46 New Hospice Rates 10/01/98
Revision E98-37 Recoupment Based on 405 IAC 1-16-4 11/06/98
Revision E98-36 Contracted Hospice Services in Nursing Facilities 11/06/98
Revision
E98-38 Issues Regarding Hospice Authorizations and
Hospice Claims Payment
11/06/98
Revision
E99-04 Election of Hospice Services by Home and
Community-Based Services Waiver Recipients
01/26/99
Revision
E99-05 Parameters and Procedures for Reimbursement for
Exceptional Circumstances for Managed Care Recipients
Who Elect Hospice
01/26/99
Revision
Medicaid Hospice Benefit Provider Manual Policy and
Procedures
02/01/99
Revision
E99-19 New Effective Date for OMPP Recoupment
Based on Non-Compliance
06/14/99
Revision E99-24 Treatment for Non-Terminal Hospice Conditions 07/30/99
Revision
E99-25 Policies and Procedures for Treatment for Non-
Terminal Conditions
07/30/99
Revision E99-40 New Rates 11/17/99
Revision E99-39 Billing Procedure and Edit 1024 12/01/99
Revision E00-11 New Policy 02/25/00
Revision E00-30 New Hospice Provider Manual 07/01/00
Review Scheduled 07/31/06
APPLICABLE INDIANAAIM EDITS AND AUDITS
387 – Service Is Not Payable
403 – Hospital “From” Date Invalid
404 – Hospital “Thru” Date Invalid
439 – Hospice Services Being Billed (Manual Payout)
499 – Claims Correction Form Not Returned Within Days
513 – Recipient’s Number Does Not Match the Recipient’s Name
01/31/2007 Hospice
Medical Policy Manual
242
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Hospice
Medical Policy Manual
243
547 – Hospital Leave Billed Without Accommodation
564 – Revenue Code Is Not Allowed for this Recipient’s Eligibility
641 – Patient Reported as Deceased
646 – Coordination With Hospice Provider
665 – Patient Enrolled Under Managed Care
1001 – Billing Provider Not Eligible to Bill on this Program
1002 – Rendering Provider Not Eligible to Render Service on this Program
1003 – Billing Provider Not Eligible to Bill on this Program for the Date of Service
1004
–
Rendering Provider Not Eligible to Render Service on this Program for the Date of
Service
1015 – Provider Not Authorized to Render this Service for this Program Without PA
1024 – Billing Provider Not Listed As Member’s LTC Provider
1025 – Billing Provider Not Eligible to Bill on this Program for the Dates of Service
1032 – Billing Provider Not Eligible to Bill this Claim
1035 – Hospice Provider Billing for Hospice Services
1036 – Rendering Provider Not Eligible
1037 – Private Duty Nurse
1039 – Services Rendered By Out of Network Provider
1042 – Certification Code Missing
2003 – Recipient Ineligible on Date of Service
2018 – Recipient Ineligible on Date(s) of Services
2023 – Recipient Ineligible on Dates of Service Due to Enrollment in a Managed Care
Organization
2024 – Patient Ineligible for Hospice Level-of-Care
2025 – Hospice Recipient Billed Without Hospice Services
2026 – Hospice Recipient Ineligible for Nursing Home
2027 – Hospice Service Not Billed Correctly
2034 – Medical and Non-Medical Supplies and Routine DME Items are Covered in the Per
Diem Rate
4014 – No Pricing Segment on File
4214 – Hospice/Waiver Duplicative Services
4233 – Date of death/discharge not covered
5001 – Duplicate of Another Claim
6748 – Hospice Respite Limited to Five Days
9064 – Hospice Pricing (Rate on File)
9069 – Room and Board Not Paid on Date of Death/Discharge
9070 – Payment is Based on the Lessor of the Billed
9090 – State Enforced Rate Reduction

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: HOSPITAL INPATIENT
DESCRIPTION:
“Inpatient” defined is a patient required to be admitted to the hospital to treat a condition
requiring close monitoring or skilled professional management.
SUMMARY OF CURRENT POLICY:
Inpatient hospital services can be covered when they are medically reasonable and
necessary and can be performed only in an inpatient hospital setting. Most inpatient
admissions are reimbursed by Diagnosis Related Groupings (DRG) methodology.
Catastrophic cases are given special consideration and are reimbursed based on outliers
of the case.
Admissions reimbursed by level of care are psychiatric admissions, physical
rehabilitation admissions, and burn admissions.
Medicaid does require prior authorization for all out-of-state admissions, in-state
psychiatric admissions, substance abuse admissions, physical rehabilitation admissions,
and burn admissions. Prior Authorization must be obtained for all admissions requiring
prior authorization wi.thin forty-eight (48) hours, excluding Saturday, Sunday, and legal
holidays. Concurrent review is necessary beyond the approved days. (Prior
Authorization requirements may be found in 405 IAC 5-3.)
MEDICAL TOPICS CROSS-REFERENCES:
Family Planning
Hospital Inpatient – Readmissions/General/Same Provider
Mental Health/Behavioral Health – Inpatient Services
Mental Health/Behavioral Health – Outpatient Services
Nursing Facilities
01/31/2007 Hospital Inpatient
Medical Policy Manual
244

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Hospital Inpatient
Medical Policy Manual
245
RULES, CITATIONS, AND SOURCES:
405 IAC 5-17 Hospital Services
Initial Policy Issue
Effective
Date
Implementation
Date
Retroactive
Date
470 IAC 5-8-9
Transferred
Hospital services 7/1/91
405 IAC 1-6-9
Repealed 8/24/97
Hospital Services
1/1/92
Revisions:
405 IAC 5-17-1
405 IAC 5-17-2
Hospital Services
8/24/97
405 IAC 5-17-3
Emergency
Inpatient
Admission
8/24/97
APPLICABLE INDIANAAIM EDITS AND AUDITS:
2020
2021
3007
3014
4081
4085
4087
4092
4099
4103
4106
4116
4202
6046
6508
6509

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Coverage Criteria
INPATIENT ACUTE CARE HOSPITAL ADMISSIONS
405 IAC 5-17 provides the rules governing hospital services.
Inpatient hospitalizations for medical conditions other than mental health, burns or
rehabilitation following a traumatic injury, are reimbursed utilizing the DRG
methodology and do not require prior authorization. (See 405 IAC 1-10.5 for DRG
reimbursement methodology)
Inpatient hospitalizations for the immediate treatment of burns DO require prior
authorization. However, considering these admissions are usually emergent in nature, the
facility has 48 hours from the time of admission, excluding weekends and holidays, in
which to request authorization. Additional days are based on medical necessity and the
percentage of the body involved.
NOTE: Effective January 10, 2001, the Office of Medicaid Policy and Planning
(OMPP) determined that the prior authorization requirement for inpatient burn
admissions was no longer required. It was determined that the prior authorization
requirement is hard coded in the IndianaAIM system and would require a Customer
Service Request (CSR) to have the hard coding removed. The CSR will not be able to be
completed until after HIPPA implementation in October 2003. In the interim, the HCE
Prior Authorization department has created a work around process that allows for
information to be received from the provider and entered in the IndianaAIM system. The
provider can be instructed that there are no further clinical updates required and request
that the provider call the department with a discharge date so that the number of days can
be added to the prior authorization approval.
Surgical procedures typically performed on an outpatient basis, when performed as an
inpatient, must be prior authorized (405 IAC 5-17-2). These may be the result of
technical or medical difficulties during the outpatient procedure; presence of physical or
mental conditions making prolonged pre or postoperative observation by skilled medical
personnel necessary, a simultaneous procedure requiring hospitalization or the likelihood
of an additional procedure requiring hospitalization.
Substance abuse admissions are also reimbursed utilizing the DRG methodology, but do
require prior authorization, as outlined under “Mental Health”.
All out-of-state services require prior authorization. (See 405 IAC 5-5-2 and Claims
Resolution Manual ESC 3010) [Exception—405 IAC 5-5-2(3)(4)]
01/31/2007 Hospital Inpatient
Medical Policy Manual
246

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Hospitalization Adult burns
(Age 10 and over)
Overview
General Information:
Prior authorization is required for all Medicaid covered burn inpatient stays that are
reimbursed under the level of care methodology described in 405 IAC 1-10-5. Days that
are not prior authorized under the level of care methodology as required by this rule will
not be covered by Medicaid.
405 IAC 5-17-3 Emergency inpatient admissions for diagnoses reimbursed under the
level of care payment methodology must be reported to the office within forty-eight (48)
hours of admission, not including Saturdays, Sundays, or legal holidays, in order to
receive Medicaid reimbursement. At that time, the same standards for prior authorization
will be applied as would have been applied if the authorization had been requested before
the admission.
First degree: Superficial
. Damage is limited to the epidermis. Erythema appears.
Between 10 and 20 % total body surface area-minor burn.
Second degree: Deep partial thickness burns of the eyes, ears, face, hands, feet or
perineum; or burns complicated by fractures or respiratory
damage; electrical burns; and all burns in poor-risk patients.
Involvement of less than 15% total body surface area-minor burn.
Involvement of 15% - 25% total body surface area-moderate burn.
Involvement of more than 25% total body surface area-major
burn.
Third degree: Full thickness burns covering less than 3% of the body, excluding
the eyes, ears, face, hands, feet or perineum = minor burn.
OR
Full thickness burns of the eyes, ears, face, hands, feet or perineum
covering > 3% and < 10% total body surface area = moderate burn.
OR
Full thickness burns of more than 10% of the total body surface-
major burn.
01/31/2007 Hospital Inpatient
Medical Policy Manual
247

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Revenue Code Narrative Description
207 Admissions for burns. One (1) unit = one (1) day.
405 IAC 5-17-2 (a)
Indicators
The admission may be approved without referral for physician review if ONE of the
following is present: (recent onset)
1. Loss or damage of skin ≥ 15% of TBS (total body surface) area.
2. High voltage burn with devitalized skin, fat, or muscle.
3. 2nd or 3rd degree burns of one of the following: Face, hands, perineal region,
encircling neck or extremities, anterior or posterior neck or limbs.
4. T ≥ 104.0° F
5. T ≥ 102.0° F and ONE of the following:
• WBC ≥ 18,000/cu.mm
• WBC ≥ 15,000/cu.mm with ≥ 7% bands
1. T ≥ 100.5° F and ONE of the following:
• Absolute neutrophil count ≤ 500/cu.mm
• WBC ≤ 1,500/cu.mm
2. Admission for an invasive procedure which necessitates an inpatient setting AND is
scheduled for the same day as admission.
AND ONE of the following treatments is being provided: (at least daily)
1. Post surgery or procedure care ≤ 3 days and at least TWO of the following:
• IV fluids ≥ 100 mL/h
• IV, IM, or ED analgesics
01/31/2007 Hospital Inpatient
Medical Policy Manual
248

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• IV or IM antiemetics
• Graft or wound care
1. Burn therapy with at least THREE of the following:
• IV electrolyte (K, Ca, Mg, P)
• IV fluids ≥ 100 mL/h
• IV plasma expanders
• O2 ≥ 28% (4L) or Hyperbaric
• TPN
OR at least THREE of the following treatments are being provided:
1. Blood or blood products
2. Complex burn, graft, or wound care
3. IV fluids ≥ 100 mL/h
4. Restorative PT or OT at least 2x/24h
5. TPN
6. IV or IM corticosteroids at least 3x/24h
7. IV or IM diuretics at least 2x/24h
8. IV or IM analgesics at least 4x/24h
9. IV or IM antiemetics at least 4x/24h
10. IV or IM anti-infectives at least 3x/24h
References
Information obtained from the Medicare MDC Criteria Manual.
01/31/2007 Hospital Inpatient
Medical Policy Manual
249

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Hospitalization Pediatric Burns
(Age 10 and under)
Overview
Prior authorization is required for all Medicaid covered burn inpatient stays that are
reimbursed under the level of care methodology described in 405 IAC 1-10-5. Days that
are not prior authorized under the level of care methodology as required by this rule will
not be covered by Medicaid.
405 IAC 5-17-3 Emergency inpatient admissions for diagnoses reimbursed under the
level of care payment methodology must be reported to the office within forty-eight (48)
hours of admission, not including Saturdays, Sundays, or legal holidays, in order to
receive Medicaid reimbursement. At that time, the same standards for prior authorization
will be applied as would have been applied if the authorization had been requested before
the admission.
The following descriptions are for age clarification purposes only.
Newborn-birth to 28 days
Infant=28 days to 18 months
Preschool=18 months to 6 years
Preadolescent=6 years to 12 years
Adolescent=12 to 18 years
Degree(s) of burns: (< 10 years old)
First degree: Superficial. Damage is limited to the epidermis. Erythema appears.
Between 10 and 20 % total body surface area-minor burn.
Second degree: Deep partial thickness
burns of the eyes, ears, face, hands, feet or
perineum; or burns complicated by fractures or respiratory
damage; electrical burns; and all burns in poor-risk patients.
9 Involvement of less than 10% total body surface area-minor
burn.
9 Involvement of 10% - 20% total body surface area-moderate
burn.
01/31/2007 Hospital Inpatient
Medical Policy Manual
250

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
9 Involvement of more than 20% total body surface area-major
burn.
Third degree: Full thickness
burns covering 2% of the body, excluding the eyes,
ears, face, hands, feet or perineum = major burn.
OR
Full thickness burns of the eyes, ears, face, hands, feet or perineum
covering > 1% and < 10% total body surface area = major burn.
Revenue Code
Narrative Description
207 Admissions for burns. One (1) unit = one (1) day.
405 IAC 5-17-2 (a)
Indicators
The admission may be approved without referral for physician review if at least ONE of
the following is present: (recent onset)
1. Electrical burns with devitalized skin, fat, or muscle
2. 1st degree burns covering 40% of TBS
3. 2nd degree burns covering 15% of TBS
4. 2nd degree burns covering face, genitalia, hands, or feet
5. 3rd degree burns covering 5% or more of TBS
AND at least ONE of the following treatments is being provided at least daily:
1. Post surgery or procedure care ≤ 2 days
2. IV electrolytes
3. Burn therapy with at least TWO of the following:
• IV fluids ≥ 30 mL/kg/24h
• IV plasma expanders
01/31/2007 Hospital Inpatient
Medical Policy Manual
251

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• O2 ≥ 28% (4L)
OR at least THREE of the following treatments are being provided:
1. Blood or blood products
2. Complex burn, graft, or wound care
3. Physical therapy
4. IV fluids ≥ 30 mL/kg/24h
5. IV plasma expanders
6. TPN or Enteral feeding
7. IV or IM corticosteroids at least 3x/24h
8. IV diuretics at least 2x/24h
9. IV or IM analgesics at least 4x/24h
10. IV or IM antiemetics at least 4x/24h
11. IV or IM anti-infectives at least 3x/24h
References
Information obtained from the Medicare MDC Criteria Manual.
01/31/2007 Hospital Inpatient
Medical Policy Manual
252

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
INPATIENT DENTAL ADMISSIONS
Revenue Code Narrative Description
120 Inpatient days (Appropriate procedure code for
dental procedure must also be authorized.)
405 IAC 5-14-18
Indicators
ONE of the following:
1. Mental incapacitation such that the recipient’s ability to cooperate with procedures is
impaired, including mental retardation, organic brain disease and behavioral problems
associated with uncooperative, but otherwise healthy, children.
2. Severe physical disorders affecting the tongue, or jaw movements.
3. Seizure disorders.
4. Significant psychiatric disorders resulting in impairment of the recipient’s ability to
cooperate with procedures.
5. Previously demonstrated idiosyncratic or severe reactions to IV sedation medication.
6. The need for oral surgery, listed in 405 IAC 5-19-17 or in extreme cases of facial
trauma, pathology, or deformity.
7. Periodontal surgery only in cases of drug-induced periodontal hyperplasia.
8. Elective oral surgery when recipient is unable to cooperate with or tolerate the
procedure.
01/31/2007 Hospital Inpatient
Medical Policy Manual
253

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Hospital Inpatient
Medical Policy Manual
254
HOSPITAL INPATIENT
FACT SHEET
ADDENDUM A
Note: This addendum contains provider notifications that have been published since the
review of the Hospital Inpatient Fact Sheet. The information in this addendum will be
incorporated into the fact sheet at the next review.
Provider Notification: BR200509 Publication Date: 03/01/2005
Subject: Hospital Stays <24 hours
Date Added to Manual: 04/29/2005
Text of Publication
As a result of changes to 405 IAC 1-10.5-3 (y), effective for admissions on or after
November 1, 2004, providers are required to bill an inpatient stay of less than 24 hours as
an outpatient service. Claims that group to diagnosis-related grouping (DRG) 637 -
Neonate, died w/in one day of birth, born here and DRG 638 - Neonate, died w/in one
day of birth, not born here are exempt from this policy because they are specific to one-
day stays. The Office of Medicaid Policy and Planning (OMPP) has received inquiries
about potential medical record compliance issues with this rule. Specifically, providers
have questioned whether billing for outpatient services when a patient has been admitted
as an inpatient will be viewed by the OMPP as non-compliance with program policies
concerning internal records and billing requirements. The OMPP will not take action
against a provider for adhering to the agency’s billing requirements for inpatient stays of
less than 24 hours, because this is in compliance with the Indiana regulation and billing
requirements. In addition, providers have questioned whether their medical records,
which originally indicated an inpatient stay of less than 24 hours, should be amended to
show that outpatient services were performed. Providers do not need to amend their
medical record keeping to comply with the changes that became effective on November
1, 2004.

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: HOSPITAL INPATIENT--READMISSIONS GENERAL/
SAME PROVIDER
DESCRIPTION:
Readmission is the term used when patients are admitted to the hospital, acute care or
other, with the same diagnosis.
SUMMARY OF CURRENT POLICY:
Readmissions are subject to medical review [currently performed by the Surveillance,
Utilization and Review Department of Health Care Excel] to determine if previous
discharge was premature. Reviews are conducted based on statistical data sets for
readmissions. If the discharge was premature and payment made, the readmission or
discharge may be subject to recoupment [currently performed by the Surveillance,
Utilization and Review Department of Health Care Excel]. Readmissions will be treated
as separate stays for payment purposes.
MEDICAL TOPICS CROSS-REFERENCES:
Hospital Inpatient
Nursing Facilities
Mental Health/Behavioral Health – Inpatient Services
Mental Health/Behavioral Health – Outpatient Services
RULES, CITATIONS, AND SOURCES:
405 IAC 1-10.5-3 Reimbursement for Inpatient Hospital Services
Indiana Health Coverage Programs Provider Manual 1999
01/31/2007 Hospital Inpatient – Readmissions/General/Same Provider
Medical Policy Manual
255

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Hospital Inpatient – Readmissions/General/Same Provider
Medical Policy Manual
256
Initial Policy Issue Effective
Date
Implementation
Date
Retroactive
Date
470 IAC 5-9-15,
5-9-38 Transferred
Hospital
Services
7/1/91
405 IAC 1-7-15,
1-7-36
Repealed
8/24/97
Hospital
Services
1/1/92
Revisions:
405 IAC 1-10.5-3 Prospective
Reimbursement
Methodology
12/27/96
405 IAC 5-17 Hospital
Services
8/24/97
APPLICABLE INDIANAAIM EDITS AND AUDITS:
6508
6509

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: HOSPITAL OUTPATIENT
DESCRIPTION
Outpatient hospital services refer to disease prevention and diagnosis and/or therapeutic
and rehabilitative services including, but not limited to, surgery, therapy, laboratory,
radiology, chemotherapy, renal dialysis, clinic, treatment room, and emergency
department care. Services must be provided by or under the direction of a physician.
This document is intended to serve as a general summary of the Indiana Health Coverage
Programs’ (IHCP) policies regarding these services. More specific information may be
found in the IHCP Provider Manual, program notices, the Indiana Administrative Code
(IAC) or other sources as appropriate. See the “Rules, Citations, and Sources” section of
this document for further information.
COVERAGE CRITERIA
The IHCP will reimburse for outpatient services provided to a member who is not
registered as an inpatient in an acute care, psychiatric, or rehabilitation hospital.
Outpatient hospital services are covered when such services are provided or prescribed by
a physician. Documentation must support medical necessity for the diagnosis and
treatment of the condition. There are four categories of service within the defined
outpatient hospital prospective payment system.
• Outpatient surgery services
• Treatment room visit services
• Standalone services
• Add-on services
Observation Room Services
Outpatient services that occur within three days preceding an inpatient admission to the
same facility for the same or related diagnosis will be considered part of the
corresponding inpatient admission.
Outpatient services within three days preceding a less than 24-hour inpatient stay should
continue to be billed as an outpatient service. Because the inpatient service was less than
24 hours, it should be billed as an outpatient service.
01/31/2007 Hospital Outpatient
257
Medical Policy Manual
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Emergency Room Services
Payments for non-emergent care that do not include surgery and that are provided in an
emergency department, treatment room, observation room, or clinic will be based on the
statewide fee schedule amount that went into effect during state fiscal year 2003.
Noncovered Services
Reimbursement shall not be made for any hospital services not covered under the IHCP.
In addition, if a service requires prior authorization (PA), which was either not obtained
or denied, reimbursement for any associated services may be denied. Separately
reimbursable DME devices may not be covered if they are not prior authorized.
PRIOR AUTHORIZATION
Surgical procedures that are usually provided on an outpatient basis, but are performed as
an inpatient service must be prior authorized (i.e., medical difficulties during the
outpatient procedure, prolonged pre- or post-operative observation, and simultaneous
procedure requiring hospitalization).
The following services require PA.
• Stress electrocardiograms except for medical conditions
• All out-of-state services
• Separately reimbursable implantable DME items
MANAGED CARE
For members enrolled in Risk-Based Managed Care (RBMC), providers must contact the
member’s managed care organization (MCO) for more specific guidelines. Refer to the IHCP
Provider Manual, Chapter 1, for detailed information about the fee-for-service (FFS), Primary
Care Case Management (PCCM), and RBMC delivery systems.
IHCP members enrolled in Medicaid Select receive the same benefit coverage, and are subject
to the same limitations as Traditional Medicaid FFS. Refer to the Medicaid Select Provider
Manual for Primary Care Providers and Office Staff for further information.
BILLING REQUIREMENTS
Outpatient Surgeries
Outpatient surgeries provided in either a hospital or an ambulatory surgical center (ASC)
are reimbursed an all-inclusive flat fee that includes all related procedures.
01/31/2007 Hospital Outpatient
258
Medical Policy Manual
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Reimbursement is available for outpatient surgeries provided in a number of settings
including an operating room, treatment room, emergency department, or clinic.
Reimbursement is based on the assignment of the Current Procedural Terminology®
(CPT) code to one of the ASC groups. Reimbursement rates have been established for
each ASC group that is reflective of the average cost for procedures within the group.
The IHCP will reimburse a maximum of two units of service regardless of the number of
incisions. The procedure with the highest ASC rate is reimbursed at 100 percent of that
rate. The procedure with the second highest ASC rate or bilateral procedure is
reimbursed at 50 percent of the respective ASC rate. All other procedures are denied. To
denote multiple surgeries, the appropriate revenue code and CPT code must be listed as
two separate detail line items on the claim form.
Surgical Revenue Codes
Surgical revenue codes are generally defined as 36X and 49X. The revenue codes for
treatment rooms, such as 45X, 51X, 52X, 70X, 71X, 72X, and 76X, are defined as
surgical revenue codes when accompanied by a surgical Healthcare Common Procedure
Coding System (HCPCS) code. These revenue codes are paid at the appropriate ASC
rate. If no surgical procedure is performed, the revenue code must be submitted without
a CPT or HCPCS code. These services are then priced at the treatment room rate.
Component billing of any related services is not appropriate and will be denied. Add-on
or standalone services are not allowed with any surgical revenue codes.
Any details billed on the claim form, not among the approved surgical revenue codes,
will be denied as services included in the ASC procedure reimbursement rate. To denote
multiple surgeries, the appropriate revenue code must be listed as two separate detail line
items on the Uniform Billing (UB-92) claim form with the applicable HCPCS surgical
procedure code.
• The primary surgical procedure is reimbursed at 100 percent of the IHCP
allowable rate.
• The second procedure is reimbursed at 50 percent of the IHCP allowable rate.
• Bilateral procedures are reimbursed at 150 percent of the IHCP allowable rate.
• Because only two procedure codes are allowed, other procedure codes will be
denied.
All outpatient services provided on the day of the surgery must be included on a single
claim. Charges for any other services provided on the day of the surgery must be
included with the charge for the surgery, as described above. Add-on or standalone
services are not separately reimbursable and will be denied.
Durable Medical Equipment
The cost of certain implantable durable medical equipment is separately reimbursable.
Some of these items require prior authorization and/or a manufacturer’s cost invoice
showing the purchase price. Claims for these items should be submitted on the CMS-
01/31/2007 Hospital Outpatient
259
Medical Policy Manual
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
1500 billing form or 837P electronic transaction. The IHCP permits only these items to
have separate reimbursement.
• Cardiac Pacemakers Single-chamber
• Cardiac Pacemakers Dual-chamber
• Implantable Loop Recorders
• Phrenic Nerve Stimulators
• New Technology Intraocular Lenses
• Vagal Nerve Stimulators
• Implantable Infusion Pumps-Non-programmable
• Implantable Infusion Pumps-Programmable
Outpatient Corneal Tissue Transplant Procedures
The cost associated with corneal tissue acquisition, HCPCS code V2785–Processing,
preserving, and transporting corneal tissue, is separately reimbursable from the ASC rate
for outpatient corneal transplant procedures. Claims for this item should be submitted on
the CMS-1500 claim form or through the 837P electronic transaction. A copy of the
invoice from the eye bank or organ procurement organization showing the actual cost of
acquiring the tissue must be attached to the claim form. Providers must follow current
policy for submitting paper attachments with the 837P electronic transaction. For
additional information about corneal tissue transplant services, please refer to the
Surgery–Transplants Medical Policy Fact Sheet.
Treatment Room Services
For purposes of the IHCP’s outpatient prospective payment system, treatment rooms
include emergency department, clinics, cast room, labor and delivery room, recovery
room, and observation room.
When surgeries are performed in a treatment room, the appropriate CPT code should
accompany the revenue code and reimbursement is based on the ASC methodology.
Otherwise, facilities should not use a surgical CPT code in addition to the treatment room
revenue code.
Treatment room services are reimbursed at a flat rate that includes most drugs and
supplies. Reimbursement is limited to one unit per day, per patient, per provider.
Services must be billed on the UB-92 claim form using the appropriate revenue code.
Standalone services may be billed in conjunction with treatment room services.
Standalone services include therapies, dialysis, radiology, and laboratory services.
Certain add-on services are allowed if billed in conjunction with a treatment room. All
other add-on services are denied if billed in conjunction with a treatment room service.
Emergency Room Services
Reimbursable emergency room services are for the treatment of ill and injured members
requiring immediate, unscheduled medical or surgical services. These claims may be
suspended for review to determine whether medical necessity, for an emergency medical
01/31/2007 Hospital Outpatient
260
Medical Policy Manual
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
condition, is met. Reimbursement is denied if services are not medically necessary. A
comprehensive list of all diagnosis codes considered emergency for outpatient
reimbursement is located in Chapter 8 of the IHCP Provider Manual. Please refer to the
Medical Policy Emergency Medicine-Emergency Services Fact Sheet for specific
coverage and billing information.
Observation Room Services
Reimbursable observation room services are furnished on the hospital’s premises,
including the use of a bed and periodic monitoring by a hospital’s nursing staff. These
services are reasonable and necessary to evaluate a patient’s condition or to determine the
need for an inpatient admission. Services are covered only when ordered by a physician
or other individual authorized by state licensure law and hospital bylaws to admit patients
or order outpatient tests.
Standalone Services
Standalone services include therapies, diagnostic testing, dialysis, laboratory, and
radiology procedures performed in an outpatient setting. Standalone services may be
billed separately, or in conjunction with treatment room visits. Standalone services are
not separately reimbursable with outpatient surgeries or on the same day as an outpatient
surgery.
Standalone services are reimbursed at an established flat statewide rate. Laboratory and
radiology services are reimbursed at the lower of the submitted charge or the fee schedule
amount. A maximum of one unit of service, per revenue code, for each date of service is
allowed, except for lab and radiology. Services must be billed on the UB-92 claim form.
Table 8.15 of Chapter 8 in the IHCP Provider Manual provides a list of revenue codes for
standalone services.
Add-on Services
Add-on services are separately reimbursable in conjunction with a standalone procedure.
Certain revenue codes for add-on services are separately reimbursable if billed in
conjunction with a treatment room. These are 255 (Drugs Incident to Radiology), 258
(IV Solutions), 29X (DME), 370 (anesthesia), 38X (Blood), 39X (Blood Storage and
Processing), and 62X (Diagnostic Supplies). All other add-on services are denied if
billed in conjunction with a treatment room visit.
Add-on services are not separately reimbursable if billed with a surgery or provided on
the same day as an outpatient surgery. Refer to table 8.14 of Chapter 8 in the IHCP
Provider Manual for specific information regarding billing add-on services.
Add-on services are complementary outpatient services provided either in a treatment
room or with a standalone service. Add-on services are reimbursed at a flat rate.
01/31/2007 Hospital Outpatient
261
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
RELATED MEDICAL TOPICS
Anesthesia Services
Consultations – Second Opinion Services
Diagnostic Services
Emergency and Evaluation Management Services
Emergency Room Services
Federally Qualified Health Centers/Rural Health Centers
Home Health Services
Inpatient Hospital Services
Laboratory Services
Managed Care Organization Services
Mental/Behavioral Health Services
Nursing Services
Observation Services
Pharmacy Services
Radiology Services
Surgery Services
Treatment Room Services
RULES, CITATIONS, AND SOURCES
Code of Federal Regulation (CFR)
42 CFR 440.20–Outpatient hospital services
Indiana Administrative Code (IAC)
405 IAC 5-1-5–Global Fee Billing
405 IAC 5-5-2–Prior authorization requirements for out-of-state services
405 IAC 5-3-13–Services requiring prior authorization
405 IAC 5-17-2–Hospital Services
405 IAC 5-28-1–Medical and Surgical Services; Reimbursement limitations
405 IAC 5-28-2–Medical and Surgical Services; Medical diagnostic procedures
405 IAC 5-29-1–Services Not Covered by Medicaid; Noncovered services
Indiana Health Coverage Programs Provider Manual
1994
1999
2005, Version 5.1
Indiana Health Coverage Programs Provider Bulletin (BT)
BT200420-Changes in DRG/LOC Inpatient and Outpatient Reimbursement
BT200114-Implantable Loop Recorders
BT200108-Phrenic Nerve Stimulators
BT200106-New Technology Intraocular Lenses
BT200032-Vagal Nerve Stimulators
Indiana Health Coverage Programs Provider Banner (BR)
BR200314-Vagal Nerve Stimulators
01/31/2007 Hospital Outpatient
262
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Hospital Outpatient
Medical Policy Manual
263
Origination Date: 08/24/1997
Revisions and
Reviews
Reason Effective Date
Revision
405 IAC 5-17-2 Hospital Services-Prior
Authorization, outpatient versus Inpatient
Procedures/Surgery
08/24/1997
Revision
405 IAC 5-3-13 Prior Authorization;
Procedures Requiring Prior Authorization
08/24/1997
Revision
405 IAC 5-16-6 Free-standing clinics and
surgical centers; limitations
08/24/1997
Review Scheduled 01/31/2007
APPLICABLE INDIANAAIM EDITS AND AUDITS
3010-Out of state provider requires prior authorization
3011-Out of state provider requires prior authorization
4000-More than two surgical units on the claim
4081-Invalid Service-Per Diem
4089-Missing or invalid HCPCS code for surgery revenue code
4095-Nonsurgical services are not reimbursed individually if performed in conjunction
with an outpatient surgery
4099-DRG not on file
4116-Diagnosis is not valid for DRG pricing
4108-No ASC on file
6514-No more than one emergency room visit per day
6515-Inpatient admit date within three days after DOS of paid outpatient claim
6516-Outpatient service rendered within three days prior to admit date of paid inpatient
claim

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: HYPERBARIC OXYGEN THERAPY
DESCRIPTION
Hyperbaric Oxygen Therapy (HBO) is a modality in which the entire body is exposed to
oxygen under increased atmospheric pressure, increasing vascular flow and improved
oxygenation of body tissue. Originally developed for the treatment of decompression
sickness, hyperbaric oxygen is adjunctive treatment for the management of select non-
healing wounds, treatment of carbon monoxide poisoning, and other conditions as noted
on pages 2 and 3, in the section entitled, “Coverage Criteria.”
RELATED MEDICAL TOPICS
Physician Services
RULES, CITATIONS, AND SOURCES
405 IAC 5-28-11
Origination Date: 1/31/05
Reviews and
Revision
Reason Date
APPLICABLE INDIANAAIM EDITS AND AUDITS
4104 – HBO Therapy Restricted by Diagnosis Code
6096 – CPT Code Billed is Not Payable According to PPS Reimbursement Methodology
6652 – Multiple Surgeries Must Be Billed on Same Claim
6751 – Hyperbaric Oxygen Therapy Greater than Two Months
6754 – Hyperbaric Oxygen Therapy
01/31/2007 Hyperbaric Oxygen Therapy
264
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
PRIOR AUTHORIZATION
Prior authorization is not required for HBO
COVERAGE CRITERIA
Reimbursement for HCPCS code C1300, Hyperbaric oxygen under pressure, full body
chamber, per 30 minute interval and CPT code 99183, Physician attendance and
supervision of hyperbaric oxygen therapy, per session is available for the following
conditions.
Table 1. – Diagnosis Codes for HBO Therapy
Diagnosis ICD 9 Codes Limitations
Acute carbon
monoxide intoxication
986
Decompression illness 993.2, 993.3
Gas embolism 958.0, 999.1
Gas gangrene 040.0
Acute traumatic
peripheral ischemia
902.53, 903.1, 903.01, 903.4,
904.0, 904.41, 996.9
Adjunctive treatment to be used
in combination with accepted
standards and therapeutic
measures.
Crush injuries and
suturing of severed
limbs
902.53, 925.1, 925.2, 926.0,
926. 1, 926.11, 926.12,
926.19, 926.8, 926.9, 927.0,
927.00, 927.01, 927.02,
927.03, 927.09, 927.10,
927.11, 927.20, 927.21,
927.3, 927.8, 927.9, 928.0,
928.00, 928.01, 928.1,
928.10, 928.11, 928.2,
928.20, 928.21, 928.3, 928.8,
928.9, 929.0, 929.9,
903.1, 903.01, 903.4, 904.0,
904.41
Adjunctive treatment to be used
in combination with accepted
standards and therapeutic
measures.
(Meleney Ulcers)
Progressive
necrotizing infections
686.0, 686.09, 728.86
Other types of cutaneous ulcers
are not covered.
Acute peripheral
arterial insufficiency
444.21, 444.22, 444.81
Compromised skin
grafts
996.52 Preparation and preservation.
Chronic Refractory
Osteomyelitis
730.10-730.19
Use when unresponsive to
conventional medical and
01/31/2007 Hyperbaric Oxygen Therapy
265
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Hyperbaric Oxygen Therapy
266
Diagnosis ICD 9 Codes Limitations
surgical management.
Osteoradionecrosis 526.89
Adjunct to conventional
treatment.
Soft tissue
radionecrosis
990
Adjunct to conventional
treatment.
Cyanide poisoning 987.7, 989.0
Actinomycosis 039.0-039.4, 039.8, 039.9
Use when unresponsive to
conventional medical and
surgical management.
Acute Cerebral Edema 348.5
Reimbursement is not available for HBO for the following conditions or services.
♦ Topical application of oxygen
♦ Cutaneous, decubitus, and stasis ulcers
♦ Chronic peripheral vascular insufficiency
♦ Anaerobic septicima and infection other than clostridial
♦ Thermal skin burns
♦ Senility
♦ Myocardial Infarction
♦ Cardiogenic shock
♦ Sickle cell crisis
♦ Acute thermal and chemical pulmonary damage including smoke
inhalation with pulmonary insufficiency
♦ Acute or chronic cerebral vascular insufficiency
♦ Hepatitis necrosis
♦ Aerobic septicemia
♦ Nonvascular causes of chronic brain syndrome, including Picks,
Alzheimers, and Korsakoff's disease
♦ Tetanus
♦ Systemic aerobic infection
♦ Organ Transplantation
♦ Organ storage
♦ Pulmonary emphysema
♦ Exceptional blood loss anemia
♦ Multiple sclerosis
♦ Arthritic diseases
Treatment may include multiple HBO sessions that may be administered over a duration
ranging from less than one week to two months, the average being two to four weeks.
Claims submitted for treatment sessions lasting more than a two-month period will be
suspended to Location 22 for submission of documentation to support medical necessity
of continued therapy.
Medical Policy Manual
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Hyperbaric Oxygen Therapy
Medical Policy Manual
267
405 IAC 5-11(c) cites “Hyperbaric therapy shall be clinically practical and shall not be a
replacement for other standard successful therapeutic measures”
BILLING REQUIREMENTS
Providers may use HCPCS code 99183 or C1300, with revenue code 413 for
reimbursement of hyperbaric oxygen therapy services as a hospital outpatient service. An
appropriate diagnosis code (see Table 1. Diagnosis codes for HBO Therapy) must be
included on claims.
Note: The evaluation and management services and/or procedures (such as wound
debridement) provided in a hyperbaric oxygen treatment should be reported separately from
CPT code 99183.

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: IMMUNIZATIONS AND VACCINES
DESCRIPTION
Immunization is the process by which a person becomes protected against a disease. This
term is often used interchangeably with vaccination or inoculation. It is defined as the
injection of a killed or weakened infectious organism in order to prevent disease.
COVERAGE CRITERIA
Vaccinations and immunizations are covered through the Vaccines for Children (VFC)
program and the Indiana Health Coverage Programs (IHCP). The VFC program provides
a variety of free vaccines to children 18 years of age and younger. The IHCP also
reimburses for vaccines not covered by the VFC program or for administration of the
vaccines to IHCP members 19 years of age and over.
Vaccines Not Available Through the Vaccines for Children Program or
Administered to Members 19 Years of Age and Older
Vaccines not available through the VFC program or vaccines administered to members
19 years of age and older are reimbursed the lesser of the provider’s usual and customary
charge, or the IHCP allowed amount for the vaccine supply. A $2.90 administration fee
is included in the reimbursement of the vaccine supply code. Influenza vaccine
instructions are revised frequently, therefore providers are to monitor program
publications for special instructions.
Vaccines for Children Program
The VFC program is a federally funded program intended to help raise childhood
immunization levels in the United States by supplying health care providers with free
vaccines to administer to children 18 years of age and younger. Recipients must meet
one or more of the following criteria.
• Be enrolled in the IHCP
• Have no health insurance
• Be identified by parent or guardian as American Indian or Alaskan native
01/31/2007 Immunizations and Vaccines
Medical Policy Manual
268
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• Be underinsured. For example, children with health insurance that does not cover
immunizations (administered in federally qualified health care centers or rural health
clinics only)
The VFC Program makes vaccines available at no cost to providers for members 18 years
of age and younger (including those 18 years of age and younger enrolled in Hoosier
Healthwise Package C). Providers are reimbursed either the lower of their submitted
charge or $8 for the administration of each vaccine. Providers are not required to
physically separate vaccine stock for children in the VFC program from the vaccine stock
for Hoosier Healthwise Package C children. No additional storage rules apply.
Currently, the VFC program offers free vaccines against the following diseases.
• Diphtheria
• Hemophilus influenza type B
• Hepatitis B
• Measles
• Mumps
• Rubella
• Pertussis
• Poliomyelitis
• Pneumococcal disease
• Tetanus
• Varicella
All vaccine ordering, distribution, and accountability processes are administered through
the Indiana State Department of Health’s (ISDH) Indiana Immunization Program. The
federal VFC program includes private and public practitioners across Indiana. Providers
should contact the ISDH to enroll in the VFC program. Under an arrangement, the ISDH
performs the VFC program administration functions, including provider enrollment,
education, and vaccine ordering and distribution. To participate in the VFC program
providers must complete the following.
1. Call the ISDH office and request VFC provider enrollment forms
2. Complete and mail the provider enrollment forms
3. Receive appropriate training and technical assistance
4. Order vaccines periodically, as needed, and maintain appropriate vaccine
supply records
Providers who choose not to participate in the VFC program must provide appropriate
vaccine referrals for the member, follow-up with the member, and document the
immunization history of the member. Hoosier Healthwise Primary Medical Providers
(PMP) who choose not to participate in the VFC program must have a procedure in place,
such as a Memorandum of Collaboration (MOC), to ensure that children are adequately
and appropriately immunized. For more information about the MOC and collaborative
agreements, contact the Hoosier Healthwise Primary Care Case Management Helpline.
01/31/2007 Immunizations and Vaccines
Medical Policy Manual
269
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
BILLING GUIDELINES
Billing Guidelines for Vaccines Not Available Through the Vaccines for Children
Program or Administered to Members 19 Years of Age and Older
Vaccines not available through the VFC program or administered to members 19 years of
age and older have an all-inclusive fee attached to the vaccine code. The IHCP calculates
the maximum allowable reimbursement based on the current average wholesale price for
the procedure code, plus $2.90 for vaccine administration to cover the costs of supplies
and staff time associated with giving the injection. The $2.90 is included in the
reimbursement for the vaccine product, and is not to be billed as a separate charge on the
claim form. The provider is reimbursed the lesser of their usual and customary charge or
the IHCP allowed amount.
Billing Guidelines in the Vaccines for Children Program
Vaccines through the VFC program are supplied to providers at no cost, and providers
are reimbursed only for the administration of the vaccine. Providers are to report the
appropriate vaccine product code, and will be reimbursed the lesser of their usual and
customary charge for administration or an $8 administration fee. In addition, providers
are to report a principal diagnosis of V20.2, Routine infant or child health check. The
IHCP permits only one administration fee per VFC vaccine injection administered. For
combined vaccines, the correct code for the combined vaccine with only one vaccine
administration fee should be billed. However, if no combination vaccine is available and
the provider must administer more than one injection, each injection is to be listed with
the appropriate CPT code and a vaccine administration fee is allowed for each. When a
specific vaccine has been removed from VFC pricing, providers are to submit claims with
charges reflective of whether the vaccine was from VFC or private stock ($8 for VFC
stock or the provider’s usual and customary charge for private stock). If the only service
performed is vaccine administration, providers must not submit a bill for an office visit.
Providers can bill an office visit in conjunction with vaccine administration only when
other services are performed during the same visit.
PRIOR AUTHORIZATION
Prior authorization is required for Synagis and Respigam for the treatment of respiratory
synctial virus. Refer to the Synagis and Respigam fact sheet for additional information.
MANAGED CARE
Coverage and billing guidelines for PrimeStep/PCCM is the same as Traditional
Medicaid fee for service. For Risk Based Managed Care, vaccines are administered to
01/31/2007 Immunizations and Vaccines
Medical Policy Manual
270

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
VFC-eligible children and billed directly to the appropriate MCO with the principal
diagnosis of V20.2, Routine infant or child health check. Managed care providers who
are not the PMP or do not have an authorization from the PMP may charge the member
for the service. The member must sign a waiver acknowledging they would not
otherwise be required to pay for the vaccination from an in-network provider. Contact
the appropriate MCO for details about reimbursement under the RBMC delivery system.
Since vaccines are provided by different funding sources, the ISDH must report the
number of doses administered to VFC members and Hoosier Healthwise Package C
members separately. Providers must indicate vaccines administered to Hoosier
Healthwise Package C members on the Patient Eligibility Screening Record and the
Vaccine Accountability Tally Sheet. Providers are to submit the forms to the ISDH by the
10th day of the following month in which the vaccines were administered. This change
standardizes the reporting time frame for all providers.
RELATED MEDICAL TOPICS
EPSDT – HealthWatch
RULES, CITATIONS, AND SOURCES:
405 IAC 5-15-3 EPSDT
Indiana Health Coverage Programs Provider Bulletins
E98-11 – Vaccines For Children Program Rate Increase
E98-21 – Vaccines For Children Program Administration Fee
BT200007 – Vaccine for Children Update
BT200151 – Revised Policy for Billing of Office-Administered Injectible Drugs
and Infusions
Indiana Health Coverage Programs Provider Banners
BR200045 – Prevnar Available Through Vaccines For Children Program
BR200144 – Influenza Vaccines
BR200233 – Administration Fee in the Vaccines For Children Program
BR200442 – Administration Fee
Indiana Health Coverage Programs Provider Newsletter
NL200404 – Vaccines for Children and Injectibles
Indiana Health Coverage Programs Provider Manual 2004
Origination Date: July 1, 1991
Revisions and Reviews Reason Date
Revision EPSDT Services 1/1/92
Revision EPSDT Periodic Screening 8/24/97
Revision Vaccines For Children (VFC) Program 1/1/98
Review Immunizations and Screenings
February
2002
01/31/2007 Immunizations and Vaccines
Medical Policy Manual
271

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Revisions and Reviews Reason Date
Revision Vaccines for Children April 2002
Review
Expansion of Vaccinations Not Reimbursed
for the Vaccines for Children Program
April 30,
2005
APPLICABLE INDIANAAIM EDITS AND AUDITS:
6018-Global Fee- Immunizations
6031-Global Immunization Not Payable When Component Paid
6096-The CPT/HCPCS code is not payable according to the PPS payment methodology
01/31/2007 Immunizations and Vaccines
Medical Policy Manual
272

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
IMMUNIZATIONS AND VACCINES FACT SHEET
ADDENDUM A
Note: This addendum contains provider notifications that have been published since the
review of the Immunizations and Vaccines Fact Sheet. The information in this
addendum will be incorporated into the fact sheet at the next review.
Provider Notification: BT200511 Publication Date: 06/01/2005
Subject: Submission of Miscellaneous Drug Injection Codes
Date Added to Manual: 07/29/2005
Text of Publication
The provider should indicate the National Drug Code (NDC) code for the drug dispensed
in the claim notes segment.
Provider Notification: NL200506 Publication Date: 06/2005
Subject: Vaccines for Children and Injectables
Date Added to Manual: 07/29/2005
Text of Publication
Provider-Purchased Vaccine
When a provider administers immunizations using the provider’s private stock, refer to
IHCP provider bulletin, BT200151, for use of the administration code 90788, as
appropriate, for the additional $2.75 rate.
Administration Fee
Separate reimbursement is allowed when the administration of the drug is the only
service billed by the practitioner. In addition, if more than one injection is given on the
same date of service and no E/M code is billed, providers may bill a separate
administration fee for each injection using 90788. When billing for privately purchased
vaccine, bill an administration code in addition to the CPT code to obtain reimbursement
for both vaccine and its administration. Do not bill an administration CPT code when
billing for VFC vaccine. VFC vaccines must be billed with the CPT code for the vaccine
and the provider’s charge (not to exceed $8) for VFC vaccine administration. Medicaid
maximum fee information can be found on the www.indianamedicaid.com Web site. Be
aware of the member’s primary medical provider assignment, managed care delivery
system assignment, and third party liability resource(s).
01/31/2007 Immunizations and Vaccines
Medical Policy Manual
273
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
RHCs and FQHCs
Note: RHC- and FQHC-specific encounter rates already include payment for
immunizations.
When submitting RHC and FQHC claims to track encounters (such claims will be
denied), bill no more than the $8 VFC administration fee for use of VFC influenza
vaccine or bill the usual and customary rate for the influenza vaccine CPT® plus the
administration CPT 90782 for use of providerpurchased meningococcal vaccine. All
immunization dollars should be included and totaled on the line specific for
immunizations in cost reports submitted to Myers & Stauffer.
01/31/2007 Immunizations and Vaccines
Medical Policy Manual
274

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
IMMUNIZATIONS AND VACCINES
ADDENDUM B
Note: This addendum contains provider notifications that have been published since the
review of the Immunizations and Vaccines Fact Sheet. The information in this addendum
will be incorporated into the fact sheet at the next review.
Provider Notification: BR200542 Publication Date: 10/18/2005
Subject: TdaP and MCV4 Vaccines
Date Added to Manual: 01/31/2006
Text of Publication
Currently, the Vaccines For Children (VFC) program cannot distribute a sufficient supply
of TdaP and MCV4 vaccines to all VFC-participating providers. Due to this shortage
crisis, the Indiana Health Coverage Programs (IHCP) is not limiting reimbursement for
TdaP, Tetanus diphtheria toxoids and acellular pertussis vaccine (CPT 90715 – Adecel
and Boostrix) and MCV4, meningococcal conjugate vaccine, tetravalent (CPT 90734 –
Menactra) to the VFC Vaccine Administration Fee of $8.00 or less. This policy allows
providers to obtain reimbursement for using privately purchased TdaP or meningococcal
vaccines if they cannot obtain a VFC vaccine. When administering privately purchased
TdaP or meningococcal vaccines, providers may bill for the cost of the vaccine plus its
administration, and the IHCP-allowable reimbursement will include payment for both.
Note: If a provider administers a free VFC vaccine, the provider should bill the appropriate TdaP or
meningococcal vaccine procedure code but not charge more than the $8.00 VFC vaccine administration
fee, and not bill the separate administration CPT code.
When a provider administers immunizations using the provider’s private stock, refer to
IHCP provider bulletin BT200151 for use of the administration code 90782, as
appropriate, for the additional $3.00 rate.
• To address an immediate need for immunizations and a shortage of available influenza
vaccines, the IHCP is not limiting reimbursement for any influenza vaccines, regardless
of availability from the VFC program. This policy will allow providers to obtain
reimbursement for using a privately purchased influenza vaccine if they do not have a
VFC vaccine due to the shortage crisis. When administering a privately purchased
influenza vaccine, providers may bill for both the cost of the vaccine plus its
administration, and the IHCP-allowable reimbursement will include payment for both.
Refer to banner page BR200442, published October 19, 2004, regarding detailed billing
instructions when administering private stock.
01/31/2007 Immunizations and Vaccines
Medical Policy Manual
275

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
IMMUNIZATIONS AND VACCINES
ADDENDUM C
Note: This addendum contains provider notifications that have been published since the
review of the Immunizations and Vaccines Fact Sheet. The information in this
addendum will be incorporated into the fact sheet at the next review.
Provider Notification: BR200627 Publication Date: 07/04/2006
Subject: Vaccines for Children
Date Added to Manual: 10/31/2006
Text of Publication
Effective July 17, 2006, the Indiana State Department of Health (ISDH) announces that
the vaccine for Hepatitis A pediatric/adolescent dosage will be available through the
Vaccines for Children program. Therefore, for dates of service on or after July 17, 2006,
reimbursement for Health Care Procedure Coding System (HCPCS) codes 90633 –
Hepatitis A vaccine, pediatric/adolescent dosage – 2-dose schedule, for intramuscular
use and 90634 – Hepatitis A vaccine, pediatric/adolescent dosage – 3-dose schedule, for
intramuscular use, is the lesser of the $8 administration fee or the billed amount.
01/31/2007 Immunizations and Vaccines
Medical Policy Manual
276

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Immunizations and Vaccines
Medical Policy Manual
277
IMMUNIZATIONS AND VACCINES
ADDENDUM D
Note: This addendum contains provider notifications that have been published since the
review of the Immunization and Vaccines Services Fact Sheet. The information in this
addendum will be incorporated into the fact sheet at the next review.
Provider Notification: BR200639 Publication Date: 09/26/2006
Subject: Vaccines for Children
Date Added to Manual: 10/31/2006
Text of Publication
Effective September 25, 2006, the Indiana State Health Department (ISDH) announces
that the vaccine CPT code 90715 - Tetanus, diphtheria toxoids and acellular pertussis
vaccine (Tdap) for use in individuals 7 years or older, for intramuscular use (Boostrix and
Adacel), is available through the Vaccines for Children (VFC) Program. Therefore, for
dates of service on or after September 25, 2006, reimbursement for CPT code 90715 is
the lesser of the $8 administration fee or the billed amount.

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: INTERMEDIATE CARE FACILITIES FOR THE
MENTALLY RETARDED
DESCRIPTION
Intermediate Care Facilities for the Mentally Retarded (ICF/MR) provide health care and
rehabilitative services to individuals who do not require acute treatment interventions;
however, as a result of mental retardation and/or a disability, these individuals do require
consistent, daily interventions to improve daily functioning.
An ICF/MR interdisciplinary team must develop a plan of care for each individual to
identify developmental strengths, functional and adaptive abilities, and deficient areas of
development. As appropriate, goals include developing appropriate interpersonal skills,
daily living skills, vocational skills, rehabilitative skills, behavior management, and other
services deemed medically necessary for the functioning of an individual. Interventions
and services are provided with sufficient intensity and frequency to achieve treatment
goals. Ongoing evaluations and revisions of the treatment plan are conducted to prevent
regression and/or loss of functional abilities.
This document is intended to serve as a general summary of the Indiana Health Coverage
Programs (IHCP) policies regarding this service. More specific information may be
found in the IHCP provider manual, program notices, the Indiana Administrative Code,
or other sources as appropriate. See the “Rules, Citations, and Sources” section of this
document for further information.
COVERAGE CRITERIA
ICF/MR services are a specific level of care (LOC) to be provided to a member who is
mentally retarded or has certain other conditions that meet medical necessity in an
institution that meets the certification standards to participate as a Medicaid provider.
ICF/MR services and/or treatment programs are delivered on an inpatient basis and under
the direction and supervision of the required professional staff. Admissions to ICF/MR
must be based upon a determination of the need for such care by an interdisciplinary
professional team. Approval by the Indiana Family and Social Services Administration –
Division of Disability, Aging, and Rehabilitative Services (DDARS)/Bureau of
Developmental Disabilities Services (BDDS) must be received by the ICF/MR institution
prior to admission, or in cases of those individuals who make application while in the
institution, prior to payment for that service. The interdisciplinary professional team
completes a comprehensive evaluation that covers physical, emotional, social, and
cognitive factors.
01/31/2007 Intermediate Care Facilities for the Mentally Retarded 278
Medical Policy Manual
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
ICF/MR reimbursement will be provided by the Medicaid program, for eligible members
who meet all of the following criteria.
• Diagnosis of mental retardation or related conditions of epilepsy, cerebral palsy, or
other developmental disability found to be closely related to mental retardation or
that require treatment similar to services required for mentally retarded individuals.
Severely or profoundly retarded, moderately retarded, severely physically
handicapped, aggressive, assaultive, security risk, or manifesting severe
hyperactive or psychotic-like behavior.
Moderately retarded and may require habit training, training and guidance in
the activities of daily living, and development of self-help skills for
maximum independence, and as needed by the member.
Participate in vocational training programs or adults who work in sheltered
workshops.
Categories of ICF/MR
There are three different categories of ICF/MR available to IHCP members. The three
categories are listed below.
1) Large private ICF/MR–greater than eight beds
2) Small ICF/MR (commonly referred to as community residential facilities
for the developmentally disabled (CRF/DD))–four to eight beds
• Basic developmental services
• Child rearing residences with specialized programs
• Developmental training
• Intensive training
• Sheltered living
• Behavioral management residences for children
• Extensive needs for adults
3) State operated facilities–greater than eight beds
Admission and Readmission Criteria for Large Private and Small ICF/MR
IHCP covers services provided by certified ICF/MR when such services are rendered to a
Medicaid member. Admissions to large private and small ICF/MR are based upon a
determination of the need for such care by the DDARS/BDDS. The interdisciplinary
professional team from the proposed placement facility reviews a comprehensive
evaluation including physical, emotional, social, and cognitive factors to ensure the
facility can meet the needs of the member. The interdisciplinary professional team
includes a physician, certified social worker, Qualified Mental Retardation Professional
(QMRP) (See 42 CFR 483.430 for further information about this professional), and other
professionals.
IHCP payment must be authorized for each member in the large private and small ICF/MR. This
process must be completed prior to the first IHCP payment. Determination of appropriate
reimbursement is based on documentation outlined by the following guidelines that are
applicable for admission and readmission of a member to a large private or small ICF/MR.
01/31/2007 Intermediate Care Facilities for the Mentally Retarded 279
Medical Policy Manual
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• Diagnostic evaluation including social and psychological components.
• A Form 450B, Physician Certification for Long-Term Care Services, completed by
the physician must submitted to DDARS/BDDS or its designee. The payment
period will not be approved for any period of time that precedes the date the
physician signs the Form 450B certifying the need for ICF/MR services.
• Both member and provider must be eligible during any period for which IHCP
reimbursement is requested.
• A physician must certify the member’s need for ICF/MR care at the time of
admission. The first recertification must take place within 12 months from the date
of admission certification. Subsequent recertifications must occur annually
thereafter, or more often, as determined by the interdisciplinary team.
• The certification must specify the level of care required by the member, and the
recertification must clearly indicate the need for care to continue at this level. The
certification must be signed by the physician and dated at the time of signature.
Subsequent recertifications must be signed by a physician, a physician assistant, or a
nurse practitioner and dated at the time of signature. (A stamped signature will
not be accepted.)
• The admission certification and the three latest recertifications must be kept in the
member’s active medical record. All other recertification must be kept on file in the
facility and be available for review purposes.
• The interdisciplinary professional team must, within 30 days after admission, review
and update the preadmission evaluation.
• The individual program plan must be reviewed by the physician or the QMRP
(Refer to 42 CFR 483.430 for further description) and revised as necessary.
• At least annually, the comprehensive functional assessment of each member must
be reviewed by the interdisciplinary team for relevancy and updated as needed.
Admission to Large State ICF/MR
Admissions to large state operated ICF/MR facilities are based upon a determination of
the need for such care by the DDARS/BDDS. The interdisciplinary professional team
from the proposed placement facility reviews the comprehensive evaluation covering
physical, emotional, social, and cognitive factors to ensure the member’s needs are met.
A physician must complete a Form 450B, Physician Certification for Long-Term Care
Services, prior to receiving the first IHCP payment.
Transfer to Another ICF/MR
A current Form 450B must be submitted for any transfer to another ICF/MR. Diagnosis
and evaluation documentation completed within the last year must be submitted as well.
For large state ICFs/MR, if the member is transferred to a noncertified unit, the admission
procedure must be followed for any readmission to the large state ICF/MR facility for
determination of appropriate reimbursement.
01/31/2007 Intermediate Care Facilities for the Mentally Retarded 280
Medical Policy Manual
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Covered Services
Multiple services are provided to IHCP members once enrolled in an ICF/MR. Facilities
provide all-inclusive per diem programs to ICF/MR enrolled members. The following
services are included in the programs.
• Room and board services
Room accommodations, all dietary services (including routine, special dietary,
and school lunches), and laundry services
• Dental services (Large state operated facilities only)
• DME services
DME, except customized items and associated repair costs, include, but not
limited, to the following items.
Ice bags
Bed rails
Canes
Walkers
Crutches
Wheelchairs
Traction equipment
• Medical and Non-medical supplies services
Medical and non-medical supplies and equipment, including items generally required
to provide adequate medical care and personal hygiene for members
• Mental health services
Behavior management, consultation, psychiatric, and psychological services
• Nursing care services (Large private and small facilities only)
Nursing services and supervision of health services
• Optometry services (Large state operated facilities only)
• Therapy services
Physical therapy, occupational therapy, speech therapy, respiratory therapy, and
audiological therapy services
• Transportation services
Transportation to vocational and habilitation services
• Vocational and habilitation services
Training in activities of daily living
Training in the development of self-help and social skills
Development of program and evaluation plans
Development and execution of activity schedules
Vocational and habilitation services must be provided in a Family and Social
Services Administration approved setting. Reimbursement is not available for
services for remediation of learning disabilities.
MANAGED CARE
Managed care members must be disenrolled from their managed care plan prior to
becoming eligible for level of care (LOC) services. Once disenrolled, IHCP coverage
continues under the fee-for-service Traditional Medicaid program.
01/31/2007 Intermediate Care Facilities for the Mentally Retarded 281
Medical Policy Manual
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
In unusual circumstances, a RBMC member may be placed in a facility on a short-term
basis. If a short-term placement becomes a long-term placement, the member is
disenrolled from the RBMC when LOC is approved and entered into IndianaAIM.
ICF/MR providers must verify eligibility upon admission of a new member, and on the
first and 15th of every month for existing members, to confirm IHCP eligibility. It is the
responsibility of the facility to verify healthcare coverage.
• If the ICF/MR facility determines, upon checking eligibility on date of admission (the
first or 15th of each month) that the member is enrolled in RBMC, the facility must
notify the RBMC within 72 hours after admission.
• If the ICF/MR facility notifies the RBMC within 72 hours, the RBMC is responsible
for charges up to 60 calendar days from the date of admission.
• If the ICF/MR facility fails to verify a member’s coverage in RBMC within 72 hours
of admission, the facility may be responsible for charges incurred until the facility has
notified the RBMC of the member’s status.
• In the case of notification past the 72-hour deadline, the RBMC is liable for charges
incurred in the ICF/MR from the date of notification and up to 60 calendar days,
beginning on the date of notification.
• If, after 60 calendar days, the member is still in the ICF/MR facility, LOC
determination has not been implemented, and the member is still enrolled in RBMC,
then the ICF/MR facility is liable for any costs associated with the member until LOC
has been implemented.
The 60 calendar day coverage requirement for RBMC is an extension of the current
managed care continuity of care policy that requires the health plan that receives the
member to honor authorizations of the previous health plan for the first 30 days. This
period is intended to allow for proper notifications and reviews to take place without
interrupting the care being delivered to the member. The overall period of 60 calendar
days is to allow sufficient time, not only for notifications and reviews, but also
preadmission screening, LOC determination, disenrollment from managed care, and to
ensure appropriate reimbursement to the facility for services rendered.
BILLING REQUIREMENTS
All ICF/MR services are covered in the per diem rate and are not to be billed separately to the
IHCP. There are reimbursement exceptions for specific services rendered to members in large
state ICF/MR facilities. The following information explains these exceptions.
• Medical services, rendered by health care providers outside a large state ICF/MR,
require prior authorization. Prior authorization will not be given for medical
services included in the per diem rate. Written evidence of physician involvement
and personal member evaluation in the progress notes and attached to the prior
authorization form is required to document the medical necessity of the service.
Documentation for prior authorization must include medical necessity of the
service, explanation of why the service cannot be rendered at the facility, and review
of criteria for the specific medical service requested.
• Hospital services rendered due to an acute illness or injury may be billed to the
IHCP directly by the hospital.
01/31/2007 Intermediate Care Facilities for the Mentally Retarded 282
Medical Policy Manual
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• Necessary dental services that cannot be provided on-site by the dental staff require
prior authorization. These prior authorized dental services must be billed directly to
the IHCP by the outside dental provider. Admission of a member to a hospital for
the purpose of performing dental services requires prior authorization. Refer to the
IHCP Provider Manual, Chapter 8, for further dental information.
• Emergency transportation is billable outside the per diem rate. The transportation
provider must bill the IHCP directly.
• Nonstandard DME services require prior authorization. The DME provider must
bill the IHCP program for reimbursement. Facilities cannot require IHCP members
to purchase or rent DME items with their personal funds.
Pharmacy Services
As a result of frequent member admissions, discharges, and readmissions, the IHCP
allows a pharmacy servicing facility residents to indicate the member’s location code in
the National Council for Prescription Drug Programs field number 307-C7. Entering 03
in this field, indicates to the claims processing system that the member resides in an
IHCP certified facility.
Personal Care Items
Routine personal care items (soap, shampoo, deodorant, and other personal hygiene
items) will be included in the per diem reimbursement. Routine personal care items do
not include personal items such as make-up, cigarettes, etc.
Client Clothing
The IHCP will not provide reimbursement for clothing items purchased by a provider for
a member.
Transportation
Transportation services furnished to members who are in ICF/MR are exempt from co-
payment requirements.
Respite Care
Respite care is not available for members residing in ICF/MR.
Reservation of Beds
IHCP reimbursement is available for reserving beds in a large private, small, or state
operated ICF/MR for IHCP members, at one-half the regular per diem rate, when one of
the following conditions is present.
• Hospitalization
Hospitalization must be ordered by the physician for treatment of an
acute condition that cannot be treated in the facility.
01/31/2007 Intermediate Care Facilities for the Mentally Retarded 283
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Intermediate Care Facilities for the Mentally Retarded 284
Medical Policy Manual
The length of time allowed for payment of a reserved bed for a single
hospital stay is 15 days.
If the member requires hospitalization longer than 15 consecutive days, the
member must be discharged from the facility.
If the member is discharged from the ICF/MR following a hospitalization in
excess of 15 consecutive days, the ICF/MR is responsible for appropriate
discharge planning if the ICF/MR does not intend to provide ongoing
services following the hospitalization. Discharge planning is provided for
those members who continue to require ICF/MR level of services.
A physician's order for hospitalization must be maintained in the member’s
medical record at the ICF/MR facility. ICF/MR providers must use revenue
code 185 when billing for bed reservations for hospitalization.
• Therapeutic leave of absence
A leave of absence must be for therapeutic reasons, as prescribed by the
attending physician and indicated in the member’s plan of care. The
length of time allotted for a therapeutic leave in any calendar year is 60
days per member residing in an ICF/MR. Leave days need not be
consecutive. If the member is absent for more than 60 days per year, no
further IHCP reimbursement will be available for reserving a bed for
that member in that year. A physician's order for the therapeutic leave
must be maintained in the member’s medical record at the ICF/MR
facility. Providers must use the revenue code 183 when billing for this
service.
When filing a claim for a hospital or therapeutic bed hold, do not use a discharge code on
the claim form. The discharge status code closes the member’s level of care segment and
all future claims are denied. Providers should use the status code 30 when billing a
hospital or therapeutic bed hold.
RELATED MEDICAL TOPICS
Dental Services
Emergency Room Services
Inpatient Hospital Services
Long-term Care Services
Mental Health Services
Mental Rehabilitation Services
Nursing Facility Services
Ophthalmological Services
Outpatient Hospital Services
Pharmacy Services
Physician Services
Surgery Services
Transportation Services
Waiver Services

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H0105
RULES, CITATIONS, AND SOURCES
Code of Federal Regulations (CFR)
42 CFR 483.400-483.480 – Intermediate Care Facilities for the Mentally Retarded
Indiana Administrative Code (IAC)
405 IAC 1-1-10 – Intermediate care for the mentally retarded; governing
provisions
405 IAC 1-1-11 – Intermediate care for the mentally retarded; eligibility
405 IAC 1-12 – Rate Setting Criteria for State-Owned Intermediate Care Facilities
for the Mentally Retarded and Community Residential Facilities
for the Developmentally Disabled
405 IAC 1-17 – Rate Setting Criteria for State-Owned Intermediate Care Facilities
for the Mentally Retarded
405 IAC 5-13 – Intermediate Care Facilities for the Mentally Retarded
Indiana Code (IC)
IC 12-15-39.6 – Long Term Care Program
IC 12-15-32 – Community Residential Facilities for the Developmentally
Disabled
Indiana Health Coverage Programs Provider Banner (BR)
BR200441 – Pharmacy & LTC update
BR200433 – LTC Adjustments made to CMI & MDS Field Audit
BR200429 – LTC Provider Specialty 030 update
BR200340 – Nursing ICF/MR
BR200312 – LTC Billing Errors
BR200309 – LTC Wheelchairs
BR200110 – Nursing Facilities and ICF/MR Claims Denying for Edit 4219
BR200107 – ICF/MR
BR199945 – LTC Forms 450B and 450B SA/DEBR
BR199812 – LTC Claim Payment Logic
Indiana Health Coverage Programs Provider Banner (BR) (cont.)
BR199805 – LTC Facilities Mass Adjustment
Indiana Health Coverage Programs Provider Bulletin (BT)
BT200412 – Waiver Audit Process
BT200371 – Documentation Standards for Home and Community-Based
Services Waiver Programs
BT200347 – Appropriate Billing Practice for IHCP Residents Discharged from a
LTC facility.
BT200343 – HCBS Waiver for Aged and Disabled and Target Case Management
for the Elderly and Disabled
BT200315 – Targeted Case Management and Requirements for Respite Care
BT200305 – Changes to the Home and Community-Based Services Waiver
Review Process
BT200252 – Home and Community Based Services Waiver Claims Information
for the Developmental Disabilities Waiver Targeted Case
01/31/2007 Intermediate Care Facilities for the Mentally Retarded
Medical Policy Manual
285

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Management for Individuals with Developmental Disabilities and
Related Home and Community-Based Services Waiver Changes
BT200123 – Initial Assessments and Annual Functional Assessments Currently
Conducted by the D&E Teams for Individuals with Developmental
Disabilities
BT200113 – New Information for Case Managers and Waiver Providers
Available on the Web
Indiana Health Coverage Programs Provider Manual – Version 5.1
Revision date: 1/31/06
Revisions and
Review
Reason Date
470 IAC 5-8-10
Transferred
ICF/MR 7/1/91
405 IAC 1-6-10
Repealed 8/24/97
ICF/MR 1/1/92
IC 12-15-32
Community Residential Facilities
for the Developmentally Disabled
2/14/92
Indiana Medicaid
Update Bulletin 95-60
Cost Reporting Issue 7/1/94
405 IAC 5-13 ICF/MR 8/24/97
405 IAC 5-13-3 ICF/MR 10/27/99
405 IAC 5-13-4
Amended
ICF/MR 10/27/99
405 IAC 5-13-7 ICF/MR 10/27/99
405 IAC 5-13-8 ICF/MR 10/27/99
405 IAC 5-13-9 ICF/MR 10/27/99
Routine Review Scheduled 1/31/06
APPLICABLE INDIANAAIM EDITS AND AUDITS
282 – Covered Days Missing
283 – Covered Days Invalid
546 – Type of Bill Invalid for Recipient Level of Care
548 – Therapeutic Leave Billed Without Accommodation Code
549 – Invalid Type of Provider to Bill for Ancillary Service
581 – Cannot Bill NDC on Home Health or Long-term Care
1018 – No Rate Segment for Level of Care
1019 – Multiple Levels of Care Per Diem on File
1023 – Level of Care Billed Not on File for This Provider
1024 – Billing Provider Is Not Member’s Listed LTC Provider, Verify Provider Number
and Resubmit
1025 – Billing Provider Not Enrolled For the Date of Service
2008 – Member Ineligible for Level of Care Billed
01/31/2007 Intermediate Care Facilities for the Mentally Retarded
Medical Policy Manual
286
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Intermediate Care Facilities for the Mentally Retarded
Medical Policy Manual
287
2011 – Medical and Non-Medical Supplies and Routine DME
2013 – Recipient Ineligible for Level of Care
4219 – Units Billed Exceed Days Covered
6047 – Excessive Therapeutic Leave Days
6067 – Excessive Therapeutic Leave Days (ICF)
6068 – Excessive Therapeutic Leave Days (ICF/MR or CRF/DD)
6081 – DME Not Payable When Patient in ICF/SNF
6511 – One Dispensing Fee Per Month for LTC Recipients

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: LABORATORY SERVICES
DESCRIPTION
A clinical laboratory is a place where materials derived from the human body are tested,
measured, or examined in order to provide information on diagnosis, monitoring,
prevention, or treatment of disease.
SUMMARY OF CURRENT POLICY
Indiana Health Coverage Programs (IHCP) reimbursement is available for most clinical
diagnostic laboratory procedures, performed in a physician's office, by an independent
laboratory, or by a hospital laboratory for its outpatients. Laboratory procedures are
subject to the Clinical Laboratories Improvement Act (CLIA) rules and regulations.
IHCP reimbursement is available for the drawing of or collection of, specimens provided
by physicians, independent laboratories, or hospital laboratories. These services are
covered only in circumstances in which a blood sample is drawn through venipuncture or
a urine sample is collected by catheterization.
RELATED MEDICAL TOPICS
Chiropractic Services
EPSDT - HealthWatch
Hematology
Laboratory Services – Group A Beta Hemolytic Streptococcal Pharyngitis Tests
Laboratory Services – HIV Testing
Laboratory Services – Microquantative Sweat Test
Podiatry
Screening Services – Newborn Screening
RULES, CITATIONS, AND SOURCES
42 CFR § 441.16 Laboratory services.
42 CFR § 440.30 Other laboratory and X-ray services.
405 IAC 5-18 Laboratory Services
Indiana State Department of Public Welfare Medical Policy Manual 1991
01/31/2007 Laboratory Services
Medical Policy Manual
288

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Indiana Medicaid Update Bulletin 95-21-- Conveyance of a Specimen to a Laboratory--
date of bulletin 05/02/95
Indiana Medical Assistance Programs Provider Manual 1994
Indiana Medicaid Update Bulletin 98-16--Clinical Laboratory Improvement Amendment
(CLIA) Requirements 06/10/98
405 IAC 5-12-4 Chiropractic Services—Laboratory Services
405 IAC 5-26-4 Podiatric Services—Laboratory or X-ray Services
405 IAC 5-18-2c Reimbursement Restrictions
IHCP 2003 Provider Manual, Chapter 7, Section 8
IHCP 2003 Provider Manual, Chapter 8, Section 3
ORIGINATION, REVISIONS, AND REVIEWS
Origination Date: 12/31/00
Reviews and Revisions Reason
Date
Indiana Medicaid Update
Bulletin 95-21
Conveyance of a Specimen to a
Laboratory
6/1/95
405 IAC 5-18 Laboratory services 8/24/97
405 IAC 5-17 Hospital services 8/24/97
405 IAC 5-12-4 Chiropractor
ordered Lab Services
8/24/97
405 IAC 5-26-4 Podiatry-Lab or X-ray services 8/24/97
Indiana Medicaid Update
Bulletin 98-16
Clinical Laboratory Improvement
Amendment (CLIA) Requirements
7/1/98
Review and Update Scheduled Review 4/30/04
APPLICABLE INDIANAAIM EDITS AND AUDITS:
4110
4112
4207
4208
6001
6007
6500
6501
6502
6503
6504
6506
6507
01/31/2007 Laboratory Services
Medical Policy Manual
289
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Laboratory Services
Medical Policy Manual
290
COVERAGE CRITERIA
Laboratory services are reimbursable only when such services are necessitated by a
condition-related diagnosis.
Clinical Diagnostic Laboratory Procedure
Introduction
The pathology and laboratory guidelines noted in the Current Procedural Terminology
(CPT) and HCFA Common Procedure Coding System (HCPCS) Level II Codes should
be used to bill lab services.
Reimbursement Methodology
Most clinical diagnostic laboratory procedures, performed in a physician’s office, by an
independent laboratory, or by a hospital laboratory for its outpatients, will be reimbursed
on the basis of the lower of the submitted charge, based on either the Medicare Clinical
Lab Fee Schedule, or the RBRVS IHCP Fee Schedule. For purposes of this fee schedule,
clinical diagnostic laboratory services include all laboratory tests listed in codes 80002 –
89399 of the CPT book except for the following categories: Blood, Blood Products,
Blood Testing, and Tests involving Physician interpretation. Providers must have a valid
Clinical Laboratory Improvement Amendment (CLIA) number on file with the IHCP in
order to be reimbursed for clinical laboratory services under CLIA regulations.
Exceptions
The following codes may be classified as clinical diagnostic laboratory procedures only
in certain circumstances. When submitted on the same claim form with codes
corresponding to blood or blood products, these codes are not subject to pricing by
Medicare fee schedules. If submitted on a claim with no charge for blood or blood
products, these services are classified as clinical diagnostic lab tests, subject to pricing by
Medicare fee schedules:
86021
86022
86880
86885
86886
86900-86911
86970-86972
86975-86978
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
60% Versus 62% Defined
Laboratory tests performed by a physician’s office or by an independent laboratory will
be reimbursed at 60% of the prevailing charge. Laboratory tests performed in a hospital
outpatient setting will be reimbursed at 62% of the prevailing charge when the tests are
performed by a qualified hospital laboratory of a sole community hospital. A qualified
laboratory is one that provides some clinical laboratory tests 24 hours a day, seven days a
week, in order to serve a hospital’s emergency room that is available to provide services
24 hours, seven days a week. A hospital with physicians and laboratory technicians on
call to handle emergencies meets the requirement. In situations where a hospital
laboratory is acting as an independent laboratory (i.e., performing tests for persons who
are non-hospital patients), the services will be reimbursed at 60% of the prevailing
charge. Additionally, laboratory tests performed by a non-qualified laboratory, or by a
qualified hospital laboratory not located in a sole community hospital, will be reimbursed
at 60% of the prevailing charge.
Hospital Outpatient Defined
A hospital outpatient is a person who has not been admitted by the hospital as an
inpatient, but is registered on the hospital records as an outpatient and receives services
directly from the hospital. If a tissue sample, blood sample, or specimen is taken by
personnel not employed by the hospital and is sent to the hospital for performance of
tests, these tests are classified as non-patient hospital services (rather than outpatient)
since the member did not directly receive services from the hospital.
Billing Procedures
When billing for clinical diagnostic tests, the appropriate CPT or HCPCS code must be
indicated on the claim form. If the procedure is administered more than one time in the
same day, it should be billed as only one line item, with an indication of the number of
units of service given in that day. Laboratories performing services must bill the IHCP
directly unless otherwise specified by the CMS.
Specimen Collection
A minimal fee will be allowed for separate charges made by physicians, independent
laboratories, or hospital laboratories for the drawing of or collection of specimens. These
services are covered only in circumstances when a blood sample is drawn through
venipuncture or where a urine sample is collected by catheterization. Specimen
collection fees must be itemized when billed. Only one charge per day for each member
will be allowed for venipuncture. A charge for catheterization will be allowed for each
patient encounter; i.e., there is no per day or per claim limitation.
01/31/2007 Laboratory Services
Medical Policy Manual
291
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Handling/Conveyance
IHCP reimburses for handling and conveyance of a specimen to a laboratory if services
are billed by a physician, chiropractor, or podiatrist [405 IAC 5-18-2(c).] Providers will
be reimbursed for no more than two conveyance fees (CPT Procedure codes 99000 and
99001) on the same date of service.
Consultative Laboratory Services
Consultative laboratory services are covered for clinical laboratory tests if the following
conditions are met:
♦ services were requested by the member’s attending physician;
♦ service relates to a test result that lies outside the clinically significant normal
or expected range in view of the condition of the member;
♦ service results in a written narrative report included in the member’s medical
record; and
♦ service requires the exercise of medical judgement by the consultant
physician.
Clinical Laboratory Improvement Amendments (CLIA)
General Information
Providers rendering laboratory services must obtain a Clinical Laboratory Improvement
Amendments (CLIA) number. Information regarding CLIA can be obtained at
www.cms.hhs.gov.
The CLIA program is intended to ensure that providers who perform laboratory
procedures do so in accordance with Federal regulations. Laboratory procedures are
defined as any procedure for the examination of materials derived from the human body.
CLIA certification types are as follows
• Certificate of Waiver—This certificate is issued to a laboratory to perform only
waived tests.
• Certificate for Provider-Performed Microscopy (PPM) Procedures—This certificate is
issued to a laboratory in which a physician, midlevel practitioner or dentist performs
no test other than the PPM procedures. This certificate permits the laboratory to also
perform waived tests.
01/31/2007 Laboratory Services
Medical Policy Manual
292
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• Certificate of Registration—This certificate is issued to a laboratory that enables the
entity to conduct moderate or high complexity laboratory testing or both until the
entity is determined by survey to be in compliance with the CLIA regulations.
• Certificate of Compliance—This certificate is issued to a laboratory after an
inspection that finds the laboratory to be in compliance with all applicable CLIA
requirements.
• Certificate of Accreditation—This is a certificate that is issued to a laboratory on the
basis of the laboratory’s accreditation by an accreditation organization approved by
HCFA.
Information regarding the procedure codes allowed to be billed by specific certificate
type can be obtained at www.cms.hhs.gov.
Edit Codes Indicating A Denied Laboratory Procedure Code
Claims for laboratory services that have been denied will post edit codes 4207 or 4208.
Edit 4207 states: CLIA Number Not On File For Date Of Service Billed. Once the
provider receives written confirmation from EDS that a CLIA Number and its effective
date have been entered into the IndianaAIM System, previously denied claims may be
resubmitted.
Edit 4208 states: Invalid CLIA Certification/Procedure Code Combination. This means
the provider billed a laboratory procedure code outside of the provider’s CLIA certificate
type and will not be reimbursed for this service.
01/31/2007 Laboratory Services
Medical Policy Manual
293

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Laboratory Services
Medical Policy Manual
294
LABORATORY SERVICES
ADDENDUM A
Note: This addendum contains provider notifications that have been published since the
review of the Laboratory Services Fact Sheet. The information in this addendum will be
incorporated into the fact sheet at the next review.
Provider Notification: BR200537 Publication Date: 09/13/2005
Subject: Lab Codes That Allow Interpretation
Date Added to Manual: 10/31/2005
Text of Publication
The IHCP reimburses the following Current Procedural Terminology (CPT®) clinical lab
codes that allow interpretation, retroactive to July 1, 2002, (retroactive to January 1,
2005, for CPT codes 84166 and 86335). The IHCP follows Medicare guidelines for the
CPT clinical lab codes that allow interpretation.
83020 83912 84165 84166 84181
84182 85390 85576 86255 86256
86320 86325 86327 86334 86335
87164 87207 88371 88372 89060
Both the technical and professional components are reported separately to ensure proper
reimbursement. Providers bill the IHCP for the technical component of the clinical lab
procedure reporting the base code only, without modifier TC. If the modifier TC is billed
at the claim detail the claim will be denied. The interpretation service is reported with the
CPT code and modifier 26. For example, providers performing both the technical
component and interpretation of CPT code 84165 report CPT code 84165 for the
technical component and the CPT code modifier combination 84165-26 for the
interpretation.
The IHCP will mass void and replace the affected claims with dates of service July 1,
2002, through August 17, 2005. The mass void and replacement of claims will begin
appearing on providers’ September 27, 2005, remittance advice statements. For any claim
that has not been submitted to the IHCP for reimbursement or may need to be voided or
replaced after the mass void or replacement of claims has been completed, providers may
use a copy of this banner page article as documentation to waive the one year filing limit.

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: LABORATORY SERVICES--GROUP A BETA
HEMOLYTIC STREPTOCOCCAL PHARYNGITIS
TESTS
DESCRIPTION
Group A Beta Hemolytic Streptococcus is a bacteria that causes sore throat, pharyngitis,
upper respiratory infection, and may cause scarlet fever. Several microbiological tests
are used to identify this organism. Infectious agents can be detected using antigen
detection, direct fluorescence microscopy, or nucleic acid probe techniques.
SUMMARY OF CURRENT POLICY
Medicaid reimbursement is available for approved laboratory tests used for the detection
of Group A Beta Streptococcus.
RELATED MEDICAL TOPICS
Laboratory Services
RULES, CITATIONS, AND SOURCES
Indiana Medicaid Update Bulletin 98-45 dated 12/01/98
Indiana Health Coverage Programs Provider Manual 1999
470 IAC 5-9-26
01/31/2007 Laboratory Services – Group A Beta Hemolytic Streptococcal Pharyngitis Tests
Medical Policy Manual
295

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Laboratory Services – Group A Beta Hemolytic Streptococcal Pharyngitis Tests
Medical Policy Manual
296
ORIGINATION, REVISIONS AND REVIEWS
Origination Date: 12/31/00
Revisions and Reviews Reason Date
405 IAC 5-18 Laboratory services 8/24/97
Bulletin 98-45 Laboratory Services 12/1/98
Reviewed Without Revision Scheduled Review 4/30/04
APPLICABLE INDIANAAIM EDITS AND AUDITS:

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: LABORATORY SERVICES - HER-2/NEU GENE
DETECTION TEST AND HER-2 PROTEIN
EXPRESSION TEST
DESCRIPTION
Many advances have been made in the treatment of breast cancer. The human HER-2/neu
gene (also known as c-erbB-2, ERBB2 or neu) encodes a protein called HER-2 protein or
p185
HER-2
. This protein receptor plays a role in controlling cell growth and division. In
25 to 30 percent of patients with breast cancer, the HER-2 protein is overexpressed as
part of the malignant transformation and tumor progression. Overexpression of HER-2
protein has been shown to contribute to the progression of the cancer and is associated
with poor clinical outcome. Targeted antibody therapy to HER-2 has played a significant
role in the treatment of metastatic breast cancer. Clinical trial results show that the HER-
2 protein on breast cancer cells is an important target for cancer therapies and that
trastuzumab (HERCEPTIN
®
) can be an effective treatment, whether used by itself or in
combination with other chemotherapy drugs.
Several types of HER-2 overexpression tests are available. One type is a
semiquantitative immunohistochemical assay that measures the overexpression of HER-2
protein (for example, the HercepTest
®
). Another type is a gene probe assay, which
detects the qualitative presence of the gene amplification in human breast tissue (for
example, Oncor’s INFORM
®
HER-2/neu Gene Detection Test). This test is a DNA probe
assay known as fluorescent in situ hybridization (FISH).
RELATED MEDICAL TOPICS
Oncology
Pathology
Laboratory
RULES, CITATIONS, AND SOURCES
BT20005 - HER-2/neu Gene Detection Test and HER-2 Protein Expression Test
01/31/2007 Laboratory Services – HER 2/neu Gene Detection Test
Medical Policy Manual
297

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Origination Date: 1/14/2000
Reviews and
Revisions
Reason Effective Date
BT200005 Fact Sheet Implementation 1/14/2000
BT200005 Review, HER-2 Test – no policy changes 7/30/04
APPLICABLE INDIANAAIM EDITS AND AUDITS
6001
6011
COVERAGE CRITERIA
Prior authorization is not required.
The FDA-approved indications for HER-2 protein overexpression and gene detection
tests are:
For HER-2 protein overexpression tests (HercepTest
®
), as an aid in assessment of
patients for whom trastuzumab (HERCEPTIN
®
) is being considered.
For HER-2/neu gene detection tests, as an adjunct to existing clinical and pathological
information and as an aid to stratify breast cancer patients with a primary, invasive,
localized breast carcinoma, and who are lymph node negative, for risk for recurrence or
disease-related death. This test is used as a prognostic indicator for these patients.
The ICD-9-CM Diagnosis Codes that support the medical necessity of HER-2 protein
overexpression and gene detection tests are as follows:
174.0−174.9 Malignant neoplasm of the female breast
175.0−175.9 Malignant neoplasm of the male breast
The ordering physician must have documentation in the member’s medical records to
support the medical necessity of the test(s) ordered. Laboratories performing the test
must have documentation that laboratory personnel education has been completed in the
proper performance of the test and reporting of the test results. Reimbursement will be
provided only to Clinical Laboratory Improvement Amendments (CLIA) certified
laboratories.
01/31/2007 Laboratory Services – HER 2/neu Gene Detection Test
Medical Policy Manual
298
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Laboratory Services – HER 2/neu Gene Detection Test
Medical Policy Manual
299
BILLING INFORMATION
A HER-2 protein overexpression test (for example, HercepTest
®
) is billed using the
following codes:
88342 Immunocytochemistry (including tissue immunoperoxidase), each
antibody
88365 Tissue in situ hybridization, interpretation and report
The HER-2/neu Gene Detection Test (for example, Oncor’s INFORM
®
) is billed using
the following codes:
83892 Enzymatic digestion
88271 Molecular cytogenetics; DNA probe, each (for example, FISH)
88274 or 88275 Interphase in situ hybridization, analyze 25-99 cells (88274) or 100-300
cells (88275). Only one (1) of these two (2) codes should be billed.
88291 Cytogenetics and molecular cytogenetics, interpretation and report

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: LABORATORY SERVICES--HUMAN
IMMUNODEFICIENCY VIRUS (HIV) TESTING
DESCRIPTION
The human immunodeficiency virus is the virus that causes acquired immunodeficiency
syndrome (AIDS). HIV progressively damages and kills cells of the body’s immune
system, destroying the body’s ability fight infections and certain cancers. HIV can be
transmitted when blood, semen, vaginal fluid, or breast milk is internally passed from an
infected person to an uninfected person. HIV is most commonly transmitted in three
ways: (1) unprotected sexual activity with an infected partner; (2) contact with infected
blood; or (3) and from mother to child during pregnancy, birth, or breast feeding.
According to the Centers for Disease Control a diagnosis of AIDS is appropriate when an
individual with HIV has a T4 lymphocyte (CD4) cell count below 200. The presence of
various opportunistic infections can also indicate the onset of AIDS.
Early HIV infection often causes no symptoms and requires a blood test to detect the
presence of HIV antibodies. HIV antibodies are often not detectable in the blood for one
to three months following infection, and can take up to six months following infection to
be detected in standard blood tests. In babies born to mothers with HIV, a definitive
diagnosis of HIV infection cannot be made using standard antibody tests until after 15
months of age.
The enzyme-linked immunoassay (EIA or ELISA) test is the standard test to detect the
presence of antibodies to HIV. If the ELISA is positive, a second test, the Western Blot,
is necessary to confirm if an individual is HIV positive. A second test may be warranted
as there are other conditions that may inaccurately produce a positive ELISA test result.
SUMMARY OF CURRENT POLICY
Testing for HIV is covered by the Indiana Health Care Programs (IHCP) when medically
necessary for establishing an HIV diagnosis. HIV testing is only covered in
circumstances when a blood sample is drawn through venipuncture or when a urine
sample is collected by catheterization. Oral HIV testing methods are not covered by the
IHCP. Testing for HIV is also covered in conjunction with family planning services and
EPSDT.
Reimbursement for HIV testing, when medically necessary for establishing an HIV
diagnosis, is available using the following codes.
01/31/2007 Laboratory Services – HIV Testing
Medical Policy Manual
300
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• 86701 – HIV-1
• 86702 – HIV-2
• 86703 – HIV-1 and HIV-2, single assay
• 86689 – HLTV or HIV antibody, confirmatory test (e.g. Western Blot)
HIV Testing of Pregnant Women and Newborns
The Indiana Code (IC) 16-41-6-8 requires that, as a routine component of prenatal care,
physicians or advanced practice nurses explain the purpose, risks, and benefits of HIV
testing and order a HIV test for pregnant women. The results of this test are confidential.
Pregnant women have the right to refuse this test. A signed statement acknowledging
that the pregnant woman was counseled and provided the information necessary to make
an informed decision regarding whether or not to be tested must be maintained in the
medical records.
If the woman provides her consent to an HIV test and the test is positive for HIV
infection, the provider must inform the pregnant woman of the test results and provide
treatment and/or referral options available to her for HIV prevention, health care, and
psychosocial services. The physician must also discuss risk reduction activities,
including methods to reduce the risk of perinatal HIV transmission and HIV transmission
through breast milk.
A physician overseeing the care of a newborn infant may offer the parent the option of a
confidential HIV test for the newborn within the first 48 hours after birth under the
following circumstances, as required in IC 16-41-6-4.
• The mother of the newborn has not been previously tested for HIV
• The mother of the newborn has refused an HIV test for the newborn
• If the physician believes that testing the newborn is medically necessary for
reasons other than those listed above.
If the parent objects to testing the newborn in writing for religious reasons, the newborn
is exempt from the testing requirement.
The results of the HIV test must be released to the mother of the newborn. If the test
results are positive, the individual who provides the test results must provide the mother
with treatment and/or referral options available to the newborn infant.
EPSDT
Reimbursement for HIV testing is available for at-risk children and youth ages 0-20.
RELATED MEDICAL TOPICS
HIV-AIDS Care Coordination
Laboratory Services
Screening Services—Newborn Screening
01/31/2007 Laboratory Services – HIV Testing
Medical Policy Manual
301

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Laboratory Services – HIV Testing
Medical Policy Manual
302
RULES, CITATIONS, AND SOURCES:
405 IAC 5-18 Laboratory Services
Indiana State Department of Public Welfare Medical Policy Manual 1991--Human
Immunodeficiency Virus Testing (86311-86314, 86687-86689)
IHCP Provider Manual, Chapter 8, Section 3, Family Planning Services
HealthWatch EPSDT Provider Manual, Section 3, Immunizations and Screenings
IndianaAIM
2004 CPT Expert
ORIGINATION, REVISIONS AND REVIEWS
Origination Date: 07/01/91
Initial Policy
Revisions and
Reviews
Reason Effective Date
Indiana State
Department of Public
Welfare Medical
Policy Manual 1991
HIV testing 7/1/91
470 IAC 5-9-26
Transferred
Laboratory services 7/1/91
405 IAC 1-7-25
Repealed 8/24/97
Laboratory services
1/1/92
405 IAC 5-18 Laboratory services 8/24/97
IHCP Provider
Manual, Chapter 8,
Section 3, Family
Planning Services
Review of HIV testing policies and
procedures
7/30/04
HealthWatch EPSDT
Provider Manual,
Section 3,
Immunizations and
Screenings
Review of HIV testing policies and
procedures
7/30/04
IndianaAIM Review of applicable HIV testing codes 7/30/04
APPLICABLE INDIANAAIM EDITS AND AUDITS:
The following edits and audits are attached to CPT codes 86701, 86702, 86703, 86689.
6001 – Complete Radiology or Pathology Procedure Payable at Reduced Amount When
Professional/Technical Component is Already Paid
6011 – Professional/Technical Component for Radiology or Pathology Not Payable
When Complete Procedure is Already Paid
6096 – CPT/HCPCS Code Billed Not Payable According to PPS Reimbursement
Methodology

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: LABORATORY SERVICES-- SWEAT CHLORIDE TEST
DESCRIPTION
The sweat chloride test (sweat test), is used to diagnose Cystic Fibrosis (CF). According
to the Merk Manual and the Cystic Fibrosis Foundation, the iontophoresis sweat test is
the only reliable test for confirming the diagnosis of CF. CF is a generalized, autosomal
recessive disorder of infants, children, and young adults, in which there is widespread
dysfunction of the exocrine glands. The disease causes excess mucus production in the
respiratory tract, signs of chronic pulmonary disease, pancreatic enzyme deficiency
resulting in steatorrhea and azotorrhea, abnormally high levels of electrolytes in the
sweat, and occasionally biliary cirrhosis.
The sweat test is ordered when an individual, usually an infant, displays symptoms of CF,
such as noticeably salty sweat, or has a close relative who has been diagnosed with CF.
It is also used to help confirm or rule out a diagnosis of CF in individuals who have tested
positive or indeterminate with other tests. The sweat test measures the amount of salt in
the sweat. A high level of salt indicates CF.
SUMMARY OF CURRENT POLICY
The sweat test is covered by the Indiana Health Care Programs (IHCP) when used to
confirm a diagnosis of CF. Reimbursement is available using the following CPT code:
• 89230 – Sweat collection by iontophoresis
RELATED MEDICAL TOPICS
Laboratory Services
Medical Supplies and Equipment (for ThAIRpy vest for C.F.)
Pediatrics
RULES, CITATIONS, AND SOURCES
Indiana Health Coverage Programs Manual 1999
405 IAC 5-18 Laboratory Services
01/31/2007 Laboratory Services – Sweat Chloride Test
Medical Policy Manual
303

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Laboratory Services – Sweat Chloride Test
Medical Policy Manual
304
ORIGINATION, REVISIONS, AND REVIEWS
Origination Date 07/01/91
Initial Policy,
Revisions, and Reviews
Reason
Effective Date
470 IAC 5-9-26 Transferred Laboratory services 7/1/91
405 IAC 1-7-25 Repealed 08/24/97 Laboratory services 1/1/92
405 IAC 5-18 Laboratory services 8/24/97
Policy Review MP Manual update 7/31/04
APPLICABLE INDIANAAIM EDITS AND AUDITS:
6001
6011
6096

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: LABORATORY SERVICES - SALIVARY ESTRIOL
TEST FOR PRETERM LABOR RISK ASSESSMENT
DESCRIPTION
Preterm labor, which is defined as labor before 37 weeks gestation, occurs in eight to 10
percent of all births and accounts for more than 85 percent of perinatal complications and
death. Several tests using either biochemical markers or endocrine assay methods are
available to predict preterm labor risk. One endocrine assay test detects and measures
salivary estriol. Estriol is most detectable in a pregnant woman’s saliva, and increases
before parturition.
The salivary estriol test is a series of tests conducted weekly or biweekly on pregnant
women between weeks 22 and 35 of gestation. Research on the salivary estriol test
indicates that it provides clinical benefits for its ability identify women at both low and
high risk for preterm birth. In the conducting serial testing on women with labor-like
symptoms, the test may ease concerns for the patient and allows the provider to use more
clinically appropriate interventions based on the member’s risk level.
SUMMARY OF CURRENT POLICY
The Indiana Health Coverage Programs (IHCP) reimburses for salivary estriol test using
CPT S3652. This test is limited to the following ICD-9-CM codes that support the
medical necessity of this test. Documentation in the member’s medical record must
support the medical necessity of the test(s) ordered.
ICD-9-CM
Code
Descriptions
V23.2 Pregnancy with history of abortion
V23.4 Pregnancy with other poor obstetric history
V23.8 Other high risk pregnancy
658.9 Other problems associated with amniotic cavity and membranes (with
V23.8)
640.9 Unspecified hemorrhage in early pregnancy (with V23.8)
644.0 Early or threatened labor
644.03 Threatened premature labor, antepartum condition, or complication
644.13 Other threatened labor, antepartum condition, or complication
01/31/2007 Laboratory Services – Salivary Estriol
Medical Policy Manual
305

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Laboratory Services – Salivary Estriol
Medical Policy Manual
306
ICD-9-CM
Code
Descriptions
654.50 Cervical incompetence
654.53 Cervical incompetence, antepartum condition, or complication
654.60 Other congenital or acquired abnormality of cervix; unspecified as to
episode of care, or
654.63 Other congenital or acquired abnormality of cervix; antepartum
condition or complication
621.0 Disorders of uterus not elsewhere classified through
621.9 Unspecified disorder of uterus
Reimbursement is available for CPT S3652, one unit per test, between gestational ages
22 to 35 weeks, every one to two weeks. Modifier U2, second trimester, or Modifier U3,
third trimester, must be indicated on the physician’s test order and on the claim.
Reimbursement for CPT S3652 will be denied when home tocolytic therapy is being
provided using CPT S9349 or 99553. Although prior authorization is not required, use of
the salivary estriol test is closely monitored for appropriateness of use.
RELATED MEDICAL TOPICS
Laboratory
Obstetrics and Gynecology
RULES, CITATIONS, AND SOURCES
Indiana Medicaid Bulletin BT200353
Indiana Medicaid Bulletin BT200014
IHCP Provider Manual, April 2003
Indiana Administrative Code 405 IAC 5-18
ORIGINATION, REVISIONS, AND REVIEWS
Origination Date – 10/1/99
Initial Policy, Revisions, and Reviews Reason Effective Date
405 IAC 5-18 Laboratory services 07/25/1997
BT200014 Original salivary estriol
policy
10/01/1999
IHCP Provider Manual Billing instructions 04/2003
BT200353 CPT Code changes 08/15/2003
MP Manual Scheduled review 07/30/2004
APPLICABLE INDIANAAIM EDITS AND AUDITS
6077
6078

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: LOCUM TENENS AND SUBSTITUTE PHYSICIAN
DESCRIPTION
Locum tenens and substitute physician are terms used to describe the relationship of a
physician who is acting as a fill-in for a member’s regular physician. The regular
physician may be the member’s primary care physician, or primary medical care
provider, (PMP). The regular physician could also be a specialist the member sees
regularly for a specific problem or chronic condition.
According to the Social Security Act 42 USC §1395x(r), the term physician includes a
doctor of medicine, osteopathy, dental surgery, dental medicine, podiatry, optometry, or
chiropractic medicine. A locum tenens or substitute physician arrangement may be used
in any one of the above disciplines; however, the locum tenens or the substitute physician
must be of the same discipline as the regular physician.
This document is intended to serve as a general summary of the Indiana Health Coverage
Programs’ (IHCP) policies regarding this service. More specific information may be
found in the IHCP provider manual, program notices, the Indiana Administrative Code
(IAC) or other sources as appropriate. See the “Rules, Citations, and Sources” section of
this document for further information.
COVERAGE CRITERIA
Locum Tenens Physicians
A locum tenens arrangement is made when the regular physician must leave a practice
due to an illness, vacation, or medical education opportunity. This arrangement is to
ensure services continue and are available to members. The locum tenens arrangement
may be used in a single practice or group practice, but the locum tenens physician cannot
be a member of the practice group where the patient’s regular physician is a member. The
locum tenens physician usually has no permanent practice and provides services in
various locations as needed. Often, this physician is reimbursed a fixed amount per diem
and is identified as an independent contractor, not an employee. The locum tenens
physician must meet all requirements for the practice of medicine in the State of Indiana
and hospital or other institutional credentialing requirements prior to providing IHCP
member services. For provision of locum tenens physician services as defined above, the
locum tenens physician is not required to be an IHCP provider. The regular physician’s
01/31/2007 Locum Tenens
Medical Policy Manual
307
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
office must maintain documentation of the locum tenens arrangement to include the
locum tenens services provided and dates of service.
Substitute Physicians
A substitute physician is requested by the regular physician to see a member in a
reciprocal arrangement. This occurs when the regular physician is unavailable to see the
member. In these instances, the IHCP member must agree to the substitute physician
providing the service. In a substitute physician arrangement, the regular physician
reciprocates the substitute physician by one of the following.
• Reimbursing the substitute the amount received for the service rendered
• Serving in the same capacity in return (in which the regular physician
might cover for the substitute in a similar situation)
For example, when an IHCP member seeks unscheduled care, a substitute physician may
be asked to see the member if the regular physician is neither available nor on-call. If a
regular physician has the member scheduled for an examination, but is called away, a
substitute physician may be asked to see the member.
PRIOR AUTHORIZATION
Prior authorization of services performed by a locum tenens or a substitute physician is
the same as for the member’s regular physician.
MANAGED CARE
For members enrolled in Risk-Based Managed Care (RBMC), providers must contact the
member’s managed care organization (MCO) for more specific guidelines. Refer to the
Provider Manual, Chapter 1, for detailed information about the fee-for-service (FFS),
Primary Care Case Management (PCCM), and RBMC delivery systems.
IHCP members enrolled in Medicaid Select PCCM receive the same benefit coverage,
and are subject to the same limitations as traditional Medicaid FFS. Refer to the
Medicaid Select Manual for Primary Medical Providers and Office Staff for further
information.
BILLING REQUIREMENTS
Locum Tenens
The regular physician’s office personnel submit claims for locum tenens services using
the regular physician’s provider number. Modifier Q6, Service furnished by a locum
tenens physician, is placed on the CMS-1500 claim form or 837P electronic transaction to
indicate services were rendered by a locum tenens physician. The payment amount is the
01/31/2007 Locum Tenens
Medical Policy Manual
308

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
lesser of the billed amount or the IHCP allowed amount for the service rendered. When
the regular physician is enrolled in IHCP as a PMP in the PCCM program, the locum
tenens physician may use the regular physician’s certification code when authorizing
services by other providers. Locum tenens arrangements should not exceed 90
consecutive days. If a physician is away from his or her office for more than 90 days,
additional locum tenens can be used to fill in during that physician absence. This means
that various physicians would be required to fill in for different 90-day periods of time.
Locum tenens should not be used to fill physician vacancies in the office. If it becomes
necessary for the same locum tenens physician to remain longer than 90 days in the same
practice for which he or she has been a temporary replacement, then he or she must enroll
as an IHCP provider.
Substitute Physician
For provision of substitute physician services as defined in this document, both the
regular physician and the substitute physician are required to be IHCP providers. The
regular physician’s office submits the claim and receives payment using the regular
physician’s IHCP provider number. The payment amount will be the lesser of the billed
amount or the IHCP allowed amount for the service rendered. If the regular physician is
enrolled in the IHCP as a primary medical provider (PMP) in PCCM, the substitute
physician may use the regular PMP’s certification code when authorizing services for
PMP members. The modifier, Q5, Services furnished by a substitute physician under a
reciprocal billing arrangement, must be reported on the CMS-1500 claim form or 837P
electronic transaction to indicate services were rendered by a substitute physician. The
substitute physician arrangement should not exceed 14 consecutive days. The substitute
physician arrangement does not apply to substitution arrangements for physicians in the
same medical group with claims submitted in the name of the medical group. For
situations in which one group member substitutes for another, the substitution is noted by
listing the substitute group member number as the rendering provider on the CMS-1500
claim form or the 837P electronic transaction and the Q5 modifier is not used. The group
number is listed as the billing provider. Table 1 compares the requirements for substitute
and locum tenens physicians.
Table 1 – Requirements for Substitute and Locum Tenens Physicians
Requirement
Substitute
Physician
Locum Tenens
Physician
Must be enrolled as an IHCP Provider Yes No
May be employed by the same group as the
regular physician
No No
Claims are submitted by the regular
physician’s office and that office receives
payment
Yes Yes
Modifier required to identify the arrangement Yes, Q5 Yes, Q6
May use the regular physician’s certification
code for PCCM PMP authorizations
Yes Yes
Maximum time frame allowed 14 days 90 days
01/31/2007 Locum Tenens
Medical Policy Manual
309

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
RELATED MEDICAL TOPICS
Physician Services
Dental Services
Chiropractic Services
Vision Care Services
RULES, CITATIONS, AND SOURCES
Social Security Act Amendments of 1994, Section 125
Omnibus Budget Reconciliation Act of 1990, Section 4110
Indiana Health Coverage Programs Provider Bulletins
BT200201
BT200115
BT200125
Indiana Health Coverage Programs Provider Manual
March 2005, Version 5.1
Origination Date: 12/31/2002
Review/Revision Reason Date
BT200201
Locum Tenens and
Substitute Physician Policy
3/5/2002
Review Scheduled 7/31/2006
APPLICABLE INDIANAAIM EDITS AND AUDITS
0231 – Rendering Provider Number Is Missing
0232 – Rendering Physician Number Not In Valid Format
0381 – Attending Physician License Number Missing
0382 – Attending Physician ID Invalid
0399 – Referring Provider I.D.# Is Not In a Valid Format
1000 – Billing Provider I.D. Number Not On File
1001 – Billing Provider Not Enrolled at SVC Location for Program Billed
1002 – Rendering Provider Not Enrolled at SVC Location for DOS
1003 – Billing Provider Not Enrolled at SVC Location on DOS
1004 – Rendering Provider Not Eligible to Render SVS on DOS
1007 – Rendering Provider Not On Provider Database
1008 – Rendering Provider Must Have an Individual Number
1010 – Rendering Provider Not Member of Group or Rendering Not Equal Billing
1028 – Rendering Provider Specialty Not Eligible to Render This Modifier
1029 – Prescribing Provider Not Eligible to Prescribe This NDC
01/31/2007 Locum Tenens
Medical Policy Manual
310
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Locum Tenens
Medical Policy Manual
311
1033 – Rendering Provider Eligible Without Specialty
1036 – Rendering Provider Not Eligible
1039 – Service Rendered by Out-Of-Network Provider
1996 – IMMIS Rendering Provider ID Number Not Enrolled
1998 – IMMIS Billing Provider ID Number Not Enrolled

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: LONG-TERM ACUTE CARE HOSPITALS
DESCRIPTION:
Long-Term Acute Care (LTAC) hospitals are designed to provide specialized acute care
for patients that require an especially long recovery period. These patients usually are in
an acute care facility, their medical condition has stabilized, but they continue to require
an acute level of care. A lesser level of care such as a skilled nursing facility (SNF) or
subacute care facility is not appropriate. Federal regulations for LTAC hospitals require
an average inpatient length of stay greater than 25 days, using Medicare program criteria
to qualify a facility as a LTAC hospital. Patients are generally discharged to home with or
without home care services, to acute inpatient rehabilitation hospitals, subacute
rehabilitation programs, or to SNFs. LTAC hospitals are licensed by state acute care
licensing standards and are accredited by the Joint Commission on Accreditation of
Healthcare Organizations (JCAHO).
MEDICAL TOPICS CROSS-REFERENCES:
Hospital Inpatient
RULES, CITATIONS, AND SOURCES:
405 IAC 1-10.5-2 (s)
405 IAC 1-10.5-3
01/31/2007 Long - Term Acute Care
Medical Policy Manual
312

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Initial Policy Issues
Effective
Date
Implementation
Date
Retroactive
Date
405 IAC 1-10.5-2
(s)
08/31/2001
BT200360
Changes in
Diagnosis-
Related Group
Relative
weights and
New Long-
Term Acute
Care Hospital
Level-of-Care
Reimbursement
Methodology
09/16/2003 11/01/2003
Revisions:
APPLICABLE INDIANAAIM EDITS AND AUDITS:
Coverage Criteria:
Prior authorization (PA) is required for LTAC hospital admissions covered by the Indiana
Health Coverage Programs (IHCP) and reimbursed under the level of care methodology
described in the Indiana Administrative Code (IAC) 405 IAC 1-10.5. Before admission to
a LTAC hospital, assessment of the patient’s current medical status and discharge goals
must be provided to Health Care Excel (HCE) for PA purposes. This information should
also be documented in the medical record. Each PA request is reviewed for medical
necessity on an individual, case-by-case basis. LTAC facilities are designated as
provider specialty type 013. LTAC facilities are paid a daily rate (per diem) for each day
of care provided. The per diem is all-inclusive. No other payments are permitted in
addition to the LTAC level-of-care (LOC) per diem. The LTAC LOC per diem payments
are in lieu of diagnosis-related group (DRG) and outlier payments. Per diem billing is
permitted on a weekly basis. LTAC LOC per diem rates are hospital specific and
implemented as budget neutral for the IHCP. LTAC LOC payments will resemble the
payments that each facility currently receives. Claims noted as statistical outliers for
each facility were not included in the development of each LTAC hospital’s LOC per
diem.
Admission Criteria
The proposed admission needs to be to a facility that meets the definition of a LTAC
hospital in 405 IAC 1-10-2(s) which states, “Long term care hospital means a
freestanding general acute care hospital licensed under Indiana Code IC 16-21 that:
(1) is designated by the Medicare program as a long term hospital; or
01/31/2007 Long - Term Acute Care
Medical Policy Manual
313
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
(2) has an average inpatient length of stay greater than twenty-five (25) days as
determined using the same criteria used by the Medicare program to determine
whether a hospital’s average length of stay is greater than twenty-five (25) days.”
“Freestanding does not mean a wing or specialized unit within a general acute care
hospital.” However, they may be licensed hospitals that operate as separate entities
within a host hospital.
The patient must be admitted directly from an acute care facility, or be a readmission
from a nursing facility or rehabilitation facility. No PA will be approved for requests for
initial admission directly from a nursing facility, physician office, or from home.
The following documentation must be included with requests for admission to a
LTAC hospital and must be available for review by HCE’s Prior Authorization
Department or Surveillance and Utilization Review (SUR) Department, as
applicable:
• A signed statement from the referring physician indicating medical necessity for
transfer to a LTAC hospital.
• The following information must accompany a request for approval and an
evaluation by the requesting facility:
– diagnosis and premorbid condition(s). If the patient is currently in an acute
care hospital, the diagnosis at discharge should be included if it has
changed from the time of admission.
– information about where the patient is being admitted from, if not
hospitalized.
– neurological assessment.
– complete listing of long and short-term goals.
– discharge plan with two options, dependent on the member’s condition
– potential date of admission.
– projected date of discharge.
– history of any previous rehabilitation therapies.
– prognosis and documentation that there is a reasonable expectation the
member’s functional and medical status will improve.
– history, physical, and discharge or case summary, if the member is
currently hospitalized.
– completed IHCP LTAC hospital pre-admission form available from the
PA contractor by contacting Health Care Excel PA Department at (317)
347-4511 in the Indianapolis local area or 1-800-457-4518.
Note: The provider is responsible for compiling and submitting the necessary
documentation for the PA request in a timely manner.
All of the following situations apply to the patient’s status and current requirements
before admission to the LTAC hospital.
01/31/2007 Long - Term Acute Care
Medical Policy Manual
314

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• The patient is medically stable.
• The initial diagnostic work up is completed.
• There are no major surgical procedures planned.
• The patient has a prognosis requiring a prolonged stay in an acute setting and
there is a reasonable expectation for an improvement in the status of his or her
medical condition.
• The patient requires interactive physician direction with daily on-site
assessment.
• The patient requires significant ancillary services dictated by complex, acute
medical needs, for example, full service and STAT laboratory, radiology,
respiratory care services.
• There is a patient-centered, outcome-focused, interdisciplinary approach
requiring a physician directed professional team, including intensive case
management, to move the patient efficiently through the continuum of care.
• Education for the patient and family must be provided to manage the patient’s
present and future health care needs.
Physician consultants are used during the PA process if assistance is needed to
determine the medical necessity of the admission. Admissions requested for
categories not specified in the following sections will be reviewed for medical
necessity and intensity of service on a case-by-case basis.
Respiratory
The patient must meet two or more of the following requirements for admission and
continued stay.
1. Requires ventilator assistance, and has failed attempts to be extubated or
maintain adequate ventilation, oxygenation, or functional level after
extubation.
2. Requires one or more of the following intravenous medications daily.
Bronchodilators Corticosteroids
Diuretics Antiviral agents
Anti-tuberculosis agents Antiprotozoal agents
Chemotherapy Antibiotics
Antifungal agents Anticoagulation medications
3. Requires frequent monitoring of tissue oxygenation (for example, pulse
oximetry), frequent respiratory therapy treatments, suctioning or inhalation
medications.
01/31/2007 Long - Term Acute Care
Medical Policy Manual
315

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Impaired Skin Integrity
Impaired skin integrity means the patient has stage three or four decubitus wounds,
infected necrotic skin conditions, surgical wounds, or burns. The patient must meet
each of the following requirements for admission or continued stay:
1. The patient has non-healing wounds that have failed to improve while
receiving home care, SNF, or acute hospital care.
2. The patient requires complex dressing changes using daily whirlpool,
debridement, frequent intramuscular or intravenous analgesics or antifungals,
frequent positioning, or hyperbaric treatments.
3. The patient requires more than one of the following intravenous medications
at least daily:
Antiviral agents Antibiotics
Antifungal agent Intravenous plasma expanders
Intravenous electrolytes Total parenteral nutrition (TPN)
Cardiac
Cardiac care is required if the member is unable to maintain adequate circulation related
to mechanical or electrical dysfunction of the cardiovascular system. The patient must
meet each of the following requirements for admission or continued stay:
1. The patient requires frequent monitoring of tissue oxygenation (for example,
pulse oximetry) and continuous telemetry.
2. The patient requires management of hemodynamic instability, cardioversion
or valsalva maneuver, temporary pacemaker, or monitoring of a functional
permanent pacemaker, monitoring for drug toxicity, defibrillation, pulmonary
artery catheterization and arterial monitoring, and monitoring of electrolyte
imbalance.
3. The patient requires two or more of the following medications intravenously
to maintain cardiovascular integrity:
Anticoagulants Antianginal agents
Antiarrhythmics Antibiotics
Anticoagulants Antihypertensives
Alpha/beta-adrenoreceptor blocking
agents
Betablockers
Calcium Channel blockers Cardiac glycosides
Class mucarinic receptor antagonists Corticosteroids
Diuretics Inotropic agents
01/31/2007 Long - Term Acute Care
Medical Policy Manual
316

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Sodium channel blockers Tissue plasminogen activators
Thrombolytic enzymes Vasodilators
Vasopressors
Continued Stay Criteria
All of the following are required to be documented for review of a continued stay in
the LTAC hospital.
• Multidisciplinary team evaluation at least weekly.
• Evidence of participation in a rehabilitation therapy program.
• Continued daily on-site direction of a qualified physician.
• Continued skilled nursing care or supervision required.
• Continued need for acute level of care as evidenced by continuing to meet the
admission criteria category requirements.
Documentation Requirements for Continued Stay
Concurrent review for approval of additional days, must be received by the PA
department at least 48 hours before the last approved day, including:
Completed IHCP LTAC hospital pre-admission form available from the PA contractor by
contacting the Health Care Excel PA Department at (317) 347-4511 in the Indianapolis
local area or 1-800-457-4518.
• A summary of the current discharge plans.
• Documentation of family or friend participation in the discharge planning
process.
• A neurological assessment update, if appropriate.
• Documentation of the member’s cooperation, participation, or progress.
Note: For review purposes, the PA contractor can request additional or updated
information at any time.
Discharge Criteria
Continued length of stay will not be authorized without the physician consultant review
when any of the following conditions occur:
• Evidence in the patient record that the patient has achieved stated goals.
• Medical complications require readmission to inpatient acute facility.
• Multidisciplinary services are no longer needed.
01/31/2007 Long - Term Acute Care
Medical Policy Manual
317
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Long - Term Acute Care
Medical Policy Manual
318
• No additional improvement is anticipated.
• Patient’s progress towards goals has remained unchanged for seven days.
Billing Guidelines
Long-term acute care facilities must submit charges on a UB-92 claim form. The revenue
code 101 –All-inclusive room and board will be utilized for the PA process and should be
reflected on the UB-92 claim form by the billing provider.
The discharging hospital must enter the patient status code 63 in Field Locator (FL) 22 on
the UB-92 claim form. This indicates the status of the patient as of the ending service
date was “discharged” or “transferred to a long-term care hospital.”

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: MEDICAL SUPPLIES AND DURABLE MEDICAL
EQUIPMENT (DME) OVERVIEW
DESCRIPTION
This document is intended to serve as a general summary of the IHCP policies regarding
this service. More specific information may be found in the IHCP provider manual,
program notices, the Indiana Administrative Code (IAC) or other sources as appropriate.
See the “Rules, Citations, and Sources” section of this document for further information.
Medical supplies are described in the Indiana Administrative Code (IAC) 405 IAC 5-19-
1 as the following.
(1) Disposable items that are not reusable and must be replaced on a frequent basis;
(2) Used primarily and customarily to serve a medical purpose;
(3) Generally not useful to a person in the absence of an illness or injury; and
(4) Covered only for the treatment of a medical condition.
405 IAC 5-19-2 defines DME as equipment that can withstand repeated use, is primarily
and customarily used to serve a medical purpose, and generally is not useful to a recipient
in the absence of illness or injury.
Coverage of specific medical supplies and DME will be addressed in individual fact
sheets.
MEDICAL SUPPLIES
Coverage Criteria
Medical supplies are items that are generally disposable and must be replaced on a
frequent basis. Medical supplies that are reimbursable by the IHCP include, but are not
limited to the following supplies.
• Antiseptics and solutions
• Bandages and dressing supplies
• Gauze
• Catheters
01/31/2007 Medical Supplies and Durable Medical Equipment (DME) Overview
Medical Policy Manual
319
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• Incontinence supplies
• Irrigation supplies
• Diabetic supplies
• Ostomy supplies
• Respiratory supplies
• Tracheotomy supplies
• Needles and syringes
The IHCP does not cover the following supplies.
• Sanitary napkins
• Cosmetics
• Dentifrice items
• Tissue
• Items generally used for personal hygiene, including but not limited to, non-
ostomy deodorizing products, soap, disposable wipes, and shampoo
Prior Authorization
Prior authorization (PA) is not required for the reimbursement of medical supplies unless
requested by an out-of-state supplier.
Billing Information
• Reimbursement is not available for medical supplies dispensed in quantities greater
than a one month supply for each calendar month, except when packaged by the
manufacturer only in larger quantities or the recipient is a Medicare beneficiary and
Medicare allows reimbursement for a larger quantity.
The IHCP does not currently define a one month quantity limitation for individual
medical supplies. The physician must determine the appropriate one month quantity
for the prescribed supply based on the member’s medical needs.
The IHCP will accept crossover claims for diabetic testing supplies with dates of
service that span 90 days. Banner BR200449 lists the procedure codes that may be
billed with a span date of 90 days, as well as the billing instructions for these
services.
• The cost of all medical and non-medical supplies, which includes those items
generally required to assure adequate medical care and personal hygiene of patients,
is included in the per diem rate for long term care (LTC) facilities. LTC facilities
include skilled nursing facilities, intermediate care facilities for the mentally retarded
(ICFs/MR), and community residential facilities for the developmentally disabled
(CRFS/DD).
01/31/2007 Medical Supplies and Durable Medical Equipment (DME) Overview
Medical Policy Manual
320

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• Medical supplies that are included as part of reimbursement for a medical or surgical
procedure are not separately reimbursable to any party.
• All traditional Medicaid claims for medical supplies are reimbursed as purchase only
items. No modifier is required to be billed with the HCPCS code. HCPCS codes
A4254 – Replacement battery, any type, for use with medically necessary home
glucose monitor owed by patient, each, A4638 – Replacement battery for patient-
owned ear pulse generator, each, and A7017 – Nebulizer, durable, glass or
autoclavable plastic, bottle type, not used with oxygen, may be billed as rental items
for crossover claims only. The RR modifier must be billed with the HCPCS code for
these rental claims to be reimbursed.
DURABLE MEDICAL EQUIPMENT (DME)
Coverage Criteria
Durable medical equipment includes equipment that can withstand repeated use, is
primarily used to serve a medical purpose, and is not useful to the recipient in the absence
of illness or injury. All DME must be ordered in writing by a physician. The written
order must be kept on file by the physician and the rendering provider for audit purposes.
DME equipment is reimbursable by the IHCP within one of the following classifications
listed in Table 1.
Table 1 - DME Classification Codes
DME
Classification
Number
DME Classification Name
1 Capped rental items
2 Inexpensive or other routinely purchased items
3 Items requiring frequent or substantial servicing
4 Customized items
5 Prosthetic and orthotic devices
6 Oxygen and oxygen equipment
Capped Rental Items
Certain procedure codes are limited to 15 months of continuous rental. Continuous rental
is defined as rental without interruption for a period of more than 60 days. A change in
provider does not cause an interruption in the rental period. Claims submitted for capped
rental items are reimbursed in the following manner.
• Claims are paid until the number of rental payments made to date reaches the
capped rental number of 15 months.
01/31/2007 Medical Supplies and Durable Medical Equipment (DME) Overview
Medical Policy Manual
321
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• Claims submitted for rental in excess of 15 total months will be denied for audit
6080, DME rentals limited to 15 months.
• Requests for approval of DME capped rental items are evaluated for
documentation of long term need. In long term situations, a decision may be
made to purchase the item.
The use of a piece of equipment during a rental period may be interrupted; however, if
the patient resumes use of the equipment within 60 days of the last payment, the original
15-month period remains active. If the interruption period exceeds the 60 day period, and
the interruption reasons are justified, a new PA request must be submitted to begin a new
15-month rental period. The supplier must document the reason for the greater-than-60-
day break in the rental period on the Indiana Prior Review and Authorization Request
form. Justification for a break in the rental period more than 60 days may include the
following.
• Change in medical necessity
• Hospitalization
• Nursing facility stay
Unless a new PA is received requesting a new rental period, the original 15-month period
remains active. If a member becomes inactive for a period of more than 60 days, a new
PA is required to resume services.
Capped rental items are also subject to replacement or servicing when certain criteria are
met. Replacement of capped rental items is not authorized more often than once every
five years per member, unless there is a change in the member’s medical needs,
documented in writing, significant enough to warrant a different type of equipment.
During the 15-month capped rental period, the supplier must supply and service the item
at no additional charge to the IHCP or the member. However, subject to prior approval
parameters, reimbursement for repair not covered by warranty is not reimbursed more
frequently than six months after the 15th month and every six months thereafter, for as
long as the equipment is medically necessary.
Capped rental items are subject to prior authorization. Providers should refer to Chapter
8, Section 3 of the Provider Manual to view the list of the capped rental codes.
Inexpensive or other routinely purchased items
Inexpensive or routinely purchased DME is defined as equipment whose purchase price
does not exceed $150, or equipment that is acquired at least 75% of the time by purchase.
Equipment in this category may be purchased or rented. The decision to rent or purchase
DME is based on the least expensive option available for the anticipated period of need.
DME items purchased with IHCP funds become the property of the OMPP.
Purchases are reimbursed in a lump sum, minus any previous rental payments. If the
equipment is rented, the IHCP will allow monthly rental payments until the rental price
01/31/2007 Medical Supplies and Durable Medical Equipment (DME) Overview
Medical Policy Manual
322
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
equals the purchase price. Any claims billed for rental payments exceeding the purchase
price will deny for audit 6065, DME total rental amount not to exceed fee for purchase.
Items requiring frequent or substantial servicing
For items requiring frequent or substantial servicing, the IHCP reimburses providers for
rental payments only, as long as the equipment is deemed medically necessary. Claims
for the purchase of these items are denied. As noted in 405 IAC 5-19-4 repair of rental
items is the responsibility of the rental provider. Providers should refer to Chapter 8,
Section 3 of the Provider Manual to view the list of items considered to require frequent
or substantial servicing.
Customized Items
Custom equipment is defined as equipment uniquely constructed or substantially
modified to meet the specific needs of an individual patient according to the description
and orders of the member’s treating physician. Due to the unique aspects, these items
cannot be grouped with similar items for purposes of payment.
Suppliers must submit documentation of the costs of the item, including the cost of labor
and types of materials used in customizing the item. A materials and labor itemization
and a manufacturer’s cost invoice must be attached to the claim when submitted for
payment. Each item on the invoice is reviewed when calculating the reimbursement
amount for all customized items.
Customized items must be billed using HCPCS code E1399 for the materials and E1340
for the labor. HCPCS code E1399 for customized equipment requires prior authorization.
The following are examples of items that are not considered customized items.
• Items that are individually constructed, but that have standard costs and charges,
and that can be billed using a national HCPCS code.
• A wheelchair that is ordered in individual parts from one or multiple
manufactures and assembled by the supplier.
• A wheelchair that is ordered from a manufacturer that makes available special
features, modifications, or components cannot be considered a customized
wheelchair.
Prosthetic and Orthotic Devices
All prosthetic and orthotic devices billed under the HCPCS L codes are paid in a lump
sum amount, and may not be rented. Prosthetic and orthotic devices billed with HCPCS
L codes do not require prior authorization.
01/31/2007 Medical Supplies and Durable Medical Equipment (DME) Overview
Medical Policy Manual
323
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Oxygen and Oxygen Equipment
The IHCP reimburses liquid and gaseous oxygen systems as rental only items, subject to
prior authorization. Reimbursement for oxygen contents is included in the reimbursement
of the oxygen system, and is not separately reimbursable for rented systems. Oxygen
contents are separately reimbursable when a third party has purchased an oxygen system,
or the IHCP or third party has rented or purchased a portable oxygen system.
Accessories, including but not limited to, cannulas, masks, and tubing, are also included
in the allowance for rented systems and are not separately reimbursable unless used with
a purchased system. See the Oxygen and Oxygen Equipment Fact Sheet for further
details.
Manually Priced Items
Reimbursement for DME services, equipment, and supplies that are billed with a
nonspecific HCPCS code with a description such as unspecified, unclassified, or
miscellaneous, is based on manual pricing. Examples of manually priced HCPCS codes
are A4649, surgical supply–miscellaneous and E1399, durable medical equipment-
miscellaneous.
Payment for manually priced HCPCS codes, related to DME services, is specific to the
item being billed. Providers must submit documentation supporting the cost of the item,
including a listing of all materials. Reimbursement is determined using the following
guidelines.
• If the provider submits an itemized sale invoice from the manufacturer listing all
materials or supplies purchased and showing the price paid for individual items,
the claim is reimbursed at the billed amount, up to 30 percent above the invoice
amount. The IHCP will not accept a manufacturer’s price list as proof of purchase
price for this level of reimbursement.
• If a provider submits retail price lists from the manufacturer, the claim is
reimbursed at 90 percent of the price on the manufacturer’s retail price list, but
will not exceed the billed amount.
• If the provider submits a copy of the provider’s own retail price list or an invoice
from their own company, which indicates the price that a provider charges the
general public for products or supplies, the claim is reimbursed at 90 percent of
the invoice or price list, not to exceed the billed amount.
A provider must not bill more than their usual and customary charge for any item. When
requesting prior authorization for miscellaneous services, an itemized list of materials
must be included in the PA request. Any item that is identified under a miscellaneous
code on the PA form must have a specific number of units identified for billing purposes
and claim adjudication.
01/31/2007 Medical Supplies and Durable Medical Equipment (DME) Overview
Medical Policy Manual
324
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Repair of DME
The IHCP reimburses for labor costs associated with the repair and servicing of DME.
Repair of DME must be billed using HCPCS code E1340, Repair or non-routine service
for DME requiring the skill of a technician, labor component, per 15 minutes. Repairs of
prosthetic and orthotic devices, hearing aids, and augmentative communication devices
should be billed using the appropriate repair codes for those devices.
The IHCP will not pay for labor for the repair of DME under the following
circumstances.
• IHCP does not pay for repair of equipment still under warranty
• No payment is authorized for repair necessitated by member misuse or abuse,
whether intentional or unintentional
• Repairs for rental equipment are the responsibility of the rental provider
• Payment for maintenance charges of properly functioning equipment is not
covered
• Repair costs for DME included in an long-term care (LTC) facility’s per diem rate
is also included in the per diem rate
In addition, the IHCP will only reimburse E1340 for tasks considered to be labor or non-
routine servicing of DME. The IHCP will not reimburse E1340 for the following types
of services.
• Evaluation of a member for a wheelchair or seating system
• Patient education in the use and care of DME
• Measurement of recipient for DME
• Initial assembly of DME
Replacement of DME
The IHCP will reimburse for the replacement of medically necessary DME under the
following circumstances.
Loss of the item due to theft or fire
• If the equipment being replaced does not require PA and does not have a limit
restriction, the provider may directly bill for the item. The provider should
maintain documentation in their records to support the reason for replacement.
This documentation would be subject to post payment review.
• If the item requires PA, the provider must submit a new PA request for the
item, including an explanation that the item was lost due to theft or fire. The
provider should maintain documentation in their records to support the reason
for replacement. This documentation would be subject to post payment
review.
• If the item has a limit restriction, whether or not the DME item requires PA,
the provider should submit a PA request for a replacement item with an
01/31/2007 Medical Supplies and Durable Medical Equipment (DME) Overview
Medical Policy Manual
325
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
explanation that the original item was lost due to fire or theft. The provider
should maintain documentation in their records to support the reason for
replacement. This documentation would be subject to post payment review.
Irreparable damage or wear
• Replacement of large DME items is not authorized more than once every five
years per member. More frequent replacement is allowed only if there is a
change in the member’s medical needs.
Change in the member’s condition that requires a change in equipment.
• These changes must be documented by the member’s physician, and a request
must be sent to the prior authorization department demonstrating a significant
change warranting new equipment.
Modifications to DME
The IHCP may make additional payment for modifications to DME. Examples of some
modifications are attachments to convert a wheelchair to a one-arm drive, or the addition
of brake extensions, wheelchair hand rims, or anti-tipping devices to a wheelchair after
the initial assembly.
Routine Maintenance
Payment for routine maintenance of properly functioning equipment is not covered by the
IHCP. Routine maintenance includes services such as testing, cleaning, regulating, and
checking of equipment that does not require the skill of a technician.
DME in Long Term Care (LTC) Facilities
DME utilized for the usual care and treatment of members in LTC facilities are
reimbursed by the IHCP in the facility’s per diem rate and may not be billed to Medicaid
by the facility, pharmacy, or other provider. Repair costs necessary to maintain
equipment reimbursed in the facility’s per diem rate is also the responsibility of the
facility. LTC facilities include skilled nursing facilities, intermediate care facilities for
the mentally retarded (ICFs/MR), and community residential facilities for the
developmentally disabled (CRFS/DD).
Nonstandard or custom/special equipment and their associated repair costs may be billed
separately to the IHCP for LTC facility members, subject to prior authorization. PA
requests for separate reimbursement of this DME for LTC facility members will be
considered on a case-by-case basis. Providers should refer to BR200307 to view specific
information regarding the prior authorization criteria for separate reimbursement of
custom wheelchairs for LTC facility members.
Package C
Package C coverage is available to certain members 19 years of age and younger.
Coverage of medical supplies and DME is included under Package C with a maximum
benefit of $2,000 per calendar year, or $5,000 per lifetime.
01/31/2007 Medical Supplies and Durable Medical Equipment (DME) Overview
Medical Policy Manual
326
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
PRIOR AUTHORIZATION
PA is required for capped rental items, selected inexpensive or other routinely purchased
items, and oxygen equipment. Providers should refer to Chapter 8, Section 3 of the IHCP
Provider Manual for a list of items that do not require PA. PA requests for DME shall be
reviewed on a case-by-case basis by the contractor, using all of the following criteria.
1. The item must be medically reasonable and necessary, as defined at 405 IAC 5-2-17,
for the treatment of an illness or injury or to improve the functioning of a body
member.
2. The item must be adequate for the medical need; however, items with unnecessary
convenience or luxury features will not be authorized.
3. The anticipated period of need, plus the cost of the item will be considered in
determining whether the item shall be rented or purchased. This decision shall be
made by the contractor based on the least expensive option available to meet the
recipient’s needs.
The following items are examples of DME that require prior authorization.
• Hospital beds
• Wheelchairs
• Ventilators
• Heated and non-heated humidifiers
• Oxygen and oxygen equipment
• Patient lifts
• Standers
• Power seating systems
• Cranial orthosis molding helmet
• Bone growth stimulators
• Enteral nutrition
Certain DME also requires a certificate of medical necessity (CMN) to be submitted with
the prior authorization request. DME that require a CMN are listed below.
• Augmentative Communication Devices
• Oxygen Equipment
• Enteral Nutrition and Parenteral and Enteral Nutrition Pumps
• Hearing Aids
• Hospital Beds
• Motorized and Nonmotorized Wheelchairs
• Negative Pressure Wound Therapy Devices
• Standers
• TENS Units
01/31/2007 Medical Supplies and Durable Medical Equipment (DME) Overview
Medical Policy Manual
327

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Out-of-State DME Providers
405 IAC 5-5-3 states that in order to be treated as an in-state provider for purposes of the
prior authorization rule, any out-of-state supplier of medical equipment must comply with
the following criteria.
a. Maintain an Indiana business office, staffed during regular business hours, with
telephone service.
b. Provide service, maintenance, and replacements for Indiana Medicaid recipients
whose equipment has malfunctioned.
c. Qualify with the Indiana Secretary of State as a foreign corporation.
Out-of-state providers who do not meet these requirements must obtain PA prior to
providing any DME of medical supplies. DME and medical supplies may not be
obtained by out-of-state or in-state providers without prior authorization by use of an
emergency indicator. If DME or medical supplies are needed in an emergency situation,
PA may be obtained by telephone.
Telephone PA
PA may be obtained by telephone for DME services under the following circumstances.
a. For medically reasonable and necessary supplies to facilitate discharge from or
prevent admission to a general hospital
b. For repair of DME when the equipment repair is necessary for life support or safe
mobility of the patient.
The DME provider must subsequently submit a properly completed prior authorization
request form signed by the ordering physician in order for the service to be approved for
reimbursement. Refer to 405 IAC 5-3-2 (b) for further instructions.
DME PROVIDER CODE SET
On November 1, 2004, the IHCP implemented the provider code set for DME providers.
The DME provider code set identifies procedure codes that are appropriate for
reimbursement by DME providers. Claims submitted by DME providers for codes not
listed on the DME code set will deny edit 1012 – Rendering provider specialty not
eligible to render procedure code. Providers must ensure that they are enrolled under the
correct provider specialty with the IHCP.
The DME code set is available on the IHCP Web site at www.indianamedicaid.com.
This code set is subject to change and will be updated accordingly based on annual and
quarterly HCPCS updates and policy changes. Providers should monitor the Web site for
changes to the DME provider code set.
01/31/2007 Medical Supplies and Durable Medical Equipment (DME) Overview
Medical Policy Manual
328

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MANAGED CARE
Primary Care Case Management (PCCM) services are subject to the same policies and
restrictions as Traditional Medicaid services. Questions regarding coverage of services in
Risk Based Managed Care (RBMC) should be directed to the appropriate Managed Care
Organization (MCO).
RELATED MEDICAL TOPICS:
Medical Supplies and Equipment - Automatic External Defibrillators
Medical Supplies and Equipment - Beds
Medical Supplies and Equipment - Gloves
Medical Supplies and Equipment - Implantable Infusion Pumps
Medical Supplies and Equipment - Incontinence Supplies
Medical Supplies and Equipment - Left Ventricular Assist Device (LVAD)
Medical Supplies and Equipment - Monitoring Devices
Medical Supplies and Equipment - Neurocybernetic Prosthesis (NCP)
Medical Supplies and Equipment - Negative Pressure Wound Therapy
Medical Supplies and Equipment - Non-Invasive Respiratory Assist Devices
Medical Supplies and Equipment - Patient-Activated Event Recorder - Implantable Loop
Recorder (ILR)
Medical Supplies and Equipment - Phrenic Nerve Stimulator
Medical Supplies and Equipment - Power Wheelchairs
Medical Supplies and Equipment - Programmable Hearing Aids
Medical Supplies and Equipment - Prothrombin Time
Medical Supplies and Equipment - Standers
Medical Supplies and Equipment - Standing Wheelchair
Medical Supplies and Equipment - ThAIRapy Vest
RULES, CITATIONS, AND SOURCES:
Indiana Administrative Code 405 IAC 5-19-1
Indiana Administrative Code 405 IAC 5-19-2
Indiana Administrative Code 405 IAC 5-19-3
Indiana Administrative Code 405 IAC 5-19-4
Indiana Administrative Code 405 IAC 5-19-5
Indiana Administrative Code 405 IAC 5-19-7
Indiana Health Coverage Program Provider Manual Chapter 8, Medical Supplies
Indiana Health Coverage Program Provider Manual Chapter 8, Oxygen and Durable
Medical Equipment
Indiana Health Coverage Program Provider Banner BR200449
Indiana Health Coverage Program Provider Banner BR200307
Indiana Health Coverage Program Website, DME Provider Code Sets
Indiana Health Coverage Program Website, DME Per Diem Table
01/31/2007 Medical Supplies and Durable Medical Equipment (DME) Overview
Medical Policy Manual
329

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Origination Date: 7/31/05
Revisions and Reviews Reason Date
405 IAC 5-19-1 Definition Medical Supplies and
Equipment
7/31/05
405 IAC 5-19-2 Definition DME 7/31/05
405 IAC 5-19-3 Reimbursement for DME 7/31/05
405 IAC 5-19-4
Repair of Purchased DME 7/31/05
405 IAC 5-19-5 Reimbursement for Replacement of
DME
7/31/05
405 IAC 5-19-7 Prior Authorization for DME 7/31/05
405 IAC 5-5-3 Out-of-State DME Services 7/31/05
405 IAC 5-3-2 Telephone Prior Authorization 7/31/05
IHCP Provider Manual
Chapter 8, Section III
Medical Supply and DME policy 7/31/05
Banner BR200449 Diabetic Test Strip Update 7/31/05
Banner BR200307 LTC Wheelchairs 7/31/05
APPLICABLE INDIANAAIM EDITS AND AUDITS:
6065 - DME total rental amount not to exceed fee for purchase
6080 - DME rentals limited to 15 months
6113 - DME limited to $2,000 per recipient per calendar year
6114 - DME limited to $5,000 per recipient per lifetime
2034 - Medical and non-medical supplies and routine DME included in the per diem
6250 - Oxygen maximum fee cut back to 1 unit/28 days
6252 - Oxygen/pound vs. oxygen maximum fee reimbursement
01/31/2007 Medical Supplies and Durable Medical Equipment (DME) Overview
Medical Policy Manual
330

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL SUPPLIES AND EQUIPMENT – (DME) OVERVIEW FACT SHEET
ADDENDUM A
Note: This addendum contains provider notifications that have been published since the
review of the (_add title of fact sheet_) Fact Sheet. The information in this addendum
will be incorporated into the fact sheet at the next review.
Provider Notification: BR200539 Publication Date: 09/27/2005
Subject: DME Billing Changes
Date Added to Manual: 10/31/2005
Text of Publication
This article notifies providers of new HCPCS code changes. Based on a recent analysis to
identify potential duplicates among temporary and permanent codes (such as, items
potentially billable under more than one HCPCS code), the OMPP has determined that
durable medical equipment (DME) codes E0953, E1000, and A4632 will no longer be
reimbursed effective November 11, 2005. Instead, the corresponding K codes (as listed
below) and their Medicare fee will be adopted.
Adopting the K codes with established Medicare fees will expedite crossover claims
processing. Direct questions about these HCPCS code changes to customer assistance at
(317) 655-3240 in the Indianapolis local area or 1-800-577-1278.
HCPCS
Code
Description Corresponding
HCPCS Code
Description Max Fee
E0953 Pneumatic Tire, EA K0067 Pneumatic Tire,
Any Size, EA
$34.77-NU
$3.41-RR
E1000 Tire, Pneumatic Caster K0074 Pneumatic Caster
Tire, Any Size
$36.00-NU
$3.96-RR
A4632 Repl Batt For External
Infusion Pump, Any
Type
K0601 Replacement Batt
For Ext Inf
Pump, Silver
Oxide, 1.5 V
$1.10
A4632 Repl Batt For External
Infusion Pump, Any
Type
K0602 Replacement Batt
For Ext Inf
Pump, Silver
Oxide, 3.0 V
$6.36
A4632 Repl Batt For External
Infusion Pump, Any
Type
K0603 Replacement Batt
For Ext Inf
Pump, Alkaline, 1.5
$0.57
01/31/2007 Medical Supplies and Durable Medical Equipment (DME) Overview
Medical Policy Manual
331

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Medical Supplies and Durable Medical Equipment (DME) Overview
Medical Policy Manual
332
HCPCS
Code
Description Corresponding
HCPCS Code
Description Max Fee
V
A4632 Repl Batt For External
Infusion Pump, Any
Type
K0604 Replacement Batt
For Ext Inf
Pump, Lithium, 3.6
V
$6.09
A4632 Repl Batt For External
Infusion Pump, Any
Type
K0605 Replacement Batt
For Ext Inf
Pump, Lithium, 4.5
V
$14.60

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: MEDICAL SUPPLIES AND EQUIPMENT,
AUTOMATIC EXTERNAL DEFIBRILLATORS (AED)
DESCRIPTION
The IHCP covers two types of automatic external defibrillators for individual use. The
first is a stand-alone model referred to as an AED. The Food and Drug Administration
(FDA) approved the AED for individual use in the home November 2003. An AED is
similar to a manual defibrillator except an AED detects and analyzes heart rhythms
automatically. A microprocessor inside the AED analyzes the victim’s heart rhythm
through adhesive electrodes and makes a determination on the need for defibrillation to
restore normal cardiac rhythm. The AED is programmed to recognize different
shockable heart rhythms such as ventricular fibrillation or ventricular tachycardia, and to
perform the defibrillation as quickly as possible. This automation reduces the amount of
training needed for effective use of the AED, and allows for people with minimal training
to perform defibrillation in emergency situations with little risk of additional injury to the
member.
To use an AED, members must have a live-in companion/spouse available to apply the
electrode pads at the time of a cardiac event and then follow the AED’s instructions. The
live-in companion or spouse needs some training with this device, as well as instruction
in CPR and access to the local Emergency Medical Service.
The second type of automatic external defibrillator is the wearable cardioverter
defibrillator (WCD). The FDA approved the WCD December 18, 2001. The equipment
is a vest-like or garment-like device worn under the member’s clothing that holds a
cardiac monitor, electrodes, and a small alarm module. The device works by monitoring
the member’s heart rhythm through the electrodes and treating abnormal heart rhythms
identified, such as ventricular fibrillation, by delivering the appropriate electrical shock to
the member. The WCD also records the member’s ECG. The alarm module alerts the
member of impending defibrillation. This allows the member to prevent unneeded
electrical shock by responding to the alarm and deactivating the device. Non-wearable
components include a battery charger, a computer modem, a modem cable, a computer
cable, WCDNET, and the diagnostic tester. WCDNET is a secure web-based data
storage and retrieval system that allows the physician to access the member's ECG data
stored on the Member Database.
01/31/2007 Medical Supplies and Equipment – Automatic External Defibrillators
Medical Policy Manual
333

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
RELATED MEDICAL TOPICS
Durable Medical Equipment
Hospital Outmember
Emergency Medicine – Cardiopulmonary Resuscitation (CPR)
RULES, CITATIONS, AND SOURCES
405 IAC 5-19-2
405 IAC 5-19-3
ORIGINATION, REVIEWS, AND REVISIONS
Origination Date: 4/30/04
Review or Revision Reason Date
Revision
ICD-9-CM Diagnosis Code
update, October 2005
01/27/05
APPLICABLE INDIANAAIM EDITS AND AUDITS:
6065 (K0606)
COVERAGE CRITERIA
The AED and the WCD are indicated for members who normally are candidates for an
implanted cardioverter defibrillator (ICD), but for whom an ICD is contraindicated, or
needs to be removed. These defibrillators are most often used for members who are
awaiting heart transplants, who have recently had a heart attack, or who have had their
ICD removed due to an ICD pocket infection. The average time of use is approximately
2-3 months, although some members awaiting transplant have used the device for over
one year.
Members who are not able to use the WCD vest due to obesity other medical conditions,
or who cannot tolerate wearing electrodes 24-hours a day, are able to utilize an AED
because the electrodes are only placed at the time of a cardiac event. Members with
limited mobility or who are confined to bed may also be unable to use the vest. The WCD
is an option for members do not have a spouse/live-in companion able and/or available to
use the device, or for members who must frequently be away from home.
01/31/2007 Medical Supplies and Equipment – Automatic External Defibrillators
Medical Policy Manual
334

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
The WCD has the capability to monitor and store ECG data as well as provide
defibrillation. The member must download the ECG data from the WCD to a computer
for their physician to access the information. The physician can obtain the data using
WCDNET, a secure web-based data storage and retrieval system. When the ECG
monitoring afforded by the WCD is not needed, or able to be utilized, the AED would be
an alternative to the WCD.
If a defibrillator is indicated, the decision to prescribe the AED vs. WCD should be the
physician’s, in consultation with the member. The option of either the AED or the WCD
is a clinical decision, influenced by the practical matters discussed above.
The IHCP covers AED and WCD and their accessories, with prior authorization, under
the following codes.
Table 1 – Wearable Cardioverter Defibrillator
HCPCS CODE DESCRIPTION
K0606 AED with integrated electrocardiogram analysis,
garment type
K0607 Replacement battery for AED, each
K0608 Replacement garment for use with AED, each
K0609 Replacement electrodes for use with AED, each
Table 2 – Automatic External Defibrillator
HCPCS CODE DESCRIPTION
E0617 Automatic External Defibrillator
K0607 Replacement battery for AED, each
K0609 Replacement electrodes for use with AED, each
PRIOR AUTHORIZATION CRITERIA FOR AUTOMATIC EXTERNAL
DEFIBRILLATORS (E0617) AND WEARABLE CARDIOVERTER
DEFIBRILLATORS (K0606)
The IHCP covers the AED (E0617) and the WCD (K0606) under the same PA criteria.
The AED or the WCD is covered for members in two circumstances below.
Members must meet either (1) BOTH criteria A and B; OR
(2) Criterion C.
A. The member has ONE of the following conditions (1-5)
1. A documented episode of cardiac arrest due to ventricular fibrillation, not due
to a transient or reversible cause ¹ (ICD-9 427.41, 427.42, 427.5);
2. A sustained, lasting 30 seconds or longer, ventricular tachyarrhythmia, either
spontaneous or induced during an electrophysiologic (EP) study, not
01/31/2007 Medical Supplies and Equipment – Automatic External Defibrillators
Medical Policy Manual
335

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
associated with acute myocardial infarction², and not due to a transient or
reversible cause (ICD-9 427.1);
3. Familial or inherited conditions with a high risk of life-threatening ventricular
tachyarrythmias such as long QT syndrome (ICD-9 426.82) or hypertrophic
cardiomyopathy (ICD-9 425.1);
4. Coronary artery disease with a documented prior myocardial infarction, (ICD-9
410.0-0-410.92) with a measured left ventricular ejection fraction² less than or
equal to 0.35, and inducible, sustained ventricular tachycardia (VT) or
ventricular fibrillation (VF) during an EP study. To meet this criterion BOTH
(a) AND (b) BELOW MUST OCCUR.
a) The myocardial infarction must have occurred more than 4 weeks
prior to the external defibrillator prescription; AND,
b) The EP test must have been performed more than 4 weeks after the
qualifying myocardial infarction.
5. Documented prior myocardial infarction (ICD-9 410.00-410.92) and a
measured left ventricular ejection fraction less than or equal to 0.30 and a
QRS duration of greater than 120 milliseconds. PATIENTS MUST NOT
HAVE:
a) New York Heart Association classification IV; OR
b) Cardiogenic shock or symptomatic hypotension while in a stable
baseline rhythm; OR
c) Had a coronary artery bypass graft (CABG) or percutaneous
transluminal coronary angioplasty (PTCA) within the past three
months; OR
d) Had an enzyme-positive MI within past month; OR
e) Clinical symptoms or findings that would make them a candidate for
coronary revascularization; OR
f) Irreversible brain damage from preexisting cerebral disease; OR
g) Any disease, other than cardiac disease (e.g., cancer, uremia, liver
failure), associated with a likelihood of survival less than one year.
B. Implantation surgery is contraindicated.
C. A previously implanted defibrillator now requires removal.
01/31/2007 Medical Supplies and Equipment – Automatic External Defibrillators
Medical Policy Manual
336

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Medical Supplies and Equipment – Automatic External Defibrillators
Medical Policy Manual
337
¹ Transient or reversible causes include conditions such as drug toxicity, severe hypoxia,
acidosis, hypocalcemia, hyperkalemia, systemic infections, and myocarditis (not all-
inclusive).
² Myocardial infarctions must be documented by elevated cardiac enzymes or Q-waves
on an electrocardiogram. Ejection fractions must be measured by angiography,
radionuclide scanning or electrocardiography.
Claims for defibrillators for other indications will be denied as not medically necessary.
The IHCP will not purchase both an AED and WCD for one member, nor rent an AED
and a WCD simultaneously for one member.
PRIOR AUTHORIZATION CRITERIA FOR ACCESSORIES K0607 – K0609
Prior authorization criteria for the accessories is based on the estimated average life
expectancies of the accessories. The accessories replacement batteries, K0607, and
replacement electrodes, K0609, are used for both the AED (E0617) and WCD (K0606).
K0607 – Replacement Battery
1. The member must currently be renting or have purchased an AED (E0617) or WCD
(K0606 with integrated electrocardiogram analysis, garment type).
2. The battery being replaced must be at least 11 months old or completely discharged.
K0608 – Replacement Garment (only for WCD)
1. The member must currently be renting or have purchased a WCD with integrated
electrocardiogram analysis, garment type, K0606.
2. The garment must be damaged or worn beyond repair and have been in use at least 5
months.
K0609 – Replacement Electrodes
1. The member must currently be renting or have purchased an AED (E0617) or the WCD
with integrated electrocardiogram analysis, garment type (K0606).
2. The electrodes being replaced must have been used for at least 22 months, or it must
be proven that the equipment is broken or damaged beyond repair.

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: MEDICAL SUPPLIES AND EQUIPMENT—HOSPITAL
BEDS/SPECIALTY BEDS
Description:
Indiana Health Coverage Programs will provide coverage for hospital beds and specialty
beds when they are medically necessary in a non-institutional setting, there is a written
physician's order, and they have been prior authorized. The purpose for the policy is to
decrease variation in utilization management by providing a guide for determining the
medical necessity of hospital beds and specialty beds.
• For purposes of the Indiana Health Coverage Programs, “hospital beds” refers to
adjustable heights, semi-electric, and total electric beds. “Specialty beds” refers to
enclosed beds, or pediatric hospital beds.
Definition of Terms:
Hospital beds:
A fixed-height hospital bed is one with manual head and leg elevation adjustments.
A variable-height hospital bed is one that has manual adjustment elevation for the head,
height, and legs.
A semi-electric hospital bed is one that has a manual height adjustment with electric
elevation for the leg and head adjustments.
A total-electric hospital bed is one that has electric adjustments for height, head, and leg
elevation.
Specialty beds:
An enclosed bed is one that is one piece of equipment, e.g., bed and mesh canopy or a
bed with padded walls and a mattress especially designed for patients with traumatic
brain injury (TBI).
01/31/2007 Medical Supplies and Equipment – Beds
Medical Policy Manual
338

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
A pediatric hospital bed has higher side rails that are close together to prevent injury from
falling through the rails and usually has a protective covering.
MEDICAL TOPICS CROSS-REFERENCES:
Home Health Services
Medical Supplies and Equipment
Physical Rehabilitation Services
RULES, CITATIONS, AND SOURCES:
405 IAC 5-19-2
Indiana Health Coverage Programs Provider Manual
Initial Policy Issues
Effective Date Implementation
Date
Retroactive Date
405 IAC 5-19-2 Medical Supplies
and Equipment
8/25/97
Provider
Bulletin 200026
Hospital Beds
and Specialty
Beds
8/01/00
8/10/00
Revisions:
405 IAC 5-19 Medical Supplies
and Equipment
10/27/99 10/27/99
APPLICABLE INDIANAAIM EDITS AND AUDITS:
6080
01/31/2007 Medical Supplies and Equipment – Beds
Medical Policy Manual
339
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
COVERAGE CRITERIA:
A hospital bed is considered medically necessary if one or more of the following
conditions are met:
• Positioning of the body that is ordered by the physician, in ways not feasible with an
ordinary bed due to a medical condition, which is expected to last at least one month.
Elevation of the head and upper body greater than 30 degrees.
• Positioning of the body that is ordered by the physician in ways to alleviate pain that
are not possible in an ordinary bed.
• Positioning of the body ordered by the physician that requires head elevation greater
than 30 degrees most of the time related to medical condition, such as congestive
heart failure, chronic pulmonary disease, or problems with aspiration. Pillows or
wedges must have been tried and failed.
• A physician orders traction that requires traction equipment, which can only be
attached to a hospital bed.
A variable height hospital bed is covered if, in addition to meeting one (1) or more of the
criteria for a hospital bed, the following condition is met:
• The physician orders a bed height different than a fixed-height hospital bed to
accommodate transfers to a chair, wheelchair, or standing position.
A semi-electric hospital bed is covered if, in addition to meeting one (1) or more of the
criteria for a hospital bed, the following condition is met:
• The physician orders frequent changes in body positioning and/or the patient has an
immediate need for a change in body position.
An enclosed bed or cubicle bed is considered medically necessary when all the following
criteria are met:
a) The patient has an appropriate diagnosis which could include, but is not limited to,
the following:
• 343.8 Other specified infantile cerebral palsy
• 343.9 Infantile cerebral palsy
• 318.1 Severe mental retardation
• 318.2 Profound mental retardation
• 780.3 Convulsions
• 345.1 Generalized convulsive epilepsy
• 345.3 Grand mal status
• 330.0 Leukodystrophy
01/31/2007 Medical Supplies and Equipment – Beds
Medical Policy Manual
340
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• 331.1 Picks disease
• 331.4 Obstructive hydrocephalus
• 348.1 Anoxic brain damage
• 854 Intracranial injury of other and unspecified nature
b) Documentation of medical necessity must include at least one (1) of the following:
• Daily seizure activity
• Uncontrolled perpetual movement related to diagnosis
• Self-injurious behavior, such as uncontrolled head banging activity
c) Documentation of safety factors tried and failed including, but not limited to, the
following:
• Chest restraints
• Side rails
• A mattress on the floor
• Protective helmet
d) Supporting documentation must include secondary diagnoses and pertinent history:
• History of injuries or falls
• High risk for fractures due to osteoporosis
• At risk for hemorrhage due to thrombocytopenia
• Frequent upper respiratory infections and or other complications related to
aspiration
• Respiratory complications related to positioning. Requires elevation of the head
and upper body greater than 30 degrees
• Requires frequent positional changes
e) A signed physician’s order for enclosed bed or cubicle bed.
f) A Medical Clearance Form completed and signed by the physician.
g) Verification that the primary caregiver is willing and able to clean and maintain the
mesh canopy per the manufacturer recommendations. Indiana Health Coverage
Programs will not pay for laundering of the mesh canopy.
Pediatric hospital beds are considered medically necessary when all the following criteria
are met:
a) Has a medically necessary diagnosis. Diagnoses could include, but are not limited to,
the following:
• V55.0 Tracheostomy
01/31/2007 Medical Supplies and Equipment – Beds
Medical Policy Manual
341
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• V55.1 Gastrostomy
• 428.0 Heart Failure
• 511.8 Other specified forms of effusion, except tuberculosis
• 511.9 Unspecified pleural effusion
• 518.81 Respiratory failure
• 518.82 Other pulmonary insufficiency, not elsewhere classified
• 518.89 Other disease of the lung, not elsewhere classified
• 519.1 Other disease of trachea and bronchus, not elsewhere classified
• 769.0 Respiratory distress syndrome
• 770.8 Other respiratory problems after birth
• 786.9 Other
b) Mandatory criteria for prior authorization (1-4)
1. A physician's order for a multi-positional bed related to frequent positioning
changes that are required.
2. Elevation of upper body and head greater than 30 degrees is required.
3. Written documentation of why a standard crib is not appropriate and what
alternative methods have been tried and failed is required.
4. A Medical Clearance Form completed and signed by the physician.
Recommended criteria (patient must meet at least one of the following criteria)
5. Documentation that indicates there is a risk for aspiration pneumonitis and/or
gastric reflux related to disease processes is present.
6. Documentation of a history of aspiration pneumonitis is present.
01/31/2007 Medical Supplies and Equipment – Beds
Medical Policy Manual
342

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
HCPCS Codes:
Fixed height beds
E0250 - Hospital bed, fixed height, with any type side rails, with mattress.
E0251 - Hospital bed, fixed height, with any type side rails, without mattress.
E0290 - Hospital bed, fixed height, without side rails, with mattress.
E0291 - Hospital bed, fixed height, without side rails, without mattress.
Variable height
• E0255 - Hospital bed variable height, (hi-lo), with any type side rails, with mattress.
E0256 - Hospital bed variable height, (hi-lo), with any type side rails, without mattress.
E0292 - Hospital bed variable height, (hi-lo), without side rails, with mattress.
E0293 - Hospital bed variable height, (hi-lo), without side rails, without mattress.
Semi-electric beds
E0260 - Hospital bed, semi- electric (head and foot adjustments), with any type side
rails, with mattress.
E0261 - Hospital bed, semi- electric (head and foot adjustments), with any type side
rails, without mattress.
E0294 - Hospital bed, semi- electric (head and foot adjustments), without side rails, with
mattress.
E0295 - Hospital bed, semi- electric (head and foot adjustments), without side rails,
without mattress.
Total electric beds
E0265 - Hospital bed, total electric (head, foot and height adjustments), with any type
side rails, with mattress.
E0266 - Hospital bed, total electric (head, foot and height adjustments), with any type
side rails, without mattress.
E0296 - Hospital bed, total electric (head, foot and height adjustments), without side
rails, with mattress.
E0297 - Hospital bed, total electric (head, foot and height adjustments), without side
rails, without mattress.
Specialty beds
Z5101 Enclosed bed/with mattress/all accessories included
Z5102 Cubicle bed/with mattress/all accessories included
Z5103 Pediatric hospital bed/with mattress/all accessories included
01/31/2007 Medical Supplies and Equipment – Beds
Medical Policy Manual
343

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Medical Supplies and Equipment – Beds
Medical Policy Manual
344
Trapeze bars and other bed accessories
E0271 - Mattress, innerspring
E0272 - Mattress, foam rubber
E0273 - Bed board
E0274 - Over-bed table
E0280 - Bed cradle, any type
E0305 - Bedside rails, half length
E0310 - Bedside rails full length
E0315 - Bed accessories: boards or tables, any type
E0910 - Trapeze bars, patient helper, attached to bed, with grab bar
E0940 - Trapeze bar, free standing, Complete with grab bar

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: MEDICAL SUPPLIES AND EQUIPMENT—GLOVES
DESCRIPTION:
The Indiana Health Coverage Programs (IHCP) provides coverage for sterile and non-
sterile gloves for use in the home by the member, family, or other non-paid caregiver.
Universal precautions are required to protect members and caregivers from exposure to
blood, body fluids, and other potentially infectious materials. Gloves, whether sterile or
non-sterile, are used as personal protective equipment (PPE), to prevent exposure. The
purpose of PPE is to prevent blood and body fluids from reaching the skin, mucous
membranes, or personal clothing. The type of glove utilized should be based on the task
to be performed, conditions present, duration of use, and the potential hazards identified.
The two main indications for the use of gloves include the following.
• To protect hands from contamination with organic matter and microorganisms
• To reduce the risks of transmission of microorganisms to both the member and
caregiver
Gloves should be worn as single use items and discarded after each care activity to
prevent transmission of microorganisms to other sites. Gloves should be worn for contact
with sterile sites, non-intact skin, mucous membranes, invasive procedures, and all
activities carrying a risk of exposure to blood, body fluids, secretions, and excretions. In
addition, they should be worn when handling contaminated instruments. The decision to
wear sterile or non-sterile gloves should be based on contact with susceptible sites or
clinical devices. Gloves should fit properly to ensure that all exposed skin is covered.
The user should inspect gloves to ensure they are not worn or torn and free of holes that
could lead to exposure.
This document is intended to serve as a general summary of the Indiana Health Coverage
Programs’ (IHCP) policies regarding this service. More specific information may be
found in the IHCP Provider Manual, program notices, the Indiana Administrative Code
(IAC) or other sources as appropriate. See the “Rules, Citations, and Sources” section of
this document for further information.
01/31/2007 Medical Supplies and Equipment – Gloves
Medical Policy Manual
345
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
COVERAGE CRITERIA
Gloves must be ordered in writing by a physician and be used only for administration of
medical treatment. Documentation of medical need will be required for all gloves, sterile
and non-sterile. The supplier should maintain a signed physician’s order in the patient
record with a start and stop date, frequency of treatment, and type of treatment for which
gloves will be used.
Documentation should indicate the reason the gloves have been ordered by the physician.
The physician order must be renewed at least every six months to ensure ongoing need
for gloves. The order should reflect any changes in the plan of care in the home
treatment setting. Providers must maintain records of the quantities supplied. If these
supplies are delivered or mailed, a record showing proof of delivery must be maintained.
Medical necessity for non-sterile gloves includes, but is not limited to, the following.
• Bowel program requiring manual evacuation
• Ostomy care program
• Wound care program
• Exposure to blood and body fluids
Sterile gloves are covered when ordered by a physician for a medically necessary
treatment. Sterile gloves are not separately reimbursed when included in sterile
procedure kits. Such kits include, but are not limited to the following.
• Catheter insertion kits
• Suture removal kits
Reasons for non-coverage
Sterile and non-sterile gloves would be non-covered for the following indications.
• If used in the home by a paid caregiver
• Not used for a medically necessary treatment
• For members who reside in a nursing facility, gloves are included in the per diem
reimbursement
• For End-Stage Renal Disease (ESRD)/dialysis services, gloves are included in the
composite reimbursement
PRIOR AUTHORIZATION
Prior Authorization (PA) is not required for sterile or non-sterile gloves.
01/31/2007 Medical Supplies and Equipment – Gloves
Medical Policy Manual
346

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MANAGED CARE
For members enrolled in a Risk-Based Managed Care (RBMC), providers must contact
the member’s managed care organization (MCO) for more specific guidelines. Refer to
the IHCP Provider Manual, Chapter 1, for detailed information about the fee-for-service
(FFS), Primary Care Case Management (PCCM), and RBMC delivery systems.
IHCP members enrolled in Medicaid Select PCCM receive the same benefit coverage and
are subject to the same limitations as traditional Medicaid FFS. Refer to the Medicaid
Select Manual for Primary Care Providers and Office Staff for further information.
BILLING REQUIREMENTS
The following Healthcare Common Procedure Coding System (HCPCS) codes are used
to report the use of sterile and non-sterile gloves.
A4927, Gloves, non-sterile, per 100
A4930, Gloves, sterile, per pair
In accordance with the provider agreement and the regulations governing this program,
providers may not bill the IHCP any amount that exceeds their usual and customary
charge to the general public.
________________________________________________________________________
RELATED MEDICAL TOPICS
Home Health Services
Hospital Outpatient
Medical Supplies and Equipment
Nursing Facilities
Pharmacy
ESRD/Dialysis Services
RULES, CITATIONS, AND SOURCES
405 IAC 5-2-17, Medically Reasonable and Necessary Service
405 IAC 5-19-1, Medical Supplies
405 IAC 5-29-1, Noncovered services
405 IAC 5-31-4, Per diem services
Indiana Health Coverage Programs Provider Banners
BR200138
BR200139
BR200322
01/31/2007 Medical Supplies and Equipment – Gloves
Medical Policy Manual
347

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Medical Supplies and Equipment – Gloves
Medical Policy Manual
348
Indiana Health Coverage Programs Provider Bulletins
BT200031-New Local Codes for Sterile and Non-Sterile Gloves
BT200353-HIPAA-Mandated Elimination of Local Codes and Local Code
Modifiers
Origination Date: 09/08/2000
Revisions and Review Reason Date
Revision Changes in Coding Requirements 08/15/2003
Review Scheduled 01/31/2007
________________________________________________________________________
APPLICABLE INDIANAAIM EDITS AND AUDITS
6096-The CPT/HCPCS Code Billed Is Not Payable According to the PPS
Reimbursement Methodology
6260-Parenteral/Enteral Kit or Supplies Denied or Payment Reduced
6261-Parenteral/Enteral Kits (Maximum Fee) Versus Supplies
6916-Global-Home Uterine Monitoring (Tocolytic Therapy)
6917-Global-Home Uterine Monitoring (Tocolytic Therapy)
6652-Multiple Surgeries Must Be Billed on Same Claim
6768-Services Not Covered for Telemedicine

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: MEDICAL SUPPLIES AND EQUIPMENT- IMPLANTABLE
INFUSION PUMPS
DESCRIPTION:
Implantable infusion pumps (IIPs) are intended to provide long-term continuous or
intermittent infusion of a drug, and are designed to deliver therapeutic levels of a drug
directly to the target compartment or organ for prolonged periods of time. IIPs are
supplied as a complete system with all the necessary components; are implanted in a
subcutaneous pocket then connected to the intra-arterial, epidural, or intrathecal catheter;
and may be programmable or nonprogrammable. The pumps are labeled by the FDA for
specified drugs and routes of administration and are usually implanted in the abdominal
area.
Non-programmable pumps deliver a predetermined constant rate of infusate, and are
limited in their ability to provide bolus or modulated patterns of delivery. Programmable
pumps have a variable delivery rate that is adjusted by radio frequency control, and have
the ability to provide bolus or modulated patterns of delivery. The reservoir volume of an
implantable infusion pump varies between 10 and 50 cc. The reservoir can be refilled as
needed through the use of specially designed needles that are inserted transcutaneously
into a self-sealing rubber septum that covers the reservoir.
This document is intended to serve as a general summary of the Indiana Health Coverage
Programs’ (IHCP) policies regarding this service. More specific information may be
found in the IHCP Provider Manual, program notices, the Indiana Administrative Code
(IAC) or other sources as appropriate. See the “Rules, Citations, and Sources” section of
this document for further information.
COVERAGE CRITERIA
The IHCP provides coverage for services considered medically necessary and reasonable
and are provided by a doctor of medicine or doctor of osteopathy related to implantable
infusion pumps provided within the scope of practice of medicine as set forth in 405 IAC
5-25-1. Prior authorization is not required for IIP services.
Test dosing or placement of a temporary catheter for trial screening can be conducted in
the outpatient setting, but is usually administered in the hospital setting. In the case of
Baclofen®, one to two intrathecal (by lumbar puncture) or epidural boluses can produce
01/31/2007 Medical Supplies and Equipment – Implantable Infusion Pumps
Medical Policy Manual
349
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
effects over a 36-hour period and the patient will need monitoring for respiratory
depression, confusion, light-headedness, vision changes, nausea and vomiting,
somulence, urinary retention, hypotension, and coma. The same or similar technique is
utilized for other medications to be administered by implanted infusion pump.
Usually, hospitalization associated with the pump implantation is required for up to three
days, to monitor for a cerebral spinal fluid (CSF) leak, to protect the surgical wound, and
to monitor the pump. The pump is activated at the time of implantation. The pump
chambers allow for approximately 30-90 days of medication administration and are
refilled at intervals dependent on pump delivery rate, usually every 25-50 days.
Members may utilize their physician, hospital, or home health agency (if they are home
bound) to have their pumps reprogrammed and filled using the appropriate coding and
billing guidelines.
The medical record should fully document the refilling of the implantable pump or
reservoir and the appropriate time, dosage, medications, and solution. The medical record
should fully document the medical history of drugs used in the pump, including adverse
reactions. IHCP reimbursement may be made for drugs necessary for the effective use of
an implantable infusion pump as long as the drug being used with the pump is itself
reasonable and necessary for the patient’s treatment.
Prior Authorization is not required for the implantable device or services. Implantable
devices for intra-arterial, epidural, and intrathecal infusions are considered medically
appropriate based on the following criteria.
1. Chemotherapy for liver cancer
The implantable infusion pump is covered for intra-arterial infusion of 5-FUDR
(Floxuridine) for the treatment of liver cancer for members with primary
hepatocellular carcinoma or Duke’s Class D colorectal cancer, in whom the
metastases are limited to the liver, and the disease is unresectable or the member
refuses surgical excision of the tumor.
2. Anti-spasmodic drugs
To administer anti-spasmodic drugs intrathecally e.g., baclofen, to treat chronic
intractable spasticity in members who have proven unresponsive to less invasive
medical therapy as determined by the following criteria.
Documented history of at least a six-week trial period on oral anti-spasmodics that
has failed to adequately control the spasticity or has produced intolerable side
effects.
Prior to pump implantation, the patient must have had a favorable response to a
trial epidural or intrathecal dose of an anti-spasmodic drug.
3. Opioid drugs
To administer opioid drugs e.g., morphine, intrathecally for treatment of severe
chronic intractable non-malignant pain and malignant pain for members who have
01/31/2007 Medical Supplies and Equipment – Implantable Infusion Pumps
Medical Policy Manual
350
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
proven unresponsive to less invasive medical therapy, the medical record must reflect
the following criteria.
An appropriate ICD-9-CM diagnosis
The member, and/or the person responsible for the member, must be fully aware
of the risks and benefits of the surgery, including the mortality and morbidity
experience of the providers
A documented medical history of less invasive medical therapy that was tried and
failed
A preliminary trial of placebo and opioid intraspinal therapy with a temporary
catheter that demonstrated adequate acceptable pain relief, degree of side effects,
and patient acceptance
4. Other uses
Coverage for other uses of implanted infusion pumps may be approved if the
practitioner has documentation maintained in the member’s medical record that
indicates all of the following.
the drug is reasonable and necessary for the treatment of the individual
it is medically necessary that the drug be administered by an implanted infusion pump
the FDA approved labeling for the pump specifies the drug being administered and
the purpose for which it is administered is an indicated use for the IIP
NOTE: Reimbursement may also be available for drugs necessary for the effective use of
an implantable infusion pump as long as the drug being used with the pump is
itself reasonable and medically necessary for the member’s treatment.
Contraindications for use of implantable infusion pump
The implantation of an infusion pump is contraindicated in the following situations.
Known allergy or hypersensitivity to the drug being used (e.g., oral baclofen,
morphine, etc)
An infection affecting the area of implantation
Body size is insufficient to support the weight and bulk of the device
Other implanted programmable devices, since crosstalk between devices may
inadvertently change the prescribed settings
Table 1, on the following page, details the procedure codes, corresponding ambulatory
surgical center (ASC) groups for each code, and IHCP coverage for IIPs. Providers
should report one of the codes detailed in Table 2, on the following page, for the device.
01/31/2007 Medical Supplies and Equipment – Implantable Infusion Pumps
Medical Policy Manual
351

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 1 – Implantable Infusion Pumps Procedure Codes,
Corresponding ASC Groups, and IHCPCoverage
CPT
Code
Description
ASC
Group
IHCP
Coverage
36260 Insertion of implantable intra-arterial infusion pump 4 Yes
36261 Revision of implantable intra-arterial infusion pump 2 Yes
36262 Removal of implantable intra-arterial infusion pump 1 Yes
62350
I
mplantation, revision or repositioning of tunneled
i
ntrathecal or epidural catheter, for long-term medication
administration via an external pump or implantable
r
eservoir/infusion pump; without laminectomy
2 Yes
62351
Implantation, revision or repositioning of tunneled
intrathecal or epidural catheter, for long-term medication
administration via an external pump or implantable
reservoir/infusion pump; with laminectomy
2 Yes
62355
Removal of previously implanted intrathecal or epidural
catheter
3 Yes
62360
Implantation or replacement of device for intrathecal or
epidural drug infusion; subcutaneous reservoir
2 Yes
62361
Implantation or replacement of device for intrathecal or
epidural drug infusion; non-programmable pump
2 Yes
62362
Implantation or replacement of device for intrathecal or
epidural drug infusion; programmable pump, including
p
reparation of pump, with or without programming
2 Yes
62365
Removal of subcutaneous reservoir or pump, previously
implanted for intrathecal or epidural infusion
2 Yes
62367
E
lectronic analysis of programmable, implanted pump for
i
ntrathecal or epidu
r
al drug infusion (includes evaluation o
f
r
eservoir status, alarm status, drug prescription status);
w
ithout reprogramming
2 Yes
62368
E
lectronic analysis of programmable, implanted pump for
i
ntrathecal or epidural drug infusion (includes evaluation o
f
r
eservoir status, alarm status, drug prescription status); with
r
eprogramming
2 Yes
Table 2 – Implantable Infusion Pump Durable Medical Equipment Codes
HCPCS
Code
Description
Prior
Authorization
Coverage
E0782
Infusion pump, implantable, non-
programmable (includes all components, e.g.,
pump, catheter, connectors, etc.)
No Yes
E0783
Infusion pump system, implantable,
programmable (includes all components, e.g.,
pump, catheter, connectors, etc.)
No Yes
01/31/2007 Medical Supplies and Equipment – Implantable Infusion Pumps
Medical Policy Manual
352

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
PRIOR AUTHORIZATION
Prior authorization is not required for the implantation or the implantable infusion pump
device.
MANAGED CARE
For members enrolled in Risk Based Managed Care (RBMC), providers must contact the
member’s managed care organization (MCO) for more specific guidelines. Refer to the
Provider Manual, Chapter 1, for detailed information about the fee-for-service (FFS),
Primary Care Case Management (PCCM), and Risk Based Managed Care (RBMC)
delivery systems.
IHCP members enrolled in Medicaid Select PCCM receive the same benefit coverage,
and are subject to the same limitations as traditional Medicaid FFS. Refer to the
Medicaid Select Manual for Primary Medical Providers and Office Staff for further
information.
BILLING REQUIREMENTS
The IHCP will provide reimbursement for implantable pump services reported with
standard billing practices.
RELATED MEDICAL TOPICS
Hospital Inpatient
Hospital Outpatient
Medical Supplies and Equipment
Pharmacy Services
Surgery
RULES, CITATIONS, AND SOURCES:
405 IAC 5-17-1
405 IAC 5-19-1
405 IAC 5-25
Indiana Health Coverage Programs Provider Manual, Version 5.1, March, 2005
01/31/2007 Medical Supplies and Equipment – Implantable Infusion Pumps
Medical Policy Manual
353

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Medical Supplies and Equipment – Implantable Infusion Pumps
Medical Policy Manual
354
Origination Date: 12/31/2003
Initial Policy Issues
Date
405 IAC 5-19-1 Medical Supplies 10/27/1999
Medical Policy Committee Meeting Implantable Infusion Pumps 10/10/2001
Review Scheduled 10/31/2006
APPLICABLE INDIANAAIM EDITS AND AUDITS:
6002 – Any Two Anesthesiology Providers Same Procedure Requires Review
6003 – Manual Pricing for Split Care Billing
6034 – Global Surgery Payable at Reduced Amount When Components of Surgical Care Paid
6035 – Components of Surgical Care Not Payable When Global Surgery Paid
6037 – Only One Assistant Surgeon Allowed for Select Surgeries
6039 – Assistant Surgeon Not Payable When Co-Surgeon Paid
6040 – Co-Surgeon Not Payable When Assistant Surgeon Paid
6096 – The CPT/HCPCS Code Billed is Not Payable According to the PPS
Reimbursement Methodology
6152 – Surgery Payable at Reduced When Consultation Paid Days Before or After Surgery
6652 – Multiple Surgeries Must be Billed on Same Claim
6768 – Services not Covered for Telemedicine
6666 – Anesthesia Services Not Allowed by Provider Billing for Surgery

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: MEDICAL SUPPLIES AND EQUIPMENT—
INCONTINENCE SUPPLIES
DESCRIPTION
The Indiana Health Coverage Programs (IHCP) covers medically necessary disposable
and reusable incontinence supplies for members three years of age or older. Disposable
incontinence supplies include diapers, briefs, protective underwear, pull-ons, liners,
shields, and underpads. Reusable products include protective underwear, pull-ons, and
underpads.
This document is intended to serve as a general summary of the IHCP policies regarding
this service. More specific information may be found in the IHCP provider manual,
program notices, the Indiana Administrative Code (IAC) or other sources as appropriate.
See the “Rules, Citations, and Sources” section of this document for further information.
COVERAGE CRITERIA
The IHCP covers incontinence supplies for members three years of age and older based
on medical necessity. A member may receive a maximum of $1,950 of incontinence
supplies per rolling calendar year. Claims billed for purchases over $1,950 per rolling
calendar year will deny for audit 6085,
Incontinence supplies limited $1,950/rolling year.
Providers may review the amount of money applied toward a member’s incontinence
supply cap on the Web interChange.
Incontinence supplies must be ordered in writing by a physician. The clinical
documentation must include a diagnosis of incontinence. The incontinence diagnosis
must also be documented on the CMS-1500 claim form, with information about the
specific quantity and description of the supplies provided. The physician’s order must
be renewed annually at minimum.
Incontinence supplies may only be provided to members in one month increments. The
supplier must maintain documentation in the members medical record of the specific
quantity and description (such as brand, type, size, and so forth) of the supplies provided.
In addition to the signed physician’s order, the supplier must maintain documentation of
proof of delivery. Documentation must include the date of delivery, address of delivery,
01/31/2007 Medical Supplies and Equipment – Incontinence Supplies
Medical Policy Manual
355

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
and signature of the IHCP member, caregiver, or family member who received the
supplies.
Incontinence supplies are included in the per diem rate for long term care (LTC) facilities
and may not be billed to Medicaid by the facility, pharmacy, or other provider. LTC
facilities include skilled nursing facilities, intermediate care facilities for the mentally
retarded (ICFs/MR), and community residential facilities for the developmentally
disabled (CRFS/DD).
NONCOVERED ITEMS
According to 405 IAC 5-29-1(5) personal comfort or convenience items are noncovered
by the IHCP. Therefore, products such as peri-wash spray, wet-wipes or baby wipes, and
soap or cleansers used for incontinence care are non-covered by the IHCP.
PRIOR AUTHORIZATION
Prior authorization is not required for the reimbursement of incontinence supplies unless
supplied by an out-of-state provider.
CODING
The IHCP adopted HCPCS T codes (T4521-T4542) for the reimbursement of
incontinence supplies effective January 1, 2005. HCPCS codes A4521-A4538 (with the
exception of A4534) were deleted December 31, 2004, as part of the annual 2005 HCPCS
update. Table 1 below lists the covered T codes for incontinence supplies. Providers
should refer to the IHCP newsletter 200504 for a crosswalk of the incontinence supply
codes.
Table 1 – Incontinence Supply Codes
Code Description
T4521 Adult sized disposable incontinence product, brief/diaper, small each
T4522 Adult sized disposable incontinence product, brief/diaper, medium each
T4523 Adult sized disposable incontinence product, brief/diaper, large, each
T4524 Adult sized disposable incontinence product, brief/diaper, extra large, each
T4525 Adult sized disposable incontinence product, protective underwear/pull-on, small size, each
T4526
Adult sized disposable incontinence product, protective underwear/pull-on, medium size,
each
T4527 Adult sized disposable incontinence product, protective underwear/pull-on, large size, each
T4528
Adult sized disposable incontinence product, protective underwear/pull-on, extra large size,
each
T4529 Pediatric sized disposable incontinence product, brief/diaper, small/medium size, each
T4530 Pediatric sized disposable incontinence product, brief/diaper, large size, each
01/31/2007 Medical Supplies and Equipment – Incontinence Supplies
Medical Policy Manual
356

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 1 – Incontinence Supply Codes
Code Description
T4531
Pediatric sized disposable incontinence product protective underwear/pull-ons, small/medium
size, each
T4532
Pediatric sized disposable incontinence product protective underwear/pull-ons, large size,
each
T4533 Youth sized disposable incontinence product, brief/diaper, each
T4534 Youth sized disposable incontinence product, protective underwear/pull-ons, each
T4535 Disposable liner/shield/guard/pad/ undergarment, for incontinence, each
T4536 Incontinence product, protective underwear/pull on reusable, any size, each
T4537 Incontinence product, protective underpad, reusable, bed size, each
T4539 Incontinence product, diaper/brief, reusable, any size, each
T4540 Incontinence product, protective underpad, reusable, chair size
CMS implemented HCPCS code A4520 – Incontinence garment, any type, (e.g. brief,
diaper), each, during the annual 2005 HCPCS update. HCPCS code A4520 is used by
third party payers for reimbursement of all incontinence supplies; however, A4520 is not
covered by the IHCP because more specific T codes are available. In addition, CMS
continued coverage of HCPCS codes A4534 – Youth-sized incontinence product, brief,
each, and A4554, Disposable underpads, all sizes (e.g., Chux’s). These A codes are non-
reimbursable by the IHCP, effective January 1, 2005.
Code A4335 may be used to obtain reimbursement for miscellaneous medically
necessary items for incontinence care that are not specifically reimbursable using the
HCPCS T codes. An example of a miscellaneous incontinence supply is a skin barrier
used to treat excoriated or reddened tissue resulting from incontinence. Only A4335 can
be used for miscellaneous supplies related to incontinence. Use of other codes may result
in claim denials or recoupment.
BILLING
Crossover claims for members with primary insurance that have been billed using A4520
must be billed to the IHCP using the appropriate T codes for the incontinence supplies on
a CMS-1500 claim form. The provider must indicate the primary payment received in
Field 29 of the CMS-1500 or 837P claim forms. All TPL claims are subject to post-
payment review.
MANAGED CARE
PrimeStep services are subject to the same policies and restrictions as Traditional
Medicaid services. Questions regarding coverage of services in Risk Based Managed
Care (RBMC) should be directed to the appropriate Managed Care Organization (MCO).
01/31/2007 Medical Supplies and Equipment – Incontinence Supplies
Medical Policy Manual
357

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Medical Supplies and Equipment – Incontinence Supplies
Medical Policy Manual
358
RELATED MEDICAL TOPICS
Medical Supplies and Durable Medical Equipment (DME) Overview
RULES, CITATIONS, AND SOURCES
Indiana Administrative Code 405 IAC 5-19-1
Indiana Health Coverage Program Provider Manual Chapter 8 Section 3
Indiana Health Coverage Program Provider Bulletin BT200430
Origination Date: 8/25/97
Revisions and Reviews Reason Date
405 IAC 5-19-1
Medical Supplies – Incontinence
Supplies policy
8/25/97
BT200130 Incontinence Supplies Update 9/17/01
BR200139 Incontinence Supplies Update 9/25/01
BT200430 Incontinence Supplies Update 7/31/05
APPLICABLE INDIANAAIM EDITS AND AUDITS:
6065 - Incontinence supplies limited $1950/rolling year

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: MEDICAL SUPPLIES AND EQUIPMENT ---
MONITORING DEVICES (APNEA MONITORS,
NONINVASIVE PULSE OXIMETRY, PNEUMOGRAMS,
AND TREND EVENT MONITORING)
DESCRIPTION
Cardiorespiratory monitoring is the observation of cardiac and respiratory activities by a
device that displays and/or records specific data. Trend event and apnea monitors are
used to monitor for apnea episodes or absences of respiration. Noninvasive pulse
oximetry is the measurement of oxygen saturation by variations of light absorption
through well-vascularized tissue during systole and diastole. A pneumogram is an
overnight recording of breathing effort, heart rate, oxygen saturation, and air flow to the
lungs during sleep.
This document is intended to serve as a general summary of the Indiana Health Coverage
Programs’ (IHCP) policies regarding these services. More specific information may be
found in the IHCP Provider Manual, program notices, the Indiana Administrative Code
(IAC) or other sources as appropriate. See the “Rules, Citations, and Sources” section of
this document for further information.
COVERAGE CRITERIA
Trend Event Monitoring and Apnea Monitors
Trend event monitoring is performed with an apnea monitor that has recording features.
The appropriate Current Procedural Terminology (CPT) code for monitoring, recording,
transmission, and interpretation must be used to bill for these services. Current coding
options are illustrated in Table 1. When an apnea monitor without a recording feature is
required, Healthcare Common Procedure Coding System (HCPCS) code E0618, Apnea
monitor, without recording feature must be used.
Table 1–Coding Options for Trend Event Monitoring and Apnea Monitors
Procedure Code Description
E0618 RR (Rental) Apnea monitor, without recording feature
E0618 NU (Purchase) Apnea monitor, without recording feature
01/31/2007 Medical Supplies and Equipment – Monitoring Devices
Medical Policy Manual
359

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 1–Coding Options for Trend Event Monitoring and Apnea Monitors
E0619 RR (Rental) Apnea monitor, with recording features
Procedure Code Description
E0619 NU (Purchase) Apnea monitor, with recording features
93268
Patient demand single or multiple event recording with pre-
symptom memory loop, 24-hour attended monitoring, per 30
day period of time; includes transmission, physician review
and interpretation
93270
Patient demand single or multiple event recording with pre-
symptom memory loop, per 30 day period of time; recording
(includes hook-up, recording, and disconnection)
93271
Patient demand single or multiple event recording with pre-
symptom memory loop, per 30 day period of time;
monitoring, receipt of transmissions, and analysis
93272
Patient demand single or multiple event recording with pre-
symptom memory loop, per 30 day period of time; physician
review and interpretation only
Pneumograms
Pneumograms should be billed using CPT code 94772, Circadian respiratory pattern
recording (pediatric pneumogram), 12 to 24 hour continuous recording, infant. This code
includes both technical (modifier TC) and professional (modifier 26) components of
service. Prior authorization (PA) for pneumograms is not required. One pneumogram,
with any number of channels, is considered one unit. Oximetry is not separately
reimbursable during a pneumogram because it is included in the pneumogram
reimbursement.
Noninvasive Pulse Oximetry
Oximetry for oxygen saturation is performed with an oximeter device that can be
appropriately billed with HCPCS code E0445, Oximeter device for measuring blood
oxygen levels noninvasively. Oximetry determination should be billed using the
appropriate CPT code. Current coding options are illustrated in Table 3, on the next
page. Noninvasive pulse oximetry reimbursement is available using the CPT codes,
94760, Noninvasive ear or pulse oximetry for oxygen saturation; single determination,
94761, Noninvasive ear or pulse oximetry for oxygen saturation; multiple determinations,
and 94762, Noninvasive ear or pulse oximetry for oxygen saturation; by continuous
overnight monitoring. PA is not required for noninvasive pulse oximetry reimbursement.
Reimbursement of codes 94760, 94761, and 94762 includes the physician interpretation
of the oximetry results and any related equipment. Noninvasive pulse oximetry is not
separately reimbursable during a pneumogram.
Noninvasive pulse oximeters are classified as capped rental items under the IHCP. The
device is available for rental using the RR modifier or purchase using the NU modifier.
Rental of noninvasive pulse oximeters with HCPCS code E0445 includes all cords,
batteries, alarms, sensors, probes, printers, and all supplies.
01/31/2007 Medical Supplies and Equipment – Monitoring Devices
Medical Policy Manual
360

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 3–Coding Options for Oximeters
Procedure Code Description
E0445 RR (Rental) Oximeter device for measuring blood oxygen levels noninvasively
E0445 NU (Purchase) Oximeter device for measuring blood oxygen levels noninvasively
94760
Noninvasive ear or pulse oximetry for oxygen saturation; single
determination
94761
Noninvasive ear or pulse oximetry for oxygen saturation; multiple
determination (e.g., during exercise)
94762
Noninvasive ear or pulse oximetry for oxygen saturation; by
continuous overnight monitoring (separate procedure)
PRIOR AUTHORIZATION
Prior Authorization (PA) is not required for apnea or trend event monitors, noninvasive
pulse oximetry or pneumograms. Please refer to the Fact Sheet that corresponds with
each additional service provided, when available, for more information on PA.
MANAGED CARE
For members enrolled in Risk Based Managed Care (RBMC), providers must contact the
member’s managed care organization (MCO) for more specific guidelines. Refer to the
IHCP Provider Manual, Chapter 1, for detailed information about the fee-for-service
(FFS), Primary Care Case Management (PCCM), and Risk Based Managed Care
(RBMC) delivery systems.
IHCP members enrolled in Medicaid Select PCCM receive the same benefit coverage,
and are subject to the same limitations as traditional Medicaid FFS. Refer to the
Medicaid Select Manual for Primary Medical Providers and Office Staff for further
information.
BILLING REQUIREMENTS
Providers are responsible for determining if Home Medical Equipment (HME) licensure
is required to submit claims for particular products or services. Information is available
on the IHCP website under Provider Code Sets.
01/31/2007 Medical Supplies and Equipment – Monitoring Devices
Medical Policy Manual
361

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Medical Supplies and Equipment – Monitoring Devices
Medical Policy Manual
362
RELATED MEDICAL TOPICS
Home Health Services
Medical Supplies and Durable Medical Equipment (DME) Overview
RULES, CITATIONS, AND SOURCES
405 Indiana Administrative Code (IAC) Rule 19 Medical Supplies and Equipment
Indiana Health Coverage Programs (IHCP) Provider Manual 2005
Origination Date: 12/31/2000
Revisions and Review Reason Date
Indiana Health Coverage
Programs Provider Bulletin –
BT200334
Procedure Code Crosswalks 06/30/2003
Review Scheduled 10/31/2006
APPLICABLE INDIANAAIM EDITS AND AUDITS
6008-Select Critical Care/Neonatal Intensive Care Components Not Payable Same Date
of Service as Global Codes
6030-Critical Care/Neonatal Intensive Care Visit Codes Payable at Reduced Amount
6065-DME Total Rental Amount Not to Exceed Fee for Purchase
6080-DME Rentals Limited to 15 Months
6096-The CPT/HCPCS Code Billed is Not Payable
6104-DME Rental From Chiropractor of More Than One Month Requires PA
6113-DME Limited to $2,000 Per Member Per Calendar Year
6114-DME Limited to $5,000 Per Member Per Lifetime
6255-Trend Event Monitor Components Not Reimbursable When Billed in Conjunction
With Trend Event Monitor
6256-Trend Event Max Fee Reimbursed at Reduced Amount When Component Services
Previously Paid
6257-Maximum Reimbursement for Oximetry
6652-Multiple Surgeries Must be Billed on Same Claim
6768-Services Not Covered for Telemedicine Services

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: MEDICAL SUPPLIES AND EQUIPMENT—
NEGATIVE PRESSURE WOUND THERAPY (NPWT)
DESCRIPTION:
NPWT is a controlled application of subatmospheric pressure to a wound. NPWT is
achieved using an electrical pump to convey, intermittently or continuously,
subatmospheric pressure through a connecting tube to a specialized wound dressing.
This specialized dressing includes a resilient open cell foam surface dressing, sealed with
an occlusive dressing that is meant to contain the subatmospheric pressure at the wound
site and promote wound healing.
Indiana Health Coverage Programs (IHCP) will provide coverage for negative pressure
wound therapy (NPWT) in a home-care setting or a long-term care setting based on the
criteria described in this policy.
MEDICAL TOPICS CROSS-REFERENCES:
Medical Supplies and Equipment
Prior Authorization
RULES, CITATIONS, AND SOURCES:
405 IAC 5-19
405 IAC 5-13
Initial Policy Issues Effective Date Implementation
Date
Retroactive
Date
BT200122 Negative
Pressure Wound
Therapy
07/07/2001
Revisions:
Policy update Pricing 04/02/2002
APPLICABLE INDIANAAIM EDITS AND AUDITS:
01/31/2007 Medical Supplies and Equipment – Negative Pressure Wound Therapy
Medical Policy Manual
363
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
COVERAGE CRITERIA:
♦ The member must be enrolled in the Indiana Health Coverage Programs.
♦ The member must have a physician's order.
♦ The NPWT must be reasonable and medically necessary.
♦ The member must have a stage III or IV pressure ulcer, neuropathic ulcer, venous or
arterial insufficiency ulcer, or chronic (being present for at least 60 days) ulcer of
mixed etiology. A complete wound program described in the criteria listed below, as
applicable depending on the type of wound, must have been tried and failed prior to
application of the NPWT.
Prior Authorization
Prior authorization is required for reimbursement of NPWT. The provider must submit a
completed prior authorization form and a completed medical clearance form signed by
the physician to the HCE Prior Authorization Unit for review of medical necessity. To be
considered medically necessary, the member must have a stage III or IV pressure ulcer,
neuropathic ulcer, venous or arterial insufficiency ulcer, or chronic (present for at least 60
days) ulcer of mixed etiology. A complete wound program described in the criteria listed
below, as applicable depending on the type of wound, must have been tried and failed
prior to application of the NPWT. Prior authorization is required, and the service will be
reimbursed as a capped rental item.
The NPWT is only authorized for four weeks at a time. Each new request requires a
statement from the treating physician describing the initial condition of the wound
including measurements, efforts taken to address wound care, and the changes in the
wound therapy being applied to affect wound healing.
♦ Each new physician's order for continued use of NPWT requires a new prior
authorization period. If a prior authorization is modified and authorized for less time
than the physician's order had requested initially, a new prior authorization form and
updated physician’s orders must be obtained before the current authorization expires.
♦ Authorization for coverage beyond four months in a home care setting will be given
individual consideration based on additional documentation that sets out the reason
for continuing us of NPWT.
01/31/2007 Medical Supplies and Equipment – Negative Pressure Wound Therapy
Medical Policy Manual
364
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Criteria:
1. For all ulcers or wounds, all of the following minimum general measures of a wound
therapy program must be addressed or applied prior to application of NPWT:
– Documentation in a patient's medical record of evaluation, care, and wound
measurements by a licensed medical professional.
– Application of dressings to maintain a moist wound environment.
– Debridement of necrotic tissue if present.
– Evaluation of and provision for adequate nutritional status.
2. In addition to criterion one, stage III or IV pressure ulcers must also be evaluated for
all of the following components:
– The patient has been appropriately turned and positioned and has a current turning
and positioning plan in place.
– If the wound is on the trunk or the pelvis, the patient has used a group 2 or 3
support surface.
– The patient's moisture and incontinence has been appropriately managed.
3. In addition to criterion one, neuropathic ulcers must also be evaluated for all of the
following components:
– The patient has been on a comprehensive diabetic or other applicable disease
management program.
– Reduction in pressure on a foot ulcer has been accomplished with appropriate
modalities.
4. In addition to criterion one, venous stasis ulcers must also be evaluated for all of the
following components:
– Compression bandages or garments have been consistently applied.
– Leg elevation and ambulation have been encouraged.
Continued Coverage:
To obtain prior authorization for continued service after the initial prior authorization of
NPWT, documentation of the following must be included with the request:
♦ Indication that a licensed medical professional has directly performed or supervised
the performance of the dressing changes.
♦ Progress and changes in the ulcer. (If there is no progress in one month, or from
month to month, the approval for the NPWT will be discontinued.)
♦ A completed NPWT medical clearance form signed and dated by the ordering
physician.
Supplies
Supplies for the NPWT must be prior authorized. Dressing sets are packaged five or ten
to a case. Each dressing set equals one unit and includes, but is not limited to, a resilient
open cell foam surface dressing, drainage tubing, and an occlusive dressing that creates a
seal around the wound site to maintain subatmospheric pressure at the wound. No more
01/31/2007 Medical Supplies and Equipment – Negative Pressure Wound Therapy
Medical Policy Manual
365

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Medical Supplies and Equipment – Negative Pressure Wound Therapy
Medical Policy Manual
366
than 15 units for dressing sets, any size, will be authorized per wound, per month. No
more than ten canisters, any size, per wound, per month, will be authorized unless
documentation is submitted with the request to identify proof of an increased amount of
supplies.
Submitting Claims
Claims must be submitted on a HCFA-1500 form using the appropriate K-codes. Health
Care Financing Administration Common Procedure Coding System (HCPCS) codes
K0538, K0539, and K0540 are described in Table 1.1 with maximum fees and monthly
allowable amounts listed.
Table 1.1 – HCPCS Codes for NPWT
HCPC
S Code
Description of Code Maximu
m Fee
Maximum Monthly Allowable
Units
K0538 Stationary portable
electrical pump that
provides controlled
subatmospheric
pressure
$1707.97
per month
A maximum of one unit will be
authorized per month. If more
than one wound exists, use a Y-
connector for the additional wound
site.
K0539 Dressing set includes,
but is not limited to, the
following: resilient
open cell foam
dressing, drainage
tubing, and occlusive
dressing
$27.28 per
dressing
set
15 per month, all sizes; per wound
K0540 Wound drainage
canister
$24.41 per
canister
Ten per month, any size; per
wound
Hospital Reimbursement
When the NPWT is used in a hospital, reimbursement for the NPWT is included in the
hospital diagnosis-related groups (DRG) rate.

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: MEDICAL SUPPLIES AND EQUIPMENT:
NON- INVASIVE RESPIRATORY ASSIST DEVICES
(CPAP AND BI-PAP)
DESCRIPTION
Non-invasive positive pressure respiratory assist devices administer positive pressure,
using a nasal and/or oral mask interface, which creates a seal, avoiding the use of more
invasive airway access (e.g., tracheostomy). These devices are sometimes applied to
assist insufficient respiratory efforts in the treatment of conditions that may involve
sleep-associated hypoventilation. It is to be distinguished from the invasive ventilation
administered via a securely intubated airway, in a member for whom interruption or
failure of ventilatory support would lead to the demise of the member. These devices are
referred to as Respiratory Assist Devices (RAD) in this document. The three types of
RAD’s are continuous positive airway pressure (CPAP) devices, bi-level positive airway
pressure (BiPAP) with a backup rate feature, and BiPAP without a backup rate feature.
MEDICAL TOPICS CROSS-REFERENCES
Medical Supplies and Equipment
RULES, CITATIONS, AND SOURCES
405 IAC 5-2-17 Medically Reasonable and Necessary Service Defined
405 IAC 5-19 Medical Supplies and Equipment
Indiana Health Coverage Programs 1999 Provider Manual
Indiana Health Coverage Programs Provider Bulletin BT 200042
Region B DMERC Supplier Manual Rev. 2, March 1995; Continuous Positive Airway
Pressure System
Region B DMERC Supplier Manual Rev. 20, December 1999; Respiratory Assist
Devices
Region B DMERC Supplier Bulletin, June 2000, 00-02; Continuous Positive Airway
Pressure Devices
Indiana Health Coverage Programs Provider Bulletin BT200401
Indiana Health Coverage Programs Provider Newsletter NL200405
01/31/2007 Medical Supplies and Equipment – Non-Invasive Respiratory Assist Devices
Medical Policy Manual
367

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
ORIGINATION, REVISIONS, AND REVIEWS
Origination Date: 12/31/00
Revisions and Reviews Reason Date
Revision BT200401 4/30/04
Revision NL200405 5/15/04
APPLICABLE INDIANAAIM EDITS AND AUDITS:
6080
COVERAGE CRITERIA:
Continuous Positive Airway Pressure System (CPAP) Coverage Criteria
CPAP is covered for members with a diagnosis of obstructive sleep apnea with
documentation of at least 30 episodes of apnea, each lasting a minimum of 10 seconds,
during 6 to 7 hours of recorded sleep (i.e., a polysomnogram).
If 30 or more episodes of apnea are documented during less than six hours of sleep (e.g.,
35 episodes in four hours), that would also meet coverage criteria. However, it is not
possible to extrapolate results if fewer than 30 episodes are observed over a shorter
period. For example, if 20 episodes of apnea are observed during three hours of sleep,
coverage criteria would not be met (i.e., it cannot be assumed that in this situation 40
episodes would have occurred if testing had been conducted during six hours of sleep).
CPAP is covered when used in members with a diagnosis of moderate or severe
obstructive sleep apnea, for whom surgery is a likely alternative to CPAP.
Copies of the member’s sleep lab evaluation, including a polysomnogram, must be
retained in the physician’s record.
The current rental maximum fee for CPAP rental is $122.25 per month. Accessories
(except humidifiers, which will be billed separately) will be included in the rental
reimbursement rate.
K0532 and K0533 Coverage Criteria
Initial Coverage Criteria for K0532 and K0533 (First Three Months of Rental)
1. Non-Invasive Positive Pressure Respiratory Assist (NPPRA) devices will be covered
only if medical necessity is documented.
2. Coverage will be considered when the physician’s documentation includes a
statement that the member is experiencing symptoms of sleep associated
01/31/2007 Medical Supplies and Equipment – Non-Invasive Respiratory Assist Devices
Medical Policy Manual
368

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
hypoventilation, such as daytime hypersomnolence, excessive fatigue, morning
headache, cognitive dysfunction, dyspnea, etc.
A respiratory assist device (K0532 and K0533), used to administer NPPRA therapy is
covered for those members with clinical disorder groups characterized as: (a)
restrictive thoracic disorders (i.e., progressive neuromuscular diseases or severe
thoracic cage abnormalities), (b) severe chronic obstructive pulmonary disease
(COPD), (c) central sleep apnea (CSA), or (d) obstructive sleep apnea (OSA) (K0532
only), and who meet the following criteria:
(a) Restrictive Thoracic Disorders:
1) There is documentation in the member’s medical record of a progressive
neuromuscular disease (for example, amyotrophic lateral sclerosis), or a severe
thoracic cage abnormality (for example, post-thoracoplasty for tuberculosis),
AND
2) An arterial blood gas PaCO
2
, done while awake and breathing the member’s
usual FIO
2
, is greater than or equal to 45 mm Hg, OR
Sleep oximetry demonstrates oxygen saturation of less than or equal to 88%,
done while breathing the member’s usual FIO
2
, OR
For a progressive neuromuscular disease only, maximal inspiratory pressure is
less than 60 cm H
2
O or forced vital capacity is less than 50% predicted, AND
3) Chronic pulmonary disease does not contribute significantly to the member’s
pulmonary limitation.
If all of the above criteria are met, either a K0532 or K0533 device (based
upon the judgment of the treating physician) will be covered for members
within this group of conditions for the first three months of NPPRA therapy.
(See below for continued coverage after the initial three months.) If all of the
above criteria are not met, then K0532 and K0533 will be denied as not
medically necessary.
(b) Severe COPD:
1) An arterial blood gas PaCO
2
, done while awake and breathing the member’s
usual FIO2, is greater than or equal to 52 mm Hg, AND
Sleep oximetry demonstrates oxygen saturation less than or equal to 88%,
done while breathing oxygen at 2 LPM or the member’s usual FIO
2
(whichever is higher
), AND
01/31/2007 Medical Supplies and Equipment – Non-Invasive Respiratory Assist Devices
Medical Policy Manual
369

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
2) Prior to initiating therapy, obstructive sleep apnea (OSA) and treatment with
CPAP has been considered and ruled out.
If all of the above criteria for members with COPD are met, a K0532 device will
be covered for the first three months of NPPRA therapy. (See below for continued
coverage after the initial three months.)
A K0533 device will usually not be covered for a member with COPD during the
first two
months, because therapy with a K0532 device with proper adjustment of
the device’s settings and member accommodation to its use will usually result in
sufficient improvement without the need of a back up rate. (See below for
coverage of a K0533 device for COPD after two months use of a K0532 device.)
If all of the above criteria are not met, K0532 and related accessories will be
denied as not medically necessary.
(c) Central Sleep Apnea
(i.e., apnea not due to airway obstruction):
Prior to initiating therapy, a complete facility-based, attended polysomnogram
must be performed documenting the following:
1) The diagnosis of central sleep apnea (CSA), AND
2) The exclusion of obstructive sleep apnea (OSA) as the predominant cause of
the sleep-associated hypoventilation, AND
3) The ruling out of CPAP as effective therapy if OSA is a component of the
sleep-associated hypoventilation, AND
4) Oxygen saturation less than or equal to 88%, done while breathing the
member’s usual FIO
2
, AND
5) Significant improvement of the sleep-associated hypoventilation with the use
of a K0532 or K0533 device on the settings that will be prescribed for initial
use at home, while breathing the member’s usual FIO
2
.
If all of the above criteria are met, either a K0532 or K0533 device (based upon
the judgment of the treating physician) will be covered for members with
documented CSA conditions for the first three months of NPPRA therapy. (See
below for continued coverage after the initial three months.) If all of the above
criteria are not met, then K0532 and K0533 and related accessories will be denied
as not medically necessary.
(d) Obstructive Sleep Apnea:
01/31/2007 Medical Supplies and Equipment – Non-Invasive Respiratory Assist Devices
Medical Policy Manual
370

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
1) A complete facility-based, attended polysomnogram has established the
diagnosis of obstructive sleep apnea, AND
A single level device (E0601 = CPAP) has been tried and proven ineffective.
If the above criteria are met, a K0532 device will be covered for the first three
months of NPPRA therapy. (See below for continued coverage after the initial
three months.) If all of the above criteria are not met, K0532 and related
accessories will be denied as not medically necessary.
A K0533 device is not medically necessary if the primary diagnosis is OSA.
Continued Coverage beyond the First Three Months of Therapy
Members covered for the first three months of a K0532 or K0533 device must be re-
evaluated to establish the medical necessity of continued coverage by Indiana Health
Coverage Programs (IHCP). While the member may need to be evaluated at earlier
intervals after the initiation of therapy, the re-evaluation upon which IHCP will base a
decision to continue coverage beyond this time must occur within 61 to 90 days of
initiating therapy. There must be documentation in the member’s medical record about
the progress of relevant symptoms and member usage of the device up to that time.
Failure of the member to consistently use the K0532 or K0533 device for an average of
four hours per 24-hour period by the time of the 61 to 90 day re-evaluation would
represent non-compliant utilization for the intended purposes and expectations of benefit
of this therapy. This would constitute reason for IHCP to deny continued service as not
medically necessary.
Aside from the above documentation in the member’s medical record, the following must
be obtained by the device supplier for continuation of coverage (beyond the initial three
months):
Documentation signed and dated by the treating physician no sooner than 61 days after
initiating use of the device, declaring that the member is compliantly using the device (an
average of four hours per 24-hour period) and that the member is benefiting from its use.
The required documentation must be submitted with the request for prior authorization
for continued service.
Criteria for Coverage of a K0533 Device for Severe COPD after Two Months Use of
a K0532 Device
1. Arterial blood gas PaCO
2
repeated no sooner than 61 days after initiation of
compliant use of K0532, done while awake and breathing the member’s usual FIO
2
,
still remains greater than or equal to 52 mm Hg, AND
01/31/2007 Medical Supplies and Equipment – Non-Invasive Respiratory Assist Devices
Medical Policy Manual
371

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
2. Sleep oximetry repeated no sooner than 61 days after initiation of compliant use of
K0532 device, and while breathing with the K0532 device, demonstrates oxygen
saturation less than or equal to 88%, done while breathing oxygen at 2 LPM or the
member’s usual FIO
2
(whichever is higher), AND
A signed and dated statement from the treating physician, completed no sooner than
61 days after the initiation of the K0532 device, declaring that the member has been
compliantly using the K0532 device [an average of four hours per 24-hour period] but
the member is NOT benefiting from its use, AND stating that the physician feels that
the member meets the listed criteria for a K0533 device.
If the above criteria for the K0533 device are not met, it will be denied as not medically
necessary.
Criteria for Coverage of E0561 and E0562 for Humidifiers
This coverage criteria is effective 5/15/04.
1. E0561 and E0562 for use with a non-invasive respiratory assistive device (RAD) will
be considered for coverage only when physician documentation supports the medical
necessity of the humidifier. Documentation must indicate that the member is
suffering from nosebleeds, extreme dryness of the upper airways, or other conditions
that interfere with compliance or use of the RAD, and that the humidifier could
improve this condition. Prior authorization is required.
2. A non-heated (E0561) or a heated (E0562) humidifier will be covered for use with a
RAD (codes E0601, K0532, and K0533), when ordered by a physician, based on
medical necessity, subject to prior authorization.
3. E0561 and E0562 are inexpensive and routinely purchased items available for purchase
only. They are single-patient use items. A rental trial is no longer required before
purchase of non-heated or heated humidifiers.
Definitions
Non-Invasive Positive Pressure Respiratory Assist (NPPRA) devices administer positive
air pressure, using a nasal and/or oral mask interface, which creates a seal, avoiding the
use of more invasive airway access (e.g., tracheostomy). It may sometimes be applied to
assist insufficient respiratory efforts in the treatment of conditions that may involve
sleep-associated hypoventilation. It is to be distinguished from the invasive ventilation
administered via a securely intubated airway, in a member for whom interruption or
failure of ventilatory support would lead to imminent demise of the member. These
devices are also referred to as Respiratory Assist Devices (RAD) in this document. Three
types of RAD’s include CPAP, BiPAP with the backup rate feature, and BiPAP without
the backup rate feature.
01/31/2007 Medical Supplies and Equipment – Non-Invasive Respiratory Assist Devices
Medical Policy Manual
372

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
A continuous positive airway pressure device (CPAP) provides air pressure by means of
a nose mask and flow generator system to prevent collapse of the oropharyngeal walls
during sleep. Code E0601 is used for a device with a single delivered pressure. Code
K0183 describes (a) a nasal mask and exhalation port or (b) nasal pillow / seal system.
A respiratory assist device (RAD) without backup rate (K0532), also known as BiPAP,
delivers adjustable, variable levels (within a single respiratory cycle) of positive airway
pressure by way of tubing and a non-invasive interface (such as a nasal or oral facial
mask) to assist spontaneous respiratory efforts and supplement the volume of inspired air
into the lungs (i.e., NPPRA).
A respiratory assist device (RAD) with backup rate (K0533), also known as BiPAP,
delivers adjustable, variable levels (within a single respiratory cycle) of positive air
pressure by way of tubing and a non-invasive interface (such as a nasal or oral facial
mask) to assist spontaneous respiratory efforts and supplement the volume of inspired air
into the lungs (i.e., NPPRA). In addition, it has a timed backup feature to deliver this air
pressure whenever spontaneous inspiratory efforts fail to occur.
Polysomnography is the continuous and simultaneous monitoring and recording of
various physiological and pathophysiological parameters of sleep for six or more hours
with physician review, interpretation, and report. It must include sleep staging which is
defined to include a one- to four-lead electroencephalogram (EEG), and electro-
oculogram (EOG), and submental electromyogram (EMG). It must also include at least
the following parameters of sleep: airflow, respiratory effort, and oxygen saturation by
oximetry. For the purpose of this policy, polysomnographic studies must be performed in
a sleep study laboratory, and not in the home or in a mobile facility. Testing must
comply with all applicable state regulatory requirements.
FIO
2
is the fractional concentration of oxygen delivered to the member for inspiration.
For the purpose of this policy, the member’s “usual FIO
2
” refers to the oxygen
concentration the member normally breathes when not undergoing testing to qualify for
coverage of NPPRA therapy. That is, if the member does not normally use supplemental
oxygen, their usual FIO
2
is that found in room air. For the purpose of this policy, a DME
supplier may not perform arterial blood gas, sleep oximetry, and polysomnographic
studies.
Reasons for Noncoverage
Respiratory assist devices are non-covered if the member does not meet the criteria for
coverage if the member is not eligible at the time of service, or if prior authorization has
not been obtained prior to the provision of service when prior authorization is required.
HCPCS Codes
K0183-K0189 are RAD (CPAP or BiPAP) accessories and will be reimbursed according
to the limitations outlined below when the RAD is member owned. Otherwise, the cost
01/31/2007 Medical Supplies and Equipment – Non-Invasive Respiratory Assist Devices
Medical Policy Manual
373
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Medical Supplies and Equipment – Non-Invasive Respiratory Assist Devices
Medical Policy Manual
374
of the accessories is included in the rental reimbursement rate. K0532 and K0533 will be
rented on a frequent and substantial servicing basis, i.e., ongoing rental. E0561 and
E0562 are inexpensive and routinely purchased items available for purchase only.
K0183-Nasal application device; maximum of one unit per three months
K0184-Nasal pillows/seals, replacement for nasal application device, per pair; maximum
of two units per one month
K0185-Headgear, used with positive pressure RAD; maximum of one unit per six months
K0186-Chin strap; maximum of one unit per six months
K0187-Tubing; maximum of one unit per one month
K0188-Filter, disposable; maximum of two units per one month
K0189-Filter, non-disposable; maximum of one unit per six months
E0561-Humidifier, non-heated, used with positive pressure airway device
E0562-Humidifier, heated, used with a positive pressure airway device
K0532-Respiratory assist device, bi-level pressure capability, without backup rate
feature, used with noninvasive interface, e.g., nasal or facial mask (intermittent assist
device with continuous positive airway pressure device)—BiPAP without backup rate
K0533-Respiratory assist device, bi-level pressure capability, with backup rate feature,
used with noninvasive interface, e.g., nasal or facial mask (intermittent assist device with
continuous positive airway pressure device)—BiPAP with backup rate
E0601-Continous positive airway pressure (CPAP) device
Approved 1/24/01

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: MEDICAL SUPPLIES AND EQUIPMENT—
PATIENT-ACTIVATED EVENT RECORDER—
IMPLANTABLE LOOP RECORDER (ILR)
DESCRIPTION:
The insertable loop recorder (ILR) is a fully implantable patient-activated event recorder
used to record the heart’s rate and rhythm at the time of a syncopal event. The
information provided by the device, in the form of an electrocardiogram (EKG), can be
used by physicians to identify, or rule out, an irregular heartbeat as the cause of such
events.
The Indiana Health Coverage Programs (IHCP) covers the patient-activated event
recorder—ILR for use after a syncopal event. The device may be implanted at any of
three places of service including inpatient, outpatient, or physician’s office. The device
may not be implanted in the same member more often than every two years or 24 months.
The recorder activator is furnished with the system and is not separately reimbursed.
COVERAGE CRITERIA
The following information includes coverage criteria for an ILR:
• The ILR device is covered only if a definitive diagnosis has not been made after
meeting all of the following conditions.
– Complete history and physical examination
– Electrocardiogram (ECG)
– Two negative or non-diagnostic 30-day pre-symptom memory loop patient demand
recordings (may be either single or multiple event recordings, with or without 24-
hour attended monitoring)
– Negative or non-diagnostic tilt table testing
– Negative or non-diagnostic electrophysiological testing
• The patient must be capable of activating the hand-held telemetry unit
• The ILR device is not covered for the following.
– Patients with presyncopal episodes
– Patients failing to fulfill the indications for coverage in this policy
– Patients for whom compliance or lifestyle make use of the external monitoring
systems inappropriate
01/31/2007 Medical Supplies and Equipment – Patient-Activated Event Recorder
Medical Policy Manual
375
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• Removal of an ILR on the same day as the insertion of a cardiac pacemaker is
considered to be part of the pacemaker insertion procedure and is not reimbursed
separately.
• The ILR is only covered for a given patient in any two-year time period (24 months).
• ECG analyses obtained during device insertion for signal quality and amplification
purposes are considered part of the implant procedure and are not reimbursed
separately.
Device Monitoring
The Current Procedure Terminology CPT code for analysis of information collected by
the recorder is 93727, Electronic analysis of implantable loop recorder (ILR) system
(includes retrieval of recorded and stored ECG data, physician review and interpretation
of retrieved ECG data and reprogramming), and should be billed only subsequent to the
date of insertion. Initial analysis and monitoring is included in the fee for insertion;
therefore, CPT code 93727 may not be billed on the date of insertion. The programmer
used to program the ILR, retrieve, display, and print stored data is furnished to the
physician, but remains the property of the manufacturer.
PRIOR AUTHORIZATION
Neither the implantation of the device nor the patient-activated event recorder—ILR
requires prior authorization (PA), but will be subject to retrospective review according to
IHCP criteria. If a replacement recorder activator is needed, PA is required.
MANAGED CARE
For members enrolled in Risk Based Managed Care (RBMC), providers must contact the
member’s managed care organization (MCO) for more specific guidelines. Refer to the
Provider Manual, Chapter 1, for detailed information about the fee-for-service (FFS),
Primary Care Case Management (PCCM), and Risk Based Managed Care (RBMC)
delivery systems.
IHCP members enrolled in Medicaid Select PCCM receive the same benefit coverage,
and are subject to the same limitations as traditional Medicaid FFS. Refer to the
Medicaid Select Manual for Primary Medical Providers and Office Staff for further
information.
BILLING REQUIREMENTS
These procedure codes have a 90-day global postoperative care designation for which
care related to the surgical procedure is not separately reimbursable unless such care is
non-routine (e.g. treatment of complications).
01/31/2007 Medical Supplies and Equipment – Patient-Activated Event Recorder
Medical Policy Manual
376

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• CPT code 33282, Implantation of patient-activated cardiac event recorder, is
used for the implantation of an ILR.
• CPT code 33284, Removal of an implantable, patient-activated cardiac event
recorder, is used for the removal of this device.
If the procedure is performed when the patient is an inpatient for a related problem,
submit a UB-92 using the International Classification of Diagnoses Ninth Edition (ICD 9-
CM) code 780.2, Syncope and collapse, as one of the diagnosis codes on the claim form.
If the procedure is performed as an outpatient, submit a UB-92 using revenue code 360,
Operating Room Services and the CPT code 33282 for implantation. The device itself
should be billed on a CMS-1500 using code E0616, Implantable cardiac event recorder
with memory, activator and programmer and 780.2, Syncope and collapse as the primary
diagnosis code. Use CPT code 33284 with revenue code 360 to bill for removal of the
device. Physician’s charges for the surgery should be billed on a HCFA-1500.
If the procedure is performed in a physician’s office, the physician should bill 33282 for
implantation and E0616 for the device on the HCFA-1500. Table 1.1, Place of Service
Codes illustrates coding for each place of service:
Table 1.1 − Place of Service Codes
Inpatient Outpatient Physician’s Office
Type of Claim
UB-92 UB-92 (and HCFA-
1500 if billing for
device)
HCFA-1500
ICD-9-CM
Diagnosis Code
780.2 – Syncope
and Collapse
780.2 – Syncope and
Collapse
780.2 – Syncope and
Collapse
Revenue and
CPT Codes
Revenue code-
360
CPT code not
necessary
Revenue code-360
CPT code-33282 for
insertion
CPT code-33284 for
removal
Revenue code not
needed
CPT code-33282 for
insertion
CPT code-33284 for
removal
HCPCS Code
Not needed On HCFA-1500 –
E0616
E0616
Table 1.2, Loop Recorder System Implantation Codes illustrates the codes for
implantation and the device. Providers must bill their usual and customary charges on
the claim form. Insertion of the device carries a 90-day global surgery designation with
no assistant surgeon required.
01/31/2007 Medical Supplies and Equipment – Patient-Activated Event Recorder
Medical Policy Manual
377

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 1.2 − Loop Recorder System Implantation Codes
Code Description
33282 Implantation of patient-activated cardiac event recorder
33284 Removal of an implantable, patient-activated cardiac event recorder
93727
Electronic analysis of ILR system (includes retrieval of recorded and
stored ECG data, physician review, and interpretation of retrieved
ECG data and reprogramming)
E0616
Implantable cardiac event recorder memory, activator, and
programmer. (The programmer is furnished by the manufacturer, to
the physician, for use in the office for reading saved information in
the recorder.)
E1399 Recorder activator (replacement)
RELATED MEDICAL TOPICS
Cardiology
Hospital Inpatient
Hospital Outpatient
Medical Supplies and Equipment
Physician Services
Surgery
RULES, CITATIONS, AND SOURCES
405 IAC 5-17-1, Reimbursement; limitations
405 IAC 5-28-1, Reimbursement; limitations
Origination Date: 10/10/01
Revisions and
Review
Reason Date
BT200114 Patient-activated Event Recorder 10/26/00
Review Scheduled 10/31/06
APPLICABLE INDIANAAIM EDITS AND AUDITS
6124-Implantable loop recorder limited to one (1) every two years
6152-Surgery payable at reduced amount when consultation paid days before or after
surgery
6649-Surgery payable at reduced amount when related postoperative care paid
6653-Postoperative care within zero to 90 days of surgery
6654-Preoperative care within one day of surgery
01/31/2007 Medical Supplies and Equipment – Patient-Activated Event Recorder
Medical Policy Manual
378
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Medical Supplies and Equipment – Patient-Activated Event Recorder
Medical Policy Manual
379
6655-Surgery payable at reduced amount when preoperative care paid
6656-Postoperative care within 10 days of select surgery
6657-Preoperative care on day of surgery
6658-Surgery payable at reduced amount when preoperative care paid same day of
service
6659-Surgery payable at reduced amount when related postoperative care paid

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: MEDICAL SUPPLIES AND EQUIPMENT—
PHRENIC NERVE STIMULATOR
(BREATHING PACEMAKER)
DESCRIPTION:
This device is an electrophrenic pacemaker for pacing of the diaphragm. It consists of an
external radio frequency transmitter, an antenna, a subcutaneous radio receiver, and a
bipolar platinum nerve electrode. Diaphragmatic pacing (intermittent electrical
stimulation of the phrenic nerves) offers patients who need long-term ventilation, and
have a functionally intact phrenic nerve and chest wall stability, freedom from
mechanical ventilation.
MEDICAL TOPICS CROSS-REFERENCES:
Home Health Services
Hospital Outpatient
Hospital Inpatient
Medical Supplies and Equipment
Nursing Facilities
Physical Rehabilitation Services
Surgery
RULES, CITATIONS, AND SOURCES:
405 IAC 5-17-1 Reimbursement; limitations
405 IAC 5-17-2 Prior authorization; generally
405 IAC 5-19-8 “Durable medical equipment” or “DME” defined
Initial Policy Issues
Effective Date Implementation
Date
Retroactive Date
BT200108 Phrenic Nerve
Stimulator
10/26/00 10/26/00
Revisions:
01/31/2007 Medical Supplies and Equipment – Phrenic Nerve Stimulator
Medical Policy Manual
380
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
APPLICABLE INDIANAAIM EDITS AND AUDITS:
6026
6027
6152
6653
6654
6655
6656
6657
6658
6659
6649
COVERAGE CRITERIA:
Patient Selection
The primary objective of implanting the phrenic nerve stimulator is to allow the member
to return to a home environment from a skilled nursing facility and be more independent.
Therefore the following criteria are mandatory for prospective candidates requesting this
device:
Functional lungs and diaphragm muscle
Absence of infection
A clear and adequate upper airway (including nasopharynx, pharynx, larynx)
Family support that includes an unpaid, physical care giver of adequate quality and the
availability of nursing and medical care
Medical Review Documentation
Prior authorization for medical necessity is required for this device and its implantation.
The equipment is costly and requires preoperative testing of the components and
thorough education of the member and his or her caregivers concerning its use.
Medical Policy Criteria
1. Members who qualify for this device will demonstrate life-threatening oxygen
depletion when respiration is unassisted.
2. For stable, non-acute quadriplegics and other spinal cord or brain stem injured
members [ICD-9-CM 344(00-09) diagnosis codes] all of the following criteria must be
met:
– Patient is oriented to name, date, and place.
– Patient’s mobility will be improved. Patient will be able to be out of bed and be
mobile per wheelchair, which may include employment or attending school.
01/31/2007 Medical Supplies and Equipment – Phrenic Nerve Stimulator
Medical Policy Manual
381
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Increased mobility will allow the patient to function without interference of large
equipment.
– Patient’s skin integrity will be better maintained because of increased mobility.
– Patient has capacity to be productive. He or she will more easily perform cognitive
tasks within physical limitations.
– Patient will be better able to eat and swallow.
3. For nonobstructive (or central) sleep apnea (ICD-9-CM 780.51, 780.53 diagnosis
codes) only when other treatments have failed, and the following criteria must be met:
– The requesting physician will present sleep studies demonstrating life- threatening
respiratory cycles when the patient is asleep.
– The member must have a diagnosis of central sleep apnea and have failed to
maintain an appropriate PO
2
level (oxygen partial pressure) with continuous
positive air pressure (CPAP) and bi-level continuous positive airway pressure
(BiPAP) treatments.
– Documentation by a specialist in otolaryngology or pulmonology of treatment
attempts will accompany the prior authorization request.
– The breathing pacemaker should never be recommended for treatment of
obstructive sleep apnea.
4. Documentation indicating medical necessity for the appropriate diagnosis will be
submitted prior to surgical implantation of the stimulator wires.
Device Monitoring
Medical device tracking regulations of the U.S. Food and Drug Administration require
that the manufacturer of the device be notified when the following occurs:
Diaphragm pacing system is implanted,
Diaphragm pacing receiver or electrode is explanted, (date, name, mailing address, and
telephone number of the explanting physician are to be included)
Diaphragm pacing patient dies,
Diaphragm pacing device is returned
Diaphragm pacing device is permanently retired from use or otherwise permanently
discarded.
Coding and Billing Instructions
For inpatient billing of the implantation of the device, the appropriate diagnosis-related
grouping (DRG) will be used. The claim for the device must be submitted as a durable
medical equipment (DME) item on a HCFA-1500 claim form. When the device is
implanted as an outpatient procedure, the revenue code 360 with CPT code 33282
should be used on the UB-92 claim form and the device billed as a DME item on a
HCFA-1500 claim form. The decision for either outpatient or inpatient status is made by
01/31/2007 Medical Supplies and Equipment – Phrenic Nerve Stimulator
Medical Policy Manual
382

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Medical Supplies and Equipment – Phrenic Nerve Stimulator
Medical Policy Manual
383
the physician and determined by the assessment of complicating factors and their severity
at the time the procedure is planned. The hospital providing the equipment for
implantation must have a DME provider number.
Table 1.1 provides the CPT codes and description information to use when submitting
claims either as an inpatient or outpatient.
Table 1.1 − CPT Codes for Inpatient and Outpatient Claims
CPT/HCPCS
Code
Description Current Pricing
64577 Incision for implantation of
neurostimulator electrodes;
autonomic nerve
RBRVS-$207.72
ASC 1-$337.08
64585 Revision or removal of
peripheral neurostimulator
electrodes
RBRVS-$83.62
ASC A-$348.20
95970 Initial programming Included in initial fee
95974 Intraoperative or subsequent
programming, first hour
Included in initial fee, provided
by manufacturer at the time of
implant then per telephone for
life of the power source at no
cost to the member.
Z5108 Implantable neurostimulator
pulse generator
Max Fee $58,299
Z5109 Radiofrequency transmitter
(external) for use with
implantable neurostimulator
radiofrequency receiver
Max Fee $37,967
Prior Authorization
Prior authorization (PA) is required for this device and its implantation whether
implanted as an inpatient or an outpatient. One or more of the following ICD-9-CM
diagnosis codes must be used when submitting requests for PA. Members with these
diagnoses who are ventilator dependent and have a tracheostomy due to partial or
complete respiratory insufficiency are considered candidates for this device subject to
review.
• 344.0-344.9 includes quadriplegia and quadraparesis of all types
• 780.51 and 780.53—nonobstructive sleep apnea
• 786.09—congenital respiratory abnormalities, other

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE MEDICAL SUPPLIES AND DURABLE MEDICAL
EQUIPMENT – MANUAL WHEELCHAIRS,
MOTORIZED/POWER WHEELCHAIRS, AND
POWER OPERATED VEHICLES
DESCRIPTION
The Indiana Health Coverage Programs (IHCP) will provide reimbursement for a manual
wheelchair, motorized/power wheelchair, or power operated vehicle (POV) when
medically necessary for IHCP members with prior authorization (PA). Certain medical
criteria must be met for the approval of each piece of equipment. The IHCP will only
reimburse for one wheelchair or POV per member, per five year period.
This document is intended to serve as a general summary of the IHCP policies regarding
this service. More specific information may be found in the IHCP provider manual,
program notices, the Indiana Administrative Code (IAC) or other sources as appropriate.
See the “Rules, Citations, and Sources” section of this document for further information.
COVERAGE CRITERIA
Manual Wheelchairs
The IHCP will reimburse a manual wheelchair, when medically necessary, subject to PA.
Requests for manual wheelchairs require that a completed medical clearance form be
submitted with the PA request.
Motorized/Power Wheelchairs
According to the Indiana Administrative Code (IAC) 405 IAC 5-19-9, “motorized
vehicles are covered only when the recipient is enrolled in a school, sheltered workshop,
or work setting, or if the recipient is left alone for significant periods of time. It must be
documented that the recipient can safely operate the vehicle and that the recipient does not
have the upper extremity function necessary to operate a manual wheelchair.”
A member who requires a motorized/power wheelchair is usually nonambulatory and has
severe weakness of the upper extremities due to a neurologic or muscular disease or
condition and would otherwise be confined to a bed or chair without the use of the power
wheelchair. A power wheelchair is covered if the member’s condition is such that the
requirement for a power wheelchair is long term (at least six months).
01/31/2007 Medical Supplies and Equipment – Power Wheelchairs
Medical Policy Manual
384
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
POVs
The IHCP will reimburse a POV, such as a scooter, subject to PA, for members who are
unable to operate a manual wheelchair and who have adequate trunk stability to safely
operate the vehicle. A POV should be considered when the member does not require the
full support or features that are provided by a power wheelchair. POVs are not covered by
the IHCP when needed for use outside the home only, or to allow the member to perform
leisure or recreational activities. Therefore, POVs that are designed, by size and features,
primarily for outdoor use, will be denied as not medically necessary.
Reimbursement for manual and motorized/power wheelchairs includes all labor charges
involved in the assembly of the wheelchair. Reimbursement of manual and
power/motorized wheelchairs and POVs also includes emergency services, delivery,
setup, and items covered under a warranty. See the Medical Supplies and Durable
Medical Equipment Overview fact sheet for further information regarding reimbursement
of labor, repairs, and replacement of durable medical equipment for wheelchairs. The
IHCP will provide reimbursement for one manual wheelchair, motorized/power
wheelchair, or POV per five year period. Any wheelchair designated for use as a backup
will be denied as not medically necessary.
PRIOR AUTHORIZATION
Manual Wheelchairs
• Providers must submit a PA request and an IHCP Non-Motorized Wheelchair
Medical Clearance form signed by a physician that documents the member’s
condition, mobility needs, and/or prognosis to support the medical necessity for a
manual wheelchair. Documentation of medical necessity must be maintained in
the member’s medical records.
Motorized/Power Wheelchairs
• For a motorized/power wheelchair to be considered for coverage, the information
submitted with the PA must be supported by documentation in the member’s
medical record that medical necessity has been met.
• A completed IHCP Motorized Wheelchair Purchase Medical Clearance Form must
be submitted with the PA request for rental or purchase of a motorized/power
wheelchair. The medical clearance form must be reviewed and signed by a
physiatrist.
• The member’s physician may prescribe a motorized/power wheelchair. However,
the medical necessity must be reviewed and the medical clearance form must be
approved and signed by a physiatrist prior to the form being submitted to the PA
department. A member is only required to see the physiatrist if the physiatrist
requests to see the member after a review of the documentation. If a physiatrist
requests to see a member after reviewing the documentation, the member would
01/31/2007 Medical Supplies and Equipment – Power Wheelchairs
Medical Policy Manual
385

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
then be required to visit the physiatrist. Providers should note that if the physiatrist
does not choose to see the member for an evaluation, the IHCP will not provide
reimbursement to the physiatrist for the chart review.
POVs
• A completed IHCP Motorized Wheelchair Purchase Medical Clearance Form
signed by a physician must be submitted with the PA request form that documents
the member’s condition, mobility needs, and/or prognosis to support the medical
necessity for a POV.
• Documentation must indicate the member’s condition that renders them unable to
operate a manual wheelchair.
• Documentation must also indicate the member is capable of safely operating a
POV, can transfer in and out of a POV, and has adequate trunk stability to safely
ride in and operate the POV.
CODING
Manual Wheelchairs
The IHCP will reimburse for both standard and nonstandard manual adult wheelchairs and
for manual pediatric wheelchairs. Manual pediatric wheelchairs are billed using HCPCS
codes E1229 and E1231-E1238.
A standard adult wheelchair is defined as a wheelchair with a base that weighs greater
than 36 lbs, with seat dimensions of 16”-18” wide, 16” deep, and greater than 19” and less
than 21” in height. A standard wheelchair includes a non-adjustable back height of 16”-
17”, fixed or detachable arm rests, fixed or detachable foot rests, and footplate extensions
of 16”-21”.
A nonstandard adult wheelchair is a wheelchair base other than a standard wheelchair or
custom wheelchair. Nonstandard wheelchair bases include, but are not limited to the
following; fully-reclining, hemi, lightweight, ultra lightweight, high strength lightweight,
semi-reclining, amputee, heavy duty, wide heavy duty, extra heavy duty, tilt-in-space, and
motorized/power wheelchairs. Table 1 lists the range of HCPCS codes available for
reporting standard and nonstandard manual wheelchairs.
Table 1 – Manual Wheelchair Codes
Code Range Description
E1130-E1160 &
E1221-E1224
Standard Manual Wheelchairs
E1031-E1039 Rollabout and Transport Chairs
E1050-E1070 Fully Reclining Wheelchairs
E1083-E1086 Hemi-Wheelchairs
E1087-E1090 High Strength Lightweight Wheelchairs
01/31/2007 Medical Supplies and Equipment – Power Wheelchairs
Medical Policy Manual
386

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 1 – Manual Wheelchair Codes
Code Range Description
E1092-E1093 Wide Heavy-Duty Wheelchairs
E1100-E1110 Semi-Reclining Wheelchairs
E1161 Tilt-in-Space Wheelchair
E1170-E1200 Amputee Wheelchairs
E1240-E1270 Lightweight Wheelchairs
E1280-E1295 Heavy-Duty Wheelchairs
Motorized/Power Wheelchairs
The IHCP will reimburse claims for adult sized motorized/power wheelchairs using
HCPCS codes K0010 through K0014 and pediatric-sized power wheelchairs using
HCPCS code E1239. See Table 2 below for a description of the “K” codes for adult-sized
motorized/power wheelchairs.
Table 2 – Codes for Motorized/Power Wheelchairs
Code Description
E1220
Wheelchair; specially sized or constructed (indicate brand name, model
number, if any, and justification)
E1239 Power wheelchair, pediatric size, not otherwise specified
K0010 Standard-weight frame motorized/power wheelchair
K0011
Standard-weight frame motorized/power wheelchair with programmable
control parameters for speed adjustment, tremor dampening, acceleration
control, and braking
K0012 Lightweight portable motorized/power wheelchair
K0014 Other motorized/power wheelchair base
POVs
POVs should be billed using HCPCS code E1230, Power operated vehicle (three or fou
wheel nonhighway), specify brand name and model number. E1230 is reimbursed at the
IHCP’s allowable rate. Specially constructed POV’s for members greater than 300
pounds is to be billed using HCPCS code E1220. Providers should submit their usual and
customary charge on the CMS 1500 or 837P electronic claim.
BILLING
Manual and Motorized/Power Wheelchairs
Providers should determine which HCPCS code listed in Tables 1 and 2 is most
appropriate to use, based on the Wheelchair Product Classification List, published by
Medicare’s Statistical Analysis Durable Medical Equipment Regional Carrier
(SADMERC). Providers are encouraged to periodically review this list for updates. If a
specific wheelchair base is not shown on the Wheelchair Product Classification List,
providers are advised to select the most appropriate code that describes the product
provided. Providers should bill the wheelchair base code and any reimbursable
modifications or upgrades on the CMS-1500 and/or 837P electronic claim.
01/31/2007 Medical Supplies and Equipment – Power Wheelchairs
Medical Policy Manual
387
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
PER DIEM
Standard wheelchairs are included in the per diem for facilities that are reimbursed on a
per diem basis. Standard wheelchairs with custom features require prior authorization and
will be reviewed on a case-by-case basis for payment outside the facility per diem.
Nonstandard wheelchairs, motorized/power wheelchair, and POVs are not included in the
facility per diem rate and are separately reimbursable by the IHCP.
MANAGED CARE
Primary Care Case Management (PCCM) services are subject to the same policies and
restrictions as Traditional Medicaid services. Questions regarding coverage of services in
Risk Based Managed Care (RBMC) should be directed to the appropriate Managed Care
Organization (MCO).
RELATED MEDICAL TOPICS
Medical Supplies and Durable Medical Equipment - Overview
Medical Supplies and Durable Medical Equipment - Wheelchair Accessories
RULES, CITATIONS, AND SOURCES
405 IAC 5-19-9
Indiana Health Coverage Programs Provider Manual 2003
Indiana Health Coverage Programs Provider Manual 2005
Indiana Health Coverage Programs Newsletter NL200402
Indiana Health Coverage Programs Bulletin BT200335
Origination Date 7/25/97
Revision and Review Reason Date
405 IAC 5-19-9 Wheelchairs and similar motorized vehicles 07/25/1997
BT200335
Motorized/Power Wheelchairs and
Programmable Electronics Bulletin
07/18/2003
2005 Provider Manual
Manual Wheelchair and Motorized/Power
Wheelchair Revisions
NL200402
Physiatrist Review of the Motorized/Power
Wheelchair Medical Clearance Form
10/30/2005
01/31/2007 Medical Supplies and Equipment – Power Wheelchairs
Medical Policy Manual
388

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
APPLICABLE INDIANAAIM EDITS AND AUDITS
6065 – DME total rental amount not to exceed fee for purchase
6082 – Nursing facility visits vs DME services
6080 – DME rentals limited to 15 months
6096 – The CPT/HCPCS code billed is not payable
6104 – DME rental from chiropractor of more than 1 month
6113 – DME limited to $2,000 per recipient per calendar year
6114 – DME limited to $5,000 per recipient per lifetime
01/31/2007 Medical Supplies and Equipment – Power Wheelchairs
Medical Policy Manual
389

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL SUPPLIES AND EQUIPMENT - POWER WHEELCHAIRS
ADDENDUM A
Note: This addendum contains provider notifications that have been published since the
review of the Medical Supplies and Equipment – Power Wheelchairs Fact Sheet. The
information in this addendum will be incorporated into the fact sheet at the next review.
Provider Notification: BR200533 Publication Date: 08/16/2005
Subject: Adjustable and Nonadjustable Seat Cushions
Date Added to Manual: 10/31/2005
Text of Publication
Currently, providers bill both adjustable and nonadjustable seat cushions using the same
HCPCS codes. Adjustable cushions have all of the characteristics of a skin protection seat
cushion (E2603 and E2604) or skin protection and positioning seat cushion (E2607 and
E2608); however, they are also adjustable. Adjustments are made by adding or removing
significant quantities of air, liquid, gel, or other fluid medium in physiologically
appropriate areas of the cushion to promote pressure reduction.
New, more descriptive, procedure code and modifier combinations (PICS) have been
developed for billing adjustable seat cushions. Medicare currently utilizes 4 definitions for
adjustable seat cushions, each billed with procedure code K0108. The IHCP has mirrored
this policy by creating 4 PICS with unique definitions for each type of adjustable seat
cushion. The new coding and pricing information for adjustable seat cushions is listed in
Table 1 below, and will be effective September 30, 2005. The coding and fee schedule for
all other wheelchair seat cushions will remain the same.
Table 1 – Adjustable Seat Cushion Codes Effective September 30, 2005
Adjustable cushions are purchase only items. Providers must attach the NU modifier when
billing adjustable seat cushions. The adjustable cushions do not have to be listed on the
Statistical Analysis Durable Medical Equipment Regional Carrier (SADMERC)
classification list in order to be reimbursed by the IHCP.
Code Description Pricing
K0108 U1
NU
Skin protection wheelchair seat cushion, adjustable, width less
than 22 inches
$330.81
K0108 U2
NU
Skin protection wheelchair seat cushion, adjustable, width
greater than or equal to 22 inches
$389.54
K0108 U3
NU
Skin protection and positioning wheelchair seat cushion,
adjustable, width less than 22 inches
$378.68
K0108 U4
NU
Skin protection and positioning wheelchair seat cushion,
adjustable, greater than or equal to 22 inches
$435.56
01/31/2007 Medical Supplies and Equipment – Power Wheelchairs
Medical Policy Manual
390
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Medical Supplies and Equipment – Power Wheelchairs
Medical Policy Manual
391

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: MEDICAL SUPPLIES AND EQUIPMENT—
PROGRAMMABLE HEARING AIDS
DESCRIPTION:
Programmable hearing aids are hearing aids that are pre-programmed specifically based
upon the member’s hearing loss. Most programmable aids can accommodate from one to
seven pre-programmed settings at a time. The device easily adjusts to the type of noise,
when a member enters into a different sound environment. It can also be re-programmed
to make adjustments to the sound quality. In addition, programmable hearing aids offer
advantages over conventional devices such as, better sound quality, flexibility, and better
clarity of speech because they are custom programmed specifically to a member’s
hearing loss. Programmable hearing aids usually would be considered a
comfort/convenience and not medically reasonable or necessary. However, in certain
circumstances, the devices are supported by medical necessity. Programmable hearing
aids require prior authorization (PA).
Indiana Health Coverage Programs (IHCP) provides coverage for programmable hearing
aids based on the criteria described in this policy. The device would be classified as a
customized item with the IHCP. Customized equipment is defined as equipment
uniquely constructed or substantially modified to meet the specific needs of an individual
member. The IHCP Provider Manual, Chapter 8, section 3 provides information
regarding durable medical equipment and hearing aids coverage and billing procedures.
This document is intended to serve as a general summary of the Indiana Health Coverage
Programs’ (IHCP) policies regarding this service. More specific information may be
found in the IHCP Provider Manual, program notices, the Indiana Administrative Code
(IAC) or other sources as appropriate. See the “Rules, Citations, and Sources” section of
this document for further information.
COVERAGE CRITERIA
Programmable hearing aids will only be prior authorized when medically necessary.
Documentation must include the significant, objective benefit to the member. Coverage
may be considered for the specific instances noted on the following page.
01/31/2007 Medical Supplies and Equipment – Programmable Hearing Aids
Medical Policy Manual
392

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• Fluctuating hearing loss (Meniere’s disease, autoimmune sensorineural hearing
loss, otogenic syphilis, large vestibular aqueduct syndrome and other entities
resulting in fluctuant hearing loss)
• Progressive hearing loss (Meniere’s disease, Alport’s syndrome, and other entities
resulting in progressive hearing loss. A retrocochlear hearing loss must be
excluded, particularly when the loss is asymmetrical)
• Severe recruitment or very narrow dynamic range
• Very young children who are hard to test or hard to fit
• Member’s with hearing loss with unusual audiometric configurations
Documentation Requirements
The PA request must be accompanied with the following documentation.
• A completed IHCP Medical Clearance and Audiometric Test (IHCP MCAT)
form. The MCAT form reflects current policy for all types of hearing aids and
can be located on the IHCP website at www.indianamedicaid.com. Medical
necessity for programmable hearing aids must be clearly documented in the
sections entitled, “Recommendation Information” and/or “Special Conditions” on
page two of the IHCP MCAT form
• A record of the audiogram obtained not more than three months from the date of
the request
• An otological examination report, signed by the physician, that includes the
medical etiology and diagnosis for the hearing loss
• A diagnosis that supports the medical necessity must be included on the PA
request and on the claim form for programmable hearing aids
• A documented case history should include at least the following information
regarding the member’s needs and life style
a. The past history of hearing aid use
b. The reason programmable hearing aid(s), rather than conventional hearing
aid(s), would be medically necessary
c. A description of the hearing environment(s) in which the member has trouble
hearing and to which the member is subjected. The frequency and duration of
exposure to these environments should also be included
d. Documentation of any other factors, such as lack of normal dexterity, should
be included
e. Documentation must be provided that supports medical necessity of the
programmable hearing aids outside of vocational needs
Programmable hearing aids may be authorized for monaural amplification (one ear) or for
binaural amplification (two ears). The Indiana Administrative Code (IAC) 405 IAC 5-
19-13 (5), Hearing aids; purchase mandates, binaural aids and CROS-type aids will be
authorized only when significant, objective benefit to the recipient can be documented.
Hearing aids come in a variety of models and styles, therefore, prices vary depending
upon not only the hearing aid model and style, but also upon the degree of hearing loss
01/31/2007 Medical Supplies and Equipment – Programmable Hearing Aids
Medical Policy Manual
393
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
and the special options chosen to personalize the instrument. Documentation should
support the number of pre-programmed settings requested. Only the least costly
alternative to meet the member’s medically necessary hearing aid needs will be approved.
Any PA request that does not or questionably meets criteria must be referred to a
consultant for review.
Additionally, some programmable aids may also be digital programmable aids. Digital
aids are typically much higher in cost than aids with analog sound. Research indicates
that digital aids improve sound quality over an analog aid much like a compact disc (CD)
improves the sound quality of a recording compared to a cassette tape. This would not
likely be medically necessary and should be denied as a comfort item as mandated in 405
IAC 5-29-1(5). The medical necessity requirement may be attained utilizing a more basic
hearing aid, whether conventional or programmable.
MANAGED CARE
For members enrolled in Risk Based Managed Care (RBMC), providers must contact the
member’s managed care organization (MCO) for more specific guidelines. Refer to the
Provider Manual, Chapter 1, for detailed information about the fee-for-service (FFS),
Primary Care Case Management (PCCM), and Risk Based Managed Care (RBMC)
delivery systems.
IHCP members enrolled in Medicaid Select PCCM receive the same benefit coverage,
and are subject to the same limitations as traditional Medicaid FFS. Refer to the
Medicaid Select Manual for Primary Medical Providers and Office Staff for further
information.
BILLING REQUIREMENTS
Coding and Reimbursement
Programmable hearing aids should only be approved using Healthcare Common
Procedure Coding System (HCPCS) code V5299, Hearing service, miscellaneous.
Utilizing the miscellaneous code for programmable hearing aids will allow the aid to be
manually priced, since the cost for the aids may vary greatly. Conventional hearing aids
should only be approved using the following HCPCS codes.
• V5050, Hearing aid, monaural, in the ear
• V5060, Hearing aid, monaural, behind the ear
• V5130, Binaural, in the ear
• V5140, Binaural, behind the ear
Unlike HCPCS codes V5130 and V5140 in which one unit is equal to two aids, one unit
of V5299 is equal to one programmable aid. However, if medical necessity is met for
binaural aids, two units may be approved.
01/31/2007 Medical Supplies and Equipment – Programmable Hearing Aids
Medical Policy Manual
394

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Medical Supplies and Equipment – Programmable Hearing Aids
Medical Policy Manual
395
Reimbursement is made at the lesser of 130% of the manufacturer’s cost invoice amount
or the provider’s usual and customary fee. A manufacturer’s cost invoice is required for
reimbursement. HCPCS code V5299 does not specifically describe programmable
hearing aids; therefore, other items may occasionally be requested under this code.
Providers should report the most appropriate code that describes the service provided, but
on an infrequent basis, it may be necessary to approve other services under this
miscellaneous code. All IHCP providers are responsible for verifying if a product or
service requires additional licensure under Home Medical Equipment (HME).
RELATED MEDICAL TOPICS
Medical Supplies and Equipment
Prior Authorization
RULES, CITATIONS, AND SOURCES
405 IAC 5-3, Prior Authorization
405 IAC 5-19, Medical Supplies and Equipment
405 IAC 5-29, Services Not Covered by Medicaid
Indiana Health Coverage Programs Provider Bulletin
BT200105-Programmable Hearing Aids
Indiana Health Coverage Programs Provider Newsletter
NL200409
Origination Date: 02/02/2001
Revisions and
Review
Reason Date
Review Scheduled 08/08/2006
APPLICABLE INDIANAAIM EDITS AND AUDITS
6000-Manual Pricing Required
6096-The CPT/HCPCS code billed is not payable
6768-Services not covered for telemedicine

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: MEDICAL SUPPLIES AND EQUIPMENT—
PROTHROMBIN TIME SELF-MANAGEMENT
MONITORS
DESCRIPTION:
Anticoagulants are increasingly being prescribed for a number of chronic and life-long
conditions. For patients who require long term anticoagulant therapy, the problems of
compliance, the use of drugs that interact with the anticoagulants, and fluctuations in
sensitivity to anticoagulants make periodic measurement of the prothrombin time (PT) or
International Normalized Ratio (INR) necessary. Studies have shown patients using home
prothrombin time monitors can achieve a degree of therapeutic effectiveness at least
comparable to patients in an anti-coagulation clinic.
MEDICAL TOPICS CROSS-REFERENCES:
Laboratory
Medical Supplies and Equipment
RULES, CITATIONS, AND SOURCES:
Initial Policy Issues
Effective Date Implementation
Date
Retroactive Date
BT200017 8/21/2000 10/5/2000 10/5/2000
405 IAC 5-18 7/25/1997 7/25/1997 7/25/1997
405 IAC 5-19 7/25/1997 7/25/1997 7/25/1997
Revisions:
APPLICABLE INDIANAAIM EDITS AND AUDITS:
6076 Limit of 4 units of strips or cuvettes per month
01/31/2007 Medical Supplies and Equipment – Prothrombin Time
Medical Policy Manual
396

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
COVERAGE CRITERIA:
Prior authorization must be obtained to purchase a home prothrombin time monitor. Prior
authorization will be granted when the physician has submitted documentation supporting
all of the following criteria, along with the appropriate medical diagnosis code(s) listed in
this bulletin. A copy of the physician’s order must accompany the request for prior
authorization.
The patient must have a medical condition requiring lifetime warfarin therapy and
monitoring of prothrombin time activity.
The patient must need to have frequent prothrombin time testing once a week or multiple
times per month.
The patient (or patient’s resident caregiver) must have the ability to use the prothrombin
time monitoring device after obtaining education on its proper use from the physician,
nurse, or appropriate health care professional. A certificate of completion of education or
training must be obtained and kept in the patient’s medical records.
The patient (or patient’s resident caregiver) must have a telephone in the home.
The patient (or patient’s resident caregiver) must agree to use the home monitoring
system in lieu of office or laboratory testing except when requested by the ordering
physician.
The patient (or patient’s resident caregiver) must not have any contraindications to or
inability to comply with anticoagulation therapy, such as:
A history of noncompliance during outpatient care;
Chronic alcoholism or other substance abuse; or
Memory impairment.
The use of self-management of oral anticoagulation can be used for, but is not limited to,
the medical conditions listed in Table 1.1.
Table 1.1 – Medical Conditions
Code(s) Description
V42.1, V42.2, and
V43.3
Prosthetic valve replacements (for example, mitral or aortic
valve replacements) or heart transplant
V42.0-V42.9 Organ or tissue replaced by transplant
V43.0-V43.89 Organ or tissue replaced by mechanical or prosthetic means
V53.31 Cardiac pacemaker
394.0-394.9 Mitral valve disease
395.0-395.9 Aortic valve disease
396-396.3, 396.8, 396.9 Mitral and aortic valve disease
401.9 HTN-hypertension
410.9 Post myocardial infarction
01/31/2007 Medical Supplies and Equipment – Prothrombin Time
Medical Policy Manual
397

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 1.1 – Medical Conditions
Code(s) Description
411.1 Angina
414.9 Chronic ischemic heart disease (coronary artery disease)
415.1-415.19 Pulmonary embolisms and infarction
427.31 Atrial fibrillation
425.4 Cardiovascular collagenosis (primarily cardiomyopathies)
427.9 Dysrhythmias
436 Acute cerebrovascular disease (includes CVA)
438 Late effects of cerebrovascular disease (old CVA)
443.9 Peripheral vascular disease, unspecified
453.0-453.9 Other venous embolism and thrombosis
Some underlying conditions that may cause venous embolism and/or thrombosis are as
follows:
Antiphospholipid antibodies
Anticardiolipin antibody
Congenital antithrombin deficiency
Hyperhomocysteinemia
Prothrombin 20210
Protein S deficiency
Systemic lupus erythematosus
Kawasaki disease with giant coronary aneurysms
Nephrotic syndrome
Cancer
Myelomeningocele
Factor V Leiden or activated protein C-resistance
Homozygous protein C deficiency
Hereditary thrombophilia
01/31/2007 Medical Supplies and Equipment – Prothrombin Time
Medical Policy Manual
398

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Medical Supplies and Equipment – Prothrombin Time
Medical Policy Manual
399
BILLING INSTRUCTIONS AND REIMBURSEMENT
Durable medical equipment providers or pharmacists supplying the monitors and testing
materials must bill only on the HCFA-1500 claim form. Only one home prothrombin
monitor per member is allowed. If multiple family members living in the same household
require use of the monitor, only one monitor will be allowed per household. Practitioners
and providers are to bill their usual and customary fee for the monitor and associated
testing supplies. The items listed in Table 1.2 are reimbursable.
Note: Rate of reimbursement may be different for risk-based managed
care members.
Table 1.2 – Reimbursable Items
Code Description Maximum
Allowed Charges
Per Unit
Z5093 Home Protime monitor, 1 unit = 1 monitor $1177
Z5094 Home Protime reagent (test) strips, 1 unit = 15 strips $75
Z5095 Home prothrombin time cuvettes, 1 unit = 6 cuvettes $48
Z5096 Batteries, standard AA, 1 unit = 1 battery $2.25
Z5097 Home Protime controls for strips, 1 unit = 1 box of
controls
$20
Z5098 Battery charger 110V, 1 unit = 1 charger $40
Codes A4258, lancet device, and A4259, lancets per box, can also be billed for blood
collecting devices when these are not included in the monitor kit or testing strips.

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: MEDICAL SUPPLIES AND EQUIPMENT—STANDERS
DESCRIPTION
“Standers” refers to a mechanical standing device that provides support and positioning,
and aids in decreasing postural instability by targeting specific muscle groups with
isokinetic exercises (a type of exercise that maintains constant torque and tension as
muscles contract).
This document is intended to serve as a general summary of the Indiana Health Coverage
Programs’ (IHCP) policies regarding this service. More specific information may be
found in the IHCP provider manual, program notices, the Indiana Administrative Code
(IAC) or other sources as appropriate. See the “Rules, Citations, and Sources” section of
this document for further information.
COVERAGE CRITERIA
IHCP will provide reimbursement for standers considered medically necessary in a non-
institutional setting. A written physician's order is required and prior authorization (PA)
is required. The purpose of this policy is to provide a guide for determining the medical
necessity of standers.
Types of Standers
Prone Standers
Prone standers support the front of the body. They lean the member forward at varying
angles to keep the member upright. Supports and straps are commonly placed at the
sides, feet, knees, buttocks, and trunk to hold the member in position. The supports can
be adjusted to accommodate growth. There are two types of prone standers, freestanding
units and lean-to units. Freestanding units have stable bases and stand independently
anywhere in the room. Lean-to units are dependent standers that lean against a stable
piece of furniture as support.
Supine Standers
Supine standers support the posterior surface of the body. The angle of most supine
standers can be adjusted from horizontal to vertical. The supine stander provides
assistance to a member who cannot stand fully upright to achieve a passive standing
position. Supine standers are usually equipped with lateral supports and anterior straps
01/31/2007 Medical Supplies and Equipment – Standers
Medical Policy Manual
400

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
positioned at the feet, knees, and trunk. Three types of supine standers include supine
frames, tables, and boards.
Vertical Standers
Vertical standers are recommended for members with good balance and trunk control.
Vertical standers provide the least amount of support of all standers and position
members in a fully upright position. Supports are placed at the knees, hips, and lower
torso. Three types of vertical standers include a vertical frame, a standing box, and a
standing table.
Multi-Positional Standers
Multi-positional standers have a full range of standing angle adjustments to supply
optimum standing positioning with the capability to convert to a prone, supine, and/or
vertical stander. Multi-positional standers typically come equipped with lateral, trunk,
and hip supports, as well as knee, foot, and body straps.
Sit-to-Stand Standers
Sit-to-stand standers allow the member to change positions from sitting to standing and
back. The stander assists the member to move to the standing position using manual
power, a hydraulic lift, or an electric lift. If the member lifts themselves manually, a
sling is commonly hooked behind the member and a foot positioner and knee block is
used to assist the member to extend their knees and hips to the standing position. The
hydraulic lift uses a gas-spring system with a push handle to assist in lifting the member’s
weight. Common sit-to stand standers are Easy Stand and Ovation products (with the
exception of the Easy Stand 6000 Glider).
PRIOR AUTHORIZATION
All initial requests for standers require PA and a completed medical clearance form
signed by the physician. A copy of a physical therapy (PT) and/or occupational therapy
(OT) evaluation within the last two months, which shows the patient’s functional and
cognitive baseline and ability to progress with therapy, will be required for the initial
prior authorization. The request for initial prior authorization must also include
documentation of medical necessity and a plan of care signed by the ordering physician.
Subsequent requests for prior authorization will require ongoing documentation
indicating progress towards goals up through the 15th month, and a completed medical
clearance form signed by a physician.
Plan of Care
The plan of care must include the following documentation.
1. Measurable goals for therapy and training
Therapy necessary to obtain a stander may be performed by a PT, OT, or family
member who has been properly trained to perform the necessary exercises.
01/31/2007 Medical Supplies and Equipment – Standers
Medical Policy Manual
401
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
2. Estimated amount of time the member is expected to stand
The member should be able to stand one hour a day or have the potential goal of
standing one hour a day. The member is not required to stand for one hour
continuously.
3. List expected benefits from utilizing the stander as an adjunctive therapy.
Examples of the benefits of passive standing include, but are not limited to, the
benefits as follows.
• Aids in the prevention of atrophy in the trunk and leg muscles
• Improves circulation to the trunk and lower extremities
• Prevents formation of decubiti (pressure sores) with changeable
positions
• Helps maintain bone integrity
• Reduces swelling in the lower extremities
• Improves range of motion
• Improves kidney and bladder function
• Decreases muscle spasms
• Strengthens the cardiovascular system and builds endurance
• Improves strength of the trunk and lower extremities
• Prevents or decreases muscle contractures
• Lessens or prevents progressive scoliosis
• Aids normal skeletal development
• Improves bowel function
General Diagnosis
The PA request must include an appropriate diagnosis demonstrating the medical
necessity for a stander. Diagnoses may include, but are not limited to, the following.
• 318.1 Severe mental retardation
• 318.2 Profound mental retardation
• 335.20 Amyotrophic lateral sclerosis
• 336.9 Unspecified disease of the spinal cord
• 340 Multiple sclerosis
• 341.9 Demyelinating disease of the central nervous system, unspecified
• 342.xx
Hemiplegia and hemiparalysis
• 343.0 Diplegia
• 343.2 Quadriplegia
• 343.9 Infantile cerebral palsy, unspecified
• 344.0x Quadriplegia
• 344.1 Paraplegia
• 348.3x Encephalopathy, not elsewhere classified
• 359.x Muscular dystrophies and other myopathies
• 741.9x Spina bifida, without mention of hydrocephalus
• 742.4 Other specified anomalies of brain
01/31/2007 Medical Supplies and Equipment – Standers
Medical Policy Manual
402
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• 783.4x Lack of expected normal physiological development in childhood
• 952.xx Spinal cord injury
• 995.55 Shaken infant syndrome
Multi-positional Stander PA criteria
When a multi-positional stander is requested, the provider must indicate the secondary
complications that justify the need for a multi-positional stander. Secondary
complications include, but are not limited to, the examples as follows.
• The member requires postural drainage
• The member requires suctioning while in the stander related to excessive
secretions
• The member has a history of postural hypotension
Additional documentation that must be included in the PA request for a multi-positional
stander includes the following.
• Specific muscle groups to be targeted for stretching and strengthening in the
stander and expected outcomes
• Specific orders indicating the proper positioning of the member in the stander
Sit-to-Stand PA Criteria
All requests for sit-to-stand standers will be considered on a case-by-case basis. All
diagnoses listed previously will be considered for sit-to-stand standers. The member
must be able to perform the following.
• Maneuver from the sitting to standing position without assistance
• Stand vertically or have the medical potential to stand vertically in the near future
Documentation of medical justification for a sit-to-stand stander must be included in the
PA request. Some examples of secondary conditions that may justify the need for a sit-
to-stand stander are as follows.
• Children who are not ready to stand fully upright, but are actively in
transition between sitting and standing
• Highly independent youth and adults who can stand vertically and safely
transfer alone
• Members who cannot stand for long periods of time due to contractures or
muscle weakness
• Members with orthostatic hypotension
Certain sit-to-stand standers, such as standers manufactured by Easy Stand, have a
mobility option. The mobility option is identified by two medium sized all-terrain tires
on the front of the stander and casters in the rear of the stander. Two maneuvering
wheels are placed at waist level and attached to a pulley system which allows the member
limited mobility in a small area. The IHCP will cover the mobility option as a
01/31/2007 Medical Supplies and Equipment – Standers
Medical Policy Manual
403
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
reimbursable accessory, included under the max fee for code E0637, Combination sit to
stand system, any size including pediatric, with seat lift feature, with or without wheels,
on a case-by-case basis. The mobility option will only be approved for members with
independent capabilities, and bilateral upper-body strength and coordination to maneuver
themselves.
Children are not required to be independent to meet the criteria for a sit-to-stand stander.
Decisions regarding approval for children will be made on a case by case basis.
NON-COVERED ITEMS
Some standers are categorized as mobile standers. These standers allow self-propulsion
in the standing position throughout large areas. Mobile standers are identified as having
large pneumatic wheels similar or identical to manual wheelchairs. Some mobile
standers are electrically powered. The IHCP will not provide reimbursement for mobile
standers. The following is a list of some mobile standers. This list is not all-inclusive.
• Rifton Mobile Stander (Rifton)
• Rifton Dynamic Stander (Rifton)
• Chameleon (Sammons Presto)
• Power Drive Stand Aid (Stand Aid of Iowa)
• Standing Dani Wheelstand (Standing Dani)
• Sprout Wheelstand (Standing Dani)
BILLING REQUIREMENTS
Suppliers should specify the brand name, model number, type of stander, and base price
of the stander on the PA request. Trays are included in the base price of a stander.
Upgraded trays will not be reimbursed. Certain supports and straps are included in the
base price of the stander, as noted previously in this document. Upgraded supports and
straps are considered on a case-by-case basis. An itemized list of any additional
attachments and accessories with the individual prices must be included with the PA
request.
The sit-to-stand stander should be billed with HCPCS code E0637. (Wheels in this
definition are considered casters by the IHCP.) Sit-to-stand standers will be suspended
for correct coding if requested with L1510. HCPCS code E0637 requires PA, and is
reimbursed at a max fee.
IHCP reimbursement is available with PA for HCPCS code E0638, Standing frame
system, one position (e.g., upright, supine or prone stander), any size, including
pediatric, with or without wheels. E0638 is a max fee priced code. Providers are to bill
their usual and customary charge for the equipment and will be reimbursed the lesser of
the submitted or max fee price.
01/31/2007 Medical Supplies and Equipment – Standers
Medical Policy Manual
404

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
The IHCP will provide reimbursement for thigh, hip, knee, ankle orthosis (THKAO)
reported with HCPCS code L1510, THKAO, standing frame, with or without tray and
accessories, for supine, prone, vertical, or multi-positional standers with PA. L1510 is a
manually priced code with a maximum cap. Providers are to bill their usual and
customary charge for the equipment and will be the reimbursed the lesser of 90% of the
manufacturer’s invoice price or the manual price cap. Attachments and accessories must
be included on the claim for the stander and will not be separately reimbursed.
Repairs and maintenance of standers are billed using E1399, Durable medical equipment,
miscellaneous, for replacement parts and E1340, Repair or non-routine service for
durable medical equipment requiring the skill of a technician, labor component, per 15
minutes, for labor charges as appropriate.
RELATED TOPICS
Medical Supplies and Equipment
RULES, CITATIONS, AND SOURCES
405 IAC 5-19-2 Medical Supplies and Equipment
Indiana Health Coverage Programs Provider Bulletins
BT200027 Standers
BT200401 New 2004 HCPCS Codes
Indiana Health Coverage Programs Provider Manual Version 5.1
Origination Date: 8/10/2000
Revisions and Reviews Reason Date
405 IAC 5-19-2 Medical Supplies and Equipment 8/25/97
Provider Bulletin 200027 Standers 8/10/00
405 IAC 5-19-2 Medical Supplies and Equipment 10/27/99
Provider Bulletin 200401 New 2004 HCPCS Codes 10/29/04
Review Update of pediatric requirements 07/31/06
APPLICABLE INDIANAAIM EDITS AND AUDITS
6065 – DME Total Rental Amount Not to Exceed Fee for Purchase
6080 – DME Rentals Limited to 15 Months
01/31/2007 Medical Supplies and Equipment – Standers
Medical Policy Manual
405
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Medical Supplies and Equipment – Standers
Medical Policy Manual
406
6096 – The CPT/HCPCS Code Billed is Not Payable According to the PPS
Reimbursement Methodology
6113 – DME Limited to $2000 Per Recipient per Calendar Year
6114 – DME Limited to $5000 Per Recipient per Lifetime
6652 – Multiple Surgeries Must be Billed on Same Claim
6768 – Services Not Covered for Telemedicine

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: MEDICAL SUPPLIES AND EQUIPMENT – STANDING
WHEELCHAIR
DESCRIPTION:
Half Power/Half Manual Standing Wheelchair
A half power/half manual standing wheelchair has a manual standing mechanism. The
manual standing mechanism functions to move the person in the chair from the sitting to
standing position. A lever is pushed to release the lift by gas cylinders while a rotation
handle is used on both sides of the chair to get to the standing position. While
transitioning to the standing position, the flip up armrests rotate inward to the chest to
provide chest support and prevent falling. Once the standing position is achieved, the
chair is stationary.
Another type of half power/half manual standing wheelchair is a manual rigid base
wheelchair that has an electric component for the standing position. The chair has the
functions as the electric/power wheelchair that has a manual standing mechanism. This
type of standing wheelchair also comes as a heavy-duty wheelchair for individuals
weighing 210 pounds or more. It is used primarily for individuals who have a high level
of quadriplegia, advanced cerebral palsy, muscular dystrophy, or multiple sclerosis. The
individual must have strong upper body strength to operate the standing mechanism.
Upper body strength is determined by evaluating if a patient can lift a 10-pound bar. An
individual must be diagnosed with a high level of paraplegia or low quadriplegia,
advanced cerebral palsy, advanced muscular dystrophy, or multiple sclerosis to qualify
for the wheelchair. In addition to significant upper body strength to operate the manual
portion of the chair, the individual must have the ability to move fingers to operate the
joystick that controls the power standing feature or the manual base of the chair.
Full Power Standing Wheelchair
A full power standing wheelchair is operated entirely by power. The standing
mechanism is controlled through the joystick. The joystick moves upward with the chair
to the standing position. Depending on the manufacturer, once the standing position is
achieved, an individual may drive the machine while in the standing position or be
stationary.
01/31/2007 Medical Supplies and Equipment – Standing Wheelchair
407
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Medical Supplies and Equipment – Standing Wheelchair
Medical Policy Manual
408
These types of wheelchairs are used primarily for individual’s who have a high level of
quadriplegia, advanced cerebral palsy, muscular dystrophy, or multiple sclerosis, have
little to no upper body strength, but do have some arm and hand control for joystick
operation.
MEDICAL TOPICS CROSS-REFERENCES:
Medical supplies and equipment
Medical supplies and equipment –standers
Medical supplies and equipment--wheelchairs
RULES, CITATIONS, AND SOURCES:
Initial Policy Issues
Effective Date
Implementation
Date
Retroactive Date
Revisions:
APPLICABLE INDIANAAIM EDITS AND AUDITS:
Not Applicable
COVERAGE DECISION:
The Indiana Health Coverage Programs (IHCP) does not cover standing wheelchairs
because there is insufficient clinical data to support the benefits of this equipment.
The manufacturers use criteria for an individual to qualify for a standing wheelchair
based upon coordination efforts with physical and occupational therapists and physician
providers.
Health Care Excel also sought the opinion of three Physical Medicine and Rehabilitation
specialists to formulate its recommendation to the IHCP, one of whom ambulates by
means of an electric wheelchair. These consultants agreed that there is insufficient
clinical research conducted on the benefits of standing wheelchairs to warrant coverage
by the IHCP.

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: MEDICAL SUPPLIES AND EQUIPMENT –
THAIRAPY
TM
VEST
DESCRIPTION:
The ThAIRapy
TM
Vest is a mechanical device that utilizes a vest and a generator to
loosen bronchial secretions and clear the airway. All requests for this durable medical
equipment device will continue to require prior authorization with an appropriate clinical
summary and physician prescription.
This document is intended to serve as a general summary of the Indiana Health Coverage
Programs’ (IHCP) policies regarding this service. More specific information may be
found in the IHCP Provider Manual, program notices, the Indiana Administrative Code
(IAC) or other sources as appropriate. See the “Rules, Citations, and Sources” section of
this document for further information.
COVERAGE CRITERIA
The following criteria must be met for the ThAIRapy
TM
Vest to be approved and covered
by the IHCP.
• A physician order
• The physician determines that the patient requires airway clearance therapy at
least once a day
• Recent pulmonary function study demonstrates
– a Forced Expiratory Volume (FEV1), 80% of predicted,
– a Forced Vital Capacity (FVC) 50% of predicted, and
– 25% decrease on small airway score (Forced Expiratory Flow [FEF] 25-
75) over one year
• Documentation supports that chest physiotherapy and/or flutter devices used
twice a day have been ineffective in managing bronchial secretions
• Documentation supports that family members and caregivers have been unable to
provide effective chest therapy
• The patient is at risk for continued hospitalization
• The patient does not have a cardiac condition
01/31/2007 Medical Supplies and Equipment – ThAIRapy Vest
Medical Policy Manual
409
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Limitations and Restrictions
Rental of the ThAIRapy
TM
Vest for three months is required before purchase of the
equipment is covered or reimbursable. At the end of three months, documentation is
required that the ThAIRapy
TM
Vest has been used at least 67% of the prescribed time.
Medical records must indicate patient compliance and tolerance before purchase will be
approved.
PRIOR AUTHORIZATION
Prior Authorization (PA) is required for the ThAIRapy vest. Please refer to the Fact Sheet
that corresponds with each additional service provided if applicable, for more information
on PA.
MANAGED CARE
For members enrolled in Risk Based Managed Care (RBMC), providers must contact the
member’s managed care organization (MCO) for more specific guidelines. Refer to the
Provider Manual, Chapter 1, for detailed information about the fee-for-service (FFS),
Primary Care Case Management (PCCM), and Risk Based Managed Care (RBMC)
delivery systems.
IHCP members enrolled in Medicaid Select PCCM receive the same benefit coverage,
and are subject to the same limitations as traditional Medicaid FFS. Refer to the
Medicaid Select Manual for Primary Medical Providers and Office Staff for further
information.
BILLING REQUIREMENTS
As of January 1, 2003, codes for the ThAIRapy vest were crosswalked from Health Care
Common Procedural Coding System national codes S8200, Chest compression vest and
S8205, Chest compression system generator and hoses (for use with chest compression
vest) to permanent code E0483, High frequency chest wall oscillation air-pulse generator
system, (includes hoses and vest), each.
Providers are responsible for determining if Home Medical Equipment (HME) licensure
is required to submit claims for particular products. Resources are available on the IHCP
website under Provider Code Sets.
01/31/2007 Medical Supplies and Equipment – ThAIRapy Vest
Medical Policy Manual
410

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Medical Supplies and Equipment – ThAIRapy Vest
Medical Policy Manual
411
RELATED MEDICAL TOPICS
Home Health Services
Medical Supplies and Equipment
RULES, CITATIONS, AND SOURCES:
405 Indiana Administrative Code (IAC) 5-19-1
405 Indiana Administrative Code (IAC) 5-25
Indiana Health Coverage Programs (IHCP) Provider Manual 2005
Origination Date: 12-31-2002
Revisions and Review Reason Date
IHCP Bulletin
BT200205
Policy Revision for Coverage of the
ThAIRapy
TM
Vest
02/01/2002
Review Scheduled 10/31/2006
APPLICABLE INDIANAAIM EDITS AND AUDITS:
6096–The CPT/HCPCS Code billed is not payable according to the PPS Reimbursement
Methodology
6065–DME total rental amount not to exceed fee for purchase
6080–DME rentals limited to 15 months
6104–DME rental from chiropractor of more than one month requires PA
6113–DME limited to $2,000 per member per calendar year
6114–DME limited to $5,000 per member per lifetime
6652–Multiple surgeries must be billed on the same claim
6768–Services not covered for telemedicine services

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: MEDICAL SUPPLIES AND EQUIPMENT—VAGUS
NERVE STIMULATOR FOR EPILEPSY
DESCRIPTION:
A vagus nerve stimulator is a small programmable device implanted under the skin. This
system works as a pacemaker for the brain. A wire lead under the skin connects the
device to the vagus nerve in the neck. The device produces weak electrical signals at
regular programmed intervals which help prevent the bursts of electrical activity in the
brain that cause seizures. The battery powered device can have the programming
adjusted by a physician as required without further surgical intervention. The vagus
nerve stimulator is indicated as an adjunctive therapy in reducing the frequency of
seizures in adults and adolescents older than 12 years old who have partial onset seizures
that are refractory to anti-epileptic medications and for which surgery has failed or is not
recommended.
This document is intended to serve as a general summary of the Indiana Health Coverage
Programs (IHCP) policies regarding this service. More specific information may be
found in the IHCP Provider Manual, program notices, the Indiana Administrative Code
(IAC), or other sources as appropriate. See the “Rules, Citations, and Sources” section of
this document for further information.
COVERAGE CRITERIA
IHCP reimbursement for implantation, revision, programming and reprogramming, and
removal of the vagus nerve stimulator is available for members older than 12 years of age
with medically intractable partial onset seizures and who are not otherwise surgical
candidates. Providers are required to perform this procedure on an outpatient basis
whenever medically possible. Implantation procedures and equipment require prior
authorization (PA) with documentation of medical necessity.
In situations where complicating factors require this procedure to be performed on an
inpatient basis, medical history and records should support the need for the inpatient
admission. PA is not required for the hospital admission or the device (reimbursement
for the device is included in the DRG payment). The device cannot be billed separately
for inpatients.
Members with an ominous prognosis or other limiting factors would not be considered
appropriate candidates for implantation of the vagus nerve stimulator (for example,
01/31/2007 Medical Supplies and Equipment – Vagus Nerve Stimulator for Epilepsy
Medical Policy Manual
412

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
members with an absent left vagus nerve, severe mental retardation, cerebral palsy,
stroke, progressive fatal neurologic disease, or progressive fatal medical disease).
Diagnosis and Procedure Codes
The International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-
9-CM) diagnosis and procedure codes listed in Tables 1 and 2 and the CPT procedure
codes noted in Table 3 are appropriate for reporting implantation, revision, programming
and reprogramming, and removal of a vagus nerve stimulator. Providers are advised to
utilize the most appropriate code for the service provided.
Table 1 – ICD-9-CM Diagnosis Codes for Reporting Vagus Nerve Stimulator
Services
Code Description
345.41 Partial epilepsy, with impairment of consciousness, with intractable epilepsy
345.51
Partial epilepsy, without mention of impairment of consciousness, with
intractable epilepsy
Table 2 – ICD-9-CM Procedure Codes for Reporting Vagus Nerve Stimulator
Services
Code Description
04.92 Implantation or replacement of peripheral neurostimulator lead(s)
04.93 Removal of peripheral neurostimulator lead(s)
86.94* Insertion or replacement of single array neurostimulator pulse generator, not
specified as rechargeable
86.95* Insertion or replacement of dual array neurostimulator pulse generator, not
specified as rechargeable
86.96* Insertion or replacement of other neurostimulator pulse generator
86.97* Insertion or replacement of single array rechargeable neurostimulator pulse
generator
86.98* Insertion or replacement of dual array rechargeable neurostimulator pulse
generator
*These codes were part of the ICD-9-CM annual update effective October 1, 2005
Table 3 – CPT Procedure Codes for Reporting Vagus Nerve Stimulator Services
Code Description
61885 Insertion or replacement of cranial neurostimulator pulse generator or
receiver, direct or inductive coupling, with connection to a single electrode
array
61888 Revision or removal of cranial neurostimulator pulse generator or receiver
64553 Percutaneous implantation of neurostimulator electrodes; cranial nerve
64573 Incision for implantation of neurostimulator electrodes; cranial nerve
64585 Revision or removal of peripheral neurostimulator electrodes
01/31/2007 Medical Supplies and Equipment – Vagus Nerve Stimulator for Epilepsy
Medical Policy Manual
413

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 3 – CPT Procedure Codes for Reporting Vagus Nerve Stimulator Services
(Cont’d)
Code Description
95970 Electronic analysis of implanted neurostimulator pulse generator system (eg,
rate, pulse amplitude and duration, configuration of wave form, battery
status, electrode selectability, output modulation, cycling, impedance, and
patient compliance measurements); simple or complex brain, spinal cord, or
peripheral (ie, cranial nerve, peripheral nerve, autonomic nerve,
neuromuscular) neurostimulator pulse generator/transmitter, without
reprogramming
95975 Electronic analysis of implanted neurostimulator pulse generator system (eg,
rate, pulse amplitude and duration, configuration of wave form, battery
status, electrode selectability, output modulation, cycling, impedance, and
patient compliance measurements); complex cranial nerve neurostimulator
pulse generator/transmitter, with intraoperative or subsequent programming,
each additional 30 minutes after first hour (List separately in addition to
code for primary procedure)
95975 Electronic analysis of implanted neurostimulator pulse generator system (eg,
rate, pulse amplitude and duration, configuration of wave form, battery
status, electrode selectability, output modulation, cycling, impedance, and
patient compliance measurements); complex cranial nerve neurostimulator
pulse generator/transmitter, with intraoperative or subsequent programming,
each additional 30 minutes after first hour
PRIOR AUTHORIZATION
PA must be obtained by the physician for the implantation procedures regardless of
setting. The following documentation must be maintained in the medical record and
submitted with the request for PA.
• Documentation that an evaluation has been made by a neurologist
• Documentation of the member’s type of epilepsy
• Documentation that the member’s seizures are medically intractable (member
continues with an unacceptable number of seizures with adequate treatment
consisting of two or more anti-epileptic drugs (AEDs) for a period of at least 12
months)
• Documentation that the member is not a surgical candidate or that surgery has
been unsuccessful (for example, the member is not a surgical candidate due to
multiple epileptic foci)
01/31/2007 Medical Supplies and Equipment – Vagus Nerve Stimulator for Epilepsy
Medical Policy Manual
414

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MANAGED CARE
For members enrolled in Risk Based Managed Care (RBMC), providers must contact the
member’s managed care organization (MCO) for more specific guidelines. Refer to the
Provider Manual, Chapter 1, for detailed information about the fee-for-service (FFS),
Primary Care Case Management (PCCM), and RBMC delivery systems.
IHCP members enrolled in Medicaid Select PCCM receive the same benefit coverage,
and are subject to the same limitations as traditional Medicaid FFS. Refer to the
Medicaid Select Manual for Primary Medical Providers and Office Staff for further
information.
BILLING GUIDELINES
Table 4, indicates the procedure codes to be used when billing for the incision,
implantation, revision, or removal of the vagus nerve stimulator. The CPT code must be
billed in conjunction with the appropriate revenue code on the UB-92 claim form. Also
included in the table are the corresponding ambulatory surgical center (ASC) groups and
the PA requirement. Claims for services provided by hospital outpatient and ambulatory
surgical centers must be billed with revenue codes 360 (Operating Room Services) or 490
(Ambulatory Surgical Care) on the UB-92 claim form.
Table 4 – Procedure Codes and Corresponding ASC Groups and Rates
Category
CPT
Code
Description
ASC
Group
PA Required
64573
Incision for implantation of
neurostimulator electrodes;
cranial nerve
Yes
64553
Percutaneous implantation of
neurostimulator electrodes;
cranial nerve
1
Yes
Implantation
61885
Insertion or replacement of
cranial neurostimulator pulse
generator or receiver, direct or
inductive coupling, with
connection to a single
electrode array
2 Yes
64585
Revision or removal of
peripheral neurostimulator
electrodes
A No
Revision/
Removal
61888
Revision or removal of cranial
neurostimulator pulse
generator or receiver
1 No
01/31/2007 Medical Supplies and Equipment – Vagus Nerve Stimulator for Epilepsy
Medical Policy Manual
415

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
The surgical procedure involves two separate incisions. Therefore, both CPT codes
64573 and 61885, or 64553 and 61885 should be used. Reimbursement is based on 100
percent of the highest ASC group and 50 percent for the second highest ASC group (no
additional reimbursement is available for three or more procedures).
Additional reimbursement, separate from the ASC rate for the implantation procedure
performed in an outpatient setting, will be allowed for the cost of the device. Providers
are to report their usual and customary charge for this device and will be reimbursed the
lesser of the submitted charges for the device or the maximum fee allowed. The device
must be billed on a CMS-1500 claim form using a DME provider number and prior
authorization must be obtained.
The appropriate HCPCS codes listed in Table 5 should be used when billing the device
and services related to the vagus nerve stimulator.
Table 5 – Codes for Additional Reimbursement for Vagus Nerve Stimulator
Devices
Code Description PA Requirement
L8680 Implantable neurostimulator electrode, each Yes
L8681
Patient programmer (external) for use with implantable
programmable neurostimulator pulse generator
Yes
L8685
Implantable neurostimulator pulse generator, single array,
rechargeable, includes extension
Yes
L8686
Implantable neurostimulator pulse generator, single array,
non-rechargeable, includes extension
Yes
L8687
Implantable neurostimulator pulse generator, dual array,
rechargeable, includes extension
Yes
L8688
Implantable neurostimulator pulse generator, dual array,
non-rechargeable, includes extension
Yes
L8689
External recharging system for implanted neurostimulator,
replacement only
Yes
Hospital Inpatient
In situations where a complicating factor is present and the patient requires admission to
the hospital for the procedure, the procedure and equipment will be reimbursed according
to the appropriate diagnosis related group (DRG) payment. PA is not required for the
admission or the device, which is included in the DRG reimbursement. However, the
physician must obtain PA for the surgical procedure. The hospital stay must be billed on
the UB-92 claim form and must include a secondary diagnosis indicating a complicating
factor that necessitated inpatient admission. Hospitals cannot receive additional
01/31/2007 Medical Supplies and Equipment – Vagus Nerve Stimulator for Epilepsy
Medical Policy Manual
416

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
reimbursement outside the DRG payment for the cost of the device. DRG payments for
inpatient procedures with complicating factors include reimbursement for the device.
Physician Billing Instructions
Physicians will bill the professional services on the CMS-1500 claim form (see Chapter 8 of
the Indiana Health Coverage Programs Provider Manual), using the appropriate procedure
codes in the following tables.
Table 6 – Surgeon CPT Procedure Codes
Category
CPT
Code
Description
PA
Required
64573
Incision for implantation of neurostimulator
electrodes; cranial nerve
Yes
64553
Percutaneous implantation of neurostimulator
electrodes; cranial nerve
Yes
Implanting
61885
Incision and subcutaneous placement of
cranial neurostimulator pulse generator and or
receiver, direct or indirect coupling with
connection to a single electrode array
Yes
64585
Revision or removal of peripheral
neurostimulator electrodes
No
Revision/
Removal
61888
Revision or removal of cranial
neurostimulator pulse generator or receiver
No
The following codes in Table 7 should be used by the physician to report interrogation
and programming services provided for members with implants.
Table 7 – Neurologist CPT Procedure Codes for Implanted Devices
Code Description
PA
Required
95970
Electronic analysis of implanted neurostimulator pulse
generator system (for example, rate, pulse amplitude and
duration, configuration of wave form, battery status, electrode
selectability, output modulation, cycling, impedance and
patient compliance measurements); simple or complex
neurostimulator pulse generator, without programming
No
95974
Complex cranial nerve neurostimulator pulse
generator/transmitter, with intraoperative or subsequent
programming, with or without nerve interface testing, first
hour
No
01/31/2007 Medical Supplies and Equipment – Vagus Nerve Stimulator for Epilepsy
Medical Policy Manual
417

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 7 – Neurologist CPT Procedure Codes for Implanted Devices
PA
Code Description
Required
95975
Complex cranial nerve neurostimulator pulse
generator/transmitter, with intraoperative or subsequent
programming, with or without nerve interface testing, each
additional 30 minutes after first hour (list separately in
addition to code for primary procedure)
No
RELATED MEDICAL TOPICS
Hospital Inpatient
Hospital Outpatient
Medical Supplies and Equipment
Surgical Services
RULES, CITATIONS, AND SOURCES
405 IAC 5-19 Medical Supplies and Equipment
405 IAC 5-28 Medical and Surgical Services
Indiana Health Coverage Programs Provider Bulletin (BT)
BT200032 – Coverage of NCP System-Vagus Nerve Stimulator
Indiana Health Coverage Programs Provider Banner (BR)
BR200537– Annual Update-ICD-9-CM
Indiana Health Coverage Programs Provider Manual
March, 2005, Version 5.1
Origination date: 04/30/2001
Reviews and
Revisions
Reason Date
BT200032
Coverage of NCP System-Vagus Nerve
Stimulator
10/23/2000
405 IAC 5-19 Medical Supplies and Equipment 8/25/1997
405 IAC 5-19 Medical Supplies and Equipment 10/27/1999
Review Scheduled 01/31/2007
APPLICABLE INDIANAAIM EDITS AND AUDITS
3003 – Procedure Code Requires Prior Authorization
6002 – Any Two Anesthesiology Providers Same Procedure Requires Review
6003 – Manual Pricing for Split Care Billing
6034 – Global Surgery Payable at Reduced Amount When Components of Surgical Care
Paid
6035 – Components of Surgical Care Not Payable When Global Surgery Paid
01/31/2007 Medical Supplies and Equipment – Vagus Nerve Stimulator for Epilepsy
Medical Policy Manual
418
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Medical Supplies and Equipment – Vagus Nerve Stimulator for Epilepsy
Medical Policy Manual
419
6037 – One Assistant Surgeon Allowed For Select Surgeries
6039 – Assistant Surgeon Not Payable When Co-Surgeon Paid
6040 – Co-Surgeon Not Payable When Assistant Surgeon Paid
6079 – Unbundling Audit for Vagus Nerve Stimulator
6096 – The CPT/HCPCS Code Billed Is Not Payable
6113 – DME Limited to $2,000 Per Recipient Per Calendar Year
6114 – DME Limited to $5,000 Per Recipient Per Calendar Year
6152 – Surgery Payable at Reduced Amount hen Consultation Paid Days Before or After
Surgery
6652 – Multiple Surgeries Must Be Billed on Same Claim
6661 – Duramorph Cannot Be Billed on Same Day as Surgery
6666 – Anesthesia Services Not Allowed By Provider Billing for Surgery
6768 – Services Not Covered for Telemedicine

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: MEDICAL SUPPLIES AND EQUIPMENT —
VENTRICULAR ASSIST DEVICE (VAD) SYSTEMS
DESCRIPTION
Ventricular Assist Devices (VADs) are mechanical circulatory assistive devices that
assist the heart in performing its pumping function. VADs can be used for short-term,
intermediate-term, and long-term support as a bridge-to-recovery, bridge-to-transplant,
and destination therapy.
VADs may be designed for use in the left ventricle (LVAD), the right ventricle (RVAD),
or for both ventricles (Bi-VAD). VADs are either pneumatic or electromechanical
pulsatile pumps that can operate in a synchronous mode (triggered by an EKG) or an
asynchronous mode. The electromechanical pumps are primarily battery operated, and
include a power hookup for battery recharging and for other necessary events.
This document is intended to serve as a general summary of the Indiana Health Coverage
Programs’ (IHCP) policies regarding this service. More specific information may be
found in the IHCP provider manual, program notices, the Indiana Administrative Code
(IAC) or other sources as appropriate. See the “Rules, Citations, and Sources” section of
this document for further information.
SUMMARY
Heart failure affects an estimated 5 million American, resulting in approximately 300,000
deaths annually. Of an estimated 16,500 Americans who would benefit from a heart
transplant each year, only 13% typically receive the surgery due to lack of available
transplant candidates. This lack of resources has increased the necessity of VADs for
qualified candidates.
VADs are used to assist the ventricles of the heart in pumping blood and therefore reduce
the heart’s workload. Due to this mechanism, VAD therapy has been shown to improve
heart function by allowing the heart to repair endocrine function and reverse remodeling.
Studies show that VAD therapy in conjunction with aggressive medical therapy may
provide increased medical benefits to cardiac patients following extended VAD use at the
time of cardiac transplantation.
01/31/2007 Medical Supplies and Equipment – VAD Device
Medical Policy Manual
420
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
VADs have been available for implantation since April 1993. The first Food and Drug
Administration (FDA) approved VAD was manufactured by Abiomed (the Abiomed
BVS 5000), and was indicated for postcardiotomy cardiogenic shock. Other VADs
indicated for cardiogenic shock include the Thoratec VAD and the TandemHeart
percutaneous transseptal ventricular assist (PTVA) device. VADs became covered by
Medicare October 18, 1993 for post-cardiotomy cardiogenic shock.
In 1994, the FDA approved VADs for bridge-to-transplant. Bridge-to-transplant is the
term used to describe an intermediate-term support by a VAD while awaiting a heart
transplant. Bridge-to-transplant is indicated for transplant candidates whose
hemodynamic status deteriorates despite maximal pharmacologic therapy or intra-aortic
balloon pump (IABP) assistance. On January 22, 1996 Medicare approved bridge-to-
transplant procedures. The Abiomed BVS 5000 may be used for bridge-to-transplant,
although its primary indication is for post-cardiotomy cardiogenic shock. Other VADs
indicated for bridge-to-transplant include the following.
• Thoratec Ventricular Assist Device System
• Novacor Ventricular Assist System
• HeartMate Left Ventricular Assistive Device (LVAD)
• HeartMate Vented-Electric VE-LVAD
• HeartMate “Sutures Not Applied” SNAP-VE
• HeartMate Implantable Pneumatic IP-VAD
• DeBakey VAD Child Left Ventricular Assist System
Initially bridge-to-transplant patients were confined to the hospital during treatment with
a VAD. In 1998, the FDA approved the VAD for use outside the hospital, and patients
were allowed to return to work during treatment with a VAD.
In July 2003, the FDA approved the HeartMate (extra long drive line) XVE-LVAD for
destination therapy based on substantial improvement in patient quality of life and
survival compared to pharmaceutical therapy. Destination therapy is permanent cardiac
support by a VAD for members who have chronic end-stage heart failure and are not
candidates for heart transplantation. Medicare approved coverage of VADs for
destination therapy October 1, 2003. The SNAP-VE, VE-LVAD, and XVE-LVAD are
approved for destination therapy.
USES OF VADS
Short-Term Support
VADs can be used for short-term support as a bridge-to-recovery (the term used to
describe a patient supported by a VAD until the heart recovers from temporary heart
failure) for patients who are experiencing postcardiotomy cardiogenic shock.
Postcardiotomy cardiogenic shock is heart failure following cardiac surgery. Conditions
that may necessitate heart surgery and ultimately lead to postcardiotomy cardiogenic
shock include myocarditis, myocardial infection, cardiomyopathy, arrhythmias, and acute
01/31/2007 Medical Supplies and Equipment – VAD Device
Medical Policy Manual
421
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
myocardial infarction (MI). Postoperatively, temporary mechanical circulation with a
VAD may be necessary to lead the patient to recovery. Patients who are placed on
cardiopulmonary bypass during surgery may also need a VAD in order to wean from
cardiopulmonary bypass.
Intermediate-Term Support
VADs are most commonly used as a bridge-to-transplant. Bridge-to-transplant is
indicated for candidates with end-stage heart failure (New York Heart Association Class
III or IV) whose hemodynamic status deteriorates despite maximal pharmacologic
therapy or intra-aortic balloon pump (IABP) assistance. The FDA lists hemodynamic
instability as noted on the next page.
• Pulmonary capillary wedge pressure less than 20mmHg
• Cardiac index less than or equal to 2L/minute/m
2
• Systolic BP less than or equal to 80mmHg
Contraindications to heart transplant, and therefore implantation of a VAD for bridge-to-
transplant, include, but are not limited to, elevated, fixed pulmonary hypertension or
severe pulmonary disease, respiratory failure, sepsis, renal failure, and severe
neurological deficit.
Improved cardiac function with extended VAD support has been reported due to
decreased demands on the heart. This creates a positive impact on mortality and the
rehabilitation potential for the transplant candidate. Reported benefits of VAD treatment
include increased cardiac output, improved cardiac index, decreased peripheral vascular
resistance, increased right ventricular ejection fraction, decreased LV diameter, improved
hemodynamic status, normalized of fluid load, improved renal and hepatic function, and
reversed passive pulmonary hypertension. Many patients will decrease from New York
Heart Association Class III or IV to Class I heart failure within three to four weeks.
Long-Term Support
LVADs are FDA approved for long-term support as destination therapy. Destination
therapy is defined as permanent mechanical cardiac support for individuals who are not
candidates for heart transplant. The individual must have New York Heart Association
Class IV, chronic end stage-heart failure, for at least 90 days, and have a life expectancy
of less than two years. The individual must have also failed to respond to medical
therapy.
The Randomized Evaluation, of Mechanical Assistance for the Treatment of Congestive
Heart Failure (REMATCH) study, comparing the use of an LVAD for destination therapy
to medical management, showed an increased one year and two year survival rate,
decreased mortality rate, and improved quality of life in the LVAD group. The LVAD
group also had a higher rate of side effects such as infection and bleeding. Members
01/31/2007 Medical Supplies and Equipment – VAD Device
Medical Policy Manual
422
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
receiving destination therapy can be treated at home and return to normal, or near normal
living, and working activities.
TYPES OF VADS
VADs can be used as left ventricular devices (LVADs), right ventricular devices
(RVADs) or biventricular devices (BI-VADS). VADs can either be pneumatic or
electromechanical. Pneumatic devices require that the patient be tethered to a large
console and remain in the hospital for the duration of treatment. Electrically powered
devices are operated by a battery pack the majority of use, and are plugged into a power
base unit during hospital stays, showering and battery recharging. Electromechanical
devices can be used in the hospital or home settings. VADs are pulsatile pumps that can
be synchronous or asynchronous. Asynchronous pumps operate independently of
cardiac rhythm and can be advantageous during potentially fatal arrhythmias.
The VAD pump is placed outside the heart while a cannula is inserted in the appropriate
ventricle(s) to pull the blood from the heart into the pump and circulate it to the body.
The VAD pump can be implanted in the body (intracorporeal) or sit outside the body
(extracorporeal).
A new type of VAD, called a percutaneous transseptal ventricular assist (PTVA), allows
implementation of mechanical support through a noninvasive technique that can be
performed in a catherization lab. The heart is accessed percutaneously by inserting a
cannula into the femoral vein, through the right atrium, into the left atrium, through a
transseptal puncture. The cannula pulls the blood from the left atrium into the VAD
pump which is externally attached to the thigh. A second cannula is placed in the
femoral artery and returns the blood for circulation throughout the body.
FDA APPROVED VADS
Abiomed Biventricular (BVS) 5000
The Abiomed BVS 5000 is a biventricular, extracorporeal, pneumatic VAD that can be
used as a LVAD, RVAD, or BI-VAD. The Abiomed BVS can be used in an
asynchronous mode for patients with arrhythmias. The Abiomed BVS is FDA approved
primarily for postcardiotomy cardiogenic shock, but is also approved for right ventricular
support for members currently using an intermediate-term LVAD. If cardiac recovery no
longer becomes possible following placement of the Abiomed BVS, the system may be
exchanged for a more permanent VAD.
Thoratec Ventricular Assist Device
The Thoratec VAD is an extracorporeal, pneumatic VAD that can be used as a LVAD,
RVAD, or Bi-VAD. The Thoratec VAD is limited to use in the hospital setting and is
FDA approved for bridge-to-recovery in postcardiotomy cardiogenic shock and also for
bridge-to-transplant.
01/31/2007 Medical Supplies and Equipment – VAD Device
Medical Policy Manual
423
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Novacor Ventricular Assist System
The Novacor Ventricular Assist System is a pulsatile, intracorporeal, electromechanical
VAD. The Novacor system is used as an LVAD for members who are candidates for
bridge-to-transplant. The Novacor system can be powered by rechargeable batteries or
an external power base unit and can be used in the hospital or home settings.
HeartMate Vented Electric Left Ventricular Systems (VE-LVAD, XVE-LVAD, and
SNAP-VE)
The HeartMate vented electric LVADs are pulsatile, intracorporeal, electromechanical
VADs. The devices have an implanted electric motor connected to a lightweight battery
pack. The vented electric LVADs can be used inside and outside the hospital setting, and
are FDA approved for bridge-to-transplant and destination therapy.
HeartMate Implantable Pneumatic Left Ventricular Assist Device (IP-LVAD)
The IP-LVAD is an implantable pump connected by a percutaneous air driveline to an
external, electrically driven air pump. The IP-LVAD is indicated for use in the hospital
setting and is FDA approved for bridge-to-transplant.
DeBakey VAD Child Left Ventricular Assist System
The DeBakey VAD has received a Humanitarian Device Exemption (February 2004) for
treatment of children ages 5-16 for bridge-to-transplant. The DeBakey VAD can be used
in the hospital or home setting.
TandemHeart Percutaneous Transseptal Ventricular Assist (PTVA) Device
The TandemHeart PTVA is an LVAD that can be implanted percutaneously in a
catheterization lab. A cannula is advanced into the femoral vein, through the right
atrium, and into the left atrium. The external pump pulls the blood from the atrium back
to the pump, then circulates the blood throughout the body through a second cannula in
the femoral artery. The TandemHeart PTVA is used in the hospital and is FDA approved
for bridge-to-recovery in postcardiotomy cardiogenic shock.
COVERAGE CRITERIA
VADs, including LVADs, RVADs, and BI-VADs, are considered medically necessary by
the IHCP under the following conditions.
1. Treatment of postcardiotomy cardiogenic shock is covered by the IHCP when
ventricular dysfunction continues after maximum medical therapy or as a means
of myocardial recovery support for individuals who are unable to be weaned off
cardiopulmonary bypass with maximal inotropic support and use of an intraaortic
balloon pump.
2. Bridge-to-transplant is covered by the IHCP for members who meet the
following criteria.
01/31/2007 Medical Supplies and Equipment – VAD Device
Medical Policy Manual
424
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
a) The member must be at risk of imminent death from nonreversible left
ventricular failure (NYHA Class III or IV).
b) The member has been prior authorized for a heart transplant (excluding dual
eligible members).
c) The member is listed as a candidate for heart transplantation by a
Medicare/Medicaid approved heart transplant center.
d) If the VAD is implanted at a different site than the Medicare/Medicaid
approved transplant center, the implanting site must receive written
permission from the Medicare/Medicaid approved center under which the
patient is listed prior to implantation of the VAD.
3. Destination therapy is covered by the IHCP for members who meet the
following criteria.
a) The member must not be a candidate for heart transplant.
b) The member must have chronic end-stage heart failure (NYHA Class IV) for
at least 90 days, and have a life expectancy of less than 2 years.
c) The member’s Class IV heart failure symptoms must have failed to respond to
optimal medical therapy for at least 60 of the last 90 days. Medical therapy
must include the treatments as listed.
• Salt restriction
• Diuretics
• Digitalis
• Beta-blockers
• ACE inhibitors (if tolerated)
d) Left ventricular ejection fraction (LVEF) must be less than 25%.
e) The member has demonstrated functional limitation with a peak oxygen
consumption of less than 12ml/kg/min; or continued need for IV inotropic
therapy due to symptomatic hypotension, decreasing renal function, or
worsening pulmonary congestion.
f) The member has the appropriate body size (greater than or equal to 1.5m
2
)
to
support the LVAD implantation.
g) VAD implantation must occur at a Medicare/Medicaid approved heart
transplant center.
A VAD is a covered service for postcardiotomy cardiogenic shock or bridge-to-transplant
only if it has received approval from the FDA for the intended purpose, and only if it is
used according to the FDA-approved labeling instructions for that intended purpose.
A VAD is a covered service for destination therapy only if it has received approval from
the FDA for destination therapy or as a bridge-to-transplant, or has been implanted as
part of an FDA investigational device exemption trial for one of these two indications.
01/31/2007 Medical Supplies and Equipment – VAD Device
Medical Policy Manual
425

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
NONCOVERED SERVICES
1. VADs are noncovered for all other conditions not listed above.
2. Use of a non-FDA approved VAD is considered investigational and is a
noncovered service.
3. The artificial heart (i.e. AbioCor, CardioWest) as a replacement heart for a
diseased heart is noncovered by the IHCP.
PRIOR AUTHORIZATION CRITERIA
VADs, including LVADs, RVADs, and BI-VADs, and their surgical implantation do not
require prior authorization. Members who receive bridge-to-transplant or destination
therapy, and who can continue therapy on an outpatient basis, will require accessory
equipment for use with the VAD. The patient supply and replacement equipment, listed
in Table 1, on the following page, require prior authorization.
Stationary Power Base and Display Module
• The power base is the electrical supply unit for the VAD. It provides
tethered functioning of the VAD by powering the VAD and
simultaneously recharging the batteries. The display module provides
pump functioning information for the physician in order to evaluate
patient status.
• The power base is purchased by the hospital or DME provider as a capital
expense and loaned to the member. The hospital or DME provider is
reimbursed a rental payment while the equipment is being used on an
outpatient basis by the member.
• The physician must submit a prior authorization request for HCPCS code
L9900 and the RR modifier.
Patient Supplies and Replacement Equipment
• Includes system controller, rechargeable batteries, a travel case, a shower
kit, and other miscellaneous supplies.
• The hospital or DME provider must supply the patient supplies and
replacement equipment.
Table 1 – VAD HCPCS Codes Requiring Prior Authorization
Code Description Effective
Date
Q0480
Drive for use with pneumatic ventricular assist device,
replacement only
2005/10/01
Q0481
Microprocessor control unit for use with electric ventricular
assist device, replacement only
2005/10/01
Q0482
Microprocessor control unit for use with electric/pneumatic
combination ventricular assist device, replacement only
2005/10/01
Q0483 Monitor/display module for use with electric ventricular assist 2005/10/01
01/31/2007 Medical Supplies and Equipment – VAD Device
Medical Policy Manual
426

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 1 – VAD HCPCS Codes Requiring Prior Authorization
Code Description Effective
Date
device, replacement only
Q0484
Monitor/display module for use with electric or
electric/pneumatic ventricular assist device, replacement only
2005/10/01
Q0485
Monitor control cable for use with electric ventricular assist
device, replacement only
2005/10/01
Q0486
Monitor control cable for use with electric/pneumatic
ventricular assist device, replacement only
2005/10/01
Q0487
Leads (pneumatic/electrical) for use with any type
electric/pneumatic ventricular assist device, replacement only
2005/10/01
Q0488
Power pack base for use with electric ventricular assist device,
replacement only
2005/10/01
Q0489
Power pack base for use with electric/pneumatic ventricular
assist device, replacement only
2005/10/01
Q0490
Emergency power source for use with electric ventricular
assist device, replacement only
2005/10/01
Q0491
Emergency power source for use with electric/pneumatic
ventricular assist device, replacement only
2005/10/01
Q0492
Emergency power supply cable for use with electric
ventricular assist device, replacement only
2005/10/01
Q0493
Emergency power supply cable for use with electric/pneumatic
ventricular assist device, replacement only
2005/10/01
Q0494
Emergency hand pump for use with electric or
electric/pneumatic ventricular assist device, replacement only
2005/10/01
Q0495
Battery/power pack charger for use with electric or
electric/pneumatic ventricular assist device, replacement only
2005/10/01
Q0496
Battery for use with electric or electric/pneumatic ventricular
assist device, replacement only
2005/10/01
Q0497
Battery clips for use with electric or electric/pneumatic
ventricular assist device, replacement only
2005/10/01
Q0498
Holster for use with electric or electric/pneumatic ventricular
assist device, replacement only
2005/10/01
Q0499
Belt/vest for use with electric or electric/pneumatic ventricular
assist device, replacement only
2005/10/01
Q0500
Filters for use with electric or electric/pneumatic ventricular
assist device, replacement only
2005/10/01
Q0501
Shower cover for use with electric or electric/pneumatic
ventricular assist device, replacement only
2005/10/01
Q0502
Mobility cart for pneumatic ventricular assist device,
replacement only
2005/10/01
Q0503
Battery for pneumatic ventricular assist device, replacement
only
2005/10/01
Q0503
Battery for pneumatic ventricular assist device, replacement
only, each
2005/10/01
01/31/2007 Medical Supplies and Equipment – VAD Device
Medical Policy Manual
427

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 1 – VAD HCPCS Codes Requiring Prior Authorization
Code Description Effective
Date
Q0504
Power adapter for pneumatic ventricular assist device,
replacement only, vehicle type
2005/10/01
Q0505
Miscellaneous supply or accessory for use with ventricular
assist device
2005/10/01
POST PAYMENT REVIEW
IHCP covered services for implantation of VADs for postcardiotomy cardiogenic shock,
bridge-to-transplant, and destination therapy are subject to post payment review.
Providers must maintain documentation in the member’s medical record that indicates
that all criteria listed under the IHCP “Coverage Criteria” have been met for
implantation of a VAD. If all of the criteria for implantation are not satisfied,
reimbursement of funds may be recouped, including surgical fees, professional fees, and
equipment costs.
MANAGED CARE
Questions regarding coverage of services to Risk Based Managed Care (RBMC)
members should be directed to the appropriate Managed Care Organization (MCO).
CODING AND BILLING INSTRUCTIONS
Tables 2-4 list the appropriate codes for billing implantation and removal of the VADs.
Table 2, on the next page, lists the diagnosis codes appropriate for implantation of a
VAD. The diagnosis code is to be billed on the UB-92 claim form with the
corresponding ICD-9-CM procedure code.
Table 2 – ICD-9-CM Diagnosis Codes
Code Description
410.xx Acute myocardial infarction
411.1 Intermediate coronary syndrome
411.81 Acute coronary occlusion without myocardial infarction
414.9 Chronic ischemic heart disease, unspecified
422.xx Acute myocarditis in disease classified elsewhere
425.x Cardiomyopathy
426.xx Conduction disorders
427.xx Cardiac dysrhythmias
428.xx Heart Failure
01/31/2007 Medical Supplies and Equipment – VAD Device
Medical Policy Manual
428

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 2 – ICD-9-CM Diagnosis Codes
Code Description
429.4 Functional disturbances following cardiac surgery
785.51 Cardiogenic shock
997.1 Cardiac complications
* xx represents diagnosis code placeholders. For example, 410.xx means all diagnoses in the 410 series are
applicable.
Table 3 lists the applicable ICD-9-CM Procedure codes for implantation, repair and
removal of a VAD. The ICD-9-CM code must be billed on the UB-92 claim form and
will be incorporated into the DRG payment.
Table 3 – ICD-9 Procedure Codes
Code Description
37.63
Repair of heart assist system
Replacement of parts of an existing VAD
37.64 Removal of heart assist system
37.65
Implant of external heart assist system
Device (outside the body but connected to heart) with external circulation and
pump
Includes: open chest procedure for cannula attachments
37.66
Implant of implantable heart assist system
Device directly connected to the heart and implanted in the upper left quadrant
of peritoneal cavity
Includes: LVAD
Pulsatile heart assist system
RVAD
Rotary pump heart assist system
Transportable, implantable heart assist system
VAD, not otherwise specified
Table 4 lists the applicable CPT codes for the physician component of the implantation
and removal of a VAD. The CPT code should be billed on a CMS-1500 claim form or
837P electronic transaction.
Table 4 – CPT Procedure Codes
Code Description
33975 Insertion of ventricular assist device; extracorporeal, single ventricle
33976 Insertion of ventricular assist device; extracorporeal, biventricular
33977 Removal of ventricular assist device; extracorporeal, single ventricle
33978 Removal of ventricular assist device; extracorporeal, biventricular
33979 Insertion of ventricular assist device, implantable, intracorporeal, single ventricle
33980 Removal of ventricular assist device, implantable intracorporeal, single ventricle
0048T
Implantation of a ventricular assist device, extracorporeal, percutaneous
transseptal access, single or dual cannulation
0049T Prolonged extracorporeal percutaneous transseptal ventricular assist device,
01/31/2007 Medical Supplies and Equipment – VAD Device
Medical Policy Manual
429

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 4 – CPT Procedure Codes
Code Description
greater than 24 hours, each subsequent 24 hours period
0050T
Removal of a ventricular assist device, extracorporeal, percutaneous transseptal
access, single or dual cannulation
Items Included in the DRG for Hospital Inpatients Utilizing the VAD System
1. ICD-9-CM Diagnoses (Primary, Secondary, Tertiary, as needed)
2. ICD-9-CM Procedures
3. VAD (included in the ICD-9-CM Procedure code)
4. Stationary Power Base and Display Module (capital purchase by the hospital)
5. Rechargeable batteries and harness (for untethered systems)
6. Miscellaneous Supplies
Billing Instructions for Outpatient Equipment Utilizing the CMS-1500 Claim Form
1. Prior authorization must be obtained for LVAD equipment.
2. The description of the Power Unit and Display Module should be entered on a
detail line with HCPCS code L9900 placed in locator 24d of the CMS-1500 claim
form. The total rental price may not exceed the purchase price.
3. The description of the accessories should be placed on a second detail line with
the appropriate HCPCS code in locator 24d of the CMS-1500 claim form.
4. An invoice for each detail must accompany the CMS-1500 claim form when
submitted.
RELATED MEDICAL TOPICS
Hospital Inpatient
Hospital Outpatient
Medical Supplies and Equipment
Physician Services
Surgery
Surgery - Transplants
Origination Date: 7/31/2001
Reviews and Revisions Reason Date
Revision
Addition of new CPT Codes and ICD-9-
CM procedure codes
10/29/2004
Revision
Addition of coverage of postcardiotomy
cardiogenic shock, destination therapy,
and a summary of types and uses of
VADS, addition of HCPCS code for
reporting replacement parts.
01/31/06
01/31/2007 Medical Supplies and Equipment – VAD Device
Medical Policy Manual
430

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Medical Supplies and Equipment – VAD Device
Medical Policy Manual
431
RULES, CITATIONS, AND SOURCES
405 IAC 5-28
405 IAC 5-19
APPLICABLE INDIANAAIM EDITS AND AUDITS
6649 – Surgery payable at reduced amount when related post-operative care paid
6653 – Post-operative care within 0-90 days of surgery
6654 – Pre-operative care within one day of surgery
6655 – Surgery payable at reduced amount when pre-operative care paid
6002 – Any two anesthesiology provider same procedure
6003 – Manual pricing for split care billing
6034 – Global surgery payable at reduced amount when components of surgical care paid
6035 – Components of surgical care not payable when global surgery paid
6039 – Assistant surgeon not payable when co-surgeon paid
6040 – Co-surgeon paid at reduced amount when assistant surgeon paid
6661 – Duramorph can not be billed on same day as surgery
6666 – Anesthesia services not allowed by provider billing for surgery
6037 – Only one assistant surgeon allowed for select surgeries
6652 – Multiple surgeries must be billed on same claim
6152 – Surgery payable at reduced amount when consult paid

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: MEDICAL SUPPLIES AND DURABLE MEDICAL
EQUIPMENT – WHEELCHAIR ACCESSORIES
DESCRIPTION
This fact sheet describes the different wheelchair accessories covered by the Indiana
Health Coverage Programs (IHCP). The following is a list of the wheelchair accessories
included in this fact sheet.
• Programmable electronic parts
• Wheelchair cushions
• Wheelchair positioning accessories
• Mounting hardware
• Universal headrest plates
• Power seating systems
• Matrix Seating System
This document is intended to serve as a general summary of the IHCP policies regarding
this service. More specific information may be found in the IHCP provider manual,
program notices, the Indiana Administrative Code (IAC) or other sources as appropriate.
See the “Rules, Citations, and Sources” section of this document for further information.
I. PROGRAMMABLE ELECTRONIC PARTS
A. Coverage Criteria
The IHCP covers programmable electronic parts for motorized/power wheelchair bases
with PA under the following circumstances.
1. The IHCP will provide separate reimbursement for medically necessary
programmable electronic system upgrades to a system that comes standard on a
specific wheelchair model for motorized/power wheelchair bases.
2. The IHCP covers adaptive switch controls, such as a sip and puff, or additional
drive controls and programming not available on the basic one-drive electronic
system for patients with degenerative diseases.
01/31/2007 Medical Supplies and Equipment – Wheelchair Accessories
Medical Policy Manual
432
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Programmable electronic systems that come standard on a specific motorized/power
wheelchair model cannot be billed separately because total reimbursement for a
motorized/power wheelchair with programmable electronics, coded as K0011, includes
the programmable electronic system.
Programmable electronic upgrades include items such as MKIV electronics. MKIV
electronics offers at least three functions over that of standard electronics, and may be
deemed medically necessary for select patients. The elements of MKIV electronics
include: smoother driving over a variety of terrain, increased speed of the chair with
quick performance selections, and the ability to program for special finger control
operation for patients who cannot effectively operate a joystick due to spasticity of limbs
or other muscular conditions.
B. Prior Authorization
The following PA criteria must be met for approval of programmable electronic parts.
• Providers must submit documentation that the member’s condition, mobility
needs, and/or prognosis supports the medical necessity for a programmable
electronic system upgrade, such as an upgrade from an Invacare MKIV RII
system, to an Invacare MKIV A system, or for an adaptive control, such as sip
and puff.
• A completed IHCP medical clearance form, to be submitted with the PA request,
for rental or purchase of the upgraded programmable electronic parts or adaptive
controls. The medical clearance form must be reviewed and signed by a
physiatrist.
• The member’s physician may prescribe a motorized/power wheelchair. However,
the medical necessity must be reviewed and the medical clearance form must be
approved and signed by a physiatrist prior to the form being submitted to the PA
department. A member is only required to see the physiatrist if the physiatrist
requests to see the member after a review of the documentation. If a physiatrist
requests to see a member after reviewing the documentation, the member would
then be required to travel to the nearest physiatrist. Providers should note that if
the physiatrist does not choose to see the member for an evaluation, the IHCP will
not provide reimbursement to the physiatrist for a chart review.
• When submitting a request for K0014, other motorized/power wheelchair base,
the PA must include documentation indicating the model, brand name, model
number and a statement documenting the medical necessity of the base for the
particular member including why customization is needed.
01/31/2007 Medical Supplies and Equipment – Wheelchair Accessories
Medical Policy Manual
433

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
C. Coding and Billing
Upgrades of programmable electronic systems are to be billed using HCPCS code
K0108, Wheelchair component or accessory not otherwise specified, with the KA
modifier, add-on option accessory. The cost of the upgraded electronics must not already
be included in the base wheelchair code. K0108 will be covered for purchase only and
will be reimbursed at the maximum fee of $1,950.
Providers must bill their usual and customary charge for the particular programmable
electronic system or joystick upgrade installed on the wheelchair base and must attach
either a provider’s cost invoice or retail price invoice to document the cost or price of the
upgraded electronic system. The invoice must include, at a minimum, supporting
information such as the item or part number, the description of the item, the quantity
provided, and the manufacturer’s name. In addition to the invoice, providers should
include supporting documentation that details the difference in the cost or price between
the electronic system that came standard on the wheelchair base and the upgraded
electronic system.
When billing K0108 for a programmable electronic system upgrade, the wheelchair base
must be billed using HCPCS code K0014. K0014 is a manually priced code. By using
K0014, the IHCP reimburses for the wheelchair base only, without the electronics.
Billing in this manner allows the IHCP to make accurate payment decisions for medically
necessary electronic system upgrades. The wheelchair base and electronic system
upgrade should be billed on the same CMS-1500 or 837 electronic transaction. Providers
may refer to IHCP bulletin BT200335 for examples of IHCP coding and reimbursement
for programmable electronic system upgrades.
Programmable adaptive controls, such as sip and puff, must be billed using the
appropriate HCPCS E code shown in Table 1. The adaptive controls are reimbursed at
the IHCP max fee on file. The wheelchair base must be billed using K0014.
Table 1 – Coding for Adaptive Controls
Code Definition
E2310
Power wheelchair accessory, electronic connection between wheelchair
controller and one power seating system motor, including all related
electronics, indicator feature, mechanical function selection switch, and fixed
mounting hardware
E2311
Power wheelchair accessory, electronic connection between wheelchair
controller and two or more power seating system motors, including all related
electronics, indicator feature, mechanical function selection switch, and fixed
mounting hardware
E2320
Power wheelchair accessory, hand or chin control interface, remote joystick or
touchpad, proportional, including all related electronics, and fixed mounting
hardware
E2321
Power wheelchair accessory, hand control interface, remote joystick,
nonproportional, including all related electronics, mechanical stop switch, and
01/31/2007 Medical Supplies and Equipment – Wheelchair Accessories
Medical Policy Manual
434

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Code Definition
fixed mounting hardware
E2322
Power wheelchair accessory, hand control interface, multiple mechanical
switches, nonproportional, including all related electronics, mechanical stop
switch, and fixed mounting hardware
E2323
Power wheelchair accessory, specialty joystick handle for hand control
interface, prefabricated
E2324 Power wheelchair accessory, chin cup for chin control interface
E2325
Power wheelchair accessory, sip and puff interface, nonproportional, including
all related electronics, mechanical stop switch, and manual swingaway
mounting hardware
E2326 Power wheelchair accessory, breath tube kit for sip and puff interface
E2327 Power wheelchair accessory, head control interface, mechanical, proportional,
including all related electronics, mechanical direction change switch, and fixed
mounting hardware
E2328
Power wheelchair accessory, head control or extremity control interface,
electronic, proportional, including all related electronics and fixed mounting
hardware
E2329
Power wheelchair accessory, head control interface, contact switch mechanism,
nonproportional, including all related electronics, mechanical stop switch,
mechanical direction change switch, head array, and fixed mounting hardware
E2330
Power wheelchair accessory, head control interface, proximity switch
mechanism, nonproportional, including all related electronics, mechanical stop
switch, mechanical direction change switch, head array, and fixed mounting
hardware
E2331
Power wheelchair accessory, attendant control, proportional, including all
related electronics and fixed mounting hardware
E2399
Power wheelchair accessory, not otherwise classified interface, including all
related electronics and any type mounting hardware
II. WHEELCHAIR CUSHIONS
A. Coverage Criteria
The IHCP reimburses wheelchair seat and back cushions for members with either a
manual or motorized/power wheelchair, when medically necessary. Reimbursable
cushions include general seat and back cushions, nonadjustable skin protection seat
cushions, adjustable skin protection seat cushions, positioning seat and back cushions,
nonadjustable skin protection and positioning seat cushions, and adjustable skin
protection and positioning seat cushions. Certain cushions require PA.
01/31/2007 Medical Supplies and Equipment – Wheelchair Accessories
Medical Policy Manual
435
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
B. Prior Authorization
General use seat cushions (E2601 and E2602) and general use back cushions (E2611 and
E2612) are covered for any member who has a wheelchair, regardless of their diagnosis.
General use cushions do not require PA.
PA is required for all other wheelchair seat and back cushions. The PA criteria for these
cushions are listed on the next page.
Nonadjustable skin protection seat cushions (E2603 and E2604) and adjustable skin
protection seat cushions (K0108 U1 and K0108 U2) are covered under the following
conditions.
1. The member must have a wheelchair.
2. The patient has either of the following conditions.
a) Current pressure ulcer of the lower back, hip, or buttock (707.03-707.05)
or past history of a pressure ulcer on one of the same areas; or
b) Absent or impaired sensation in the area of contact with the seating
surface or inability to carry out a functional weight shift due to one or
more of the following diagnoses:
• Spinal cord injury resulting in quadriplegia or paraplegia
(344.00-344.1)
• Other spinal cord disease (336.0-336.3)
• Multiple sclerosis (340)
• Other demylinating disease (341.0-341.9)
• Cerebral palsy (343.0-343.9)
• Anterior horn cell disease including amyotrophic lateral sclerosis
(335.0-335.21, 335.23-335.9)
• Post polio paralysis (138)
• Traumatic brain injury resulting in quadriplegia (344.09)
• Spina bifida (741.00-741.93)
• Childhood cerebral degeneration (330.0-330.9)
• Alzheimer’s disease (331.0)
• Parkinson’s disease (332.0)
Positioning seat cushions (E2605 and E2606) and positioning back cushions (E2613-
E2616, E2620) are covered under the following conditions.
1. The member must have a wheelchair.
01/31/2007 Medical Supplies and Equipment – Wheelchair Accessories
Medical Policy Manual
436
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
2. The member has significant postural asymmetries that are due to one or more of
the diagnoses listed in 2b above, or of the following diagnoses:
a) Monoplegia of the lower limb (344.30-344.32, 438.40-438.42)
b) Hemiplegia due to stroke, TBI or other injury (342.00-342.92, 438.20-
438.22)
c) Muscular dystrophy (359.0, 359.1)
d) Torsion dystonias (333.4, 333.6, 333.7)
e) Spinocerebellar disease (334.0-334.9)
Nonadjustable skin protection and positioning seat cushions (E2607 and E2608) and
adjustable skin protection and positioning seat cushions (K0108 U3 and K0108 U4) are
covered under the following conditions.
1. The member must have a wheelchair
2. The member must meet the criteria for coverage of both a skin protection seat
cushion and a positioning seat cushion.
A custom fabricated seat cushion (E2609) and a custom fabricated back cushion (E2617)
are covered under the following conditions.
1. The member must have a wheelchair.
2. The cushion must meet the IHCP guidelines for custom DME.
3. The prescribing physician must submit comprehensive documentation that
explains what other prefabricated seat or back cushions have been tried, and why
these items have not been sufficient to meet the patients needs.
4. The member must meet all criteria necessary for the approval of a prefabricated
seat or back cushion.
C. Coding and Billing
Table 2 lists the codes available for reporting wheelchair seat and back cushions. The
provider should choose the most appropriate code for billing on the CMS-1500 and/or the
837P electronic claim. Providers may refer to the Statistical Analysis Durable Medical
Equipment Regional Carrier (SADMERC) as a guideline for coding wheelchair cushions.
The IHCP does not limit reimbursement of seat or back cushions to the cushions listed in
the SADMERC classification list. If a provider chooses to supply a cushion that is not
listed on the SADMERC classification list, it is the provider’s responsibility to submit the
HCPCS code that most appropriately represents the type of cushion being supplied.
01/31/2007 Medical Supplies and Equipment – Wheelchair Accessories
Medical Policy Manual
437

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 2 – Wheelchair Cushion Codes
Code Description
E2601 General use wheelchair seat cushion, width less than 22 inches, any depth
E2602 General use wheelchair seat cushion, width 22 inches or greater, any depth
E2603
Skin protection wheelchair seat cushion, nonadjustable, width less than 22 inches,
any depth
K0108
U1
Skin protection wheelchair seat cushion, adjustable, width less than 22 inches
E2604
Skin protection wheelchair seat cushion, nonadjustable, width 22 inches or
greater, any depth
K0108
U2
Skin protection wheelchair seat cushion, adjustable, width greater than or equal
to 22 inches
E2605 Positioning wheelchair seat cushion, width less than 22 inches, any depth
E2606 Positioning wheelchair seat cushion, width 22 inches or greater, any depth
E2607
Skin protection and positioning wheelchair seat cushion, nonadjustable, width
less than 22 inches, any depth
K0108
U3
Skin protection and positioning wheelchair seat cushion, adjustable, width less
than 22 inches
E2608
Skin protection and positioning wheelchair seat cushion, nonadjustable, width
22 inches or greater, any depth
K0108
U4
Skin protection and positioning wheelchair seat cushion, adjustable, width
greater than or equal to 22 inches
E2609 Custom fabricated wheelchair seat cushion, any size
E2611
General use wheelchair back cushion, width less than 22 inches, any height,
including any type mounting hardware
E2612
General use wheelchair back cushion, width 22 inches or greater, any height,
including any type mounting hardware
E2613
Positioning wheelchair back cushion, posterior width less than 22 inches, any
height, including any type mounting hardware
E2614
Positioning wheelchair back cushion, posterior width 22 inches or greater, any
height, including any type mounting hardware
E2615
Positioning wheelchair back cushion, posterior-lateral width less than 22
inches, any height, including any type mounting hardware
E2616
Positioning wheelchair back cushion, posterior-lateral width 22 inches or
greater, any height, including any type mounting hardware
E2617
Custom fabricated wheelchair back cushion, any size, including any type
mounting hardware
E2620
Positioning wheelchair back cushion, planar back with lateral supports, width
less than 22 inches, any height, including any type mounting hardware
E2621
Positioning wheelchair back cushion, planar back with lateral supports, 22
inches or greater, any height, including any type mounting hardware
01/31/2007 Medical Supplies and Equipment – Wheelchair Accessories
Medical Policy Manual
438
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
III. WHEELCHAIR POSITIONING ACCESSORY
A. Coverage Criteria
Positioning accessories include cushioned headrests, lateral trunk or hip supports, medial
and lateral thigh supports, and shoulder harnesses or chest straps. The IHCP covers
wheelchair positioning accessories (HCPCS codes E0955-E0957, and E0960) when
medically necessary for IHCP members with postural disorders. The cushioned
headrests, lateral trunk or hip supports, and medial and lateral thigh supports require PA.
Shoulder harnesses and chest straps (E0960) do not require PA.
B. Prior Authorization
Positioning accessories E0955-E0957 will be approved if the member has
1) A wheelchair; and
2) Significant postural asymmetries that are due to one or more of the following
diagnoses.
• Spinal cord injury resulting in quadriplegia or paraplegia (344.00-344.1)
• Other spinal cord disease (336.0-336.3)
• Multiple sclerosis (340)
• Other demeylinating disease (341.0-341.9)
• Cerebral palsy (343.0-343.9)
• Anterior horn cell diseases including amyotrophic lateral sclerosis (335.0-335.21,
335.23-335.9)
• Post polio paralysis (138)
• Traumatic brain injury resulting in quadriplegia (344.09)
• Spina bifida (741.00-741.93)
• Childhood cerebral degeneration (330.0-330.9)
• Alzheimer’s disease (331.0)
• Parkinson’s disease (332.0)
• Monoplegia of the lower limb (344.30-344.32, 438.40-438.42)
• Hemiplegia due to stroke, TBI or other injury (342.00-342.92, 438.20-438.22)
• Muscular dystrophy (359.0, 359.1)
• Torsion dystonias (333.4, 333.6, 333.7)
• Spinocerebellar disease (334.0-334.9)
C. Coding and Billing
Providers must report the codes listed in Table 3 for positioning accessories. Positioning
accessories E0955-E0957 include fixed mounting hardware in the cost of the equipment.
If adjustable or swingaway mounting hardware is medically necessary for these
accessories, providers may bill for the hardware separately using HCPCS code E1028
01/31/2007 Medical Supplies and Equipment – Wheelchair Accessories
Medical Policy Manual
439

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
(See IHCP policy for Mounting Hardware on the following page). Providers may bill for
lateral thigh supports utilizing HCPCS code K0108.
Table 3 – Codes for Positioning Accessories
Code Description
E0955 Wheelchair accessory, headrest, cushioned, any types, including fixed
mounting hardware
E0956 Wheelchair accessory, lateral trunk or hip support, any type, including fixed
mounting hardware
E0957 Wheelchair accessory, medial thigh support, any type, including fixed
mounting hardware
E0960 Wheelchair accessory, shoulder harness/straps or chest strap, including any
type mounting hardware
K0108 Wheelchair component or accessory not otherwise specified
IV. MOUNTING HARDWARE
A. Coverage Criteria
The IHCP allows reimbursement of swingaway or retractable mounting hardware, when
fixed mounting hardware is not adequate to meet the member’s medical needs, using
HCPCS code E1028, Wheelchair accessory, manual swingaway, retractable or
removable mounting hardware for joystick, other control interface or positioning
accessory. E1028 may only be reimbursed under the circumstances on the following
page.
• May be reimbursed in addition to positioning accessories (E0955-E0957) when
swingaway, or removable type adjustable hardware is required.
• Reimbursable with lateral pediatric supports (E1025-E1027) when swingaway
and removable hardware are required.
• For swingaway hardware used with hand and chin control interfaces (E2320 and
E2321), swingaway or flip-down hardware for head control interfaces (E2327-
E2330) and swingaway hardware for an indicator display box that is related to the
multi-motor electronic connection (E2310 or E2311).
• HCPCS Code E1028 is not to be used for swing away hardware used with sip and
puff interface (E2325) because swingaway hardware is included in the allowance
for that code.
B. Prior Authorization
PA is required for mounting hardware. Providers must indicate the mounting hardware
that is required, including the brand name, model name and number, and the type of
equipment the mounting hardware is being used with. Documentation of medical
01/31/2007 Medical Supplies and Equipment – Wheelchair Accessories
Medical Policy Manual
440
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
necessity for the swingaway, retractable, or removable mounting hardware must be
included with the PA request.
C. Coding and Billing
Providers must use HCPCS code E1028 to bill mounting hardware. Requests for
mounting hardware using HCPCS code E1399 will be denied. Requests for swingaway,
retractable, or removable mounting hardware for use with equipment other than those
specified in this policy will be denied.
V. UNIVERSAL HEADREST PLATES
A. Coverage Criteria
The IHCP will reimburse a universal headrest plate when the initial headrest ordered for a
new wheelchair does not meet the member’s needs upon the first or subsequent fittings,
and a replacement headrest is required. The universal headrest plate is necessary to fit a
headrest from one manufacturer to the wheelchair back from a different manufacturer.
Universal headrest plates will not be covered for the initial headrest ordered for use on a
new wheelchair. The wheelchair back should be pre-drilled to accommodate the headrest
initially ordered with the wheelchair.
B. Prior Authorization
Reimbursement of the universal headrest plates will be subject to the PA criteria on the
next page.
1. Universal headrest plates will be covered when the initial headrest ordered for a
new wheelchair does not meet the member’s medical needs and a replacement
headrest is required. The provider must document on the PA request, the brand
name and model of the original headrest and include an explanation of why the
headrest did not meet the member’s needs. In addition, the provider must indicate
the brand name and model of the subsequent headrest that will be used on the
wheelchair.
2. Universal headrest plates will be covered for a used wheelchair if the member’s
condition changes and the wheelchair back is not pre-drilled for the headrest. The
provider must provide documentation of the medical necessity for the headrest.
3. Replacement universal headrest plates will be covered with documentation of an
explanation for the replacement (i.e. plate is damaged due to high tone/spasticity
of the patient).
01/31/2007 Medical Supplies and Equipment – Wheelchair Accessories
Medical Policy Manual
441
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
C. Coding and Billing
HCPCS code E1028, Wheelchair accessory, manual swingaway, retractable or
removable mounting hardware for joystick, other control interface or positioning
accessory, must be used for PA and billing. Requests for approval of the universal
headrest plate using HCPCS code E1399, Durable medical equipment, miscellaneous;
will be denied for appropriate coding. Providers should submit their usual and customary
charge using HCPCS code E1028.
VI. POWER SEATING SYSTEMS
A. Coverage Criteria
The IHCP covers power seating systems including power tilt only, power recline only
with or without sheer reduction, and the combination of power tilt and recline with or
without sheer reduction. In addition, the IHCP will reimburse for power leg elevation
systems to be used with power seating systems. The IHCP does not provide
reimbursement for a power seat elevator, as this equipment is not considered medically
necessary.
B. Prior Authorization
Power seating systems require PA. Providers must submit documentation of medical
necessity detailing the member’s condition that requires tilt and/or recline.
Documentation must also be submitted that indicates the member requires assistance to
use a manual tilt and/or recline and is left unattended for extended periods of time, or
would be otherwise able to independently care for themselves with the power system.
Power tilt-in-space seating systems may be considered for the following medical
conditions.
• History of skin breakdown or current indication of skin breakdown that cannot be
controlled by less costly modalities such as pressure relief cushions or manual
pressure relief techniques
• Excessive extensor or flexor muscle tone that requires a constant seat-to-back
angle, when automatic position changes are necessary for the member
• Upper body instability that causes dependent sitting balance requiring gravity
assisting positioning
• Orthostatic hypotension
Power recline seating systems may be considered for the following medical conditions.
01/31/2007 Medical Supplies and Equipment – Wheelchair Accessories
Medical Policy Manual
442

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• History of skin breakdown or current indication of skin breakdown that cannot be
controlled by less costly modalities such as pressure relief cushions or manual
pressure relief techniques
• Hip extension contractures that do not require a constant seat-to-back angle, when
automatic position changes are necessary for the member
• Orthostatic hypotension
• Bladder management, such as intermittent catheterization
Shear reduction may be approved when documentation indicates the level of possible
skin breakdown justifies sheer reduction.
C. Coding and Billing
Table 4 lists the codes available for reporting power seating systems. Providers should
bill the appropriate code on the CMS-1500 or 837P electronic transaction.
Table 4 – Power Seating System Codes
Code Description
E1002 Wheelchair accessory, power seating system, tilt only
E1003
Wheelchair accessory, power seating system, recline only, without shear
reduction
E1004
Wheelchair accessory, power seating system, recline only, with mechanical
shear reduction
E1005
Wheelchair accessory, power seating system, recline only, with power shear
reduction
E1006 Wheelchair accessory, power seating system, combination tilt and recline,
without shear reduction
E1007 Wheelchair accessory, power seating system, combination tilt and recline, with
mechanical shear reduction
E1008 Wheelchair accessory, power seating system, combination tilt and recline, with
power shear reduction
E1009 Wheelchair accessory, addition to power seating system, mechanically linked
leg elevation system, including pushrod and leg rest, each
E1010 Wheelchair accessory, addition to power seating system, power leg elevation
system, including leg rest, each
VII. MATRIX SEATING SYSTEM
A. Coverage Criteria
The IHCP will provide reimbursement for the purchase of, any medically necessary
revisions to, and repairs of the Matrix seating system, with PA. Coverage of the Matrix
seating system includes coverage of the Matrix TMX Composite Shell, the Matrix Extra
Rigid Support Frame, the Matrix TMX Seat Cover, the Matrix Mobility Base, the TMX
Quik Release Interface, or other Matrix accessories.
01/31/2007 Medical Supplies and Equipment – Wheelchair Accessories
Medical Policy Manual
443
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
B. Prior Authorization
PA requests for the Matrix TMX Composite Shell and the Matrix Extra Rigid Support
Frame will be considered for approval if medical necessity for custom seating is
established by a physiatrist or orthopedic surgeon and all criteria listed below are met.
1. The member has tried multiple seating systems in the past three years (replacement
every 6-12 months) showing unsuccessful seating attempts by other means, or it is
expected that the member will have frequent change of seating needs (every 6-12
months) due to growth or anticipated surgeries.
2. The TMX is prescribed for the treatment of severe musculoskeletal deformity, severe
weakness of the trunk muscles, or severe spasticity/extremely high tone.
3. The member must weigh less than 300 pounds with room for growth (should only be
given prior approval on a case-by-case basis if weight could reasonably exceed 300
pounds within next three years).
4. The member is in the wheelchair six to eight hours daily, or is expected to be, with
use of the TMX seating system.
5. The TMX must be the most cost-effective of the available options (documentation
must be provided that identifies all seating options that would meet medical necessity,
including cost, and address why each was not selected).
A photograph showing current seating may be required with the PA request. Members
must meet the above PA criteria to be approved for other Matrix accessories or the
Matrix Mobility Base. In addition, providers requesting the Matrix Mobility Base must
document that the Matrix TMX system has an expected use of a minimum of 10 years.
Providers requesting the TMX Quik Release Interface must meet the above criteria and
include documentation of the need for portability.
D. Coding and Billing
The Matrix TMX Composite Shell is billed with HCPCS code E1399 U1, Matrix
Composite Shell, and includes the reimbursement of a custom contoured insert, rigid
support frame, wheelchair interface mounting kit, all hardware needed for the seat/back,
and all refitting/readjustments needed within six months (unless necessitated by a
significant change in medical condition). Reimbursement for the composite shell does
not include reimbursement of an extra rigid support frame, seat covers, headrests,
armrests, footrests, leg troughs, footboxes, covers for these special items, or a mobility
base. The Matrix Extra Rigid Support Frame is billed with HCPCS code E1399 U2,
Matrix Extra Rigid Support Frame, when medically necessary for increased stability, and
includes reimbursement of the frame and necessary hardware.
01/31/2007 Medical Supplies and Equipment – Wheelchair Accessories
Medical Policy Manual
444
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Revisions to the Matrix Composite Shell are accomplished with simple hand tools, such
as an electric screwdriver. A revision may involve minor adjustments to relieve specific
pressure points or to add more support in specific areas by changing the shape of the
Matrix TMX fabric at that site. In other cases, a revision may entail complete
disassembly of the seat and reshaping to the current needs of the member. A complete
disassembly and refitting is usually done for growth or significant posture changes (such
as with Herrington rod placement for scoliosis). Often, with a complete disassembly and
refitting, extra parts are needed to enlarge the seat. This is usually accomplished by
inserting extra TMX fabric into the center of the seat. These extra parts do not represent
an additional cost to the provider since many parts are left over from the initial fitting.
The manufacturer has indicated that any extra parts needed as part of a revision should
not receive reimbursement. Therefore, no reimbursement will be made for extra fabric,
hardware, or framing components necessary to make such modifications.
Revisions (regardless of the intensity or degree of modification required) are reimbursed
only as labor by a technician using code E1340, Repair or non-routine service, requiring
the skill of a technician, per 15 minutes. Units billed for labor must reflect actual time
spent and a log of time spent must be maintained in the member’s record, which is
subject to post-payment review. The term “revision” applies only to the reshaping of the
Matrix TMX shell to meet current medical needs of the member. For addition of any
ancillary accessories, these items should be coded using the most appropriate HCPCS
code.
Matrix TMX seat covers (whether provided with a new system or as replacement covers)
are billed using HCPCS code E2605, Positioning wheelchair cushion, width less than 22
in, any depth. Only half-covers will be covered by the IHCP since full covers are
aesthetic in nature and not medically necessary. With normal wear and tear, new covers
will rarely be approved more often then every 18 months. Replacement of covers at an
interval less than 18 months may be considered in cases such as theft, fire, or irreparable
damage from an auto accident or similar circumstances. Cases of extreme wear may be
considered on a case by case basis for replacement more frequently than the specified
time frame (pictures may be required along with a detailed explanation of the excessive
wear and the care for the items). Two covers may be allowed in cases of incontinence
because the covers must be air-dried when washed, which would cause the entire chair to
be non-usable for 24 to 72 hours.
The TMX Mobility Base is billed using HCPCS code E1220, Wheelchair; specially sized
or constructed, and includes all refitting and readjustments needed within six months
(unless necessitated by a significant change in medical condition). Claims submitted for
the TMX Mobility Base must reflect the provider’s usual and customary charge. The
mobility base can be adapted to fit the changing needs of the patient. Parts needed for
growth will receive no additional reimbursement if “left-over” parts from previous
fittings are available for use. Labor for growth modifications may be billed using
E1340.
01/31/2007 Medical Supplies and Equipment – Wheelchair Accessories
Medical Policy Manual
445

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
As all components of the TMX are supported by a lifetime warranty, replacement of an
entire Matrix TMX Mobility Base will rarely be approved. An entire mobility base may
be considered for replacement only in cases of unusual or unavoidable circumstances,
such as theft, fire, or irreparable damage from an auto accident or similar circumstance.
No additional reimbursement will be made for TMX Mobility Base framing components
or any component under warranty. Additional reimbursement is made for parts not under
lifetime warranty, such as tires or casters.
The Matrix Quik Release Interface is billed using HCPCS code E1399. Additional
Matrix accessories such as headrests, armrests, footrests, etc. should be billed using the
most appropriate HCPCS code. Only if no code is available for the accessory should
E1399 be used for PA requests or billing.
VIII. MANAGED CARE
Primary Care Case Management (PCCM) services are subject to the same policies and
restrictions as Traditional Medicaid services. Questions regarding coverage of services in
Risk Based Managed Care (RBMC) should be directed to the appropriate Managed Care
Organization (MCO).
RELATED MEDICAL TOPICS
Medical Supplies and Equipment – Manual Wheelchairs and Motorized/Power
Wheelchairs
RULES, CITATIONS, AND SOURCES
IHCP Provider Bulletin
BT200335 – Motorized Wheelchairs
BT200401 – HCPCS Updates
IHCP Provider Banner
BR200509 – Matrix Seating System
BR200525 – Universal Headrests
Origination Date: 01/31/06
Revisions and
Review
Reason Date
APPLICABLE INDIANAAIM EDITS AND AUDITS
6065 - DME total rental amount not to exceed fee for purchase
01/31/2007 Medical Supplies and Equipment – Wheelchair Accessories
Medical Policy Manual
446
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
6080 - DME rentals limited to 15 months
6113 - DME limited to $2,000 per recipient per calendar year
6114 - DME limited to $5,000 per recipient per lifetime
01/31/2007 Medical Supplies and Equipment – Wheelchair Accessories
Medical Policy Manual
447

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Medical Supplies and Equipment – Wheelchair Accessories
Medical Policy Manual
448
MEDICAL SUPPLIES AND EQUIPMENT – WHEELCHAIR ACCESSORIES
ADDENDUM A
Note: This addendum contains provider notifications that have been published since the
review of the Medical Supplies and Equipment-Wheelchair Accessories Fact Sheet. The
information in this addendum will be incorporated into the fact sheet at the next review.
Provider Notification: BR200627 Publication Date: 07/04/2006
Subject: July 2006, Quarterly HCPCS Codes Update
Date Added to Manual: 10/31/2006
Text of Publication
HCPCS codes K0734 through K0737 are new codes to report adjustable skin protection
and positioning seat cushions currently reported with HCPCS code K0108 and modifier
U1, U2, U3, or U4. The IHCP created the procedure code to modifier combinations in
order to mirror Medicare policy for the use of HCPCS code K0108 for adjustable seat
cushions as published in provider banner BR200536. Adjustable cushions are purchase-
only items by the IHCP, and providers must attach the NU modifier when billing to the
IHCP. Pricing established for adjustable and positioning seat cushions as published in
BR200536 is applied to HCPCS codes K0734 through K0737, effective for dates of
service on or after September 1, 2006. Providers are allowed to report procedure code
K0108 and modifier U1, U2, U3, or U4 for skin protection and positioning seat cushions
for dates of service through August 31, 2006.

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: MENTAL HEALTH/BEHAVIORAL HEALTH—
INPATIENT SERVICES
DESCRIPTION
Acute psychiatric and substance abuse inpatient services are mental health interventions
used to stabilize and manage people with severe symptoms and behaviors that have or
may result in harm to self and/or others. The following information describes presenting
factors that may meet medical necessity for inpatient services.
• Current and/or recent serious suicide ideation with plan and potential means with
lethal intent
• Current and/or recent serious, violent, impulsive, and unpredictably dangerous
homicidal ideation with plan and potential means with lethal intent
• Current and or recent harm to self or others with plan and potential means with
lethal intent
• Unable to care for self due to a psychiatric condition so that imminent life-
threatening deterioration has occurred
• Acute psychotic symptoms, severely bizarre thinking and/or psychomotor
agitation/retardation that cannot be safely treated in a less restrictive level-of-care
Depending on the needs of the patient, acute psychiatric and substance abuse inpatient
services often include, but are not limited to, 24-hour psychiatric and medical services,
continuous monitoring, medication management, treatment planning, individual therapy,
family therapy, and group therapy.
This document is intended to serve as a general summary of the Indiana Health Coverage
Programs (IHCP) policies regarding this service. More specific information may be
found in the IHCP provider manual, program notices, the Indiana Administrative Code,
or other sources as appropriate. See the “Rules, Citations, and Sources” section of this
document for further information.
PRIOR AUTHORIZATION
Prior authorization (PA) is required for all acute inpatient admissions, emergency and
non-emergency. The facility is responsible for initiating the PA review process.
Providers should contact the Health Care Excel (HCE) PA department for the initial pre-
certification and concurrent review. For IHCP members enrolled in Managed Care, refer
01/31/2007 Mental Health/Behavioral Health – Inpatient Services
Medical Policy Manual
449
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
to the Managed Care section for PA requirements. PA is not a guarantee of payment for
services rendered.
Reimbursement is available for inpatient care provided on the psychiatric unit of an acute
care hospital only when the need for admission has been certified. The Division of
Family and Children State Form 1261A, Certification Plan of Care for Inpatient
Psychiatric Hospital Services Determination of Medicaid Eligibility, fulfills the written
certification of need requirements.
The HCE PA department reviews each 1261A form and determines whether the requested
acute inpatient services meet medical necessity. Reimbursement is denied for any days
the facility cannot justify a need for inpatient care. If the provider fails to complete a
telephone PA pre-certification, reimbursement will be denied for the period of time from the
admission to the actual date of notification.
Emergency Admissions
• A telephone PA must be completed within 48 hours of the admission date, not
including Saturdays, Sundays, and legal holidays
• A completed 1261A form must be received, via U.S. mail, within 14 working days of
the admission date, not including Saturdays, Sundays, and legal holidays
Non-Emergency Admissions
• A telephone PA must be completed prior to admission
• A completed 1261A form, via U.S. mail, must be received within ten working days of
the admission date, not including Saturdays, Sundays, and legal holidays
When an individual applies to become an IHCP member after admission to a facility,
providers must notify HCE in writing within ten days of receiving a notification of IHCP
eligibility. At that time, providers may request coverage for the entire period of service for
which reimbursement is being sought.
COVERAGE CRITERIA
Admission Criteria
Members must meet medical necessity to be eligible for acute IP psychiatric and
substance abuse inpatient services. Members must present with the following criteria at
the time of admission.
• Acute psychiatric inpatient admissions are available for members with a sudden
onset of a psychiatric condition manifesting itself by acute symptoms of such
severity that the absence of immediate medical attention could reasonably be
expected to result in one or more of the following.
01/31/2007 Mental Health/Behavioral Health – Inpatient Services
Medical Policy Manual
450
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Danger to the individual
Danger to others
Death of the individual
• Substance abuse inpatient admissions must be to a psychiatric facility or unit.
Admissions to a general hospital floor are only appropriate when medical services are
required for life-support and cannot be rendered in a substance abuse treatment facility
or unit. These inpatient detoxification, rehabilitation, and aftercare admissions are
available for members when the following criteria have been determined.
Evaluation, treatment, and detoxification are based on the stated
medical condition and/or primary diagnosis for inpatient admission
Need for safe withdrawal from alcohol and/or other drugs is indicated
Reasonable evidence that detoxification and aftercare cannot be
accomplished in an outpatient setting
History of recent convulsions or poorly controlled convulsive disorder
Plan of Care
Each Medicaid member admitted to an acute psychiatric facility or unit must have an
individually developed plan of care. For members between 22 and 65 years of age in a
psychiatric facility of 16 beds or less, plans of care must be developed by the attending or
staff physician. For members under 21 years of age, plans of care must be developed by
a physician and interdisciplinary team. All plans of care must be developed within 14
days of the admission date regardless of the age of the member. The following
components must be documented in each member’s plan of care.
• Treatment objectives and goals including an integrated program of appropriate
therapies, activities, and experiences designed to meet these objectives
• Discharge and coordination plans for inpatient services with partial discharge plans
including appropriate services in the community to ensure continuity of care (i.e.,
family and school)
The plan of care is developed as a result of a diagnostic evaluation that includes an
examination of the medical, psychological, social, and behavioral aspects of the
member’s presenting problem and previous treatment interventions. The plan of care must
be reviewed and updated at least every 90 days for members between 22 and 65 years old by
the attending or staff physician to ensure appropriate services being provided continue to be
medically necessary. An interdisciplinary team is required to develop and direct the plan
of care for all members 21 years and younger. This team is responsible for developing
and updating plans of care at least every 30 days. One of the following professionals or
combination of professionals must be active in the treatment planning process.
• A board certified or eligible psychiatrist
• A psychologist endorsed as a health service provider in psychology (HSPP) and a
physician licensed to practice medicine or osteopathy
01/31/2007 Mental Health/Behavioral Health – Inpatient Services
Medical Policy Manual
451
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• A physician licensed to practice medicine or osteopathy with specialized training
and experience in the diagnosis and treatment of mental diseases and a psychologist
endorsed as an HSPP or licensed psychologist
A professional, who is qualified to make determinations regarding mental health
conditions and treatments, must be part of the interdisciplinary team as well. At least one
of the following professionals must be active in the treatment planning and
implementation process.
• A licensed clinical social worker, licensed marital and family therapist, licensed
mental health counselor, or a person holding a master’s degree in social work,
marital and family therapy, or mental health counseling
• An advanced practice nurse or registered nurse who has specialized training or one
year experience in treating people with mental illnesses
• An occupational therapist registered with the National Association of Occupational
Therapists who has specialized training or one year of experience treating people
with mental illnesses
• A psychologist endorsed as an HSPP or a licensed psychologist
Package C Members
Inpatient mental health and substance abuse services are covered when the services are
medically necessary for the diagnosis or treatment of the member’s condition except
when provided in a mental health institution with more than 16 beds.
Restricted Card Program Members
Mental and behavioral health services are included in the carved-out Medicaid services;
therefore, a written referral is not required for members of the Restricted Card Program to
access these services.
REIMBURSEMENT
Inpatient Services
Reimbursement will be denied for any days during which the acute psychiatric inpatient
hospitalization is found to lack medical necessity. Telephone certifications of medical
necessity provide a basis for reimbursement and must be supported by the written
certification of need. If the required written documentation is not submitted within the
specified time frame and/or does not support medical necessity, reimbursement will be
denied.
Acute inpatient care is reimbursed based on a level of care (LOC) and a diagnosis related
group (DRG) methodology. The following information describes reimbursement for
psychiatric and substance abuse inpatient services.
01/31/2007 Mental Health/Behavioral Health – Inpatient Services
Medical Policy Manual
452
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• Acute psychiatric inpatient services are reimbursed on a LOC reimbursement
methodology; therefore, these services are paid on a per diem basis. The per diem
rate includes routine, ancillary, and capital costs. Direct care physicians services are
excluded from the per diem rate and are separately billable. All other supplies and
services provided to members, including services provided by health service
providers in psychology (HSPP) and clinical psychologists, salaried, contracted, and
independent providers, are included in the per diem rate and cannot be billed
separately.
• Substance abuse inpatient services are reimbursed on a DRG reimbursement
methodology; therefore, this service is paid on a per case basis.
OTHER INPATIENT SERVICES
Readmission
A readmission is defined as a hospital admission within three days following a previous
hospital admission and discharge for the same or related condition. Same or related
condition refers to the primary diagnosis code which is based on the first three digits of
the ICD-9-CM code. Readmissions are treated as separate stays for payment purposes,
but are subject to medical review. Providers should bill one inpatient claim when a
patient is readmitted to their facility within three days of a previous inpatient discharge
(the stays should be consolidated on one claim) for the same or related diagnosis. If it is
determined that a discharge is premature, payment made as a result of the discharge or
readmission may be subject to recoupment. Additionally, post=payment review of
readmissions will be conducted to ensure providers are appropriately following the
readmission policies and guidelines.
Observation Stays
Psychiatric and substance/chemical abuse observation stays in acute care hospitals and
freestanding psychiatric hospitals are reimbursable. The observation period must last no
more than three days (72 hours). If the member meets the criteria for inpatient admission
prior to the end of the observation period, the member’s status may be changed to
inpatient at that time. IHCP members may qualify for observation status meeting both of
the following criteria.
• The criteria for inpatient admission have not been met
• The treating physician or mental health provider has determined that
allowing the member to leave the facility would likely put the member at
serious risk
Observation stays are reimbursed according to outpatient mental health services. Refer to
the IHCP Provider Manual Outpatient services for more information regarding these
services.
01/31/2007 Mental Health/Behavioral Health – Inpatient Services
Medical Policy Manual
453
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Less Than 24-Hour Stays
Providers should bill any inpatient stay that is less than 24 hours as an outpatient service.
Inpatient stays less than 24 hours that are billed as an inpatient service will be denied, or
will be subject to retrospective review.
Outpatient Service Within Three Days of an Inpatient Stay
Outpatient services that occur within three days preceding an inpatient admission to the
same facility for the same or related diagnosis are considered part of the corresponding
inpatient admission. Providers are required to submit an inpatient claim only when both
of the services, outpatient and inpatient, occur at same facility.
If an outpatient claim is paid before the inpatient claim is submitted, the inpatient claim
will deny with an explanation of benefits code indicating that the provider should bill
services on the inpatient claim. The provider should adjust the outpatient claim
(complete adjustment) and resubmit one inpatient claim.
Reserving Beds
Reimbursement is available for reserving beds in psychiatric hospitals; it is not available
in general acute care hospitals. Hospitalization must be ordered by a physician for the
treatment of an acute condition that cannot be treated in a psychiatric facility. Physician
orders must be maintained in the member’s file at the facility. The total length of time
reimbursable per inpatient stay is 15 days. If a member requires more than 15 days
consecutively, the member must be discharged from the psychiatric facility. Facilities are
reimbursed at one-half the regular per diem rate.
Therapeutic Leave of Absence
Reimbursement is available for therapeutic leave of absence from psychiatric hospitals; it
is not available from general acute hospitals. Leaves of absence must be for therapeutic
reasons and ordered by a physician as indicated in the member’s plan of care. Physician
orders must be maintained in the member’s file at the facility. The total length of time
available for therapeutic leaves of absence is 60 days per calendar year per member. If a
member is absent from a psychiatric hospital for more than 60 days per year, no further
reimbursement will be available for reserving a bed for that member in that year.
Facilities are reimbursed at one-half the regular per diem rate.
MANAGED CARE
While the MCO retains responsibility for the delivery and payment of most care for its
members, certain services are not paid by the MCO. These services remain the financial
01/31/2007 Mental Health/Behavioral Health – Inpatient Services
Medical Policy Manual
454

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
responsibility of the State and reimbursed on a fee-for-service basis. These carve-out
services include most mental health and substance abuse services from IHCP enrolled
providers with a mental health specialty. The following information describes who
providers should notify when a RBMC member is admitted for acute psychiatric or
substance abuse inpatient services.
• Mental health care provided to an MCO-enrolled member in an acute care
facility is the responsibility of the MCO and is authorized and reimbursed by
the MCO.
• Mental health services provided to an MCO-enrolled member in a freestanding
mental health facility or by mental health specialties are not the MCO’s
responsibility and are subject to all PA and reimbursement criteria established
for IHCP fee-for-service billing.
RELATED MEDICAL TOPICS
Assertive Community Treatment Services
Community Mental Health Centers Services
Developmental, Neurological, and Psychological Testing Services
Emergency Medicine—Emergency Room
Emergency Medicine—Emergency Services
Evaluation and Management Services
Intermediate Care Facility for the Mentally Retarded
Inpatient Hospital Services
Outpatient Hospital Services
Outpatient Mental Health Services
Managed Care Services
Mental Health Rehabilitation Services
Pharmacy Services
Physician Services
Psychiatric Residential Treatment Facility Services
Smoking Cessation Services
Waiver Services
RULES, CITATIONS, AND SOURCES
Indiana Administrative Code (IAC)
405 IAC 1-8-2 – Hospital and ambulatory surgical center reimbursement for
outpatient services
405 IAC 1-10.5-3 – Perspective reimbursement methodology
405 IAC 5-2-19 – “Outpatient services” defined
405 IAC 5-3 – Prior authorization
405 IAC 5-2-17 – “Medically reasonable and necessary service” defined
405 IAC 5-20-8 – Outpatient mental health services
405 IAC 5-21 – Community mental health rehabilitation services
01/31/2007 Mental Health/Behavioral Health – Inpatient Services
Medical Policy Manual
455
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
405 IAC 5-25 – Physician services
405 IAC 5-29 – Services not covered by Medicaid
405 IAC 5-37 – Smoking cessation treatment policy
440 IAC 5.2-2-3 – Assertive community treatment services
Indiana Code (IC)
IC 12-15-12 – Managed care
IC 25-23-1-1(b)(3) – Advance practice nurses credentialed in psychiatric or
mental health nursing by the American Nurses
Credentialing Center
Indiana Health Coverage Programs Provider Banner (BR)
BR200532 – Supervision of Mental Health or Managed Care Organization
Services
BR200523 – Assertive Community Treatment Community Mental Health Centers
H0040 – ACT Services, Per Diem
BR200514 – Hoosier Healthwise Mandatory Risk-based Managed Care (RBMC)
Enrollment
BR200451 – Mental Health Cross Walked Local Codes
BR200430 – EDS Made Changes to HCPCS Code H0040–Assertive Community
Treatment Program, Per Diem
BR200424 – Explanation of benefits (EOB) code 5007
BR200439 – CPT Codes 90805, 90807, 90809, 90810, 90813, and 90815 for
Psychotherapy with Medical Evaluation and Management, and
CPT Code 90862 for Pharmacological Management
BR200244 – System Modification Affecting Medicare Part B Crossover Claims
Billed by a Health Service Provider in Psychology (HSPP) with an
AH Modifier
BR200421 – Mid-level Practitioner Modifiers
BR200240 – Explanation of Benefit (EOB) Code 4116–Diagnosis Code is Not
Valid for DRG Pricing.
BR199952 – Supervision Requirements
BR199950 – Risked-based Managed Care Inpatient Psychiatric Services
BR199946 – Implementation of Smoking Cessation Treatment Services
BR199920 – Changes in Diagnostic Related Groups/Level of Care Inpatient and
Outpatient Methodologies
BR199810 – Modifications to the Computer System to Allow Psychiatric
Medicare Part B Crossover Claims
Indiana Health Coverage Programs Provider Bulletin (BT)
BT200506 – State-wide Hoosier Healthwise Mandatory Managed Care
Organization Transition
BT200432 – Annual Notice for Hoosier Healthwise Managed Care Members
BT200429 – Psychiatric Residential Treatment Facility Services for Patients
Younger than Six Years Old
BT200415 – Clarification of Mental Health Modifiers, Mental Health Clinics,
and Community Mental Health Centers
BT200413 – Assertive Community Treatment Service Coverage, Standards, and
Requirements
01/31/2007 Mental Health/Behavioral Health – Inpatient Services
Medical Policy Manual
456
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
BT200404 – Acute Care Hospitals, Psychiatric Hospitals, Psychiatrists, Health
Services Providers in Psychology, Mental Health Clinics,
Physicians, Primary Medical Providers for Primary Care Case
Management and Community Mental Health Centers
BT200360 – Changes in Diagnosis-Related Group Relative Weights and New
Long-Term Acute Care Hospital Level-of-care Reimbursement
Methodology
BT200362 – Prior Authorization for Hoosier Healthwise Managed Care
Organizations
BT200340 – Hoosier Healthwise Mandatory Managed Care Organization
Transition
BT200262 – Hoosier Healthwise Managed Care Certification Code Requirements
BT200240 – MDwise Managed Care Organization Service Area Expansion
BT200231 – Managed Care Carve-out and Self-referral Education
BT200208 – Changes to the Medicaid Medical Policy Rule Regarding Outpatient
Mental Health Services (405 IAC 5-20 and 405 IAC 5-21)
BT200206 – Medicaid Rehabilitation Option Issues
BT200145 – Community Mental Health Centers Third Party Liability Edits
BT200140 – Mandatory Managed Care Organization Enrollment Update
BT200129 – Changes in Diagnostic-Related Groups/Level-of-care
Inpatient and Outpatient Reimbursement Methodologies
BT200124 – Acute Care and Freestanding Psychiatric Hospitals Reimbursement
for Child Abuse and Neglect Cases
BT200121 – Important Information about Hoosier Healthwise Managed Care
Entity Contact Information
BT200049 – Procurement of Hoosier Healthwise MCO Contract
BT200028 – Changes to the Medicaid Medical Policy Rule Regarding
Outpatient Mental Health Services (405 IAC 5-20 and 405 IAC 5-
21)
BT199951 – House Enrolled Act (HEA) 1396, OMPP is Amending the Mental
Health Services Sections of the Covered Services Rule (405 IAC 5-
20 and 405 IAC 5-21)
BT199950 – Free-Standing Psychiatric Hospitals Are Carved-out of Risk-based
Managed Care (RBMC) and Paid on a Fee-for-service Basis
BT199946 – Implementation of Smoking Cessation Treatment Services
BT199930 – Participation in the Indiana Medicaid Hospital Care for the Indigent
(HCI) Payment, Municipal County Hospital Indiana Medicaid
Shortfall Payment and Indiana Medicaid Disproportionate Share
Hospital (DSH) Payment Programs
BT199917 – Acute Care and Freestanding Psychiatric Hospitals Child Abuse and
Neglect Cases
BT199902 – Changes in Diagnostic-Related Groups/Level-of-care Inpatient and
Outpatient Reimbursement Methodologies
BT199822 – Mental Health Crossover Claims
BT199819 – New Mental Health Rehabilitation Option Procedure Code–Z5025
Indiana Health Coverage Programs Provider Newsletter (NL)
01/31/2007 Mental Health/Behavioral Health – Inpatient Services
Medical Policy Manual
457

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
NL200506 – Psychiatric Residential Treatment Facility Update PRTF
NL200501 – Cross Walked Local Codes
NL200405 – Cognitive Therapy Services
NL200406 – Mental Health Billing Modifiers
NL200410 – Medicaid Behavioral/Physical Health Coordination Form
Indiana Health Coverage Programs Provider Manual
1999
2005 Version 5.1
Revision: December 15, 2005
Revision and Review Reason Date
Indiana State Department of Public
Welfare Medical Policy Manual 1991
Inpatient Psychiatric
Reimbursement
7/01/91
470 IAC 5-9-22 Transferred Mental Health Services 7/01/91
405 IAC 1-6-13;1-7-20 Repealed Mental Health Services 1/01/92
405 IAC 5-20 Mental Health Services 8/24/97
Routine Review Scheduled 1/31/06
APPLICABLE INDIANAAIM EDITS AND AUDITS
0437 – Psych Adjustment Amount Invalid
0636 – Enrolled in the RBMC of the Hoosier Health Program
2028 – Non-IHCP Member Ineligible for Dates of Services
2032 – Therapy and Hospital are Only Leave Days Valid on Psychiatric Unit
2033 – Package C Recipients Not Eligible for Clone Dates
3000 – Units Exceed Prior Authorization Master
3001 – Dates of Service Not on Prior Authorization Database
3007 – No Prior Authorization Segment for Level of Care
3008 – Prior Authorization Units Equal Zero
4082 – Bed Reservation in Psychiatric Hospital
4083 – Inpatient Mental Health Facility
4085 – Inpatient Care in a Psychiatric Hospital for Members Ages 22-64
4086 – Therapies After More than 30 Days from Hospital
4204 – Invalid Diagnosis for Procedure Code/ Modifier
5000 – Possible Duplication
5001 – Exact Duplication
5009 – Suspect Duplication Different Provider Allowed
6120 – Outpatient Mental Health/ Substance Abuse Services Office Visits, Maximum 30
Per Calendar Year Without Prior Authorization
6121 – Outpatient Mental Health/ Substance Abuse Services Office Visits, Maximum 50
Per Calendar Year Without Prior Authorization
6125 – Cognitive Rehabilitation is Limited to Procedure and Diagnosis
6258 – Inpatient Psych Leave Days Only Allowed 60 Per Year
6270 – Smoking Cessation Counseling Limited to Ten 15-minute Units Per Calendar
Year
6508 – Same Day Discharge
6515 – Inpatient Admit Date Within Three Days After DOS of Paid Outpatient Claim
01/31/2007 Mental Health/Behavioral Health – Inpatient Services
Medical Policy Manual
458
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Mental Health/Behavioral Health – Inpatient Services
Medical Policy Manual
459
6516 – Outpatient Services Rendered Within Three Days Prior to Admit Date of Paid
Inpatient Claim
6517 – Inpatient Claim Discharge Date Within Three Days Prior to Admit Date Paid
Inpatient Claim
6518 – Inpatient Claim Admit Date Within Three Days After Discharge Date of Paid
Inpatient Claim
6615 – Assertive Community Treatment Is Limited One Per Day
6631 – Medical Leave cannot Exceed Four Consecutive Days
6632 – T2048 Is Only Reimbursable for Provider Specialty 033
6633 – 25180 and T2048 Limited to One Psychiatric Treatment Per Day
6635 – Treatment Leave Days Exceed 14 Per Year
6636 – Mid-level Services Not Reimbursable the Same Day a Paid PRTF Service
6673 – Psychiatric Per Diem Medical Leave Days Cannot Exceed Four Consecutive Days
6900 – Outpatient Mental health Services More Than 20/Year Without Prior
Authorization
6901 – Outpatient Psychiatric Testing Exceeds Two Units Per Year
6902 – Outpatient Therapies Over 80 Units Requires Prior Authorization
6921 – One Diagnostic Evaluation Per Recipient Every Six Months

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: MENTAL HEALTH/BEHAVIORAL HEALTH—
OUTPATIENT SERVICES
DESCRIPTION
Outpatient mental health services are interventions intending to reduce and/or alleviate
symptoms, improve the level of functioning, and prevent further and/or recurrent
deterioration. After clients are assessed, a determination is made as to what forms of
therapy will most likely be beneficial. Common interventions of outpatient treatment
include individual, family, couple, and group counseling.
Therapy is a collaborative process; therefore, the client is expected to be active and
cooperative with establishing the treatment plan. Treatment plans include specific goals,
methods to accomplish goals, and methods to measure the progress of treatment goals.
Measurable goals are also necessary to determine when improvement or deterioration of a
client’s functioning has occurred. Treatment plans must be reviewed and updated on a
regular basis to reflect continued needs and identify new goals of the client.
This document is intended to serve as a general summary of the Indiana Health Coverage
Programs (IHCP) policies regarding this service. More specific information may be
found in the IHCP provider manual, program notices, the Indiana Administrative Code,
or other sources as appropriate. See the “Rules, Citations, and Sources” section of this
document for further information.
COVERAGE CRITERIA
The IHCP covers outpatient (OP) mental health services provided by a licensed medical
doctor, doctor of osteopathy, psychologist endorsed as a Health service provider in
psychology (HSPP), psychiatric hospitals, psychiatric wings of acute care hospitals, and
OP mental health facilities. Reimbursement is also available for services provided by
mid-level practitioners when services are supervised by a physician or a HSPP. Mid-
level practitioners who are eligible to provide OP mental health services must have
obtained one of the following credentials.
• Academy of Certified Social Workers
• Advanced Practice Nurses credentialed in psychiatric or mental health nursing
by the American Nurses Credentialing Center
• Certified Clinical Social Worker
01/31/2007 Mental Health/Behavioral Health – Outpatient Services
Medical Policy Manual
460
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
•
Certified Psychologist
• Independent Practice School Psychologist
• Licensed Clinical Social Worker
• Licensed Marriage and Family Therapist
• Licensed Mental Health Counselor
• Licensed Psychiatric and Mental Health Clinical Nurse Specialist
• Licensed Psychologist
• Psychologist with a basic certificate
• Registered Nurse with a master’s degree in nursing with a major in psychiatric
and mental health nursing from an accredited school of nursing
• Master’s degree in social work, marital and family therapy, or mental health
counseling
The physician, psychiatrist, or HSPP is responsible for certifying the diagnosis and
supervising the plan of treatment. They are responsible for seeing the member during the
intake process or reviewing the medical information obtained by the mid-level
practitioner within seven days of the intake process. Also, the physician, psychiatrist, or
HSPP must see the member or review the medical information and certify medical
necessity on the basis of medical information provided by the mid-level practitioner at
intervals not to exceed 90 days. Both reviews must be documented in writing; co-
signatures alone are not sufficient.
I. Outpatient Services
Medicaid reimbursement is available for one psychiatric diagnostic interview
examination without prior authorization (PA) per member, per provider, per rolling
calendar year (Refer to Chapter 2 of the IHCP Provider Manual for an explanation of
rolling calendar year). A maximum of two diagnostic interview examinations per
member, per rolling calendar year is allowed without PA when one examination is
provided by a physician or HSPP and one examination is provided by a mid-level
practitioner. All additional examinations require PA.
Prior authorization is required for mental health services provided in an OP facility or
office setting that exceed 20 units per member, per provider, per rolling calendar year.
A current treatment plan and progress notes, outlining the necessity and effectiveness of
therapy, must be attached to the PA form and available for audit purposes. The
following CPT codes are subject to the 20 units per member, per provider, per rolling
calendar year.
• 90801–90802
• 90804–90815
• 90845–90857
Noncovered Services
• Day care
• Hypnosis
01/31/2007 Mental Health/Behavioral Health – Outpatient Services
Medical Policy Manual
461

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
•
Biofeedback
• Missed appointments
• Experimental drugs, treatments, procedures, and all related services
• Services for the remediation of learning disabilities
• Treatments or therapies of an educational nature
• Acupuncture
• Hyperthermia
• Hypnotherapy
• Day care or partial day care or partial hospitalization
• Cognitive rehabilitation, except for treatment of traumatic brain injury
Package C Members
Package C members are eligible for outpatient mental health services. Reimbursement is
available for 30 visits per member, per rolling calendar year. An additional 20 visits may
be covered with PA for a maximum of 50 visits per year.
Reimbursement
All rendered outpatient services must be identified and itemized on the CMS-1500.
The medical record documentation must identify the services and the length of time
of each therapy session. This information must be available for audit purposes. Mid-
level services should be billed using the rendering provider number of the supervising
practitioner (physician or HSPP), following by the appropriate modifier, and the
billing provider number of the outpatient mental health clinic or facility. Table 1 lists
mid-level practitioners and the appropriate corresponding modifiers for billing
purposes.
Table 1 – Outpatient Mental Health Modifiers for Mid-level Practitioners
Mid-level Practitioner Modifier
Clinical Psychologist AH
Clinical Social Worker AJ
Nurse Practitioner or Clinical Nurse Specialist HE/ SA
Nurse Practitioner or Clinical Nurse Specialist in a
non-mental health arena
SA
Any other mid-level practitioner HE
Claims billed for services provided by mid-level practitioners and billed with the
appropriate modifier will reimburse 75% of the IHCP allowed amount. HSPPs do not use
modifiers.
II. Mental Health Rehabilitation Option Services
Mental Health Rehabilitation Option (MRO) services are restricted to providers
enrolled as Community Mental Health Centers (CMHC). These services are provided
to individuals, families, or groups living in the community who need aid
intermittently for emotional disturbances or mental illnesses. MRO services include
01/31/2007 Mental Health/Behavioral Health – Outpatient Services
Medical Policy Manual
462
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
traditional IHCP outpatient mental health services, partial hospitalization services,
and case management services. Outpatient mental health services may include, but
not limited to, services provided in the member’s home, workplace, mental health
facility, or emergency department. These services must be rendered by a qualified
mental health professional. Reimbursement is available for the following MRO
outpatient mental health services.
• Case management services
• Conjoint counseling or psychotherapy
• Crisis intervention
• Diagnostic assessment and pre-hospitalization screening
• Family counseling or psychotherapy
• Group counseling or psychotherapy
• Individual counseling or psychotherapy
• Medication or somatic treatment
• Partial hospitalization
• Training in activities in daily living
Partial Hospitalization
Partial hospitalization is a series of structured group activities with components of
two or more hours in duration, but less than full-time hospitalization. For billing
purposes, one unit of service is equal to 15-minutes. Actual time per day is to be
totaled and rounded up to the closest one-quarter hour.
Reimbursement
Community Mental Health Centers must continue to use the HW modifier to denote
MRO services in addition to the modifiers that identify the qualifications or the
individual rendering the service. Community Mental Health Center providers are to
bill using the CPT code H0031 HW, Mental health assessment, by non-physician (one
unit equals one-quarter hour), for physicians performing mental health assessments.
Mid-level practitioners should bill using the CPT code H0031 HW with the
appropriate mid-level practitioner modifier.
Services Not Reimbursable
• Case management services will not be provided as a means for enrollment in
the Medicaid program or verification of IHCP benefits
• Case management services will not be reimbursed for activities which are
vocational in nature or job skill oriented
• MRO services are not covered for Package C members
III. Assertive Community Treatment Service
01/31/2007 Mental Health/Behavioral Health – Outpatient Services
Medical Policy Manual
463
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Assertive Community Treatment (ACT) is an intensive mental health service for
members discharged from a hospital after multiple or extended stays, or who are
difficult to engage in treatment. ACT services are provided by an interdisciplinary
staff team and must be ordered by a physician. Services are provided through
intensive community supports. The goal of ACT services is to decrease the frequency
of hospitalizations, length of hospitalizations, and/or need of crisis services. ACT
services must be available 24-hours a day, seven days a week, with emergency
response coverage, including availability of a psychiatrist. The ACT team must meet
and discuss the services rendered, schedule services, and progress of ACT members
on a daily basis during the five-day work week to meet program review standards.
ACT teams should have a procedure in place to track daily team meeting attendance
and client therapy participation.
Prior authorization (PA) is required for ACT services covered by the IHCP. It is not
necessary to submit PA requests to HCE. The Division of Mental Health and
Addiction reviews random samples of ACT medical records for appropriateness and
ensures that the required services and documentation are maintained. The provider is
responsible for compiling and maintaining the necessary documentation for PA.
ACT services are only reimbursable for members who meet the criteria for ACT
services and for whom the ACT team psychiatrist has documented medical necessity.
Eligibility for ACT services is determined from the current medical status, psychiatric
history, and status at time of consideration for ACT services. Treatment plan goals
must be documented and reviewed by the ACT team psychiatrist. The following
illustrates additional information which must be documented in the medical record
and is required for the PA process.
• Member's level of functioning factor score at the date of most recent assessment and
the date of that assessment
• Clinical summary including documentation of any institutionalizations and hospital
visits related to the member's condition in the past two years
• Documentation supporting the member's severe limitations with activities of daily
living, a current treatment plan, and documentation supporting how the member
meets the CMHC requirements for ACT participation
• Individual treatment plans, which need to be reviewed and updated every 90 days,
must be developed and include the following documentation: medication
administrating and monitoring; self-medication monitoring; crisis assessment and
intervention; symptom assessment, management and individual supportive therapy;
substance abuse training and counseling; psychosocial rehabilitation and skill
development; personal, social, and interpersonal skill training; and coordination
with case management, consultation, and psycho-educational support for
individuals and their families
• Signature of the ACT team psychiatrist is considered the clinical review required for
PA
01/31/2007 Mental Health/Behavioral Health – Outpatient Services
Medical Policy Manual
464
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Reimbursement
ACT services must be billed using procedure code H0040, ACT Program, per diem,
and modifier HW, Funded by state mental health agency. One unit of ACT service
equals one 24-hour day. The ACT team psychiatrist or a health service provider in
psychology (HSPP) who is an ACT team member must be present at the daily team
meetings for the service code to be reimbursed at 100% of the Medicaid allowable
amount. Additional mid-level practitioner modifiers are necessary, when the ACT
team psychiatrist or HSPP is not in attendance at the daily team meeting, to obtain
reimbursement at 75% of the allowed rate.
IV. Waiver Services
Waiver members may receive MRO services; however, services may not be
duplicated. The waiver case manager and the CMHC must coordinate services to
ensure duplication does not occur.
V. Other Outpatient Mental Health Services
Testing Services
PA is required for all units of testing which includes codes CPT 96101, Psychological
testing (includes psychodiagnostic assessment of personality, psychopathology,
emotionality, intellectual abilities, eg, WAIS-R, Rorschach, MMPI) with
interpretation and report, per hour; 96110, Developmental testing; limited (eg,
Developmental Screening Test II, Early Language Milestone Screen), with
interpretation and report; 96111, Developmental testing; extended (includes
assessment of motor language, social, adaptive, and/ or cognitive functioning by
standardized developmental instruments) with interpretation and report; and 96116,
Neurobehavioral status exam (clinical assessment of thinking, reasoning and
judgment, eg, acquired knowledge, attention, memory, visual spatial abilities,
language functions, planning) with interpretation and report, per hour, and 96118,
Neuropsychological testing battery (eg, Halstead-Reitan, Luria, WAIS-R) with
interpretation and report, per hour. A physician or an HSPP must provide all testing
services, as well as, interpretation and reporting.
Medication Management Services
During the initial assessment with the member, there must be face-to-face contact
with a physician; however, at the three-month review, the face-to-face contact may be
made with the physician or an advanced practice nurse with prescription authority
providing services within the scope of practice. Table 2 includes covered medication
management services that are reimbursable only when rendered by a physician or an
advanced practice nurse with prescription authority.
01/31/2007 Mental Health/Behavioral Health – Outpatient Services
Medical Policy Manual
465

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 2 – Medication Management Services
CPT
Code
Description
90805
Individual psychotherapy, insight oriented, behavior modifying and/or
supportive, in an office or outpatient facility, approximately 20 to 30 minutes
face-to-face with the patient; with medical evaluation and management services
90807
Individual psychotherapy, insight oriented, behavior modifying and/or
supportive, in an office or outpatient facility, approximately 45 to 50
minutes face-to-face with the patient; with medical evaluation and
management services
90809
Individual psychotherapy, insight oriented, behavior modifying and/or
supportive, in an office or outpatient facility, approximately 75 to 80
minutes face-to-face with the patient; with medical evaluation and
management services
90811
Individual psychotherapy, interactive, using play equipment, physical
devices, language interpreter, or other mechanisms of nonverbal
communication, in an office or outpatient facility, approximately 20 to 30
minutes face-to-face with the patient; with medical evaluation and
management services
90813
Individual psychotherapy, interactive, using play equipment, physical
devices, language interpreter, or other mechanisms of nonverbal
communication, in an office or outpatient facility, approximately 45 to 50
minutes face-to-face with the patient; with medical evaluation and
management services
90815
Individual psychotherapy, interactive, using play equipment, physical
devices, language interpreter, or other mechanisms of nonverbal
communication, in an office or outpatient facility, approximately 75 to 80
minutes face-to-face with the patient; with medical evaluation and
management services
90862
Pharmacologic management, including prescription, use, and review of
medication with no more than minimal medical psychotherapy
RELATED MEDICAL TOPICS
Assertive Community Treatment Services
Community Mental Health Centers Services
Developmental, Neurological, and Psychological Testing Services
Evaluation and Management Services
Intermediate Intensive Care Facility for the Mentally Retarded
Inpatient Hospital Services
Managed Care Services
Mental Health Rehabilitation Services
Outpatient Services
Pharmacy Services
Physician Services
01/31/2007 Mental Health/Behavioral Health – Outpatient Services
Medical Policy Manual
466

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Psychiatric Residential Treatment Facility Services
Smoking Cessation Services
Waiver Services
RULES, CITATIONS, AND SOURCES
Indiana Administrative Code (IAC)
405 IAC 1-8-2 – Hospital and ambulatory surgical center reimbursement for
outpatient services
405 IAC 1-10.5-3 – Perspective reimbursement methodology
405 IAC 5-2-19 – “Outpatient services” defined
405 IAC 5-3 – Prior authorization
405 IAC 5-2-17 – “Medically reasonable and necessary service” defined
405 IAC 5-20-8 – Outpatient mental health services
405 IAC 5-21 – Community mental health rehabilitation services
405 IAC 5-25 – Physician services
405 IAC 5-29 – Services not covered by Medicaid
405 IAC 5-37 – Smoking cessation treatment policy
440 IAC 5.2-2-3 – Assertive community treatment services
Indiana Code (IC)
IC 12-15-12 – Managed care
IC 25-23-1-1(b) (3) – Advance practice nurses credentialed in psychiatric or
mental health nursing by the American Nurses
Credentialing Center
Indiana Health Coverage Programs Banner (BR)
BR200532 – Supervision of mental health or managed care organization services
BR200523 – Assertive community treatment community mental health centers
H0040–ACT services, per diem
BR200514 – Hoosier Healthwise mandatory risk-based managed care (RBMC)
enrollment
BR200451 – Mental health cross walked local codes
BR200439 – CPT codes 90805, 90807, 90809, 90811, 90813, and 90815 for
psychotherapy with medical evaluation and management, and CPT
code 90862 for pharmacological management
BR200430 – EDS made changes to HCPCS code H0040–Assertive community
treatment program, per diem
BR200424 – Explanation of benefits (EOB) code 5007
BR200244 – System modification affecting Medicare Part B crossover claims
billed by a health service provider in psychology (HSPP) with an AH
modifier
BR200421 – Mid-level practitioner modifiers
BR200240 – Explanation of benefit (EOB) code 4116–diagnosis code is not valid
for DRG pricing.
BR199952 – Supervision requirements
BR199950 – Risked based managed care inpatient psychiatric services
BR199946 – Implementation of smoking cessation treatment services
01/31/2007 Mental Health/Behavioral Health – Outpatient Services
Medical Policy Manual
467
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
BR199920 – Changes in diagnostic related groups/level of care inpatient and
outpatient methodologies
Indiana Health Coverage Programs Provider Bulletin (BT)
BT200506 – State-wide Hoosier Healthwise mandatory managed care
organization transition
BT200432 – Annual notice for Hoosier Healthwise managed care members
BT200429 – Psychiatric residential treatment facility services for patients
younger than six years old
BT200415 – Clarification of mental health modifiers, mental health clinics,
and community mental health centers
BT200413 – Assertive community treatment service coverage, standards, and
requirements
BT200404 – Acute care hospitals, psychiatric hospitals, psychiatrists, health
services providers in psychology, mental health clinics,
physicians, primary medical providers for primary care case
management, and community mental health centers
BT200362 – Prior authorization for Hoosier Healthwise managed care
organizations
BT200360 – Changes in diagnosis-related group relative weights and new long-
term acute care hospital level-of-care reimbursement methodology
BT200340 – Hoosier Healthwise mandatory managed care organization transition
BT200262 – Hoosier Healthwise managed care certification code requirements
BT200240 – MDwise managed care organization service area expansion
BT200231 – Managed care carve-out and self-referral education
BT200208 – Modifications to prior authorization requirement
BT200206 – Medicaid rehabilitation option issues
BT200145 – Community mental health centers third party liability edits
BT200140 – Mandatory managed care organization enrollment update
BT200129 – Changes in diagnostic-related groups/level-of-care inpatient and
outpatient reimbursement methodologies
BT200124 – Acute care and freestanding psychiatric hospitals reimbursement
for child abuse and neglect cases
BT200121 – Important information about Hoosier Healthwise managed care
entity contact information
BT200049 – Procurement of Hoosier Healthwise MCO contract
BT200028 – Changes to the Medicaid medical policy rule regarding outpatient
mental health services (405 IAC 5-20 and 405 IAC 5-21)
BT199946 – Implementation of smoking cessation treatment services
BT199930 – Participation in the Indiana Medicaid hospital care for the indigent
(HCI) payment, Municipal County Hospital Indiana Medicaid
shortfall payment and Indiana Medicaid disproportionate share
hospital (DSH) payment programs
BT199917 – Acute care and freestanding psychiatric hospitals child abuse and
neglect cases
01/31/2007 Mental Health/Behavioral Health – Outpatient Services
Medical Policy Manual
468

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
BT199902 – Changes in diagnostic-related groups/level-of-care inpatient and
outpatient reimbursement methodologies
Indiana Health Coverage Programs Provider Newsletter (NL)
NL200506 – Psychiatric residential treatment facility update PRTF
NL200501 – Crosswalked local codes
NL200410 – Medicaid behavioral/physical health coordination form
NL200406 – Mental health billing modifiers
NL200405 – Cognitive therapy services
Indiana Health Coverage Programs Manual
1999
2005 Version 5.1
Revisions: December 16, 2005
Revision and
Review
Reason Date
470 IAC 5-9-22 Mental Health Services 7/01/91
405 IAC 1-6-13;
1-6-13.1; & 1-7-20
Mental Health Services (Repealed) 1/01/92
405 IAC 5-20-8 Outpatient Mental Health Services 8/24/97
405 IAC 5-21-1
Community Mental Health Rehabilitation
Services
8/24/97
Indiana Medicaid
Update Bulletin
Mental Health Rehabilitation Option 7/01/98
Indiana Health
Coverage Programs
Mental Health Providers, Physicians, and
Psychologists
12/28/99
Indiana Medicaid
Update Bulletin
Psychiatrists, Physicians, Health Service
Providers in Psychology, and Mental Health
Facilities Clinics
8/04/00
Routine Review Scheduled 1/31/06
APPLICABLE INDIANAAIM EDITS AND AUDITS
1040 – MRO Services Can Only be Billed On a HCFA 1500 Form By A CMHC
2202 – Recipient Not Enrolled With Billing MCO
4203 – Denial Modifier for Non Covered MRO Service- Y8
4204 – Invalid Diagnosis for Procedure Code/Modifier Combination
6120 – Outpatient Mental Health/ Substance Abuse Services Office Visits, Maximum 30
Per Calendar Year Without Prior Authorization
6121 – Outpatient Mental Health/ Substance Abuse Services Office Visits, Maximum 50
Per Calendar Year Without Prior Authorization
6125 – Cognitive Rehabilitation is Limited to Procedure and Diagnosis
6270 – Smoking Cessation Counseling Limited to 10 15-minute Units Per Calendar
Year
6515 – Inpatient Services Preformed 3 Days After DOS of Paid Outpatient Claim
6516 – Outpatient Services Rendered Within 3 Days Prior to Admit
01/31/2007 Mental Health/Behavioral Health – Outpatient Services
Medical Policy Manual
469
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Mental Health/Behavioral Health – Outpatient Services
Medical Policy Manual
470
6517 – Inpatient Claim Discharge Date Within 3 Days Prior to Admit Date of Paid
Inpatient Claim
6518 – Inpatient Claim Admit Date Within 3 Days After Discharge Date of Paid Inpatient
Claim
6615 – Assertive Community Treatment Is Limited One Per Day
6632 – T2048 Is Only Reimbursable for Provider Specialty 033
6633 – Z5180 and T2048 Limited to One Psychiatric Treatment Per Day
6636 – Mid-level Services Not Reimbursable the Same Day a Paid PRTF Service
6673 – Psychiatric Per Diem Medical Leave Days Cannot Exceed Four Consecutive Days
6900 – Outpatient Mental health Services More Than 20/Year Without Prior
Authorization
6902 – Outpatient Therapies Over 80 Units Requires Prior Authorization
6921 – One Diagnostic Evaluation Per Recipient Every 6 Months

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: NURSING FACILITIES
DESCRIPTION:
A nursing facility [long-term care facility (LTC)] is an extended-care facility for persons who
require medical attention of the type and complexity that does not require hospitalization.
Nursing facilities provide 24-hour nursing supervision, rehabilitation services, activity and social
services, a restraint-appropriate environment, careful attention to nutritional needs, and measures
to prevent complications of decreased mobility. (Taber’s Cyclopedic Medical Dictionary, Edition
18, Copyright © 1997 by F.A. Davis Co., Philadelphia, PA).
This document is intended to serve as a general summary of the Indiana Health Coverage
Programs’ (IHCP) policies regarding this service. More specific information may be found in
the IHCP Provider Manual, program notices, the Indiana Administrative Code (IAC) or other
sources as appropriate. See the “Rules, Citations, and Sources” section of this document for
further information.
COVERAGE CRITERIA
Services and products furnished by a nursing facility for the usual care and treatment of IHCP
members are reimbursed in the per diem rate. The per diem rate for nursing facilities includes room
and board, nursing care, the cost of all medical and nonmedical supplies and equipment, medically
necessary and reasonable therapy services, which include physical, occupational, respiratory, and
speech pathology services, and transportation to vocational/habilitation service programs. Routine
nursing services must be provided by a registered nurse, a licensed practical nurse, or a nurse's
aide.
IHCP reimbursement is not available for personal care or comfort items. The IHCP member
receives a personal needs allowance to be used at the member’s discretion for items such as
clothing, makeup, or other personal items. Providers may not utilize these funds without the
member’s expressed consent.
Hospice Services
Providers are advised to consult the Medical Policy Fact Sheet regarding Hospice Services and
the IHCP Hospice Provider Manual for information regarding hospice services provided to an
IHCP member that is residing in a nursing facility.
01/31/2007 Nursing Facilities
471
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Transportation Services
Providers are advised to consult the Medical Policy Fact Sheet/Transportation Services for
further information.
Pharmacy Services
The IHCP does not provide reimbursement for pharmacy services provided to a dually eligible
IHCP member in a nursing facility during a Medicare covered post-hospitalization period. Drug
products dispensed for these members should be billed to the nursing facility only. Providers are
advised to consult the Medical Policy Fact Sheet/Pharmacy Services for further information.
Case Mix Reimbursement
Under the case mix reimbursement system, each nursing facility has one nursing facility level of
care designation in IndianaAIM and one per diem rate calculated on resource usage for residents
within the facility. The per diem rate changes quarterly and is calculated by IHCP’s rate setting
contractor.
INDIANA PRE-ADMISSION SCREENING
PRE-ADMISSION SCREENING AND RESIDENT REVIEW
Indiana pre-admission screening (IPAS) refers to the assessment and determination of a
member’s eligibility prior to admission to a nursing facility. Resident review (RR) refers to the
annual evaluation used to determine the necessity to continue services due to a change in
condition. Initial medical information is submitted for all nursing facility admissions utilizing
the Form 450B, described on the following page.
All individuals applying for admission to Medicaid-certified nursing facilities, regardless of their
source of payment, must be pre-screened through a two level screening process, Pre-Admission
Screening and Resident Review (PASRR)
5
. Level I identifies individuals who may be mentally
ill (MI) or Mentally Retarded/Developmentally Disabled (MR/DD). A PASRR Level II
assessment is conducted by the Community Mental Health Centers (CMHCs) for nursing facility
residents who may be MI. Nursing facility residents who may be MR/DD receive the PASRR
Level II assessment by the Diagnostic and Evaluation (D&E) Team. Nursing facility residents
may also require assessment under the Resident Review (RR) Level II process if they are
identified as possibly being MI or MR/DD under one of the following circumstances.
• The resident was not assessed through the PASRR program prior to admission
• The resident has a substantial change in condition related to their MI or MR/DD
condition, which may require a change in services or placement
Diagnostic and Evaluation Teams
Diagnostic and Evaluation (D&E) Teams must be contracted and approved by the Division of
Disability, Rehabilitative Services (DDARS), and the Bureau of Developmental Disabilities
01/31/2007 Nursing Facilities
472
5
PASRR is a Federally required administrative program that requires all individuals with mental illness (MI) and/or
mental retardation/developmental disabilities (MR/DD), who make application for Level II placement, must be
admitted to a Medicaid-certified nursing facility.
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Services (BDDS) to conduct the PASRR Level II MR/DD assessments and be enrolled with the
IHCP to be eligible to submit Level II MR/DD claims. Providers may obtain a list of contracted
and authorized D&E Teams from DDARS.
Community Mental Health Centers
CMHCs are contracted and approved by the Division of Mental Health and Addiction (DMHA)
to conduct the PASRR Level II MR/DD assessments and are enrolled with the IHCP to be
eligible to submit Level II MR/DD claims. Providers may obtain a list of contracted and
authorized CMHCs from the DMHA.
Form 450B/Nursing Facility Level of Care
The Form 450B, Physician Certification for Long Term Care Services, must be submitted by the
nursing facility to gain State authorization for admissions, transfers between levels of care, re-
admissions, Medicare-to-Medicaid transfers, and new Medicaid eligibility. This form is
submitted by the nursing facility to the Office of Medicaid Policy and Planning (OMPP). There
are currently three variations of Form 450B. Facilities can order Form 450B and OMPP 450B
SA/DE (State Form 49120) from the Department of Administration, Forms Distribution Center.
Table 2 in Appendix A describes the use of the forms listed below.
• Form 450B (State Form 38143) is used for physician certification for LTC services.
• OMPP 450B SA/DE (State Form 49120) is used for nursing facility level of services,
State authorization, and data entry.
• OMPP 450B SA/DE (computer-generated) (State Form 49210) is used for nursing
facility level of service, State authorization, and data entry. This computer-generated
OMPP 450B SA/DE includes an Indiana Family Social Services Administration signature
and is considered to be the official Form 450B and must be maintained in the resident’s
medical records.
Form 450B is not required for any readmission following a hospitalization exceeding the bed-
hold policy, as long as the resident was approved for nursing facility care prior to the
hospitalization. This applies to readmissions from a hospital to the same or another nursing
facility; however, there must be no break in medical care. Nursing facilities are required to
submit the OMPP 450B SA/DE (computer-generated) to the OMPP in place of the paper Form
450B, for purposes of data entry of the resident’s readmission date and the appropriate facility
provider number in IndianaAIM to reinstate nursing facility reimbursement. If the facility
intends to reflect other changes, such as new Medicaid eligibility, readmission, or a transfer from
another nursing facility, this information should not be entered in the “Level of Care Transfer
Date” box.
Resident Level of Care Information on the IndianaAIM System
Under case mix, the reimbursement rate is based on the information submitted on the Minimum
Data Set (MDS) 2.0 form
6
for each resident. This information is then to be used to compute a
facility-average case mix index and rate. Therefore, residents certified for nursing facility
01/31/2007 Nursing Facilities
473
6
MDS is a comprehensive assessment for all residents of LTC facilities certified to participate in the Medicaid
program.
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
placement by the Office of Medicaid Policy and Planning (OMPP) will be denoted with the ‘N’
level of care indicator.
Billing Requirements for PASRR
All PASRR claims are reviewed with the State PASRR database prior to reimbursement.
CMHCs and D&E Teams may submit claims for PASRR Level II assessments conducted as a
result of a Level I referral. Providers should terminate a PASRR Level II assessment
immediately if it is determined that the Level I referral was not appropriate, such as, applicant is
not DD or has a primary diagnosis of dementia. The submitted claim should reflect a reduced
fee as appropriate for the individual assessment. PASRR claims use normal claim processing
billing procedures, with the following minor differences.
• D&E Teams and CMHCs are only approved to conduct PASRR Level II assessments
through contractual arrangements with DDARS and DMHA. PASRR providers must be
enrolled as IHCP providers.
• PASRR applicant(s) or member(s) may be dually-eligible in the IHCP.
• Providers are advised to submit claims for the member using the PASRR member
identification number that begins with 800 and the member’s social security number. If
an applicant does not have or refuses to provide a social security number, providers may
contact the EDS Customer Assistance Unit.
• Providers may not bill members for a PASRR Level II assessment.
• Services cannot be combined with other non-PASRR service type(s), even if the
service(s) are rendered on the same day, or same visit. For example, a claim for PASRR
services cannot be combined with a claim for other IHCP services.
• PASRR claims are subject to all edits and audits not excluded by PASRR program
requirements. If a claim encounters an edit or audit for missing or invalid information,
the claim suspends or denies.
• Provider reimbursement for rendered services is determined by the procedure codes,
modifiers as defined in Table 1, and the associated maximum (max) fee rate. Procedure
code(s), modifier(s), and max fee rate(s) must accompany all PASRR claim submissions.
• Providers may void or replace PASRR claims.
• PASRR financial information is available on the 835 Remittance Advice transaction.
Table 1 – CPT Codes for Reporting PASRR Services
CPT Code Description
T2011 U1 UA
T2011, Preadmission screening and resident review (PASRR) level II
evaluation, per evaluation
U1, PAS (Preadmission screening)
UA, Mental retardation/developmental disability
T2011 U1 UA HI
T2011, Preadmission screening and resident review (PASRR) level II
evaluation, per evaluation
U1, PAS (Preadmission screening)
UA, Mental retardation/developmental disability
HI, Integrated mental health and mental retardation/developmental
disabilities program
01/31/2007 Nursing Facilities
474
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Nursing Facilities
475
Table 1 – CPT Codes for Reporting PASRR Services
CPT Code Description
T2011 U2 UA
T2011, Preadmission screening and resident review (PASRR) level II
evaluation, per evaluation
U2, RR (Resident review)
UA, Mental retardation/developmental disability
T2011 U2 UA HI
T2011, Preadmission screening and resident review (PASRR) level II
evaluation, per evaluation
U2, RR (Resident review)
UA, Mental retardation/developmental disability
HI, Integrated mental health and mental retardation/developmental
disabilities program
T2011 U1 UB
T2011, Preadmission screening and resident review (PASRR) level II
evaluation, per evaluation
U1, PAS (Preadmission screening)
UB, Mental illness
T2011 U1 UB TS
T2011, Preadmission screening and resident review (PASRR) level II
evaluation, per evaluation
U1, PAS (Preadmission screening)
UB, Mental illness
T2011 U2 UB
T2011, Preadmission screening and resident review (PASRR) level II
evaluation, per evaluation
U2, RR, (Resident review)
UB, Mental illness
BILLING REQUIREMENTS
The IHCP does not provide separate reimbursement for medical supplies, non-medical supplies,
and routine durable medical equipment (DME) items for members residing in LTC facilities.
LTC facilities include nursing facilities, intermediate care facilities for the mentally retarded
(ICFs/MR), and community residential facilities for the developmentally disabled (CRFs/DD).
The costs for these services are included in the facility per diem rate, and the medical supplier, or
DME supplier should bill the long-term care facility directly for such services.
Therapeutic Leave Days/Hospital Leave Days
The revenue code specific to the leave must be utilized for billing purposes. Revenue code 183
must be used for a therapeutic leave of absence, and revenue code 185 must be used for the
hospitalization bed hold. A supporting physician’s order for all leave of absences must be
maintained in the member’s medical record.
Ancillary Charges
The IHCP does not provide separate reimbursement for medical, nonmedical supplies, and
routine medical equipment. These items are included in the nursing facility per diem rate. Food
supplements, nutritional supplements, and infant formulas are also excluded from separate
billing and reimbursement.
Medical Policy Manual
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Therapy Services
All therapy services provided to IHCP members by nursing facilities are included in the
established per diem rate. Therefore, a provider may not bill the IHCP for therapies in addition
to the established per diem rate.
NURSING FACILITY AUDITS
The IHCP conducts on-site audits in nursing facilities to review the continuing need for IHCP
reimbursement and to ensure that PASRR requirements are met. The on-site audit process
includes a verification of the MDS 2.0 responses transmitted to the IHCP rate setting contractor,
through a review of documentation in the member’s medical record. A sample of all residents in
the nursing facility is reviewed, including those residents whose care is not directly funded by
the Indiana Medicaid program.
MANAGED CARE
The IHCP pays for room and board under the IHCP hospice benefit for dually-eligible
Medicare/Medicaid nursing facility residents and Medicaid-only nursing facility residents who
elect the hospice benefit. Each provider group must complete their respective responsibilities to
disenroll the member from managed care to ensure that the hospice provider may successfully
bill the IHCP for room and board under the hospice benefit. Each provider group must comply
with the IHCP Provider Agreement and regularly verify IHCP eligibility.
Nursing facilities and the Area Agency on Aging must notify the specific managed care
organization (MCO) immediately when an MCO member is admitted to a facility or undergoes
IPAS/PASRR. The MCO is financially responsible for all care provided to its members until
enrollment termination is effective. IHCP fee-for-service is financially responsible for nursing
facility reimbursement when the member is approved for intermediate level of care (LOC),
skilled LOC, or general case mix and the member is disenrolled from the MCO.
Nursing facilities must coordinate with the MCO to allow members to use appropriate in-
network service during the period when the member is assigned to the MCO. Providers are
advised to contact the individual MCO for further information.
RELATED MEDICAL TOPICS
Evaluation and Management Services
Hospice
Hospital, Inpatient
Hospital, Outpatient
Intermediate Care Facilities for the Mentally Retarded
Nursing Services
Physical Rehabilitation Services
Transportation Services
01/31/2007 Nursing Facilities
476
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
RULES, CITATIONS, AND SOURCES
405 IAC 1-12.2 Rate setting
405 IAC 1-12-5 New provider; interim rate setting
405 IAC 1-12-7 Request for rate review
405 IAC 1-14.5 Rate setting criteria for HIV nursing facilities
405 IAC 1-14.6 Rate setting criteria for nursing facilities
405 IAC 1-17 Rate setting criteria for state-owned intermediate facilities for the mentally
retarded
405 IAC 5-13-3 Services included in the per diem for large private and small ICFs/MR
405 IAC 5-31 Nursing Facility Services
405 IAC 5-5-2 Out-of-State Services
405 IAC 5-30-1 Transportation Services
Indiana Medical Assistance Program Provider Manual 1994
Indiana Medicaid Update Bulletin
95-81 Transportation
98-06 Utilization Review Update
98-26 Case Mix
98-29 Verification
98-30 Hospice Benefit
98-40 450B SA/DE
99-21 Personal Needs Allowance
Indiana Health Coverage Programs Banners
BR200302 – Pharmacy services during Medicare Post-Hospitalization Period
BR200506 – Billing
BR200524 – PASRR and Medical Review Team
Indiana Health Coverage Programs Bulletins
BT200002 – Use of Forms 450B and OMPP 450B SA/DE
BT200501 – Excessive Nursing Home Visits or More Than One per 27 Days, Pre-
Admission Screening and Resident Review
BT200513 – Pre-Admission Screening and Resident Review Level II Claims
Processing Change
Indiana Health Coverage Programs Provider Newsletters
NL200503 – Disenrolling a Member from an IHCP Managed Care Program
Origination Date: 12/31/2000
Revisions and
Review
Reason Date
Indiana Medicaid
Update Bulletin
95-81
Transportation Trip Limit Policy 12/20/1995
405 IAC 5-31, 5-5-2,
5-24-7, 5-30-1
Nursing Facility Services, Out-of-State Services, Pharmacy
Services, Transportation Services
08/24/1997
01/31/2007 Nursing Facilities
477
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Nursing Facilities
Medical Policy Manual
478
Revisions and
Review
Reason Date
Indiana Medicaid
Update Bulletin
98-40
Form 450B SA/DE, Case Mix Reimbursement,
Verification of Eligibility for Medicaid Member in
Residing in Nursing Facilities
10/01/1998
Indiana Medicaid
Update Bulletin
99-21
Personal Needs Allowance (PNA) 07/01/1999
405 IAC 1-12-5 and
405 IAC 1-12-7
ICF/MR and Nursing Facility Services 10/01/1999
405 IAC 5-5-2, and
5-30-1, 5-31-1, 5-31-
5, 5-31-8, Amended;
405 IAC 5-31-1.1,
Added; 405 IAC 5-
31-2, 5-31-3,
Repealed
Out of State Services, Transportation Services, and
Nursing Facility Services
10/27/1999
Review/Revision Scheduled Review and Update 10/31/2006
APPLICABLE INDIANAAIM EDITS AND AUDITS
1017 – No Rate Segment for Level of Care (Case Mix)
1018 – No Rate Segment for Level of Care
1019 – Multiple Levels of Care Per Diem on File
1023 – Level of Care Billed Not On File for This Provider
1030 – Ancillary Service Not Covered
2008 – Recipient Ineligible for Level of Care Billed
3015 – Out of State Noncovered Services – LTC
3016 – Out of State Home Health Services are Non Covered
4015 – PASRR Assessments
4113 – Unit Dose Packaging Covered for Residents of Long Term Care
6047 – Excessive Therapeutic Days
6067 – Excessive Therapeutic Leave Day (ICF)
6068 – Excessive Therapeutic Leave Days (ICF/MRO or CFR)

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Appendix A
Table 2 – Use of Forms 450B, 450B SA/DE (Paper or Computer Generated) for
IHCP Members
Scenario Qualifier Form Required
Accompanied
Information
Official Form for
Medical Record
Initial Admission
to NF
7
(IPAS and
PASRR)
All cases of
IPAS/PASRR
Entire 450B
(Sections I and II)
completed
Complete
IPAS/PASRR
packet
Computer generated
OMPP 450B SA/DE
or Form 450B with
Section III completed
NF to hospital and
return same NF
(with existing
effective Medicaid
reimbursement
date)
Not exceeding
bed hold policy
None None
Existing 450B with
effective Medicaid
reimbursement date
NF to hospital and
return same NF
(with existing
effective Medicaid
reimbursement
date)
Exceeding bed
hold policy
450B (Section I
only) or 450B
SA/DE
None
Returned 450B with
effective Medicaid
reimbursement date
NF to hospital and
return to another
NF (with effective
Medicaid
reimbursement
date)
Following any
length of
hospitalization
450B (Section I
only) or 450B
SA/DE
None
Returned 450B with
effective Medicaid
reimbursement date
Transfer from NF
to NF (no
intervening
hospitalization)
None
Entire 450B
(Section I and II)
completed or 450B
Sa/DE with fully
completed MDS
8
Copy of PAS 4B
from the
previous NF
Returned 450B with
effective Medicaid
reimbursement date
Resident change
from private pay to
Medicaid member
Including
changes in
eligibility status
from Medicaid
MCO to regular
Medicaid
Entire 450B
(Section I and II)
completed or 450B
Sa/DE with fully
completed MDS³ or
computer-generated
OMPP 450B
SA/DE***
9
Copy of PAS 4B
Returned 450B with
effective Medicaid
reimbursement date
or computer-
generated OMPP
450B SA/DE.
7
NF-Nursing Facility
8
The fully completed MDS for the period under review should be submitted with the Form 450B
SA/DE only. The day of the MDS observation period must be within 90 days of Medicaid
effective date or requested start date.
01/31/2007 Nursing Facilities
479
9
Resubmit an updated (RID, dates, provider number) State-generated OMPP 450B SA/DE if a
resident became Medicaid eligible and the requested effective date for Medicaid reimbursement
is within 90 days of the state-authorized signature on the OMPP 450B SA/DE.
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Nursing Facilities
Medical Policy Manual
480
Scenario Qualifier Form Required
Accompanied
Information
Official Form for
Medical Record
Change from
Medicare primary
payer to Medicaid
primary payer
(without existing
effective Medicaid
reimbursement
date
When Medicare
coverage ends
Entire 450B
(Section I and II)
completed or 450B
Sa/DE with fully
completed MDS³
Copy of PAS 4B
Returned 450B with
effective Medicaid
reimbursement date or
computer-generated
OMPP 450B SA/DE
Change from
Medicare payer to
Medicaid primary
payer (with
existing effective
Medicaid
reimbursement
date)
When Medicare
coverage ends
450B (Section I
only) or 450B
SA/DE
None
Returned 450B with
effective Medicaid
reimbursement date or
computer-generated
OMPP 450B SA/DE

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: NURSING SERVICES
DESCRIPTION:
General nursing services for this policy include those services rendered by registered
nurses (RN) and licensed practical nurses (LPN), in an office setting, in-patient or out-
patient hospital setting, clinic, or home health setting. Other nursing services included in
this policy are services rendered by advance practice nurses.
SUMMARY OF CURRENT POLICY
Medicaid reimbursement is available for nursing services rendered by registered nurses,
licensed practical nurses, and home health agencies who are Medicaid providers, subject
to the following limitations.
• The nurse must possess a current and active license from the Health Professions
Bureau.
• Services must be rendered under the supervision and orders of a physician.
• The IHCP no longer enrolls RNs and LPNs in the IHCP program as rendering
providers. However, RNs and LPNs are allowed to be the rendering provider of
appropriate services in settings including but not limited to the physician office,
in-patient or out-patient hospital setting, clinic, and home health settings.
• The IHCP will reimburse nursing services to the billing provider supervising the
nursing services (e.g. physician, home health agency).
• Nursing services provided by a home health agency require prior authorization
subject to the Indiana Administrative Code (IAC) 405 IAC 5-22-2. For further
information, see the Home Health Fact Sheet.
IHCP reimbursement is available for appropriately licensed and certified advanced
practice nurses enrolled in the IHCP, according to the scope of the applicable license and
certification. The Indiana Code (IC) IC 25-23-1-1 lists three categories of advanced
practice nurses, including nurse practitioners, nurse midwives, and clinical nurse
specialists. Other types of nurse practitioners enrolled in the IHCP include: certified
registered nurse anesthetists, family practice nurse practitioners, pediatric nurse
practitioners, and obstetric nurse practitioners.
01/31/2007 Nursing Services
481
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
RELATED MEDICAL TOPICS
Anesthesia Services
Home Health Services
Mental Health
RULES, CITATIONS, AND SOURCES
405 IAC 5-22 Nursing and Therapy Services
405 IAC 5-10 Anesthesia Services
Indiana Medical Assistance Program Provider Manual 1994
Indiana Health Coverage Programs Provider Manual, Version 5.0, 2004
Indiana Health Coverage Programs Banner, BN200351
848 IAC 2-2
848 IAC 2-3
IC 25-23-1-1.1
IC 25-23-1-1.2
IC 25-23-1-1.3
848 IAC 4-1-3
848 IAC 5-1
IC 25-23-1.19.6
848 IAC 4-2-1
848 IAC 4-3-1
848 IAC 3-1-2
IC 25-23-1-30
NL200406
Origination Date: 12/31/00
Review and Revisions Reason Date
407 IAC 5-8-19-Nursing and Therapy
Services
Transferred 7/1/91
405 IAC 1-6-20; 1-7-21 Nursing and
Therapy Services
Repealed 8/24/97 1/1/92
405 IAC 5-22 Nursing and Therapy Services 8/24/97
405 IAC 5-22-2 Nursing and Therapy Services 10/27/99
405 IAC 5-22-2
Nursing and Therapy Services
Revision
1/30/05
Provider Enrollment Provider Specialty
Matrix
Provider Specialties Revision 1/30/05
848 IAC 2-2 Registered Nursing 1/30/05
848 IAC 2-3 Licensed Practical Nursing 1/30/05
IC 25-23-1-1.1 Registered Nurse 1/30/05
01/31/2007 Nursing Services
482
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Nursing Services
483
Review and Revisions Reason Date
IC 25-23-1-1.2
Licensed Practical Nurse
Defined
1/30/05
IC 25-23-1-1.3 Practical Nursing Defined 1/30/05
848 IAC 4-1-3
Advanced Practice Nurse
Defined
1/30/05
848 IAC 5-1 and IC 25-23-1.19.6.
Prescriptive Authorities of
Advanced Practice Nurses
1/30/05
848 IAC 4-2-1.
Nurse Practitioner Scope of
Practice
1/30/05
848 IAC 4-3-1
Clinical Nurse Specialist Scope
of Practice
1/30/05
848 IAC 3-1-2 Nurse-Midwife Defined 1/30/05
IC 25-23-1-30 CRNAs 1/30/05
2004 Provider Manual Ch. 8 Section 3 Nurse Practitioner Billing 1/30/05
BN200351 CRNA Billing 1/30/05
NL200406
Mid-Level Practitioner Billing in
Mental Health
1/30/05
APPLICABLE INDIANAAIM EDITS AND AUDITS
None
PROVIDER TYPE AND SPECIALTY
Provider Type Provider Specialty
09 Advance practice nurse 092 Pediatric nurse practitioner
091 Obstetric nurse practitioner
092 Family nurse practitioner
093 Nurse practitioner, other (Clinical
nurse specialist)
094 Certified registered
nurse anesthetist (CRNA)
095 Certified nurse midwife
COVERAGE CRITERIA
Registered Nurse
The IHCP will provide coverage of services performed by an RN in accordance with the
criteria set in IC 25-23-1-1.1 provided below. An RN is not enrolled as a billing provider
in the IHCP and may not directly bill the IHCP for any services provided. An RN does
not have medical diagnostic or prescriptive privileges and may not sign prescriptions or
member records on behalf of the physician.
Medical Policy Manual
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Sec. 1.1. (a) As used in this chapter, "registered nurse" means a person who holds a valid
license issued:
(1) under this chapter; or
(2) by a party state (as defined in IC 25-23.2-1-11); and who bears primary
responsibility and accountability for nursing practices based on
specialized knowledge, judgment, and skill derived from the principles
of biological, physical, and behavioral sciences.
(b) As used in this chapter, "registered nursing" means performance of services
which include but are not limited to:
(1) assessing health conditions;
(2) deriving a nursing diagnosis;
(3) executing a nursing regimen through the selection, performance,
and management of nursing actions based on nursing diagnoses;
(4) advocating the provision of health care services through
collaboration with or referral to other health professionals
(5) executing regimens delegated by a physician with an unlimited license to
practice medicine or osteopathic medicine, a licensed dentist, a licensed
chiropractor, a licensed optometrist, or a licensed podiatrist;
(6) teaching, administering, supervising, delegating, and evaluating nursing
practice;
(7) delegating tasks which assist in implementing the nursing, medical, or
dental regimen; or
(8) performing acts which are approved by the board or by the board in
collaboration with the medical licensing board of Indiana.
As stated in the IC, an RN may derive a nursing diagnosis. A nursing diagnosis consists
of identifying needs that may be performed by an RN including appropriate preventative,
restorative, maintenance, and promotional activities. These actives may be performed by
meeting or assisting with self-care needs, counseling and teaching. For further
information on the responsibilities of an RN, refer to 848 IAC 2-2.
Licensed Practical Nurse
The IHCP will provide coverage of services performed by an LPN in accordance with the
criteria set in IC 25-23-1-1.2 and IC 25-23-1-1.3 provided below. An LPN is not enrolled
as a billing provider in the IHCP and may not directly bill the IHCP for any services
provided. An LPN does not have medical diagnostic or prescriptive privileges and may
not sign prescriptions or member records on behalf of the physician.
Sec. 1.2. As used in this chapter, "licensed practical nurse" means a person who holds a
valid license issued under this chapter or by a party state (as defined in IC 25-
23.2-1-11) and who functions at the direction of:
(1) a registered nurse;
(2) a physician with an unlimited license to practice medicine or osteopathic
medicine;
(3) a licensed dentist;
01/31/2007 Nursing Services
484
Medical Policy Manual
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
(4) a licensed chiropractor;
(5) a licensed optometrist; or
(6) a licensed podiatrist;
in the performance of activities commonly performed by practical nurses and
requiring special knowledge or skill.
Sec. 1.3. As used in this chapter, "practical nursing" means the performance of services
commonly performed by practical nurses, including:
(1) contributing to the assessment of the health status of individuals or groups;
(2) participating in the development and modification of the strategy of care;
(3) implementing the appropriate aspects of the strategy of care;
(4) maintaining safe and effective nursing care; and
(5) participating in the evaluation of responses to the strategy of care.
For further information on the responsibilities of a LPN, refer to 848 IAC 2-3.
Advanced Practice Nurse
The IHCP will provide coverage of services performed by an advanced practice nurse in
accordance with the criteria set in 848 IAC 4-1-3 provided below. An advanced practice
nurse can enroll as a billing provider. See the billing section of this document for
specific details. An advanced practice nurse has prescriptive authorities as described in
848 IAC 5-1 and IC 25-23-1.19.6. The advanced practice nurse must include their
signature, credentials, and identification number on each prescription in order for the
prescription to be valid.
Sec. 3. (a) “Advanced practice nurse” means a registered nurse holding a current
license in Indiana who:
(1) has obtained additional knowledge and skill through a formal,
organized program of study and clinical experience, or its equivalent,
as determined by the board;
(2) functions in an expanded role of nursing at a specialized level through
the application of advanced knowledge and skills to provide healthcare
to individuals, families, or groups in a variety of settings, including, but
not limited to:
(A) homes;
(B) institutions;
(C) offices;
(D) industries;
(E) schools;
(F) community agencies;
(G) private practice;
(H) hospital outpatient clinics; and
(I) health maintenance organizations; and
(3) makes independent decisions about the nursing needs of clients.
01/31/2007 Nursing Services
485
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Nurse Practitioner
One type of advanced practice nurse is a nurse practitioner. The IHCP will provide
coverage of services performed by a nurse practitioner under the same criteria as
advanced practice nurses as provided in 848 IAC 4-1-3. The scope of practice of a nurse
practitioner is outlined in 848 IAC 4-2-1 below.
Sec. 1. A nurse practitioner shall perform as an independent and interdependent
member of the health team as defined in 848 IAC 2-1-3. The following are
standards for each nurse practitioner:
(1) Assess clients by using advanced knowledge and skills to:
(A) identify abnormal conditions;
(B) diagnose health problems;
(C) develop and implement nursing treatment plans;
(D) evaluate patient outcomes; and
(E) collaborate with or refer to a practitioner, as defined in IC 25-23-1-19.4,
in managing the plan of care.
(2) Use advanced knowledge and skills in teaching and guiding clients and other
health team members.
(3) Use appropriate critical thinking skills to make independent decisions,
commensurate with the autonomy, authority, and responsibility of a nurse
practitioner.
(4) Function within the legal boundaries of their advanced practice area and shall
have and utilize knowledge of the statutes and rules governing their
advanced practice area, including the following:
(A) State and federal drug laws and regulations.
(B) State and federal confidentiality laws and regulations.
(C) State and federal medical records access laws.
(5) Consult and collaborate with other members of the health team as appropriate
to provide reasonable client care, both acute and ongoing.
(6) Recognize the limits of individual knowledge and experience, and consult
with or refer clients to other health care providers as appropriate.
(7) Retain professional accountability for any delegated intervention, and
delegate interventions only as authorized by IC 25-23-1 and this title.
(8) Maintain current knowledge and skills in the nurse practitioner area.
(9) Conduct an assessment of clients and families which may include health
history, family history, physical examination, and evaluation of health risk
factors.
(10) Assess normal and abnormal findings obtained from the history, physical
examination, and laboratory results.
(11) Evaluate clients and families regarding development, coping ability, and
emotional and social well-being.
(12) Plan, implement, and evaluate care.
(13) Develop individualized teaching plans with each client based on health needs.
(14) Counsel individuals, families, and groups about health and illness and
01/31/2007 Nursing Services
486
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
promote attention to wellness.
(15) Participate in periodic or joint evaluations of service rendered, including, but
not limited to, the following:
(A) Chart reviews.
(B) Client evaluations.
(C) Outcome statistics.
(16) Conduct and apply research findings appropriate to the area of practice.
(17) Participate, when appropriate, in the joint review of the plan of care.
Clinical Nurse Specialist
A second type of advanced practice nurse is a clinical nurse specialist. The IHCP will
enroll a clinical nurse specialist under specialty type 093, nurse practitioner, other. The
IHCP will provide coverage of services performed by a clinical nurse specialist under the
same criteria as advanced practice nurses as provided in 848 IAC 4-1-3. The scope of
practice of a clinical nurse specialist is outlined in 848 IAC 4-3-1 below.
Sec. 1. A clinical nurse specialist shall perform as an independent and interdependent
member of the health care team as defined in 848 IAC 2-1-3. The following are
standards for each clinical nurse specialist:
(1) Assess clients by using advanced knowledge and skills to:
(A) identify abnormal conditions;
(B) diagnose health problems;
(C) develop and implement nursing treatment plans;
(D) evaluate patient outcomes
(2) Use advanced knowledge and skills in teaching and guiding clients and other
health team members.
(3) Use appropriate critical thinking skills to make independent decisions,
commensurate with the autonomy, authority, and responsibility of the
clinical nurse specialist
(4) Function within the legal boundaries of their advanced practice area and shall
have and utilize knowledge of the statutes and rules governing their
advanced practice area, including the following:
(A) State and federal drug laws and regulations.
(B) State and federal confidentiality laws and regulations.
(C) State and federal medical records access laws.
(5) Consult and collaborate with other members of the health team as appropriate
to provide reasonable client care.
(6) Recognize the limits of individual knowledge and experience, and consult
with or refer clients to other health care providers as appropriate.
(7) Retain professional accountability for any delegated intervention, and
delegate interventions only as authorized by IC 25-23-1 and this title.
(8) Maintain current knowledge and skills in the nurse practitioner area.
(9) Provide direct nursing care utilizing advanced scientific knowledge, nursing
theory, and nursing skills in the assessment, planning, implementation, and
evaluation of health and nursing care of individual clients.
01/31/2007 Nursing Services
487
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
(10) Provide indirect nursing care through planning, guiding, evaluating, and
directing nursing care delivered by nursing and ancillary personnel as
authorized by IC 25-23-1 and this title.
(11) Conduct nursing research, including methods of nursing intervention and
healthcare in the area of specialization, and apply research findings
appropriate to the area of practice.
(12) Teach and counsel individuals or groups by utilizing communication skills
and teaching or learning theories to increase knowledge or functioning of
individuals or groups, nursing personnel, students, and other members of the
health care team.
(13) Serve as a consultant and as a resource, utilizing advanced health knowledge
and skills, to those who are directly and indirectly involved in patient care.
(14) Participate in periodic or joint evaluations of service rendered, including, but
not limited to, the following:
(A) Chart reviews.
(B) Client evaluations.
(C) Outcome statistics.
Nurse-Midwife
A nurse-midwife is another type of advanced practice nurse. Medicaid reimbursement is
available for services rendered by a certified nurse-midwife under the same criteria as
advanced practice nurses as provided in 848 IAC 4-1-3. 848 IAC 3-1-2 states that the
practice of nurse-midwifery means the practice of nursing and the extension of that
practice, including well-woman gynecological healthcare, family planning, and care to
the normal and expanding family throughout pregnancy, labor, delivery, and post -
delivery. For further details on the scope of practice for nurse-midwives, see 848 IAC 3-
3-1.
Certified Registered Nurse Anesthetist (CRNA)
A CRNA is a type of nurse practitioner. Like nurse practitioners, Medicaid
reimbursement is available for services rendered by a CRNA under the same criteria as
advanced practice nurses as provided in 848 IAC 4-1-3. A CRNA must graduate from
an accredited Nurse Anesthesia Educational program and be properly licensed and
certified to practice to be reimbursed by the IHCP. IC 25-23-1-30 notes that CRNAs do
not have to obtain prescriptive authority to administer anesthesia. See the billing section
of this document entitled “Billing Requirements” for billing instructions and
reimbursement information for CRNAs.
BILLING REQUIREMENTS
Nurse Practitioners
Reimbursement is available for medically necessary services or preventative health care
services provided by a nurse practitioner enrolled either as a billing, group or dual provider.
01/31/2007 Nursing Services
488
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
The Provider Manual Chapter 8, Section 3 lists the IHCP instructions for proper billing of
nurse practitioner procedures as follows. The term nurse practitioner, as indicated in the billing
section of the provider manual, refers to all advanced practice nurses (family practice nurse
practitioners, pediatric nurse practitioners, obstetric nurse practitioners, nurse midwives, clinical
nurse specialists) except certified registered nurse anesthetists.
• Independently practicing nurse practitioners are reimbursed at 75 percent of the
rate on file. The nurse practitioner provider number is included in Locators 24K
and 33 of the CMS-1500 Claim Form.
• Nurse practitioners, not individually enrolled in the IHCP, and clinical nurse
specialists employed by physicians, in a physician directed group or clinic, bill
services with the SA modifier and the physician number in locators 24K and 33
and are reimbursed at 100 percent of the Medicaid allowed amount.
• Nurse practitioners, with an individual provider number, and employed by a
physician(s) should bill using their own provider number in locator 24K and the
billing group number in locator 33 and are reimbursed at 100 percent of the
Medicaid allowed amount.
• Nurse practitioner services in outpatient hospital settings are not separately
billable and are included in the hospital outpatient reimbursement rate.
Further information for billing nurse practitioner services are found in the IHCP Provider
Manual, Chapter 8, Section 3.
Certified Registered Nurse Anesthetists
CRNAs must use anesthesia CPT codes (00100-01999) and bill with the appropriate
modifier. The table below lists the only modifiers that can be used by CRNAs. The AD
modifier may not be used. One of the anesthesia procedure code modifiers listed in the
table below must be reported to identify services rendered by the CRNA and the
anesthesiologist providing medical direction. CRNAs use the same physical status
modifiers that apply to the anesthesiologist. Anesthesia details submitted by a CRNA are
reimbursed at 60 percent of the allowed amount. Further instructions for billing services
of CRNAs can be found in the Provider Manual Chapter 8.
Modifier Description
QS Monitored anesthesia care service
QX CRNA service: with medical direction by a physician
QZ CRNA service: without medical direction by a physician
QK
Medical direction of two, three, or four concurrent anesthesia procedures involving
qualified individuals
Nurse Practitioners and Clinical Nurse Specialists in Mental Health
Mental health services provided by a nurse practitioner or clinical nurse specialist under
the supervision of a physician, psychiatrist, or HSPP must be billed with the HE modifier
in conjunction with the SA modifier, as indicated in field 24K of the CMS-1500 claim
form. Claims billed for nurse practitioner and clinical nurse specialist mental health
01/31/2007 Nursing Services
489
Medical Policy Manual
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Nursing Services
Medical Policy Manual
490
services will reimburse 75 percent of the IHCP allowed amount for the procedure code
identified.

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: OBSTETRIC CARE
DESCRIPTION
Obstetric care includes the care of and services provided to a member during pregnancy
and childbirth (including the immediate postpartum period).
This document is intended to serve as a general summary of the IHCP policies regarding
this service. More specific information may be found in the IHCP provider manual,
program notices, the Indiana Administrative Code (IAC) or other sources as appropriate.
See the “Rules, Citations, and Sources” section of this document for further information.
SUMMARY OF CURRENT POLICY
The Indiana Health Coverage Programs (IHCP) provide reimbursement for various
services for obstetric care, which may include, but are not limited to the following.
Pregnancy care coordination services are available for eligible Medicaid
members. All eligible pregnant women may receive initial assessment services.
IHCP reimbursement is available for sonography procedures performed during
pregnancy with the restrictions noted in 405 IAC 5-27-6.
Home tocolytic infusion therapy utilizing a home uterine monitoring device is
covered with limitations.
IHCP reimbursement is available for anesthesia services provided during labor
and delivery.
The IHCP covers HIV testing for pregnant members and newborns. Please refer to the
Medical Policy fact sheet for Lab Services, Human Immunodeficiency Virus (HIV)
testing for additional information.
01/31/2007 Obstetric Care
491
Medical Policy Manual
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
COVERAGE CRITERIA
Pharmacy Services
The IHCP does not require a copayment for drugs dispensed to a pregnant member.
Family planning services and supplies furnished to individuals of a child bearing age do
not require a copayment.
Transportation Services
No copayment is required for transportation provided to pregnant members; however,
transportation exceeding the 20-mile one-way trip limitation is subject to prior
authorization. Refer to the Medical Policy fact sheet for Transportation Services for
additional information.
Antepartum Services
The IHCP follows the guidelines of the American College of Obstetricians and
Gynecologists (ACOG) which separates antepartum care from delivery and postpartum
care. This enables the Indiana Medicaid Program to more effectively encourage
antepartum care and track its impact on reducing poor pregnancy outcomes.
Recommended Visit Schedule
The IHCP reimburses up to 14 visits for normal antepartum care, one visit more than the
13 visits recommended by the American College of Obstetricians and Gynecologists
(ACOG). Providers are reimbursed for the following number of visits in a normal
pregnancy:
• Three visits in trimester one
• Three visits in trimester two
• Eight visits in trimester three
Additional antepartum care visits are allowed for members considered to have a
medically high-risk pregnancy. See this fact sheet’s section entitled “Medically High-
Risk Pregnancies” for additional information.
Other Outpatient Office Visits
CPT procedure codes 99211-99215 or 99241-99245 may be billed for outpatient office
visits rendered to pregnant members if related to a concurrent medical condition requiring
medical care or consultative referral. The concurrent condition must be identified as
either a primary or secondary condition by a valid ICD-9-CM diagnosis code and the
appropriate diagnosis reference number (1, 2, 3, or 4) must be indicated in form Locator
24E of the CMS-1500 claim form.
01/31/2007 Obstetric Care
492
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Billing for Antepartum Visits
The last menstrual period (LMP) date, indicated in a MM/YY/DD format, must be
indicated in form locator 14, on the CMS-1500 claim form or field 28 of the 837P
electronic transaction. Claims submitted without the LMP will not be processed for
payment. The provider’s charge for each antepartum visit must be entered in form
Locator 24F. The expected date of delivery (EDD) must be indicated in form Locator
14, and the appropriate diagnosis codes entered in form Locator 21 and referred to in form
Locator 24E on the CMS-1500 claim form or the 837P transaction for pregnancy-related
services.
Antepartum care for pregnant members must be billed separately from the delivery and
postpartum visits. Each antepartum visit must be individually listed on the CMS-1500 claim
form or the 837P transaction. Table 1 – Antepartum Trimester Billing Requirements
describes billing requirements specific to each trimester.
Table 1 – Antepartum Trimester Billing Requirements
Trimester Visit No. CPT
codes
Modifier
required for
all codes
visit codes.
Comments
1
59425 or
99201
through
99215
*U1,
Trimester
one (0
through 14
weeks 0
days)
Providers may use a new or
established patient E/M code,
99201–99215, for the first
antepartum visit to reflect the
initial assessment, testing,
counseling, and other services
typically performed during
this time. Claims may be
submitted after each visit or
at the end of the respective
trimester. Required
antepartum tests and
screenings for the first
trimester should be billed
along with the first trimester
visits. Services within the
first trimester should be billed
within 30 days of the end of
the trimester.
First
Trimester
2 – 3 59425
*U1,
Trimester
one (0
through 14
weeks 0
days)
Claims may be submitted
after each visit or at the end
of the respective trimester.
Required antepartum tests
and screenings for the first
trimester should be billed
01/31/2007 Obstetric Care
493
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Obstetric Care
494
Table 1 – Antepartum Trimester Billing Requirements
Trimester Visit No. CPT
codes
Modifier Comments
required for
all codes
visit codes.
along with each visit.
Services provided within the
trimester should be billed
within 30 days of the end of
the trimester.
Second
Trimester
4-6 59425
*U2,
Trimester
Two (14
weeks 1day
through 28
weeks 0
days)
Claims may be submitted
after each individual visit or
at the end of the respective
trimester. Required
antepartum tests and
screenings for the second
trimester should be billed
along with each visit.
Services provided within the
second trimester should be
billed within 30 days of the
end of the trimester.
Third
Trimester
7-12
(Up to eight
antepartum visits
during the third
trimester are
allowed for a
normal
pregnancy.)
59426
*U3,
Trimester
Three (28
weeks 1 day
through
delivery
Third trimester antepartum
visits can be billed with the
delivery and postpartum
services on the same CMS
1500 claim form or the 837P
transaction. Required
antepartum tests and
screenings for the third
trimester should be billed
along with each visit.
*The modifier is placed in the modifier space following the CPT code in form Locator
24D of the CMS-1500.
Antepartum Tests and Screenings Schedule
In addition to the schedule for antepartum visits, the OMPP has developed a schedule of
tests and screenings highly recommended to be provided to pregnant members within
each respective trimester. Other tests and screenings, such as those defined as optional,
should be rendered only when the provider determines the procedure is medically
necessary. The tests and screenings in Table 2, on the following pages, may be billed
with the appropriate antepartum care visit code on the same CMS-1500 claim form or
837P transaction. The trimester schedules are uniform with the standards established by
the ACOG and the American Academy of Pediatrics (AAP).
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 2 – Antepartum Tests and Screenings Schedule
Trimester One (three total visits)
HCPCS Code Procedure
59425*
59426* - U1
First trimester visits = 3
59015
Chorionic Villa Sampling (CVS), optional
for women older than 35
81000 (includes microscopy for suspected
urinary tract infection)
OR
81002 (without microscopy)
OR
81001 (Urinalysis, automated with
microscopy)
OR
81003 (Urinalysis, automated without
microscopy
Urinalysis, by dipstick, performed each
visit
The use of the automated urinalysis is to be
based on medical necessity as determined
by the physician.
86644 CMV antibody titer
86694 Herpes simplex test
86701 HIV test (optional)
86777 Toxoplasma antibody titer
88150, 88152 - 88155 Cervical cytology (Pap smear)
80055
Total obstetrical panel includes:
• CBC with complete differential
• Hepatitis B surface antigen
• Rubella antibody titer
• Syphilis test
• Antibody screen, RBC
• Blood typing (ABO)
• Blood typing (RhD)
or instead of 80055 use the following:
85025 CBC with complete differential
87340 Hepatitis B surface antigen
86762 Rubella antibody titer
86592 Syphilis Test; Qualitative (eg, VDRL,
RPR, ART)
86850 Antibody screen, RBC
86900 Blood typing (ABO)
86901 Blood typing (RhD)
01/31/2007 Obstetric Care
495
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
* Use the appropriate CPT code for the number of antepartum visits:
59425 Antepartum care only; 4-6 visits
59426 Antepartum care only, 7 or more visits
01/31/2007 Obstetric Care
496
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 2 – Antepartum Tests and Screenings Schedule
(continued)
Trimester Two (three total visits)
HCPCS Code Procedure
59425*
59426* - U2
Second trimester visits = 3
59000 Amniocentesis, optional for women older
than 35
81000 (includes microscopy for suspected
urinary tract infection)
OR
81002 (without microscopy)
OR
81001 (Urinalysis, automated with
microscopy)
OR
81003 (Urinalysis, automated without
microscopy
Urinalysis, by dipstick, performed each
visit
The use of the automated urinalysis is to be
based on medical necessity as determined
by the physician.
82105 Serum alpha-fetoprotein
82947 Diabetic screening
82951 Glucose tolerance test
86644 CMV antibody titer
86694 Herpes simplex test
86777 Toxoplasma antibody titer
80055 Total obstetrical panel includes:
• CBC with complete differential
• Hepatitis B surface antigen
• Rubella antibody titer
• Syphilis test
• Antibody screen, RBC
• Blood typing (ABO)
• Blood typing (RhD)
Or instead of 80055, use the following (if not done in first trimester due to late entry into
prenatal care)
85025 CBC with differential
87340 Hepatitis B surface antigen
86762 Rubella antibody titer
86592 Syphilis test: Qualitative (eg, VDRL,
RPR, ART)
86850 Antibody Screen (RBC)
86900 Blood Typing (ABO)
86901 Blood Typing (RhD)
01/31/2007 Obstetric Care
497
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
* Use the appropriate CPT code for the number of antepartum visits:
59425 Antepartum care only; 4-6 visits
59426 Antepartum care only, 7 or more visits
Table 2 – Antepartum Tests and Screenings Schedule
(Continued)
Trimester Three (eight total visits)
HCPCS Code Procedure
59425*
59426* - U3
Third trimester visit = 8
81000 (includes microscopy for suspected
urinary tract infection)
OR
81002 (without microscopy)
OR
81001 (Urinalysis, automated with
microscopy)
OR
81003 (Urinalysis, automated without
microscopy
Urinalysis, by dipstick, performed each
visit
The use of the automated urinalysis is to be
based on medical necessity as determined
by the physician.
85025 CBC with differential
86592
Syphilis test, repeat test for patients who
tested positive in first trimester
86850 Antibody Screen (RBC) - repeat for
patients who tested negative in first
trimester
86644 CMV antibody titer
86694 Herpes simplex test
86777 Toxoplasma antibody titer
80055 Total obstetrical panel includes:
• CBC with complete differential
• Hepatitis B surface antigen
• Rubella antibody titer
• Syphilis test
• Antibody screen, RBC
• Blood typing (ABO)
• Blood typing (RhD)
Or instead of 80055, use the following (if not done in first trimester due to late entry into
prenatal care)
85025 CBC with differential
87340 Hepatitis B surface antigen
01/31/2007 Obstetric Care
498
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
86850 Antibody Screen (RBC)
86900 Blood Typing (ABO)
86901 Blood Typing (RhD)
* Use the appropriate CPT code for the number of antepartum visits:
59425 Antepartum care only; 4-6 visits
59426 Antepartum care only, 7 or more visits
Normal Pregnancies
A normal pregnancy is one in which the physician determines that the pregnant member
is not at risk of a preterm birth or poor pregnancy outcome due to medical or
psychosocial reasons. The following diagnosis codes indicate a normal, low-risk
pregnancy:
♦ V22.0 – Supervision of normal first pregnancy
♦ V22.1 – Supervision of other normal pregnancy
Psychosocially High-Risk Pregnancies
High-risk pregnancies identified for psychosocial reasons are limited to the IHCP
standard maximum of 14 antepartum care visits. Psychosocial high-risk pregnancies do
not automatically qualify for higher reimbursement unless another medical complication
exists that is listed under the ICD-9-CM codes for a high-risk pregnancy. Pregnant
women with psychosocial factors identified that may affect the pregnancy may require
care coordination. Prenatal Care Coordination services are described on page 11 of this
fact sheet. ICD-9-CM diagnosis codes V15.82, V23.7, V60.0 through V62.9, 305.1,
648.33, 995.80, and 995.81 are typically used to indicate a high-risk pregnancy for
psychosocial reasons.
Medically High-Risk Pregnancies
Some pregnant members have medical complications that may adversely affect the
outcome of the pregnancy if not adequately addressed. These complications, usually
identified during the prenatal assessment, may place the member and the fetus in a high-
risk pregnancy category that requires additional primary care management. Only
physicians (MDs or DOs) may be reimbursed for medically high-risk pregnancy care.
Providers may refer members identified as having medically high-risk pregnancies only
to appropriate physicians. Referrals to non-physicians for high-risk pregnancy-related
services are not permitted. Providers in the PrimeStep PCCM delivery system
participating in a Memorandum of Collaboration agreement may provide care for patients
as defined in the agreement.
A pregnant woman may be considered high-risk if at least one medical condition is
identified in her current pregnancy or obstetrical history that places her at risk for a
preterm birth or poor pregnancy outcome. Further information regarding the Prenatal
Risk Assessment Form is located in Chapter 8, of the Indiana Health Coverage Programs
Provider Manual. The form can be printed from the Forms section of the IHCP website,
www.indianamedicaid.com
, and is to be used by the provider as a tool for identifying
01/31/2007 Obstetric Care
499
Medical Policy Manual
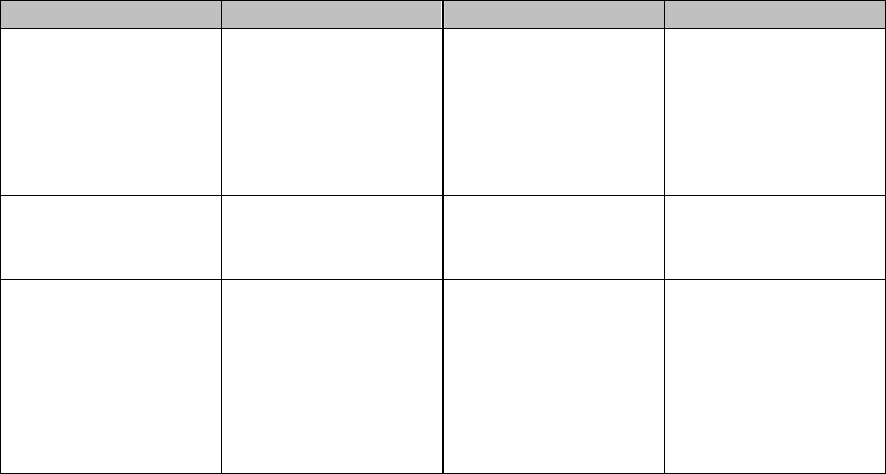
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
pregnant members at risk of a preterm birth or poor pregnancy outcomes due to medical
or psychosocial reasons.
Reimbursement for Medically High-Risk Pregnancies
Members identified as having a medically high-risk pregnancy may receive additional
antepartum care visits, beyond the maximum of 14 allowed for a normal pregnancy. The
IHCP recognizes that the care of pregnant women in the medically high-risk category
requires greater physician management. Higher reimbursement is available when
providers bill with prenatal office visit procedure codes (CPT codes 59425 and 59426)
and an ICD-9-CM diagnosis code that is listed in Table 3 – ICD-9-CM Diagnosis Codes
for Medically High-Risk Pregnancy. Each trimester should be billed on a separate
claim form. To receive additional reimbursement, the provider must document the
specific medical high-risk factors in the medical record and indicate the high-risk
diagnosis when submitting claims. This information must be easily identifiable on the
medical record for audit purposes. This requirement may be met if the provider
completes a Prenatal Risk Assessment form, and retains a copy of the form in the
member’s medical record.
The IHCP does not determine conditions that may or may not complicate a pregnancy.
Therefore, if a physician determines that an illness or injury could complicate a
pregnancy or have an adverse effect on the outcome of the pregnancy, the IHCP allows
billing for covered services provided to treat that illness or injury. Physicians must use
one of the diagnosis codes listed in Table 3 as the primary diagnosis on the claim. If none
of the diagnosis codes are appropriate for the situation, a pregnancy diagnosis code
should be listed as the primary diagnosis code, and the illness or injury being treated
should be identified as the secondary diagnosis code.
Table 3 - ICD-9-CM Diagnosis Codes for Medically High-Risk Pregnancy
Medical Factor Code Medical Factor Code
Anemias, Acquired
and Hereditary
282.0 – 282.9,
283.1X – 283.9,
284.0, 284.9, 285.0
– 285.9, 287X,
288X, 648.20,
648.23
Other (for medical
high-risk-
pregnancy)
Examples include
V23.1, V23.4X,
V23.8X, and V23.9
Current Drug or
Alcohol Abuse
304.00 – 304.93,
648.30, 648.33
Other Specified
Complications of
Pregnancy
646.80, 646.83
Current Malignancy
or Leukemia
140.0 – 174.9,
176.0 – 184.9,
188.0 – 214.3,
214.8 – 221.9,
223.0 – 233.3,
233.7 – 236.3,
236.7 – 239.9
Pregnancy with
History of Abortion
646.30, 646.33,
V23.2
01/31/2007 Obstetric Care
500
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Obstetric Care
501
Table 3 - ICD-9-CM Diagnosis Codes for Medically High-Risk Pregnancy
Medical Factor Code Medical Factor Code
Diabetes
648.00, 648.03,
648.80, 648.83
Preterm
Complications,
History of or with
Current Pregnancy
640.00, 640.03,
640.80, 640.83,
640.90, 640.93,
641.00, 641.03,
641.10, 641.13,
641.20, 641.23,
641.30, 641.33,
641.80, 641.83,
641.90, 641.93,
658.10, 658.13,
671.30, 671.33,
760.5
Excessive Vomiting
in Pregnancy
643.00, 643.03,
643.10, 643.13,
643.20, 643.23,
643.80, 643.83,
643.90, 643.93
Preterm Labor in
Current Pregnancy
or Previous
Pregnancy
644.00, 644.03,
644.10, 644.13,
644.20, 654.50,
654.53, V13.21
History of a
Previous Pregnancy
Resulting in a
Congenital
Anomaly or
Complication to
Infant
286.0 – 286.4, 317,
318.X, 319, V19.5,
V21.30 – V21.35,
V23.41
Potential Structural
Complications of
Pregnancy or
Delivery
629.23, 648.70,
648.73, 654.00,
654.03, 654.10,
654.13, 654.20,
654.23, 654.50,
654.53, 654.60,
654.63, 657.00,
657.03, 658.00,
658.03, V67.00
Infections Affecting
Pregnancy
041.02, 042,
079.5X, 090.X –
099.X, 616.10,
647.33, 647.53,
655.33, 795.71,
V08, V01.6
Primigravida, less
than 17 years or
more than 35 years
659.50, 659.53,
659.80, 659.83,
V23.81 – V23.84
Hypertension and
Related Disorders in
Current or Previous
Pregnancy
642.00, 642.03,
642.10, 642.13,
642.20, 642.23,
642.30, 642.33,
642.40, 642.43,
642.50, 642.53,
642.60, 642.63,
642.70, 642.73,
642.90, 642.93
Renal
Complications and
Infections
580.0 – 593.9,
639.3, 646.20,
646.23, 646.60,
646.63
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Obstetric Care
502
Table 3 - ICD-9-CM Diagnosis Codes for Medically High-Risk Pregnancy
Medical Factor Code Medical Factor Code
Maternal Diseases
or History Affecting
Pregnancy
345.00 – 345.91,
523.0 – 523.9,
646.13, 646.70,
646.73, 646.80,
646.83, 648.10,
648.13, 648.50,
648.53, 648.60,
648.63, 656.23,
V23.82, V23.84,
V42.0 – V42.9
Respiratory Disease,
History of or
Acquired
480.0 – 487.0,
491.0 – 491.9,
493.0X – 493.9X,
V46.1X
Multiple
Gestation/Grand
Multipara
651.00, 651.03,
651.10, 651.13,
651.20, 651.23,
651.30, 651.33,
651.40, 651.43,
651.50, 651.53,
651.60, 651.63,
651.80, 651.83,
651.90, 651.93,
659.40, 659.43,
659.60, 659.63
V23.3
Smoking, more than
10 cigarettes per day
305.11, 648.33,
V15.82
POSTPARTUM SERVICES
Postpartum Reimbursement
Up to two postpartum visits are allowed within 60 days post delivery. The provider may
be reimbursed for up to two postpartum visits using CPT code 59430, Postpartum care
only (separate procedure). Services are subject to post payment review. If CPT codes
59410, Vaginal delivery only (with or without episiotomy and/or forceps); including
postpartum care or 59515, Cesarean delivery only; including post partum care are used
when billing, only one additional postpartum visit may be billed using procedure code
59430, Postpartum care only (separate procedure).
PRENATAL CARE COORDINATION
Care coordination for pregnant women is an active, ongoing process of assisting the
individual to identify, access, and utilize community resources, and coordinating the
services to meet individual needs. This includes locating service sources, making
appointments for services, and following up to verify or reschedule appointments for
eligible women whose pregnancies are at risk for low birth weight or poor pregnancy
outcomes. Members identified by the Prenatal Risk Assessment form (see Appendix A)
Medical Policy Manual
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
as having a high-risk pregnancy due to medical or psychosocial conditions, may also
receive pregnancy care coordination services through the Indiana Health Coverage
Programs (IHCP).
After an initial evaluation by the obstetrician, IHCP members may be referred to a
Prenatal Care Coordinator that conducts an assessment to determine the necessary
services. The Prenatal Risk Assessment form must be used by Prenatal Care
Coordinators during the initial visit to assess for potential high-risk pregnancy issues.
Prenatal care coordination services are designed to combat preterm or poor pregnancy
outcomes by linking the pregnant member to all necessary services, including medical,
health promotion, and social services. These services, available on a trimester basis, may
be provided by physicians, registered nurses, social workers, and registered dietitians
with certified training in pregnancy case management to combat poor pregnancy
outcomes, and include:
♦ Home visits, including the initial and postpartum home visit,
♦ Referral to social service agencies,
♦ Follow-up activities to ensure services were received.
If the initial prenatal risk assessment indicates a high-risk condition that could result in a
poor pregnancy outcome, the pregnancy care coordinators should provide intensive
intervention services including up to two additional prenatal reassessments, a postpartum
assessment, and the required home visit following delivery, to gather vital information
about the pregnancy outcome and assess the newborn’s care needs.
Additional information regarding Care Coordination Services for pregnant women can be
found in Indiana Health Coverage Programs Provider Manual, Chapter 8, and in the
Medical Policy fact sheet for Case Management – Pregnant Women.
EMERGENCY LABOR AND DELIVERY SERVICES
Certain undocumented persons and persons living in Indiana legally but not meeting
Medicaid citizenship eligibility criteria are entitled to IHCP coverage of treatment for
medical emergency conditions. Hoosier Healthwise Package E provides services defined
by the Omnibus Budget Reconciliation Act of 1986 as “a medical condition of sufficient
severity (including severe pain) that the absence of medical attention could result in
placing the patient’s health in serious jeopardy, serious impairment of bodily functions,
or serious dysfunction of any organ or part.” In the case of pregnant women eligible for
such emergency coverage, reimbursement is available for labor and delivery services up
to the time the mother is stable. A child born in Indiana is eligible at birth for full IHCP
coverage, regardless of the mother’s immigration status, if all other Medicaid eligibility
criteria are met.
01/31/2007 Obstetric Care
503
Medical Policy Manual
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
ECHOGRAPHY
The IHCP does not reimburse for routine echographies. The diagnosis of a normal
pregnancy does not substantiate an echography. Documentation in the patient’s medical
record must substantiate the medical need for an echography. Echographies performed to
detect fetal malformations or intrauterine growth retardation should have an ICD-9-CM
code from the V22 series, Normal pregnancy as the primary diagnosis and an ICD-9-CM
diagnosis code from the V28 series, antenatal screening, listed as the secondary
diagnosis. Pregnancy-related echographies billed without a secondary diagnosis to
support medical necessity of the echography are subject to payment recovery. The
secondary codes are as follows:
• V28.3 – Screening for malformation using ultrasonics
• V28.4 – Screening for fetal growth retardation using ultrasonics
The IHCP does not provide reimbursement for echographies performed for gender
determination.
SONOGRAPHY
Reimbursement is available for sonography services performed during pregnancy when
indicated by one or more of the following conditions.
• Early diagnosis of ectopic or molar pregnancy
• Fetal age determination if necessitated by the following.
o Discrepancy in size versus fetal age
o Lack of fetal growth or suspected fetal death
o Fetal postmaturity syndrome
o Guide for amniocentesis
o Placental localization associated with abnormal bleeding
o Polyhydramnios or oligohydramnios
o Suspected multiple births
o Suspected congenital anomaly
Reimbursement is available for sonography for fetal age determination prior to
therapeutic, non-elective, abortions when the age of a fetus cannot be determined by the
patient’s history and physical examination in the case of fetal demise, or for a missed
abortion (miscarriage). The information may also be essential for the selection of an
abortion method when a procedure is being considered and the conditions meet the
requirements of IC 16-10-3-3 for an elective abortion.
Reimbursement is also available for ultrasound guidance to perform a procedure that
improves fetal status. Reimbursement is not available for CPT code 59072, Fetal
01/31/2007 Obstetric Care
504
Medical Policy Manual
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
umbilical cord occlusion, including ultrasound guidance because this procedure is
designed to terminate a fetus.
HOME TOCOLYTIC INFUSION THERAPY
The IHCP provides reimbursement for home tocolytic infusion therapy utilizing a home
uterine monitoring device. Refer to the Medical Policy fact sheet for Home Health Care
for further information, including prior authorization requirements.
To qualify for this therapy, the member must meet the following criteria.
• Be at least 24 to 34 weeks gestation
• Be in current preterm labor. Preterm labor is defined as greater than or equal to
six contractions per hour
• Have a cervical dilation of greater than or equal to one centimeter, or an
effacement of greater than or equal to 75 percent
• Have direct home telephone access to providers, which means having a working
telephone
• Have experienced secondary failure to wean from infused tocolytics, or have
failed oral therapy and require continued infusion therapy
• Have an obstetrician or gynecologist (OB/GYN) as the referring physician, or
have had a consultation with an OB/GYN
Cases of premature labor treated with oral medication only, or requests for home uterine
monitoring devices alone for the purpose of screening high-risk pregnancies will not be
approved. Members who receive only oral medications or who only require home uterine
monitoring devices do not qualify for tocolytic infusion therapy.
SALIVARY ESTRIOL
One of the endocrine assay tests developed to predict preterm labor risk is a test that
detects and measures salivary estriol (for example, the SalEst™ test). The Indiana Health
Coverage Programs (IHCP) covers salivary estriol testing using CPT code S3652, saliva
test, hormone level; to assess preterm labor risk. However, reimbursement is limited to
one unit per test, between gestational ages 22 to 35 weeks, every one to two weeks. This
test gives the physician/practitioner additional information to assess the risk for preterm
delivery and identify high-risk members, even when the patient does not appear to be
high-risk by traditional assessment methods. Refer to the Medical Policy fact sheet for
Laboratory Services – Salivary Estriol Test for further information.
ANESTHESIA FOR VAGINAL OR CESAREAN DELIVERY
The IHCP does provide reimbursement for anesthesia services for a vaginal or cesarean
delivery. Refer to the Medical Policy fact sheet for Anesthesia Services for further
information.
01/31/2007 Obstetric Care
505
Medical Policy Manual
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
General, regional or epidural anesthesia administered by the same provider who performs
the surgical or obstetrical delivery procedure will be denied because it is included in the
surgical delivery fee.
PRIOR AUTHORIZATION
Prior authorization (PA) is required for a pregnant member’s transportation services
exceeding the 20-mile one-way trip limitation.
PA is required by the IHCP for CPT code 59897, Unlisted fetal invasive procedure,
including ultrasound guidance. The PA request must document the ultrasound guided
procedure being performed, the purpose or goal of the procedure, and the medical
necessity of the procedure. This information must also be documented in the medical
record. Each request will be reviewed on a case-by-case basis. PA will be approved for
procedures that are medically necessary and have the goal of preserving or improving
fetal status. Procedures with the goal of fetal demise will be denied. Prior authorization
can be received for this procedure up to six months after the ultrasound guided procedure
is performed.
MANAGED CARE
IHCP members enrolled in Hoosier Healthwise PrimeStep (PCCM) must select primary
medical provider (PMP). If the member does not select a PMP, one is assigned to the
member. An OB/GYN may choose to be a PMP for pregnant women only, or for all
women. The PMP is responsible for providing or authorizing most primary and preventive
services, and for reviewing and authorizing necessary specialty care and hospital
admissions. Members enrolled in PrimeStep receive the same benefit coverage and are
subject to the same limitations as traditional Medicaid Fee-for-Service. Refer to the
Hoosier Healthwise Manual for Primary Medical Providers and Office Staff for further
information.
Hoosier Healthwise Package B provides medically necessary services to pregnant women
to meet the health needs of the pregnancy. In addition to drug coverage, transportation,
family planning, routine prenatal, delivery, and postpartum care, the IHCP reimburses
providers for a condition that may complicate the pregnancy. A condition that may
complicate the pregnancy is defined as a condition manifesting itself by symptoms of
sufficient severity that the absence of medical attention could reasonably be expected to
result in a deterioration of the patient’s condition or a need for a higher level of care. As
with all claims, Hoosier Healthwise Package B members’ benefits are reimbursed in
accordance with the IAC.
01/31/2007 Obstetric Care
506
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Services for Hoosier Healthwise Package B members must comply with the following
restrictions.
Reimbursement is not available for any service other than pregnancy-related
services.
The IHCP pays for drugs prescribed for indications directly related to the
pregnancy in accordance with IAC restrictions
Drugs or services necessary to prevent complications related to the pregnancy.
Chiropractors may receive reimbursement for services provided to Package B members.
Claims must be submitted with one of the diagnosis codes in Table 4 and the appropriate
chiropractic diagnosis code and chiropractic procedure code.
Table 4
ICD-9-CM Diagnosis Codes – Chiropractic Services for Package B Members
Diagnosis
Codes
Description
646.93 Unspecified complication of pregnancy – antepartum condition or
complication
648.73 Bone and joint disorders of the back, pelvis, and lower limbs –
antepartum condition or complication
648.93 Other current conditions classified elsewhere – antepartum condition or
complication
Following termination or loss of the pregnancy or following delivery, a Package B member is
eligible solely for transportation, family planning, and postpartum care services. Urgent
care services unrelated to complications of the puerperium are not reimbursed. Eligibility
for these services begins on the last day of pregnancy and extends through the end of the
month in which the last day of the 60-day period ends.
All children born to mothers covered by Hoosier Healthwise Package B are covered
under Fee-For-Service, PrimeStep, or Risk Based Managed Care (RBMC) for at least the
first year of life. Pregnant Hoosier Healthwise members are encouraged to select, prior to
delivery, a pediatric provider to serve as the PMP for the newborn.
When billing for urgent care services for Hoosier Healthwise Package B, claims must be
appropriately marked and coded as an emergency. The primary diagnosis code must be
pregnancy related, or the claim will be denied. If a pregnancy and urgent care only
member receives a sterilization procedure following delivery, the primary diagnosis code
should be pregnancy with voluntary sterilization as a secondary diagnosis. The
appropriate consent forms must be completed and sent with the claim.
Questions regarding coverage of services to RBMC members should be directed to the
appropriate Managed Care Organization (MCO).
01/31/2007 Obstetric Care
507
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
RELATED MEDICAL TOPICS
Abortion
Anesthesia Services
Case Management-Pregnant Women
Chiropractic Services
Family Planning
Gynecology-Laminaria
Gynecology-Pelvic Exam Under Anesthesia
HIV Care Coordination
Home Health Services
Lab Services – Human Immunodeficiency Virus (HIV) testing
Lab Services-Salivary Estriol
Screening Services-Newborn Screening
Transportation
RULES, CITATIONS, AND SOURCES
405 IAC 5-22-3 Nursing and Therapy Services--Certified nurse midwife services
405 IAC 5-11 Case Management Services for Pregnant Women
405 IAC 5-24-7 Obstetric Services--Copayment for legend and nonlegend drugs
405 IAC 5-27-2 Radiology Services--Utilization criteria
405 IAC 5-27-6 Sonography
Indiana Code 16-41-6 Communicable Disease: Mandatory Testing of Individuals with
Communicable or Dangerous Diseases
Indiana Medical Assistance Program Provider Manual 1994
Indiana State Department of Public Welfare Medical Policy Manual 1991
Indiana Medicaid Update Bulletins 97-14 and 95-21
Indiana State Medicaid Plan 01/01/92 Nurse-Midwife Services
Indiana Health Coverage Programs Provider Bulletins
BT2000014 – Salivary Estriol Test
BT200137 – Care Coordination Outcome Report Form
Indiana Health Coverage Programs Provider Newsletters
NL200402 – Prenatal Risk Assessment
NL200405 – Home Tocolytic Infusion Therapy and Sonography
NL200409 – Chiropractic Services for Package B members
Indiana Health Coverage Programs Provider Manual, Version 5.0, July 2004
01/31/2007 Obstetric Care
508
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Origination Date: 12/31/00
Revisions and
Reviews
Reason Date
405 IAC 5-22-3
Nursing and Therapy Services-Certified nurse
midwife services
08/24/97
405 IAC 5-27
Obstetric Services-Copayment for legend and
nonlegend drugs
8/24/97
Indiana Medicaid
Bulletin BT200353
(dated 8/15/03)
Radiology Services 8/24/97
405 IAC 5-11-1
Amended
Case Management Services for Pregnant Women 8/24/97
405 IAC 5-11-7
Amended
Case Management Services for Pregnant Women 10/27/99
Amended Case Management Services for Pregnant Women 10/27/99
Indiana Health
Coverage Programs
Newsletter 200405
Home Tocolytic Infusion Therapy and
Sonography
1/1/04
Review Scheduled Review 4/29/05
APPLICABLE INDIANAAIM EDITS AND AUDITS
504 – Expected Delivery Date Missing
535 – Invalid Expected Date of Delivery/Trimester Combination
1031 – High-risk Prenatal Care May Only Be Rendered by a Physician
2005 – SOBRA Pregnant Women (Detail)
2012 – Pregnant and Urgent Care Only
6002 – Any Two Anesthesiology Providers Same Procedure
6003 – Manual Pricing for Split Care Billing
6034 – Global Surgery Payable at Reduced Amount When Component Paid
6035 – Component of Surgical Care Not Payable When Global Paid
6037 – Only One Assistant at Surgery Allowed for Select Surgeries
6039 – Assistant at Surgery Not Payable When Co-Surgeon Paid
6040 – Co-Surgeon Not Payable When Assistant Surgeon Paid
6041 – E&M Codes Not Reimbursable with Prenatal Codes
6042 – Prenatal Codes Not Reimbursable with E&M Codes
6043 – Prenatal Visits Limited to 14 in a 10 Month Period
6044 – Prenatal Visits Limited to Three in Second Trimester
6045 – Prenatal Visits Limited to Eight
6050 – Care Coordinator - Reassessment
6051 – Care Coordinator – Initial Assessment
6052 – Care Coordination Post-Partum Assessment/Outcome
6064 – Components Not Payable when Global Paid – Medical System
01/31/2007 Obstetric Care
509
Medical Policy Manual
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
6070 – Prenatal Visits Limited to Four in the First Trimester
6096 – CPT/HCPCS Code Billed is Not Payable According to the PPS Reimbursement
Methodology
6660 – Preoperative and Postoperative Care Billed with Unlisted Surgeries Requires
Review
6666 – Anesthesia Service Not allowed by Provider Billing for Surgery
6702 – Newborn Screening Limited to One per Lifetime
6703 – Maternity/One Within Nine Month Period
6704 – Family Planning Service/One Every 12 Months
6800 – Care Coordination Transportation for Home Visit
6801 – Care Coordination – Transportation for Home Visit
6802 – Care Coordination – Transportation (Postpartum)
6916 – Global – Home Uterine Monitoring
6917 – Components-Home Uterine Monitoring
01/31/2007 Obstetric Care
510
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
APPENDIX A
Indiana Health Coverage Programs
PRENATAL RISK ASSESSMENT FORM
Patient Name RID Number LMP
Provider Name Medicaid Provider ID Number EDD
Provider Telephone Number Plan (check one) FFS PCCM
MCO Name______________________________________________
*At Risk of Preterm Birth or Poor Pregnancy Outcome
Medical Factors (Please check all that apply)
1. Anemias, Acquired and Hereditary 11. Other (for medical high risk – pregnancy)
2. Current Drug or Alcohol Abuse
12. Other Specified Complications of Pregnancy
3. Current Malignancy or Leukemia 13. Pregnancy with History of Abortion
4. Diabetes
14. Preterm Complications, History of or with Current Pregnancy
5. Excessive Vomiting in Pregnancy
15. Preterm Labor in Current Pregnancy or Previous Pregnancy
6. History of a Previous Pregnancy Resulting in a Congenital
Anomaly or Complication to Infant
16. Potential Structural Complications of Pregnancy or Delivery
7. Infections Affecting Pregnancy
17. Primigravida, less than 17 years or more than 35 years
8. Hypertension and Related Disorders in
Current or Previous Pregnancy
18. Renal Complications and Infections
9. Maternal Diseases or History Affecting Pregnancy
19. Respiratory Disease, History of or Acquired
10. Multiple Gestation/Grand Multipara
20. Smoking, more than 10 cigarettes per day
Psychosocial Factors That May Affect Current Pregnancy Outcome
Please check all that apply
21. Acute Reaction to Stress
27. Missed Prenatal Appointment s, consecutive
22. Domestic Violence
28. Other and Unspecified Disorders of Eating
23. High Risk Sexual Behavior
29. Other Personal History Presenting Hazards to Health
24. Lack of Housing Resources
30. Other Psychosocial Circumstances
25. Late Initial Visit, after 14 weeks of pregnancy
31. Prenatal Care Non-compliance, most recent pregnancy
26. Lead Exposure
32. Unwanted Pregnancy
Other Risk Factors Affecting Medical or Psychosocial Condition Not Described in Any Above Listing
(Include ICD-9 Diagnosis Codes)
Provider Signature Date
*NOTE: Refer to provider notifications for update information.
01/31/2007 Obstetric Care
511
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
OBSTETRIC SERVICES
ADDENDUM A
Note: This addendum contains provider notifications that have been published since the
review of the Obstetric Services Fact Sheet. The information in this addendum will be
incorporated into the fact sheet at the next review.
Provider Notification: NL200606 Publication Date: 06/2006
Subject: Appropriate Billing of Professional Services for Multiple Births
Date Added to Manual: 10/31/2006
Text of Publication
This article outlines the appropriate billing of professional services for multiple births.
Multiple birth deliveries are subject to multiple surgery reimbursement. The current
reimbursement policy indicated in 405 IAC 5-28-1 (g) for pricing multiple surgical
procedures states that 100 percent of the global fee is reimbursed for the most expensive
procedure. The second most expensive procedure is reimbursed at 50 percent of the
global fee and remaining procedures are reimbursed at 25 percent of the global fee. The
IHCP only reimburses for one cesarean procedure regardless of the number of babies
delivered during the cesarean section. Therefore, only one detail line with one unit of
service is billed for cesarean delivery procedures codes. The IHCP only reimburses for
one delivery procedure code that includes postpartum care. If there are multiple births
during one delivery, the first delivery code can include postpartum care; however, any
subsequent deliveries are billed with a procedure code that does not include postpartum
care.
If billing for multiple births when all births are vaginal deliveries, providers bill the first
birth using procedure code 59409 – Vaginal delivery only (with or without episiotomy
and/or forceps); 59410 – Vaginal delivery only (with or without episiotomy and/or
forceps); including postpartum care; 59612 – Vaginal delivery only, after previous
cesarean delivery (with or without episiotomy and/or forceps), or 59614 – Vaginal
delivery only, after previous cesarean delivery (with or without episiotomy and/or
forceps); including postpartum care. The second birth and any subsequent births are
billed using procedure codes 59409 or 59612 with modifier 51 – Multiple procedures.
When billing for one vaginal birth and one or more births by cesarean section; the
cesarean birth is billed with procedure code 59514 – Cesarean delivery only or 59515 –
Cesarean delivery only; including postpartum care and the vaginal birth is billed using
procedure code 59409 or 59612 with modifier 51.
When billing for two or more vaginal births and one or more births by cesarean; the
cesarean birth(s) are billed on one detail line with one unit of service using procedure
01/31/2007 Obstetric Care
512
Medical Policy Manual
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Obstetric Care
Medical Policy Manual
513
code 59514 or 59515. The vaginal birth(s) are billed as separate details using procedure
code 59409 or 59612 with modifier 51.
If all births are delivered by cesarean; the cesarean birth(s) are billed using the
appropriate procedure code 59514, 59515, 59620, or 59622 and one unit of service.
If an assistant surgeon aids in the cesarean delivery the service is billed using modifiers
80 and 82 to indicate the service was performed by an assistant surgeon. The
reimbursement for the assistant surgeon’s services is 20 percent of the allowed amount
for the cesarean delivery. Providers cannot bill the same rendering provider number for
both the surgeon and assistant surgeon details when billing for a cesarean delivery. If
billing for assistant surgery services provided by a physician assistant, providers can bill
the same rendering provider number for both the surgeon and physician assistant surgery
details. The detail for the physician assistant is billed with the AS modifier to indicate the
service was provided by the physician assistant. The reimbursement for the physician
assistant’s services is 20 percent of the allowed amount for the cesarean delivery.

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: ONCOLOGY
DESCRIPTION:
As defined in Dorland’s Medical Dictionary, 1994-Edition 28, oncology is the study of
tumors; a tumor is a morbid enlargement or a new growth of tissue in which the
multiplication of cells is uncontrolled and progressive; also called neoplasm.
SUMMARY OF CURRENT POLICY:
Oncology services are covered if the services are medically necessary and reasonable
services and are provided by a doctor of medicine or doctor of osteopathy for diagnostic,
preventive, therapeutic, rehabilitative, or palliative services provided within the scope of
the practice of medicine as set forth in 405 IAC 5-25-1. Prior authorization is required
for bone marrow or stem cell transplants. Per 405 IAC 5-28-10, outpatient administration
of chemotherapy and costs related to this therapy, including catheterization, physician’s
visits, cost of drug and solutions, pump regulators, and servicing, will be covered and do
not require prior authorization. . Chemotherapy services provided by a home health
agency are subject to the prior authorization criteria. Prior authorization is not required
for parenteral infusion pumps when used in conjunction with parenteral
hyperalimentation, including central venous catheters.
MEDICAL TOPICS CROSS-REFERENCES:
Hematology
Hospital Outpatient
Laboratory Services
Laboratory Services-HER-2/neu Gene Detection Test and HER2 Protein Expression
TestPathology
Pharmacy
01/31/2007 Oncology
Medical Policy Manual
514

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
RULES, CITATIONS, AND SOURCES:
405 IAC 5-25 Physician Services
405 IAC 5-3 Prior Authorization-exceptions
405 IAC 5-29-1 Noncovered services
405 IAC 5-28-10 Chemotherapy
405 IAC 5-19-6 Durable Medical Equipment subject to prior authorization
Indiana Medicaid Update Bulletin 96-25—Billing procedures for chemotherapy
Indiana Health Coverage Programs Provider Manual 1999
Initial Policy Issue Effective
Date
Implementatio
n Date
Retroactiv
e Date
405 IAC 1-7-12
Repealed 07/25/97
Medical Services 1/1/92
Indiana Medicaid
Update Bulletin
96-25
Billing Procedures
For Chemotherapy
7/18/96
405 IAC 5-3
Prior
Authorization
Exceptions
8/24/97
405 IAC 5-29-1 Noncovered
Services
8/24/97
405 IAC 5-28-10 Chemotherapy 8/24/97
405 IAC 5-19-6 Durable Medical
Equipment subject
to Prior
Authorization
8/24/98
Revisions:
405 IAC 5-3-12
Amended
Prior
Authorizations
Exceptions
10/27/99
405 IAC 5-25 Physician Services 8/24/97
405 IAC 5-29-1
Amended
Noncovered
Services
10/27/99
01/31/2007 Oncology
Medical Policy Manual
515
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
APPLICABLE INDIANAAIM EDITS AND AUDITS:
4090
4091
COVERAGE CRITERIA:
Billing Procedures for Chemotherapy and Radiation Treatment Services
Chemotherapy
All outpatient hospital chemotherapy and radiation treatment services are billed on the
UB-92 claim form.
Chemotherapy services consist of four components: treatment room services,
administration of chemotherapy agent, chemotherapy agent, and intravenous (IV)
solution and IV equipment. Each of these four components can be separately billed when
chemotherapy is administered. In order to bill for chemotherapy services, providers
should adhere to the following guidelines:
Treatment room services-Bill using revenue codes 45X, 51X or 76X.
Administration of chemotherapy agent--Bill using revenue codes 331, (Description
Chemotherapy–Oral is not listed in IndianaAIM as attached to any of the CPT
chemotherapy administration codes listed below) or 335. The appropriate CPT
chemotherapy administration codes (96400-96549) should be listed along with revenue
codes. Preparation of chemotherapy agents is included in the service for administration
of the agent. [American Medical Association (AMA) Current Procedural Terminology
(CPT) 2002]
Chemotherapy agent--Bill using revenue code 636 (Drugs requiring detailed coding)
along with the appropriate HCPCS J code(s) (J9000-J9390).
Intravenous solution and IV equipment--Bill using revenue code 258 for the IV solution
and revenue code 261 for IV equipment. No reimbursement will be made for other
revenue codes associated with supplies.
01/31/2007 Oncology
Medical Policy Manual
516
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Oncology
Medical Policy Manual
517
Radiation Treatment
Radiation Treatment services consist of two components: Treatment room services and
administration of radiation treatment. In order to bill for radiation treatment services,
providers should adhere to the following guidelines: Treatment room services—Bill using
revenue codes 45X, 51X, or 76X.
Administration of radiation treatment—Bill using revenue codes 330, 333, or 339 along
with the appropriate CPT radiation treatment code(s) (77261-77799). When
chemotherapy and radiation treatment services are rendered on the same day, all
applicable components may be billed to Medicaid.

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: ONCOLOGY—BREAST AND CERVICAL CANCER
PROGRAM
DESCRIPTION:
United States Code Title 42, Chapter 6A, Subchapter XIII, Section 300k makes grants
available to states to screen women for breast and cervical cancer as a preventative health
measure. The State Department of Health is responsible for implementing the Indiana
Breast and Cervical Cancer Program. Effective July 1, 2001, patients diagnosed with
breast or cervical cancer, including pre-cancerous lesions, through the Indiana Breast and
Cervical Cancer Program of the State Department of Health, are eligible for Medicaid
during the course of their treatment if they meet the eligibility requirements in Indiana
Code 12-15-2-13.5.
This document is intended to serve as a general summary of the Indiana Health Coverage
Programs’ (IHCP) policies regarding this service. More specific information may be
found in the IHCP Provider Manual, program notices, the Indiana Administrative Code
(IAC) or other sources as appropriate. See the “Rules, Citations, and Sources” section of
this document for further information.
COVERAGE CRITERIA
In order to be eligible for Medicaid while receiving treatment for breast or cervical
cancer, a woman must meet the following criteria.
1. A woman may not be eligible for Medicaid under any other section of IC 12-15.
2. A woman must be under 65 years of age.
3. A woman has been screened for breast or cervical cancer through the breast and
cervical cancer screening program under the Federal Breast and Cervical Cancer
Mortality Prevention Act of 1990 (42 U.S.C. 300k) and determined to need
treatment for breast or cervical cancer.
4. A woman is not otherwise covered under credible coverage as defined in 42
U.S.C. 300gg(c).
5. A woman’s family income does not exceed 200% of the federal income poverty
level for the same size family.
A woman eligible for Medicaid under this provision is limited to coverage for the
duration of treatment required for breast or cervical cancer. The woman is entitled to full
Medicaid coverage as specified in the State Plan.
01/31/2007 Oncology – Breast and Cervical Cancer
Medical Policy Manual
518
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
PRIOR AUTHORIZATION
Requirements for prior authorization (PA) are contingent upon each service rendered to
participants of the Breast and Cervical Cancer Program. Please refer to the Fact Sheet
that corresponds with each service rendered, when available, for more information on
PA.
MANAGED CARE
For members enrolled in Risk Based Managed Care (RBMC), providers must contact the
member’s managed care organization (MCO) for more specific guidelines. Refer to the
Provider Manual, Chapter 1, for detailed information about the fee-for-service (FFS),
Primary Care Case Management (PCCM), and Risk Based Managed Care (RBMC)
delivery systems.
IHCP members enrolled in Medicaid Select PCCM receive the same benefit coverage,
and are subject to the same limitations as traditional Medicaid FFS. Refer to the
Medicaid Select Manual for Primary Medical Providers and Office Staff for further
information.
BILLING REQUIREMENTS
IHCP providers are responsible for following correct coding procedures in accordance
with standard billing practices. Please refer to the Fact Sheet that corresponds with each
service rendered, when available, for more information on billing requirements.
RELATED MEDICAL TOPICS
Genetic Testing-BRCA1 and BRCA2 for Breast and Ovarian Cancer
Gynecology-Hysterectomy
Hospice
Hospital Inpatient Hospital Outpatient
Laboratory Services
Laboratory Services-HER-2-/Neu Gene Detection and HER2 Protein Expression Tests
Medical Supplies and Equipment
Oncology
Pathology
Pharmacy
Transportation
01/31/2007 Oncology – Breast and Cervical Cancer
Medical Policy Manual
519

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Oncology – Breast and Cervical Cancer
Medical Policy Manual
520
RULES, CITATIONS, AND SOURCES
Indiana Code 12-15-2-13.5
Indiana Code 12-15-2.3
Indiana Health Coverage Programs Provider Banner
BR200134 - Eligibility
Indiana Health Coverage Programs Provider Bulletin
BT200605 – Billing Requirements and Prior Authorization Criteria for Genetic
Testing for Breast and Ovarian Cancer
Indiana Health Coverage Programs Provider Manual Version 5.1, March, 2005
Origination Date: 07/01/2001
Revisions and Review Reason Date
Indiana Code 12-15-2-13.5
12-15-2.3
Eligibility
Presumptive Eligibility for Women
with Breast of Cervical Cancer
07/01/2001
42 USC 1396r-1b
Presumptive Eligibility for Certain
Breast or Cervical Cancer Patients
10/01/2000
Review Scheduled 10/31/2006
APPLICABLE INDIANAAIM EDITS AND AUDITS
IndianaAIM Edits and Audits are dependent on procedure codes corresponding to
services rendered to Breast and Cervical Cancer Program participants. Please refer to the
Fact Sheet that corresponds with each service rendered, when available, for more
information on applicable IndianaAIM edits and audits.

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: OPHTHALMOLOGIC SERVICES
DESCRIPTION
Ophthalmology is the branch of medicine pertaining to the eye that includes anatomy,
physiology, and pathology. Ophthalmologists are licensed medical physicians or osteopathic
physicians who have the ability and credentials to perform surgical procedures on the eye and
related structures.
Optometry is the professional practice concerned with the eye and related structures to
determine the presence of vision problems and eye disorders. (Stedman Electronic Medical
Dictionary v. 5.0, 2005). Other vision related services such as pharmaceutical, surgeries, and
diabetes self management training (DSMT) are covered services when determined to be
medically necessary.
This document is intended to serve as a general summary of the IHCP policies regarding
this service. More specific information may be found in the IHCP provider manual,
program notices, the Indiana Administrative Code (IAC), or other sources as appropriate.
See the “Rules, Citations, and Sources” section of this document for further information.
COVERAGE CRITERIA
Ophthalmology services must be provided by an ophthalmologist or an optometrist within the
scope of their licensure. The IHCP provides reimbursement for vision care services subject to
the following restrictions.
• One routine vision care examination and refraction is covered for members 18 years
old and younger, per rolling calendar year
• One routine vision care examination and refraction is covered for members 19 years
old and older every two years
• For eyeglasses, including frame and lenses, documentation of medical necessity must
include the following.
• A change of 0.75 diopters for members six to 42 years old
• A change of 0.50 diopters prescription or change for members more than 42
years old
• An axis change of at least 15 degrees
01/31/2007 Ophthalmologic Services
Medical Policy Manual
521
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• Replacement frames and lenses are only covered when medical necessity guidelines
are met or when necessitated by loss, theft, or damage beyond repair
PRIOR AUTHORIZATION
Most vision care services do not require prior authorization; however, prior authorization is
required for the following services.
• Blepharoplasty for a significant obstructive vision problem
• Prosthetic device, except eyeglasses
• Reconstructive or plastic surgery
*Prior authorization is required for all vision services provided to 590 members when an
amount greater than $500.00 per procedure is billed.
INITIAL EXAMINATIONS
IHCP coverage for vision examinations is limited to specific criteria. Documentation of
medical necessity must be maintained in the provider’s office and is subject to post payment
review and audit. Initial examinations may include the following.
• Eye examination, including history
• Visual acuity determination
• External eye examination
• Biocular measure
• Routine ophthalmoscopy
• Tonometry and gross visual field testing, including color vision, depth perception, or
stereopsis
• Any additional examination must be medically necessary
DIAGNOSTIC SERVICES
Diagnostic services, if medically necessary, may be submitted for reimbursement in addition to
the eye examination. These services may include the following.
• Supplemental evaluation
• Multiple pattern fields, including Roberts, Harrington, or Flods
• Central field study
• Peripheral field study
• Tangent screen study
• Color field study
• Binocular ophthalmoscope
• Other supplemental testing
01/31/2007 Ophthalmologic Services
Medical Policy Manual
522

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• Visual skills study
• Clinical photography
• Bifocal determination
• Trifocal determination
• Definitive fundus evaluation
• Electrophysiology
• Gonioscopy
• Neutralization of lens or lenses
• Extended ophthalmoscopy
• Serial tonometry
• Refractions
• Office visit
• Consultation
• Visual skills testing
The Medicare program does not cover refractions because the service is considered statutorily
excluded. For dually-eligible members, this service may be billed directly to the IHCP for
consideration on the CMS-1500 or 837P. Providers are not required to submit refraction
claims to Medicare first.
Lenses
The prescription of lenses, when required, is included in the HCPCS code 92015–
Determination of refractive state. It includes specification of lens type, monofocal, bifocal,
lens power, axis, prism, absorptive factor, impact resistance, and other factors. The IHCP does
not provide coverage for all lenses. If a member chooses to upgrade to non-covered lenses, the
basic lens V code can be billed to the IHCP. The upgrade portion can be billed to the member,
only if the member was given an appropriate advance notification of the non-covered service
and a separate procedure code for the service exists. Safety lenses are covered only for corneal
lacerations and other severe intractable ocular or ocular adnexal diseases. Table 1 lists non-
covered lens CPT codes.
TABLE 1—Non-covered Vision Codes
CPT Code Code Description
V2702 Deluxe lens feature
V2744 Tint, photochromatic, per lens
V2745
Additions to lens, tint, any color, solid, gradient or equal, excluded
photochromatic, any lens material, per lens
V2750 Antireflective coating, per lens
V2760* Scratch resistant coating, per lens
V2781 Progressive lens, per lens
V2782*
Lens, index 1.54 to 1.65 plastic or 1.60 to 1.79 glass, excludes polycarbonate,
per lens
V2783*
Lens, index greater than or equal to 1.66 plastic, or greater than or equal to 1.80
glass, excludes polycarbonate, per lens
V2786* Specialty occupational multifocal lens, per lens
*These codes are non-covered effective December 1, 2004.
01/31/2007 Ophthalmologic Services
Medical Policy Manual
523
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Polycarbonate Lenses
The IHCP has developed specific criteria for polycarbonate lenses to ensure that they are used
only for medically necessary conditions that require additional ocular protection for members.
HCPCS code V2784—Lens, polycarbonate or equal, any index, per lens may be billed when a
corrective lens is medically necessary and if one or more of the following criteria is met.
• Member has carcinoma in one eye and the healthy eye requires a corrective lens
• Member has only one eye which requires a corrective lens
• Member has had eye surgery and still requires the use of a corrective lens
• Member has retinal detachment or is post-surgery for retinal detachment and
requires a lens to correct a refractive error of one or both eyes
• Member has a cataract in one eye or is post-cataract surgery and requires a lens to
correct a refractive error of one or both eyes
• Member has low vision or legal blindness in one eye with normal or near normal
vision in the other eye
• Other conditions deemed medically necessary by the optometrist or
ophthalmologist. These conditions must be such that one eye is affected by an
intractable ocular condition and the polycarbonate lens is being used to protect the
remaining vision of the healthy eye.
Frames
Reimbursement is available for frames, including but not limited to, plastic or metal. Providers
should bill for frames using V2020—Frames, purchase. Providers who receive payment from
the IHCP for the frames, may not bill the member for any additional cost above the IHCP
reimbursement, except in circumstances where a member requests an upgrade that has a
separately billable code or for non-covered services, instances where the member signed a
waiver.
The IHCP does not cover any portion of a deluxe or fancy frame purchase, except when
medically necessary. Charges for medically necessary deluxe frames must be submitted with
procedure code V2025—Deluxe frame. All Medicaid claim forms submitted for a more
expensive frame must be accompanied by documentation supporting medical necessity.
Situations where medical necessity for a more expensive frame may be indicated include, but
are not limited to, the following.
• Frames to accommodate facial deformity or anomaly
• Allergy to standard frame materials
• Infant or child frames
If a member chooses to upgrade to a deluxe frame without medical necessity, the entire frame
is considered to be non-covered and may be billed to the member, if proper advance notice of
non-coverage was provided and signed by the member. In these situations, only the claim for
the lenses should be submitted to the IHCP for reimbursement.
01/31/2007 Ophthalmologic Services
Medical Policy Manual
524
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Replacement Eyeglasses
Repair or replacement services refer to the part of the eyeglasses that is broken or damaged.
Members are not entitled to a new pair of eyeglasses if the lenses and/or frames can be
repaired. The following information describes instances that support medical necessity for the
replacement of the eyeglasses.
• Members 19 years of age and older that have met the medical necessity for the
replacement of the eyeglasses may be eligible for a new pair of eyeglasses two years
from the date the replacement eyeglasses were provided.
• If a member needs replacement eyeglasses due to loss, theft, or damage beyond
repair, prior to the established limitations, the modifier RP—Replacement or repair ,
must be used to bill for this service.
• If a member needs replacement eyeglasses due to a change in the prescription and it
is prior to the established limitations, the modifier SC—Medically necessary service
or supply, must be used to bill for this service.
• Documentation must be present in the member’s medical record to substantiate the
need for replacement frames or lenses. Documentation that eyeglasses have been
lost, stolen, or damaged beyond repair must include a signed statement by the
member detailing how the eyeglasses were lost, stolen, or damaged beyond repair.
* The modifiers are only needed on claims for replacement of frames or lenses within the one
or two year period, based on the member’s age at the time of service. However, all eyeglasses
dispensed must meet the minimum prescription requirements for the initial dispensing and each
subsequent dispensing of eyeglasses.
Contact Lenses
Contact lenses are covered when medically necessary. Documentation is not required with the
claim, but must be maintained in the member’s medical record for post-payment review.
Medical necessity for contact lenses include, but is not limited to, members with a severe facial
deformity who are physically unable to wear eyeglasses or members who have a severe allergy
to all frame materials. The prescription of contact lenses includes the specification and physical
characteristics such as power, size, curvature, flexibility, and gas permeability. Fitting contact
lenses includes instruction, training, and incidental revision of the lenses during the training
period. Follow-up and documentation of successful fitting of the extended wear lenses is
necessary as well.
OPHTHALMOLOGIC SURGERIES
Documentation must be maintained in their member’s medical records to support medical
necessity for all ophthalmologic surgeries, including Argon and Krypton LASER Beam
therapy, YAG LASER, Intraocular Lenses (IOLs), New Technology Intraocular Lenses
(NTIOLs) and Vitrectomy. If performed in the office, the submitted billing code should reflect
the location. If performed in another location, the global surgery billing/payment fee will
apply. Other related information regarding these surgeries may be found in the Surgery and
Transplant Fact Sheets.
01/31/2007 Ophthalmologic Services
Medical Policy Manual
525
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Argon and Krypton LASER Beam Therapy
Argon and Krypton LASER beam therapy uses a variety of gases to produce light beams to
provide therapy for multiple conditions. Argon and Krypton LASER Beam treatment may be
used to weld the retina to the back of the eye in the case of small retinal detachment or may be
used to incise tissue to provide a new avenue for the aqueous humor to drain as part of the
treatment of glaucoma.
Intraocular Lenses (IOLs) and New Technology Intraocular Lenses (NTIOLs)
IOLs and NTIOLs are intraocular lenses that are implanted to replace the natural lenses
following procedures such as cataract surgery. Other diagnoses that support medical necessity
of NTIOLs include, but are not limited to, the following.
• Glaucoma
• Iris melanoma
• Cilliary boy melanoma
• Choroids melanoma
Any facility reimbursed at an ASC rate should submit claims for surgical insertions of
IOLs using the CPT code 66983, 66984, 66985, or 66986 and the appropriate revenue
code on a UB-92 claim form. The NTIOLs claim must be submitted on a separate HCFA-
1500 claim form using the facility’s durable medical equipment (DME) provider number.
The appropriate Q-Code will be reimbursed at an allowed rate of $50 for each implanted
NTIOLs lens.
Vitrectomy
A vitrectomy is the removal of the vitreous humor when it is diseased or damaged. Diagnoses
that may support medical necessity of vitrectomy as a sight-saving procedure include, but are
not limited to, the following.
• Vitreal hemorrhage
• Retinal detachment
• Scarring or fibrosis of vitreous
• Proliferative retinopathy
Documentation must be maintained in the member’s medical record. The operative report
should be reviewed and the claim paid as follows.
• If the vitrectomy is performed through the pars plana, the vitrectomy and the
appropriate cataract extraction code will be paid according to the multiple surgical
procedure payment guidelines.
• If the claim states “restorations of anterior chamber,” the cataract extraction will be
paid, and the vitrectomy is included in the procedure and will not be reimbursed
separately.
01/31/2007 Ophthalmologic Services
Medical Policy Manual
526

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• If an open sky vitrectomy is performed with the cataract extraction, the vitrectomy
and the cataract extraction will be paid according to the multiple surgical procedure
payment guidelines.
Vitrectomy services billed with corneal transplant on the same eye should be denied if the
service is to restore the anterior chamber. Vitrectomy through the pars plana or the open sky
technique with the corneal transplant should be paid according to the guidelines for vitrectomy
with cataract surgery. A pars plana vitrectomy and photocoagulation billed separately should
be combined and coded appropriately.
YAG LASER
The YAG LASER treatment is the laser separation of the posterior capsule that is used when
there is a blockage between the lens and the vitreous humor of the eye. This treatment allows
the passage of light through the media to the retina which was initially retracted and obstructed.
The YAG LASER may be used for the surgical removal of pathological tissue, i.e. brain
tumors, hemorrhoids, and chondylmata. When used in conjunction with a surgery other than
specific to ophthalmological treatments, it should be included in the global fee billing schedule
when filing a claim.
BILLING INFORMATION
Vision care reimbursement is not available for more than one unit for eye exams and other
ophthalmologic procedures. IHCP providers may only bill one unit, per member, per day for
the procedures indicated in Table 2. Claims that have more than one unit per day for these
codes will automatically pay for one unit. Examinations where counseling and coordination of
care are the dominant services may be coded with the appropriate evaluation and management
(E/M) code using the time factor associated with the code. Documentation in the member’s
record must include the total time of the encounter, synopsis of the counseling topics, and
information regarding the coordination of care efforts. The following services are included in
the eye examination and are not separately billable.
• Biocular measurement
• Routine ophthalmoscopy and external eye examination
• Gross visual field testing including color vision, depth perception, or stereopsis
• Tonometry
• Visual acuity determination
Table 2—Ophthalmologic Services
CPT
Code
Description
9200
2
Ophthalmological services: medical examination and evaluation with initiation of
diagnostic and treatment program; intermediate, new patient
01/31/2007 Ophthalmologic Services
Medical Policy Manual
527

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 2—Ophthalmologic Services
CPT
Code
Description
9200
4
Ophthalmological services: medical examination and evaluation with initiation of
diagnostic and treatment program; comprehensive, new patient, one or more visits
9201
2
Ophthalmological services: medical examination and evaluation, with initiation or
continuation of diagnostic and treatment program; intermediate, established patient
9201
4
Ophthalmological services: medical examination and evaluation, with initiation or
continuation of diagnostic and treatment program; comprehensive, established patient,
one or more visits
92018
Ophthalmological examination and evaluation, under general anesthesia, with or
without manipulation of globe for passive range of motion or other manipulation to
facilitate diagnostic examination; complete
9201
9
Ophthalmological examination and evaluation, under general anesthesia, with or
without manipulation of globe for passive range of motion or other manipulation to
facilitate diagnostic examination; limited
9202
0
Gonioscopy (separate procedure)
9206
0
Sensorimotor examination with multiple measurements of ocular deviation (i.e.,
restrictive or paretic muscle with diplopia) with interpretation and report (separate
procedure)
92065
Orthoptic and/or pleoptic training, with continuing medical direction and evaluation
92081
Visual field examination, unilateral or bilateral, with interpretation and report; limited
examination (i.e., tangent screen, Autoplot, arc perimeter, or single stimulus level
automated test, such as Octopus 3 or 7 equivalent)
9208
2
Visual field examination, unilateral or bilateral, with interpretation and report;
intermediate examination (i.e., at least 2 isopters on Goldmann perimeter, or
semiquantitative, automated suprathreshold screening program, Humphrey
suprathreshold automatic diagnostic test, Octopus program 33)
92083
Visual field examination, unilateral or bilateral, with interpretation and report; extended
examination (i.e., Goldmann visual fields with at least 3 isopters plotted and static
determination within the central 30 degrees, or quantitative, automated threshold
perimetry, Octopus programs G-1, 32 or 42, Humphrey visual field analyzer full
threshold programs 30-2, 24-2, or 30/60-2)
9210
0
Serial tonometry (separate procedure) with multiple measurements of intraocular
pressure over an extended time period with interpretation and report, same day (i.e.,
diurnal curve or medical treatment of acute elevation of intraocular pressure)
9212
0
Tonography with interpretation and report, recording indentation tonometer method or
perilimbal suction method
9213
0
Tonography with water provocation
9214
0
Provocative tests for glaucoma, with interpretation and report, without tonography
92250
Fundus photography with interpretation and report
01/31/2007 Ophthalmologic Services
Medical Policy Manual
528

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 2—Ophthalmologic Services
CPT
Code
Description
92260
Ophthalmodynamometry
92265
Needle oculoelectromyography, one or more extraocular muscles, one or both eyes, with
interpretation and report
92270
Electro-oculography with interpretation and report
92275
Electroretinography with interpretation and report
92284
Dark adaptation examination, with interpretation and report
92285
External ocular photography with interpretation and report for documentation of medical
progress (i.e., close-up photography, slit lamp photography, goniophotography, stereo-
photography)
92286
Special anterior segment photography with interpretation and report; with specular
endothelial microscopy and cell count
92287
Special anterior segment photography with interpretation and report; with fluorescein
angiography
92311
Prescription of optical and physical characteristics of and fitting of contact lens, with
medical supervision of adaptation; corneal lens for aphakia, one eye
92312
Prescription of optical and physical characteristics of and fitting of contact lens, with
medical supervision of adaptation; corneal lens for aphakia, both eyes
92313
Prescription of optical and physical characteristics of and fitting of contact lens, with
medical supervision of adaptation; corneoscleral lens
92315
Prescription of optical and physical characteristics of contact lens, with medical
supervision of adaptation and direction of fitting by independent technician; corneal lens
for aphakia, one eye
92316
Prescription of optical and physical characteristics of contact lens, with medical
supervision of adaptation and direction of fitting by independent technician; corneal lens
for aphakia, both eyes
ORTHOPTIC OR PLEOPTIC TRAINING, VISION TRAINING, AND THERAPIES
All vision training therapies are covered under CPT code 92065—Orthoptic and/ or pleoptic
training, with continuing medical direction and evaluation. The medical record must be
maintained to support medical necessity and include the following coverage criteria for these
services.
• CPT code 92065—Orthoptic and /or pleoptic training, with continuing medical
direction and evaluation is limited to one unit or visit per day
• Vision therapy services must be ordered by a physician or an optometrist
• The physician or optometrist must document a diagnosis, treatment plan, and the
need for continued treatment in the medical record
01/31/2007 Ophthalmologic Services
Medical Policy Manual
529

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• Vision therapy services can be performed by an optometrist, a physician, or
supervised staff. Staff must be trained or certified to provide these vision services
• Staff trained or certified in vision training may perform orthoptic and pleoptic
training only under the direct supervision of an optometrist or physician. Direct
supervision requires that the supervising physician or optometrist must be physically
available at the time and the location of where the vision therapy services are
rendered
• All documentation of directly supervised vision therapy services rendered by staff
must be cosigned by the supervising optometrist or physician in the medical record
VISION CARE FOR MANAGED CARE ORGANIZATIONS
MANAGED CARE
For members enrolled in Risk Based Managed Care (RBMC), providers must contact the
member’s managed care organization (MCO) for more specific guidelines. Refer to the
Provider Manual, Chapter 1, for detailed information about the fee-for-service (FFS), Primary
Care Case Management (PCCM), and Risk Based Managed Care (RBMC) delivery
systems.
IHCP members enrolled in Hoosier Healthwise PrimeStep (PCCM) receive the same
benefit coverage, and are subject to the same limitations, as traditional Medicaid FFS.
Refer to the Hoosier Healthwise Manual for Primary Medical Providers and Office Staff
for further information.
Ophthalmologic Surgeries
Providers furnishing optical or ophthalmology services to members enrolled in MCO delivery
systems must contact the appropriate organization and refer the appropriate manual for specific
guidelines for surgical services.
RELATED MEDICAL TOPICS:
Anesthesia Services
Clinic Services—FQHC and Rural Health Clinic Services
Diabetes Self Management Treatment (DSMT)
Hoosier Healthwise Managed Care Organizations
Hospital Inpatient Services
Hospital Outpatient Services
Physician Services
Prior Authorization
Podiatry Services
Surgery Services
Transplant Services
01/31/2007 Ophthalmologic Services
Medical Policy Manual
530

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
RULES, CITATIONS, AND SOURCES:
Code of Federal Regulations
42 CFR 440.120 Subpart A—Definitions
42 CFR 441.30 Subpart A—General Provisions—Optometric services
Indiana Administration Code
405 IAC 5-9-1—Evaluation Management (E/M) services
405 IAC 1-6-14 Repealed 8/24/97—Optometrist and Optician services
405 IAC 1-7-1 Repealed 8/24/97—Global Fee Billing
405 IAC 5-3-13—Prior Authorization
405 IAC 5-16-6—Home Health Agency and Clinic Services
405 IAC 5-19-11—Prosthetic Devices
405 IAC 5-23—Vision Care Services
405 IAC 5-28-1(a), (g)—Medical and Surgical Services
405 IAC 5-36-1—Diabetes Self Management Treatment
470 IAC 5-8-14 Transferred —Optometrist and Optician Services
470 IAC 5-9-1 Transferred—Global Fee Billing
Indiana Health Coverage Programs Bulletin
E96-01—Indiana Medicaid Ophthalmologists, Optometrists, and Opticians
E96-20—Prior Authorization Rule Changes
E96-26—Coverage Issues: Metal Frames for Eyeglasses
E96-37—Vision Services in Hoosier Healthwise
E98-05—Vision Coverage
E98-09—Vision Training
BT199909—Removal of Services from Prior Authorization
BT199916—Changes in Policy and Billing of Vision Services
BT200103—New Technology Intraocular Lenses
BT200217—Orthoptic and Pleoptic Training, Vision Training, and Therapy Changes
BT200427—Hoosier Healthwise Mandatory Managed Care Organization Transition
BT200506—State-wide Hoosier Healthwise Managed Care Organization Transition
BT200507—New 2005 Quarterly HCPCS Codes
Indiana Health Coverage Programs Banner (continued)
BR199952—Coverage
BR200237—Limits for replacement eyeglasses
BR200238—Replacement eyeglasses
BR200433—Provider code sets
BR200434—Provider code sets
BR200435—Billing and claims
Indiana Health Coverage Programs Provider Manual
1999
Version 5.1, March 2005
Indiana Health Coverage Programs Provider Monthly Newsletter
NL200505—Vision Services
Indiana State Department of Public Welfare Medical Policy Manual 1991
Origination Date: 1/31/2000
01/31/2007 Ophthalmologic Services
Medical Policy Manual
531

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Revisions and
Reviews
Reason Date
470 IAC 5-8-14
Transferred
Optometrist and Optician Services 7/1/91
470 IAC 5-9-1
Transferred 1/1/92
Global fee billing 7/1/91
405 IAC 1-6-14
Repealed 8/24/97
Optometrist and Optician Services 1/1/92
405 IAC 1-7-1
Repealed
Global Fee Billing 1/1/92
Indiana Medicaid
Update Bulletin
96-01
Indiana Medicaid Ophthalmologists,
Optometrists, and Opticians
1996
Indiana Medicaid
Update Bulletin
96-20
Prior Authorization 7/22/96
Indiana Medicaid
Update Bulletin
96-26
Coverage Issues 7/26/96
Indiana Medicaid
Update Bulletin
96-37
Vision Services in Hoosier Healthwise 1/1/97
405 IAC 5-3-13
Services Requiring
Prior Authorization
8/24/97
405 IAC 5-23 Vision Care Services 8/24/97
42 CFR 440.120 Subpart A–Definitions 10/1/97
42 CFR 441.30
Subpart A–General
Provisions–Optometric services
10/1/97
Indiana Medicaid
Update Bulletin
98-09
Vision Training 3/27/98
01/31/2007 Ophthalmologic Services
Medical Policy Manual
532

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Origination Date: 1/31/2000 (continued)
Revisions and
Reviews
Reason Date
Indiana Medicaid
Update Bulletin
BT199909
Removal of Services from Prior
Authorization
4/19/99
405 IAC 5-23-3
Amended
Vision Care Services –
Reimbursement limitations
10/27/99
405 IAC 5-16-6
Home Health Agency and
Clinic Services
11/1/99
Vision Services Code
Set Optician (180)
Changes in Policy and
Billing of Vision Services
10/1/04
Vision Services Code
Set Optometrist (190)
Changes in Policy and
Billing of Vision Services
10/1/04
Revision
Ophthalmological Service Fact Sheet:
Vision care, Surgeries, Vision training,
and Managed care organizations
7/29/05
APPLICABLE INDIANAAIM EDITS AND AUDITS:
6006—Only One New Patient Visit per 3 Years
6012—Medical Services 20 per Year
6014—Global Payable at a Reduced Fee When Components Paid- Medical Services
6017—Global Payable at a Reduced Fee When Components Paid- Endocrine/Nervous/
Eye/Ear
6048—Components Not Payable When Global Paid- Endocrine/ Nervous/ Eye/ Ear
6069—Office Visits 30 per Year
6096—CPT/ HCPCS Code Billed is not Payable According to the PPS Reimbursement
Methodology
6600—Frames Initial or Replacement- Member 18 years or younger
6601—Lenses Initial or Replacement- Member 18 years or younger
6602—Postoperative Cataract Lens Replacement/ One per Year
6603—Frames Initial or Repair/Replacement – Member Over 18 years
6604—Lenses Initial or Repair/Replacement – Member Over 18 years
6605—Frames Replacement Versus Frame Repair on Same Date of Service
6606—Frame Replacements Parts in Excess of $20
6607—Frames Repair Versus Frame Replacement
6608—Frame- One Replacement Allowed per Day
6610—Routine Vision Exam Limited to One Exam Per Twelve (12) Months for Ages 1 to 18
Years
6611—Routine Vision Exam Limited to One Exam per Twenty-four (24) Months for Ages 19
to 999 Years
6649—Surgery Payable at Reduced Amount When Related Postoperative Care Paid
6651—Surgical Cutback Procedures are Limited to One per Lifetime
6652—Multiple Surgeries Must be Billed on Same Claim
01/31/2007 Ophthalmologic Services
Medical Policy Manual
533
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Ophthalmologic Services
Medical Policy Manual
534
6653—Postoperative Care Within Zero to 90 Days of Surgery
6654—Postoperative Care Within One Day of Surgery
6655—Surgery Payable at Reduced Amount When Preoperative Care Paid
6656—Postoperative Care Within 10 Days of Select Surgery
6657—Postoperative Care on Day of Surgery
6658—Surgery Payable at Reduced Amount When Preoperative Care Paid Same Date of
Service
6659—Surgery Payable at Reduced Amount When Related Postoperative Care Paid
6660—Preoperative and Postoperative are Billed with Unlisted Surgeries Requires Review
6665—Bilateral Versus Unilateral Surgeries
6920—Diabetes Management Limited to 8 Units in 12 months

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: OSTEOGENIC BONE GROWTH STIMULATOR
DESCRIPTION
Electrical stimulation produces calcification and mineralization of the fibrocartilage
repair tissue (bone growth) at a fracture site and helps to increase vascularity. Electrical
stimulators are used for nonhealing or hard to heal fractures, usually of the long bones,
and also for spinal fusions.
There are several types of bone growth stimulators available to deliver therapy by
different methods.
• Implantable direct current stimulators are most commonly used in spinal fusions, at
the time of the surgical procedure, but can be implanted surgically for bone grafting
of nonunion and stress fractures. Invasive electric stimulators can be either fully or
partially implantable. A second surgical procedure is necessary at the end of
treatment to remove the device. The advantages of the implantable stimulators are
constant current treatment and patient compliance. Disadvantages are the high cost
and the requirement of two surgical procedures.
• One type of non-invasive external device uses electrodes that are applied to the skin
at the fracture site. The electrodes can be placed under a cast when necessary. The
device operates on an external battery pack and eliminates the need for surgical
procedures to initiate treatment. The unit may be operated up to 24 hours per day, but
the 9-volt battery must be changed daily. Disadvantages of this device are possible
skin irritation from the electrodes and frequent battery replacement.
• A second type of external device is a coil that is placed under the cast or on the
outside of the cast. The coil attaches to a battery pack and a control unit that is worn
externally. This device is recommended for treatment no more than 10 hours per day.
Patient compliance is a concern with this device because the unit is heavy and larger
than most other options.
• The newest option is an ultrasonic osteogenic stimulator, a noninvasive unit that may
be applied directly to the skin. This OBGS produces pulsed ultrasound, which
increases vascularity to speed healing. The ultrasound signal, comparable to that used
in conventional fetal monitoring, is transmitted to the skin via a conductive coupling
01/31/2007 Osteogenic Bone Growth Stimulator
Medical Policy Manual
535

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Osteogenic Bone Growth Stimulator
Medical Policy Manual
536
gel, which coats the skin. In the event a cast is present, a hole is made in the cast so
the device can be applied to the skin. Overlaying the fracture site or through a
window in the cast, this device is used for only 20 minutes per day. The disadvantage
of the ultrasound OBGS is it is larger than all other options.
The IHCP covers osteogenic stimulators with prior authorization under the following
codes:
Table 1 – Osteogenic Bone Stimulators
HCPCS CODE DESCRIPTION
E0747 Osteogenesis Stimulator, noninvasive
E0748 Osteogenesis Stimulator, noninvasive, spinal applications
E0749 Osteogenesis Stimulator, surgically implanted
E0760 Osteogenesis Stimulator, low intensity ultrasound
RELATED MEDICAL TOPICS
Physician Services
Durable Medical Equipment
Surgery
Hospital Inpatient
Hospital Outpatient
RULES, CITATIONS, AND SOURCES
405 IAC 5-3-5
405 IAC 5-17-1
405 IAC 5-17-2
405 IAC 5-19-1
405 IAC 5-19-2
405 IAC 5-19-3
405 IAC 5-19-6
405 IAC 5-19-7
405 IAC 5-19-8
ORIGINATION, REVIEWS AND REVISIONS
Origination date: 7/30/04
Review and Revisions Reason Date
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
APPLICABLE INDIANAAIM EDITS AND AUDITS
6002
6003
6034
6035
6039
6040
6065
6080
6096
6104
6152
6162
6661
6666
01/31/2007 Osteogenic Bone Growth Stimulator
Medical Policy Manual
537
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
COVERAGE CRITERIA
The PA criteria are as follows.
Noninvasive Stimulators (E0747 and E0748) The noninvasive stimulator devices are
covered only for the following indications.
• Nonunion of long bone fractures;
• Congenital pseudoarthroses; and
• As an adjunct to spinal fusion surgery for patients at high risk of
pseudarthrosis due to previously failed spinal fusion at the same site or for
those undergoing multiple level fusion. A multiple level fusion involves three
(3) or more vertebrae (e.g. L3-L5, L4-S1, etc.)
Invasive (Implantable) Stimulator (E0749) The implantable invasive stimulator is
covered only for the following indications.
• Nonunion of long bone fractures; and
• As an adjunct to spinal fusion surgery for patients at high risk of
pseudarthrosis due to previously failed spinal fusion at the same site or for
those undergoing multiple level fusion. A multiple level fusion involves three
(3) or more vertebrae (e.g. L3-L5, L4-S1, etc.)
Ultrasound Stimulator (E0760) The ultrasound stimulator is covered for the following
indications.
• Nonunion of a fracture documented by a minimum of two sets of radiographs
obtained prior to starting treatment with the ultrasound stimulator, separated
by a minimum of 90 days, each including multiple views of the fracture site,
and with a written interpretation by a physician stating that there has been no
clinically significant evidence of fracture healing between the two sets of
radiographs.
• The member must have documented failure of at least one surgical
intervention for the treatment of the fracture.
• The ultrasonic osteogenic stimulator may not be used concurrently with other
non-invasive osteogenic devices.
The above policy relates to nonunion fractures as defined below. The diagnosis for
nonunion of a fracture must meet the following criteria.
1. Serial radiographs must have confirmed that fracture healing has ceased for three
or more months prior to starting treatment with an osteogenic stimulator.
2. Serial radiographs must include a minimum of two sets of radiographs, each
including multiple views of the fracture site separated by a minimum of 90 days.
01/31/2007 Osteogenic Bone Growth Stimulator
Medical Policy Manual
538
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Osteogenic Bone Growth Stimulator
Medical Policy Manual
539
Non-unions of the skull, vertebrae, and those that are tumor-related are excluded from
coverage. Treatment for fresh fractures and non-union associated with osteomyelitis is
not covered.

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: OUT-OF-STATE SERVICES
DESCRIPTION:
Recipients of Indiana Medicaid may require healthcare services when they are outside the
state of Indiana. Out-of-state healthcare providers can enroll in the Indiana Medicaid
Program. If an Indiana Medicaid member requires healthcare services, he/she should
inquire prior to receipt of service if the service organization is enrolled as an Indiana
Medicaid provider, if possible.
MEDICAL TOPICS CROSS-REFERENCES:
Potentially All Medical Policies
RULES, CITATIONS, AND SOURCES:
405 IAC 5-5 Out-of-State Services
405 IAC 5-13-5 Prior authorization for services rendered outside of the large state
ICF/MR
Provider Bulletin 96-12
Provider Bulletin 95-79
HCFA 10-1-97, 42 CFR 431.52
Indiana Health Coverage Programs Provider Manual 1999
01/31/2007 Out-of-State Services
Medical Policy Manual
540

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Initial Policy Issues
Effective
Date
Implementation
Date
Retroactive Date
470 IAC 5-9-4
Transferred
Out-of-state
medical services
7/1/91
405 IAC 1-7-4
Repealed
8/24/97
Out-of-state
medical services
1/1/92
Bulletin 95-79 Out-of-state
Prescriptions
1995
Bulletin 96-12 Out-of-state
Prescriptions
1996
Revisions:
405 IAC 5-5 Out-of-state
Services
8/24/97
405 IAC 5-5-1
Amended
Out-of-state
Services
4/10/98
405 IAC 5-5-2
Amended
Out-of-state
Services
10/27/99
405 IAC 5-13-5 PA for services
rendered outside
of large state
ICFMR
8/24/97
BT200317 Pharmacy
Processor
Change
Reminders
3/23/03
APPLICABLE INDIANAAIM EDITS AND AUDITS:
3010
3011
3015
3016
01/31/2007 Out-of-State Services
Medical Policy Manual
541
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
COVERAGE CRITERIA:
IHCP reimbursement is available for the following services provided outside the state of
Indiana.
• Acute general hospital care
• Physician services
• Dental services
• Pharmacy services
• Transportation services
• Therapy services
• Podiatry services
• Chiropractic services
• Durable medical equipment and supplies
• Hospice services, subject to the conditions in 405 IAC 5-34-3
PRIOR AUTHORIZATION
Prior authorization may be granted for any time period from one day to one year for out-
of-state medical services listed above if the service meets criteria for medical necessity
and any one of the following criteria is met.
• The service is not available in Indiana. Care provided by out-of-state
Veteran’s Administration and Shrine hospitals is an exception to this
requirement.
• The member has received services from the provider previously.
• Transportation to an appropriate Indiana facility would cause undue exposure
or hardship to the recipient or the Medicaid program.
• The out-of-state provider is a regional treatment center or distributor.
• The out-of-state provider is significantly less expensive than the Indiana
providers, for example, large laboratories versus an individual pathologist.
All out-of-state service provided to IHCP members require prior authorization (PA) with
the following exceptions.
• Emergency services. Continuation of inpatient treatment and hospitalization is
subject to IHCP prior authorization requirements.
• Recipients of the adoption assistance program placed outside of Indiana will receive
approval for all routine medical and dental care provided out-of-state.
01/31/2007 Out-of-State Services
Medical Policy Manual
542

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
The following out-of-state cities are designated as having the same PA requirements as
in-state service providers.
Designated Areas for In-state PA Requirements
State City
Danville Illinois
Watseka
Louisville Kentucky
Owensboro
Michigan Sturgis
Cincinnati
Hamilton
Harrison
Ohio
Oxford
Members can receive services in Chicago, Illinois, subject to all of the following
conditions.
• The member’s physician determines the service is medically necessary.
• Transportation to an appropriate Indiana facility would cause undue hardship
to the patient or the patient’s family.
• The service is not available in the immediate area.
• The member’s physician complies with all of the criteria set forth in this
article, in accordance with the state plan and 42 CFR 456.3.
Prior authorization of the following will not be approved and the services are not covered
when by any out-of-state provider or a designated out-of-state service provider.
• Nursing facilities or Intermediate care facilities for the mentally retarded
(ICFs/MR).
• Any other long-term care facility, including facilities directly associated with, or part
of, an acute general hospital.
• Any provider type that is not eligible for enrollment in the IHCP.
OUT–OF-STATE SUPPLIERS OF MEDICAL EQUIPMENT
In order to be treated as an in-state provider, any out-of-state supplier of medical
equipment must comply with the following.
01/31/2007 Out-of-State Services
Medical Policy Manual
543
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Out-of-State Services
Medical Policy Manual
544
1. Maintain an Indiana business office, staffed during regular business hours,
with telephone service.
2. Provide service, maintenance, and replacements for Indiana Medicaid
members whose equipment has malfunctioned.
3. Qualify with the Indiana Secretary of State as a foreign corporation.
REIMBURSEMENT
Enrolled out-of-state hospital providers are reimbursed for inpatient acute care services at
diagnosis related group (DRG) in-state rates or the established reimbursement
methodology for Medicaid members in the provider’s state. All other out-of-state
hospital procedures and reimbursement methodologies are the same as for enrolled in-
state hospital providers. Providers are reimbursed according to the IHCP reimbursement
policy.
Provider Bulletin BT200317 announced that ACS Healthcare, the Pharmacy Benefits
Manager (PBM) would assume processing of the IHCP pharmacy claims. As part of this
information, ASC provided notification of the requirements on the IHCP Pharmacy
Claim-NCPCP 3.2(3C). Field number 411 (prescribe ID) is keyed with the following
numbers for out-of-state prescribers.
91111111-Illinois
92222222-Kentucky
93333333- Ohio
94444444- Michigan
95555555- all other states
The bulletin also provides information regarding claims that are denied for a restricted or
lock-in member with an out-of-state prescriber number that has denied for an invalid
lock-in prescriber. If the member indicates an out-of-state prescriber is a valid referral,
the pharmacy must call HCE to receive an override for payment of the claim.

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: PHARMACY
DESCRIPTION:
Pharmacy is the practice of preparing and preserving drugs, and of compounding and
dispensing medicines according to prescriptions of physicians or other professionals as
authorized by State and Federal laws.
SUMMARY OF CURRENT POLICY
Pharmacy policies cover prescribed drugs for the cure, mitigation, prevention of disease,
or health maintenance and are prescribed by a physician, dispensed by licensed
pharmacists after receipt of a written prescription. Pharmacy, in this instance, may be
considered a collection of services that are covered by Medicaid, with some restrictions.
The Indiana Health Coverage Programs (IHCP) has developed a Preferred Drug List
(PDL). This listing is reviewed and updated quarterly based upon recommendations
submitted by the Therapeutics Committee to the Drug Utilization Review Board. Non-
preferred drugs require prior authorization.
RELATED MEDICAL TOPICS
Diabetes Self-Management Training
EPSDT – HealthWatch
Family Planning
Home Health Services
Hospital Inpatient
Hospice
Immunizations
Intermediate Care Facilities for the Mentally Retarded
Medical Supplies and Equipment
Nursing Facilities
Oncology
Smoking Cessation Treatment
01/31/2007 Pharmacy
Medical Policy Manual
545

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
RULES, CITATIONS, AND SOURCES
405 IAC 5-24 Pharmacy Services
405 IAC 5-25-3 Physician Services
405 IAC 5-28-10 Medical and Surgical Services
405 IAC 5-31-5 Nursing Facility Services
405 IAC 5-36 Diabetes Self Management Training Policy
405 IAC 5-37 Smoking Cessation Treatment Policy
405 IAC 5-34 Hospice
42 CFR 440.120
Indiana Medicaid Update Bulletin 96-04
Indiana Medicaid Update Bulletin 98-08
BT200132 Brand Medically Necessary PA
BT200148 Indiana Rational Drug Program
BT200151 Revised Policy for Office-Administered Injectable Drugs and Infusions
BT200210 Indiana Rational Drug Program Phase 2
BT200221 ProDUR Alerts
BT200225 Indiana Rational Drug Program Phase 3
BT200235 Preferred Drug List Phase 1
BT200243 Preferred Drug List Phase 2
BT200247 Preferred Drug List Phase 3
BT200255 Preferred Drug List Phase 4 and 5
BT200261 Preferred Drug List Phase 6
BT200307 Preferred Drug List Phase 7
BT200319 Preferred Drug List Phase 8
BT200333 Preferred Drug List Phase 9
BT200342 Preferred Drug List Phase 10
BT200351 PDL Re-Review
BT200359 PDL Re-Review
BT200365 ProDUR Alerts
BT200403 PDL Changes to BT200359
BT200406 Updated Pharmacy Copayment Information
BT200407 PDL Changes
BT200411 PDL Changes
BT200422 PDL Changes
Indiana Health Coverage Programs Provider Manual 2004
01/31/2007 Pharmacy
Medical Policy Manual
546

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Origination Date : 12/31/2000
Revisions and Reviews Reason Date
470 IAC 5-8-20
Transferred
Pharmacy Services 7/1/91
405 IAC 1-6-21 Repealed 8/24/97 Pharmacy Services 1/1/92
405 IAC 5-24 Pharmacy Services 8/24/97
405 IAC 5-25-3
Injections administered by
physician
8/24/97
405 IAC 5-28-10 Chemotherapy 8/24/97
405 IAC 5-31-5
Nursing Facility Services:
Legend and prescription items
8/24/97
405 IAC 5-24-3
Amended
Pharmacy Services 10/27/99
405 IAC 5-24-5
Amended
Pharmacy Services 10/27/99
405 IAC 5-24-8
Amended
Pharmacy Services 10/27/99
405 IAC 5-31-5
Amended
Nursing Facility Services:
Legend and prescription items
10/27/99
405 IAC 5-24-8.5
Prior authorization; other
drugs
01/07/02
405 IAC 5-24-11
Limitations on quantities
dispensed and frequency of
refills
01/07/02
405 IAC 5-24-12 Risk Based Managed Care 01/07/02
405 IAC 5-24-8.6
Prior authorization limitations
and other; antianxiety,
antidepressant, or
antipsychotic agents
02/14/02
405 IAC 5-24-4
Amended
Reimbursement of Legend
Drugs
04/30/02
405 IAC 5-24-5
Amended
Reimbursement of Non-
Legend Drugs
04/30/02
405 IAC 5-24-6
Amended
Dispensing Fee 04/30/02
405 IAC 5-31-4
Amended
Nursing Facility Services; Per
Diem Services
06/05/03
405 IAC 5-24-7
Amended
Copayment for legend and
non-legend drugs
02/24/04
01/31/2007 Pharmacy
Medical Policy Manual
547

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
APPLICABLE INDIANAAIM EDITS AND AUDITS
Effective March 23, 2003, ACS State Healthcare assumed the responsibility for
pharmacy claims processing. IndianaAIM edits and audits for pharmacy claims are
effectively no longer active; however, shadow pharmacy claims are shared by ACS and
IndianaAIM edits and audits ‘post and pay’ on these shadow claims. In 2005, the
pharmacy processor is subject to change.
COVERAGE CRITERIA
Drug Utilization Review Board
The Drug Utilization Review Board was originated in the Ominibus Reconciliation Act
(OBRA) of 1990, coincident and ancillary to federal rebate provisions that require each
state to have a Drug Utilization Review (DUR) program. The Indiana DUR program is
comprised of two components. Prospective DUR (Pro-DUR) is enabled by the Medicaid
contractor’s point-of-sale (POS) system and performed by the pharmacist at the service
location. Retroactive DUR (Retro-DUR) is based on paid claims data that is reviewed
by the state DUR board or by Medicaid contractor(s). The Indiana DUR board is
required to file an annual report of Pro-DUR and Retro-DUR activities with the Centers
for Medicare and Medicaid Services (CMS). Copies of this report are available upon
request from CMS.
Medicaid Therapeutics Committee and Preferred Drug List
Senate Enrolled Act No. 228 of the 2002 General Assembly created a requirement for a
preferred drug list (PDL) under Indiana Medicaid. In accordance with law, drugs that are
included on the Indiana Medicaid PDL do not require prior authorization, and drugs that
are not included on the PDL do require prior authorization. Basic criteria for prior
authorization requests for drugs not included on the PDL are therapeutic failure or
adverse reaction with the preferred drug.
In accordance with the new law, a Medicaid Therapeutics Committee, which is a
subcommittee of the state’s Medicaid Drug Utilization Review (DUR) Board, was
selected. The Therapeutics Committee, which is comprised of five physicians and two
pharmacists, has the responsibility of assisting the DUR Board in the Board’s
development and recommendation of a PDL to the Office of Medicaid Policy and
Planning.
The Therapeutics Committee makes recommendations to the DUR Board for changes to
the PDL quarterly. Changes to the PDL are published in provider newsletters, bulletins,
banners, and the website for pharmacy services, www.indianapbm.com
.
Prior Authorization
Prior authorization is required for the following pharmacy services.
01/31/2007 Pharmacy
Medical Policy Manual
548
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
(1) Brand name drugs that are subject to the generic substitution per IC 16-22-42-4.
(2) Drugs not included in the Indiana Medicaid PDL (non-preferred drugs).
(3) Other drugs that the Indiana Medicaid DUR Board places on prior authorization,
include, but are not limited to, the following.
• Cox-II Inhibitors for patients less than 70 years of age
• Cytotec
• Toradol (if greater than 20 tablets for 5 days per month)
• Growth Hormone
• Synagis and Respigam
• Actiq
• Forteo
Limitations to prior authorization are identified by 405 IAC 5-24-8.6. The following
drugs are exempt from prior authorization requirements.
(1) Central nervous system drugs classified by Drugs Facts and Comparisons as any
of the following.
(a) antianxiety
(b) antidepressant
(c) antipsychotic
(d) any drugs “cross-indicated” to classifications (a) through (c) and used for
the purpose generally held to be reasonable, appropriate, and within
community standards of practice
(2) Brand name drugs where the physician has written in his own hand-writing ,
“Brand Medically Necessary.”
Pro-DUR
The purpose of Pro-DUR is to improve the quality and cost-effectiveness of drug use by
ensuring that prescriptions are appropriate, medically necessary, and not likely to result in
adverse medical reactions. In addition to the OBRA ’90 requirements for program
agencies, the act required pharmacy providers to perform the following activities for all
patients.
(1) Prospective Drug Utilization Review (Pro-DUR)
(2) Patient counseling
(3) Proper patient records maintenance
(4) Evaluation of the following drug therapy problems before filling prescriptions
(a) therapeutic duplication
(b) drug-disease contraindications
(c) drug-drug interactions
(d) incorrect drug dosage or duration of drug treatment
(e) drug-allergy interactions
(f) evidence of clinical abuse or misuse
01/31/2007 Pharmacy
Medical Policy Manual
549
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
In addition, therapeutic screening takes place though computer based software programs
set up by the IHCP. The IHCP therapeutic screening system alerts pharmacists when the
member’s claims profile detects the following potential conflicts.
(1) drug-drug interaction
(2) drug age precaution
(3) drug disease alert
(4) drug pregnancy alert
(5) high and low dosage alerts
(6) overuse and underuse precaution
(7) therapeutic duplication
The claims processing software sends an alert to the pharmacist including the following
information with the rejected claim response.
(1) Drug conflict code
(2) Clinical significance code, or severity
(3) Other pharmacy indicator
(4) Previous fill date
(5) Quantity of previous fill
(6) Database indicator
(7) Other prescriber indicator
(8) Free text for drug-drug and therapeutic duplication that contains the name of the
drug in the related claim history
(9) DUR overflow
DESI (LTE) Drugs
The Federal Food, Drug, and Cosmetics Act of 1938 established the requirement that a
manufacturer prove the safety of a drug before it could be marketed in the United States.
In 1962, this act was amended to require that drugs sold in the United States be regulated
more closely. All new drugs must demonstrate, via adequate studies, both safety and
efficacy before introduction into the market. Federal law prohibits payment under the
Indiana Medicaid Program for any drug that is considered “less than effective” (LTE) or
“identical, related, or similar” (IRS) to an LTE drug. Comprehensive listings of updated
and revised DESI listings are published regularly and available from the Medicaid
pharmacy benefit management contractor.
Mandatory Generic Substitution and “Brand Medically Necessary”
Generic substitution under the Medicaid program is mandatory per IC 16-42-22-10.
Pharmacy providers must dispense in accordance with the law, and failure to do so can
result in a risk of recoupment.
In addition, pharmacy providers must be aware of and dispense in accordance to the
provisions in 405 IAC 5-24-8. Prior authorization is required for a brand name drug that
is subject to generic substitution under IC 16-42-22-10. The prescriber must indicate
“brand medically necessary” on the written prescription drug order and substantiate the
medical necessity of the brand name drug to the PBM.
01/31/2007 Pharmacy
Medical Policy Manual
550
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
The following drugs are exempt form the brand medically necessary prior authorization
requirement; however, the prescription must indicate “brand medically necessary” in the
prescriber’s own handwriting.
• Dilantin
• Coumadin
• Lanoxin
• Premarin
• Tegretol
• Provera
• Synthroid
Non-Legend or Over-the-Counter (OTC) Drugs
The IHCP provides for coverage and reimbursement of OTC drugs in accordance with
405 IAC 5-24-3. The IHCP has an OTC drug formulary that is updated regularly and
published for providers.
Usual and Customary Charge
Providers are to bill the program for covered services with only the provider’s usual and
customary charge to the general public for the covered service. The provider’s usual and
customary charge includes any dispensing fee that the provider may charge to the general
public.
Drug Copayment
Indiana Medicaid drug copayment is set out in 405 IAC 5-24-7. Under IC 12-15-6, a
copayment is required for legend and nonlegend drugs and insulin in accordance with the
following.
(1) The copayment amount is deducted from the Medicaid reimbursement to the
provider.
(2) In accordance with 42 CFR 447.15, the provider may not deny services to an
eligible member due to the member’s inability to pay. This service guarantee
does not apply to an individual who is able to pay nor does this eliminate the
individual’s liability for the copayment.
(3) The amount of the copayment is $3.00 for each covered drug dispensed and is
collected by the pharmacy provider.
(4) Individuals enrolled in the Children’s Health Insurance Program (CHIP), or
Hoosier Healthwise Package C, are responsible for a copayment of $3.00 for
generic, compound, single source legend drugs, and $10.00 for brand name drugs.
The following pharmacy services are exempt from the copayment requirement.
(1) Emergency services provided in a hospital, clinic, office, or other facility
equipped to furnish emergency care.
01/31/2007 Pharmacy
Medical Policy Manual
551
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
(2) Services furnished to individuals less than eighteen years of age.
(3) Services furnished to pregnant women, if such services are related to the
pregnancy or any other medical condition that may complicate the pregnancy.
(4) Services furnished to individuals who are inpatient in hospitals, nursing facilities,
intermediate care facilities for the mental retarded, or other medical institutions.
(5) Family planning services and supplies furnished to individuals of child bearing
age.
(6) Health maintenance organization (HMO) pharmacy services.
Long Term Care Facilities and Pharmacy Services
Pharmacy providers are not entitled to separate reimbursement for any Medicaid covered
service that is reimbursed through per diem reimbursement. Medical and non-medical
supplies and legend and non-legend water products are included in the per diem rate for
nursing facilities. These supplies and water products when dispensed to the nursing
facility residents are not separately billable to Medicaid.
Covered, manufacturer packaged, unit dose medications are covered by Medicaid for
long term care facility residents only. However, the program does not provide
reimbursement for costs associated with unit dose packaging when the pharmacy
provider packages or repackages medications. In addition, FMAC and SMAC rates apply
to manufacturer packaged unit dose medications.
State laws IC 25-26-13-25 (h) and 856 IAC 1-21-1 allow for the return of medications
from long term care facilities to the dispensing pharmacy under certain circumstances.
Medications returned to the dispensing pharmacy that are put back in stock for re-
dispensing must be credited to the program within 30 days of being returned to the
pharmacy.
Hospice
Pharmacy services, for conditions related to the patient’s terminal illness are included the
hospice per diem rate. In addition, pharmacy services not related to the patient’s terminal
condition are covered outside the hospice per diem rate. All pharmacy services not
related to the patient’s terminal condition are subject to PDL limitations and PA is
required for hospice patients in instances where a non-preferred drug is prescribed or
PDL limitations have been exceeded.
Reimbursement Methodology
Pharmacy services are reimbursed at the lowest of the following.
1. Estimated Acquisition Cost (EAC),
2. Federal Upper Limit (FUL, FMAC),
3. State Maximum Allowable Cost (SMAC), or
4. the provider’s usual and customary (U&C) charge.
01/31/2007 Pharmacy
Medical Policy Manual
552

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
PHARMACY SERVICES
ADDENDUM A
Note: This addendum contains provider notifications that have been published since the
review of the Pharmacy Services Fact Sheet. The information in this addendum will be
incorporated into the fact sheet at the next review.
Provider Notification: BR200532 Publication Date: 08/09/2005
Subject: HEA 1325 and Medicare Part D Drug Prescription
Date Added to Manual: 10/31/2005
Text of Publication
The passage of House Enrolled Act (HEA) 1325 has created some confusion. Some
advocates and industry representatives have been disseminating information that Prior
Authorizations and other clinical edits on behavioral health drugs covered through the
Hoosier Healthwise Managed Care Organizations (MCO) are invalid as of July 1, 2005.
However, that information is inaccurate.
HEA 1325 confers upon the Mental Health Quality Assurance Committee the
responsibility to make recommendations to the Office of Medicaid Policy and Planning
(OMPP) regarding access to behavioral health drugs through the Indiana Medicaid
program. The OMPP has the ultimate responsibility for implementing any restrictions
with the advice of the Committee. The Mental Health Quality Assurance Committee is
currently being assembled in accordance with the guidelines set forth in HEA 1325.
Until the committee is formed and the OMPP issues guidance regarding access to
behavioral health drugs by Hoosier Healthwise members in the Risk-Based Managed
Care program, all MCO preferred drug lists (PDL) clinical edits will remain in effect.
• Effective January 1, 2006, the Centers for Medicare and Medicaid Services
(CMS) is implementing the new Medicare prescription drug coverage. This coverage,
also known as Medicare Part D, is a new benefit to help Medicare members pay for
prescription drugs.
The IHCP will provide information as it becomes available with banner pages, the IHCP
provider newsletter, bulletins, and the IHCP Web site. The annual IHCP Seminar and
fourth quarter provider workshops will include materials and training about the new
Medicare Prescription Drug Benefit.
For more information about the Medicare Prescription Drug Benefit visit the CMS Web
site at http://www.cms.gov/medicarereform/
01/31/2007 Pharmacy
Medical Policy Manual
553

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Provider Notification: 200531 Publication Date: 08/02/2005
Subject: State Maximum Allowable Cost
Date Added to Manual: 10/31/2005
Text of Publication
Effective September 9, 2005, the following drug groups will be added to the State
Maximum Allowable Cost (State MAC) for legend drugs rate list.
Drug Name State MAC Rate
CILOSTAZOL 100 MG TABLET 0.70570
GABAPENTIN 800 MG TABLET 1.52230
MOMETASONE FUROATE 0.1% CREAM 1.1577
QUINAPRIL HCL 20 MG TABLET 0.78670
QUINAPRIL HCL 40 MG TABLET 0.93950
Effective July 15, 2005, State MAC rates for the following drugs will be increased
as listed below.
Drug Name State MAC Rate
CYCLOSPORINE 100 MG SOFTGEL 4.25370
PAROXETINE HCL 10 MG TABLET 0.99107
PAROXETINE HCL 20 MG TABLET 0.76272
PAROXETINE HCL 30 MG TABLET 1.00320
PAROXETINE HCL 40 MG TABLET 0.88560
PROMETHAZINE 25 MG TABLET 0.38430
TRETINOIN 0.01% GEL 1.50720
Effective September 9, 2005, State MAC rates for the following drugs will be decreased
as listed below.
Drug Name State MAC Rate
CLINDAMYCIN PHOS 1% GEL 0.41748
PROMETHAZINE W/COD SYRUP 0.02316
TRIAMCINOLONE 0.1% CREAM 0.03528
OXYCODONE/APAP 7.5/325 MG TABLET 0.92724
01/31/2007 Pharmacy
Medical Policy Manual
554

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
PROMETHAZINE 25 MG SUPPOSITORY 0.69876
Effective June 24, 2005, the State MAC rate for Bupropion SR 150mg Tab - AB2
(Zyban) will be removed. The State MAC rate for Bupropion SR 150mg Tab - AB1
(Wellbutrin) will remain active. Please direct any questions regarding the State MAC for
legend drugs to the Myers and Stauffer pharmacy unit at (317) 816-4136 or 1-800-591-
1183, or e-mail at [email protected]
Effective January 1, 2006, the Centers for Medicare and Medicaid Services (CMS) is
implementing a Medicare prescription drug benefit. This coverage, also known as
Medicare Part D, is a new benefit to help Medicare members pay for prescription drugs.
The IHCP will provide information as it becomes available with banner pages, the IHCP
provider newsletter, bulletins, and the IHCP Web site. The annual IHCP Seminar and
fourth quarter provider workshops will include materials and training about the new
Medicare Prescription Drug Benefit.
For more information about the Medicare Prescription Drug Benefit visit the CMS Web
site at http://www.cms.gov/medicarereform/
This is to advise providers that the new drug Revatio® (sildenafil citrate—Pfizer) will
require prior authorization under the Traditional Indiana Medicaid pharmacy benefit.
Criteria for approval of prior authorization requests is limited to the labeled indication,
diagnosed pulmonary arterial hypertension to improve exercise ability. While the drug is
on the Preferred Drug List, prior authorization is being required in order to prevent fraud,
abuse, waste, overutilization, or inappropriate utilization, specifically due to its similarity
to Viagra®, which is also a sildenafil citrate product. Please call ACS for prior
authorization questions at 1-866-879-0106.
Provider Notification: BT200515 Publication Date: 07/25/2005
Subject: Post Payment Auditing
Date Added to Manual: 10/31/2005
Text of Publication
Post-payment auditing of pharmacy claims identifies instances where providers have
incorrectly billed the Indiana Health Coverage Programs (IHCP), resulting in
overpayments. Sometimes the incorrectly billed claim can be corrected by adjusting the
claim. In other instances, Prudent Rx cannot adjust the fields containing incorrect
information.
For example, a provider submitted a claim for 75 cc of albuterol solution, 5 mg/cc that is
available in a 20 cc bottle. The prescription, however, was written and dispensed for a
total of 75 cc, or 25 vials of the 3 cc premixed vials. Because these are entirely different
products and the submitted NDC is incorrect, the claim cannot be adjusted by Prudent
01/31/2007 Pharmacy
Medical Policy Manual
555

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Rx. The claim must be reversed via the audit process and subsequently a replacement
claim must be submitted by the provider to accurately reflect what was dispensed.
To resolve an audit, a provider must repay the incorrectly billed claim and then submit a
new claim that will replace the incorrectly billed claim. If the audited claim is less than
one year old at the time that the provider agrees to the overpayment, the provider may
submit the replacement claim with the correct information via POS, after repayment has
been made via the audit process.
For those claims with dates of service more than one year old, the following procedure
has been established that will allow providers to submit a replacement claim for payment:
1. Prepare the paper claim for the rendered service with the correct billing information.
2. Sign the overpayment acceptance form, return it and indicate acceptance of the
recovery of the inappropriately billed claim.
3. Send the new replacement claim and the overpayment acceptance form to Prudent Rx
as instructed in the audit letter.
4. Clearly indicate on all new replacement claims the Transaction Control Number
(TCN) of the audited claim that is being replaced.
Prudent Rx will send the audited claim to ACS to be reversed. After the overpayment has
been collected and the audited claim reversed, Prudent Rx will send the replacement
claim to ACS to be paid.
It is important the providers remember the following:
• This process must be followed in this order to prevent the replacement
claims from being denied as duplicates.
• Replacement claims will not be processed unless the provider has agreed
to recovery of the overpayment.
• Providers must correct the error identified on the audited claim.
Submitting the replacement claim exactly as it was originally billed will
result in the claim being re-audited.
• Prudent Rx will only accept replacement paper claims that are:
More than one year old
Subject to the audit and recovery process
• Prudent Rx will not accept claims for services that are more than one year
old and were not subject to the audit process. Prudent Rx will return these
claims to the provider. Error Code RD (Missing/Invalid Date on the
Prescription)
• Prudent Rx will no longer be recovering funds for claims found to have an
error code of RD (missing/invalid date on prescription) during the audit
process. Instead, these errors will be referred to the Indiana Board of
Pharmacy.
01/31/2007 Pharmacy
Medical Policy Manual
556

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Provider Notification: BT200515 Publication Date: 07/25/2005
Subject: Post Payment Auditing
Date Added to Manual: 10/31/2005
Text of Publication
This notice advises providers that, in response to rapidly escalating expenditures for
Medicaid-covered drugs, and in order to stay within available appropriations while
maintaining beneficiary access to services, the office will be adopting an emergency rule
that amends pharmacy reimbursement for Medicaid and HoosierRx. Specifically,
estimated acquisition cost (EAC) for brand name legend drugs will change from Average
Wholesale Price (AWP) minus 13.5 percent to AWP minus 19 percent. At the same time,
to bring consistency to reimbursement policy for insulins, OTC insulins will commence
being paid in accordance with applicable legend drug EAC methodology. These changes
will be effective October 1, 2005.
• Effective January 1, 2006, the CMS is implementing the new Medicare prescription
drug coverage. This coverage, also known as Medicare Part D, is a new benefit to help
Medicare members pay for prescription drugs. The IHCP Web site now includes a new
section titled Medicare Prescription Drug Coverage. Providers should visit this section
periodically at http://www.indianamedicaid.com/ihcp/ProviderServices/medicareD.asp
for the latest information.
The annual IHCP Seminar and fourth quarter provider workshops will include materials
and training about the new Medicare prescription drug benefit.
For more information about the Medicare prescription drug benefit visit the CMS Web
site at http://www.cms.gov/medicarereform/
01/31/2007 Pharmacy
Medical Policy Manual
557

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
PHARMACY SERVICES
ADDENDUM B
Note: This addendum contains provider notifications that have been published since the
review of the Pharmacy Services Fact Sheet. The information in this addendum will be
incorporated into the fact sheet at the next review.
Provider Notification: BT200531 Publication Date: 12/21/2005
Subject: Medicaid Prescription Drug Coverage Changes Due to the New
Medicare Prescription Drug Coverage
Date Added to Manual: 01/31/2006
Text of Publication
Beginning Sunday, January 1, 2006, Medicaid can no longer pay for prescription drugs
for people who have both Medicare and Medicaid, commonly referred to as dual
eligibles.
Medicaid Prescription Drug Coverage Changes for Medicare Beneficiaries
Instead, your prescription drugs will be covered by a Medicare prescription drug plan
(PDP). This plan is sometimes called Medicare Part D. If you continue to be eligible for
Medicaid, Medicaid will continue to pay for other Medicaid-covered health care for
which you are eligible that Medicare does not cover.
What This Change Means For You
Because Medicaid can no longer cover your prescription drugs, you must enroll in a
Medicare PDP to continue getting prescription drug coverage. Medicare is working with
companies that offer these PDPs. You can choose from 13 plans available in your area
and not have a premium. Some drug plans may not cover all the drugs you take, some
may limit what pharmacies you can use, or they may require small co-payments. For
these reasons, you must pick a plan that best meets your needs. You can get help
choosing a Medicare PDP by contacting Medicare at 1-800-MEDICARE (1-800-633-
4227). TTY users should call 1-877-486-2048 or visit the Medicare Web site at
www.medicare.gov
If you do not enroll yourself in a Medicare PDP by December 31, 2005, Medicare will
automatically enroll you in a low-cost PDP in your area. This guarantees that you will
still have prescription drug coverage on January 1, 2006. You can choose another
Medicare PDP at any time. The change will take effect the first day of the month
following the receipt of the change.
Extra Help with Medicare Prescription Drug Plan Costs
Because you get full Medicaid benefits, Medicare will give you extra help paying for
your PDP costs. The extra help you get from Medicare will pay no more than $35.69
toward your monthly premium for prescription drug coverage. If you choose a Medicare
01/31/2007 Pharmacy
Medical Policy Manual
558
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
PDP with a higher premium, you will have to pay out of pocket the difference between
the higher premium amount and $35.69. Please note that some members will have no
premium to pay at all. Depending on the drug plan you choose, you will pay:
• No monthly premium or a low monthly premium
• No yearly deductible
• $0 to $5 co-payments for your prescription drugs
If you are in a long-term care facility (nursing facility), Intermediate Care Facility for the
Mentally Retarded, or Community Residential Facility for the Developmentally Disabled
(group home) you will have no co-payment while you are in the facility. If you are in an
assisted living facility or adult living facility you will have a small co-payment.
Choose a Prescription Drug Plan by December 31, 2005
The Medicare & You 2006 handbook issued in October 2005, has information about
Medicare PDPs, and it lists the plans in your area and how to enroll. You can enroll in a
Medicare PDP on your own or you can stay with the plan that Medicare chose for you. If
you do not enroll yourself in a Medicare PDP by December 31, 2005, you will remain
with the plan chosen for you by Medicare. This guarantees that you still have prescription
drug coverage on January 1, 2006.
Prescription Drug Coverage through an Employer or Union
If you already have prescription drug coverage through your past or current employer or
union, you may not need to enroll in a Medicare PDP. Some retirement plans may
discount retiree coverage if you join a Medicare PDP. Talk with your employer or union
benefits administrator to see if your current prescription drug coverage is at least as good
as the Medicare drug plan. If your current prescription drug coverage is not better, then
choose a Medicare plan that meets your needs. You can get help choosing a Medicare
PDP that meets your needs by contacting Medicare at 1-800-MEDICARE (1-800-633-
4227). TTY users should call 1-877-486-2048 or visit the Medicare Web site at
www.medicare.gov
Effects of Coverage Change for People with Medicare and Medicaid
It is important for you to know that, beginning January 1, 2006, Indiana Medicaid will no
longer cover your prescription drugs, except for a few types of prescription drugs that are
excluded from the new Medicare prescription drug benefit and are covered by Indiana
Medicaid. Although Medicare will cover most prescription drugs for people who have
both Medicaid and Medicare, Indiana Medicaid will still pay for benzodiazepines
(examples include Xanax, Ativan, Klonopin and Valium), barbiturates (examples include
Seconal and phenobarbital), and over-the-counter (OTC) drugs that are on the State of
Indiana Over-the-Counter Drug Formulary.
Medicaid will continue to pay for all your other Medicaid-covered health care, such as
hospital, physician, and home health care services. When appropriate, Medicaid will also
continue to pay your monthly premiums for Medicare Part A (hospital) and Medicare
Part B (medical) coverage.
01/31/2007 Pharmacy
Medical Policy Manual
559

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
These coverage changes are necessary due to the passage of the Medicare Modernization
Act (MMA) and federal rules supporting the MMA. This change in law affects all people
who are eligible for both Medicare and Medicaid. You are eligible for both Medicare and
Medicaid. You have the right to request an appeal, but because you are eligible for
Medicare and Medicaid, your appeal will be considered without a hearing. Full Medicaid
drug benefits can only continue for those not eligible to enroll in Medicare. You will
continue to receive Medicaid coverage, but most of your drugs will now be covered by
Medicare.
Provider Notification: BT200532 Publication Date: 12/29/2005
Subject: Updated Notice Of Program Change Due To The New Medicare
Prescription Drug Coverage
Date Added to Manual: 01/31/2006
Text of Publication
Note: The information in this bulletin is not directed to those providers rendering
services in the risk-based managed care (RBMC) delivery system. The information in this
bulletin is directed to the service delivery and billing staff of all fee-for-service and
pharmacy providers. Please distribute appropriately. We also request that the associations
distribute this information to their members and/or place this information in the materials
that they publish.
This bulletin provides additional information and clarification concerning Provider
Bulletin BT200526 released on November 15, 2005. Effective January 1, 2006, the
Centers for Medicare and Medicaid Services (CMS) is implementing the new Medicare
prescription drug coverage, also known as Medicare Part D. With the implementation of
this new coverage, Medicaid can no longer pay for Medicare-covered prescription drugs
for members who also have Medicare.
Members entitled to receive traditional Medicare, and who receive full Medicaid benefits,
are eligible for Medicare Part D. People who receive Medicare benefits and full Medicaid
benefits were automatically enrolled in Medicare prescription drug coverage in October
2005. These members can choose a Medicare prescription drug plan (PDP) on their own
prior to December 31, 2005, or remain with the PDP chosen for them by Medicare.
Medicare will pay for the majority of prescription drugs for these members.
CMS Solution For People Not Auto-Enrolled
CMS is contracting with a single national plan, Wellpoint, to manage payment of
prescription drug claims for people who may not have been auto-enrolled. This single
national plan is for the very limited number of dual eligible beneficiaries who have not
yet been auto-enrolled into a Medicare Part D plan. CMS will provide additional
information to pharmacies about this process.
01/31/2007 Pharmacy
Medical Policy Manual
560

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
E1 Eligibility Function Testing
CMS advised pharmacy providers to begin testing the new E1 eligibility function that will be
available to pharmacies for the implementation of the new Medicare Part D plan. Testing may
help to eliminate many of the pharmacy insurance and billing issues concerning third party
liability. Free test transactions are available at:
http://medifacd.ndchealth.com/Pharmacies/MediFacD_Pharmacies_Testing.htm
http://medifacd.ndchealth.com/home/MediFacd_home.htm
CMS Fax Procedures for Multiple LTC Resident PDP Enrollment Information
Long-term care (LTC) facilities may need PDP enrollment information for residents of
their facility. Nursing homes without Internet access or who need Medicare PDP
enrollment information for multiple residents can now use a special CMS fax-based
procedure. Nursing home representatives must provide the required authentication
information for each of their Medicare residents using the appropriate authentication
form. Nursing homes are required to fax the completed form, along with the appropriate
cover sheet including the name and telephone number of a voice contact, to Medicare at
(785) 830-2593. Providers must use these forms to expedite fax requests for PDP
information to CMS. Failure to follow these procedures will result in a delay in response
time. The Medicare customer service representatives will process the requests and fax
them back to the nursing home. To access these forms, cover sheets, and instructions,
refer to:
http://www.indianamedicaid.com/ihcp/Forms/LTC_Nursing_Home_Administrators_FA
X_Procedures.doc
or call 1-800-MEDICARE to request the forms.
Medicare Part D Coverage and Exclusions
This section provides coverage and exclusion information for Medicare Part D. Some
Medicare-excluded drugs are also excluded from coverage by Medicaid. Certain drugs,
including but not limited to over-the counter drugs excluded by Medicare, may be
covered by Medicaid, if the drug is part of the member’s covered Medicaid benefits.
Medicare Part D Coverage
Medicare drug plans will cover brand name and generic drugs as follows:
• Prescription drugs
• Biological products
• Insulin as described in specified paragraphs of section 1927(K) of the Medicare
Modernization Act
• Medical supplies for injection of insulin such as syringes, needles, alcohol
swabs, and gauze
• Vaccines licensed under section 351 of the Public Health Service Act. Vaccines
not covered by Medicare Part B that are determined to be medically necessary are
covered under Medicare Part D.
Note: PDPs are not required to cover all Medicare Part D covered drugs. Refer to the
PDP specific drug formulary for coverage information.
01/31/2007 Pharmacy
Medical Policy Manual
561
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Home Infusion Therapy
Medicare Part D drugs that are a component of home infusion therapy are not to be billed
to Indiana Medicaid for dual eligibles. Provider adherence to this policy will be
monitored via post payment review and recoupment will follow in cases of violation of
this directive.
Medicare Part D Exclusions
After December 31, 2005, Medicaid cannot pay for Medicare-covered prescription drugs
for people with Medicare and Medicaid, the Medicare PDP deductibles, or co-payments.
Medicare Part D coverage excludes the following drugs:
• Drugs for anorexia, weight loss, or weight gain
• Drugs used to promote fertility
• Drugs used for cosmetic purposes or for hair growth
• Drugs from a manufacturer who requires that any associated tests and
monitoring services be purchased exclusively from the manufacturer or the
manufacturer’s designee
• Benzodiazepines
• Barbiturates
• Drugs used for symptomatic relief of cough and colds
• Prescription vitamins and mineral products, except prenatal vitamins and
fluoride preparation products
• Any drug covered through Medicare Part A or Medicare Part B
• Supplies, equipment, and services involved in the delivery of home infusion
Indiana Medicaid Coverage of Medicare Part D Excluded Drugs
Indiana Medicaid will pay for Medicare Part D - excluded drugs to the extent that they
are a covered Indiana Medicaid benefit. Any OTC drug covered by Indiana Medicaid
must be on the State of Indiana OTC Drug Formulary. Medicare Part D - excluded
prescription drugs are subject to the preferred drug list (PDL), existing PDL limits, and
any other applicable pharmacy program restrictions. The Medicaid-covered, Medicare Part
D - excluded drug listing can be viewed at:
http://www.indianamedicaid.com/ihcp/ProviderServices/medicareD.asp. This listing is
subject to change based on changes to program policy. The OTC Drug Formulary and
PDL can be viewed at http://www.indianapbm.com/. Pharmacists and prescribing
practitioners should contact ACS with any questions related to the PDL or OTC Drug
Formulary by calling 1-866-879-0106.
Medicaid Spenddown and Medicare Part D
Indiana Medicaid members with a spenddown must still satisfy their monthly spenddown
obligation before providers will be reimbursed for Medicaid. Medicare Part D co-
payments and out-of-pocket expenses for Medicare-excluded drugs covered by Medicaid
will apply to spenddown, in addition to other Medicaid covered medical expenses.
Pharmacists will be notified of the amount the member owes for his or her remaining
spenddown balance at the time the POS claim adjudicates. Pharmacists may collect
payment from the member at the point of dispensing. Members will be responsible for
retaining bills or receipts for Medicare Part D out-of-pocket expenses that count toward
01/31/2007 Pharmacy
Medical Policy Manual
562

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
their monthly Medicaid spenddown. Members must take the receipts or bills to their
local Office of Family Resources to apply to their spenddown. For general information
about spenddown, providers should refer to the provider manual, bulletins, newsletter
articles, and banners. For additional information about spenddown, refer to BT200527
available on the IHCP Web site at:
http://www.indianamedicaid.com/ihcp/Bulletins/BT200527.pdf.
Medicare Part D and Medicaid Eligibility Verification
An upgrade to the IHCP Eligibility Verification System (EVS) will be implemented
along with Medicare Part D. A new Medicare indicator of ‘D’ will display on the
eligibility verification response if a member is eligible for Medicare Part D. All forms of
IHCP eligibility verification – Web interChange, Automated Voice Response (AVR), and
the Omni swipe card system – are included in this upgrade.
Providers who use OMNI for eligibility verification must download the new version of
OMNI software on or after January 1, 2006, to display the Part D indicator. There is no
cost associated with this download. The IHCP provider bulletin, BT200303, published
January 31, 2003, provides complete download instructions. The bulletin is available
from the IHCP Web site at www.indianamedicaid.com. Direct questions about the
OMNI device download to the OMNI Help Desk at (317) 488-5051 in the
Indianapolis local area or 1-800-284-3548. The OMNI Help Desk telephone lines are
available from 8 a.m. to 5 p.m. Monday through Friday, excluding State holidays.
Additional Information
Medicare Part D is a federal program implemented by CMS. The information in this
bulletin is meant to address changes to the Indiana Medicaid program as a result of
implementation of the Medicare Part D benefit. The following are additional sources of
information about Medicare Part D:
• Medicare & You 2006 handbook
• Medicare Web site at www.medicare.gov
• 1-800-MEDICARE (1-800-633-4227) or 1-877-486-2048 for TTY users
• Medicare Part B versus Part D Information at
http://www.cms.hhs.gov/pdps/PartbandPartDdoc-revised7-27-05.pdf
01/31/2007 Pharmacy
Medical Policy Manual
563

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
PHARMACY SERVICES
ADDENDUM C
Note: This addendum contains provider notifications that have been published since the
review of the Pharmacy Services Fact Sheet. The information in this addendum will be
incorporated into the fact sheet at the next review.
Provider Notification: NL200602 Publication Date: 02/2006
Subject: New Pharmacy Reimbursement Rule
Date Added to Manual: 04/28/2006
Text of Publication
This notice is to advise providers that, in response to rapidly escalating expenditures for
Medicaid-covered drugs, and in order to stay within available appropriations while
maintaining beneficiary access to services, the office will be adopting an emergency rule
that amends pharmacy reimbursement for Medicaid and HoosierRx. Specifically, EAC
for brand name legend drugs will change from AWP minus 13.5 percent to AWP minus
16 percent with a dispensing fee of $4.90. (This change also applies to HoosierRx.) At
the same time, to bring consistency to reimbursement policy for insulins, OTC insulins
are now paid in accordance with applicable legend drug EAC methodology. These
changes are effective for dates of service on or after October 1, 2005.
01/31/2007 Pharmacy
Medical Policy Manual
564

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
PHARMACY SERVICES
ADDENDUM D
Note: This addendum contains provider notifications that have been published since the
review of the Pharmacy Services Fact Sheet. The information in this addendum will be
incorporated into the fact sheet at the next review.
Provider Notification: BT200613 Publication Date: 04/28/2006
Subject: Billing and Policy Reminders: Emergency Supply,
Third Party Liability Codes
Date Added to Manual: 10/31/2006
Text of Publication
Emergency Supply
The purpose of the Emergency Supply Policy is to comply with federal emergency
supply provisions, and ensure that patients do not go without covered outpatient drugs in
emergency situations. Emergency situations are being construed as including instances
when it is not possible to obtain prior authorization (PA) due to PA offices being closed.
It is not the intent to allow pharmacy providers to circumvent otherwise applicable
program parameters, such as PDL status, “brand medically necessary” requirements, PDL
step therapy edits, or early refill edits. It has been determined from the recent review, that
some providers are misusing the emergency supply provisions for such purposes and
perhaps in other inappropriate situations.
EDS intends to make compliance with the emergency supply provisions of the Medicaid
program a primary focus of review by the pharmacy audit contractor, Prudent Rx. Those
providers found to be violating the Emergency Supply Billing Policy face possible
recoupment of funds associated with the misbillings, as well as other applicable
sanctions.
Third Party Liability Codes
Some pharmacy providers are misusing third party liability (TPL) codes, in particular,
TPL Override Code 2. This code is intended to: (1.) be utilized by a pharmacy to indicate
that they have billed the primary payer, and (2.) reflect the actual dollar amount that the
primary payer has paid towards the claim. In particular, when using TPL Override Code
2, the pharmacy provider must report a non-zero amount in National Council for
Prescription Drug Programs (NCPDP) field 431-DV – Other Payer Amount Paid. If the
primary payer has been billed and has not paid any amount, a TPL Override Code 2
should never be used. There are more appropriate codes to report the situation.
Attachment 1 of this bulletin is a quick reference of all TPL Override Codes available for
use with the NCPDP versions 5.1 and 1.1 transaction sets. For the full descriptions of
these override codes and the corresponding NCPDP field numbers for all TPL
01/31/2007 Pharmacy
Medical Policy Manual
565

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Pharmacy
Medical Policy Manual
566
information, refer to the Companion Guide: NCPDP Versions 1.1 and 5.1 Transaction
Payer Sheet available on the IHCP Web site at http://www.indianamedicaid.com/
ihcp/TradingPartner/CompanionGuides/payersheet.pdf. Review your utilization of the
TPL Override Codes to ensure that in all instances the codes are being used solely as
specified.
Third Party Liability (TPL) Override Codes Quick Reference
Code and Description Explanation
TPL Override Code 2 –
Other payment exists-
payment collected
This code should be used when other insurance exists and
payment is collected. The other payer amount collected
[National Council for Prescription Drug Programs (NCPDP)
field 431-DV] and other payer date (NCPDP field 443-E8)
must be populated with a non-zero amount.
TPL Override Code 3 –
Other coverage exists-
this claim not covered
This code should be used when the primary insurance does not
cover any portion of the claim. Examples of this include over-
the-counter (OTC) items and any other item that is covered by
the Indiana Health Coverage Programs (IHCP) that is not
covered by the primary insurance.
TPL Override Code 4 –
Other coverage exists-
payment not collected
This code should only be used in cases in which a patient has
active TPL coverage, but the claim is not paid. Deductibles
and exhausted benefits are examples of such situations.
TPL Override Code 5 –
Managed Care plan
denial
This code should not be used for risk-based managed care
(RBMC) IHCP denials; rather, it is to be used when the
primary insurance is a managed care organization that denies
the claim.
TPL Override Code 6 –
Other coverage exists,
not a participating
provider
This code should be used when the dispensing pharmacy or
prescribing physician is not a participating provider in the
primary insurance company’s network.
TPL Override Code 7 –
Other coverage exists,
not in effect at time of
service
The dispensing pharmacy should use this code only if a denial
has been received from the primary insurance company stating
the coverage for the participant has been terminated or if it has
been otherwise verified that there is no other existing third
party coverage.
TPL Override Code 8-
Claim is billing for a
copay
This code should be used when the pharmacy is billing the
IHCP for a fixed copayment required by another insurer. The
copay amount should be submitted in the Gross Amount Due
and Other Amount Claimed submitted fields (NCPDP fields
430-DU and 480-H9, respectively)

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: PHARMACY – BOTULINUM TOXIN INJECTIONS
DESCRIPTION
The United States Food & Drug Administration (FDA) has approved two types of
botulinum toxin injections; botulinum toxin type A, (Botox) and botulinum toxin type B
(Myobloc). These injections are approved for symptomatic treatment of certain
neuromuscular conditions. Botox and Myobloc are neurotoxins that interfere with
neuromuscular transmission. They are injected directly into the affected muscle, through
a procedure called chemodenervation, to temporarily paralyze the muscle by blocking the
nerve for the affected muscle. Each treatment lasts approximately three months. The
affected muscle is identified by electrode stimulation of the muscle, manual examination
of the affected area, or by electromyography (EMG). Botox and Myobloc provide
symptomatic treatment of focal dystonias, such as blepharospasm, cervical dystonia,
hemifacial spasms, and other neurological conditions that cause excessive muscle
contractions. In addition, research has shown these drugs to be successful in improving
neuromuscular function, relieving pain, improving range of motion, and enhancing the
effectiveness of physical therapy.
SUMMARY OF CURRENT POLICY
The Indiana Health Coverage Programs (IHCP) provides reimbursement for
chemodenervation using Botox and Myobloc for the treatment of certain neuromuscular
conditions including cervical dystonia, cerebral palsy, multiple sclerosis and other
muscular and neurological conditions that cause excessive muscle contractions.
Reimbursement is only available when administered in a physician’s office, consistent
with IHCP policy concerning reimbursement for injectable pharmaceutical products.
Prior authorization (PA) is not required for this service. The IHCP does not provide
reimbursement for Botox and Myobloc for cosmetic purposes, as indicated in 405 IAC 5-
24-3.
COVERAGE CRITERIA
Botox and Myobloc are neurotoxins that interfere with neuromuscular transmission.
IHCP reimbursement is available for Botox and Myobloc injections when determined
medically necessary. Botox and Myobloc are not covered by the IHCP and for cosmetic
purposes. Reimbursement is limited to one injection every three months. TABLE 1, on
the next page, shows the HCPCS codes that are available for billing Botox and Myobloc.
01/31/2007 Pharmacy – Botulinum Toxin Type A (BOTOX)
Medical Policy Manual
567

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
TABLE 1
HCPCS Coding for Botox and Myobloc Injections
HCPCS Code Definition
J0585
Botulinum toxin type A, per unit
(Botox)
J0587
Botulinum toxin type B, per 100 units
(Myobloc)
Botox and Myobloc are injected directly into the affected muscle, through a procedure
called chemodenervation, to temporarily paralyze the muscle by blocking the release of
acetylcholine to the nerve of the affected muscle. The affected muscle is identified by
electrode stimulation of the muscle, manual examination of the affected area, or by
electromyography (EMG). IHCP reimbursement for Botox and Myobloc must include
one of the following CPT codes available for billing chemodenervation, listed in TABLE
2.
TABLE 2
CPT Codes for Chemodenervation for Use with
Botox and Myobloc Injections
CPT Code Definition
64612
Chemodenervation of muscle(s); muscle(s) innervated by facial nerve
(e.g., for blepharospasm, hemifacial spasm)
64613
Chemodenervation of muscle(s); cervical spinal muscles(s)
(e.g. for spasmodic torticollis)
64614
Chemodenervation of muscle(s); extremity(s) and/or trunk muscle(s)
(e.g., for dystonia, cerebral palsy, multiple sclerosis)
64640 Destruction by neurolytic agent; other peripheral nerve or branch
95860
Needle electromyography; one extremity with or without
related paraspinal areas
95861
Needle electromyography; two extremities with or without
related paraspinal areas
95867 Needle electromyography; cranial nerve supplied muscle(s), unilateral
95868 Needle electromyography; cranial nerve supplied muscle(s), bilateral
95869
Needle electromyography; thoracic paraspinal muscles
(excluding T1 or T12)
95870
Needle electromyography; limited study of muscles in on extremity
non-limb (axial) muscles (unilateral or bilateral), other than thoracic
paraspinal, cranial nerve supplied muscles or sphincters
Treatment with botulinum toxin injections provides temporary relief of symptoms
associated with these conditions and is indicated for use when conventional treatment has
failed and/or in conjunction with physical therapy or other therapeutic techniques. Botox
and Myobloc have been shown to be medically necessary in the treatment of the
following conditions.
• Blepharospasm
• Cervical Dystonia
01/31/2007 Pharmacy – Botulinum Toxin Type A (BOTOX)
Medical Policy Manual
568
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• Hyperhidrosis
• Strabismus
• Oromandibular Dystonia
• Lingual Dystonia
• Hemifacial Spasms
• Myofascial Pain Syndrome
• Spasticity related to stroke, cerebral palsy, multiple sclerosis, and spinal cord
injury
• Paraplegia, hemiplegia, quadriplegia, or monoplegia
IHCP reimbursement for Botox and Myobloc injections is limited to specific diagnosis
codes to ensure that injections are medically necessary. TABLE 2 shows the ICD-9-CM
codes that are available for reimbursement of Botox and Myobloc injections.
TABLE 2
ICD-9-CM Codes Available for
Reimbursement of Botox and Myobloc Injections
ICD-9-CM
Code
Definition
333.6 Idiopathic torsion dystonia
333.7 Symptomatic torsion dystonia
333.81 Blepharospasm
333.82 Orofacial dyskinesia
333.83 Spasmodic torticollis
333.84 Organic writers’ cramp
333.89 Other fragments of torsion dystonia
334.1 Hereditary spastic paraplegia
340 Multiple sclerosis
341.0 Neuromyelitis optica
341.1 Schilder’s disease
341.8 Other demyelinating diseases of central nervous system
341.9 Demyelinating disease of central nervous system, unspecified
342 Hemiplegia and hemiparesis
342.10 Spastic hemiplegia and hemiparesis affecting unspecified side
342.11 Spastic hemiplegia and hemiparesis affecting dominant side
342.12 Spastic hemiplegia and hemiparesis affecting nondominant side
343.0 Diplegic
343.1 Hemiplegia
343.2 Quadriplegic
343.3 Monoplegic
343.4 Infantile hemiplegia
343.8 Other specified infantile cerebral palsy
343.9 Infantile cerebral palsy, unspecified
351.8 Other facial nerve disorders
378.00 Esotropia, unspecified
01/31/2007 Pharmacy – Botulinum Toxin Type A (BOTOX)
Medical Policy Manual
569

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
TABLE 2
ICD-9-CM Codes Available for
Reimbursement of Botox and Myobloc Injections
ICD-9-CM
Definition
Code
378.01 Monocular estropia
378.02 Monocular esotropia with A pattern
378.03 Monocular esotropia with V pattern
378.04 Monocular estropia with other noncomitances
378.05 Alternating esotropia
378.06 Alternating estropia with A pattern
378.07 Alternating estropia with V pattern
378.08 Alternating esotropia with other noncomitances
378.10 Exotropia, unspecified
378.11 Monocular exotropia
378.12 Monocular exotropia with A pattern
378.13 Monocular exotropia with V pattern
378.14 Monocular exotropia with other noncomitances
378.15 Alternating exotropia
378.16 Alternating exotropia with A pattern
378.17 Alternating exotropia with V pattern
378.18 Alternating exotropia with other noncomitances
378.20 Intermittent heterotropia, unspecified
378.21 Intermittent esotropia, monocular
378.22 Intermittent esotropia, alternating
378.23 Intermittent exotropia, monocular
378.24 Intermittent exotropia, alternating
378.30 Heterotropia, unspecified
378.31 Hypertropia
378.32 Hypotropia
378.33 Cyclotropia
378.34 Monofixation syndrome
378.35 Accommodative component in esotropia
378.40 Heterophoria, unspecified
378.41 Esophoria
378.42 Exophoria
378.43 Vertical heterophoria
378.45 Alternating hyperphoria
378.50 Paralytic strabismus, unspecified
378.51 Third or oculomotor nerve palsy, partial
378.52 Third or oculomotor nerve palsy, total
378.53 Fourth or trochlear nerve palsy
378.54 Sixth or abducens nerve palsy
378.55 External ophthalmoplegia
378.56 Total ophthalmoplegia
01/31/2007 Pharmacy – Botulinum Toxin Type A (BOTOX)
Medical Policy Manual
570

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
TABLE 2
ICD-9-CM Codes Available for
Reimbursement of Botox and Myobloc Injections
ICD-9-CM
Definition
Code
378.6 Mechanical strabismus
378.60 Mechanical strabismus, unspecified
378.61 Brown’s (tendon) sheath syndrome
378.62 Mechanical strabismus from other musculofascial disorders
378.63 Limited duction associated with other conditions
378.71 Duane’s syndrome
378.72 Progressive external ophthalmoplegia
378.73 Strabismus in other neuromuscular disorders
378.81 Palsy of conjugate gaze
378.82 Spasm of conjugate gaze
378.83 Convergence insufficiency or palsy
378.84 Convergence excess or spasm
378.85 Anomalies of divergence
378.86 Internuclear ophthalmoplegia
378.87 Other dissociated deviation of eye movements
378.9 Unspecified disorder of eye movements
478.29 Other diseases of pharynx or nasopharynx
478.75 Laryngeal spasm
478.79 Other diseases of larynx
530.0 Achalasia and cardiospasm
565.0 Anal fissure
705.21 Primary focal hyperhydrosis
723.5 Torticollis, unspecified
729.1 Myalgia and myositis, unspecified
754.1
Certain congenital musculoskeletal deformities of sternocleidomastoid
muscle
Billing Partial Units
The IHCP recognizes that Botox is distributed in single-dose vials of 100 units. Botox
has an extremely short shelf life, once reconstituted, and because of this short shelf life,
some wastage of the product may be unavoidable. The IHCP has adopted the following
Medicare carrier’s policy for billing unused units of Botox.
IHCP providers may bill the entire 100 units to the IHCP in cases in which less than 100
units are injected in a single treatment session AND the balance of the product is
discarded. If more than 100 units are injected in a single treatment session, and the
remainder is not used for another member, the billed amount on the claim should be
rounded up to the nearest 100 units. Whenever unused Botox is billed, both the amount
01/31/2007 Pharmacy – Botulinum Toxin Type A (BOTOX)
Medical Policy Manual
571

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Pharmacy – Botulinum Toxin Type A (BOTOX)
Medical Policy Manual
572
of the agent actually administered and the amount discarded are to be documented in the
member’s medical record.
Similarly, Myobloc has a short shelf life, and wastage of the product may be unavoidable.
The IHCP has adopted the following Medicare carrier’s policy for billing unused units of
Myobloc.
Myobloc is supplied in 2,500, 5,000, and 10,000 units. Billing for Myobloc must show
the number of units given on the claim form. If a vial is split between two or more
members, the amount of the Myobloc used for each member is billed and the unused can
be billed as wastage on the claim for the last member injected. If the vial is not split
between two or more members, the discarded portion may be billed to the IHCP.
Whenever unused Myobloc is billed, both the amount of agent actually administered and
the amount discarded are to be documented in the member’s medical record.
RELATED MEDICAL TOPICS
Pharmacy
Injectables
RULES, CITATIONS, AND SOURCES
405 IAC 5-24 Pharmacy Services
BR199935 – Botox injection billing
BT200207 – 2002 New HCPCS Codes
IHCP Provider Manual, July 2004 - Injections
ORIGINATION, REVISIONS, AND REVIEWS
Origination date: 5/25/97
Revisions and Reviews Reason Date
405 IAC 5-24 Pharmacy Services 7/25/1997
BR199935 Appropriate Billing Guidelines 8/19/1999
BT200207
New HCPCS Codes –Cover New HCPCS Code
J0587 (Myobloc)
2/15/2002
IHCP Provider Manual Injections July 2004
Medical Policy Manual 4
th
Quarter 2004 Update 1/31/05
APPLICABLE INDIANAAIM EDITS AND AUDITS
6096 – The CPT/HCPCS code billed is not payable in PPS

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: PHARMACY - SYNAGIS® AND RESPIGAM®
DESCRIPTION
Respiratory syncytial virus (RSV) is a cause of serious respiratory tract infections in
infants and children under four years of age. The symptoms are similar to the common
cold, although they may lead to more serious infections such as bronchiolitis, pneumonia,
and severe lower respiratory tract disease. Synagis® (palivizumab) and RespiGam®
(RSV-IGIV) are prophylactic treatment options for people at risk for RSV. Synagis® is a
monoclonal antibody given as a monthly intramuscular injection. RespiGam® is a blood
product given as a monthly infusion.
This document is intended to serve as a general summary of the Indiana Health Coverage
Programs (IHCP) policies regarding this service. More specific information may be
found in the IHCP Provider Manual, program notices, the Indiana Administrative Code
(IAC) or other sources as appropriate. See the “Rules, Citations, and Sources” section of
this document for further information.
COVERAGE CRITERIA
Synagis® and RespiGam® are reimbursed by the IHCP for prophylactic treatment of
RSV. A member will not be approved for Synagis® or RespiGam® if he or she is
currently receiving immunoglobulin infusions. Immunity should be acquired through
those infusions. Synagis® is the preferred product for prophylaxis; however, Synagis®
or RespiGam® will be reimbursed if prior authorization (PA) criteria are met.
PRIOR AUTHORIZATION
Synagis® and RespiGam® require PA. The following criteria reflect the conditions
under which Synagis® and RespiGam® will be prior authorized.
PA Submission
Non-pharmacy providers submit PA requests for Synagis® (90378) or RespiGam®
(90379 or J1565) to the Health Care Excel (HCE) PA Department. The HCE PA
Department may be contacted by calling 1-800-457-4518 or (317) 345-4511. It is
recommended that providers submitting PA requests attach the Synagis Prior
01/31/2007 Pharmacy – Synagis and RespiGam
Medical Policy Manual
573

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Authorization Form for Synagis® or RespiGam® available for download at
www.indianamedicaid.com. In addition, providers solely enrolled as durable medical
equipment (DME) providers are not eligible for the reimbursement of pharmaceuticals.
Approval Period and Quantity
The approval period for Synagis® and RespiGam® is October 1 through April 30, the
RSV season. Approval consists of a total of six doses of Synagis® or RespiGam®. The
administration of a seventh dose requires a separate PA.
Location of Service
Synagis® may be administered in any setting where intramuscular injections are
appropriate, including home administration. RespiGam® may be administered in a
clinic, physician’s office, or hospital. RespiGam® is not approved for home
administration.
PA Criteria
Synagis® and RespiGam® may be administered to members at risk for RSV. At least
one of the following criteria must be met before the patient is considered at risk for RSV.
1. The member is less than 24 months old at the start of therapy and has chronic lung
disease, especially if on oxygen chronically or off oxygen less than three to six
months.
2. The member is younger than one year old and has a history of accompanying
medical problems, for example, caffeine administration for respiratory stimulation
within the last year.
3. The member is younger than six months old at the start of therapy with a
gestational age of 29 to 32 weeks.
4. The member is less than three months old at the start of therapy with a gestational
age of 33 to 36 weeks and accompanying medical problems.
5. The member is six months old at the start of therapy with a gestational age of 33
to 36 weeks and has one of the following risk factors; school age siblings,
crowding in the home, daycare attendance, exposure to tobacco smoke in the
home, multiple births, neurological disease, anticipated cardiac surgery, distance
to or availability of hospital care.
MANAGED CARE
For members enrolled in Risk Based Managed Care (RBMC), providers must contact the
member’s managed care organization (MCO) for more specific guidelines. Refer to the
01/31/2007 Pharmacy – Synagis and RespiGam
Medical Policy Manual
574

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
IHCP Provider Manual, Chapter 1, for detailed information about the fee-for-service
(FFS), PrimeStep, and RBMC delivery systems.
IHCP members enrolled in PrimeStep receive the same benefit coverage, and are subject
to the same limitations, as traditional Medicaid FFS. Refer to the Hoosier Healthwise
Manual for Primary Medical Providers and Office Staff for further information.
BILLING REQUIREMENTS
Physicians, hospitals, and clinics that provide Synagis® or RespiGam® in an office,
home, or outpatient setting must bill the appropriate claim format with CPT code 90378
for Synagis®, or CPT code 90379 or HCPCS code J1565 for RespiGam®. Providers
must report the most specific code that represents the immune globulin product. The
administration of the immune globulin product is separately billable. A pharmacy, or a
provider dually enrolled as a pharmacy, must submit claims for Synagis® or RespiGam®
with the NDC on the pharmacy claim form.
Table 1 - Codes for Reporting Immune Globulin Product for Synagis or RespiGam
Drug Code Description
Synagis® 90378
Respiratory syncytial virus immune globulin (RSV-
IgIM), for intramuscular use, 50 mg, each
RespiGam® 90379
Respiratory syncytial virus immune globulin (RSV-
IgIV), human, for intravenous use
RespiGam® J1565
Injection, respiratory syncytial virus immune globulin,
intravenous, 50 mg
RELATED MEDICAL TOPICS
Immunizations and Vaccines
Pharmacy
RULES, CITATIONS, AND SOURCES
Indiana Code
15 IC 12-15-21-3 Subjects for Which Rules to Be Adopted
Indiana Administrative Code
405 IAC 5-2-17 Medically Reasonable and Necessary Service Defined
405 IAC 5-3 Prior Authorization
405 IAC 5-24-8.5 Prior Authorization; Other Drugs
Indiana Health Coverage Programs Provider Newsletters
NL200410 Prior Authorization Instructions
Indiana Health Coverage Programs Provider Bulletins
BT200210 Prior Authorization Changes
01/31/2007 Pharmacy – Synagis and RespiGam
Medical Policy Manual
575

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Pharmacy – Synagis and RespiGam
Medical Policy Manual
576
Indiana Health Coverage Programs Provider Banners
BR200240 Prior Authorization Changes
BR200239 Prior Authorization Changes
BR200238 Prior Authorization Changes
BR199919 Billing Instructions
BR199918 Billing Instructions
Origination Date: 07/31/05
Revisions and
Review
Reason Date
APPLICABLE INDIANAAIM EDITS AND AUDITS
3000 Units Exceed PA Master
3001 Dates of Service Not On PA Database
3003 Procedure Code Requires PA
3008 Prior Authorized Units Equals Zero
6096 The CPT/HCPCS Code Billed Is Not Payable According to the PPS
Reimbursement Methodology

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: PLASMAPHERESIS
DESCRIPTION
Plasmapheresis is the most common type of apheresis procedure, and involves the
removal of a prescribed amount of plasma from the circulating blood. A plasmapheresis
treatment takes several hours, and an average course is six to ten treatments over a two to
ten week period. Plasmapheresis can also involve plasma exchange. During plasma
exchange, plasma is removed and discarded, and replaced with allogenic (genetically
different) plasma or another substitution fluid, such as albumin.
Plasmapheresis is prescribed for patients with acute, self-limited diseases, where plasma
exchange is used to acutely lower the circulating pathogenic substances; or for patients
with chronic diseases that produce an overabundance of pathogenic autoantibodies, such
as systemic lupus erythmatosus (SLE).
COVERAGE CRITERIA
Plasmapheresis is covered when performed in a hospital setting (either inpatient or
outpatient); or in a nonhospital setting (e.g. a physician directed clinic), when the
following conditions are met.
A physician is available to perform medical services and to respond to medical
emergencies at all times during patient care hours
The member is under the care of a physician
All nonphysician services are furnished under the direct responsibility of a
physician
Plasmapheresis is considered medically necessary for the following indications.
Plasma exchange for acquired myasthenia gravis
Plasmapheresis in the treatment of primary macroglobulinemia (Waldenstrom)
Plasmapheresis and plasma exchange for the treatment of hyperglobulinemias,
including (but not limited to) multiple myelomas, cryoglobulinemia and
hyperviscosity syndromes
01/31/2007 Plasmapheresis
577
Medical Policy Manual
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Plasmapheresis or plasma exchange as a last resort treatment of thrombotic
thrombocytopenic purpura (TTP)
Plasmapheresis or plasma exchange as the last resort treatment of life threatening
rheumatoid vasculitis
Plasma exchange in the treatment of Goodpasture’s Syndrome
Plasma exchange in the treatment of glomerulonephritis, associated with
antiglomerular basement membrane antibodies and advancing renal failure, or
pulmonary hemorrhage
Plasmapheresis, with plasma exchange, for treatment of chronic relapsing
polyneuropathy for members with severe or life threatening symptoms, who have
failed to respond to conventional therapy
Plasmapheresis, with plasma exchange, for treatment of life threatening
scleroderma and polymyositis that is unresponsive to conventional therapy
Plasmapheresis for treatment of Guillain-Barre Syndrome
Plasmapheresis, as a last resort, for life threatening systemic lupus erythematous
(SLE), when conventional therapy has failed to prevent clinical deterioration
PRIOR AUTHORIZATION
Plasmapheresis does not require prior authorization.
MANAGED CARE
For members enrolled in Risk Based Managed Care (RBMC), providers must contact the
member’s managed care organization (MCO) for more specific guidelines. Refer to the
Provider Manual, Chapter 1, for detailed information about the fee-for-service (FFS), Primary
Care Case Management (PCCM), and Risk Based Managed Care (RBMC) delivery
systems.
IHCP members enrolled in Hoosier Healthwise PrimeStep (PCCM) receive the same
benefit coverage, and are subject to the same limitations, as traditional Medicaid FFS.
Refer to the Hoosier Healthwise Manual for Primary Medical Providers and Office Staff
for further information.
BILLING REQUIREMENTS
The IHCP reimburses plasmapheresis services when IHCP providers bill with CPT code
36514, Therapeutic apheresis; for plasmapheresis. The appropriate ICD-9-CM procedure
code is 99.71, Therapeutic plasmapheresis. Prior authorization is not required.
Plasmapheresis services are subject to post payment review. Providers must maintain
documentation in the member’s medical record that indicates the IHCP coverage criteria
has been met.
01/31/2007 Plasmapheresis
578
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Plasmapheresis
Medical Policy Manual
579
RELATED MEDICAL TOPICS
Physician Services
Hospital Services – Outpatient
Hospital Services – Inpatient
RULES, CITATIONS, AND SOURCES
405 IAC 5-3-13 – Services Requiring Prior Authorization
Indiana Health Coverage Programs Provider Manual, July 2004, Version 5.0
Origination Date: 4/29/05
Revisions and
Review
Reason Date
APPLICABLE INDIANAAIM EDITS AND AUDITS
6002 – Any Two Anesthesiology Providers
6003 – Manual Pricing for Split Care Billing
6034 – Global Surgery Payable at Reduced Amount
6035 – Components of Surgical Care Not Payable When Global Surgery Paid
6039 – Assistant Surgeon Not Payable when Co-Surgeon Paid
6040 – Co-Surgeon Not Payable When Assistant Surgeon Paid
6096 – The CPT/HCPCS Code Billed Is Not Payable

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: PODIATRY
DESCRIPTION
Podiatry is a specialized practice focusing on the study and care of the foot and related
structures, including its anatomy, pathology, and medical and surgical treatment.
SUMMARY OF CURRENT POLICY
The Indiana Health Coverage Programs (IHCP) covers services and procedures
associated with the diagnosis of foot disorders and mechanical, medical, or surgical
treatment of such disorders, subject to restrictions and limitations set in the Indiana
Administrative Code (IAC), 405 IAC 5-26.
RELATED MEDICAL TOPICS
Consultations – Second Opinion
Diagnostic Studies
Hospital Outpatient
Nursing Facilities
S
urgery – Surgical Services
RULES, CITATIONS, AND SOURCES
405 IAC 5-5 Out of State Services
405 IAC 5-17 Hospital Services
405 IAC 5-26 Podiatric Services
Indiana State Department of Public Welfare Medical Policy Manual 1991
Indiana Medical Assistance Program Provider Manual 1994
Indiana Medicaid Update Bulletins, 95-21; 96-38
Indiana Medicaid Bulletin, 091598
Indiana Health Coverage Programs Provider Manual 1999
IHCP Provider Manual, July 2004
01/31/2007 Podiatry
580
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
ORIGINATION, REVISIONS, AND REVIEWS
Origination date: 12/31/04 Revisions and
Reviews
Reason Date
Indiana State Department of Public
Welfare Medical Policy Manual, 1991
Podiatric Services 7/1/91
470 IAC 5-8-16;
470 IAC 5-9-13
Transferred
Podiatric Services 7/1/91
405 IAC 1-7-13;
405 IAC 1-6-16
Repealed 8/24/97
Podiatric Services 1/1/92
405 IAC 5-26 Podiatric Services 8/24/97
Indian Medicaid Update Bulletin
Prior Authorization Rule
Changes
6/3/96
Indiana Medicaid Update Bulletin, 96-38 Hoosier Healthwise Changes 11/16/96
Indiana Medicaid Update Bulletin, 091598
Podiatry Coding for Routine
Foot Care
4/1/98
Indiana Medicaid Bulletin, BT199909
Removal of Services from Prior
Authorization
3/15/98
Indiana Medicaid Bulletin, BT200208
Modifications to Prior
Authorization Requirement
2/19/02
IHCP Provider Manual Manual Update 7/2004
APPLICABLE INDIANAAIM EDITS AND AUDITS
6049 – Components not payable when global paid
6090 – Office visits limited to one per year – podiatrist
6091 – One initial office visit per recipient – podiatrist
6651 – Surgical cutback procedure – 50%
6664 – Global vs. Components – Integumentary/neuromuscular
6665 – Bilateral vs. Unilateral surgery
6855 – More than 6 routine foot care treatments per year
6857 – Preoperative Doppler studies payable to podiatrist
01/31/2007 Podiatry
581
Medical Policy Manual
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
COVERAGE CRITERIA
General Restrictions
As indicated in 405 IAC 5-26-2, podiatric services are subject to the following general
restrictions:
1. In an emergency situation, when services require prior authorization (PA), the
authorization must be obtained within 48 hours of the time the service was provided,
not including Saturdays, Sundays, and legal holidays.
2. Any podiatrist services rendered during inpatient days that do not receive appropriate
PA, or are subsequently found not to be medically necessary will not be reimbursed.
PA is required for hospital stays as outlined in 405 IAC 5-17 (b).
3. Any podiatrist services rendered during an outpatient visit that do not receive
appropriate PA, or are subsequently found not to be medically necessary will not be
reimbursed.
4. Consultation services rendered by a podiatrist in a nursing facility are not covered
when performed on members on a routine basis for screening purposes, except in
those cases where a specific foot ailment is involved.
Prior Authorization (PA)
PA is required for the following services:
• Orthotics and related devices for the foot as outlined in 405 IAC 5-19.
• Palliative or hygienic care for the removal or trimming of corns, calluses, and
nails covered for members with painful keratosis, diseased nails, or deformed
nails. PA is provided for six treatments per year.
• Routine foot care in excess of six services per year for members with diabetes
mellitus, peripheral vascular disease, or peripheral neuropathy
Routine Foot Care
Routine foot care, as described in the IHCP Provider Manual, includes the following.
• Cutting or removal of corns, calluses or warts, including plantar warts
• Trimming of nails, including mycotic nails
• Treatment of fungal, mycotic infection of the toenail with clinical evidence
toenail infection and compelling medical evidence that the member has a marked
limitation of ambulation requiring active treatment of the foot or will result in
significant medical complications.
01/31/2007 Podiatry
582
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Routine foot care is only reimbursable by IHCP if the member is being treated by a
medical doctor or doctor of osteopathy for treatment and evaluation of a systemic disease
during the six months prior to rendering routine foot care services. A maximum of six
routine foot care services per rolling 12-month period are covered only when: (1) a
member has a diagnosis of systemic disease of sufficient severity that treatment of the
disease may pose a hazard when performed by a non professional such procedure would
be hazardous, and (2) the condition has resulted in severe circulatory embarrassment or
areas of desensitization in the legs or feet. Routine foot care, when performed in these
situations does not require PA. Table 1 shows the ICD-9 codes that represent conditions
that justify IHCP coverage for routine foot care.
Table 1: ICD-9 Codes for Routine Foot Care
ICD-9 Code(s) Description
250.00 – 250.91 Diabetes mellitus
440.20 – 440.29 Arteriosclerotic vascular disease of the lower extremities
443.1 Thromboangiitis obliterans
459.1 Post-phlebitis syndrome
357.1 – 357.7 Peripheral neuropathies of the feet
Podiatric Office Visits
The IHCP covers one podiatric office visit per year, using the following evaluation and
management (E/M) codes: 99211, 99212, and 99213. The IHCP does not reimburse for
the following types of extended or comprehensive office visits: (1) new patient
comprehensive, CPT codes 99204 and 99205; (2) established patient detailed, CPT code
99214; and, (3) established patient comprehensive, CPT code 99215.
New patient podiatric office visits are limited to one visit, per provider, within a three
year period. A new patient is, “one who has not receive professional services from the
provider or another provider of the same specialty who belongs to the same practice
within the last three years,” per 405 IAC 5-26-7. Reimbursement for subsequent office
visits is included in the procedure performed on that date and is not billed separately.
However, if a significant problem is addressed on a subsequent visit, the visit code may
be reported with the -25 modifier.
Laboratory Services, X-Rays and Doppler Evaluations
The IHCP reimburses a podiatrist for laboratory or X-ray services only when rendered by
or under the personal supervision of a podiatrist. Services ordered by a podiatrist, but
performed by a laboratory or x-ray facility can be directly billed by the laboratory or x-
ray facility. Comparative foot x-rays are only reimbursed when prior authorization is
obtained under Reimbursement is available for the following lab and x-ray services.
• Cultures of foot infections and mycotic fungal nails for diagnostic purposes.
• Medically necessary presurgical testing.
• Sensitivity studies for treatment of infection processes.
01/31/2007 Podiatry
583
Medical Policy Manual
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
The IHCP will reimburse one ultrasonic measurement of blood flow (Doppler evaluation)
per year, when prior authorized for the proposed medical procedure. Coverage is
available, subject to the following limitations.
• A preoperative diagnosis of diabetes mellitus, peripheral vascular disease or
peripheral neuropathy.
• The ultrasonic measurement is for preoperative podiatric evaluation.
• The ultrasonic measurement cannot be used for routine screening purposes.
• The ultrasonic measurement cannot be used as an evaluation of routine foot care
procedures, including such services as removal or trimming of corns, calluses, and
nails.
Surgical Procedures
The IHCP will reimburse for the following podiatric surgical procedures without prior
authorization.
• Drainage of skin abscesses of the foot
• Drainage or injections of a joint or bursa of the foot
• Surgical cleansing of the skin
• Trimming of skin lesions of the foot, other than those identified as included in
routine foot care services
Podiatrists may be required to obtain a confirmatory consultation, in accordance with the
guidelines for consultations and second opinions at 405 IAC 5-8-4, in order to establish
medical necessity for the following podiatric surgical procedures.
• Bunionectomy procedures
• All surgical procedures involving the foot
A confirmatory consultation is required regardless of the setting in which the surgery is
performed, including ambulatory surgical centers, hospitals, clinics, or in the office.
All services provided by the podiatrist must be performed within the scope of practice for
podiatric medicine. Reimbursement for other surgical procedures performed within the
scope of the podiatrist’s license is available subject to the prior authorization
requirements of 405 IAC 5-3.
Surgical procedures on one or both feet performed on the same date are paid at 100% of
the IHCP allowance for the major procedure and 50 percent of the IHCP allowance for
subsequent procedures. If surgery is performed on both feet and the surgery for the
second foot is performed at least five days after the first, 100% allowance is payable for
the second surgery.
01/31/2007 Podiatry
584
Medical Policy Manual
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
If the major surgical procedure is performed on one toe, a period of five days must pass
before subsequent surgery on the same toe would again be paid at 100% of the IHCP
allowable reimbursement. Surgery performed sooner than five days is paid at 50% of the
IHCP allowable reimbursement.
If the major surgical procedure is performed on one toe, a period of 30 days must elapse
before a subsequent surgery on the same toe would again be paid at 100% of the IHCP
allowable reimbursement. Surgery performed sooner than 30 days is reimbursed at 50%
of the IHCP allowable reimbursement.
Podiatric surgical procedures, including diagnostic surgical procedures, cannot be
fragmented and billed separately, as they are included in the major procedure.
The following surgical procedures are generally included in major surgical procedures:
• Arthroscopy or arthrotomy procedures in the same area as a major joint procedure
unless the claim documents a second incision was made
• Local anesthesia administered to perform the surgical or diagnostic procedure
• Scope procedures used for the surgical procedure approach
01/31/2007 Podiatry
585
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Podiatry
Medical Policy Manual
586
PODIATRY SERVICES
ADDENDUM A
Note: This addendum contains provider notifications that have been published since the
review of the Podiatry Services Fact Sheet. The information in this addendum will be
incorporated into the fact sheet at the next review.
Provider Notification: BR200544 Publication Date: 11/01/2005
Subject: Podiatry Multiple Unit Billing
Date Added to Manual: 01/31/2006
Text of Publication
The Health Care Excel Surveillance and Utilization Review (SUR) Department identified
utilization issues related to podiatrists inappropriately billing multiple units of Current
Procedural Terminology (CPT®) codes 99201-99203) for new patient visits and CPT
codes 99211-99213 for established patient visits.
Office visits for podiatry services are limited to the following:
• New patient office visits – Limited to one visit per member, per provider, every
three years, using CPT codes 99201, 99202, or 99203.
• Office visits – Limited to one visit per member, per 12 months, without obtaining PA,
using CPT codes 99211, 99212 or 99213.
This information can be found in the IHCP Provider Manual, Chapter 8, Section 3, and
in the Indiana Administrative Code (IAC), 405 IAC 5-26-7.
SUR is advising all providers to carefully review claims submitted to the IHCP to ensure
proper billing of units for these services. The SUR Department is conducting a review of
claims to determine any inappropriate reimbursement and recoup overpayments. If a
provider identifies overpayments related to these errors, the provider should file an
adjustment or contact the SUR Department to arrange for repayment.

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: RADIOIMMUNOTHERAPY
DESCRIPTION
The Indiana Health Coverage Programs (IHCP) provides reimbursement for
radioimmunotherapy for the treatment of refractory low-grade B-cell non-Hodgkin’s
lymphoma utilizing Zevalin®, effective May 1, 2003, and Bexxar®, effective January 1,
2004. The IHCP will provide reimbursement for additional radioimmunotherapy
regimens as approved by the Food and Drug Administration (FDA).
This document is intended to serve as a general summary of the IHCP policies regarding
this service. More specific information may be found in the IHCP Provider Manual,
program notices, the Indiana Administrative Code (IAC) or other sources as appropriate.
See the “Rules, Citations, and Sources” section of this document for further information.
COVERAGE CRITERIA
Radioimmunotherapy is utilized for the treatment of low-grade B-cell non-Hodgkin’s
lymphoma in patients that have not responded to or failed other chemotherapy treatments,
and should not be used for the first line of treatment. The patient’s medical record must
support the medical necessity of the radioimmunotherapy regimen. Zevalin® and
Bexxar® are monoclonal antibodies that target lymphocytes, including malignant B-cells
involved in the disease. Radiation-carrying antibodies infused into a patient circulate
throughout the body, bind to specific cells, and deliver cytotoxic radiation directly to
cancerous cells. This treatment methodology may result in significant tumor shrinkage
and avoidance of larger full body treatment doses of radiation.
The radioimmunotherapy regimen is administered in two separate steps. The first step is
diagnostic to determine the radiopharmaceutical biodistribution of radiolabeled
antibodies. The second step is the therapeutic administration of targeted radiolabeled
antibodies. The published criteria for determining appropriate biodistribution involve
making a qualitative comparison of isotope uptake in several organ systems between at
least two nuclear medicine scans. Therefore, these scans cannot be read in isolation and
should be reported once regardless of the number of scans performed during the
treatment regimen. Rituximab® and its infusion prior to the administration of Zevalin®
01/31/2007 Radioimmunotherapy
587
Medical Policy Manual
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
and the infusion of tositumomab prior to the administration of Bexxar® are separately
reimbursable.
Currently radioimmunotherapy is not a procedure typically performed more than once.
Codes specific to the radioimmunotherapy procedure are limited to one unit per lifetime.
The IHCP will re-examine the issue if future research determines that the coverage and
reimbursement of additional radioimmunotherapy services would be appropriate.
PRIOR AUTHORIZATION
Radioimmunotherapy services do not require prior authorization (PA).
MANAGED CARE
For PrimeStep, the same policies apply as those applicable to the Traditional Fee-For-
Service (FFS) program. For Risk-Based Managed Care (RBMC) specific policies, please
consult the appropriate Managed Care Organization (MCO).
BILLING REQUIREMENTS
Billing for Outpatient Facility Setting – Effective January 1, 2004
Table 1.1 provides information regarding billing radioimmunotherapy services provided
in an outpatient facility utilizing Zevalin® or Bexxar® for dates of service on or after
January 1, 2004. Billing instructions changed in 2004, and the radiopharmaceutical is
separately reimbursable from the nuclear medicine scanning procedure. Specific codes
were developed to represent the diagnostic and therapeutic supply of Bexxar® and the
infusion and supply of tositumomab in the Bexxar® regimen. New codes are used to
report the scanning procedure, and HCPCS codes G0273 and G0274 are not reimbursable
on or after January 1, 2004. HCPCS code G0273 has been replaced by CPT code 78804
for the diagnostic portion, and HCPCS code G0274 has been replaced by CPT code
79403 for the therapeutic portion of the scanning procedure. The outpatient facility will
bill the technical component of the procedure using CPT code 78804 or 79403, the
radiopharmaceutical using HCPCS code C1080, C1081, C1082, or C1083, and the
appropriate revenue code on the UB-92 claim form or 837I transaction. Rituximab®
(J9310) and its infusion (Q0084), prior to the administration of Zevalin®, or the supply
and infusion of tositumomab (G3001), prior to the administration of Bexxar®, are
separately reimbursable to the facility. Effective January 1, 2005, providers may report
the appropriate code(s) for the infusion of Rituximab® - Q0084, 96410, 96414, 96422,
and 96425. The physician will bill the professional component with the code-modifier
combination 78804-26 or 79403-26 on a CMS-1500 claim form or 837P transaction.
01/31/2007 Radioimmunotherapy
588
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
*Limited to one unit per lifetime
Table 1.1 - Zevalin® and Bexxar® Therapy
Provided in an Outpatient Facility (effective 1/1/04)
Code Revenue Code Description
78804*
(outpatient
facility)
341
Radiopharmaceutical localization of tumor or
distribution of radiopharmaceutical agent(s);
whole body, requiring two or more days imaging
79403*
(outpatient
facility)
340, 342
Radiopharmaceutical therapy, radiolabeled
monoclonal antibody by intravenous infusion
78804-26*
(physician)
N/A
Radiopharmaceutical localization of tumor or
distribution of radiopharmaceutical agent(s);
whole body, requiring two or more days imaging
79403-26*
(physician)
N/A
Radiopharmaceutical therapy, radiolabeled
monoclonal antibody by intravenous infusion
C1080*
(Bexxar®)
or
C1082*
(Zevalin®)
343, 636
Supply of radiopharmaceutical diagnostic
imaging agent, I-131 tositumomab, per dose
Supply of radiopharmaceutical diagnostic
imaging agent, indium-111 ibritumomab
tiuxetan, per dose
C1081*
(Bexxar®)
or
C1083*
(Zevalin®)
343, 344, 636
344, 636
Supply of radiopharmaceutical therapeutic
imaging agent, I-131 tositumomab, per dose
Supply of radiopharmaceutical therapeutic
imaging agent, yttrium 90 ibritumomab tiuxetan,
per dose
01/31/2007 Radioimmunotherapy
589
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 1.1 - Zevalin® and Bexxar® Therap
y
Provided in an Outpatient
Facility (effective 1/1/04) continued
Code Revenue Code Description
J9310
and as
appropriate
Q0084
96410
(as of 1/1/05)
96414
(as of 1/1/05)
96422
(as of 1/1/05)
96425
(as of 1/1/05)
(Zevalin®
regimen)
636
335
335
335
335
335
Rituximab, 100 mg
Chemotherapy administration by infusion
technique only, per visit
Chemotherapy administration, intravenous;
infusion technique, up to one hour
; infusion technique, initiation of prolonged
infusion (more than 8 hours), requiring the
use of a portable or implantable pump
Chemotherapy administration, intra-arterial;
infusion technique, up to one hour
; infusion technique, initiation of prolonged
infusion (more than 8 hours), requiring the
use of a portable or implantable pump
G3001
(Bexxar®
regimen )
333, 34x
Administration and supply of tositumomab,
450 mg
Billing for Physician Office Setting – Effective January 1, 2004
Table 1.2 provides information regarding billing radioimmunotherapy services provided
in a physician office setting utilizing Zevalin® or Bexxar® for dates of service on or after
January 1, 2004. Billing instructions have changed for 2004, and new codes are used to
report the radiopharmaceutical and nuclear medicine scanning procedures. HCPCS code
G0273 has been replaced by CPT code 78804 for the diagnostic portion, and HCPCS
code G0274 has been replaced by CPT code 79403 for the therapeutic portion of the
scanning procedure. HCPCS code A9522 has been replaced by code C1082 for the
supply of the diagnostic imaging agent, and HCPCS code A9523 has been replaced by
HCPCS code C1083 for the supply of the therapeutic imaging agent in the Zevalin®
regimen. Specific codes have also been developed to represent the diagnostic and
01/31/2007 Radioimmunotherapy
590
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
therapeutic supply of Bexxar® and the infusion and supply of tositumomab in the
Bexxar® regimen. The physician office provider will bill the global scanning procedure
with CPT code 78804 or 79403 and the radiopharmaceutical with HCPCS code C1080,
C1081, C1082, or C1083 on a CMS-1500 claim form or 837P transaction. Rituximab®
(J9310) and its infusion (Q0084) prior to the administration of Zevalin® and the supply
and infusion of tositumomab (G3001) prior to the administration of Bexxar® are
separately reimbursable. Effective January 1, 2005, providers may report the appropriate
code(s) for the infusion of Rituximab® - Q0084, 96410, 96414, 96422, and 96425.
Table 1.2 - Zevalin® and Bexxar® Therapy Provided in a
Physician Office (effective 1/1/04)
Code Description
78804*
Radiopharmaceutical localization of tumor or distribution
of radiopharmaceutical agent(s); whole body, requiring
two or more days imaging
79403*
Radiopharmaceutical therapy, radiolabeled monoclonal
antibody by intravenous infusion
C1080*
(Bexxar®)
or
C1082*
(Zevalin®)
Supply of radiopharmaceutical diagnostic imaging agent,
I-131 tositumomab, per dose
Supply of radiopharmaceutical diagnostic imaging agent,
indium-111 ibritumomab tiuxetan, per dose
C1081*
(Bexxar®)
or
C1083*
(Zevalin®)
Supply of radiopharmaceutical therapeutic imaging agent,
I-131 tositumomab, per dose
Supply of radiopharmaceutical therapeutic imaging agent,
yttrium 90 ibritumomab tiuxetan, per dose
*Limited to one unit per lifetime
01/31/2007 Radioimmunotherapy
591
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 1.2 - Zevalin® and Bexxar® Therapy
Provided in a Physician Office (effective 1/1/04) continued
Code Description
J9310
and as
appropriate
Q0084
96410
(as of 1/1/05)
96414
(as of 1/1/05)
96422
(as of 1/1/05)
96425
(as of 1/1/05)
(Zevalin®
regimen)
Rituximab, 100 mg
Chemotherapy administration by infusion technique only,
per visit
Chemotherapy administration, intravenous; infusion
technique, up to one hour
; infusion technique, initiation of prolonged infusion
(more than 8 hours), requiring the use of a portable or
implantable pump
Chemotherapy administration, intra-arterial; infusion
technique, up to one hour
; infusion technique, initiation of prolonged infusion
(more than 8 hours), requiring the use of a portable or
implantable pump
G3001
(Bexxar®
regimen)
Administration and supply of tositumomab, 450 mg
Billing for Outpatient Facility Setting – May 1, 2003 through December 31, 2003
Table 2.1 provides information regarding billing for outpatient facility treatment utilizing
Zevalin® for dates of service May 1, 2003 through December 31, 2003. The outpatient
facility will bill HCPCS code G0273 for the diagnostic portion or HCPCS code G0274
for the therapeutic portion of the procedure with revenue code 333 on a UB-92 claim
form or 837I transaction. Reimbursement for the radiopharmaceutical is included in the
reimbursement for the procedure when performed by an outpatient facility. Rituxumab®
(J9310) and its infusion (Q0084) prior to the administration of Zevalin® are separately
reimbursable to the facility. The physician will bill the professional component with
HCPCS code G0273-26 or G0274-26 on a CMS-1500 claim form or 837P transaction.
01/31/2007 Radioimmunotherapy
592
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 2.1- Zevalin® Therapy Provided in an Outpatient
Facility (effective 5/1/2003-12/31/2003)
Code Revenue Code Description
G0273*
(outpatient
facility)
333
Radiopharmaceutical biodistribution, single or
multiple scans on one or more days, pre-treatment
planning for radiopharmaceutical therapy of non-
hodgkin’s lymphoma, includes administration of
radiopharmaceutical (e.g., radiolabeled antibodies)
G0274*
(outpatient
facility)
333
Radiopharmaceutical therapy, non-hodgkin’s
lymphoma, includes administration of
radiopharmaceutical (e.g., radiolabeled antibodies)
G0273-26*
(physician)
N/A
Radiopharmaceutical biodistribution, single or
multiple scans on one or more days, pre-treatment
planning for radiopharmaceutical therapy of non-
hodgkin’s lymphoma, includes administration of
radiopharmaceutical (e.g., radiolabeled antibodies)
G0274-26*
(physician)
N/A
Radiopharmaceutical therapy, non-hodgkin’s
lymphoma, includes administration of
radiopharmaceutical (e.g., radiolabeled antibodies)
J9310
and
Q0084
636
335
Rituximab, 100 mg
Chemotherapy administration by infusion
technique only, per visit
*Limited to one unit per lifetime
01/31/2007 Radioimmunotherapy
593
Medical Policy Manual
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Billing for Physician Office Setting - May 1, 2003 through December 31, 2003
Table 2.2 provides information regarding billing for radiopharmaceutical therapy services
performed in the physician office setting utilizing Zevalin® for dates of service May 1,
2003 through December 31, 2003. The radiopharmaceutical is separately reimbursable
from the procedure when performed in a physician office setting. The supply of the
radiopharmaceutical, HCPCS codes A9522 and A9523, is to be reported once during the
treatment regimen, regardless of the dosage administered, as reimbursement is based on
the supply required for the procedure and not the per millicurie rate. The physician office
provider will bill HCPCS codes G0273 and A9522 for the diagnostic portion or HCPCS
codes G0274 and A9523 for the therapeutic portion on a CMS-1500 claim form or 837P
transaction. Rituxumab® (J9310) and its infusion (Q0084) prior to the administration of
Zevalin® are separately reimbursable.
*Limited to one unit per lifetime
Table 2.2- Zevalin® Therapy Provided in a Physician Office
(effective 5/1/2003-12/31/2003)
Code Description
G0273*
Radiopharmaceutical biodistribution, single or multiple
scans on one or more days, pre-treatment planning for
radiopharmaceutical therapy of non-hodgkin’s lymphoma,
includes administration of radiopharmaceutical (e.g.,
radiolabeled antibodies)
G0274*
Radiopharmaceutical therapy, non-hodgkin’s lymphoma,
includes administration of radiopharmaceutical (e.g.,
radiolabeled antibodies)
A9522*
Supply of radiopharmaceutical diagnostic imaging agent,
indium-111 ibritumomab tiuxetan, per mci
A9523*
Supply of radiopharmaceutical therapeutic imaging agent,
yttrium 90 ibritumomab tiuxetan, per mci
J9310
and
Q0084
Rituximab, 100 mg
Chemotherapy administration by infusion technique only,
per visit
01/31/2007 Radioimmunotherapy
594
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Radioimmunotherapy
Medical Policy Manual
595
RULES, CITATIONS, AND SOURCES
Indiana Health Coverage Programs Provider Newsletters
NL200408 Radioimmunotherapy billing for Zevalin®, effective May 1, 2003-
December 31, 2003
NL200411 Radioimmunotherapy billing for Zevalin® and Bexxar®, effective
January 1, 2004
NL200505 Revisions to Radioimmunotherapy Services
Indiana Health Coverage Programs Provider Banners
BR200446 Pricing for CPT code 78804
BR200513 Addition of revenue codes 343 and 344, and new codes for
the administration of Rituximab®
Origination date 1/31/05
Revisions and Reviews Reason Date
Revision
Changes in Billing
Requirements
7/31/05
APPLICABLE INDIANAAIM EDITS AND AUDITS
6140 Radioimmunotherapy service (78804) is limited to one unit per lifetime
6141 Radioimmunotherapy service (79403) is limited to one unit per lifetime
6142 Radioimmunotherapy service (C1080) is limited to one unit per lifetime
6143 Radioimmunotherapy service (C1081) is limited to one unit per lifetime
6144 Radioimmunotherapy service (C1082) is limited to one unit per lifetime
6145 Radioimmunotherapy service (C1083) is limited to one unit per lifetime
6146* Radioimmunotherapy service (A9523) is limited to one unit per 14 days
6147* Radioimmunotherapy service (G0274) is limited to one unit per 14 days
6148* Radioimmunotherapy service (G0273) is limited to one unit per 14 days
6149* Radioimmunotherapy service (A9522) is limited to one unit per 14 days
*Audits 6146, 6147, 6148, and 6149 are effective 2003/05/01 and end dated 2003/12/31.
The audits were established in IndianaAIM for one unit per fourteen days to correspond to
the timeframe of the radioimmunotherapy regimen; however provider publications state
one unit per lifetime.

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: RADIOLOGY
DESCRIPTION:
Radiology is the branch of medicine concerned with the diagnosis and treatment of
disease by using ionizing radiation, radionuclides, nuclear magnetic resonance, and
ultrasound.
SUMMARY OF CURRENT POLICY
Medicaid reimbursement is available to radiology inpatient and outpatient facilities,
freestanding clinics, and surgical centers for services provided to IHCP members.
Radiological services must be ordered in writing by a physician or other practitioner
authorized to do so under state law. The Indiana Administrative Code 405 IAC 5-27-1
allows for prior authorization (PA) of radiological services that exceed utilization
parameters set forth by the IHCP; however, there are no PA restrictions in place.
RELATED MEDICAL TOPICS
Diagnostic Studies
Laboratory Services
Oncology
Physician Services
CT scans
Radionuclide Bone Scans
Hospice
01/31/2007 Radiology
596
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
RULES, CITATIONS, AND SOURCES
405 IAC 5-27 Radiology Services
405 IAC 5-8-2 "Consultation" defined
405 IAC 5-12-3 Chiropractic x-ray services
405 IAC 5-26-4 Laboratory or x-ray services [Podiatric Services]
Indiana Medical Assistance Program Provider Manual 1994
Indiana State Medicaid Plan--Other Laboratory and X-ray Services
Indiana State Department of Public Welfare Medical Policy Manual 1991
Indiana Health Coverage Programs Provider Manual 1999
IHCP Bulletin BT199925
IHCP Bulletin BT200127
IHCP Bulletin BT200144
Origination Date: 7/1/91
Reviews and Revisions Reason Date
405 IAC 5-9-21,
Transferred to
470 IAC 5-9-21
Radiology Services 7/1/91
470 IAC 5-8-16, 5-9-13 Podiatric Services 7/1/91
IHCP Provider Manual
1991
Laboratory and X-Ray Services,
Radiology Services, and Radiation
Therapy
7/1/91
470 IAC 5-8-9 Hospital Services 7/1/91
405 IAC 5-27 Radiology Services Revision 8/24/97
405 IAC 5-26-4 Podiatric Servies Revision 8/24/97
405 IAC 5-12-3 Chiropractic Services Revision 8/24/97
IHCP Provider Manual
2004
Radiology Services Revision
(chiropractic services removed)
10/29/04
APPLICABLE INDIANAAIM EDITS AND AUDITS:
6001 – Complete Radiology And Pathology Procedures Payable At Reduced Amount
When Profesional/Technical Components Already Paid
6011 – Professional/Technical Components Not Payable When Complete Procedure
Already Paid
6110 – Component Procedures Not Payable When Global Procedure Paid - Radiology
Services
01/31/2007 Radiology
597
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
COVERAGE CRITERIA
Provider Type and Specialty Type
Table 1 lists the provider types and specialties that are authorized to bill radiology
services to the IHCP. Radiology clinics can be enrolled as a billing provider or as a
group with members. A radiology group with rendering members are enrolled with
provider type 31 – Physician, with provider speciality 341 (radiologist). Radiology clinics
can either be enrolled as a freestanding clinic or a mobile X-ray clinic.
Table 1
Provider Type Provider Specialty
29 Radiology Provider
290 Freestanding x-ray clinic
291 Mobile x-ray clinic
31 Physician 341 Radiologist
Radiology providers are required to submit a copy of their Registration Certificate, ISDH
Notice of Compliance, and operator certificates for all employee operators except PET
CT scanner operators. Please note that PET and MRI services do not require certification
or a Notice of Compliance.
Out-of-state mobile radiology providers performing services in Indiana must be certified
in Indiana and possess a Notice of Compliance in Indiana. All operators must be certified
in the state of Indiana.
Coverage Limitations
Reimbursement is available to radiology inpatient and outpatient facilities, freestanding
clinics, and surgical centers for services provided to members subject to the following
limitations.
♦ A radiological service must be ordered in writing by a physician or other practitioner
authorized to do so under state law.
♦ The radiological service facility must bill the IHCP directly for components
provided by the facility. When two practitioners each provide a portion of the
radiology service, each practitioner may bill the IHCP separately for the
component provided. The IHCP reimburses a physician or other practitioner for
radiological services only when such services are performed by or under direct
supervision of the physician or practitioner.
♦ A physician is reimbursed for the professional component of a radiological
service by billing the appropriate CPT code along with Modifier 26, Professional
component. When billing only the technical component, Modifier TC, Technical
01/31/2007 Radiology
598
Medical Policy Manual
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
component, must be used with the appropriate CPT code. When billing for both
professional and technical components of service, modifiers should not be used.
CPT codes billed using these modifiers are listed in the Federal Register under
RVUs and related information.
♦ Radiology procedures cannot be unbundled and billed separately. Such
circumstances may include, but are not limited to the following:
• CPT codes for supervision and interpretation procedures are not
reimbursed when the same provider bills for the complete procedure
CPT code.
• If two provider specialties are performing a radiology procedure, the
radiologist bills for the supervision and interpretation procedure and
the second physician bills the appropriate injection, aspiration, or biopsy
procedure.
• Angiographic procedures, when performed as an integral component of a
surgical procedure by the operating physician, are not reimbursed
separately. Such procedures include, but are not limited to the following:
Angiographic injection procedures during coronary artery bypass
graft
Peripheral, percutaneous transluminal angioplasty procedures
Radiology services are available to PCCM and RBMC members on a self-referral basis.
RBMC member claims should be submitted to the member’s MCO for payment. Services
that require PA furnished to members enrolled in RBMC must be prior authorized by the
member’s MCO in accordance with the MCO’s guidelines.
Utilization Criteria for General Radiological Services
Criteria for the use of radiological services include consideration of the following.
♦ Evidence that this radiologic procedure is necessary for the appropriate treatment
of illness or injury.
♦ X-rays of the spinal column limited to cases of acute documented injury or a
medical condition in which interpretation of X-rays would make a direct impact
on the medical/surgical treatments.
♦ Reimbursement is available for X-rays of the extremities and spine for the study
of neuromusculoskeletal conditions.
♦ Reimbursement is not available for radiology examinations of any body part taken
as a routine study not necessary for the diagnosis or treatment of a medical
condition. Situations generally not needing radiology services include, but are not
limited to the following:
01/31/2007 Radiology
599
Medical Policy Manual
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• Fluoroscopy without films
• Pregnancy
• Premarital examinations
• Research studies
• Routine physical examinations or check-ups
• Screening, pre-operative chest X-ray
Radiation Treatment Services Revenue Codes
Radiation Treatment Services consist of two components which are separately
reimbursable using the following code combinations.
♦ Administration of radiation treatment
• Bill using revenue codes 330, 333, or 339 along with the appropriate CPT
radiation treatment code, 77261 through 77799.
♦ Treatment room services
• Bill using revenue codes 45X, 51X, 52X, or 76X.
When chemotherapy and radiation treatment services are rendered on the same day, all
applicable components are billed to the IHCP.
Computerized Tomography Scans
Reimbursement may be available for diagnostic examination of the head and of other
parts of the body, head scans, and body scans, performed by computerized tomography
(CT) scanners, subject to the following restrictions:
♦ The scan should be reasonable and necessary for the individual patient.
♦ The use of a CT scan must be found to be medically appropriate considering the
patient’s symptoms and preliminary diagnosis.
♦ Reimbursement is made only for CT scans performed with equipment certified
by the FDA.
♦ Whole abdomen, or whole pelvis scans on greater than 20 cuts is not
reimbursed, except in staging cancer for treatment evaluation.
♦ PA is not required for CT scans.
01/31/2007 Radiology
600
Medical Policy Manual
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Radionuclide Bone Scans
Reimbursement is available for radionuclide bone scans when performed for the
detection and evaluation of suspected or documented bone disease.
Gastrointestinal Studies
Reimbursement is available for upper gastrointestinal (GI) studies when performed for
detection and evaluation of diseases of the esophagus, stomach, and duodenum. An upper
GI study is not a covered service for a patient with a history of duodenal or gastric ulcer
disease unless recently symptomatic. An upper GI study is not a covered service in the
preoperative cholecystectomy patient unless symptoms indicate an upper GI abnormality
in addition to cholelithiasis, or if the etiology of the abdominal pain is uncertain.
X-ray Services While in Hospice
Costs for services such as X-rays and laboratory are not included on the attending
physician’s billed charges. These costs are included in the daily hospice care rates paid
and are expressly the responsibility of the hospice provider. However, if the Medicaid
hospice member requires radiological services not related to the terminal illness, the
hospice provider is not responsible for these radiological services. IHCP will allow for
separate reimbursement of the non-hospice related radiological treatment in these
circumstances. The Medicaid provider billing for the treatment of the non-terminal
condition is reminded that they are responsible for obtaining Medicaid prior authorization
for any non-hospice services that are subject to prior authorization.
01/31/2007 Radiology
601
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Radiology
Medical Policy Manual
602
RADIOLOGY FACT SHEET
ADDENDUM A
Note: This addendum contains provider notifications that have been published since the
review of the Radiology Fact Sheet. The information in this addendum will be
incorporated into the fact sheet at the next review.
Provider Notification: BR200509 Publication Date: 03/01/2005
Subject: Transportation of Portable EKG or X-ray Machine
Date Added to Manual: 04/29/2005
Text of Publication
As of April 15, 2005, HCPCS code R0076 - Transportation of portable EKG to facility
or location, per patient, is a non-covered service for the IHCP. Providers will no longer
be reimbursed for this service. HCPCS code R0070 -Transportation of portable x-ray
equipment and personnel to home or nursing home, per trip to facility or location, one
patient seen, is reported for one patient served. HCPCS code R0070 must not be reported
with modifiers UN, UP, UQ, UR, or US. One unit must be reported for the trip. When
more than one patient is served, providers should report HCPCS code R0075 -
Transportation of portable x-ray equipment and personnel to home or nursing home, per
trip to facility or location, more than one patient seen, with the appropriate modifier
representing the number of patients served. One unit must be reported for the trip. The
service must be reported on each member’s claim with the appropriate modifier.
Reimbursement will be prorated according to how many patients are served, as
represented by modifiers UN, UP, UQ, UR, and US. The table below lists the percentage
of the fee schedule amount that each modifier will reimburse when reported with HCPCS
code R0075.
Modifier Description Fee Schedule
Percentage
UN Two patients served 50%
UP Three patients served 33%
UQ Four patients served 25%
UR Five patients served 20%
US Six or more patients served 16%

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
RADIOLOGY SERVICES
ADDENDUM B
Note: This addendum contains provider notifications that have been published since the
review of the Radiology Services Fact Sheet. The information in this addendum will be
incorporated into the fact sheet at the next review.
Provider Notification: BR200544 Publication Date: 11/01/2005
Subject: Stereotactic Radiosurgery
Date Added to Manual: 01/31/2006
Text of Publication
The Indiana Health Coverage Programs (IHCP) currently covers three types of
stereotactic radiosurgery (SRS) as represented by Healthcare Common Procedure Coding
System (HCPCS) codes G0173, G0242, and G0251. In addition, the IHCP covers pre-
operative planning under HCPCS code G0243 or G0338. Reimbursement for physician
services is bundled into the pre-operative planning service.
Currently, all SRS procedures are manually priced by the IHCP. In order to more closely
align IHCP pricing with Medicare, the IHCP will amend pricing for SRS procedures as
reflected in Table 1 below, effective December 15, 2005. Providers must bill SRS
therapy and pre-operative planning with revenue code 333 on the UB-92 claim form or
837I transaction.
Table 1 – New IHCP SRS Reimbursement Rates, Effective December 15, 2005
Code Description Reimbursement
G0173
Linear accelerator based stereotactic radiosurgery, complete
course of therapy in one session
$5,250
G0242
Multi-source photon stereotactic radiosurgery (cobalt 60
multi-source converging beams) plan, including dose volume
histograms for target and critical structure tolerances, plan
optimization performed for highly conformal distributions,
plan positional accuracy and dose verification, all lesions
treated, per course of treatment
$1,450
G0243
Multi-source photon stereotactic radiosurgery, delivery
including collimator changes and custom plugging, complete
course of treatment, all lesions
$5,250
G0251
Linear accelerator based stereotactic radiosurgery, delivery
including collimator changes and custom plugging,
fractionated treatment, all lesions, per session, maximum five
sessions per course of treatment
$1,150
G0338 LINAC based stereotactic radiosurgery plan, including dose $1,450
01/31/2007 Radiology
603
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Radiology
604
Code Description Reimbursement
volume histograms for target and critical structure tolerances,
plan optimization performed for highly conformal
distributions, plan positional accuracy and dose verification,
all lesions treated, per course of treatment
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
RADIOLOGY SERVICES
ADDENDUM C
Note: This addendum contains provider notifications that have been published since the
review of the Radiology Services Fact Sheet. The information in this addendum will be
incorporated into the fact sheet at the next review.
Provider Notification: BR200606 Publication Date: 02/07/2006
Subject: Changes to Billing Requirements for Stereotactic Radiosurgery
Date Added to Manual: 04/28/2006
Text of Publication
The purpose of this article is to advise IHCP providers of changes to billing requirements
for physician’s services for stereotactic radiosurgery (SRS). IHCP bulletin BT200528
advises providers to report the physician’s professional services that had previously been
billed with HCPCS codes G0242 and G0338, with Common Procedural Terminology
(CPT
©
) code 77301; however, services previously billed with these two codes should be
billed with procedure identification code 77301 U5. The HCPCS codes listed in Table 2
are end-dated December 31, 2005. Effective for dates of service on and after January 1,
2006, providers are advised to bill the SRS services as reflected in Table 3. All other
billing requirements for SRS therapy remain unchanged.
Table 1 – HCPCS Codes Non-Covered by Medicare, Effective
February 7, 2006
92630 92633 A6530 A6533 A6534 A6535 A6536
A6537 A6538 A6539 A6540 A6541 A6542 A6543
A6544 A6549 E0172 E0641 J7306 S2078 S2079
Table 2 – SRS HCPCS Codes End-Dated December 31, 2005
Code Description
G0242
Multi-source photon stereotactic radiosurgery (cobalt 60 multi-source
converging beams) plan, including dose volume histograms for target and
critical structure tolerances, plan optimization performed for highly conformal
distributions, plan positional accuracy and dose verification, all lesions treated,
per course of treatment
G0338
LINAC-based stereotactic radiosurgery plan, including dose volume histograms
for target and critical structure tolerances, plan optimization performed for
highly conformal distributions, plan positional accuracy and dose verification;
all lesions treated, per course of treatment
01/31/2007 Radiology
605
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 3 – SRS CPT Codes Effective January 1, 2006
Code Description Reimbursement
77301
Intensity modulated radiotherapy plan, including dose-
volume histograms for target and critical structure partial
tolerance specifications
$1,014.63
77301
U5
Intensity modulated radiotherapy plan, including dose-
volume histograms for target and critical structure partial
tolerance specifications; multi-source photon or linear
accelerator based stereotactic radiosurgery plan
optimization for highly conformal distributions, plan
positional accuracy and dose verification, all lesions
treated, per course of treatment
$1,450.00
01/31/2007 Radiology
606
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Radiology
Medical Policy Manual
607
RADIOLOGY SERV ICES
ADDENDUM D
Note: This addendum contains provider notifications that have been published since the
review of the Radiology Services Fact Sheet. The information in this addendum will be
incorporated into the fact sheet at the next review.
Provider Notification: BR200639 Publication Date: 09/26/2006
Subject: Proton Treatment Billing
Date Added to Manual: 10/31/2006
Text of Publication
The purpose of this article is to inform providers that the Indiana Health Coverage
Programs (IHCP) has determined that it is appropriate for providers to use the Current
Procedural Terminology (CPT®) codes listed in Table 1 to report the technical
component only of the CPT codes noted in Table 1 for reporting proton treatment
delivery. Therefore, effective for dates of service on or after December 1, 2006, the IHCP
will not reimburse providers services reported using the CPT codes listed in Table 1 and
billed with modifiers 26 – Professional component, and TC – Technical component.
Providers are advised to bill CPT codes 77520, 77522, and 77525 for the technical
component only. Additionally, providers are advised to report the professional services
using an appropriate CPT procedure code.
Table 1 – CPT Codes Reporting Proton Treatment Delivery
CPT Code Description
77520 Proton treatment; simple, without compensation
77522 Proton treatment; simple, with compensation
77525 Proton treatment delivery; complex

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE RADIOLOGY - POSITRON EMISSION TOMOGRAPHY
(PET) SCANS
DESCRIPTION
Positron Emission Tomography (PET) is a noninvasive diagnostic imaging procedure that
assesses the level of metabolic activity and perfusion in various organ systems of the human
body. A positron camera (tomograph) is used to produce cross-sectional tomographic
images, which are obtained from positron emitting, radioactive tracer substances
(radiopharmaceuticals) that are administered intravenously to the patient.
COVERAGE CRITERIA
The Indiana Health Coverage Programs (IHCP) reimbursement policy currently uses the
same criteria and coding methodology as Medicare. Providers must bill ICD-9-CM
codes supporting medical necessity and the appropriate HCPCS codes. Appropriate ICD-
9-CM diagnoses for billing purposes are included in TABLE 1 – ICD-9-CM CODES
SUPPORTING MEDICAL NECESSITY. Professional and technical components are
billable for PET scans.
TABLE 1 – ICD-9-CM CODES SUPPORTING MEDICAL NECESSITY
PET
SCAN
IMAGING
CPT CODE ICD-9-CM CODE
Breast
Cancer
78811, 78812,
78814, 78815
174.0, 174.1, 174.2, 174.3, 174.4, 174.5, 174.6
174.8, 147.9, 175.0, 175.9, 195.0, 195.1, 195.2,
195.3, 195.4, 195.5, 195.8, 196.0, 196.1, 196.2,
196.3, 196.5, 196.6, 196.8, 196.9, 197.0, 197.1,
197.2, 197.3, 197.4, 197.5, 197.6, 197.7, 197.8,
198.0, 198.1, 198.2, 198.3, 198.4, 198.5, 198.6,
198.7, 198.81, 198.82, 198.89, 199.0, 199.1
Myocardial
perfusion
imaging
78459, 78491,
78492
411.1, 411.81, 411.89, 412, 413.0, 413.1, 413.9,
414.8, 414.00, 414.01, 414.02, 414.03, 414.04,
414.05
01/31/2007 Radiology – PET Scans
608
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Radiology – PET Scans
609
TABLE 1 – ICD-9-CM CODES SUPPORTING MEDICAL NECESSITY
PET
SCAN
CPT CODE ICD-9-CM CODE
IMAGING
Refractory
seizures
78608, 78609
345.01, 345.11, 345.2, 345.3, 345.41, 345.51,
345.61, 345.71, 345.81, 345.91
Regional or
whole
body, for
single
pulmonary
nodule
78811, 78812,
78813, 78814,
78815, 78816
235.7, 239.1, 793.1, V71.1
Thyroid
Cancer
78811, 78812,
78814, 78815
193
Whole
body, for
colorectal
cancer
78813, 78816
153.0, 153.1, 153.2, 153.3, 153.4, 153.5, 153.6,
153.7, 153.8, 153.9, 154.0, 154.1, 154.2, 197.5,
V10.05, V10.06, V71.1
Whole
body, for
esophageal
cancer
78813, 78816
150.0, 150.1, 150.2, 150.3, 150.4, 150.5, 150.8,
150.9, V10.03, V71.1
Whole
body, for
melanoma
78813, 78816
172.0, 172.1, 172.2, 172.3, 172.4, 172.5, 172.6,
172.7, 172.8, 172.9, V10.82, V71.1
Whole
body, for
non-small
cell lung
carcinoma
78813, 78816
162.2, 162.3, 162.4, 162.5, 162.8, 162.9, 196.1,
V10.11, V71.1
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Radiology – PET Scans
610
TABLE 1 – ICD-9-CM CODES SUPPORTING MEDICAL NECESSITY
PET
SCAN
CPT CODE ICD-9-CM CODE
IMAGING
Whole
body, for
lymphoma
78813, 78816
200.00, 200.01, 200.02, 200.03, 200.04, 200.05,
200.06, 200.07, 200.08, 200.10, 200.11, 200.12,
200.13, 200.14, 200.15, 200.16, 200.17, 200.18,
200.20, 200.21, 200.22, 200.23, 200.24, 200.25,
200.26, 200.27, 200.28, 200.80, 200.81, 200.82,
200.83, 200.84, 200.85, 200.86, 200.87, 200.88,
201.00, 201.01, 201.02, 201.03, 201.04, 201.05,
201.06, 201.07, 201.08, 201.10, 201.11, 201.12,
201.13, 201.14, 201.15, 201.16, 201.17, 201.18,
201.20, 201.21, 201.22, 201.23, 201.24, 201.25,
201.26, 201.27, 201.28, 201.40, 201.41, 201.42,
201.43, 201.44, 201.45, 201.46, 201.47, 201.48,
201.50, 201.51, 201.52, 201.53, 201.54, 201.55,
201.56, 201.57, 201.58, 201.60, 201.61, 201.62,
201.63, 201.64, 201.65, 201.66, 201.67, 201.68,
201.70, 201.71, 201.72, 201.73, 201.74, 201.75,
201.76, 201.77, 201.78, 201.90, 201.91, 201.92,
201.93, 201.94, 201.95, 201.96, 201.97, 201.98,
202.00, 202.01, 202.02, 202.03, 202.04, 202.05,
202.06, 202.07, 202.08, 202.10, 202.11, 202.12,
202.13, 202.14, 202.15, 202.16, 202.17, 202.18,
202.20, 202.21, 202.22, 202.23, 202.24, 202.25,
202.26, 202.27, 202.28, 202.30, 202.31, 202.32,
202.33, 202.34, 202.35, 202.36, 202.37, 202.38,
202.40, 202.41, 202.42, 202.43, 202.44, 202.45,
202.46, 202.47, 202.48, 202.50, 202.51, 202.52,
202.53, 202.54, 202.55, 202.56, 202.57, 202.58,
202.60, 202.61, 202.62, 202.63, 202.64, 202.65,
202.66, 202.67, 202.68, 202.80, 202.81, 202.82,
202.83, 202.84, 202.85, 202.86, 202.87, 202.88,
202.90, 202.91, 202.92, 202.93, 202.94, 202.95,
202.96, 202.97, 202.98, V10.71, V10.72, V10.79,
V71.1
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Radiology – PET Scans
611
TABLE 1 – ICD-9-CM CODES SUPPORTING MEDICAL NECESSITY
PET
SCAN
CPT CODE ICD-9-CM CODE
IMAGING
Whole
body, or
regional,
for head
and neck
cancer
78811, 78812,
78813, 78815,
78816
140.0, 140.1, 140.3, 140.4, 140.5, 140.6, 140.8,
140.9, 141.0, 141.1, 141.2, 141.3, 141.4, 141.5,
141.6, 141.8, 141.9, 142.0, 142.1, 142.2, 142.8,
142.9, 143.0, 143.1, 143.8, 143.9, 144.0, 144.1,
144.8, 144.9, 145.0, 145.1, 145.2, 145.3, 145.4,
145.6, 145.8, 145.9, 146.0, 146.1, 146.2, 146.3,
146.4, 146.5, 146.6, 146.7, 146.8, 146.9, 147.0,
147.1, 147.2, 147.3, 147.8, 147.9, 148.0, 148.1,
148.2, 148.3, 148.8, 148.9, 149.0, 149.1, 149.8,
149.9, 160.0, 160.1, 160.2, 160.3, 160.4, 160.5,
160.8, 160.9, 161.0, 161.1, 161.2, 161.3, 161.8,
161.9, 162.0, 170.0, 170.1, 171.0, 173.0, 173.1,
173.2, 173.3, 173.4, 190.0, 190.1, 190.2, 190.3,
190.4, 190.5, 190.6, 190.7, 190.8, 190.9, 194.1,
194.3, 194.4, 194.5, 195.0, V10.01, V10.02, V10.12,
V10.21, V10.22, V10.81, V10.83, V10.84, V10.89,
V71.1
Prior Authorization Requirements
The IHCP does not require prior authorization for PET scans.
Managed Care
PET scan services are reimbursed and billed the same for Fee-for-Service and PrimeStep.
Radiology services do not require a PMP’s two digit certification code for payment.
However, the eight-digit PMP license number continues to be required for claim
reimbursement. For more information, see provider bulletin BT200262 or the IHCP
Provider Manual.
For Risk Based Managed Care (RBMC), providers are to contact the individual MCO(s)
to determine coverage, billing, and authorization requirements for PET scans.
Billing for PET Scans
If the member is an inpatient, the PET scan will be covered in the diagnosis-related
grouping (DRG) payment to the hospital. The CPT codes for PET scans represent the
global service. The provider performing just one component of the test should report the
component performed.
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
If the member has services performed in the outpatient area of the hospital or a
freestanding facility, the PET scan billing should be completed as follows.
IHCP requires that providers bill specific ICD-9-CM diagnosis codes (Table 1) and
the appropriate CPT codes when submitting claims. Claims for PET scans that do
not include the appropriate ICD-9-CM diagnosis code will be denied.
A radiologist should bill the professional services with the appropriate CPT codes and
the 26 modifier on the CMS-1500 claim form or 837P electronic transaction.
A facility should bill the technical component with the appropriate CPT Codes on the
UB-92 claim form.
RELATED MEDICAL TOPICS
Radiology
Oncology
Oncology-Breast and Cervical Cancer
RULES, CITATIONS, AND SOURCES
Hoosier Healthwise Manual for Primary Medical Providers and Office Staff, January
2003
Indiana Administrative Code – 405 IAC 5-27-1
Indiana Health Coverage Programs Provider Bulletins
BT200339 – Criteria and Billing Information
BT200262 – PrimeStep PMP Certification Code Changes
BT200516 – PET Scan Changes
Indiana Health Coverage Programs Provider Banner BR200505 – ICD-9-CM Diagnosis
Code Restriction
Indiana Health Coverage Programs –Provider Manual, Version 5.0, July, 2004
Origination Date: 10/30/03
Revisions and
Reviews
Reason Date
405-IAC-5-27-1 Radiology, Reimbursement Limitations 7/25/97
BT200339 Coverage Criteria and Billing Information 6/9/2003
BT200505 ICD-9-CM Diagnosis Code Restriction 3/1/05
Revision Review Project Completion 4/29/05
Revision Change from HCPCS G codes to CPT Codes 7/29/05
01/31/2007 Radiology – PET Scans
612
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
APPLICABLE INDIANAAIM EDITS AND AUDITS
6001 – Complete procedure payable at reduced rate
6011 – Radiology/technical component for radiology or pathology
Table 19- X-ray
6137 – Pet Scan Procedures Limited to Diagnosis
6138 – Procedure Code G0296 Limited to Diagnosis Codes
6139 – Pet Scan Procedures Limited to Diagnosis
6160 – Pet Scan Procedures Limited to Diagnosis
6161 – Pet Scan Procedures Limited to Diagnosis Codes
6162 – Pet Scans Limited to Diagnosis and Procedures
6163 – Pet Scans Limited to Certain Procedures Codes
6164 – Pet Scans Limited to Diagnosis Codes
6165 – Pet Scans Limited to Diagnosis Codes
6166 – Pet Scans Limited to Diagnosis Codes
6167 – Pet Scans Limited to Diagnosis Codes
01/31/2007 Radiology – PET Scans
613
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Radiology – PET Scans
Medical Policy Manual
614
RADIOLOGY PET SCANS
ADDENDUM A
Note: This addendum contains provider notifications that have been published since the
review of the Radiology PET Scans Fact Sheet. The information in this addendum will
be incorporated into the fact sheet at the next review.
Provider Notification: BR200602 Publication Date: 01/10/2006
Subject: PET Scan Coding
Date Added to Manual: 01/31/2006
Text of Publication
The IHCP bulletin BT200516 provided billing guidelines for Positron Emission
Tomography (PET) scans. BT200516 advised providers to bill PET scans using an
appropriate Common Procedural Terminology (CPT®) code and an appropriate
International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM)
diagnosis code. Table 1 lists additional coding updates for PET scans. CPT codes 78811,
78812, and 78815 are added to PET scan imaging for non-small cell lung carcinoma.
CPT code 78815 is added to PET scan imaging for colorectal and esophageal cancer. All
other billing requirements remain unchanged.
Note: Reimbursement for PET scan services remains unchanged.
Reimbursement for the appropriate CPT code, billed with the
technical component (TC) and appropriate ICD-9-CM code, on a
UB-92 claim form, is $829.09. Reimbursement for professional
services, reported with the appropriate CPT code, modifier 26
(professional services) and the appropriate ICD-9-CM code, and
billed on a CMS-1500 or 837P electronic transaction, reimburses
from the resource-based relative value scale (RBRVS) fee schedule.
Table 1 – ICD-9-CM Codes Supporting Medical Necessity
PET Scan Imaging CPT Code ICD-9-CM Code
Whole body, for non-
small cell lung
carcinoma
78811, 78812,
78813, 78815,
78816
162.2, 162.3, 162.4, 162.5, 162.8, 162.9,
196.1, V10.11, V71.1
Whole body, for
colorectal cancer
78813, 78815,
78816
153.0, 153.1, 153.2, 153.3, 153.4, 153.5,
153.6, 153.7, 153.8, 153.9, 154.0, 154.1,
154.2, 197.5, V10.05, V10.06, V71.1
Whole body, for
esophageal cancer
78813, 78815,
78816
150.0, 150.1, 150.2, 150.3, 150.4, 150.5,
150.8, 150.9, V10.03, V71.1

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: SCREENING SERVICES—NEWBORN SCREENING
DESCRIPTION:
Screening Services are various methods and procedures used to determine disease or the
potential for developing an illness. Screening is usually done on a large number of
persons who are thought to be at risk for disease. To screen is defined as “to examine,
using physical and mental examinations and laboratory tests; to determine the presence of
certain diseases or characteristics.”
MEDICAL TOPICS CROSS-REFERENCES:
EPSDT – HealthWatch
A. HIV-AIDs Care Coordination
B. Laboratory Services
C. Obstetric Care
RULES, CITATIONS, AND SOURCES:
D. Indiana Code 16-41-6 Communicable Disease: Mandatory Testing of Individuals
with Communicable or Dangerous Diseases
E. Indiana Medicaid Update Bulletin 95-21 dated May 2, 1995
Indiana Health Coverage Programs Provider Manual 1999
01/31/2007 Screening Services – Newborn Screening
615
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Initial Policy Issue Effective
Date
Implementatio
n Date
Retroactive
Date
Indiana Medicaid
Update bulletin
95-21
Newborn Screening
5/2/95 5/1/95
410 IAC 3-3-3 Newborn Screening
Log
11/7/86
405 IAC 5-15 Early and Periodic
Screening,
Diagnostic, and
Treatment Services
8/24/97
IC16-41-17-2 Prevention and
Treatment
Programs:
Examination of
Infants for
Phenylketonuria,
Hypothyroidism,
and Other Disorders
1993
F. IC 16-41-6 Communicable
Disease: Mandatory
Testing of
Individuals with
Communicable or
Dangerous Diseases
1993;1996;
1998
Revisions:
405 IAC 3-2
Amended
Prior Authorization 10/27/99
405 IAC 3-3-3
Amended
Prior Authorization 10/27/99
410 IAC 3-3-3
Amended
Newborn Screening
Log
07/11/01
IC 16-41-17-2
Prevention and
Treatment
Programs:
Examination of
Infants for
Phenylketonuria,
Hypothyroidism,
and Other Disorders
1999;2001
APPLICABLE INDIANAAIM EDITS AND AUDITS:
6028
6702
01/31/2007 Screening Services – Newborn Screening
616
Medical Policy Manual
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
COVERAGE CRITERIA:
Medicaid reimbursement is available for services provided to eligible children under the
EPSDT program. This program is set out in 405 IAC 5-15.
Indiana Code (IC), IC 16-41-17-2 and Indiana Administrative Code (IAC), 410-3-3-3
provides information regarding the screening examinations that are required for all
newborn infants that are born in the state of Indiana.
♦ phenylketonuria (PKU)
♦ galactosemia
♦ hypothyroidism
♦ homocystinuria
♦ maple syrup urine disease
♦ hemoglobinopathies (which includes sickle cell anemia),
♦ congenital adrenal hyperplasia
♦ biotinidase deficiency
♦ disorders detected by tandem mass spectrometry or other technologies with
the same or greater detection capabilities as tandem mass spectrometry if the
state department determines that the technology is available for use by
designated laboratory under section 7 of this chapter (IC 16-41-17-2).
This is a required procedure for every infant not earlier than forty-eight hours
after birth and not before the infant has been on a protein diet for at least twenty-
four hours, and no later than one hundred twenty hours after birth. This usually
occurs before discharge from the hospital and the physician or midwife is
responsible for making the referral to an appropriate facility to make sure that the
blood specimen is obtained and submitted in accordance with the law. For
preterm infants the specimen may be taken on the day of discharge or on the sixth
day if the nursery stay is prolonged beyond six days. Medicaid pays for the
newborn hospitalization, and therefore, the screening is already included in the
DRG. Hospitals are not permitted to bill separately for newborn screening.
Every infant may receive a physiologic hearing screening examination at the earliest
feasible time for detection of hearing impairments.
If a parent of an infant objects in writing for reasons pertaining to religious beliefs only,
the infant is exempt from the examinations required by IC 16-41-17-2.
State law also provides that if a physician believes that testing the newborn infant is
medically necessary; the physician may order a confidential test for the newborn infant in
order to detect the human immunodeficiency virus (HIV) or the antibody or antigen to
HIV. The test must be ordered at the earliest feasible time not exceeding 48 hours after
the birth of the infant.
01/31/2007 Screening Services – Newborn Screening
617
Medical Policy Manual
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Screening Services – Newborn Screening
Medical Policy Manual
618
Newborn screening results must be recorded in the patient record for infants younger than
one year old.

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: SMOKING CESSATION
DESCRIPTION:
Smoking cessation refers to a course of treatment designed to assist individuals in
decreasing or stopping the use of tobacco products.
MEDICAL TOPICS CROSS-REFERENCES:
Pharmacy
RULES, CITATIONS, AND SOURCES:
Initial Policy Issues
Effective Date Implementation
Date
Retroactive Date
405 IAC 5-37 Smoking
cessation
treatment
policy
10/27/99
Revisions:
405 IAC 5-37
Smoking
cessation
treatment
policy
10/31/01
APPLICABLE INDIANAAIM EDITS AND AUDITS:
6270
1040—on hold
01/31/2007 Smoking Cessation
Medical Policy Manual
619
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
COVERAGE CRITERIA:
Coverage of Smoking Cessation Treatment services is for dates of service on or after
October 27, 1999.
Reimbursement for smoking cessation is available for one (1) twelve (12) week course of
treatment per member per calendar year.
Treatment may include prescription of any combination of smoking cessation products
and counseling. One (1) or more modalities of treatment may be prescribed. Counseling
must be included in any combination of treatment.
Prior authorization is not required for reimbursement for smoking cessation products or
counseling.
Hoosier Healthwise
Providers of smoking cessation treatment services must obtain the Primary Medical
Provider (PMP) certification for Hoosier Healthwise enrollees.
Smoking Cessation Products
Reimbursement is available to pharmacy providers for smoking cessation products when
prescribed by a licensed practitioner within the scope of his/her license under Indiana
law.
Only patients who agree to participate in smoking cessation counseling are to receive
prescriptions for smoking cessation products. The prescribing practitioner may want to
have the patient sign a commitment to establish a “quit date” and to participate in
counseling as the first step in smoking cessation treatment. A prescription for such
products will serve as documentation that the prescribing practitioner has prescribed or
obtained assurance from the patient that counseling will concomitantly occur with the
receipt of smoking cessation products.
Products covered by Indiana Medicaid include, but are not limited to, the following:
♦ Sustained release buproprion products.
♦ Nicotine replacement drug products (patch, gum, inhaler).
Smoking Cessation Counseling
Counseling services must be prescribed by a licensed practitioner within the scope of
his/her license under Indiana law. Reimbursement is available for smoking cessation
counseling services rendered by the following licensed practitioners participating in the
Indiana Medicaid Program.
01/31/2007 Smoking Cessation
Medical Policy Manual
620
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
♦ A physician
♦ A physician’s assistant
♦ A nurse practitioner
♦ A registered nurse
♦ A psychologist
♦ A pharmacist
♦ A dentist
Counseling must be provided as follows: A minimum of thirty (30) minutes [two (2)
units] and a maximum of one hundred fifty (150) minutes [ten (10) units] within the
twelve (12) weeks. Counseling will be billed in fifteen (15) minute increments.
BILLING INSTRUCTIONS AND REIMBURSEMENT
Counseling Services
Providers/practitioners of counseling services must bill only on the CMS 1500 claim
form (please see applicable provider manual section for full details), utilizing procedure
code Z5064 (smoking cessation treatment counseling) with a primary diagnosis code of
305.1 (Tobacco use disorder). As previously noted herein, one unit of Z5064 will be
considered as fifteen (15) minutes of service. Fractional units of service cannot be billed
on the CMS1500, so providers/practitioners should accumulate billable time equivalent to
whole units, before billing. Counseling must be provided within the twelve (12) week
course of treatment and must be a minimum of thirty (30) minutes [two (2) units] with a
maximum of one hundred fifty (150) minutes [ten (10) units].
The Indiana Medicaid maximum allowable rate for code Z5064, smoking cessation
treatment counseling services is $22.08 per unit, regardless of the type of practitioner
rendering the service. PLEASE NOTE: Providers/practitioners are to bill their “usual
and customary charge” for the units of service rendered, and Medicaid will calculate the
final reimbursement amount.
Practitioners eligible to provide smoking cessation treatment counseling services, but not
currently enrolled as an Indiana Medicaid provider, should contact the EDS Customer
Assistance Unit for instructions on how to proceed. Eligible practitioners such as
pharmacists, who work for or own Medicaid-enrolled pharmacies, will bill for counseling
services they render through the enrolled entity in which they provide services.
Physician’s assistants, registered nurses, and psychologists who are not health service
providers in psychology (HSPP) will bill for counseling services they render through the
enrolled entity in which they provide services.
Providers/practitioners are reminded that they are NOT entitled to Medicaid
reimbursement for a service which they provide to the general public at no charge,
including smoking cessation counseling services. The Health Care Excel (HCE)
Surveillance Utilization and Review (SUR) department will closely monitor adherence to
this program limitation.
01/31/2007 Smoking Cessation
Medical Policy Manual
621
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Smoking Cessation
Medical Policy Manual
622
Both ordering and rendering practitioners should maintain sufficient documentation of
their respective functions to substantiate the medical necessity of the service rendered,
and the provision of the service itself; this requirement is consistent with existing
Medicaid policies and regulations.
Smoking Cessation Products
Smoking cessation products, previously mentioned herein, will be covered under the
Indiana Medicaid Program for products provided on October 27, 1999, and later.
Reimbursement is available to pharmacy providers for smoking cessation products when
prescribed by a licensed practitioner within the scope of his/her license under Indiana
law.
Over-the-counter smoking cessation products must still be prescribed by a licensed
practitioner in order for the pharmacy to be reimbursed by Medicaid. All smoking
cessation products must be prescribed by a licensed practitioner for use, along with
counseling, within the twelve (12) week treatment timeframe.
Pharmacies will bill for reimbursement according to the normal procedures as outlined in
the provider manual (please see applicable provider manual section for full details).

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: SPEECH AND HEARING
DESCRIPTION
Speech and Hearing Services are provided for Indiana Health Coverage Programs (IHCP)
members with speech, hearing, and/or language disorders. These services include
diagnostic, screening, preventive, or corrective services provided by or under the
direction of a speech pathologist or audiologist.
This document is intended to serve as a general summary of the Indiana Health Coverage
Programs (IHCP) policies regarding this service. More specific information may be
found in the IHCP provider manual, program notices, the Indiana Administrative Code
(IAC) or other sources as appropriate. See the “Rules, Citations, and Sources” section of
this document for further information.
COVERAGE CRITERIA
Medically necessary speech pathology and audiology services are available to IHCP
members within the limitations set forth in the Indiana Administrative Code (IAC).
IHCP reimbursement is available for therapy services provided outside the State, and are
subject to the same limitations as in-state services. Refer to the Out-Of-State Services
Medical Policy fact sheet.
SPEECH PATHOLOGY
Speech pathology services are allowed with the following restrictions.
• Evaluations and re-evaluations are limited to three hours of service per
evaluation.
• Reevaluation will not be authorized more than one time yearly unless
there is a significant change in the member’s condition. Documentation
must be submitted.
• Group therapy is covered in conjunction with individual therapy only.
• Physician involvement and personal patient evaluation is required.
Documentation in the member’s medical record must be maintained.
Therapy must be ordered by a physician. A current plan of treatment and
01/31/2007 Speech and Hearing
Medical Policy Manual
623
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
progress notes, substantiating medical necessity and the effectiveness of
the prescribed therapy, must be attached to the prior authorization request
and is subject to post payment review.
• Therapy must be provided by a licensed therapist or qualified assistant
under the direct supervision of the therapist as appropriate.
• Therapy must be of such a level of complexity and sophistication and the
condition of the member must be such that the judgment, knowledge, and
skills of a licensed therapist are required.
• IHCP reimbursement is available only for medically reasonable and
necessary therapy.
• Therapy rendered for diversional, recreational, vocational, or avocational
purposes, or for the remediation of learning disabilities or for
developmental activities that can be conducted by nonmedical personnel,
is not covered by the IHCP.
• Therapy for rehabilitative services will be covered for a member no longer
than two (2) years from the initiation of the therapy unless there is
significant change in medical condition. Documentation must be
maintained in the medical record.
• Maintenance therapy is not a covered service.
• When an IHCP member is enrolled in therapy, ongoing evaluations to
assess progress and redefine therapy goals are part of the therapy program.
These ongoing evaluations are not separately reimbursed by the IHCP
program.
• One hour of billed therapy service must include a minimum of forty-five
minutes of direct patient care with the balance of the hour spent in related
patient services.
• Therapy services will not be approved for more than one hour per day per
type of therapy.
• A request for therapy services, which would duplicate other services
provided to a patient, will not be prior authorized.
• Speech therapy is covered for a maximum of 50 visits per rolling 12
months per type of therapy (Package C only).
Prior Authorization for Speech Pathology
Requests for prior authorization (PA) are reviewed for medical necessity as well as the
member’s abilities and/or progress. A speech pathologist consultant is available to
review documentation for medical necessity. Prior authorization is required for speech
pathology services with the following exceptions.
• Initial evaluations.
• Any combination of therapy ordered in writing prior to a member’s
discharge from an inpatient hospital that may continue for a period not to
exceed thirty (30) units in thirty (30) calendar days.
• Therapy services provided by a nursing facility or large private or small
intermediate care facility for the mentally retarded (ICF/MR), which are
included in the facility’s per diem rate.
01/31/2007 Speech and Hearing
Medical Policy Manual
624
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• Out-of-state services require prior authorization. (See 405 IAC 5-5-2).
Refer to the Medical Policy fact sheet for Out-Of-State Services for
further information.
AUDIOLOGY/HEARING TESTS
Audiology services are allowed with restrictions.
• The physician must certify in writing the need for an audiological
assessment or evaluation.
• The audiology service must be rendered by a licensed audiologist or a
person registered for his clinical fellowship year who is supervised by a
licensed audiologist. A registered audiology aide can provide services
under the direct on-site supervision of a licensed audiologist under 880
IAC.
• When a member is to be fitted with a hearing amplification device, by
either the audiologist or a registered hearing aide specialist, a medical
clearance and audiometric test form must be completed in accordance with
instructions and submitted with the request for PA. This form must be
complete and must include the proper signatures where indicated before
the PA request will be reviewed.
• Initial audiological assessments are limited to one assessment every three
years per member. If more frequent audiological assessments are
necessary, PA is required.
Provisions of audiology services are subject to the following criteria.
• The member’s history must be completed by any involved professional.
• The referring physician must complete Part 2 of the Medical Clearance
and Audiometric Test Form no earlier than six months prior to the
provision of the hearing aid. Children fourteen years of age and under
must be examined by an otolaryngologist; members 14 years of age and
older may be examined by a licensed physician if an otolaryngologist is
not available.
• All testing must be conducted in a sound-free enclosure. If a member is
institutionalized and his or her physical or medical condition precludes
testing in a sound-free enclosure, the ordering physician must verify
medical confinement in the initial order for audiological testing. The
audiological assessment must be conducted by a licensed audiologist,
clinical fellowship year audiologist, or otolaryngologist. Testing
conducted by other professionals and cosigned by an audiologist or
otolaryngologist will not be reimbursed by the IHCP. If the audiological
evaluation reveals one or more of the following conditions, the member
must be referred to an otolaryngologist for further evaluation.
o Speech discrimination testing indicates a score of less than 60% in
either ear.
01/31/2007 Speech and Hearing
Medical Policy Manual
625

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
o Pure tone testing indicates an air bone gap of fifteen decibels or more
for two adjacent frequencies in the same ear.
• The hearing aid evaluation may be completed by the audiologist or
registered hearing aid specialist.
• The hearing aid contract portion of the audiometric test form must be
signed by a registered hearing aid specialist.
• Audiological assessments rendered more frequently than every three years
will be assessed on a case-by-care basis, based upon documented
otological disease.
Audiologic procedures cannot be fragmented and billed separately. Hearing tests, such as
whispered voice and tuning fork, are considered part of the general otolaryngology
services and cannot be reported separately.
• Basic comprehensive audiometry includes pure tone, air and bone threshold, and
discrimination; testing provided for both ears.
• All other audiometric testing procedures will be reimbursed on an individual
basis, based on the medical necessity for such test procedures.
Prior Authorization for Audiology/Hearing Tests
All requests for PA will be reviewed on a case-by-case basis. The following audiological
services do not require PA.
• A screening test indicating the need for additional medical examination
(screenings are not reimbursed separately under the IHCP).
• Initial assessment of hearing;
• Determination of suitability of amplification and the recommendation
regarding a hearing aid;
• The determination of functional benefit to be gained by the use of a
hearing aid;
• Audiology services provided by a nursing facility or large ICF/MR, which
are included in the facility’s established per diem rate.
Out-of-state services require prior authorization. (See 405 IAC 5-5-2). Refer to the MP
fact sheet for Out-Of-States Services for further information.
Health Watch Early Periodic Screening Diagnosis, and Treatment (EPSDT)
Ensuring that all children in the IHCP receive age-appropriate, comprehensive,
preventive services is the primary goal of the HealthWatch/EPSDT program.
Components of screening examinations and the recommended frequency of screenings
are listed in the EPSDT Provider Manual, HealthWatch Periodicity and Screening
Schedule found in Appendix A., 18-23 months, 24-35 months, and 3 years. Audiometric
screening should be provided yearly for ages 4 through 14, and at 16, 18, and 20 years of
age. Refer to the EPSDT Medical Policy fact sheet.
Hearing Aids Prior Authorization and Documentation Criteria
Hearing Aids
01/31/2007 Speech and Hearing
Medical Policy Manual
626
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
IHCP reimbursement is available for the purchase, repair, or replacement of hearing aids,
including, air conduction or conventional hearing aids, bone anchored or bond
conduction hearing aids (BAHA), and programmable hearing aids, under the following
conditions.
• PA is required for hearing aids.
• A medical clearance form and audiometric test form must be completed and
submitted with the PA request form.
• Any involved professional must complete a member history. The referring
physician must complete Part 2 of the Medical Clearance and Audiometric Test
Form no earlier than six months prior to the provision of the hearing aid.
• Hearing aid fitting may be provided by either the audiologist or a registered
hearing aid specialist. Services must be performed in accordance with the
appropriate provisions of 405 IAC 5-22.
• Hearing aids purchased by the IHCP become the property of the Office. All
hearing aids purchased by the Office, which are no longer needed by a member,
must be returned to the County Office of Family and Children.
• Hearing aids are not covered for members with a unilateral pure tone average loss
(500, 1000, 2000, or 3000 hertz) equal to or less than thirty decibels.
• Binaural aids and CROS-type aids will be authorized only when significant, objective
benefit to the member can be documented.
• Canal hearing aids are non-covered.
Air Conduction Hearing Aid
The air conduction hearing aid, or conventional hearing aid, amplifies and sends sound
through the earmold, into the ear canal, through the middle ear and to the inner ear. This
type of hearing aid is not appropriate for a child with Atresia, as the ear canal is blocked
and the sound cannot get through. Conventional hearing aids will be authorized only if
they are medically necessary and significant, and objective benefit to the member is
documented.
Bone-Anchored Hearing Aids/BAHA
A bone conduction hearing aid is different from a conventional air conduction hearing
aid. Unlike other hearing aids, BAHA hearing aids transmit sound through the bone of
the skull rather than to the ear canal. This process is called direct bone conduction. Bone
conduction hearing aids will be authorized only if they are medically necessary and
significant, and objective benefit to the member is documented. Indications for BAHA’s
include the following.
• Chronic ear infection.
• Congenital hearing loss.
• Single-sided deafness (SSD).
• History of middle ear damage.
CROS/BICROS Hearing Aids
01/31/2007 Speech and Hearing
Medical Policy Manual
627
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
CROS is an acronym for Contralateral Routing of Signals. A CROS hearing aid is fit to a
person who has normal hearing in one ear and one ear that is unaidable. The unaidable
ear may be unaidable due to the severity of the loss, a physical malformation of the ear, a
chronic medical condition that causes occlusion of the ear canal or any combination of
the three. A CROS hearing aid consists of a microphone at the level of the unaidable ear
which transmits via a wire or FM to a receiver in (or at) the normal hearing ear. BI
CROS stands for bilateral-CROS. This refers to a hearing aid system which incorporates
two microphones, one in (or at) each ear and a single amplifier and receiver. In this case,
the BI CROS device is fit to an individual who has a hearing loss in both ears, but one ear
is unaidable, allowing the individual to receive sounds from both sides of their head in
their “good” ear.
Programmable Hearing Aids
Programmable hearing aids will be authorized only if they are medically necessary and
objective evidence of significant benefit to the member is documented. Indications for
programmable hearing aids include the following.
• Fluctuating hearing loss (Meniere’s disease, autoimmune sensorineural hearing
loss, otogenic syphilis, large vestibular aqueduct syndrome, and other conditions
resulting in fluctuant hearing loss).
• Progressive hearing loss (Meniere’s disease, Alport’s syndrome, and other
conditions resulting in progressive hearing loss. A retrocochlear hearing loss
must be excluded, particularly when the loss is asymmetrical).
• Severe recruitment or very narrow dynamic range.
• Very young children who are hard to test or hard to fit.
• Patients with hearing loss with unusual audiometric configurations.
Documentation Requirements for Programmable Hearing Aids
• An IHCP Medical Clearance and Audiometric Test (IHCP MCAT) form must be
completed. Medical necessity for programmable hearing aids must be clearly
documented in the sections entitled, “Recommendation Information” and/or
“Special Conditions” on page two of the IHCP MCAT form.
• A record of the audiogram obtained not more than three months before the date of
the request.
• An otological examination report signed by the physician that includes the
medical etiology of or diagnosis for the hearing loss.
• A diagnosis that supports medical necessity must be included on the PA request
and on the claim for programmable hearing aids.
• A documented case history of the member’s needs and lifestyle that include at the
following:
o Past history or hearing aid use.
o Reason the programmable hearing aids(s) rather than a
conventional hearing aid(s) is medically necessary.
o Description of the hearing environment(s) in which the member
has trouble hearing and to which he or she is subjected. The
01/31/2007 Speech and Hearing
Medical Policy Manual
628
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
frequency and duration of exposure to these environments should
also be included.
o Documentation of any other factors, such as lack of normal
dexterity, should be included.
o Documentation must be provided that supports medical necessity
of the programmable hearing aids outside of vocational needs.
Hearing Aid Maintenance and Repair
The IHCP provides reimbursement for the maintenance or repair of hearing aids under
the following conditions.
• Repairs for hearing aids and ear molds do not require PA; however,
reimbursement for such repairs cannot be made more often than once every 12
months. Repairs may be prior authorized more frequently for members under 18
years of age if circumstances justifying need are documented.
• Batteries, sound hooks, tubing, and cords do not require PA.
• The IHCP will not reimburse for repair of hearing aids still under warranty.
• Routine servicing of functioning hearing aids is not covered by the IHCP.
• No payment shall be made for repair or replacement of hearing aids necessitated
by member misuse or abuse whether intentional or unintentional.
Hearing Aid Replacement
IHCP reimbursement is available for the replacement of hearing aids under the following
conditions.
• Replacement of hearing aids is subject to 405 IAC 5-19-14, Hearing aids;
maintenance and repair.
• Requests for replacement of hearing aids must document a change in the
member’s hearing status and must state the purchase date and condition of the
current hearing aid.
• Hearing aids shall not be replaced prior to five years from the purchase date.
Replacements may be prior authorized more frequently for members under 18
years of age, if circumstances justifying medical necessity are documented.
Cochlear Implants
A cochlear implant device is an electronic instrument. Part of the device is implanted
surgically to stimulate auditory nerve fibers, and the other part is worn or carried by the
individual to capture, analyze, and code sound. Cochlear implant devices are available in
single-channel and multi-channel models. The purpose of the implanted device is to
provide awareness and identification of sounds to facilitate communication for persons
who are moderately to profoundly hearing impaired.
Cochlear Implants will be authorized only if they are medically necessary and objective
evidence of significant benefit to the member is documented. Indications for cochlear
implants include the following.
01/31/2007 Speech and Hearing
Medical Policy Manual
629
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• Cochlear implantation will be covered for treatment of bilateral pre-or-post-
linguistic, sensorineural, moderate-to-profound hearing loss in individuals who
demonstrate limited benefit from amplification.
• Post-linguistically deafened adults must demonstrate test scores of less than or
equal to 40% on sentence recognition scores from tape recorded tests in the
patient’s best listening condition.
• Cochlear implants are covered for children between ages 2-17 where there is
demonstrated by the ability to improve on age appropriate closed-set word
identification tasks with amplification.
Coverage is provided only for those patients who meet all of the following selection
guidelines.
o Diagnosis of bilateral moderate-to-profound sensorineural hearing
impairment with limited benefit from appropriate hearing (or vibrotactile)
aids;
o Cognitive ability to use auditory clues and a willingness to undergo an
extended program of rehabilitation;
o Freedom from middle ear infection, an accessible cochlear lumen that is
structurally suited to implantation, and freedom from lesions in the
auditory nerve and acoustic areas of the central nervous system;
o No contraindications to surgery; and
o The device must be used in accordance with Food and Drug
Administration (FDA)-approved labeling.
Individuals must have hearing test scores of greater than 40% and less than or equal
to 60% only when the provider is participating in, and patients are enrolled in, either
an FDA-approved category B investigational device exemption clinical trial as
defined at 42 CFR 405.201, a trial under the Centers for Medicare & Medicaid (CMS)
Clinical Trial Policy as defined at section 310.1 of the National Coverage
Determinations Manual, or a prospective, controlled comparative trial approved by
CMS as consistent with the evidentiary requirements for National Coverage Analyses
and meeting specific quality standards. See the Medical Policy Fact Sheet for
Clinical Trails for further information.
Cochlear Implant Maintenance and Repair
The IHCP provides reimbursement for the maintenance or repair of cochlear implants
under the following conditions.
• Repairs for cochlear implants do not require PA; however, reimbursement for
such repairs cannot be made more often than once every 12 months. Repairs may
be prior authorized more frequently for members under 18 years of age if
circumstances justifying need are documented.
• Batteries do not require PA.
• Headset/headpiece, microphone, and transmitting coil/cable do require PA.
• Payment is not available for repair of a cochlear implant device still under
warranty.
01/31/2007 Speech and Hearing
Medical Policy Manual
630
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• Routine servicing of a functioning cochlear implant is not covered under the
IHCP.
• No payment shall be made for repair or replacement of hearing aids necessitated
by member misuse or abuse whether intentional or unintentional.
• The device is in continuous use and still meets the medical necessity needs of the
beneficiary.
• All charges for cochlear implant parts and repairs are to reflect no more than the
usual and customary (U&C) charge to the public.
Cochlear Implant Replacement
The IHCP provides reimbursement for the replacement of cochlear implants under the
following conditions.
• Replacement of a cochlear implant is subject to repair and maintenance criteria.
• Requests for replacement of cochlear implants must document a change in the
member’s status and must state the purchase date and condition of the current
cochlear implant.
• Cochlear implants shall not be replaced prior to five years from the purchase date.
Replacements may be prior authorized more frequently for members under 18
years of age, if circumstances justifying medical necessity are documented.
• For replacement of a cochlear implant with an upgraded model:
o Documentation substantiates that the newer generation technology
provides additional capacity.
o The current implant has been worn for at least four years.
BILLING REQUIREMENTS
The IHCP provides reimbursement to IHCP enrolled providers for services billed with
the appropriate HCPCS procedure code. Programmable hearing aids should be submitted
for reimbursement using HCPCS procedure code V5299, Hearing Service,
Miscellaneous. All other hearing aids will be submitted using the appropriate HCPCS V
code.
A hearing services code set was implemented for audiologists, provider specialty 200,
and hearing aid dealers, provider specialty 220. Audiologists and hearing aid dealers will
be reimbursed from a designated list of procedure codes effective October 1, 2004.
Codes billed by audiologists and hearing aid dealers that are not on the list will deny for
edit 1012 - Rendering
provider specialty not eligible to render procedure code. The code set is subject to
change based
on policy and coverage changes.
Programmable hearing aids are manually priced and require that an invoice accompany
the claim. Providers should bill their usual and customary charge to the IHCP; however,
reimbursement will be made at the lower of the provider’s usual and customary charge or
130 percent of the manufacturer’s invoice price.
01/31/2007 Speech and Hearing
Medical Policy Manual
631

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MANAGED CARE
For PrimeStep, the same policies apply as those applicable to the Traditional Fee-For-
Service program. For Risk-Based Managed Care (RBMC) specific policies, please
consult the appropriate MCO.
RELATED MEDICAL TOPICS
EPSDT HealthWatch
Home Health Services
Intermediate Care Facilities for the Mentally Retarded
Medical Supplies and Equipment
Medical Supplies and Equipment – Programmable Hearing Aids
Nursing Facilities
Physician Services
Surgery Global Billing/Payment Guidelines
Therapies
Transportation Services
Out-Of-State Services
RULES, CITATIONS, AND SOURCES
42 CFR 405.201
42 CFR 440.110
IC 12-15-34 Home Health Services
IC 12-15-39 Long Term Care
405 IAC 5-3 Prior Authorization
405 IAC 5-4 Provider Enrollment
405 IAC 5-13 Intermediate Care Facilities for the Mentally Retarded
405 IAC 5-15 Early and Periodic Screening, Diagnostic, and Treatment Services
405 IAC 5-16 Home Health Agency and Clinic Services
405 IAC 5-19 Medical Supplies and Equipment
405 IAC 5-22 Nursing and Therapy Services
405 IAC 5-25 Physician Services
405 IAC 5-31 Nursing Facility Services
405 IAC 5-32 Rehabilitation Unit
405 IAC 5-33 Acute Care Hospital
405 IAC 5-34 Hospice Services
405 IAC 5-36 Diabetes Self Management Training
880 IAC Speech-Language Pathology and Audiology Board
Indiana Health Coverage Programs Provider Manual 2004
Indiana Health Coverage Programs Provider Bulletins
BT200105 - Programmable Hearing Aids
Indiana Health Coverage Programs Provider Banners
BR01-06-1998 – Hearing Aids PA Codes 96105, 96111
01/31/2007 Speech and Hearing
Medical Policy Manual
632

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
BR12-29-1998 – Hearing Test Audit Update
BR200435 – Audiologist and Hearing Aid Dealers Code Set
Indiana Health Coverage Programs Provider Newsletters
NL200409 – Audiology and Hearing Aid Services
Indiana Health Coverage Programs HealthWatch Early Periodic Screening, Diagnosis,
and Treatment (EPSDT) Provider Manual, 2002
Origination Date: 12/31/02
Revisions and Review Reason Date
IHCP Web site Hearing Services Provider Code
Set
10/01/04
42 CFR 440.110 Physical Therapy, Occupational
Therapy, and Services for
Individuals with Speech,
Hearing, and Language
Disorders
05/28/04
405 IAC 5-31-4 Nursing Facility Services 06/05/03
405 IAC 5-3-12 Prior Authorization 08/28/01
405 IAC 5-4-1 Provider Enrollment 06/27/01
405 IAC 5-13-3 Intermediate Care Facilities for
the Mentally Retarded
06/27/01
405 IAC 5-15-3 Early and Periodic Screening,
Diagnostic, and Treatment
Services
06/27/01
405 IAC 5-16-2, 5-16-3.1
Home Health Agency and Clinic
Services
06/27/01
405 IAC 5-19-13, 5-19-14, 5-19-15, 5-
19-16, 5-19-17
Medical Supplies and Equipment 06/27/01
405 IAC 5-22-5, 5-22-6, 5-22-7, 5-22-9 Nursing and Therapy Services 06/27/01
405 IAC 5-25-3 Physician Services 06/27/01
405 IAC 5-32-2 Rehabilitation Unit 06/27/01
405 IAC 5-33-1, 5-33-2 Acute Care Hospital Admission 06/27/01
405 IAC 5-34-8 Hospice Services 06/27/01
405 IAC 5-36-1 Diabetes Self Management
Training
06/27/01
IC 12-15-39.6-1 Long Term Care Defined 01/01/97
IC 12-15-34-2 Home Health Services Defined 1995
IC 12-15-34-4 Items and Services Included
Under IC 12-15-34-2 Definition
of Home Health Services
1992
Review Scheduled 04/29/05
Review Cochlear Implant Criteria Added 10/29/05
APPLICABLE INDIANAAIM EDITS AND AUDITS:
1012 – Rendering Provider Specialty Not Eligible to Render Procedure Code
01/31/2007 Speech and Hearing
Medical Policy Manual
633
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Speech and Hearing
Medical Policy Manual
634
6054 – Only One Hearing Test per 36 Months Without PA
6056 – Only One Hearing Aid Repair per 12 months Allowed for Members 18 and Older
6057 – Only One Hearing Aid Repair per 12 Months Allowed for Members Under 18
Years
Without PA
6058 – Hearing Aid Earmolds Repair per 12 Months – Members 18 Years or Older
6059 – Hearing Aid Earmolds Repair per 12 Months – Member Under 18 Years
6060 – Speech Therapy Evaluations – One per Year
6750 – Therapies Within 30 Days From Hospital Discharge Date Without Approved PA

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: SUBSTANCE ABUSE
DESCRIPTION:
Substance abuse services covered by the IHCP include services for inpatient
detoxification, rehabilitation, and after care for chemical dependency and outpatient
services for individuals who have a substance-related disorder. Substance abuse, as
defined by the Diagnostic and Statistical Manual of Mental Disorders, fourth edition
(DSM-IV), is a maladaptive pattern of substance use manifested by recurrent and
significant adverse consequences related to the repeated use of substance. The terms
substance abuse, chemical dependency, and substance-related disorder are used
interchangeably in this document.
SUMMARY OF CURRENT POLICY
The Indiana Health Coverage Programs (IHCP) provides reimbursement for inpatient
substance abuse services and outpatient substance abuse services to members who meet
the criteria for services. Inpatient substance abuse treatment is available, subject to prior
authorization (PA). Outpatient substance abuse services are available to members
enrolled in the Medicaid Rehabilitation Option (MRO), who meet the substance-related
disorder guidelines for case management services. Case management services under
MRO are not subject to PA.
RELATED MEDICAL TOPICS
Emergency Medicine – Emergency Room
Mental Health/Behavioral Health – Inpatient Services
Mental Health/Behavioral Health – Outpatient Services
Community Mental Health Rehabilitation Services
RULES, CITATIONS, AND SOURCES
405 IAC 5-17 Hospital Services
405 IAC 5-20 Mental Health Services
405 IAC 5-21 Community Mental Rehabilitation Services
Indiana Health Coverage Programs Provider Manual 1999
Medicaid Rehabilitation Option Provider Manual, March 2004
01/31/2007 Substance Abuse
Medical Policy Manual
635

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
ORIGINATION, REVIEWS AND REVISIONS
Origination Date: August 24, 1997
Revisions and
Reviews
Reasons Date
405 IAC 5-17-3 Emergency Admissions 8/24/97
405 IAC 5-17-5
Inpatient detoxification, rehabilitation, and after care for
chemical dependency
8/24/97
405 IAC 5-20 Mental Health Services 7/25/97
405 IAC 5-21-5
Community Mental Health Rehabilitation Services
Case Management Services
7/25/97
BT199923
Appropriate Procedures for Submitting the Certification of
Ned (Form 1261A for Inpatient Psychiatric Services
8/24/99
BR199950 MCO Financial Responsibility 12/14/99
IHCP Provider
Manual
Chapter 8
Inpatient Mental Health Services
7/04
IHCP Provider
Manual
Chapter 6
Institutional Prior Authorization Policy Requirements
7/04
MRO Provider
Manual
Section 4
Procedure Codes – Case Management
9/04
Medical Policy
Manual
4
th
Quarter 2004 Update 1/05
APPLICABLE INDIANAAIM EDITS AND AUDITS
6096 – The CPT/HCPCS Code Billed Not Payable In PPS
3014 – Substance Abuse DRG Requires Prior Authorization
01/31/2007 Substance Abuse
Medical Policy Manual
636
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
COVERAGE CRITERIA
Inpatient Substance Abuse Services
The IHCP provides reimbursement for inpatient substance abuse and chemical
dependency treatment, as outlined in 405 IAC 5-17-3. Substance abuse and chemical
dependency admissions are reimbursed based on the DRG payment methodology.
Admission must be to a psychiatric setting, unless medical services are required for life
support and cannot be rendered in a substance abuse treatment unit or facility.
Inpatient substance abuse services are covered for Package C members when the services
are medically necessary for the diagnosis or treatment of a member’s condition.
Managed care organizations (MCO) are financially responsible for all facility, ancillary,
and professional services related to carved-out mental health services, including
substance abuse related services, when rendered in an acute care hospital, by the primary
medical provider, or any other specialty not enrolled as a psychiatrist, health services
provider in psychology, or mental health services provider.
PA is considered for approval on a case-by-case basis for inpatient detoxification,
rehabilitation, and aftercare under the following circumstances.
• Treatment, evaluation, and detoxification is necessary based on the stated medical
condition
• Need for safe withdrawal from alcohol or other drugs
• History of recent convulsions or poorly controlled convulsive disorder
• Reasonable evidence that detoxification and aftercare cannot be accomplished in
an outpatient setting.
A Certification of Need, Form 1261A is required for all mental health admissions,
including admissions for substance abuse and chemical dependency, regardless of setting.
For non-emergency admissions, this form must be received within ten working days of
admissions. For emergency psychiatric admissions, as defined in 405 IAC 5-20-6, this
form must be received within 14 days of admission. This form must include detailed
information to document the necessity of the admission. In the event that the form does
not meet the requirements, any claim(s) associated with the admission will be denied.
Detoxification is defined as treatment requiring physician assessment and supervision and
skilled nursing care to restore physiological functioning impaired by prolonged and
excessive use of alcohol and/or drugs. IHCP members must meet the following criteria
for inpatient detoxification.
1. Evidence of symptoms of withdrawal that require close medical monitoring or
continuous observation.
a. Three (3) or more of the following conditions.
• Delirium tremens
01/31/2007 Substance Abuse
Medical Policy Manual
637
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• Hypertension of recent onset
• Impaired or absence of gag reflex
• Tachycardia
• Elevated temperature
• Diaphoresis
• Piloerection (goose bumps)
b. OR one (1) of the following conditions.
• Seizures
• Hallucinations of recent onset
• Disorientation or confusion
2. History of severe withdrawal reaction, such as seizures, delirium tremens or
psychotic episode.
3. Intoxicated with a history of recent, severe idiosyncratic intoxication, such as
violence or blackouts while under the influence.
4. In addition to alcohol/drug condition, member has a co-existing medical and/or
psychiatric condition that requires medical and psychiatric services.
5. Recent history of alcohol or other drug abuse and is currently unable to control
abuse outside of a restrictive 24-hour care environment that is demonstrated by
documented recent failed attempts.
6. Dependency or abuse must be contributing to severe social and/or emotional
dysfunction in one or more life spheres, e.g., vocational, familial, or social.
Outpatient Substance Abuse Services
Outpatient substance abuse services are covered under the case management service of
the Medicaid Rehabilitation Option (MRO) program. PA is not required for MRO case
management services. Case management services are available for IHCP members ages
18 to 64 years of age and 17 years of age and younger, who are determined to have a
substance-related disorder, as defined in 405 IAC 5-21-5. In order to receive case
management services for a substance-related disorder, IHCP members must meet all of
the following criteria.
1. A substance-related disorder, as defined in the DSM-IV.
2. Significant functional impairments in two of the following areas.
a. Activities of daily living
b. Interpersonal functioning
c. Ability to live without recurrent use of chemicals
d. Psychological functioning
01/31/2007 Substance Abuse
Medical Policy Manual
638
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Substance Abuse
Medical Policy Manual
639
3. The duration of the addiction has been in excess of twelve months. However,
members who have blackouts, convulsions, or other serious medical
consequences of withdrawal from substance abuse and/or chemical dependency
do not have to meet the durational requirement of these criteria.

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: SURGERY—MULTIPLE PROCEDURES/SAME
OPERATIVE SESSION
DESCRIPTION:
Multiple surgical procedures may be performed on the same patient on the same day
when it is determined a benefit to the surgeons and patient for the best outcome for the
patient.
SUMMARY OF CURRENT POLICY:
Reimbursement for two or more surgeries performed during the same operative period
will be subject to the rules set forth in 405 IAC 5-28-1(g). The most expensive procedure
being paid at the full-allowed amount (100%), the second procedure at one-half (50%) of
its allowed amount and the third procedure at twenty-five percent (25%) of its allowed
amount. Documentation may be required to indicate medical necessity for the multiple
procedures.
Many surgeries require prior authorization (see 405 IAC 5-3). Prior authorization for
multiple surgeries on the same day does not override the restrictions of 405 IAC 5-28.
MEDICAL TOPICS CROSS-REFERENCES:
Gynecology -- Hysterectomy
Obstetric Care
Physician Services
Surgery-Global Billing/Payment Guidelines
Surgery-Office Visits
Surgery-Removal of Implants
Surgery-Services Requiring Prior Authorization
Surgery-Surgery and Anesthesia By the Same Provider
Surgery-Surgeon and Assistant Surgeon, Same Provider
Surgery-Surgical Services
Surgery-Suture of Wounds
01/31/2007 Surgery – Multiple Procedures/Same Operative Session
Medical Policy Manual
640

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Surgery – Multiple Procedures/Same Operative Session
Medical Policy Manual
641
RULES, CITATIONS, AND SOURCES:
405 IAC 5-28 Medical and Surgical Services
405 IAC 5-1-5 Global Fee Billing
Indiana Health Coverage Programs Provider Manual 1999
Initial Policy
Issues
Effective
Date
Implementatio
n Date
Retroactive Date
470 IAC 5-9-1
Transferred
Global fee billing 7/1/91
405 IAC 1-7
Repealed 8/24/97
Global fee billing 1/1/92
Revisions:
405 IAC 5-28 Medical and
surgical services
8/24/97
405 IAC 5-1-5 Global fee billing 8/24/97
APPLICABLE INDIANAAIM EDITS AND AUDITS:
6651
6665
COVERAGE CRITERIA:

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: SURGERY—OFFICE VISITS
DESCRIPTION:
Prior to the performance of a surgical procedure, either inpatient or outpatient, the patient
consults with the surgeon who will be performing the procedure. The visit can occur in
the physician’s office, in the emergency room, or in the out-patient surgery area.
SUMMARY OF CURRENT POLICY:
Office visits made with the surgeon prior to the scheduling of surgery are billed under the
global surgery payment/billing rules. Certain modifiers may be used to distinguish a
preoperative visit from a more in-depth visit at which time the decision was made for
surgery, a significant, separately identifiable evaluation and management visit was made
the same day of surgery, the surgeon served as a consultant for a second or third opinion,
or an unrelated procedure or service by the same physician during the post-operative
period. The appropriate modifier should be used to claim payment for these services.
MEDICAL TOPICS CROSS-REFERENCES:
Emergency Medicine
Surgery; Orthopedic, Otolaryngology, Ophthalmology, Thoracic, Urology, Vascular
Physician Office Services
RULES, CITATIONS, AND SOURCES:
405 IAC 5-25-1
405 IAC 5-25-2
IC 12-8-6-3
IC 12-8-6-5
IC 12-15-1-10
IC 12-15-21-2
IC 12-15-21-3
Indiana Health Coverage Programs Provider Manual 1999
01/31/2007 Surgery – Office Visits
Medical Policy Manual
642

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Surgery – Office Visits
Medical Policy Manual
643
Initial Policy Issues
Date Approved
By Authority
Date Applied
To Program
Date
Implemented
In Program
405 IAC 5-9-1;
5-9-11
Transferred
01/01/92
Revisions:
405 IAC 1-7-9
Repealed
01/01/92
405 IAC 5-25-
1;5-58-2
08/24/97
APPLICABLE INDIANAAIM EDITS AND AUDITS:
764
765
766
770
COVERAGE CRITERIA:

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: SURGERY - PLASTIC/RECONSTRUCTIVE
GENITOURINARY AND BREAST
DESCRIPTION
Plastic or reconstructive surgery is intended to restore the normal appearance or function
of tissues or body structures that are missing, defective, damaged, misshapen, or that
have been significantly altered due to disease, trauma, surgery, or congenital anomalies.
When a significant functional impairment is present, reconstructive services may be
considered medically necessary; however, if significant functional impairment is not
present, reconstructive services may not be considered medically necessary.
This document is intended to serve as a general summary of the Indiana Health Coverage
Programs (IHCP) policies regarding this service. More specific information may be
found in the IHCP provider manual, program notices, the Indiana Administrative Code
(IAC) or other sources as appropriate. See the “Rules, Citations, and Sources” section of
this document for further information.
COVERAGE CRITERIA
The IHCP will provide reimbursement for reconstructive or plastic surgery for congenital
defects, developmental anomalies, trauma, infection, tumors, or disease. Reconstructive
or plastic surgery is generally performed to improve function, but may also be done to
approximate a normal appearance. Prior authorization (PA) must be obtained.
Breast reconstruction is surgery performed to revise or change the configuration of the
breast (male or female). Reduction mammoplasty is the surgical removal of a substantial
portion of the breast(s), including the skin and underlying glandular tissue, until a
clinically normal size is obtained. Bilateral surgery is usually performed; however, when
there is significant hypertrophy of one breast, resulting in an abnormal appearance
between the member’s breasts, a unilateral breast reduction may be performed. Such a
procedure may also be needed to achieve symmetry of the contralateral side when the
opposite breast has been reconstructed after mastectomy.
IHCP reimbursement is not available for breast reconstruction to reshape the normal
structure to improve appearance or self-esteem. IHCP reimbursement is not available for
cosmetic symptoms including ptosis, poorly fitting clothing, unacceptable appearance, or
01/31/2007 Surgery – Plastic / Reconstructive Genitourinary and Breast 644
Medical Policy Manual
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Surgery – Plastic / Reconstructive Genitourinary and Breast 645
Medical Policy Manual
nipple-areolar distortion. In addition, the use of liposuction to perform breast reduction is
considered investigational. Therefore, IHCP reimbursement is not available.
Reconstructive surgery is considered medically necessary for missing, defective,
damaged, or misshapen structures of the genitourinary system. Additionally, the IHCP
will provide reimbursement if a member has had significant alterations due to disease,
trauma, surgery, or congenital anomalies. Prior authorization is required for
reconstructive surgery.
The IHCP does not provide reimbursement for the following.
1. Scar removal or tattoo removals by excision or abrasion
2. Penile implants
3. Perineoplasty for sexual dysfunction
4. Tubal reanastomosis for the purpose of infertility
The IHCP defines intersex surgery as surgical intervention for members having
congenital anomalies, resulting in both male and female characteristics. The IHCP
considers intersex surgery medically necessary for congenital anomalies that result in a
member having ambiguous genitalia. Documentation in the member’s medical record is
required to support medical necessity. All other intersex surgery is not covered. Intersex
surgeries require prior authorization.
PRIOR AUTHORIZATION
Breast Reduction
The IHCP will provide reimbursement for breast reduction surgery in females.
Documentation must be maintained in the member’s medical record. Prior authorization
criteria is as follows.
1. History of the member’s symptoms for at least six months related to the large, pendulous
breasts must include the following.
• Neck and shoulder pain
• Low back pain
• Strap mark indentation
• Restriction of physical activities
• Poor posture
• Skin irritation (submammary
intertigo)

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
2. Asymmetry of the breasts will not be authorized unless it is done to achieve
symmetry of the contralateral side when the opposite breast has been reconstructed
after mastectomy for cancer.
3. Specific weight guidelines vary with the height and weight of the member. Table 1,
on the following page, displays the height and weight categories with the minimum
amount of breast tissue expected to be removed. Variances may occur which are not
accommodated by this table; these variances will be reviewed on an individual basis.
Table 1. Expected Minimum Amount of Breast Tissue to be Removed
Height Weight
Expected Amount of Breast
Tissue to be Removed
Under 5’ Less than 140 lbs 300 Gm per breast
5’ – 5’4” Up to 180 lbs 350 Gm per breast
5’4” – 5’7” Up to 220 lbs 400 Gm per breast
5’7” and up 211 lbs and greater 500 Gm per breast
The IHCP will provide reimbursement for breast reduction surgery in males that are over
age 18 years of age or 18 months after the end of puberty, for gynecomastia, when
medically necessary. PA criteria is as follows.
1. The tissue to be removed is glandular breast tissue and not the result of obesity,
adolescence, or reversible effects of a drug treatment which can be discontinued.
Documentation must be maintained in the medical record.
2. Documentation in the medical record indicates the conditions which may be
associated with gynecomastia and includes, but is not limited, to the following.
• Documented androgen deficiency
• Chronic liver disease that causes decreased androgen availability
• Klinefelter’s syndrome (Chromosome 47XYY Syndrome)
• Adrenal tumors that cause androgen deficiency or increased secretion of
estrogen
• Brain tumors that cause androgen deficiency
• Testicular tumors causing androgen deficiency or tumor secretion of estrogen
• Endocrine disorders, such as hyperthyroidism
Table 2 below lists the codes for reporting breast reduction and reconstruction surgeries.
Additionally, Table 2 reflects the PA requirement for the individual codes.
Table 2 - Codes for Reporting Breast Reduction and Reconstruction Surgeries
CPT
Code
Description
PA
Requirement
19140 Mastectomy for gynecomastia Yes
19316 Mastopexy No
19318 Reduction mammaplasty Yes
19325 Mammaplasty, augmentation; with prosthetic implant Yes
01/31/2007 Surgery – Plastic / Reconstructive Genitourinary and Breast
Medical Policy Manual
646

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 2 - Codes for Reporting Breast Reduction and Reconstruction Surgeries
CPT
Code
PA
Description
Requirement
19328 Removal of intact mammary implant No
19330 Removal of mammary implant material No
19340
Immediate insertion of breast prosthesis following
mastopexy, mastectomy or in reconstruction
Yes
19342
Delayed insertion of breast prosthesis following mastopexy,
mastectomy, or in reconstruction
Yes
19350 Nipple/areola reconstruction No
19357
Breast reconstruction, immediate or delayed, with tissue
expander, including subsequent expansion
No
19361
Breast reconstruction with latissimus dorsi flap, with or
without prosthetic implant
No
19364 Breast reconstruction with free flap No
19366 Breast reconstruction with other technique No
19367
Breast reconstruction with transverse rectus abdominis
myocutaneous flap (TRAM), single pedicle, including
closure of donor site;
No
19368
Breast reconstruction with transverse rectus abdominis
myocutaneous flap (TRAM) single pedicle, including closure
of donor site; with microvascular anastomosis
(supercharging)
No
19369
Breast reconstruction with transverse rectus abdominis
myocutaneous flap (TRAM), double pedicle, including
closure of donor site
No
19370 Open periprosthetic capsulotomy, breast No
19371 Periprosthetic capsulectomy, breast No
19380 Revision of reconstructed breast No
Genitourinary Surgery
The IHCP provides reimbursement for female reconstructive surgery with PA for one of
the following conditions. Documentation supporting medical necessity must be
maintained in the medical record.
1. Agenesis of the vagina
2. Post-trauma
3. Post cancer therapy
The IHCP provides reimbursement for male reconstructive surgery with PA in the
following circumstances. Documentation supporting medical necessity must be
maintained in the medical record.
1. Absence of testicle as a result of illness, injury, or congenital anomaly
2. No evidence of active infection, malignancy, or current treatment for
malignancy
01/31/2007 Surgery – Plastic / Reconstructive Genitourinary and Breast
Medical Policy Manual
647

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
The IHCP provides reimbursement for intersex surgery with PA for congenital
anomalies, resulting in a member having ambiguous genitalia. Documentation
supporting medical necessity must be maintained in the medical record.
Table 3 below reflects the codes for reporting female and male reconstructive surgery
and intersex surgeries. Additionally, Table 3 reflects the PA requirement for each code.
Table 3 - CPT Codes for Reporting Reconstructive Genitourinary Surgery
CPT
Code
Description
PA
Requirement
54660 Insertion of testicular prosthesis (separate procedure) Yes
54670 Suture or repair of testicular injury No
55970 Intersex surgery; male to female Yes
55980 Intersex surgery; female to male Yes
56805 Clitoroplasty for intersex state No
57291 Construction of artificial vagina; without graft No
57292 Construction of artificial vagina; with graft No
57335 Vaginoplasty for intersex state No
MANAGED CARE
For members enrolled in Risk Based Managed Care (RBMC), providers must contact the
member’s managed care organization (MCO) for more specific guidelines. IHCP
members enrolled in Hoosier Healthwise PrimeStep [Primary Care Case Management
(PCCM)] receive the same benefit coverage, and are subject to the same limitations as
traditional Medicaid Fee-For-Service (FFS). Refer to the Hoosier Healthwise Manual for
Primary Medical Providers and Office Staff for further information.
Refer to the IHCP Provider Manual, Chapter 1, for detailed information about the FFS,
PCCM, and RBMC delivery systems.
BILLING REQUIREMENTS
PA does not guarantee reimbursement for services. Documentation supporting medical
necessity must be maintained in the medical records. Providers are advised to report the
procedure code that best describes the services rendered.
Providers submitting claims for CPT codes 55970 and 55980, reporting intersex
surgeries, must submit documentation to substantiate the procedure performed. The
physician’s notes or the operative notes are to be submitted with these submitted claims.
01/31/2007 Surgery – Plastic / Reconstructive Genitourinary and Breast
Medical Policy Manual
648

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Surgery – Plastic / Reconstructive Genitourinary and Breast
Medical Policy Manual
649
RELATED MEDICAL TOPICS
Consultations & Second Opinions
Hospital Inpatient
Hospital Outpatient
RULES, CITATIONS, AND SOURCES
Indiana Administrative Code
405 IAC 5-13 – Services requiring prior authorization
405 IAC 5-29-1 – Noncovered services
Indiana Health Coverage Program Provider Manual
Version 5.1, March, 2005
Indiana Health Coverage Programs Provider Newsletters
NL200501 – Physician Services
Indiana Health Coverage Programs Provider Bulletins
BT200208 – Modifications to Prior Authorization Requirements
Origination Date: 7/29/05
Revisions and
Review
Reason Date
Revision
Scheduled review and separation of the Global
Surgery Fact Sheet into multiple fact sheets
07/29/05
APPLICABLE INDIANAAIM EDITS AND AUDITS
6002 – Any Two Anesthesiology Providers Same Procedure Requires Review
6003 – Manual Pricing for Split Care Billing
6034 – Global Surgery Payable at Reduced Amount When Components of Surgical Care
Paid
6035 – Components of Surgical Care Not Payable When Global Surgery Paid
6037 – Only One Assistant Surgeon Allowed for Select Surgeries
6039 – Assistant Surgeon Not Payable When Co-Surgeon Paid
6040 – Co-Surgeon Not Payable When Assistant Surgeon Paid
6061 – Components Not Payable When Global Paid – Genital Urinary/Reproductive
Systems
6096 – The CPT/HCPCS Code Billed Is Not Payable According to the PPS
Reimbursement Methodology
6152 – Surgery Payable at Reduced Amount When Consultation Paid Days Before or
After Surgery
6650 – Lifetime Procedures Are Limited to One Per Lifetime
6652 – Multiple Surgeries Must Be Billed on Same Claim
6661 – Duramorph Cannot Be Billed on the Same Day As Surgery
6665 – Bilateral Versus Unilateral Surgeries
6666 – Anesthesia Services Not Allowed By Provider Billing for Surgery
6706 – Global Payable at Reduced Fee When Components Paid – Genital
Urinary/Reproductive Systems

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: SURGERY - PLASTIC AND RECONSTRUCTIVE SURGERY
FACIAL AND MAXILLOFACIAL
DESCRIPTION
Facial plastic surgery is a general term for any surgery that proposes to alter the appearance of
the face and includes the restoration of appearance after accidental injury or correction of a
physical functional impairment caused by an accidental injury, a congenital anomaly, disease, or
previous therapeutic process. Reconstructive surgery may require completion in staged
procedures.
Maxillofacial surgery includes the diagnosis and surgical treatment of congenital or acquired
diseases, dysfunction, defects, or injuries of the mouth, jaws, face, neck, and associated regions.
Maxillofacial surgical services are provided by individuals licensed to practice dentistry that
have completed an approved residency in oral surgery and eligible for certification by the Board
of Oral and Maxillofacial Surgery or physicians who have completed residency training in
plastic surgery and are eligible for certification by the American Board of Plastic Surgery.
This document is intended to serve as a general summary of the Indiana Health Coverage
Programs’ (IHCP) policies regarding these services. More specific information may be found in
the IHCP Provider Manual, program notices, the Indiana Administrative Code (IAC), or other
sources as appropriate. See the “Rules, Citations, and Sources” section of this document for
further information.
I. FACIAL PLASTIC AND RECONSTRUCTIVE SURGERIES
The IHCP provides reimbursement for facial plastic or reconstructive surgery related to disease
or trauma, specifically for surgery that alters the appearance of the lower face including the
upper jaw, lower jaw, and chin. Surgery for these portions of the face may be considered
cosmetic, or may be indicated when severe abnormalities result in functional impairment
affecting the ability to eat, swallow, or breathe. Procedures may be indicated to correct or
restore appearance following traumatic injuries or following medical or surgical treatments that
result in anatomical changes.
The IHCP will provide reimbursement with prior authorization for surgical procedures to remove
excess skin from the face and tighten the muscles of the face to correct a facial abnormality; for
example, following burn injury or facial palsy from neurologic disease. IHCP reimbursement is
01/31/2007 Surgery – Plastic / Reconstructive Surgery – Facial and Mazillofacial
Medical Policy Manual
650

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
not available for surgical procedures to remove excess skin from the face and tighten the muscles
of the face for members with no functional impairments, disease, or injury-related facial changes.
A. Craniofacial Deformities
Coverage Criteria for Craniofacial Deformities
Major mid-face craniofacial deformities of the facial bones or skull may require corrective
reconstructive surgeries due to abnormal development in position, size, or shape. There are
multiple diagnoses, such as Crouzon, Apert, Pfeiffer, Saethre-Chotzen, Carpenter, and
Antley-Bixler that cause restricted growth of the midface. Members with these craniofacial
midface diseases often require long-term monitoring of the ears, nose, and throat for
occurrence of the following conditions that are frequently related to mid-face anomalies.
• Vision, hearing, speech, and language disabilities
• Learning disabilities
• Orthodontic problems due to abnormal shape and position of the jaw
• Abnormalities in the facial skeleton about the orbits, maxilla, and mandible
• Skull deformities, including a narrow width and an elongation from front to back
• Triangularly shaped skull, often with ridging in the midline of the forehead
• Flattening of the forehead on one side with bulging on the opposite side
• Proptosis (bulging eyes)
• Strabismus (wandering eye)
• Dry eyes
• Corneal ulcers
• Blindness (if corneal damage is untreated)
• Upper airway obstruction with sleep apnea (partial or complete cessation of
respiration during sleep)
Nasal deformities may be congenital or acquired. Rhinoplasty is to change the shape or size
of the nose is considered medically necessary when performed as a result of disease,
structural abnormality, previous therapeutic process or reconstruction due to trauma.
Septoplasty is the surgical procedure to correct defects or deformities of the nasal septum,
often by alteration or partial removal of skeletal structures. Septoplasty is considered
medically necessary when there is a functional impairment that does not respond to medical
management treatment. Documentation must support failed conservative, medical
interventions for severe airway obstruction.
Prior Authorization for Craniofacial Deformities
PA is required for most rhinoplasty and septoplasty services. However, PA is not required
for members receiving rhinoplasty surgery related to a documented, primary diagnosis of
cleft lip and/or cleft palate. Please refer to the section of this fact sheet that describes cleft lip
and cleft palate services for additional information.
Congenital birth defects have a variety of presentations including cleft nasal deformity,
which may be associated with cleft lip and/or cleft palate, characterized by distorted,
01/31/2007 Surgery – Plastic / Reconstructive Surgery – Facial and Mazillofacial
Medical Policy Manual
651

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
abnormally developed nasal structures. Deviations in the septum can alter normal airflow,
which may result in mucosal changes. This interference in airflow may cause middle or
inferior turbinate abnormalities. Additionally, sinus drainage may be compromised by
deviation of the septum and can result in recurrent or chronic sinusitis. Surgical correction of
congenital birth defects may involve staged procedures, flaps, or grafts. Table 1, on page 5,
contains CPT codes utilized to report rhinoplasty services.
• Rhinoplasty is medically necessary when performed for correction or repair of the following
conditions.
• Nasal deformity secondary to a cleft lip/palate or other congenital craniofacial
deformity causing functional impairment
• Chronic, non-septal, nasal obstruction due to vestibular stenosis (collapsed internal
valves) secondary to trauma, disease, or congenital defect, when both of the
following criteria are met:
• Documentation of the member’s condition
• Nasal airway obstruction unresponsive to a recent trial of conservative
medical management that either has not resolved or would not be
expected to resolve with septoplasty/turbinectomy alone
• Septoplasty is medically necessary when performed for the following conditions.
• Recurrent epistaxis related to septal deformity
• Asymptomatic septal deformity that prevents access to other transnasal areas when
such access is required to perform medically necessary procedures (e.g.,
ethmoidectomy)
• In association with cleft lip or cleft palate repair
• Obstructed nasal breathing due to septal deformity or deviation that is
unresponsive to medical management and is interfering with the effective use of
medically necessary continuous positive airway pressure (CPAP) for treatment of
obstructive sleep disorder
B. External Ear Disorders
Coverage Criteria for External Ear Disorders
Microtia is a condition defined by an external ear that is not fully formed. Often, there is an
associated malformation of the external auditory canal and the middle ear bones which
transmit vibration of the eardrum to the cochlea. These anomalies occur as a part of many
developmental anomalies of the head and neck. Conductive hearing loss may be associated
with an abnormality of the external ear canal or middle ear. If hearing loss occurs in both
ears, hearing aid devices may be considered to regain functional hearing ability.
Prior Authorization for External Ear Disorders
The IHCP will provide reimbursement for, but is not limited to, the CPT codes included in
Table 1, on page 5, for members with repair and/or reconstruction of the external ear. The
01/31/2007 Surgery – Plastic / Reconstructive Surgery – Facial and Mazillofacial
Medical Policy Manual
652

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
codes in Table 1 are not meant to be all inclusive. Prior authorization will be granted based
on documentation of medical necessity maintained in the member’s medical record.
• History of the etiology of the external ear deformity
• Any other medical documentation that supports the member’s need of this service that
will assist in the review process
• Absence of any additional medical condition jeopardizing the end result of the
surgery or surgeries. These might include, for example, a suppressed immune
system, a current infection that is unresponsive to medical management, or medical
instability following illness or injury
C. Facial Nerve Disorders
Coverage Criteria for Facial Nerve Disorders
Facial nerve disorders often result from inflammation, infection, injury, or tumors in the
nerve tissue. Common symptoms of facial nerve disorders include, but are not limited to, the
conditions listed below.
• Numbness of the face
• Dryness of the eye secondary to reduced tear production
• Dryness of the mouth secondary to reduced saliva production
• Eyelid retraction and poor blinking mechanism
• Malpositioned eyelid and eyebrow
• Abnormal facial movement
• Facial twitching and/or spasms
• Disturbance of taste
• Restricted breathing
• Weakness of the arm, fingers, and hand on the same side as the facial weakness
Many types of nerve anomalies necessitate multiple operative procedures occurring at
different stages of skeletal development. When facial nerve injury occurs, treatment may
include repair or grafting of nerve tissue. The treatment of neurologic disorders may require
surgery that includes the following procedures.
• Facial nerve decompression
• Nerve repair and grafting
• Reinnervation techniques
• Regional muscle transfers
• Free muscle transfers
Physical therapy will improve functional outcomes allowing surgical repair of facial nerves,
especially for patients requiring tissue transfer. Physical therapy utilizes facial neuromuscular
retraining to optimize the motor control of facial muscles. Refer to the Therapy Services fact
sheet for physical therapy PA requirements.
01/31/2007 Surgery – Plastic / Reconstructive Surgery – Facial and Mazillofacial
Medical Policy Manual
653

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
PA Criteria for Facial Disorders
The IHCP will provide reimbursement for, but is not limited to, the CPT codes included in
Table 1, on page five, for the members with midface disorders, nasal deformities, external
ear disorders, and facial disorders. Providers are advised to report the most appropriate code
for the procedure performed. The prior authorization requirement is indicated for each CPT
code. The following information on the must be maintained in the member’s medical record.
• History of the presenting problem
• Symptoms related to the facial disorder
• Previously attempted, less-invasive medical management treatment that has failed
• Any other medical documentation that supports the member’s need of this service
• Absence of any additional medical condition jeopardizing the end result of the
surgery. These might include; for example, a suppressed immune system, a current
infection unresponsive to medical management, or medical instability following
illness or injury.
Table 1 – CPT Codes for Midface Disorders, Nasal Deformities,
External Ear Disorders, and Facial Disorders
CPT
Code
Description
PA
Requirement
21137 Reduction forehead; contouring only Yes
21138
Reduction forehead; contouring and application of prosthetic material
or bone graft
Yes
21139
Reduction forehead; contouring and setback of anterior frontal sinus
wall
Yes
21230
Graft; rib cartilage, autogenous, to face, chin, nose or ear (includes
obtaining graft
Yes
21235
Graft; ear cartilage, autogenous, to nose or ear (includes obtaining
graft)
Yes
21244
Reconstruction of mandible, extraoral, with transosteal bone plate (e.g.,
mandibular staple bone plate)
Yes
21245 Reconstruction of mandible or maxilla, subperiosteal implant; partial Yes
21246 Reconstruction of mandible or maxilla, subperiosteal implant; complete Yes
21247
Reconstruction of mandibular condyle with bone and cartilage
autografts (includes obtaining grafts) (eg, for hemifacial microsomia)
Yes
21248
Reconstruction of mandible or maxilla, endosteal implant (e.g., blade,
cylinder); partial
Yes
21249
Reconstruction of mandible or maxilla, endosteal implant (e.g., blade,
cylinder); complete
Yes
21255
Reconstruction of zygomatic arch and glenoid fossa with bone and
cartilage (includes obtaining autografts)
Yes
01/31/2007 Surgery – Plastic / Reconstructive Surgery – Facial and Mazillofacial
Medical Policy Manual
654

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 1 – CPT Codes for Midface Disorders, Nasal Deformities,
External Ear Disorders, and Facial Disorders
01/31/2007 Surgery – Plastic / Reconstructive Surgery – Facial and Mazillofacial
Medical Policy Manual
655
CPT
Code
PA
Requirement
Description
21256
Reconstruction of orbit with osteotomies (extracranial) and with bone
grafts (includes obtaining autografts) (eg, micro-ophthalmia)
No
21270 Malar augmentation, prosthetic material Yes
21295
Reduction of masseter muscle and bone (e.g., for treatment of benign
masseteric hypertrophy); extraoral approach
Yes
21296
Reduction of masseter muscle and bone (e.g., for treatment of benign
masseteric hypertrophy); intraoral approach
Yes
21299 Unlisted craniofacial and maxillofacial procedure Yes
30400
Rhinoplasty, primary; lateral and alar cartilages and/or elevation of
nasal tip
Yes
30410
Rhinoplasty complete, external parts including bony pyramid, lateral
and alar cartilages, and/or elevation of nasal tip
Yes
30420
Rhinoplasty complete, external parts including bony pyramid, lateral
and alar cartilages, and/or elevation of nasal tip including major septal
repair
Yes
30430
Rhinoplasty, secondary; minor revision (small amount of nasal tip
work)
Yes
30435 Rhinoplasty, intermediate revision (bony work with osteotomies) Yes
30450 Rhinoplasty, major revision (nasal tip work and osteotomies) Yes
D. Blepharoplasty
Coverage Criteria for Blepharoplasty
Blepharoplasty is a surgical procedure that removes excess skin and fatty tissue around the
eyes. The IHCP provides reimbursement for blepharoplasties to improve abnormal function
resulting in significant visual field loss or to reconstruct deformity due to trauma or a disease
process. Reimbursement is not provided for blepharoplasties to enhance the appearance of
the eyes.
PA Criteria for Blepharoplasty
PA is required for all blepharoplasties and documentation must support the medical necessity
to improve abnormal function that has resulted in significant visual field loss or to
reconstruct deformity due to trauma or a disease process as described on the next page.
IHCP reimbursement for reporting services related to blepharoplasty includes, but is not
limited to, the codes listed in Table 2.

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 2 – Codes for Reporting Blepharoplasty
CPT
Code
Description
PA
Requirement
15820 Blepharoplasty, lower eyelid; Yes
15821 Blepharoplasty, lower eyelid; with extensive herniated fat pad Yes
15822 Blepharoplasty, upper eyelid; Yes
15823
Blepharoplasty, upper eyelid; with excessive skin weighting down
lid
Yes
67900
Repair of brow ptosis (supraciliary, mid-forehead or coronal
approach)
Yes
67901
Repair of blepharoptosis; frontalis muscle technique with suture or
other material
Yes
67902
Repair of blepharoptosis; frontalis muscle technique with fascial
sling (includes obtaining fascia)
Yes
67903
Repair of blepharoptosis; (tarso)levator resection or advancement,
internal approach
Yes
67904
Repair of blepharoptosis; (tarso)levator resection or advancement,
external approach
Yes
67906
Repair of blepharoptosis; superior rectus technique with fascial
sling (includes obtaining fascia)
No
67911 Correction of lid retraction No
67912
Correction of lagophthalmos, with implantation of upper eyelid lid
load (eg, gold weight)
Yes
67914 Repair of ectropion; suture No
PA will be granted for blepharoplasty under the following indications.
• Upper eyelid blepharoplasty to relieve obstruction of central vision when ALL of the
following criteria are met.
o Visual field test without the eyelid or brow taped shows points of visual loss
inside the 25° circle of the superior field AND
o Visual field test with the eyelid or brow taped shows improvement in the superior
field with no visual loss inside the 40° circle of the superior field; AND
o A photograph of the patient looking straight ahead shows the eyelid at or below
the upper edge of the pupil
• Upper eyelid blepharoplasty for upper eyelid position that is contributing to prosthesis
difficulties in an anophthalmic (complete absence of the eye) socket
• Lower eyelid blepharoplasty to relieve excessive lower lid bulk secondary to systemic
corticosteroid therapy, myxedema, Graves’ disease, nephritic syndrome, or other
metabolic or inflammatory disorders that precludes proper positioning of eyeglasses
• Upper or lower eyelid Blepharoplasty to treat chronic corneal exposure and/or recurrent
corneal abrasions caused by conditions such as; ectropion (eyelid turning outward) or
entropion (eyelid turning inward)
01/31/2007 Surgery – Plastic / Reconstructive Surgery – Facial and Mazillofacial
Medical Policy Manual
656

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
II. Maxillofacial Services
The IHCP provides coverage for maxillofacial surgery services. PA is required for certain
maxillofacial surgeries as described elsewhere in this fact sheet. Providers may be required,
based upon the facts of the case, to obtain a second or third opinion substantiating the medical
necessity or approach for maxillofacial surgery related to disease and conditions of the jaw and
contiguous structures, regardless of the setting in which the procedure is performed. The
following maxillofacial services are covered by the IHCP.
• Orthognathic (jaw realignment) surgery with or without osteotomy
• Treatment of temporomandibular joint (TMJ) syndrome
• Removal of non-cancerous cysts, tumors, and growths of the oral and facial region
• Surgical removal of impacted teeth
• Treatment of facial fractures
• Treatment of soft tissue trauma
• Osseointegrated (bone anchored) implants, including dental and craniofacial implants
• Adjunctive treatment of sleep apnea including mandibular advancement splints and jaw
advancement surgery
• Reconstructive surgery for disease or trauma
• Salivary gland surgery
• Radiology services for evaluation of maxillofacial anomalies
• Anesthesia services for maxillofacial surgery
A. Orthognathic (Jaw Realignment) Surgery
Orthognathic surgery is the revision of the upper jaw (maxilla) and/or the lower jaw
(mandible) by ostectomy, osteotomy, or osteoplasty, and is intended to alter the relationship
of the jaws and teeth. These surgical procedures are intended to correct jaw and cranio-facial
deformities that are associated with significant functional impairment, or to reposition the
jaws when conventional orthodontic therapy is unable to correct dental malocclusion.
Coverage Criteria for Orthognathic (Jaw Realignment) Surgery
The IHCP reimburses orthognathic surgery to correct jaw and craniofacial deformities
causing significant functional impairment for members with congenital abnormality present
at birth, or to treat a significant accidental injury, infection, or tumor when ONE OR MORE
of the following clinical indications are met.
1. Anteroposterior discrepancies
• Maxillary/mandibular incisor relationship: overjet of 5 mm or more, or a value
less than or equal to zero (norm 2 mm)
• Maxillary/mandibular anteroposterior molar relationship discrepancy of 4 mm or
more (norm 0 to 1 mm)
• These values represent two or more standard deviations from published norms
01/31/2007 Surgery – Plastic / Reconstructive Surgery – Facial and Mazillofacial
Medical Policy Manual
657
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
2. Vertical discrepancies
• Presence of a vertical facial skeletal deformity which is two or more standard
deviations from published norms for accepted skeletal landmarks
• Open bite
o No vertical overlap of anterior teeth
o Unilateral or bilateral posterior open bite greater than 2 mm
• Deep overbite with impingement or irritation of buccal or lingual soft tissues of the
opposing arch
• Supraeruption of a dentoalveolar segment due to lack of occlusion
3. Transverse discrepancies
• Presence of a transverse skeletal discrepancy which is two or more standard
deviations from published norms
• Total bilateral maxillary palatal cusp to mandibular fossa discrepancy of 4 mm or
greater, or a unilateral discrepancy of 3 mm or greater, given normal axial inclination
of the posterior teeth
4. Asymmetries
• Presence of anteroposterior, transverse or lateral asymmetries greater than 3 mm with
concomitant occlusal asymmetry. ONE of the following symptoms must be present
due to the malocclusion.
• Difficulty swallowing and/or choking, or ability to chew only soft or liquid food;
• Symptoms must be documented in the medical record, must be significant, and must
persist for at least four months
• Other causes of swallowing/choking problems must have been ruled out by history,
physical exam, and/or appropriate diagnostic study including, but not limited to,
allergies, neurologic or metabolic diseases, and hypothyroidism
• Speech abnormalities have been determined by a speech pathologist or therapist to be
due to the malocclusions and not improved by speech therapy or orthodontia
• Malnutrition must be related to the inability to masticate, significant weight loss must
be documented over 4 months, and a low serum albumin exists that is related to
malnutrition
• Presence of intra-oral trauma while chewing related to malocclusion or recurrent
damage to the soft tissues of the mouth during mastication)
• Documentation of significant obstructive sleep apnea that is not responsive or
treatable by conservative means
An oral surgeon or a plastic surgeon may provide orthognathic surgical services as the
maxillofacial specialist performing the procedure. Other specialists, such as an orthodontist
or otolaryngologist may be required to assist with the procedure. The procedure may be
performed in an inpatient hospital, outpatient hospital, or ambulatory surgery center (ASC).
Orthognathic services are considered medical/professional services, and providers bill these
services using appropriate CPT codes on a CMS 1500 claim form or 837P electronic
transaction.
01/31/2007 Surgery – Plastic / Reconstructive Surgery – Facial and Mazillofacial
Medical Policy Manual
658

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Anesthesia for orthognathic surgery may be performed by an anesthesiologist or certified
nurse anesthetist, as medically appropriate. The anesthesia service should be billed with the
appropriate anesthesia CPT code for the head or neck (00100-00352) on the CMS 1500 claim
form or 837P electronic transaction.
Prior Authorization for Orthognathic Surgery and Related Procedures
PA is required for certain maxillofacial procedures related to diseases or conditions of the
jaw and contiguous structures, sliding mandibular osteotomies for prognathism (projected
jaw) or micrognathism (protracted jaw), and for certain reconstructive or plastic surgeries,
including genioplasty. The IHCP provides reimbursement for the codes listed in Table 3 for
orthognathic surgery and related procedures necessary for facial aesthetic surgery. The table
indicates whether PA is required for each procedure.
Table 3 – Orthognathic Surgery Codes
CPT
Code
Description
PA
Requirement
21110
Application of interdental fixation device for conditions other than
fracture or dislocation, includes removal
Yes
21125 Augmentation, mandibular body or angle; prosthetic material Yes
21127
Augmentation, mandibular body or angle; with bone graft, onlay
or interpositional (include obtaining autograft)
Yes
21141
Reconstruction midface, LeFort I; single piece, segment
movement in any direction (eg, Long Face syndrome), without
bone graft
No
21142
Reconstruction midface, LeFort I; two pieces, segment movement
in any direction, without bone graft
No
21143
Reconstruction midface, LeFort I; three or more pieces, segment
movement in any direction, without bone graft
No
21145
Reconstruction midface, LeFort I; single piece, segment
movement in any direction, requiring bone grafts (includes
obtaining autografts)
No
21146
Reconstruction midface, LeFort I; two pieces, segment movement
in any direction, requiring bone grafts (includes obtaining
autografts) (eg, ungrafted unilateral alveolar cleft)
No
21147
Reconstruction midface, LeFort I; three or more pieces, segment
movement in any direction requiring bone grafts (includes
obtaining autografts) (eg, ungrafted bilateral alveolar cleft or
multiple osteotomies)
No
21150
Reconstruction midface, LeFort II; anterior intrusion (e.g.,
Treacher-Collins Syndrome)
No
21151
Reconstruction midface, LeFort II; any direction, requiring bone
grafts (includes obtaining autografts)
No
21154
Reconstruction midface, LeFort III (extracranial), any type,
requiring bone grafts (includes obtaining autografts); without
LeFort I
No
01/31/2007 Surgery – Plastic / Reconstructive Surgery – Facial and Mazillofacial
Medical Policy Manual
659

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Surgery – Plastic / Reconstructive Surgery – Facial and Mazillofacial
Medical Policy Manual
660
Table 3 – Orthognathic Surgery Codes
CPT
Code
PA
Description
Requirement
21155
Reconstruction midface, LeFort III (extracranial), any type,
requiring bone grafts (includes obtaining autografts); with LeFort I
No
21193
Reconstruction of mandibular rami, horizontal, vertical, C, or L
osteotomy; without bone graft
No
21194
Reconstruction of mandibular rami, horizontal, vertical, C, or L
osteotomy; with bone graft (includes obtaining autograft)
No
21195
Reconstruction of mandibular rami and/or body, sagittal split;
without internal rigid fixation
No
21196
Reconstruction of mandibular rami and/or body, sagittal split; with
internal rigid fixation
No
21198 Osteotomy, mandible, segmental No
21206 Osteotomy, maxilla, segmental (eg, Wassmund or Schuchard) Yes
21208
Osteoplasty, facial bones; augmentation (autograft, allograft, or
prosthetic implant)
No
21209 Osteoplasty, facial bones, reduction Yes
21210
Graft, bone, nasal, maxillary, or malar areas ( includes obtaining
graft)
Yes
21215 Graft, bone, mandible (includes obtaining graft) Yes
21247
Reconstruction of mandibular condyle with bone and cartilage
autografts (includes obtaining grafts) (eg, for hemifacial
microsomia)
Yes
B. Temporomandibular Joint Syndrome
Temporomandibular joint syndrome or temporomandibular joint (TMJ) disorder is a
condition resulting from macro-trauma or micro-trauma to the temporomandibular jaw joint
and its surrounding muscles and tissues. Macro-trauma is usually a result of direct trauma to
the TMJ. Micro-trauma is usually a chronic, indirect process that can be associated with
conditions such as stress, anxiety, sleep disorders, dysfunctional occlusions, and myofascial
disorders. Causes of TMJ include acute injury, clenching or grinding of the teeth, muscle
spasms, dental occlusions, and degenerative joint disease. Common symptoms of TMJ
include the following.
• Clicking, popping, grating, or grinding sounds when moving the jaw
• TMJ pain or stiffness while chewing, talking, or yawning
• Facial pain, especially in the ear region
• Jaw locking open or closed
• Difficulty in opening the jaw wide
• Uncomfortable bite
• Headache or earache

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Coverage Criteria for Treatment of TMJ
The IHCP will cover both non-surgical and surgical treatments for TMJ. There are several
approaches to treatment for TMJ based on the cause and severity of the disease; however,
most members can be treated without surgery. IHCP members must receive a trial of
conservative therapy before surgical treatment for TMJ will be prior authorized.
Nonsurgical Treatment of TMJ
Documentation in the member’s medical records must indicate that at least two of the
following forms of non-surgical interventions have been performed for a total of three to six
months without adequate relief of symptoms prior to being evaluated for surgical treatment
of TMJ.
1. Medical management may include non-opiate analgesics and non-steroidal anti-
inflammatory drugs (NSAIDS) for mild-to-moderate inflammatory conditions and pain.
Low-dosage tricyclic antidepressants may be prescribed for chronic pain, sleep
disturbances, and nocturnal bruxism. Adjuvant pharmacologic therapies may include
anticonvulsants, membrane stabilizers, and sympatholytic agents for unremitting pain,
and opiate analgesics, corticosteroids, anxiolytics, and muscle relaxants for refractory
pain. Osteopathic manipulative therapy may be included as part of the medical
management prescribed. Medical management may be prescribed by a dentist,
orthodontist, an ear nose and throat (ENT) specialist, psychiatrist, or oral surgeon.
2. Physical therapy (PT) for TMJ may include active and passive jaw exercises, thermal
modalities, manipulation modalities, electrogalvanic stimulation, and transcutaneous
electrical nerve stimulation (TENS). Table 4, on the next page, lists the appropriate
codes to report PT for TMJ. Cranial manipulation, continuous passive motion, diathermy,
infrared, and ultrasound treatment, hydrotherapy, myofunctional therapy, iontophoresis,
and neuromuscular re-education are not considered medically necessary physical therapy
treatments for TMJ. PA is required for physical therapy as designated below. Refer to
the Medical Policy fact sheet entitled Therapy Services for further information regarding
physical therapy services.
Table 4 – Physical Therapy Codes for TMJ
CPT
Code
Description
PA
Requirement
64550 Application of surface (transcutaneous) neurostimulator No
97010
Application of a modality to one or more areas; hot or cold
packs
Yes
97032
Application of a modality to one or more areas; electrical
stimulation (manual), each 15 minutes
No
97110
Therapeutic procedure, one or more areas, each 15 minutes;
therapeutic exercises to develop strength and endurance,
range of motion and flexibility
Yes
97140
Manual therapy techniques (eg, mobilization/manipulation,
manual lymphatic drainage, manual traction), one or more
regions, each 15 minutes
No
01/31/2007 Surgery – Plastic / Reconstructive Surgery – Facial and Mazillofacial
Medical Policy Manual
661

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
3. Psychiatric/psychological therapy may be initiated when TMJ is caused by a
psychosomatic condition due to stress or anxiety. For example, bruxism, or teeth
grinding, considered a psychophysiological disorder, is a common tension habit that can
lead to TMJ. IHCP members without other obvious causative factors for TMJ symptoms
(such as major trauma, arthritis, and jaw misalignment) should be evaluated for
psychosomatic causes, and treated as appropriate. The IHCP will reimburse for the
psychiatric codes listed in Table 5, in relation to treatment of TMJ.
Table 5 – Psychiatric Codes for TMJ Therapy
CPT
Code
Range
Description
PA
Requirement
90801 Psychiatric diagnostic interview No
90804-
90809
Behavior modifying and/or supportive psychotherapy
(office/outpatient)
No
90816-
90822
Behavior modifying and/or supportive psychotherapy
(inpatient, residential care)
No
90846-
90853
Family and group psychotherapy No
4. Mechanical therapy is provided through removable intra-oral appliances. Intra-oral
appliances are used to treat members with dysfunctional occlusions. Treatment generally
lasts for up to six months. The IHCP will provide reimbursement for intra-oral
appliances, using HCPCS code E1700, Jaw motion rehabilitation system. No PA is
required. Intra-oral appliances may be provided by a dentist, ENT specialist,
orthodontist, or oral surgeon.
Surgical Treatment of TMJ
Treatment of TMJ by maxillofacial surgery will be covered if both of the following
conditions are met.
1. A physical exam, diagnostic X-rays (70328-70330), arthrography (70332), or
orthopantogram (70355), and diagnostic imaging (CT, MRI, or arthroscopy) indicate an
intra-articular cause of TMJ.
2. At least two non-surgical methods of treatment, as described previously, have been tried
and have failed to adequately relieve the member’s symptoms. Documentation in the
medical record must establish that non-surgical treatment has been attempted for a period
of three to six months prior to a request for prior authorization for surgery.
Prior Authorization for the Surgical Treatment of TMJ
PA is required for a diagnostic arthroscopy (29800) and an arthrotomy of the TMJ joint
(21010). Table 6, on page 14, lists the radiology codes for evaluation of TMJ that are
covered by the IHCP. Table 7, on page 14, lists the codes for reporting treatment of TMJ
that are covered by the IHCP. PA requirements are noted in both tables.
01/31/2007 Surgery – Plastic / Reconstructive Surgery – Facial and Mazillofacial
Medical Policy Manual
662
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Prior authorization will be granted for surgical treatment of TMJ under the following
circumstances. Documentation must be maintained in the member’s medical record and be
submitted for prior authorization of these procedures.
1. Arthrocentesis is covered when the following conditions are met.
• Persistent pain for more than three to six months that cannot be controlled by non-
surgical treatment
• Clinical examination and/or diagnostic imaging that indicates the presence of
hypomobility of the TMJ joint
• Medically necessary instillation of therapeutic drugs into the joint
2. Arthroscopy is covered when the following conditions are met.
• Persistent pain for more than three to six months that cannot be controlled by non-
surgical treatment
• Clinical examination and/or diagnostic imaging indicates joint pathology such as
internal derangement, hypomobility, or hypermobility that requires internal structural
modification
3. Arthrotomy, disc plication, discectomy, and arthroplasty with or without autograft and
allograft are covered when the following conditions are met.
• Persistent pain for more than three to six months that cannot be controlled by non-
surgical treatment
• Severe, unremitting pain
• Clinical examination and/or diagnostic imaging indicates joint pathology, such as
internal derangement, hypomobility, or hypermobility that requires internal structural
modification where minimally invasive surgery, such as arthrocentesis or
arthroscopy, is not appropriate or has failed
4. Arthroplasty with total prosthetic joint replacement is covered when the following
conditions are met.
• Inflammatory arthritis involving the TMJ which is not responsive to other modalities
of treatment
• Recurrent fibrosis and/or bony ankylosis not responsive to other modalities of
treatment
• Failed tissue graft
• Failed previous joint reconstruction
• Loss of vertical mandibular condylar height due to bone reabsorption, trauma,
developmental abnormality, or pathologic lesion
01/31/2007 Surgery – Plastic / Reconstructive Surgery – Facial and Mazillofacial
Medical Policy Manual
663

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 6 – Radiology Codes for the Evaluation of TMJ
CPT
Codes
Description
PA
Requirement
70328
Radiology examination, temporomandibular joint, open and closed
mouth; unilateral
No
70330
Radiology examination, temporomandibular joint, open and closed
mouth; bilateral
No
70332
Temporomandibular joint arthrography, radiological supervision and
interpretation
No
70336 Magnetic resonance (e.g., proton) imaging, temporomandibular joints No
70450 Computed tomography, head or brain; without contrast material No
70460 Computed tomography, head or brain; with contrast material(s) No
70470
Computed tomography, head or brain; without contrast material,
followed by contrast material(s) and further sections
No
70486 Computed tomography, maxillofacial areas; without contrast material No
70487 Computed tomography, maxillofacial areas; with contrast material(s) No
70488
Computed tomography, maxillofacial areas; without contrast material,
followed by contrast material(s) and further sections
No
Table 7 – Surgery Codes for the Treatment of TMJ
CPT
Codes
Description PA
Requirement
20605
Arthrocentesis, aspiration and/or injection; intermediate joint or bursa
(eg, temporomandibular, acromioclavicular, wrist, elbow, or ankle,
olecranon bursa)
No
21010 Arthrotomy, temporomandibular joint Yes
21050 Condylectomy temporomandibular joint No
21060
Meniscectomy, partial or complete, temporomandibular joint (separate
procedure)
No
21070 Coronoidectomy (separate procedure) No
21116 Injection procedure for temporomandibular joint arthrography No
21240
Arthroplasty, temporomandibular joint, with or without autograft
(includes obtaining graft)
No
21242 Arthroplasty, temporomandibular joint, with allograft No
21243
Arthroplasty, temporomandibular joint, with prosthetic joint
replacement
No
21480
Closed treatment of temporomandibular dislocation; initial or
subsequent
No
21485
Closed treatment of temporomandibular dislocation; complicated (e.g.,
recurrent requiring intermaxillary fixation or splinting), initial or
subsequent
No
21490 Open treatment of temporomandibular dislocation No
29800
Arthroscopy, temporomandibular joint, diagnostic, with or without
synovial biopsy (separate procedure)
Yes
29804 Arthroscopy, temporomandibular joint, surgical No
01/31/2007 Surgery – Plastic / Reconstructive Surgery – Facial and Mazillofacial
Medical Policy Manual
664

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Surgery – Plastic / Reconstructive Surgery – Facial and Mazillofacial
Medical Policy Manual
665
Anesthesia for the Surgical Treatment of TMJ
Local anesthesia is usually adequate arthrocentesis and arthroscopic TMJ procedures. The local
anesthesia is included in the reimbursement for the procedure. General anesthesia may be
necessary for other TMJ procedures. General anesthesia may be performed by an
anesthesiologist or certified nurse anesthetist, as medically appropriate. IHCP providers are
advised to report professional service for anesthesia with the appropriate anesthesia CPT code
for the head or neck (00100-00352) on the CMS 1500 claim form or 837P electronic transaction.
Billing for the Surgical Treatment of TMJ
An oral surgeon or plastic surgeon may provide radiologic and surgical services as the
maxillofacial specialist treating TMJ. Surgical treatments for TMJ are considered
medical/professional services; therefore, providers must bill these services using the appropriate
CPT code on a CMS 1500 or 837P electronic transaction. Arthrocentesis, arthroscopy, and
uncomplicated, closed treatment of TMJ dislocation may be performed in an office setting, as
well as an outpatient or ASC setting. The remaining procedures may be performed in an
inpatient hospital, outpatient hospital, or ASC setting.
III. CLEFT LIP AND CLEFT PALATE
Cleft lip and cleft palate are congenital defects which occur during in-utero stages of
development. Cleft lip is a separation of the two sides of the lip. This separation may include
the bones of the upper jaw and/or upper tissue of the gum. Cleft palate is an opening in the roof
of the mouth in which the two sides of the palate did not fuse or join together. Either defect may
occur unilaterally or bilaterally.
Cleft palate and cleft lip may cause problems related to eating, speaking, and facial structure.
The following findings are common problems resulting from these developmental defects.
• Swallowing difficulties
• Middle ear dysfunction
• Speech difficulties
• Poor facial muscle control
• Abnormal dentition
Coverage Criteria for Cleft Lip and Cleft Palate
The IHCP stipulates that the treatment of cleft lip and cleft palate must be provided by a
craniofacial interdisciplinary team of health care professionals. According to the American Cleft
Palate-Craniofacial Association, the following health disciplines may be included in the overall
treatment process.
• Anesthesiology
• Audiology
• Dentistry
• Genetic counseling
• Neurology
• Ophthalmology
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• Oral and maxillofacial surgery
• Orthodontics
• Otolaryngology
• Plastic surgery
• Prosthodontics
• Radiology
• Speech-language pathology
• Social services
The craniofacial team monitors the member's condition throughout treatment and provides any
required interventions. Consultation with other professionals may be necessary depending on the
member’s needs. Specific aspects that are monitored may include, but not be limited to, the
following.
• Height and weight
• Nutritional intake and feeding
disorder symptoms
• Growth, motor, cognitive, and
social development
• Speech and language
development
• Hearing status
• Dentition
• Genetic diagnoses
01/31/2007 Surgery – Plastic / Reconstructive Surgery – Facial and Mazillofacial
Medical Policy Manual
666

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Otolaryngologists, plastic surgeons, and oral surgeons usually recommend surgery to
correct left lip and cleft palate deformities. Depending on condition of the member,
secondary surgical procedures may be required involving the lip, nose, palate, and jaw.
These procedures usually are staged over a period of several years.
Prior Authorization for Cleft Lip and Cleft Palate Services
PA is not needed for cleft lip and cleft palate services, except orthodontic services related
to cleft palate. Orthodontic PA requests must be submitted on the Indiana Prior Review
and Authorization Request Form, not the Indiana Prior Review and Authorization Dental
Form. Documentation for services must be maintained in the member’s dental or medical
record and treatment plan. Members are expected to continue treatment with the same
practitioner for the period of treatment time that is prior authorized. If the member must
discontinue treatment with one practitioner and begin treatment with another practitioner,
the practitioner continuing the treatment must submit a new PA request. The first
practitioner must refund part of the reimbursement to the IHCP. Generally, one third of
the reimbursement is for the evaluation and treatment plan, and two thirds of the
reimbursement is for the actual treatment. Based upon the time remaining in the
treatment rendered by a new practitioner, the first practitioner must prorate the amount to
be refunded to the program.
Rhinoplasty may be required to treat a cleft lip or cleft palate. Appropriate HCPCS codes
for these services include HCPCS code 30460, Rhinoplasty for nasal deformity secondary
to congenital cleft lip and/or palate, including columellar lengthening; tip only, and
HCPCS code 30462, Rhinoplasty for nasal deformity secondary to congenital cleft lip
and/ or palate, including columellar lengthening; tip, septum, osteotomies. All of the
following criteria must be documented in the member’s medical record to confirm the
medical necessity for these services.
• Documentation of the extent of the deformity and associated symptoms
• Documentation of the plan for surgical correction
• Photographs to verify a plan that includes multiple surgeries
• History and physical including any problems/congenital deformities that could
potentially affect the outcome of the requested procedure
• Documented evidence of family/caregiver education about the plan of care
and
special health care needs of the member, pre and post op
• The requested surgery is expected to correct a specified portion of the
deformity
• The requested surgery is expected to improve the member’s functional status
Prior Authorization of Orthodontic Services for Craniofacial Deformity or Cleft
Palate
01/31/2007 Surgery – Plastic / Reconstructive Surgery – Facial and Mazillofacial
Medical Policy Manual
667

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Orthodontic services are covered for members with documentation of diagnoses of
craniofacial anomaly and malocclusion. All appliances, retainers, and repair or
replacement of retainers are included in the fee for the comprehensive treatment and may
not be billed separately if comprehensive treatment is rendered. Table 8 below contains
appropriate orthodontic HCPCS codes and descriptions for craniofacial services. All of
the following criteria are required to meet medical necessity criteria.
• A member must be diagnosed by a practitioner of a recognized craniofacial team
• The member must be treated by a licensed practitioner, who accepts routine
craniofacial members for orthodontic services
• A signed statement from the practitioner, who is a member of a hospital based
craniofacial team, will certify the correct craniofacial diagnosis and
malocclusion
• The diagnosis must include information a description of facial and soft tissue,
skeletal, dental, occlusal, functional, and applicable medical conditions
• A step-wise treatment plan must be submitted with the treatment phase and an
approximate length of time for treatment identified
• The PA request must be for the time period specified
Table 8 – Craniofacial Orthodontic HCPCS Codes
HCPCS
Code
Description
D8010 Limited orthodontic treatment of the primary dentition
D8020 Limited orthodontic treatment of the transitional dentition
D8030 Limited orthodontic treatment of the adolescent dentition
D8040 Limited orthodontic treatment of the adult dentition
D8050 Interceptive orthodontic treatment of the primary dentition
D8060 Interceptive orthodontic treatment of the transitional dentition
D8070 Comprehensive orthodontic treatment of the transitional dentition
D8080 Comprehensive orthodontic treatment of the adolescent dentition
D8090 Comprehensive orthodontic treatment of the adult dentition
D8210 Removable appliance therapy
D8220 Fixed appliance therapy
NONCOVERED SERVICES
The IHCP does not provide reimbursement for the following services.
• Blepharoplasties when not related to a significant obstructive vision problem
• Dermabrasion surgery for acne pitting or marsupialization
• Ear piercing
• Ear lobe reconstruction
• Otoplasty for protruding ears unless one of the following applies
01/31/2007 Surgery – Plastic / Reconstructive Surgery – Facial and Mazillofacial
Medical Policy Manual
668

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
o Multifaceted craniofacial abnormalities due to congenital
malformation or maldevelopment, such as Pierre Robin Syndrome
o A member has pending or actual employment where protruding ears
would interfere with the wearing of required protective devices
• Removal of keloids caused from pierced ears unless one of the following is
present
o Keloids are larger than three centimeters
o Obstruction of the ear canal is fifty percent or more
• Rhinoplasty or bridge repair of the nose in the absence of a significant
obstructive breathing problem
• Rhytidectomy
• Scar removals or tattoo removals by excision or abrasion
MANAGED CARE
For members enrolled in Risk Based Managed Care (RBMC), providers must contact the
member’s managed care organization (MCO) for more specific guidelines. IHCP
members enrolled in Hoosier Healthwise PrimeStep primary care case management
(PCCM) receive the same benefit coverage, and are subject to the same limitations as
traditional Medicaid fee-for-service (FFS) members. Refer to the Hoosier Healthwise
Manual for Primary Medical Providers and Office Staff for further information.
Additionally, refer to the IHCP Provider Manual, Chapter 1, for detailed information
about the FFS, PCCM, and RBMC delivery systems.
BILLING REQUIREMENTS
PA does not guarantee reimbursement for services. Documentation supporting medical
necessity must be maintained in the medical records. Providers are advised to report the
procedure code that best describes the services rendered. Billing requirements for
specific procedures are included in this fact sheet.
Reimbursement for cleft lip, cleft palate, and orthodontic services for craniofacial
deformity is determined by diagnosis-related group (DRG) relative weights and average
lengths of stay. Hospitals cannot bill IHCP members for the difference between
payments and actual charges, except for those conditions stated in the IHCP Provider
Manual, Chapter 4. Refer to the IHCP Provider Manual for specific information
regarding reimbursement for these services.
RELATED MEDICAL TOPICS
Anesthesia Services
Dental Services
01/31/2007 Surgery – Plastic / Reconstructive Surgery – Facial and Mazillofacial
Medical Policy Manual
669

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Mental Health Services
Pharmacy – Botulinum Toxin A (BOTOX)
Speech and Hearing Services
Surgical Services
Therapy Services
RULES, CITATIONS, AND SOURCES
Indiana Administrative Code
405 IAC 5-3-13 – Services Requiring Prior Authorization
405 IAC 5-14-3 – Diagnostic Services
405 IAC 5-14-21 – Maxillofacial Surgery
405 IAC 5-28 – Medical and Surgical Services
405 IAC 5-29-1 – Services Not Covered by Medicaid
IHCP Provider Banners
BR200519 – Flexible Base Dentures
IHCP Provider Bulletins
BT19909 – Removal of Services from Prior Authorization
BT200109 – HCPCS Code Updates
BT200208 – Modifications to Prior Authorization Requirement
BT200230 – Criteria for Orthodontic Services
BT200231 – Carve Out and Self-Referral Education
BT200321 – Correct Codes for Billing of IHCP Dental Services
BT200360 – Changes in DRG Relative Weights and New Long Term Acute Care
Level- of-Care Reimbursement Methodology
BT200362 – Prior Authorization for Hoosier Healthwise Managed Care
Organizations
IHCP Provider Newsletters
NL200505 – RBMC Carve Out Dental Guidelines
IHCP Provider Manual – March 2005, Version 5.1
Origination Date: 01/31/06
Revisions and
Review
Reason Date
Revision
Scheduled review and separation of the Global
Surgery Fact Sheet into multiple fact sheets
1/31/06
APPLICABLE INDIANAAIM EDITS AND AUDITS
6001 – Complete Radiology & Pathology Procedures Payable at Reduced Amt When
Professional/Technical Components Already Paid
01/31/2007 Surgery – Plastic / Reconstructive Surgery – Facial and Mazillofacial
Medical Policy Manual
670
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Surgery – Plastic / Reconstructive Surgery – Facial and Mazillofacial
Medical Policy Manual
671
6002 – CRNA/AA and Anesthesiologist Same Procedure Requires Review
6003 – Manual Pricing with Split Care Billing
6011 – Professional/Technical Components for Radiology or Pathology Not Payable
When Complete Procedure Already Paid
6034 – Global Surgery Payable at Reduced Amount When Components of Surgical Care
Paid
6035 – Components of Surgical Care Not Payable When Global Surgery Paid
6037 – Only One Assistant Surgeon Allowed for Select Surgeries
6039 – Assistant Surgeon Not Payable When Co-Surgeon Paid
6040 – Co-Surgeon Not Payable When Assistant Surgeon Paid
6049 – Components Not Payable When Global Paid – Integumentary and Neuromuscular
System
6096 – The CPT/HCPCS Code Billed Is Not Payable According to the PPS
Reimbursement Methodology
6098 – Chiropractic Services Limited to Procedure and Diagnosis Codes Identified
6099 – Reimbursement is Limited to 50 Chiropractic Visits per Calendar Year
6100 – Maximum of 60 Chiropractic Therapeutic Physical Medicine Treatments per
Calendar Year
6110 – Component Procedures Not Payable When Global Procedure Paid – Radiology
Services
6112 – Maximum of 14 Chiropractic Therapeutic Physical Medicine Treatments per
Calendar Year
6115 – Physical Therapy Services Limited to 50 Visits per Calendar Year
6118 – Occupational Therapy Services Limited to 50 Visits per Calendar Year
6120 – Outpatient Mental Health/Substance Abuse Services-Office Visits, Maximum 30
per Calendar Year W/Out Prior Authorization
6121 – Outpatient Mental Health/Substance Abuse Services-Office Visits, Maximum
Fifty (50) per Calendar Year with Prior Authorization
6122 – Chiropractic Therapeutic Physical Medicine Treatments 15 through 50 Require
Prior Authorization
6171 – One MRT Service per Lifetime
6403 – Respiratory Mutually Exclusive Codes CCI
6652 – Multiple Surgeries Must Be Billed on Same Claim
6661 – Duramorph Cannot Be Billed on Same Day as Surgery
6666 – Anesthesia Services Not Allowed By Provider Billing for Surgery
6900 – Outpatient Mental Health
Services More Than 20 per 27 Days

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: SURGERY - PLASTIC AND RECONSTRUCTIVE -
PANNICULECTOMY
DESCRIPTION
Dorlands Medical Dictionary defines a panniculectomy as the surgical removal of
excessive skin, subcutaneous tissue and fat of the abdominal wall. While it is similar to
abdominoplasty, which is typically used for cosmetic purposes, panniculectomy involves
the removal of a hanging apron of excess skin, the pannus or panniculus, in a transverse
or vertical wedge, but does not include muscle plication, neoumbilicoplasty or flap
elevation.
This document is intended to serve as a general summary of the Indiana Health Coverage
Programs’ (IHCP) policies regarding this service. More specific information may be
found in the IHCP Provider Manual, program notices, the Indiana Administrative Code
(IAC) or other sources as appropriate. See the “Rules, Citations, and Sources” section of
this document for further information.
COVERAGE CRITERIA
All requests for panniculectomy will require prior authorization with an appropriate
clinical summary and physician documentation of medical necessity. According to the
IHCP Provider Manual, Version 5.1, “Morbid Obesity is a disorder associated with
medical complications, and it, by itself, is considered a disease.” The International
Classification of Diseases 9
th
Edition Clinical Modification (ICD-9-CM) diagnosis codes
for this disease are 278.01, Morbid Obesity or 278.00, Obesity, unspecified.”
Massive weight loss, defined as loss of 50 percent of excess weight, often results in laxity
and redundancy of the abdominal skin termed a panniculus. Patients with a large
panniculus may experience functional and hygiene problems. At extremes, a panniculus
may have recurrent infections, ulcerate and even become necrotic. Panniculectomy for
medical necessity should be considered when ALL the following criteria are met.
• Pannus hangs at or below the level of the symphysis pubis, as demonstrated on
pre-operative photographs.
• Pannus causes a chronic and persistent skin condition (e.g. intertriginous
dermatitis, panniculitis, cellulitis, or skin ulcerations) that is refractory to at least
six months of medical treatment in addition to good hygiene practices. Treatment
01/31/2007 Surgery – Plastic / Reconstructive Surgery – Panniculectomy
Medical Policy Manual
672

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
should include topical antifungals; topical and/or systemic corticsteroids; and/or
local or systemic antibiotics.
• Pannus interferes with activities of daily living.
Current Procedural Terminology (CPT) code for panniculectomy may be found in Table
1, Panniculectomy Procedure Code.
Table 1 – Panniculectomy Procedure Code
CPT Code Description
15831
Excision, excessive skin and subcutaneous tissue (including
lipectomy); abdomen (abdominoplasty)
The diagnosis codes which may be appropriate in determining medical necessity for
panniculectomy may be found in Table 2, Panniculectomy Diagnosis Codes.
Table 2 – Panniculectomy Diagnosis Codes
Diagnosis Code Description
278.1 Localized adiposity
682.2 Other cellulitis and abscess, trunk
692.9 Contact dermatitis and other eczema, unspecified
695.89 Unspecified erythematous condition
701.8
Other specified hypertrophic and atrophic conditions of
skin
701.9 Unspecified hypertrophic and atrophic conditions of skin
707.8 Chronic ulcer of other specified site
707.9 Chronic ulcer of unspecified site
729.30 Panniculitis, unspecified site
729.39 Panniculitis, other sites
According to the Medical Policy (MP) Bariatric Surgery Fact Sheet, “Panniculectomy
following gastric bypass procedures performed for cosmetic reasons, even if performed
incidentally to a ventral herniorrhaphy, is a non-covered service.”
PRIOR AUTHORIZATION
Many surgeries require prior authorization, as required in the Indiana Administrative
Code (IAC) citation 405 IAC 5-3. Prior authorization for multiple surgeries on the same
day does not override the restrictions of 405 IAC 5-28. Prior authorization is required for
panniculectomy. Information that should be included along with the request for prior
authorization includes the following.
• The member’s diagnosis(es)
• The member’s current weight and height
• Preoperative photograph(s) front and lateral views
01/31/2007 Surgery – Plastic / Reconstructive Surgery – Panniculectomy
Medical Policy Manual
673

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• History and physical including all previous surgeries and the member’s weight
loss history
• Medical documentation of medical conditions and complications of infections
outlining all treatments, including duration and responses.
• Documentation of limitations on mobility and daily activities due to the pannus or
resulting complications.
MANAGED CARE
For members enrolled in Risk Based Managed Care (RBMC), providers must contact the
member’s managed care organization (MCO) for more specific guidelines. Refer to the
Provider Manual, Chapter 1, for detailed information about the fee-for-service (FFS),
Primary Care Case Management (PCCM), and Risk Based Managed Care (RBMC)
delivery systems.
IHCP members enrolled in Medicaid Select PCCM receive the same benefit coverage,
and are subject to the same limitations as traditional Medicaid FFS. Refer to the
Medicaid Select Manual for Primary Medical Providers and Office Staff for further
information.
BILLING REQUIREMENTS
Reimbursement requires compliance with all IHCP guidelines including obtaining
appropriate referrals for recipients enrolled in Medicaid Managed Care programs.
Physicians bill professional services on the CMS-1500 claim form. Providers must bill
the ICD-9-CM diagnosis code to the highest level of specificity that supports medical
necessity.
RELATED MEDICAL TOPICS
Surgical Services
Anesthesia Services
Gastroenterology
Consultations-Second Opinion
RULES, CITATIONS, AND SOURCES
405 IAC 5-3-13 Services requiring prior authorization
405 IAC 5-28-1 Reimbursement limitations
405 IAC 5-29-1 Services not covered by Medicaid
Provider Manual, Version 5.1
01/31/2007 Surgery – Plastic / Reconstructive Surgery – Panniculectomy
Medical Policy Manual
674

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Surgery – Plastic / Reconstructive Surgery – Panniculectomy
Medical Policy Manual
675
Origination Date: October 1, 2006
Revisions and
Review
Reason Date
Origination 10/01/ 2006
APPLICABLE INDIANAAIM EDITS AND AUDITS
6002- Any Two Anesthesiology Providers Same Procedure Required
6003- Manual Pricing for Split Care Billing
6034- Global Surgery Payable at Reduced Amount When Components of Surgical Care
Paid
6035- Components of Surgical Care Not Payable When Global Surgery Paid
6039- Assistant Surgeon Not Payable When Co-Surgeon Paid
6040- Co-Surgeon Not Payable When Assistant Surgeon Paid
6661- Duramorph Can Not be Billed on Same Day as Surgery
6666- Anesthesia Services Not Allowed by Provider Billing for Surgery

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: SURGERY—REMOVAL OF IMPLANTS
DESCRIPTION:
Medical implants, i.e. pins, screws, rods, plates, etc., many times will need to be removed
when the fracture has healed or the symptoms that required implantation of the device
abate. The implant may cause pain and restrict movement. The treatment is to remove
the implant.
SUMMARY OF CURRENT POLICY:
Implant removals requiring an operating room are usually considered minor procedures
and the rules governing minor procedures in 405 IAC 5-28-1 apply. Some implants may
be removed in the physician’s office and should be included in the office fee claim.
MEDICAL TOPICS CROSS-REFERENCES:
Anesthesia Services
Hospital Outpatient
Ophthalmology
Radiology
Physical Rehabilitation Services
Surgery-Global Billing/Payment Guidelines
Surgery-Multiple Procedures/Same Operative Session
Surgery-Office Visits
Surgery-Services Requiring Prior Authorization
Surgery-Surgery and Anesthesia By the Same Provider
Surgery-Surgeon and Assistant Surgeon, Same Provider
Surgery-Surgical Services
Surgery-Suture of Wounds
Urology
01/31/2007 Surgery – Removal of Implants
Medical Policy Manual
676

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
RULES, CITATIONS, AND SOURCES:
405 IAC 5-28 Medical and Surgical Services
Indiana State Department of Public Welfare Medical Policy Manual 1991
Indiana Health Coverage Programs Provider Manual 1999
Initial Policy
Issues
Effective Date Implementation
Date
Retroactive Date
Indiana State
Department of
Public Welfare
Medical Policy
Manual 1991
Removal of
Implants
7/1/91
470 5-9-1
Transferred
Global Fee
Billing
7/1//91
405 IAC 1-7-1
Repealed 8/24/97
Global Fee
Billing
1/1/92
Revisions:
405 IAC 5-1-5 Global Fee
Billing
8/24/97
405 IAC 5-28 Medical and
Surgical
Services
8/24/97
APPLICABLE INDIANAAIM EDITS AND AUDITS:
6014
6015
6017
6022
6023
6034
6035
6048
6049
6061
6063
6064
6071
6072
6630
6634
01/31/2007 Surgery – Removal of Implants
Medical Policy Manual
677
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Surgery – Removal of Implants
Medical Policy Manual
678
6649
6650
6653
6654
6655
6656
6657
6658
6659
6660
6664
6706
COVERAGE CRITERIA:

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: SURGERY—SERVICES REQUIRING PRIOR
AUTHORIZATION
DESCRIPTION:
Some specifically listed surgical procedures, according to guidelines in 405 IAC 5-3-13,
require prior authorization. Criteria for coverage must be documented in the request for
prior authorization and approval received before the service is provided in order to
receive reimbursement for those specified surgical services.
SUMMARY OF CURRENT POLICY:
Medicaid reimbursement is available for certain surgeries, as listed in 405 IAC 5-3-13,
when prior authorization is received in accordance with the prior authorization
guidelines, as outlined below. Presently, the list includes, such surgical procedures as
reduction mammoplasties, rhinoplasty or bridge repair of the nose when related to a
significant obstructive breathing problem, intersex surgery, blepharoplasties for a
significant obstructive vision problem, sliding mandibular osteotomies for prognathism or
micrognathism, reconstructive or plastic surgery, bone marrow or stem cell transplants,
all organ transplants covered by the Medicaid program, plasmapheresis, strabismus
surgery for patients over ten (10) years of age, maxillofacial surgeries related to diseases
and conditions of the jaws and contiguous structures, temporomandibular joint surgery,
submucous resection of nasal septum and septoplasty when associated with significant
obstruction, hysterectomy, tonsillectomy, adenoidectomy, cataract extraction, surgery to
the foot, and all dental admissions.
MEDICAL TOPICS CROSS-REFERENCES:
Surgery-Global Billing/Payment Guidelines
Surgery-Multiple Procedures/Same Operative Session
Surgery-Office Visits
Surgery-Removal of Implants
Surgery-Surgery and Anesthesia By the Same Provider
Surgery-Surgeon and Assistant Surgeon, Same Provider
Surgery-Surgical Services
Surgery-Suture of Wounds
01/31/2007 Surgery – Services Requiring Prior Authorization
Medical Policy Manual
679

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Surgery – Services Requiring Prior Authorization
Medical Policy Manual
680
RULES, CITATIONS, AND SOURCES:
405 IAC 5-3 Prior Authorization
Indiana Health Coverage Programs Provider Manual 1999
Initial Policy
Issues
Effective Date Implementation
Date
Retroactive Date
470 5-9-3;
5-9-9; 5-9-14
Transferred
Prior
Authorization
7/1/91
405 IAC 1-7-3
Repealed 8/24/97
Prior
Authorization
1/1/92
Revisions:
405 IAC 5-3 Prior
Authorization
8/24/97
405 IAC 5-3-2
Amended
Prior
Authorization
10/27/99
405 IAC 5-3-5
Amended
Prior
Authorization
10/27/99
405 IAC 5-3-6
Amended
Prior
Authorization
10/27/99
405 IAC 5-3-12
Amended
Prior
Authorization
10/27/99
APPLICABLE INDIANAAIM EDITS AND AUDITS:
3003
COVERAGE CRITERIA:

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: SURGERY—SURGEON AND ASSISTANT SURGEON,
SAME PROVIDER
DESCRIPTION:
A patient may have the need for two procedures coincidentally by two surgical
specialists. One may serve as the assistant surgeon during the other specialist’s
procedure and then bill as the primary surgeon for the portion of which he/she is the
specialist with the first surgeon remaining as an assistant.
SUMMARY OF CURRENT POLICY:
Another surgeon may be requested to assist the performing surgeon during a complex
surgical procedure. Documentation explaining the need should accompany the claim and
the modifier 80 should be used. The global fee rule will be used to assess appropriate
payment.
MEDICAL TOPICS CROSS-REFERENCES:
Consultations – Second Opinion
Gynecology – Hysterectomy
Obstetric Care
Physician Services
Surgery-Global Billing/Payment Guidelines
Surgery-Multiple Procedures/Same Operative Session
Surgery-Removal of Implants
Surgery-Services Requiring Prior Authorization
Surgery-Surgery and Anesthesia By the Same Provider
Surgery-Surgical Services
Surgery-Suture of Wounds
RULES, CITATIONS, AND SOURCES:
405 IAC 5-25-2 Physician Services
Indiana State Department of Public Welfare Medical Policy Manual 1991
01/31/2007 Surgery – Surgeon and Assistant Surgeon, Same Provider
Medical Policy Manual
681

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Surgery – Surgeon and Assistant Surgeon, Same Provider
Medical Policy Manual
682
Indiana Health Coverage Programs Provider Manual 1999
Initial Policy
Issues
Effective Date Implementation
Date
Retroactive Date
Indiana State
Department of
Public Welfare
Medical Policy
Manual 1991
Assistant
Surgeon
7/1/91
470 IAC 5-8-15
Transferred
Surgeon and
Assistant
Surgeon by
Same Physician
7/1/91
405 IAC 1-6-15
Repealed
8/24/97
Physician
Services
1/1/92
Revisions:
405 IAC 5-25-2 Physician
Services
8/24/97
APPLICABLE INDIANAAIM EDITS AND AUDITS:
6037
6038
COVERAGE CRITERIA:

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: SURGERY—SURGERY AND ANESTHESIA BY THE
SAME PROVIDER
DESCRIPTION:
The performing surgeon may provide local anesthesia or conscious sedation with and
without local anesthesia to perform minor procedures and endoscopic examinations.
SUMMARY OF CURRENT POLICY:
Reimbursement for anesthesia administered by the surgeon in conjunction with a surgical
procedure is included in the fee for the surgical procedure.
MEDICAL TOPICS CROSS-REFERENCES:
Anesthesia Services
Clinic Services—FQHC and Rural Health Clinic Services
Obstetric Care
Opthalmology—Vitrectomy
Opthalmology—YAG Laser
Surgery—Global Billing/Payment Guidelines
Surgery—Multiple Procedures/Same Day Operative Session
Surgery—Office Visits
Surgery—Removal of Implants
Surgery—Services Requiring Prior Authorization
Surgery—Surgeon and Assistant Surgeon, Same Provider
Surgery—Surgical Services
Surgery—Suture of Wounds
Urology
RULES, CITATIONS, AND SOURCES:
405 IAC 5-10 Anesthesia Services
Indiana State Department of Public Welfare Medical Policy Manual 1991
IC 12-15-1-10
IC 12-15-21-2
IC 12-15-21-3
01/31/2007 Surgery – Surgery Anesthesia by the Same Provider
Medical Policy Manual
683

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Surgery – Surgery Anesthesia by the Same Provider
Medical Policy Manual
684
Indiana Health Coverage Programs Provider Manual 1999
Initial Policy Issues
Effective Date Implementation
Date
Retroactive Date
Indiana State
Department of
Public Welfare
Medical Policy
Manual 1991
Surgery and
anesthesia by
the same
provider
7/1/91
470 IAC 5-9-27
Transferred
Anesthesia
Services
7/1/91
405 IAC 1-7-26
Repealed
Anesthesia
Services
1/1/92
Revisions:
405 IAC 5-10 Anesthesia
Services
8/24/97
APPLICABLE INDIANAAIM EDITS AND AUDITS:
COVERAGE CRITERIA:
Surgery and Anesthesia by the Same Provider
Indiana State Department of Public Welfare Medical Policy Manual 1991
If a physician bills separate charges for anesthesia, whether local or regional (such as
saddle block), and surgery on the same day, it should be combined and coded as the
surgical procedure.

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: SURGERY—SURGICAL SERVICES
DESCRIPTION:
Surgical services are services for a member requiring or seeking medically necessary
perioperative care. These include, but are not limited to, the operating room, recovery room,
outpatient admitting and discharge, and preoperative preparation. Prior to the performance of a
surgical procedure, either inpatient or outpatient, the member consults with the surgeon who will
be performing the procedure. The visit can occur in the physician’s office, in the emergency
room, in the outpatient surgery area, or an ambulatory surgery center (ASC).
This document is intended to serve as a general summary of the Indiana Health Coverage
Programs (IHCP) policies regarding surgical services. More specific information may be found
in the IHCP Provider Manual, program notices, the Indiana Administrative Code (IAC), or other
sources as appropriate. See the “Rules, Citations, and Sources” section of this document for
further information.
COVERAGE CRITERIA
The IHCP provides reimbursement for surgical services performed by IHCP enrolled providers
when reported with the appropriate Current Procedural Terminology (CPT) or Health Care
Common Procedure Coding System (HCPCS) procedure codes. Physician reimbursement for a
surgical procedure generally includes the preoperative visits performed on the same day or the
day prior to the surgery for major surgical procedures, and the day of the surgical procedure for
minor surgical procedures.
Prior authorization (PA) is required for a scheduled inpatient surgical procedure if that procedure
is usually performed on an outpatient basis. Reimbursement for the inpatient admission is
determined by the appropriate Diagnostic Related Group (DRG), but may be subject to
retrospective review of the medical necessity for the inpatient stay. The following criteria is
used for determining the medical necessity for an inpatient admission.
• Technical or medical difficulty during the outpatient procedure as documented in the
medical record
• Presence of physical or mental conditions which make prolonged preoperative or
postoperative observations by a nurse or other skilled medical personnel medically
necessary
• Performance of another procedure simultaneously, which itself requires hospitalization
01/31/2007 Surgery – Suegical SServices
Medical Policy Manual
685
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• Anticipation of an additonal procedure, which would require hospitalization following
the initial procedure
• Documentation must be maintained in the medical record and clearly document any
complications and services provided
Office Visits
Office visits made with the surgeon prior to the scheduling of surgery are billed under the global
surgery payment/billing rules. Certain modifiers may be used to distinguish a preoperative visit
from a more in-depth visit at which time the decision was made for surgery; a significant,
separately identifiable evaluation and management visit was made the same day of surgery; the
surgeon served as a consultant for a second or third opinion; or an unrelated procedure or service
by the same physician during the postoperative period. The appropriate modifier should be used
for claim payment of these services. Documentation must be maintained in the medical record.
Postoperative Care
Global postoperative care days for surgical procedures include a 90-day period following a major
surgical procedure and a 10-day period following a minor surgical procedure. Separate
reimbursement is available for care provided during these global postoperative periods that is
unrelated to the surgical procedure, or for care given not considered routine and postoperative
care for the surgical condition, such as complications. The medical visits are billed separately
from the surgical fee. Complications may include, but are not limited to, the following
examples.
• Cardiovascular complications
• Comatose conditions
• Elevated temperature above 38.4 degrees Celsius, or 101 degrees Fahrenheit, for two or
more consecutive days
• Medical complications due to anesthesia, other than nausea and vomiting
• Post operative wound infection requiring specialized treatment
• Renal failure
Surgery and Anesthesia, Same Provider
Reimbursement for anesthesia administered by the surgeon in conjunction with a surgical
procedure is included in the fee for the surgical procedure.
Multiple Procedures, Same Operative Session
Multiple surgical procedures may be performed on the same patient on the same day when it is
determined to be beneficial for the surgeon(s) and patient, and provides the best outcome for the
patient. Documentation is required to indicate the medical necessity for multiple procedures.
When two or more surgeries are performed during the same operative period reimbursement will
be subject to the following multiple surgery reductions.
• 100 percent of the global fee for the most expensive procedure
• 50 percent of the global fee for the second most expensive procedure
• 25 percent of the global fee for the remaining procedures
01/31/2007 Surgery – Suegical SServices
Medical Policy Manual
686
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Removal of Implants
The IHCP provides reimbursement for removal of medical implants (i.e., pins, screws, rods,
plates, etc.) when a fracture has healed or the symptoms that required implantation of the device
abate. Implant removals requiring an operating room are usually considered minor procedures
and the rules governing minor procedures in 405 IAC 5-28-1 apply. Some implants may be
removed in the physician’s office and should be included in the office fee claim.
Surgeon and Assistant Surgeon
A member may require two procedures coincidentally by two different surgical specialists. Each
surgeon may serve as the assistant surgeon during the other surgeon’s procedure. The surgeons
may bill as primary surgeon for that portion of surgery for which he/she was responsible. Refer
to the Billing Requirements section of this fact sheet for billing guidelines.
Wound Closure
There are times that it is not advisable to close an operative incision at the time of the initial
surgical procedure, such as, infectious drainage or gangrenous bowel. The patient may remain in
the hospital for observation and return to the operating room for secondary wound closure.
When a dehiscence occurs in the immediate postoperative period, the patient may return to the
operating room for suturing as an emergency procedure.
• Secondary closure after the initial surgical procedure may be considered part of the initial
surgery and part of the global fee schedule
• Dehiscence of a wound is considered a complication of the primary procedure. As an
emergency procedure, the rules pertaining to emergency procedures will apply
PRIOR AUTHORIZATION
IHCP reimbursement is available for certain surgeries, as listed in 405 IAC 5-3-13, when PA is
received in accordance with the PA guidelines. Further information regarding PA criteria for the
following services can be located in the corresponding Medical Policy fact sheets. Currently, PA
is required for the following surgical procedures.
• Reduction mammoplasty
• Rhinoplasty or bridge repair of the nose when related to a significant obstructive
breathing problem
• Intersex surgery
• Blepharoplasties for a significant obstructive vision problem
• Sliding mandibular osteotomies for prognathism or micrognathism
• Reconstructive or plastic surgery
• Bone marrow or stem cell transplant
• Organ transplants
• Maxillofacial surgeries related to diseases and conditions of the jaws and contiguous
structures
• Temporomandibular joint surgery
01/31/2007 Surgery – Suegical SServices
Medical Policy Manual
687
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• Submucous resection of nasal septum and septoplasty when associated with significant
obstruction
• Weight reduction surgery, including gastroplasty and related gastrointestinal surgery
• Orthodontic procedures for members under 21 years of age for cases of craniofacial
deformity or cleft palate
• All dental procedures requiring hospital admission
• Out-of-state procedures
PA is required for surgical procedures usually performed on an outpatient basis, when scheduled
as an inpatient. Notification following emergency care must be done within 48 hours or the first
working day following the weekend or holiday. If authorization is not received, the claim will be
denied.
MANAGED CARE
For members enrolled in Risk Based Managed Care (RBMC), providers must contact the
member’s managed care organization (MCO) for more specific guidelines. Refer to the Provider
Manual, Chapter 1, for detailed information about the fee-for-service (FFS), Primary Care Case
Management (PCCM), and RBMC delivery systems.
IHCP members enrolled in Medicaid Select PCCM receive the same benefit coverage and are
subject to the same limitations as traditional Medicaid FFS. Refer to the Medicaid Select Manual
for Primary Care Providers and Office Staff for further information.
BILLING REQUIREMENTS
The following modifiers must be indicated on the CMS 1500 claim or the 837P electronic
transaction when applicable to the procedure performed.
• Modifier 54, Surgical care only, indicates the physician performed only the surgical care
• Modifier 55, Postoperative management only, indicates the physician only performed on
the postoperative care
• Modifier 56, Preoperative management only, indicates the physician only performed on
the preoperative care
• Modifier 57, Decision for surgery, indicates the decision for surgery was made on the
same day as the surgery
All surgical procedures performed on the same day, by the same rendering physician, must be
billed on the same claim form; otherwise, the claim may be denied and the original claim will
require adjustment for additional payment.
Providers submitting CMS 1500 claims or 837P electronic transaction using modifier 50,
indicating a bilateral procedure, must report only one unit. The use of modifier 50 ensures that
the procedure code is reimbursed at the lower of 150 percent of the billed charge or the rate on
01/31/2007 Surgery – Suegical SServices
Medical Policy Manual
688
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Surgery – Suegical SServices
Medical Policy Manual
689
file. Modifier 50 is not to be utilized if the CPT code description specifies the procedure as
bilateral.
A surgeon may be requested to assist the performing surgeon as an assistant surgeon during a
complex surgical procedure. Documentation explaining the need for an assistant should
accompany the claim and modifier 80 should be used.
Additional Surgical Billing Requirements
The following perioperative encounters require additional/specific documentation.
• Surgery Payable at Reduced Amount When Related Post-Operative Care Paid
• Post-Operative Care Within 0-90 days of Surgery
• Pre-Operative Care on Day of Surgery
• Surgery Payable at Reduced Amount When Pre-Operative Care Paid Same Date of
Service
To explain the above situations, the IHCP requires that the provider submit the following
documentation.
• Medical reason and unusual circumstances for the separate evaluation and management
(E/M) visit
• The medical necessity of visit occurring due to a complication, such as cardiovascular
complications, comatose conditions, elevated temperature for two or more consecutive
days, medical complications due to anesthesia other than nausea and vomiting, post-
operative wound infection requiring specialized treatment, or renal failure
RELATED MEDICAL TOPICS
Anesthesia Services
Clinic Services—FQHC and Rural Health
Clinic Services
Consultations – Second Opinion
Emergency Medicine – Emergency Room
Emergency Medicine – Emergency Services
Emergency Services
Gastroenterology
Gynecological Services
Hospital Inpatient
Hospital Outpatient
Obstetric Care
Ophthalmology
Therapy Services
Radiology Services

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
RULES, CITATIONS, AND SOURCES
Indiana Administrative Code
405 IAC 5-1-5 Global Fee Billing
405 IAC 5-3 Prior Authorization
405 IAC 5-10 Anesthesia Services
405 IAC 5-25 Physician Services
405 IAC 5-28 Medical and Surgical Services
Indiana Code
IC 12-8-6-3 Administration of state program
IC 12-8-6-5 Rules
IC 12-15-1-10 Administrative actions and directions
IC 12-15-21-2 Acceptance by provider of Medicaid claim payment
Indiana Health Coverage Programs Provider Manual
1999
December, 2006 Version 6.0
Indiana State Department of Public Welfare Medical Policy Manual 1991
Indiana Health Coverage Programs Bulletins
BT200208 – Modifications to Prior Authorization Requirement
BT200216 – Split Billing of Global Surgery Postoperative Care Days
BT200511 – HIPAA Modifications
Origination date: 12/31/2000
Review or Revision Reason Date
470 IAC 5-9-1, 5-9-
3,
5-9-9, 5-9-12, 5-9-
14, 5-9-27
Transferred
Anesthesia Services, Global fee billing, Medical
Services, Prior Authorization, Surgical Services
Indiana State
Department of
Public Welfare
Medical Policy
Manual 1991
Removal of Implants, Suture of Wounds
07/01/1991
405 IAC 1-7, 1-7-3,
1-7-12; 1-7-14, 1-7-
26
Repealed 8/24/97
Anesthesia Services, Global fee billing, Medical
Services, Prior Authorization, Surgical Services
01/01/1992
405 IAC 5-1-5, 5-3,
5-10, 5-25, 5-28,
Anesthesia Services, Global fee billing, Medical
Services, Prior Authorization, Surgical Services
08/24/1997
405 IAC 5-3-2, 5-3-
5, 5-3-6, 5-3-12
Amended
Prior Authorization 10/27/1999
01/31/2007 Surgery – Surgical Services
Medical Policy Manual
690

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Review or Revision Reason Date
Revision
Scheduled review and consolidation of fact
sheets
• Surgery-Multiple Procedures/Same Operation
• Surgery-Office Visits
• Surgery-Removal of Implants
• Surgery-Services Requiring PA
• Surgery-Surgeon and Asst. Surgeon, Same
Provider
• Surgery-Surgical Services
• Surgery-Suture of Wounds
• Surgery/Anesthesia Same Provider
01/31/07
APPLICABLE INDIANAAIM EDITS AND AUDIT
0764 – Missing or Invalid Compound Product ID Qualifier
0765 – Improper Order of Dispensing Status Code on Partial Fill Transaction
0766 – Missing or Invalid Associated Prescription or Service Reference Number on
Completion Transaction
0770– Completion Transaction Not Permitted with Same Date of Service as Partial
Transaction
3003 – Procedure Code Requires PA
4000 – More Than Two Surgical Units on the Claim
6014 – Global Payable at a Reduced Fee When Components Paid – Medical Services
6015 – Global Payable at a Reduced Fee When Components Paid – Respiratory Services
6017 – Global Payable at a Reduced Fee When Components Paid – Endocrine/Nervous
/Eye/Ear
6022 – Components Not Payable When Global Paid – Digestive System
6023 – Global Payable at a Reduced Fee When Components Paid – Digestive System
6034 – Global Surgery Payable at Reduced Amount When Components of Surgical Care
Paid
6035 – Components of Surgical Care Not Payable When Global Surgery Paid
6037 – Only One Assistant Surgeon Allowed for Select Surgeries
6038 – Two Assistant Surgeons Allowed Only for Select Surgeries
6039 – Assistant Surgeon Not Payable hen Co-Surgeon Paid
6040 – Co-Surgeon Not Payable When Assistant Surgeon Paid
6048 – Components Not Payable When Global Paid- Endocrine/Nervous/Eye/Ear
6049 – Components Not Payable When Global Paid – Endocrine/Nervous/Eye/Ear
6061– Components Not Payable When Global Paid – Genital Urinary/Reproductive
Systems
6063 – Components Not Payable When Global Paid – Respiratory System
6064 – Components Not Payable When Global Paid – Medical System
6071 – Components Not Payable When Global Paid Cardiovascular/Lymphatic System
01/31/2007 Surgery – Surgical Services
Medical Policy Manual
691
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Surgery – Surgical Services
Medical Policy Manual
692
6072 – Global Payable at Reduced Fee When Components Paid – Cardiovascular/Lymphatic
System
6630 – Professional/Technical Components for Cardiac Catheterization Versus the Complete
Procedure
6634 – Complete Procedure for Cardiac Catheterization Versus Technical/Professional
Components
6649 – Surgery Payable at Reduced Amount When Related Postoperative Care Paid
6650 – Lifetime Procedures are Limited to One Per Lifetime
6651 – Surgical Cutback Procedure 50 Percent
6652 – Multiple Surgeries Must be Billed on Same Claim
6653 – Postoperative Care Within Zero to 90 Days of Surgery
6654 – Preoperative Care Within One Day of Surgery
6655 – Surgery Payable at Reduced Amount When Preoperative Care Paid
6656 – Postoperative Care Within 10 Days of Select Surgery
6657 – Preoperative Care On Day of Surgery
6658 – Surgery Payable at Reduced Amount When Preoperative Care Paid Same Date of
Service
6659 – Surgery Payable at Reduced Amount Related Care Paid
6660 – Preoperative and Postoperative Care Billed With Unlisted Surgeries Requires
Review
6664 – Global Payable at a Reduced Fee When Components Paid–Integumentary and
Neuromuscular
6665 – Bilateral Versus Unilateral Surgeries
6706 – Global Payable at a Reduced Fee When Components Paid–Genital
Urinary/Reproductive Systems

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: SURGERY—SUTURE OF WOUNDS
DESCRIPTION:
There are times that it is unadvisable to close an operative incision at the time of the
initial surgical procedure, (i.e. infectious drainage, gangrenous bowel). The patient
remains in the hospital for close observation and must return to the operating room for an
intermediate closure. When a dehiscence occurs in the immediate postoperative period,
the patient must return to the operating room for suturing as an emergency procedure.
SUMMARY OF CURRENT POLICY:
The suture of wounds is a covered Medicaid service under the following circumstances:
1) A secondary closure after the initial surgical procedure may be considered part of the
initial surgery and part of the global fee schedule.
A dehiscence of a wound is considered a complication of the primary procedure. As an
emergency procedure, the rules pertaining to emergency procedures will apply.
MEDICAL TOPICS CROSS-REFERENCES:
Emergency Medicine – Emergency Room
Emergency Medicine – Emergency Services
Gynecology -- Hysterectomy
Hospital Inpatient
Hospital Outpatient
Surgery – Global Billing/Payment Guidelines
Surgery – Services Requiring Prior Authorization
Surgery – Surgery and Anesthesia By the Same Provider
RULES, CITATIONS, AND SOURCES:
405 IAC 5-28-1
Indiana Health Coverage Programs Provider Manual 1999
01/31/2007 Surgery – Suture of Wounds
Medical Policy Manual
693

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Initial Policy Issues
Effective Date Implementation
Date
Retroactive Date
Indiana State
Department of
Public Welfare
Medical Policy
Manual 1991
Suture of Wounds 7/1/91
470 IAC 5-9-
12; 5-9-14
Transferred
Medical Services;
Surgical Services
7/1/91
405 IAC 1-7-
12; 1-7-14
Repealed
8/24/97
Medical Services;
Surgical Services
1/1/92
Revisions:
405 IAC 5-28-1 Medical and
Surgical Services
8/24/97
APPLICABLE INDIANAAIM EDITS AND AUDITS:
4000
6014
6015
6017
6022
6023
6034
6035
6037
6038
6039
6040
6048
6049
6061
6063
6064
6071
6072
6630
6634
6649
6650
6651
6653
6654
01/31/2007 Surgery – Suture of Wounds
Medical Policy Manual
694
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
6655
6656
6657
6658
6659
6660
6664
6706
01/31/2007 Surgery – Suture of Wounds
Medical Policy Manual
695
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Surgery – Suture of Wounds
Medical Policy Manual
696
COVERAGE CRITERIA:
Indiana State Department of Public Welfare Medical Policy Manual 1991
1. Claims for intermediate repair of wounds should include the location and size of the
wound repaired. If the size of the wound is not indicated, use the smallest code for
the location of the wound.
2. Multiple wounds on the same day should be paid the full amount for the major
(largest) wound and half for all other lacerations.
3. Claims for multiple complex repairs done on the same day should be routed to
Medical Policy for review and pricing.

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: SURGERY—TRANSPLANTS
DESCRIPTION
Stedman’s Medical Dictionary defines a transplant as a transfer from one part to another, such
as tissue or an organ, in grafting and transplantation. Autologous transplants involve tissue or
organ transferred into a new position in the body of the same individual. Allogenic transplant
pertains to transfer of human tissue or an organ from one person to another; allogenic indicates
it is genetically different but still within the same species.
This document is intended to serve as a general summary of the Indiana Health Coverage
Programs (IHCP) policies regarding this service. More specific information may be found in the
IHCP provider manual, program notices, the Indiana Administrative Code (IAC) or other sources
as appropriate. See the “Rules, Citations, and Sources” section of this document for further
information.
COVERAGE CRITERIA
The IHCP provides reimbursement for the following transplants when medically necessary with
prior authorization (PA). Reimbursement is also available for the cost of procuring the organs or
tissue.
Bone Marrow and Stem Cell
Lung, cadaver and live donor
Heart
Heart/Lung
Liver, cadaver and live donor
Renal, cadaver and live donor
Pancreas, cadaver and live donor
Autologous Islet Cell
Intestinal, cadaver
Multi-visceral
The IHCP provides reimbursement for corneal tissue transplantation when medically necessary.
Corneal tissue transplantation was removed from the Indiana Administrative Code (IAC) citation
405 IAC 5-3-13, Services Requiring Prior Authorization, in 2005.
Reimbursement for the transplant donor’s hospital and surgical expenses for the removal of the
donor tissue or organ during the inpatient admission will be provided when the recipient of the
transplant is an IHCP member, the member meets criteria for the transplant, and the transplant is
considered medically necessary.
01/31/2007 Surgery – Transplants
Medical Policy Manual
697

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Each type of transplantation has been included in separate sections of this document and includes
specific information regarding prior authorization criteria, coding information, and
documentation requirements. Additionally, information regarding the removal of transplanted
tissue and transplantation billing in general is included in separate sections of this document.
A. Bone Marrow or Stem Cell Transplantations (Other than for Breast Cancer)
1. Coding for Bone Marrow or Stem Cell Transplantations, Autologous or Allogenic
The IHCP will provide reimbursement for autologous or allogenic bone marrow or stem cell
transplants listed in Table 1. These codes should be used for bone marrow or stem cell
transplants with or without the diagnosis of breast cancer.
Table 1 - CPT Codes for Bone Marrow or Stem Cell Transplantations
CPT
CODE
DESCRIPTION
38204
Management of recipient hematopoietic progenitor cell donor search and cell
acquisition
38205
Blood-derived hematopoietic progenitor cell harvesting for transplantation, per
collection; allogenic
38230 Bone marrow harvesting for transplantation
38240 Bone marrow or blood-derived peripheral stem cell transplantation; allogenic
38241 Bone marrow or blood-derived peripheral stem cell transplantation; autologous
38242
Bone marrow or blood-derived peripheral stem cell transplantation; allogenic
donor lymphocyte infusions
2. Indications for Bone Marrow or Stem Cell Transplantations (Other Than
Breast Cancer)
The IHCP will provide reimbursement for bone marrow transplants with prior authorization
for ONE of the following indications other than for breast cancer.
• Adult or childhood acute myeloid leukemia (includes nonlymphocytic or
nonlymphoblastic)
• Adult or high risk childhood lymphocytic (lymphoblastic) leukemia in remission
• Myelodysplastic syndromes
• Acute lymphocytic or non-lymphocytic leukemia in remission
•
Non-Hodgkin’s lymphoma of intermediate and high grade (stage 3 or 4) in remission or
with evidence of chemotherapy responsive disease
• Hodgkin’s Disease (lymphoma) in second remission or refractory to primary therapy
• Neuroblastoma: High risk disease by the International Neuroblastoma Staging System
criteria with no evidence of disease progression at the time of transplant
• Congenital Marrow Failure Syndromes unresponsive to medical therapy
• Severe Aplastic Anemia
• Severe Combined Immunodeficiency Disease
01/31/2007 Surgery – Transplants
Medical Policy Manual
698
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• Multiple Myeloma (tandem stem cell transplants for treatment of Multiple Myeloma must
receive PA as two separate procedures). Tandem stem cell transplants are considered
medically necessary in patients who fail to achieve a complete remission or a good partial
remission (at least 50% reduction in tumor cells) after the first transplant.
• Germ-cell Cancer: recurrent or refractory to primary therapy (tandem stem cell
transplants should be considered for relapsed patients and must receive PA as two
separate procedures)
• Ovarian cancer
• Hurler’s Syndrome (other inherited metabolic diseases will be considered based on
published literature)
• Ewing’s Sarcoma limited to pulmonary relapse only
• Sickle cell anemia
• Thalassemia major or transfusion dependent thalassemia intermedia
AND when a member meets ALL of the following criteria.
• The life expectancy following the transplant can reasonably be expected to be one year or
more, measured by current standards.
• The member, or his or her guardian, demonstrates a reasonable ability to comply with
physician-directed postoperative treatment meant to reduce the chance of organ rejection.
• The adult member is competent and understands the risks and benefits of the transplant.
• The member has normal or treatable cardiovascular, pulmonary, hepatic, and renal
function.
3. Contraindications to Bone Marrow or Stem Cell Transplantations (Other Than Breast
Cancer)
Prior authorization will not be given for bone marrow transplants in the following
circumstances.
• The member is a juvenile and has no identifiable caretaker or no adequate family support
structure
• The member is septic or has an active infection
• The member has a frank relapse or progression of leukemia or disease
• The member has a condition preventing rehabilitation
• The member exceeds 175% of normal weight for height and age
• The member has an abnormal central nervous system (CNS) condition, e.g., CVA,
Organic Brain Syndrome, or dementia. (CNS metastasis as a consequence of the primary
diagnosis for which the transplant is being requested would be excluded from this
restriction.)
• The member has another active malignancy or history of active malignancy within two
years, excluding skin cancers cured by simple excision. Documentation will be required
at the time of PA request.
• Transplant is contraindicated for acquired immune deficiency syndrome (AIDS) as
defined by a CD4 count of less than 200 cells/mm³ unless the following are noted.
o CD4 count greater than 200 cells/mm³
o HIV-1 RNA undetectable
01/31/2007 Surgery – Transplants
Medical Policy Manual
699
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
o Stable anti-retroviral therapy for more than 90 days
o No other complications from AIDS (e.g., opportunistic infection, including
aspergillus, tuberculosis, coccidioidomycosis, or antimicrobial resistant fungal
infections, or Kaposi’s sarcoma or other neoplasms)
• The member is moribund
• The member has an active illegal drug or alcohol dependence within the six months prior
to the submission of the request
• The member has two or more significant abnormal lab or x-ray results, non-disease
related
4. Documentation for Bone Marrow Transplantations (Other than Breast Cancer)
The following documentation must be maintained in the member’s medical record.
• History and physical examination (H&P) signed by a physician and includes the
member’s height, weight and gender, completed within a medically reasonable timeframe
prior to the submission of the request. Additionally, for members with a history of
depression, suicide attempts or drug dependence, the H&P should include documentation
of psychiatric or psychological evaluation signed by a psychiatrist or health services
provider in psychology (HSPP).
• Clear documentation of the member’s disease status including copies of all recent results
of imaging studies, bone marrow testing, cytogenetics, and molecular studies when
indicated
• CBC, UA, complete metabolic profile (CMP), and EKG within a medically reasonable
timeframe prior to submission of the request.
• Urine or serum B-HCG (females only)
• Urine creatinine clearance or glomerular filteration rate (GFR)
• Chest X-ray within 90 days prior to submission of the request
• Urine drug screen within a medically reasonable timeframe prior to submission of the
request for members > 18 years of age or based on physician discretion
• Appropriate screening for colon cancer, for members over 40 years of age
• Thallium stress test results, or suitable alternative per a cardiologist, for members with
history of significant cardiac risk factors.
• HIV and hepatitis B virus (HBV), hepatitis C virus (HCV), cytomegalovirus (CMV), and
any other serology testing, including toxoplasmosis, syphilis, and Epstein-Barr virus
(EBV) results within a medically reasonable timeframe of the submission of the request.
Positive results may be a relative contraindication.
• Results of arterial blood gases (ABGs) and pulmonary function tests if member was (is) a
smoker or has a history of lung disease. Forced expired volume (FEV) less than 60% of
normal and forced vital capacity (FVC) less than 50% of normal may be a
contraindication to transplant. Pulmonary function studies in pediatric members may
vary depending on testing capabilities.
• Dental evaluation with treatment of any significant dental disease
01/31/2007 Surgery – Transplants
Medical Policy Manual
700

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
B. Bone Marrow and Stem Cell Transplantations for Breast Cancer
1. Coding for Bone Marrow and Stem Cell Transplantations for Breast Cancer
The IHCP advises providers to report the appropriate CPT code, as listed previously in this
document in Table 1 for bone marrow and stem cell transplants for breast cancer.
2. Indications for Bone Marrow and Stem Cell Transplantations for Breast Cancer
The IHCP will provide bone marrow transplants with prior authorization for confirmed
cancer of the breast, Stage II with >10 positive axillary nodes, Stage III B, or Stage IV, with
all of the following indications.
• Documentation of no other organ disease that interferes with his/her health
• Life expectancy of less than twelve months without procedure, documented by oncologist
• Life expectancy of 18 months or greater with procedure, documented by oncologist
• Breast cancer staging, overall physical status, and response to past therapy documented
by the attending oncologist within three months of the procedure request
• Documentation of the completion of induction therapy without disease progression,
within three months prior to the procedure request
• Documentation of a Karnofsky Performance status >70 (a measurement of rehabilitation
potential) within three months prior to the procedure request
• No history of previous chemotherapy if Stage III B, or only adjuvant therapy if Stage IV
• Documentation of no history of a second active malignancy or > five years from initial
diagnosis and treatment without evidence of recurrence
• Documentation of one failed hormonal therapy, if tumor estrogen receptor (ER) level is
>10 femtomoles/mg and Stage IV
• Documentation of no brain metastases as evidenced by CT scan
• Documentation of no central nervous system involvement
• Documentation of a bone marrow aspirate and bilateral ischial bone biopsies with no
evidence of marrow involvement with breast cancer
3. Contraindications for Bone Marrow and Stem Cell Transplantations for Breast Cancer
IHCP reimbursement for bone marrow and stem cell transplants will not be provided when
any of the following clinical situations are present.
• An active malignancy, other than breast cancer
• Active illegal drug, tobacco, or alcohol dependence within the last six months
• Documentation of irreversible primary organ disease (e.g., heart, lung or kidney)
• Two or more documented abnormal lab or x-ray results not related to the breast cancer
may be a relative contraindication
01/31/2007 Surgery – Transplants
Medical Policy Manual
701

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Surgery – Transplants
Medical Policy Manual
702
4. Documentation for Bone Marrow and Stem Cell Transplantations for Breast Cancer
The following studies are to be completed within a medically reasonable timeframe prior to
the PA request and documentation must be maintained in the member’s medical record.
• History and physical (H&P) examination signed by a physician that includes the
member’s height, weight and gender. Additionally, the H&P should include psychiatric
or psychological evaluation in cases having a history of depression, suicide attempts or
drug dependence signed by a psychiatrist or HSPP.
• Clear documentation of the member’s disease status including copies of all recent results
of imaging studies, bone marrow testing, cytogenetics, and molecular studies when
indicated
• Abdominal and chest CT
• Bone scan
• Chest X-ray, posteroanterior view
• Bone marrow aspirate and bilateral ischial bone biopsy and cellularity (pathology reports)
• Pulmonary function, including DLCO > 60% predicted
• Thallium stress test results, or suitable alternative per a cardiologist, for members with
history of significant cardiac risk factors
• Karnofsky Performance Status > 70
• Dental evaluation with treatment of any significant dental disease
• Urine drug screen within 90 days prior to submission of the request for members >18
years of age or based on physician discretion
• HIV, hepatitis B virus (HBV), hepatitis C virus (HCV), syphilis and cytomegalovirus
(CMV) serologies
• Laboratory values
o CBC
o CMT
o Creatinine Clearance (CC)
o Urinalysis
o Carcinoembryonic antigen
(CEA)
o PT/INR, PTT
o Creatine Kinase (CK)
o Lactic Acid Dehydrogenase
C. Lung Transplantations
1. Coding for Lung Transplantations
The IHCP provides reimbursement for three components of lung transplantation, with PA as
listed below. The IHCP provides reimbursement for the CPT codes for lung transplants
listed in Table 2 that follows on the next page.
• Harvesting
of the lung includes cold preservation (see CPT code 32850).
• Backbench work
consists of preparation of cadaver donor single lung or both lungs prior
to transplantation. This includes dissection of the lung from tissue around it and
preparation of the pulmonary venous/atrial cuff, pulmonary artery and bronchus
bilaterally (see CPT codes 32855 and 32856).

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• Recipient transplantation includes transplanting a single lung or both lungs into the
patient (see CPT codes 32851-32854).
Table 2 - CPT Codes for Reporting Lung Transplantations
CPT
Code
Description
32850 Donor pneumonectomy (including cold preservation), from cadaver donor
32851 Lung transplant, single; without cardiopulmonary bypass
32852 Lung transplant, single; with cardiopulmonary bypass
32853
Lung transplant, double (bilateral sequential or en bloc); without cardiopulmonary
bypass
32854 Lung transplant, double (bilateral sequential or en bloc); with cardiopulmonary bypass
32855
Backbench standard preparation of cadaver donor lung allograft prior to
transplantation, including dissection of allograft from surrounding soft tissues to
prepare pulmonary venous/atrial cuff, pulmonary artery, and bronchus; unilateral
32856
Backbench standard preparation of cadaver donor lung allograft prior to
transplantation, including dissection of allograft from surrounding soft tissues to
prepare pulmonary venous/atrial cuff, pulmonary artery, and bronchus; bilateral
2. Indications for Lung Transplantations
The IHCP considers lung transplants medically necessary with prior authorization for ONE
of the following indications.
• Primary pulmonary hypertension
• Alpha-1 antitrypsin deficiency
• Pulmonary fibrosis (primary or secondary)
• Cystic fibrosis
• Surfactant deficiency
• Bronchopulmonary dysplasia
• Pulmonary berylliosis (with chronic interstitial granulomatous fibrosis)
• Atrioventricular canal
• Bronchiectasis
• Pulmonary vascular disease
3. Contraindications for Lung Transplantation
IHCP reimbursement for lung transplantation will not be provided when any of the following
clinical situations are present.
• Active illegal drug, tobacco, or alcohol dependence within the last six months
• Active malignancy or other organ disease
• Acquired immune deficiency syndrome (AIDS) as defined by a CD4 count of less than
200 cells/mm³ unless the following are noted
o CD4 count greater than 200 cells/mm³
01/31/2007 Surgery – Transplants
Medical Policy Manual
703
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Surgery – Transplants
Medical Policy Manual
704
o HIV-1 RNA undetectable
o Stable anti-retroviral therapy for more than 90 days
o No other complications from AIDS (e.g., opportunistic infection, including
aspergillus, tuberculosis, coccidioidomycosis, antimicrobial resistant fungal
infections, or Kaposi’s sarcoma or other neoplasms)
4. Documentation for Lung Transplantation
Documentation must indicate the following information was obtained within a medically
reasonable timeframe prior to the request.
• History and physical (H&P) examination signed by a physician that includes the
member’s height, weight and gender. Additionally, the H&P should include psychiatric
or psychological evaluation in cases having a history of depression, suicide attempts or
drug dependence signed by a psychiatrist or HSPP.
• Clear documentation of the disease status of the member including copies of all recent
results of imaging studies, bone marrow testing (when indicated), cytogenetics, molecular
studies, etc.
• No use of tobacco products for a period of six months prior to request or transplant
• Life expectancy without transplant is expected to be 18 months or less
• Life expectancy with transplant is expected to be 24 months or greater
• Ventilator dependency
• Current Prednisone use of less than 20 mg/day. Chronic high dose steroids for
extrapulmonary disease are a contraindication. Prednisone dosage >5mg/day for a child
with cystic fibrosis may be considered a contraindication.
• Karnofsky performance status > 70
• Chest X-ray, posteroanterior view
• Urine drug screen within 90 days prior to submission of the request for members > 18
years of age or based on physician discretion
• CT scans of lungs (other CT scans as applicable)
• Thallium stress test results, or suitable alternative per a cardiologist, for members with
history of significant cardiac risk factors
• Results of arterial blood gases (ABGs) and carboxyhemoglobin, and pulmonary function,
including forced eixred volume (FEV) of 25% normal and decreasing forced volume
capacity (FVC) of 40% normal or less.
• HIV, hepatitis B virus (HBV), hepatitis C virus (HCV), syphilis, and cytomegalovirus
(CMV) serologies
• Lab values within normal limits
o CBC, urine, CEA, CMP
o Plasma ammonia
o Creatine Kinase (CK)
o Serum magnesium
o Lactic Acid Dehydrogenase
o Serum phosphate
o Platelet count

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• Dental evaluation with treatment of any significant dental disease
D. Heart Transplantations
1. Coding for Heart Transplantations
The IHCP provides reimbursement for the following three components of heart
transplantation with or without lung transplant. Providers are advised to report the
appropriate CPT code from Table 3 for reimbursement of heart transplants.
• Cadaver donor cardiectomy consists of harvesting and cold preservation of the graft prior
to transport (see CPT code 33940).
• Backbench work consists of dissection of the donor heart from surrounding soft tissue
prior to transplantation and preparation of aorta, superior vena cava, inferior vena cava,
pulmonary artery, and left atrium for transplantation (see CPT code 33944).
• Recipient transplantation includes transplanting the heart/lungs into the patient (see CPT
codes 33935 and 33945).
Repair or resection procedures of the donor heart should be reported using CPT codes 33300,
33310, 33320, 33400, 33463, 33464, 33510, 33641, 35216, 35276, and 35685 as appropriate.
These procedures do not require prior authorization.
Table 3 - CPT Codes for Reporting Heart Transplantations
CPT
Codes
Description
33940 Donor cardiectomy (including cold preservation)
33944 Backbench standard preparation of cadaver donor heart allograft prior to
transplantation, including dissection of allograft from surrounding soft tissues to
prepare aorta, superior vena cava, inferior vena cava, and pulmonary artery, and left
atrium for implantation
33945 Heart transplant, with or without recipient cardiectomy
2. Indications for Heart Transplantation
The IHCP considers heart transplants medically necessary with prior authorization for ONE
of the following indications.
• Eisenmenger’s syndrome (ventricular septal defect - cardiac failure with significant right-
to-left shunt producing cyanosis)
• Other complex congenital defects
• Myocardial failure unresponsive to medical management
• End-stage cardiomyopathy
• Inability to be weaned from temporary ventricular-assist devices after myocardial
infarction or non-transplant cardiac surgery
• Valvular heart disease
01/31/2007 Surgery – Transplants
705
Medical Policy Manual
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
AND meets ALL of the following criteria.
• Life expectancy with current medical management is expected to be 12 months or less
• Life expectancy after transplant expected to be two years
• The present degree of disability severely limits the member’s activity. (New York Heart
Association Classification III or IV)
• Member or guardian demonstrates a reasonable ability to comply with postoperative
treatments meant to reduce the possibility of organ rejection, including medication
administration and cardiac biopsies
• Adult member understands the risk and benefits of the transplant
• Member is one week of age or older and less than 70 years of age
3. Contraindications to Heart Transplantation
IHCP reimbursement for heart transplantation will not be provided when any of the
following clinical situations are present.
• A juvenile with no identifiable caretaker or adequate family (social) support structure
• The member is moribund
• Fixed pulmonary hypertension or severe pulmonary disease (unless receiving combined
heart/lung transplantation)
• Diabetes or uncontrolled hypertension
• Hepatic fibrosis or cirrhosis
• Hepatitis C with histological evidence of hepatic disease
• Uncorrected abdominal aneurysm greater than 4 centimeters
• The member exceeds 175% of normal weight for height and age
• Abnormal central nervous system (CNS) condition (e.g., CVA)
• Active malignancy or infection
• Active systemic disease that would not be alleviated by the requested transplant or that
severely limits life expectancy or precludes adequate post-transplant rehabilitation such
as autoimmune or collagen vascular disease
• Active gastrointestinal disease, such as bleeding peptic ulcer or diverticulitis
• Active illegal drug, tobacco, or alcohol dependence within the last six months
• Two or more documented, significant, abnormal lab or x-ray results
• Acquired immune deficiency syndrome (AIDS) as defined by a CD4 count of less than
200 cells/mm³ unless the following are noted
o CD4 count greater than 200 cells/mm³
o HIV-1 RNA undetectable
o Stable anti-retroviral therapy for more than 90 days
o No other complications from AIDS (e.g., opportunistic infection, including
aspergillus, tuberculosis, coccidioidomycosis, antimicrobial resistant fungal
infections, or Kaposi’s sarcoma or other neoplasms).
• Uncontrolled or untreated psychiatric disorders that interfere with compliance to a strict
treatment regimen
01/31/2007 Surgery – Transplants
706
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
4. Documentation for Heart Transplantation
Documentation in the member’s medical record must indicate the following information was
obtained within a medically reasonable timeframe prior to the request for PA.
• History and physical (H&P) examination signed by a physician that includes the
member’s height, weight and gender: Additionally, the H&P should include psychiatric
or psychological evaluation in cases having a history of depression, suicide attempts or
drug dependence signed by a psychiatrist or HSPP.
• Clear documentation of the member’s disease status including copies of all recent results
of imaging studies, bone marrow testing, cytogenetics, and molecular studies when
indicated
• CBC, UA, and CMP
• Chest X-ray, posteroanterior view
• Urine drug screen within 90 days prior to submission of the request for members > 18
years of age or based on physician discretion
• Appropriate screening for colon cancer, if member is greater than 40 years of age
• HIV, hepatitis B virus (HBV), hepatitis C virus (HCV), syphilis, and cytomegalovirus
(CMV) serologies
• Results of EKG, multigated-heart scan (MUGA scan), heart catheterization(s), or
electrophysiological studies
• Results of arterial blood gases (ABGs) and pulmonary function tests if member was (is) a
smoker or has a history of lung disease. Forced expired volume (FEV) less than 60% of
normal, and forced vital capacity (FVC) less than 50% of normal may be a
contraindication to transplant.
• Dental evaluation with treatment of any significant dental disease
E. Heart/Lung Transplantation
1. Coding for Heart/Lung Transplantation
The IHCP provides reimbursement for the following three components of a heart/lung
transplantation, with PA. Table 4, on the next page, lists the codes to report for
reimbursement of a heart/lung transplantation.
• Cadaver donor cardiectomy with pneumonectomy
consists of harvesting and cold
preservation of the graft prior to transport (see CPT code 33930).
• Backbench work includes dissection of the tissue around the heart and lungs and
preparation of aorta, superior vena cava, inferior vena cava, and trachea for
transplantation (see CPT code 33933).
• Recipient transplantation
includes transplanting the heart/lungs into the patient (see CPT
code 33935).
Repair or resection procedures of the donor heart should be reported using CPT codes 33300,
33310, 33320, 33400, 33463, 33464, 33510, 33641, 35216, 35276, and 35685 as appropriate.
These procedures do not require prior authorization.
01/31/2007 Surgery – Transplants
707
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 4 - CPT Codes for Reporting Heart/Lung Transplantations
CPT
Codes
Description
33930 Donor cardiectomy-pneumonectomy (including cold preservation)
33933 Backbench standard preparation of cadaver donor heart/lung allograft prior to
transplantation, including dissection of allograft from surrounding soft tissues to
prepare aorta, superior vena cava, inferior vena cava, and trachea for implantation
33935 Heart-lung transplant with recipient cardiectomy-pneumonectomy
2. Indications for Heart/Lung Transplantation
The IHCP considers heart/lung transplants medically necessary with prior authorization if
criteria for both heart and lung transplantation are met.
3. Contraindications for Heart/Lung Transplantation
IHCP reimbursement for heart/lung transplantation will not be provided when any of the
contraindications for either a heart or lung transplantation, indicated previously in this
document, are present.
4. Documentation for Heart/Lung Transplantation
The IHCP requires that documentation for heart lung transplantation meet the same criteria
required for both heart and lung transplantation.
F. Hepatic (Liver) Transplantation
1. Coding for Liver Transplantation
The IHCP reimburses for three different components of liver transplantation as indicated
below. Table 5, on the next page, lists the codes available for reporting liver transplantation.
• Cadaver or living donor hepatectomy
consists of harvesting and cold preservation of the
graft prior to transplantation and care of the donor, in the case of living donor
hepatectomy (see CPT codes 47133, 47140-47142).
• Backbench work
consists of preparation of donor liver prior to transplantation. This
includes preparation of whole liver graft, including dissection and removal of
surrounding tissue and soft tissue, preparation of the vena cava, portal vein, hepatic artery
and common bile duct. Also included is preparation of the whole liver with splitting of
the liver for partial grafts. Additional reconstruction of the liver graft including venous
and arterial anastomosis(es) may also be performed (see CPT codes 47143-47147).
• Recipient transplantation
includes transplanting the liver into the patient and care of the
recipient (see CPT codes 47135-47136).
01/31/2007 Surgery – Transplants
708
Medical Policy Manual

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Surgery – Transplants
Medical Policy Manual
709
Table 5 - CPT Codes for Hepatic Transplantations
CPT Code Description
47133 Donor hepatectomy (including cold preservation), from cadaver donor
47135
Liver allotransplantation; orthotopic, partial or whole, from cadaver or living
donor, any age
47136
Liver allotransplantation; heterotopic, partial or whole, from cadaver or living
donor, any age
47140
Donor hepatectomy (including cold preservation), from living donor; left lateral
segment only (segments II and III)
47141
Donor hepatectomy (including cold preservation), from living donor; total left
lobectomy (segments II, III and IV)
47142
Donor hepatectomy (including cold preservation), from living donor; total right
lobectomy (segments V, VI, VII and VIII)
47143
Backbench standard preparation of cadaver donor whole liver graft prior to
allotransplantation, including cholecystectomy, if necessary, and dissection and
removal of surrounding soft tissues to prepare the vena cava, portal vein, hepatic
artery, and common bile duct for implantation; without trisegment or lobe split
47144
Backbench standard preparation of cadaver donor whole liver graft prior to
allotransplantation, including cholecystectomy, if necessary, and dissection and
removal of surrounding soft tissues to prepare the vena cava, portal vein, hepatic
artery, and common bile duct for implantation; with trisegment split of whole
liver graft into two partial liver grafts (ie, left lateral segment (segments II and
III) and right trisegment (segments I and IV through VIII)
47145
Backbench standard preparation of cadaver donor whole liver graft prior to
allotransplantation, including cholecystectomy, if necessary, and dissection and
removal of surrounding soft tissues to prepare the vena cava, portal vein, hepatic
artery, and common bile duct for implantation; with lobe split of whole liver
graft into two partial liver grafts (ie, left lobe (segments II, III, and IV) and right
lobe (segments I and V through VIII)
47146
Backbench reconstruction of cadaver or living donor liver graft prior to
allotransplantation; venous anastomosis, each
47147
Backbench reconstruction of cadaver or living donor liver graft prior to
allotransplantation; arterial anastomosis, each
2. Indications for Liver Transplantation
The IHCP considers liver transplants medically necessary with prior authorization for the
ONE of the following indications.
• Acute liver failure due to viral hepatitis, drug reactions or toxins
• Chronic liver failure due to ONE of the following
o primary biliary cirrhosis
o chronic active hepatitis
o autoimmune hepatitis
o sclerosing cholangitis
o biliary atresia
o Budd-Chiari syndrome
o alcoholic cirrhosis
o cryptogenic cirrhosis
o toxin induced cirrhosis
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• Non-resectable, primary tumors of the liver, such as primary hepatomas and
cholangiocarcinomas
• The development of life-threatening complications, such as variceal hemorrhage,
encephalopathy, spontaneous bacterial peritonitis or intractable ascites
• Inborn errors of metabolism, such as Alpha-1 antitrypsin deficiency, Wilson’s
disease, primary hyperoxaluria, primary hypercholesterolemia, or tyrosinosis
• Traumatic or inflammatory, non-infectious conditions, other than metastatic
cancer, which has resulted in the destruction of the liver or in the inability of the
liver to function
AND meets ALL of the following criteria.
• Member, or guardian, demonstrates a reasonable ability to comply with post-
operative treatments meant to reduce the possibility of organ rejection
• Life expectancy following transplant is expected to be two years
• Adult member is competent and understands the risks and benefits of the
procedure
• Juveniles, or guardians, understand the likelihood of growth retardation as a result
of the liver condition
• Member has normal or reversible cardiac, pulmonary, and renal function
3. Contraindications to Liver Transplantation
IHCP reimbursement will not be provided for liver transplantation when any of the
following clinical situations are present.
• A juvenile with no identifiable caretaker or adequate family (social) support
system
• Sepsis
• Age greater than 70 years of age and/or less than 90 days of age
• Any condition that would prevent rehabilitation
• Any severe, uncorrectable, pulmonary, cardiovascular, or renal dysfunction
• The member exceeds 175% of normal weight for height and age
• Active extrahepatic infection
• An abnormal central nervous system disorder, e.g., CVA
• An active extrahepatic malignancy
• Acquired immune deficiency syndrome (AIDS) as defined by a CD4 count of less
than 200 cells/mm³ unless the following are noted.
o CD4 count greater than 200 cells/mm³
o HIV-1 RNA undetectable
o Stable anti-retroviral therapy for more than 90 days
o No other complications from AIDS (e.g., opportunistic infection, including
aspergillus, tuberculosis, coccidioidomycosis, antimicrobial resistant fungal
infections, or Kaposi’s sarcoma or other neoplasms)
01/31/2007 Surgery – Transplants
Medical Policy Manual
710
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• Active systemic disease, other than diabetes, that would not be alleviated by the
requested transplant or would limit life expectancy or compromise recovery, e.g.,
systemic vasculitis
• Active gastrointestinal disease, such as bleeding peptic ulcer or diverticulitis
• The member is moribund
• Active illegal drug or alcohol dependence within the previous six months
• Two or more significant, non-liver associated, abnormal lab or x-ray results
• Relative contraindications include, but are not limited to, previous extensive
upper abdominal surgery, and thrombosis involving portal, superior mesenteric or
splenic veins
4. Documentation for Liver Transplantation
Documentation in the member’s medical record must indicate the following
information was obtained within a medically reasonable timeframe prior to the
request for PA.
• History and physical (H&P) examination signed by a physician that includes the
member’s height, weight and gender. Additionally, the H&P should include
psychiatric or psychological evaluation in cases having a history of depression,
suicide attempts or drug dependence signed by a psychiatrist or HSPP.
• Clearly document the disease status of the member including copies of all recent
results of imaging studies, bone marrow testing (when indicated), cytogenetics,
molecular studies, etc., if done, including CT scans or nuclear scans when
appropriate for the work-up.
• CBC, UA, CMP, and EKG
• Chest X-ray, posteroanterior view
• Urine drug screen within 90 days prior to submission of the request for members
> 18 years of age or based on physician discretion
• Appropriate screening for colon cancer if the member is over 50 years of age
• Thallium stress test results, or suitable alternative per a cardiologist, if the
member has a history of significant cardiac risk factors.
• HIV, hepatitis B virus (HBV), hepatitis C virus (HCV), syphilis, and
cytomegalovirus (CMV) serologies
• Results of arterial blood gases (ABGs) and pulmonary function tests if member
was (is) a smoker or has a history of lung disease. Forced expired volume (FEV)
less than 60% of normal and forced vital capacity (FVC) less than 50% of normal
may be a contraindication to transplant.
• Dental evaluation with treatment of any significant dental disease
01/31/2007 Surgery – Transplants
Medical Policy Manual
711

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
G. Renal Transplantation
1. Coding for Renal Transplantation
The IHCP provides reimbursement for the following three different components of renal
transplantation. Table 6 lists the codes available for reporting renal transplantation.
• Cadaver or living donor nephrectomy consists of harvesting and cold preservation
of the graft prior to transplantation and care of the donor (see CPT codes 50300,
50320, and 50547).
• Backbench work consists of preparation of the donor kidney prior to
transplantation. This includes removal of perinephretic fat, diaphragmatic and
retroperitoneal attachments, excision of adrenal gland; and preparation of
ureter(s), renal vein(s), renal artery(s), and ligating branches as necessary. Other
reconstruction procedures may involve venous, arterial, or ureteral
anastomosis(es) necessary for the transplant (see CPT codes 50323, 50325,
50327-50329).
• Recipient transplantation includes transplanting the kidney into the patient (see
CPT codes 50360, 50365).
Table 6 - CPT Codes Renal Transplantations
CPT Codes Description
50300
Donor nephrectomy (including cold preservation); from cadaver donor,
unilateral or bilateral;
50320 Donor nephrectomy (including cold preservation); open, from living donor
50323
Back bench standard preparation of cadaver donor renal allograft prior
to transplantation, including dissection and removal of perinephric fat,
diaphragmatic and retroperitoneal attachments, excision of adrenal
gland, and preparation of ureter(s), renal vein(s), and renal artery(s),
ligating branches, as necessary
50325
Backbench standard preparation of living donor renal allograft (open or
laparoscopic) prior to transplantation, including dissection and removal
of perinephric fat and preparation of ureter(s), renal vein(s), and renal
artery(s), ligating branches, as necessary
50327
Backbench reconstruction of cadaver or living donor renal allograft
prior to transplantation; venous anastomosis, each
50328
Backbench reconstruction of cadaver or living donor renal allograft
prior to transplantation; arterial anastomosis, each
50329
Backbench reconstruction of cadaver or living donor renal allograft
prior to transplantation; ureteral anastomosis, each
50340 Recipient nephrectomy (separate procedure)
50360
Renal allotransplantation, implantation of graft; without recipient
nephrectomy;
50365
Renal allotransplantation, implantation of graft; with recipient
nephrectomy
50380 Renal autotransplantation, reimplantation of kidney
01/31/2007 Surgery – Transplants
Medical Policy Manual
712

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
2. Indications for Renal Transplantation
The IHCP considers kidney transplantations medically necessary with prior
authorization for ONE or more of the following indications.
• Severe chronic renal failure with anticipated progression to end stage renal
disease. Severe chronic renal failure is defined as a creatinine clearance of less
than 30cc/min.
• Post-nephrectomy for pyonephrosis (infected hydronephrosis) due to chronic
infection; infection must be resolved.
• Arteriovenous fistula with intractable hematuria not amenable to renal artery
occlusive procedures
• Urothelial tumor of the renal pelvis
• Post-nephrectomy of atrophic kidney to treat uncontrolled hypertension
• Uncontrollable post transplant hypertension
• End-stage renal disease and availability of an acceptable donor kidney
AND meets ALL of the following criteria.
• Member has completed an evaluation and been accepted by the transplant
committee at the kidney transplant center. Documentation must include a
summary letter from the transplant center indicating acceptance and outlining the
preoperative tests and their results.
• Absence of malignancy, or malignancy that has had curative therapy (e.g., surgical
resection of non-invasive squamous cell or basal cell skin cancer), or the
estimated risk of recurrence of the malignancy is less than 10% within the next
two years. For example, renal cell carcinoma treated by nephrectomy with no
evidence of metastatic disease two years after the nephrectomy, prostate cancer
with negative prostate-specific antigen (PSA) levels after treatment, surgically
treated colon cancer, thyroid cancer with normal thyroglobulin levels after
therapy, and others. Women should have a negative Pap smear and
mammography within the last year.
• The life expectancy following the transplant can reasonably be expected to be one
year or more, measured by current standards.
• The member, or guardian, demonstrates a reasonable ability to comply with post-
operative treatments meant to reduce the chance of organ rejection.
• The adult member is competent and understands the risks and benefits of the
transplant.
3. Contraindications to Renal Transplantation
IHCP reimbursement will not be provided for renal transplantation when any of the
following clinical situations are present.
• A juvenile with no identifiable caretaker or adequate family support structure
01/31/2007 Surgery – Transplants
Medical Policy Manual
713
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• Severe neurological or mental impairment in persons without adequate social
support, such that the person is unable to adhere to the regimen necessary to
preserve the transplant
• Oxalosis
• Recurrent uncorrectable lower urinary tract infections
• Any condition preventing rehabilitation
• Fixed pulmonary hypertension or severe pulmonary disease
• The member exceeds 175% of normal weight for height and age
• Progressive or deteriorating neurologic disease
• Persistent, uncontrolled coagulation disorder
• Active malignancy currently or within the past two years
• Active infection
• Active systemic disease other than renal, e.g., vasculitis, causing significant
comorbidities
• Active gastrointestinal disease, such as bleeding peptic ulcer or diverticulitis
• The member is moribund
• Active illegal drug, or alcohol dependence within the last six months
• Two or more abnormal non-renal labs or x-rays without adequate explanation by
the physician
• Acquired immune deficiency syndrome (AIDS) as defined by a CD4 count of less
than 200 cells/ mm³ unless the following are noted
o CD4 count greater than 200 cells/mm³
o HIV-1 RNA undetectable
o Stable anti-retroviral therapy for more than 90 days
o No other complications from AIDS (e.g., opportunistic infection, including
aspergillus, tuberculosis, coccidioidomycosis, antimicrobial resistant fungal
infections, or Kaposi’s sarcoma or other neoplasms)
4. Documentation for Renal Transplantation
Documentation must indicate that the following information was obtained within a
medically reasonable timeframe prior to the request.
• History and physical (H&P) examination signed by a physician that includes the
member’s height, weight and gender. Additionally, the H&P should include
psychiatric or psychological evaluation signed by a psychiatrist or HSPP in cases
having a history of depression, suicide attempts or drug dependence.
• CBC, UA, CMP, and EKG
• Chest X-ray, posteroanterior view
• CT scans or nuclear scan results when appropriate for the work-up
• Urine drug screen within 90 days prior to submission of the request for members
> 18 years of age or based on physician discretion
• Appropriate screening for colon cancer, for members over 50 years of age
• Thallium stress test results, or suitable alternative per a cardiologist, for members
with history of significant cardiac risk factors
01/31/2007 Surgery – Transplants
Medical Policy Manual
714

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• HIV, hepatitis B virus (HBV), hepatitis C virus (HCV), syphilis, and
cytomegalovirus (CMV) serologies
• Dental evaluation with treatment of any significant dental disease
• Results of arterial blood gases (ABGs) and pulmonary function tests if member
was (is) a smoker or has a history of lung disease. Forced expired volume (FEV)
less than 60% of normal and forced vital capacity (FVC) less than 50% of normal
may be a contraindication to transplant.
H. Pancreatic Transplantation
1. Coding for Pancreatic Transplantation
IHCP provides reimbursement for three different components of pancreatic
transplants with PA. Pancreatic transplantation that is performed at the same time as
kidney transplantation is to be reported with the appropriate CPT code for each organ
transplanted. Table 7 lists the CPT codes that are available for reporting pancreatic
transplantation.
• Cadaver pancreatectomy
consists of harvesting and cold preservation of the graft
prior to transplantation (see CPT code 48550).
• Backbench work consists of preparation of the donor pancreas prior to
transplantation. This includes preparation of the pancreas by dissecting the soft
tissues surrounding the pancreas, splenectomy, duodenotomy, ligation of the bile
duct, ligation of the mesenteric vessels, and Y-graft arterial anastomosis from the
iliac artery to the superior mesenteric artery and to the splenic artery. Venous
anastomosis(es) may also be included in reconstruction of the donor pancreas.
(See CPT codes 48551 and 48552).
• Recipient transplantation includes transplanting the pancreas into the patient (see
CPT code 48554).
Table 7 - CPT Codes for Pancreatic Transplantation
CPT Codes Description
48550
Donor pancreatectomy, (including cold preservation), with or without
duodenal segment for transplantation
48551
Backbench standard preparation of cadaver donor pancreas allograft prior
to transplantation, including dissection of allograft from surrounding soft
tissues, splenectomy, duodenotomy, ligation of bile duct, ligation of
mesenteric vessels, and Y-graft arterial anastomosis from iliac artery to
superior mesenteric artery and to splenic artery
48552
Backbench reconstruction of cadaver donor pancreas allograft prior to
transplantation, venous anastomosis, each
48554 Transplantation of pancreatic allograft
01/31/2007 Surgery – Transplants
Medical Policy Manual
715
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
2. Indications for Pancreatic Transplantation
The IHCP considers pancreatic transplantation medically necessary with PA when the
following criteria are met.
ONE of the following.
• Type I diabetes mellitus
• Diabetic nephropathy with deteriorating or poor status
• Diabetic neuropathy
• Diabetic enteropathy
• Diabetic retinopathy, such as proliferative retinitis
• Diabetics who fail aggressive medical management of blood sugar
• Diabetics who demonstrate multiple episodes of ketoacidosis or hypoglycemia
despite rigorous control and compliance
• Traumatic or inflammatory conditions, other than cancer, which has resulted in
the destruction of the functional ability of the pancreas
AND meets ALL of the following criteria.
• Life expectancy following the transplant can reasonably be expected to be one
year, measured by current standards and the transplant results of the institution
doing the procedure
• Member, or guardian, demonstrates a reasonable ability to comply with the
postoperative treatments meant to reduce the chance of organ rejection
• Adult member is competent and understands the risks and benefits of the
transplant
4. Contraindications to Pancreas Transplantation
The IHCP will not provide reimbursement for pancreatic transplantation for the
following clinical situations.
• Member is a juvenile with no identifiable caretaker or family support structure
• Type II diabetes
• The member is moribund
• Any condition preventing rehabilitation
• Severe, uncorrectable pulmonary, cardiac, renal, or hepatic dysfunction
• The member exceeds 175% of normal weight for height and age
• Abnormal CNS condition, e.g., CVA
• Active malignancy currently, or within past two years
• Active infection
• Active systemic disease other than diabetes, e.g., systemic vasculitis
• Active gastrointestinal disease, such as bleeding peptic ulcer or diverticulitis
• Active illegal drug, tobacco, or alcohol dependence within the last six months
• Two or more abnormal lab or x-ray reports, non-disease related
01/31/2007 Surgery – Transplants
Medical Policy Manual
716

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• Acquired immune deficiency syndrome (AIDS) as defined by a CD4 count of less
than 200 cells/mm³ unless the following are noted
o CD4 count greater than 200 cells/mm³
o HIV-1 RNA undetectable
o Stable anti-retroviral therapy for more than 90 days
o No other complications from AIDS (e.g., opportunistic infection, including
aspergillus, tuberculosis, coccidioidomycosis, antimicrobial resistant fungal
infections, or Kaposi’s sarcoma or other neoplasm)
5. Documentation for Pancreas Transplantation
Documentation must indicate the following information was obtained within a
medically reasonable timeframe prior to the request.
• History and physical (H&P) examination signed by a physician that includes the
member’s height, weight and gender. Additionally, the H&P should include
psychiatric or psychological evaluation signed by a psychiatrist or HSPP in cases
having a history of depression, suicide attempts, or drug dependence.
• Clear documentation of the member’s disease status including copies of all recent
results of imaging studies, bone marrow testing, cytogenetics, and molecular
studies when indicated
• CBC, UA, CMP, and EKG
• HIV, hepatitis B virus (HBV), hepatitis C virus (HCV), syphilis, and
cytomegalovirus (CMV) serologies
• Chest X-ray, posteroanterior and lateral views
• CT scans or nuclear scan results when appropriate for the work-up
• Urine drug screen within 90 days prior to submission of the request for members
> 18 years of age or based on physician discretion
• Appropriate screening for colon cancer, results for members greater than 50 years
of age
• Thallium stress test results, or suitable alternative per a cardiologist, for members
with history of significant cardiac risk factors
• Results of arterial blood gases (ABGs) and pulmonary function tests if member
was (is) a smoker or has a history of lung disease. Forced expired volume (FEV)
less than 60% of normal and forced vital capacity (FVC) less than 50% of normal
may be a contraindication to transplant.
• Dental evaluation with treatment of existing caries
I. Islet Cell Transplantation
1. Codes for Islet Cell Transplantation
The IHCP provides reimbursement for autologous islet cell transplantation with PA
under CPT code 48160, Pancreatectomy, total or subtotal, with autologous
transplantation of pancreas or pancreatic islet cells. Allogenic islet cell
01/31/2007 Surgery – Transplants
Medical Policy Manual
717

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
transplantation, reported with HCPCS codes G0341, G0342, and G0343, are
noncovered services, and are considered investigational.
2. Indications for Islet Cell Transplantation
An islet cell transplantation is indicated as an adjunct to a total or near total
pancreatectomy in patients with chronic pancreatitis.
3. Contraindications for Islet Cell Transplantation
The IHCP will not provide reimbursement for pancreatic islet cell transplantation for
the following clinical situations.
• Allogenic islet cell transplantation
• Treatment of type I diabetes
• Other applications for allogenic transplantation
• Active illegal drug, tobacco, or alcohol dependence within the last six months
4. Documentation for Islet Cell Transplantation
Documentation must indicate the following information was obtained within a
medically reasonable timeframe prior to the request
• History and physical (H&P) examination signed by a physician that includes the
member’s height, weight and gender
• Clear documentation of the member’s disease status including copies of all recent
results of imaging studies, bone marrow testing, cytogenetics, and molecular
studies when indicated
• HIV, hepatitis B virus (HBV), hepatitis C virus (HCV), syphilis, and
cytomegalovirus (CMV) serologies
• Urine drug screen within 90 days prior to submission of the request for members
> 18 years of age or based on physician discretion
J. Intestinal (or Small Bowel) Transplantation
1. Codes for Intestinal Transplantation
The IHCP provides reimbursement for three different components of intestinal (or
small bowel) transplantation with PA. Table 8 lists the CPT codes available for
reporting the transplantations.
• Cadaver or living donor enterectomy
consists of harvesting and cold preservation
of the graft prior to transplantation and care of the donor (see CPT codes 44132
and 44133).
01/31/2007 Surgery – Transplants
Medical Policy Manual
718

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• Backbench work consists of preparation of donor intestine prior to
transplantation. This includes mobilizing and developing the superior mesenteric
artery and vein (see CPT code 44715). Also included is any additional
reconstruction of graft including venous and arterial anastomosis(es) (see CPT
codes 44720-44721) prior to transplantation.
• Recipient transplantation includes transplanting the intestine into the patient (see
CPT codes 44135 and 44136).
Table 8 - CPT Codes for Reporting Intestinal and Small Bowel Transplantation
CPT Code Description
44132 Donor enterectomy (including cold preservation), open; from cadaver
donor
44133 Donor enterectomy (including cold preservation), open; partial, from
living donor
44135 Intestinal allotransplantation; from cadaver donor
44136 Intestinal allotransplantation; from living donor
44715 Backbench standard preparation of cadaver or living donor intestine
allograft prior to transplantation, including mobilization and fashioning of
the superior mesenteric artery and vein
44720 Backbench reconstruction of cadaver or living donor intestine allograft
prior to transplantation; venous anastomosis, each
44721 Backbench reconstruction of cadaver or living donor intestine allograft
prior to transplantation; arterial anastomosis, each
2. Indications for Intestinal Transplantation
The IHCP considers intestinal transplant medically necessary with PA for members
with irreversible intestinal failure who can no longer be maintained on total parenteral
nutrition (TPN). PA may be given for small bowel or intestinal transplantation for
the indications listed in Table 9, below. Clinical indications of TPN failure are listed
in Table 10.
Members must meet both of the following criteria.
• The member must be capable of following a complex medical regimen post-
transplantation.
• The member must be emotionally stable with a realistic attitude demonstrated
during past and current illness.
Table 9 - Indications for Intestinal Transplantation
Pediatric Adult
Aganglionosis (Hirschsprung’s disease) Crohn’s disease
Congenital epithelial mucosal disease
(microvillus inclusion disease, tufting
enteropathy)
Desmoid tumors
01/31/2007 Surgery – Transplants
Medical Policy Manual
719

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 9 - Indications for Intestinal Transplantation (continued)
Pediatric Adult
Gastroschisis Gardner’s syndrome/familial polyposis
Intestinal atresia Ischemia
Necrotizing enterocolitis Trauma
Pseudo-obstruction Volvulus
Volvulus Surgical adhesions
Radiation enteritis Hollow visceral myopathy
Short gut syndrome Inflammatory bowel disease
Short Gut Syndrome
Table 10 - Clinical Indications of TPN Failure
Clinical Indications
Impending or overt liver failure due to TPN. Symptoms include:
• Elevated bilirubin and/or liver enzymes
• Gastroesophageal varicies
• Coagulopathy
• Splenomegaly
• Thrombocytopenia
• Stomal bleeding
• Hepatic fibrosis/cirrhosis
Central line access failure as evidenced by:
• Thrombosis of two or more of the major central channels (jugular, subclavian, and
femoral veins)
• Pulmonary embolism
• Superior vena cava syndrome
• Chronic venous insufficiency
Two or more episodes of systemic sepsis due to line infection per year that requires
hospitalization or a single episode of line-related fungemia, septic shock or acute
respiratory distress syndrome
Frequent episodes of severe dehydration despite IV fluid supplementation
3. Contraindications for Intestinal Transplantation
The IHCP will not provide reimbursement for intestinal transplantation for the
following clinical situations.
• Absolute Contraindications
Members with the following absolute contraindications will not be approved for
intestinal transplantation.
o Active malignancy, with the exception of squamous or basal cell carcinoma
o Ongoing, recurring, or unsuccessfully treated infections
o Serious cardiac insufficiencies that create an inability to tolerate transplantation
o Active systemic illness
01/31/2007 Surgery – Transplants
Medical Policy Manual
720

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
o Active illegal drug, tobacco, or alcohol dependence within the last six months
o Demonstrated patient noncompliance with medical recommendations
o Acquired immune deficiency syndrome (AIDS) as defined by a CD4 count of
less than 200 cells/mm³ unless all of the following are noted
(1) CD4 count greater than 200 cells/mm³ for greater than 5 months
(2) HIV-1 RNA undetectable
(3) Stable anti-retroviral therapy for more than 90 days
(4) No other complications from AIDS (e.g., opportunistic infection, including
aspergillus, tuberculosis, coccidioidomycosis, antimicrobial resistant fungal
infections, Kaposi’s sarcoma or other neoplasm)
• Relative Contraindications
Members meeting any ONE of the following general or disease specific relative
contraindications must be evaluated carefully.
o Potential complications from immunosuppressive medications
o Cerebrovascular disease or accident, or progressive neuropathy or myopathy
that is not amenable to rehabilitation
o Malnutrition defined by a body mass index (BMI) of less than 17 or greater
than 33
o Uncontrolled co-morbid conditions such as diabetes mellitus, hypertension,
autoimmune disease, or cytopenia
o Untreated osteoporosis with a T-score greater than 2.5 standard deviations
from mean or a Z-score greater than 2 standard deviations from mean
o Uncorrected abdominal aortic aneurysm greater than four centimeters
o Diabetes with end-organ damage such as neuropathy, nephropathy, and
retinopathy
o The member is greater than 70 years of age
o Peripheral vascular disease not amenable to surgical or percutaneous therapy
4. Documentation for Intestinal Transplantation
Documentation must indicate that the following information was obtained within a
medically reasonable timeframe prior to the request.
• History and physical (H&P) examination signed by a physician that includes the
member’s height, weight and gender. Additionally, the H&P should include
psychiatric or psychological evaluation signed by a psychiatrist or HSPP in cases
having a history of depression, suicide attempts or drug dependence.
• All current medication and treatment plans
• Clear documentation of the member’s disease status including copies of all recent
results of imaging studies, bone marrow testing, cytogenetics, and molecular
studies when indicated
• Chemistries, including CBC, CMP, UA and creatinine clearance (if creatinine is
greater than 2.0)
• Lipid and hepatic function panels
01/31/2007 Surgery – Transplants
Medical Policy Manual
721

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• HIV, hepatitis B virus (HBV), hepatitis C virus (HCV), syphilis, and
cytomegalovirus (CMV) serologies
• Urine drug screen within 90 days prior to submission of the request for members
> 18 years of age or based on physician discretion.
• Recent electrocardiogram (EKG) and chest X-ray, posteroanterior view
• Psychosocial evaluation, performed at the transplant center
• Dental evaluation with treatment of any significant dental disease
K. Multi-Visceral Transplantation
1. Coding for Multi-Visceral Transplantation
Multi-visceral transplantation includes transplantation of the intestine, pancreas, and
liver. Additional organs could include the stomach and colon. Table 11 lists the codes
available for reporting multi-visceral transplantation. Providers are advised to report
the appropriate CPT code for the organs transplanted.
The IHCP reimburses for the three components (removal of donor organ, backbench
work and recipient transplantation) for each organ included in the multi-visceral
transplant.
• Cadaver or living donor enterectomy consists of harvesting and cold preservation
of the organs prior to transplantation and care of the donor.
• Backbench work consists of preparation of donor organs prior to transplantation.
• Recipient transplantation includes transplanting the organs into the patient.
Table 11 -Codes for Reporting Multi-Visceral Transplantation
Codes Description Reference
44132-44133 Donor enterectomy (including cold preservation), open
44135-44136 Intestinal allotransplantation
44715,
44720-44721
Backbench work – intestine allograft
See Table 8
47133,
47140-47142
Donor hepatectomy
47135-47136 Liver transplantation
47143-47147 Backbench work – liver graft
See Table 4
48550 Donor pancreatectomy
48554 Pancreatic transplant
48551-48552 Backbench work – pancreas allograft
See Table 7
2. Indications for Multi-Visceral Transplantation
The IHCP considers multi-visceral transplant medically necessary with PA when
ONE of the following criteria are met.
01/31/2007 Surgery – Transplants
Medical Policy Manual
722

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• Irreversible intestinal and multi-visceral organ failure that can no longer be
maintained with TPN
• Total occlusion of the splanchnic circulation
• Extensive GI polyposis
• Myopathy or neuropathy of the hollow viscera
• Abdominal malignancy
NOTE:
1. Members must meet the PA criteria listed in this fact sheet for intestinal, liver and/or
pancreatic transplantation in order to qualify for multi-visceral transplantation of these
organs.
2. Providers should refer to the intestinal transplantation criteria for indications of
intestinal failure and TPN failure.
AND both of the following criteria.
• The patient must be capable of following a complex medical regimen post-
transplantation.
• Emotionally stable with realistic attitude demonstrated during past and current
illness.
3. Contraindications to Multi-Visceral Transplantation
The IHCP will not provide reimbursement for multi-visceral transplantation for the
following clinical situations.
• Absolute Contraindications
Members with the absolute contraindications as listed below will not be approved
for multi-visceral transplantation.
o Active malignancy, with the exception of squamous or basal cell
carcinoma
o Ongoing, recurring, or unsuccessfully treated infections
o Serious cardiac insufficiencies that create an inability to tolerate
transplantation
o Active systemic illness
o Active illegal drug, tobacco, or alcohol dependence within the last
six months
o Demonstrated patient noncompliance with medical
recommendations
o Acquired immune deficiency syndrome (AIDS) as defined by a
CD4 count of less than 200 cells/mm³ unless all of the following
are noted
(1) CD4 count greater than 200 cells/mm³
(2) HIV-1 RNA undetectable
(3) Stable anti-retroviral therapy for more than 90 days
01/31/2007 Surgery – Transplants
Medical Policy Manual
723

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
(4) No other complications from AIDS (e.g., opportunistic
infection, including aspergillus, tuberculosis,
coccidioidomycosis, antimicrobial resistant fungal infections,
Kaposi’s sarcoma or other neoplasm)
• Relative Contraindications
Members meeting any ONE of the following general or disease specific relative
contraindications must be evaluated carefully.
o Potential complications from immunosuppressive medications
o Cerebrovascular disease or accident, or progressive neuropathy or
myopathy that is not amenable to rehabilitation
o Malnutrition defined by a BMI of less than 17 or greater than 33
o Uncontrolled co-morbid conditions such as diabetes mellitus,
hypertension, autoimmune disease, or cytopenia
o Untreated osteoporosis with a T-score greater than 2.5 standard
deviations from mean or a Z-score greater than 2 standard
deviations from mean
o Uncorrected abdominal aortic aneurysm greater than four
centimeters
o Diabetes with end-organ damage such as neuropathy, nephropathy,
and retinopathy
o The member is greater than 70 years of age
o Peripheral vascular disease not amenable to surgical or
percutaneous therapy
4. Documentation for Multi-Visceral Transplantation
Documentation in the member’s medical record must indicate the following
information was obtained within a medically reasonable timeframe prior to the
request for PA.
• History and physical (H&P) examination signed by a physician that includes the
member’s height, weight and gender. Additionally, the H&P should include
psychiatric or psychological evaluation signed by a psychiatrist or HSPP in cases
having a history of depression, suicide attempts or drug dependence.
• All current medication and treatment plans
• Clear documentation of the member’s disease status including copies of all recent
results of imaging studies, bone marrow testing, cytogenetics, and molecular
studies when indicated
• Chemistries, including CBC, CMP, UA, and creatinine clearance (if creatinine is
greater than 2.0)
• Lipid and hepatic function panel
• HIV, hepatitis B virus (HBV), hepatitis C virus (HCV), syphilis, and
cytomegalovirus (CMV) serologies
01/31/2007 Surgery – Transplants
Medical Policy Manual
724

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• Urine drug screen within 90 days prior to submission of the request for members
> 18 years of age or based on physician discretion
• Recent electrocardiogram (EKG) and chest X-ray, posteroanterior view
• Psychosocial evaluation, performed at the transplant center
• Dental evaluation with treatment of any significant dental disease
REMOVAL OF TRANSPLANTED ORGANS
Certain organs may require removal following transplantation due to organ rejection.
Removal of a transplanted organ does not require prior authorization. Transplantation of
another organ does require a new PA request. The CPT codes available for reporting
transplant removal are listed in Table 12.
Table 12 - CPT Codes for Reporting Removal of Transplanted Organs
CPT Code Description
44137 Removal of transplanted intestinal allograft, complete
48556 Removal of transplanted pancreatic allograft
50370 Removal of transplanted renal allograft
BILLING REQUIREMENTS
The IHCP provides reimbursement for coverage of costs related to donor testing and
harvesting. Additionally, the IHCP allows reimbursement for the donor’s medications that
are typically covered by the IHCP. The donor costs are billed under the IHCP member’s
name and recipient identification number (RID).
Transplantation of multiple organs at the same time is to be reported with the appropriate
CPT code for each organ. Claims submitted for multiple organ transplantations will be
subject to the multiple procedure reduction.
Transportation services for the member and caregiver to and from the transplant center are
provided following guidelines for transportation services in the Medical Policy fact sheet for
Transportation Services.
Routine post-operative surgical care during the first 90 days is included in the physician
reimbursement for surgical procedures. Separate reimbursement is available for care
provided that is not considered routine for the surgical condition, such as complications.
Organ transplants are not covered for Hoosier Healthwise Package C members. Inpatient
claims submitted to the IHCP that group to experimental organ transplant DRGs are denied.
Refer to the Medical Policy Fact Sheet for Clinical Trials for further information regarding
any experimental or investigational procedure. DRGs for non-experimental organ transplants
are 103, 302, 480, 795, 803, 804, and 805.
01/31/2007 Surgery – Transplants
Medical Policy Manual
725

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
OUT-OF-STATE TRANSPLANTATIONS
The IHCP will provide for transplant surgeries in out-of-state facilities when the hospital
specializes in the particular transplantation procedure, or if the hospital is one of a limited
number of hospitals that can perform the procedure. All out-of-state services must be prior
authorized. The requests for these procedures are reviewed on an individual basis. Refer to
the Medical Policy Fact Sheet for Out-of-State Services for further information.
Out-of-state providers who receive approval from OMPP for transplantation will receive a
written notification regarding how the claim will be reimbursed (either by the IHCP
statewide rate or a percentage of the provider’s usual and customary), and the coverage
period (such as 365 days from transplant). The provider will be assigned a point of contact at
EDS to assist with tracking expenditures, and processing of payment for services. Outpatient
lab services are paid at the IHCP rate on file, with no additional payment unless specific
approval is given by OMPP.
MANAGED CARE
Organ transplantations require the Primary Medical Providers (PMP) to obtain prior
authorization for transplants. This should not be confused with PMP referral. Hoosier
Healthwise managed care organizations (MCOs) have their own authorization requirements.
Please contact the appropriate MCO for further information.
TRANSPLANTATIONS THAT DO NOT REQUIRE PRIOR AUTHORIZATION
A Corneal Tissue Transplantation
1. Coding for Corneal Tissue Transplantation
The IHCP provides reimbursement for corneal tissue transplants when medically
necessary without prior authorization for services provided in-state. However, all
procedures provided out-of-state must be prior authorized. Corneal tissue
transplantation was removed from 405 IAC 5-3-13, services that require PA, in 2005.
Table 13 lists the covered CPT codes for the reimbursement of corneal tissue
transplants.
Table 13 - CPT Codes for Reporting Corneal Transplantations
CPT Codes Description
65710 Keratoplasty (corneal transplant); lamellar
65730 Keratoplasty (corneal transplant); penetrating (except in aphakia)
65750 Keratoplasty (corneal transplant); penetrating (in aphakia)
01/31/2007 Surgery – Transplants
Medical Policy Manual
726

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 13 - CPT Codes for Reporting Corneal Transplantations (continued)
CPT Codes Description
65755 Keratoplasty (corneal transplant); penetrating (in pseudophakia)
65780 Ocular surface reconstruction; amniotic membrane transplantation
65781
Ocular surface reconstruction; limbal stem cell allograft (e.g.
cadaveric or living donor)
65782
Ocular surface reconstruction; limbal conjunctival autograft
(includes obtaining graft
2. Indications for Corneal Tissue Transplantation
IHCP provides reimbursement for corneal transplantation for full thickness corneal
disease for the following medical conditions.
• Bullous keratopathy
• Corneal opacity
• Corneal thinning with potential for corneal perforation
• Keratoconus with ≥ 2 episodes of corneal hydrops
• Keratoconus (conical protrusion of cornea caused by thinning of the stroma)
with potential for corneal perforation
Reimbursement is available for corneal transplantation for partial thickness corneal
disease for ONE of the following medical conditions.
• Superficial stromal opacification
• Marginal corneal thinning or infiltration
• Localized corneal thinning or descemetocele formation
The IHCP provides reimbursement for transplantation of new tissue to the cornea for
the treatment of severe corneal surface disease, reported with CPT codes 65780,
65781, and 65782 for the following medical conditions.
• Corneal pannus or superficial corneal scarring
• Persistent corneal epithelial defects
• Corneal perforation
• Neurotrophic keratitis
• Persistent corneal epithelial defects
• Bullous keratopathy
• Corneal thinning
• Corneal ulcer
• Chemical burns of the ocular surface
• Erythema multiforme, including Stevens-Johnson syndrome
01/31/2007 Surgery – Transplants
Medical Policy Manual
727

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
3. Billing for Corneal Tissue
The IHCP provides reimbursement for donated corneal tissue under HCPCS code
V2785 – processing, preserving, and transporting of corneal tissue. The IHCP
requires providers to bill HCPCS code V2785 on the CMS-1500 claim form or the
837P transaction for reimbursement separate from the Ambulatory Surgery Center
(ASC) rate for outpatient corneal transplant procedures. A copy of the invoice from
the eye bank or organ procurement organization showing the actual cost of acquiring
the tissue must be attached to the claim form. When submitting paper attachments
with an 837P transaction, providers must follow the instructions in the IHCP Provider
Manual, Chapter 8, Section 1. The IHCP will reimburse providers 90% of the invoice
amount.
RELATED MEDICAL TOPICS
Hospital Inpatient
Hospital Outpatient
Out of State Services
Transportation
Surgical Services
RULES, CITATIONS, AND SOURCES
Indiana Administrative Code
405 IAC 5-3-13 – Services Requiring Prior Authorization
405 IAC 5-29-1 – Services not Covered by Medicaid; Noncovered Services
Indiana Code
IC 29-2-16-12 – Donation Costs
Indiana Health Coverage Programs Provider Bulletins
BT199928 – Hoosier Healthwise Package C Overview
BT200018 – Package C Claim Update
BT200231 – Carve Out and Self-Referral Education
BT200420 – Changes in Hospital Reimbursement Methodologies and Updated
DRG/LOC Reimbursement Rates
Indiana Health Coverage Programs Provider Banners
BR200506 – Corneal Tissue Reimbursement
Indiana Health Coverage Programs Provider Manual
Version 5.1, March, 2005
01/31/2007 Surgery – Transplants
Medical Policy Manual
728

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Surgery – Transplants
Medical Policy Manual
729
Origination Date: 12/31/2000
Revisions and
Review
Reason Date
Revision
Revision of 405-IAC-5-3-13 Services requiring prior
authorization, Revision of PA criteria
07/29/05
APPLICABLE INDIANAAIM EDITS AND AUDITS
4062–Organ Transplants are Non-covered for Package C.
6002–Any Two Anesthesiology Providers Same Procedure Requires Review
6003–Manual Pricing for Split Care Billing
6015–Global Payable at a Reduced Fee When Components Paid – Respiratory System
6034–Global Surgery Payable at Reduced Amount When Components of Surgical Care Paid
6035–Components of Surgical Care not Payable When Global Surgery Paid
6037–One Assistant Surgeon Allowed for Select Surgeries
6038–Two Assistant Surgeons Allowed Only for Select Surgeries
6039–Assistant Surgeon Not Payable When Co-Surgeon Paid
6040–Co-Surgeon Paid at Reduced Amount when Assistant Surgeon Paid
6063–Components Not Payable When Global Paid – Respiratory System
6096–The CPT/HCPCS Code Billed is Not Payable According to the PPS
Reimbursement Methodology
6152–Surgery Payable at Reduced Amount When Consultation Paid Days Before or
After Surgery
6649–Surgery Payable at Reduced Amount When Related Postoperative Care Paid
6652–Multiple Surgeries Must be Billed on Same Claim
6661–Duramorph Can Not Be Billed on Same Day as Surgery
6666–Anesthesia Services Not Allowed By Provider Billing for Surgery

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: THERAPY SERVICES
DESCRIPTION
The Indiana Health Coverage Programs (IHCP) covers therapy services for its members.
Therapy services, as described in this fact sheet, encompass occupational therapy,
respiratory therapy, and physical therapy.
SUMMARY OF CURRENT POLICY
Reimbursement is available only for medically reasonable and necessary therapy services
provided by professionally trained staff, within the scope of their professional license
and/or credentials. Medically necessary therapy services, as defined in 405 IAC 5-22-1
are, “for the restoration of an impaired level of function caused by an acute change in
medical condition.” Therapy services must be complex enough to require the judgment,
knowledge, and skills of a qualified therapist. The IHCP will only cover rehabilitative
services for up to two years from the initiation of the therapy unless there is a significant
change in the medical condition. Therapy services may be provided in inpatient and
outpatient settings such as the home, outpatient clinics, rehabilitation hospitals, inpatient
hospitals, long term care facilities, and ICF/MR facilities.
RELATED MEDICAL TOPICS
Aged and Disabled – Waiver Services
Autism – Waiver Services
Consultations – Second Opinions
Emergency Medicine – Cardiopulmonary Resuscitation (CPR)
Emergency Medicine – Emergency Room
Emergency Medicine – Emergency Services
EPSDT - HealthWatch
Home Health Services
Hospital Inpatient
Intermediate Care Facilities for the Mentally Retarded
Medical Supplies and Equipment
Medically Fragile Children – Waiver Services
01/31/2007 Therapy Services
Medical Policy Manual
730

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
RULES, CITATIONS, AND SOURCES
IC 25-23.5-1-4
IC 25-23.5-1-5
IC 25-23.5-1-5.5
IC 25-23.5-1-6
IC 25-23.5-5-1
IC 25-23.5-5-2
IC 25-27-1-1
IC 25-27-1-6
IC 25-34.5-1-6
IC 25-34.5-1-7
IC 25-34.5-2-8
IC 25-35.6-1-2
IC 25-35.6-1-3
IC 25-35.6-1-4
IC 25-35.6-1-5
405 IAC 5-3-12 Prior Authorization; exceptions
405 IAC 5-5 Out of State Services
405 IAC 5-16 Home Health Agency and Clinic Services
405 IAC 5-16-4 Rehabilitation center services; limitations
405 IAC 5-17 Hospital Services
405 IAC 5-17-4 Physical rehabilitation services
405 IAC 5-22 Nursing and Therapy Services
405 IAC 5-32 Rehabilitation Unit
Indiana Medicaid Update Bulletin 96-26
Indiana Health Coverage Programs Provider Manual 1999
Indiana Health Coverage Programs Provider Manual, July 2004
ORGANIZATION, REVISIONS, AND REVIEWS
Origination Date: July 1, 1991
Revisions and Reviews Reason Date
IC 25-23.5 Occupational Therapy 1989
IC 25-27 Physical Therapy 1989
IC 25-34.5 Respiratory Therapy 1989
470 IAC 5-8-19; 5-9-23,
24, 25, & 37 Transferred
Nursing and Therapy Services; Inpatient, Outpatient,
and Allied Health Services; Rehabilitation Unit
7/1/91
405 IAC 1-6-20; 1-7-21;
1-7-22; 1-7-23; 1-7-35
Repealed 8/24/97
Nursing and Therapy Services; Inpatient Therapy,
Outpatient Therapy, and Allied Health Services;
Rehabilitation Unit
1/1/92
01/31/2007 Therapy Services
Medical Policy Manual
731

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Revisions and Reviews Reason Date
Indiana Medicaid Update
Bulletin
Bypassing of Occurrence Code “50” for OT and
PT
7/29/96
405 IAC 5-3-12 Prior Authorization; exceptions 8/24/97
405 IAC 5-5-1 Out of State Services, Therapy Services 8/24/97
405 IAC 5-16 Home Health Agency Services
Medicaid Reimbursement for OT, PT, and RT
8/24/97
405 IAC 5-16-4 Rehabilitation Center Services; Limitations 8/24/97
405 IAC 5-22-5 Occupational, Physical, and Respiratory Therapy
and Speech Pathology; Reimbursement
8/24/97
405 IAC-5-22-6 Occupational, Physical, and Respiratory Therapy
and Speech Pathology; Criteria for Prior
Authorization
8/24/97
405 IAC 5-22-8 Physical Therapy Services 8/24/97
405 IAC 5-22-10 Respiratory Therapy Services 8/24/97
405 IAC 5-22-11 Occupational Therapy Services 8/24/97
405 IAC 5-29-1 Non-Covered Services 8/24/97
405 IAC 5-5-1 Out of State Services, Therapy Services
10/27/99
405 IAC 5-16-2 Home Health Agency Services
Medicaid Reimbursement for OT, PT, and RT
10/27/99
405 IAC 5-16-3 Home Health Agency Services
Medicaid Reimbursement for OT, PT, and RT
10/27/99
405 IAC 5-16-3.1 Home Health Agency Services; Limitations 10/27/99
405 IAC 5-22-6 Occupational, Physical, and Respiratory Therapy
and Speech Pathology; Criteria for Prior
Authorization
10/27/99
405 IAC 5-29-1 Non-Covered Services 10/27/99
Scheduled Review IHCP Provider Manual, Chapter 8 Therapy
Services
1/31/2005
APPLICABLE INDIANAAIM EDITS AND AUDITS
4086 – Therapies More Than 30 Days from Hospital Discharge
6750 – No More Than 30 Hours Within 30 Days from Hospital Discharge
6752 – Physical Therapy Evaluation Limited to One per 12 Month
6753 – Occupational Therapy Evaluation Limited to One per 12 Month
6755 – Outpatient Therapies Exceeded 80 Units per Year
01/31/2007 Therapy Services
Medical Policy Manual
732
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
COVERAGE CRITERIA
General Therapy Service Limitations
Prior authorization (PA) is required for all therapy services, as indicated in 405 IAC 5-
22-6 and outlined in the IHCP Provider Manual. The following are exceptions to the PA
requirement for occupational, physical, and respiratory therapy services.
• Initial evaluations
• Any combination of occupational, physical, and respiratory therapy ordered in
writing prior to a recipient’s discharge from inpatient hospital care, may continue
for a period not to exceed 30 hours, sessions, visits in 30 calendar day with PA
• Deductible and copayment for services covered by Medicare Part B
• Oxygen equipment and supplies necessary for the delivery of oxygen with the
exception of concentrators
Physical therapy and occupational therapy ordered in writing by a physician to treat an
acute medical condition requires PA, except in the following instances, as required in 405
IAC 5-22-8, 10, and 11.
• Physical therapy services ordered in writing by a physician in an outpatient setting
may continue for a period not to exceed 12 hours, sessions, or visits in 30
calendar days without PA. This includes the provision of splints, crutches, and
canes. Additional services require PA.
• Occupational therapy services ordered in writing by a physician may continue for
a period not exceeding 12 hours, sessions, or visits in 30 calendar days without
PA. This includes the provision of splints, crutches, and canes.
• Respiratory therapy services ordered in writing for the acute medical diagnosis of
asthma, pneumonia, bronchitis, and upper respiratory infection may be provided
for a period not to exceed 14 hours or 14 calendar days without PA. Additional
services require PA.
The following criteria must be met for PA of physical, respiratory, and occupational
therapy, when provided outside of the exceptions previously stated.
• Written evidence of physician involvement and personal member evaluation will
be required to document acute medical needs. Therapy must be ordered by a
physician.
• A current plan of treatment, including clearly stated and measurable goals and
progress.
• Once a member fails to progress or has reached his/her potential, services are
discontinued.
• Therapies must be provided by a qualified therapist or qualified assistant under
direct supervision of the therapist as appropriate.
• Therapy must be complex enough to require the judgment, knowledge and skills
of a qualified therapist.
01/31/2007 Therapy Services
Medical Policy Manual
733
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• Therapy for diversional, recreational, vocational, or avocational purpose or for the
remediation of learning disabilities or for developmental activities that can be
conducted by nonmedical personnel is non-covered.
• Therapy for rehabilitative services will be covered for a member no longer than
two years from the initiation of the therapy unless there is a significant change in
medical condition.
• Ongoing evaluations to assess progress and redefine therapy goals are part of the
therapy program. Ongoing evaluations are not separately billable under the
IHCP.
• One hour of therapy must include a minimum of 45 minutes of direct care with
the member. Only one hour per day, per type of therapy will be approved.
• Therapies which duplicate other services provided to a patient will not be
authorized (e.g., nursing services).
Therapy services provided by a nursing facility (NF) or large private or small
intermediate care facility for the mentally retarded are included in the facility’s per diem
rate. Therapy services provided in these settings is not separately reimbursable.
Occupational therapy psychiatric services are non-covered. In addition, general
strengthening exercise programs for recuperative purposes and passive range of motion
services as the only or primary modality are non-covered.
The initial evaluation does not require prior authorization; however, any additional re-
evaluations require PA, unless it is conducted during the initial 30 days after a member
has been discharged from the hospital and those orders include physical or occupational
therapy. Re-evaluations will only be authorized one time per year, unless the provider
submits documentation showing a significant change in the member’s condition.
Therapy assistants may only perform the following activities. Reimbursement for these
activities is included in the IHCP rate for the particular modality provided by the licensed
therapist and may not be billed separately.
• Assisting member’s in preparation for, as necessary during, and at the conclusion
of the treatment
• Assembling and disassembling equipment
• Assisting the physical therapist in the performance of appropriate activities related
to the treatment of the member
• Following established procedures pertaining to the care of equipment and supplies
• Preparing, maintaining, and cleaning treatment areas and maintaining supportive
areas
• Transporting patients, records, equipment, and supplies in accordance with
established policies and procedures
• Performing established clerical procedures
01/31/2007 Therapy Services
Medical Policy Manual
734
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Occupational therapy
Occupational therapy, as defined in the Indiana Code (IC) 25.23-5-1-5, is the planning
and directing exercises and programs to enhance sensory and motor skills to achieve and
maintain an individual’s optimal functional ability in activities of daily living and to
further prevent disability.
Occupational therapy is reimbursed when performed by an occupational therapist or by a
certified occupational therapy assistant under the direct, on-site supervision of a
registered occupational therapist. Reimbursement for occupational therapy evaluations is
only available when performed by a registered occupational therapist. Occupational
therapy psychiatric services are non-covered.
Physical Therapy
Physical therapy, as defined in IC 25-27-1-1, is, “the evaluation of, treatment of, or
instruction in physical rehabilitative and habilitative techniques and procedures to
evaluate, prevent, correct, treat, alleviate, and limit physical disability;
pathokinesiological function, bodily malfunction, pain from injury, disease, and any other
physical disability or mental disorder.”
Physical therapy is reimbursed in the IHCP when performed by a licensed physical
therapist or certified therapy assistance under the direct, on-site supervision of a licensed
physical therapist.
Respiratory Therapy
Respiratory care, as defined in IC 25-34.5-1-6 is an allied health specialty designed to
assist the supervising physician or osteopath in the treatment, management, diagnostic
testing, control, and care of patients with deficiencies and abnormalities associated with
the cardiopulmonary system. A respiratory therapy practioner is someone who meets the
licensing requirements under IC 25-34.5.
Respiratory therapy services are reimbursed by the IHCP only when performed by a
licensed respiratory therapist or certified respiratory therapy technician who is an
employee or contractor of a hospital, medical agency, or clinic. Respiratory therapists
are not recognized providers by the IHCP. Services performed by a respiratory therapist
must be billed by the supervising physician.
Inpatient Rehabilitation Services
The IHCP provides reimbursement for medically necessary inpatient rehabilitation
services provided by licensed, certified, or registered staff members. All rehabilitation
center services require PA. A written plan of care is required for all rehabilitation
01/31/2007 Therapy Services
Medical Policy Manual
735
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
services. The therapist or psychologist and the attending physician must cooperatively
develop the plan of care.
Prior to admission to a physical rehabilitation unit, the member’s total rehabilitative
potential must be evaluated. Documentation in the medical record must include the
member’s condition, IHCP criteria, and level of care necessary in the rehabilitation unit.
The following conditions must be met for reimbursement for a physical rehabilitation
admission.
• The member must be medically stable.
• The member must be responsive to verbal or visual stimuli.
• The member must have sufficient mental alertness to participate in the program.
• The member’s premorbid condition(s) indicates a potential for rehabilitation.
• The expectation for improvement is reasonable.
In addition to these conditions, the member must be able to demonstrate the inability to
function independently as defined in 405 IAC 5-32-1. The following are evaluated to
determine the member’s ability or inability to function independently.
• Cognitive function (attention span, memory, or intelligence)
• Communication (aphasia with major receptive or expressive dysfunction)
• Continence (bladder or bowel)
• Mobility (transfer, walk, climb stairs, or wheelchair)
• Pain management (pain behavior limits functional performance)
• Perceptual motor function (spatial orientation or depth or distance perception)
• Self-care activities (drink or feed, dress, maintain personal hygiene, brace or
prosthesis)
The following intensity of service criteria must be met for reimbursement for services
provided in a rehabilitation center.
• Multidisciplinary team evaluation at least every two weeks.
• Physical therapy must be provided in conjunction with occupational and/or speech
therapy.
• Participation in a rehabilitation program must be under the direction of a qualified
physician.
• Daily skilled rehabilitative nursing care or supervision.
01/31/2007 Therapy Services
Medical Policy Manual
736

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
THERAPY SERVICES
ADDENDUM A
Note: This addendum contains provider notifications that have been published since the
review of the Therapy Services Fact Sheet. The information in this addendum will be
incorporated into the fact sheet at the next review.
Provider Notification: BR200539 Publication Date: 09/27/2005
Subject: Hippotherapy for Physical Therapy
Date Added to Manual: 10/31/2005
Text of Publication
The IHCP has initiated coverage of hippotherapy for physical therapy effective April 1,
2005. To be covered, services must be provided by a licensed physical therapist and
should be billed using the appropriate HCPCS code from the following list:
97110 – Therapeutic exercises to develop strength and endurance, range of
motion, and flexibility
97112 – Neuromuscular re-education of movement, balance, coordination,
kinesthetic sense, posture, and/or proprioception for sitting and/or
standing activities
97530 – Therapeutic activities to improve functional performance
97533 – Sensory integrative techniques to enhance sensory processing and
promote adaptive responses to environmental demands
Services must be ordered by a physician and included in the patient’s treatment plan.
Existing PA requirements for physical therapy apply to hippotherapy.
Note: Procedure code S8940 (hippotherapy per person, equestrian, hippotherapy, per
session) was a newHCPCS code effective January 1, 2005, and is not covered by the
IHCP.
01/31/2007 Therapy Services
Medical Policy Manual
737

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
THERAPY SERVICES
ADDENDUM B
Note: This addendum contains provider notifications that have been published since the
review of the Therapy Services Fact Sheet. The information in this addendum will be
incorporated into the fact sheet at the next review.
Provider Notification: BT200611 Publication Date: 04/20/2006
Subject: Notification of Physical Therapist Assistant’s Rule Change
Date Added to Manual: 04/28/2006
Text of Publication
The purpose of this bulletin is to advise providers that the Indiana Administrative Code
(IAC) 405 IAC 1-11.5-2 was amended to allow for the reimbursement of services
provided by certified physical therapists’ assistants (PTA). This rule amends 405 IAC 5-
22-8 regarding supervision requirements for services provided by certified physical
therapists’ assistants. The PTA is precluded from performing and interpreting tests,
conducting initial or subsequent assessments, and developing treatment plans. Under
direct supervision, a PTA is still required to consult with the supervising physical
therapist daily to review treatment. The consultation can be either face-to-face or by
telephone.
Covered Procedures for PTAs
Effective April 1, 2006, the Indiana Health Coverage Programs (IHCP) has identified
procedures that can be performed by a PTA and are eligible for reimbursement. Providers
must bill these services with the modifier HM–Less than a bachelor’s degree. Pricing for
these services will reimburse at 75 percent of the reimbursement level for a physical
therapist. Table 1 lists the physical therapy services that PTAs may perform. Evaluation
and testing codes are excluded from this list as PTAs may not administer tests or perform
evaluations.
Table 1–Physical Therapy Services that May Be Performed by a PTA Current
Procedural Terminology® Code Description
Code Description
29505 Application of long leg splint (thigh to ankle or toes)
29515 Application of short leg splint (calf to foot)
29520 Strapping; hip
29530 Strapping; knee
29540 Strapping; ankle and/or foot
29550 Strapping; toes
01/31/2007 Therapy Services
Medical Policy Manual
738

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 1–Physical Therapy Services that May Be Performed by a PTA Current
Procedural Terminology® Code Description
Code Description
29580 Strapping; Unna Boot
29590 Denis-Browne splint strapping
97010 Application of a modality to one or more areas; hot or cold compacts
97012 Application of a modality to one or more areas; traction, mechanical
97014
Application of a modality to one or more areas; electrical stimulation
(unattended)
97016 Application of a modality to one or more areas; vasopneumatic devices
97018 Application of a modality to one or more areas; paraffin bath
97022 Application of a modality to one or more areas; whirlpool
97024 Application of a modality to one or more areas; diathermy
97026 Application of a modality to one or more areas; infrared
97028 Application of a modality to one or more areas; ultraviolet
97032
Application of a modality to one or more areas; electrical stimulation (manual),
each 15 minutes
97033 Application of a modality to one or more areas; iontophoresis, each 15 minutes
97034 Application of a modality to one or more areas; contrast baths, each 15 minutes
97035 Application of a modality to one or more areas; ultrasound, each 15 minutes
97036 Application of a modality to one or more areas; Hubbard tank, each 15 minutes
97110
Therapeutic procedure, one or more areas, each 15 minutes; therapeutic exercises
to develop strength and endurance, range of motion and flexibility
97112
Therapeutic procedure, one or more areas, each 15 minutes; neuromuscular
reeducation of movement, balance, coordination, kinesthetic sense, posture,
and/or proprioception for sitting and/or standing activities
97113
Therapeutic procedure, one or more areas, each 15 minutes; aquatic therapy with
therapeutic exercise
97116
Therapeutic procedure, one or more areas, each 15 minutes; gait training
(includes stair climbing)
97124
Therapeutic procedure, one or more areas, each 15 minutes; massage, including
effleurage, petrissage, and/or tapotement (stroking, compression, percussion)
97140
Manual therapy techniques (e.g., mobilization/manipulation, manual lymphatic
drainage, manual traction), one or more regions, each 15 minutes
97150 Therapeutic procedure(s), group (two or more individuals)
97760
Orthotic(s) management and training, (including assessment and fitting when not
otherwise reported), upper extremity (s) and/or trunk, each 15 minutes
97761 Prosthetic training, upper and/or lower extremity (s), each 15 minutes
01/31/2007 Therapy Services
Medical Policy Manual
739

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Therapy Services
Medical Policy Manual
740
Table 1–Physical Therapy Services that May Be Performed by a PTA Current
Procedural Terminology® Code Description
Code Description
97530
Therapeutic activities, direct (one on one) patient contact by the provider (use of
dynamic activities to improve functional performance), each 15 minutes.

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
MEDICAL POLICY FACT SHEET
TITLE: TRANSPORTATION SERVICES
DESCRIPTION
Transportation services enable Indiana Health Coverage Program (IHCP) members to get
to and from medically necessary services. General categories within this section include;
types of transportation services, definition of a trip, prior authorization requirements and
exemptions, covered services, provider requirements, and the transportation code set.
COVERAGE CRITERIA
Transportation services must be for transportation to and/or from an IHCP covered
service. In addition, the member being transported for treatment must be present in the
vehicle in order for IHCP reimbursement to be available. The transportation provided
must be the least expensive type of transportation that meets the medical needs of the
member. IHCP reimbursement is available for emergency and non-emergency
transportation services, subject to program restrictions. These limitations and restrictions
are set out in the Indiana Code (IC), the Indiana Administrative Code (IAC), and IHCP,
newsletters, bulletins, and banners.
TYPES OF TRANSPORTATION SERVICES AND DEFINITIONS
Advanced Life Support – ALS
The Indiana Emergency Medical Services Commission (EMSC), Title 836 of the Indiana
Administrative Code (IAC), defines advanced life support (ALS) as follows:
• Care given at the scene of an accident, act of terrorism, or illness, care given during
transport, or care given at the hospital by a paramedic, emergency medical technician-
intermediate, and care that is more advanced than the care usually provided by an
emergency medical technician or an emergency medical technician-basic advanced.
The term advanced life support may include any of the following acts of care.
• Defibrillation
• Endotracheal intubation
• Parenteral injection of appropriate medications
• Electrocardiogram interpretation
• Emergency management of trauma and illness
01/31/2007 Transportation Services
Medical Policy Manual
741

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
The IHCP provides reimbursement for medically necessary emergency and non-
emergency ALS ambulance services when the level of service rendered meets the EMSC
definition of ALS.
Note: In accordance with Indiana Code (IC) 16-1-31, vehicles and staff that provide
emergency services must be certified by the EMSC to be eligible for
reimbursement for transports involving either ALS or basic life support (BLS)
services.
Basic Life Support – BLS
BLS is defined by the EMSC as the following:
• Assessment of emergency patients
• Administration of oxygen
• Use of mechanical breathing devices
• Application of antishock trousers
• Performance of cardiopulmonary resuscitation (CPR)
• Application of dressings and bandage materials
• Application of splinting and immobilization devices
• Use of lifting and moving devices to ensure safe transport
• Use an automatic or semiautomatic defibrillator
• Administration of epinephrine through an auto-injector
• An emergency medical technician-basic advanced may perform the following.
– Electrocardiogram interpretation
– Manual external defibrillation
– Intravenous fluid therapy
The term basic life support and BLS services do not include invasive medical care
techniques or advanced life support. The IHCP provides reimbursement for medically
necessary emergency and non-emergency BLS ambulance services when the level-of-
service rendered meets the EMSC definition of BLS.
Commercial or Common Ambulatory Service – CAS
The IHCP provides reimbursement for transportation of ambulatory (walking) members
to or from an IHCP-covered service. Commercial or Common Ambulatory Service
(CAS) transportation may be provided in any type of vehicle; however, providers must
bill all transportation services according to the level of service rendered. For example, if
transportation of an ambulatory member is provided by an ambulance, but no ALS or
BLS services are medically necessary for the transport of the member, the ambulance
01/31/2007 Transportation Services
Medical Policy Manual
742
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
provider must bill the CAS charges. Base rate, waiting time, and mileage are separately
billable and reimbursed for CAS transportation.
Non-Ambulatory Service (Wheelchair Van) – NAS
Non-ambulatory services (NAS) or wheelchair services are reimbursable when a member
must travel in a wheelchair to or from an IHCP-covered service. Claims for ambulatory
members transported in a vehicle equipped to transport non-ambulatory members must be
billed according to the CAS level of service and rate, and not billed according to the
vehicle type. Base rate, waiting time, and mileage are separately billable and reimbursed
for NAS transportation.
Taxi
Taxi providers transport ambulatory members and may operate under authority from a
local governing body (city taxi or livery license). Taxi providers whose rates are
regulated by local ordinance must bill the metered or zoned rate, as established by local
ordinance, and are reimbursed up to the maximum allowable fee. Taxi providers whose
rates are not regulated by local ordinance are reimbursed the lower of their submitted
charge or the maximum allowable fee based on trip length. Taxi providers are not
separately reimbursed for mileage above the maximum allowable rate for the trip;
however, mileage must be documented on the driver’s ticket by odometer readings or
mapping software.
Definition of a Trip
For billing purposes, a trip is defined as transporting a member from the initial point of
pick-up to the drop off point at the final destination. Transportation must be the least
expensive type of transportation available that meets the medical needs of the member.
Trips must be billed according to the level of service rendered and not according to the
vehicle type. Providers must bill for all transportation services provided to the same
member on the same date of service on one claim form.
If the provider makes a round trip for the same member, same date of service, and same
level of base code, both runs should be submitted on the same detail with two units of
service to indicate a round trip. Additionally, all mileage for the trip must be billed on the
one detail with the total number of miles associated for the round trip.
If the provider transports a member on the same date of service, but different trip levels,
for example the ‘to’ trip was a CAS trip, and the ‘return’ trip was a NAS trip with
mileage for each base. These base trips must be billed on two different claim forms with
the corresponding mileage for each base.
01/31/2007 Transportation Services
Medical Policy Manual
743

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Note: In the Units field on the CMS-1500 or Service Unit Count field on the 837P,
the provider must use a 1 with the base unit code to indicate a one-way trip and
a 2 to indicates a two-way trip. The transportation modifiers must be used to
indicate the place of origin and destination for each service.
Multiple Destinations
If the member is transported to multiple points in succession, the provider may not bill
for a trip between each point of the destination. The following examples offer
explanations of this concept:
• Example 1: A vehicle picks up a member at home and transports the member to the
physician’s office. This is a one-way trip.
• Example 2: A vehicle picks up a member from home and transports the member to
the physician’s office. The provider leaves, and later the same vehicle picks the
member up from the physician’s office and transports the member back to the
member’s home. This is considered two one-way trips.
• Example 3: A vehicle picks the member up from the physician’s office and transports
the member to the laboratory for a blood draw, waits outside the laboratory for the
member, and then transports the member home. This is a one-way trip, even though
there was a stop along the way. A stop along the way is not considered a separate
trip.
• Example 4: A vehicle picks up Member A at the member’s home and begins to
transport the Member A to the dialysis center. Along the way, a stop is made to pick
up Member B at a nursing home and both Member A and Member B are transported to
the dialysis center. The stop at the nursing home is not considered a separate trip and
the transportation of Member A from home to the dialysis center is considered a one-
way trip.
Prior Authorization
Prior authorization (PA) is required for the following transportation services:
• Trips exceeding 20 one-way trips per member, per rolling 12-month period, with
certain exceptions as described in this billing guide
• Trips of 50 miles or more one way, including all codes associated with the trip (wait
time, parent or attendant, additional attendant, and mileage)
• Interstate transportation or transportation services rendered by a provider located out-
of-state in a non-designated area
• Train or bus services
• Airline or air ambulance services
01/31/2007 Transportation Services
Medical Policy Manual
744

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
PA requests must include a brief description of the anticipated care and description of the
clinical circumstances necessitating the need for the transportation. The PA requests are
reviewed and a PA decision letter is sent to the member and the requesting provider.
Transportation providers may request authorization for members that exceed 20 one-way
trips. Examples of situations that require frequent medical intervention include, but are
not limited to, prenatal care, chemotherapy, and other therapy services. PA may be
granted up to one year following the date of service.
Twenty One-Way Trip Limitation and Exemptions
Transportation is limited to 20 one-way trips per member, per rolling calendar year.
Providers must request PA for members who exceed 20 one-way trips if frequent medical
intervention is required. However, some services are exempt from the 20 one-way trip
limitation. Information about those services is included in the following sections.
Emergency Transportation Services
Emergency ambulance transportation is exempt from the 20 one-way trip limitation.
Providers must indicate that the transportation was an emergency by using the Y
indicator in Field 24I on the CMS-1500 or in the Emergency Indicator on the 837P.
Additional information about ambulance transportation services, including emergency
transportation, is included on page 10 of this billing guide.
Hospital Admission or Discharge
Transportation services for transporting a member to a hospital for admission or for
transporting the member home following discharge from the hospital are exempt from the
20 one-way trip limitation. This includes inter-hospital transportation when the member
is discharged from one hospital for the purpose of admission to another hospital. The
transportation modifiers must be used to indicate the place of origin and destination for
each service.
Note: Transporting an IHCP member to or from a hospital for any reason unrelated
to an admission or discharge is not exempt from the 20-trip limitation.
Members on Renal Dialysis or Members Residing in Nursing Homes
Members on renal dialysis and members residing in nursing homes are exempt from the
20 one-way trip limitation. Claims for members undergoing dialysis or members in
nursing homes must be filed with one of the diagnosis codes listed in Table 1.1 on the
next page. The diagnosis code should be entered on the CMS-1500 or 837P, and a 1
should be placed in Field 24E of the CMS-1500 claim form or the Diagnosis Code
Pointer on the 837P, to indicate that the first diagnosis code applies.
01/31/2007 Transportation Services
Medical Policy Manual
745

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 1.1 – Diagnosis Codes for Transportation of Renal Dialysis Patients and
Patients Residing in Nursing Homes
Diagnosis Code Usage
V56.0, V56.1, or V56.8 Patient undergoing renal dialysis
V70.5 Patient residing in nursing facility
Accompanying Parent/Attendant
Procedure codes for accompanying parent or attendant are not applied to the member’s
20 one-way trip limitation. Prior authorization is required for an accompanying parent or
attendant only when the trip exceeds 50 miles one-way.
Additional Attendant
Procedure codes A0424 – Extra ambulance attendant, ground (ALS or BLS) or air (rotary
or fixed wing) and A0130 U6 – Non-emergency transportation; wheelchair van,
additional attendant, are not applied to the member’s 20 one-way trip limitation. Prior
authorization is required for procedure codes A0424 and A0130 U6 when the trip
exceeds 50 miles one-way.
Mileage
Transportation providers are expected to transport members along the shortest most
efficient route to and from a destination. All transportation providers must document
mileage on the driver’s ticket using odometer readings or mapping software programs.
Reimbursement is available for mileage, in addition to the base rate, under the following
circumstances:
• Ambulance providers are reimbursed for loaded mileage for each mile of the trip
regardless of the type level of service being billed.
• CAS and NAS providers are reimbursed for loaded mileage when the member is
transported more than ten miles one way.
• Taxi providers are not reimbursed for mileage and are not required to submit mileage
with their claim. However, mileage must be documented on the driver’s ticket using
odometer readings or mapping software, as outlined in the documentation
requirements section of this billing guide.
• Although the first 10 miles of a CAS or NAS trip are automatically deducted from
each one-way trip, CAS and NAS providers must bill for all mileage, including the
first 10 miles to ensure proper reimbursement. For trips less than 10 miles, the
provider is not required to bill mileage; however, if mileage is billed, the mileage will
process as a denied line item.
• Trips and associated mileage in excess of 50 miles one way require PA. If PA has not
been obtained, reimbursement for mileage, the base rate, and any other transportation
01/31/2007 Transportation Services
Medical Policy Manual
746

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
services related to the trip are denied. Providers must bill for all transportation
services provided to the same member on the same date of service on one claim form.
• Providers must report mileage using procedure code A0425 and the appropriate U
modifier for transportation services in conjunction with ALS, BLS, CAS, or NAS base
rates. Mileage must not be fragmented. Mileage for round trips must be submitted on
one detail line using the appropriate code listed in Table 1.2.
• Effective July 1, 2004, procedure code S0215 Non-emergency transportation;
mileage, per mile was made non-reimbursable. Providers must bill the appropriate
mileage code listed in Table 1.2. In addition, procedure code S0215 must not be
reported with the codes listed in Table 1.2, or providers may be reimbursed
incorrectly.
Table 1.2 – Mileage Codes and Descriptions
Code Description
A0425 U1 ALS ground mileage, per statute mile
A0425 U2 BLS ground mileage, per statute mile
A0425 U3 CAS ground mileage, per statute mile
A0425 U5 NAS ground mileage, per statute mile
Mileage Units and Rounding
Providers must bill the IHCP for whole units only. Partial mileage units must be rounded
to the nearest whole unit. For example, if the provider transports a member between 15.5
miles and 16.0 miles, the provider must bill 16 miles. If the provider transports the
member between 15.0 and 15.4 miles, the provider must bill 15 miles.
Multiple Passengers
When two or more members are transported simultaneously from the same county to the
same vicinity for medical services, the second and subsequent member transported for
medical services in a single CAS or NAS vehicle is reimbursed at one-half the base rate.
The full base code, mileage, and waiting time are reimbursed for the first member only.
For example, no mileage should be billed in conjunction with T2004 - Non-emergency
transport; commercial carrier, multi-pass, individualized service provided to more than
one patient in the same setting.
The IHCP does not provide reimbursement for multiple passengers in ambulances or
family member vehicles. Additional reimbursement is not available for multiple
passengers when the billing provider does not bill non-IHCP customers for these
services. Table 1.3, on the next page, shows the correct coding methods for multiple
passengers.
01/31/2007 Transportation Services
Medical Policy Manual
747

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 1.3 Coding Transportation for Multiple Passengers
Type of
Transportation
First Member
Second and Subsequent
Members
Commercial
Ambulatory Services
T2003 for base rate
A0425 U3 for mileage
T2007 U3 for waiting time, if
applicable
T2004 for base rate
No reimbursement for
mileage
No reimbursement for waiting
time
Non-Ambulatory
Services
A0130 for base rate
A0425 U5 for mileage
T2007 U5 for waiting time, if
applicable
A0130 TT for base rate
No reimbursement for
mileage
No reimbursement for waiting
time
Taxi, non-regulated, 0-
5 miles
A0100 UA (no mileage) A0100 UA TT (no mileage)
Taxi, non-regulated, 6-
10 miles
A0100 UB (no mileage) A0100 UB TT (no mileage)
Taxi, non-regulated,
11 or more miles
A0100 UC (no mileage) A0100 UC TT (no mileage)
Note: PA for a base code includes both the base code and the multiple passenger
code that corresponds to the approved base code. When last minute changes in
scheduling modify the service from a single passenger to a multiple passenger,
the provider must use the appropriate code.
Accompanying Parent or Attendant
Accompanying parent – When members younger than 18 years of age needs an adult to
accompany them to a medical service, the provider should bill the appropriate
accompanying parent or attendant code.
Accompanying attendant – When adult members need an attendant to travel or stay
with them for a medical service, the provider should bill the appropriate accompanying
parent or attendant code.
The following are guidelines for billing the accompanying parent or attendant codes:
• The procedure code for the base rate and the accompanying parent or attendant is
billed under the IHCP member’s identification number (RID).
• Additional reimbursement is not available for accompanying parent or attendant when
the billing provider does not bill non-IHCP customers for like services.
01/31/2007 Transportation Services
Medical Policy Manual
748

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• The provider must maintain documentation on the driver’s ticket to support that the
accompanying parent or attendant was transported with the IHCP member. This
documentation must include the name, signature, and relation of the accompanying
parent or attendant.
Table 1.4 lists the base rates and the applicable accompanying parent or attendant code.
The provider must bill both the base code and the accompanying parent or attendant code
using the member’s information.
Table 1.4 – Procedure Codes for Accompanying Parent or Attendant
Type of Transportation Base Code
Accompanying
Parent/Attendant
Commercial Ambulatory Services T2003 T2001
Non-Ambulatory Services A0130 A0130 TK
Taxi, non-regulated, 0-5 miles A0100 UA A0100 UA TK
Taxi, non-regulated, 6-10 miles A0100 UB A0100 UB TK
Taxi, non-regulated, 11 or more
miles
A0100 UC A0100 UC TK
Additional Attendant
Transportation providers sometimes need an additional attendant to help load a member.
An additional attendant is needed in situations where the driver cannot load the member
without help, such as when wheelchair-bound member lives upstairs and the residence
has no wheelchair ramp. This code is not subject the 20-trip limit; however, if the trip
exceeds 50 miles one-way prior authorization is required for all procedure codes,
including additional attendant codes. The additional attendant who assists must be an
employee of the billing provider and is not required to remain for the trip.
Providers must document the need for an additional attendant on the driver’s ticket. The
documentation is subject to post-payment review. The additional attendant is limited to a
maximum of two extra units; although, usually one attendant is sufficient.
Reimbursement for an additional attendant is limited to NAS or wheelchair van and
ambulance transportation. For ambulance providers, the additional attendant is the third
or fourth attendant, as ambulances are required to have two attendants.
Prior to the January 1, 2004, providers were instructed to use procedure code Z5023 –
Additional attendant transportation. Local code Z5023 was crosswalked to national code
A0424 – Extra ambulance attendant, ground (ALS or BLS) or air (fixed or rotary
winged); (requires medical review). Procedure code A0424 did not include NAS or
wheelchair van transportation. Effective immediately, procedure code A0130 U6 – Non-
ambulatory transportation; wheelchair van, additional attendant is covered for NAS or
wheelchair van additional attendant transportation. Procedure code A0130 U6 is covered
retroactively to January 1, 2004, when the local code Z5023 was end-dated. Procedure
01/31/2007 Transportation Services
Medical Policy Manual
749

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
code A0424 will continue to be covered for ambulance transportation when an additional
attendant is required. Table 1.5, on the next page, includes the procedure codes for
additional attendant.
Table 1.5 – Procedure Codes for Additional Attendant
Type of Transportation
Procedure
Code
Description
Non-ambulatory or
wheelchair van
transportation
A0130 U6
Non-ambulatory transportation;
wheelchair van, U6 = additional
attendant
Ambulance transportation
(ALS and BLS)
A0424
Extra ambulance attendant, ground
(ALS or BLS) or air (fixed or rotary
winged); (requires medical review)
Waiting Time
Waiting time in excess of 30 minutes is reimbursable only when the vehicle is parked
outside the medical service provider, awaiting the return of the member to the vehicle
and if the member is transported 50 miles or more one-way. PA must be obtained for all
codes associated with trips of 50 miles or more one-way, including waiting time. The
IHCP does not cover the first 30 minutes of waiting time; however, the total waiting time
must be included on the claim, or the claim will not be paid appropriately.
For all procedure codes used to bill waiting time, one unit of service is billed for every 30
minutes of waiting time. When the provider has waited between 15 to 30 minutes, partial
30-minute increments should be rounded up to the next unit. For example, if the provider
has waited 45 minutes, the units of service billed would be two or 2.0. Partial 30-minute
increments less than 15 minutes, must be rounded down. For example, if the provider has
waited one hour and ten minutes, the units of service billed for waiting time would be
two or 2.0. Documentation, including start and stop times, must be maintained on the
driver’s ticket to support the waiting time billed.
Ambulance Transportation Services
The IHCP covers both emergency and non-emergency ALS and BLS ambulance
transport services. Emergency ambulance services are exempt from the 20 one-way trip
limit and do not require PA. In addition, emergency ambulance services are exempt from
the copayment requirement. Providers must bill emergency services by using the Y
indicator in Field 24I on the CMS-1500 or in the Emergency Indicator on the 837P, to
indicate that the service rendered was an emergency. As a reminder, transportation must
be the least expensive type of transportation available that meets the medical needs of the
member.
01/31/2007 Transportation Services
Medical Policy Manual
750

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Note: Air ambulance and interstate transportation services require PA. In addition,
any transportation services provided by a provider located in an out-of-state,
non-designated area require PA.
Level of Service Rendered Versus Level of Response
All transportation services must be billed according to the level of service rendered and
not the provider’s level of response or vehicle type. The IHCP provides reimbursement
for the both emergency and non-emergency ambulance services; however ALS services
are only covered when the level of service is medically necessary and BLS services are
not appropriate due to the medical conditions of the member being transported.
Ambulance providers should refer to the Indiana EMSC definitions of ALS and BLS
services listed in Title 836 of the IAC. Ambulance providers must bill the IHCP
according to the level of service rendered. The following examples explain the level of
service policy:
• Example 1: ALS personnel and ambulance are dispatched. On arrival, the member is
found to need emergency medical transport, but no ALS services. The BLS
emergency transport code must be used. Subsequently, if no emergency is present, the
non-emergency BLS ambulance transport code should be used to transport the
member.
• Example 2: An ambulance is called to transport a member to a scheduled
appointment. Upon arrival it is discovered that the member can instead be transported
by a CAS service or wheelchair van. The ambulance provider can either call for the
appropriate vehicle or transport the patient in the ambulance. If the ambulance
provider transports the member, the appropriate CAS or NAS transportation code(s)
must be used to bill the IHCP.
A complete listing of ambulance transportation codes is included in Table 1.11. The
procedure codes listed in Tables 1.6 and 1.7 are valid for ambulance providers when
used to bill for CAS or NAS level of service. Effective May 1, 2005, procedure codes
A0426 U3, A0428 U3, A0426 U5, and A0428 U5 will no longer be reimbursable.
Ambulance providers must bill the most appropriate CAS or NAS code listed in Tables
1.6 and 1.7 if the level of service does not meet the EMSC definition of ALS or BLS
services. Ambulance providers are still permitted to bill A0425 U1 or A0425 U2 to be
reimbursed for mileage.
Table 1.6– Valid CAS Codes for Ambulance Providers
Procedur
e Code
Reimbursemen
t
Description
T2003 $10.00 Non-emergency transportation, encounter/trip
T2007 U3 $4.25
Transportation waiting time, air ambulance and non-
emergency vehicle, one-half (1/2) hour increments; CAS
01/31/2007 Transportation Services
Medical Policy Manual
751

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 1.6– Valid CAS Codes for Ambulance Providers
Procedur Reimbursemen
Description
e Code t
T2003 $10.00 Non-emergency transportation, encounter/trip
A0426 U3 $10.00
Ambulance service, advanced life support, non-
emergency transport, level 1 (ALS1); CAS
A0428 U3 $10.00
Ambulance service, basic life support, non-emergency
transport; CAS
Table 1.7– Valid NAS Codes for Ambulance Providers
Procedur
e Code
Reimbursemen
t
Description
A0130 $20.00 Non-emergency transportation, wheel chair van base rate
A0130 U6 $5.00
Non-emergency transportation, wheel chair van base
rate; additional attendant
T2007 U5 $4.25
Transportation waiting time, air ambulance and non-
emergency vehicle, one-half (1/2) hour increments; NAS
A0426 U5 $20.00
Ambulance service, advanced life support, non-
emergency transport, level 1 (ALS1); NAS
A0428 U5 $20.00
Ambulance service, basic life support, non-emergency
transport; NAS
Note: Effective May 1, 2005, procedure codes A0426 U3, A0426 U5, A0428 U3,
and A0428 U5 are no longer reimbursable. Procedure codes T2003 and T2007
U3 must be billed by ambulance providers when the level of service rendered
is that of a CAS provider. Procedure codes A0130, A0130 U6, and T2007 U5
must be billed by ambulance providers when the level of service rendered is
that of a NAS or wheelchair van provider. Ambulance providers are still
permitted to bill A0425 U1 or A0425 U2 to be reimbursed for mileage.
Ambulance Mileage
Only loaded ambulance mileage is reimbursed for each mile of the trip. The provider’s
documentation must contain mileage from mapping software or odometer readings
indicating starting and ending trip mileage. Ambulance mileage must be billed using
A0425 U1 – Ground mileage, per statute mile; ALS or A0425 U2 – Ground mileage, per
statute mile; BLS. The U1 and U2 modifier are used to differentiate between ALS and
BLS mileage. Claims billed without the U1 or U2 modifier will deny, and providers will
be required to resubmit with the appropriate modifier.
01/31/2007 Transportation Services
Medical Policy Manual
752

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Neonatal Ambulance Transportation
Reimbursement is available for specialized neonatal ambulance services specially
equipped for inter-hospital transfers of high-risk or premature infants only when the
member has been discharged from one hospital for admission to another hospital.
Procedure code A0225 – Ambulance service, neonatal transport, base rate, emergency
transport, one-way must be used only for neonatal ambulance transport.
Oxygen and Oxygen Supplies
Procedure code A0422 – Ambulance (ALS or BLS) oxygen, and oxygen supplies, life
sustaining situation must not be billed with ALS codes A0426, A0427, and A0433.
These base codes for ALS transport include the reimbursement for supplies and oxygen
in an ALS situation.
Procedure code A0422 can be billed with BLS codes A0428 or A0429, if medically
necessary. Emergency Medical Technicians (EMTs) and paramedics must document the
medical necessity for oxygen use in the medical record maintained by the provider.
Member Copayments
Transportation services require a copayment. Providers are advised to review 405 IAC 5-
30-2 for complete copayment narratives.
The determination of the member's copayment amount is to be based on the
reimbursement for the base rate or loading fee only. No copayment is required for an
accompanying parent or attendant. Transportation providers may collect a copayment
amount from the IHCP member equal to those listed in Table 1.8.
Table 1.8– Transportation Copayments
Transportation Service Member Copayment
Transportation services that pay $10.00 or
less
$0.50 each one way trip
Transportation services that pay $10.01 to
$50.00
$1 each one way trip
Transportation services that pay $50.01 or
more
$2 each one way trip
Exemptions to Copayments for Transportation Services
The following services are exempt from the copayment requirement:
• Emergency ambulance services
• Services furnished to members younger than 18 years old
01/31/2007 Transportation Services
Medical Policy Manual
753
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• Services furnished to pregnant women
• Services furnished to members who are in hospitals, nursing facilities (NFs),
intermediate care facilities for the mentally retarded (ICFs/MR), or other medical
institutions. This includes instances where a member is being transported for the
purpose of admission or discharge.
• Transportation services provided under a Managed Care Organization (MCO) to its
Hoosier Healthwise enrollees
Federal Guidelines for Copayment Policy
According to 42 CFR 447.15, providers may not deny services to any member due to the
member’s inability to pay the copayment amount on the date of service. Pursuant to this
federal requirement, this service guarantee does not apply to a member who is able to
pay, nor does a member’s inability to pay eliminate his or her liability for the copayment.
It is the member's responsibility to inform the provider that he or she cannot afford to pay
the copayment on the date of service. The provider may bill the member for copayments
not paid on the date of service.
Package C Transportation Services
Hoosier Healthwise Package C members are eligible to receive emergency ambulance
services, subject to the prudent layperson definition of emergency in 407 IAC 1-1-6.
Non-emergency ambulance transportation between medical facilities is a covered service
when ordered by the treating physician.
Risk Based Managed Care Hoosier Healthwise Services
Transportation services for Risk Based Managed Care (RBMC) members are the
responsibility of the MCO. Providers must contact the appropriate MCO for more
information about transportation guidelines for RBMC members.
Non-covered Transportation Services
Reimbursement is not available for the following transportation services:
• One-way trips exceeding 20 per member, per rolling 12-month period, except when
medically necessity for additional trips is documented through the PA process
• Trips of 50 miles or more one way, unless PA is obtained
• First 30 minutes of waiting time for any type of conveyance, including ambulance
• Non-emergency transportation provided by any of the following:
– A volunteer with no vested or personal interest in the member
– An interested individual or neighbor of the member
– A caseworker or social worker
01/31/2007 Transportation Services
Medical Policy Manual
754

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
• Ancillary, non-emergency transportation charges including, but not limited to, the
following:
– Parking fees
– Tolls
– Member meals or lodging
– Escort meals or lodging
• Disposable medical supplies, other than oxygen, provided by a transportation provider
• Transfer of durable medical equipment, either from the member’s residence to place of
storage, or from the place of storage to the member’s residence
• Use of red lights and siren for an emergency ambulance call
• All inter-hospital transportation services, except when the member has been
discharged from one hospital for admission to another hospital
• Delivery services for prescribed drugs, including transporting a member to or from a
pharmacy to pick up a prescribed drug
Documentation Requirements for Transportation Services
Each claim must be supported with the following documentation on the driver’s ticket or
run sheet:
• Complete date of service, including day, month, and year of service, such as 3/15/04
• Complete member name and address of pick-up, including street address, city, county,
state, and ZIP
• Member identification number
• Member signature - If the member is unable to sign, the driver should document that
“the patient was unable to sign” and the reason for the inability
• Waiting time including the actual start and stop time of the waiting period, such as
wait time from 1 p.m. to 3:20 p.m.
• Complete service provider name and address, including street address, city, county,
state, and ZIP
Note: If the service provider’s name is abbreviated on the driver’s ticket, the
provider must document the complete provider name or maintain a facility
abbreviation listing. This will help to expedite the post-payment review
process.
• Name of the driver who provided transportation service
• Vehicle odometer reading at the beginning and end of the trip or mileage from
mapping software, including the date the transportation service was provided and the
specific starting and destination address. If mapping software is used, it must indicate
the shortest route.
01/31/2007 Transportation Services
Medical Policy Manual
755
IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Note: All providers, including taxi providers, must document mileage using either
odometer readings or mapping software. Taxi providers must document the
distance traveled to support the metered or zoned rate or mileage code billed.
• Indication of a one-way or round trip
• Indication of CAS or NAS transportation
• Name and relationship of any accompanying parent or attendant to support the
accompanying parent or attendant code billed, if applicable
Note: When an attendant or parent is billed as part of the transport, the parent or
attendant must also sign the driver’s ticket.
It is the provider’s responsibility to verify that the member is being transported to or from
a covered service. It is the provider’s responsibility to maintain documentation that
supports each transport and/or service provided. Transportation providers put themselves
at risk of recoupment of payment if the required documentation is not maintained or
covered services cannot be verified.
Registration Requirements
• Commercial or Common Ambulatory and Non-Ambulatory Providers
– All for profit only CAS and NAS providers are required to certify annually through
the Indiana Motor Carrier Services (MCS) and obtain a Motor Carrier Certification.
– Providers must keep a copy of the certification for their records.
• Taxi Providers
– Providers must have documentation showing operating authority from a local
governing body (city taxi or livery license), if applicable.
– Providers must keep a copy of the documentation for their records.
• Ambulance
– Providers must have an Emergency Medical Services (EMS) Commission
certification.
– Providers must keep a copy of the certification for their records.
– In accordance with IC 16-1-31, vehicles and staff that provide ambulance services
must be certified by the EMS Commission to be eligible for reimbursement for
transports involving either advanced life support or basic life support services.
Failure to maintain the EMS Commission certification on all vehicles involved in
transporting members results in termination of the IHCP Provider Agreement.
• Bus
– Providers must have a MCS certificate from the Indiana Department of Revenue.
– Providers must keep a copy of the certification for their records.
• Family Member
– Providers must have an authorization letter from the local Office of Family and
Children (OFC) (contact caseworker).
01/31/2007 Transportation Services
Medical Policy Manual
756

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
– Providers must keep a copy of the authorization letter for their records.
• Air Ambulance
– Providers must have EMS Commission Air Ambulance certification.
– Providers must keep a copy of the certification for their records.
Chapter 4 of the IHCP Provider Manual includes detailed information about
enrollment requirements and responsibilities. Providers who fail to maintain the
required registration documentation may be referred to the appropriate governing
agencies.
Transportation Code Sets
Effective July 1, 2004, transportation providers are limited to specific codes based on the
provider specialty listed on the provider enrollment file. Table 1.9 through 1.15 list the
procedures codes allowed for each transportation provider specialty. The transportation
HCPCS code (or local code), the national code(s), reimbursement rates, and the
procedure code description are listed for each provider specialty. As a reminder, local
HCPCS codes were end-dated effective December 31, 2003. The applicable national
HCPCS code is listed for each end-dated local code. Due to several coverage changes
that were made in 2004, the coverage dates are indicated, where applicable.
Commercial Ambulatory Service Provider
Table 1.9 – CAS Provider Code Set
264 Commercial Ambulatory Service (CAS) Provider
Transportation HCPCS Code Rate National HCPCS Code Rate Description
S0215
(Non-reimbursable effective June
30, 2004)
$1.25 A0425 U3
(January 1, 2004 – present)
$1.25
Ground mileage, per statute mile;
CAS
X3028
(End-dated December 31, 2003)
$10.00 T2003 U9
(January 1, 2004 – June 30, 2004)
T2003
(July 1, 2004 – present)
$10.00
Non-emergency transportation,
encounter/trip (CAS)
X3029
(End-dated December 31, 2003)
$5.00 T2004 TT
(January 1, 2004 – June 30, 2004)
T2004
(July 1, 2004 – present)
$5.00
Non-emergency transportation,
commercial carrier, multi-pass
(CAS)
01/31/2007 Transportation Services
Medical Policy Manual
757

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 1.9 – CAS Provider Code Set
264 Commercial Ambulatory Service (CAS) Provider
Transportation HCPCS Code Rate National HCPCS Code Rate Description
X3030
(End-dated December 31, 2003)
$5.00 T2001 TK
(January 1, 2004 – June 30, 2004)
T2001
(July 1, 2004 – present)
$5.00
Non-emergency transportation,
patient attendant/escort (CAS)
Y9009
(End-dated December 31, 2003)
$4.25 T2007 U3
(January 1, 2004 – present)
$4.25
Transportation waiting time, air
ambulance and non-emergency
vehicle, one-half (1/2) hour
increments; CAS
Note: As of July 1, 2004, T2003 U9, T2004 TT, and T2001 TK no longer require a modifier.
Additional information is available in IHCP provider newsletter, NL200409, published
September 15, 2004.
Non-Ambulatory Service Provider
Note: Ambulatory members transported in a vehicle equipped to transport non-ambulatory members
must be billed according to the CAS level of service and rate, and not billed according to the
vehicle type. CAS codes are included in the NAS provider code set and listed at the end of
Table 1.10.
Table 1.10 – NAS Provider Code Set
265 Non-Ambulatory Service (NAS) Provider
Transportation HCPCS Code Rate National HCPCS Code Rate Description
S0215
(Non-reimbursable effective
June 30, 2004)
$1.25 A0425 U5
(January 1, 2004 - present)
$1.25
Ground mileage, per statute mile;
NAS
Y9001
(End-dated December 31, 2003)
$20.00 A0130
(January 1, 2004 - present)
$20.00
Non-emergency transportation, wheel
chair van base rate
X3039
(End-dated December 31, 2003)
$10.00 A0130 TK
(January 1, 2004 - present)
$10.00
Non-emergency transportation, wheel
chair van base rate; extra patient or
passenger, non-ambulance
Y9201
(End-dated December 31, 2003)
$10.00 A0130 TT
(January 1, 2004 - present)
$10.00
Non-emergency transportation, wheel
chair van base rate; individualized
service provided to more than one
patient in same setting
Z5023
(End-dated December 31, 2003)
$5.00 A0130 U6
(January 1, 2004 - present)
$5.00
Non-emergency transportation, wheel
chair van base rate; additional
attendant
Y9009
(End-dated December 31, 2003)
$4.25 T2007 U5
(January 1, 2004 - present)
$4.25
Transportation waiting time, air
ambulance and non-emergency
vehicle, one-half (1/2) hour
increments; NAS
01/31/2007 Transportation Services
Medical Policy Manual
758

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 1.10 – NAS Provider Code Set
265 Non-Ambulatory Service (NAS) Provider
Transportation HCPCS Code Rate National HCPCS Code Rate Description
S0215
(Non-reimbursable effective
June 30, 2004)
$1.25 A0425 U3
(January 1, 2004 - present)
$1.25
Ground mileage, per statute mile;
CAS
X3028
(End-dated December 31, 2003)
$10.00 T2003 U9
(January 1, 2004 – June 30, 2004)
T2003
(July 1, 2004 – present)
$10.00
Non-emergency transportation,
encounter/trip (CAS)
X3029
(End-dated December 31, 2003)
$5.00 T2004 TT
(January 1, 2004 – June 30, 2004)
T2004
(July 1, 2004 – present)
$5.00
Non-emergency transportation,
commercial carrier, multi-pass (CAS)
X3030
(End-dated December 31, 2003)
$5.00 T2001 TK
(January 1, 2004 – June 30, 2004)
T2001
(July 1, 2004 – present)
$5.00
Non-emergency transportation,
patient attendant/escort (CAS)
Y9009
(End-dated December 31, 2003)
$4.25 T2007 U3
(January 1, 2004 – present)
$4.25
Transportation waiting time, air
ambulance and non-emergency
vehicle, one-half (1/2) hour
increments; CAS
Note: Ambulatory members transported in a vehicle equipped to transport non-ambulatory members
must be billed according to the CAS level of service and rate, and not billed according to the
vehicle type. CAS codes are included in the NAS provider code set and are listed in Table
1.10.
Ambulance (ALS and BLS) Provider
Note: Transportation must be billed according to the level of service rendered. Therefore, CAS and
NAS codes are included in the Ambulance (ALS and BLS) provider code set and are listed in
Table 1.11. More information about coverage and billing of ambulance services is included on
page 10 of this billing guide.
Table 1.11– Ambulance Provider Code Set
260 Ambulance (ALS and BLS) Provider
Transportation HCPCS Code Rate National HCPCS Code Rate Description
A0070
(End-dated December 31, 2003)
$15.00
A0422
(January 1, 2004 – present)
$15.00
Ambulance (ALS and BLS)
oxygen and oxygen supplies, life-
sustaining situation
01/31/2007 Transportation Services
Medical Policy Manual
759

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 1.11– Ambulance Provider Code Set
260 Ambulance (ALS and BLS) Provider
Transportation HCPCS Code Rate National HCPCS Code Rate Description
A0390
(Non-reimbursable effective
March 31, 2004)
$4.00
A0425 U1
(April 1, 2004 – present)
$4.00
Ground mileage, per statute mile;
ALS
A0380
(Non-reimbursable effective
March 31, 2004)
$3.50
A0425 U2
(April 1, 2004 – present)
$3.00
Ground mileage, per statute mile;
BLS
A0420
(Non-reimbursable effective
March 31, 2004)
$20.00
A0420 U1
(April 1, 2004 – present)
$20.00
Ambulance waiting time ALS,
one-half (1/2) hour increments
A0420
(Non-reimbursable effective
March 31, 2004)
$20.00
A0420 U2
(April 1, 2004 – present)
$20.00
Ambulance waiting time BLS,
one-half (1/2) hour increments
A0426
(No changes)
$85.00
A0426
(No changes)
$85.00
Ambulance service, advanced life
support, non-emergency
transport, level 1 (ALS1)
A0427
(No changes)
$150.00
A0427
(No changes)
$150.00
Ambulance service, advanced life
support, emergency, level 1
(ALS1-emergency)
A0428
(No changes)
$85.00
A0428
(No changes)
$85.00
Ambulance service, basic life
support, non-emergency
transport; (BLS)
A0429
(No changes)
$100.00
A0429
(No changes)
$100.00
Ambulance service, basic life
support, emergency transport,
(BLS-emergency)
A0433
(No changes)
$150.00
A0433
(No changes)
$150.00 Advanced ALS (Level 2)
A0434
(Non-reimbursable effective
March 31, 2004)
$158.30
A0225
(April 1, 2004 – present)
$150.00
Ambulance service, neonatal
transport, base rate, emergency
transport, one-way
A0999
(No changes)
Manual
A0999
(No changes)
Manual Unlisted ambulance service
Z5023
(End-dated December 31, 2003)
$5.00
A0424
(January 1, 2004 – present)
$5.00
Extra ambulance attendant,
ground (ALS or BLS) or air
(rotary and fixed wing)
N/A N/A
A0426 U3
(January 1, 2004 – May 1, 2005)
Use T2003 effective May 1, 2005.
$10.00
Ambulance service, advanced life
support, non-emergency
transport, level 1 (ALS1); CAS
N/A N/A
A0426 U5
(January 1, 2004 – May 1, 2005)
Use A0130 effective May 1, 2005.
$20.00
Ambulance service, advanced life
support, non-emergency
transport, level 1 (ALS1); NAS
01/31/2007 Transportation Services
Medical Policy Manual
760

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 1.11– Ambulance Provider Code Set
260 Ambulance (ALS and BLS) Provider
Transportation HCPCS Code Rate National HCPCS Code Rate Description
N/A N/A
A0428 U3
(January 1, 2004 – May 1, 2005)
Use T2003 effective May 1, 2005.
$10.00
Ambulance service, basic life
support, non-emergency
transport; CAS
N/A N/A
A0428 U5
(January 1, 2004 – May 1, 2005)
Use T2003 effective May 1, 2005.
$20.00
Ambulance service, basic life
support, non-emergency
transport; NAS
N/A N/A
T2003
(Replacement code for A0426 U3 and
A0428 U3, effective May 1, 2005.)
$10.00
Non-emergency transportation,
encounter/trip (CAS)
N/A N/A
A0130
(Replacement code for A0426 U5 and
A0428 U5, effective May 1, 2005.)
$20.00
Non-emergency transportation,
wheel chair van base rate (NAS)
N/A N/A
T2007 U3
(Use this code for waiting time when the
transport is a CAS level of service.)
$4.25
Transportation waiting time, air
ambulance and non-emergency
vehicle, one-half (1/2) hour
increments; CAS
Z5023
(End-dated December 31, 2003)
$5.00 A0130 U6
(January 1, 2004 - present)
$5.00
Non-emergency transportation,
wheel chair van base rate;
additional attendant
N/A N/A
T2007 U5
(Use this code for waiting time when the
transport is a NAS level of service.)
$4.25
Transportation waiting time, air
ambulance and non-emergency
vehicle, one-half (1/2) hour
increments; NAS
Note: Transportation must be billed according to the level of service rendered. Therefore, CAS and
NAS codes are included in the Ambulance (ALS and BLS) provider code set and are listed in
Table 1.11. More information about coverage and billing of ambulance services is included on
page 10 of this billing guide.
Air Ambulance
Table 1.12– Air Ambulance Code Set
261 Air Ambulance
Transportation HCPCS Code Rate National HCPCS Code Rate Description
A0140
(No changes)
Manual
A0140
(No changes)
Manual
Non-emergency transportation and
air travel (private or commercial),
intra or interstate
A0430
(No changes)
Manual
A0430
(No changes)
Manual
Ambulance service, conventional
air service transport, one way
(fixed wing)
A0431
(No changes)
Manual
A0431
(No changes)
Manual
Ambulance service, conventional
air service, transport, one way
(rotary wing)
01/31/2007 Transportation Services
Medical Policy Manual
761

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Table 1.12– Air Ambulance Code Set
261 Air Ambulance
Transportation HCPCS Code Rate National HCPCS Code Rate Description
A0999
(No changes)
Manual
A0999
(No changes)
Manual Unlisted ambulance service
Taxi Provider
Table 1.13– Taxi Code Set
263 Taxi Provider
Transportation HCPCS Code Rate National HCPCS Code Rate Description
X3031
(End-dated December 31, 2003)
$6.00
A0100 UA
(January 1, 2004 – present)
$6.00
Taxi, rates non-regulated, 0-5
miles
X3032
(End-dated December 31, 2003)
$10.00
A0100 UB
(January 1, 2004 – present)
$10.00
Taxi, rates non-regulated, 6-10
miles
X3033
(End-dated December 31, 2003)
$15.00
A0100 UC
(January 1, 2004 – present)
$15.00
Taxi, rates non-regulated, 11 or
more miles
X3034
(End-dated December 31, 2003)
$3.00
A0100 TK UA
(January 1, 2004 – present)
$3.00
Taxi, rates non-regulated, 0-5
miles for accompanying
parent/attendant
X3036
(End-dated December 31, 2003)
$5.00
A0100 TK UB
(January 1, 2004 – present)
$5.00
Taxi, rates non-regulated, 6-10
miles for accompanying
parent/attendant
X3038
(End-dated December 31, 2003)
$7.50
A0100 TK UC
(January 1, 2004 – present)
$7.50
Taxi, rates non-regulated, 11 or
more miles for accompanying
parent/attendant
X3035
(End-dated December 31, 2003)
$3.00
A0100 TT UA
(January 1, 2004 – present)
$3.00
Taxi, rates non-regulated, 0-5
miles for multiple passengers
X3037
(End-dated December 31, 2003)
$5.00
A0100 TT UB
(January 1, 2004 – present)
$5.00
Taxi, rates non-regulated, 6-10
miles for multiple passengers
Y9210
(End-dated December 31, 2003)
$7.50
A0100 TT UC
(January 1, 2004 – present)
$7.50
Taxi, rates non-regulated, 11 or
more miles for multiple
passengers
Y9010
(End-dated December 31, 2003)
$15.00
A0100 U4
(January 1, 2004 – present)
$15.00
Non-emergency transportation;
taxi, suburban territory
Family Member Transportation Provider
Table 1.14– Family Member Transportation Provider Code Set
266 Family Member Provider
Transportation HCPCS Code Rate National HCPCS Code Rate Description
Y9012
(End-dated December 31, 2003)
$0.28
A0090
(January 1, 2004 – present)
$0.28
Non-emergency transportation,
per mile-vehicle provided by
individual (family member, self,
neighbor) with vested interest
01/31/2007 Transportation Services
Medical Policy Manual
762

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
Bus Provider
Table 1.15 – Bus Provider Code Set
262 Bus Provider
Transportation HCPCS Code Rate
National HCPCS
Code
Rate Description
N/A N/A A0110
Max fee $25.00
(January 1, 2004 – June 30, 2004)
Manual
(June 30, 2004 – present)
Non-emergency transportation
and bus, intra or interstate carrier
RELATED MEDICAL TOPICS
Case Management – Pregnant Women
Emergency Medicine – Emergency Room
Emergency Medicine – Emergency Services
Intermediate Care Facilities for the Mentally Retarded
Nursing Facilities
Obstetric Care
Out-of-State-Services
Physical Rehabilitation Services
RULES, CITATIONS, AND SOURCES
405 IAC 5-3-9 (4) PA
405 IAC 5-4-2 Provider agreement requirements for transportation services
405 IAC 5-4-3 Enrollment of a family member as a transportation provider
405 IAC 5-5-2 PA requirements for out-of-state services
405 IAC 5-5-1 Out-of-state services; general
405 IAC 5-30 Transportation Services
405 IAC 5-30-4 PA
Indiana Health Coverage Programs Banners
BR199935
BR200027
BR200138
BR200408
BR200412
BR200415
Indiana Medicaid Update Bulletin
92-05
92-26
93-05
01/31/2007 Transportation Services
Medical Policy Manual
763

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
93-12
93-19
95-36
95-53
97-05
98-04
BT200353
BT200505
Indiana Health Coverage Programs Newsletter
NL200405
ORIGINATION, REVISIONS, AND REVIEWS
Origination Date: 07/01/91
Revisions and
Reviews
Reason
Date
470 IAC 5-8-17;
5-8-17.1; & 5-8-
17.2 Transferred
Transportation 07/1/91
405 IAC 1-6-17
Repealed
8/24/97
Transportation 07/1/91
Bulletin 92-21 Transportation 10/22/92
Bulletin 92-15 Transportation 07/13/92
Bulletin 93-05
Transportation 03/24/93
Bulletin 93-12
Transportation
05/01/93
Bulletin 93-19 Transportation 07/01/93
01/31/2007 Transportation Services
Medical Policy Manual
764

IHCP Policy and Review Services Library Item #: MP10004 Document Control #: H20070007
01/31/2007 Transportation Services
Medical Policy Manual
765
Revisions and
Reviews
Reason
Date
Bulletin 93-20 Transportation 10/15/93
Bulletin 97-05 Transportation
01/29/97
405 IAC 5-30 Transportation 08/24/97
405 IAC 5-5-2 PA for out-of-state services 08/24/97
405 IAC 5-4 Provider Enrollment 08/24/97
Bulletin 98-04
Transportation 02/09/98
405 IAC 5-5-2
Amended
PA for out-of-state services 10/27/99
405 IAC 5-30-1
Amended
Transportation 10/27/99
BR200138 Transportation 09/18/01
Newsletter
NL200405
Transportation Code Set and Policy
Updates
05/15/04
Scheduled Review 07/30/04
BT200505
Transportation Billing Guide and Policy
Updates
03/04/05
APPLICABLE INDIANAAIM EDITS AND AUDITS
1012 – Rendering provider specialty not eligible to render procedure
3012 – Transportation exceeding fifty miles requires PA
4016 – Miles per trip equal to zero
4017 – Waiting time not payable with less than 50 miles
4068 – Mileage and other services will only paid when billed with base rate
4069 – No mileage for multiple passenger base rate
4078 – 30 minutes of waiting time not reimbursable
4079 – Waiting time reimbursable only when recipient is transported 25 miles or more per5
one way trip
4080 – Ten miles not reimbursable per one way trip
6803 – Transportation: one way trip in excess of twenty miles requires PA
6804 – Mileage is not payable when billed with a taxi base
6805 – Reimbursement for taxi base rate reduced
