
DRAFT FOR TESTING
Guidance for Industry on Providing Regulatory
Information in Electronic Format:
eCTD electronic Submissions
This document is published under the auspices of the
EU Telematic Implementation Group - electronic submissions (TIGes)
Please note that this document is being published on the EMEA eSubmission
website so that both agencies and applicants can gain practical experience of
building, submitting and receiving eCTDs.
The Topic Group consider that through this process we will gain valuable
experience of what works and what additional information is required to ensure
that eCTDs become the de facto submission standard across the EU.
Version 1.0
May 2009
Guidance for Industry on Providing Regulatory Information in Electronic Format: eCTD Applications
Version: 1.0 May 2009
Page 1 of 41

Document Control
Change Record
Version Author(s) Comments
1.0 eCTD Topic Group This document has been prepared by the eCTD Guidance Topic
Group of the TIGes. It is largely based on the NeeS guidance
document 1.4.
Coming into Operation
Version Date in operation Comment
1.0 May 2009 This document is specifically called a “Draft for Testing”. The
Topic Group fully anticipate comments from NCAs and
applicants which will enable future versions to reflect practical
experience of users. In this way the document will evolve to
become an essential work of reference in this area.
Guidance for Industry on Providing Regulatory Information in Electronic Format: eCTD Applications
Version: 1.0 May 2009
Page 2 of 41
Table of Contents
1. INTRODUCTION ..................................................................................................................4
2. GENERAL CONSIDERATIONS...........................................................................................6
2.1 Scope..................................................................................................................................6
2.2 Structure of submissions ....................................................................................................6
2.3 Transitional arrangements..................................................................................................7
2.4 Moving to eCTD format from paper or NeeS type applications ..........................................7
2.5 General Submission Considerations ..................................................................................7
2.6 Correspondence .................................................................................................................7
2.7 Paper requirements ............................................................................................................8
2.8 Hardware............................................................................................................................8
2.9 General Technical eCTD Information .................................................................................8
2.10 Other Technical Information ...........................................................................................10
2.11 Archiving and working copies .........................................................................................12
2.12 Technical baseline applications......................................................................................12
3. MODULE SPECIFIC INFORMATION ................................................................................13
3.1 General information ..........................................................................................................13
3.2 Module 1 eCTD envelope, administrative information and prescribing information folder13
3.3 Module 2 overviews and summaries folder ......................................................................15
3.4 Module 3 quality folder......................................................................................................15
3.5 Module 4 Nonclinical study reports folder.........................................................................16
3.6 Module 5 clinical study reports folder ...............................................................................16
4. ADVICE ON SPECIFIC APPLICATION TYPES ................................................................18
4.1 Initial MA Applications.......................................................................................................18
4.2 Variation Applications.......................................................................................................18
4.3 Extension Submissions.....................................................................................................20
4.4 Renewal Submissions ......................................................................................................20
4.5 PSURs..............................................................................................................................21
4.6 MR and DCP Applications ................................................................................................21
4.7 Referrals...........................................................................................................................21
4.8 Active Substance Master Files .........................................................................................22
4.9 Vaccine Antigen Master Files ...........................................................................................22
4.10 Plasma Master Files .......................................................................................................22
4.11 Applicant Initiated Action ................................................................................................23
4.12 Duplicate Applications ....................................................................................................23
ANNEX 1 ECTD REFERENCE DOCUMENTS ................................................................................24
ANNEX 2 GUIDANCE ON TEXT SEARCHABLE DOCUMENTS .........................................................25
1. General...............................................................................................................................25
2. Documents that must always be text searchable ...............................................................25
3. Documents that do not need to be text searchable ............................................................26
4. Further Information .............................................................................................................26
ANNEX 3 GUIDANCE AND BEST PRACTICE ON THE STRUCTURE OF MODULE 3 - CTD-QUALITY
CONSIDERATIONS FOR ECTD SUBMISSIONS IN EUROPE ............................................................27
1. Introduction.........................................................................................................................27
2. General Principles ..............................................................................................................27
3. Module 3 XML Attributes in the eCTD ................................................................................30
Guidance for Industry on Providing Regulatory Information in Electronic Format: eCTD Applications
Version: 1.0 May 2009
Page 3 of 41

1. INTRODUCTION
In 2005 the Heads of Medicines Agencies agreed that all Member States would be able to accept
eCTD (electronic Common Technical Document) applications, without accompanying paper copies, by
the end of 2009. The benefits of moving to e-working are considered to be:
• Reduction in administrative overheads from less paper movement,
• Reduction of physical archiving space,
• Facilitation of the review process.
This Guidance Document is intended to assist pharmaceutical companies with the submission of
regulatory information in electronic format to the National Competent Authorities (hereinafter referred
to as NCAs) and the European Medicines Agency (hereinafter referred to as EMEA). This document
details the requirements for the submission of eCTD electronic submissions. A separate document,
covering NeeS (Non-eCTD electronic Submissions), has already been published on the EMEA’s
eSubmission website and is available via this
link.
This document has been created by the eGuidance Topic Group, a sub-group of the Telematics
Implementation Group – Electronic Submissions (TIGes), consisting of agency representatives from
Belgium, Denmark, EMEA, France, Germany, Hungary, The Netherlands, Portugal, Sweden and the
United Kingdom, together with industry representatives from EFPIA and EGA. It has also been
endorsed by the TIGes. National Competent Authorities have been strongly recommended to adopt
this guidance as the basis for their dealings with applicants.
It should be stressed that this Guidance Document reflects the current situation and will be regularly
updated in the light of changes in national and/or European legislation together with further experience
gained within NCAs and the EMEA of using information submitted in electronic format. It should be
emphasised that eCTD applications should now be regarded as the principal submission format in the
EU.
This document assumes a certain basic understanding of eCTD applications. A list of background
publications can be found in
Annex 1. Applicants should pay special attention to the recommendations
of the ICH M2 Expert Working Group on the eCTD and the TIGes in the EU. Consolidated
specifications can also be found on the Commission Eudralex
website. International standards
development through ICH, ISO and HL7 will eventually lead to the eCTD becoming part of a wider
group of regulated product submissions, covering medical devices, veterinary products and food
additives as well as medicinal products.
This Guidance Document consists of four parts: Introduction, General Considerations, Module Specific
Information and Advice on Specific Application Types together with associated annexes. In addition
please refer to Chapter 7 of the
Notice to Applicants, Volume 2A for specific NCA requirements.
Guidance for Industry on Providing Regulatory Information in Electronic Format: eCTD Applications
Version: 1.0 May 2009
Page 4 of 41
A brief glossary of terms (for the purpose of this document only) is indicated below
:
Term
Definition
Applicant A pharmaceutical company or its agent that is submitting
information in support of an application.
Applicant’s information Regulatory information submitted by an applicant for, or
to maintain, a marketing authorisation that falls within the
scope of this guidance document.
eCTD application A collection of electronic documents compiled by a
pharmaceutical company or its agent in compliance with
European legislation and guidelines in order to seek a
marketing authorisation or any amendments thereof. An
eCTD application may comprise a number of
sequences. In the EU an eCTD application may comprise
several dosage
forms and strengths, all under one
invented product name. This is understood to be
equivalent to a Global Marketing Authorisation according
to Art. 6 para 2 Dir. 2001/83/EC as amended. Some
review tools describe such a collection as a dossier.
Procedure A Community registration procedure for the authorisation
of medicinal products in the European Community. There
are 4 types of procedure that operate within the EC –
Centralised, Decentralised, Mutual Recognition and
National.
Submission or Sequence A single set of information and/or electronic documents
supplied at one particular time by the applicant as a part
of, or the complete, eCTD Application. In the context of
eCTD, this is equivalent to a sequence.
Regulatory activity A collection of sequences covering the start to the end of
a specific business process, e.g. an initial MA application
or Type II variation. It is a concept used in some review
tools to group together several business related
sequences.
Guidance for Industry on Providing Regulatory Information in Electronic Format: eCTD Applications
Version: 1.0 May 2009
Page 5 of 41

2. GENERAL CONSIDERATIONS
2.1 Scope
2.1.1 Types of product
This guidance covers the submission of electronic regulatory information for all human medicinal
products falling within the competence of NCAs and the EMEA. This includes prescription, over the
counter medicines, innovative and generic product submissions. The product types include small
molecules, biotech products, herbals, vaccines, homeopathics and blood products.
2.1.2 Types of submission
This guidance applies to all submissions related to the authorisation and maintenance of medicinal
products, including new marketing authorisations, variations, renewals, PSURs, active substance
master files.
2.1.3 Types of procedures
This guidance covers applications made in any of the applicable Community procedures (National,
Mutual Recognition, Decentralised and Centralised).
2.1.4 Exceptions
This guidance does not apply to the electronic submission of pre-MA information such as scientific
advice, clinical trial applications and related submission correspondence.
2.2 Structure of submissions
This document provides guidance on how to organise application information for electronic submission
using the eCTD specifications. Guidance on the detailed information to be included is described in the
Common Technical Document (CTD), and relevant ICH and EU Q&A documents.
The structure and organisation of an eCTD submission is defined by the following standards:
• ICH M2 eCTD Specification
• EU Module 1 Specification
• Relevant ICH and EU Q&A docs
Annex 1 contains links to the currently approved version of these documents.
Typically, an eCTD application will cover all dosage forms and strengths of a product with any one
invented name. In the centralised procedure, this will be equivalent to all dosage forms and strengths
covered by an EMEA application number (e.g. EMEA/H/123). In MRP/DCP, a single eCTD application
should preferably be used for the procedure. However if an applicant decides not to apply for all
strengths and dosage forms in every member state in the procedure, the possibility of having one
eCTD application per strength should be considered. Applicants should carefully consider what an
eCTD application should cover before submitting the first sequence, as the choice could have
implications for workload for the lifespan of the product. For example, if the applicant decides to have
one eCTD per strength or dosage form, it is expected that each of these eCTD applications will be
maintained individually, such that submission of a single sequence that covers more than one strength
or dosage form will no longer be possible if very good reasons are not presented for a change over. In
these rare cases, please contact the NCA/RMS/EMEA concerned at an early planning stage.
For further details on the pros and cons of the different approaches to dossier structure, see Annex 3,
Table 1.
Guidance for Industry on Providing Regulatory Information in Electronic Format: eCTD Applications
Version: 1.0 May 2009
Page 6 of 41

Please check for specific NCA guidance when preparing national eCTDs. .
2.3 Transitional arrangements
The specifications mentioned in section 2.2 above will change over time and are likely to affect both
eCTD building tools and the applicant’s internal business processes as well as the agencies review
tools and processes. Once a new specification has been agreed and endorsed by the appropriate EU
body, eCTD building tools will need to be updated. Specific transitional guidance will be provided on
each occasion that the ICH and/or EU specifications are updated.
Please note that it should not be necessary to reformat and resubmit previously submitted applications
to reflect such changes.
2.4 Moving to eCTD format from paper or NeeS type applications
An eCTD product life cycle can be started with an initial, variation or renewal MA application. However,
changing to eCTD format should not be performed in the middle of an on-going regulatory activity (i.e.
do not submit responses to questions as an eCTD if the corresponding application to which they relate
has not been submitted as an eCTD).
Where a repeat use procedure in eCTD format is planned, the change of format should be made
before the start of the process.
Where an eCTD application is being used for the first time for a variation or renewal application,
applicants are encouraged to submit a technical baseline for the product as this will greatly aid the
review process. Please see
section 2.12 for further information on the content of baseline applications.
2.5 General Submission Considerations
2.5.1 Document granularity
Submissions are a collection of documents and each document should be provided as a separate file.
The detailed structure of the eCTD should conform to the
ICH Granularity Document and EU M1
specifications.
2.5.2 File Naming
The eCTD file naming conventions described in the ICH M2 eCTD Specification and EU Module 1
Specification are highly recommended. If an applicant wishes to submit multiple files in one section,
where only one highly recommended name is available, this can be achieved using a suffix to the
filename, using the file name-var.pdf convention as described in the EU Module 1 Specification, where
the -var component has no dashes or illegal characters (e.g. pharmaceutical-development-
container.pdf).
2.5.3 Placement of Documents
Guidance on the placement of documents within the eCTD structure for particular submission types
can be found in the
EU-CTD Notice to Applicants and/or in the EMEA post-authorisation guidance for
centralised applications.
2.6 Correspondence
In addition to the eCTD application information may need to be exchanged to assist the processing or
handling of the application. Not all that correspondence should be included in the eCTD. This is
because the eCTD exchange is currently one way only, from applicant to Agency, and not all
correspondence is directly relevant to the application dossier.
Guidance for Industry on Providing Regulatory Information in Electronic Format: eCTD Applications
Version: 1.0 May 2009
Page 7 of 41

Accordingly, only the minimum amount of correspondence that relates directly to the content of the
dossier should be included in eCTD submissions to NCAs and the EMEA. All other correspondence
should be exchanged outside the eCTD via the usual electronic means (email, Eudralink etc). Such
documentation is likely to be handled in different ways within the authorities and normally not within the
eCTD review system.
Where correspondence acknowledges the final change to details submitted in the body of the dossier,
the agreement cannot be documented by that correspondence alone. The revised information should
be provided in an accompanying replacement document situated in the appropriate place in the body
of the dossier, with the relevant information contained in a covering letter. See further detailed
information in
section 3.2.3
The eCTD is designed to ensure that users have a current view of the information submitted in the
appropriate place in the dossier at all times.
2.7 Paper requirements
An overview of the requirements for paper and electronic copies is specified for each NCA and EMEA
in the
Notice to Applicants, Volume 2A, Chapter 7.
Guidance on the minimum requirements to produce a paper submission from an eCTD has also been
published in the
Notice to Applicants, Volume 2B
2.8 Hardware
NCAs and the EMEA will not accept any hardware (laptops, desktops, zip drives, etc.) from applicants
in connection with the submission of information in electronic format. The electronic information should
be directly readable and usable on NCAs and EMEA hardware and software.
2.9 General Technical eCTD Information
2.9.1 File formats
In general terms the majority of documents included in electronic submissions should be in PDF
format, file version 1.4 (see next section on the use of later PDF file versions).
The use of XML for applications forms in particular is likely to increase as agency systems develop the
functionality to handle it in their own business processes. See
Section 3.2.4 for further information.
Further detailed guidance on file formats can be found in the ICH eCTD specification document and EU
Module 1 specifications.
2.9.2 Portable Document Format (PDF)
Portable Document Format (PDF) is an open, de facto, electronic publishing standard. Although
created by Adobe Systems Incorporated there are several alternative suppliers of PDF software.
Applicants need to check that their PDF documents meet the following key requirements:
• Files should be legible with Acrobat Reader, version 5.0 or higher.
• Only PDF file version 1.4 should be used, except where there is an agency specific requirement
for a later version for application forms.
• Documents should be generated from electronic source documents and not from scanned
material, except where access to the source electronic file is unavailable or where a signature is
required. See Annex 2 for further guidance on text searchable documents.
Additional details on PDF, including those relating to the good presentation of tables, can be found in
the
ICH eCTD Specification, Appendix 7.
Guidance for Industry on Providing Regulatory Information in Electronic Format: eCTD Applications
Version: 1.0 May 2009
Page 8 of 41

2.9.3 Sequence Numbers
Sequence numbers are used to differentiate between different submissions of the same application
over the life cycle of the product. The review tools being used by most NCAs and the EMEA are able
to handle sequences submitted out of numerical order, i.e. 0003 submitted after 0004.This can occur
when the preparation of a sequence is delayed. However, it is recommended that,sequence numbers
should normally follow the order of submission of the sequences. A Sequence Tracking Table should
always be included as an annex to the cover letter in every submission within MRP/DCP (see
CMD(h)
recommendations on the cover letter). Specific recommendations for MR and DC Procedures are
given in the CMD(h)
guidance on e-submission. A similar tracking table is recommended for national
applications.
The initial eCTD life cycle submission should normally have a sequence number of 0000. If applicants
consider that there are good reasons to use another number they should explain this in the cover
letter.
As additional data is submitted in response to questions, the sequence number of the submission will
advance, 0001, 0002, etc. Only in the case of a technically invalid submission can a sequence be
replaced with one using the same number, e.g. the initial sequence “0000” will be replaced by another
“0000”.
When starting an eCTD product life cycle at a point other than with an initial MA application, such as a
variation or renewal, the initial eCTD sequence serving as the baseline, should preferably be
numbered as “0000”.
The relationship of one sequence to another is managed using the related sequence number. This
allows sequences to be grouped together that make up an application or a regulatory activity.
2.9.4 Bookmarks and hypertext links
Navigation through an electronic submission is greatly enhanced by the intelligent use of bookmarks
and hypertext links. ICH guidance states “It is expected that any document that has a Table of
Contents (TOC) will have bookmarks (see the eCTD specification for details). Documents without
TOCs should have bookmarks included where it aids in the navigation around the document content.
For example, a 4 page document summarising findings could require bookmarks to aid navigation.
However, a 300 page file containing a single data listing might not require bookmarks as there is no
further internal structure. Please consult regional guidance documents for further details.”
In general terms, bookmarks and hyperlinks should be used to aid navigation. The overuse of
hyperlinks may confuse rather than help assessors and may cause problems later in life cycle
management.
Additional details on creating bookmarks and hypertext links in PDF documents can be found in the
ICH eCTD Specification, Appendix 7.
2.9.5 Extensible Mark-up Language (XML)
XML is the format for the backbone files for the eCTD. Details on XML can be found in the ICH eCTD
Specification Document, Appendix 7. Initiatives on the use of XML structured information are
supported by NCAs and the EMEA for the Product Information Management (PIM) system and e-
application forms. Please refer to EMEA e-submission website for further details.
Guidance for Industry on Providing Regulatory Information in Electronic Format: eCTD Applications
Version: 1.0 May 2009
Page 9 of 41

2.9.6 Other File Formats
Other file formats such as rich text (RTF) or MS Word formats may be required in addition to the PDF
requirement of the eCTD, by specific NCAs or the EMEA, especially for the provision of product
information documents or the Module 2 documents. Please refer to the
Notice to Applicants, Volume
2A, Chapter 7 for further details.
These files should not be added as leaf elements within the eCTD
structure. They should be provided in a separate folder called,
e.g.“<sequence>-workingdocuments” on the CD/DVD containing
the eCTD.
2.9.7 Technical validation of eCTD submissions
The technical validation of an eCTD is a separate activity to the content validation of a submission and
takes place irrespective of the type of the submission. NCAs and the EMEA have adopted a
common
set of technical criteria against which all eCTDs can be checked using eCTD review and validation
tools.
The general process for the validation of eCTD submissions is divided into two parts: technical and
content validation. An eCTD must pass technical validation before it undergoes content validation.
Technical validation errors of category A will result in the applicant being asked to resubmit the eCTD
sequence with the same sequence number, category B errors will normally not require resubmission
but may require fixing in a subsequent sequence, category C do not require resubmission but may be
fixed in subsequent sequences at the applicant’s discretion.
Errors found during the content validation should be resolved through the submission of a new eCTD
sequence. These errors must never be resolved by resubmitting an existing sequence.
2.10 Other Technical Information
2.10.1 Security issues
The physical security of the submission during transportation is the responsibility of the applicant.
Once received by NCAs and the EMEA, security and submission integrity is the sole responsibility of
NCAs and the EMEA.
2.10.2 Password protection
Submission or file level security is not permitted. If one-time security settings or password protection of
electronic submissions are used this could constitute grounds for the rejection of the submission.
2.10.3 Virus protection
The applicant is responsible for checking the submission for viruses. Checking should be performed
with an up-to-date virus checker and be confirmed in the cover letter. After receipt at NCAs and the
Guidance for Industry on Providing Regulatory Information in Electronic Format: eCTD Applications
Version: 1.0 May 2009
Page 10 of 41

EMEA, a similar internal virus check will be performed. If a virus is detected it will constitute grounds
for rejection of the electronic submission.
2.10.4 Electronic signatures
Although electronic signatures are currently accepted in the EU as being legally equivalent to
handwritten signatures (Directive 1999/93/EC), the majority of NCAs and EMEA do not have a system
for that yet and therefore require that certain specific documents (covering letters, Application Forms)
are authenticated by separate signed paper copies. Please refer to each NCA for detailed guidance on
this matter or the
Notice to Applicants, Volume 2A, Chapter 7.
2.10.5 Transmission Media
Currently CD-ROM, CD-R, DVD-R are considered acceptable media standards. Applicants should
provide the electronic information on the smallest number of discs possible, taking into consideration
the size of the submission.
If an individual eCTD submission is of such a size as to span several CDs, the provision of a DVD is
recommended. However, if CD-R must be used, when large applications are submitted it is inevitable
that the application will necessarily span multiple CDs. Where possible, individual modules should not
be split over multiple CDs (e.g. if possible, a single CD should contain Module 1, Module 2, if too large
to fit on the same CD should then go onto the next CD even if this requires CD 1 not to be filled to
capacity and so on). If, in the case of larger modules, where a split over multiple CDs is inevitably
necessary, subfolders should be distributed in sequence, and these subfolders should not be split
between CDs, even if this requires a CD to be sent not full to capacity.
It is the choice of the applicant if a separate CD/DVD is provided for each new sequence or if several
sequences (e.g. concerning several variations) for the same medicinal product (same eCTD) is
provided on the same CD/DVD. This should be clearly described in the cover letter and indicated on
the disc (see 2.10.6).
2.10.6 Labelling of Media:
Each CD or DVD submitted with an eCTD should include the following label information, clearly
presented and printed on the media:
¾ Format: eCTD
¾ The applicant’s name
¾ The product (invented) name(s)
¾ The International Non-proprietary Name (INN) of the active substance(s)
¾ The full application number(s) (if known)
¾ The sequence number(s) of the eCTD submissions contained on the CD/DVD
¾ Number of media units per full set and an indication of the place of the individual CD/DVD
within this set (e.g. 1(5), 2(5), etc.
¾ The submission type(s) of each eCTD submission(s) contained on the CD/DVD (e.g. Initial
Application, Variation Type II), as per the eCTD envelope information
2.10.7 Procedure for sending electronic information
Some NCAs are able to accept eCTDs submitted via their portals. Generally only small (<100MB)
applications can be handled this way. Applicants should check with individual NCAs for details of this
process. If submissions are uploaded via a portal no data corruption should occur as a result of the
process.
In all other cases the eCTD submission should be sent to the address referred to in the
Notice to
Applicants, Volume 2A, Chapter 7.
Guidance for Industry on Providing Regulatory Information in Electronic Format: eCTD Applications
Version: 1.0 May 2009
Page 11 of 41

Electronic media sets should be submitted at the same time as any required paper documentation.
The electronic media should be packed adequately to prevent damage and the package should
include a cover letter. Please see
section 3.2.3 for details on the format of cover letters.
2.11 Archiving and working copies
Please refer to the Notice to Applicants, Volume 2A, Chapter 7 for details of the number of copies of
electronic submissions required for archiving and review purposes. Many NCAs destroy discs after
data has been uploaded into their systems. Where an NCA requires the disc to be archived they may
have additional requirements.. Note: The current standard to burn CDs / DVDs is
UDF, which has
replaced the former
ISO standard 9660.
2.12 Technical baseline applications
These should normally be submitted as sequence 0000. It should be clearly stated in the cover letter of
the “baseline eCTD” that the content of the current dossier has not been changed, only its format.
Consequently the first variation in eCTD format should then be submitted as sequence 0001. It is not
necessary for the baseline eCTD to contain document hyperlinks as it would not normally be
assessed.
The technical baseline application can also be used by applicants to switch from one eCTD sequence
per strength, to one eCTD sequence covering multiple strengths. For the switch the pros and cons of
the different approaches to dossier structure, as described in Annex 3, Table 1, should be taken into
consideration.
The switch from one approach to another should only be allowed once during the life
cycle.
Guidance for Industry on Providing Regulatory Information in Electronic Format: eCTD Applications
Version: 1.0 May 2009
Page 12 of 41

3. MODULE SPECIFIC INFORMATION
3.1 General information
The following subfolders should be used to organise the files for each module in a submission: m1,
m2, m3, m4, and m5 following the principles set out for the CTD in
Notice to Applicants, Volume 2B.
There is also a subfolder util to organise eCTD technical files in the submission. If a module is not
appropriate for a particular submission it should be omitted. Empty subfolders should not be included.
Each document should be provided as an individual PDF file, except those specifically requested in a
different format, e.g. e-Application Form or PIM data.
A single eCTD application can cover multiple drug substances (e.g. in case of fixed combination
products), multiple manufacturing sites, multiple medicinal products based on one invented name
(different pharmaceutical forms or strengths). Careful planning is required to ensure that the dossier
can be expanded as the product range is expanded or reduced by the submission of later sequences.
Please see Annex 3 for further details.
Currently it is outside the scope of current eCTD specifications to allow cross references to
documents, sections or modules in other eCTD dossiers.
3.2 Module 1 eCTD envelope, administrative information and prescribing
information folder
3.2.1 General considerations
In the case of country specific files or folders the country code should appear in the file and folder
name as the differentiating marking.
Module 1 “Not Applicable (N/A)” documents should not be included in the eCTD. However, when a
justification for the absence of a certain document in module 1 is required, such justification should be
provided in its corresponding section in the eCTD structure. In any case, all section titles should
always appear in the module 1 eCTD backbone, displayed by the style sheet, even if these sections
are not populated.
3.2.2 Creation and Management of Envelope Information
The eCTD envelope should be used to describe the eCTD sequence:
Country In the centralised procedure, there should only be one envelope with the
entry ‘emea’. For MRP/DCP, each country in the procedure needs to have
a separate envelope entry. Common must not be used as a country
identifier in the envelope.
Application number The application number should be the centralised procedure/application
number (EMEA/H/C/...), MRP/DCP procedure number (e.g. SE/H/1234...)
or other national application or licence number as required by NCAs.
Multiple entries are possible. The full application number including the
regulatory activity identifier should be used if known.
Applicant Entries for ‘applicant’ should be consistent for all eCTDs from any single
applicant (legal entity), as they define where eCTDs are stored in internal
systems. Consistency of spelling is also relevant over time to allocate the
eCTD correctly.
Agency-name Self explanatory, from picklist in the most recent EU m1 eCTD
specification. Assure that Country and Agency name will be consistent.
Guidance for Industry on Providing Regulatory Information in Electronic Format: eCTD Applications
Version: 1.0 May 2009
Page 13 of 41

ATC If unknown at the time of submission, the entry can say ‘to be confirmed’.
Submission type From picklist, see m1 specification for further details.
Procedure type From picklist, see m1 specification for further details.
Invented-name The trade name/invented name for the medicinal product covered by the
application. If the eCTD covers multiple strengths or dosage forms, this
entry does not need to describe the complete name, a simple entry, for
example, ‘Wonderdrug’ will suffice.
INN The International non-Proprietary name for the drug substance.
Sequence The sequence number here must match the sequence number in the folder
structure.
Related-sequence For a description and example of how to use the ‘related sequence’ entry,
see the EU m1 specification, p14.
Submission-description This element is used to describe this particular eCTD sequence
3.2.3 Cover letter
The cover letter should, at least for MRP and DCP applications, include as a minimum, the information
specified in the
CMDh Guidance document. There is also a template that can be used. Please see the
Notice to Applicants, Volume 2A Chapter 7, for details on the provision of signed paper copies of the
cover letter and application form. The omission of submitted data from certain sections can also be
explained here.
The cover letter should also mention if the product information is being provided as PIM data..
The cover letter should be submitted with the document operation attribute “new” which is in line with
EU M1 specifications. As eCTD viewing tools will display all "new" leaf elements in a current or
cumulative view, it is recommended that you place additional descriptive text in the leaf title to help you
identify specific cover letters. This will help identify each cover letter leaf and the submission it is in,
rather than having the cover letters named the same in each sequence. Some examples for the leaf
titles could be:
Cover Letter for Sequence 0000
Cover Letter for Germany for Sequence 0000
Cover Letter for France for Type II Variation 028 (0023)
3.2.4 XML application forms
Some NCAs request that applicants create an application form on their portals which assist in their
internal case creation process. Specific NCA advice should be obtained on where to locate them in the
eCTD structure.
The majority of NCAs and EMEA require the application form to be provided as a PDF file together
with separate signed paper copies. Please refer to Chapter 7 of the
Notice to Applicants volume 2A for
further details.
3.2.5 Product information
Product information should normally be supplied as PDF files but some NCAs require an RTF or Word
file in addition to facilitate assessment. Details can be found in section 2.9.6. If the Product Information
Management (PIM) system is being used files are required in XML format. Please refer to the
Notice to
Applicants, Volume 2A, Chapter 7 for details.
3.2.6 Use of response documents section
The submission of electronic information in response to a list of questions from NCAs and EMEA
should follow the same basic principles as the first submission. The written response should be
submitted following the EU recommended response folder and file structure. Please note that all data
Guidance for Industry on Providing Regulatory Information in Electronic Format: eCTD Applications
Version: 1.0 May 2009
Page 14 of 41
related documents are aligned with the CTD structure, refer to EU CTD question 4.c. using the
operation attributes of “new”, “replace”, “append” or “delete” as appropriate.
To help in the management of responses over the lifecycle of the eCTD, the responses relating to a
particular regulatory activity should be grouped under a node-extension in the eu-regional.xml file. The
title of the node-extension should identify the regulatory activity (e.g. Responses to Questions for the
Initial Application, Responses to Questions for Type II Variation 028, etc.). It is recommended that you
provide a full copy of the list of questions received from the agencies as the first leaf in this section.
It is recommended that the responses be split up into separate files for each major section of the
submission (e.g. Quality, Non-clinical and Clinical). You should use the leaf title to identify the
particular set of responses (e.g. Response to Major Objections - Quality). If responses to more than
one question are submitted in a single file then you should use bookmarks within the PDF file to
clearly identify each response. It is possible to submit the response to each question in a separate file
but if you choose to do so then you must use node-extensions and leaf titles to group and identify the
responses under the top level node-extension.
All of the files for the response documents should be placed in the folder m1/eu/responses/CC, where
CC is the appropriate country identifier code for use in MRP/DCP.
3.2.7 Use of the additional data section
The 'Additional Data’ section should only be used for nationally required information in National, MR
and Decentralised Procedures.
In addition this section can be used for all procedures when an old version of a DTD is being used
during an agreed transition period, to support inclusion of a newly defined section of Notice to
Applicants (refer to transition guidance issued with specification).
3.3 Module 2 overviews and summaries folder
3.3.1 General considerations
Each document should be provided as an individual PDF.
3.3.2 Structure of Module 2 Documents
Documents in module 2 should normally be submitted with the document operation attribute “new” as it
would help clarifying what to assess with each submission. As eCTD viewing tools will display all
"new" leaf elements in a current or cumulative view, it is recommended that you place additional
descriptive text in the leaf title to help identifying the documents related to each submission.
New information in module 2 could also be integrated into the former document and then replace the
former one with the operation attribute “replace”
The summaries should be used to justify the absence of data in module 3-5 instead of submitting place
holder files stating “No data submitted” or N/A.
3.4 Module 3 quality folder
3.4.1 Module 32S drug substance
If the product contains multiple drug substances, then documentation for each substance should be
provided in its own m32s section. If a drug substance is manufactured at multiple sites or by multiple
different manufacturing companies, documentation can be provided in multiple m32s sections.
Guidance for Industry on Providing Regulatory Information in Electronic Format: eCTD Applications
Version: 1.0 May 2009
Page 15 of 41

However, it may be possible to write documentation that covers multiple manufacturers in one CTD
section – the way the information is provided is left up to the applicant. For further details, please see
Annex 3.
3.4.2 Module 32p drug product
Each dosage form covered by an eCTD application should be described in its own m32p section. If an
application describes multiple strengths of any one dosage form, then documentation that covers all
strengths can be provided in a single m32p section, or alternatively each strength can be covered by
its own strength-specific documents in multiple strength-specific CTD sections. For further details, see
Annex 3.
3.5 Module 4 Nonclinical study reports folder
3.5.1 Guidance on the handling of granular study reports
Submissions created in eCTD format for the use within the FDA may provide more granular study
reports using study tagging files. There is no need to re-organise the reports for submission to the
EMEA or NCAs. See 3.6.2.below for further information.
3.6 Module 5 clinical study reports folder
3.6.1 Management and handling of multiple indications
In cases where the application includes multiple therapeutic indications, the reports should be
organized in a separate Section m535 for each indication. In such cases, if a clinical efficacy study is
relevant to only one of the indications included in the application, it should be included in the
appropriate section in m5 (eg m5\53-clin-stud-rep\535-rep-effic-safety-stud\anxiety\5351-stud-rep-
contr). If a clinical efficacy study is relevant to multiple indications, the study report should be included
in the most appropriate subsection of m535 and referenced as necessary in the equivalent section
under the different indication. In Module 2, a separate “Summary of Clinical Efficacy” module should be
submitted for each indication, although closely related indications can be within a single document.
Regardless of which way is chosen, it is important to give clear guidance to the assessor when the
supportive data/study report documents are applicable to more then one indication.
3.6.2 Management and handling of granular clinical study reports
ICH Q&A 22 recommends use of E3 granularity for clinical study reports. In Europe, node extensions
should be used to group together individual files. STFs from submissions in the US are not required
but a submission will not be rejected if they are included. If a US NDA is repurposed for submission in
the EU, the study content (the study report and any relevant appendices) should be placed under a
node extension. The STF xml file itself and any content not usually provided in Europe (eg datasets)
should be removed. In order to keep the cumulative and current dossier views of the eCTD consistent,
applicants are advised to use node extensions for all clinical study reports, regardless of the
granularity of the content (i.e. even reports that consist of only one document should also be presented
in node extensions). For further details see
EU eCTD Q&A 10.
3.6.3 Provision of CRFs and data when requested
If case report forms and individual patient data listings are submitted in m537 (as appendices 16.3 and
16.4 in the ICH clinical study report guideline E 3) they should be placed in the same order as the
clinical study reports appearing in m535 and should be indexed by study. Please note that bookmarks
will not be required as there will be no further internal structure.
3.6.4 Provision of synopses of individual studies
It is acceptable either to include copies of the synopses for each study in Section 2.7.6 or to provide
hyperlinks to synopses located in Module 5 without providing copies in section 2.7.6. In either case a
Guidance for Industry on Providing Regulatory Information in Electronic Format: eCTD Applications
Version: 1.0 May 2009
Page 16 of 41
Listing of Clinical Studies should be provided and this should include hyperlinks to the first page of
each synopsis.
Guidance for Industry on Providing Regulatory Information in Electronic Format: eCTD Applications
Version: 1.0 May 2009
Page 17 of 41

4. ADVICE ON SPECIFIC APPLICATION TYPES
4.1 Initial MA Applications
The recommended start for an eCTD life cycle is the initial MA application. It should normally be
provided as sequence 0000. Any other number should be justified in the cover letter. All documents
included should have the operation attribute “New” and be placed in the relevant sections in line with
the different eCTD specifications.
The submission type should be initial-maa. The procedure type depends on the application that is
made. Please find below an overview of the different application types:
Procedure Procedure type
Centralised Procedure (CP) centralised
Decentralised Procedure (DCP) decentralised
Mutual Recognition Procedure (MRP) mutual-recognition
National national
Repeat Use Procedure (RUP) mutual-recognition
For responses to questions documents, see
section 3.2.6.
The following milestones of the procedures are proposed as appropriate sequences to be submitted
during the assessment of an initial new application. Although the example relates to the centralised
procedure similar principles can be applied to other procedures.
Day Number eCTD milestone sequence Notes
-14 Initial submission
-1 Response to business validation issues
if required
121 Response to List of Questions (LoQ)
181 Response to List of Outstanding Issues
(LoOI)
210 Final English
Application as agreed at opinion
215 Provision of translations
245 Provision of final agreed translations
following linguistic review
It is not also required to send interim
working versions of the product
information before this point as eCTD
270 Decision
I.e. final amended documentation if
any changes occur during the
Standing Committee
phase
4.2 Variation Applications
All types of variations should be submitted within the eCTD as new sequences. The cover letter and
application form should be included in the eCTD but the majority of NCAs also require these items to
be submitted in signed paper format. Please refer to the
Notice to Applicants, Volume 2A, Chapter 7
for details.
In the eCTD, the documents should be included as electronic sourced document and not as
a scanned copy of the signed documents.
Documents related to the variation should be included in relevant sections or be deleted by use of the
appropriate document operation attribute.
Guidance for Industry on Providing Regulatory Information in Electronic Format: eCTD Applications
Version: 1.0 May 2009
Page 18 of 41

The submission type for the sequence has to be for:
Variation type Submission type
Type IA variation var-type1a
Type IB variation var-type1b
Type II variation var-type2
The procedure type should be for variation of the procedure:
Procedure Procedure type
Centralised Procedure (CP) centralised
Mutual Recognition Procedure (MRP) mutual-recognition
National national
However, in relation to the first variation in eCTD format, the applicant is strongly encouraged to also
submit a so called “baseline eCTD”, i.e. to submit the current dossier in an eCTD format, at least
Module 1, the cover letter and product information as a minimum, and Module 3. This ”baseline eCTD”
would then be submitted as sequence 0000 and the first variation in eCTD format submitted as
sequence 0001.
It should be clearly stated in the cover letter of the “baseline eCTD” that the content of the current
dossier has not been changed but only the dossier format.
Parallel variations should be submitted as individual sequences. Problems can occur in cases where a
variation is not approved whilst a subsequent variation is approved. In such case a new sequence
could be submitted containing the old section by replacing or deleting the submitted sections
reaffirming the registered status of the modules.
The following milestones of the procedures are proposed as appropriate sequences to be submitted
during the assessment of variations. Although the example relates to the centralised procedure it could
be applied to other procedures:
Type IA & IB Variations
Day Number eCTD milestone sequence Notes
1 Start of the procedure <description>
e.g. “Start of the procedure phone
number changes”
Type II Variations
Day Number eCTD milestone sequence Notes
1 Start of the procedure <description>
e.g. “Start of the procedure indication
enlargement”
91 Response to RSI
If applicable
91 Final English
+5 Provision of translations
+30 Provision of final agreed translations
following linguistic review
It is also not required to send interim
working versions of the product
information before this point as eCTD
Guidance for Industry on Providing Regulatory Information in Electronic Format: eCTD Applications
Version: 1.0 May 2009
Page 19 of 41

4.3 Extension Submissions
Several dosage forms can be managed within a single eCTD application, and this helps avoid
submission of data multiple times (e.g. active substance changes). Submissions for an extension can
either be submitted within an existing eCTD application, as a new sequence (continuous sequence
numbering), or as a new eCTD application (sequence 0000), depending on the procedure. In
MRP/DCP, an extension will be submitted within the same procedure, but with a different product
number, and as such, the recommendation is to submit the line extension as a new sequence within
the original eCTD application, submitting a new module 1, an updated module 2 and new or updated
32P section. If m32p is combined for all previous existing strength/dosage form(s), an updated section
should be provided, replacing existing documents where necessary. If a separate m32p is being
provided for the additional strength/dosage form to describe the extension, then all documents should
have the operation attribute of 'new'.
For Extension applications, only new data should be submitted as a new sequence in the already
submitted eCTD. The submission type has to be “extension”. If single eCTDs are used for each
strength or form of a product, full data concerning the extension applied for has to be included in the
submitted eCTD and therefore clear information should be given to the assessor on what is new
compared to earlier submitted data for the product to avoid unnecessary assessment.
In the centralised procedure, extensions are typically managed under the same procedure number as
the original dosage form, and again the recommendation is to submit the extension as a new
sequence within the original eCTD application, using a new m32p to describe the different dosage
form.
For national applications, the applicant should discuss with the relevant NCA.
4.4 Renewal Submissions
Please note that a renewal application can be used as the first eCTD in a product lifecycle in a similar
manner to variations. The recommendation given in the section above applies likewise.
The submission type has to be “renewal”. The procedure type depends on the procedure:
Procedure Procedure type
Centralised Procedure (CP) centralised
Mutual Recognition Procedure (MRP) mutual-recognition
National national
The following milestones of the procedures are proposed as appropriate sequences to be submitted
during the assessment of renewals:
Day Number
eCTD milestone sequence
Notes
1 Start of the procedure <description>
e.g. “Start of the procedure annual
reassessment”
61 Final English
+5 Provision of translations
+30 Provision of final agreed translations
following linguistic review
It is not also required to send interim
working versions of the product
information before this point as eCTD
Guidance for Industry on Providing Regulatory Information in Electronic Format: eCTD Applications
Version: 1.0 May 2009
Page 20 of 41

4.5 PSURs
The submission of a Periodic Safety Update Report (PSUR) should consist of a cover letter, eventually
(depending upon national requirements) an application form and the report itself as a new document in
m536 as well as perhaps some amendments on m25 The structure for a PSUR should follow the
respective
guidance documents. The naming of the leaf element shall indicate the number of the
PSUR or the period covered.
The submission type has to be “psur”. The procedure type depends on the procedure:
Procedure Procedure type
Centralised Procedure (CP) centralised
Mutual Recognition Procedure (MRP) mutual-recognition
National national
4.6 MR and DCP Applications
Please refer to the following specific CMDh guidance document on the use of eCTDs in MRP and DCP
procedures. In general, it is expected that the application covers the full dossier without cross-
referencing to other applications using the same dossier.
4.7 Referrals
4.7.1 CMD referral:
The response that the applicant has to prepare to the list of questions prepared by the CMD(h) will be
sent as an eCTD sequence to all CMD(h) members, according the timelines as defined. The applicant
will create this new sequence in which the documentation is stored according to the recommended
CTD format.
The type of submission of the new sequence should be “referral”. The procedure type depends on the
procedure:
Procedure Procedure type
Centralised Procedure (CP) centralised
Mutual Recognition Procedure (MRP) mutual-recognition
National national
4.7.2
CHMP referral:
In case of a CHMP referral and the applicant wants to submit documentation/information, a new eCTD
sequence will be created and submitted. The applicant doesn’t submit the entire history of all
sequences, but a new sequence with only the information/documentation that concerns the referral.
This sequence will be sent out to all involved parties (as defined in CMD(h) Standard Operation
Procedure).
Only in case the authority/EMEA request the documentation of previous submitted sequences, the
applicant will send a copy of the relevant sequence(s).
The type of submission of the new sequence should be “referral”. The procedure type depends on the
procedure:
Guidance for Industry on Providing Regulatory Information in Electronic Format: eCTD Applications
Version: 1.0 May 2009
Page 21 of 41

Procedure Procedure type
Centralised Procedure (CP) centralised
Mutual Recognition Procedure (MRP) mutual-recognition
National national
In case of a newly created/submitted sequence, the cover letter contains background information for
the reason of the referral. Any other document, which concerns the referral, has to be included
according to the CTD structure. Any additional documentation that doesn’t have a place in the dossier,
for example overview of the registrations/applications involved in the referral, should be placed in m10-
cover.
4.8 Active Substance Master Files
The ASMF consists of two parts, applicant’s (“open”) and restricted (“closed”) part, and both should be
submitted by the ASMF holder. The ASMF can be submitted as an eCTD regardless if an application
for Marketing Authorisation for a medicinal product referring to the ASMF is submitted in eCTD format
or not. It should follow the structure of Module 3.2.S of the CTD. All “not applicable” place holders of
the eCTD should be left empty in accordance with the eCTD specifications. The ASMF will be a stand-
alone eCTD with possibilities for LCM.
The applicant's "open" part of the ASMF should be included in section m3/32S of the eCTD for the
medicinal product application. If there are more than one ASMF used for the active substance(s), each
ASMF “open” part should be provided in its own m32S section, clearly distinguished by appropriate file
names.
A copy of the "Letter of Access" addressed to the regulatory authority shall be included in Annex 6.10
of the application form and be placed in m12/cc (i.e. in the respective folder for each concerned NCA).
Even if an application for Marketing Authorisation for a medicinal product is submitted in eCTD format
and there is a reference to an ASMF, the ASMF submitted by the ASMF holder does not have to be
provided in eCTD format. Documents from the “open” part ASMF may be included in the eCTD for the
medicinal product.
The submission type has to be “asmf”. The procedure type normally depends on the procedure.
However as one ASMF application can be used for different types of procedure (national, CP, MRP,
DCP and RUP), the procedure type “national”/”centralised”: should be used.
4.9 Vaccine Antigen Master Files
The VAMF consists of the scientific data according to Part III of Annex I of Commission Directive
2001/83/EC as amended. To support the life cycle on the one hand side, to keep the documents
manageable and to assure the correct alignment of the complete VAMF on the other hand side it is
required that the manufacturer submits the VAMF (including versioning), preferably in an electronic
format following the principles of eCTD, The complete VAMF can be processed with its own
submission / case / procedure number separately.
The application of a medicinal product will contain the same data package including the certificate of
compliance with Community legislation, together with the evaluation report attached.
4.10 Plasma Master Files
The PMF consists of the scientific data according to Part III of Annex I of Commission Directive
2001/83/EC as amended. To support the life cycle on the one hand side, to keep the documents
manageable and to assure the correct alignment of the complete PMF on the other hand side it is
required that the manufacturer submits the PMF (including versioning), preferably in [eCTD] format,
The complete PMF can be processed with its own submission / case / procedure number separately.
Guidance for Industry on Providing Regulatory Information in Electronic Format: eCTD Applications
Version: 1.0 May 2009
Page 22 of 41

The application of a medicinal product will contain the same data package including the certificate of
compliance with Community legislation, together with the evaluation report attached.
.
4.11 Applicant Initiated Action
Applicants may decide to withdraw their application during any stage of the product life cycle and this
section explains the general principles that should be followed.
Withdrawal of the whole product covered by an eCTD should preferably be submitted as a new
sequence only including a cover letter. The operation attribute “delete” is not required to be used for
the documents.
However, if the application for withdrawal only concerns for example one strength or dosage form out
of several covered by the same eCTD, the application should be submitted as a new sequence, but
normally include the operation attribute “delete” for documents only relevant for this strength.
Furthermore, if relevant it should also include updated documents with the operation attribute “replace”
for documents that covered several other strengths and that now need to be revised to exclude from
the document the strength or dosage form to be withdrawn.
The submission type has to be “withdrawal”. The procedure type is depending on the procedure:
Procedure Procedure type
Centralised Procedure (CP) centralised
Mutual Recognition Procedure (MRP) mutual-recognition
National national
4.12 Duplicate Applications
As a duplicate is an independently authorised medicinal product, there is no definition of a “duplicate”
in the pharmaceutical legislation. However, for practical purpose, a duplicate application is defined by
reference to the first application or marketing authorisation as follows based on
CMD(h)
recommendations on multiple / duplicate applications:
- same dossier (copy of modules 1, 2, 3, 4 and 5);
- same legal basis according to Directive 2001/83/EC, as amended;
- different tradename;
- same or different applicant/marketing authorisation holder.
Since this is the case only at the time of submission and can later on lead to different, independent
dossiers, one eCTD per duplicate application has to be submitted (with the possibility of including
several strengths, pharmaceutical forms etc. if relevant). It should however be clearly written in the
cover letter that it is exactly the same content (with the only exemption of different tradename and
maybe different MAH), so that redundant review work is avoided.
Guidance for Industry on Providing Regulatory Information in Electronic Format: eCTD Applications
Version: 1.0 May 2009
Page 23 of 41

Annex 1 eCTD Reference Documents
A number of relevant documents can be found on the Documentation tab on the
e-Submission website
at the EMEA. It is recommended that owing to the speed that information changes the following
websites should be consulted regularly:
http://estri.ich.org or http://estri.ich.org/eCTD/index.htm
http://ec.europa.eu/enterprise/pharmaceuticals/eudralex/eudralex_en.htm
http://esubmission.emea.europa.eu/
http://www.hma.eu/27.html
Most important documents to be considered are the following (as of October 1
st
, 2008):
•
http://estri.ich.org/eCTD/eCTD_Specification_v3_2_2.pdf
•
http://estri.ich.org/eCTD/eCTDQAV1_16.xls
•
http://ec.europa.eu/enterprise/pharmaceuticals/eudralex/homev2.htm
EMEA Q&As
• http://www.emea.europa.eu/htms/human/genguidance/genreg.htm
ICH M4
•
http://www.ich.org/LOB/media/MEDIA554.pdf
ICH M4 Q&As:
•
http://www.ich.org/LOB/media/MEDIA1189.pdf
EU CTD Q&As:
•
http://ec.europa.eu/enterprise/pharmaceuticals/eudralex/vol-2/b/ctd_qa_05_2006.pdf
Guidance for Industry on Providing Regulatory Information in Electronic Format: eCTD Applications
Version: 1.0 May 2009
Page 24 of 41
Annex 2 Guidance on Text Searchable Documents
1. General
Applicants are requested to ensure that all submissions contain the maximum amount of text
searchable content. Documents with searchable text will aid the assessor, or any other user, in
searching for specific terms and also in copying and pasting information into another document, such
as an assessment report.
We recognize that not all documents need to be text searchable. This short document provides some
guidance about what must be text searchable and the ways to ensure that files are created
appropriately.
1.1 Creating Text Searchable Files
PDF files with searchable text can be created by all PDF tools from a source file in a text format (e.g.
MS Word, SAS, MS Powerpoint, Rich Text Files, etc.). When created in this way, the file will usually be
the smallest in size (measured in kilobytes or megabytes) that they can be.
If the only version of a document available is in paper, then scanning to PDF and using an Optical
Character Recognition (OCR) routine is the only way to create searchable text. PDF files created in
this way tend to be much larger in size, for the same number of pages (from 10 to 100 times as large),
and the quality of the text that is created will almost certainly not be a 100% match to the original text.
It is noted that tools for checking and correcting this text tend to be somewhat cumbersome. For these
reasons, applicants are recommended to use scanning/OCR only as a last resort.
Applicants are reminded that the text produced by the OCR routine should be “hidden” behind the
image of the original page so that the user can refer to the picture of the page and the text on it as final
verification of the data. As a result, the applicant should ensure that, as a minimum, the text on the
scanned image is legible to the user. Poor quality images should not be provided and you should note
that these can only inevitably lead to poor quality OCR text.
2. Documents that must always be text searchable
(i.e. the PDF should be produced wherever possible from a text source, such as MS Word, but if
sourced from a scanned original then they must be OCR’d.)
• Key administrative documents in Module 1 including, the cover letter, application form, product
information documents
o Applicants are reminded that some NCAs regard logging in through a portal as
sufficient to establish a users identity and do not require handwritten signatures on
Cover Letters and Application Forms submitted this way.
o This also covers similar documents provided in non-MAA submissions.
• Any document in Module 2 of the MAA (QOS, Preclinical Overview and Summaries, Clinical
Overview and Summaries).
o This also covers similar documents provided in non-MAA submissions.
• The main body of text and main tables in any preclinical or clinical report required to support the
main claim of the application.
o This also covers similar documents provided in non-MAA submissions.
• The main body of text in any reports, methods, analytical procedures, etc. supplied in Module 3 of
the MAA
o This also covers similar documents provided in non-MAA submissions.
• The main body of text of Periodic Safety Update Reports (PSURs)
• The main body of text of Risk Management Plans
• The main body of text of Environmental Risk Assessment
• Any English translation of a document originally written in a foreign language (see also below)
Guidance for Industry on Providing Regulatory Information in Electronic Format: eCTD Applications
Version: 1.0 May 2009
Page 25 of 41
3. Documents that do not need to be text searchable
(i.e. the PDF should be produced wherever possible from a text source, such as MS Word, but if
sourced from a scanned original then there is no need for OCR.)
• Any original GMP certificate
• Any original certificate of analysis
• Any manufacturer’s licences
• Any certificate’s of suitability
• Any Manufacturing Authorisation
• Any document written in a foreign language where a translation is provided in English (however,
the translation should be text searchable, see above)
• Any literature references sourced from journals, periodicals and books (except when these are
used in a bibliographic application to support the main claims of the application).
• The blank CRF in a Clinical Study Report
• Patient data listings (when supplied)
• CRFs (when supplied)
• Any page with a signature that does not contain other information key to the understanding of the
submission
• Applicants should consider providing signatures on separate pages from key text in reports,
overviews, etc.
4. Further Information
If applicants are uncertain whether or not a particular document should be text searchable, they should
contact their NCA for guidance.
Guidance for Industry on Providing Regulatory Information in Electronic Format: eCTD Applications
Version: 1.0 May 2009
Page 26 of 41

Annex 3 Guidance and Best Practice on the Structure of Module 3 -
CTD-Quality Considerations for eCTD Submissions in Europe
1. Introduction
The ICH eCTD Specification allows the applicant to manage eCTDs at different levels in Module 3.
The normal choice should be one single eCTD application that covers multiple drug substances,
multiple manufacturers, multiple drug products (components), multiple dosage forms, presentations,
invented names and strengths. If the applicant for some reason needs to have one eCTD per strength
or dosage form, this should be explained and guidance should be given in the cover letter about which
documentation differs to prevent duplicate of work during assessment.
This Annex is based on the use of the ICH eCTD specification v3.2. Refer to the Glossary for an
explanation of terms.
1.1 Electronic information in the eCTD
In addition to CTD-Q documents, eCTD applications provide quality information in various locations:
• Module 1 XML attributes: Envelope – INN, Invented Name (Trade Name)
• Leaf XML attribute: eCTD Title
• Module 3 XML Attributes
o m3-2-s-drug-substance: substance
o m3-2-s-drug-substance: manufacturer
o m3-2-p–drug-product: product-name
o m3-2-p–drug-product: dosage form
o m3-2-p–drug-product: manufacturer
o m3-2-p-4–control-of-excipients: excipient
More than 1 entry for each of the XML Attributes above generally results in the replication of the
relevant portion of both the XML and the folder architecture, (e.g., 3.2.S Drug Substance, 3.2.P Drug
Product, 3.2.P.4 Control of Excipients)
1
.
2. General Principles
2.1 Document Granularity
eCTD applications can handle different authoring strategies for CTD-Q information. For any given
CTD-Q topic (e.g., P.1 Description and Composition of the Drug Product), either a single document
can be provided that covers multiple strengths and manufacturers, or multiple documents can be
provided, e.g. per strength and/or per manufacturer. Regardless of the XML attributes, when there are
significant differences in content it is best practice to provide multiple documents, to realise the
lifecycle benefit that eCTD offers. If there are multiple files in the same element, the title of each leaf
should be used to distinguish the scope of each document’s content.
2.2 Identifying to an agency what the application covers
The regulatory procedure drives what options are available for how many eCTD applications to provide
per product range. Generally speaking, multiple eCTD applications can be provided for different
strengths and dosage forms. However, a single eCTD is preferred, see Section
1. Introduction. A key
factor in making this decision is that in Europe the applicant cannot cross-refer from one eCTD to
another (e.g., for drug substance).
Guidance for Industry on Providing Regulatory Information in Electronic Format: eCTD Applications
Version: 1.0 May 2009
Page 27 of 41
1
See ‘Manufacturer of Drug Product’ as an exception.

For the Centralised Procedure, a single eCTD application should cover all strengths and dosage forms
within the procedure, as illustrated in Figure 1.
In MRP/DCP, a single eCTD is needed per procedure that covers all countries regardless of the
invented names. However, different dosage forms or strengths can be managed in separate eCTDs at
the applicant’s discretion, even if one combined eCTD is preferred. Applicants should carefully
consider what an eCTD application will cover before submitting the first sequence. Refer to Section 2.2
Structure of Submissions, and
Table 1 – Advantages and disadvantages of eCTD application
structures
.
Figure 1 – Illustration of what an eCTD covers for a product with the invented name
‘Wonderdrug’, Centralised Procedure
Duplicate/2nd brand injection
Duplicate/2nd brand tablets
Wonderdrug
Wonderdrug injection
Wonderdrug tablets
100mg
400mg
300mg
200mg
diluent
iv powder
Duplicate/2
n
d
brand
100mg
400mg 300mg
diluent
iv powder
eCTD Application 1 (0000, 0001, 0002 etc)
eCTD Application 2 (0000, 0001, 0002 etc)
200mg
Guidance for Industry on Providing Regulatory Information in Electronic Format: eCTD Applications
Version: 1.0 May 2009
Page 28 of 41

Table 1 – Advantages and disadvantages of eCTD application structures
One Combined eCTD For Multiple Strengths And Dosage Forms
Advantages Disadvantages
Clinical and non-clinical documentation is
provided only once
Any change to any strength/dosage form will add another
sequence to the application, and therefore the application
in general will eventually contain a larger number of
sequences. Some of these would cover all products
covered by the eCTD application, others may affect only
one strength or dosage form. Applicants need to use the
submission description to describe what each sequence
covers.
Any changes to drug substance, or safety
related changes that affect the product,
will require only one sequence
Adding a new strength (line extension) could involve
replacing all ‘common’ documents with documents
covering existing strengths plus the new strength, and
also adding new additional strength-specific documents
Common documents can be included only
once (e.g., Pharmaceutical Development
for multiple tablet strengths)
Life cycle management becomes more complex in the
following situations:
• In MRP, an applicant applies for only certain
strengths, in certain countries (e.g. strength 1 and 3
in CMS X and strength 2 and 4 in CMS Y, etc)
• An applicant wants to transfer a certain marketing
authorisation (certain strength) within one eCTD
application to another MAH.
• An applicant wishes to withdraw one strength
• Variations may be only applicable for one specific
strength, and result in the creation of strength
specific documents. These would have to be added
to the lifecycle and managed alongside the existing
documentation, which, if originally ‘common’, would
then only cover the existing (non-affected) strengths
All lifecycle is in one place Could get complex (e.g., multiple SmPCs)
Documents that are common are
presented only once and therefore read
only once by the assessor
Guidance for Industry on Providing Regulatory Information in Electronic Format: eCTD Applications
Version: 1.0 May 2009
Page 29 of 41
One eCTD Application Per Strength Or Dosage Form
Advantages Disadvantages
A new strength (line extension) could be
handled in a new eCTD and would not
affect existing lifecycle
All clinical and non-clinical reports are provided for each
strength or dosage form (cannot cross reference across
different eCTDs in the EU)
Life cycle management can be
maintained per strength so fewer issues
when applying for only certain strengths in
certain countries within MRP/DCP, or MA-
transfer or withdrawals, line extensions,
variations, etc.
Any changes to the drug substance or changes that
affect all strengths/dosage forms of the product (eg
safety related changes to the labelling) would mean
building and submitting multiple eCTD sequences, one
within each eCTD application.
Lifecycle is maintained separately, and would need to be
managed across multiple potentially identical eCTD
applications
If all strengths/dosage forms are not Common documents must be included in each eCTD

marketed in every country in an MRP
Procedure, then a unique application per
strength will avoid the possibility that one
CMS will not accept the dossier because
it contains data on a product which is not
being marketed in that country.
application, (cannot cross reference from one eCTD to
another in the EU)
Difficult for the assessor to know what to read/what is
unique. This needs therefore to be thoroughly described
in each submission, which will typically consist of multiple
identical sequences in different eCTD application
lifecycles.
This alternative goes against a founding principle for the
management of electronic data insofar as it means :
- loss of storage place : the same information will be
archived several times at different places, sent several
time for long-term filing, and saved several times in the
everyday back-up of servers.
- multiple data entry : data concerning the common part
of the multiple dossiers (i.e. the major part of the
dossiers) will have to be entered several times in the
document management systems, reviewing systems and
workflows, both in NCAs and in Pharmaceutical
companies
At NCAs, uncertainty on whether a MA for a dosage form
is granted on the basis of assessment of data pertaining
to the dossier of another dosage form
2.2.1 EU Envelope
The Module 1 EU envelope provides the trade name (invented name) of the drug product. The
application number element, which is repeatable, lists all of the product licences or application
numbers covered by the eCTD. Applicants should ensure that entries for invented name, INN,
Applicant and Application Number in the EU envelope are complete and consistent. NB The
application number and INN may not be known at the time of the first submission and may have to be
substituted in later sequences.
3. Module 3 XML Attributes in the eCTD
3.1 Choosing Module 3 XML attributes
The XML attributes reflect the document granularity used in Module 3. The actual words for the
attributes need not be an exact match of the words used in the content of Module 3 documents. Many
eCTD building tool vendors have based their tools on the ICH style sheet, and this means that the
original Module 3 XML attributes cannot be changed with later submissions within the same application
without losing the lifecycle benefit that eCTD offers. (i.e. if a Module 3 XML attribute is altered mid
lifecycle, it will no longer be possible to replace, append to or delete content provided with the previous
attribute value.)
More than 1 entry for any attribute generally results in the replication of the relevant portion of both the
XML and the folder architecture, (e.g., 3.2.S Drug Substance, 3.2.P Drug Product, 3.2.P.4 Control of
Excipients).
Guidance for Industry on Providing Regulatory Information in Electronic Format: eCTD Applications
Version: 1.0 May 2009
Page 30 of 41

3.2 Drug Substance (32s) Attributes – Substance-1, Manufacturer-1
3.2.1 Drug substance
The entry for the drug substance name attribute can be an abbreviation of the INN, or the company’s
code for the drug substance.
If there is more than 1 drug substance in the product, there is a separate set of 3.2.S.1 to 3.2.S.7
folders and corresponding XML for each drug substance. This also applies for the open (applicant’s)
parts of Active Substance Master Files (ASMFs).
If a drug substance is covered by a Certificate of Suitability (CEP), the certificate is to be provided in
3.2.Regional Information (and in Module 1.2 for annex 5.10). Only relevant sections of its 3.2.S.1 to
3.2.S.7 folders are used, if needed (e.g., for information not covered by the CEP). See EU CTD Q&As
Question 12 (
http://ec.europa.eu/enterprise/pharmaceuticals/eudralex/vol-2/b/ctd-qa-updatev3_2008-
02.pdf).
3.2.2 Manufacturer of drug substance
In conjunction with the drug substance attribute, each additional manufacturer entry results in
additional 3.2.S.1 to 3.2.S.7 XML elements and folders, where there is content provided.
Various approaches are possible depending on the number of manufacturing
companies/manufacturing sites and the quantity of documentation that is manufacturer-specific. The
general principle is that the resulting XML is a reflection of the document granularity (rather than the
eCTD specification driving the attributes).
3.2.2.1 Approach 1 – Single XML section covering all Manufacturers of the Drug Substance
Where drug substance documentation is identical or very similar for all manufacturers (and hence
there are a minimal number of manufacturer-dependent documents), then a non-specific manufacturer
attribute can be used (such as the parent name of a group of companies, or ‘applicant’ or ‘all’). For
CTD topics that are manufacturer-specific, having separate documents enables the applicant to
manage lifecycles as-needed. In such cases, the title and file name of each leaf is to be customised to
differentiate the files, e.g., leaf title of “Batch Analysis – [manufacturer 1]” where the entry is either the
[current company name] or [current manufacturing town] and file name of batch-analyses-
manufacturer1.pdf. Using leaf titles and filenames to distinguish manufacturers does not involve
adding any extra XML attributes for drug substance manufacturer. As an illustration, see
Figure 2,
where the specification is manufacturer-independent but stability data documentation has been
separated by manufacturer.
This approach does not prevent a future scenario when a new manufacturer may have its own XML
attribute (due to a significant volume of manufacturer-specific documentation). Note that a known
limitation of ICH eCTD specification v3.2 is that the original, non-specific XML attribute cannot then be
modified in the application.
2
Guidance for Industry on Providing Regulatory Information in Electronic Format: eCTD Applications
Version: 1.0 May 2009
Page 31 of 41
2
When any XML attributes are no longer accurate nor in accordance with this guidance, it is
acceptable to retain original entries. It is not desirable to correct the XML attributes (i.e., applicants
need not apply an operation attribute of DELETE to previously-submitted files and re-submit the latest
versions with new XML attributes).

Figure 2 - Single Drug Substance, 2 Manufacturers with similar documentation, the few site/manufacturer-
specific documents identified by the XML title (not by adding an additional XML section):
XML Files and folders (directory
Arrows indicate destination of xlink:hrefs
32 of 41
3.2.2.2 Approach 2 – New XML sections for each Manufacturer of the Drug Substance
When there are many manufacturer-specific documents, it is helpful to have additional XML attributes
and equivalent folders for each manufacturer, see
Figure 3. Since these files are located in separate
elements, the leaf titles and filenames do not need to be customised per manufacturer. In this
illustration, since the ‘specification’ document is manufacturer-independent, it appears only once in the
folder structure. Additional XML attribute entries are not expected for each intermediate manufacturing
site or packaging site, but can be used.
As an alternative to Approach 1 and Approach 2 (but not illustrated here), an additional entry of
‘common’ may be used for manufacturer-independent documents (e.g., those in 3.2.S.1 General
Information), such that both the XML and the folder structure contain a ‘common’ entry. If this
approach is used, files do not need to be linked from ‘common’ folders to the named manufacturer
folder(s), i.e., these files appear once in the XML and once in the folder directory.
For example, a drug substance section could contain three 32s:
32s-aspirin-manufacturer-1 (containing information specific to the manufacturer e.g. 3.2.S.2)
32s-aspirin-manufacturer-2 (containing information specific to the manufacturer e.g. 3.2.S.2)
32s-aspirin-common (containing manufacturer-independent information)
33 of 41

Figure 3 Single Drug Substance, 2 Manufacturers with significant volume of different documentation (one
section for Acme Chemicals, another for Pharmasupply).
XML Files and Folders (directory)
34 of 41
3.3 Drug Product (32p) – Product/Dosage Form/Manufacturer
3.3.1 Drug product name
Since the M1 EU envelope contains the invented name, it is not necessary to use this name in the
product name XML attribute that is used in Module 3. Avoiding the trade name in Module 3 could
prove beneficial for original MAAs, since the proposed name is not always accepted and/or is not
always applicable to all EU member states.
The entry can be a general term such as ‘active’, or ‘product’ that then produces a full set of 3.2.P.1 to
3.2.P.8 XML elements and folders. Alternatively, the internal company code of the drug product name
may be used. If applicable, another full set of XML elements and folders can be used as needed, e.g.,
for ‘diluent’.
3.3.2 Dosage form
In conjunction with the above product name, each additional dosage form entry results in an additional
full set of 3.2.P.1 to 3.2.P.8 XML elements and folders. When deciding on the degree of detail (e.g.,
‘tablet’ vs. ‘film-coated tablet’, 'frozen" vs. "refrigerated" formulation for vaccines), consider the
potential for future line extensions and the proportion of drug product documents that could be re-used.
If that proportion is small, then consider an initial specific dosage form entry.
3.3.3 Strength
Not all 3.2.P documents are, nor need to be, strength-dependent. For example, for a common
granulation for six strengths, many documents would have nearly identical content; little benefit would
be derived from having strength-specific documentation.
3.3.3.1 Approach 1 – Single General XML Section Covering All Strengths
If there is a limited number of documents in the submission that are strength-specific, there can be a
single 3.2.P, with a non-specific XML attribute such as ‘tablet’. Where there are multiple files under the
same element, the XML title and file name of each leaf is used to differentiate any documents where
the content is strength-specific, e.g. ‘Stability Data – 100 mg’ and ‘Stability Data – 200 mg’ and
‘stability-data-100mg.pdf’ and ‘stability-data-200mg.pdf’, respectively. In this case, the term ‘all
strengths’ can be used to concatenate with the dosage form entry for the XML attribute for the single
3.2.P. This approach does not prevent a future scenario where a new strength has its own XML
attribute. However, a known limitation of the ICH eCTD specification v3.2 is that the original, non-
specific XML attribute (eg ‘all strengths’) cannot then be modified - see note below under
Figure 4
below.
Figure 4 illustrates this approach, where the Pharmaceutical Development document is strength-
independent but Stability Data documentation has been split by strength.
35 of 41

Figure 4 - Approach 1 - One 32p for all Strengths, any strength specific documents identified by the XML title,
not by adding an additional XML section
XML Files and Folders (directory)
Note: The use of the term ‘all-strengths’ will mean that if the applicant subsequently submits a line extension for an additional strength (e.g.
1000mg) where the documentation is significantly different, and approach 2 is preferred for the new strength, then the attribute ‘all-strengths’
will not include the documentation for the 1000mg tablet.
36 of 41
3.3.3.2 Approach 2 – Separate XML section covering one Strength or Dosage Form
If a strength or dosage form is manufactured in a significantly different way from other
strength(s)/dosage forms and has a large volume of its own 3.2.P documentation, then a separate
3.2.P branch with appropriate subsections applicable to that manufacturer can be justified. In this
case, the dosage form XML attribute and folder includes the strength (e.g., Tablet 5 mg and tablet-
5mg, respectively). Documentation that pertains to all strengths should only be included once.
Previously-submitted documents or documents that are applicable to more than one strength can be
referred to in new XML leaves under each strength specific XML branch, without re-providing the
content files themselves, see
Figure 5.
37 of 41

Figure 5 – Approach 2- Separate XML and documents for Strengths – significant content differences, but
Pharmaceutical Development only provided once in the folder structure and referred to from the XML twice
XML Files and Folders (directory)
38 of 41
3.3.3.3 Approach 3 – Different XML sections covering each Strength
Some applicants may find it beneficial to have different XML sections for each strength. Even though
many of the leaves in these XML sections may point to the same document, there is no need to
provide a document more than once. See
Figure 6 where the Pharmaceutical Development and
Stability Data documents are provided one time in the folders, but referenced multiple times from the
XML.
However, it should be noted that many eCTD building tools do not include functionality to allow an
applicant to use multiple XML leaves to repeatedly reference to a single document in the folder
directory, and therefore this approach is not therefore recommended unless the user has significant
eCTD experience.
39 of 41
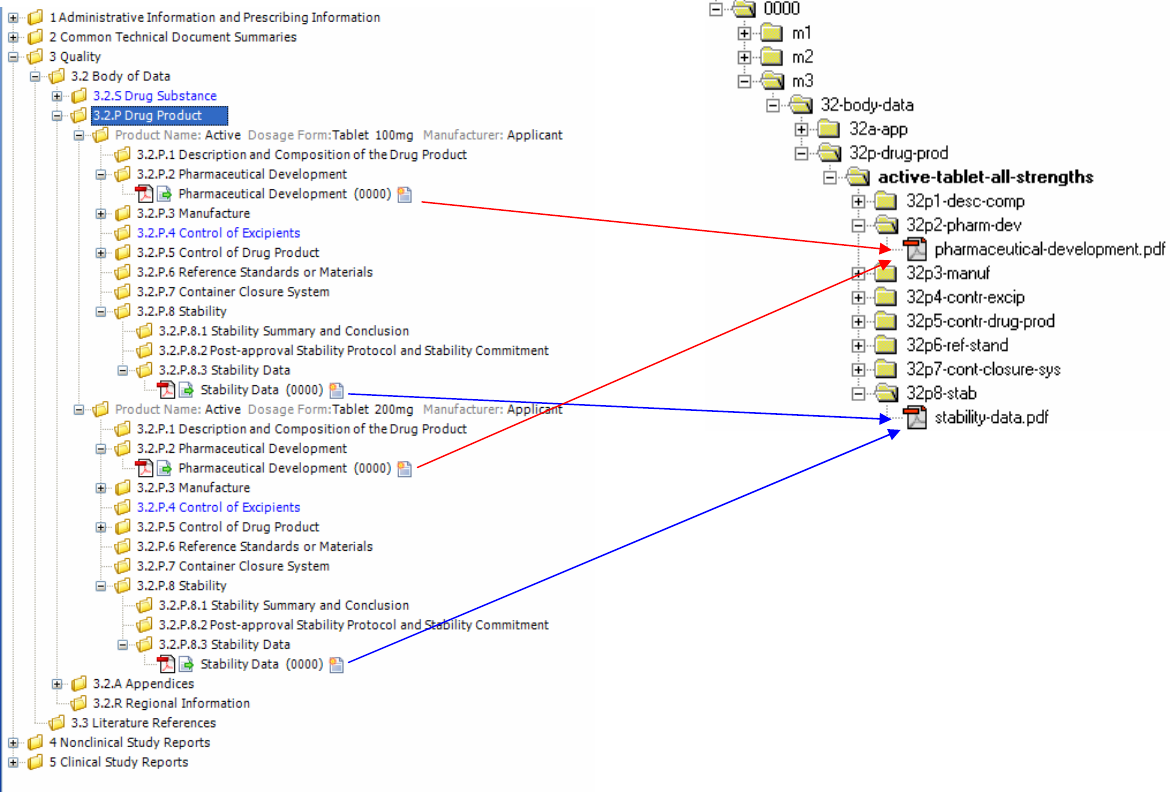
Figure 6 - Approach 3 - Multiple XML leaves per Strength, one document per Strength – this uses the XML to
differentiate between strengths, without requiring the applicant to write multiple similar documents – all
xlink:href entries point to shared documents in ‘all-strengths’ folder
XML Files and Folders (directory
40 of 41

3.3.4 Manufacturer of drug product
In conjunction with the above product name and dosage form, each manufacturer entry results in
a set of 3.2.P.1 to 3.2.P.8 and 3.2.A.1 and 3.2.A.2 XML elements. However, in many eCTD
building tools, entries for drug product manufacturer do not result in additional directory folders.
Hence it is practical to use high-level descriptors such as ‘common’, ‘applicant’, or ‘not required’.
If specific manufacturer entries are provided, then the guidance is similar to that for the
‘
Manufacturer of Drug Substance’. If the building tool did not generate a set of directory folders
per manufacturer of drug product, then corresponding filenames should be customised per
manufacturer. Alternatively, experienced applicants may wish to manually produce a second set
of 3.2.P.1 to 3.2.P.8 folders, which will involve either adding ‘manufacturer’ to the name of the
directory folder, (e.g. tablet-5mg-site1), and editing all xlink:hrefs in the corresponding XML, or
editing xlink:hrefs before publishing the eCTD. Applicants should consult their eCTD tool vendor
for further details
.
3.4 Excipients
Each excipient entry generally produces a full set of 3.2.P.4.1 to 3.2.P.4.6 XML elements and
folders. This XML attribute need not be used at all (it is not required per ICH M4Q). The
description below reflects current industry practice.
3.4.1 Compendial excipients
There is generally little to register for pharmacopoeial excipients. In this case, an XML attribute
entry of ‘compendial’ is acceptable. A single file that addresses CTD topics 3.2.P.4.1 to 3.2.P.4.4
for all excipients can be provided e.g., in 3.2.P.4.1 with a title for the leaf that accurately describes
its content. Alternatively, an entry of ‘compendial – [named excipient]’ is acceptable, e.g.,
‘compendial - purified water’. In this case a single file addresses CTD topics 3.2.P.4.1 to
3.2.P.4.4 for each pharmacopoeial excipient. In most cases, these documents will simply consist
of statements referring to the relevant pharmacopoeia.
In either case, if additional tests are performed on the pharmacopoeial excipient, these can be
located alongside the above file in the relevant folder (e.g., 3.2.P.4.2, 3.2.P.4.3) and with an
appropriate title.
3.4.2 Non-compendial excipients
For non-pharmacopoeial excipients, the excipient XML attribute entry can be a general term, e.g.,
‘coating agent’, ‘flavouring agent’, ‘sweetening agent’. In this case, the title and filename of the
leaf provides details and differentiates files, e.g., title ‘Validation of Assay – Opadry Yellow’.
Alternatively, a more specific attribute entry such as ‘non-compendial opadry yellow’ can be used.
41 of 41
