
Dear Colleagues,
At Pfizer, we are committed to our purpose of breakthroughs that change patients’ lives, which means
upholding the highest standards when we interact with physicians, healthcare organizations, patients, and
other stakeholders. Our
Healthcare Law Compliance Guide (commonly known as the White Guide)
provides an overview of the laws, regulations, and Pfizer policies and guidelines that govern our U.S.
-
based biopharmaceutical business. It is essential that you familiarize yourself with the White Guide.
Every co
lleague is accountable for understanding and meeting our company’s compliance requirements.
We encourage you to bookmark the White Guide as a reference to help ensure that you remain in
compliance with all policies and procedures applicable to your work. D
o not hesitate to consult with your
team attorney if you have any questions or e
-mail the White Guide team at
By acting with integrity every day and always emb
odying our values of Courage, Excellence,
Equity and Joy, we believe we will make great progress in leading the conversation and
becoming known as the most patient
-centric company. To access the tools and resources you
need to act with integrity, visit
integrity.pfizer.com.
Rady A. Johnson
Douglas M. Lankler

The White
Guide
Living our value of Equity requires that we act with integrity. One way we demonstrate our
commitment to integrity is by complying with laws and the rules governing our business.
Compliance with these laws builds trust with patients, Healthcare Professionals, institutions,
purchasers, and the government. It is also critical to achieving our purpose of breakthroughs
that change patients’ lives.
Copyright © 2020 Pfizer Inc. All rights reserved

White Guide – Chapter 1: Overview and Key Principles
Rev. 12/20
Page 2 of 405
CONTENTS
CHAPTER #1 – OVERVIEW AND KEY PRINCIPLES ................................................................................. 2
CHAPTER #2 – ADVERTISING AND PROMOTIONAL LABELING .......................................................... 21
CHAPTER #3 – PROMOTIONAL INTERACTIONS WITH HEALTHCARE PROFESSIONALS ............... 44
CHAPTER #4 – MARKETING PROGRAMS ............................................................................................. 52
CHAPTER #5 – HCP AND GOVERNMENT OFFICIAL CONSULTING ENGAGEMENTS ...................... 69
CHAPTER #6 – GOVERNMENT HEALTHCARE PROGRAMS ................................................................ 89
CHAPTER #7 – SUPPORT OF EXTERNAL ORGANIZATIONS ............................................................. 104
CHAPTER #8 – NON-PROMOTIONAL AND MEDIA ACTIVITIES .......................................................... 125
CHAPTER #9 – PFIZER-SPONSORED RESEARCH AND CLINICAL RESEARCH
COLLABORATIONS (CRCs) ......................................................................................... 144
CHAPTER #10 – THE PFIZER PATIENT ASSISTANCE PROGRAM (PAP) AND DONATIONS
TO INDEPENDENT CHARITABLE PATIENT ASSISTANCE PROGRAMS (ICPAPS) .. 169
CHAPTER #11 – PRIVACY: PROTECTING PERSONAL INFORMATION ............................................. 187
CHAPTER #12 – PROMOTIONAL INTERACTIONS WITH CONSUMERS ............................................ 209
CHAPTER #13 – PROMOTIONAL INTERACTIONS WITH EMPLOYER GROUPS ............................... 221
CHAPTER #14 – STARTERS .................................................................................................................. 227
CHAPTER #15 – STATE LAWS: HCP AND STATE EMPLOYEE RESTRICTIONS ............................... 243
CHAPTER #16 – FEDERAL EMPLOYEE INTERACTIONS AND LOBBYING ........................................ 281
CHAPTER #17 – PUBLICATIONS ........................................................................................................... 306
CHAPTER #18 – MEALS, EDUCATIONAL ITEMS, GREENSTONE GIVEAWAYS, AND HCP
PAYMENT DISCLOSURE .............................................................................................. 316
CHAPTER #19 – SAVINGS AND FREE TRIAL PROGRAMS ................................................................. 339
CHAPTER #20 – NON-DISCOUNT ARRANGEMENTS WITH SPECIALTY PHARMACIES AND
OTHER ACCOUNTS ...................................................................................................... 351
CHAPTER #21 – PATIENT SUPPORT ROLES ...................................................................................... 362
CHAPTER #22 – INDEPENDENT MEDICAL GRANTS .......................................................................... 371
APPENDIX – ACRONYM LIST ................................................................................................................. 385
Chapter and Appendix

Rev. 12/20
Page 2 of 405
CHAPTER #1 – OVERVIEW AND KEY
PRINCIPLES

White Guide – Chapter 1: Overview and Key Principles
Rev. 12/20
Page 4 of 405
Chapter
#1
OVERVIEW AND KEY PRINCIPLES
CONTENTS
Introduction.................................................................................................................................................... 6
Patient Support Roles ................................................................................................................................... 7
Overview of Key Healthcare Laws and Regulations ..................................................................................... 9
Anti-Kickback Laws ................................................................................................................................ 9
Safe Harbors from the Federal Anti-Kickback Statute.......................................................................... 10
Federal Health Care Programs ................................................................................................................... 11
Medicaid Best Price Law ...................................................................................................................... 12
Medicare Part D Regulations ................................................................................................................ 12
FDA Laws and Regulations......................................................................................................................... 13
Advertising................................................................................................................................................... 13
Promotional Labeling .................................................................................................................................. 14
Starters (Samples) ...................................................................................................................................... 14
Federal and State Pharmaceutical Disclosure and Compliance Laws ....................................................... 15
Overview of Other Key Laws and Regulations ........................................................................................... 15
False Claims Act ................................................................................................................................... 15
Privacy Laws ......................................................................................................................................... 16
State Consumer Protection Laws ......................................................................................................... 16
Foreign Corrupt Practices Act .............................................................................................................. 16
Industry Codes, Guidance, and Our Government Agreements .................................................................. 17
PhRMA Code ........................................................................................................................................ 17
PhRMA Guiding Principles – Direct To Consumer Advertisements About Prescription
Medicines .............................................................................................................................................. 17
OIG Compliance Program Guidance for Pharmaceutical Manufacturers ............................................ 18
Pfizer’s Government Agreements ......................................................................................................... 18
Pfizer’s Corporate Integrity Agreements .............................................................................................. 18
Pfizer’s State Attorneys General Agreements ...................................................................................... 19
Overview and Key Principles

White Guide – Chapter 1: Overview and Key Principles
Rev. 12/20
Page 5 of 405
Violations and Penalties .............................................................................................................................. 19
Pfizer’s Compliance Program...................................................................................................................... 20
For More Information ................................................................................................................................... 20

White Guide – Chapter 1: Overview and Key Principles
Rev. 12/20
Page 6 of 405
Chapter #1 Overview and Key Principles
Living out our value of Equity requires that we act with integrity. One way we demonstrate our commitment
to integrity is by complying with laws and regulations that govern our business. Compliance with these laws
builds trust with patients, Healthcare Professionals (HCPs), institutions, purchasers, and the government.
It is also critical to achieving our purpose of breakthroughs that change patients’ lives.
All Pfizer colleagues must understand how laws, regulations, guidance, and industry codes that govern our
business apply to their roles; including, but not limited to:
Key Healthcare Laws
• Anti-Kickback Laws (state and federal);
• Medicaid Best Price Law & Medicare Part D Regulations;
• FDA Laws & Regulations; and
• Federal and State Pharmaceutical Disclosure and Compliance Laws.
Other Key Laws
• False Claims Act;
• Privacy Laws;
• State Consumer Protection Laws; and
• Foreign Corrupt Practices Act.
Industry Codes, Guidance, and Government Agreements
• PhRMA Code on Interactions with Healthcare Professionals;
• PhRMA Guiding Principles on Direct to Consumer Advertising;
• OIG Compliance Program Guidance for Pharmaceutical Manufacturers; and
• Pfizer's Corporate Integrity Agreement and State Attorneys General Agreements.
Introduction

White Guide – Chapter 1: Overview and Key Principles
Rev. 12/20
Page 7 of 405
This Chapter provides an overview of some of the key laws, regulations, guidance, and industry
codes that apply to our business. The policies contained in this Guide are designed to help ensure
that your activities comply with these laws, regulations, guidance, industry codes, any applicable
CIAs and State Attorneys General Agreements. Alternative approaches may be permissible in
particular circumstances if approved by Legal.
Non-compliance with these policies can subject Pfizer colleagues to disciplinary action up to and
including termination of employment. Further, improper activities that violate one or more of these
laws and regulations could result in criminal and civil penalties for you and the Company.
If the application of any policy is unclear to you, discuss the issue with your manager or team
attorney.
Pfizer is committed to supporting patient access to the medicines prescribed by HCPs in a manner
consistent with all applicable laws and regulations. As part of this commitment, some Pfizer brands may
offer brand-specific reimbursement and patient support activities that are carried out by field-based
colleagues (hereinafter “Patient Support Roles”). Generally, Patient Support Roles are field-based roles
that seek to expand access to, reimbursement of and education about Pfizer products in a non-promotional
Makes it illegal to offer to pay or provide anything of value knowingly and willfully in order to
induce an individual or entity to recommend or prescribe a product or service that is
reimbursed by the government. It is also illegal to ask for or receive a payment in exchange
for prescribing or recommending a product or service that is reimbursed by the government.
Anti-Kickback Laws:
Prohibits charging Medicaid more than the lowest price (i.e., “best price”) at which Pfizer
offers a product to any other customer. Pfizer must calculate and report to the federal
government our “best price” for each product.
Best Price Law:
Prohibits making, or inducing someone else to make, a false claim for reimbursement from
the federal government.
False Claims Act:
Patient Support Roles

White Guide – Chapter 1: Overview and Key Principles
Rev. 12/20
Page 8 of 405
manner. As of the publication of this chapter, Patient Support roles include Field Reimbursement Managers
(“FRMs”), Clinical Educators (“CEs”) and Patient Affairs Liaisons (“PALs”). Patient Support Role activities
are intended to facilitate patient access to Pfizer medicines and associated patient support programs when
a Pfizer medicine is prescribed by a patient’s HCP, or to provide training and/or education regarding
relevant Pfizer products or therapeutic areas. Although Patient Support Roles are externally facing roles,
they are separate from the sales organization and are not intended to promote Pfizer products.
Typical Characteristics of Roles
Sales Colleague
Account Manager
Compensation
Includes Sales Credit and
Quota (i.e. a product Rx sales
target for assigned
customers).
Exclusively Business Goals and
Objectives (without an assigned Sales
Credit and Quota).
Customers
Individual HCP.
Health Systems, IDNs, Health Plans,
Employers, Group Purchasing
Organizations.
Tools (examples)
Promotional Visual Aid.
Organized Customer Tool (see Orange
Guide Chapter 14: Organized Customer
and Payer Tools and Resources).
Engagements
(examples)
Face-to-face or virtual product
detail to educate HCPs about
the benefits and risks Pfizer
products may have for
individual patients.
Generally, C-Suite level interactions to
discuss population health,
Collaborations (see Orange Guide
Chapter 5: Interactions with Health
Systems), Contracting (see Orange
Guide Chapter 12: Discount and Rebate
Contracting), and Quality Engagements
(see Orange Guide Chapter 14:
Organized Customer and Payer Tools
and Resources).
In addition, the differences between the Sales Colleague and Account Manager roles require that they
interact with internal Pfizer colleagues, and particularly with Field Medical Colleagues, differently. For
purposes of this guide, “Field Medical Colleagues” include Field-Based Medical Directors (FMDs),
Medical Outcome Specialists (MOSs), and Health Data Analytics Specialists (HDAS). Interactions between
Field Commercial Colleagues and Field Medical Colleagues must be limited so as to preserve the

White Guide – Chapter 1: Overview and Key Principles
Rev. 12/20
Page 9 of 405
independence of Field Medical Colleagues. Field Commercial Colleagues may not, nor should they appear
to, direct the activities of Field Medical Colleagues. For this reason, internal interactions between Field
Commercial Colleagues and Field Medical Colleagues and external interactions between such colleagues
and Pfizer customers must be carefully considered to ensure the content and context of the medical activity
is appropriate. Further distinctions in how Sales Colleagues and Account Managers may interact with Field
Medical Colleagues are discussed throughout the Orange Guide.
The differences between Sales Colleagues and Account Managers are driven not only by business needs
but also the need to mitigate inherent risks specific to each role in customer interactions. Therefore, while
the laws and policies discussed in the Orange Guide apply to all Field Commercial Colleagues, application
of those laws and policies may differ depending on whether the Field Commercial Colleague is a Sales
Colleague or an Account Manager. Where relevant, the Orange Guide tailors its guidance for the two
distinct Field Commercial Colleague roles. Except where a unique policy or application of a policy to one
of the roles is called out, one should assume that the Orange Guide policy applies in the same manner to
both Sales Colleagues and Account Managers.
The Orange Guide is an overview of the laws, regulations, and policies that govern the activities of
Field Commercial Colleagues. It is not intended to cover every activity or issue that may present
itself. Just because an activity is not specifically prohibited by the Orange Guide does not mean it
is permissible or compliant. As compliance is everyone’s responsibility you are expected to apply
the principles in the Orange Guide broadly and seek guidance from your manager or team attorney
if you have a question.
Anti-Kickback Laws
HCP’s treatment decisions should not be tainted by motives of personal gain or enrichment. Federal and
state anti-kickback laws seek to eliminate improper influences on healthcare decisions, to reduce the
overutilization of services and to prevent patient harm. These laws make it illegal to, knowingly and willfully,
offer, pay or provide anything of value to induce an individual or entity to recommend or prescribe a product
or service that is reimbursed by the government. It is also illegal to ask for or receive a payment in exchange
for prescribing or recommending a product or service that is reimbursed by the government. In certain
states, relevant anti-kickback laws also punish the transfer of remuneration to induce business that is
payable by a commercial insurer (not just government-funded healthcare plans). The anti-kickback laws
prohibit such activities as:
• Providing a gift, payment, or anything of value to an HCP (including a pharmacist) intended to influence
the prescribing, dispensing, or recommending of pharmaceutical products;
Overview of Key Healthcare Laws and Regulations

White Guide – Chapter 1: Overview and Key Principles
Rev. 12/20
Page 10 of 405
• Providing a gift, payment, or anything of value to a retail or wholesale customer to influence the
purchase of pharmaceutical products;
• Providing an educational or research grant to a managed care organization to influence the formulary
position of a product;
• Paying for the services (e.g., consulting services) of an HCP or other customer at a fee above the
reasonable, fair market value for such services in exchange for prescribing or giving favorable
treatment to a Pfizer drug; and
• Providing valuable services for free or below fair market value to an HCP or other customer with intent
to induce prescriptions for Pfizer products.
Similarly, U.S. law provides for the imposition of civil monetary penalties against any person who offers or
transfers "remuneration" to a Medicare or state healthcare program (including Medicaid) beneficiary that is
likely to influence the beneficiary's selection of a particular provider or supplier of healthcare product or
service that is reimbursed by a federal healthcare program.
Fair Market Value (FMV)
Price at which an asset or service passes from a willing seller to a willing buyer based on market
demand and supply. Pfizer is required to pay any person or entity in a position to purchase,
prescribe, endorse, or recommend our products fair market value for the good or service Pfizer
receives in return. For example, Pfizer must pay HCPs fair market value compensation for
speaking and consulting services. Similarly, for example, Pfizer must pay a Specialty Pharmacy
fair market value compensation for any prescribing data Pfizer wishes to purchase from it.
Pfizer treats all HCPs and other customers as if they are subject to the anti-kickback laws, even though
they may not participate in government healthcare programs. Indeed, as noted above, certain states punish
exchanges of value with HCPs and other customers even where the services are paid for by commercial
insurers (and not just by government healthcare programs).
Safe Harbors from the Federal Anti-Kickback Statute
The federal Anti-Kickback Statute is so broad that, if read literally, it could restrict many otherwise legitimate
marketing activities and even some non-promotional activities. Recognizing this, the U.S. Department of
Health and Human Services (HHS) Office of Inspector General (OIG) has defined certain “safe harbors.”
Activities that fall entirely within a safe harbor, do not violate the Anti-Kickback Statute. Because the federal
Anti-Kickback Statute is an intent-based statute, failure to satisfy a safe harbor does not mean the conduct

White Guide – Chapter 1: Overview and Key Principles
Rev. 12/20
Page 11 of 405
is illegal. Because of this, the Pfizer Legal Division should be contacted to discuss each arrangement or
activity that potentially implicates the Anti-Kickback statute.
U.S. Department of Health and Human Services (HHS)
United States federal administrative agency that oversees Medicaid, Medicare, and other federally
funded healthcare programs.
Office of Inspector General (OIG)
A legal department within HHS charged with enforcing federal healthcare laws and regulations and
negotiating and overseeing Corporate Integrity Agreements.
A number of safe harbors are relevant to our business activities, but three are especially important:
• Discount safe harbor: Allows Pfizer to discount the price of a product to make it competitive with other
products, provided that the discount is properly reported to the government and complies with other
safe harbor requirements.
• Managed Care safe harbor: Permits Pfizer to provide a wide array of discounted items or services to
certain eligible managed care organizations under specified circumstances.
• Personal Services and Management Contracts safe harbor: Protects legitimate service
arrangements recorded in a written agreement where the compensation is determined in advance and
is based on fair market value for the service. This safe harbor is applicable in Pfizer’s engagement of
HCPs for consulting and speaking services as well as other entities from whom Pfizer may purchase
services and that are in a position to purchase, prescribe, endorse, or recommend Pfizer products.
Many federal healthcare programs, such as Medicaid and Medicare, purchase prescription drug products
or reimburse for their purchase. Under Medicaid, the government covers the cost of prescription medicines
for low income and disabled patients. Since 2006, Medicare coverage has included outpatient prescription
medicines purchased by eligible senior citizens through a pharmacy.
Pharmaceutical manufacturers additionally provide preferred prescription drug pricing to federal customers
generally via the Federal Supply Schedule and to specific federal purchasers, including the Department
of Veterans Affairs (VA) and the Department of Defense (DoD), as required by statute. Companies also
provide discounts under the Public Health Services 340B Drug Pricing Program, as well as through
certain state-supported programs, including State Pharmaceutical Assistance Programs and AIDS Drug
Assistance Programs.
Federal Health Care Programs

White Guide – Chapter 1: Overview and Key Principles
Rev. 12/20
Page 12 of 405
Paying or providing benefits to healthcare providers or beneficiaries to prescribe or utilize products
ultimately reimbursed by federal healthcare programs potentially implicates the federal Anti-Kickback
Statute and state all-payor laws. Similarly, a failure to provide the government with preferential pricing in
certain situations may expose a manufacturer to liability under various federal and state laws. The
government’s increased role in purchasing or reimbursing for pharmaceuticals has heightened its attention
to certain federal laws, including the False Claims Act (further described below), to ensure that entities are
not submitting false claims to the government for reimbursement. It is critical that Pfizer remain vigilant of
– and responsive to – all federal and state laws that may be implicated while doing business with the
government.
Medicaid Best Price Law
Under federal law, Medicaid is entitled to quarterly rebates based on the lowest price a pharmaceutical
company offers on a product to a customer (excluding certain customer types). This is generally referred to
as the “best price” for the product. Pfizer is responsible for calculating and reporting to the federal
government the metrics that are utilized to calculate these rebates.
A failure to account for discounts or other price concessions accurately could result in inaccurate price
reporting to the federal government. This could occur if, for example, Pfizer mischaracterizes discounts
provided to a managed care or retail customer, such as through a rebate disguised as an educational grant
or by paying more than fair market value for a service that Pfizer purchases from a Specialty Pharmacy in
order to reduce the net cost of the Pfizer products that organization purchases. If Pfizer reduces the net
cost in this way without accurately reporting such discounts to the federal government, Medicaid could end
up paying more for the Pfizer products than the managed care or retail customer, a violation of the Medicaid
Best Price Law. Violating this law could result in a company having to pay significant penalties and being
subjected to operating restrictions. For more information on issues pertaining to discounting and price
reporting, see Orange Guide Chapter 12: Discount and Rebate Contracting and White Guide Chapter 6:
Government Healthcare Programs.
Medicare Part D Regulations
The Medicare program provides an outpatient drug benefit to Medicare beneficiaries through Medicare
“Part D.” There are two types of Medicare health plans. “Medicare Advantage Prescription Drug” plans
(MA-PD) provide both medical coverage (for hospital and physician charges) as well as drug coverage.
Alternatively, stand-alone “Prescription Drug Plans” (PDPs) provide drug coverage only. Beneficiaries
who enroll in PDPs can still receive broader medical coverage through Medicare.
MA-PDs and PDPs are private health plans that contract with the Centers for Medicare and Medicaid
Services (CMS), the federal agency that administers Medicare and Medicaid. CMS regulates these health
plans closely and has become increasingly vigilant in monitoring their interactions with manufacturers. In

White Guide – Chapter 1: Overview and Key Principles
Rev. 12/20
Page 13 of 405
particular, CMS has expressed concern that Medicare health plans not be overcharged for prescription
drugs and that all formulary placement and prescribing decisions be made based on appropriate
considerations. As a result, MA-PDs and PDPs are required to report their costs to the government and,
in so doing, must disclose any “direct or indirect remuneration” that they receive from pharmaceutical
companies. Accordingly, Pfizer must be vigilant in monitoring the payments that it makes to MA-PDs and
PDPs, as well as in its general relationship with these plans.
The Food and Drug Administration (FDA) regulates almost every aspect of our business, from research
and development to sales and marketing. FDA regulation of product advertising and promotional labeling
directly affects our customer relationships. Therefore, all colleagues must understand the basic rules we
must follow to ensure compliance with FDA laws and regulations.
Food and Drug Administration (FDA)
United States federal agency responsible for regulation of most foods, dietary supplements, drugs,
vaccines, biological medical products, blood products, medical devices, radiation-emitting devices,
veterinary products, and cosmetics.
The FDA also strictly regulates the “advertising” of all prescription drug products marketed in the United
States.
Advertising
Includes advertisements published in journals, magazines, newspapers, and other periodicals, as
well as broadcast media such as radio, television, and telephone.
All Pfizer promotional materials (whether in print or electronic form)– including all visual aids, brochures,
journal advertising, promotional programs, and other sales aids –must be consistent with the product’s
FDA-approved labeling, contain balanced statements about the product’s benefits as well as risks, be
truthful and not misleading, and supported by substantial evidence. In addition, all promotional materials,
unless Reminder Advertisements or Reminder Labeling, must also include the product’s Prescribing
FDA Laws and Regulations
Advertising

White Guide – Chapter 1: Overview and Key Principles
Rev. 12/20
Page 14 of 405
Information (PI) or, for print advertisements making product claims, a Brief Summary that includes a
drug’s side effects, contraindications, and effectiveness.
Field Commercial Colleagues must adhere to the policies set forth in Orange Guide Chapter 2: Interactions
with HCPs and Health Systems, and all other colleagues must adhere to the policies set forth in White
Guide Chapter 2: Advertising and Promotional Labeling, White Guide Chapter 3: Promotional Interactions
with Healthcare Professionals, White Guide Chapter 12: Promotional Interactions with Consumers, and
White Guide Chapter 13: Promotional Interactions with Employer Groups, and any other relevant policies
and guidance.
The FDA strictly regulates the “labeling” of all prescription drug products that Pfizer markets in the United
States, including “promotional labeling.”
Labeling
Includes all “labels and other printed, written or graphic matter: (1) upon any article or any of its
containers or wrappers, or (2) accompanying such article” including sales materials in the Veeva
CRM system and other promotional materials.
The Prescription Drug Marketing Act of 1987 (PDMA) prohibits the sale, purchase, or trade of drug
samples (called “starters” at Pfizer). It is illegal for any individual (including physicians) to sell or seek
reimbursement for a free starter. Individuals who engage in or encourage such conduct are subject to
criminal prosecution. Drug samples could be considered “remuneration” under the anti-kickback laws if
provided to an HCP for the wrong reason. Starters should never be distributed to benefit an HCP personally
or to induce an HCP to prescribe our products. Prescription decisions should be based solely on patient
need.
In addition, several states have laws that affect whether and to whom starters may be distributed. For
example, some states have particular limitations on distributing starters for controlled substances and some
have requirements on when starters that were lost or stolen must be reported. Depending on state law, not
all HCPs may accept starters. For more information on how to develop a compliant starter strategy, see
the Orange Guide Chapter 10, White Guide, Chapter 14.
Promotional Labeling
Starters (Samples)

White Guide – Chapter 1: Overview and Key Principles
Rev. 12/20
Page 15 of 405
Pharmaceutical manufacturers operating in the United States are required to submit reports to the
government regarding payments and other transfers of value made to U.S.-licensed physicians, mid-levels
and teaching hospitals under the transparency provisions of the Sunshine Act and the SUPPORT Act. In
addition, a growing number of states and even municipalities regulate pharmaceutical companies’
interactions with HCPs. These state and municipal laws and regulations include disclosure of payments
made to HCPs, restrictions or prohibitions on gifts and meals, and reporting of data such as Average
Manufacturing Price and Best Price. Some of these restrictions may even extend to interactions that occur
outside of the geographic boundaries of the state that enacted the law or regulation.
For more information on whether your activities are affected by federal or state pharmaceutical disclosure
requirements or state compliance laws, see the Meals, Educational Items, and HCP Payment Disclosure
Chapter and the State Laws: HCP and State Employee Restrictions Chapter in this Guide.
False Claims Act
The False Claims Act (FCA) prohibits entities and individuals from submitting or inducing another to submit
a false claim for reimbursement from the federal government. The federal government has used the FCA
to investigate and prosecute pharmaceutical companies for falsely reporting best price, paying kickbacks
to healthcare providers, and encouraging physicians to seek reimbursement from the government for free
samples of prescription drug products.
For example, if a company pays a kickback to a HCP to prescribe its product, the government can allege
that when the claim was submitted to the government for the product, the claim was false because it was
the result of an illegal kickback. The government has also used the FCA to combat instances of off-label
promotion. Under the government’s reasoning, when a pharmaceutical company engages in off-label
marketing, the company puts into motion a series of events in which a prescription will be reimbursed by a
government program even though it was not eligible for reimbursement (e.g., physician writes a prescription
for an off-label use, pharmacist fills the prescription, pharmacist then seeks reimbursement for the off-label
prescription). In so doing, the government has argued that the pharmaceutical company has “induced”
another party to submit a false claim, resulting in an alleged violation by the pharmaceutical company.
Sales Colleagues must ensure that all HCP interactions comply with Orange Guide Chapter 2: Interactions
with HCPs and Health Systems, and all other colleagues must ensure that marketing materials and other
commercial activities comply with White Guide Chapter 2: Advertising and Promotional Labeling and White
Guide Chapter 3: Promotional Interactions with Healthcare Professionals, and any other relevant policies
and guidance.
Federal and State Pharmaceutical Disclosure and Compliance Laws
Overview of Other Key Laws and Regulations

White Guide – Chapter 1: Overview and Key Principles
Rev. 12/20
Page 16 of 405
Privacy Laws
Pfizer and its partners and service providers perform various services (e.g., advertising and promotion
agencies) that may collect and process various types of personal information (e.g., healthcare data). Also,
colleagues may encounter sensitive personal information in the course of their visits to meet with HCPs.
Colleagues are responsible for ensuring that the data is handled carefully and in compliance with Pfizer’s
policies and applicable federal and state privacy laws and regulations, including data breach notification
laws.
For more information about your obligations to maintain patient privacy, see Orange Guide Chapter 8:
Privacy: Protecting Personal Information and White Guide Chapter 11: Privacy: Protecting Personal
Information.
State Consumer Protection Laws
Many states have laws that seek to protect consumers from inappropriate marketing and sales practices.
For example, virtually all states have broad laws prohibiting “unfair” or “deceptive” trade practices. Some
state Attorneys General further contend that state consumer protection laws encompass off-label
promotion. You should direct any questions regarding state consumer protection laws and their impact on
your activities to your team attorney.
Foreign Corrupt Practices Act
The Foreign Corrupt Practices Act (FCPA) is a U.S. federal law that prohibits corrupt or improper
payments to non-U.S. government officials. The definition of “government official” includes any officer
or employee of, or acting on behalf of, a non-U.S. government (any department, agency, or instrumentality)
or public international organization. HCPs at foreign government-owned hospitals, for example, may qualify
as foreign officials under the FCPA.
The FCPA contains both anti-bribery and accounting provisions. Violations of the FCPA may result in
criminal prosecution and/or civil sanctions against Pfizer and any of its individual employees, including for
the misconduct of third parties acting on Pfizer’s behalf.
The anti-bribery section of the FCPA prohibits U.S.-based companies from, directly or indirectly, offering,
paying, promising to pay, or authorizing payment of anything of value to a non-U.S. government official to
improperly or corruptly influence that official to take any governmental act or decision to assist a company
in obtaining or retaining business, or gaining an improper advantage (examples of such decisions could
include influencing clinical trials, writing prescriptions, awarding business contracts or regulatory approvals,
or not enforcing requirements such as mandatory inspections). The FCPA contains no minimum threshold
and “anything of value” can be considered a bribe (e.g., gifts, a contract, meals, employment for a family
member). Additionally, a bribe need not actually be paid in order to violate the law.

White Guide – Chapter 1: Overview and Key Principles
Rev. 12/20
Page 17 of 405
The accounting provision requires companies with securities listed on U.S. stock exchanges to make and
keep books and records that accurately and fairly reflect the transactions of the corporation and to devise
and maintain an adequate system of internal accounting controls.
Pfizer colleagues who are permitted to enter into any interaction in which a payment or other benefit may
be given to a non-U.S. HCP (e.g., engaging the individual as a consultant), must follow My Anti-Corruption
Policy and Procedures (MAPP). MAPP sets forth Pfizer’s global policy and procedures that are designed
to help colleagues use good judgment and comply with the anti-bribery and anti-corruption laws of the U.S.
and the other countries in which we operate. For more information, see White Guide Chapter 5: HCP and
Government Official Consulting Engagements and MAPP.
PhRMA Code
The Pharmaceutical Research and Manufacturers of America Code on Interactions with Healthcare
Professionals (PhRMA Code) was developed and adopted by many of the country’s leading research-
based pharmaceutical and biotechnology companies including Pfizer. It applies to relationships with
physicians and other HCPs. Pfizer is committed to following its principles.
The PhRMA Code is intended, among other things, to protect patients from undue influences on healthcare
decision-making and reaffirm that interactions between company representatives and HCPs should be
ethical and focused on informing HCPs about the benefits and risks of medicines in order to help enhance
patient care.
The PhRMA Code, as well as “Frequently Asked Questions,” can be viewed on Global Policy Xchange on
GCO On Demand.
PhRMA Guiding Principles – Direct To Consumer Advertisements About Prescription
Medicines
PhRMA Guiding Principles – Direct to Consumer Advertisements About Prescription Medicines set forth
the industry’s commitment to use of DTC advertising as a means to increase the awareness of various
diseases and conditions, inform patients about potential treatment options, motivate patients to talk to their
physician, and help patients communicate more effectively with their physician. In 2018, PhRMA updated
these Principles by adding that all product-related DTC television advertising should direct patients to
information about the cost of the medicine being advertised— the list price and average, estimated or typical
patient out-of-pocket costs, or other context about the potential cost of the medicine. Pfizer provides this
information through a website. Pfizer Guidance for the Implementation of the Updated PhRMA DTC
Principles must be followed when developing DTC advertising. When developing DTC advertising,
Industry Codes, Guidance, and Our Government Agreements

White Guide – Chapter 1: Overview and Key Principles
Rev. 12/20
Page 18 of 405
Marketing colleagues must also adhere to the policies set forth in White Guide Chapter 2: Advertising and
Promotional Labeling.
Pfizer adopts and follows the PhRMA Code on Interactions with HCPs and the guiding principles on Direct
to Consumer Advertising.
OIG Compliance Program Guidance for Pharmaceutical Manufacturers
The OIG Compliance Program Guidance for Pharmaceutical Manufacturers sets forth its general views on
the value and fundamental principles of compliance programs for pharmaceutical companies and the
specific elements that pharmaceutical companies should consider when developing and implementing
effective compliance programs. The Guidance states that the following seven elements are recognized as
fundamental to an effective compliance program: (1) implementing written policies and procedures;
(2) designating a compliance officer and compliance committee; (3) conducting effective training and
education; (4) developing effective lines of communication; (5) conducting internal monitoring and auditing;
(6) enforcing standards through well publicized disciplinary guidelines; and (7) responding promptly to
detected problems and undertaking corrective action. All seven elements are embedded in Pfizer’s
compliance program.
Pfizer’s Government Agreements
A Corporate Integrity Agreement (CIA) is a written agreement with the OIG that typically imposes upon
a company certain integrity obligations (e.g., training, reporting, or audits) for a specified period of time,
typically five (5) years from the date the CIA is executed.
A State Attorney General Agreement is a written agreement with one or more state Attorneys General
that imposes certain integrity obligations for a specified period of time or as an ongoing obligation. We may
also enter into agreements with city or municipal governments or regulatory agencies that require certain
integrity obligations.
Pfizer’s Corporate Integrity Agreements
In 2018, Pfizer paid $23.5 million to resolve civil claims by the U.S. government and entered into a five-year
CIA. The government alleged that Pfizer's donations to charitable foundations that provided copay
assistance to patients did not comply with federal law.
Pfizer’s CIA Requirements: The CIA sets certain compliance-related requirements, most of which were
already reflected in Pfizer's Compliance Program. Some of our CIA obligations include:
• Annual compliance training for U.S. colleagues;
• CEO, CCQRO, Management and other certifications;
• Disclosure of certain violations of company policy or law;

White Guide – Chapter 1: Overview and Key Principles
Rev. 12/20
Page 19 of 405
• Annual third party reviews of certain systems, policies, processes, and transactions;
• Policies and procedures regarding donations to Independent Charity Patient Assistance Programs,
Pfizer's free drug program, and financial assistance in the form of cost-sharing (co-pay coupons or
co-pay cards); and
• Monitoring of certain activities associated with donations to Independent Charity Patient Assistance
Programs.
Pfizer’s State Attorneys General Agreements
Pfizer has entered into written agreements directly with several state Attorneys General, cities, and
municipalities, which impose certain integrity obligations upon Pfizer. Because these agreements are
entered into with individual states, cities or municipalities, the obligations can and do vary among
agreements and may be more restrictive than applicable law. Generally, these agreements include
obligations related to promotional activities, incentive compensation, medical information, reprints, and
physician payment posting. While some obligations exist only for a pre-specified time period, some of the
obligations do not expire. As applicable, obligations impacting Pfizer colleague activities are implemented
through policies and procedures governing the relevant activities.
For additional information regarding these agreements, please visit the State AG Agreements page on the
Corporate Compliance Division website.
The OIG, the U.S. Department of Justice, the FDA, state Attorneys General and certain local governments
aggressively enforce the anti-kickback and other laws and regulations discussed in this Overview. In
addition to violating our obligations under our government agreements, any violation of law is subject to
prosecution and potentially punishable by a fine and/or imprisonment, as well as civil monetary penalties.
Conviction under these laws can also result in Pfizer’s exclusion from participation in federal and state
healthcare programs, as well as imprisonment of officers and/or employees responsible for each violation.
Failure to adhere to FDA advertising and promotion regulations, in particular, can result in the need to run
corrective advertising or to “pre-clear” future promotional materials. Violations of the PDMA, which can
include failing to follow starter management requirements, may result in criminal sanctions, including
imprisonment.
In addition, Pfizer may face regulatory investigations, significant fines and litigation for failure to comply with
applicable privacy laws and regulations, including state data breach notification laws.
Violations and Penalties

White Guide – Chapter 1: Overview and Key Principles
Rev. 12/20
Page 20 of 405
Pfizer takes compliance with these laws, regulations, and agreements very seriously and expects every
colleague to do the same. Pfizer’s Compliance Program is regularly enhanced to help ensure that we meet
or exceed the complex and evolving legal, regulatory and industry requirements, as well as the expectations
of patients and providers. Your commitment to integrity and owning compliance is essential to achieving
our purpose of breakthroughs that change patients’ lives and is critical to Pfizer’s success. Acting with
integrity requires that colleagues promptly disclose potential violations and cooperate with investigations of
possible violations. Each colleague has a Duty to Act by reporting suspected compliance violations to
your manager, another manager, Human Resources, Legal, Compliance or via the Compliance Helpline
(1-866-866-7349 or online at https://pfizer.ethicspoint.com), or directly via e-mail at
corporate.complian[email protected], or by phone (1-212-733-3026).
If you are involved in a compliance investigation in any capacity (for example, as a witness or complaining
party), you are expected to keep the details of the investigation confidential. Maintaining confidentiality
helps to preserve the integrity of the process and protects the individuals participating in the investigation.
Unless prohibited by local law, any exceptions to confidentiality must first be discussed with the Compliance
Division.
Duty to Act
If you reasonably believe that an employee has violated the law or Pfizer policy, you have a duty
to report that information immediately to your manager, another manager, Human Resources,
Legal, or the Compliance Division. Pfizer has open door, anti-retaliation, and confidentiality policies
to encourage and protect all Pfizer colleagues who raise valid concerns.
• Colleagues must be familiar with and abide by all of the policies and guidance in this Guide.
• Questions may be referred to your manager or team attorney.
Pfizer’s Compliance Program
For More Information

Rev. 01/20
Page 21 of 405
CHAPTER #2 – ADVERTISING AND
PROMOTIONAL LABELING

White Guide – Chapter 2: Advertising and Promotional Labeling
Rev. 12/20
Page 22 of 405
Chapter
#2
ADVERTISING AND PROMOTIONAL
LABELING
CONTENTS
Introduction.................................................................................................................................................. 23
Key Points to Ensure Compliance ........................................................................................................ 24
Core Compliance Principles for Professional and Consumer Promotional Materials ................................. 24
Pre-Approval Communication ............................................................................................................... 24
Pre-Approval Interactions with Healthcare Decision Makers ............................................................... 25
Post-Approval Communication ............................................................................................................. 26
Core Principle #1: Ensure Statements are Consistent with Product Labeling ............................... 26
Core Principle #2: Support All Claims with Adequately Substantiated Evidence .......................... 27
Core Principle #3: Be Accurate, Truthful, and Not Misleading....................................................... 29
Core Principle #4: Provide Balanced Information about Benefits and Risks ................................. 30
Core Principle #5: All Promotional Materials Must Be Approved through Review Committee ...... 31
Core Principle #6: Don’t Make Claims About Investigational Products or Investigational Uses
of Marketed Products ..................................................................................................................... 32
Requirements of Promotional Labeling and Advertising ............................................................................. 32
Use of Reprints in Product Promotion ......................................................................................................... 36
Direct-To-Consumer and Internet Advertising ............................................................................................ 37
Direct-To-Consumer Advertising .......................................................................................................... 37
Patient Testimonials ............................................................................................................................. 38
Usage of Animals in Advertising ........................................................................................................... 38
Internet and Social Media Promotion ................................................................................................... 39
Pfizer Biopharmaceuticals Group: Paid Media Policy ................................................................................. 40
For More Information ................................................................................................................................... 43
Advertising and Promotional Labeling

White Guide – Chapter 2: Advertising and Promotional Labeling
Rev. 12/20
Page 23 of 405
Chapter #2 Advertising and Promotional Labeling
A fundamental basis for our promotional interactions with Healthcare Professionals (HCPs) and
consumers is to promote our products and educate about the disease states they treat. Pfizer’s Guiding
Principles pertaining to Promotional Materials and Communications are intended to ensure that the
development, review, approval and use of those materials comply with all applicable laws, regulations,
policies, and procedures while optimizing efficiency, effectively mitigating risks, and leveraging Pfizer’s
scale.
These Guiding Principles should always be followed when developing, reviewing, approving, and using
promotional materials:
• Ensure Statements are Consistent with Product Labeling;
• Support All Claims with Adequately Substantiated Evidence;
• Be Accurate, Truthful, and Not Misleading;
• Provide Balanced Information about Benefits and Risks;
• All Promotional Materials Must Be Approved through Review Committee;
• Don’t Make Claims About Investigational Products or Investigational Uses of Marketed Products.
In addition to these guiding principles, each Local Country is accountable for establishing and employing
processes that will ensure that its promotional activities and materials comply with this Policy, all applicable
local laws and regulations and industry standards. Additionally, the local country is accountable to ensure
that local procedures are implemented and documented to support this policy.
These principles and process requirements are set forth in detail in Pfizer’s Global Content Policy:
Commercial Standards for Promotional Materials.
This Chapter summarizes Pfizer policy regarding the development, review, and approval of
advertising and promotional labeling for the U.S. human biopharmaceutical business. Non-
compliance with these policies puts the Company at risk and can subject Pfizer colleagues to
disciplinary action up to and including termination of employment.
Any exceptions to the following policies/principles must be approved in writing by the BU Chief
Counsel.
Introduction

White Guide – Chapter 2: Advertising and Promotional Labeling
Rev. 12/20
Page 24 of 405
Pre-Approval Communication
Prior to approval, the Food and Drug Administration (FDA) permits only two types of advertisements for
drugs: “Institutional Advertising” and “Coming Soon” advertising. Institutional Advertising may
announce that a drug company is conducting research in a particular therapeutic area to develop a new
drug, but the name of the investigational drug must not be mentioned and any representation (written,
verbal, or graphic) that directly or indirectly identifies the drug must not be included in the advertisement.
Coming Soon advertising, announces the name of the product that will be available soon without any
information (written, verbal, or graphic) relating to the therapeutic area, safety, efficacy, or intended use of
the drug. Coming Soon advertisements are permissible only if the drug is not expected to have a boxed
warning. Coming Soon advertisements must meet the requirements of a reminder advertisement
Key Points to Ensure Compliance
• A brand Review Committee (RC) may approve clinical reprints for promotional use by Field
Commercial Colleagues only if they are consistent with the product’s label, as detailed later
in this Chapter.
• Like other forms of promotion, Direct-to-Consumer (DTC)
communications must comply
with FDA regulations and Pfizer’s five core principles as well as PhRMA’s Guiding
Principles. DTC communications should educate patients and consumers and encourage
them to seek guidance from healthcare professionals.
• As outlined in White Guide Chapter 4: Marketing Programs
, Customer Engagement
Programs (CEPs) must be designed, reviewed, approved, and conducted in compliance
with
Corporate Policy (CP) #902: Management of Safety Information for Customer
Engagement Programs (CEPs) and
Corporate Procedure (CP) #902a: Management of
Safety Information for CEPs.
•
As it does with other forms of advertising and promotional labeling, the FDA regulates
Pfizer’s use of the Internet and social media to promote its products. Websites that contain
promotional product information must comply with all the laws, regulations, and principles
that govern promotional materials created for traditional media in addition to relevant Pfizer
policies and guidelines. See DRT.pfizer.com
for guidelines associated with the appropriate
use of the Internet and social media channels in advertising and promotional labeling.
Core Compliance Principles for Professional
and Consumer Promotional Materials

White Guide – Chapter 2: Advertising and Promotional Labeling
Rev. 12/20
Page 25 of 405
(described further below) and therefore must not contain any representations about the product. For a
particular product, Pfizer can choose only one of these two types of advertising during the pre-approval
time period. Companies are not permitted to use both types simultaneously or to alternate between these
approaches during the pre-approval time period. Other than these two types of advertising, no promotion
may be conducted for a product prior to its approval.
Pre-Approval Communication
When can I meet with customers to begin discussing a new product or new indication?
Pfizer is not permitted to promote a new product or indication prior to receiving FDA
approval. This means that Pfizer is not permitted to make claims about the safety and
efficacy profile of the product until after FDA approval. In limited circumstances it may
be appropriate to discuss an unapproved product or indication with a customer as part
of a non-promotional interaction (e.g., advisory board, scientific exchange, and certain
payer communications). All colleagues must receive appropriate approvals before
proactively discussing any unapproved product or indication with an HCP or consumer
or other customer. See White Guide Chapter 8: Non-Promotional and Media Activities,
and U.S. Guidance for Interactions with Healthcare Decision-Makers and Payers Pre-
FDA Approval for more information.
Pre-Approval Interactions with Healthcare Decision Makers
In limited circumstances, Medical Colleagues and Commercial Colleagues (not including sales
representatives) may engage in communications with Healthcare Decision-Makers (e.g., formulary
decision-makers, payers, and other similar entities) related to investigational assets and Investigational
Uses of Approved Products (PIE - Preapproval Information Exchange). Communication of PIE must be
designed and executed for the specific purpose of facilitating a Healthcare Decision-Maker’s planning and
budgeting in preparation for the potential approval and market availability of a Pfizer investigational product
or use. Examples of a Healthcare Decision-Maker’s business planning and budgeting processes may
include, among others: benefit design analysis and modification, identification of disease prevalence within
a population, potential medical policy modification, treatment pathway considerations, and evaluation of
new to market policies.
Whether Pfizer should execute a PIE should be determined on a case-by-case basis, in consultation with
the relevant Global Product Counsel (GPC).
Any PIE communications to a Healthcare Decision-Maker must be unbiased, factual, accurate, truthful,
non-misleading, objective and non-promotional in content, presentation, and tone. Material presented
during a PIE communication is not considered promotional in nature; therefore, the post-marketing

White Guide – Chapter 2: Advertising and Promotional Labeling
Rev. 12/20
Page 26 of 405
submission requirements pertaining to promotional materials [submission of materials at time of first use to
FDA’s Office of Prescription Drug Promotion (OPDP)] do not apply.
Disclosures:
The investigational nature of the product or use must always be clearly and prominently disclosed and
should be unambiguous throughout the PIE communication.
The regulatory status of the investigational product or use should be similarly disclosed (e.g., disclosure
that FDA has not evaluated the safety and effectiveness of the product or use, or that the product or use is
currently under FDA review).
A caveat should be included (as applicable) that the sought-after label and indication(s) for the product are
subject to change, should the product or use be FDA approved at all.
PIE must not be used to:
• claim that an investigational product or use is effective or safe
• recommend or encourage off-label uses
• encourage future prescribing o engage in actual or perceived quid pro quo
Please refer to U.S. Guidance for Interactions with Healthcare Decision-Makers and Payers Pre-FDA
Approval for more information on how to compliantly engage in a PIE and the process and procedure for
obtaining approval to engage in a PIE.
Post-Approval Communication
Core Principle #1: Ensure Statements are Consistent with Product Labeling
Regulatory authorities in each country typically dictate the content of the approved product labeling.
Colleagues must ensure that all statements in Pfizer Promotional materials and communications are
consistent with the locally approved product label and local regulations, or otherwise authorized by law.
When creating global and/or regional materials (any materials created for more than one country) that will
be distributed to multiple local markets for their review and approval, the Core Data Sheets may be used
as the reference label.
Pfizer, like all pharmaceutical companies, is permitted to promote only FDA-approved uses of its products
in the United States. All promotional statements made about a Pfizer drug must be consistent with the
information contained in the product’s labeling. In certain circumstance RC may approve content that is
not specifically contained in the label but is not inconsistent with the label. Uses that remain under
investigation or that are under FDA review, but have not been approved, are considered off-label and claims
about such uses are not to be made in promotion.

White Guide – Chapter 2: Advertising and Promotional Labeling
Rev. 12/20
Page 27 of 405
Core Principle #2: Support All Claims with Adequately Substantiated Evidence
Promotional materials and the claims they contain must be directly substantiated either by reference to the
approved labeling or by scientific evidence. Safety and effectiveness claims must be supported by an up-
to-date evaluation of evidence that is scientifically valid and consistent with the approved product label.
Quotations must be referenced. Claims must not be stronger than the evidence warrants. Any non-clinical
data or studies must be identified clearly as non-clinical and not used in a misleading manner (i.e., to
suggest or imply clinical relevance where none can be established). When substantiation consists of data-
on-file, the data must be readily available and retrievable to be provided upon request. Such requests are
subject to review and approval by Legal.
Standards for Claims
“Claims” are characterizations of a Pfizer product, whether related to effectiveness, safety, dosing, or drug
class. All product claims must be consistent with label and supported by adequately substantiated
evidence. Local laws and regulations may also have additional standards or requirements for making
claims and the level of scientific substantiation required to make a claim.
Under FDA regulations, a drug is considered “misbranded” if its labeling or advertising contain claims that
are not supported by substantial evidence. Substantial evidence generally means two randomized,
double-blind, placebo-controlled clinical trials, although the required evidence may vary in certain disease
areas or situations (for example, in rare diseases or oncology). These are often referred to as “adequate
and well controlled” clinical trials. In most cases, any statement that could impact an HCP’s decision to
prescribe a Pfizer product, or not to prescribe a competing product, should be considered a claim that needs
to be supported by substantial evidence. Moreover, consistent with core principle #1, such a claim must
be consistent with the approved labeling. Additionally, RC teams should consider all FDA feedback (e.g.,
from labeling discussions, OPDP or APLB “preclearance”/advisory comments, etc.) when determining the
appropriateness of a specific claim.
The chart on the following page sets out examples of typical claims and the generally accepted evidence
to support the claim being made in approved promotional materials:
Type of Claim
Example
Generally Accepted
Supporting Evidence
Efficacy or Safety Claim
“Product X has been shown to
reduce blood pressure by 30%
in most adult patients”
2 adequate and well controlled
clinical trials and/or direct
support in FDA-approved label
Comparative Claim:
Comparing any attribute of the
“In two studies, Product X
reduced high blood pressure as
well as competing Product Y”
2 adequate and well controlled
clinical trials comparing Product
X and Product Y head-to-head
(or, in certain circumstances, 1
White Guide – Chapter 2: Advertising and Promotional Labeling
Rev. 12/20
Page 28 of 405
Type of Claim
Example
Generally Accepted
Supporting Evidence
Pfizer product with a competing
product
large, well-controlled head-to-
head study) using comparable,
approved dosage regimens
Superiority Claim: Claiming an
attribute of the Pfizer product is
better or superior to a
competing product
“Product X demonstrated
superiority in reducing blood
pressure over Product Y in two
studies”
2 adequate and well controlled
clinical trials comparing Product
X and Product Y head-to-head
(or, in certain circumstances, 1
large, well-controlled head-to-
head study) using comparable,
approved dosage regimens
Healthcare Economic or
Pharmacoeconomic Claim
1
:
Claiming use of a Pfizer product
results in lower healthcare costs
“Over the course of treatment,
Product X may (or on average)
reduce hospital costs by Y%”
“Competent and reliable”
scientific evidence is required
for claims that are made to
formulary committees and that
are related to the product’s
indication
Quality of Life (QoL) Claim:
Claiming use of a Pfizer product
improves one’s overall quality of
life or an aspect of one’s life
“Patients on Product X showed
improved daily physical
function”
2 adequate and well-controlled
clinical trials using an
appropriate FDA-agreed upon
validated Quality of Life
instrument
1
Healthcare Economics or Pharmacoeconomic Claims are generally limited to use with formulary decision makers
In addition, as a general rule, product claims must have clinical as well as statistical significance. Any
exceptions to this rule must be carefully reviewed to ensure the claim does not inappropriately imply greater
efficacy or fewer risks than otherwise established by scientific or medical evidence. It is also important to
ensure that each claim is only as strong as the evidence that supports it. In other words, each product
claim must be narrowly tailored to match the findings of the data.
Superlative Claims
Is it ever appropriate to use superlatives like “best” or “safest?”
It is almost never appropriate to use unqualified superlatives such as “best” or “safest”
since such claims can rarely, if ever, be supported by substantial evidence. For
example, to establish that a product is the best or safest would require successful
head-to-head trials against all existing therapies.

White Guide – Chapter 2: Advertising and Promotional Labeling
Rev. 12/20
Page 29 of 405
Core Principle #3: Be Accurate, Truthful, and Not Misleading
• First and foremost, promotional materials must be accurate, truthful, not misleading and sufficiently
complete to help enable the recipient to form his or her own opinion of the therapeutic value of the
Pfizer product.
• Information contained in Promotional Materials and Promotional Communications must be based
on all relevant evidence and should reflect that evidence clearly. Materials must not mislead by
distortion, exaggeration, undue emphasis, omission or in any other way. Every effort should be
made to avoid ambiguity.
• Visual representations, including graphics in promotional materials must not be used in a
misleading manner. Graphics and images selected for use should also reflect appropriate local
market cultural sensitivities.
Advertising and promotional labeling must not be false or misleading. Accordingly, all Pfizer promotional
materials must accurately and truthfully present all material information, which includes the product’s
important risk and safety information. Materials are false and misleading when they make a claim that is
not supported by appropriate data or that is not consistent with the product label.
Promotional material may be considered false or misleading if, for example, the material:
• Promotes the drug for an unapproved use or indication;
• Overstates the product’s efficacy or claims it is effective in a broader range of conditions or patients
than has been demonstrated by substantial evidence;
• Uses favorable data derived from patients treated with dosages different from those recommended
in the approved labeling;
• Minimizes the product’s safety risks;
• Suggests that a drug is safer or more effective than another drug when the claim has not been
demonstrated by substantial evidence;
• Markets two or more products in a way that falsely or misleadingly conflates the various properties
of the respective products;
• Contains or relies on outdated or selective (“cherry-picked”) clinical or other data;
• Inaccurately reflects the methodology used to conduct the clinical study;
• Provides favorable information or conclusions from a study that is inadequate in design, scope, or
conduct to furnish significant support for such information or conclusions;
• Uses the concept of statistical significance to support a claim that has not been demonstrated to
have clinical significance or validity;
• Fails to reveal the range of variations around quoted average results;
• Uses statistical analyses and techniques on a retrospective basis to discover and cite findings not
soundly supported by the study or to suggest scientific validity and rigor for data from studies, the
design, or protocol of which are not amendable to formal statistical evaluations;

White Guide – Chapter 2: Advertising and Promotional Labeling
Rev. 12/20
Page 30 of 405
• Presents information from a study in a way that implies that the study represents larger or more
general experience with the drug than it actually does; or
• Uses statistics on numbers of patients or counts of favorable results or side effects derived from
pooled data from various insignificant or dissimilar studies, in a way that suggests that such
statistics are valid even if they are not.
Visual Representations
A brand team wants to include photographs of families (children and parents) in their
promotional materials. Are there any concerns with doing this?
Visual representations, artwork, and graphics must be taken into consideration when
determining whether material may be deemed false and misleading. Visuals can imply
claims about the product and must be consistent with the product’s labeling. For
example, if a product is indicated for adults, including pictures focusing on children in
the advertising could lead viewers to mistakenly believe that the product is indicated
for use in children. Accordingly, all visuals must be reviewed to ensure they are not
misleading in light of the product’s indication or any claim made about the product.
Core Principle #4: Provide Balanced Information about Benefits and Risks
Promotional Materials and Promotional Communications must present an appropriate balance between
information relating to efficacy and information relating to safety of the product.
Each piece must have fair balance as a whole in terms of content and format. The word “safe” must not be
used without qualification. A product may be described as being “well tolerated”, provided that this claim
can be substantiated and includes safety context and balance, such as warnings, contraindications, and
other significant safety information.
Essential product safety information, such as contraindications, precautions, and side effects, must be
presented fairly, accurately, and according to local laws and regulations.
To be truthful and not misleading, all advertising and promotional labeling must present a “fair balance”
of the promoted product’s potential benefits and risks. This means that significant risk and safety
information must be presented together with efficacy claims in comparable prominence.
As a general rule, promotional materials are judged in their entirety to determine whether the advertised
products are portrayed with fair balance. However, an individual spread (e.g., set of facing pages expected
to be viewed together), must still be evaluated together to ensure that it is accurate, fair, and balanced. To
be appropriately balanced, the prominence (based on the typeset, font size, color, use of white space, etc.)
of efficacy claims must be “reasonably comparable” to the presentation of information related to boxed

White Guide – Chapter 2: Advertising and Promotional Labeling
Rev. 12/20
Page 31 of 405
warnings (where applicable), contraindications, warnings/precautions, side effects, and other important
safety information. Appropriate product labeling must also be included.
Fair Balance
Can promotional materials for a product claim that the product is “safe?”
No. The word “safe” cannot be used without qualification since all products have risks.
A product may, however, be described as having a “well-studied safety profile” if that
can be substantiated by medical evidence. Appropriate safety information, such as
boxed warnings, contraindications, warnings/precautions, and side effects must also
always be provided to balance and provide context to such a statement.
Core Principle #5: All Promotional Materials Must Be Approved through Review
Committee
All materials intended to promote our products for use in the United States, including materials required to
be filed with the FDA’s Office of Prescription Drug Promotion (OPDP) or Advertising and Promotional
Labeling Branch (APLB) by Date Of First Use, all pieces being submitted to OPDP or APLB for advisory
comments, and disease awareness and pre-launch materials prepared in anticipation of FDA approval must
be approved through RC (or a comparable review process for corporate unbranded messaging) prior to
use. For more information on the RC Process, see CMCD REG08-WI-US01: Process Governing Review
and Approval of United States Product Team Advertising and Promotional Materials. The Review
Committee tab on GCO Policy Xchange on GCO on Demand includes helpful documents. The GCO Policy
Xchange on GCO on Demand also provides links to general and platform specific guidelines as well as
communications from Pfizer’s U.S. Advertising and Promotional Policy Committee (PPC).
Sales Colleagues on Veeva CRM are expected to utilize the digital materials on their approved device (i.e.,
tablet or iPad) whenever possible when engaging in detailing HCPs (see Orange Guide Chapter 2:
Interactions with HCPs and Health Systems). Pfizer product teams requesting exceptions from this general
rule must seek approval from Legal (i.e., Global Product Counsel) and Compliance. In addition, Pfizer
product teams seeking to utilize paper materials only and not develop any digital materials for detailing
purposes must also seek approval from Legal and Compliance.
Pfizer RC teams are encouraged to initiate an “In-Context Training” platform to provide specific guidance
regarding key promotional pieces, such as visual aids and clinical reprints, outlining the boundaries of what
representatives “can and cannot say” about a product based on the content of the piece. For more
information regarding what types of pieces must include in-context training and how it should be provided,
consult the brand’s team attorney.

White Guide – Chapter 2: Advertising and Promotional Labeling
Rev. 12/20
Page 32 of 405
Core Principle #6: Don’t Make Claims About Investigational Products or Investigational
Uses of Marketed Products
Neither Pfizer nor any person acting on its behalf may make a promotional claim about an investigational
product, or an investigational use of a marketed product, before the product or indication/use has been
approved by a local Regulatory Authority unless otherwise permitted by local law. This prohibition applies
even if the medical community widely accepts the use of an approved product for an unapproved indication.
This requirement is not intended to restrict non-promotional communications and activities as permitted by
local law and regulation.
The FDA regulates two categories of promotional materials which have slightly different requirements:
promotional labeling and advertisements. The FDA uses the term promotional labeling to apply to a
broad array of materials used in marketing a product, including, for example, brochures, mailing pieces,
detailing pieces, websites, social media platforms, exhibits, literature reprints, and similar pieces of printed,
audio, or visual matter descriptive of a drug.
In contrast to labeling, FDA regulations define advertising to include the following: advertisements in
published journals, magazines, other periodicals, newspapers, and advertisements broadcast through
media such as radio and television.
Both promotional labeling and advertising for a drug must include a fair balance between efficacy and risk
information and must not be false or misleading in any respect. With some exceptions, promotional labeling
and advertisements must also typically include:
• Proprietary Name & Established (Generic) Name;
• Approved indication(s) for use (including any limitations of use);
• Dosage form(s) and dosage(s);
• Quantitative amounts of active ingredients in combination products;
• Name of the company responsible for marketing the product and its agent (co-promote partner);
• Boxed warning (where applicable), contraindications, warnings/precautions, and side effects; and
• Appropriate labeling: 1) Full prescribing information, including Patient Package Insert (or
Medication Guide and/or Instructions for Use) for promotional labeling; 2) appropriate brief
summary for print advertisements; and 3) adequate provision for broadcast advertisements such
as television, radio, and telephone. Pfizer strongly encourages the use of Consumer-friendly
language in consumer materials. Consumer Brief Summary should be used in lieu of the full
prescribing information in consumer print material with RC agreement.
Requirements of Promotional Labeling and Advertising

White Guide – Chapter 2: Advertising and Promotional Labeling
Rev. 12/20
Page 33 of 405
Specific requirements apply to advertisements in certain media or directed to certain audiences:
• Professional print advertisements must include a Professional Brief Summary.
• Consumer print advertisements must include the Consumer Brief Summary, Important Facts Brief
Summary, the Patient Package Insert, or the Medication Guide, as determined by the RC.
• Broadcast advertisements (television, radio, or telephone) must include the Major Statement and
ensure adequate provision of the full prescribing information.
Moreover, the full prescribing information – both the Package Insert (PI) and the Patient Package Insert
(PPI) or Medication Guide and/or Instructions for Use, as appropriate – must accompany promotional
labeling directed to HCP’s (with the exception of some reminder ads). Consumer Brief Summary should
be used in lieu of the full prescribing information in consumer print material with RC agreement. These
concepts are explained in the tables on the following pages.
• Proprietary (Brand) Name & Established (Generic) Name is required on all promotional
labeling and advertising. The established (generic) name must be included at least once
with the proprietary (brand) name where the proprietary name is featured and at least once
per page or spread.
o
There must be no intervening matter between the brand and generic name. The
established name must be used in type at least half as large as the type used for the
most prominent presentation of the proprietary name. For example, in a logo, the
generic name must be included, and the type size used must be at least half the size
of the type used for the brand name.
o
On any page of an advertisement or promotional labeling in which the proprietary
name or designation is not featured but is used in the running text, the established
name shall be used at least once in the running text, typically at first mention or
otherwise prominently.
o
In television advertisements, the generic name should be included immediately
following the most prominent display of the brand name on the screen (i.e., through
“supers” that are used as headlines or taglines).
o
In radio advertisements and telephone scripts, the generic name should be included
at the first mention of the brand name.
o
In electronic media, the generic name should accompany the brand name at the most
prominent mention, and the generic name should also appear at least once in the
running text. For electronic media, including websites and presentations, the generic
name must be visible on the screen at all times.

White Guide – Chapter 2: Advertising and Promotional Labeling
Rev. 12/20
Page 34 of 405
•
Professional Brief Summary
typically includes all risk information from the full
prescribing information regarding the product including, but not limited to, boxed warning
(where applicable), contraindications, warnings/precautions, and side effects, and
information under headings such as cautions, special considerations. The Brief Summary
typically excludes the pharmacokinetics, pharmacology, and dosage information from the
full prescribing information unless there is important risk information included in these
sections. Consult with your brand Regulatory team member for further guidance.
•
The Consumer Brief Summary is generally derived from the PPI (or Medication Guide)
and is used in consumer print DTC advertisements and promotional labeling.
Labeling
Requirements for Consumer Directed Print Material.
• Major Statement
conveys a drug’s most important risk information in consumer-friendly
language during a broadcast advertisement. A product’s Major Statement is typically
crafted with significant input from the FDA’s OPDP or APLB through their responses to
requests for advisory comments.
• Adequate Provision
is applicable in the context of broadcast advertisements only. The
term refers to providing the audience with a reasonably convenient way to obtain the
drug’s full prescribing information. Pfizer’s approach to disseminating the product’s
approved labeling for broadcast advertisements is to include each of the following
components:
o
Providing a toll-free telephone number in the advertisement for consumers to call to
request the full prescribing information or to have it read to them over the phone;
o
Providing an Internet web page (URL) address where the full prescribing information
can be viewed;
o Disclosing that HCPs may provide additional product information.
Please note that Pfizer no longer requires reference to a print publication provided that the
three components delineated above are satisfied. For further information regarding Adequate
Provision and alternative approaches to fulfilling that requirement, see the
PPC memorandum
regarding “’Adequate Provision’ and the ‘Book of Record’ in Broadcast Advertisements” dated
August 26, 2016 available on GCO Policy Xchange on GCO on Demand.
For telephone advertisements, see
“PI/PPI Treatment in Consumer Labeling, Multicultural
Items, and IVRs” for more information on GCO Policy Xchange on GCO on Demand.
White Guide – Chapter 2: Advertising and Promotional Labeling
Rev. 12/20
Page 35 of 405
FDA Submission of Promotional Materials
When do promotional materials need to be sent to the FDA?
All branded promotional materials for Pfizer drugs must be submitted to the FDA’s
OPDP or APLB before or at the time that Pfizer first uses the materials. Except in the
case of drugs approved via the Subpart H accelerated approval process or biologics
and vaccines approved under Subpart E, Pfizer is not required to submit any materials
to OPDP or APLB prior to first use. A company may choose to seek advisory
comments from the FDA on materials prior to use. This is typically done prior to the
launch of a new product or new indication so that the company may receive guidance
from OPDP or APLB on the promotional presentation including efficacy claims and fair
balance when particular claims are made. Moreover, pursuant to the PhRMA DTC
Guiding Principles, Pfizer has committed to seek advisory comments on new television
broadcast advertising campaigns.
•
Reminder Promotional Materials
are short promotional pieces that contain a drug’s
proprietary (brand) name and established (generic) name and may contain dosage form
and strength, as well as pricing information or formulary coverage. A reminder cannot
mention or imply the drug’s indication, effectiveness, safety, uses, or dosing regimen. Nor
can a reminder give any representation of the drug, either direct or implied. The inclusion
of any such information could transform a reminder into a full advertisement or promotional
labeling. Because reminders call attention to the name of the drug product but do not
include indications or dosage recommendations, they are not required to carry the full
prescribing information or brief summary. Reminder promotional materials also do not
include safety disclosures since there are no efficacy or other claims to balance.
Pursuant
to FDA regulations (with a limited exception for “price reminders” subject to strict
requirements including disclosure of the price paid by the consumer), reminder
promotional materials cannot be used for products that carry a boxed warning.
• Help Seeking or Disease Awareness Advertisements
are communications
disseminated to consumers or HCPs that discuss a particular disease or health condition,
but do not mention any Pfizer drug or make any representation or suggestion concerning
a particular Pfizer drug.

White Guide – Chapter 2: Advertising and Promotional Labeling
Rev. 12/20
Page 36 of 405
A brand Review Committee may approve clinical reprints for promotional use by Sales Colleagues only if
they are consistent with the product’s label. In order for a reprint to qualify as “consistent with the product’s
label,” (for indication, efficacy, and safety) it must satisfy ALL of the following conditions:
The primary message must fall within the product’s label;
It contains, at most, only an insignificant amount of information that is inconsistent with the label; and
Any information that is inconsistent with the label must not be reasonably likely to be used to support an
inappropriate promotional message.
Further, in accordance with agreements between Pfizer and certain state Attorneys General, the following
additional requirements and restrictions apply when an RC is considering approval of a reprint for
promotional use:
Pfizer is prohibited from disseminating information regarding an off-label use of a Pfizer product if that use
was submitted to the FDA for approval and the FDA either: (1) refused to approve the application; or (2)
indicated that FDA-identified deficiencies must be resolved before approval can be granted, unless the
information clearly and conspicuously discloses to the recipient that the FDA has issued that advice
regarding the off-label use.
Pfizer is prohibited from distributing reprints containing off-label information about any Pfizer product to
physician specialties who do not customarily prescribe the product if the distribution of the reprint, combined
with other promotional activities, promotes off-label use of the product.
Zyvox, and Atgam specific restrictions: Only certain Medical colleagues may identify, select, approve,
and disseminate reprints containing off-label information (beyond insignificant references to off-label
information). Also, these reprints shall be accompanied by FDA-approved labeling, include a prominent
disclosure on the cover or first page that the article may discuss off-label information, and not be referred
to in a promotional manner. MOS colleagues may disseminate reprints relating to pharmacoeconomic or
health outcomes to healthcare organizations. Commercial Colleagues are prohibited from disseminating
such reprints.
Opioid-specific restrictions: Reprints relating to the use of opioids for chronic pain should be accompanied
by information relating to the potential risks of addiction, abuse, and misuse associated with extended-
release opioids.
Use of Reprints in Product Promotion

White Guide – Chapter 2: Advertising and Promotional Labeling
Rev. 12/20
Page 37 of 405
Before approving a reprint, the product Review Committee should carefully consider additional risk
mitigation measures that may be appropriate, such as, implementation guides, carriers, wrappers,
backgrounders, and/or enhanced training, on a case-by-case basis. In accordance with CMCD REG08-
WI-US01: Process Governing Review and Approval of United States Product Team Advertising and
Promotional Materials, any reprint reviewed for approval under this guidance may also be referred by the
product Review Committee to the relevant Business Unit Review Committee (BURC).
Promotional use by sales personnel of any reprint that does not satisfy this guidance must be
approved by the BU Chief Counsel.
Direct-To-Consumer Advertising
Pfizer has adopted the PhRMA Guiding Principles on Direct to Consumer Advertisements about
Prescription Medicines and Pfizer’s Guidance for the Implementation of the Updated PhRMA DTC
Principles. These principles support the use of DTC advertising to communicate information about medical
conditions and potential treatments so that patients can make informed choices. Like all promotion, DTC
communications must comply with FDA regulations and Pfizer’s five core principles, as stated above.
In addition to the five core principles, PhRMA’s Guiding DTC Principles serve to ensure that DTC
communications educate patients and consumers and encourage them to seek guidance from their
healthcare professionals. All Pfizer DTC materials should be consistent with the PhRMA Principles. In the
event of any inconsistency, Pfizer guidance takes priority over the PhRMA Principles.
Opioid-specific requirements: Advertising of Pfizer opioids should also include information concerning
the potential risks of addiction, abuse, and misuse of the products when used in accordance with their FDA-
approved prescribing information.
FDA Submission of Promotional Materials
The PhRMA Guiding Principles for DTC Communications do not specify a time that
companies need to wait after submitting television advertising to the FDA for review.
Why are we required to wait 45 days?
As part of Pfizer’s settlement with several state Attorneys General, Pfizer agreed to
undertake additional obligations with respect to its television advertising. One of those
obligations was to submit all new television advertising to the FDA for review and wait
at least 45 days for comments. Pfizer is obligated to modify its advertising consistent
with written comments it receives.
Direct-To-Consumer and Internet Advertising

White Guide – Chapter 2: Advertising and Promotional Labeling
Rev. 12/20
Page 38 of 405
Patient Testimonials
Like all other advertising and promotion, testimonials must follow the core principles outlined above. Any
testimonial used by Pfizer must be consistent with the product label and must include and/or be
accompanied by fair balance. Testimonials must not include any claims that Pfizer could not make directly.
Moreover, in accordance with our agreements with state Attorneys General, Pfizer cannot disseminate in a
promotional context any patient testimonial relating to a Pfizer product that does not clearly and
conspicuously disclose what the generally expected performance would be in the depicted circumstances
or clearly and conspicuously disclose the limited applicability of the experience described by the patient
testimonial to what consumers may generally expect to achieve. Please refer to the Guidance for the
Implementation of the Updated PhRMA DTC Principles. Please also refer to Testimonials: Patient
Recruitment and Engagement, available on GCO Policy Xchange on GCO on Demand, for further guidance
on specific requirements for using patient testimonials in promotion.
Usage of Animals in Advertising
Introduction
Because respect is a key tenet in our use of animals, Pfizer has established standards regarding the use
of animals in the marketing of Pfizer products. If advertisements (print, digital or television) featuring
animals are used, any animal shown should be healthy and in a natural or appropriate setting. Non-human
primates must not be used in the advertising of Pfizer products, and other wild animals will also not be used
unless they are shown in their natural setting or portrayed through animation or computer-generated
graphics. These standards are part of Pfizer’s Corporate Policy # 901. The summary below is intended
only as an overview, and colleagues are responsible for referring to and following all applicable portions of
CP #901.
Planning Guidance
For promotional planning, if you are considering a campaign or promotional tactic that may feature an
animal or animals the following steps are required:
Animals in TV Advertising: If a Pfizer television promotional campaign will contain any use of animal
(including domesticated animals such as dogs, cats, birds, etc.), please include Gloria Gaito, Global Animal
Welfare, at the concept stage – before market research or testing. All storyboards must be reviewed and
approved by Gloria Gaito in conjunction with Global Product Counsel.

White Guide – Chapter 2: Advertising and Promotional Labeling
Rev. 12/20
Page 39 of 405
Animals in Print and Digital Promotion: If a Pfizer promotional print or digital campaign will feature any
animal, and in the absence of a storyboard, please outline use as follows:
• Provide a detailed overview paragraph outlining animal(s) use;
• Specify the medium: print, digital, or both;
• Clarify whether the animal(s) depicted are from stock photography, existing Pfizer photo archive,
from an existing DTC TV advertisement that went through the process for TV advertising above, or
will be part of a new photo shoot;
• List the type and number of animal(s) being used (itemize all animals being depicted – dog, cat,
horse, etc.);
• Describe the setting (for non-wild animals this can be a natural or appropriate setting);
• Describe what the animal will be doing;
• Explain the objective of the promotional tactic; and
• All outlines must be reviewed and approved by Gloria Gaito, Global Animal Welfare in conjunction
with Global Product Counsel.
Use of Wild Animals in Any Promotion: If any wild animal is being considered for use in promotion
(including bears, porcupines, sea lions, etc.) these animals must only be shown in their natural setting (a
zoo is not considered a natural setting) or portrayed through animation or computer-generated graphics.
At concept stage and prior to market research, their use for TV advertising should be drafted in a
Storyboard, and for print and digital work (in the absence of a storyboard) should be provided in a detailed
overview. Both require a concept review by Gloria Gaito, Global Animal Welfare, in conjunction with Global
Product Counsel.
To view Corporate Policy # 901, please click here.
If you have additional questions, please contact Gloria Gaito or your Pfizer legal contact for your
promotional work.
Internet and Social Media Promotion
Like other forms of promotion, the FDA governs Pfizer’s use of the Internet and social media to promote its
products. This includes PfizerPro, product websites and Facebook, as well as banner and other Internet
advertisements, such as sponsored search (or search-engine marketing).
Pfizer websites that contain product information that is deemed promotional must comply with the laws,
regulations, and principles that govern promotional materials created for traditional media. This means that
any discussion of the product’s uses or indications on websites must adhere to FDA-approved labeling.

White Guide – Chapter 2: Advertising and Promotional Labeling
Rev. 12/20
Page 40 of 405
Websites must appropriately balance any claims of efficacy with the relevant risk information, and the risk
information should be presented in a manner similar to the presentation of efficacy information. For
example, if the efficacy presentation is active (e.g., an audio component), then the risk information should
likewise have an active element. Similarly, if efficacy or benefits claims are made on a page, balancing
safety information must be incorporated into that page with comparable prominence. Having risk
information “one click away” is not generally acceptable; rather, risk information must be incorporated into
the body of any webpage that includes any benefit claims. Branded webpages may not include or imply
any product claims (including the indication) unless fair balance is provided on that same page. In addition,
the webpage must include a link to the product’s package insert.
Detailed information on the requirements of Internet promotion, including Facebook (social media), can be
found under the Advertising & Promotion Guidelines tab on Global Policy Xchange on GCO on Demand.
This information can also be found at DRT.Pfizer.com. Promotional teams with questions regarding
implementation of new social media initiatives can seek a concept review with the Digital Review Team
(DRT).
As part of our advertising and promotional efforts, the Pfizer Biopharmaceuticals Group (PBG) purchases
media placements for advertisements or other marketing content. This activity is referred to as PAID
MEDIA and includes related media services and vendors. Examples of paid media include placing a TV
commercial on CBS or YouTube, banner ads on Medscape, a sponsored article in The New York Times,
paid search on Google, or newsfeed placements on Facebook. Media services includes data and tools for
media activation and optimizations, and Pfizer’s external media agencies. See table below for additional
details on paid media related definitions.
While paid media efforts are an important tool to reach patients and HCPs, there are also multiple risks
including compliance, fraud, privacy, and reputation. To protect Pfizer, PBG policy requires that PAID
MEDIA be centrally led and governed by the Pfizer MEDIA LAB. Centralized media ensures media activity
adheres to Pfizer policies and guidelines, and achieves the following Pfizer requirements:
• Control and Tracking: visibility of all media spend and practices, and ability to pause/stop.
• Protection of Media Spend: fair market value, auditing, appropriate terms and conditions.
• Media Vendor Management: single Pfizer voice and process, and proper vendor vetting.
• Respect Privacy: adherence to Pfizer’s Privacy Policy, and privacy laws and regulations.
• Reputation: media practices and placements consistent with Pfizer values and policies.
Pfizer Biopharmaceuticals Group: Paid Media
Policy

White Guide – Chapter 2: Advertising and Promotional Labeling
Rev. 12/20
Page 41 of 405
Paid Media Procedures
This policy applies to all colleagues (across functional areas/BUs/divisions) that are engaging in paid media
and media services that are funded by and for the benefit of PBG. To be compliant with this policy,
colleagues are required to follow these standard operating procedures.
OVERALL
All PBG paid media and media services must be initiated and implemented through
the Media Lab and its processes, guidelines, and tracking systems.
PURCHASING
Only the Media Lab can implement, negotiate or purchase paid media or media
services on behalf of PBG.
• Colleagues must not sign contracts for paid media or media services
VENDORS
Relative to media vendors for paid media and media services:
• The Media Lab is the single point of contact for all media vendors
• The Media Lab is responsible for vetting and approving media vendors
VENDORS WITH
MULTIPLE
OFFERINGS
For vendors that have media and non-media offerings:
• Refer vendors to the Media Lab for paid media and media services offerings
• Non-media colleagues must not engage in media related discussions, sign
contracts, or bundle media with non-media offerings
AGENCIES
The Media Lab selects and manages external media agency resources which must
be utilized for all PBG paid media and media services.
• Other agencies (i.e., creative, digital, PR) cannot engage in media activity
DATA & TOOLS
The Media Lab is responsible for all media services related to data, tools, and
analytics used for media activation, optimizations, reporting, and measurement.
CO-PROMOTES
For co-promotes, where the co-promote’s media agency is leading media (not the
Pfizer Media Lab), Pfizer brand teams are responsible for ensuring core media
requirements are met for compliance, privacy, auditing, FMV, and vendors.

White Guide – Chapter 2: Advertising and Promotional Labeling
Rev. 12/20
Page 42 of 405
Key activities that are out-of-scope for this policy include Pfizer Corporate funded paid media, paid media
that is included in HCP congresses/conferences, and in-pharmacy.
For questions related to the Paid Media policy, please contact Ken Haener (Media Lab) or Kyle
Peckens (Procurement).
Media Related Definitions
Paid Media: refers to efforts, where Pfizer is paying to place ads or other content, on media and
other platforms not owned or controlled by Pfizer. Paid media is used to reach patients, HCPs
or other targets.
• Pfizer Content for paid media includes branded or unbranded communications, in standard
ad units (i.e., TV commercial, banner ad) or non-standard/custom formats.
• Paid Media Platforms: includes all existing and emerging media platforms and tactics:
Media Services: related to paid media, includes the following
• Data and Analytics for media targeting, optimizations, reporting, and return-on-investment
• Programmatic Technology such as demand side and data management platforms
• Implementation Tools for ad serving, verification, fraud detection
• Emerging Media Applications such as artificial intelligence, machine learning, automatic
content recognition, blockchain, and automation tools
• Media Agencies-of-Record used for planning and purchasing PBG media
Media Vendors: are companies or organization and their respective sales representatives, that
sell paid media or media services.

White Guide – Chapter 2: Advertising and Promotional Labeling
Rev. 12/20
Page 43 of 405
• Refer any questions to your team’s Regulatory colleague or product attorney.
• CMCD Global REG08-POL: Requirements for the Content and Approval of Promotional Activities
and/or Materials.
• CMCD REG08-WI-US01: Process Governing Review and Approval of United States Product Team
Advertising and Promotional Materials.
• PhRMA Guiding Principles on Direct to Consumer Advertisements about Prescription Medicines.
• Pfizer’s Guidance for the Implementation of the Updated PhRMA DTC Principles.
• CEP Resource Center at http://cep.pfizer.com.
• Global Policy Xchange on GCO on Demand internal website here.
• The Digital Review Team website at http://drt.pfizer.com/.
• Compliance Division internal website (for state Attorney General agreements) at
http://corporatecompliance.pfizer.com/Resources/Pages/StateAGAgreements.aspx.
For More Information

CHAPTER #3 – PROMOTIONAL
INTERACTIONS WITH HEALTHCARE
PROFESSIONALS

White Guide – Chapter 3: Promotional Interactions
with Healthcare Professionals
Rev.12/20
Page 45 of 405
Chapter
#3
PROMOTIONAL INTERACTIONS WITH
HEALTHCARE PROFESSIONALS
CONTENTS
Introduction.................................................................................................................................................. 46
Key Points to Ensure Compliance ........................................................................................................ 46
Four Core Compliance Principles for Successful Product Promotion ........................................................ 47
Use Only RC-Approved Materials and Selling Statements .................................................................. 48
Stay On-Label and Discuss Only Approved Products and Indications ................................................ 48
Provide an Accurate and Balanced Presentation ................................................................................. 50
Patient Access / Reimbursement Support Resources ................................................................................ 50
Never Engage in Actual or Perceived Quid Pro Quo Arrangements .................................................... 50
Key Point Regarding Meals, Educational Items, and Other Transfers of Value to HCPs .................... 51
For More Information ................................................................................................................................... 51
Promotional Interactions with Healthcare Professionals
IntroductionPromotional Interactions with Healthcare
Professionals
Introduction
IntroductionPromotional Interactions with Healthcare
Professionals
IntroductionPromotional Interactions with Healthcare
Professionals
Introduction
Introduction
Introduction
IntroductionPromotional Interactions with Healthcare
Professionals
IntroductionPromotional Interactions with Healthcare
Professionals
Introduction
IntroductionPromotional Interactions with Healthcare
Professionals
IntroductionPromotional Interactions with Healthcare
Professionals

White Guide – Chapter 3: Promotional Interactions
with Healthcare Professionals
Rev.12/20
Page 46 of 405
Chapter #3 Promotional Interactions With Healthcare
Professionals
Pfizer Sales Colleagues have primary responsibility for promoting our products to Healthcare
Professionals (HCPs). However, non-Sales colleagues, including Marketing and Medical colleagues, may
also interact with HCPs in various settings where activities governed by promotional standards may take
place. These settings may include congresses, conventions, symposia, and field rides with Field
Commercial Colleagues. Other interactions with HCPs may also be considered promotional, depending on
the content and context of the interaction. The “Four Core Compliance Principles” reviewed in this
Chapter are applicable to any Pfizer colleague engaged in a promotional interaction with an HCP. For a
more detailed discussion of the policies applicable to Sales Colleagues, see Orange Guide Chapter 2:
Interactions with HCPs and Health Systems.
• Use only Pfizer Review Committee (RC) approved materials when engaging in promotional
interactions with HCPs.
•
All promotional statements must be on-label (consistent with the product’s package insert)
and consistent with RC-approved materials/messaging. All inquiries about off-label
information or unapproved clinical data must be referred to Pfizer U.S. Medical Information
(USMI) (1-800-438-1985)
•
Do not discuss new products or indications before they are FDA-approved unless you have
approval to do so.
•
Always ensure accurate and balanced presentations of the risks and benefits of any Pfizer
product for the relevant approved indication are given.
• Never engage in any actual or perceived quid pro quo arrangement.
•
Key Points to Ensure Compliance use only Pfizer Review Committee (RC) approved materials
when engaging in promotional interactions with HCPs.
•
All promotional statements must be on-label (consistent with the product’s package insert)
and consistent with RC-approved materials/messaging. All inquiries about off-label
information or unapproved clinical data must be referred to Pfizer U.S. Medical Information
(USMI) (1-800-438-1985)
•
Do not discuss new products or indications before they are FDA-approved unless you have
approval to do so
•
Always ensure accurate and balanced presentations of the risks and benefits of any Pfizer
product for the relevant approved indication are given
• Never engage in any actual or perceived quid pro quo arrangement
Key Points to Ensure Compliance
Introduction

White Guide – Chapter 3: Promotional Interactions
with Healthcare Professionals
Rev.12/20
Page 47 of 405
Your interactions with HCPs always must be based on providing accurate and balanced information. Pfizer
has Four Core Compliance Principles that protect you and the Company when you are engaged in
promotional interactions with HCPs:
• Use only RC-approved materials and selling statements;
• Stay on-label and discuss only approved products and indications;
• Provide an accurate and balanced presentation; and
• Never engage in actual or perceived quid pro quo arrangements.
• Four Core Compliance Principles for Successful Product PromotionKey Points to Ensure
ComplianceUse only Pfizer Review Committee (RC) approved materials when engaging in
promotional interactions with HCPs
•
All promotional statements must be on-label (consistent with the product's package insert)
and consistent with RC-approved materials/messaging. All inquiries about off-label
information or unapproved clinical data must be referred to Pfizer U.S. Medical Information
(USMI) (1-800-438-1985)
•
Do not discuss new products or indications before they are FDA-approved unless you have
approval to do so
•
Always ensure accurate and balanced presentations of the risks and benefits of any Pfizer
product for the relevant approved indication are given
• Never engage in any actual or perceived quid pro quo arrangement
•
Key Points to Ensure ComplianceUse only Pfizer Review Committee (RC) approved materials
when engaging in promotional interactions with HCPs
•
All promotional statements must be on-label (consistent with the product's package insert)
and consistent with RC-approved materials/messaging. All inquiries about off-label
information or unapproved clinical data must be referred to Pfizer U.S. Medical Information
(USMI) (1-800-438-1985)
•
Do not discuss new products or indications before they are FDA-approved unless you have
approval to do so
•
Always ensure accurate and balanced presentations of the risks and benefits of any Pfizer
product for the relevant approved indication are given
• Never engage in any actual or perceived quid pro quo arrangement
Key Points to Ensure Compliance
Four Core Compliance Principles for Successful Product Promotion

White Guide – Chapter 3: Promotional Interactions
with Healthcare Professionals
Rev.12/20
Page 48 of 405
Use Only RC-Approved Materials and Selling Statements
Each promoted Pfizer product has a multi-disciplinary Pfizer Review Committee (RC) that reviews and
approves all sales and marketing materials for the product and associated disease states. Any written
materials that you use in a promotional interaction, whether a marketing visual aid, a clinical reprint, or any
other resource or tool, must be approved by the relevant RC. RC-approved materials are prepared in
accordance with FDA-approved product labeling. You may not alter RC-approved materials in any way. In
addition, any materials marked “DO NOT DETAIL” or “Internal Use Only” must not be shared with HCPs or
other customers. For more information on the review and approval of promotional materials, see White
Guide Chapter 2: Advertising and Promotional Materials.
It is critical that you only make promotional statements that are RC-approved, and follow all guidance and
direction contained in any relevant product Implementation Guide or other RC-approved guidance to help
ensure appropriate execution.
Stay On-Label and Discuss Only Approved Products and Indications
All promotional statements about a drug must be consistent with the product’s labeling and must be
consistent with information contained in RC-approved materials. Off-label promotion is taken extremely
seriously by Pfizer and the government.
Examples of appropriate on-label and impermissible off-label promotional claims are provided in the table
on the following page.
Detailing and Promotional Materials: Examples of On-label vs. Off-label Claims
On-label Claims (Appropriate)
Off-label Claims (Inappropriate)
Statements that accurately reflect an approved
indication
Statements that inappropriately broaden an
indication
E.g., “Lyrica is effective across the full spectrum of
painful neuropathic conditions”
Statements about a product’s efficacy for the
approved indication, consistent with labeling
Statements about a product’s efficacy for an
unapproved use
E.g., “Toviaz can help your patients with insomnia
to sleep better”

White Guide – Chapter 3: Promotional Interactions
with Healthcare Professionals
Rev.12/20
Page 49 of 405
Detailing and Promotional Materials: Examples of On-label vs. Off-label Claims
On-label Claims (Appropriate)
Off-label Claims (Inappropriate)
Statements about a product’s efficacy within a
population of patients specifically identified in the
package label
Statements about a product’s efficacy within a
population of patients that are not consistent with
the product labeling or where the safety and
efficacy in that population has not been
established
E.g., “Pristiq can be used in pediatric patients”
Statements about the safety of a product that are
consistent with the information in the package
insert
Statements about the safety of a product that
misstate, minimize, or are inconsistent with the
information in the package insert
E.g., “Patients taking Xeljanz do not really
experience side effects”
Prior to FDA approval of a product or the approval of a new indication for the product, a claim by the
manufacturer (or its representatives) that the product is efficacious and/or safe for such use could be
deemed illegal. Pre-approval promotion may result in severe penalties. Therefore, you may only discuss
approved products and indications in accordance with RC-approved promotional materials. No matter how
compelling the scientific evidence, you must not discuss any product or indication with customers in
promotion until it is approved by the FDA.
Additionally, you may only make comparative claims (comparing an attribute of one product to an attribute
of another product) when you use Pfizer RC-approved promotional materials that expressly make such
claims and you follow all directions provided in any applicable implementation guidance. The FDA
considers promotional materials and claims to be false and misleading if they state or imply that a drug’s
safety or efficacy is comparable or superior to that of another drug without “substantial evidence” to support
such statements or suggestions. It is not appropriate to make comparative safety or efficacy claims based
solely on the data in products’ package inserts (i.e. “label-to-label” comparison). Similarly, because of
differences in clinical trial designs, inclusion criteria, and other factors, it generally is not permissible to
compare results from two separate clinical trials.
If an HCP asks you an unsolicited question about an unapproved product or use, or asks for information
outside of, or inconsistent with, a product’s approved labeling or Pfizer RC-approved materials, the question
must be referred to Pfizer U.S. Medical Information (1-800-438-1985).

White Guide – Chapter 3: Promotional Interactions
with Healthcare Professionals
Rev.12/20
Page 50 of 405
Questions submitted to Pfizer’s U.S. Medical Information must be unsolicited. Pfizer colleagues are not
permitted to solicit or otherwise prompt HCPs to ask questions about unapproved products or off-label uses
of a product in any promotional interaction.
Provide an Accurate and Balanced Presentation
All promotional materials, selling statements, and presentations about Pfizer products must be truthful,
accurate and not misleading, be supported by substantial scientific evidence, and appropriately “balance”
product claims with risk and safety information. Promotion is false or misleading if it does not include
relevant risk and safety information or if it is not supported by appropriate scientific evidence.
The FDA requires “fair balance” in the presentation of a product’s benefits and risks and it is necessary
to provide this information so that HCPs can make informed treatment decisions. The more robust the
efficacy statements, the more risk information needs to be provided to balance the presentation. This
means providing the relevant boxed warning(s) (where applicable), contraindications,
warnings/precautions, side effects, and other material information, such as relevant clinical trial exclusion
criteria or limitations, that allow a prescriber to make an informed decision about whether to prescribe the
product. Balanced presentations demonstrate Pfizer’s commitment to patient care and are required under
the law.
Pfizer is committed to supporting access to the Pfizer medicines prescribed to patients by their doctors. As
part of this commitment, some Pfizer brands offer reimbursement and patient access resources such as
copay cards, hubs, and other programs to help patients get access to their prescribed Pfizer medicine.
When communicating about these resources to doctors, you should not communicate that these offerings
are a reason to prescribe the product, that they differentiate the product from competitors, or that these
resources provide independent value to a doctor by relieving administrative burden or otherwise providing
a service to the doctor. As with efficacy and safety claims, all communications about these resources must
be consistent with RC-approved materials and implementation guides.
Some Pfizer brands may offer brand-specific reimbursement and patient support activities that are carried
out by field-based colleagues. See White Guide Chapter 21 Patient Support Roles for further guidance on
appropriate interactions regarding these patient support activities.
Never Engage in Actual or Perceived Quid Pro Quo Arrangements
Never offer or appear to offer any payment or item of value in exchange for prescribing or formulary
placement of a Pfizer product. The decision of an HCP to prescribe or recommend a Pfizer product, or put
it on a formulary, must be based on the clinical data and the best interest of the patient, and not on any
payment or value offered by Pfizer.
Patient Access / Reimbursement Support Resources

White Guide – Chapter 3: Promotional Interactions
with Healthcare Professionals
Rev.12/20
Page 51 of 405
Never give something of value – even something of nominal value – to influence an HCP, directly
or indirectly, to prescribe or recommend a Pfizer product, or to influence its formulary positioning.
Doing so could put both you and Pfizer at substantial risk and subject you to disciplinary action.
• Orange Guide Chapter 2: Interactions with HCPs and Health Systems.
• Green Guide: Governance for External Medical Activities.
• White Guide Chapter 5: HCP and Government Official Consulting Engagements.
• White Guide Chapter 15: State Laws: HCP and Government Employee Restrictions.
• White Guide Chapter 18: Meals, Educational Items, and HCP Payment Disclosure.
For More InformationKey Point Regarding Meals, Educational Items, and Other Transfers
of Value to HCPs
On occasion, in the course of promotional and other interactions such as consultant meetings and
conventions, Pfizer colleagues may have a bona fide reason to provide a meal or other item of
value to an HCP.
All colleagues (including HQ/Marketing colleagues) are required to comply
with Pfizer policies and laws (including state law restrictions) regarding when, how, and by
whom meals, educational items, or other items of value may be provided to HCPs
. For
further guidance, please see
White Guide Chapter 5: HCP and Government Official Consulting
Engagements; White Guide Chapter 15: State Laws: HCP and Government Employee Restrictions;
White
Guide Chapter 18: Meals, Educational Items, and HCP Payment Disclosure
Key Point Regarding Meals, Educational Items, and Other Transfers of Value
to HCPs
For More Information

Rev.12/20
Page 52 of 405
CHAPTER #4 MARKETING
PROGRAMS

White Guide – Chapter 4: Marketing Programs
Rev. 12/20
Page 53 of 405
Chapter
#4
MARKETING PROGRAMS
CONTENTS
Introduction.................................................................................................................................................. 54
Key Points to Ensure Compliance ........................................................................................................ 54
Speaker Programs ...................................................................................................................................... 55
Content Development ........................................................................................................................... 57
Speaker Programs Topics and Invitations ............................................................................................ 58
Speaker Recruitment & Contracting ..................................................................................................... 59
Speaker Training .................................................................................................................................. 60
Program Execution ............................................................................................................................... 60
Promotional Opportunities at Third-Party Meetings and Conventions ........................................................ 61
Symposia Programs ............................................................................................................................. 61
Exhibit/Display & Other Advertising Opportunities ............................................................................... 63
External Websites and Other Digital Activities ............................................................................................ 63
Customer Engagement Programs (CEPs) .................................................................................................. 64
Savings and Free Trial Programs ............................................................................................................... 64
Hub Support Programs ............................................................................................................................... 65
Pfizer Patient Assistance Programs and RxPathways ............................................................................... 65
Quality Programs ........................................................................................................................................ 66
Commercial E-mails .................................................................................................................................... 67
For More Information ................................................................................................................................... 68
Marketing Programs

White Guide – Chapter 4: Marketing Programs
Rev. 12/20
Page 54 of 405
Chapter #4 Marketing Programs
The general term “marketing programs” is used in this Chapter to describe activities that promote Pfizer
products by providing HCPs or consumers with educational, scientific, and clinical information consistent
with FDA regulations. Marketing programs include speaker programs, symposia, congress and convention
exhibits and displays, and any other activities designed to promote Pfizer or its products. Pfizer marketing
programs, including those executed by an advertising agency or other vendor working on Pfizer’s behalf,
must adhere to FDA regulations and other rules governing promotion and must be approved by the relevant
brand Review Committee (RC). This Chapter also includes information about “quality programs,” which
may not be used to promote products. For more information on the development of promotional materials
used as a part of a marketing program, see White Guide Chapter 2: Advertising and Promotional Materials.
This Chapter is relevant to all Pfizer Marketing colleagues and other colleagues who are responsible for
developing and executing speaker programs and other marketing initiatives. Non-compliance with these
policies puts the Company at risk and can subject Pfizer colleagues to disciplinary action up to and including
termination of employment.
•
Pfizer marketing programs, including those executed by an advertising agency or other vendor
working on Pfizer’s behalf, must adhere to FDA regulations and other rules governing
promotion and must be approved by the relevant Review Committee.
•
The main objective of all speaker programs must be to meet an educational need by providing
truthful and non-misleading, scientific and educational information consistent with FDA
guidelines on the appropriate utilization of Pfizer products and/or on relevant disease areas.
•
Each brand team must follow the “Speaker Program Needs Assessment Guidance” to obtain
approval and funding for the use of speaker programs as a promotional tool.
•
Pfizer policy requires that speakers engage attendees at an external venue for a minimum of
45 minutes, inclusive of Q&A, or a minimum of 30 minutes for programs inclusive of Q&A in
an in-office setting. Marketing must be mindful of these requirements when developing
content in order to ensure that a sufficient amount of content is provided to support program.
duration requirements.
Key Points to Ensure Compliance
Introduction

White Guide – Chapter 4: Marketing Programs
Rev. 12/20
Page 55 of 405
A speaker program is a promotional activity controlled by Pfizer in which a speaker (typically an external
HCP) presents educational information on a product, disease state, or other healthcare topic, consistent
with Pfizer’s policies on advertising and promotion, to a group of invited HCPs or other appropriate
attendees. Even though an external individual is engaged to speak, Pfizer is responsible for the conduct
and content at speaker programs since the FDA considers speakers to be representatives of Pfizer. This
section focuses on speaker programs for HCPs. For more information on speaker programs with a
consumer audience, see White Guide Chapter 12: Promotional Interactions with Consumers.
• Speakers may only be selected based on their expertise, credentials, and ability to
communicate with the target audience.
•
Prior to providing any speaking services, all speakers must be screened as part of a vetting
process and must have a signed agreement with Pfizer which documents the fair market value
rate to be paid.
•
Prior to engaging in any speaker program, all new speakers are required to complete training
on: (1) the brand's core product or topic slide kit (as applicable); and (2) Pfizer compliance
policies (annually).
•
Brand teams should design, review, approve, and conduct Customer Engagement Programs
in compliance with Corporate Policy (CP) #902: Management of Safety Information for CEPs
and Corporate Policy (CP) #902a: Management of Safety Information for CEPs Procedure.
•
At many third--party meetings and conventions, Pfizer may pay for space for an opportunity to
promote its products (or, in some cases, to promote Pfizer), but Pfizer must not pay more than
fair market value for the opportunity.
Copay savings programs,discount programs, direct purchase programs, voucher
programs and free trial programs (collectively “Savings and Free Trial Programs”), and
other similar programs offered by U.S. teams (including brand teams and non-brand teams,
such as the U.S. Trade Group) must be developed and implemented in accordance with
Chapter 19 of the White Guide: Savings and Free Trial Programs and the relevant standard
operating procedure.
Key Points to Ensure Compliance
Speaker Programs

White Guide – Chapter 4: Marketing Programs
Rev. 12/20
Page 56 of 405
Speaker Programs are a valuable way to present information on products, a disease state related to our
products, or other healthcare topics to a group of HCPs and/or other appropriate attendees as applicable
under local laws, regulations and industry codes. However, Speaker Programs also present a risk to Pfizer.
Transfer of value to an HCP, such as speaker fees or meals to attendees, could be perceived as an attempt
to improperly influence prescribing decisions. Presentation of inaccurate information (e.g. downplaying
safety issues) can result in perceived or actual patient harm. Pfizer is responsible for Speaker Program
activities conducted on its behalf, including all information the speaker presents, any payments related to
the program, attendance by appropriate attendees, and the venue. As a result, minimum standards have
been developed to ensure consistency across U.S. businesses:
• A legitimate business purpose exists for both individual programs and a brand’s Speaker
Program strategy
• Speaker selection is based on qualification and experience
• Appropriate HCP attendance
• Appropriate materials and content
• Appropriate event execution
• Appropriate expenditures are made
• Appropriate transparency of transfers of value
For more information regarding Global Speaker Program Policy minimum standards, please click on
the link.
If a brand team wishes to conduct speaker programs, it must coordinate with Legal, Compliance, and
Medical to prepare a Speaker Program Needs Assessment (SPNA). The SPNA must be approved and
submitted along with the brand team’s request for funding (typically as part of their proposed Operating
Plan for the upcoming year) following the requirements and processes outlined in the Speaker Program
Needs Assessment Guidance (SPNA Guidance). Given the heightened risk associated with speaker
programs, Brand Teams should first consider whether other promotional strategies with lower compliance
risk would be sufficient to accomplish the Brand Team’s educational goal. In the SPNA, each speaker
program series must set forth a valid educational objective (as defined in the SPNA Guidance) tied to a
defined event in the product’s lifecycle or supporting data to support the need to conduct the speaker
program series and the proposed volume of programs. In general, the goal of all speaker program initiatives
may only be to meet an educational need by providing truthful and non-misleading, scientific and
educational information on the appropriate utilization of Pfizer products and/or on relevant disease states.
It is against Pfizer policy to design a speaker program strategy for the purpose of inducing speakers
to prescribe Pfizer products or to affect their placement on a formulary.

White Guide – Chapter 4: Marketing Programs
Rev. 12/20
Page 57 of 405
Sales and Marketing can both plan speaker programs, although programs for most brands are more
typically executed by Sales. All speaker programs, regardless of whether they are Marketing programs or
Sales programs, must be implemented and executed in accordance with the SPNA and Pfizer policies and
procedures.
Speaker Programs
If a Pfizer Sales or Marketing colleague initiates a speaker program, what
responsibilities does he or she have?
Regardless of who funds the event (Sales or Marketing), the program host is
responsible for the overall compliant management of the event. Generally, the Sales
or Marketing colleague chooses an appropriate venue and presentation topic (from
the list of RC-approved topics in Centris), selects an appropriate speaker, and contacts
that speaker. The program host must review Pfizer’s speaker policies and the
speaker’s slide deck with the speaker prior to the event to ensure that the speaker
understands that he or she must present the entire deck in accordance with the
product’s approved labeling and is using an approved slide deck without any
additional, unapproved slides. Colleagues may e-mail slide decks to the speaker for
the sole purpose of the pre-program review discussion with the speaker only if the
speaker is not able to download the deck. The slide deck in this instance must be
already RC-approved, locked, and available in Centris. The program host must
monitor the program, make any needed corrections or clarifications, and identify any
potential compliance violations that occurred at the program as part of the Centris
close-out process. For more information on program host responsibilities when
conducting a speaker program, see Orange Guide Chapter 9: Speaker Programs for
HCPs.
Content Development
All speaker program initiatives must be aligned to an approved SPNA which identifies the legitimate
educational objectives (as defined in the SPNA Guidance) for a proposed speaker program series.
Marketing, with input from Medical, is responsible for developing speaker program content which must be
designed to meet the educational objectives identified in the SPNA. Examples of legitimate educational
needs are:
• A gap in knowledge about a Pfizer product or related disease state within an appropriate target
audience, as supported by appropriate data or objective information; or
• Promotional education for HCPs about a new product, new indication, significant change to a
product’s risk/benefit profile, or significant new safety or efficacy data.
If there is no identifiable legitimate educational need for the speaker program initiative – for example, in
cases in which the information is already well known and understood by the target audience – then it may

White Guide – Chapter 4: Marketing Programs
Rev. 12/20
Page 58 of 405
not be appropriate to include the initiative in the SPNA toto execute the initiative. For example, if the product
has been on the market for many years and HCPs are generally familiar with the information proposed to
be presented and there is no data or information demonstrating a need for more education, then the
likelihood of identifying appropriate audiences for programs and, therefore, an unmet educational need
decreases. If a Brand Team has significant changes to their speaker program strategy (i.e. new information
or new deck that will create a significant increase in funding or number of programs) the SPNA will need to
be amended and submitted in GCMA for review by Legal, Compliance and Medical.
All speaker program content must be reviewed and approved by the relevant RC, be designed to meet the
educational objective identified in the SPNA and comply with Pfizer policies on advertising and promotion.
All speaker program decks must also include a mandatory introductory compliance slide which notifies
attendees of certain key Pfizer speaker program policies (e.g., speakers are presenting on Pfizer’s behalf;
content is required to be consistent with FDA-approved labeling; etc.). Even when Pfizer hires a third-party
vendor to assist with the development or execution of a program or series of programs, Pfizer remains
responsible for the content and message.
Speakers must use only Pfizer RC-approved slides when speaking on behalf of Pfizer, and the slides used
must be approved for the audience (HCPs or consumers). Pfizer policy requires that speakers engage
attendees for a minimum of 45 minutes, inclusive of Q&A, for external venue programs, or a minimum of
30 minutes for programs in an in-office setting. Marketing colleagues must be mindful of these requirements
when developing content in order to ensure that a sufficient amount of content is provided to support
program duration requirements. For more information on content development, see White Guide Chapter
2: Advertising and Promotional Materials.
Pfizer policy prohibits speakers from modifying slides or inserting their own slides (including introductory,
speaker bio, case study, and disease state slides). In limited circumstances, a speaker may present slides
that are not contained in the standard approved speaker deck so long as RC approval of the speaker’s
slides is received prior to the speaker program. All slides for which a speaker seeks RC approval must be
consistent with product labeling, accurate and truthful, supported by substantiated and scientifically-sound
data, and appropriately balanced with information on both benefits and risks.
Speaker Programs Topics and Invitations
As part of the RC approval process, brand teams are required to provide a speaker program topic name
for each presentation. This topic name typically mirrors the title within the slide deck itself. The topic name
is also used to populate various materials and systems associated with speaker programs, including
program invitations generated on behalf of colleagues hosting speaker programs, the program name that
displays in Centris, as well as logistical communications used by the scheduling vendor when working with
both speakers and Sales Colleagues.

White Guide – Chapter 4: Marketing Programs
Rev. 12/20
Page 59 of 405
Brand teams should generally avoid using product names (either branded or generic) in speaker
program topics, since such references can trigger additional legal and regulatory requirements which
Pfizer’s systems and processes are not routinely set up to manage. To be clear, although a speaker
program title reflecting a product name (e.g., “Calmia: A Treatment for Mild Anxiety”) may be appropriate
as part of a slide deck containing balance and Important Safety Information, such a title could constitute an
unbalanced product claim if it were populated into a stand-alone branded speaker program invitation that
lacks Important Safety Information or is not accompanied by a PI.
If a brand team believes there is a compelling justification to use a product name in a speaker program
topic/invitation (e.g., in the case of a new product launch), please discuss the matter beforehand with your
team attorney, Regulatory, and the Meetings & Events (M&E) team to ensure that all legal, regulatory,
and operational requirements are satisfied.
Regarding development and finalization of all program invitations for Marketing-Led Programs, including
when working with an agency, it is imperative to adhere to the requirements outlined in the “Preparing and
Distributing Invitations” section in Orange Guide Chapter 9: Speaker Programs for HCPs.
Speaker Recruitment & Contracting
A list of active and appropriate Pfizer speakers for given products and topics will appear in Centris. These
are speakers that: (1) have been vetted by a third-party against an objective set of criteria that reflects
evolving industry standards and accounts for reputational risks; (2) have been assessed based on their
qualifications, license status and presence on internal and exclusion lists; (3) have a signed contract with
Pfizer; (4) have completed compliance training; (5) have completed training on a core product or topic slide
kit (either live or online, as applicable); and (6) have not yet reached Pfizer’s annual promotional speaker
payment cap or caps on frequency of utilization by individual sales representatives or sales district
members. An HCP’s promotional speaking contract with Pfizer is valid for one year and is typically renewed
automatically.
The two main analyses relevant to the speaker recruitment process are: (1) determination of the number of
speakers reasonably required to execute the expected number of speaker programs (in order to determine
if new speakers need to be recruited for the initiative in addition to speakers already trained and active);
and (2) identification of the qualifications and expertise of the speakers necessary to execute the planned
programs.
Speakers may only be selected based on their expertise, credentials, and ability to communicate with the
target audience. Speakers may not be selected to: establish, maintain, or improve a relationship with the
speaker; gain or improve access to the speaker; or reward past prescribing or induce future prescribing.
Although the speaker should have experience with the product or disease state, prescribing volume may
not specifically be considered when selecting a speaker. In addition to doctors, speakers may be nurses,

White Guide – Chapter 4: Marketing Programs
Rev. 12/20
Page 60 of 405
pharmacists, or any other person with the requisite subject matter expertise and credibility to speak on a
particular topic. Consult the M&E team for more information on speaker nominations and validation.
Prior to providing any speaking services, all speakers must have a signed agreement in place with Pfizer
that documents the speaker’s fair market value (FMV) payment rate. FMV and appropriate tier status will
be determined for each speaker during the nomination and vetting process. Speakers enter the speaker
bureau with an annual speaker payment cap of $50,000. Brand teams, with mandatory Medical
consultation, must review and approve any Annual Cap Reclassification increase request prior to submitting
the request through M&E to Legal and/or Compliance for final approval.
Only speakers may be paid in connection with speaker programs; attendees may not be compensated in
any manner. Speakers may also be reimbursed for reasonable business-related expenses associated with
speaking at the program, such as out-of-pocket lodging, transportation, or parking costs, in accordance
with the U.S. Consultant – Speaker Business Travel and Expense SOP. Pfizer’s HCP Payment Disclosure
and State Reporting SOP applies to all speaker and consulting fees, travel expenses, meals, and other
items of value provided in connection with speaker programs.
Speaker Training
Prior to engaging in any speaking engagements, all speakers are required to complete training on: (1) Pfizer
Promotional Speaker Compliance Guidelines Training (annually); and (2) the brand’s Core Product or Topic
Training Slide Kit, as applicable. Accordingly, all Pfizer brands that execute speaker programs must create
Core Product and/or Topic Training Slide Kits that cover the key aspects of the product or topic, including
Important Safety Information.
Depending on factors including the specific needs of the brand team, a speaker may complete training
either online via Centris, via WebEx, or live in-person. In limited instances, separate individual training may
be conducted by a Field Medical Director (FMD) for speakers who cannot complete training through other
available means – consult your team attorney for guidance. If a speaker is paid for participation in training,
he or she will be required to complete at least two speaker programs on the relevant product within a year
of the paid training. For more information on speaker training, see Training resources available on the
Speaker Programs tab in Global Policy Xchange on GCO on Demand. Speaker training sessions should
be held in venues and locations (typically limited to the speaker’s country of practice unless there are
security or logistical concerns) that are appropriate and conducive to informational communication and
training about medical information. Specifically, resorts are not appropriate venues.
Program Execution
Regardless of whether it is a Marketing- or Sales-led program, all speaker programs must be executed
consistent with the requirements outlined in Orange Guide Chapter 9: Speaker Programs for HCPs and
Orange Guide Chapter 16: Consumer, Patient, and Employee Interactions.

White Guide – Chapter 4: Marketing Programs
Rev. 12/20
Page 61 of 405
Pfizer brand teams are often provided the opportunity to promote Pfizer products by paying for promotional
opportunities at third-party meetings and conventions. These opportunities must always be Fair Market
Value (FMV) and satisfy our tangible benefits requirements Common promotional opportunities include, but
are not limited to:
• Symposia Programs/Product Theaters;
• Exhibit/Booth Display Space;
• Advertisement Space in Conference Brochure;
• Delegate Bag Inserts.
Financial support in exchange for these opportunities can occur at a variety of venues and programs, but
the key principle is that Pfizer is paying for the space or opportunity to promote its products (or, in some
cases, to promote Pfizer) and must not pay more than fair market value for the opportunity.
There are several factors to consider when deciding fair market value with respect to promotional
opportunities. Examples include the following:
• The opportunity to promote Pfizer or a Pfizer product to a relevant and appropriate population of
HCPs or consumers;
• The opportunity for Pfizer colleagues to interact with conference attendees;
• The length of time given to Pfizer to exhibit, display, speak, or interact;
• The physical location of the table or booth in relation to those attending an event; and
• The extent of the internet traffic associated with a conference organizer’s website.
In addition, Pfizer should be sure that other companies providing financial support in exchange for
promotional opportunities are charged the same amount for the same type of opportunity. Often, the event
brochure lists the levels of support opportunities available and describes the space and services that are
available at each level. This type of brochure should accompany the request for financial support whenever
possible because it helps to validate the fair market value of the opportunity. Follow the procedures outlined
in the Funding Requests for Not-for-Profit Organizations SOP (External Funding Requests SOP) to facilitate
funding for promotional opportunities at third-party meetings and conventions. All promotional materials
used at a marketing program, such as exhibit panels, professional advertising, and consumer materials,
must be approved by the appropriate brand RC.
Symposia Programs
Pfizer defines symposia as Pfizer-initiated and/or controlled live events held in conjunction with a congress
or convention. (Note that external organizations may use the term “symposia” for other types of events;
Promotional Opportunities at Third-Party Meetings and Conventions

White Guide – Chapter 4: Marketing Programs
Rev. 12/20
Page 62 of 405
however, the preceding definition is used for purposes of this Pfizer policy.) The content is typically
customized for the event and delivered by a Pfizer-paid faculty speaker and is subject to RC approval.
Attendees are not paid and are generally not asked to provide formal feedback.
• Symposia may be open-door, at which any congress/convention participant may attend, or
closed-door, invitation-only events. For both, attendance is controlled with logistic support
provided by meeting planners on the M&E team.
Open-door symposia take place at third-party events, such as congresses or conventions, with logistical
support provided by Convention Housing & Logistics Meeting Manager on the Meetings and Events Team,
and in partnership with the Global Congress Center of Excellence (CoE) group within Global
Commercial Operations (GCO). Closed-door symposia may coincide with, but typically do not take place
at, third-party events such as congresses or conventions, with logistical support provided by the M&E team.
There are three types of symposia:
• Promotional Symposia (also commonly known as “product theaters”) are programs where
product-specific information is provided consistent with the product label;
• Non-promotional Symposia are symposia where no promotional content or product-specific
information is mentioned; the intent is to foster unbranded disease awareness; and
• Scientific-exchange Symposia are symposia where non-promotional scientific or medical
information about an unapproved product (e.g., a pipeline product) may be presented. Marketing
colleagues are not permitted to execute these programs and thus they are not discussed in this
Chapter.
Initiating Symposia Programs
The Global Congress CoE and Marketing teams are responsible for determining the annual Pfizer congress
and convention open-door symposia plan. Any symposium, however, can be proposed, initiated, and
conducted by any appropriately trained Pfizer colleague (“Project Owner”) responsible for the project
management of symposia. The Project Owner must document the need for a symposium on a Business
Rationale Form and follow the rest of the steps required by the HCP Engagements SOP.
Except for scientific-exchange symposia, fees paid to speakers at other symposia are included in, and
subject to, Pfizer’s annual speaking fee cap (also applicable to traditional speaker programs). Colleagues
wishing to engage a speaker for a symposium event should first check the status of the speaker’s cap.
Content Development
The content of a symposium, which includes any promotional materials that will be presented or handed
out at the event, must be RC-approved. The Project Owner and the vendor are responsible for ensuring
the symposium faculty follows Pfizer’s content requirements and processes. For symposia managed with

White Guide – Chapter 4: Marketing Programs
Rev. 12/20
Page 63 of 405
the support of the Global Congress CoE: Convention Housing & Logistics team, the Symposia Specialists
are responsible for ensuring that all speakers have received compliance training.
Invitations, Logistics, and Meals
The Project Owner and the Symposia Specialist or M&E Manager (as applicable) are responsible for
logistics related to the program. Travel and lodging expenses may be provided for Pfizer speakers but not
for attendees. Modest meals and refreshments may be provided, where appropriate. These and any other
items of value conferred to certain HCPs are subject to disclosure in accordance with Pfizer’s HCP Payment
Disclosure and State Reporting SOP and may also be subject to disclosure or further restrictions in
accordance with applicable state law. HCP attendees who are licensed to practice in Minnesota or
Vermont must not be provided a meal by Pfizer at these programs (although coffee or other light
snacks at the convention/congress booth are permissible for Vermont HCPs). For closed-door
symposia events where a meal will be provided to all attendees, potential invitees should be screened in
advance using the HCP License Lookup Tool on GCO Policy Xchange on GCO on Demand so as not to
invite those holding an MN or VT license. For additional information, see White Guide Chapter 15: State
Laws: HCP and Government Employee Restrictions and White Guide Chapter 18: Meals, Educational
Items, and HCP Payment Disclosure.
Exhibit/Display & Other Advertising Opportunities
Funding for an exhibit or display or other promotional opportunity at a congress or convention must not
be greater than the fair market value of the opportunity. Likewise, brand teams cannot bypass the grant
process administered by Global Medical Grants (GMG) by funding a promotional opportunity when the
funding request is really for non-promotional aspects of a program. Promotional and non-promotional
funding must always be separated, easily identifiable, and able to be tracked for auditing purposes. In
addition, if an opportunity involves the distribution or provision of any items to conference attendees, brand
teams may only fund opportunities involving PhRMA Code compliant educational items.
The process for funding sponsorship opportunities is outlined in the Funding Requests for Not-for-Profit
Organizations SOP (External Funding Requests SOP), which is described in more detail in White Guide
Chapter 7: Support of External Organizations. Applicable Foreign Corrupt Practices Act due diligence must
also be conducted by the FRF owner for sponsorships involving non-U.S. third-party congresses,
conventions, and open-door symposia. In addition, all requests from managed care customers, regardless
of amount, must be reviewed and approved by Legal before the date of the event and before Pfizer may
pay for the exhibit/display or undertake any activities associated with the exhibit or display opportunity.
External Websites and Other Digital Activities

White Guide – Chapter 4: Marketing Programs
Rev. 12/20
Page 64 of 405
Like other forms of promotion, the FDA regulates Pfizer’s use of the Internet to promote its products in the
United States. This includes PfizerPro and product websites, as well as social media, banner, and other
Internet advertisements. For more information, see White Guide Chapter 2: Advertising and Promotional
Materials. Detailed information on the requirements of Internet promotion, including on social media
platforms, such as YouTube, Twitter and Facebook, as well as search engine optimization and search
engine marketing can be found at the Digital Review Team site.
Pfizer has legal, regulatory, and ethical responsibilities to monitor the safety profile of its products through
the collection, evaluation, and timely reporting of safety information to regulatory authorities. To meet these
responsibilities, colleagues are required to submit Reportable Safety Information within 24 hours of
becoming aware of any such information concerning Pfizer products, as stated in CP #903: Your
Responsibility to Report Information about the Safety, Quality, and Performance of Pfizer Products Policy
and associated training “Your Reporting Responsibilities: Monitoring the Safety, Performance and Quality
of Pfizer Products” (YRR).
CEPs are broadly defined as Pfizer-sponsored programs that allow for two-way communication between
Pfizer and its customers for Pfizer to gain insight from, or provide information or support to, its customers.
Examples of CEPs are Pfizer-sponsored programs, such as: patient support programs, market research,
disease awareness and screening programs, Pfizer-sponsored digital media with open text fields, and
Pfizer-sponsored customer outreach programs. Because CEPs are a potential source of Reportable Safety
Information, CEPs require a process to identify and report such information within required timelines.
CEP Program Owners are accountable for ensuring that the design, review, approval, and conduct of the
CEP comply with CP #902: Management of Safety Information for CEPs Policy and CP #902a:
Management of Safety Information for CEPs Procedure, which also contain more detail on the types of
programs that may be considered CEPs. See the CEP Resource Center at http://CEP.Pfizer.com for tools
and other information.
Pfizer is committed to encouraging patients to talk to their doctors about available treatment options and to
helping patients better afford Pfizer medications. As part of this commitment, Pfizer has developed various
copay savings programs, discount programs, direct purchase programs, and voucher programs and free
trial programs (collectively, “Savings and Free Trial Programs”) to help patients access or evaluate the
safety and tolerability of their prescribed Pfizer medications. All communications about Pfizer Savings and
Free Trial Programs are subject to RC approval.
Customer Engagement Programs (CEPs)
Savings and Free Trial Programs

White Guide – Chapter 4: Marketing Programs
Rev. 12/20
Page 65 of 405
Even though such programs are designed to benefit patients, if not carefully developed and implemented,
they may raise a number of significant legal risks (such as under federal and state kickback laws, consumer
protection laws, the “Best Price” Medicaid Drug Rebate Statute, state contract law, and state pharmacy
laws). Savings and Free Trial Programs offered by U.S. teams (including brand teams and non-brand
teams, such as the U.S. Trade Group) must therefore be structured and implemented in accordance with
Chapter 19 of the White Guide, Savings and Free Trial Programs.
In addition to Savings and Free Trial Programs, Pfizer also contracts with third-party vendors to operate
product-specific Hubs that support patients with certain prescription access and treatment needs. Hub
offerings include, for example, reimbursement support (benefits verification, prior authorizations, appeals),
product and disease state education, and nursing support. As with Savings and Free Trial Programs, Pfizer
Hub services are designed to support patients, and Pfizer structures these programs to provide only limited
support (in accordance with applicable Department of Health and Human Services Office of Inspector
General guidance). Communications about Pfizer Hubs and their services are subject to RC approval. For
more information about Hub operations and Pfizer’s compliance efforts, please refer to Chapter 10 of the
White Guide.
Marketing materials that reference programs operated by Global Health & Patient Access (formerly
Corporate Responsibility) (e.g., Pfizer Patient Assistance Programs (PAPs), Pfizer Institutional Patient
Assistance Program (IPAP), and Pfizer RxPathways (PRxP), including implementation guides, must be
created in line with the below requirements:
• The PRxP team will make available through PROMOs Prime a set of unbranded PRxP materials
that can be used by Marketing for purposes of discussing PRxP. The team will also make limited
materials available on Pfizer PAPs.
• Marketing teams may include in their marketing materials the PRxP logo and PRxP pre-approved
taglines and logo lock-ups without requiring the approval of the PRxP RC. The placement of the
logo and tagline must be either at the bottom of the piece or in an area where it can be separated
from the brand, therapeutic area or other messaging in the materials. Marketing teams should
send samples of these materials to PRxP to keep on file.
• If a Marketing team wishes to include PRxP and/or Pfizer PAPs/IPAP information beyond the
standard PRxP logo and tagline, those materials must be reviewed by the PRxP team and require
the approval of both the PRxP RC and the brand, therapeutic area, or other relevant RC that
normally approves these materials.
• Brand RCs must review and approve materials related to product-specific patient support
hubs. Content that mentions services managed by Global Health & Patient Access (e.g. patient
Hub Support Programs
Pfizer Patient Assistance Programs and RxPathways

White Guide – Chapter 4: Marketing Programs
Rev. 12/20
Page 66 of 405
assistance programs, reimbursement support services) must be reviewed by a member of the
PRxP Team, and PRxP RC if the content deviates from standard language provided. At a
minimum, such materials must: (i) accurately and transparently describe the program offerings;
(ii) clearly outline eligibility criteria; (iii) not guarantee coverage; (iv) not include unsubstantiated
claims comparing Pfizer and competitor programs; and (v) not position the program as an
inducement or reward for prescribing the relevant medication.
A note on Independent Charity Patient Assistance Programs (ICPAPs)
With the exception of certain colleagues engaged in reimbursement support and approved in advance by
Legal, Commercial and Medical Affairs Colleagues must not discuss the following with Healthcare
Professionals (HCPs) or patients: specific ICPAPs; the availability of funding in relevant disease states from
ICPAPs; or that ICPAPs can overcome copay barriers. Approved reimbursement support colleagues may
provide to HCPs materials approved by the relevant Brand RC that discuss generally the range of
assistance options to which Pfizer RxPathways (or the relevant product Hub connects patients (including
information about ICPAPs). See Corporate Policy and Procedure #803, Contributions to ICPAPs, for
additional information.
Quality programs refer to RC-approved activities that offer information and other resources relating to
therapeutic areas, disease states, and patient care to healthcare organizations, such as medical groups,
long term care, health maintenance organizations (HMOs), U.S. Department of Veterans Affairs (VA),
U.S. Department of Defense (DoD), and pharmacy benefit managers. Quality programs focus on
addressing the overall quality of healthcare rather than promoting Pfizer products.
Under Pfizer standards, quality programs can be used to support the following objectives:
• Enhance the quality of patient care or clinical research;
• Enhance Pfizer’s corporate image, visibility, name recognition, and general goodwill;
• Offer free information with broad and general applicability to the target audience; and
• Provide scientifically sound information.
Quality programs improve patient care by providing customers with information about, for example, quality
accreditation standards, HCP’s patient interaction skills, and management of medical conditions.
Quality programs must never be offered in exchange for increased prescribing or improved formulary status.
Although customers may alter prescribing habits based on information provided at a quality program, Pfizer
employees must never require such changes as a condition of the program.
Pfizer’s quality programs may never be offered to:
Quality Programs

White Guide – Chapter 4: Marketing Programs
Rev. 12/20
Page 67 of 405
• Establish or improve Pfizer’s relationship with that HCP or institution;
• Gain or improve access to an HCP or institution;
• Reward past prescribing or induce future prescribing;
• Influence an upcoming formulary decision; and/or
• Offer an implied discount on the price of Pfizer products.
Every quality program must receive approval from the relevant RC before it is made available publicly.
The CAN-SPAM Act of 2003 establishes an opt-out framework for commercial e-mail and pre-empts state
commercial e-mail statutes. The Act is enforced by the Federal Trade Commission (FTC), state Attorneys
General, and Internet Service Providers (ISPs).
All commercial e-mail must include the following:
• A clear and conspicuous notice that the consumer can opt-out of receiving future e-mails.
• An Internet-based mechanism for opting out, such as a reply e-mail address or a link to a
website. This mechanism must remain in effect for at least 30 days after the commercial e-mail is
sent and an opt-out request must be honored within ten (10) business days of receipt. Brand
teams are not allowed to share or sell an e-mail address of someone who has opted out.
• A clear and conspicuous identification that the e-mail is an advertisement. The Act does not
require specific language, so teams may choose how to describe the e-mail as an advertisement
(e.g., use of words such as, promotional, marketing, announcement or advertisement are all
acceptable). Commercial e-mail sent to a consumer who has specifically opted-in to receive
commercial e-mail from the Marketer does not need to be identified as an advertisement.
• The sender’s physical postal address. The Direct Marketing Association requires that the
address be a street address.
There is an exception from these requirements for certain specified transactional e-mails, including e-mails
that: (i) facilitate or confirm an agreed-to commercial transaction; (ii) give warranty, recall, safety or security
information; (iii) give information about a change in terms, features, or account balances for ongoing
relationships; (iv) provide information about employment relationships or benefits; or (v) deliver already
purchased goods or services. A transactional e-mail may contain advertising if the primary purpose of the
e-mail is transactional, not promotional. E-mails that are not primarily commercial or transactional (other”
e-mails) are also exempt from the above requirements.
The Act prohibits false or misleading information in the “From” and “Subject” lines of company e-mails. The
“Subject” line should accurately reflect the content of the e-mail and the “From” line should accurately
indicate the sender. This requirement can be challenging for some affiliate marketing and “forward to a
Commercial E-mails

White Guide – Chapter 4: Marketing Programs
Rev. 12/20
Page 68 of 405
friend” referral e-mails. There are also special rules for multi-party promotional content e-mails that enable
one entity to be deemed the sole sender for disclosure and opt-out purposes. Consult with your team
attorney if your program involves referral or multi-party e-mails.
The Act also prohibits falsifying header information, harvesting e-mail addresses, opening multiple e-mail
accounts using false information and using open relays to transmit commercial e-mail. It pre-empts state
commercial e-mail laws but does not pre-empt state fraud and trespass laws that can be applied to
commercial e-mail.
Colleagues who are responsible for sending commercial e-mail should coordinate with GCO’s
HCP/Patient Engagement Center and their team attorney to ensure compliance with all applicable
laws and regulations.
• For speaker programs, consult the Meetings & Events (M&E) team, and for conventions /
congresses / symposia consult the Global Congress CoE Leads.
• Speaker Programs tab on GCO Policy Xchange on GCO on Demand.
• Orange Guide Chapter 9: Speaker Programs for HCPs.
• Orange Guide Chapter 16: Consumer, Patient, and Employee Interactions.
• Co-Pay Savings and Free Trial Programs page on GCO Policy Xchange on GCO on Demand:
o Savings and Free Trial Policy and SOP.
o New Limitations Regarding Free Trial Voucher Programs (Restrictions on Free Trial Vouchers –
July 2011).
o Massachusetts Update on Loosened Copay, Coupon, and Free Trial Voucher restrictions.
• HCP Consulting guidelines and resources available on the ENGAGE and HCP Engagements tabs on
GCO Policy Xchange on GCO on Demand.
• Digital Review Team E-mail Guidelines.
• CEP Resource Center at Customer Engagement Programs home page at http://CEP.Pfizer.com.
• White Guide Chapter 2: Advertising and Promotional Materials.
• White Guide Chapter 5: HCP and Government Official Consulting Engagements.
• White Guide Chapter 7: Support of External Organizations.
• White Guide Chapter 12: Promotional Interactions with Consumers.
• White Guide Chapter 15: State Laws: HCP and Government Employee Restrictions.
• White Guide Chapter 18: Meals, Educational Items, and HCP Payment Disclosure.
• Refer other questions to your team’s Regulatory colleague or team attorney.
For More Information

Rev. 12/20
Page 69 of 405
CHAPTER #5 – HCP AND
GOVERNMENT OFFICIAL
CONSULTING ENGAGEMENTS

White Guide – Chapter 5: HCP and Government Official Consulting
Engagements
Rev. 12/20
Page 70 of 405
Chapter
#5
HCP AND GOVERNMENT OFFICIAL
CONSULTING ENGAGEMENTS
CONTENTS
Introduction.................................................................................................................................................. 71
Key Points to Help Achieve Compliance .............................................................................................. 73
Consulting Engagement Controls Overview ............................................................................................... 75
Requirements for a Bona Fide Consulting Arrangement ............................................................................ 76
Legitimate Need for Services ............................................................................................................... 76
Consultant Qualifications ...................................................................................................................... 77
Consultant Screening Requirements .................................................................................................... 77
Restricted Markets ................................................................................................................................ 78
Export Controlled Technology .............................................................................................................. 78
Written Agreement ................................................................................................................................ 80
Meeting Venue...................................................................................................................................... 81
Output / Deliverables ............................................................................................................................ 81
Reimbursement of Expenses ............................................................................................................... 82
Types of Consulting Arrangements ............................................................................................................. 82
Advisory Board Meeting ....................................................................................................................... 82
Live Speaker Training Meeting ............................................................................................................. 83
Focus Groups and Market Research ................................................................................................... 83
Preceptorships and Mentorships .......................................................................................................... 84
Retaining Government Employees as Speakers or Consultants ................................................................ 84
Non-U.S. HCPs and Government Officials ........................................................................................... 84
U.S. State and Federal Government Officials ...................................................................................... 86
Retaining Government Employees in Connection with Their Official Duties ................................. 86
Retaining Government Employees Outside of Their Official Duties .............................................. 86
For More Information ................................................................................................................................... 88
HCP and Government Official Consulting Engagements

White Guide – Chapter 5: HCP and Government Official Consulting
Engagements
Rev. 12/20
Page 71 of 405
Chapter #5 HCP and Government Official
Consulting Engagements
Pfizer enters into consulting engagements with Healthcare Professionals (HCPs) for a range of services
including business counseling, Pfizer colleague training, external HCP education and training, pre-clinical
and clinical program design, post-launch regulatory compliance assistance, and marketing program
development, among others. For this Chapter HCP is defined as members of the medical, dental, pharmacy
and nursing professions and anyone else, who may in the course of their professional activities recommend,
influence, manage or directly prescribe, supply, administer, or buy any medicines; researchers and
investigators; and any other individuals engaged in healthcare-related practices or employed by a
healthcare institution, as noted in the HCP Engagements SOP.
For U.S.-based business units, Corporate Affairs, and Medical colleagues, the HCP Engagements SOP is
applicable to most HCP and non-U.S. HCP and non-U.S. government official (GO) consulting
engagements. However, that SOP does not apply to Marketing and Sales speaker programs, clinical
services, and other activities subject to other policies (see the Scope section of the SOP for more
information). For U.S.- based WRDM, GPD, and Upjohn R&D colleagues (collectively, R&D colleagues),
HCP engagements in support of Pfizer’s Research and Development (R&D) activities are generally
subject to the policies and procedures set forth in in SOP 201: R&D HCP/GO Engagements & Interactions.
While all of Pfizer’s HCP engagement policies reflect the same core principles and the core requirements
are common across activity types, some of the specific requirements and controls detailed in this Chapter
relate most directly to engagements covered by the HCP Engagements SOP, which is applicable to the
U.S.-based business units noted above. Consult the relevant SOP for the specific requirements applicable
to a particular engagement.
Because these interactions potentially implicate federal and state anti-kickback laws and other U.S. and
international anti-corruption laws, it is important for Pfizer colleagues to establish that a proposed consulting
relationship is bona fide prior to engaging the consultant. Any HCP consulting arrangement must meet the
following requirements:
• The consultant is not a Restricted Party
1
, in a Restricted Market, or otherwise prohibited from being
engaged by Pfizer
2
;
1
See Corporate Policy (CP) #206, Compliance with Global Trade Control Laws
(http://policysource.pfizer.com/Corporate/PDFDocuments/206.GlobalTradePolicy.pdf).
2
Not on any applicable internal Pfizer exclusion lists; or lists of HCPs/GOs subject to state disciplinary
actions, state licensing suspension or revocation, FDA Warning Letters, Independent Oversight Committee
membership, or any international equivalent to the foregoing.
Introduction

White Guide – Chapter 5: HCP and Government Official Consulting
Engagements
Rev. 12/20
Page 72 of 405
• There is a legitimate business need for the services;
• Each consultant is selected based on his or her expertise and knowledge and not to gain access or to
influence prescribing habits;
• The number of consultants and duration of the engagement are appropriate to the business need;
• A written contract is executed that specifies the nature of the services and the basis of payment for
those services;
• The term of the agreement is for at least one year (unless a shorter term is approved by a Legal
colleague);
• The services are provided as outlined in the written contract; and
• Any compensation does not exceed Fair Market Value (FMV).
Consultants must provide an actual service. For example, passive activities, such as time spent merely
listening to a marketing presentation, are not considered bona fide services and are not compensable. You
must select consultants who possess experience or expertise relevant to the engagement. Consultants
should never be selected to influence or reward their prescribing or recommendation of Pfizer products or
to provide Pfizer with any other improper benefit or advantage. Consulting fee payments must not be
determined in a manner that takes account of the past, present, or future volume or value of business
generated by consultants for Pfizer. The written consulting agreement should specify that there is no
connection between the compensation provided and the prescribing of Pfizer products.
Your objective in entering into a consulting arrangement with an HCP must never be to:
• Establish or improve a relationship with the HCP;
• Gain or improve access to the HCP;
• Reward past prescribing;
• Induce future prescribing;
• Influence formulary decision making; or
• Gain any other improper business advantage.
Corporate Policy (CP) #207: Global Policy on Interactions with Healthcare Professionals (GPIHP) governs
relationships with HCPs, including interactions with physicians, nurses, pharmacists, and others, who
administer, prescribe, purchase, or recommend prescription medicines. The process for fair market value
analysis of HCP payments is described in the HCP Engagements SOP and discussed in detail in the US
Fair Market Value (FMV) – Consulting and Speaking SOP. The process for conducting meetings and
consultancy engagements with individuals who hold employment outside the U.S. is outlined in My Anti-
Corruption Policy and Procedures (MAPP). You should consult these SOPs, as applicable, to identify the
specific steps that are necessary to plan and execute a compliant consulting engagement. The GCO Policy

White Guide – Chapter 5: HCP and Government Official Consulting
Engagements
Rev. 12/20
Page 73 of 405
Xchange on GCO on Demand website also contains job aids, guidelines, and other useful documents to
help ensure a compliant consulting arrangement.
For R&D colleagues, the HCP engagements process is detailed in SOP 201: R&D HCP/GO
Engagements & Interactions and R&D’s Compliance CNTR contains guidance, tools and resources
conducting compliant interactions with the external scientific community. The Pfizer Enterprise Travel &
Meetings Americas team is generally responsible and should always be consulted for managing the
logistics for meetings that involve HCP consultants.
This Chapter summarizes certain key Pfizer policies regarding HCP and non-U.S. GO consulting
engagements and is relevant to all U.S.-based business units who are involved with initiating and
executing these engagements. Non-compliance with these policies puts the Company at risk and
can subject Pfizer colleagues to disciplinary action up to and including termination of employment.
All HCP/GO consulting engagements must meet all of the following requirements:
• Further a legitimate business need that has been adequately documented.
• Involve HCPs/GOs who are:
o
Selected based on documented expertise and knowledge that meets that business need;
o
Not selected to gain access or influence prescribing or recommendation of a product, nor
to promote off-label use (or make other impermissible claims); and
o
Cleared through the Restricted Party Screening (RPS) process (even if the engagement
is occurring solely within the United States);
▪
For HCPs/GOs processed through the ENGAGE system, automated RPS occurs in
ENGAGE.
Key Points to Help Achieve Compliance

White Guide – Chapter 5: HCP and Government Official Consulting
Engagements
Rev. 12/20
Page 74 of 405
Key Points to Help Achieve Compliance
▪ HCPs/GOs not processed through Engage Plus must be screened through the RPS
OnDemand Portal
3
by the colleague wishing to engage the consultant (or someone
else assigned to do so).
o Not on any applicable internal Pfizer exclusion lists or any lists of HCPs/GOs subject to
state disciplinary actions, state licensing suspension or revocation, FDA Warning Letters,
Independent Oversight Committee membership, or any international equivalent to the
foregoing.
• Be memorialized in a written contract that:
o Specifies the nature and scope of the services and the amount and basis of payment for
those services;
o Has a term of agreement for at least one year (unless Legal approves a shorter term); and
o For non-U.S. HCPs and GOs, includes required MAPP terms and conditions.
• Not involve a payment in excess of fair market value; and
• Not involve either of the following, unless required licenses or other authorizations have been
obtained, the issuance of which is not guaranteed, and written approval received from an
attorney in the Global Trade Controls Center of Excellence (GTC CoE).
o Activities in a Restricted Market, organizations or governmental entities from a Restricted
Market, or individuals ordinarily resident in a Restricted Market; or
o The exchange of Technology that is controlled for export (see CP #206 and
gtc.pfizer.com).
Additionally, Pfizer colleagues must ensure that:
• The output/work product of a consulting engagement is collected and retained, and for
engagements subject to the HCP Engagements SOP, it is documented how such output/work
product was used to aid the business;
• The output/work product is consistent with the terms of the consulting agreement; and
• Where a Business Rationale Form (BRF) is required by the applicable SOP, the engagement
was consistent with the relevant BRF (or ensure that a legitimate rationale for any significant
differences, as well as any approval thereof, are documented).
3
RPS OnDemand.

White Guide – Chapter 5: HCP and Government Official Consulting
Engagements
Rev. 12/20
Page 75 of 405
Key Points to Help Achieve Compliance
The GCO Meetings and Events (M&E) North America team oversees organization of meetings
involving HCP consultants. For additional guidance on engaging an HCP or GO, consult the HCP
Engagements tab on GCO Policy Xchange on GCO on Demand.
Pfizer has developed, implemented, and continued to maintain controls to manage HCP consulting
engagements, including:
• Annual Consultant Needs Assessment: For engagements subject to the HCP Engagements SOP,
on an annual basis, U.S. business unit Compliance, Global Product Counsel, and their respective
brand teams will develop Annual Consultant Needs Assessments (ACNAs) relating to each product
in accordance with each brand’s operating plan. Generally, an ACNA must be completed for all
marketed products and products within 18 months of anticipated approval date. Each ACNA must
identify the estimated number of, expenses associated with, and the business rationale for, HCP
consultant engagements and activities intended to occur during the year in connection with
government-reimbursed products. R&D SOP 201 does not require completion of an ACNA.
• Business Rationale Form Requirements: Prior to each engagement under the HCP Engagement
SOP (and certain engagements related to FDA-approved Pfizer products under R&D SOP #201), Pfizer
must ensure that a Business Rationale Form (BRF) is completed describing the justification for
retention of a consultant by Pfizer. The BRF must identify the business need for the services of the
consultant and must provide specific details including qualifications of the consultant(s) to be engaged,
the scope of services to be provided, and the expected work product/information to be generated from
the engagement. The relevant approver will review the BRF for consistency with Pfizer policy and with
the relevant ACNA if applicable. The team attorney will ONLY review BRFs that include at least one
US HCP and the project will have any impact on advancing US business directly or indirectly.
Explanation for any variance from the ACNA (if applicable) should be documented.
• Contract Requirements: Pfizer must execute written agreements with the consultants it engages prior
to work beginning. The agreement must describe the scope of work to be performed as well as the
consultant fees to be paid. Fees are determined based on a centrally managed rate structure that
represents fair market value. The agreement must also describe the compliance obligations of the
consultant, including consent to and cooperation with Pfizer’s public disclosure of payment to the HCP.
The agreement must also obligate the consultant to adhere to any disclosure requirements regarding
their relationship with Pfizer, such as requirements of any healthcare institution, medical committee, or
other medical or scientific organization with which the consultants are affiliated. If the consultant is a
non-U.S. HCP or Government Official, the My Anti-Corruption Policy and Procedures (MAPP) process
must be completed before the contract (with MAPP required clauses for consultant engagements) is
executed.
Consulting Engagement Controls Overview

White Guide – Chapter 5: HCP and Government Official Consulting
Engagements
Rev. 12/20
Page 76 of 405
• Work Product: Any work product created as a result of a consultant engagement must be collected,
retained, and assessed to verify consistency with what the consultant was engaged to provide/do, as
set forth in the BRF, if applicable. Other documentation of completion of services may be appropriate.
This assessment and verification must be documented in an Engagement End Document (EED).
The following section provides an overview of the key compliance requirements pertaining to the process
for engaging HCP consultants as outlined in the HCP Engagements SOP or R&D SOP 201.
Legitimate Need for Services
Because of the inherent kickback risk that many HCP consulting arrangements pose, a legitimate need for
services must always be established and documented. For engagements under the HCP Engagements
SOP (and engagements under R&D SOP 201 related to an FDA-approved product), Pfizer colleagues must
complete a BRF to document and obtain approval that a legitimate need exists for a proposed consultant
service. This involves:
• Identifying the business need to retain the consultant (e.g., the gap in knowledge, understanding, or
expertise that the consultant will be able to fill);
• Identifying the necessary and substantive services that the consultant will provide; and
• Describing how the output or deliverable(s) of the proposed arrangement will benefit Pfizer.
The relevant team attorney must review each BRF associated with any proposed consulting engagements
prior to the retention of consultants. The attorney will review BRFs for consistency with Pfizer policy and
with the relevant ACNA, if applicable, and will work with the team to ensure that any variance from the
ACNA has been appropriately documented.
Legitimate Need
A Marketing team would like to organize a series of four advisory board meetings with
various specialties to gain a better understanding how its pain medication is used in
different clinical settings. The team would like to engage 15 HCPs for each meeting
and intends to use the information to improve and tailor the promotional message for
each specialty. Is this an acceptable initiative?
Maybe. It is permissible to engage consultants to gain a better understanding of how
a promotional strategy or campaign may be received by HCPs. However, it is
important that such initiatives involve the minimum number of HCPs necessary to meet
the business objectives of the team. Here, it is not clear whether it is necessary to
hold four separate advisory board meetings involving a total number of 60 HCPs.
Requirements for a Bona Fide Consulting Arrangement

White Guide – Chapter 5: HCP and Government Official Consulting
Engagements
Rev. 12/20
Page 77 of 405
Legitimate Need
Depending on the nature of the information sought, it may indeed be necessary and
appropriate, but it is also possible that a smaller number of meetings and consultants
would be able to provide the same information. The Marketing team must provide
specific details in the BRF explaining why this approach is necessary.
Consultant Qualifications
It is essential that the qualifications of a proposed consultant meet the identified business need. You
are prohibited from selecting an HCP because he or she is a “high prescriber” or because you are seeking
to influence his or her prescribing or recommendation of Pfizer products, or to gain any other improper
business advantage. Though a consultant’s experience with a particular class of drugs may be taken into
consideration in determining whether he or she is qualified to provide the requested services, prescribing
habits may not be the basis for selection. For engagements subject to the HCP Engagements SOP,
requiring a BRF the following must be addressed:
• The number of consultants necessary for the project or meeting must be supported objectively; and
• The qualifications of the consultants needed to meet the identified business need.
For engagements subject to the HCP Engagements SOP, project managers should work with a Pfizer
Medical colleague to define the required qualifications and specifications for consultant selection.
Consultant Screening Requirements
Pfizer colleagues must submit a request to screen prospective consultants before proceeding with any
engagement or signing a related contract. HCPs/GOs processed through Engage Plus are automatically
subjected to applicable screenings.
• Restricted Party Screening (RPS): As discussed in CP #206, when HCPs/GOs are subjected to RPS,
they are compared to over 70 Restricted Party Lists maintained by various governmental entities around
the world. Individuals and entities are placed on such lists for a variety of reasons, including
participation in criminal activity and support for such activity. Pfizer is prohibited from any interactions
with these Restricted Parties, even if the engagement in question is occurring solely within the United
States. Parties appearing on a Restricted Party List may not be engaged as consultants or speakers
for Pfizer. These requirements apply to both institutions and entities, as well as individual HCPs
engaging with Pfizer.
o HCPs/GOs not processed through Engage Plus must be screened through RPS OnDemand
4
,
by the colleague wishing to engage the consultant (or someone else assigned to do so).
4
For information on how to use RPS OnDemand, see the GTC CoE website.

White Guide – Chapter 5: HCP and Government Official Consulting
Engagements
Rev. 12/20
Page 78 of 405
o A consultant agreement may only be signed after the consultant clears the RPS process.
• State Discipline and FDA Warning Letter Screening: Pfizer actively screens its HCP speakers and
consultants for disciplinary actions by state medical boards, FDA warning letters, and other misconduct
(In rare cases, exceptions may be granted by the BU Chief Counsel or BU Compliance Counsel.)
• IOC Member List: Per Clinical and Medical Controlled Document (CMCD) CT22-GSOP-RF08 2.0:
Independent Oversight Committees Conflicts of Interest, depending on their role, current members of
an active Independent Oversight Committee (IOC) for a Pfizer trial (including Data Monitoring
Committees) may not be engaged in certain financial relationships with Pfizer, including as paid
consultants, advisors, or speakers for Pfizer. The IOC Database can be accessed using this
link: http://dmc.pfizer.com/.
o For additional information regarding permissible activities of IOC members, please consult
CMCD CT22-GSOP-RF08 2.0: Independent Oversight Committees Conflicts of Interest and
White Guide Chapter 9: Pfizer-Sponsored and Non-Sponsored Clinical Research Including
Investigator-Initiated Research Studies (IIRs).
• Minnesota-Licensed Prescribers: Per Pfizer policy, Minnesota-licensed prescribers may only be
engaged as consultants in certain circumstances. For more information see White Guide Chapter 15:
State Laws: HCP and Government Employee Restrictions.
Restricted Markets
An engagement cannot involve any of the following, unless required licenses or other authorizations have
been obtained, the issuance of which is not guaranteed, and written approval received from an attorney in
the GTC CoE:
• Activities in a Restricted Market;
• Organizations or Governmental Entities from a Restricted Market; or
• Individuals ordinarily resident in a Restricted Market.
The list of Restricted Markets is available on gtc.pfizer.com, under the “Restricted Markets” section
5
.
Export Controlled Technology
Most Pfizer projects do not involve transfers of Technology controlled for export. However, if there will be
any exchange of Technology that is controlled for export (see full definition in CP #206), you must contact
the GTC CoE for further guidance prior to proceeding. Export controlled Technology can include
information regarding certain viruses, toxins, bacteria, or pathogens; information regarding medical
5
http://ecfd13.pfizer.com/sites/GTC/Pages/Restricted-Markets.aspx.

White Guide – Chapter 5: HCP and Government Official Consulting
Engagements
Rev. 12/20
Page 79 of 405
countermeasures to treat nerve agent poisoning; certain military or defense related items, services, or
projects; and certain sensitive or sophisticated manufacturing or equipment processing information.
Fair Market Value Compensation
Pfizer may only provide compensation that does not exceed fair market value for consultant services and
in a manner that does not account for the volume or value of business that may be generated by the
consultant for Pfizer. Generally, colleagues should determine appropriate fees by utilizing the US Fair
Market Value Calculator for U.S. consultants and the “Consultancy/Service Arrangements” tab (subsection
“FMV”) of the Country Profiles for non-U.S. HCPs and/or GOs, or by going to the FMV webpage on Pfizer’s
GCO Policy Xchange on GCO on Demand. For U.S. consultancies, if the proposed FMV for an HCP
exceeds the high end of the system-generated range for a particular tier (e.g., based on the unique and
relevant credentials and attributes of that HCP), the responsible colleague for the engagement must receive
legal and business approval prior to committing to the higher rate. Note that colleagues should make every
effort to ensure consistency in FMV when engaging an HCP (i.e., the rate should be generally consistent
for a specific activity regardless of which business or function is engaging a particular HCP). The FMV rate
must then be reflected in the written agreement. Pfizer must pay all US and International Consultant fees
directly to all consultants/institution i.e., vendors may not make payments to consultants on behalf of Pfizer.
(this would not apply to market research that is blinded to Pfizer, where a vendor may pay the HCPs
directly).
Zero Fee Engagements
I would like to discuss a new marketing initiative with an HCP, and she does not wish
to be paid anything for the meeting, including no travel expenses. Do I still need to
treat this as a consulting engagement, subject to the various required controls (e.g.,
BRF, contract, etc.)?
Maybe. If an HCP interaction will be merely exploratory to a business relationship and
no compensation of any kind will be provided, it probably does not constitute a
consultant engagement triggering the controls described in this Chapter (although
colleagues should consider whether a confidentiality agreement is appropriate).
However, when the activities are such that compensation would normally be provided
but for an HCP’s request not to be compensated, and/or if Pfizer will cover or reimburse
an HCP’s travel expenses (e.g., hotel; airfare; taxi), the interaction should be
processed as a formal consulting engagement. For further guidance, consult your
team attorney.
Consulting Engagements with Non-HCPs

White Guide – Chapter 5: HCP and Government Official Consulting
Engagements
Rev. 12/20
Page 80 of 405
Do the HCP Engagements SOP and the requirements in this Chapter apply to
interactions and payments to patients or U.S.-based non-healthcare professionals?
No. But colleagues must understand that the definition of “HCP” in the HCP
Engagements SOP is very broad and includes categories of individuals who may
influence prescribing behavior without being prescribers themselves. For a list of
specialties and categories considered to be HCPs for purposes of these requirements,
consult the HCP Consulting: U.S. Fair Market Value SOP. Further, these requirements
apply to interactions with any non-U.S. Government Officials.
Written Agreement
Consultants must execute a written consulting agreement with Pfizer prior to the services being provided.
The requirements below apply specifically to engagements subject to the HCP Engagements SOP, but the
requirements for engagements subject to R&D SOP 201 are similar. The written agreement must:
• Include a detailed description of the services that the consultant will provide including the project
deliverables or other appropriate milestones;
• Specify the fee and that payment is contingent on full participation in meetings and/or completion of
any work product or other deliverables;
• State why the consultant was selected (i.e., why his/her expertise is needed);
• Indicate that the consulting fee was not determined in a manner which accounts for past, present, or
future volume or value of business generated by the consultant for Pfizer;
• Specify that only reasonable, documented expenses may be reimbursed;
• Describe the compliance obligations of the consultant;
• Contain the consultant’s consent to an agreement to cooperate with Pfizer’s disclosure of payments
and other items of value provided in connection with the engagement, in accordance with Pfizer policies
on HCP payment disclosure (including U.S. HCP Payment Disclosure Policy) and applicable laws;
• Require the consultant to disclose his/her relationship with Pfizer and to adhere to the disclosure
requirements of any healthcare institution, medical committee, or other medical or scientific
organization with which the consultant is affiliated;
• Contain the consultant’s representation that he/she has not been, and is not, subject to government
discipline or criminal sanction unknown to Pfizer;
• Include the Standard Anti-Corruption Contract Provisions for Consultancy or Services Arrangements,
set forth in MAPP Appendix 8, if a non-U.S. HCP or GO is being engaged; and

White Guide – Chapter 5: HCP and Government Official Consulting
Engagements
Rev. 12/20
Page 81 of 405
• With respect to the RPS and Restricted Market points noted above, the GTC CoE maintains template
contract provisions that are recommended for any Consultancy or Services agreements, or contracts.
(Contract templates in the Engage Plus system include GTC provisions. The GTC CoE maintains the
latest version of the provisions on gtc.pfizer.com, under the Guidance and Contract Language tab.
HCP Consultant Engagements with Employer Institutions
An HCP that I wish to engage as a consultant has advised me that her employer-
Institution requires that her consulting fees be paid to the Institution, not her. Is this OK?
Yes. In these cases, the Consulting Agreement should generally be between Pfizer
and the Institution (or other employer entity) directly, with the HCP identified in the
contract as the Institution employee performing the services. Although Pfizer contracts
with and provides payment to the Institution rather than the HCP individually, all of the
consulting arrangement compliance principles outlined in this Chapter apply. If the
consultant is a U.S.-licensed prescriber, the data Pfizer reports to the government will
identify the Institution receiving the payment and the individual HCP associated with the
payment. Finally, if you are engaging a non-U.S. HCP/Institution, MAPP/FCPA due
diligence requirements will apply. Contact ENGAGE2@pfizer.com,
have questions about a particular arrangement.
Meeting Venue
The venue for any consultant meeting, including a live speaker training meeting, must be conducive to the
business purpose of the meeting, commercially reasonable, and not susceptible to characterization by third
parties as “resort-like” or “lavish”. If a colleague plans an HCP engagement at a Congress, or as part of a
sponsorship of a Congress, that is held at a “resort-like” destination, the Legal approver must be made
aware of this location prior to BRF approval in order to discuss the appropriateness and need for the ad
board with the team and BU Compliance, as needed. Pfizer colleagues should generally utilize the
Enterprise Travel & Meetings Americas team to organize meetings involving HCP consultants.
Output / Deliverables
The Project Manager is responsible for ensuring the retention of any work product generated from the
engagements and for completing an Engagement End Document (EED) which:
• Describes the information or work product (e.g., advice, slides, meeting minutes, and agendas)
collected from or generated by or with the consultants;
• Provides recommendations/incorporation of the information learned or advice obtained from the
consultant, if applicable; and

White Guide – Chapter 5: HCP and Government Official Consulting
Engagements
Rev. 12/20
Page 82 of 405
• Where a BRF was required, assesses whether the work product is consistent with what was identified
in the BRF and required under the consulting agreement. If there are inconsistencies, they must be
noted and explained in the EED.
Reimbursement of Expenses
Consultants may be reimbursed for (or Pfizer will directly arrange) reasonable travel (e.g., coach airfare for
flights lasting less than 5 hours) and lodging expenses incurred in connection with the consulting services.
Consultants may not be reimbursed for extended or non-business-related stays at a hotel prior to or after a
meeting, or for travel or additional lodging costs for spouses or other guests.
All Pfizer HCP consulting arrangements must adhere to the guidelines outlined above. Certain HCP
consulting arrangements, however, entail specific compliance risks which are discussed further below.
Advisory Board Meeting
Advisory Boards are a specific type of Consultancy or Services Arrangement involving meetings with
consultants to obtain advice and feedback on scientific, commercial, and/or healthcare-related issues to
help Pfizer better understand the external environment, therapeutic areas, data and use of products
(approved or in development), commercial, clinical, and medical asset strategies, payer landscape, or
unmet medical needs. Advisory board meetings may pose risk because they can involve larger numbers
of HCPs and potentially entail discussion of off-label information about Pfizer products. (If off-label
information is presented at an advisory board meeting, it must bear a direct relationship to the legitimate
purpose of the meeting. For additional information, see White Guide Chapter 8: Non-Promotional and
Media Activities.) The purpose of an advisory board meeting must be to gain needed feedback or advice,
and not to provide a forum for product promotion.
Certain minimum standards applicable to the compliant execution of Advisory Board interactions must be
addressed when organizing an Advisory Board. These global requirements are set forth in the Global
Advisory Board Guidelines which must be adhered to in addition to local requirements and laws Pfizer
colleagues should ensure that advisory board participants clearly understand that they are being retained
to provide a service and not to passively receive promotional presentations. An advisory board meeting
cannot be designed:
• To reward, influence, or induce the invited consultants to prescribe, recommend, supply, sell,
administer, or buy any Pfizer products or to affect the outcome of any clinical trial inappropriately;
• To provide physicians with an opportunity to meet and mingle with their peers;
• To solicit confidential competitive information; or
Types of Consulting Arrangements

White Guide – Chapter 5: HCP and Government Official Consulting
Engagements
Rev. 12/20
Page 83 of 405
• To convey product information where Pfizer is not obtaining appropriate advice or information.
Input from Sales Colleagues
A brand team is planning an advisory board meeting to solicit feedback and learn about
a disease state related to a pending new indication for the product. Can the Brand team
seek assistance from Sales to identify possible advisory board consultants?
Yes. Sales can be a valuable resource in assisting brand teams with the identification
of HCP experts. Sales may suggest possible consultants based on specific criteria
provided by the brand team that would meet the needs for the advisory board. Sales
Colleagues, however, may not be involved with any communications with HCPs
regarding the proposed advisory board, e.g., offering an invitation to participate, and
may not participate in the advisory board itself.
If the meeting involves a non-U.S. HCP or GO, Pfizer colleagues must also complete the necessary due
diligence in compliance with MAPP.
Live Speaker Training Meeting
Prior to conducting any speaking engagements, all Pfizer promotional speakers are required to complete
training on: (1) the brand’s core product training slide kit; and (2) Pfizer’s compliance requirements. Contact
DL-Speaker-Operat[email protected]om to verify compliance training completion or to setup a speaker for
online compliance training. Paid speaker training activities are treated as consulting arrangements.
When your speaker program initiative requires speakers to be trained, you should consult with your brand
RC to determine whether a live training program is appropriate. In many cases, a training method other
than a live meeting may be sufficient. If an HCP is compensated for participating in speaker training, the
speaker contract must obligate the HCP to speak twice within 12 months about the product on which he or
she received speaker training. For more information on speaker recruitment, contracting, and training, see
White Guide Chapter 4: Marketing Programs, and Orange Guide Chapter 9: Speaker Programs for HCPs.
Focus Groups and Market Research
Market research initiatives typically involve canvassing randomly selected HCPs (or those selected on
the basis of objective criteria) to obtain representative information via a “focus group” meeting or a
telephone or online survey. Pfizer conducts market research for a number of purposes, including to help
us gain a better understanding of customer needs, to assess how Pfizer and competitor products are
perceived and used in clinical practice, and to develop and test promotional messages.
Typically, market research initiatives are conducted in a manner which “blinds” Pfizer and the HCPs from
knowing each other’s identities. In order to prevent Pfizer from learning the identity of individual market

White Guide – Chapter 5: HCP and Government Official Consulting
Engagements
Rev. 12/20
Page 84 of 405
research respondents and to protect respondent-identifiable information, the final set of respondents is
generally a randomly selected or screened subset of a larger sampling universe, and outside vendors are
typically utilized to conduct the research. Such “blinded” market research does not constitute a consultant
engagement and is specifically excluded from the scope of the HCP Engagements SOP.
To help achieve compliance with Pfizer policies and procedures governing the conduct of market research,
Pfizer colleagues should generally execute any market research activities through Business Analytics.
All market research activities must be conducted in accordance with the CASRO Code of Standards and
Ethics for Survey Research. Further, no detailing or other dissemination of promotional information is
permitted, except for the purpose of legitimately testing a particular promotional message or strategy.
Preceptorships and Mentorships
A preceptorship is a training program for Pfizer colleagues, usually provided and hosted/managed by
university or teaching hospitals, which addresses a therapeutic area or the clinical use of one or more Pfizer
product(s) in professional practice. Occasionally, a preceptorship may also be conducted by one or more
HCPs directly engaged by Pfizer at a Pfizer-organized/managed training event. All of the consulting
arrangement compliance principles outlined in this Chapter apply to preceptorship programs regardless of
whether Pfizer engages with or pays an institution or an individual HCP.
Preceptorships should not be confused with mentorship programs, which are one-on-one observational
teaching sessions where a Pfizer colleague (usually a Sales Representative) observes or “shadows” an
HCP (usually a physician) at his or her office or institutional practice. No compensation of any kind may be
provided to an HCP mentor. Mentorships are not considered consultantships subject to the HCP
engagement process; however, a letter agreement describing the purpose of the mentorship and setting
forth patient privacy and confidentiality obligations must be executed. For additional information regarding
mentorships, please consult the Mentorship Guidelines and Forms available on MyPfieldNet.
Preceptorship institutions and HCP mentors must be selected based on their expertise and qualifications.
These programs may not be used as selling opportunities or offered to influence prescribing practices or
formulary decisions. For information on the privacy considerations of these activities, see White Guide
Chapter 11: Privacy: Protecting Personal Information.
For activities specific to R&D, see R&D’s Compliance CNTR and R&D SOP 201.
Non-U.S. HCPs and Government Officials
The Foreign Corrupt Practices Act (FCPA) is a U.S. law that prohibits corrupt or improper payments to
non-U.S. GOs. The FCPA prohibits offering, paying, promising to pay, or authorizing payment or the
Retaining Government Employees as Speakers or Consultants

White Guide – Chapter 5: HCP and Government Official Consulting
Engagements
Rev. 12/20
Page 85 of 405
provision of anything of value to a foreign official with the intent of influencing the official or gaining an
improper advantage. The statute broadly covers “anything of value,” which includes cash payments, gifts,
meals, and any other item that may have value to the recipient. Further, the definition of “foreign official” is
very broad and includes any officer or employee of a non-U.S. government (any department, agency, or
instrumentality) or public international organization. Due to public funding of many health systems outside
the United States, many non-U.S. individuals employed outside the U.S. could fall within this definition.
HCPs working at foreign government-owned hospitals, for example, qualify as government officials under
the FCPA. If you intend to engage as a consultant an individual who is employed outside the U.S. or enter
into any other interaction in which a payment or other benefit (monetary or non-monetary) may be given to
the individual, you must follow all applicable Pfizer procedures as outlined in the U.S. Regional MAPP SOP.
Note also that, in addition to the FCPA, other anti-bribery/anti-corruption laws govern interactions with both
U.S. and non-U.S. government officials, including the UK Bribery Act. It is critical that colleagues fully
comply with all applicable Pfizer policies and procedures on interactions with government officials.
Non-U.S. Government Official
May I engage an HCP who may be a Government Official in his or her home country to
attend an advisory board?
Maybe. Pfizer does not prohibit engaging GOs, but MAPP requires that whenever a
non-U.S. HCP or GO is being engaged as a consultant for an ad board, certain due
diligence and approvals are required. Further, additional approvals are needed when
a GO is in a position where he/she could influence Pfizer’s business (called a
“potentially-influencing government official” or “PIGO”) to ensure there is no appearance
of impropriety with respect to the engagement. The due diligence and approvals are
initiated in the FCPA/MAPP pre-approval system (Ariba ACM). The FCPA
Requirements Project form must be completed in ACM prior to the engagement. The
form will help guide you through the correct MAPP process, including the determination
of whether a GO is a PIGO and, if so, will route the form for required additional
approvals. Remember that any engagement must also comply with local law. Consult
the Country Profile for the HCP’s country of employment for local law and restrictions,
notify the consultant’s employer if required, and use the non-U.S. Consultant
Agreement template.

White Guide – Chapter 5: HCP and Government Official Consulting
Engagements
Rev. 12/20
Page 86 of 405
U.S. State and Federal Government Officials
Many state and federal government agencies require their employees to obtain approval prior to engaging
in consulting activities with outside organizations. Pfizer’s standard consulting template includes a clause
requiring proposed HCPs consultants who are government employees to warrant that, if required, they have
obtained any prior approvals required by their relevant government agency and/or ethics officer to provide
consulting services and to accept any fees and expense reimbursements.
Part-time State or Federal Employees
May I engage an HCP who works part-time at a federal government institution to be a
consultant?
Yes, but HCPs who work part-time for a federal government agency are required to
follow the policies of that agency. Every consultant agreement with a government
employee, whether employed full-time or part-time, will generally include the
government employee’s representation that he/she has been approved to act by the
relevant agency and/or the agency’s ethics officer, and specifically state whether the
employee may accept a fee as well as expense reimbursement.
Retaining Government Employees in Connection with Their Official Duties
Federal laws, regulations, and agency policies generally prohibit federal executive branch employees from
receiving anything of value in return for performing outside activities related to the employee’s official
position. Therefore, there are only limited circumstances in which Pfizer can engage federal employees in
connection with their official duties. Also, a government employee may never consult with Pfizer on any
matter pending before the employee’s government agency, unless the agency wishes the individual to do
so as part of his/her official duties. In general, however, a federal employee cleared to work with Pfizer on
an official basis may receive expense reimbursement but not a consulting fee.
Retaining Government Employees Outside of Their Official Duties
At times, Pfizer may retain a federal employee to perform services in his/her individual capacity outside of
his/her official duties. Services that may not relate to an employee’s official duties should conform to the
following parameters:
• Employee is advising on matters about which he/she is a subject matter expert and is not being
engaged because of his/her official position, but rather based on that expertise;
• Employee is not advising in relation to a matter pending before his/her government agency;
• Employee is taking personal time to participate rather than participating during employer/government
time (in which case he/she must be acting in an official capacity); and

White Guide – Chapter 5: HCP and Government Official Consulting
Engagements
Rev. 12/20
Page 87 of 405
• Employee is not conveying information that draws on ideas or official data that is not public information.
The rules on the acceptance of a fee in such circumstances are interpreted differently by different agencies.
The individual agency that employs the individual must therefore determine whether or not the federal
employee can accept a fee from Pfizer. If Pfizer engages a federal employee outside of his/her official
duties, the federal employee may not use his or her official title or position to identify himself or herself in
connection with the services, including teaching, speaking, or writing on behalf of Pfizer or in conjunction
with Pfizer colleagues. An employee’s title or position may, however, be included as part of his or her
general biographical details when teaching, speaking, or writing. The employee’s title or position may also
be used in connection with the publication of an article in a scientific or professional journal; however, a
disclaimer must be printed acknowledging that the views expressed in the article do not necessarily
represent those of the employee’s agency or the United States.
Promotional Speakers
Can a VA employee be a speaker for Pfizer?
Yes, with appropriate approvals from the VA entity that employs the individual, as long
as Pfizer complies with the entity’s requirements pertaining to its employees. Every
consultant agreement with a government employee must include the representation that
he/she has been approved to enter into it by the relevant agency and/or the agency’s
ethics officer and specifically state whether the employee may accept a fee as well as
expense reimbursement.
National Institutes of Health
Can National Institutes of Health (NIH) employees work for Pfizer as consultants, if
they have their employer’s permission? May we offer them payment for speaking at a
Pfizer event?
The NIH, as well as most other government agencies, has special conflict of interest
rules. Part-time and full-time NIH employees are prohibited from working or consulting
for industry, with or without compensation, unless they have been granted prior written
approval. Therefore, Pfizer may not directly retain NIH employees as consultants in
their personal capacity without written approval from an authorized representative of
NIH, and may not compensate NIH employees for teaching, speaking, writing, or
editing.

White Guide – Chapter 5: HCP and Government Official Consulting
Engagements
Rev. 12/20
Page 88 of 405
• GCO Policy Xchange on GCO on Demand.
• HCP Engagements SOP.
• HCP Consulting: U.S. Fair Market Value SOP.
• PolicySource:
o CP #206: Compliance with Global Trade Control Laws
o US-CS-BTE-SOP-01 US Consultant/Speaker Business Travel and Expense (T&E)
o CP #207: Global Policy on Interactions with Healthcare Professionals (GPIHP)
o CP #301: Travel, Entertainment and Other Business-Related Expenses
o CP #304: Global Meetings and Congresses Policy and Procedure
o My Anti-Corruption Policy and Procedures (MAPP)
• Orange Guide Chapter 9: Speaker Programs for HCPs.
• White Guide Chapter 4: Marketing Programs.
• White Guide Chapter 8: Non-Promotional and Media Activities.
• White Guide Chapter 9: Pfizer-Sponsored and Non-Sponsored Clinical Research Including
Investigator-Initiated Research Studies (IIRs).
• White Guide Chapter 11: Privacy: Protecting Personal Information.
• White Guide Chapter 15: State Laws: HCP and Government Employee Restrictions.
• White Guide Chapter 18: Meals, Educational Items, and HCP Payment Disclosure.
• CMCD CT 22: Use of Data Monitoring Committees and Conduct of Interim Analysis.
• SOP 201: R&D HCP/GO Engagements & Interactions.
• Global Advisory Board Guidelines .
• E-mail Contacts:
o Refer FCPA questions to FCPAQuestions@pfizer.com
o Refer GTC, RPS, and Restricted Market questions to [email protected]om
For More Information

Rev. 12/20
Page 89 of 405
CHAPTER #6 – GOVERNMENT
HEALTHCARE PROGRAMS

White Guide – Chapter 6: Government Healthcare Programs
Rev. 12/20
Page 90 of 405
Chapter
#6
GOVERNMENT HEALTHCARE
PROGRAMS
CONTENTS
Introduction.................................................................................................................................................. 91
Key Points to Ensure Compliance ........................................................................................................ 92
Medicare...................................................................................................................................................... 92
Medicare Part D .................................................................................................................................... 93
Pharmacy and Therapeutic (P&T) Committee Members ..................................................................... 95
Medication Therapy Management Programs ................................................................................. 95
Medicaid ............................................................................................................................................... 96
Medicaid Drug Rebate Program .................................................................................................... 96
Medicaid Risk Areas ............................................................................................................................. 98
Inaccurate Price or Product Reporting and Concealing Best Price ............................................... 98
Health Insurance Exchanges ...................................................................................................................... 98
Section 340B Pricing Program .................................................................................................................... 99
Federal Supply Schedule .......................................................................................................................... 100
Federal Ceiling Price ................................................................................................................................. 101
Department of Veterans Affairs and the Department of Defense ............................................................. 101
State Pharmaceutical Assistance Programs ............................................................................................. 102
340B AIDS Drug Assistance Programs .................................................................................................... 102
For More Information ................................................................................................................................. 103
Government Healthcare Programs

White Guide – Chapter 6: Government Healthcare Programs
Rev. 12/20
Page 91 of 405
Chapter #6 Government Healthcare Programs
Pharmaceutical manufacturers have become increasingly involved with government customers and
stakeholders. For example, many federal and state healthcare programs, including Medicare and
Medicaid, purchase Pfizer medicines or reimburse for their purchase. Prior to the passage of the Medicare
Prescription Drug, Improvement, and Modernization Act (MMA), the Medicare program only covered
the cost of certain prescription medicines dispensed either in a doctor’s office or in a hospital setting. Now,
the program provides comprehensive prescription drug coverage for eligible individuals. The government,
historically, also has covered the cost of prescription drugs for low income and disabled patients under
Medicaid.
Pharmaceutical manufacturers additionally provide preferred prescription drug pricing to federal customers
generally via the Federal Supply Schedule and to specific federal purchasers, including the Department
of Veterans Affairs (VA) and the Department of Defense (DoD), as required by statute. Companies also
provide discounts under the Public Health Services 340B Outpatient Drug Pricing Program, as well as
through certain state-supported programs, including State Pharmaceutical Assistance Programs and
AIDS Drug Assistance Programs.
Paying or providing benefits to healthcare providers or beneficiaries to prescribe or utilize products
ultimately reimbursed by federal healthcare programs potentially implicates the federal Anti-Kickback
Statute and state all-payor laws. Similarly, failure to provide the government with preferential pricing in
certain situations may expose a manufacturer to liability under various federal and state laws. It is critical
that Pfizer remain vigilant of – and responsive to – all federal and state laws that may be implicated while
doing business with the government.
This Chapter summarizes key Pfizer policies regarding government healthcare programs. Non-
compliance with these policies puts the Company at risk and can subject Pfizer colleagues to
disciplinary action up to and including termination of employment.
Introduction

White Guide – Chapter 6: Government Healthcare Programs
Rev. 12/20
Page 92 of 405
Medicare is a federally-funded and administered healthcare program. In general, individuals are eligible
for Medicare if they are 65 years or older, under 65 with certain disabilities, or any age with permanent
kidney failure. Notably, Medicare does not cover all healthcare services, nor does it pay for the entire cost
of the services that it does cover. Additionally, Medicare does not pay program beneficiaries directly under
any of these parts; rather Medicare reimburses healthcare providers and professionals for the services and
products provided to beneficiaries.
The original Medicare program had two parts: Part A (Hospital Insurance) and Part B (Supplemental
Medical Insurance). Medicare Part A helps defray the costs of inpatient care received in a hospital, skilled
nursing facility, or hospice. Medicare Part B helps pay for medically-necessary healthcare professional
services and other outpatient care not covered under Part A. Part B also covers some preventive services
such as screening exams and lab tests to detect, prevent, or manage a medical condition. Under the
original Medicare program, the government reimburses the provider (e.g., a doctor or an institution) for
certain drugs used in certain settings as part of payment for the patient’s overall care. Medicare
beneficiaries may also enroll in the Medicare Advantage (MA) Program, otherwise known as Medicare
Part C. MA Plans are managed care Medicare plans that generally provide a wider range of services than
those covered under the original Medicare program.
• Pfizer must not link or reference the terms of a commercial rebate agreement with a
Medicare Part D agreement or leverage a commercial arrangement to secure a Medicare
Part D agreement.
•
Pfizer must not provide P&T Committee members with "special treatment." In addition,
Pfizer colleagues must take special care not to link any financial transaction (other than
disclosed rebate or discount arrangements) to formulary decisions or formulary placement
of a Pfizer product.
•
Pfizer may not provide any substantial assistance in the structuring of a Part D sponsor's
Medication Therapy Management Program (MTMP). In addition, Pfizer may not provide any
substantial resources to, or work with, a Plan D sponsor for the purpose of helping such a
customer fulfill its MTMP obligations.
•
Generally, if Pfizer provides anything of monetary value to its customers as part of price
negotiations, it must be reflected in Pfizer's reported discounts to Medicaid. Under no
circumstances may Pfizer conceal information to avoid paying higher Medicaid rebates.
Key Points to Ensure Compliance
Medicare

White Guide – Chapter 6: Government Healthcare Programs
Rev. 12/20
Page 93 of 405
In addition, with the changes introduced by the MMA, individuals covered under Medicare are also eligible
for outpatient prescription drug coverage under Medicare Part D. Operationally, beneficiaries may obtain
prescription drug coverage through Part D stand-alone Prescription Drug Plans (also called PDPs) or
through Medicare Advantage-Prescription Drug Plans (also called MA-PD plans) under Part C. Part
D enrollees incur cost-sharing obligations (including deductibles and copayments), although many low-
income individuals are eligible for subsidies. A Medicare beneficiary who wants prescription drug coverage
can choose to enroll in either a stand-alone PDP under Part D (for drug coverage only), or, if the beneficiary
wants health and drug coverage, he/she can enroll in a MA-PD plan.
Medicare Part D
The Medicare Prescription Drug Benefit functions as an insurance program, with private companies
providing prescription drug coverage and administering the Part D benefit. The Centers for Medicare &
Medicaid Services (CMS) oversees the Part D program and contract with private health insurance
companies and Pharmacy Benefit Managers to act as PDP or MA-PDs (under Part C), respectively, and
administer the Part D prescription drug benefit. Because the federal government funds the Part D benefit,
CMS regulates these plans closely. In particular, CMS seeks to ensure that the Part D program is not
overcharged for prescription drugs and that all prescribing decisions are based on appropriate
considerations. Thus, Part D plans must report their costs to the government, and in doing so, must disclose
any “direct or indirect remuneration” (including rebates) that they receive from pharmaceutical
manufacturers. Accordingly, Pfizer must carefully track all payments to Part D plans in the event that CMS
requests verification of cost data provided by a Medicare Part D plan.
A Managed Care Customer is a non-governmental entity whose principal business is to manage or provide
health benefits, including prescription drug coverage. Such customers include traditional indemnity
insurance plans, Health Maintenance Organizations (HMOs), Preferred Provider Organizations
(PPOs), and Pharmacy Benefit Managers (PBMs). Because Medicare Part D contracts with private
insurance companies to implement the drug benefit program, many of Pfizer’s Managed Care Customers
administer prescription benefit coverage for both Medicare Part D beneficiaries as well as non-Medicare
(commercial) beneficiaries. In so doing, these Managed Care Customers frequently negotiate discounts
with pharmaceutical manufacturers on behalf of both governmental and commercial plans.
The government has expressed concern that Managed Care Customers may use access to Medicare Part
D enrollees as leverage in negotiations with pharmaceutical companies in order to obtain preferential terms
under their commercial agreements. This practice is known as “swapping.” Here are some examples of
possible swapping scenarios:
• A pharmaceutical manufacturer and a Managed Care Customer have a commercial agreement that
provides the Managed Care Customer with an average 10% rebate on all products. The parties enter
into negotiations on new commercial and Part D agreements. In exchange for the Managed Care

White Guide – Chapter 6: Government Healthcare Programs
Rev. 12/20
Page 94 of 405
Customer placing a pharmaceutical manufacturer’s products on the new Part D formulary, the
manufacturer offers to increase its rebate on the commercial agreement to an average 12.5% rebate.
The additional 2.5% rebate is the swap and may be considered an improper reward to the Managed
Care Customer for providing the pharmaceutical company with access to the Managed Care
Customer's Part D plan. In the government’s eyes, this could be a problem, because Medicare
beneficiaries or the government would have been cheated out of the additional 2.5% rebate provided
on the commercial side.
• A pharmaceutical manufacturer and a Managed Care Customer have no existing contractual
relationship and seek to negotiate new commercial and Part D rebate agreements. During the
negotiations, the parties reference and compare the terms of both agreements. Since the agreements
were negotiated at the same time, any concessions made by the Managed Care Customer to accept
lower rebates on the Part D agreement could be construed to have occurred in order to improperly
compensate the pharmaceutical company for providing the Managed Care Customer with greater
rebates on its commercial plans. Additionally, even if the rebate rates were equivalent under both
contracts, the fact that there were commingling and the comparison of terms might prompt the
government to scrutinize any concessions made to identify whether the commercial deal was made at
the expense of Medicare Part D.
In short, “swapping” exists where a Managed Care Customer and a pharmaceutical company agree to
“swap” concessions under the Part D agreement to the detriment of Part D beneficiaries or the government.
This may lead to higher costs under Part D, in exchange for more favorable terms for the Managed Care
Customer’s commercial agreement. Indeed, Managed Care Customers may be willing to accept higher
Part D costs in exchange for lower commercial plan costs because the government subsidizes the majority
of the Part D plan costs. Thus, it is important that Pfizer colleagues negotiating with Managed Care
Customers separate discussions and negotiations of commercial agreements from discussions and
negotiations of Part D agreements. Pfizer colleagues must take particular care to ensure that they do not
link or reference the terms of the commercial rebate agreement with the Part D agreement or leverage the
commercial arrangement to secure a Part D agreement. Payments to Managed Care Customers who act
as Part D sponsors may also implicate the Anti-Kickback Statute and thus Pfizer should ensure that all
arrangements are properly structured.
Contract Negotiations
May discussions regarding a commercial contract and a Part D contract occur in the
same meeting with a Managed Care Customer?
Discussions of a commercial contract and a Part D contract may occur in the same
meeting with a Managed Care Customer, so long as the two are not discussed
contemporaneously (i.e., the discussion regarding commercial agreements must be

White Guide – Chapter 6: Government Healthcare Programs
Rev. 12/20
Page 95 of 405
clearly separate and apart from the discussion of Part D arrangements). For example,
a Pfizer colleague may discuss the commercial contract in the first half of the meeting
and then indicate to the customer that the latter part of the meeting is devoted solely
to Part D contract discussions.
Pharmacy and Therapeutic (P&T) Committee Members
Many healthcare organizations and PBMs, including Managed Care Customers administering Part D drug
plans, maintain lists of preferred drugs (commonly referred to as formularies) that healthcare professionals
within that organization may prescribe, or which are eligible for reimbursement by the organization.
Decisions about which pharmaceutical products are included on a formulary are determined by that
organization’s Pharmacy and Therapeutics (P&T) Committee. P&T Committees typically make
formulary decisions based upon assessments of safety, efficacy, tolerability, and increasingly, cost-
effectiveness. Those organizations with P&T Committees frequently make decisions regarding the drugs
that are covered under Medicare Part D, Medicaid, or other government healthcare programs.
P&T Committee members are charged with an important responsibility and therefore are expected to avoid
both actual and perceived conflicts of interest when making formulary decisions. It is Pfizer policy not to
engage in any activity that could be construed as improperly influencing the independent judgment of a
P&T Committee member. In fact, consistent with the PhRMA Code on Interactions with Healthcare
Professionals, any HCPs engaged by Pfizer as speakers or consultants who also serve as members of a
P&T Committee must disclose to the Committee the existence and nature of their relationship with Pfizer.
This requirement should generally extend for at least two years beyond the termination of any speaker or
consulting arrangement.
It is important that Pfizer colleagues not give P&T Committee members anything that might be considered
“special treatment.” In addition, Pfizer colleagues must take special care not to link any financial transaction
(other than disclosed rebate or discount arrangements) to Part D formulary decisions or Part D formulary
placement of a Pfizer product. For additional information on interactions with P&T Committee Members,
see Orange Guide Chapter 7: P & T Committee Interactions addressing Sales Colleagues’ promotional
P&T committee interactions and the Green Guide: Governance for External Medical Activities, addressing
Field Medical Director (FMD) activities.
Medication Therapy Management Programs
The MMA mandated the institution of Medication Therapy Management Programs (MTMPs), which must
be offered to targeted Medicare beneficiaries and are intended to provide a wide range of services designed
to improve patient outcomes, reduce the risk of adverse events, and control the cost of drug therapy.
Targeted beneficiaries generally include Part D enrollees who have multiple chronic diseases, are taking
White Guide – Chapter 6: Government Healthcare Programs
Rev. 12/20
Page 96 of 405
multiple Part D drugs, and are likely to incur annual costs for Part D drugs that exceed a pre-established
threshold.
Currently, Part D sponsors have the flexibility to develop and implement a MTMP that best serves the needs
of their specific patient populations. Pfizer customers often seek help in developing a MTMP. Since MTMPs
are mandated by law, any substantial assistance provided by Pfizer in this area could be construed as
remuneration or a subsidy of that customer’s business expenses, which would constitute a violation of the
Anti-Kickback Statute. Therefore, Pfizer may not provide any substantial assistance in the structuring of a
Part D sponsor’s MTMP. In addition, Pfizer may not provide any substantial resources to, or work with, a
Part D sponsor for the purpose of helping such a customer fulfill its MTMP obligations. For additional
information on permissible and impermissible activities with respect to MTMPs, consult the Pricing & Access
Legal team.
Managed Care Customer Resources
May Pfizer provide approved patient care materials in order to help satisfy a Pfizer
customer’s MTMP obligations?
No. Pfizer may not provide Pfizer materials (including Pfizer quality programs and
quality care pyramids) with the intent that a customer uses them to satisfy its MTMP
requirements. Pfizer may not assist in the structuring of MTMPs or encourage the use
of Pfizer materials in MTMPs. For additional information regarding MTMPs, consult
the Pricing & Access Legal team.
Medicaid
Medicaid is a governmental healthcare program jointly funded by federal and state governments. Medicaid
offers healthcare benefits, including prescription drug coverage, for the nation’s indigent and disabled
persons. Although the federal government establishes general guidelines for the program, including
minimum coverage requirements and certain quality standards, Medicaid is administered at the state level,
with each state setting its own guidelines regarding eligibility and services. Like Medicare, the Medicaid
program does not pay program beneficiaries directly but rather reimburses healthcare professionals and
pharmacies for medical services and prescription medicines provided.
Medicaid Drug Rebate Program
In order for its outpatient drugs to be covered by the Medicaid program, a manufacturer must enter into a
national rebate agreement with the Secretary of HHS. This agreement generally requires manufacturers
to offer Medicaid agencies the mandated discounts for covered prescription drugs. Pfizer is responsible for
calculating and reporting to the federal government on a monthly and quarterly basis various metrics for

White Guide – Chapter 6: Government Healthcare Programs
Rev. 12/20
Page 97 of 405
each of Pfizer’s products and, ultimately, for paying corresponding rebates based on Medicaid recipients’
purchases of the company’s covered drugs. In return for these rebates, state Medicaid agencies must pay
for all of the drug company’s covered drugs (with certain limited exceptions). If the price of the
manufacturer’s drug rises faster than the inflation rate, states may require an additional rebate. Pfizer
and/or its predecessor entities have signed a Rebate Agreement with HHS for all Pfizer labeler codes and
Pfizer remains vigilant of its obligations under the Medicaid Drug Rebate Program.
For single-source (non-generic) drugs, the basic rebate amount per unit is either:
• 23.1% of the Average Manufacturer Price (AMP) for such unit; or
• If greater, the difference between the AMP and the manufacturer’s Best Price for such unit.
The Patient Protection and Affordable Care Act (PPACA) additionally revised the statutory minimum
rebates for pediatric, clotting, and generic drug products.
AMP and Best Price are key terms under the Medicaid Rebate Program and are both statutorily defined.
Pursuant to PPACA, AMP was redefined to mean the average price paid by wholesalers in the United
States to the manufacturer for a drug that is distributed to the retail pharmacy class of trade. A
manufacturer’s Best Price is the single lowest unit price at which the manufacturer sells the covered
outpatient drug to any eligible customer in the United States. Best Price generally includes all sales and
associated rebates, discounts, and other price concessions provided by the manufacturer to any entity,
unless statutorily excluded.
Generally, if Pfizer provides anything of monetary value to its customers as part of price negotiations, it
must be reflected in Pfizer’s reported price points. When submitting government price reports to the
government, Pfizer must therefore take into consideration all cash discounts, free goods contingent upon a
purchase requirement, volume discounts, and rebates (other than rebates under the Medicaid Drug Rebate
Program itself). In addition, free or reduced-price services, grants, other price concessions, or other
benefits offered to induce a sale may also be considered pricing terms.
The following transactions are excluded from the Best Price calculation:
• Sales at “nominal prices” (defined as prices less than 10% of AMP) if made to “covered entities” under
Section 340B of the Public Health Service Act (see discussion below), intermediate care facilities for
the mentally handicapped, and certain state-owned or operated nursing facilities;
• Prices paid by Medicare Part D Plans;
• Prices charged to the Indian Health Service, the Department of Veterans Affairs, the Department of
Defense, the Public Health Service, and entities entitled to discounts which include federally-qualified
and migrant health centers and certain high-indigent care hospitals;

White Guide – Chapter 6: Government Healthcare Programs
Rev. 12/20
Page 98 of 405
• Prices charged under the Federal Supply Schedule of the United States General Services
Administration and qualifying single award contract price of any federal agency;
• Prices negotiated from drug manufacturers for covered drugs under a qualifying discount card program;
and
• Any prices used under a qualified state pharmaceutical assistance program.
CMS uses AMP and Best Price data to calculate the Rebate Per Unit (RPU) (also called Unit Rebate
Amount (URA) values). The RPU is the amount that is owed by the pharmaceutical manufacturer for each
unit of its product reimbursed by state Medicaid agencies to dispensing pharmacies. For more information
on Pfizer’s Medicaid Best Price determinations and AMP and rebate calculations, consult the Pricing &
Access Legal team.
Notably, under the Medicaid Drug Rebate Program, pharmaceutical manufacturers must provide quarterly
AMP, Best Price, customary prompt pay discounts, and nominal price reports to CMS. Manufacturers also
must provide monthly AMP data to CMS. Pfizer is committed to reporting its AMP and Best Price values
within the mandated 30-day period. Some States also require Pfizer to report certain pricing information.
Medicaid Risk Areas
Inaccurate Price or Product Reporting and Concealing Best Price
The government has become increasingly focused on manufacturers’ pricing and price reporting to ensure
that its programs are receiving the greatest benefit for taxpayer-funded healthcare dollars. Therefore, the
government expects companies to provide complete and accurate sales and discount data when reporting
AMP and Best Price. The government also expects companies to accurately identify and report the required
product and package size information. Misclassification or incorrect product information reported to the
government may cause inaccurate rebate payments to states and may also subject a manufacturer to
penalties. Under no circumstances may Pfizer conceal information to avoid paying higher Medicaid
rebates. Indeed, reporting false or inaccurate information to the government could lead to significant liability
under the federal False Claims Act (FCA). In addition, inaccurate or incomplete reporting could be used
to prove criminal liability under the False Claims Act and/or a violation of the Medicaid Drug Rebate
Agreement, respectively. Significantly, liability under any of these statutes could subject Pfizer to exclusion
from federal healthcare programs.
In 2010, the PPACA went into effect and seeks to expand coverage, control healthcare costs, and improve
healthcare delivery in part by: (1) requiring most U.S. citizens to have health insurance; (2) creating state-
based health insurance exchanges where individuals can purchase coverage; and (3) providing premium
Health Insurance Exchanges

White Guide – Chapter 6: Government Healthcare Programs
Rev. 12/20
Page 99 of 405
and cost-sharing credits to individuals/families with income between 133-400% of the federal poverty level
who purchase insurance on the exchanges.
The state-based exchanges created by the PPACA, called Health Insurance Exchanges (HIEs or
Exchanges) are intended to be a marketplace where individuals can compare health insurance benefit
programs and costs and buy insurance. The PPACA requires that health insurance plans provide a
minimum package of services in 10 categories called Essential Health Benefits (EHB), including
prescription drug coverage. Individuals who purchase insurance through an Exchange may be eligible for
premium credits and cost-sharing subsidies depending on their income. The premium credits offset an
individual’s premium payments so that they do not exceed a certain percentage of their income (e.g., an
individual whose income is 133-150% of the Federal Poverty Limit may receive credits so that their premium
payments won’t exceed 4% of their income). Cost-sharing subsidies are intended to reduce the amounts
individuals will have to pay for out-of-pocket costs.
Because Pfizer products may be covered under a plan purchased on an Exchange and by an individual
eligible for premium credits and costs-sharing subsidies, kickback risks may be heightened. For information
on permissible and impermissible activities with respect to HIEs, consult the Pricing & Access Legal team.
Section 340B of the Public Health Service Act, established under sections 601 and 602 of the Veterans
Healthcare Act of 1992, requires pharmaceutical manufacturers participating in the Medicaid program to
enter into a second agreement called a “pharmaceutical pricing agreement” with HHS and provide
discounts to certain entities as a condition of reimbursement. Specifically, the Section 340B Pricing
Program requires that manufacturers make covered outpatient drugs available to certain purchasers
(referred to as “Covered Entities”) at discounted prices that are approximately equal to the price for such
drugs under state Medicaid programs.
Covered Entities include federally qualified health centers, community health centers (including migrant,
homeless, family planning, and AIDS health centers), and other clinics receiving Public Health Service Act
funding, and qualifying acute care hospitals that provide a disproportionate share of indigent care. Further,
pursuant to the Deficit Reduction Act and PPACA, certain additional hospitals and health centers may be
eligible to enroll in the 340B Pricing Program.
Section 340B pricing discounts are calculated using the Medicaid rebate formula and notably are excluded
from Best Price calculations. These discounts are deducted from the manufacturer’s selling price at time
of purchase by an eligible covered entity. Any subsequent price reductions necessitated by Medicaid
pricing data restatements are paid as rebates. To determine these discounts, each quarter Pfizer calculates
the Section 340B Ceiling Price (the statutorily defined maximum price that can be charged to Covered
Entities) for every covered drug marketed by Pfizer using the same pricing data submitted to CMS for the
Section 340B Pricing Program

White Guide – Chapter 6: Government Healthcare Programs
Rev. 12/20
Page 100 of 405
Medicaid Rebate Program. For additional information on Section 340B and Pfizer’s pricing policy, consult
the Pricing & Access Legal team.
The Federal Supply Schedule (FSS) program provides federal agencies with a simplified process of
acquiring almost everything the federal government uses, including pharmaceutical products, at a
discounted price.
The Department of Veterans Affairs (VA) negotiates FSS contracts with drug manufacturers to establish
FSS Prices. Under the Veterans Healthcare Act of 1992, drug manufacturers must list their drugs on the
FSS to receive payment for the purchase of those drugs by federal agencies. In general, those prices must
be no greater than certain statutorily set ceiling prices or, in certain instances, the prices manufacturers
charge selected commercial customers. Furthermore, FSS Prices may not increase faster than inflation
during a multi-year contract period.
FSS Prices are available to federal purchasers of prescription drugs, including the “Big Four” – the
Department of Veterans Affairs (VA), the Public Health Service (PHS, including the Indian Health
Service), the Department of Defense (DoD), and the Coast Guard — which are the four largest
purchasers of pharmaceutical drugs within the federal government.
Federal Supply Schedule

White Guide – Chapter 6: Government Healthcare Programs
Rev. 12/20
Page 101 of 405
The Big Four federal agencies have the right to purchase their pharmaceutical drugs from the FSS like
every other federal agency. Under the Veterans Healthcare Act of 1992, however, manufacturers must
also make covered outpatient drugs available to the Big Four at a statutorily discounted price, known as
the Federal Ceiling Price, which is at a minimum 24% below the Non-Federal Average Manufacturer
Price (non-FAMP). Non-FAMP is conceptually similar to the Medicaid AMP but is calculated based on
prices paid by a different class of customers. (AMP is based on prices paid by U.S. wholesalers for drugs
to be distributed to the retail pharmacy class of trade, but non-FAMP is the average of actual prices paid
by U.S. wholesalers to the manufacturer for drugs to be distributed to non-federal purchasers generally.)
Manufacturers must report their non-FAMP on a quarterly basis. As with Best Price, in calculating the non-
FAMP, a manufacturer must take into consideration any eligible cash discount or similar price reduction to
eligible customers during the reporting period. “Nominal” prices and prices paid by the federal government
are categorically excluded from non-FAMP calculations. The government also requires an additional
discount if the Federal Ceiling Price increases faster than inflation.
In addition to purchasing prescription drugs from FSS or from the manufacturer at the Federal Ceiling Price,
the VA and DoD may negotiate lower prices through competitively bid arrangements.
Blanket Purchase Agreements: Blanket Purchase Agreements (BPAs) are awarded under an existing FSS
contract and per regulations issued by GSA, BPAs must be competed. DoD uses BPAs to obtain sub-FSS
pricing for its Military Treatment Facilities and Mail Order Pharmacy in exchange for favorable placement
of drugs on the DoD Uniform Formulary. DoD BPAs are competed as part of the Uniform Formulary review
process and each manufacturer with drugs under review has an opportunity to submit a BPA offer. To
facilitate and standardize BPA offers, DoD issues various formulary scenarios or “condition sets” for
manufacturers to bid on. BPAs are awarded to the companies whose products are selected for formulary
placement consistent with the condition set.
In recent years, the VA has limited its use of BPAs, because the VA’s former process for awarding BPAs
did not comply with the requirement that BPAs be competed.
FSS Temporary Price Reductions: Manufacturers that wish to voluntarily offer FSS purchasers a more
favorable price can do so through a voluntary temporary price reduction (TPR) to the FSS contract. TPRs
are put in place through the FSS modification process. Manufacturers can control which FSS agencies are
able to access the TPR and how long it will be in place.
Federal Ceiling Price
Department of Veterans Affairs and the Department of Defense

White Guide – Chapter 6: Government Healthcare Programs
Rev. 12/20
Page 102 of 405
National Contracts: Another mechanism for the VA or DoD to obtain lower pricing is through a National
Contract. The agencies seek competitive bids from manufacturers for products that have multiple generics
on the market and award the National Contract to the manufacturer that offers the lowest price. Because a
National Contract is a requirements contract, the agencies that have access to the contract will buy the
product only from the National Contract holder (unless a particular patient is unable to tolerate that product).
State pharmaceutical assistance programs (SPAPs) generally provide pharmaceutical benefits or
assistance to a defined population that usually consists of disabled, indigent, or low-income elderly persons.
These subsidy programs utilize a combination of state and local funds to pay for a portion of the SPAPs’
costs. SPAPs usually obtain discounts or rebates on drugs either through negotiations with drug companies
or because such discounts or rebates are mandated under state law.
Pfizer generally only pays rebates to SPAPs if they have been formally qualified by CMS as a SPAP. Pricing
discounts offered to an unofficial SPAP may impact Pfizer’s Best Price.
AIDS Drug Assistance Programs (ADAPs) are state-operated programs, federally funded through the
Ryan White HIV/AIDS Treatment Modernization Act, intended to help HIV positive patients have access to
HIV treatments. Notably, ADAPs are covered entities under the 340B Drug Pricing Program and, thus, are
able to receive 340B pricing on covered outpatient drugs. There are 57 jurisdictions that operate ADAPs,
including Puerto Rico, the U.S. Virgin Islands, and other associated territories. Each individual state or
territory decides which medications will be covered and how they will be distributed, as well as the clinical
and income eligibility for participation in the programs. Reimbursement models include the following:
• Rebate Eligible States are states that submit utilization data via invoices.
• Hybrid States are states that contract through a central pharmacy that orders and dispenses
medication for them.
• Direct Purchase States are states that receive an upfront discount from the wholesaler in lieu of a
rebate. These customers purchase through a Pfizer-approved authorized wholesaler.
• Indirect Purchase States are states that receive a rebate. The rebate and discount are based off of
the wholesale acquisition cost (WAC) in effect on the last day of the reporting quarter.
• Combo States are states that receive rebates in part, but also act as direct purchase states.
Because of these various models, Pfizer ADAP customers include states and private entities that sell to
and/or act on behalf of the states.
State Pharmaceutical Assistance Programs
340B AIDS Drug Assistance Programs

White Guide – Chapter 6: Government Healthcare Programs
Rev. 12/20
Page 103 of 405
• Orange Guide Chapter 7: P&T Committee Interactions
• Green Guide: Governance for External Medical Activities
• http://www.pfizerrxpathways.com
• Refer any other questions to your team attorney or the Pricing & Access Legal team.
For More Information

Rev. 12/20
Page 104 of 405
CHAPTER #7 – SUPPORT OF
EXTERNAL ORGANIZATIONS

White Guide – Chapter 7: Support of External Organizations
Rev. 12/20
Page 105 of 405
Chapter
#7
SUPPORT OF EXTERNAL
ORGANIZATIONS
CONTENTS
Introduction................................................................................................................................................ 106
Key Points to Ensure Compliance ...................................................................................................... 108
Sponsorships and Charitable Contributions: All Divisions ........................................................................ 111
General ............................................................................................................................................... 111
Sponsorships and Charitable Contributions: WRDM and GPD ......................................................... 111
Sponsorships and Charitable Contributions: BUs, CBO, WMS, and Corporate Affairs ..................... 111
Sponsorships ...................................................................................................................................... 112
Charitable Contributions ..................................................................................................................... 114
Collaborations ........................................................................................................................................... 119
Overview ............................................................................................................................................. 120
Collaborations – Tangible Benefit and Disclosure of Pfizer Involvement ........................................... 121
Awards, Scholarships, and Fellowships .................................................................................................... 121
Overview ............................................................................................................................................. 121
Non-Financial Support .............................................................................................................................. 122
Personal Volunteering ........................................................................................................................ 122
Regular Membership and Board Membership .................................................................................... 123
For More Information ................................................................................................................................. 124
Support of External Organizations

White Guide – Chapter 7: Support of External Organizations
Rev. 12/20
Page 106 of 405
Chapter #7 Support of External Organizations
Pfizer is often asked to provide funding or other support to external organizations including for-profit and
not-for-profit entities. Pfizer provides external funding through sponsorships and charitable contributions.
Pfizer also supports joint collaborations with external organizations to advance shared objectives. Pfizer
additionally sponsors awards, scholarships, fellowships, and similar funding in support or recognition of the
education and professional accomplishments of healthcare professionals and students. Such Pfizer
funding and support is a demonstration of the commitment to fund programs and initiatives that have broad
public benefit, advance medical care, and improve patient outcomes.
As with any other interactions between Pfizer and entities involved in healthcare-related industries,
providing funding or other support to external organizations can present legal and perception risks if
applicable laws, regulations, and Pfizer policies are not followed. All such interactions and the provision of
financial support must be conducted appropriately to ensure that payments will not be perceived as an
attempt to inappropriately influence the prescribing or recommendation of Pfizer products and to ensure
the preservation of external organizations’ independence. In addition, Pfizer’s policy requires that
promotional materials, and certain other materials provided by colleagues through collaborations with
external organizations, be reviewed and approved by the applicable Review Committee.
Pfizer must also comply with certain reporting and disclosure requirements of the Sunshine Act and State
Laws. Included in scope for reporting are any payments or transfers of value that are made directly or
indirectly to a Covered Recipient.
“Covered Recipient” Defined
A Covered Recipient means any U.S. licensed physician, dentist, podiatrist, optometrist,
chiropractor, physician assistant, nurse practitioner, clinical nurse specialist, certified registered
nurse anesthetist and anesthesiologist assistant, certified nurse-midwife or teaching hospital as
defined under section 6002 of the U.S. Affordable Care Act (“Sunshine Act“) and section 6111 of
the Substance Use-Disorder Prevention that Promotes Opioid Recovery and Treatment
(“SUPPORT”) Act.
Introduction

White Guide – Chapter 7: Support of External Organizations
Rev. 12/20
Page 107 of 405
A payment or transfer of value is considered indirect if it is known that the organization receiving the funding
will be conveying a benefit to a Covered Recipient even if Pfizer does not direct or influence the selection
of the recipient or have knowledge of the identity of the recipient.
If Pfizer has agreed to an organization’s use of funds that includes a payment or transfer of value to Covered
Recipients in any form of direct, indirect, or in-kind payment or transfer of value, then the Pfizer
project manager is responsible to collect all relevant information for each Covered Recipient required
for disclosure using the Sunshine Data Template available at
http://ecfd13.pfizer.com/sites/sunshinetracker/SiteContent/Sunshine%20Tracker%20Quick%20Reference
%20Guide_07262013%20V1.pdf.
The Centers for Medicare and Medicaid Services (CMS) discloses the data on a publicly available website.
CMS discloses calendar year data on June 30
th
of the following year. Please refer to Orange Guide
Chapter 18: Meals, Educational Items, Greenstone Giveaways, and HCP Payment Disclosure for
more information on our disclosure obligations under the Sunshine Act.
This Chapter summarizes key Pfizer policies regarding specified types of funding and support of
external organizations. Non-compliance with these policies puts the Company at risk and can
subject Pfizer colleagues to disciplinary actions up to and including termination of employment.

White Guide – Chapter 7: Support of External Organizations
Rev. 12/20
Page 108 of 405
• Understand the policies that apply to your group. Funding to not-for-profit organizations by
U.S.-based (and non-U.S. based when charged to U.S. cost centers) colleagues within
Pfizer Biopharmaceutical Group, Upjohn (excluding Upjohn R&D colleagues), collectively
Business Units or BUs, Worldwide Medical & Safety (comprised of legacy Pfizer Medical
division and Safety group), Chief Business Office (CBO), and Corporate Affairs must follow
the policy and procedures outlined in the
SOP on Funding Requests for Not-for-Profit
Organizations. For questions relating to this SOP, e-mail USFundingRequest@Pfizer.com
.
•
Funding to external organizations by U.S.-based colleagues in WRDM (excluding
Worldwide Medical & Safety (WMS)), Global Product Development (GPD) and Upjohn R&D
(collectively, “R&D colleagues”) must follow R&D SOP 202.
• Pfizer colleagues in other divisions must follow Corporate Procedure 801
and also the
review, approval and documentation requirements applicable to their division.
•
Funding under this Chapter is not intended to provide support for Independent Medical
Education activities or research activities such as Investigator Sponsored Research (ISR)
and Clinical Research Collaborations (CRCs).
•
Understand the types of activities your group is permitted to fund by reviewing the relevant
SOP or reaching out to the supporting teams identified in the relevant SOP.
•
For U.S.-based (and non-U.S. based when charged to a U.S. cost center) colleagues in the
BUs, WMS, CBO, and Corporate Affairs, the following table summarizes permitted funding
by group:
Key Points to Ensure Compliance
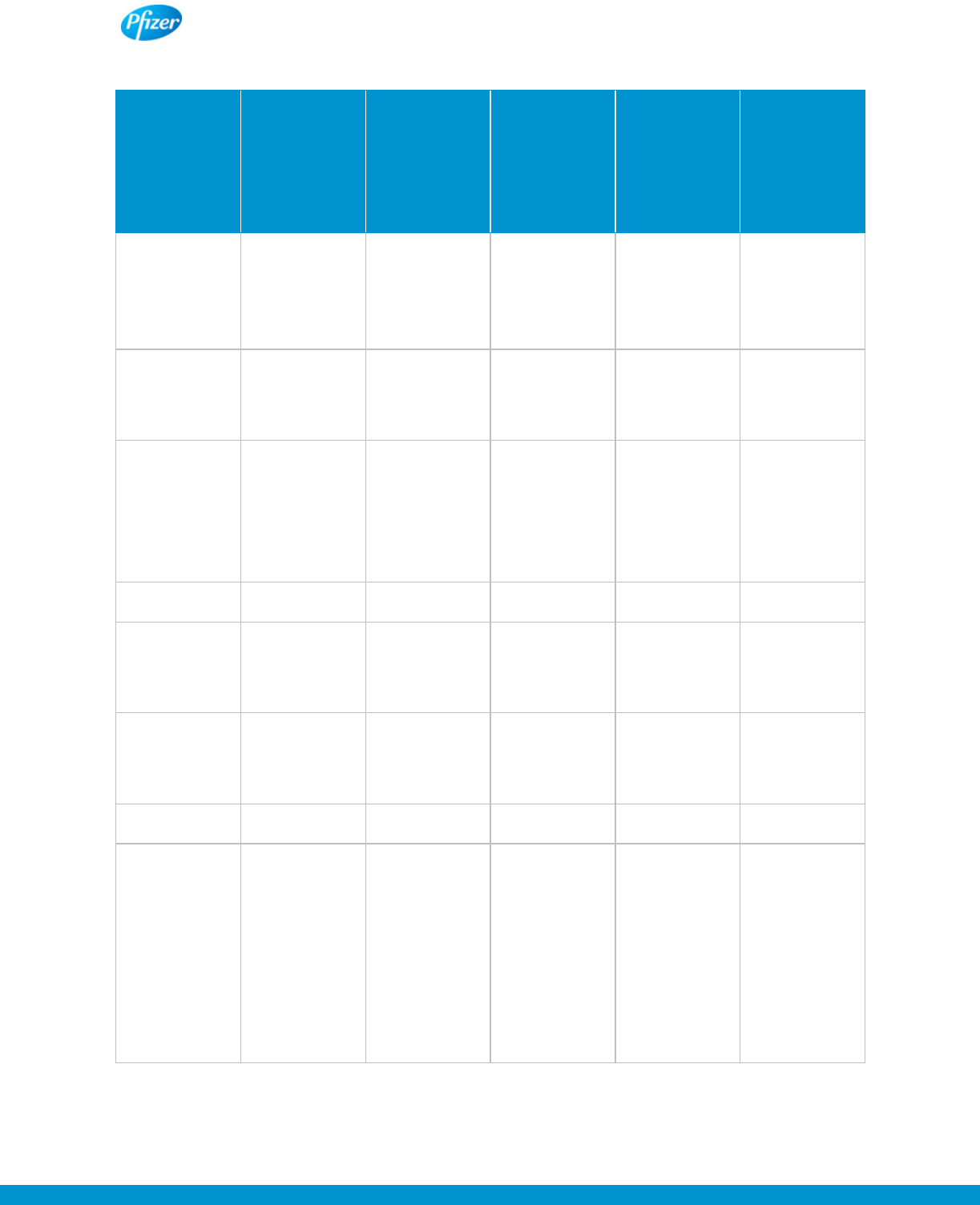
White Guide – Chapter 7: Support of External Organizations
Rev. 12/20
Page 109 of 405
Type of
Funding
Sales
Non-Sales
(including
PCA and
Account
Managers)
Corporate
Affairs
BU Medical,
WMS, and
CBO
Global
Medical
Grants (GMG)
Non-
Healthcare
Charitable
Contribution
Yes*
Yes
Yes
Healthcare
Charitable
Contribution
Yes
Policy
Focused
Healthcare
Charitable
Contribution
Yes
Special Event
Yes
Sponsorship
Yes, but DBM
and above
only
Yes
Yes
Yes
Yes
Collaboration
Yes, but DBM
and above
only
Yes
Yes
Yes
Yes
Fellowship
Yes**
Independent
Charity
Patient
Assistance
Programs
Only certain
Colleagues
within Global
Health &
Patient Access
(legacy
Corporate
Responsibility)

White Guide – Chapter 7: Support of External Organizations
Rev. 12/20
Page 110 of 405
* To remain consistent with, and for purposes of this chart found in the SOP on Funding Requests for Not-
for-Profit Organizations, “PCA” and Account Managers includesallAccount Management roles e.g. Account
Directors, Key Account Managers (KAMs), HIT Specialists and Vaccine Account Management roles. PCA
& Account Management colleagues must consult their Team Attorney before proceedng to support a Non-
Healthcare Charitable Contribution. **Patient & Health Impact (PHI) colleagues involved in designing and
conducting research related to health economics and real world data are the only CBO colleagues permitted
to fund Fellowships.
• Field Commercial Colleagues, as defined in Chapter 1 of this Guide, may fund sponsorships
that provide an appropriate “tangible benefit” (as defined later in this Chapter) to Pfizer. For
BU Sales Colleagues, these sponsorships may only be provided at the DBM level or higher.
•
A funding request that does not include a “tangible benefit” will not be treated as a
sponsorship but rather as a charitable contribution. Charitable contributions are not eligible
for funding by Sales Colleagues.
•
External organizations will often submit funding requests using key terms (e.g., “charitable
contribution,” “grant,” and “sponsorship”) interchangeably and inconsistently. Pfizer
colleagues must identify the substantive nature of each request based on Pfizer definitions
and ensure that it is a type of request they are permitted to fund, under relevant SOPs.
•
Never offer or provide funding: (i) as a “quid pro quo” to inappropriately influence the
formulary positioning, recommendation, or increased prescribing of a Pfizer product; or (ii)
to gain improper favor with a healthcare professional, government official, or any other
individual or organization.
•
Never provide individual HCPs or group practices with grant funding or donations unless
approved in advance by Legal.
• Never link charitable funding to a commercial transaction or interaction.
•
Never provide funding to an organization in a manner that undermines the organization’s
independence or mission, or for capital support or “start-up” costs.
•
Never provide funding for any activity that may result in inappropriate promotion of Pfizer
products or where there is a likelihood that treatment options will not be presented in a fair
and balanced manner.
• Never sign an agreement or contract until the funding request is fully approved.
• Never make a verbal or written commitment until the funding request is fully approved.
Key Points to Ensure Compliance

White Guide – Chapter 7: Support of External Organizations
Rev. 12/20
Page 111 of 405
General
Not-for-profit organizations, including but not limited to qualified 501(c)(3) charitable organizations, may
offer Pfizer the opportunity to provide funding for sponsorships or charitable contributions. Colleagues must
follow the review, approval, and documentation requirements applicable to their division prior to making
any commitment of funding.
Sponsorships and Charitable Contributions: WRDM and GPD
Funding and non-financial support in the form of volunteering, membership and board membership to
external organizations by U.S. WRDM (excluding WMS), GPD , and Upjohn R&D colleagues (collectively,
“R&D colleagues”) must follow R&D SOP 202 and R&D SOP 203. Guidance and support can be found on
the R&D Compliance CNTR.
Sponsorships and Charitable Contributions: BUs, CBO, WMS, and Corporate Affairs
The remainder of this section describes the policy that applies to U.S.-based (and non-U.S. based when
charged to U.S. cost centers) colleagues in the BUs, including Field Commercial Colleagues, WMS, CBO,
and Corporate Affairs. Colleagues in these divisions should refer to the SOP on Funding Requests for Not-
for-Profit Organizations (“External Funding SOP”) to determine whether a funding opportunity is a
sponsorship or a charitable contribution. This Chapter does not comprehensively address the activities that
may be funded by BU Leadership and the Medical Lead for each BU. Those activities are addressed in the
External Funding SOP.
Determining the appropriate funding type will determine which colleague groups are permitted to
fund them. How a third party defines or describes the funding request does not determine Pfizer’s
classification. In fact, external organizations will often submit funding requests using key terms
interchangeably and inconsistently (e.g., “charitable contributions,” “grants,” and “sponsorships”). Each
colleague must identify the substantive nature of each request, based on Pfizer’s standard definitions
summarized below, to ensure that a request represents the type of opportunity that they can appropriately
fund. Such guidance can be found in the External Funding SOP.
Sponsorships and Charitable Contributions: All Divisions

White Guide – Chapter 7: Support of External Organizations
Rev. 12/20
Page 112 of 405
“Not-for-Profit” Defined
A “not-for-profit” (also referred to as a “non-profit”) organization is an organization that does not
distribute its profits to its owners and is typically organized for educational, charitable, or scientific
purposes. The External Funding SOP applies to entities that have been designated as not-for-
profit by appropriate state and federal agencies, including but not limited to: 1) certain charities and
patient advocacy groups designated by a 501(c)(3) status; 2) professional medical associations or
chambers of commerce (501(c)(6) status); and 3) cultural and civic organizations (501(c)(4) status).
Sponsorships
Sponsorships are funding opportunities provided by either for-profit or not-for-profit organizations that
present a “tangible benefit” to Pfizer. They can be funded by most Pfizer groups in accordance with the
processes and requirements described in this Chapter. A tangible benefit is any legitimate, appropriate,
and business-oriented benefit to the proprietary interests, business, or public policy goals of Pfizer or its
products, services, or programs. The receipt of general recognition or incidental goods or services that do
not directly promote Pfizer business goals in and of itself does not constitute a tangible benefit. A tangible
benefit must provide the opportunity to truly advertise or advance Pfizer business interests, e.g., to educate
customers and/or prescribers about the specific attributes of our products and/or services.
A funding request characterized as a sponsorship that does not include a tangible benefit in return
for funding will not be treated as a sponsorship but rather as a charitable contribution. As
discussed in the next section, Sales Colleagues are not permitted to make any charitable
contributions. All other colleagues (including PCA*) are not permitted to make healthcare charitable
contributions but are permitted to make appropriate non-healthcare charitable contributions.
Colleagues may not ask a requesting organization to change the associated benefits being offered
for funding in order to impact the classification or source of funding within Pfizer.
* To remain consistent with the SOP on Funding Requests for Not-for-Profit Organizations, “PCA” includes
Account Managers, including but not limited to Account Directors, Key Account Managers (KAMs),
Oncology KAMs, HIT Specialists, and Vaccine Account Managers (VAMs).

White Guide – Chapter 7: Support of External Organizations
Rev. 12/20
Page 113 of 405
Tangible Benefit Examples*
Fair Recognition Examples
(Not Considered A Tangible Benefit)
• An activity provides a Tangible Benefit where
Pfizer is a direct recipient of the activity output
(e.g., funding the development of literature that
will then be used by Pfizer) or where Pfizer has
any input with respect to the execution or
content of the activity (e.g., providing strategic
direction or message development).
• Distribution of branded materials or
dissemination of information on specific
products.
• Promotional placement of product logos on a
podium or in literature aimed at HCPs or
patients.
• Opportunity to promote Pfizer products (e.g.,
via branded materials or a booth at an
exhibition).
• Opportunity to promote Pfizer's programs or
services (e.g., Pfizer RxPathways).
• Providing or selecting a speaker (including for
a policy topic).
• Opportunity to promote Pfizer unbranded
programs (such as smoking cessation which
may have related branded or unbranded
materials).
• Opportunity to promote specific businesses,
portfolios, or franchises within Pfizer (e.g.,
Pfizer Oncology, Pfizer Women’s Health,
Pfizer Vaccines), provided that such promotion
involves activities beyond mere promotional
placement of its name/logo, such as the ability
to distribute materials or information related to
such business, portfolio, or franchise and/or
products within such business, portfolio, or
franchise.
• Placement of a Pfizer corporate logo by itself
on a podium, in literature, or on a purchased
table at an event.
• Honorable mentions and announcement of
thanks, written or verbal.
• Tickets to an event.
• Recognition in conference brochure/program
(such as listing as Gold Sponsor).
* Subject to meeting all relevant review committee approval requirements.

White Guide – Chapter 7: Support of External Organizations
Rev. 12/20
Page 114 of 405
If a not-for-profit sponsorship opportunity satisfies the above key characteristics, U.S.-based (and
non-U.S. based when charged to U.S. cost centers) colleagues in BUs, WMS, CBO, and Corporate
Affairs may submit a funding request using the Funding Request Form (FRF) available at
https://aribaprime.pfizer.com/Sourcing/Main.
Evaluate Substantive Nature of Funding Request
Can a colleague in a BU, WMS, CBO, or Corporate Affairs, fund a sponsorship as long
as the tangible benefit criteria is met?
Not necessarily. When evaluating the substantive nature of a funding request for a
sponsorship, colleagues must differentiate the tangible benefit offered from the
activity/event. For example, at times organizations may offer exhibit space in return
for providing support for a medical education conference. While the exhibit space is
considered a tangible benefit, only GMG is permitted to support the medical education
conference through an independent medical education (IME) grant. In order to fund a
sponsorship for the exhibit space, the funding request must clearly outline support is
being provided for the exhibit space and not for the medical education conference.
Submission of Funding Requests by Sales Colleagues
Sponsorships may be funded only by Sales Colleagues at the District Business Manager
(DBM) level or higher. The purchase of exhibit and display space by U.S. Sales Colleagues is
covered by the Exhibit and Displays SOP (ED SOP2-01) and is processed through Ariba ACM.
However, if a U.S. Sales Colleague funds a sponsorship that provides for a package of benefits
(i.e., in addition to the exhibit and display space) then the SOP on Funding Requests for Not-for-
Profit Organizations should be followed.
Before submitting any requests using the FRF, colleagues must complete the Funding Request
training module in order to gain access to the FRF in Ariba. All funding requests are subject to
review and approval by the designated reviewers aligned to each BU/Division/Function, unless
otherwise noted. Contact USFundingRequest@Pfizer.com for more information about training.
Charitable Contributions
Generally, charitable contributions are expenditures that are intended to fund a qualified 501(c)(3)
organization in the United States (or non-U.S.-based not-for-profit entity equivalently recognized by the
respective country’s local government) for its broad charitable purpose or mission. As described above,
any funding opportunity that does not include a direct tangible benefit to Pfizer will be treated as a charitable

White Guide – Chapter 7: Support of External Organizations
Rev. 12/20
Page 115 of 405
contribution (for purposes of determining whether specified colleagues can fund it). When permitted,
charitable contributions must be made for a bona fide charitable purpose and without any ulterior
commercial motive. Charitable contributions may include some benefit to Pfizer, but any benefit given to
Pfizer must be incidental to the donation itself. Pfizer may not provide input into the content or strategic
direction of the activity being funded, nor receive rights to use the results of the activity being funded. Due
to limited funding, not all charitable contribution requests will be approved.
Pfizer broadly distinguishes between four categories of charitable contributions: non-healthcare,
healthcare, policy-focused healthcare, and Special Events. This section contains definitions and examples
of each type of charitable contribution, a description of the groups that may provide funding and an overview
of the relevant approval process.
Non-healthcare charitable contributions are the donation of money, goods, or services to organizations
or programs that exist for broad public benefit not related to products or healthcare topics.
• Examples: Contribution for disaster relief; contribution for a school fundraiser.
• Colleagues who May Provide Funding: U.S.-based (and non-U.S. based when charged to U.S. cost
centers) colleagues in the following Pfizer divisions: BU non-Sales Functions (including PCA),
Corporate Affairs, CBO, and WMS. For purposes of the External Funding SOP, PCA includes Account
Managers, as defined above.
• Approval Process: Requests for non-healthcare charitable contributions may be submitted using the
Funding Request Form at https://aribaprime.pfizer.com/Sourcing/Main. All such requests are subject
to review and approval by designated reviewers aligned to each BU/Division/Function.
Healthcare charitable contributions (non-policy focused) are charitable contributions to healthcare-
related organizations or non-healthcare related organizations for healthcare-related programs. Field
Commercial Colleagues may not fund healthcare charitable contributions. GMG funds charitable
contributions related to the following: disease state focused patient or community education or advocacy;
health screening and surveying; improved patient access to care (e.g., transportation costs); and other
programs provided by organizations whose general mission is to benefit specific patient groups. If the
target audience of a patient/community education program also includes HCPs, the request may not be
supported as a charitable contribution—the request must be processed as an Independent Medical
Education (IME) grant (refer to the chapter titled “Independent Medical Grants”).
• Examples: Contribution to the Arthritis Foundation for patient education on lifestyle changes that can
help them manage their condition; contribution to CancerCare for improved access to care—
transportation to/from medical appointments for patients with breast cancer.
• Colleagues who May Provide Funding: GMG

White Guide – Chapter 7: Support of External Organizations
Rev. 12/20
Page 116 of 405
• Approval Process: Requests for (non-policy-focused) healthcare charitable contributions that meet
the criteria above must be submitted directly by the 501(c)(3) not-for-profit organization to GMG via
Pfizer’s online Grant Management System (GMS). Colleagues may not submit requests to GMG on
an organization’s behalf. This website includes a list of criteria that any request must meet to be eligible
for GMG charitable funding. Funding from GMG may not be used to support food or beverages for
learners/participants. GMG will review submissions for completeness, alignment with clinical areas of
interest, compliance with Pfizer policies, and other requirements. Organizations will receive an e-mail
notification when the request is approved or denied.
Policy-focused healthcare charitable contributions are contributions to organizations where the funds
are to be used for the organization’s specific mission-related activities that align with Pfizer’s public policy
goals. This includes, but is not limited to, patient education on public policy issues, policy-related access
to healthcare issues, and support of charities whose general mission is to further healthcare policy (and
does not include continuing medical education/continuing education (CME/CE) or disease state, medical,
or clinically-focused activities).
• Example: Charitable contribution to the Georgia Medical Society for education of members on
healthcare reform.
• Colleagues who May Provide Funding: Corporate Affairs
• Approval Process: Requests must be submitted by appropriate colleagues using the Funding Request
Form. All such requests are subject to review and approval by designated reviewers aligned to each
BU/Division/Function.
“Special Events” are contributions to organizations whose goals align with Pfizer’s public policy goals to
help fund their fundraising dinners, walks, biking and golf events, galas, awards ceremonies, and other
similar events. Special Events are activities that do not present tangible benefits to Pfizer (and are therefore
ineligible for sponsorship funding).
• Examples: Financial support of a Multiple Sclerosis Society walkathon.
• Colleagues who May Provide Funding: Corporate Affairs
• Approval Process: All requests must be submitted by appropriate colleagues using the Funding
Request Form through Ariba-ACM. All such requests are subject to review and approval by designated
reviewers aligned to each BU/Division/Function.
• Internal Coordination: Involvement of BU colleagues in policy-focused healthcare charitable
contributions and Special Events must be strictly limited. Certain designated BU colleagues are
permitted to present therapeutic area strategies and priorities to Corporate Affairs so that Corporate
Affairs has access to the most comprehensive information in determining how best to work with
requesting organizations. These presentations may not focus on specific events or funding

White Guide – Chapter 7: Support of External Organizations
Rev. 12/20
Page 117 of 405
opportunities and may occur only during development of operating plans and strategic planning
discussions.
• Additional Assistance: If a Special Event includes or requires Pfizer participation, such as volunteers
to hand out materials or seats at a gala table, Corporate Affairs may invite colleagues to participate
only if there is no branded or promotional interaction with the organization, and discussions with
attendees must not involve Pfizer brands or products. Colleagues are not permitted to invite HCPs to
these events.
Key Characteristics: Sponsorships vs. Charitable Contributions
Characteristic
Sponsorship
Charitable
Contributions
Promotional in nature
Yes
No
Payee must be a not-for-profit organization
(501(c)(3) or similar designation)
Yes (except for Exhibit
and Displays)
Yes (but only 501(c)(3)
organizations are
eligible)
Pfizer must receive a “tangible benefit”
Yes
No
Payment can be made to an individual HCP
or private practice group
No
No
Tickets or invitations received as a result
can be offered to Healthcare Professionals
No
No
Agreement documenting terms and
conditions of Pfizer funding
Yes (agreement must
clearly indicate the
“tangible benefit”)
Yes
Information on Pfizer’s External Funding SOP
Where can Pfizer colleagues in the BUs, WMS, CBO, and Corporate Affairs get help
and information on Pfizer’s policy regarding funding to not-for-profit organizations?
Funding requests must be initiated at Ariba-ACM under the Create menu, select
“Funding Request Project”. Additional resources are also available at GCO
PolicyXchange under the Funding Requests. Global Policy Xchange on GCO On
Demand also includes a funding request “wizard” and other tools that can help you
determine whether a proposed funding activity is permissible for you to support. You
can direct any questions about the process to USFundingRequest@Pfizer.com.

White Guide – Chapter 7: Support of External Organizations
Rev. 12/20
Page 118 of 405
Purchase of a single ticket to a Gala/Fundraiser
The External Funding SOP prohibits Field Commercial Colleagues from funding a
table at a gala or fundraiser for a not-for-profit organization. But can these colleagues
purchase a single ticket to this type of event?
Yes. The SOP permits these colleagues to purchase single tickets to fundraising
events for legitimate business purposes. The ticket fee may be submitted as an
invoice and charged to your department’s payment process. However, remember that
colleagues in these groups are not permitted to purchase entire tables at such events.
Colleagues must operate within the spirit of these guidelines and not purchase
individual tickets in a manner that result in the purchase of a whole table in order to
circumvent the SOP.
Funding Request from For-Profit Organizations
Does the External Funding SOP apply to funding requests from for-profit
organizations?
No. These requests are evaluated under similar standards but are not covered by the
External Funding SOP. However, colleagues should submit the funding request using
the Funding Request Form (FRF) in Ariba-ACM with a note clearly indicating the
organization is for-profit for the reviewer’s attention
Sales-Funded Exhibit and Display Requests
Are Exhibit and Display Fees made payable to not-for-profit organizations covered by
the External Funding SOP?
Sales-funded Exhibits and Displays are subject to a different SOP –
ED SOP2-01 available on Global Policy Xchange on GCO On Demand under the
Funding Requests tab which is separate from the External Funding SOP. Sales
colleagues should submit Exhibit and Display requests through Ariba ACM using the
Funding Request Form (FRF) which will be routed to the aligned program activity
coordinator for review and follow the applicable policies (available in Global Policy
Xchange on GCO On Demand under the “Funding Requests” tab). However, if an
Exhibit and Display request is part of a larger promotional sponsorship package that
includes other benefits (in addition to an exhibit and display space), then the External
Funding SOP should be followed.

White Guide – Chapter 7: Support of External Organizations
Rev. 12/20
Page 119 of 405
Appropriate Pfizer Foundation Referrals
Can a customer’s request for a charitable contribution be forwarded to the Pfizer
Foundation for consideration?
No. The Pfizer Foundation is an independent, tax exempt organization established by
Pfizer Inc. and does not accept unsolicited funding requests. The Pfizer Foundation
provides funding through targeted initiatives focused primarily on healthcare and
science education such as the Pfizer Foundation Matching Gifts Program or the Pfizer
Foundation Healthy Families, Healthy Futures program.
Funding Virtual Activities
During the coronavirus pandemic, activities may be converted from live (or in-person)
to virtual. How is funding support for virtual activities impacted?
The tangible benefit requirements remain in effect when supporting sponsorships for
virtual activities. While some uncertainty as to virtual attendance is understandable
given the novelty of these virtual activities, it is the responsibility of colleagues to
ensure that for any such virtual activity there is a realistic expectation of a legitimate,
appropriate, and business-oriented benefit to the proprietary interests, business, or
public policy goals of Pfizer or its products, services, or programs. More importantly,
colleagues should also consider after each virtual event how valuable the event was
in terms of opportunity to truly advertise or advance Pfizer business interests (e.g., to
educate customers and/or prescribers about the specific attributes of our products
and/or services), including consideration of the number of attendees and that the
anticipated opportunities actually materialized consistent with the basis of approval by
Pfizer. For sponsorships already approved/paid that result in significantly revised
tangible benefits due to cancellation/postponement, please refer to Guidance for
Activities Cancelled/Postponed due to COVID-19. You can direct any questions about
the process to USFunding[email protected]m.
Another way that Pfizer supports external organizations is by participating in collaborations or joining
coalitions to advance shared objectives (this chapter does not include guidance regarding Clinical Research
Collaborations). Colleagues must follow the review, approval, and documentation requirements applicable
to their division. The requirements for BUs, WMS, CBO, and Corporate Affairs are described below.
Collaborations

White Guide – Chapter 7: Support of External Organizations
Rev. 12/20
Page 120 of 405
Overview
A Collaboration is an activity or project undertaken by Pfizer with one or more external organizations
(either for-profit or not-for-profit) to advance specified shared objectives, where all parties participate as
equal partners. Pfizer must not only support the organization with funding (in cash or in-kind resources or
expertise) but must also make a substantial intellectual contribution to the project. “Substantial intellectual
contribution” means conceiving and designing a project, acquiring data, or analyzing and interpreting data.
If the organization creates materials that are published, this must occur in conjunction with Pfizer. In a
Collaboration, Pfizer is involved with the creation of the output, provides feedback on suggested
publications, and has the right to use the materials being created. For BU colleagues, all materials
developed for distribution must go through a Pfizer RC evaluation to check the content for factual accuracy
and compliance with applicable laws, regulations and Pfizer policies.
Pfizer’s involvement in a Collaboration must be disclosed clearly in all resulting materials in a manner that
does not imply that the materials were funded through an unrestricted grant or Charitable Contribution.
Such disclosure should state “Developed in collaboration with Pfizer” or similar terms.
• Examples: A brand team may collaborate with cancer survivor organizations on a pamphlet about
effective patient–physician dialogue; “Campaign to Quit” conducted jointly with the American Lung
Association.
• Colleagues who May Provide Funding: U.S.-based (and non-U.S. based when charged to U.S. cost
centers) colleagues in the BUs, WMS, CBO, and Corporate Affairs.
• Approval Process: Colleagues should discuss all pertinent facts about a collaboration with Legal prior
to submitting the Funding Request Project for approval. After consulting with Legal, requests to
participate in a collaboration must be submitted by appropriate colleagues by creating a Funding
Request Project in Ariba-ACM, which are subject to formal review and approval by designated
reviewers aligned to each BU/Division/Function.
• One type of collaboration involves Pfizer working with two or more separate entities to achieve a
common objective (e.g., public policy development). This type of collaboration is commonly known as
a coalition. Pfizer’s membership in a coalition may involve monetary funding or a donation in-kind of
resources or expertise but must always include Pfizer’s involvement in the development of the mission
and goals and the advancement of the aims of the collective group. Due to a high degree of legal risk
in healthcare-related coalitions, the majority of the group’s members must be non-commercial, non-
manufacturer organizations and they should be the partners who have ultimate control over the coalition
and its messaging, subject to Pfizer’s rights to review the content for factual accuracy and to ensure
compliance with applicable laws, regulations, and Pfizer policies.

White Guide – Chapter 7: Support of External Organizations
Rev. 12/20
Page 121 of 405
Collaborations – Tangible Benefit and Disclosure of Pfizer Involvement
Given the nature of Pfizer’s involvement in collaborations, including the provision of strategic input and
often the rights to use the output of the activities, this category must provide Pfizer with a tangible
benefit and should not be considered a charitable contribution even if the receiving organization is
a not-for-profit entity.
Pfizer’s participation in collaborations must also be appropriately disclosed in all resulting materials in a
manner that does not imply that funding was provided via an unrestricted grant or charitable contribution
(e.g., “Developed in partnership with Pfizer” rather than “Funding support provided by Pfizer”).
Overview
Pfizer sponsors awards, scholarships, fellowships, and similar funding in support or recognition of HCPs
and students. WMS and BU Medical are permitted to fund awards, fellowships, and scholarships. Certain
PHI colleagues are also permitted to fund fellowships. The requirements and process in this section relate
to the US External Funding SOP. R&D colleagues should refer to R&D SOP 202 for requirements related
to Fellowship funding.
Awards are programs developed with an independent professional group to provide funds or other
recognition to an individual demonstrating professional excellence in the field of medical science or
healthcare leadership or an outstanding commitment to public health or patient care. Fellowships are
generally funds paid to medical schools; academic medical centers; teaching hospitals; schools of nursing,
pharmacy, or public health; and other healthcare-related organizations to support junior faculty or emerging
leaders in medical science for one or more years of research or study. Scholarships are funds awarded
to students engaged in a full-time academic activity (normally a medical degree) to aid with education costs.
Pfizer also sponsors awards, scholarships, fellowships, and similar funding that: (1) permit medical
students, residents, fellows, and other healthcare professionals in training to attend carefully selected
educational conferences; or (2) support clinical or research fellowships.
• Colleagues who May Provide Funding: Awards, scholarships, and fellowships are permitted to be
funded only by WMS and BU Medical colleagues. PHI colleagues involved in designing and
conducting research related to health economics and real world data are the only CBO colleagues
permitted to fund fellowships.
• Approval Process: All such funding requests are subject to review and approval by designated
reviewers aligned to each BU/Division/Function.
• Requirements: Pfizer funding of awards, scholarships, and fellowships is permissible only under the
following circumstances:
Awards, Scholarships, and Fellowships

White Guide – Chapter 7: Support of External Organizations
Rev. 12/20
Page 122 of 405
o The selection of awardees is independent of direct and indirect Pfizer influence, which includes
direct selection of awardees as well as choosing the selection committee that makes the ultimate
decision about individual awardees;
o The application is competitive and open to all relevant institutions and candidates in a given
geographic area or therapeutic area;
o Resulting programs are not related to any Pfizer product;
o Pfizer receives an unsolicited request from an organization to fund a fellowship program that
already exists, or is being developed, and will be operated by, the organization; and
o Such awards, scholarships, and fellowships comply with applicable state laws and regulations.
In addition, awards, scholarships, and fellowships must be provided directly to requesting organizations
(e.g., academic medical center; professional association) that independently select final individual
awardees. It is permissible to assemble and retain a selection committee to evaluate requesting
organizations when such expertise is required; provided that such requesting organizations independently
select the individual student or HCP ultimately to receive the award, scholarship, or fellowship. Whenever
possible, programs should be co-sponsored with non-profit medical societies, professional groups, or
similar organizations.
Awarded funds must be used only for the direct expenses of the program and may not be used to subsidize
the requesting organization’s existing, routine, or ordinary business expenses. Fellowships must be paid
directly to the awardee’s institution and cannot be paid directly to the awardee. In addition, individuals who
already have positions at the requesting organization are not prohibited from consideration as long as the
pool of candidates is wide-ranging. Fellowship funds cannot be used to cover a salary for a position that
bills services, or for that portion of a position that bills services. If a position includes both billable services
and research or teaching, the award must be pro-rated based on the amount of time the awardee will devote
to non-billable teaching and research. Also, funding cannot be used to cover the salaries of other individuals
assisting the awardee.
Personal Volunteering
With the exception of manager-approved team building activities or site-led hands-on volunteer activities,
volunteering activities by Pfizer colleagues must be done during a colleague's personal time. Please review
CP801 to review guidance on volunteering. Personal volunteering should not be linked to commercial goals
or objectives or otherwise be part of promotional activities or business plans.
Non-Financial Support

White Guide – Chapter 7: Support of External Organizations
Rev. 12/20
Page 123 of 405
This prohibition, however, does not apply to activities approved by the relevant BU or division that are
undertaken with organizations to promote Pfizer’s products or advance Pfizer’s business interests
appropriately. For example, an Account Manager can join an employer coalition for the purpose of
advocating for Pfizer’s position on formulary benefit design (assuming necessary approvals are obtained).
Regular Membership and Board Membership
Colleagues should exercise caution when participating as a regular member, officer, trustee or board
member of an external organization, particularly if the organization is likely to request funding from Pfizer.
Colleagues must always ensure that their participation in external organizations is consistent with this
Chapter, the Summary of Pfizer Policies on Business Conduct (the “Blue Book”), Corporate Policy 203: -
Conflicts of Interest, and other applicable Pfizer policies that address conflicts of interest.
Pfizer colleagues participating as officers or board members must recuse themselves from joining
in any decisions or activities relating to Pfizer, Pfizer products, or competitor products.
While Pfizer encourages you to be active in the community in which you live and work, some activities,
such as serving on a board of directors or trustees, advisory board or committee, may present a conflict of
interest in some situations. Colleagues must ensure that such activities do not present a conflict of interest
or create the appearance of one, pursuant to Corporate Policy 203: Conflicts of Interest. With limited
exception (as described in CP203), before accepting a role with an outside organization, inform your
manager to determine if any specific review or approvals are required. In some situations, consultation with
Legal and Compliance may be appropriate and additional approvals required.
Accordingly, every colleague who participates as a regular member, officer, trustee or board member of an
external organization that requests funding from Pfizer (in the form of a sponsorship, charitable contribution,
Special Event, or otherwise) must obtain approval from Corporate Responsibility prior to making a financial
commitment. In addition:
1. Make appropriate disclosures to the reviewer responsible for reviewing the funding request. These
disclosures must identify the colleague’s role in the organization and his or her involvement in the
activity for which funding is being solicited (for example, participation on an event planning committee);
and
2. Disclose to the organization, prior to the submission of a funding request that he or she is not
participating in Pfizer’s review or approval of the request.

White Guide – Chapter 7: Support of External Organizations
Rev. 12/20
Page 124 of 405
• Sales Colleagues who need information about policies for funding Exhibit and Display opportunities
can review Orange Guide Chapter 2: Interactions with HCPs and ED SOP2-01 – Exhibits and Displays
Standard Operating Procedure available in Global Policy Xchange on GCO On Demand under
“Funding Requests”.
• SOP on Funding Requests for Not-for-Profit Organizations applies to U.S.-based (and non-U.S. based
when using U.S. cost centers) colleagues in the BUs, WMS, CBO, and Corporate Affairs. For questions
relating to this SOP, e-mail USFundingRequest@Pfizer.com.
• For other general information and training materials regarding Funding Requests, consult the Funding
Requests tab on GCO Policy Xchange.
• For questions regarding (non-policy-focused) healthcare charitable contributions, e-mail
healthcharitables@Pfizer.com or visit www.pfizer.com/healthcharitables.
• R&D colleagues must refer to R&D’s Compliance CNTR for guidance and support.
• Please refer to Orange Guide Chapter 18: Meals, Educational Items, Greenstone Giveaways, and HCP
Payment Disclosure for more information on our funding disclosure obligations under the Sunshine Act.
• For more information on the Pfizer Foundation, please refer to
https://www.pfizer.com/purpose/responsibility/the-pfizer-foundation.
• For information about Pfizer’s disclosure of external funding activities, please visit
https://www.pfizer.com/purpose/transparency/transparency-in-grants.
• For information regarding Pfizer’s policies related to donations to, and interactions with, Independent
Charity Patient Assistance Programs (ICPAPs) organizations, as it relates to Pfizer Colleagues is
described in more detail in Corporate Policy and Procedure #803 – Contributions to Independent
Charity Patient Assistance Programs.
• Refer other questions to your Legal support.
For More Information

Rev. 12/20
Page 125 of 405
CHAPTER #8 – NON-PROMOTIONAL
AND MEDIA ACTIVITIES

White Guide – Chapter 8: Non-Promotional and Media Activities
Rev. 12/20
Page 126 of 405
Chapter
#8
NON-PROMOTIONAL AND MEDIA
ACTIVITIES
CONTENTS
Introduction................................................................................................................................................ 128
Key Points to Ensure Compliance ...................................................................................................... 128
Service-Based Relationships .................................................................................................................... 129
Bona Fide Consulting Engagements .................................................................................................. 130
Speaker Programs .............................................................................................................................. 130
Non-Promotional Communications ........................................................................................................... 131
Scientific Exchange ............................................................................................................................ 132
Third Party Scientific Meetings ........................................................................................................... 133
Transactional Communications .......................................................................................................... 134
Responding to Unsolicited Requests for Medical Information .................................................................. 135
Specified Roles with Respect to Non-Promotional Communications ................................................. 135
Pfizer Medical Information Department ........................................................................................ 135
External Promotional Speakers ................................................................................................... 135
Field Medical Directors and Similar Field-Based Medical Colleagues ........................................ 136
MOS Colleagues and Similar Field-Based Medical Colleagues .................................................. 136
Other Pfizer Medical Colleagues ................................................................................................. 136
Press Releases and Other Media Communications ................................................................................. 137
Disclosures of “Material” Developments ............................................................................................. 137
Corporate Press Releases ................................................................................................................. 139
Press Releases relating to new clinical study results ......................................................................... 139
Promotional Press Releases .............................................................................................................. 140
Product-Specific Press Kits and Other Media Materials..................................................................... 140
Contact Information & Disclaimer ....................................................................................................... 141
Non-Promotional and Media Activities

White Guide – Chapter 8: Non-Promotional and Media Activities
Rev. 12/20
Page 127 of 405
Non-Promotional External Speaking Engagements and Publications ...................................................... 141
Interviews and Other Requests for Information ........................................................................................ 142
For More Information ................................................................................................................................. 142
White Guide – Chapter 8: Non-Promotional and Media Activities
Rev. 12/20
Page 128 of 405
Chapter #8 Non-Promotional and Media Activities
In the United States, the Food and Drug Administration (FDA) regulates all advertising and promotional
labeling that Pfizer disseminates for its products. For more information on Pfizer policy regarding the
development, review, and approval of advertising and promotional labeling, see White Guide Chapter 2:
Advertising and Promotional Materials. The FDA does recognize, however, that certain activities and the
provision of information about current research and scientific data may be neither advertising nor
promotional labeling. Thus, manufacturers may distribute certain information, and make some
communications, without being subject to FDA rules. Such non-promotional activities can generally be
characterized as either service-based relationships or non-promotional communications.
This Chapter summarizes certain key Pfizer policies regarding key non-promotional activities,
including certain media activities. Non-compliance with these policies puts the Company at risk
and can subject Pfizer colleagues to disciplinary action up to and including termination of
employment.
• Non-promotional communications are those which are not designed or intended to promote
the use of a Pfizer product in order to impact prescribing, purchase, or recommendation. They
must be: truthful, accurate, and not misleading; supported by relevant scientific data (including
any relevant safety data) where applicable, and complete (i.e., not "cherry-picked"); narrowly
tailored to the topic being discussed; and void of any promotional claims or promotional
context.
•
Within a service-based relationship, both on-label and unapproved uses for an approved
product as well as unapproved products may be presented or discussed with an HCP during
his or her performance of a service for Pfizer so long as any off-label information is relevant
and narrowly tailored to the specific bona fide purpose of the service arrangement. All
applicable policies, procedures, and approval processes for engaging HCPs for services must
be followed.
Key Points to Ensure Compliance
Introduction

White Guide – Chapter 8: Non-Promotional and Media Activities
Rev. 12/20
Page 129 of 405
Key Points to Ensure Compliance
• Transactional communications are those that are generally administrative or "business to
business" in nature, do not involve a clinical discussion and do not contain any promotional
claims.
• Scientific exchange is a type of medical communication, defined as "the communication of
medical information in a non-promotional manner and may include unapproved information."
Scientific exchange is generally regarded by Pfizer as an infrequent activity in which
authorized Medical colleagues engage and which requires approval by the BU Medical Asset
Lead, BU Chief Counsel, and the Medical Governance Committee.
• All Pfizer colleagues (including Medical colleagues engaged in scientific exchange) are
prohibited from making any claims of safety or efficacy about an unapproved product (e.g., a
pipeline product) or about an unapproved use for an approved product.
• Pfizer policy only permits certain Pfizer Medical colleagues to respond to unsolicited requests
for medical information about unapproved products or uses. All other colleagues must refer
unsolicited medical requests to Pfizer's Medical Information Department (1-800-438-1985) or
www.pfizermedinfo.com.
• All press releases must be coordinated with and issued by Pfizer Global Media Relations (1-
212-573-1226). A press release discussing an unapproved product or use or other information
that may be considered inconsistent with product labeling must be non-promotional in tone and
must comply with the principles of scientific exchange. It may not state that an unapproved
product (or an unapproved use of an approved product) is "safe" or "effective."
• Material nonpublic information (i.e., information that could reasonably be expected to impact
the Company’s stock price) must be communicated only in a press release, a filing with the
U.S. Securities and Exchange Commission, and/or a webcast presentation to which the public
has been invited in advance.
• All media inquiries must be directed to Pfizer Global Media Relations (1-212-573-1226) and all
inquiries from investors and investment analysts must be directed to Pfizer Investor Relations
(1-212-573-2668).
Pfizer engages HCPs and others (such as consumers and advocates) to perform services necessary for
the operation of Pfizer business. Generally, such service-based relationships are performed under a
service/consultant agreement, and any compensation provided to the engaged individual in return for
services performed must be at fair market value. At times, an individual may be willing to provide services
Service-Based Relationships

White Guide – Chapter 8: Non-Promotional and Media Activities
Rev. 12/20
Page 130 of 405
to Pfizer without compensation. Regardless, in all service-based relationships, Pfizer must have a
legitimate, good-faith business need for the services being performed and an agreement in place.
When HCPs are engaged to provide bona fide services, communications directly related to the service-
based relationship are considered non-promotional. Although service-based relationships must never be
used as a pretext for communicating information that would otherwise be impermissible to disseminate,
information about unapproved products or indications may be shared so long as it is relevant and narrowly
tailored to the specific bona fide purpose of the service arrangement. Unapproved information may also
be discussed prior to the service-based relationship for the purpose of proposing a service-based
relationship; however, any such information must be limited to that which is essential to enable a decision
on whether to enter into the service arrangement and must not be a pretext for a discussion that would
otherwise be impermissible. A non-disclosure agreement may be required before any such communication.
Consult your team attorney before sharing any potentially sensitive information without a non-disclosure
agreement in place.
Bona Fide Consulting Engagements
Consulting engagements are one type of service-based relationship. For instance, Pfizer may engage
HCPs, consumers, advocates, and formulary decision makers to serve as consultants in their individual
capacity, as well as to serve on advisory boards with other consultants. All applicable policies, procedures,
and approval processes for engaging consultants must be followed. For more information, see White Guide
Chapter 5: HCP and Government Official Consulting Engagements.
Bona Fide Consulting Engagement
Pfizer is planning to pursue a new indication for an oncology product. The clinical
team lead for the product would like to engage a consultant to assist with clinical trial
design, which would involve discussion of unapproved uses for the product. Is this
permissible?
Yes. In order to obtain services in connection with clinical trial strategy for a new
indication, the clinical team would have to discuss unapproved uses for the product.
Of course, the interaction must always be scientific and objective in tone and
substance and follow relevant Pfizer guidelines.
Speaker Programs
Although speaker programs involve a Pfizer service-based relationship with a speaker, speaker programs
are promotional activities because they are intended to influence the prescribing of the HCPs who attend
the programs. To ensure compliance, all speakers must be trained and contractually agree to abide by

White Guide – Chapter 8: Non-Promotional and Media Activities
Rev. 12/20
Page 131 of 405
FDA regulations and Pfizer policies governing promotion. These policies require that all Pfizer promotional
speakers use RC-approved materials and provide information consistent with product labeling. Remember
that it is not permissible to engage a particular HCP as a speaker in order to influence his or her prescribing.
In strictly limited circumstances, Pfizer permits speakers to respond to unsolicited questions from the
audience requesting specific information outside of product labeling. The speaker may respond briefly to
the specific question but must note that the use/information under discussion is unapproved and that he or
she is answering the question based upon his or her own knowledge or experience and that their response
may not represent the view of Pfizer. For more information, see White Guide Chapter 4: Marketing
Programs and Orange Guide Chapter 9: Speaker Programs for HCPs.
The FDA regulates promotional labeling – including both printed and oral statements designed or intended
to promote the use of a Pfizer product in order to impact prescribing – regardless of whether the promotional
statement is made by a Sales or Marketing colleague or someone from another function. All promotional
statements must be consistent with a product’s approved labeling and Global Commercial Content: Review
and Approval Procedure – U.S. Addendum. In contrast, non-promotional communications are those that
are not designed or intended to promote the use of a Pfizer product in order to impact prescribing.
Non-promotional communications outside of service-based relationships are generally divided into several
distinct categories:
• Responses to unsolicited medical requests from HCPs or other customers;
• Proactive communication of clinical or scientific information that is new and/or urgently important
to particular HCPs/customers (“scientific exchange”) (See EMC01 – LSOP 3.0 Medical Review
Committee for review and approval process) EMC01 – LSOP 3.0 Medical Review Committee;
Publications in peer-reviewed journals; and
• Transactional Communications.
Each category has specific rules that govern its appropriate use, but in general, non-promotional
communications must be:
• Truthful, accurate, and not misleading;
• Supported by relevant scientific data where applicable, including any relevant safety data, and
complete (i.e., not “cherry-picked”);
• Narrowly tailored to the purpose and/or topic being discussed; and
• Void of any promotional claims or promotional context.
Non-Promotional Communications

White Guide – Chapter 8: Non-Promotional and Media Activities
Rev. 12/20
Page 132 of 405
Scientific Exchange
In limited circumstances, Pfizer may proactively provide scientific and medical information about
unapproved products or uses, or information inconsistent with an approved product’s labeling under the
principle of “scientific exchange.” Scientific exchange includes the proactive communication by Medical
colleagues of medical information in a non-promotional manner. Whether a communication will be
considered non-promotional depends on the content of the communication as well as the context in which
the information is presented. Given the infrequent nature of Scientific Exchange, any proposed medical
communication of this type must first be approved by the BU Medical product/asset lead and BU Chief
Legal Counsel, in consultation with Global Medical Governance.
Key factors that BU Medical, BU Chief Legal Counsel, and Global Medical Governance will consider when
evaluating a proposal for Scientific Exchange include the following:
• Whether the data proposed to be communicated is novel and/or urgently important to particular
HCPs/customers. Providing previously disclosed information that is no longer new or is already
known within the medical community is more likely to be viewed as promotional, while providing
new, robust, important scientific information that is not widely known in the medical community is
more likely to be viewed as non-promotional;
o Often such data will include new safety information;
• Proposed frequency, duration, and reach of the medical communication;
o Frequency and duration should be limited and HCPs/customers receiving the information
should be narrowly selected on a need to know basis;
• Proposed execution of the communication. Non-promotional communications must not be
promotional in tone (i.e., they must be devoid of brand logos and colors, promotional slogans, and
other content promoting a Pfizer product). Claims about the safety or efficacy of an unapproved
product or for an unapproved indication are likely to be considered promotional and are not
permitted to be proactively delivered under the guise of scientific exchange.
In terms of context:
• The involvement of Sales or Marketing functions makes a communication more likely to be viewed
as promotional, while involvement limited to Medical colleagues or clinical investigators may make
the communication more likely to be viewed as non-promotional.
• If the activity is part of a commercial strategy, it is more likely to be viewed as promotional than if it
were an activity initiated and led by Medical (without Sales and Marketing involvement).

White Guide – Chapter 8: Non-Promotional and Media Activities
Rev. 12/20
Page 133 of 405
Scientific exchange is generally regarded by Pfizer as an infrequent activity in which authorized Medical
colleagues engage. Scientific exchange must be approved by the BU Medical Asset Lead, BU Chief
Counsel, and Medical Governance Committee.
All Pfizer colleagues (including Medical colleagues engaged in scientific exchange) are prohibited from
making claims of safety or efficacy about an unapproved product (e.g., a pipeline product) or about an
unapproved indication for an approved product.
Even within the context of scientific exchange, all information disseminated must be truthful, accurate, and
non-misleading. Similarly, any communications, including those under scientific exchange, that are viewed
by the government as concerted activity to promote unapproved use of a company's product, and/or
concerted activity intended to result in improper claims for government reimbursement, could lead to civil
or criminal prosecution under the federal False Claims Act (FCA).
Third Party Scientific Meetings
Third party scientific meetings and congresses provide an important venue at which Pfizer Medical and
other authorized colleagues can present, critically review, and discuss ongoing or completed research
among a professional peer group. Even so, not all activities at scientific meetings qualify as legitimate
scientific exchange or other non-promotional communication. As a result, individual activities must be
considered to determine whether the content and context of the activity qualify as non-promotional.
The table on the following page provides details and examples of factors that can help determine whether
an activity at a third-party meeting is likely to be viewed as promotional or non-promotional.
More likely to be viewed as
Content / Context
Promotional
Non-promotional
Type of
Presentation
Company-sponsored satellite
symposia
Peer-reviewed podium or poster
presentation in bona fide scientific
session of a medical congress
Originality of
Content
Previously disclosed information that is
no longer new or is already known
within the medical community
New, important scientific information
that is not widely known in the medical
community
Peer Review
Information has not undergone formal
peer review
Information has undergone formal peer
review
Location of
Activity
Commercial booth
Medical Information booth separated
from any commercial space or activity

White Guide – Chapter 8: Non-Promotional and Media Activities
Rev. 12/20
Page 134 of 405
More likely to be viewed as
Content / Context
Promotional
Non-promotional
Speaker
An individual with no direct involvement
in the research being presented
A Medical colleague or investigator
with direct involvement in the research
being presented
Role of Sales and
Marketing
Sales or Marketing involvement
No involvement by Sales or Marketing
Since no one factor is determinative, the totality of the circumstances must be considered when assessing
whether a particular presentation or activity constitutes legitimate scientific exchange or other non-
promotional communication not subject to promotional standards. For more information please consult
your team attorney.
Transactional Communications
Transactional communications are those that are generally administrative or “business to business” in
nature, do not involve a clinical discussion and do not contain any promotional claims. Examples of
transactional communications are:
• Communications with customers that contain only a factual statement of matters such as product
price, availability, formulary status, coding, etc. and do not contain any clinical information;
• Administrative communications with consumers or HCP customers regarding copay, patient
assistance or similar approved programs, that contain no promotional claims (such as letters
enclosing reimbursement checks; letters confirming eligibility or denial in copay programs; websites
that only administer reimbursement or copay programs with no promotional claims; etc.); and
• Communications to “C-suite” or similar level customers that are not intended to promote product
use or formulary placement but have separate business purposes such as a potential business
collaboration or joint initiative with a customer.
Any disease state or product related information contained within these Transactional Communications
should be non-promotional in content and tone and should be the minimum necessary to meet the non-
promotional purpose of the communication.

White Guide – Chapter 8: Non-Promotional and Media Activities
Rev. 12/20
Page 135 of 405
To help ensure that responses to unsolicited questions seeking unapproved information are considered
non-promotional communications, Pfizer policy permits only certain Pfizer Medical colleagues to respond
to such requests for information. For these colleagues, the provision of off-label information in response to
a question is appropriate so long as the question is unsolicited, and the response is:
• Truthful, accurate, balanced, and not misleading;
• Supported by relevant scientific data, including any safety data, and complete (i.e., not “cherry-
picked”);
• Narrowly tailored to answer the question asked;
• Void of any promotional claims; and
• Documented in accordance with relevant Pfizer policy (e.g., the Green Guide).
For more information on whether Medical colleagues not identified below are permitted to respond to a
request for unapproved medical information, please consult your team attorney.
Specified Roles with Respect to Non-Promotional Communications
Pfizer Medical Information Department
The Pfizer Medical Information Department provides accurate, timely, and balanced medical information to
customers, including responses to unsolicited customer requests. Medical Information is structured to
enable Pfizer to respond appropriately to inquiries that may require reference to both on-label and
unapproved data. If a colleague, including a Medical colleague, is involved in a promotional interaction with
an HCP who has unsolicited questions about unapproved products or indications, the colleague must refer
the HCP to Pfizer's Medical Information Department (1-800-438-1985).
External Promotional Speakers
HCPs retained as promotional speakers cannot solicit unapproved questions or initiate unapproved
discussions of our products with other HCPs at Pfizer speaker programs. If a promotional speaker is asked
an unsolicited question regarding unapproved information by an audience member, however, he or she
may briefly respond to the specific question. Speakers must note that the use/information under discussion
is unapproved, that he/she is answering the question based upon his/her own knowledge or experience,
and that his/her views may not represent the views of Pfizer. A promotional speaker retained by Pfizer is
“speaking for Pfizer” when he or she presents, and failure to adhere to these guidelines could expose Pfizer
(and the speaker) to the risk of prosecution and penalties.
Responding to Unsolicited Requests for Medical Information

White Guide – Chapter 8: Non-Promotional and Media Activities
Rev. 12/20
Page 136 of 405
Field Medical Directors and Similar Field-Based Medical Colleagues
Field Medical Directors (FMD) provide approved (through the Medical Review Committee process EMC01
– LSOP 3.0 Medical Review Committee), non-promotional medical and scientific information to HCPs
regarding the safe and appropriate use of Pfizer medicines for approved indications. FMDs may also
provide support for Pfizer-sponsored research activities (e.g., facilitation of research site selection and study
placement) and interact, where appropriate, with Investigator-Sponsored Research investigators. Please
consult the Green Guide: Governance for External Medical Activities, for policy on responding to requests
for unapproved information and other non-promotional activities. The Green Guide is applicable to FMD,
Medical Outcomes Specialists (MOS), and other field-based Medical colleagues in the United States, as
well as U.S. Business Unit (BU) Medical Affairs colleagues, when interacting with HCPs.
MOS Colleagues and Similar Field-Based Medical Colleagues
Medical Outcomes Specialists (MOS) is a group within U.S. Medical Affairs that primarily works with
organized customers such as payers (including formulary and P&T committees), Integrated Delivery
Networks, medical groups, and colleges of pharmacy. In general, MOS responsibilities include:
(a) demonstrating the pharmacoeconomic value of Pfizer’s in-line products; (b) collaborating with
customers to advance the quality of patient care in areas of interest to Pfizer; (c) working with customers
on outcomes research to identify provider or patient knowledge gaps and areas for quality improvement
interventions; and (d) providing Pfizer brand teams with customer perspectives to enable the development
of appropriate customer-focused tools and medical communications to support patient access to medicines.
The MOS group may respond to unsolicited requests for: on-label data; pharmacoeconomic information
related to an approved indication, whether or not included in product labeling; and information consistent
with the product label and approved by a Medical Review Committee (MRC). MOS are not permitted to
respond to unsolicited requests for unapproved data.
All unsolicited requests received by MOS for unapproved data, including those seeking information on the
general safety or efficacy of Pfizer products, must be referred to Pfizer’s Medical Information Department.
The MOS group and other similar field-based medical groups must adhere to the Green Guide.
Other Pfizer Medical Colleagues
As mentioned above, FDA laws and regulations apply to promotional statements made by Pfizer Medical
colleagues about our products in much the same way that they apply to statements by Sales
representatives and other Pfizer colleagues. However, there may be limited circumstances in which it is
permissible for Pfizer Medical colleagues to respond to an unsolicited request for medical information. For
more information on whether it is permissible to respond to a request for medical information, Medical
colleagues should consult their team attorney.
Unsolicited Request for Medical Information

White Guide – Chapter 8: Non-Promotional and Media Activities
Rev. 12/20
Page 137 of 405
A physician contacts Pfizer seeking information regarding the unapproved use of the
Pfizer product. Can the Pfizer Medical colleague who supports the product provide
this information?
Yes. It is permissible for the Medical colleague to provide the requested information
as long as the information provided is: (1) truthful, accurate, and not misleading; (2)
supported by the relevant scientific data, including any safety data; (3) narrowly
tailored to answer the question asked; and (4) void of any promotional claims.
Press releases provide timely updates on an array of topics, such as new business alliances, significant
regulatory decisions, major recalls or safety issues, financial performance, and significant clinical trial
results. They are typically disseminated over a paid news distribution service (e.g., BusinessWire) and to
print, broadcast, and online news sources, as well as posted on www.Pfizer.com. Pfizer Global Media
Relations oversees all communications intended for release to the media, whether written, verbal, or
electronic (including press releases, video news releases, submissions for newspapers, and media FAQ
documents). For guidance regarding dissemination of press releases and other information via corporate
social media channel(s), please see the Pfizer Twitter Guidelines and CP #407: Social Media Policy.
Disclosures of “Material” Developments
Because Pfizer is a publicly traded company, Pfizer generally seeks to inform the investment community of
“material” developments (i.e., developments that could reasonably be expected to impact the Company’s
stock price). Press releases help Pfizer to accomplish this goal. Our press releases must provide balanced,
accurate, complete, and non-misleading information. Failure to do so can trigger lawsuits. For example,
investors might seek damages based on a claim that they were not provided adequate information about
events that negatively impacted the Company’s stock price. Pfizer Global Media Relations will consult with
Corporate Governance to determine if a disclaimer is required regarding forward looking information (see
“Contact Information & Disclaimer” below).
Material nonpublic information may not be disclosed selectively – meaning it may not be disclosed in
nonpublic conversations, meetings, or written materials or other means – to financial market participants.
Such information may be disclosed only for legitimate business reasons on a need-to-know basis internally
to Pfizer colleagues or to engaged consultants or advisors who are bound by an obligation to maintain
confidentiality. At the time of public disclosure, such information must be disclosed to the entire investment
community in a press release, a filing with the U.S. Securities and Exchange Commission (SEC), a
webcast presentation to which the public has been invited in advance, and/or another method reasonably
designed to provide broad dissemination. Only information that has been previously disclosed publicly may
Press Releases and Other Media Communications

White Guide – Chapter 8: Non-Promotional and Media Activities
Rev. 12/20
Page 138 of 405
be discussed in nonpublic settings, such as in meetings or calls with investors or investment analysts. For
more information, see CP #604: Treatment of Material Nonpublic Information.
Corporate Governance, in consultation with investor relations/media (as well as, if appropriate, certain
internal stakeholders) will make an assessment as to whether a press release is material (i.e., whether the
press release discloses developments/information that could reasonably be expected to impact the
Company’s stock price). A determination will also be made regarding whether a blackout notice (which
may restrict trading of Pfizer securities by certain colleagues) should be sent to, and/or preclearance
procedures imposed upon, those colleagues “in the know” prior to public disclosure of the development.
Individuals that are “in the know” should not trade in Pfizer securities prior to the determination of whether
the information included in the press release is material and whether a blackout notice is required. Whether
or not a blackout notice is imposed, pursuant to CP 604, colleagues must not trade in Pfizer securities while
aware of material nonpublic information relating to the Company or trade in the securities of any other
company while aware of material nonpublic information relating to that company which was obtained in the
course of their employment with Pfizer.
When Pfizer issues a press release related to products under investigation for new, unapproved uses (even
if the product is approved and marketed for other indications), the Company must strike an appropriate
balance to comply with both regulatory restrictions against pre-approval promotion and Pfizer’s obligations
as a publicly traded company to disclose material developments to the investment community. As a general
rule, press releases addressing new, unapproved uses must be scientific and objective, not promotional in
tone, and must clearly indicate that the product is not approved for the studied use by the FDA or regulatory
authorities in other jurisdictions. There should be no promotion of an unapproved use for a marketed
product (i.e., a press release should not claim that a drug is safe and effective for an unapproved indication
and any unapproved uses should be described as “investigational”).
• Press releases disclosing “material” developments are typically non-promotional and must be
approved by Pfizer Global Media Relations in consultation with Finance, Investor Relations,
Corporate Governance, as well as the Legal, Regulatory, and Medical colleagues responsible for
the product/therapeutic area, if applicable.
If you receive an inquiry from investors or investment analysts, you must refer them to Pfizer Investor
Relations (1-212-573-2668). Any inquiry from the media should be forwarded to Pfizer Global Media
Relations (1-212-573-1226).
Following is additional information regarding Corporate, New Data, and Promotional press releases,
each of which must be assessed for materiality, blackout notice, etc. in accordance with the
procedures set forth above.

White Guide – Chapter 8: Non-Promotional and Media Activities
Rev. 12/20
Page 139 of 405
Corporate Press Releases
Pfizer typically announces new business alliances, significant regulatory decisions, major recalls or safety
communications, and information regarding financial performance, among other things, via “Corporate”
press releases. A Corporate press release may not contradict product labeling or promote an unapproved
use. Similarly, it should not claim that a product is “safe.” If an unapproved use is discussed, it must be
described as investigational in the press release.
• Corporate press releases must be approved by Pfizer Global Media Relations in consultation with
Investor Relations, Corporate Governance, as well as the Legal, Regulatory, and Medical
colleagues responsible for the product/therapeutic area.
Pre-approval Communications / Press Releases
Is it permissible to issue a press release to the investment community claiming that a
new study demonstrates that a product (or a new use) that has not yet been FDA-
approved is safe and effective?
No. Press releases that provide details about unapproved products or uses must be
objectively factual and should avoid the use of promotional adjectives or conclusory
comments about safety or efficacy (as the regulators have not yet made
determinations about those issues). They should also describe such uses as
“investigational.”
Press Releases relating to new clinical study results
Press releases announcing new clinical trial results must describe the size of the study, the study design,
and the primary endpoints. If a decision is made to include results of secondary endpoints, all of the
secondary endpoints must be included. Furthermore, if the new data release contains disclosure of Phase
3, Phase 3B and certain Phase 4 study results subject to Clinical and Medical Controlled Document (CMCD)
CT20-POL: Public Disclosure of Pfizer Clinical Study Data and Authorship, the requirements of that policy
should be met. For more information, see CMCD CT20-POL: Public Disclosure of Pfizer Clinical Study
Data and Authorship.
If Pfizer decides to disseminate previously released study data on a subsequent occasion via a press
release, then it would generally be considered a “promotional” press release, which is discussed in more
detail below. Similarly, if promotional language or tone is used, then the press release needs to be treated
as a promotional press release.
• “New data” press releases are initiated by Global Media Relations and must be approved by the
Product Counsel in consultation with Clinical Development Legal; Regulatory, and Medical

White Guide – Chapter 8: Non-Promotional and Media Activities
Rev. 12/20
Page 140 of 405
colleagues responsible for the product/therapeutic area in consultation with Investor Relations and
Corporate Governance.
Promotional Press Releases
Press releases that discuss marketed products may be subject to FDA standards for promotional labeling.
Therefore, a product’s approved indication(s), a fair balance of risk information, and a link to the approved
product label must be included if a promotional press release includes claims of safety and efficacy.
Risk information typically includes contraindications, warnings, precautions, adverse events, and other
material information. Unless the press release is targeted to media outlets that primarily reach scientific or
professional audiences, a consumer-friendly version of safety information should be included. In addition,
the FDA-approved full prescribing information should be supplied with all press releases involving marketed
products (paper copies should include a copy of the approved prescribing information and electronic copies
should reference the location of the prescribing information on https://www.pfizer.com). A promotional
press release may not contradict FDA-approved labeling or promote an unapproved use. In addition, the
FDA views promotional product-related press releases as subject to submission at time of first use. Thus,
such press releases must be reviewed and approved by the relevant Review Committee (RC) and
submitted to the FDA’s Office of Prescription Drug Promotion (OPDP) or Advertising and Promotional
Labeling Branch (APLB) (for biologics, including vaccines) for filing on or before date of first use (DOFU),
prior to dissemination.
• Promotional press releases must be approved by the Product Review Committee (Medical,
Regulatory, Legal, and Marketing) and Pfizer Global Media Relations in consultation with Investor
Relations and Corporate Governance.
Product-Specific Press Kits and Other Media Materials
Product-specific “press kits” are subject to the same FDA regulatory requirements as written promotional
materials. Thus, a press kit must meet promotional standards (e.g., not misleading, consistent with product
labeling, and including appropriate safety information) and must be RC-approved.
For components within the press kit that may be distributed further, the appropriate balance must be
included within those components. A press kit must also contain a copy of the full Prescribing Information
for any Pfizer product that is referenced in the press release.
As with press kits, other media materials, such as audio/video news releases, are generally regarded as
promotional labeling and therefore must meet promotional standards and be RC approved. For more
information on such standards and the review and approval process for promotional materials please refer
to Global Commercial Content: Review and Approval Procedure – U.S. Addendum and White Guide
Chapter 2: Advertising and Promotional Materials.

White Guide – Chapter 8: Non-Promotional and Media Activities
Rev. 12/20
Page 141 of 405
Post-approval Communications
Do we need to submit for internal review a press release that highlights newly
published clinical trial data for an approved Pfizer product?
Yes. Any release that discusses data about an approved product or unapproved
product must be approved by Legal, Medical, and Regulatory, as well as Pfizer Global
Media Relations in consultation with Investor Relations and Corporate Governance.
Contact Information & Disclaimer
Press releases must be dated and should contain contact information for the appropriate person in Media
and/or Investor Relations (and any other appropriate persons).
If a press release could be seen as including any “forward-looking” information (e.g., information that
describes or suggests future events or results), it should include a disclosure notice. Colleagues should
work with their legal representative and contact the Corporate Governance team as early as possible in
order to confirm whether information may be considered forward-looking and to work together to draft and
approve an appropriate disclosure notice, as needed. The press release’s disclosure language will be
customized to the information included in the press release.
Pfizer colleagues may participate in external non-promotional speaking engagements and contribute to
articles and publications relevant to their areas of expertise. As representatives of Pfizer, colleagues must,
however, ensure that any Company information disclosed in presentation materials, handouts, Q&A
sessions, articles, etc., is truthful, accurate, complete, timely, and not proprietary or otherwise confidential.
Further, such external communications should generally be consistent with Pfizer’s publicly stated position
on related issues.
When invited to speak at a third-party sponsored meeting, seminar, workshop, conference, etc., or to author
a document for publication, you must obtain the approval of your manager. Your manager must determine
whether it is appropriate for you to participate and should consult Legal, if necessary. (If you are unclear
whether the content of your proposed activity is likely to be perceived as promotional, you should consult
your team attorney for further guidance).
Colleagues approved to participate in external speaking engagements are not required to obtain prior
review and approval of their presentation materials (including pre-read materials, PowerPoint
presentations, handouts, etc.) unless requested by the approving manager, but must be sure not to disclose
any confidential information or material non-public information and to include appropriate disclaimers in
Non-Promotional External Speaking Engagements and Publications

White Guide – Chapter 8: Non-Promotional and Media Activities
Rev. 12/20
Page 142 of 405
their presentations (including that you are an employee of Pfizer and that views expressed by you may not
represent the views of Pfizer).
If you have any uncertainty regarding what information may be considered confidential or material (or if the
nature of the engagement involves discussion related to Pfizer or Pfizer products) you should consult with
your manager or Legal, as appropriate. If you are asserting any personal opinions in a talk or speaking
engagement, you must clarify with the audience that the opinions expressed are yours and not necessarily
those of Pfizer. If the press, other media, and/or analysts or investors are reasonably likely to be present
at a third-party sponsored event, you must contact Pfizer Global Media Relations and Pfizer Investor
Relations (as applicable) well in advance of the event to ensure effective preparation.
From time to time, Pfizer colleagues may be approached by the media or federal, state, or local officials to
answer questions regarding Pfizer or Pfizer products.
• If you receive any type of inquiry or request for information from the media (including verbal or
telephone, written or electronic requests): direct the inquiry or request to Pfizer Global Media
Relations (1-212-573-1226). Unless specifically directed by a member of Pfizer Global Media
Relations, you may not answer any questions or supply any information directly to the media or
conduct interviews with the media. For more information, see CP #409: Relations with the News
Media.
• If you receive any type of inquiry from investors or investment analysts: direct the inquiry to Pfizer
Investor Relations (1-212-573-2668).
• If you receive any type of inquiry or request for information from any federal, state, or local
government entity: promptly seek guidance from the Legal Division before responding.
• Refer any questions to your Regulatory Affairs or Legal team colleague, Pfizer Global Media Relations
& Digital Communications (1-212-573-1226), or Pfizer Investor Relations (1-212-573-2668).
• Green Guide Green Guide – Governance for External Medical Activities
• Medical Review Committee USA-EMC01-LSOP..
• CMCD GNT-01 Independent Medical Grants.
• Orange Guide Chapter 9: Speaker Programs for HCPs.
• White Guide Chapter 2: Advertising and Promotional Materials.
Interviews and Other Requests for Information
For More Information

White Guide – Chapter 8: Non-Promotional and Media Activities
Rev. 12/20
Page 143 of 405
• White Guide Chapter 4: Marketing Programs.
• White Guide Chapter 5: HCP and Government Official Consulting Engagements.
• CMCD CT20-POL: Public Disclosure of Pfizer Clinical Study Data and Authorship.
• CP #407: Social Media Policy.
• CP #409: Relations with the News Media.
• CP #604: Treatment of Material Nonpublic Information.
• Pfizer Twitter Guidelines.
• Requests for medical information should be directed to Global Medical Information at 1-800-438-1985
or www.pfizermedinfo.com.

Rev. 12/20
Page 144 of 405
CHAPTER #9 – PFIZER-SPONSORED
RESEARCH AND CLINICAL
RESEARCH COLLABORATIONS
(CRCs)

White Guide – Chapter 9: Pfizer-Sponsored Research
and Clinical Research Collaborations (CRCs)
Rev. 12/20
Page 145 of 405
Chapter
#9
PFIZER-SPONSORED RESEARCH AND
CLINICAL RESEARCH COLLABORATIONS
CONTENTS
Introduction................................................................................................................................................ 147
Key Points to Ensure Compliance ...................................................................................................... 148
Healthcare Law Compliance Issues .......................................................................................................... 149
Pfizer-Sponsored Clinical Studies ............................................................................................................. 149
Regulatory and Ethical Framework .................................................................................................... 149
IND Requirements ........................................................................................................................ 149
IDE Requirements ........................................................................................................................ 149
Privacy Rules ............................................................................................................................... 150
Good Clinical Practice .................................................................................................................. 150
Interactions with HCPs and Government Employees .................................................................. 150
Additional Requirements .............................................................................................................. 151
Scientific Validity and Value to Pfizer ................................................................................................. 151
Selection of Investigators ................................................................................................................... 151
Study Design, Conduct, and Monitoring ............................................................................................. 153
Managing Study Conduct Quality Issues ........................................................................................... 153
Compensating Investigators ............................................................................................................... 154
Investigator Meetings ......................................................................................................................... 155
Public Disclosure and Access to Study Data ..................................................................................... 157
Expanded Access ............................................................................................................................... 158
Clinical Research Collaborations .............................................................................................................. 158
General Requirements ....................................................................................................................... 158
CRC Support ...................................................................................................................................... 160
Evaluation of Potential CRCs ............................................................................................................. 160
Scientific Review ....................................................................................................................................... 160
Monitoring, Auditing, Tracking and Data Collection by Pfizer ............................................................ 160
Pfizer-Sponsored Research and Clinical Research Collaborations (CRCs)

White Guide – Chapter 9: Pfizer-Sponsored Research
and Clinical Research Collaborations (CRCs)
Rev. 12/20
Page 146 of 405
Collaborators Roles and Responsibilities ................................................................................................. 160
Regulatory and Ethical Obligations .................................................................................................... 161
Regulatory Requirements ................................................................................................................... 161
Protocol, Informed Consent and other Study Document Development ............................................. 162
Study Monitoring ....................................................................................................................................... 162
Document Reviews and Site Visits ........................................................................................................... 162
Regulatory Inspections and Audits ........................................................................................................... 162
Reporting of Safety Data .................................................................................................................... 162
Drug Supply ........................................................................................................................................ 163
Insurance ............................................................................................................................................ 163
Study Budget ...................................................................................................................................... 163
Governance ............................................................................................................................................... 163
Governance ............................................................................................................................................... 163
Confidentiality Agreement .................................................................................................................. 164
CRC Agreement ................................................................................................................................. 164
Joint Study Team Meetings....................................................................................................................... 164
Study Deliverables .................................................................................................................................... 164
Study Results ...................................................................................................................................... 164
Clinical Study Report (CSR) ............................................................................................................... 164
Study Data .......................................................................................................................................... 165
Publications ............................................................................................................................................... 165
Primary Submission ............................................................................................................................ 165
Publication Quality .............................................................................................................................. 165
Pre-publication Review by Pfizer ........................................................................................................ 166
Redaction of Confidential Information ................................................................................................ 166
Authorship Standards ......................................................................................................................... 166
Disclosure of Support ......................................................................................................................... 166
Secondary Publications ...................................................................................................................... 167
For More Information ................................................................................................................................. 167

White Guide – Chapter 9: Pfizer-Sponsored Research
and Clinical Research Collaborations (CRCs)
Rev. 12/20
Page 147 of 405
Chapter #9: Pfizer-Sponsored Research and Clinical
Research Collaborations (CRCs)
Pfizer engages scientists, Healthcare Professionals (HCPs), academic and other research institutions,
for-profit co-development partners, as well as government agencies to conduct research and
development projects and studies. These include in vitro experiments (discovery), in vivo studies
(preclinical animal), human clinical studies, and consultancies and services related to these areas. This
research can generate important information about Pfizer products as well as valuable medical and
scientific information that can lead to improvements in clinical care, the development of new treatments,
and better delivery of healthcare to patients.
Pfizer-sponsored clinical studies are frequently a key part of the development of medicinal products and
devices. Pfizer-Sponsored clinical studies are designed, conducted, and overseen by Pfizer or on behalf
of Pfizer. When Pfizer is the sponsor, it is generally responsible for the regulatory obligations applicable in
the geographies where these studies are conducted. Pfizer-sponsored studies may be intended to support
a new product, a significant change in the labeling of a product, a new indication, a proposed advertising
claim, or post-marketing commitments. The company may engage the services of Contract Research
Organizations (CROs) or other service providers to assist in execution of some or all elements of clinical
trial conduct including study design, start-up, study management, data monitoring, data analysis, and
reporting. There are various types of Pfizer-sponsored clinical trials, which are covered by different SOPs,
CMCD CT02-GSOP Protocol Development for Interventional Studies, CMCD CT24-GSOP Non-
Interventional Studies and CMCD CT45-GSOP Interventional Studies With Minimal Risk. Contact the
Business Process Owners (BPOs) for these SOPs.
Clinical Research Collaborations or CRCs are engagements under which Pfizer collaborates with an
external party to perform a clinical study and/or other clinical research activities. CRCs allow Pfizer to
partner with investigators and organizations (Collaborators) to generate innovative research of potential
scientific value to patients, physicians and the greater scientific community. Collaborators may be
academic institutions, research networks, cooperative groups, government agencies or other entities.
Pfizer may provide Collaborator with one or more types of support, such as funding, product(s), device(s),
equipment or other types of support such as in-kind services like publication writing support,
Pharmacokinetic (PK) analysis, etc. In addition, Pfizer can contribute to the development of the protocol,
Informed Consent Documents (ICDs), Statistical Analysis Plan (SAP), and other relevant study
documents. In certain CRCs, Pfizer will want to obtain access to and/or request data to be transferred to
Pfizer.
Introduction

White Guide – Chapter 9: Pfizer-Sponsored Research
and Clinical Research Collaborations (CRCs)
Rev. 12/20
Page 148 of 405
CRCs facilitated and managed by the CRC team, that is part of the Global Medical Grants group, are subject
to the requirements of CMCD CT44-GSOP: Clinical Research Collaborations, see link. For more
information, please contact the BPO for CT44 or a member of the Clinical Development Legal team.
This Chapter is relevant to all Pfizer colleagues who have responsibility for Pfizer-sponsored
clinical studies and CRCs. Non-compliance with policies applicable to those activities puts the
Company at risk and can subject Pfizer colleagues to disciplinary action up to and including
termination of employment.
• The decision to engage an HCP as a clinical investigator in a Pfizer-sponsored study or in a
CRC study, must be made by Pfizer colleagues in a Medical, Clinical, or R&D function (i.e.,
not Commercial). Commercial colleagues may not attempt to influence a decision to engage
the services of an HCP as a clinical investigator, or to collaborate in a CRC.
• Funding or other support for medical research must never be provided to:
o Establish or improve Pfizer's relationship with an HCP or health care organization;
o Gain or improve access to an HCP;
o Reward past prescribing practices or influence or induce future prescribing practices; or
o Reward a past formulary decision or influence a future formulary decision.
• Research sponsored or supported by Pfizer must:
o Have genuine scientific and/or medical value;
o
Involve investigators or institutions selected on the basis of criteria relevant to the
research;
o Involve compensation that reflects "fair market value" for the services provided; and
o
Be conducted in compliance with recognized scientific and ethical standards, as well as
applicable laws and regulations.
Pfizer colleagues must follow all Pfizer policies and procedures in establishing and administering
Pfizer-sponsored studies and in the support of CRCs.
Key Points to Ensure Compliance

White Guide – Chapter 9: Pfizer-Sponsored Research
and Clinical Research Collaborations (CRCs)
Rev. 12/20
Page 149 of 405
Payments to HCPs may violate certain international, federal, and/or state anti-kickback statutes if such
payments are offered or made to reward or influence the recipient’s prescribing or formulary practices or to
establish, maintain, or improve Pfizer’s relationship with an HCP or formulary decision maker. In addition,
both the Pharmaceutical Research and Manufacturers of America (PhRMA) Code on Interactions with
Healthcare Professionals and the Department of Health and Human Services Office of Inspector General
(OIG) Compliance Program Guidance for Pharmaceutical Manufacturers forbid the use of “token” consulting
arrangements. An example of a “token” consulting arrangement would be one involving payment to an
investigator to encourage the use of a Pfizer product or to reward an investigator for previous use of a Pfizer
product, rather than to address a genuine scientific issue or obtain meaningful clinical information.
If a clinical study involves the performance of bona fide research in return for fair market value
compensation and conforms to the ethical requirements for clinical studies, the study should pass scrutiny
under the various healthcare laws; Pfizer policies and procedures, including global Clinical and Medical
Controlled Documents (CMCDs); regulatory requirements; ethical standards; and Pfizer-endorsed industry
guidelines.
Regulatory and Ethical Framework
IND Requirements
Clinical studies of drugs and biological products in the United States must be conducted under an
Investigational New Drug (IND) application, unless an exemption applies. An IND is required for clinical
studies involving an unapproved compound and, generally, for those studies that involve an FDA-approved
product if the study will be used to support a new indication, advertising claim, or significant change in
product labeling, or if the study involves an increased level of risk associated with the use of the drug (21
CFR 312.2). The study team must secure approval from the appropriate regulatory colleagues in order to
proceed without an IND.
In certain instances, Pfizer may choose to conduct studies at clinical trial sites outside of the U.S. under an
IND application to facilitate acceptance of the results of those studies by the FDA. Those studies would
then be subject to FDA regulations. In addition, all non-U.S. studies must comply with applicable local laws,
regulations, guidelines, and ethical codes.
IDE Requirements
Clinical studies of investigational devices in the United States, unless exempt, must be conducted under
an approved Investigational Device Exemption (IDE) to support a Premarket Approval (PMA)
Healthcare Law Compliance Issues
Pfizer-Sponsored Clinical Studies

White Guide – Chapter 9: Pfizer-Sponsored Research
and Clinical Research Collaborations (CRCs)
Rev. 12/20
Page 150 of 405
application or a premarket notification 510(k) submission to the FDA. Clinical studies are most often
conducted to collect safety and effectiveness data to support a PMA, as few 510(k)s require clinical data to
support the application. Investigational use also may include clinical evaluation of modifications or new
intended uses of legally marketed devices.
Privacy Rules
Global and U.S. data privacy rules require investigators to protect the confidentiality of any identifiable
health information about a study participant that they obtain in connection with the study and to secure
appropriate informed consents from study participants before disclosing such information to Pfizer. The
Privacy Rule of the Health Insurance Portability and Accountability Act of 1996 (HIPAA) also impacts
the conduct of Pfizer-sponsored clinical studies in the United States. While HIPAA is not directly applicable
to Pfizer in its role as study sponsor, it applies to most of Pfizer’s contracted U.S. investigators with respect
to their use and disclosure of Protected Health Information collected in studies. Pfizer personnel must
always ensure that appropriate measures are taken to protect any study participant information that they
access or review in accordance with Corporate Policy (CP) #404: Protecting the Privacy of Personal
Information. For a more detailed discussion of Protected Health Information, please see White Guide
Chapter 11: Privacy: Protecting Personal Information.
Good Clinical Practice
All Pfizer supported studies must be conducted in accordance with the principles of recognized international
ethical and data integrity standards, including the International Council on Harmonization Good Clinical
Practice (ICH GCP) guidelines and applicable regulatory standards. CMCD CT19-POL: Global Standards
for Interventional Studies describes Pfizer clinical study standards that are applicable worldwide, including
in those countries that do not have established laws or practices for protection of human subjects.
Interactions with HCPs and Government Employees
All interactions with HCPs in connection with Pfizer supported studies must comply with CP #207: Global
Policy on Interactions with Healthcare Professionals (GPIHP). Pfizer is also committed to compliance with
relevant industry standards, including PhRMA’s Principles for Conduct of Clinical Trials and Communication
of Clinical Trial Results. In addition, all interactions with government officials or persons likely to interact
with government officials in Pfizer supported studies must comply with My Anti-Corruption Policies and
Procedures (MAPP). See White Guide Chapter 5: HCP and Government Official Consulting Engagements,
for additional information on interactions with government officials. In addition there are useful materials
available at the R&D’s Compliance CNTR http://ecfd13.pfizer.com/sites/wrdcompliance/Pages/Home.aspx)
and on GCO Policy Xchange on GCO on Demand, such as the U.S. Healthcare Professional Payment
Disclosure, State Law and Physician Payment Sunshine Act Reporting SOP.

White Guide – Chapter 9: Pfizer-Sponsored Research
and Clinical Research Collaborations (CRCs)
Rev. 12/20
Page 151 of 405
Additional Requirements
Unless an exemption applies, all Pfizer supported clinical studies must be reviewed and approved by a
qualified Institutional Review Board (IRB) or Independent Ethics Committee (IEC) to help ensure the
protection of the rights and welfare of study participants. Clinical investigators must also secure voluntary
and fully informed consent from each study participant or, in appropriate circumstances, his or her legal
representative.
In controlled studies, Pfizer policy also requires that the medical care provided to the control group is
medically and ethically appropriate. Placebo-controlled studies are appropriate only in certain limited
circumstances (e.g., when use of a placebo does not present undue risk to the health or well-being of the
study participants), and in all cases the IRB or IEC must review and approve the appropriateness of the
proposed treatment for the control group. Where applicable, Pfizer-sponsored clinical study teams also
coordinate the provision of study drug to study participants after the study concludes (i.e. post-study access)
in accordance with local laws and regulations. Applicable regulatory and ethical requirements and industry
standards for Pfizer-sponsored clinical studies are reflected in the CMCD Policies and SOPs on Clinical
Trials.
Scientific Validity and Value to Pfizer
A Pfizer-sponsored clinical study must be a bona fide research project; that is, it must be scientifically valid
and have a clear and appropriate purpose, with goals that are relevant to product development or other
legitimate Pfizer research or business needs. Before study teams develop a study protocol, they must
establish the purpose of the study and how the study deliverables (e.g., study data or report; biological
samples) are likely to be used.
In contrast, so-called “studies” that are intended to familiarize clinicians with a new drug rather than to
collect scientifically important information are not acceptable. Such projects are likely to be viewed as
“sham” or “seeding” studies, and compensation to participating HCPs could violate anti-kickback laws.
Selection of Investigators
As the study sponsor, Pfizer must select only those investigators who possess the appropriate professional
qualifications, training, experience, time, and resources to conduct the study adequately. Investigators
must also be evaluated to ensure that they are appropriately licensed, are not disqualified from conducting
clinical research by any relevant regulatory body and have not been previously assessed by Pfizer as
unacceptable for any other reason. Under no circumstances may Pfizer select study investigators or
institutions on any improper basis, such as to reward or influence prescribing practices or formulary
decisions.

White Guide – Chapter 9: Pfizer-Sponsored Research
and Clinical Research Collaborations (CRCs)
Rev. 12/20
Page 152 of 405
To reduce the risk of bias and ensure data integrity, investigators must also be free from significant conflicts
of interest. For those “covered studies” used to support a U.S. regulatory application, FDA regulations
require investigators to disclose any significant financial interests in Pfizer, any proprietary interest in the
study drug, and any compensation affected by the outcome of the study. Significant payments (exceeding
$25,000) to the investigator or institution that are in addition to the costs of conducting the clinical study
must also be disclosed.
The roles and responsibilities of Pfizer clinical investigators in a Pfizer-sponsored study are documented in
a Clinical Study Agreement between Pfizer and/or Pfizer’s CRO and the investigator or his or her
institution. The Clinical Study Agreement also memorializes the investigator’s commitment to conduct the
study in accordance with an approved protocol, comply with all regulatory obligations, report to Pfizer any
adverse experiences that occur over the course of the study, and secure study participant informed consent.
Pfizer policies and procedures relating to selection of investigators and financial disclosures are described
in CMCD INV02-GSOP: Investigational Site Selection Preparation and Initiation, CMCD INV04-GSOP:
Investigational Site Management and Monitoring, CMCD INV02-INV04-WI-GL02: Managing Investigational
Site Documents, and CMCD REG32-WI-GL03: Preparation of Financial Disclosure Information for U.S.
FDA Submissions.
Conflict of Interest
Why does Pfizer need to ask investigators and sub-investigators to disclose financial
interests they, their spouses and/or their dependent children have in Pfizer?
Per FDA regulation, when we submit a marketing application to FDA for approval of a
drug, device or biologic, we will need either to certify to the absence of certain financial
interests and arrangements of clinical investigators and sub-investigators that could
affect the reliability of data submitted to FDA, or disclose those financial interests and
arrangements to the agency and identify steps taken to minimize the potential for bias.
If an applicant does not include certification and/or disclosure with its application or
does not certify that it was unable to obtain the information despite exercising due
diligence, the agency may refuse to file the application.

White Guide – Chapter 9: Pfizer-Sponsored Research
and Clinical Research Collaborations (CRCs)
Rev. 12/20
Page 153 of 405
Data Monitoring Committee Members
May a member of a Data Monitoring Committee (DMC) for a Pfizer-sponsored study
be engaged as an investigator for another Pfizer study? May a DMC member be
engaged for other services, such as consulting or speaking for Pfizer?
Members of a DMC or other Independent Oversight Committee (IOC) for Pfizer
sponsored studies relating to a particular product are not permitted to serve
(concurrently or within the prior 12 months) as an investigator on a study relating to
the same product. They are permitted, however, to serve as an IOC member for one
product and simultaneously be an investigator for a different product. With limited
exceptions, individuals may not contract with Pfizer in any other capacity (e.g., on an
advisory board, as a speaker, or as a consultant) while serving as an IOC member for
a Pfizer study. Furthermore, a former or current DMC member may not author a
publication about that study, even if the study has been completed and the DMC
disbanded. For further details, see CMCD CT22-GSOP: Independent Oversight
Committees, and White Guide Chapter 5: HCP and Government Official Consulting
Engagements
Study Design, Conduct, and Monitoring
Pfizer-sponsored studies are conducted according to a general study plan and clinical protocol developed
and documented by Pfizer. Pfizer CMCD Policies and SOPs on Clinical Trials identify Pfizer requirements
for the preparation of clinical protocols, as well as the requirements for securing IRB or IEC approval,
informed consent, study participant recruitment, participation and compensation criteria, data collection and
privacy, and study documentation and monitoring practices, including adverse event monitoring and
reporting.
Managing Study Conduct Quality Issues
All Pfizer colleagues and parties with whom Pfizer contracts in support of clinical trial activities must report
within 1 business day, to the appropriate Pfizer Quality Assurance group any suspected Quality Events
(QEs). QEs are nonconformities to applicable policy, procedure, protocol or Good Practices
Quality Guidelines and Regulations (GxP). Examples of QEs are those that involve the safety or
rights of participants, or non-compliance with accepted ethical research norms that is likely to impact the
integrity of the study data, such as significant departures from the clinical protocol or falsification of research
records. It is Pfizer policy to investigate promptly any suspected quality issue related to a clinical study.
Pfizer will take appropriate action to investigate the quality issue, remedy it, when possible, and prevent
future recurrence. Pfizer’s requirements and procedures for reporting and handling QEs are described in
CMCD QMS01-GSOP: Reporting and Management of Quality Events .
White Guide – Chapter 9: Pfizer-Sponsored Research
and Clinical Research Collaborations (CRCs)
Rev. 12/20
Page 154 of 405
Safety Information & Adverse Event Monitoring – Pfizer-Sponsored Studies
Are Pfizer study teams obligated to report safety information from Pfizer-sponsored
studies? Can Pfizer choose what type of information it reports to regulatory
authorities?
Study sponsors cannot choose what safety information they report to regulatory
authorities. As a study sponsor, Pfizer is required to record and evaluate all safety
information received from any source and to provide expedited reports to regulatory
authorities of adverse events that are both serious and unexpected. Pfizer study
teams must immediately notify all investigators, IRBs, and IECs, as well as the relevant
regulatory authorities of significant unanticipated problems such as new safety
information, in accordance with CMCD AEM01-POL: Adverse Event Monitoring (AEM)
System. If significant safety information is discovered after study participants have
agreed to be involved in the study, the study participants must be provided this new
information, regardless of whether it may affect their willingness to continue to be
involved in the study.
>
Compensating Investigators
Pfizer compensates its investigators and study sites for performing services necessary to conduct a study.
Compensation must reflect the fair market value of the services performed. The rate of compensation
may take into consideration factors such as investigator expertise, required procedures, time commitment,
study complexity, and locale. Pfizer does not, under any circumstances, provide compensation to reward
or influence prescribing or formulary decisions or to influence the data generated by the study.
Requirements relating to investigator compensation are set out in CMCD CT18-POL: Compensation to
Investigators, and include the following:
• Compensation must be linked to specific protocol-related services or associated activities (e.g.,
booking or reimbursement of reasonable travel, lodging, and meal expenses associated with
attendance at investigator meetings). Investigators cannot be paid for the development of the
primary publication (abstract, poster, presentation or manuscript) related to the study).
• The basis of compensation must be documented in a study budget that serves as an attachment
to the Clinical Study Agreement;
• Compensation must be reasonable when compared to compensation for similar clinical studies
sponsored by the pharmaceutical/biotechnology industry in the country where the study is
conducted; and

White Guide – Chapter 9: Pfizer-Sponsored Research
and Clinical Research Collaborations (CRCs)
Rev. 12/20
Page 155 of 405
• Study participants should be informed, as part of the informed consent process, that Pfizer is
providing compensation to the investigator or institution for involvement in the study.
• Under no circumstances may financial compensation to investigators in Pfizer-sponsored studies:
• Be tied to the outcome of the study;
• Include Pfizer stock or stock options;
• Include payments to HCPs outside the study for referring potential study participants;
• Include special incentives such as enrollment bonuses, awards, or gift certificates designed to
reward the achievement of participant enrollment goals within a specified time period; or
• Include any other type of additional incentives or rewards, except those prospectively identified in
the Clinical Study Agreement or approved by the IRB or IEC.
Investigator Compensation
If enrollment is lagging in a sponsored study, can Pfizer offer investigators increased
compensation or gifts to help expedite enrollment? For example, can we pay
investigators an extra $700 per enrolled study participant or give them an iPad if a
certain enrollment figure is reached?
While Pfizer may compensate investigators with fair market value payments for their
participation in a clinical study, offering investigators increased per-participant
incentives to accelerate enrollment is not permitted. Investigator compensation must
be linked to bona fide services. If enrollment is difficult, Pfizer can make arrangements
to cover the cost of additional advertising, staff time, or bona fide recruitment efforts
by the investigator or others. These additional payments will need to be made to an
investigator’s institution or clinical trial office, rather than to an individual investigator
or his or her staff. If a study team has questions about whether a particular type of
additional compensation is acceptable, the team should consult the relevant attorney
supporting the asset on the Clinical Development Legal team.
Investigator Meetings
Pfizer routinely invites investigators and key research staff working on Pfizer-sponsored studies to study-
related meetings. Such meetings are usually held at the launch of a study and, as needed, intermittently
as the study progresses. These investigator meetings provide information about the drug and study
protocol, as well as opportunities for training and other activities designed to increase the consistency and
quality of study conduct. Any Reimbursement to investigators and staff for travel to investigator meetings
and associated expenses must comply with CP #301: Travel, Entertainment and Other Business Related

White Guide – Chapter 9: Pfizer-Sponsored Research
and Clinical Research Collaborations (CRCs)
Rev. 12/20
Page 156 of 405
Expenses. The venue of investigator meetings should be conducive to the business purpose of the
meeting, convenient for the participants, and not “resort-like” or “lavish.” International investigator meetings
must comply with Pfizer’s MAPP. The Meetings & Events Team within Global Commercial Operations is
typically responsible for organizing such investigator meetings. Financial Support
In a Pfizer-sponsored study, Pfizer covers the cost of the investigational aspects of the study. This
includes any treatments, procedures, or tests that are required by the protocol and that the study participant
would not have received had he or she not participated in the study. In studies involving the use of a Pfizer
product as the study drug, Pfizer generally provides or covers the cost for all study drugs. In the United
States, the value of the study drug may be captured for reporting under the Physician Payments Sunshine
Act.
Some studies also include certain protocol-required Standard of Care (SOC) services. SOC services are
medically necessary treatments, procedures, or tests that would be administered to the patient even if
he/she had not enrolled in the study, consistent with good medical practice. Under certain circumstances,
the costs of SOC services are not required to be covered by the study sponsor. However, Pfizer generally
will not charge study participants for the costs of a Pfizer drug used in a Pfizer-sponsored study, even if the
use of that drug is standard of care. For studies conducted in the United States, the determination of
whether SOC costs may be charged to the study participant/insurer is governed by CMCD CT48-WI-GL01
Development of Per Subject Cost and CMCD INV02-INV04-CT24-WI-GL01 Clinical Study Agreements and
Site Investigator Payments. For studies conducted outside the United States, this determination requires
consultation with local Legal and Regulatory.
Generally, Pfizer also covers the costs of medical treatment and diagnosis for any study-related research
injury. A research injury is a physical injury caused by treatments or procedures required by the protocol
that the study participant would not have sustained if he or she had not participated in the study. Pfizer
does not offer compensation for lost wages, pain and suffering, or expenses other than medical care.
Pfizer’s research injury compensation practices for non-U.S. studies may differ based on the impact of local
law or conformance to generally accepted local or regional guidelines. Study participants must be free to
withdraw from a study at any time without penalty or loss of benefits to which they are otherwise entitled.
Participant Compensation
May Pfizer compensate research participants for their time and any reasonable
expenses incurred during their participation in a sponsored clinical study? Can any
payment be made contingent upon the completion of the study?
Pfizer is committed to compensating research participants fairly. Study participants
should not have to bear unduly burdensome costs as a result of their participation in a
Pfizer-sponsored study but should also not be offered compensation that could be
seen as excessive and, therefore, undermine the principle of voluntary informed

White Guide – Chapter 9: Pfizer-Sponsored Research
and Clinical Research Collaborations (CRCs)
Rev. 12/20
Page 157 of 405
Participant Compensation
consent. Pfizer may offer a reasonable payment to research participants so long as
the payment has been reviewed and approved by an IRB or IEC prior to the
commencement of the clinical study. Payments must also be based on consistent
criteria and must not be contingent on completion of the study. For more information
please see CMCD CT17-POL, Compensation to Research Subjects.
Public Disclosure and Access to Study Data
Pfizer believes that it is important for researchers, trial participants, regulators, and others acting in the best
interest of patients to have access to clinical trial information to advance medical understanding and
progress. It is also important that this access works in ways that protect patient privacy, preserve regulatory
authority, and maintain incentives for those who generate data to conduct new research. Pfizer publicly
shares results from its clinical trials, whether the results are neutral, negative or positive.
Pfizer recognizes that there are public health benefits associated with making clinical study information
widely available to HCPs and study participants through clinical study registries and results databases. On
ClinicalTrials.gov, Pfizer prospectively registers Pfizer-sponsored interventional studies in human subjects
that evaluate the safety and/or efficacy of a product, as well as Pfizer-sponsored non-interventional studies
(regardless of study design or data source) in which the safety and/or efficacy of a Pfizer product will be
assessed. ClinicalTrials.gov is a publicly-available study registry provided as a service by the United States
National Institutes of Health. Pfizer posts results of studies on ClinicalTrials.gov (and on EudraCT, a
publicly-available portal managed by the European Medicines Agency) within the timeframes
specified in CMCD CT28-GSOP: Public Disclosure of Pfizer-Sponsored Studies.
Pfizer is committed to compliance with all federal and state requirements regarding access to clinical study
information and results.
Pfizer also voluntarily complies with PhRMA’s Principles for Conduct of Clinical Trials and Communication
of Clinical Trial Results and encourages the publication of the results of its sponsored studies whether or
not the results are favorable to the Pfizer product. Under those principles and Pfizer policy, study results
must be reported in an objective, accurate, balanced, and complete manner and must discuss study
strengths and limitations. Reports must also disclose Pfizer’s financial support. Pfizer reserves the right
to review, prospectively, any proposed publication or other disclosure of the results of a Pfizer-sponsored
study to prevent inadvertent disclosure of Pfizer proprietary information, and may request a delay in
publication, if necessary, to protect intellectual property rights. In addition, all investigators who participate
in the conduct of a single or multi-site clinical study are entitled to review relevant statistical tables, figures,
and reports for the entire study at a designated Pfizer facility or other mutually-agreeable location.

White Guide – Chapter 9: Pfizer-Sponsored Research
and Clinical Research Collaborations (CRCs)
Rev. 12/20
Page 158 of 405
Pfizer applies the authorship criteria established by the International Committee of Medical Journal
Editors (ICMJE), which ensures that only those individuals who deserve authorship credit based on their
contributions to a publication are identified as authors. Individuals, who contribute to the publication in
other roles, including technical writers, should be appropriately acknowledged, and sources of financial
support for the study should be disclosed. Pfizer’s policy on the public disclosure of information, access to
data, and publications related to Pfizer-sponsored studies is outlined in CMCD CT20-POL: Public
Disclosure of Pfizer Clinical Study Data and Authorship, CMCD CT28-GSOP: Public Disclosure of Pfizer-
Sponsored Studies, CMCD CT37-GSOP: Development of Pfizer Publications and CMCD CT37-SOP-
POC01: Development of Pfizer Regional Offices and Country Offices Publications. Authorship of
publications, including the standards for acknowledgment as an “author” or “contributor,” is also discussed
more fully in White Guide Chapter 17: Publications.
Expanded Access
Pfizer is sometimes asked to provide an investigational product that has not yet received regulatory
approval to treat a seriously ill patient who has exhausted approved treatment options and is ineligible to
participate in any ongoing clinical study. Such requests should be submitted to Pfizer’s external online
portal, PfizerCAReS.com. CMCD CT16-GSOP: Expanded Access Programs identifies the criteria that
must be met for Pfizer to consider a “Expanded Access” request. Expanded Access requests are reviewed
by our medical experts inside the company, and we strive to respond within 5 business days. Some of the
criteria include that the investigational product is being investigated under an appropriate regulatory
authorization and there is meaningful human clinical data to support the determination that the potential
benefits to the patient outweigh the risks. Non-clinical factors, such as the identity of the patient or the
requestor, must not play a determinative role in the consideration of a compassionate access request. The
relevant study team is responsible for evaluating expanded access requests, and the clinical lead will make
the final determination. See CT16-GSOP for more information and to review Pfizer’s Expanded Access
policy, and e-mail PfizerCAReS@pfizer.com with any questions.
General Requirements
CRCs can be initiated either by Pfizer (i.e., Pfizer approaches an external party to propose a collaboration),
or by the external (i.e., external party approaches Pfizer to propose a collaboration). If confidential
information will be shared during the initial discussions with the collaborator, a Confidentiality Disclosure
Agreement (CDA) must be executed prior to such discussion.
It is important to understand the similarities and differences between a CRC and Investigator Sponsored
Research (ISR) (also known as investigator-initiated research (IIR) or an investigator-sponsored trial).
Similar to an ISR, a Collaborator is responsible for carrying out the research in accordance with the study
Clinical Research Collaborations

White Guide – Chapter 9: Pfizer-Sponsored Research
and Clinical Research Collaborations (CRCs)
Rev. 12/20
Page 159 of 405
protocol and, unless country-specific laws require otherwise, acting as the regulatory sponsor of the study.
Pfizer’s contribution may include funding, product(s), device(s), equipment or other forms of support such
as in-kind services like publication writing support, Pharmacokinetic (PK) analysis, etc. However, unlike
an ISR in the CRC context, Pfizer can contribute to protocol design and statistical plan, reviews and
approves the study protocol and any informed consent documents prior to submission to the Institutional
Review Board (IRB)/Independent Ethics Committee (IEC), has study oversight capabilities and may
access patient-level data to facilitate its own research, development, commercialization and other activities.
For example, Pfizer may use the data to support an application to a regulatory authority or to fulfill a
regulatory commitment.
CRCs must only be used for medical or clinical research that is scientifically sound, supports Pfizer strategy,
and is compliant with relevant regulations.
All industry standards, local laws, regulations, and professional standards appropriate to a country or region
also apply to CRCs. In the event that those standards are more stringent than those described in this SOP,
the more restrictive standard applies.
It is important to engage the CRC group as early as possible to determine feasibility & resource needs
across the enterprise prior to engaging fully with a potential collaborator.
If a CRC is an option for clinical development of an asset, then it is important to have proactive
communications with key internal stakeholders and partner lines regarding expectations concerning the
process, timelines and roles and responsibilities. It is also important to consider integrating CRC into asset
planning (i.e., life-cycle, operating plan) discussions.
• The Principal Investigator (PI) as well as the regulatory sponsor of record for the research must be
determined and documented and collectively are considered the “Collaborator”. CRCs must have a
protocol that outlines the details of the research activities being conducted. There should be a clear
rationale for selecting a collaborator (e.g., an external partner like an Academic Research Organization)
that is based upon their medical and clinical research expertise (e.g., therapeutic area knowledge and
experience, efficient clinical trial execution, global filing capabilities) and is confirmed through the
diligence process.
• There are several important questions that should be discussed early on with the potential
collaborator(s) in order to assess their ability and willingness to engage in certain aspects of the
collaboration as applicable such as (but not limited to):
o Pfizer review and contribution to protocol, Informed consent documents, statistical analysis plan,
monitoring plan, data capture elements or tools, data analysis, publications etc.)
o Pfizer access to and/or a copy of the raw data, tables and listings, samples, regulatory
documentation etc.

White Guide – Chapter 9: Pfizer-Sponsored Research
and Clinical Research Collaborations (CRCs)
Rev. 12/20
Page 160 of 405
o Pfizer review of systems, trainings and processes
o Auditing, monitoring and termination rights
OLE AND RESPONSIBILITIES
CRC Support
Pfizer may provide a Collaborator with one or more types of support, such as funding, product(s), device(s),
equipment or other types of support such as in-kind services like publication writing support,
Pharmacokinetic (PK) analysis, etc. In addition, Pfizer can contribute to the development of the protocol,
Informed Consent Documents (ICDs), Statistical Analysis Plan (SAP), and other relevant study documents.
Evaluation of Potential CRCs
Pfizer’s evaluation of a proposed CRC is based on scientific merit, strategic fit and the Collaborator’s
qualifications and ability to perform the study. All proposed CRCs undergo scientific review and a standard
due diligence review, each described below.
Scientific review of proposed CRCs is carried out by Pfizer representatives with medical, clinical and
statistical expertise. Decisions are based on the scientific merit and quality of the proposed research,
technical feasibility and strategic alignment of the research with Pfizer projects.
Due diligence review is a thorough, cross-functional process designed to evaluate CRCs from a risk-
mitigation perspective, as well as to assist in the development of the collaboration framework. Reviewers
include representatives from Pfizer’s functional lines such as Quality Assurance, Clinical Informatics,
Clinical Operations, Medicinal Sciences, Pharmacovigilance, Regulatory, Legal and other subject matter
experts as needed. In order to facilitate Pfizer due diligence activities and understand the collaborators
research facility(ies) and capabilities, Pfizer requests the collaborator to complete questions around
feasibility, site process and procedures, systems and training procedures
Monitoring, Auditing, Tracking and Data Collection by Pfizer
Pfizer and its representatives may review Collaborator’s study conduct and monitoring practices and
general procedures to verify data quality and integrity. Collaborator has the obligation to cooperate with
such activities and ensure reasonable access. If Pfizer notifies Collaborator of any concerns, Collaborator
must promptly take appropriate steps to ensure the integrity of the study data.
Scientific Review
Collaborators Roles and Responsibilities

White Guide – Chapter 9: Pfizer-Sponsored Research
and Clinical Research Collaborations (CRCs)
Rev. 12/20
Page 161 of 405
Unless the parties otherwise agree, the Collaborator may conduct the research using its own system,
policies and procedures. The Trial Master File (TMF) resides with the collaborator.
Regulatory and Ethical Obligations
A Collaborator acts as the research sponsor and must ensure that the study is conducted in accordance
with the protocol, all applicable laws and regulations, and good practice quality guidelines such as, Good
Clinical Practices and Good Laboratory Practices (collectively, GxPs). As sponsor, a Collaborator
assumes all regulatory responsibilities, including ensuring approval and oversight of the study by an
IRB/IEC, obtaining necessary regulatory approvals (e.g., submitting and holding the clinical trial
authorization or obtaining an investigational new drug (IND) application), and all reporting obligations to
the appropriate regulatory authorities.
Unless an exemption applies, all applicable CRC studies must be reviewed and approved by a qualified
Institutional Review Board (IRB) or Independent Ethics Committee (IEC) to help ensure the protection
of the rights and welfare of study participants. Pfizer requires the provision of IRB/IEC approval and renewal
letters. Continuation of support by Pfizer requires timely submission of a copy of IRB/IEC renewal
documentation subsequent to the original IRB/IEC approval (as required per local regulations).
Regulatory Requirements
As with Pfizer-sponsored studies, CRC studies of drugs and biological products in the United States must
be conducted under an Investigational New Drug (IND) application, unless an exemption applies. An IND
is required for clinical studies involving an unapproved product and, generally, for those studies that involve
an FDA-approved product if the study will be used to support a new indication, advertising claim, or
significant change in product labeling, or if the study involves an increased level of risk associated with the
use of the drug (21 CFR 312.2). For clinical trials in the United States utilizing a Pfizer product, Pfizer
requires documentation of IND submission or exemption from the investigator-sponsor.
For studies conducted in the European Union, conduct under a clinical trial application (CTA) is required,
a copy of the submission letter to the CTA, must be provided to Pfizer in English. If Pfizer will provide
packaged and labeled Pfizer product, then Pfizer must receive a copy of the approved CTA, with Section
4.2 (IMPD or Letter of Access from Pfizer) and Section D (in its entirety) must be translated in English,
before Pfizer can provide Qualified Person release of product. For more information regarding CTAs,
please consult http://eudract.emea.europa.eu/document.html.
Collaborator confirms study registration on applicable registries such as ClinicialTrials.gov or
www.clinicaltrialsregister.eu.

White Guide – Chapter 9: Pfizer-Sponsored Research
and Clinical Research Collaborations (CRCs)
Rev. 12/20
Page 162 of 405
Protocol, Informed Consent and other Study Document Development
Collaborator and Pfizer can jointly develop study documents including but not limited to the Protocol, SAP,
ICDs, and data capture forms as agreed to. It is important that Pfizer have the opportunity to review and
approve all study documents prior to submission to regulatory and/or ethic committees and must receive
the final IRB/IEC approved protocol and informed consent documents. It is also important to engage Pfizer
when considering any amendments to such documents as continuation of support by Pfizer for a CRC study
will be contingent on Pfizer’s review and acceptance of these changes.
For studies with sites in the European Union (EU) where drug support is being requested, the final study
protocol must be signed by the principal investigator and is required for Qualified Person (QP) release of
drug supplies.
Collaborator monitors the study in accordance with GxPs and all applicable law and provides Pfizer with
access to information relating to study deviations, including any significant GxP protocol compliance issues.
Collaborator and Pfizer agree to a monitoring plan prior to study commencement.
Upon reasonable notice and during regular business hours, Collaborator shall permit Pfizer personnel and
representatives access to study premises, facilities, systems and processes including Standard Operating
Procedures (SOPs) and study records, as well as investigators and other research staff.
To satisfy its duties as study sponsor, Collaborator may conduct quality oversight reviews and audits of the
facilities, services, systems and processes of third parties, such as labs, who are involved in study
execution. Collaborator provides Pfizer, at Pfizer’s request, with copies of all findings from such reviews.
Reporting of Safety Data
As study sponsor, the Collaborator reports required safety information (such as serious adverse events
(SAEs)) to the appropriate regulatory authorities and to the IRBs/IECs. Pfizer also requires the provision
of certain safety information as outlined in the Safety Reporting Reference Manual for IIR or Clinical
Research Collaboration Studies provided to the Collaborator prior to study commencement. Collaborator
and all investigators and other study staff must understand and fully comply with the adverse event reporting
requirements.
Study Monitoring
Document Reviews and Site Visits
Regulatory Inspections and Audits

White Guide – Chapter 9: Pfizer-Sponsored Research
and Clinical Research Collaborations (CRCs)
Rev. 12/20
Page 163 of 405
Drug Supply
The Collaborator ensures that each study site has appropriate procedures, training and controls in place to
receive, store, dispense and destroy or return the Pfizer product as required for the proposed study. Any
drug product provided by Pfizer for an approved study must only be used in accordance with the approved
protocol.
Collaborator maintains a record of any drug product that was received, dispensed, returned or destroyed
throughout the duration of the study. Unless otherwise instructed by Pfizer, Collaborator arranges for the
destruction of any expired or unused drug product in accordance with all applicable laws and regulations
and institutional policies.
Insurance
Each Collaborator carries and maintains, at its sole expense, insurance coverage of the kind and with
liability limits appropriate to the collaboration to protect itself and Pfizer against any claims or liabilities that
may arise from the conduct of the study with insurers with a minimum “A‑” A.M. Best rating. Any deductibles
or retentions for such insurance policies are assumed solely by Collaborator. Collaborator’s insurance
policies must be primary and non‑contributing with respect to any other similar insurance policies available
to Pfizer or its affiliates.
Study Budget
Detailed budgets should be provided as soon as possible during protocol development and are subject to
review and fair market value evaluation. Pfizer provides Collaborator with a budget template to ensure that
all study-related expenses have been appropriately itemized and included.
Pfizer does not pay or reimburse Collaborator for (i) services not performed, or services performed that are
not in compliance with the CRC Agreement; (ii) Standard of Care services; or (iii) any attorneys’ fees
incurred by Collaborator in the negotiation of the CRC Agreement or any related agreement. “Standard of
Care” means any medically necessary treatments, procedures or tests administered in a manner that is
consistent with current medical practice and that would be expected to be performed on or provided to a
subject even if the subject were not participating in the study.
Pfizer Inc. complies with state, federal and voluntary ethics code reporting requirements worldwide (e.g.,
Open Payments f/k/a Sunshine Act; EFPIA Code). This includes the reporting of payments and drug supply
costs provided to Collaborator and any investigators by Pfizer in support of collaborative studies.
Governance
Governance

White Guide – Chapter 9: Pfizer-Sponsored Research
and Clinical Research Collaborations (CRCs)
Rev. 12/20
Page 164 of 405
Confidentiality Agreement
Prior to engaging in CRC discussions, confidential information may be exchanged. Therefore, it is generally
necessary to enter into a confidentiality agreement with Collaborators to ensure that any confidential
information is protected. Each Collaborator ensures that its employees, agents and subcontractors are
aware of Collaborator’s confidentiality obligations, and that they are able to abide by the same restrictions
in carrying out study-related activities.
CRC Agreement
The CRC Agreement outlines each party’s roles and responsibilities, as well as expectations regarding
study conduct, milestones and deliverables, publications, intellectual property, term/termination,
safety/pharmacovigilance, confidentiality and other provisions. Study-related activities do not commence
until a CRC Agreement is signed by all parties.
It is important for the Collaborator’s study personnel and Pfizer colleagues to keep the lines of
communication open as to the status of study activities from the beginning. Pfizer and the Collaborator will
determine the appropriate meeting format and frequency to communicate study progress which address
(among other things) the progress of research activities, reported patient outcomes/clinical activity, safety
updates, projected publication dates, and projected and completed milestones.
Study Results
Pfizer requires the provision of study results at the conclusion in accordance with the agreed statistical
analysis plan for this study. Collaborator will provide to Pfizer the study results in the form of a written,
succinct summary of key study results, whether such study results arise from interim analyses or final
analysis.
Clinical Study Report (CSR)
In some cases, Pfizer may also require the provision of a CSR. The final study report should reflect the
results of the study as a whole. The CSR will be organized in a mutually agreeable manner and should
include at a minimum, the information identified below. Collaborator should provide Pfizer with an
opportunity to review and comment on the CSR, including but without limitation the proposed list of tables,
and Collaborator will incorporate any comments reasonably requested by Pfizer.
The CSR should address:
Joint Study Team Meetings
Study Deliverables

White Guide – Chapter 9: Pfizer-Sponsored Research
and Clinical Research Collaborations (CRCs)
Rev. 12/20
Page 165 of 405
• Ethics;
• Investigators and study administration;
• Study objectives;
• Investigational plan;
• Study subjects;
• Efficacy evaluation;
• Safety evaluation; and
• Discussion and overall conclusions; supportive tables, figures and graphs.
Study Data
In certain CRCs, Pfizer will want to obtain access to and/or request data to be transferred to Pfizer. To
ensure that study data are collected in a way that facilitates analysis and use by both Parties, Collaborator
will provide Pfizer an opportunity to review and comment in advance on the case report forms on which
study data will be recorded. Pfizer and Collaborator will discuss the use of a mutually agreeable electronic
transmission method that is demonstrated to be compatible with uploading to the targeted Pfizer database
and that protects the security and integrity of the data.
Pfizer supports the exercise of academic freedom and encourages the Collaborator(s) to publish the study
results, whether or not they are favorable to Pfizer or any Pfizer product. It is encouraged that both the
Collaborator and Pfizer discuss publication plans, including but not limited to, the targeted Congress or
journal, publication type, proposed publication title, proposed authors and writing support. When selecting
a peer-reviewed journal, it is recommended the authors choose one which offers Open Access publishing.
Primary Submission
Unless the Collaborator and Pfizer decide there is a reason a Publication is not needed, the Collaborator is
responsible for ensuring submission of one or more manuscripts reporting study results for the primary
endpoint(s) to a peer-reviewed medical or scientific journal and congresses (e.g. manuscripts, abstracts,
posters, and presentations) within 18 months of the last subject last visit or the primary completion date,
whichever is earlier.
Publication Quality
Publications should not be based on preliminary or interim data analyses, unless (i) such analysis(es) was
specified in the study’s SAP prior to commencement of the study and approved by both Collaborator and
Publications

White Guide – Chapter 9: Pfizer-Sponsored Research
and Clinical Research Collaborations (CRCs)
Rev. 12/20
Page 166 of 405
Pfizer, and (ii) a contemporaneous copy of the relevant interim data has been archived. Publications should
only be based on applicable data quality standards as agreed between the Parties in writing and will be
generated from data that meet data quality standards consistent with Pfizer-provided guidance.
Pre-publication Review by Pfizer
Neither Collaborator nor Principal Investigator will submit a Publication without providing Pfizer at least 30
days to review and comment on the proposed Publication and any data that supports it. Collaborator will
provide Pfizer with any study data that supports the Primary Publication, or any interim Publication at least
30 days in advance of any public disclosure (including disclosure to journals, scientific congresses, etc.).
Pfizer shall provide its comments with respect to such Publication within 30 days of its receipt of such written
copy. The review period may be extended upon the request of Pfizer for an additional period of 45 days in
the event that Pfizer wishes to prepare and file any patent applications relating to any of the Confidential
Information or Inventions. Collaborator is responsible for ensuring that the Principal Investigator consider
Pfizer's comments on any such proposed Publication in good faith, including a recommendation not to
publish if the data or proposed Publication does not satisfy the standards set forth in the Pfizer policies or
standard operating procedures.
Redaction of Confidential Information
Collaborator will, and will ensure that the authors, on request, remove any confidential information that has
not previously been publicly disclosed from the Publication before disclosure to third parties and submission
to a journal. If Collaborator deems disclosure of such confidential information necessary for appropriate
scientific presentation or understanding of the study results, Collaborator will initiate discussions with Pfizer
regarding this issue.
Authorship Standards
For all Publications, Collaborator will ensure all authors comply with standard academic practice and the
International Committee of Medical Journal Editors (ICMJE) authorship guidelines and with any additional
professional, published standards of the journal or professional society where they seek to publish their
study findings. Pfizer personnel may be included as appropriate authors for primary and secondary
publications if they qualify under ICMJE guidelines.
Disclosure of Support
In any Publication, authors will disclose Pfizer support (and support by any other external entity) of the
study. Collaborator will also adhere to any journal guidelines for disclosing industry support and conflicts
of interest in a Publication.

White Guide – Chapter 9: Pfizer-Sponsored Research
and Clinical Research Collaborations (CRCs)
Rev. 12/20
Page 167 of 405
Secondary Publications
Both Parties have the right to publish or present any aspect of the study results, after the Primary Publication
of the study results as a whole. Secondary Publications will acknowledge Collaborator’s sponsorship and
Pfizer’s support of the study and any participating sites. Secondary Publications will be submitted to the
other Party for review and comment 30 days before any public disclosure. Each Party is responsible for
ensuring it considers the other Party’s comments on any such proposed Publication in good faith, including
a recommendation not to publish if the data or proposed Publication does not satisfy the standards set forth
in Pfizer’s policies or standard operating procedures.
Safety Information & Adverse Event Monitoring – Clinical Research Collaborations
Are Pfizer study teams obligated to report safety information from CRCs? Can the
CRC sponsor choose what type of information to report to regulatory authorities?
CRC sponsors cannot choose what safety information they report to regulatory
authorities. The CRC, sponsor/investigator is required to record and evaluate all
safety information received from any source and provide expedited reports to
regulatory authorities of adverse events that are both serious and unexpected.
However if a Pfizer colleague becomes aware of an adverse event, Pfizer must also
report it in accordance with CMCD AEM01-POL: Adverse Event Monitoring (AEM)
System. For CRCs, all investigators, IRBs and IECs, as well as the relevant regulatory
authorities, should be immediately informed of significant unanticipated problems such
as new safety information. If significant safety information is discovered after study
participants have agreed to be involved in the study, the study participants must be
provided this new information, regardless of whether it may affect their willingness to
continue to be involved in the study.
• My Anti-Corruption Policy and Procedures (MAPP)
• CP #207: Global Policy on Interactions With Healthcare Professionals (GPIHP)
• CP #301: Travel, Entertainment and Other Business Related Expenses
• CP #404: Protecting the Privacy of Personal Information
• PhRMA’s Principles for Conduct of Clinical Trials and Communication of Clinical Trial Results
• Consult the following Clinical and Medical Controlled Documents (CMCD) Policies and SOPs
o CMCD AEM01-POL: Adverse Event Monitoring (AEM) System
For More Information

White Guide – Chapter 9: Pfizer-Sponsored Research
and Clinical Research Collaborations (CRCs)
Rev. 12/20
Page 168 of 405
o CMCD CT18-POL: Compensation to Investigators
o CMCD CT19-POL: Global Standards for Interventional Clinical Studies
o CMCD CT20-POL: Public Disclosure of Pfizer Clinical Study Data and Authorship
o CMCD CT22-GSOP: Independent Oversight Committees
o CMCD CT28-GSOP: Public Disclosure of Pfizer-Sponsored Studies
o CMCD CT37-GSOP: Development of Pfizer Publications
o CMCD CT44-GSOP: Clinical Research Collaborations
o CMCD GNT01-GSOP: Independent Medical Grants
o CMCD INV02-GSOP: Investigational Site Selection Preparation and Initiation
o CMCD INV04-GSOP: Investigational Site Management and Monitoring
o CMCD QMS01-GSOP: Reporting and Management of Quality Events
• Orange Guide Chapter 6: Clinical Research and Clinical Research Collaborations
• White Guide Chapter 2: Advertising and Promotional Materials
• White Guide Chapter 5: HCP and Government Official Consulting Engagements
• White Guide Chapter 11: Privacy: Protecting Personal Information
• White Guide Chapter 17: Publications

Rev. 12/20
Page 169 of 405
CHAPTER #10 – THE PFIZER
PATIENT ASSISTANCE PROGRAM
(PAP) AND DONATIONS TO
INDEPENDENT CHARITABLE
PATIENT ASSISTANCE PROGRAMS
(ICPAPS)

White Guide – Chapter 10: The Pfizer Patient Assistance Program (PAP) and
Donations to Independent Charitable Patient Assistance Programs (ICPAPs)
Rev. 12/20
Page 170 of 405
Chapter
#10
THE PFIZER PATIENT ASSISTANCE
PROGRAM (PAP) AND DONATIONS TO
INDEPENDENT CHARITY PATIENT
ASSISTANCE PROGRAMS (ICPAPs)
CONTENTS
Introduction................................................................................................................................................ 171
Donations to Independent Charity Patient Assistance Programs (ICPAPs) ...................................... 171
Patient Support Hubs and Pfizer RxPathways ................................................................................... 172
Core Compliance Principles ...................................................................................................................... 172
Key Points to Ensure Compliance ...................................................................................................... 173
Pfizer Patient Assistance Program (PAP) ................................................................................................ 175
The Pfizer Patient Assistance Program (i.e., Free Drug Program) .................................................... 175
Medicare Part D Patients and the Pfizer Patient Assistance Program ............................................... 177
Institutional Patient Assistance Program (IPAP) ................................................................................ 178
Compliance Core Principles – Pfizer PAP ................................................................................................ 179
PAP Guidance for Pfizer Non-Field Colleagues ................................................................................. 180
Interactions and Communications with Pfizer PAP/IPAP Vendors .................................................... 181
External Communications Regarding Pfizer Foundation PAP/IPAP .................................................. 182
Independent Charity Patient Assistance Programs (ICPAPs) .................................................................. 183
ICPAP Guidance for Pfizer Non-Field Colleagues .................................................................................... 183
Pfizer RxPathways® and Product-Specific Patient Support Hubs ........................................................... 185
For More Information ................................................................................................................................. 186
The Pfizer Patient Assistance Program (PAP) and Donations to
Independent Charity Patient Assistance Programs (ICPAPS)

White Guide – Chapter 10: The Pfizer Patient Assistance Program (PAP) and
Donations to Independent Charitable Patient Assistance Programs (ICPAPs)
Rev. 12/20
Page 171 of 405
Chapter #10 The Pfizer Patient Assistance Program (PAP)
and Donations to Independent Charitable Patient
Assistance Programs (ICPAPS)
Pfizer believes that all patients should have access to the medicines prescribed by their Healthcare
Providers (HCPs). For decades, Pfizer has partnered with HCPs, community health centers, free clinics,
and pharmacies to help eligible patients access the medicines they need through a number of programs.
This Chapter describes key Pfizer policies regarding Pfizer’s charitable activities to support patients’ access
to their prescribed medications, including Pfizer’s internal free drug Patient Assistance Program (PAP),
which includes its Institutional Patient Assistance Program (IPAP), and Pfizer’s donations to
Independent Charity Patient Assistance Programs (ICPAPs). This Chapter also briefly describes the
activities of Pfizer’s product-specific or therapeutic-area specific Patient Support Hubs (Hubs) and Pfizer
RxPathways, through which patients may access the Pfizer PAP as well as certain other patient support
programs (e.g., copay cards, discount cash pay cards, vouchers, free trial programs, prior authorization
assistance, benefits verification) that help eligible patients in the United States, Puerto Rico, and U.S. Virgin
Islands access the Pfizer medications prescribed by their HCPs. See White Guide Chapter 19 for
information regarding these programs.
As part of its commitment to improving patient access to medicines, Pfizer established a charitable internal
free drug program that provides commercially-available Products
6
to nearly 300 Federally Qualified Health
Centers (FQHC), free clinics and disproportionate share hospitals. Through this initiative, Pfizer donates
applicable Products to participating institutions that in turn provide the Products for free to eligible patients
treated at the facilities, based on eligibility requirements determined by Pfizer. Pfizer operates the PAP,
which includes the IPAP, on behalf of the Pfizer Patient Assistance Foundation (PPAF), a Pfizer non-
profit 501(c)(3) private operating foundation. Information regarding Pfizer’s policies related to the PAP,
including the IPAP, is provided in this Chapter. Information regarding the PAP and IPAP processes and
procedures is available in the Pfizer Patient Assistance Program & Institutional Patient Assistance
Program Standard Operating Procedure (PAP/IPAP SOP).
Donations to Independent Charity Patient Assistance Programs (ICPAPs)
In addition to the programs described above, Pfizer also may make charitable donations to ICPAPs, which
are independent, U.S. 501(c)(3) non-profit organizations that operate patient assistance programs to help
financially needy patients, including federal healthcare beneficiaries (e.g., Medicare patients), access their
6
Product availability varies by institution.
Introduction

White Guide – Chapter 10: The Pfizer Patient Assistance Program (PAP) and
Donations to Independent Charitable Patient Assistance Programs (ICPAPs)
Rev. 12/20
Page 172 of 405
medicines by assisting such patients with their out-of-pocket copay obligations. ICPAPs may establish
disease state funds that provide financial assistance with copay obligations associated with treatment for
specific disease states, including copay obligations for branded and generic drugs or other treatments
associated with the disease state. ICPAPs operate independently from Pfizer and award assistance to
patients based on their independently-developed eligibility criteria. Information regarding Pfizer’s policies
related to donations to, and interactions with, ICPAPs as it relates to Pfizer Colleagues is described in this
Chapter and in more detail in Corporate Policy and Procedure #803.
Patient Support Hubs and Pfizer RxPathways
Patients may access the Pfizer PAP, information regarding other financial assistance options (including
ICPAPs), and a variety of patient support programs by contacting either Pfizer RxPathways or a Hub.
Information regarding Pfizer’s policies related to Hubs and Pfizer RxPathways is described in this Chapter.
The Pfizer PAP (including IPAP), donations to ICPAPs, and other patient support programs
7
play an
important role in assisting patients with accessing medically necessary products that are prescribed by their
HCPs. However, several federal and state laws and other regulatory guidance are implicated in connection
with the operation of these programs, including, for example, federal and state anti-kickback statutes, the
federal Beneficiary Inducement Statute, the federal False Claims Act, government price reporting
obligations, federal and state privacy laws, and U.S. Department of Health and Human Services’ Office of
Inspector General (OIG) guidance. It is Pfizer’s policy to establish and implement these programs and
activities consistent with all applicable laws, regulations, and guidance issued by the OIG.
These programs and activities are intended to support appropriate patient access to independently-
prescribed Pfizer Products (or to other prescribed medicines in the case of ICPAP donations) and are not
intended to: (i) induce a patient to select a Product; (ii) induce an HCP to prescribe, or reward an HCP for
prescribing Products; or (iii) reduce economic or administrative burdens for an HCP (or related practice or
office staff). Pfizer Colleagues are not permitted to promote Pfizer’s patient support programs as a reason
to prescribe a Product.
Pfizer offers its programs in a non-discriminatory fashion to all eligible patients who are prescribed an
applicable Pfizer Product and the availability of these offerings is unrelated to the volume or value of
business generated by any HCP or healthcare facility.
To ensure that Pfizer meets these obligations, Pricing & Access Legal Team reviews and provides guidance
regarding the programs and activities covered in this Chapter, including PAP, donations to ICPAPs,
7
These programs include copay cards, discount cash pay cards, vouchers, free trial programs, and the Pfizer
Savings Program.
Core Compliance Principles

White Guide – Chapter 10: The Pfizer Patient Assistance Program (PAP) and
Donations to Independent Charitable Patient Assistance Programs (ICPAPs)
Rev. 12/20
Page 173 of 405
RxPathways and Hub activities in the United States, Puerto Rico and the U.S. Virgin Islands. In addition,
Pricing & Access Legal must review and approve the inclusion, removal and exclusion of Products from the
Pfizer PAP and IPAP. The ICPAP Review Committee (RC) must approve all donations to ICPAPs.
NOTE: Third-party vendors acting on Pfizer’s behalf in administering the Hubs, PAP, and IPAP
must certify/warrant compliance with applicable state and federal healthcare laws and regulations
and Pfizer policies and procedures.
Consult Pricing & Access Legal for additional information on Pfizer’s PAP, IPAP, and interactions with
ICPAPs, and your team attorney on the design and implementation of other patient support programs. Non-
compliance with these policies puts the Company at risk and can subject Pfizer Colleagues to disciplinary
actions up to and including termination of employment.
• Pfizer colleagues must follow the requirements described in this Chapter when: (i) engaging
in activities related to the Pfizer PAP; (ii) interacting with ICPAPs, to the extent appropriate;
(iii) discussing patient support programs and resources with HCP customers
• Pfizer PAP/IPAP:
•
On behalf of PPAF, a non-profit 501(c)(3) private operating foundation, the Global
Partnerships & Social Impact team and other authorized Pfizer colleagues operate the Pfizer
PAP and IPAP consistent with their charitable purpose.
o
Free Product is provided without the intent to induce, reward, or influence a patient’s use
of a Product; to induce, reward, or influence an HCP’s prescribing decisions; and/or to
endorse or recommend the purchase of a Product.
o
Free Product is provided only after prescribers have made an independent clinical
decision that the Product is medically appropriate for the individual patient.
o
Free Product is awarded based on reasonable, uniform, and consistent measures of
financial need and without regard to the providers, practitioners, or suppliers used by the
patient or the insurance plan (if any) in which the patient is enrolled.
o Free Product is provided outside of any insurance benefit.
Key Points to Ensure Compliance
White Guide – Chapter 10: The Pfizer Patient Assistance Program (PAP) and
Donations to Independent Charitable Patient Assistance Programs (ICPAPs)
Rev. 12/20
Page 174 of 405
• ICPAPs (See also Corporate Policy and Procedure #803):
o
Global Partnerships & Social Impact team (with ICPAP Review Committee oversight) is
solely responsible for ICPAP donations and related activities and, with limited
exceptions, Global Partnerships & Social Impact team must not share information related
to ICPAP donations with other Pfizer colleagues.
o Colleagues outside of Global Partnerships & Social Impact team must not:
•
Discuss patient need, business interests, or funding decisions related to donations
to ICPAPs for copay assistance with the Global Partnerships & Social Impact team
for the purpose of influencing donations; or
•
Seek to influence, or be involved in, any communications between the Global
Partnerships & Social Impact team and the ICPAPs related to donations for copay
assistance.
o
Except for certain Colleagues engaged in reimbursement support and approved by
Legal, Pfizer colleagues must not discuss with HCPs or patients:
• Specific ICPAPs;
• The availability of funding in relevant disease states; or
• That ICPAPs can overcome copay barriers.
• Patient Support Programs Offered through Hubs or Pfizer Rx Pathways:
o
Pfizer's patient support programs are intended to support patient access to
independently-prescribed Products.
o
Pfizer colleagues are not permitted to promote Pfizer's patient support programs as a
reason to use or prescribe a Pfizer Product.
o
Because Pfizer's patient support programs are operated to assist patients with accessing
prescribed Products, Pfizer colleagues must not state or suggest that these programs
provide independent value to any HCP or reduce economic or administrative burdens for
an HCP (or related practice or office staff).
•
Patients and HCPs may visit the Pfizer RxPathways website (PfizerRxPathways.com), the
PAP Connect website (PfizerPAPConnect.com) and/or the relevant Hub websites to learn
more about patient assistance and patient support programs offered by Pfizer.
•
If you have questions about any of the guidance provided in this Chapter, please contact
Legal or your Pfizer team attorney.
Key Points to Ensure Compliance

White Guide – Chapter 10: The Pfizer Patient Assistance Program (PAP) and
Donations to Independent Charitable Patient Assistance Programs (ICPAPs)
Rev. 12/20
Page 175 of 405
The Pfizer PAP (including IPAP) is operated by the Pfizer Patient Assistance Foundation (PPAF), which
is a non-profit 501(c)(3) private operating foundation. PPAF is funded through cash and in-kind (i.e.,
Product) donations from Pfizer. Pfizer also donates services, facilities, equipment, supplies, and
Colleagues’ time to the extent necessary for PPAF to conduct its charitable activities related to the Pfizer
PAP. PPAF operates consistent with its certificate of incorporation and bylaws. Pfizer Colleagues elected
to PPAF’s Board of Directors and Pfizer’s Global Partnerships & Social Impact team, some of whom serve
as officers of PPAF, have primary responsibility for managing the Pfizer PAP operations on behalf PPAF,
with support from certain other functions (e.g., Legal, Compliance, Privacy, Global Procurement, Finance,
Pfizer Global Supply).
Pfizer’s Global Partnerships & Social Impact team, on behalf of PPAF, is responsible for the day-to-day
operations of the Pfizer PAP, including establishing patient and institution eligibility criteria and determining
Product inclusion, exclusion, and removal criteria. Global Partnerships & Social Impact team must
complete a PAP Program Approval for Pricing & Access Legal review and approval of product inclusion,
exclusion, or removal from the PAP.
All Pfizer Colleagues that conduct business related to the Pfizer PAP work on behalf of PPAF. As such,
they must fulfill the independent charitable objectives of PPAF.
The Pfizer Patient Assistance Program (i.e., Free Drug Program)
Overview: The Pfizer PAP provides eligible uninsured and underinsured patients who meet program-
specific financial need criteria and other eligibility requirements with Products prescribed by their HCPs for
free. Eligible uninsured patients are enrolled in the program for 12 months. Eligible underinsured patients,
which include both commercially and government insured patients, are enrolled through the end of the
calendar year. Patients can re-apply as often as needed once their enrollment period expires. The free
Product is delivered to enrolled patients via doctors’ offices, home delivery, or retail pharmacies –
depending on the Product.
To learn more about the Pfizer PAP and whether they may be eligible for free Product, patients or their
advocates may contact Pfizer RxPathways or a Hub, if a Hub is available for the Product prescribed.
Covered Products: A large number of Pfizer Products are available for free through the Pfizer PAP. A
full list of Products available through the Pfizer PAP is available on the PfizerRxPathways.com website.
Pfizer Patient Assistance Program (PAP)

White Guide – Chapter 10: The Pfizer Patient Assistance Program (PAP) and
Donations to Independent Charitable Patient Assistance Programs (ICPAPs)
Rev. 12/20
Page 176 of 405
In general, the majority of Pfizer Products are available through the Pfizer PAP, EXCEPT the following:
• Products that are typically administered in an inpatient hospital setting (the Pfizer PAP is for
outpatients only);
• Products that are classified as opioids; and
• Products that have lost their patent exclusivity and have affordable multi-sourced generics available
(with affordable defined as $30 or less for a 30-day supply), except as available by medical
exception.
Eligibility Requirements: In order to qualify for free Product from the Pfizer PAP, patients and their HCPs
must meet the following eligibility requirements:
• Patients must have a valid prescription for the Product for which they are seeking assistance.
• Patients must have no prescription coverage (uninsured) or not enough coverage (underinsured)
to pay for the Product.
• Patients must complete an application that asks for basic patient information (e.g., name, address,
phone number, e-mail address, annual gross household income, household size, and insurance
status (e.g., uninsured, commercial insurance, government insurance)). The patient’s HCP must
also complete a section of the PAP application form that asks for basic information about the HCP,
including name, address, and DEA number. Note: The information requested in the PAP
application form may include Personal Information or Sensitive Personal Information and must not
be used or disclosed unless certain conditions are met. For more information on Personal
Information and Pfizer’s policies for protecting patient privacy, see Orange Guide Chapter 8:
Privacy: Protecting Personal Information.
• Patients must demonstrate financial need by meeting specific household income requirements,
which vary by Product, but start at 400% of the Federal Poverty Level, adjusted for family size.
Patients must provide proof of income, such as a W2 form, a paystub, or prior year’s tax return with
their PAP application form or consent to e-verification
• Patients must live in the United States, U.S. Virgin Islands, or Puerto Rico.
• Patients must be treated by a healthcare provider licensed in the United States, U.S. Virgin Islands,
or Puerto Rico.
• Patients prescribed certain Products may be required to seek alternate forms of coverage or
financial assistance, such as Pfizer copay cards (for commercially insured patients only), Medicaid,
Medicare Part D Low Income Subsidies, or ICPAP support, before they can be enrolled in the Pfizer
PAP.

White Guide – Chapter 10: The Pfizer Patient Assistance Program (PAP) and
Donations to Independent Charitable Patient Assistance Programs (ICPAPs)
Rev. 12/20
Page 177 of 405
Referring Patients to the Pfizer PAP
You are a Sales representative and one of your HCP customers tells you that he has
Xeljanz patients who are uninsured. He asks you whether Pfizer can provide these
patients with financial assistance to cover their out-of-pocket costs for Xeljanz. Xeljanz
is included in the Pfizer PAP. Should you refer him to the Pfizer RxPathways website
and tell him to have his patients apply to the Pfizer PAP?
Yes, you may inform the HCP that he can refer patients to the Pfizer RxPathways
website or its toll-free number (1-844-989-PATH) for information about the Pfizer PAP
and other available assistance programs. You may also inform the HCP that he can
refer the patient to a Hub, if available for the Product. Field sales representatives must
not imply or guarantee that Pfizer will provide any specific assistance to patients. Field
sales colleagues also must not answer patient-specific questions regarding the Pfizer
PAP and should direct HCPs with such questions to the applicable PAP vendor or
other resource (e.g., applicable Pfizer website) for additional information.
Medicare Part D Patients and the Pfizer Patient Assistance Program
As described above, patients with prescription drug coverage through commercial plans or government
healthcare programs, like Medicare Part D, can apply to receive Products for free through the Pfizer PAP
if such patients are having difficulty paying for their medicines. The Pfizer PAP provides free drug to eligible
patients enrolled in government healthcare programs, including Medicare Part D, as described below.
According to guidance issued by the OIG, manufacturers may not subsidize the copay or other out-of-
pocket expenses of Medicare Part D beneficiaries. Such subsidies, according to OIG, are likely to implicate
the federal Anti-Kickback Statute. This is why Pfizer often prohibits federal health care program
beneficiaries from using copay coupons/cards and Pfizer copay card/coupon rules always prohibit their use
for any products reimbursed by federal healthcare programs (See White Guide, Chapter 19, Savings and
Free Trial Programs, for more information about copay cards and other Pfizer savings programs). In
contrast, OIG has stated that manufacturers may provide free medications to Medicare Part D beneficiaries
so long as several safeguards are met (see list below), including that manufacturers provide such free
medications entirely outside the patients’ Part D benefits. This means that if a Part D beneficiary receives
free Product through a PAP no claims for payment can be filed with the Part D plan associated with such
Product. The free drug provided to such patients also must not count toward the beneficiary’s true out-of-
pocket costs (TrOOP) or overall Part D spending.

White Guide – Chapter 10: The Pfizer Patient Assistance Program (PAP) and
Donations to Independent Charitable Patient Assistance Programs (ICPAPs)
Rev. 12/20
Page 178 of 405
In order to help ensure compliance with all applicable legal requirements, the Pfizer PAP must meet the
following requirements:
• Notification to Part D plans that the Product is being provided to a Part D beneficiary outside the
Part D benefit;
• Provision of free Product for the whole Part D coverage year or the portion of the year remaining
after the beneficiary received patient assistance;
• Provision of free Product even if the beneficiary’s use of the drug is periodic;
• Maintenance of accurate and timely records to verify the provision of the free Product outside the
Part D benefit;
• Award free Product based on reasonable, uniform, and consistent measures of financial need and
without regard to providers, practitioners, or suppliers; and
• Compliance with any applicable guidance issued by the Centers for Medicare and Medicaid
Services.
Pfizer Patient Assistance Program and Medicare Part D
A patient with Medicare Part D prescription coverage is having difficulty paying for her
Pfizer primary care medicine. Can she apply for assistance through the Pfizer PAP?
Yes. Patients with prescription coverage – such as Medicare Part D, Medicaid, or
commercial insurance – who are having difficulty paying for their Pfizer prescription
medicines can apply to receive free drug from the Pfizer PAP. Patients should call
Pfizer RxPathways or the relevant Hub to learn more. If eligible, a patient will receive
her Product for free through the end of the calendar year. Pfizer’s PAP vendor will
instruct the patient that she must not file any claims for payment with her Part D plan
or count the free Product that she receives from the PAP towards her TrOOP or overall
Part D spending. In addition, Pfizer’s PAP vendor will instruct the patient that she must
provide notification to her Part D plan that the Product is being provided outside of her
benefit. The Vendor will also directly notify Part D plan sponsors when their members
are enrolled in the PAP.
Institutional Patient Assistance Program (IPAP)
Overview of Program: The IPAP provides select Products to eligible, financially needy, uninsured patients
through nearly 300 federally-qualified community health centers, disproportionate share hospitals, free

White Guide – Chapter 10: The Pfizer Patient Assistance Program (PAP) and
Donations to Independent Charitable Patient Assistance Programs (ICPAPs)
Rev. 12/20
Page 179 of 405
clinics, and state pharmacy programs.
8
Through this initiative, Pfizer donates the participating Products to
participating institutions that in turn provide the Products for free to eligible patients treated at the facilities,
based on eligibility requirements determined by Pfizer.
Covered Products: At present, many Pfizer Products are available for free through the IPAP. For a
complete list of Products available, visit PfizerRxPathways.com.
Eligibility Requirements: To qualify to receive Product for free through the IPAP, patients must:
• Receive their care at one of the nearly 300 institutions that participate in the program;
• Have no prescription coverage (unlike the Pfizer PAP, which helps both uninsured and
underinsured, the IPAP is for uninsured patients only); and
• Have a household income of at or below 400% of the Federal Poverty Level, adjusted for family
size.
The institutions that participate in the IPAP are responsible for ensuring that patients meet the program
eligibility guidelines. Pfizer audits participating institutions on a regular basis to ensure compliance with
program rules.
In order to help ensure compliance with all applicable legal requirements, all Pfizer Colleagues must adhere
to the following core principles:
• Free Product will be awarded based on reasonable, uniform, and consistent measures of financial
need and without regard to the providers, practitioners, or suppliers used by the patient or the
insurance plan (if any) in which the patient is enrolled.
• Free Product will be provided outside of any insurance benefit.
• Free Product will be provided only after prescribers have made an independent clinical decision
that the Product is medically appropriate for the individual patient.
• Free Product must be provided without the intent to induce, reward, or influence a patient’s use of
any Product, to induce, reward, or influence an HCP’s prescribing decisions, and/or to endorse or
recommend the purchase of a Product.
• Pfizer will operate the Pfizer PAP consistent with its charitable purposes and without undue
influence from Pfizer Commercial Colleagues.
8
Product availability varies by institution and eligibility.
Compliance Core Principles – Pfizer PAP

White Guide – Chapter 10: The Pfizer Patient Assistance Program (PAP) and
Donations to Independent Charitable Patient Assistance Programs (ICPAPs)
Rev. 12/20
Page 180 of 405
PAP Guidance for Pfizer Non-Field Colleagues
Pfizer’s Global Partnerships & Social Impact team is responsible for administering the Pfizer PAP on behalf
of PPAF a non-profit 501(c)(3) private operating foundation. Except as described below or in the PAP/IPAP
SOP, or as otherwise approved in advance by Pricing & Access Legal, Pfizer Colleagues must not be
involved in the development, operation or management of the Pfizer PAP.
• Budget – The Pfizer PAP budget is a component of the Global Health & Patient Access budget
within the Corporate Affairs budget and, consistent with Company procedures related to budget
approval, is approved by the Pfizer Executive Leadership Team. Brand teams indirectly fund the
Pfizer PAP operations through either periodic forecast budget transfers or permanent budget
transfers to Global Partnerships & Social Impact team. Global Partnerships & Social Impact team
may request brand teams to provide such budget transfers as needed. Commercial Colleagues
involved in the review and approval of brand budgets may review and approve such budget
transfers, as appropriate. As part of this process, relevant Commercial Colleagues may
communicate with Global Partnerships & Social Impact Colleagues to understand Global
Partnerships & Social Impacts’ requests for budget transfers. As part of these communications,
Global Partnerships & Social Impact team may provide relevant Pfizer PAP/IPAP data (e.g., PAP
utilization or forecasting data, and information about expected administrative costs).
• Operations – Except for Pfizer Colleagues acting on behalf of or in service of PPAF, or
appropriately involved in establishing and approving the Pfizer PAP budget in accordance with the
PAP/IPAP SOP, Pfizer Colleagues may not be involved in the management or operations of the
Pfizer PAP. Further, Pfizer Colleagues must not seek to influence Global Partnerships & Social
Impact’ management or operation of the Pfizer PAP, including but not limited to decisions regarding
whether to include a Product in the Pfizer PAP, the patient or institutional eligibility criteria, and
other program terms and conditions.
Commercial Colleagues may provide to Global Partnerships & Social Impact team information about the
timing of new Product launches and Product acquisitions and may request that Global Partnerships & Social
Impact team consider adding any such new Product to the Pfizer PAP. In addition, at the request of Global
Partnerships & Social Impact team, relevant Pfizer Colleagues (including Commercial Colleagues) may
provide information about Products, relevant diseases, and patient populations to allow Global Partnerships
& Social Impact team to develop the PAP budget and establish patient need.
• PAP Data – Pfizer Colleagues may request reports containing Pfizer PAP data from Global
Partnerships & Social Impact team. Global Partnerships & Social Impact team must consult with
Pricing & Access Legal prior to distributing any new report type/data to anyone not working on
behalf of PPAF. Consult your team attorney for review and approval of Pfizer PAP data reports
from co-promote partners. Pfizer Colleagues may use these reports for operational purposes only,

White Guide – Chapter 10: The Pfizer Patient Assistance Program (PAP) and
Donations to Independent Charitable Patient Assistance Programs (ICPAPs)
Rev. 12/20
Page 181 of 405
including but not limited to, financial forecasting and budgeting, evaluating current and projected
Product utilization, and compliance monitoring and program auditing. Pfizer Colleagues must not
use Pfizer PAP data and reports to drive commercial objectives (e.g., to increase product utilization
and any related strategy).
o Pfizer Colleagues must never conduct Return on Investment (ROI) analyses on the provision
of free drug through the Pfizer PAP , attempt to correlate Pfizer’s donations to ICPAPs with
Pfizer PAP utilization, or use PAP data to conduct any analysis prohibited by the Corporate
Policy and Procedure #803 Contributions to ICPAPs or any other Pfizer policy.
o Pfizer Colleagues should contact Legal and Compliance with any questions on the appropriate
use of PAP/IPAP data and reports.
• Patient Data – Pfizer Colleagues and vendors who must access patient data to perform services
related to administration of the Pfizer PAP must keep such data confidential and must ensure that
they do not provide such data to any other person who should not have access to it, consistent with
all data privacy requirements described in White Guide Chapter 11: Privacy: Protecting Personal
Information. Other than those Pfizer Colleagues who must receive patient data to administer the
Pfizer PAP, no Pfizer employee or contractor may receive identifiable data from PAP vendors
related to the Pfizer PAP. IPAP institutions must not provide individually identifiable data, including
personal health information, to the IPAP vendor, PPAF, a non-profit 501(c)(3) private operating
foundation, or Pfizer, except as may be required for program auditing purposes.
• Use of Patient and HCP Personal and Contract Information – Patients and their prescribing
HCPs must be notified in writing and acknowledge how patient and HCP information may be used
when a patient applies for the Pfizer PAP. Patient and HCP contact information gathered through
the PAP application and enrollment process may not be used to promote or market other Pfizer
programs or Products. Patients and HCPs must not be required to enroll in any other program or
opt-in to receive Pfizer marketing materials as a condition of enrolling in the Pfizer PAP. If
information regarding such patients or HCPs is gathered through other means (e.g., Hub or
PfizerPro enrollment), Pfizer may use that information for purposes consistent with other Pfizer
policies and SOPs.
Interactions and Communications with Pfizer PAP/IPAP Vendors
• Other than Pfizer Colleagues authorized to act on behalf or in service of PPAF, a non-profit
501(c)(3) private operating foundation, and Field Reimbursement Managers (FRMs), in limited
circumstances, Pfizer Colleagues must not communicate with the vendors that administer the Pfizer
PAP and IPAP for any reason.
o This prohibition does not prevent the SAS CoE and other appropriate Colleagues from
communicating with Hub vendors who also administer the Pfizer PAP regarding other patient

White Guide – Chapter 10: The Pfizer Patient Assistance Program (PAP) and
Donations to Independent Charitable Patient Assistance Programs (ICPAPs)
Rev. 12/20
Page 182 of 405
support programs or activities. Pfizer Colleagues should refer all questions or concerns
regarding vendors’ operation of the Pfizer PAP and IPAP to Global Partnerships & Social
Impact team.
External Communications Regarding Pfizer Foundation PAP/IPAP
Communications by Pfizer Colleagues, contractors, or third-party vendors with patients and/or HCPs
regarding the Pfizer PAP and IPAP must be factual and non-promotional. All communications must be
truthful, non-misleading, and consistent with Pfizer policies and procedures and applicable laws and
regulations.
In general, all external communications should comply with the following guidelines:
• The Pfizer PAP and IPAP must not be used as a tool to promote Products, to differentiate Products
from competitor products, or to influence HCP prescribing habits.
• Although the Pfizer PAP and IPAP are available to all eligible patients irrespective of their
diagnosis, Commercial Colleagues, contractors, and third-party vendors must not promote the
availability of the Pfizer PAP or IPAP for any off-label use of a Product.
• Pfizer Colleagues, contractors, and third-party vendors must not describe the Pfizer PAP and IPAP
as a way to fill gaps in Product coverage (e.g., Medicare Part D donut hole).
• Pfizer Colleagues, contractors, and third-party vendors must not make any statements about the
potential outcome of an application or guarantee enrollment in, or provision of, free Product through
the Pfizer PAP or IPAP.
• Pfizer Colleagues, contractors, and third-party vendors (other than those contracted to administer
the Pfizer PAP and IPAP) must not fill out or submit PAP applications on behalf of patients or HCPs.
• All marketing materials that reference the Pfizer PAP and/or IPAP must be approved through all
applicable Pfizer materials review processes.
A Note about Field Commercial Colleagues: The above guidance also applies to all Field
Commercial Colleagues. Therefore, it is particularly important to keep in mind the rules surrounding
external communications when creating marketing materials that reference the Pfizer PAP and
IPAP. For additional guidance on creating marketing materials, see White Guide Chapter 4:
Marketing Programs.

White Guide – Chapter 10: The Pfizer Patient Assistance Program (PAP) and
Donations to Independent Charitable Patient Assistance Programs (ICPAPs)
Rev. 12/20
Page 183 of 405
In addition to the Pfizer free drug programs described above, Pfizer also may make charitable contributions
to ICPAPs through its Global Partnerships & Social Impact team. Pfizer believes all individuals deserve
access to quality healthcare and all medicines prescribed by their physicians. Charitable contributions to
ICPAPs can provide a means to help patients access their medicines by providing significant financial
assistance to patients for copay, deductible, and/or premium obligations for prescriptions (collectively,
“copay assistance”). ICPAPs may focus financial assistance on costs associated with treatment for specific
disease states, and generally have disease-state funds that provide copay assistance for all branded and
generic drugs or other treatments associated with the disease state. ICPAPs must operate entirely
independently from Pfizer and award patient assistance based on their independently-developed eligibility
criteria.
Patients who apply for free Product through the Pfizer PAP may be required to seek alternate forms of
coverage or financial assistance before they can be enrolled in the Pfizer PAP, including for example,
ICPAP support.
While federal healthcare program beneficiaries can obtain copay assistance through independent, third-
party ICPAPs, Pfizer may not directly subsidize the copay or other out-of-pocket expenses of Medicare Part
D beneficiaries or other federal healthcare program patients. Given this restriction on Pfizer directly
subsidizing the out-of-pocket expenses of federal healthcare program beneficiaries, donations to ICPAPs
may implicate the federal Anti-Kickback Statute. The OIG, however, has issued guidance permitting
ICPAPs to provide copay assistance to federal healthcare program beneficiaries using donations from
manufacturers if sufficient safeguards exist. It is Pfizer’s policy to comply with government guidance and
laws in making contributions to ICPAPs to ensure those safeguards are met.
For additional guidance on interactions with ICPAPs, please see Corporate Policy and Procedure
#803 Contributions to Independent Charity Patient Assistance Programs.
All Pfizer Non-Field Colleagues must understand and operate according to the following standards in
relation to ICPAPs:
• Communications with ICPAPs. Only the Global Partnerships & Social Impact team (including Legal
and Compliance Colleagues advising Global Partnerships & Social Impact) may communicate with,
ICPAP Guidance for Pfizer Non-Field Colleagues
Independent Charity Patient Assistance Programs (ICPAPs)

White Guide – Chapter 10: The Pfizer Patient Assistance Program (PAP) and
Donations to Independent Charitable Patient Assistance Programs (ICPAPs)
Rev. 12/20
Page 184 of 405
and receive information and data from, ICPAPs regarding donations to ICPAPs for copayment
assistance.
• Data from Other Third Parties. Hubs, Pfizer RxPathways, and specialty pharmacies may assist
patients with searching for available ICPAP funding. Data received from these third parties (whether
incorporated into a Pfizer business report or otherwise) must be limited in frequency (i.e., not more than
monthly), may be shared internally only as necessary, and the nature and type of report that will be
shared must be approved by Legal prior to distribution. Under no circumstances should Pfizer obtain
information about: (1) other donors or other donations made to the ICPAP except for general
information on total donations received or funding available; or (2) use the data to correlate the amount
or frequency of Pfizer’s donations to ICPAPs with the ICPAP’s support of patients prescribed Pfizer
Products. Subject to the one exception listed below, data received from third parties must not be: (1)
disaggregated and/or patient-specific; or (2) whether or not in the aggregate, related to the identity or
amount of subsidized drugs.
• Exception: Vendors may provide certain patient-specific or disaggregated information to Pfizer
Colleagues responsible for administering the Pfizer PAP (e.g., Global Partnerships & Social Impact or
SAS CoE Colleagues) or engaged in reimbursement support (e.g., Field Reimbursement Managers) in
the event such information is critical to the Colleagues’ job responsibilities with respect to operating the
Pfizer PAP or assisting patients access their medicines. Other Pfizer Colleagues must not seek to
obtain or be provided with such information.
• Independence of ICPAPs. Pfizer Colleagues, including the Global Partnerships & Social Impact team,
must not exert or attempt to exert any direct or indirect control over an ICPAP or the entity operating
the ICPAP regarding establishing new disease state funds, the scope of a new or proposed disease
state fund, the modification of a disease state fund, or criteria for determining eligibility of patients who
qualify for assistance.
• Information Related to ICPAPs. The Global Partnerships & Social Impact team must not share
information related to donations to ICPAPs for copay assistance with any other Pfizer Colleagues,
except as specifically provided in Corporate Policy and Procedure # 803 Contributions to Independent
Charity Patient Assistance Programs.
• ROI Analysis. Pfizer Colleagues are prohibited from undertaking any “Return on Investment” analysis
or other analysis that seeks to correlate a past or future donation to ICPAPs to the number of subsidized
prescriptions for Pfizer Products including, for example, to determine the amount to donate to ICPAPs.
• Undue Influence. The Global Partnerships & Social Impact team has sole responsibility for
determining the allocation of the approved budget for donations to ICPAPs, subject to review and
approval by the ICPAP Review Committee. Pfizer Colleagues are prohibited from discussing patient
need, business interests, or funding decisions related to donations to ICPAPs for copay assistance with
Global Partnerships & Social Impact team for purposes of influencing donations decisions.

White Guide – Chapter 10: The Pfizer Patient Assistance Program (PAP) and
Donations to Independent Charitable Patient Assistance Programs (ICPAPs)
Rev. 12/20
Page 185 of 405
• Co-Promote Agreements. If Pfizer collaborates with a third party in the marketing or promotion of a
drug, it will be responsible, through Global Partnerships & Social Impact team, for making its own
decisions regarding the provision of donations to ICPAPs for copay assistance in accordance with
Pfizer’s policies and procedures and co-promote partners will make their own donations separately.
Pfizer must not provide any funding or reimbursement to its co-promote partners for donations to
ICPAPs for copay assistance and must not share information about its donations with its co-promote
partners. Pfizer Colleagues with responsibility for co-promote partnerships should consult Corporate
Policy and Procedure # 803 and Legal.
Pfizer has established Pfizer RxPathways and Hubs to connect eligible patients to, and in the case of Hubs
to provide patients with, a range of resources such as benefits investigation and verification, prior
authorizations and appeals support, drug delivery and administration support, copay support, financial
assistance, and patient education.
• Pfizer RxPathways® is not brand-specific, and serves as a single point of access that connects
patients, regardless of their insurance status, to available financial assistance and other patient support
programs, such as the Pfizer PAP, IPAP, Hubs, copay and savings offers, free trial programs, and other
resources. Pfizer RxPathways is run by the Global Partnerships & Social Impact team.
• Hubs provide Product-specific or disease-state specific patient support and offer eligible patients a
single point of access for a range of financial assistance and other patient support programs. Hubs are
jointly managed by the SAS CoE and Global Partnerships & Social Impact teams. The offerings that
the Hub provides, or to which the Hub connects patients, are overseen by different teams depending
on the offering (e.g., Global Partnerships & Social Impact team is responsible for Reimbursement
Support and the Pfizer Patient Assistance Program services, while Pfizer brand teams oversee most
Savings and Free Trial Programs).
It is Pfizer’s policy to establish and implement Pfizer RxPathways and the Hubs consistent with all
applicable laws and regulations. To that end, Pfizer RxPathways and the Hubs provide no more than limited
reimbursement support to patients who are prescribed a Pfizer Product. RxPathways and the Hubs are
intended to support patient access to independently-prescribed Pfizer Products and are not intended to
reward or induce an HCP for past, present or future prescribing of Products or to reduce economic or
administrative burdens for an HCP (or related practice or office staff).
Pfizer offers its RxPathways and Hub activities in a non-discriminatory fashion to all eligible patients after
they are prescribed an applicable Pfizer Product by their HCP. The availability of RxPathways and Hub
support is unrelated to the volume or value of business generated by any HCP or healthcare facility. To
ensure that Pfizer meets these obligations, the Pfizer Pricing & Access Legal Team must review and provide
Pfizer RxPathways® and Product-Specific Patient Support Hubs

White Guide – Chapter 10: The Pfizer Patient Assistance Program (PAP) and
Donations to Independent Charitable Patient Assistance Programs (ICPAPs)
Rev. 12/20
Page 186 of 405
guidance for RxPathways and each Hub operating in the United States. Additionally, Pfizer annually re-
evaluates the need for specific Hub activities on a Product-by-Product basis to substantiate the need for
their continued offering.
Pfizer Colleagues must not promote Pfizer’s Hub activities as a reason to prescribe a Pfizer Product. In
addition, because the Hubs are operated to assist patients with accessing prescribed Products and offer
no substantial and independent value from the Product, Pfizer Hub programs are not a means to reduce
economic or administrative burdens for an HCP and her staff. And, Pfizer Colleagues should not suggest
otherwise.
Pfizer Colleagues must follow the guidance summarized below when engaging their HCP customers in
discussions regarding RxPathways or the Hubs:
• Pfizer Colleagues must limit promotion of the availability of RxPathways and Hub activities to
Review Committee-approved information about RxPathways and any Hub program.
• Pfizer Colleagues must not promote RxPathways or Hub programs and activities to induce HCPs
to prescribe Products or to discourage HCPs from prescribing alternative therapies.
• Pfizer Colleagues (with the exception of Field Reimbursement Managers) should refer all inquiries
from their HCP customers regarding the status of a particular patient case to the applicable Field
Reimbursement Manager through the appropriate channel or refer the HCP or office staff to
RxPathways or the applicable Hub.
In addition, Pfizer Colleagues should not contact RxPathways or any Hub directly to discuss patient cases
or receive any data from RxPathways or any Hub without previous direction approved by the Pricing &
Access Legal Team.
• For more information about Pfizer RxPathways, the Pfizer PAP and IPAP, or other patient support
programs, please contact The Pfizer RxPathways Team at PfizerRxPathways@pfizer.com.
• For more information about Hub programs, please see the Standard Operating Procedure for Patient
and Reimbursement Support Hubs including, for example, guidance to govern the initiation,
management, and execution of Hubs / Hub activities
• For RC-approved FAQs and Talking Points, visit MyPfieldNet.
• If you have additional questions about the information covered in this Chapter, please contact your
team attorney.
For More Information

Rev. 12/20
Page 187 of 405
CHAPTER #11 – PRIVACY:
PROTECTING PERSONAL
INFORMATION

White Guide – Chapter 11: Privacy: Protecting Personal Information
Rev. 12/20
Page 188 of 405
Chapter
#11
PRIVACY: PROTECTING PERSONAL
INFORMATION
CONTENTS
Introduction................................................................................................................................................ 190
Governing Laws and Pfizer Policies ................................................................................................... 190
Key Points to Ensure Compliance ...................................................................................................... 192
The Legal Landscape ................................................................................................................................ 194
Health Insurance Portability and Accountability Act of 1996 (HIPAA) ................................................ 194
Pfizer is Not a Covered Entity under HIPAA ................................................................................ 194
Pfizer is Generally Not a Business Associate under HIPAA ........................................................ 194
HIPAA is Still Relevant for Pfizer ................................................................................................. 195
State Medical Information Privacy Laws ............................................................................................ 195
Federal and State Privacy and Security Laws .................................................................................... 195
Federal and State Breach Notification Laws ...................................................................................... 196
Laws Protecting the Personal Information of Children ....................................................................... 197
Requirements for Transparency, Notice, and Consent ...................................................................... 197
Pfizer’s Policies Relating to Privacy and Personal Information ................................................................ 197
Notice and Consent ............................................................................................................................ 198
Aggregated, Anonymized or De-identified Data ................................................................................. 198
Avoiding Exposure to Protected Health Information........................................................................... 199
Adverse Event Reporting .................................................................................................................... 199
Vendor Obligations ............................................................................................................................. 199
Activities That May Result in the Use and Disclosure of Personal Information ........................................ 200
Marketing Initiatives and Other Communications ............................................................................... 200
Pfizer-Sponsored Third-Party Communications .......................................................................... 200
Digital Marketing Initiatives .......................................................................................................... 201
Pfizer’s Patient Programs ................................................................................................................... 201
Working with HCPs ............................................................................................................................. 202
Mentorships and Preceptorships ........................................................................................................ 203
Consumer Health Fairs or Screenings ............................................................................................... 204
Privacy: Protecting Personal Information

White Guide – Chapter 11: Privacy: Protecting Personal Information
Rev. 12/20
Page 189 of 405
Patient Information and Clinical Trials ................................................................................................ 205
Other Privacy Issues ................................................................................................................................. 206
Healthcare Professional Prescriber Data ........................................................................................... 206
Handling Personal Information of Healthcare Professionals and Other ............................................. 207
Pfizer Policy on Your Responsibility for Safeguarding Personal Information ........................................... 207
For More Information ................................................................................................................................. 208

White Guide – Chapter 11: Privacy: Protecting Personal Information
Rev. 12/20
Page 190 of 405
Chapter #11 Privacy: Protecting Personal Information
This Chapter highlights key Pfizer policies regarding the protection of Personal Information (defined
below). Activities that involve collection or access to Personal Information include health
screenings, surveys, clinical research, and mentorships as well as Personal Information in your
possession—such as on your computer.
Non-compliance with these policies puts the Company at risk and can result in disciplinary action
up to and including termination of employment.
Privacy is often described as an individual’s desire to keep his/her Personal Information confidential and,
by extension, to determine when, how, and to what extent Personal Information is shared with others.
Personal information (PI) includes any information that alone or in combination with other data identifies,
links or relates to, or can be used to identify or link to a person or household, such as name or initials,
address, phone number, e-mail address, preferences, unique online identifiers or IP addresses. Sensitive
Personal Information (SPI) is a subset of Personal Information that is generally considered to include
more private details about an individual and may trigger additional requirements under the law. Sensitive
Personal Information may include geolocation data, financial information, national identifiers such as social
security number, information about an individual’s race, ethnicity, religion, sex life/sexual orientation, and
information about a person’s physical or mental health (e.g., a person’s medical history, physical or mental
condition, diagnosis or treatment protocol) or under certain state laws biometric data.
Governing Laws and Pfizer Policies
The United States does not have a comprehensive data protection law. Instead there are a number of
sectoral data protection and security laws at the federal level. There are also many state data protection
and security laws, mainly focused on data breaches. California has recently enacted a new privacy law
called the California Consumer Privacy Act (CCPA) which applies to all California Residents and is the
closest resemblance to a comprehensive privacy law in the United States. Many other countries around
the world have enacted more stringent protections on the use, access, or transfer of Personal Information
and Sensitive Personal Information. The European Union (EU) is widely regarded as having some of the
most stringent privacy protections for individuals in the world. In 2018 the EU privacy law called the General
Data Protection Regulator (GDPR) went into effect granting EU residents the most comprehensive privacy
protections to date. GDPR requires a number of actions by a company including; prompt review of
individual rights claims such as the “right to be forgotten”, companies to maintain reasonable security
protections of personal data, inventories of personal data processing activities, specific contracts with
Introduction

White Guide – Chapter 11: Privacy: Protecting Personal Information
Rev. 12/20
Page 191 of 405
service providers, adequate protections for transfers of personal data outside the EU, 72 hour data breach
reporting, appointment of a Data Protection Officer and other requirements. Other countries with some
form of a comprehensive or more robust privacy law(s) include; Argentina, Australia, Brazil, Canada,
Colombia, Israel, Japan, Mexico, Peru, Singapore, South Korea, and Uruguay.
Although this Chapter is focused largely on certain U.S. privacy topics, it is important to consider whether
any sales and marketing activities conducted in the United States may have privacy implications for
complying with the laws of other countries. Consult your team attorney or the Global Privacy Office (GPO)
if a proposed activity presents potential privacy implications for individuals outside of the United States or
involves the transmission of Personal Information collected in one country to another country. A privacy
implication includes any collection, use, transfer, storage, or deletion of personal information of any kind. It
is important to note that merely accessing Personal Information about an individual in another country via
your computer or a database is likely considered an international transfer of personal information.
The goal of data privacy laws is to ensure that companies like Pfizer handle Personal Information in a way
that is transparent, fair and reasonable. For example, when an individual chooses to share such information
with a person or entity they trust, regardless of the circumstances under which Personal Information is
shared, he or she generally expects that the person or entity to use that information for limited purposes,
hold that information in confidence, and keep it reasonably protected. Pfizer respects this expectation and
is committed to appropriately protecting all Personal Information in its care in compliance with applicable
privacy laws and regulations and Pfizer’s corporate policies and procedures. Pfizer also recognizes that in
many countries this is more than an expectation but rather an individual fundamental right. Pfizer’s policy
is to safeguard all Personal Information it receives and maintains, regardless of the form, format, location,
or use. For additional information, see Corporate Policy (CP) #404: Protecting the Privacy of Personal
Information.
During the global pandemic resulting from COVID19 many new processes were implemented to facilitate
telecommuting, telemedicine and other measures in order to ensure social distancing and help prevent
spreading of the deadly virus. Please be mindful and consider the ways in which uses of personal data
may have been impacted by the pandemic. Many of these new processes involved sharing of personal
data while people worked remotely. If you have a new practice that needs review related to COVID19
please consult your team attorney. It is unclear what will happen with these new processes once the
pandemic is over.
White Guide – Chapter 11: Privacy: Protecting Personal Information
Rev. 12/20
Page 192 of 405
• Corporate Policy (CP) #404: Protecting the Privacy of Personal Information requires all Pfizer
colleagues and contractors to protect Personal Information collected or used by or on behalf
of Pfizer. Before you initially collect any Personal Information (directly or via any third-party
service providers), your team attorney must be consulted and approve any collection, use,
sharing or storage of personal information.
•
Access to Personal Information, including Sensitive Personal Information, should be limited to
individuals who need to know the information in order to perform their job duties.
•
All Service Providers that process Personal Information must have appropriate contractual
privacy and security terms and conditions in place between with Pfizer.
•
Sensitive Personal Information should only be processed when it is necessary for an
authorized business purpose. If Pfizer or its business partner or service provider will be
processing Sensitive Personal Information, consult your team attorney and make them aware
of this fact. Pfizer colleagues and contractors must ensure that such information is received
in compliance with applicable.
•
If you become aware that Pfizer, a business partner, or service provider hasprocessed
Sensitive Personal Information or more extensive Personal Information than intended,
expected, or necessary for the business purpose, immedately notify your team attorney and
report per CP #411: Information Incident Response Policy.
All Pfizer-sponsored third-party
communications to patients, healthcare professionals (HCPs)
, and other customers must
be approved by the appropriate Pfizer Review Committee (RC)
, which will consider issues of
privacy and consent as part of its review process.
•
Do not sign a document that is called a “Business Associate Agreement” or otherwise relates
to “Business Associate” status without receiving explicit written approval to do so by your team
attorney or the GPO.
•
When using Personal Information to identify and communicate with current and potential Pfizer
customers (HCPs, patients or other consumers) it is important to work with your team attorney,
the GPO, and Digital Channel Enablement (DCE)
to ensure compliance with applicable legal
requirements and Pfizer policies and procedures.
•
When setting up a mentorship or preceptorship, Pfizer colleagues must inform physicians
serving as mentors or preceptors that they are required to obtain their patients’ written
authorization before Pfizer colleagues may be allowed to observe any consultation,
examination, and/or treatment of any patient
•
Personal Information should generally only be processed in a manner that is transparent and
within the reasonable expectations of the individual, whose information is being processed.
Key Points to Ensure Compliance

White Guide – Chapter 11: Privacy: Protecting Personal Information
Rev. 12/20
Page 193 of 405
• Avoid situations likely to lead to the inadvertent disclosure of Personal Information, including
Sensitive Personal Information, such as being present at or near private conversations
between HCPs and patients.
•
Pfizer colleagues should not engage health fair attendees in discussions regarding a specific
patient's health.
•
Always disclose that you are a Pfizer employee or representative when interacting with
patients. For example, wear your Pfizer branded name tag at all times when attending a
consumer health fair or during a mentorship or preceptorship.
•
Safeguard the confidentiality of prescriber data as you would any other Personal Information.
As a general rule, prescriber data should be used only for internal business purposes and not
in interactions with Pfizer's customers, including the HCPs themselves.
o o Only share an HCP’s prescriber data with Pfizer personnel and properly
contracted and on-boarded vendors who are assisting with your initiative. Consult
your team attorney before sharing HCP prescriber data with anyone outside of
Pfizer.
•
HCPs are permitted to disclose Protected Health Information (defined below) about patients
to persons “subject to FDA jurisdiction” for activities related to the quality, safety, or
effectiveness of an FDA-regulated product or activity for which the person has responsibility.
(see
CP #903: Your Responsibility to Report Information about Safety, Quality, or
Performance of Pfizer Products).
•
Any suspected breach of the security of Personal Information, including Sensitive Personal
Information, must be immediately reported as an “Incident”. Pfizer colleagues should avoid
using the term “breach” when reporting a potential incident involvling Personal Infoarmtion.
Do not use the word “breach” in e-mail subject lines. A “Breach” is a legal definiton that varies
by jurisdction and only determined by legal counsel. Lost or stolen computers or other devices
containing Pfizer data must be reported to the user’s local Service Desk/Help Desk (the
worldwide list of contact telephone numbers is available online at http://ITSupport.pfizer.com
).
Any other incidents of potential unauthorized access to Pfizer data must be reported to the
pursuant to CP #411: Information Incident Response Policy.
You should also notify your team
attorney.
Key Points to Ensure Compliance

White Guide – Chapter 11: Privacy: Protecting Personal Information
Rev. 12/20
Page 194 of 405
Health Insurance Portability and Accountability Act of 1996 (HIPAA)
The Health Insurance Portability and Accountability Act of 1996, as amended by the Health
Information Technology for Economic and Clinical Health Act (HITECH Act) and their implementing
regulations, (collectively, HIPAA), imposes strict limitations on the use and disclosure of Protected Health
Information (PHI) by “Covered Entities” and their “Business Associates,” as defined below.
Pfizer is Not a Covered Entity under HIPAA
Under HIPAA, the term “Covered Entity” includes HCPs that engage in electronic transactions for which a
standard has been adopted under HIPAA, as well as health plans and healthcare clearinghouses. HIPAA
requires Covered Entities to take certain reasonable steps to protect the privacy and security of PHI. To
accomplish this, HCPs and other Covered Entities must maintain appropriate administrative, technical, and
physical safeguards to protect PHI. Pfizer’s employee group health plan is deemed a Covered Entity under
HIPAA. However, Pfizer itself is not a Covered Entity under HIPAA.
Pfizer is Generally Not a Business Associate under HIPAA
In addition to protecting PHI in the hands of a Covered Entity, HIPAA also protects PHI created, received,
maintained, or transmitted by a Covered Entity’s “Business Associate.” A Business Associate is a person
or entity that creates, receives, maintains, or transmits PHI for certain functions, activities, or services it
conducts for or on behalf of a Covered Entity. Under HIPAA, Covered Entities are obligated to enter into a
written contract called a Business Associate Agreement with a Business Associate before any PHI is
disclosed to the Business Associate. Business Associates are required to comply with a variety of
requirements under HIPAA and Business Associate Agreements, including safeguarding PHI, limiting the
use and disclosure of PHI in connection with the functions performed or services it provides, and requiring
notifications of breaches (i.e., impermissible uses or disclosures) of PHI. In the vast majority of situations,
Pfizer does not perform work on behalf of an HCP or other Covered Entity and does not function as a
Business Associate. It is unlikely that a situation will arise in which Pfizer will act as a Business Associate.
Business Associates are required to enter in a Business Associate Agreement (BAA) when they perform
Business Associate functions for a Covered Entity. No Pfizer colleague or contractor may enter into a
Business Associate Agreement without the express written consent of the team attorney or the
GPO.
For more information about Business Associate Agreements, see the section on Working with HCPs within
this Chapter.
The Legal Landscape

White Guide – Chapter 11: Privacy: Protecting Personal Information
Rev. 12/20
Page 195 of 405
HIPAA is Still Relevant for Pfizer
Although Pfizer is generally not a Covered Entity or Business Associate, HIPAA is still relevant for Pfizer.
There are several HIPAA requirements with important implications for pharmaceutical manufacturer sales
and marketing activities, such as manufacturer-sponsored third-party communications, disease
management and health outcomes activities, and manufacturer-sponsored online health tracking tools.
Please consult your team attorney and/or the GPO for advice on whether HIPAA may have implications for
any proposed business arrangement or program involving health information (even if Pfizer is not receiving
such information), such as point-of-sale marketing communications at the pharmacy or marketing
communications distributed by a health plan or plan benefits administrator.
State Medical Information Privacy Laws
Nearly every state has its own laws protecting the privacy of health or medical information. Some of these
state laws may be more stringent than HIPAA in certain respects. For example, California’s medical privacy
law is more restrictive than HIPAA in terms of permissible uses and disclosures of medical information and,
unlike HIPAA, allows class action lawsuits for significant damages for the negligent release of confidential
medical information regulated by law.
HIPAA does not preempt (override) state privacy laws that do not conflict with HIPAA standards, or state
privacy laws that are more stringent than HIPAA standards. Furthermore, these state laws sometimes
regulate entities that are not subject to HIPAA. Therefore, Pfizer should take steps to ensure compliance
with both HIPAA and state laws in connection with Pfizer programs or initiatives, even if Pfizer is not directly
subject to the laws itself.
Federal and State Privacy and Security Laws
Federal Law.
In the United States, the Federal Trade Commission (FTC) has operated as a “de facto” privacy regulator.
Section 5 of the FTC Act prohibits “unfair and deceptive acts or practices in or affecting commerce”.
“Deceptive” practices are defined as involving a material representation, omission or practice that is likely
to mislead a consumer acting reasonably in the circumstances. This could be a company stating in their
privacy policy that they do one thing with regard to personal information and in practice they do not,
including, for example, the statement “we encrypt all sensitive data” when in fact not all sensitive data is
encrypted. An act or practice is “unfair” if it causes or is likely to cause substantial injury to consumers
which is not reasonably avoidable by consumers themselves and not outweighed by countervailing benefits
to consumers or to competition. This has been interpreted as companies that process personal information
must implement a reasonable information security program and related practices to appropriately protect
personal information. Many Companies have faced significant enforcement actions, related costs and
public perception implications along with fines for failing to comply with Section 5 of the FTC Act.

White Guide – Chapter 11: Privacy: Protecting Personal Information
Rev. 12/20
Page 196 of 405
State Laws.
In addition to federal laws, some states have their own data protection laws. For example,
The California Consumer Privacy Act of 2018 (CCPA) is a sweeping new law that introduces a host of
privacy rights for California residents and creates robust obligations for many businesses that collect
personal information about California consumers. For example, businesses will need to consider
implementing processes and procedures to authenticate and respond to verifiable consumer requests. The
CCPA also sets forth certain provisions businesses should include in their contracts with service providers
and other privacy requirements under this new law.
Massachusetts has implemented information security requirements applicable to certain types of Personal
Information (e.g., social security number, driver’s license number, and financial account information) about
Massachusetts residents. These requirements include encryption of portable devices, e-mail, and back-up
tapes that contain such classes of Personal Information. Since information related to Massachusetts
residents could be intermingled with data relating to residents of other states, this law has effectively
imposed information security requirements beyond its borders.
Federal and State Breach Notification Laws
HITECH added new breach notification requirements under HIPAA for Covered Entities and Business
Associates related to “unsecured” health information. If PHI is acquired, accessed, used, or disclosed in a
manner not permitted by the HIPAA Privacy Rule or in a manner that compromises the security or privacy
of the PHI, the HIPAA Breach Notification Rule requires notification to affected individuals, the U.S.
Department of Health & Human Services (HHS), and in some cases, the media.
In addition to the federal requirements, every state has its own breach notification law. State breach
notification laws require that, under certain circumstances, the individuals whose data has been
compromised (unauthorized access or disclosure) be notified of the breach and/or that state government
officials be notified. These laws do not contain uniform requirements from one state to the next. The
growing trend is expanding the types of matters which are defined as a reportable breach. Many state laws
include health data as a trigger for notification. Consequently, managing even a relatively small breach
(e.g., a lost laptop containing Personal Information) can be complex, time-consuming, and costly. Some
notification periods under these breach notification laws are very short. Therefore, it is critical that any
suspected breach be reported immediately to Pfizer’s Global Security Operations Center (GSOC) (1-212-
733-7900 or GSOCwatchro[email protected]) pursuant to CP #411: Information Incident Response Policy.
You should also notify your team attorney. Lost or stolen computers or other devices containing Pfizer data
must be reported to your local Service Desk/Help Desk (the worldwide list of contact telephone numbers is
available online at http://ITSupport.pfizer.com).

White Guide – Chapter 11: Privacy: Protecting Personal Information
Rev. 12/20
Page 197 of 405
Laws Protecting the Personal Information of Children
The federal Children’s Online Privacy Protection Act (COPPA) prohibits the collection, use or disclosure
of a broad range of Personal Information collected online from children younger than 13 without the
verifiable consent of a parent or guardian and is enforced by the U.S. Federal Trade Commission (FTC).
Most Pfizer programs and services do not target children. If your program does intend to target children,
or could be perceived to appeal to children (e.g., use of cartoons or games), please consult your team
attorney and/or the GPO.
Under at least one state law, Pfizer may not sell the Personal Information of minors without depending on
age, either parental opt-in consent or consent of the minor. Such state laws may define “Sales” is very
broadly defined to include any selling, providing, making available or disclosing personal information in
exchange for any consideration or thing of value, i.e. not only sales for money.
Requirements for Transparency, Notice, and Consent
There is a strong trend toward transparency, notice, and opt-in consent with respect to the collection and
use of Personal Information. There also is an expansion of what is defined as “personal information” as
technologies and privacy awareness expand. In many cases personal information can now include IP
addresses, device identifiers and anything that could reasonably identify an individual. No longer is the
definition of personal information limited to “name plus” such as full name plus your social security number.
The types of harms or injuries are no longer just financial injuries such as having your credit card number
stolen but now often include remedial injuries such as disclosure of racial identity, sexual orientation,
political affiliation and more depending on the location.
As privacy laws are constantly evolving with greater and greater protections and rights for individuals, Pfizer
colleagues and contractors should consult their team attorney before engaging in any activity that that
contemplates the collection, use, sharing or storing of personal information of any kind, whether that
individual is an HCP, a patient, or another type of consumer. Special attention should be paid to initiatives
that involve collection by Pfizer or third-party vendors of patient cell phone numbers, text messaging, email
addresses, DOB, diagnosis or treatment information and dates.
Pfizer respects the privacy of individuals, including patients, caregivers, and HCPs. Pfizer’s has enacted
appropriate safeguards designed to protect Personal Information it processes of all kinds.
Every colleague and contractor have the obligation to play his or her role in helping to protect Personal
Information in light of the Personal Information he or she possesses or accesses, as well as any initiatives
involving Personal Information that he or she is handling. This includes understanding any Personal
Information that such initiatives or campaigns will collect, use, or share, and the lifecycle of that data (e.g.,
Pfizer’s Policies Relating to Privacy and Personal Information

White Guide – Chapter 11: Privacy: Protecting Personal Information
Rev. 12/20
Page 198 of 405
to whom it will flow, how it will be stored and retained, etc.), and ensuring that all such Personal Information
is handled and safeguarded in compliance with all applicable Pfizer policies and procedures.
Notice and Consent
Pfizer may obtain access to Personal Information as part of critical business activities such as:
• Communicating directly to patients through approved Pfizer-sponsored third-party communications;
• Engaging in a mentorship or preceptorship involving patient contact;
• Collecting Personal Information as part of an approved survey, screening tool, or other similar activity;
• Collecting Personal Information from HCPs in connection with enrollment in marketing programs;
• Collecting Personal Information from consumers in connection with coupon/copay programs, Internet
websites, and other consumer offerings;
• Collecting Personal Information in connection with patient assistance programs;
• Collecting Personal Information in the course of recruiting patients as speakers or to provide
testimonials; and
• Analyzing HCP prescriber information in connection with sales and marketing activities.
To be compliant with law and Pfizer policy, it is critical that the appropriate disclosures and, in some cases,
an affirmative consent be in place prior to accessing, collecting, or using Personal Information. Before your
team collects any Personal Information or designs any program which could result in Personal Information
being directly or inadvertently disclosed to Pfizer, you must first consult your team attorney to confirm that
any required notice and consent have been provided and/or obtained. To the extent you are using a third-
party service provider to assist with your program, consult the relevant Legal colleague who may consult
with the GPO to determine whether appropriate contractual terms are in place.
Aggregated, Anonymized or De-identified Data
It is sometimes permissible for Pfizer to obtain previously personally identifiable information from an HCP
or health plan administrator without an individual’s consent if the information has been aggregated or
anonymized. “Aggregated” data is information about multiple individuals that is compiled and does not
allow for the re-identification of any one individual. “Anonymized” data is data that cannot be identified
as belonging to any specific individual and usually involves removing certain key identifiers (including the
individual’s name, many elements of the individual’s address, telephone number, date of birth, patient ID,
and social security number), which either alone or in combination, could link the information with a specific
individual. The standard for “anonymizing” data varies between countries. Therefore, always consult your
team attorney before assuming information has been properly “anonymized” Who may reach out to GPO.
White Guide – Chapter 11: Privacy: Protecting Personal Information
Rev. 12/20
Page 199 of 405
Avoiding Exposure to Protected Health Information
Pfizer colleagues must avoid situations in which they may be exposed to PHI without an individual’s
consent. With certain exceptions, HCPs are not permitted to use or disclose an individual’s PHI unless the
individual has authorized the use or disclosure in writing in advance. In the event an HCP or other person
inadvertently or intentionally exposes you to the PHI of others, you should not document or reproduce the
information in any media or form. You must also strictly maintain the confidentiality of the information in
accordance with Pfizer’s policy of safeguarding the privacy of all patient-related data, and consult your team
attorney to determine whether any additional steps should be taken.
Adverse Event Reporting
HCPs are permitted to share PHI about their patients without a Business Associate Agreement or patient
authorization in limited circumstances. HCPs are permitted to disclose PHI to persons “subject to the
jurisdiction of the FDA,” such as Pfizer, for activities related to the quality, safety, or effectiveness of an
FDA-regulated product or activity for which the person has responsibility. Therefore, if an HCP reports an
adverse event or other safety or product information, continue to follow the process established for
collecting information about and reporting these events pursuant to Corporate Policy 903 – Your
Responsibility to Report Information about Safety, Quality or Performance of Pfizer Products.
Vendor Obligations
All Pfizer vendors who will have access to Personal Information of or on behalf of Pfizer must follow our
policy of safeguarding Personal Information and the Pfizer colleague must ensure contractual terms are in
place with the vendor to protect personal information. To this end, Pfizer has a Privacy Exhibit and the-
Third Party Security Requirements that may be included as part of contracts with such vendors following
consultation with Procurement and/or the GPO. If Personal Information is processed by the Service
Provider, the Privacy Exhibit is required. To qualify as a service provider to Pfizer, vendor contracts need
certain language to be included under; otherwise disclosures of Personal Information to the vendor may
constitute sales, of which a consumer may opt-out. Please note that in addition to the contractual
requirements, any vendors that will have access to or process Personal Information on behalf of Pfizer may
be required to complete and pass appropriate Pfizer vendor vetting processes managed by BT Audit and
Assessment. For more information about Pfizer’s Vendor Compliance Assessment Service (VCAS)
please visit: http://ecfd13.pfizer.com/sites/BTCompliance.

White Guide – Chapter 11: Privacy: Protecting Personal Information
Rev. 12/20
Page 200 of 405
When using Personal Information to communicate with current or potential Pfizer customers (HCPs or
consumers) for promotional purposes, it is important to work with Digital Channel Enablement (DCE) to
ensure compliance with applicable legal requirements as well as Pfizer policies and procedures.
Marketing Initiatives and Other Communications
Pfizer-Sponsored Third-Party Communications
A variety of marketing initiatives and other communications may raise privacy concerns. For example,
Pfizer may want to sponsor a medication compliance/adherence program to be provided by or through a
customer (e.g., a Managed Care Organization (MCO) or a pharmacy). These programs usually involve
sending scheduled mailings to patients to remind them to fill or refill a prescription.
Under certain limited circumstances, PHI may be used by HCPs such as pharmacists to tailor
communications for treatment of the individual. Occasionally, and subject to strict limitations and legal
review, Pfizer may pay for certain communications to be made to patients. For example, such
communications may include MCOs and retail pharmacies sending Pfizer-approved disease management,
educational materials, or medication compliance mailings to inform or remind patients of the schedule to fill
or refill a prescription for a chronic medication. When considering such arrangements, you must consult
with your team attorney, who may consult with the GPO as appropriate, to determine compliance with
applicable privacy laws and regulations and appropriate contractual terms.
Importantly, Pfizer-sponsored third-party communications to patients must be the subject of a Pfizer-
approved service agreement between Pfizer and the MCO, pharmacy, or intermediary service provider.
Depending on the origin of the service agreement (Headquarters or the field), the appropriate team attorney
must review it and, if the relationship involves an MCO customer, the agreement must also undergo
Organized Customer Legal Team review and approval.
A key reason to enter into the service agreement is to ensure the protection of patient privacy as well as
compliance with applicable laws and Pfizer policy. Pfizer should not receive any patient names, addresses,
or other Sensitive Personal Information. All materials sent to patients must be approved by the appropriate
Review Committee, which will consider issues of patient privacy and patient consent as part of its review
process. The RC may consult with the GPO on such issues as appropriate.
Activities That May Result in the Use and
Disclosure of Personal Information

White Guide – Chapter 11: Privacy: Protecting Personal Information
Rev. 12/20
Page 201 of 405
Digital Marketing Initiatives
Pfizer must implement reasonable measures designed to protect Personal Information collected and
transmitted via the Internet. Additionally, laws and other guidance restrict the use of Personal Information
for interest-based advertising (sometimes called “online behavioral advertising”).
Pfizer teams proposing to conduct web-based marketing and promotional activities (e.g., advertising,
websites, Facebook pages, etc.) that use or collect Personal Information should consult their team attorney
or the GPO to determine whether such activities raise privacy concerns. In addition, any externally-facing
Internet application (such as a website, mobile device application, or Facebook page) must undergo and
pass Vulnerability and Threat Management Testing, which may be accessed at http://websecurity.
pfizer.com. For more information about Pfizer policies on Internet promotion, see White Guide Chapter 2:
Advertising and Promotional Materials.
Pfizer’s Patient Programs
As a general policy, Pfizer does not communicate directly with patients based on their health information
unless, among other requirements, the patient has affirmatively consented (or “opted in”) to receiving such
communications.
Pfizer has a standardized Privacy and Consent Policy for all U.S.-based consumer activities that involve
the collection and use of consumers’ Personal Information by any channel, including hard copy or online
forms, business reply cards, telephone and fax. To obtain the Privacy and Consent Policy and related
requirements, see the Privacy and Consent Policy section under the Patient & Physician Marketing Group
tab in GCO Policy Xchange on GCO on Demand. These activities include, but are not limited to, disease
management program enrollment forms, coupons and rebate offers, and sweepstakes offers. The
guidelines apply only when the consumer provides Personal Information, such as name and address or e-
mail address. Pfizer may not discriminate against or exclude consumers from participating in programs
based on the fact that consumers do not opt-in or opt-out of providing their Personal Information. The same
applies to the subsequent selling of the consumer’s Personal Information. Offering financial or other
valuable incentives in exchange for data may be allowed in limited circumstances; any such programs or
offers require review by the Global Privacy Office. Whenever a Pfizer program requires a consumer to
provide such information, the program must also include a simple, timely mechanism (e.g., a toll-free
telephone number or a mailing address) that allows participating individuals to promptly discontinue or “opt-
out” of the program. Before engaging in any loyalty or rewards, or other incentive programs, you must
consult with your team attorney. It is also especially important to work with your team attorney and the
GPO to ensure that all programs contain appropriate privacy language in both the program terms as well
as the opt-out because requirements vary depending upon the communication channel used. The Privacy
and Consent Policy must also be communicated to, and followed by, any vendors preparing materials on
behalf of Pfizer. Therefore, it is important that Pfizer teams considering programs that would collect

White Guide – Chapter 11: Privacy: Protecting Personal Information
Rev. 12/20
Page 202 of 405
Personal Information consult the DCE team to determine whether appropriate authorizations and guidelines
are in place. In addition, it is important to work through DCE supported processes and vendors to
ensure compliance with “Do Not Contact” lists and appropriate management of data.
Working with HCPs
When interacting with HCPs, you may find yourself in situations in which you may access Personal
Information, including Sensitive Personal Information. As noted above, these situations should be avoided
to the extent possible. If such exposure cannot be avoided or is a routine, unavoidable element of the
engagement with the HCP, be sure to follow these guidelines.
HCPs may incorrectly request that you sign a Business Associate Agreement. As noted above, Pfizer
generally does not function as a Business Associate, and therefore signing such an agreement is prohibited
absent the express written approval of your team attorney or the GPO. The protections HCPs seek can
more appropriately be provided through a confidentiality agreement. A confidentiality agreement commits
you and Pfizer to treat the Personal Information you may have access to with care and safeguard its
confidentiality. To address this need and provide an alternative to a Business Associate Agreement, Pfizer
has developed two Pfizer template forms, either of which you are permitted to offer to the HCP as assurance
of your intent to keep Personal Information confidential:
1. The Privacy Pledge can be signed and provided to HCPs or customers who might have general
concerns about Pfizer’s position on HIPAA as it relates to its representatives.
2. The Patient Health Information Confidentiality Agreement can be signed and provided to an HCP
or institution that would like a specific agreement to cover situations where a Pfizer representative might
inadvertently come into contact with patient health information.
No changes can be made to these templates before signing them unless your team attorney or the GPO
has approved the change in advance.
A copy of the Privacy Pledge and Patient Health Information Confidentiality Agreement can be downloaded
from MyPfieldNet under the “Compliance” tab.
Business Associate Agreements
What should I do if a physician insists that I sign a Business Associate Agreement
before I enter a patient clinic? Can I sign the Business Associate Agreement?
No. You must not sign a Business Associate Agreement, even if required by an HCP
in order to be allowed access to a facility. Colleagues are able to sign the Pfizer
Privacy Pledge or Patient Health Information Confidentiality Agreement using the
templates found on MyPfieldNet. Providing a copy of one of these documents with
your signature is typically enough to satisfy the HCP’s concerns about patient privacy.

White Guide – Chapter 11: Privacy: Protecting Personal Information
Rev. 12/20
Page 203 of 405
Business Associate Agreements
If the HCP continues to insist on a Business Associate Agreement, please promptly
contact your team attorney for assistance.
Signing Customer Confidentiality Agreements
If an HCP insists that I sign a facility’s Confidentiality Agreement, even after I sign and
show him or her Pfizer’s Privacy Pledge and Patient Health Information Confidentiality
Agreement, can I sign the version the HCP wants me to sign?
Maybe. While it is advised that you rely on one of the Pfizer templates, in certain
instances a confidentiality agreement provided by a customer may be acceptable to
sign. However, you should never do so unless your team attorney has first reviewed
and approved the agreement.
Mentorships and Preceptorships
A mentorship allows a Pfizer colleague to observe or “shadow” an HCP (usually a physician) at his or her
office or institutional practice. A preceptorship, on the other hand, is a training presentation by an HCP to
a team or group of Pfizer colleagues about a particular therapeutic area or the clinical use of one or more
Pfizer products in professional practice.
Mentorships and preceptorships can be valuable educational tools, but may impact patient privacy if Pfizer
colleagues are permitted to observe treatment and/or consultation sessions with a patient, or if Pfizer
colleagues discuss an individual’s treatment with a patient’s HCP.
When setting up a mentorship or preceptorship, Pfizer colleagues must ensure that physicians serving as
mentors or preceptors know they must obtain their patient’s written authorization before Pfizer colleagues
may be allowed to observe any consultation, examination, and/or treatment. You may offer Pfizer’s sample
Patient Authorization Form (available on MyPfieldNet) to an HCP; however, they are not required to use
the Pfizer form. This form includes language required by HIPAA and may not be altered without the
advance approval of your team attorney or the GPO. The requesting HCP should maintain the signed
Patient Authorization Form as part of the patient’s record and provide a copy of the form to the patient. You
should not retain a copy of a signed Patient Authorization Form.
For more information on these activities, see White Guide Chapter 5: HCP and Government Official
Consulting Engagements and the Mentorship Guidelines and Forms available on MyPfieldNet.

White Guide – Chapter 11: Privacy: Protecting Personal Information
Rev. 12/20
Page 204 of 405
Patient Consents Regarding Mentorships and Preceptorships
Does a patient have to sign an authorization form before a Pfizer Sales Colleague can
observe an examination or treatment as part of a mentorship or preceptorship, or is
oral permission sufficient?
It is the HCP’s responsibility to secure appropriate patient authorization in a
mentorship or preceptorship. Pfizer has developed a form authorization for the HCP
to use in the event there is no existing authorization. Under HIPAA, with limited
exceptions, a patient must authorize in writing the disclosure of his or her PHI. Oral
permission is generally not acceptable under HIPPA or Pfizer guidelines. It is also
important to remember that once proper authorization is obtained from the patient, the
Pfizer colleague participating in the mentorship or preceptorship must identify himself
or herself as an employee or contractor of Pfizer, as the case may be. A name badge
identifying the colleague as a Pfizer employee must be worn at all times when
interacting with a patient.
Chart Reviews
Is it permissible to conduct chart reviews as part of our collaborative studies/programs
with customers?
No. Pfizer colleagues should never conduct a chart review.
Consumer Health Fairs or Screenings
Consumer health fairs and screenings may raise patient privacy concerns because Personal Information is
often communicated in the presence of Sales Colleagues or other Pfizer colleagues at the health fair. Pfizer
colleagues should not engage health fair attendees in discussions regarding a specific patient’s health.
These discussions should occur between the patient and an appropriate HCP. Should a patient attempt to
initiate such a discussion, the Pfizer colleague should make clear that he or she is not an HCP and is not
providing medical advice, and should redirect the patient to an HCP at the fair or state that the patient
should discuss the matter with his or her physician.
For more information on health fairs and screenings, see White Guide Chapter 12: Promotional Interactions
with Consumers.

White Guide – Chapter 11: Privacy: Protecting Personal Information
Rev. 12/20
Page 205 of 405
Medical Colleague (e.g., MOS, FMD) Interactions with Consumers at Health Fairs and
Screenings
May a colleague, with a medical background, counsel consumers on how to interpret
their screening results at a Pfizer-sponsored health screening?
No. Pfizer colleagues are not permitted to practice medicine or provide clinical advice
to patients in the course of their work for Pfizer.
Patient Assistance Programs and Protected Health Information
May Pfizer receive Protected Health Information from health plans for the purposes of
Pfizer’s Patient Assistance Programs?
Pfizer’s policy is that it may receive PHI from health plans in order to verify an
individual’s eligibility for Pfizer’s Patient Assistance Programs only if the information is
transferred to Pfizer with the patient’s written authorization and the information is used
solely for the program or other appropriate use explicitly identified on the authorization
form. For more information on Pfizer’s Patient Assistance Programs, see White Guide
Chapter 10: Patient Assistance Programs.
Patient Information and Clinical Trials
Pfizer is committed to protecting the privacy and security of the Personal Information generated in clinical
trials, including with respect to the electronic transmission of clinical trial data. Pfizer has established
technical, physical, and administrative security measures, which include integrity controls and encryption
(where appropriate), to guard against unauthorized access to Personal Information that it electronically
transmits or receives.
Teams involved with Pfizer-sponsored and investigator-initiated studies are responsible for securing
appropriate consent for the use of patient information obtained from clinical trials.
In accordance with Clinical and Medical Controlled Document (CMCD) INV04-GSOP: Clinical Site
Management and Monitoring , clinical study team members must always protect the confidential nature of
the Personal Information that they review. If Personal Information is copied or referenced in monitoring
reports, appropriate written authorizations must generally be obtained from patients. Although Pfizer is not
directly covered by HIPAA, it is subject to other laws which protect the confidentiality of subjects’ Personal
Information.

White Guide – Chapter 11: Privacy: Protecting Personal Information
Rev. 12/20
Page 206 of 405
Use of Data from Clinical Studies
May Pfizer use records from its sponsored clinical studies for marketing purposes?
No. The use of medical records is strictly regulated. Pfizer’s policy is that Personal
Information in clinical study records may never be used for marketing purposes. Prior
to patient enrollment in a clinical study, investigators are required to explain to patients
what health information will be collected, how that information will be used, and to
whom and for what purposes it may be disclosed. In general, study participant medical
data is generated or received and maintained by the clinical study investigator during
the course of the study. Pfizer does receive a report of study-related data; however,
the clinical investigator “key-codes” the data by replacing the identities of the
participants with unique codes. Pfizer does not receive the keys to these codes, nor
does the Company receive the names or other contact information of study participants
except in very limited circumstances, such as when necessary to report adverse
events.
Healthcare Professional Prescriber Data
From time to time, Pfizer uses prescriber data to facilitate effective marketing communications with
HCPs. Prescriber data serves a variety of purposes, including the tracking of Pfizer-product adverse events
and the ability to focus marketing initiatives on HCPs who would most likely benefit from information about
a particular Pfizer product. This information is confidential, however, so it is vital not to use this prescriber
data in a manner that compromises its confidential nature or your integrity as a Pfizer colleague.
You are prohibited from sharing an HCP’s prescriber data with other individuals and entities outside of
Pfizer as that would compromise its confidentiality. HCP prescriber data must only be used for legitimate
business purposes, such as the development of your team’s promotional strategy. Access to HCP
prescriber data must be limited to individuals with a legitimate business need. When developing reports
that contain HCP prescriber data, colleagues should remove any extraneous data prior to distributing the
report, if possible. In the event that removing extraneous data is not possible, you must provide instructions
to recipients that in reviewing the report, that they must filter for HCPs that are on their TCLs or within their
territory or area of responsibility prior to reviewing the data.
Pfizer respects the confidentiality of this data and the wishes of any HCP who asks that his or her prescriber
data not be made available to Pfizer Sales Colleagues. Pfizer also has designated the Global Information
Other Privacy Issues

White Guide – Chapter 11: Privacy: Protecting Personal Information
Rev. 12/20
Page 207 of 405
Stewardship Lead in Global Business Analytics as the internal contact to respond to inquiries regarding
Pfizer’s policy on the use of prescriber data. Given that this area of law is quickly evolving, Pfizer colleagues
must consult with their team attorney or the GPO before engaging in an activity that involves the use or
disclosure of prescriber data for marketing or promotional purposes. Under many privacy laws business
contact information still amounts to what is defined as personal information.
Handling Personal Information of Healthcare Professionals and Other
As a general policy, Pfizer restricts access to Personal Information and other sensitive information to
individuals who need to know the information to perform their job duties. In general, most Pfizer colleagues,
including Sales Colleagues, do not need access to Personal Information about HCPs for any reason and
should not request, collect, or retain any such information. This type of information includes, but is not
limited to:
• Social Security or other government-issued numbers;
• Driver’s license numbers;
• Health insurance identification numbers;
• Credit card, debit card, bank account numbers, or any other financial account identifiers (with or without
associated security numbers);
• Employment identification numbers; and
• Biometric data (fingerprints, voiceprints, or retinal scans).
Access to and collection of Personal Information imposes an obligation to keep that information confidential
and secure and to tell stakeholders when such information is lost or stolen or there has been a breach of
security. Disclosure of certain types of Personal Information, even if accidental, can expose Pfizer,
colleagues, and contractors to legal liability, create a risk of fraud or even identity theft for the information
owner, and erode confidence in Pfizer and its commitment to privacy and information security.
You are responsible for handling Personal Information in accordance with all applicable Pfizer policies and
procedures. You should familiarize yourself with:
• CP #403: Acceptable Use of Information Systems;
• CP #404: Protecting the Privacy of Personal Information;
• CP #405: Records and Information Management Policy and Procedure
• CP #411: Information Incident Response Policy; and
Pfizer Policy on Your Responsibility for
Safeguarding Personal Information

White Guide – Chapter 11: Privacy: Protecting Personal Information
Rev. 12/20
Page 208 of 405
• CP #903: Your Responsibility to Report Information about Safety, Quality or Performance of Pfizer
Products .
Pfizer’s Handling Sensitive Information (HSI) Guidelines – Procedures for Handling PI and SPI for
Colleagues and Contractors provides important guidance about appropriate information handling and
security procedures, which include, but are not limited to:
• Encrypting your computer and using only encrypted USB flash drives;
• Properly destroying media or paper containing Personal Information;
• Promptly reporting lost or stolen Pfizer equipment, Personal Information, and other potential data
incidents to Pfizer’s Global Security Operations Center (GSOC) (1-212-733-7900 or GSOC
IT Service Desk (The worldwide list of contact telephone numbers is available online at
http://ITSupport.pfizer.com); and
• Never using unencrypted e-mail to transfer Personal Information outside of the Pfizer network.
If you have additional questions about appropriate information handling and security procedures, you
should consult the Handling Sensitive Information Guidelines or speak with your team attorney or the GPO.
• CP #403: Acceptable Use of Information Systems
• CP #404: Protecting the Privacy of Personal Information
• CP #405: Records and Information Management Policy and Procedure
• CP #411: Information Incident Response Policy
• Handling Sensitive Information (HSI) Guidelines – Procedures for Handling PI and SPI for Colleagues
and Contractors, see link
• Clinical and Medical Controlled Document (CMCD) INV04-GSOP: Clinical Site Management and
Monitoring
• Refer any questions to the Enterprise Multi-Channel Marketing team, your team attorney, or the Global
Privacy Office (privacy.officer@pfizer.com)
For More Information

Rev. 12/20
Page 209 of 405
CHAPTER #12 – PROMOTIONAL
INTERACTIONS WITH CONSUMERS

White Guide – Chapter 12: Promotional Interactions with Consumers
Rev. 12/20
Page 210 of 405
Chapter
#12
PROMOTIONAL INTERACTIONS WITH
CONSUMERS
CONTENTS
Introduction................................................................................................................................................ 211
Key Points to Ensure Compliance ...................................................................................................... 211
Meals and Items of Value to Consumers .................................................................................................. 212
Meals .................................................................................................................................................. 212
Items of Value ..................................................................................................................................... 212
Exhibits and Displays ................................................................................................................................ 213
Consumer Speaker Programs................................................................................................................... 213
Health Fairs and Public Screenings .......................................................................................................... 214
Screenings Offered to Employees of a Single Employer ................................................................... 214
Screenings Offered to the Public at Large ......................................................................................... 215
Additional Guidelines for All Screenings ............................................................................................ 215
Product Support Programs........................................................................................................................ 217
Disease Management Programs ........................................................................................................ 217
Unbranded Communications ....................................................................................................... 217
Branded Communications ............................................................................................................ 218
Medication Compliance Programs...................................................................................................... 218
Employees as Consumers ........................................................................................................................ 219
For More Information ................................................................................................................................. 220
Promotional Interactions with Consumers

White Guide – Chapter 12: Promotional Interactions with Consumers
Rev. 12/20
Page 211 of 405
Chapter #12 Promotional Interactions with Consumers
Pfizer interacts with consumers (non-HCPs) at various types of events including speaker programs, health
fairs, public screenings, disease management programs, and other Pfizer or non-Pfizer events. Laws and
industry standards specifically govern promotional interactions with consumers and require that Pfizer treat
promotional interactions and activities with consumers differently than those with HCPs. Like interactions
with HCPs, interactions with consumers can involve promotional risks. The U.S. Department of Health
and Human Services (HHS) Office of Inspector General (OIG) has warned that offering incentives to
consumers, such as remuneration or free services, may implicate the federal Anti-Kickback Statute.
Consumer protection laws that prohibit unfair or deceptive trade practices have been interpreted by some
state Attorneys General to encompass off-label promotion.
The FDA has established stringent requirements regarding direct-to-consumer communications. Also,
PhRMA has adopted its Guiding Principles on Direct to Consumer Advertisements About Prescription
Medicines to provide guidance to Pfizer and other member companies on ways to ensure that DTC
communications provide accurate, accessible, and useful information to patients and consumers. Pfizer
has committed to follow this guidance and has adopted its own Guidance for the Implementation of the
Updated PhRMA DTC Principles. For more information on the development of DTC promotional materials,
see White Guide Chapter 2: Advertising and Promotional Materials.
This Chapter summarizes certain Pfizer policies regarding promotional interactions with non-HCP
consumers. Non-compliance with these policies puts the Company at risk and can subject Pfizer
colleagues to disciplinary action up to and including termination of employment.
• Pfizer colleagues may provide occasional meals of minimal value to consumers ($50 or less
per person, including tax and tip). Meals may never, however, be provided to solicit business
or in a manner that might suggest that the recipient is being bribed or improperly influenced.
•
As with speaker programs for HCPs, Pfizer is responsible for the conduct of speakers and the
content of presentations at speaker programs for consumers. The program and speaker must
follow all applicable Centris requirements. The content of a consumer program should be
appropriate for a “lay” audience consistent with Pfizer Principles for Clear Health
Communication found here.
Key Points to Ensure Compliance
Introduction

White Guide – Chapter 12: Promotional Interactions with Consumers
Rev. 12/20
Page 212 of 405
Meals
Pfizer may provide meals of limited value ($50 or less per person, including food, beverage, tax, and tip) or
approved education items to consumers. Some examples of such items include providing a modest snack
or refreshment (e.g., fruit, granola bars, bottled water) to consumers that visit a Pfizer exhibit or display, or
providing a modest meal to attendees at a Pfizer consumer speaker program. However, meals may never
be provided to solicit business or in a manner that might suggest that the recipient is being bribed or
improperly influenced.
Items of Value
Pfizer may provide items of nominal value to consumers at exhibits, displays or public events. Examples
of such items include mugs, water bottles and stress balls, which may be provided to consumers that visit
a Pfizer exhibit or display, or at a public event where Pfizer sponsors a table. However, items of value may
never be provided to solicit business or in a manner that might suggest that the recipient is being bribed or
• Pfizer Sales Colleagues may promote Pfizer products at health screenings as long as the
exhibit and display booth is physically separate and apart from the screening area.
•
If any Pfizer colleague is present during a patient/consumer interaction at a health fair or
screening, he or she must clearly identify themself as a Pfizer employee and may not offer
any medical opinions, advice, or consultation, even if the colleague has a license to practice
medicine or is any type of healthcare professional.
•
The Pricing & Access Legal Team must approve all disease management program
arrangements with Managed Care Organizations (MCOs)
. Such arrangements must be
documented in a service agreement that sets forth the basis for payment, as well as the
program materials.
•
Employees of customer organizations may also be considered consumers. Pfizer interactions
with such employees (such as at a health fair) must conform to the same principles applicable
to consumer interactions.
• As outlined in White Guide Chapter 4: Marketing Programs
, Customer Engagement Programs
(CEPs) must be designed, reviewed, approved, and conducted in compliance with
CP #902:
Management of Safety Information for CEPs Policy and CP #902a: Management of Safety
Information for CEPs Procedure.
Key Points to Ensure Compliance
Meals and Items of Value to Consumers

White Guide – Chapter 12: Promotional Interactions with Consumers
Rev. 12/20
Page 213 of 405
improperly influenced. The OIG has defined items of “nominal value” as having a retail value of no more
than $15 per item or $75 in the aggregate per recipient, on an annual basis.
Pfizer is routinely offered the opportunity to purchase display space (booths) at medical meetings or to
sponsor health-related meetings that allow booths or displays. Such events may include health fairs where
consumers can be educated about Pfizer and its products.
As long as the Pfizer exhibit booth is separate and not joined with the health screening, Pfizer can provide
approved consumer materials at a health fair where Pfizer is also conducting a health screening. However,
it should never appear or be the case that Pfizer is conducting the screening in order to drive people to ask
their doctors about Pfizer products. Health fairs and public screenings are discussed in further detail later
in this Chapter. For more information regarding exhibit and display space, see White Guide Chapter 4:
Marketing Programs.
Providing Food to Consumers at a Display
I have a display table at a community health fair next week. Can I provide food at my
table? What about covering the cost of sandwiches for all the heath fair attendees?
You can provide modest hospitality snacks at a display table where you are interacting
with consumers. Any food you provide to consumers must be consistent with the level
of interaction you are having with them. In this case, because you are interacting at a
display table, it would be acceptable to provide modest snack items like fruit, granola
bars, and drinks. It would not be appropriate for you to cover the costs of sandwiches
or other food items for all attendees since you are permitted to provide food only to
those consumers with whom you interact. Remember, even when you have more
extensive interactions with consumers (e.g., at a speaker program) the cost of food,
beverage, tax, and tip should never exceed $50 per attendee.
A speaker program for consumer audiences is a promotional activity controlled by Pfizer at which a speaker
presents a Pfizer RC-approved slide deck intended for consumers. As with a speaker program for an HCP
audience, Pfizer is responsible for the conduct of the speaker and the content of the presentation to
consumers. Pfizer colleagues must adhere to Pfizer policies regarding consumer presentations and should
follow any applicable procedures in the speaker program system (Centris) for program set up. Prior to
engaging in any speaking engagements, speakers are required to complete training on: (1) Pfizer
Exhibits and Displays
Consumer Speaker Programs

White Guide – Chapter 12: Promotional Interactions with Consumers
Rev. 12/20
Page 214 of 405
Promotional Speaker Compliance Guidelines; and (2) the brand’s core product training or topic training
slide kit, as applicable.
The content of a consumer program should be appropriate for a “lay” audience, consistent with Pfizer
Principles for Clear Health Communication found here. When developing a consumer speaker program
slide deck, Pfizer must be mindful that many consumers have different educational backgrounds and their
ability to understand medical information varies.
Consumer programs should be broadly advertised such that each program will likely result in an audience
of at least three consumers. The chosen venue for the program must be conducive to providing educational
information, and Pfizer may not offer entertainment or recreation. An HCP engaged to speak at the program
must not provide specific medical advice to a consumer attendee, nor may the speaker use the Pfizer
program as an opportunity to promote his or her medical services or practice, or to recruit new patients.
Host colleagues are required to monitor all aspects of a consumer program, including content delivered,
and make corrections if needed. For more information on speaker programs to consumer audiences, see
Orange Guide Chapter 16: Consumer, Patient, and Employee Interactions, and for more information on
speaker programs generally, see White Guide Chapter 4: Marketing Programs.
Pfizer colleagues may interact with consumers at health fairs and, at times, organize public screenings.
Screenings promote the early detection of diseases and offer patients a meaningful opportunity to treat a
disease or condition.
Health screenings fall under two major categories: (1) screenings offered to employees of a single
employer; and (2) screenings offered to the public at large. For both types of screenings, Pfizer colleagues
that are present during any patient interactions must clearly identify themselves as Pfizer employees.
Wearing a Pfizer name tag at all times is a good way to provide identification. Also, under no circumstances
may Pfizer colleagues offer any medical opinions, advice, or consultation, even if the colleague has a
license to practice medicine or is a healthcare professional.
Screenings Offered to Employees of a Single Employer
Pfizer health screenings offered to employees of a single employer promote Pfizer goodwill. The
screenings must be conducted by an approved third-party vendor that routinely conducts such screenings
and that has entered into an appropriate contract with Pfizer.
These screenings may not be offered for employees of healthcare providers or payers of healthcare items
and services, including hospitals, medical practice groups, or MCOs that seek reimbursement from the
federal government. The screenings must be limited to current employees and their beneficiaries only and
Health Fairs and Public Screenings

White Guide – Chapter 12: Promotional Interactions with Consumers
Rev. 12/20
Page 215 of 405
must expressly exclude retirees who are beneficiaries under the employer’s retiree health plan. Also, the
screenings cannot be organized or designed in any way to generate referrals for any particular customer.
Pfizer Sales Colleagues may promote Pfizer products at the screenings as long as the exhibit and display
booth is physically separate and apart from the screening area. Further, no financial Return-On-
Investment (ROI) or similar analyses can be tied to the screening event. Any advertising or publicity
materials for the event that are created by Pfizer – or for which Pfizer colleagues provide input – should be
approved by the relevant product Review Committee (RC) or the Payer Channel Access (PCA) RC.
Screenings Offered to the Public at Large
Screenings offered to the public at large may be organized by a third-party or Pfizer directly. If an IRS
501(c) (3) healthcare-related charitable organization requests Pfizer support for a screening, the request
must be submitted directly by the organization to Pfizer’s office of Global Medical Grants (GMG) via the
charitable contribution website at www.pfizer.com/healthcarecharitables. See White Guide Chapter 7:
Support of External Organizations, for additional information about healthcare-related charitable
contributions.
If a public health screening is organized by Pfizer, the screening proposal must be approved by the
management of the team organizing the screening. As with screenings offered to an employer for its
employees, the screening cannot be organized or designed in any way to generate referrals for any
particular customer. The screening must be conducted by a third-party vendor that is not a healthcare
provider/payer and that routinely conducts such screenings. The vendor must enter into an appropriate
contract with Pfizer.
Sales Colleagues can promote Pfizer products at these screenings with an exhibit and display as long as
the exhibit and display booth is physically separate and apart from the screening area. Again, however, no
ROI-type analysis can be tied to the screening event.
Screenings offered to the public must be advertised and open to the community at large. This means the
screening should have a broad, community audience and should not be targeted to members of any
particular group. This does not mean that an entire city must be invited or that the event must be advertised
in a city newspaper. However, the public screening must be advertised in a broad manner and not merely
at a particular hospital or in particular medical offices. All advertising and publicity materials must be
approved by the relevant product RC or the PCA RC.
Additional Guidelines for All Screenings
Consumer health fairs and screenings raise privacy issues whenever they involve obtaining Personal
Information from individuals, including details that relate to an individual’s health status. If an individual’s
affiliation with Pfizer and Pfizer’s sponsorship of the screening are disclosed and apparent, a consumer’s
White Guide – Chapter 12: Promotional Interactions with Consumers
Rev. 12/20
Page 216 of 405
participation in the event is deemed to be his/her consent to share this Personal Information with a Pfizer
representative.
Pfizer’s ability to use or disclose data obtained at consumer health fairs or public screenings is strictly
limited by the terms specified on Pfizer’s Patient Authorization and Release form, which the screening
vendor must require that all screening participants sign. You may obtain a copy of the form under the
Compliance tab on MyPfieldNet. Aggregated de-identified data can be provided to an employer and/or
managed care customer only if the screening participant has signed a Patient Authorization and Release
form which specifically authorizes that the data can be provided to the employer and/or MCO managing the
prescription drug benefit. For more information on the topic of patient consent, see White Guide Chapter
11: Privacy: Protecting Personal Information.
Health fairs and screenings also raise concerns regarding the doctor/patient relationship. An HCP who
works for the screening vendor and provides disease screening services may explain the test results but
cannot prescribe a specific drug or treatment even if licensed to do so. In all cases, consumers should be
encouraged to speak to their individual HCPs about the results of the screening.
Managed Care Customer Health Screening
An MCO would like Pfizer to conduct a disease screening for employees of an
employer to which the MCO provides pharmacy benefit services. The MCO would
also like Pfizer to provide it with the de-identified, aggregate data from the screening.
Can Pfizer organize the screening and provide the data?
Maybe. The only reason Pfizer may conduct a disease screening is to improve
employee health. Pfizer cannot subsidize the operating expenses of the MCO or the
employer by conducting a screening that the MCO or employer would do on its own.
If there is an independent, valid reason for Pfizer to fund the screening, Pfizer can
organize it. If, for example, the employer suggested by the MCO is one of the larger
employers in an area, Pfizer would have an independent, valid reason to be screening
such a large employee population. If conducted, Pfizer may provide aggregated, de-
identified data from the screening to the MCO only if Pfizer’s Patient Authorization and
Release form has been signed by screening participants and it specifically authorizes
Pfizer to provide the data to the MCO administering the drug benefit. Employees of
the MCO are not eligible to participate in the screening and the MCO should not appear
as a co-sponsor of the event unless the MCO independently provides funding or
services.

White Guide – Chapter 12: Promotional Interactions with Consumers
Rev. 12/20
Page 217 of 405
Health Screening Vendors
Is there a list of approved vendors that can be used to conduct health screenings?
No. Some national vendors that have been used in recent years include Vitalogy and
Cardinal, but Pfizer does not require that these vendors, or even a national vendor, be
used. Pfizer does prohibit the use of vendors that are healthcare providers/payers.
This policy is intended to protect against the potential risks involved when making
payments to such providers/payers, as well as the risks that the use of such
providers/payers could be perceived as being aimed at generating patient referrals for
such providers/payers. If you are unsure about whether a vendor is a healthcare
provider/payer, contact your team attorney.
HCP Screener
Can a doctor or nurse from a healthcare provider/payer, such as a hospital or private
practice, conduct the screening free of charge if Pfizer pays for screening materials?
No, the screening must always be conducted by a vendor that is not a healthcare
provider/payer, even where no payment is being made to the screener.
Disease Management Programs
Pfizer or an MCO may at times mail Pfizer RC-approved disease management materials, patient education
materials, or other types of branded materials to healthcare providers and/or patients, subject to certain
payment and authorization requirements under HIPAA.
Unbranded Communications
Unless prior authorizations are obtained from MCO’s members, Pfizer is limited to providing unbranded
health information to the MCO’s members, if Protected Health Information will be used by the MCO in
making the communication.
Product Support Programs

White Guide – Chapter 12: Promotional Interactions with Consumers
Rev. 12/20
Page 218 of 405
Branded Communications
If Pfizer seeks to compensate an MCO for sending branded health information and Protected Health
Information will be used by the MCO in making the communication, the following must be met:
• Only RC-approved patient education materials may be used;
• There must be a written service agreement between Pfizer and the MCO that clearly states the
services to be provided and the basis for payment, which must be equal to the fair market value
cost of developing and/or conducting the services to be provided;
• The MCO must secure authorization from its members before making the communication if the
communication does not relate to a drug or biologic that is currently prescribed to the patient
(discussed in the following section);
• The Organized Customer Legal team must approve the proposed arrangement and agreement
before any commitment can be made to the MCO;
• The amount paid must be directly attributable to an invoice for mailing costs and calculated on a
per-unit (e.g., per letter) basis;
• A lump sum payment to the MCO in excess of actual project costs is not permissible because any
excess payment could be interpreted as an attempt to enrich the MCO and as an illegal
inducement;
• The proposed mailing must conspicuously disclose Pfizer’s financial support; and
• It is preferable, but not required, that a third-party mailing operation perform the services and
receive the payment. If a third-party is used, the third-party may only receive fair market value for
its services and it may not pass through any additional payment beyond that required to cover
direct costs of the mailing to the MCO.
Please consult your team attorney if you have questions on the permitted scope of communications with
MCOs and their members.
Finally, because of privacy concerns, disease management program customer mailings must not involve
disclosure to Pfizer of patient names, addresses, or other Personal Information. All logistics that could lead
to disclosure of Personal Information must be handled through the MCO or a third-party mailing operation
that has been retained by the MCO.
Medication Compliance Programs
From time to time, Pfizer may want to pay for a medication compliance program (sometimes referred to as
a “refill reminder” or “adherence” program) to be provided by or through a customer (e.g., an MCO or a
pharmacy). These programs typically involve sending scheduled mailings or other communications (e.g.,
text messages) to patients to remind them to fill or refill a current prescription. Such programs are

White Guide – Chapter 12: Promotional Interactions with Consumers
Rev. 12/20
Page 219 of 405
appropriate promotional activities, and Pfizer may implement these programs without individual patient
authorizations if Pfizer and the customer comply with the terms of the marketing “refill reminder exception”
under the HIPAA Privacy Rule. The type of compensation permitted under the refill reminder exception
depends on whether the compensation is provided directly by Pfizer to either the customer or a business
associate of the customer for the relevant communications. The communications must conspicuously
disclose Pfizer’s support.
If Pfizer pays a customer directly, Pfizer may reimburse the customer only for the reasonable direct or
indirect costs related to the labor, materials, supply, and capital and overhead costs of making the
communications. If Pfizer pays a customer’s business associate, Pfizer may compensate the business
associate up to the fair market value of the services provided.
Please note that the following activities are not permitted under the refill reminder exception:
• Communications regarding new formulations of a currently-prescribed drug or biologic;
• Communications about a drug that may be used in conjunction with a currently prescribed drug or
biologic, also known as an adjunctive drug; and
• Communications encouraging an individual to switch from a currently prescribed drug or biologic.
For arrangements that do not comply with the requirements of the HIPPA refill reminder exception, the
customer must obtain HIPAA-compliant patient authorizations before disseminating the communications.
The team RC must approve any medication compliance program, each of which must also be documented
in a service agreement that sets forth the basis for payment, as well as the program materials. If the
customer is an MCO, the Organized Customer Legal team must review and approve the proposed
arrangement. Because the use of confidential patient medical information to communicate with patients
has privacy implications even if patient-identifiable information is not disclosed, please consult the section
on Pfizer-Sponsored Third-Party Communications in White Guide Chapter 11: Privacy: Protecting Personal
Information.
Employers are increasingly making decisions regarding the access their employees have to medicine. As
a result, Pfizer colleagues may have an interest in calling on employers to present information about Pfizer
products relevant to the employer in making these decisions. It is important to understand that working
with employers has both business and legal risks, which require careful attention.
Employers will often request that Pfizer interact directly with their employees in the interest of providing
health education. It is important that Pfizer treat these employees as consumers. Accordingly, Pfizer must
ensure that it applies the same principles set forth in this Chapter to its interactions with employees.
Employees as Consumers

White Guide – Chapter 12: Promotional Interactions with Consumers
Rev. 12/20
Page 220 of 405
Also note that discussions with employees, as consumers, must comply with FDA regulations. As noted
earlier in this Chapter, additional considerations and limitations may apply to employees of healthcare
providers or payers of healthcare items and services, including hospitals, medical practice groups, or MCOs
that seek reimbursement from the federal government. For more information on interactions with employer
representatives (such as benefit managers and medical/non-medical personnel who play a role in
administering health benefits for an employer), see White Guide Chapter 11: Privacy: Protecting Personal
Information.
• Guidance for the Implementation of the Updated PhRMA DTC Principles.
• Pfizer Principles for Clear Health Communication found here.
• CP #902: Management of Safety Information for CEPs Policy .
• CP #902a: Management of Safety Information for CEPs Procedure.
• Orange Guide Chapter 16: Consumer, Patient, and Employee Interactions.
• White Guide Chapter 2: Advertising and Promotional Materials.
• White Guide Chapter 4: Marketing Programs.
• White Guide Chapter 7: Support of External Organizations.
• White Guide Chapter 11: Privacy: Protecting Personal Information.
• White Guide Chapter 13: Promotional Interactions with Employer Groups..
• Refer any other questions to Regulatory or your team attorney.
For More Information

Rev. 12/20
Page 221 of 405
CHAPTER #13 – PROMOTIONAL
INTERACTIONS WITH EMPLOYER
GROUPS

White Guide – Chapter 13: Promotional Interactions with Employer Groups
Rev. 12/20
Page 222 of 405
Chapter
#13
PROMOTIONAL INTERACTIONS WITH
EMPLOYER GROUPS
CONTENTS
Introduction................................................................................................................................................ 223
Key Points to Ensure Compliance ...................................................................................................... 223
Coordinate with Director, Employers (DE) ................................................................................................ 224
Treat Employer Representatives (Decision Makers) as HCPs ................................................................. 224
Tailor Discussion to Individual Employer Representative ......................................................................... 224
Benefit Managers................................................................................................................................ 225
State/Municipal Employees ................................................................................................................ 225
Unions ................................................................................................................................................. 225
Brokers and Consultants .................................................................................................................... 225
For More Information ................................................................................................................................. 226
Promotional Interactions with Employer Groups

White Guide – Chapter 13: Promotional Interactions with Employer Groups
Rev. 12/20
Page 223 of 405
Chapter #13 Promotional Interactions with Employer Groups
Employers are increasingly involved with decisions regarding their employees’ prescription drug benefits.
As a result, Pfizer colleagues may at times address the benefits and risks of Pfizer products with employers.
It is important to understand that working with employers has both business and legal risks if not done in
an appropriate manner. It is also important to distinguish between interactions with employer
representatives who make formulary or coverage decisions regarding Pfizer products and interactions with
employees who also may be patients taking a Pfizer product.
This Chapter summarizes certain key Pfizer policies regarding interactions with employers and
employer representatives. Non-compliance with these policies puts the Company at risk and can
subject Pfizer colleagues to disciplinary action up to and including termination of employment.
•
Coordinate all employer-related activities with the relevant Director, Employers (DE).
• Treat employees as consumers.
•
Always provide fair and balanced presentations to employer representatives that include the
proven benefits of the product along with relevant safety information.
•
Treat all employer representatives as if they are subject to federal healthcare laws, including
the Anti-Kickback Statute, even those employers that may not participate in government
programs.
•
When interacting with employer representatives, tailor any product discussion carefully to
the representative’s background, especially if the employer representative does not have a
medical background.
•
Pfizer colleagues may not direct employers to a specific PBM/HMO or encourage an
employer to switch to a different PBM/HMO.
Key Points to Ensure Compliance
Introduction

White Guide – Chapter 13: Promotional Interactions with Employer Groups
Rev. 12/20
Page 224 of 405
In order to best leverage existing relationships and avoid providing inconsistent messages, all employer
activities should be coordinated with the relevant Director, Employers (DE). DEs (formerly called NEAMs)
are Pfizer colleagues in the Payer and Channel Access (PCA) group who are dedicated to working with
employers. DEs work directly with national employers, brokers, employee benefit consultants, unions, and
national associations and coalitions, and they also coordinate with regional account management with
respect to regional employers and associations. DEs work to understand the employer market, develop
clear plans, and coordinate implementation of those plans with other colleagues. In many cases, DEs have
established relationships with employers, unions, or other associations, and have a clear understanding of
permissible and impermissible discussions and activities with these individuals and entities.
Pfizer colleagues may interact with medical and non-medical employer representatives, such as CEOs,
CFOs, CMDs, and benefit managers. In some cases, these employer representatives play a role in the
treatment of patients by influencing the recommendation, purchase, or reimbursement of products. When
interacting with these employer representatives, Pfizer colleagues must always give a fair and balanced
presentation and, for product information, include the proven benefits of the product along with relevant risk
information. All unsolicited inquiries requesting off-label information about unapproved products or uses
must be referred to U.S. Medical Information. Pfizer colleagues must treat all employer representatives as
if they are subject to federal healthcare laws, including the Anti-Kickback Statute, even those employers
that may not participate in government programs. As a result, Pfizer colleagues may never engage in any
actual or perceived quid pro quo, including offering or appearing to offer any remuneration or item of value
in exchange for prescription or formulary recommendations or referrals.
Employers and Employees
Should Pfizer employees treat employer representatives (decision makers) and
employees in the same manner?
No. Pfizer colleagues must treat employer representatives as HCPs. Employees
should be treated as consumers.
When interacting with employer representatives, Pfizer colleagues must tailor any product discussion
carefully to the representative’s background, especially if the employer representative does not have a
Coordinate with Director, Employers (DE)
Treat Employer Representatives (Decision Makers) as HCPs
Tailor Discussion to Individual Employer Representative

White Guide – Chapter 13: Promotional Interactions with Employer Groups
Rev. 12/20
Page 225 of 405
medical background. Use appropriate, approved employer market-specific tools when working with
employers as resources that are designed for other audiences may not resonate with these customers.
Benefit Managers
Benefit managers may want to discuss the coverage offerings and access availability for Pfizer products.
As with HCPs at medical groups or hospitals, Pfizer colleagues may engage in discussions about coverage
and access, provided that their statements are truthful, accurate, and not misleading, and that Pfizer
colleagues only use materials approved for that purpose, such as Pfizer-approved access grids. Pfizer
colleagues may not direct employers to a specific PBM/HMO or encourage an employer to switch to a
different PBM/HMO. Pfizer colleagues may not discuss confidential information between Pfizer and a
PBM/HMO, including whether or not Pfizer has a rebate agreement with a particular PBM/HMO or any of
the contractual terms with any employer, even if the employer is a customer of the PBM/HMO in question.
State/Municipal Employees
Some of the larger employers in an area may be public entities, such as state universities, state agencies,
or municipalities. Interacting with these employers may subject you to additional guidelines relevant to
interacting with public employees, such as restrictions on gifts or meals or reporting obligations arising from
lobbying laws. For more information, see White Guide Chapter 15: State Laws: HCP and Government
Employee Restrictions and White Guide Chapter 16: Federal Employee Interactions and Lobbying. Pfizer
colleagues should consult with the Government Relations Director or Legal before interacting with a state
or municipal employer.
Unions
Certain interactions with unions are subject to federal reporting obligations and possibly other limitations.
Pfizer colleagues should check with a DE and Legal before interacting with any union representative.
Brokers and Consultants
When interacting with employer groups, Pfizer colleagues may come in contact with employee benefit
consultants or brokers. There are national DE leads specifically assigned to work with brokers and
consultants. To ensure that Pfizer presents a consistent message, Pfizer colleagues must consult with
their DE before interacting with any broker or consultant. Pfizer colleagues may not direct or influence
employers to work with a specific broker or consultant.
Materials Used With Employers
What type of information may Pfizer provide to employer representatives?

White Guide – Chapter 13: Promotional Interactions with Employer Groups
Rev. 12/20
Page 226 of 405
Pfizer may only use RC-approved materials when interacting with employer
representatives. However, keep the employer representative’s background in mind
when deciding which materials to use, especially if the employer representative does
not have a medical background. Use the tools that have been specially developed for
use with employers. As always, all product information provided must be on-label, fair
and balanced, and must include the proven benefits of the product along with relevant
safety information.
• Contact a member of the Director, Employers team or an attorney from the Pricing & Access Legal
Team.
• White Guide – Chapter 3: Promotional Interactions with Healthcare Professionals.
• White Guide Chapter 12: Promotional Interactions with Consumers.
• White Guide Chapter 15: State Laws: HCP and Government Employee Restrictions.
• White Guide Chapter 16: Federal Employee Interactions and Lobbying.
• The Orange Guide.
For More Information

Rev. 12/20
Page 227 of 405
CHAPTER #14 – STARTERS

White Guide - Chapter 14: Starters
Rev. 12/20
Page 228 of 405
Chapter
#14
STARTERS
CONTENTS
Introduction................................................................................................................................................ 229
Key Points to Ensure Compliance ...................................................................................................... 230
Starter Allocation ....................................................................................................................................... 231
Starter Packaging ...................................................................................................................................... 231
Key Points: Basic Rules Regarding Handling of Starter Packaging ................................................... 233
Inclusion of Materials with Starters ........................................................................................................... 233
Distribution of Starters to Approved Recipients ........................................................................................ 233
Hospitals, VA, and DoD Institutions.................................................................................................... 235
Starters May Not Be Distributed for Research, Charitable Activities, or To Defray Patients’
Pharmacy Expenses ........................................................................................................................... 236
Managing Starters ..................................................................................................................................... 237
Starter Storage Requirements ............................................................................................................ 237
Accurately Document Receipt and Delivery of Starters ..................................................................... 237
Completion of eSAFs and SAFs ......................................................................................................... 238
Reconciling Starter Inventory ............................................................................................................. 239
Reminder on Expired Starters ............................................................................................................ 240
Free Trial Vouchers: An Alternative to Starter Distribution ....................................................................... 240
Key Points for Developing a Voucher Program and Distributing Vouchers ....................................... 241
For More Information ................................................................................................................................. 242
Starters

White Guide - Chapter 14: Starters
Rev. 12/20
Page 229 of 405
Chapter #14 Starters
Pfizer provides healthcare professionals (HCPs) with free pharmaceutical drug product samples
(referred to as “starters”) to give to patients so that they can evaluate the efficacy and tolerability of our
products for the patient before filling a prescription. Starters also provide HCPs an opportunity to become
familiar with a drug and its properties, thereby enhancing their ability to make appropriate prescribing
decisions. The distribution of starters is highly regulated under federal and state law, and the misuse
of starters can have severe implications for both individual colleagues and Pfizer.
The Prescription Drug Marketing Act of 1987 (PDMA) is the key federal law governing the distribution
of drug samples. Pfizer policies for complying with the PDMA are described in the Starter Compliance
Manual, and the key points are summarized in this Chapter. The distribution of starters is also impacted
by other healthcare laws such as those dealing with fraud and abuse and off-label promotion.
In addition, several states have laws that affect whether and to whom starters may be distributed.
For example, some states have limitations on distributing starters for controlled substances.
Likewise, some states impose requirements (that differ from federal law) on when lost or stolen
starters must be reported, as well as which mid-level practitioners (e.g., nurse practitioners,
physician assistants) may prescribe drugs and are authorized to accept starters. This Chapter
summarizes certain key Pfizer policies regarding distribution of human biopharmaceutical starters.
Non-compliance with these policies puts the Company at risk and can subject Pfizer colleagues to
disciplinary action up to and including termination of employment.
Introduction

White Guide - Chapter 14: Starters
Rev. 12/20
Page 230 of 405
•
It is illegal to sell, purchase, or trade, or offer to sell, purchase, or trade, starters. Starters
may be provided only to licensed HCPs eligible to receive starters and only if they are
expected to distribute them for free, on-label use by their patients.
• The amount of starters allocated by each brand team must be based on the expected on-
label use of the product. Starters must not be provided to HCPs in quantities that may
appear to be intended as an inducement to use Pfizer products (i.e., a kickback).
Providing starters in quantities or dosages based on off-label use is not permitted.
• Requests for starters from a Sales Colleague may be initiated during in-person visits, or
remotely over approved channels that allow Sales Colleagues to view the HCP and obtain
the HCP's signature on an electronic starter request form.
• Starters may be packaged separately or in kits that may include PhRMA Code compliant
educational items. All patient and provider materials packaged with starters must be
reviewed and approved by the applicable Review Committee (RC) prior to distribution.
Individual starter units cannot be altered in any way either before or after they are delivered
to an HCP.
• Only licensed HCPs authorized by their states' laws to receive and prescribe medications
may sign a request for starters. Pfizer policy requires Sales Colleagues to witness the
signature personally on every starter request.
• Sales Colleagues using Veeva are required to use the electronic Starter Activity Form
(eSAF) within Veeva for starter transactions - a paper Starter Activity Form (SAF) may
only be used in the very limited circumstances described in this Chapter.
• All starter transactions must be documented completely and accurately at the time of the
transaction. (Those limited transactions that use paper SAFs must be entered into Veeva
as soon as possible after the call is made.) - Except for shipment acknowledgements which
are handled in STORK.
• Starters may not be provided to HCPs for use in clinical trials, other research activities,
or for distribution to patients in order to mitigate the cost of their treatment. HCPs seeking
to assist patients who cannot afford their medications should be referred to Pfizer
RxPathways. Starters may not be provided for charitable activities or an HCP's other
philanthropic endeavors, nor may they be provided to missions or nonprofit organizations
under any circumstances.
Key Points to Ensure Compliance

White Guide - Chapter 14: Starters
Rev. 12/20
Page 231 of 405
A prescription drug starter sample is defined under the PDMA as a unit of a drug that is not intended
to be sold and is intended to promote the sale of the drug. Such items must be clearly labeled
to reflect their intended use. Off-label uses of a product should not be considered for starter allocations.
Although HCPs may prescribe our products for off-label uses, our products cannot be promoted outside
the approved labeling and therefore, Pfizer may not knowingly provide starters for such uses.
When Sales Colleagues distribute starters, they are engaging in product promotion. Leaving a starter
with an HCP implicitly delivers a message that the product is appropriate for its labeled use. When an
HCP states or implies that he or she is using a Pfizer product for an off-label use, providing starters to
that HCP for such off-label use may be considered off-label promotion and could subject Pfizer to
prosecution.
Teams determining starter allocations should also consider the potential demand for a product on the
black/grey market and/or the potential risk of diversion. If the product has a greater diversion potential,
teams should consider limiting the number of starters distributed to the minimum amount necessary.
On-Label Use Starter Allocation and Distribution
I am on a product team reviewing starter allocations for a product that HCPs often
prescribe for off-label uses. I would like to take the market for these uses into
consideration when planning starter allocations, even though Sales Colleagues will
not detail these uses. Is this permissible?
No. Off-label uses should not be considered when determining starter allocations.
When Pfizer distributes starters, it is engaging in product promotion. Providing starters
to HCPs in quantities or at dosages that might be deemed to support off-label uses
could be considered off-label promotion. Off-label use can also be implied if Pfizer
provides starters to a specialist who does not treat the condition for which the product
is indicated (e.g., Eliquis to Oncology Specialist, Xtandi to OBGYN Specialists).
Separate starter packaging, including the sample identification on the label (i.e., “Sample – Not for
Sale”), is required by the FDA. Also, the OIG Compliance Program Guidance for Pharmaceutical
Manufacturers notes that companies should clearly and conspicuously label individual samples as units
that may not be sold (thus minimizing the ability of recipients to intentionally or inadvertently sell
samples).
Starter Allocation
Starter Packaging

White Guide - Chapter 14: Starters
Rev. 12/20
Page 232 of 405
Starter “packaging” includes all product containers (e.g., blister cards and bottles), individual unit boxes
(e.g., the box containing a single sample bottle) and starter packs. Starter packages must remain intact
and, as the labeling on starters is FDA-approved, Pfizer Sales Colleagues may not alter starter labeling or
packaging. Applying stickers or writing on starter packaging is not permitted. Any alteration or removal
of starter packaging can render the product “misbranded” under the law.
However, the outer shelf display packaging that holds together product containers with individual unit boxes
or starter packs typically does not contain the FDA-approved labeling. Its removal does not, therefore,
result in the misbranding of the product. If asked to do so by the recipient HCP or on the colleague’s
own initiative, a Sales Colleague may remove the product containers or starter packs from the outer display
packaging if it will allow the starters to more easily fit in the space available. Sales Colleagues must
ensure that at least one package insert is left with each unit of product starter left behind.
Stickers
Can a Sales Colleague place Pfizer Review Committee-approved (i.e., RC- approved)
product stickers on starters?
No. Stickers or labels may not be affixed to any starter packaging. Starter
packaging has been approved by the FDA and altering it by affixing stickers or labels
could “misbrand” the package, rendering it in violation of the law. If an HCP
requests adhesive tracking labels for use in recording his or her practice’s receipt
of starters or distribution to individual patients, Sales Colleagues may follow the
instructions found in the Starter Compliance Manual and use the accompanying
template to create them. Please note, however, that while these adhesive tracking
labels can be left with the starters they are not, under any circumstances, to be affixed
to the starters by a Pfizer colleague.
Appropriate Use of Formulary Stickers
Can a Sales Colleague put “Now on Formulary” or other approved stickers in the
sample closet?
Yes. With the approval of the HCP’s office staff, a Sales Colleague can place RC-
approved stickers in the sample closet to identify Pfizer’s starters, but the stickers
cannot be placed on starter packaging itself and may never be placed on a competitor’s
product or product packaging.
If a colleague has any questions about what may be done with respect to a particular product’s
starter packaging, he or she should consult his or her manager, NA GCO NA HCP/Patient Sample
Operations, or the relevant team attorney.

White Guide - Chapter 14: Starters
Rev. 12/20
Page 233 of 405
Provided that starter product packaging remains intact, starters may be offered in kits that include PhRMA
Code compliant educational items, such as patient journals or other disease state educational booklets.
Starter kits may also include co-pay coupons, co-pay cards, savings cards, and other similar offerings to
consumers for the specific starter product.
Before such materials may be distributed in a starter kit, they must be reviewed and approved for such use
by the applicable brand RC. When presenting such items for review, the RC team must be advised that
the items will accompany starters as part of a starter kit or other promotional program. These additional
materials must be submitted to the FDA at the time of first use. As with any promotional materials, Sales
Colleagues may not alter these additional materials in any way or add their own promotional materials to
them.
Adding Materials to Starter Packages
Can a Sales Colleague insert RC-approved promotional items such as a packet of co-
pay cards or vouchers into a starter package for the relevant product?
No. Promotional materials must be specifically approved by RC for distribution as part
of a starter package. If a Sales Colleague independently adds materials to a starter
package – even if those materials are themselves RC-approved – it could constitute an
impermissible alteration of the starter packaging.
Detailed procedures for starter accountability and compliance are set forth in the Starter Compliance
Manual. Sales Colleagues and other colleagues involved directly in starter distribution should be familiar
with the policies and procedures set forth in this manual.
•
DO NOT alter or remove product packaging as it contains information required by law and
approved by the FDA;
•
DO NOT remove starter bottles from the individual unit boxes in which they were provided
(if applicable); and
•
DO NOT apply stickers or labels to any starter packaging, including the individual unit
boxes, product containers, sample packs, and outer display packaging.
Key Points: Basic Rules Regarding Handling of Starter Packaging
Inclusion of Materials with Starters
Distribution of Starters to Approved Recipients

White Guide - Chapter 14: Starters
Rev. 12/20
Page 234 of 405
By law, pharmaceutical companies may provide starters only to licensed HCPs with authority to prescribe
medication or, at the prescriber’s direction, to the pharmacy of the institution in which the licensed HCP
works. Only a licensed HCP may sign a request for starters. The authority to prescribe and/or accept
starters varies by state. Certain restrictions may apply to mid-level HCPs (e.g., NPs and PAs) and their
ability to prescribe and/or receive starters within their state.
In addition, some states have particular limitations on distributing starters for controlled substances like
Lyrica. Sales Colleagues should check with their manager, GCO NA HCP/Patient Sample Operations,
or their team attorney if they have questions about who can receive particular Pfizer starters in their state.
Starters cannot, under any circumstances, be provided to an HCP:
• If the HCP intends to seek reimbursement from the government for the starter;
• If the HCP is within an excluded medical specialty;
• If the HCP intends to use the starter for his or her personal use;
• To reward the HCP for past prescribing or as a financial inducement for future prescribing;
• If it is reasonably certain that the HCP intends to provide the starters for an off-label use; or
• If the prescriber’s license number has not been verified in Veeva.
In the past, other pharmaceutical companies and individuals have been charged under the Federal False
Claims Act and the Anti-Kickback Statute and fined hundreds of millions of dollars for encouraging
HCPs to bill government programs for starters. For this reason, HCPs must confirm their understanding
and acceptance of the fact that starters “cannot be sold, traded, bartered, returned for credit, or
utilized to seek reimbursement” by signing the eSAF (or paper SAF, in those limited circumstances
where paper SAFs are permitted).
Pfizer policy further provides that Sales Colleagues must personally witness the signature on all starter
requests.
If a Sales Colleague suspects that an HCP is charging the government or patients for starters, the
colleague must immediately stop providing starters to that HCP and discuss the situation with his or her
manager, GCO NA HCP/Patient Sample Operations, or relevant team attorney.
Pharmaceutical companies are required to maintain records tracking the movement of all starters from the
time they leave the distribution facility to the time they are delivered to an HCP. Significant losses,
including inventories with unacceptably large negative variances and all thefts of starters, must be
reported by GCO NA HCP/Patient Sample Operations to the FDA within five business days. Some states
also have reporting obligations that are more stringent than federal law. It is essential, therefore, that Sales

White Guide - Chapter 14: Starters
Rev. 12/20
Page 235 of 405
Colleagues notify NA Sample Operations of all thefts and starter losses immediately upon becoming aware
of them. Record falsification and diversion of starters must also be reported to the FDA.
Pfizer GCO NA HCP/Patient Sample Operations handles all PDMA-mandated FDA reporting, as well as
compliance with the reporting requirements set forth in Section 6004 of the federal Affordable Care Act
(with support from the Pfizer Transparency Team). It is critical that Sales Colleagues adhere to all
policies, procedures, recordkeeping, and system requirements pertaining to starter distribution to ensure
compliance with all applicable tracking and reporting laws.
Additionally, Pfizer routinely conducts reviews and audits of Sales Colleagues’ starter activities. Failure to
comply with applicable laws and Pfizer’s policies may result in disciplinary action, up to and including
termination of employment, and may cause both a Sales Colleague and Pfizer to be liable for substantial
penalties.
On-label Use of Starter
If a starter package containing a particular dosage of a product is not used on-label by
a particular specialty because that specialty would never see the appropriate type of
patient, but there is another starter dosage that would typically be used on-label by the
same specialty, is there any limitation on what Sales Colleagues can distribute to that
specialty?
Yes. Sales Colleagues may only distribute starter packages which are consistent with
the on-label use of the product for each particular specialty. Thus, if a Pfizer product
has different approved dosages for individual indications, Sales Colleagues may only
distribute those starter dosages that are indicated for the treatment of conditions that
the prescribers they call on are likely to see among their patient population.
Distribution of Starters to Physicians for Personal Use
If an HCP asks a Sales Colleague for additional Lyrica starters because the HCP’s
spouse suffers from fibromyalgia, can the colleague give them to the HCP?
No. Federal and state laws, as well as industry guidelines (the PhRMA Code on
Interactions with Healthcare Professionals and the American Medical Association’s
Code of Ethics) prohibit the distribution of starters to HCPs for their own or their family’s
personal use.
Hospitals, VA, and DoD Institutions
Sales Colleagues are permitted to provide starters to hospitals and other healthcare institutions that use
them in the treatment of their patients. In all cases, Sales Colleagues must deliver the starters to an HCP

White Guide - Chapter 14: Starters
Rev. 12/20
Page 236 of 405
eligible to receive the starters on behalf of the hospital or other institution (this may include the pharmacist
in charge of handling starters for the institution).
Some hospitals and healthcare institutions have policies that require starters to be left in the pharmacy and
not with the individual physicians who have requested them. Sales Colleagues may do this only after
completing a paper dual-signature “In House Pharmacy” Starter Activity Form. This form is used
to document the physician’s request for starters and the pharmacist’s receipt of the starters in the institution
pharmacy. The “In House Pharmacy” Starter Activity Form can be ordered from GCO NA HCP/Patient
Sample Operations by logging on to PROMOSprime and choosing that item under the order category
“Starter Ops Forms.” As further described in this Chapter, for Sales Colleagues using Veeva, this is one
of only two very limited exceptions under which a paper SAF may be used.
Meanwhile, many government institutions, such as Department of Veterans Affairs (VA) clinics and
hospitals, prohibit pharmaceutical companies from leaving starters. Other government institutions that
do accept starters generally require them to be provided to the Chief of Pharmacy and not to individual
physicians. Even if intended for use in private practice, starters should not be left for VA or Department
of Defense (DoD) physicians at the government institution in which they work. For more information on
the distribution of starters in these government institutions, see the Federal Employee Interactions and
Lobbying Chapter in this Guide.
Sales Colleagues must learn the sample policies of any institution that they call on and follow those
rules, unless they conflict with Pfizer policy or the PDMA. If there are any questions about whether
a customer’s sample policies are consistent with Pfizer policies on starter distribution, Sales
Colleagues should contact GCO NA HCP/Patient Sample Operations or their team attorney before
leaving starters with that customer.
Starters May Not Be Distributed for Research, Charitable Activities, or To Defray Patients’
Pharmacy Expenses
Starters may not be used for clinical trials or other research activities; nor may they be provided to non-
profit organizations for missions or other charitable activities or to HCPs for distribution to patients as a
means of mitigating their medication costs. A request for medication or other clinical supplies to
support legitimate scientific investigations must be referred to the relevant Medical team for
consideration as an Investigator-Sponsored Research (ISR) grant. (For more information on scientific
research, see the Pfizer-Sponsored and Non-Sponsored Clinical Research Including Investigator-
Sponsored Research Studies (ISRs) Chapter in this Guide.) HCPs seeking to assist their patients in
mitigating their medication costs should be referred to Pfizer RxPathways. (For more information, see the
Patient Assistance Programs Chapter in this Guide.)
Requests for medication from charities or from healthcare providers for charitable missions should be
directed to the Global Health & Patient Access team.

White Guide - Chapter 14: Starters
Rev. 12/20
Page 237 of 405
As required by law and Pfizer policy, Sales Colleagues must adhere to strict requirements regarding
documentation of their receipt and delivery of starters, and management of their starter inventory.
Starter Storage Requirements
Starters must be stored securely and under thermostatically-maintained, temperature-controlled conditions
in accordance with the product’s labeling to maintain the starters’ integrity, stability, and efficacy. Starters
must be stored away from hazardous materials and any other substances that could cause contamination
or otherwise degrade them.
Starters may be transported in an automobile trunk during the business day but should never be left there
overnight. For this reason, only the number of starters that are expected to be distributed on a particular
day should be carried in a Sales Colleague’s trunk, with any remaining quantities removed and returned to
storage at the end of the day.
If starters are stored in a commercial warehouse unit, the lease contract for that space should contain
language confirming that it is artificially temperature-controlled and be in Pfizer’s name with access made
available to both the Sales Colleague and his/her manager during normal hours of operation. Starters
should be stored off the floor on shelves or pallets. In addition, Sales Colleagues should confirm that the
facilities in which they lease space either use an onsite generator to maintain their unit’s ambient
temperature in the event of a power outage or will call them if such an outage lasts 24 hours or longer.
Sales Colleagues whose storage facilities sustain an unmitigated power outage lasting more than 24 hours
should suspend sampling and contact Starter Compliance via e-mail (StarterCompliance@pfizer.com) for
further instructions.
Accurately Document Receipt and Delivery of Starters
To accurately document receipt and delivery of starters, Sales Colleagues must strictly adhere to the
policies and procedures in the Starter Compliance Manual, including:
• Guidelines for acknowledging the receipt of starter shipments immediately upon acceptance;
• Documentation of the starters delivered to licensed HCPs;
• Procedures for transferring starters between Sales Colleagues; and
• Entry of starter transactions into Veeva at the time of their occurrence.
Failure to adhere to these policies and procedures can place Sales Colleagues and Pfizer at risk under the
PDMA and other applicable laws, distort their on-hand reported inventory balance, and undermine the
reconciliation of their annual starter inventory.
Managing Starters

White Guide - Chapter 14: Starters
Rev. 12/20
Page 238 of 405
Completion of eSAFs and SAFs
Sales Colleagues using Veeva must use their approved device (i.e., tablet or iPad) for every starter
transaction – subject to two very limited exceptions outlined below. A paper Starter Activity Form
(SAF) may only be used:
• When a Sales Colleague is delivering starters at an institution that requires starters to be left with
its pharmacy and not with the individual HCPs requesting them (in this case, the dual-signature
“In House Pharmacy” SAF described in this Chapter must be used); or
• With prior written approval from GCO NA HCP/Patient Sample Operations in very limited circumstances
while the Veeva system is inoperable due to significant hardware or software malfunctions for an
extended period of time, until such time as the malfunction is resolved. (Sales Colleagues should
ensure that their approved devices (i.e., tablets or iPads) are charged; drained batteries do not qualify
as a device malfunction.) Written requests may only be submitted by Sales Colleagues by e-
mailing a description of the issue, including information provided as part of the CSC Help Center
assigned ticket, to StarterComplianc[email protected].
If a paper SAF is used as permitted above, Sales Colleagues must enter the relevant information into
Veeva as soon as possible after completing the paper SAF transaction.
The Veeva and paper SAF starter call records are designed to document requests for starters and
confirm receipt of provided starters. The Veeva (and paper SAF) starter transactions are Pfizer’s legal
record of each starter transaction and must accurately reflect the date on which the request and delivery
occurred, the name, address, license number, and professional designation of the prescriber, and the
products and quantities that they are given.
The Veeva eSAF (or paper SAF) must be completed in its entirety before it is presented to the prescriber
for signature. If a prescriber does not provide his/her signature to confirm request/receipt of starters, the
Sales Colleague must not provide him/her with starters. A receipt form may be provided to a physician
when using the Sales Colleague’s approved device (i.e., tablet or iPad) by checking the receipt
requested by mailbox option on the screen. (If using a paper SAF in the limited circumstances described
above, the yellow copy of the form must be left with the recipient to retain for their records.)
In the limited instances described in the Starter Compliance Manual, paper SAFs may be used to document
your starter transactions subject to the same requirements for documenting starter transactions electronically
(e.g., Sales Colleague must witness signature of HCP), with the exception of the preceding rule concerning
the capture of recipients’ signatures electronically using Veeva.

White Guide - Chapter 14: Starters
Rev. 12/20
Page 239 of 405
Witnessing Signatures for Starters
When a Sales Colleague delivers starters to a HCP’s office, can the receptionist take
the approved device (i.e., tablet or iPad) to the HCP for signature?
No. The Sales Colleague’s device should always remain in the Sales Colleague’s
immediate proximity. Pfizer policy requires that the Sales Colleague always personally
witness the HCP signing the starter request. (In the limited circumstances where a
paper SAF is permitted, a receptionist may take the SAF to the HCP for signature as
long as the Sales Colleague can clearly see the HCP signing the form.)
Is it permissible to accept a request for starters from an HCP at a location other than
the one to which the starters will be sent?
No, not when the HCP has made the request during an in-person visit by a Sales
Colleague. Sales Colleagues are required to confirm that the locations to which starters
are shipped are medical offices where patients are treated, and it is Pfizer’s policy that
this verification be performed in person. When accepting requests for starters for
controlled substances, it is essential that Sales Colleagues also confirm that the HCP
is registered with the DEA at the office where he/she is called on and to which those
items will be sent.
Note that when a request for starters is made over a VCC call, starters may be sent to
a location other than the HCP’s current location. In other words, starters may be sent
to the HCP’s office, even though the HCP is located at home for the VCC call. Starters
may not be sent to an HCP’s home.
Reconciling Starter Inventory
The PDMA requires that every Sales Colleague have at least one physical inventory count of their
starters taken within each 12-month period. Successful reconciliation requires accurate starter recording
in Veeva, timely call reporting, routine synchronization with the Veeva server, and the correction of any
errors or discrepancies found in the course of recording starter information.
Sales Colleagues should regularly review their weekly Veeva Starter Activity Reports (SARs) and
periodically conduct their own physical inventory count. This count should be reconciled against the
Ending Balance Report that is sent to each Sales Colleague with their SAR. If a Sales Colleague finds an
error or discrepancy when reconciling starters, he or she should immediately contact GCO NA HCP/Patient
Sample Operations for further guidance.
In addition, all starter losses and thefts should be reported to GCO NA HCP/Patient Sample Operations
immediately so that the required notification can be submitted to the FDA within five days.

White Guide - Chapter 14: Starters
Rev. 12/20
Page 240 of 405
Reminder on Expired Starters
Expired starters cannot be given to a healthcare provider under any circumstances and should be returned
promptly to Pfizer’s authorized destruction facility. Sales Colleagues should rotate their starters upon
receiving each delivery, placing those closest to their date of expiration in front to ensure that they distribute
them first.
You can still deliver "soon-to-expire" starters (i.e., starters that still have more than 30 days before
expiration) to an HCP, but once actually expired, they must be returned to Pfizer's authorized destruction
facility.
HCPs seeking to return expired or damaged starters should be directed to call Pfizer’s Starter Customer
Service Team (1-800-533-4535) to schedule an appointment for the pickup of those items.
Paying for Bins in Starter Closets
Can a Sales Colleague pay for bins or space in starter closets in HCPs’ offices?
No. Paying for bins or for space in starter closets could violate anti-kickback laws.
Some product teams use free trial voucher programs as a substitute for, or alternative to, the physical
distribution of starters.
In a voucher program Pfizer (via Sales Colleagues and/or through Pfizer’s patient websites, for example)
provides HCPs or patients with certificates (vouchers) that patients can redeem at a pharmacy for a free
“trial prescription” of a medicine. Vouchers, like Starters, are intended to allow appropriate patients to utilize
a product for a limited time for the purpose of allowing the prescribing HCP to evaluate efficacy and safety.
The HCP must give the patient a prescription for the amount of product covered by the voucher. The patient
takes the prescription and voucher to the pharmacy, where he/she receives the product free of charge. A
third-party administrator that contracts with pharmacy networks then reimburses the pharmacy.
Brand teams may offer both Starters and vouchers. Sales representatives may distribute both starters and
vouchers to the same HCP office in accordance with the above principles. Prior to distributing the resources
to a given HCP, however, sales representatives should carefully consider the needs of a particular HCP or
office. For example, it may be appropriate to leave vouchers at a health system with restrictions on drug
sampling. It may also be appropriate to leave starters with an HCP who desires to start treatment
Free Trial Vouchers: An Alternative to Starter Distribution

White Guide - Chapter 14: Starters
Rev. 12/20
Page 241 of 405
immediately without waiting for the patient to redeem a voucher. Sales representatives should clearly
indicate the appropriate use of these resources to HCPs, including that: (1) vouchers are not intended to
address financial hardship and insurance delays; and (2) an individual patient should receive either a
voucher or starter, but not both. The intent is to prevent a patient from receiving both Starters and vouchers
to extend beyond a reasonable trial period (i.e., stacking) and for HCPs to direct patients to the appropriate
resources to address financial hardship and insurance delays.
Additional requirements for Pfizer teams implementing voucher programs may be found in Chapter 19 of
the White Guide.
Improper use of vouchers can implicate the state and federal false claims acts and anti-kickback laws and
could also be deemed to impact the “best price” of a product (i.e., the discount the Company is required to
give the Medicaid program on every unit of product it reimburses). For more information, see White Guide
– Chapter 6: Government Healthcare Programs.
• Vouchers are intended to allow appropriate patients to utilize a product for a limited time for
the purpose of allowing the prescribing HCP to evaluate efficacy and safety. Do not position
vouchers to HCPs for the purpose of addressing long term issues such as patient access
or financial need.
•
Voucher disbursements must be recorded completely and accurately in Veeva to ensure
compliance with all applicable federal and state reporting requirements;
•
Vouchers must never be offered or provided to HCPs contingent upon the HCP's past,
current, or future prescribing practices;
• Vouchers may not be offered to HCPs for personal use; and
• Vouchers are a form of product promotion. They may not be offered to HCPs for off-label
uses; nor may they be offered to an HCP that practices in a specialty that is excluded for
that specific product.
Key Points for Developing a Voucher Program and Distributing Vouchers

White Guide - Chapter 14: Starters
Rev. 12/20
Page 242 of 405
• Questions may be referred to GCO NA HCP/Patient Sample Operations, the relevant Sales
Manager, or team attorney.
• For Pfizer’s policies for complying with the PDMA, see the Starter Compliance Manual.
• Sales Colleagues who need to order “In House Pharmacy” Starter Activity Forms can obtain them
by calling Standard Register at 1-800-313-8263.
• For more information on the use of product in scientific investigations, see the Pfizer- Sponsored
and Non-Sponsored Clinical Research Including Investigator-Sponsored Research Studies (ISRs)
Chapter.
• For more information on distributing starters in government institutions, see Orange Guide: Chapter
4 - Federal Employee Interactions and Lobbying.
For More Information

Rev. 12/20
Page 243 of 405
CHAPTER #15 – STATE LAWS:
HCP AND STATE EMPLOYEE
RESTRICTIONS

White Guide – Chapter 15: State Laws: HCP and State Employee Restrictions
Rev. 12/20
Page 244 of 405
Chapter
#15
STATE LAWS: HCP AND
STATE EMPLOYEE RESTRICTIONS
CONTENTS
Introduction................................................................................................................................................ 247
Summary of Key State/City HCP-Related Healthcare Compliance Laws ................................................ 248
Summary of Key State Employee Gift Laws ............................................................................................. 254
Key Points to Ensure Compliance ...................................................................................................... 255
California ................................................................................................................................................... 256
The Law: The California Drug Marketing Practices Law .................................................................... 256
Definition of Healthcare Professional ................................................................................................. 256
How the Law Impacts Pfizer Colleague Activities .............................................................................. 256
The value of the following items must be included when calculating the annual aggregate
limit: .................................................................................................................................................... 256
The value of the following items are not included when calculating the annual aggregate limit: ....... 256
City of Chicago .......................................................................................................................................... 257
The Law: Pharmaceutical Representative Licensing Ordinance ........................................................ 257
How the Law Impacts Pfizer Colleague Activities .............................................................................. 257
Colorado .................................................................................................................................................... 258
The Law: Restrictions on Gifts to State Employees ........................................................................... 258
Definition of Healthcare Professional State Employee under the law ................................................ 259
How the Law Impacts Pfizer Colleague Activities .............................................................................. 259
Colorado Pricing Disclosure Requirements ........................................................................................ 259
Connecticut ............................................................................................................................................... 260
The Law: Connecticut Compliance Program Law & APRN Disclosure Law ..................................... 260
How the Law Impacts Pfizer Colleague Activities .............................................................................. 260
District of Columbia ................................................................................................................................... 261
The Law: Prescription Drug Marketing Costs Disclosure Law ........................................................... 261
Definition of Healthcare Professional ................................................................................................. 261
How the Law Impacts Pfizer Colleague Activities .............................................................................. 262
State Laws: HCP and State Employee Restrictions

White Guide – Chapter 15: State Laws: HCP and State Employee Restrictions
Rev. 12/20
Page 245 of 405
The Law: Pharmaceutical Detailer Licensing Law ............................................................................. 262
Gifts to D.C. Medication Advisory Committee Prohibited ................................................................... 262
How the Law Impacts Pfizer Colleague Activities .............................................................................. 262
Louisiana ................................................................................................................................................... 263
The Law: Code of Governmental Ethics ............................................................................................. 263
Definition of "Public Servant" .............................................................................................................. 264
How the Law Impacts Pfizer Colleague Activities .............................................................................. 264
Massachusetts .......................................................................................................................................... 265
The Law: Pharmaceutical and Medical Device Manufacturer Conduct Law (Massachusetts
Marketing Code of Conduct) ............................................................................................................... 265
Definition of Healthcare Professional ................................................................................................. 266
How the Law Impacts Pfizer Colleague Activities .............................................................................. 266
Meals .................................................................................................................................................. 266
Helpful Points ...................................................................................................................................... 267
Other Prohibited Items of Value and Activities ................................................................................... 267
Disclosure ........................................................................................................................................... 268
Non-patient Identified Prescriber Data ............................................................................................... 268
Michigan .................................................................................................................................................... 269
Starters Policy for Mid-Level Practitioners ......................................................................................... 269
Minnesota .................................................................................................................................................. 269
The Law: Gift Restriction Law ............................................................................................................. 269
Educational Items ............................................................................................................................... 270
Meals .................................................................................................................................................. 270
Consulting Engagements with MN HCPs ........................................................................................... 270
Definition of Practitioner ..................................................................................................................... 271
How the Law Impacts Pfizer Colleague Activities .............................................................................. 271
Nevada ...................................................................................................................................................... 272
The Law: Nevada Marketing Code of Conduct .................................................................................. 272
Pharmaceutical Sales Representatives Registration ......................................................................... 273
Pharmaceutical Sales Representative Annual Disclosure Report ..................................................... 273
New Jersey................................................................................................................................................ 273
How the Law Impacts Pfizer Colleague Activities .............................................................................. 274
Definition of a New Jersey Prescriber ................................................................................................ 274
Meals .................................................................................................................................................. 274
Consulting Engagements with New Jersey Prescribers ..................................................................... 275

White Guide – Chapter 15: State Laws: HCP and State Employee Restrictions
Rev. 12/20
Page 246 of 405
New York ................................................................................................................................................... 275
The Law: Restrictions on Gifts to State and Local Officers and Employees ...................................... 275
Definition of Officer or Employee ........................................................................................................ 275
How the Law Impacts Pfizer Colleague Activities .............................................................................. 275
Vermont ..................................................................................................................................................... 276
The Law: The Prescribed Products Law ............................................................................................ 276
Definition of Healthcare Provider ........................................................................................................ 276
How the Law Impacts Pfizer Colleague Activities .............................................................................. 277
Meals .................................................................................................................................................. 277
Gift Ban ............................................................................................................................................... 278
Marketing Research ........................................................................................................................... 278
Disclosure of Expenditures to Vermont HCPs .................................................................................... 279
The Law: Vermont Price Disclosure Law ........................................................................................... 279
For More Information ................................................................................................................................. 280

White Guide – Chapter 15: State Laws: HCP and State Employee Restrictions
Rev. 12/20
Page 247 of 405
Chapter #15 State Laws: HCP and State Employee
Restrictions
States are increasingly enacting laws and regulations that impact our business and restrict our activities,
including your interactions with HCPs and state employees. Many of these state laws are more restrictive
than federal law and the generally applicable Pfizer policies set forth elsewhere in this Guide. Pfizer will
continue reporting all meals and other transfers of value required under the Sunshine Act and SUPPORT
Act to the Federal Government. All colleagues must ensure that their records on these expenditures are
accurate and complete.
It is important that all colleagues understand all applicable state laws and policies– and not only the ones
applicable to the states where they work because certain state laws may apply regardless of where
an interaction occurs. Activities that violate these laws may result in criminal and civil penalties for you
and Pfizer.
This Chapter is relevant to all colleagues but particularly those who may interact with HCPs with an active
license in the states discussed in this Chapter and with state employees. This includes Account Managers
who interact with various customer employees. Depending on the state, the law may apply to interactions
with Account employees even when they are not practicing physicians, by virtue of their continuing to be
licensed in the state or their responsibilities in the Account. Non-compliance with these policies puts the
Company at risk and can subject Pfizer colleagues to disciplinary actions up to and including termination
of employment.
If a HCP is licensed in multiple states, the most restrictive state’s rules will apply.
If you have any questions about state healthcare compliance laws and HCP-related restrictions:
• Consult the State Healthcare Law Compliance section on Global Policy Xchange on GCO On Demand;
• Send questions to StateHealthcareLawCompliance@pfizer.com; or
• Consult your team attorney.
If you have any questions about state employee gift restrictions:
• Consult with the appropriate Government Relations Director (GRD); or
• Consult your team attorney.
Introduction

White Guide – Chapter 15: State Laws: HCP and State Employee Restrictions
Rev. 12/20
Page 248 of 405
State
Important Provisions of
the State Law
Key Points to
Ensure Compliance
California
Companies shall adopt a
comprehensive compliance program
which sets specific dollar limits on gifts,
promotional materials, and activities.
Accurately and completely record all
expenditures on HCPs.
Monitor expenditures per HCP and
coordinate with your colleagues to
ensure compliance with Pfizer’s annual
limit of $3,500 per California HCP.
Chicago
Individuals who market or promote
prescription drugs to HCPs in Chicago
must obtain a license. Individuals who
promote prescription drugs in Chicago
for fewer than 15 days per calendar
year are exempt from the licensing
requirement.
Licensed pharmaceutical
representatives who market or promote
pharmaceuticals listed on the CDPH
website would need to provide a
disclosure report.
Colleagues responsible for Chicago and
who promote Pfizer products in Chicago
for 15 days or more per calendar year
must obtain a license. Licenses will be
required starting July 1, 2017. Licenses
must be renewed every year and
continuing education requirements must
be satisfied. Licensees will also be
required to record certain information
about their interactions with HCPs.
Connecticut
The Connecticut Compliance Program
Law requires companies to adopt a
marketing code of conduct – the
PhRMA Code is acceptable.
Starting in 2016, companies must begin
tracking payments or other transfers of
value provided to Advanced Practice
Registered Nurses (‘APRN’) authorized
to practice independently (i.e., not in
collaboration with a physician) for
reporting.
Follow all Pfizer policies and
procedures and the PhRMA Code.
Accurately and completely record all
expenditures to all HCPs, including
APRNs.
Summary of Key State/City HCP-
Related Healthcare Compliance Laws

White Guide – Chapter 15: State Laws: HCP and State Employee Restrictions
Rev. 12/20
Page 249 of 405
State
Important Provisions of
the State Law
Key Points to
Ensure Compliance
District of
Columbia
Individuals engaged in the practice of
“pharmaceutical detailing” must secure
a license to detail in person in D.C.
Individuals who practice
“pharmaceutical detailing” in D.C. less
than 30 days per calendar year are
exempt from this requirement. The
D.C. Board of Pharmacy believes that
the exemption may be claimed only by
individuals detailing in D.C. “once a
year for a short duration of time of less
than 30 consecutive days.”
Companies must report certain
marketing costs.
Members of the D.C. Medication
Advisory Committee must not receive
gifts, including meals or remuneration
for speaking or consulting.
Colleagues whose territory or
geographic responsibilities include D.C.
must obtain a detailer license from the
D.C. Board of Pharmacy, renew it every
even numbered year, and attend
Continuing Education courses.
For Sales Colleagues providing meals
in Washington, D.C., where the total
cost per person exceeds $25, all
individuals partaking in the meal must
be listed individually.
Do not provide any gift or meal to any
member of the Medication Advisory
Committee, no matter how nominal the
value.
Massachusetts
Adopt a marketing code of conduct
consistent with Massachusetts
regulations.
Companies may not provide meals
(including snacks or other
refreshments) to MA-licensed HCPs
except in the office or hospital setting
when accompanied by an informational
presentation or if provided in
connection with a speaker program or
symposia (limited exception for MA
HCPs under bona fide service contracts
with Pfizer, in connection with job
interview, or at exhibit booths at large-
scale conferences.).
In-office or in-hospital meals are
permissible during educational
presentations.
Out-of-office “snacks” (as defined in
Orange Guide Chapter 18) are
prohibited.
Pfizer may also provide modest meals
at out-of-office speaker programs as
well as at symposia taking place at a
convention or congress setting.
Pfizer may provide modest meals to
MA-licensed HCPs in connection with
bona service contracts or in connection
with a job interview for prospective
employment.

White Guide – Chapter 15: State Laws: HCP and State Employee Restrictions
Rev. 12/20
Page 250 of 405
State
Important Provisions of
the State Law
Key Points to
Ensure Compliance
Pfizer must give HCPs the opportunity
to withhold prescriber data.
Pfizer must annually report certain HCP
expenditures to Massachusetts.
Refreshments or snacks at conference
exhibit booths are permissible. If you
are unsure whether an HCP has a MA
license, check the HCP Lookup Tool.
You can also check Veeva CRM, which
flags most (but not all) MA HCPs.
You must make a good faith effort to
determine whether an HCP is
licensed in Massachusetts.
Michigan
Michigan state healthcare regulations
require pharmaceutical manufacturers
to link mid-level practitioners to
supervising physician when requesting
starters.
All starter requests recorded for
Michigan Registered Nurses and
Advanced Practice Registered Nurses
(NP, CNS, CRNA, CNM, AN) must
include the supervising physician’s
name in the transaction’s call
notes in Veeva. The Veeva system
does not allow us to distinguish
between APRNs and Registered
Nurses in Michigan, therefore, we must
record the supervising physician’s
name for all nurses that request
starters.
When starters for controlled substances
are included, the supervising
physician’s name and his or her DEA
registration number must also be added
to the transaction’s call notes in Veeva.
Michigan state law now permits a
Physician’s Assistant to order and
receive starters directly, without
recording a supervising physician’s
name or DEA registration number.

White Guide – Chapter 15: State Laws: HCP and State Employee Restrictions
Rev. 12/20
Page 251 of 405
State
Important Provisions of
the State Law
Key Points to
Ensure Compliance
Minnesota
Gifts to practitioners are prohibited.
Pfizer policy prohibits HCP meals to MN
practitioners, including nominal meals
and snacks. There is limited exception
for: (i) for MN HCPs under bona fide
service contracts with Pfizer; (ii)
refreshments or other snacks at a
convention/congress exhibit booth.
Pfizer policy prohibits providing text
books, journal subscriptions, online
subscription services (e.g., Epocrates),
and anatomical models, to MN
practitioners.
Pfizer policy also prohibits engaging MN
practitioners as paid consultants, except
for the following type of projects:
• Reasonable honoraria and
expenses of a practitioner who
serves on the faculty at a
professional or educational
conference or meeting
• Substantial professional or
consulting services of a practitioner
in connection with a genuine
research project
• Speaking and speaker training
Pfizer must report permissible non-
gift expenditures that exceed
$100/year.
Do not invite MN practitioners to any
speaker programs that provide meals
(even if the program is outside of MN).
Unless an exception applies, do not
provide MN practitioners meals or
snacks.
Do not provide MN practitioners text
books, journal subscriptions, online
subscription services (e.g., Epocrates,
including trial memberships), or
anatomical models.
Do not engage MN HCPs as
commercial consultants.
Accurately and completely record all
practitioner expenditures.
If you are unsure of whether an HCP is
a MN licensed prescriber, you can
check the HCP Lookup Tool. Also,
Veeva CRM flags most (but not all)
practitioners with MN licenses.
You must make a good faith effort to
determine whether a practitioner is
licensed in Minnesota.
Nevada
Nevada Marketing Code of Conduct
requires companies to adopt a
marketing code of conduct – the
PhRMA Code is acceptable.
Follow all Pfizer policies and
procedures and the PhRMA Code.

White Guide – Chapter 15: State Laws: HCP and State Employee Restrictions
Rev. 12/20
Page 252 of 405
State
Important Provisions of
the State Law
Key Points to
Ensure Compliance
Manufacturers must provide to the
Nevada Department of Health and
Human Services (DHHS) a list of
pharmaceutical sales representatives
who market prescription drugs on behalf
of the manufacturer to licensed,
certified, or registered health care
providers, pharmacies and pharmacy
employees, and operators or
employees of medical facilities in the
state. The license registry applies to
individuals who physically reside in or
visit Nevada five (5) days or more
annually in order to communicate with
HCPs.
Manufacturers must annually report to
Nevada DHHS information about
transfers of value and samples provided
to Nevada covered recipients by
registered pharmaceutical sales
representatives.
Accurately and completely record all
expenditures, as well as samples to NV
HCPs.
New Jersey
Meals to a New Jersey prescriber must
not exceed $15 for breakfast or lunch
promotional meetings and $30 for
dinner promotional meetings. These
limits do not apply to: (I) Speaker
Programs; (ii) Symposia; (iii) Bona fide
service contract; (iv) Job interview with
Pfizer; and (v) Nominal snacks provided
at convention/congress exhibit booth.
The restriction applies to Prescribers
that practice in New Jersey or have
New Jersey patients, regardless of the
prescriber’s practice site.
Do not provide NJ prescribers with
meals over $15 for breakfast and lunch
and $30 for dinner unless on occasions
otherwise exempted.
You must make a good faith effort to
determine whether a prescriber is
licensed in New Jersey.
If you are unsure of whether a
prescriber has a NJ license, you can
check the HCP Lookup Tool. Also,
Veeva CRM flags most (but not all)
prescribers with NJ licenses.

White Guide – Chapter 15: State Laws: HCP and State Employee Restrictions
Rev. 12/20
Page 253 of 405
State
Important Provisions of
the State Law
Key Points to
Ensure Compliance
A New Jersey prescriber shall not
accept more than $10,000 in the
aggregate from all pharmaceutical
manufacturers in a calendar year for
certain Bona Fide Services. Bona Fide
Services impacted by the cap include:
(1) promotional activities (does not
include Speaker Programs); (2)
participation on advisory boards; and
(3) consulting arrangements. Payments
for research activities and/or
remuneration for travel, lodging, and
other personal expenses associated
with the impacted Bona Fide Services
are not subject to the $10,000 annual
aggregate cap.
Vermont
Vermont prohibits all HCP meals,
including in-office meals and meals of
nominal value (there is a limited
exception for: (i) bona fide service
contracts; (ii) refreshments or other
snacks at a convention/congress exhibit
booth; and (iii) in connection with a job
interview for prospective employment.
Vermont also prohibits paid market
research surveys involving VT-licensed
HCPs. The restriction applies whether
the survey is conducted directly by
Pfizer or through an independent third
party survey research organization.
Pfizer must report certain HCP
expenditures, as well as samples,
copay cards, coupons, and vouchers, to
Vermont.
Do not invite VT HCPs to any speaker
programs that provide meals or snacks
(even if the program is conducted
outside of VT).
Do not provide VT HCPs with meals or
snacks (except in connection with a
bona fide service contract, job interview
or snacks at a convention exhibit
booth).
Do not engage VT HCPs as part of any
paid marketing research surveys.
Accurately and completely record all
HCP expenditures, as well as samples,
copay cards, coupons, and vouchers
provided to VT-licensed HCPs.
Provide VT Price Disclosure Forms to
HCPs as appropriate (available on
www.pfizer.com/vtprescribers).

White Guide – Chapter 15: State Laws: HCP and State Employee Restrictions
Rev. 12/20
Page 254 of 405
State
Important Provisions of
the State Law
Key Points to
Ensure Compliance
Price Disclosure Forms must be
provided to HCPs when detailing and
posted on Pfizer’s website.
If you are unsure of whether an HCP
has a VT license, you can check the
HCP Lookup Tool. Also, Veeva CRM
flags most (but not all) VT HCPs.
You must make a good faith effort to
determine whether an HCP is licensed
in Vermont.
Almost all states have restrictions on interactions with state employees (including HCPs employed by state
institutions). Consult the appropriate Government Relations Director (GRD) for the state employee
restrictions in your state. A summary of the most significant state restrictions is provided below.
State
Important Provisions of
the State Law
Key Points to
Ensure Compliance
Colorado
State employees may not receive
anything of value worth more than $65
from a company (as a whole, not by
employee) per year.
Accurately and completely record all
expenditures on state employees.
Monitor spending per state employee
and coordinate with your colleagues to
ensure Pfizer is not spending beyond
the $65 annual limit.
Louisiana
State employees are prohibited from
performing certain compensated
services for pharmaceutical companies.
State employees have a $62 cap on
food, drinks, and refreshments provided
during a single event.
Before considering engaging a state
employee to perform a compensated
service, consult with your manager.
Before providing a meal or
refreshments to state employees,
coordinate with your colleagues to
ensure the employee is not receiving
value greater than $62 during the
event.
New York
State and local employees are
prohibited from receiving gifts.
Do not provide meals or educational
items to state or local employees.
However, state and local employees
may receive food items of nominal
Summary of Key State Employee Gift Laws

White Guide – Chapter 15: State Laws: HCP and State Employee Restrictions
Rev. 12/20
Page 255 of 405
State
Important Provisions of
the State Law
Key Points to
Ensure Compliance
value (which the state interprets as no
more than $15) as long as they are not
part of a meal.
• Understand the laws and policies of the states in which you work and the states where the
HCPs with whom you interact hold licenses.
•
Always remember that several state laws may apply regardless of where an interaction
occurs.
•
Before providing a meal or educational item to an HCP, know where the HCP is licensed
and follow any applicable state restrictions. For example, regardless of where the
interaction takes place, significant restrictions apply to HCPs with active VT, MA, MN, and
NJ licenses. These restrictions apply to all Pfizer colleagues.
•
Conduct your activities in accordance with the relevant state laws described in this Chapter,
as well as general Pfizer policy found in this Guide.
• Be aware of and abide by all spending limits and restrictions.
•
Remember that federal government employees, such as those working for the VA or DoD,
must follow federal gift restrictions, which include restrictions on meals. For further
information on these restrictions, see the Federal Employee Interactions and Lobbying
Chapter in this Guide.
•
Almost all states impose restrictions on what may be provided to state and local employees
(including HCPs employed by state institutions). You can direct any specific questions on
state laws that are not addressed in this Guide to the relevant team attorney or to
. For information about state employee
restrictions, consult with your Government Relations Director.
Key Points to Ensure Compliance

White Guide – Chapter 15: State Laws: HCP and State Employee Restrictions
Rev. 12/20
Page 256 of 405
The Law: The California Drug Marketing Practices Law
The California Drug Marketing Practices Law requires that each pharmaceutical company:
• Establish, at a minimum, a comprehensive compliance program that complies with the requirements
set forth in the OIG’s Compliance Program Guidance for Pharmaceutical Manufacturers and PhRMA’s
Code on Interactions with Health Care Professionals;
• Set an annual aggregate limit for spending on meals, promotional items, and other activities provided
to covered HCPs; and
• Declare annually, on its public website, that it is in compliance with California Law.
Definition of Healthcare Professional
Covered HCPs include any CA-licensed prescriber of human drugs, medical student, or member of a
formulary committee. Non-prescribing pharmacists, nurses, and office staff, who are not medical students
or formulary committee members, are not included in the annual aggregate limit on spending to covered
HCPs.
How the Law Impacts Pfizer Colleague Activities
Pfizer has set its annual aggregate limit on covered promotional expenditures at $3,500 per covered
California HCP. This limit does not apply to CA-licensed HCPs practicing in other states.
The value of the following items must be included when calculating the annual aggregate
limit:
• PhRMA Code compliant meals, including all food and beverage in and outside a medical office or
hospital, in connection with any promotional activity; and Pfizer Review Committee (RC) approved
educational items. (Like text books, anatomical models etc.)
The value of the following items are not included when calculating the annual aggregate
limit:
• Starters;
• Fair market value payments for services, such as speaking and consulting payments;
• RC-approved promotional literature such as clinical reprints and slim jims;
• Independent educational grants (financial support for continuing education forums);
• Financial support for educational scholarships; and
California

White Guide – Chapter 15: State Laws: HCP and State Employee Restrictions
Rev. 12/20
Page 257 of 405
• Pfizer RC-approved marketing material.
All colleagues who engage in activities in California should be aware that their expenditures which meet
the criteria above will be included when calculating the annual aggregate limit. Colleagues must ensure
that their records on these expenditures are accurate and complete.
The State of California can impose significant penalties on Pfizer for failure to comply with this law. If you
have any questions concerning the California Pharmaceutical Sales and Marketing Disclosure Law, please
contact the team attorney with responsibility for California.
The Law: Pharmaceutical Representative Licensing Ordinance
The Chicago Pharmaceutical Representative Licensing Ordinance requires individuals who market or
promote prescription drugs to HCPs, while both are physically within the City of Chicago, to obtain a license.
Individuals who promote prescription drugs in Chicago for fewer than 15 days per calendar year are exempt
from the licensing requirement. Licenses will be required beginning July 1, 2017.
E.g. If an Inside Sales Representative (ISR) is calling on a Chicago HCP via telephone while the ISR is
physically in Chicago, then he/she should apply for a license (assuming he/she is doing this for 15 days or
more a year). If the ISR is never physically in Chicago while making the telephone calls, then the ordinance
does not apply.
How the Law Impacts Pfizer Colleague Activities
Colleagues who promote Pfizer products in Chicago for 15 days or more per calendar year must obtain a
license. It is the colleague’s responsibility to renew the license annually and to remain in compliance with
continuing education requirements. License applications will require the following:
• The applicant’s full name, residence address, and residence telephone number;
• The applicant’s business address and business telephone number;
• A description of the type of work in which the applicant will engage;
• Affirmation that the applicant completed the required professional education course; and
• $750 licensing fee.
The initial professional education course and application are available on the Chicago Department of Public
Health (“CDPH”) website.
Licensees will be required to abide by a code of ethics.
City of Chicago

White Guide – Chapter 15: State Laws: HCP and State Employee Restrictions
Rev. 12/20
Page 258 of 405
Pharmaceutical sales representatives who market or promote a drug listed on the CDPH webpage during
the month that the representative is licensed must track their interactions with health care
professionals regarding those drugs for potential disclosure, including:
• A list of health care professionals within Chicago contacted;
• The dates the health care professionals were contacted;
• The location and duration of contact;
• The pharmaceuticals promoted;
• Whether product samples were provided to the health care professional and the quantity provided;
• Whether promotional materials (e.g. brochures, demo models) were provided to the health care
professional and the value of those materials; and
• The value of meals provided to the health care professional.
As of July 2017, the disclosure list includes only the category of Schedule II medications. Sales
representatives who obtain licenses as of October 15, 2017 and do not promote or market a Schedule II
drug will not have to track any interactions for the next year until license renewal, at which point they
must again see what drugs or drug categories are listed on the website. The Pfizer Transparency team will
submit any required disclosures on behalf of the sales representative.
Chicago can impose significant penalties on Pfizer colleagues for failure to comply with this law, which may
include fines of no less than $1,000 and no more than $3,000 per violation. If you have any questions
concerning the Chicago Pharmaceutical Representative Licensing Ordinance, please contact the Sales and
Marketing Attorney with responsibility for Chicago.
The Law: Restrictions on Gifts to State Employees
Colorado law prohibits any state employee from soliciting, accepting, or receiving, directly or indirectly, any
gift or other item of value (including meals), regardless of form (e.g., money, service loan, travel,
entertainment, hospitality, or promise) worth more than $65 in any calendar year.
As with any other customer, colleagues may not provide any type of gift, regardless of value, to a Colorado
state employee if the gift is intended or expected to influence or reward that employee in the performance
of any activity related to his or her official duties.
Colorado

White Guide – Chapter 15: State Laws: HCP and State Employee Restrictions
Rev. 12/20
Page 259 of 405
Definition of Healthcare Professional State Employee under the law
A Colorado state employee includes any HCP employed, either full-time or part-time, by the State of
Colorado, any community healthcare providers employed by a Colorado county or municipal government,
and any physicians employed at the University of Colorado Health Sciences Center.
How the Law Impacts Pfizer Colleague Activities
Collectively, Pfizer colleagues are prohibited from providing gifts, including meals, which have a total value
over $65 to a Colorado state employee in any calendar year. This means that colleagues must coordinate
to ensure that no employee of the State of Colorado receives more than $65 in items and meals from Pfizer
as a company during any calendar year. (The $65 annual limit is not per Pfizer colleague.) Pfizer RC-
approved educational items of more than nominal value (e.g., anatomical models) may not be provided to
Colorado state employees who are healthcare providers, even though they are RC-approved items. This
limitation applies to all Pfizer colleagues who interact with employees of the State of Colorado.
The following items are exceptions to the annual $65 limit for Colorado state employees:
• Unsolicited PhRMA Code compliant food and beverage snack items of nominal value (e.g., doughnuts
and non-alcoholic beverages such as soft drinks and coffee) which are not part of a meal;
• Unsolicited RC-approved educational items of nominal intrinsic value; and
• Fair market value payments for an employee’s provision of services, such as speaking or consulting
services.
Helpful Point
If you are not sure whether an HCP is employed by the State of Colorado or just affiliated with a
state institution, you must confirm his or her relationship with the state prior to providing any meals
or items of more than nominal value to the HCP. If the HCP receives regular compensation directly
from a state institution, he or she is likely considered a state employee and is therefore subject to
the restrictions discussed in this section.
Colorado Pricing Disclosure Requirements
Colorado passed a Price Transparency law, effective August 2, 2019, requiring manufacturers to provide
Colorado Licensed Prescribers, the Wholesale Acquisition Cost (WAC) price of their products, and at least
3 generic products in the same Therapeutic Class for any marketed product. Therapeutic Class is defined
as “ a group of similar drugs that have the same or similar mechanisms of action and are used to treat a
specific condition”.

White Guide – Chapter 15: State Laws: HCP and State Employee Restrictions
Rev. 12/20
Page 260 of 405
As a result of this new law, we are putting the WAC price for each product and any generic information on
our website for it to be available publicly. The information can be found at www.pfizer.com/coprescribers.
Sales Representatives in Colorado are required to do the following:
• Show Colorado Prescribers the landing page of the website at first contact and at every detail; and
• Advise Colorado Prescribers that this is the landing page where they can get the most up to date
information on WAC prices and any generic information relating to our products.
If you have any questions, please contact the team attorney with responsibility for Colorado.
The Law: Connecticut Compliance Program Law & APRN Disclosure Law
• Requires pharmaceutical, biological, and medical device companies to adopt and implement a
marketing code that is at least as restrictive as the PhRMA Code and a comprehensive compliance
program.
• Connecticut Department of Consumer Protection has authority to investigate alleged violations of the
code-adoption requirement and alleged failures to conduct any training program or regular audit for
compliance with the adopted code. Violations of the provisions would subject a company to a civil
penalty of up to $5,000.
Connecticut law also requires manufacturers to disclose payments and transfers of value provided to
Connecticut-licensed Advanced Practice Registered Nurses (APRNs) who practice not in collaboration with
a physician (i.e., independently). Definition of Advanced Practice Registered Nurse below for purposes of
the Connecticut disclosure law is defined as:
• An APRN who practices “not in collaboration with a physician” (i.e., an APRN who practices
independently); and
• Who appears in the Connecticut Department of Public Health annual APRN list, available at
https://portal.ct.gov/DCP/Drug-Control-Division/Drug-Control/Expenditure-Disclosure-Form.
How the Law Impacts Pfizer Colleague Activities
All colleagues who engage in activities with Connecticut APRNs should be aware that their expenditures
on APRNs will be reported and ensure that transfers of value, including their reporting of attendees at
speaker programs, is accurate and complete.
Connecticut

White Guide – Chapter 15: State Laws: HCP and State Employee Restrictions
Rev. 12/20
Page 261 of 405
The Law: Prescription Drug Marketing Costs Disclosure Law
The District of Columbia (D.C.) Prescription Drug Marketing Costs Disclosure Law requires Pfizer to report
all marketing costs for prescription drugs to the D.C. Department of Health, including the value, nature,
purpose, and recipient of all expenses associated with advertising, marketing, and direct promotion to D.C.
residents through radio, television, magazine, newspaper, direct mail, and telephone.
Specifically, costs associated with the following activities are required to be reported:
• Direct-to-consumer advertisements targeting D.C. residents;
• Educational or informational programs, materials, or seminars provided to healthcare professionals,
pharmacies, clinics, health plans, and other healthcare providers;
• Remuneration for promoting or participating in educational or informational sessions;
• Food, entertainment, gifts, and anything else provided to HCPs valued at more than $25 or provided
for less than market value;
• All expenses associated with HCP trips and travel;
• Starters (unless they are for distribution to patients at no charge); and
• The aggregate cost of all employees and contractors engaging in drug advertising and promotion in
D.C.
The following marketing expenses do not have to be reported:
• Food, gifts and other expenses of $25 or less;
• Compensation for bona fide clinical trial activities;
• Scholarships and expenses for attending educational, scientific, or policy conferences if attendee is
selected by the sponsoring organization; and
• Payments to D.C.-licensed HCPs for participating in blinded market research, if: a) the research is
conducted by an “independent survey research organization;” b) the pharmaceutical client does not
know the identity of the practitioners participating in the research; and c) the payments are determined
and made by the survey research organization.
Definition of Healthcare Professional
The D.C. definition of a Healthcare Professional (HCP) is broad. The law applies to expenditures provided
to persons and entities who are licensed to provide healthcare in D.C., including healthcare professionals
and persons employed by them who work in D.C., licensed insurance carriers, health plans and benefit
District of Columbia

White Guide – Chapter 15: State Laws: HCP and State Employee Restrictions
Rev. 12/20
Page 262 of 405
managers, pharmacies, hospitals, nursing facilities, clinics, and other entities licensed to provide healthcare
in D.C.
How the Law Impacts Pfizer Colleague Activities
All colleagues who engage in activities in D.C. should be aware that expenditures which meet the criteria
above will be reported to the D.C. Department of Health. Colleagues must take special care to ensure that
their reporting of attendees is accurate and complete. As a result, T&E submissions for meals over $25
per person to D.C. HCPs, must list all recipients partaking in the meal individually. D.C. can impose
significant penalties on Pfizer for failure to comply with this law.
The Law: Pharmaceutical Detailer Licensing Law
The Pharmaceutical Detailer Licensing Law requires licensure for any colleague or speaker who
communicates with a licensed HCP located in D.C. for the purpose of promoting a pharmaceutical product.
However, the law exempts individuals who engage in “pharmaceutical detailing” less than 30 days per
calendar year from the requirement to obtain licensure.
The D.C. Board of Pharmacy interprets the exemption as only applying to individuals detailing in D.C. “once
a year for a short duration of time of less than 30 consecutive days.”
Gifts to D.C. Medication Advisory Committee Prohibited
D.C. law also prohibits offering a gift or remuneration of any kind to a member of the D.C. Medication
Advisory Committee (DCMAC). Colleagues must not give anything of value to any DCMAC member (even
if the item is RC-approved or would be acceptable for non-DCMAC members), including:
• Speaking and consulting fees;
• Food or beverage, whether inside or outside the office, or in connection with a promotional program or
otherwise; and
• Educational items (e.g., textbooks and anatomical models).
However, colleagues may provide starters to DCMAC members who are licensed physicians engaged in
the practice of medicine and who intend to distribute them free of charge to patients.
For a list of DCMAC members, please consult the Department of Health Care Finance FAQ
(Question 27).
How the Law Impacts Pfizer Colleague Activities
Colleagues whose territory or geographic responsibilities include D.C. and who detail HCPs in D.C. must
complete and submit a license application to the D.C. Board of Pharmacy. These colleagues must have a

White Guide – Chapter 15: State Laws: HCP and State Employee Restrictions
Rev. 12/20
Page 263 of 405
valid pharmaceutical detailer license before calling on an HCP in D.C. It is your responsibility to apply for
your license, and application costs will be reimbursed by Pfizer.
The license application materials are available online at the DC Safe Rx Pharmaceutical Detailers License
website. The license application requires submission of an affidavit to abide by a Code of Ethics.
Impacted colleagues will need to renew their license each even numbered year prior to the end of February.
Colleagues should plan to submit their application by December 31st of the preceding year to allow
adequate time for review and processing of your application prior to the deadline. As part of the license
renewal application, you will need to attest that you have completed a minimum of 15 hours of continuing
education during the two year period preceding the date the license expires. You must register for a
“SafeRx Pharmaceutical Detail Licensing CE Program” through P2L. Once registered, you will receive a
list of CMR training courses that are approved for CE under the SafeRx Pharmaceutical Detail Licensing
Program. It can take up to two months to complete each course offered, and Pfizer will pay directly for
home study courses taken with the CMR SafeRx Pharmaceutical Detail Licensing CE Program. If you have
completed a CMR Certification or CMR Flex course post receipt of your pharmaceutical detailer’s license,
you should contact CMR at (800)328–2615 or program@cmrinstitute.org to determine if you already
received renewal credit.
The District of Columbia can impose significant penalties on Pfizer colleagues for failure to comply with this
law, which may include a fine of up to $10,000 as well as penalties and sanctions. If you have any questions
concerning the D.C. Prescription Drug Marketing Costs Disclosure Law or SafeRx please contact the Sales
and Marketing Attorney with responsibility for the District of Columbia.
The Pharmaceutical Detailer Licensing Law requires licensure for any colleague or speaker who
communicates with a licensed HCP located in DC, for the purpose of promoting a pharmaceutical product.
Colleagues whose territory or geographic responsibilities include DC and who detail HCPs in DC must
complete and submit a license application to the DC Board of Pharmacy.
The Pharmaceutical Detailer Licensing Law requires that any Speaker we engage to speak in DC obtain a
Pharmaceutical Detailer License if they plan to speak more than once in DC, in a calendar year.
The Law: Code of Governmental Ethics
The Louisiana Code of Governmental Ethics prohibits HCPs who are “public servants” from performing
certain compensated services for Pfizer, such as receiving fees for speaking services or reimbursement for
associated expenses. In addition, Louisiana imposes a $63 cap on food, drink, or refreshment provided to
a public servant for a single event. The amount should be calculated by dividing the total cost of the food
by the total number of persons (including non-public servants) at the event.
Louisiana

White Guide – Chapter 15: State Laws: HCP and State Employee Restrictions
Rev. 12/20
Page 264 of 405
Definition of "Public Servant"
“Public servants” are either public employees, or elected officials. They include, amongst others, persons
who are employees at any of the following institutions:
• Louisiana State University (LSU) and affiliated hospitals and clinics;
• Charity hospitals and other state hospitals;
• Medicaid P&T Committee members;
• State prisons; and
• State rural health clinics.
Public employee is anyone, whether compensated or not, who is:
• An administrative officer or official of a governmental entity who is not filling an elective office;
• Appointed by any elected official when acting in an official capacity and the appointment is to a post or
position the appointee is to serve either as a member or employee of the government or a governmental
agency;
• Engaged in the performance of a governmental function; or
• Under the supervision or authority of an elected official or another employee of the governmental entity.
How the Law Impacts Pfizer Colleague Activities
Louisiana public servants cannot be engaged as promotional speakers for Pfizer.
The Louisiana Board of Ethics has stated, however, that a public employee can serve as a consultant (e.g.,
at a marketing advisory board) as long as the consultant services are related to his or her academic
discipline or area of expertise and prior approval has been granted. For example, at LSU, the LSU chief
administrative officer would need to approve such a consultancy. Further, if a public servant is involved in
research with Pfizer, he or she can in most circumstances receive reimbursement for travel expenses for a
Pfizer-sponsored clinical trial. Lastly, the Code of Governmental Ethics and Board of Ethics’ rulings do not
prohibit a public servant from speaking at a conference where Pfizer has provided an independent
educational grant since Pfizer does not control the selection of the speaker or the content of the
presentation, and the expenses at such an event would be paid by the conference organizer directly.

White Guide – Chapter 15: State Laws: HCP and State Employee Restrictions
Rev. 12/20
Page 265 of 405
Helpful Point
If you are not sure whether a potential speaker is a Louisiana public servant, you must confirm
their status prior to engaging the person as a speaker. If the person receives regular compensation
directly from one of the institutions above, they are probably a “public servant” and would be
prohibited from receiving compensation from Pfizer for speaking.
The cap on meal expenditures at any program in Louisiana where Pfizer is providing a meal and where
there is at least one public servant present is $63.
This Louisiana law applies to any event where Pfizer is providing food or drink, and where a public servant
is present, including speaker programs, advisory board meetings, and speaker training meetings. It would
not, however, apply to an event funded through an independent educational grant, where Pfizer provides
financial support for the event and the grant recipient provides the meal.
The State of Louisiana can impose significant penalties on Pfizer and individual Pfizer employees for failure
to comply with the law.
If you have any questions concerning the Louisiana laws discussed here, please contact the team attorney
with responsibility for Louisiana.
The Law: Pharmaceutical and Medical Device Manufacturer Conduct Law (Massachusetts
Marketing Code of Conduct)
The Massachusetts Marketing Code of Conduct restricts Pfizer’s ability to provide meals and other items of
value to HCPs licensed in Massachusetts (MA). The law also requires Pfizer to disclose payments and
items provided to “Covered Recipients” (further defined below) that have a value of $50 or more.
(Remember, Pfizer policy has a $40 restriction on in-office or in-hospital meals which you need to comply
with. Office staff are not required to be listed by name for in-office or in-hospital meals since our threshold
is $40 per meal.) These laws are more restrictive than the PhRMA Code. They apply to all colleagues and
extend to interactions with Massachusetts HCPs that occur outside of Massachusetts.
In summary, the law requires Pfizer to:
• Adopt the Massachusetts Marketing Code of Conduct;
• Establish a compliance program and conduct an annual audit and training;
• Disclose annually certain financial interactions between Pfizer and Covered Recipients; and
Massachusetts

White Guide – Chapter 15: State Laws: HCP and State Employee Restrictions
Rev. 12/20
Page 266 of 405
• Provide Massachusetts HCPs the opportunity to withhold their prescriber data from use by sales and
marketing.
Failure to comply with any provision of the law can subject Pfizer to a penalty of $5,000 per violation.
Definition of Healthcare Professional
The Massachusetts definition of a healthcare professional (HCP) is broad. It includes any person who
prescribes prescription drugs and is licensed to provide healthcare in Massachusetts, including a
partnership or corporation comprised of such persons, as well as employees and agents of such persons
(e.g., nurses, office staff, etc.). Examples of Massachusetts HCPs include:
• Physicians;
• Physician Assistants;
• Certified nurse midwife;
• Psychiatric nurse mental health specialists;
• Nurse Practitioners; and
• Employees and agents of such persons (e.g., nurses, office staff, etc.).
Massachusetts HCPs do not include hospitals, nursing homes, pharmacists, health benefit plan
administrators, healthcare professionals not licensed in Massachusetts, and other entities if they are not
agents, employees, etc. of a MA-licensed HCP. However, such entities and individuals are considered
Covered Recipients for MA disclosure, as described below.
How the Law Impacts Pfizer Colleague Activities
All colleagues (regardless of division, business unit, or role) who engage in activities with Massachusetts-
licensed HCPs, regardless of where the HCP practices or where the interaction occurs, should be aware
that Massachusetts laws restrict Pfizer’s ability to provide meals and other items of value to Massachusetts
HCPs. In addition, certain expenditures have to be reported, so all colleagues must ensure that their
records on these expenditures are accurate and complete.
You must make a good faith effort to determine whether an HCP is licensed in Massachusetts. To help
you determine whether an HCP holds a MA license, you should check the HCP Lookup Tool. Sales
Colleagues can also access this information on Veeva CRM.
Meals
The Massachusetts Marketing Code of Conduct is more restrictive than the PhRMA Code with respect to
the provision of meals to HCPs. Subject to the other requirements of Pfizer’s policies, meals may be
provided to MA HCPs in certain limited situations that are specifically identified in the following guidance.

White Guide – Chapter 15: State Laws: HCP and State Employee Restrictions
Rev. 12/20
Page 267 of 405
• In-office or in-hospital meals are permissible during educational presentations.
• Out-of-office meals and “snacks” (as defined in Orange Guide Chapter 18) are prohibited.
• Pfizer may also provide modest meals at out-of-office speaker programs and at symposia taking place
at a convention or congress setting.
• Refreshments or snacks at convention or congress exhibit booths are permissible.
• There is a limited exception for meals provided as compensation to Massachusetts HCPs who are
consulting pursuant to a bona fide contract or meals provided at an investigator meeting whereby such
costs are covered within the clinical study agreement or meals provided in connection with a job
interview.
• FMDs or MOSs may not provide out of office meals to MA HCPs as the interactions they have do not
meet the definition of “scientific exchange” in MA.
As a general matter meals are prohibited in all other situations that are not specifically identified in the
guidance above.
Please see the Disclosure section below for T&E requirements for meals provided to Massachusetts HCPs
and Covered Persons.
Other Prohibited Items of Value and Activities
Generally, educational items may be provided to Massachusetts-licensed HCPs as long as they are RC-
approved and consistent with the PhRMA Code.
Colleagues are prohibited from making expenditures on behalf of any Massachusetts HCP for:
Colleagues may provide modest meals to Massachusetts-licensed HCPs at Pfizer Speaker
Programs or as part of an informational presentation in an HCP’s office or a hospital setting.
There are also exceptions for meals provided as compensation under valid consulting or other
contractual agreements, meals provided in connection with a job interview, and refreshments
provided in a convention/congress exhibit booth.
Colleagues must make a good faith effort to determine whether an HCP is licensed in MA and
can consult the HCP Lookup Tool
or Veeva CRM. The meal and gift restrictions apply even
when a Massachusetts-licensed HCP is located in another state.
Helpful Points

White Guide – Chapter 15: State Laws: HCP and State Employee Restrictions
Rev. 12/20
Page 268 of 405
• Entertainment or recreational items of any value;
• Grants, scholarships, subsidies, or educational items offered with the intent to encourage or modify
prescribing behavior; or
• Residents, fellows, and HCPs to attend educational conferences (where funding comes directly from
Pfizer and Pfizer chooses the recipient).
In addition, Pfizer may only provide CME support (through the process and standards associated with
Independent Grants for Learning and Change (IGLC)) to conference organizers that meet ACCME
standards or equivalent standards. Pfizer may not, however, provide funding directly to support meals for
HCPs or compensate HCPs for attending CME events.
Disclosure
Pfizer must track and report annually all expenditures made to Massachusetts Covered Recipients for sales
and marketing activities that are $50 or greater (per transaction). The definition of “Covered Recipients”
is broader than the definition of HCPs and includes hospitals, nursing homes, pharmacists, and health
benefit administrators. Therefore, even though pharmacists are not subject to the meal restrictions set forth
above (because they are not included in the definition of HCP), they are subject to the disclosure
requirements since they are considered Covered Recipients, so certain payments to pharmacists must be
disclosed. Expenditures that do not need to be disclosed include those associated with rebates and
discounts, genuine research, clinical trials, demonstration units, and starters. Disclosed data will be made
publicly available on the state’s website.
Co-pay cards, coupons and free trial vouchers may be provided to MA residents or to providers or
pharmacies for distribution to MA residents, subject to the following:
• Distribution of these offerings is prohibited for drugs that have an AB-rated generic equivalent (e.g.,
Lipitor).
• Colleagues must accurately record and track in Veeva CRM the distribution of these items to any HCPs.
• Coupon offers for all schedule II opioids, including Embeda, are prohibited.
• Marketing and other HQ teams developing these programs must abide with the other parameters
outlined in the Massachusetts Update on Loosened Co-pay, Coupon and Free Trial Voucher
restrictions, dated August 8, 2012.
Non-patient Identified Prescriber Data
Before using non-patient-identified prescriber data, Pfizer must give Massachusetts HCPs the opportunity
to request that their prescriber data be withheld from Sales and Marketing and not be used for marketing

White Guide – Chapter 15: State Laws: HCP and State Employee Restrictions
Rev. 12/20
Page 269 of 405
purposes. The Commercial Operations group within Pfizer is responsible for ensuring that any prescriber
data provided by Pfizer to Sales representatives complies with state law.
Starters Policy for Mid-Level Practitioners
Michigan state healthcare regulations require pharmaceutical manufacturers to link mid-level practitioners
to supervising physician when requesting starters.
• All starter requests recorded for Michigan Registered Nurses and Advanced Practice Registered Nurses
(NP, CNS, CRNA, CNM, AN) must include the supervising physician’s name in the transaction’s call
notes in Veeva. The Veeva system does not allow us to distinguish between APRNs and Registered
Nurses in Michigan, therefore, we must record the supervising physician’s name for all nurses that
request starters.
• When starters for controlled substances are included, the supervising physician’s name and his or her
DEA registration number must also be added to the transaction’s call notes in Veeva.
• Michigan state law now permits a Physician’s Assistant to order and receive starters directly, without
recording a supervising physician’s name or DEA registration number.
The Law: Gift Restriction Law
Minnesota prohibits Pfizer from offering or giving any gift of value to a Minnesota healthcare practitioner,
as defined below in this section. The definition of “gift” includes any thing or service that is given and
received for less than fair market value unless it is specifically permitted under the statute. The restrictions
apply to all colleagues (not only Sales) and extend to interactions with Minnesota practitioners that occur
outside of Minnesota.
The following are not considered “gifts” under the statute and may be given to Minnesota practitioners:
• Free drug samples for free distribution to patients (i.e. starters);
• Payment to sponsor a medical conference, professional meeting, or other educational program,
provided no payment is made directly to a practitioner;
• Reasonable fees and expenses of a practitioner who serves on the faculty at a professional or
educational conference or meeting;
Michigan
Minnesota

White Guide – Chapter 15: State Laws: HCP and State Employee Restrictions
Rev. 12/20
Page 270 of 405
• Compensation at fair market value in connection with a genuine research project;
• Certain publications and educational materials, including most (but not all) RC-approved educational
materials (e.g., Pfizer-created branded and unbranded promotional materials, reprints, literature, and
other printed materials); and
• Salaries or other benefits paid to employees.
Educational Items
Educational items which provide general medical or drug information are not considered to be “publications
and educational materials” and may not be provided. Examples of prohibited items include textbooks,
journal subscriptions, online subscription services (such as trial memberships for Epocrates), and
anatomical models. If you are unsure about whether an RC-approved item can be provided to a Minnesota
practitioner, check with your manager or your team attorney.
Meals
As of May 31, 2010, Pfizer prohibits all colleagues from providing meals to Minnesota practitioners, subject
to a very limited exception for meals provided as a reasonable expense to practitioners who serve on the
faculty at a Pfizer professional or educational conference or meeting who are receiving compensation for
services pursuant to a contract with Pfizer. A modest meal is not considered a “gift” under the law in these
circumstances. Where a Minnesota practitioner is serving as a speaker at a Pfizer promotional program,
for example, his or her meal does not constitute a gift and may be provided. Additionally, nominal snacks
provided at educational/scientific conventions/congress exhibit booths are allowable and not considered
banned gifts. All meals must, however, comply with all Pfizer policies on providing meals to HCPs, including
the policy that meals should be modest and not exceed $135 in value.
Companies are required to submit annual reports to the Minnesota Board of Pharmacy of non-gift payments
to practitioners, such as consulting fees, speaking honoraria, and related expenses, if the payments total
$100 or more per year per practitioner.
Consulting Engagements with MN HCPs
Pfizer policy prohibits engaging Minnesota-licensed practitioners as consultants except with respect to the
following types of projects:
• Reasonable honoraria and payment of the reasonable expenses of a practitioner who serves on the
faculty at a professional or educational conference or meeting. This does not include internal Pfizer
meeting where the audience are Pfizer Colleagues; and
• Compensation for the substantial professional or consulting services of a practitioner in connection with
a genuine research project.

White Guide – Chapter 15: State Laws: HCP and State Employee Restrictions
Rev. 12/20
Page 271 of 405
Engaging Minnesota practitioners as consultants for any other purposes is prohibited without prior
Legal approval.
Definition of Practitioner
A “practitioner” is essentially anyone who is able to prescribe a prescription drug in Minnesota regardless
of whether the practitioner actively prescribes. Physicians, nurse practitioners, physician assistants,
dentists, dental therapists, optometrists, podiatrists and veterinarians are all included in the definition of
practitioner in Minnesota. Pharmacists, however, are not included in the definition of practitioner and are
therefore not subject to the gift restrictions but are considered covered recipients for state disclosure.
You should treat any Minnesota practitioner as if they are subject to the Minnesota gift law regardless of
the state in which the practitioner works or where the practitioner is geographically located. For example,
if a Minnesota-based practitioner is attending a speaker program in another state, the Minnesota state gift
law still applies. If a physician who lives and practices in Florida is dual licensed in Minnesota, the
Minnesota gift law is deemed to apply. Therefore, meals cannot be provided to any Minnesota-licensed
practitioner, regardless of his or her location except as noted herein.
How the Law Impacts Pfizer Colleague Activities
All colleagues are prohibited from providing meals to Minnesota-licensed practitioners, unless the meal is
provided as a reasonable expense to a practitioner in connection with serving on the faculty at a Pfizer
professional or educational conference or meeting or performing bona fide services under one of the
permitted consulting engagements, and who is receiving compensation for services pursuant to a contract
with Pfizer. Refreshments and snacks provided at educational/scientific conventions/congress exhibit
booths are also allowed. These types of meals are not considered a “gift” under the state statute.
You must make a good faith effort to determine whether a practitioner is licensed in Minnesota. To help
you determine whether a practitioner holds a Minnesota license, you can check the HCP Lookup Tool.
Sales Colleagues can also access this information by looking up the HCP on their Veeva CRM tablet or
iPad. Note that Veeva CRM flags most (but not all) MN HCPs.
Minnesota can impose significant penalties on Pfizer as well as criminal misdemeanor penalties for failure
to comply with this law. If you have any questions concerning the Minnesota Gift Law, please contact the
team attorney with responsibility for Minnesota.

White Guide – Chapter 15: State Laws: HCP and State Employee Restrictions
Rev. 12/20
Page 272 of 405
Helpful Points
Colleagues must not offer or give any gift of value to a Minnesota HCP, including certain
educational items (e.g. textbooks).
Colleagues must not provide meals or refreshments to Minnesota HCPs, except in the limited
instance for certain HCPs under contract with Pfizer or at a congress/convention exhibit booth, as
detailed above.
Colleagues must not engage Minnesota HCPs as consultants, except under the limited
circumstances detailed in this Chapter.
You are required to make a good faith effort to determine whether an HCP is licensed in Minnesota
before providing a gift or a meal to the HCP. You can consult the HCP Lookup Tool for a list of
Minnesota HCPs, as noted above.
The meal and gift restrictions apply even when a Minnesota HCP is located in another state.
The Law: Nevada Marketing Code of Conduct
The Nevada Marketing Code of Conduct requires all manufacturers and wholesalers who sell or market a
drug in Nevada to:
• Adopt a written marketing code of conduct (the current PhRMA Code is acceptable);
• Adopt a training program to provide regular training to appropriate employees on the marketing code
of conduct;
• Conduct annual audits to monitor compliance with the marketing code of conduct;
• Adopt policies and procedures for investigating instances of noncompliance with the marketing code of
conduct;
• Identify a compliance officer responsible for the marketing code of conduct; and
• Submit certain information annually to the Nevada State Board of Pharmacy (including the marketing
code of conduct, description of the training program; description of the investigation policies; contact
information for the Compliance Officer; and certification of the company’s annual audit and compliance
with its marketing code of conduct).
Nevada

White Guide – Chapter 15: State Laws: HCP and State Employee Restrictions
Rev. 12/20
Page 273 of 405
Pharmaceutical Sales Representatives Registration
• Pharmaceutical manufacturers are required to provide Nevada DHHS with a list of sales
representatives that market prescription drugs to Nevada Covered Recipients (including, but not limited
to, Nevada HCPs, pharmacies or employees thereof, and employees of medical facilities). The updated
guidance applies to Sales Representatives and District Business Managers (“DBMs”) only. Sales
Representatives include Inside Sales Representatives (ISRs) and Contracted Inside Sales
Representative (CISRs). These are colleagues who detail customers remotely over the phone/web.
• Sales Representatives who reside in Nevada or visit Nevada for 5 days or more annually must be listed
on the Nevada Registry prior to conducting business in Nevada.
• ISRs and CISRs must register in NV only if they physically reside or visit NV 5 days or more annually
or reside in NV.
• Manufacturers must submit a complete list of all Sales Representatives who are in scope for the Nevada
registry employed during the previous calendar year annually by January 15. Additionally,
manufacturers must provide updates to the Department, as personnel changes occur.
Pharmaceutical Sales Representative Annual Disclosure Report
On or before March 1 of each year, Pfizer, on behalf of each Sales Representative or District Manager
listed on the Nevada Registry, is required to submit a report listing Nevada covered recipients who have
been provided a sample or transfer of value greater than $10 or total transfer of value that exceeds $100
aggregate for the previous year per sales rep per HCP.
The information provided in the Disclosure Report includes:
• The Sales Representative registry ID;
• The name, credential, NPI, and zip code of the NV covered recipient;
• The date of the interaction;
• The type and amount of transfer of value provided; and
• The product, NDC and quantity of the sample provided.
The Law: The state of New Jersey has placed restrictions on Meals and Consulting Arrangements
between New Jersey Prescribers and Pharmaceutical Manufacturers.
New Jersey

White Guide – Chapter 15: State Laws: HCP and State Employee Restrictions
Rev. 12/20
Page 274 of 405
The law impacts the way Pfizer engages New Jersey Prescribers and restrict Pfizer’s ability to
provide meals to a New Jersey Prescriber. The law applies to all Pfizer colleagues who interact with
New Jersey Prescribers who practice in NJ or who have NJ patients.
How the Law Impacts Pfizer Colleague Activities
You must make a good faith effort to determine whether an HCP is a Prescriber in New Jersey. To help
you determine whether an HCP is a prescriber in New Jersey, you can check the HCP Lookup Tool. Sales
Colleagues can also access this information by looking up the HCP in Veeva CRM.
Definition of a New Jersey Prescriber
The definition of a New Jersey Prescriber is broad. It includes any New Jersey Prescriber who holds an
active New Jersey license and, either practices in New Jersey or has New Jersey patients, regardless of
the Prescriber’s practice site. New Jersey Prescribers include:
• Physicians;
• Physician assistants;
• Podiatrists;
• Advanced Practice Nurses;
• Dentists; and
• Optometrists.
Meals
Providing meals to New Jersey Prescribers must meet the following conditions:
• Meals provided at promotional meetings may not exceed $15 for breakfast or lunch and $30 for dinner.
The $15 or $30 must include the tax, tip and any delivery charge.
• The above meal limits apply to in-office, in-hospital and out of office meals but do not apply to Speaker
Programs and Symposia as these are considered educational events exempt from the restriction.
• The restriction applies to all Pfizer colleagues, not just Field Commercial Colleagues.
There are limited exceptions for meals provided to New Jersey Prescribers that are under a Bona Fide
Services contract with Pfizer, if the Prescriber is provided a meal as part of a job recruiting process or if
refreshments and snacks are provided at educational/scientific conventions/congress exhibit booths.

White Guide – Chapter 15: State Laws: HCP and State Employee Restrictions
Rev. 12/20
Page 275 of 405
Consulting Engagements with New Jersey Prescribers
New Jersey Prescribers are also subject to the following restrictions with respect to Bona Fide
Services they provide:
• A New Jersey Prescriber shall not accept more than $10,000 in the aggregate from all pharmaceutical
manufacturers in a calendar year, for Bona Fide Services.
• Bona Fide Services include participation on advisory boards and consulting arrangements.
• Being the speaker at a Speaker Program is educational and not considered a promotional activity and
thus not subject to the cap. (A Speaker Program is where an approved speaker, typically an external
healthcare professional under contract with Pfizer, presents information on products, disease states,
or other healthcare topics to a group of appropriate attendees.)
• Payment or remuneration for travel, lodging, and other personal expenses associated with Bona Fide
Services are not included in the $10,000 aggregate cap.
The Law: Restrictions on Gifts to State and Local Officers and Employees
New York prohibits all NY elected officials, state officers and employees, state legislators, state legislative
employees, municipal officers, and municipal employees from receiving (directly or indirectly) any gift. “Gift”
includes anything of value given in any form, including any money, service, loan, travel, entertainment,
hospitality, or promise, unless an exception applies. Colleagues may not provide any item to a New York
State or local officer or employee if the item is intended or expected to influence or reward the New York
State or local officer or employee in the performance of any activity related to his or her official duties.
Definition of Officer or Employee
A New York officer or employee includes, amongst others, any HCP employed, either full-time or part-time,
by any New York State or county hospital, New York State Medicaid Board, or any other New York State
or county agency. Bear in mind that an HCP with a private practice could also be a New York officer or
employee.
How the Law Impacts Pfizer Colleague Activities
Pfizer colleagues may not provide any gift, including meals, to a New York State officer or employee.
Additionally, Pfizer colleagues may not provide gifts, including meals, to any New York local officer or
employee. In addition, even PhRMA Code compliant RC-approved educational items such as anatomical
models or textbooks may not be provided.
New York

White Guide – Chapter 15: State Laws: HCP and State Employee Restrictions
Rev. 12/20
Page 276 of 405
Pfizer colleagues may continue to provide PhRMA-compliant food and beverage items of nominal value
(e.g., doughnuts, cookies, and non-alcoholic beverages such as soft drinks and coffee) which are not part
of a meal. New York interprets “nominal” as a value of $15 or less.
Helpful Point
If you are not sure whether an HCP is employed by the State of New York or a municipal institution,
or is just affiliated with such an institution, you must determine the relationship prior to providing
any item of value to the HCP. If the HCP receives regular compensation directly from one of these
institutions, he or she is likely a state official and would be governed by the restrictions discussed
in this section.
If you have any questions, please contact the team attorney with responsibility for New York.
The Law: The Prescribed Products Law
The Vermont Prescribed Products Law significantly restricts Pfizer’s ability to provide meals and other items
of value to Vermont healthcare providers (HCPs). These laws are more restrictive than the PhRMA Code.
They apply to all colleagues and extend to interactions with Vermont HCPs occurring outside of the State
of Vermont. Pfizer is required to disclose these expenditures to the State of Vermont.
In certain circumstances, Pfizer has an obligation to self-report to the State of Vermont
if any colleague inadvertently provides a prohibited gift or meal to a Vermont HCP. If you become
aware of any such occurrence, you must report it immediately to
StateHealthcareLawCompliance@pfizer.com.
Definition of Healthcare Provider
Healthcare provider is defined very broadly in Vermont. It includes:
• Any person licensed to prescribe products or authorized to recommend prescribed products
(“healthcare professionals”);
• Partnerships and corporations comprised of healthcare professionals;
• Officers, agents, and employees of healthcare professionals (e.g., nurses, office staff, etc.); and
• Hospitals, nursing homes, pharmacists, and any other person authorized to dispense or purchase for
distribution prescribed products.
Vermont

White Guide – Chapter 15: State Laws: HCP and State Employee Restrictions
Rev. 12/20
Page 277 of 405
Examples of HCPs in Vermont include:
• Physicians;
• Nursing Homes;
• Nurse Practitioners;
• Dentists;
• Nurses and Healthcare professional office staff;
• Physician assistants;
• Hospitals;
• Pharmacists;
• Licensed Clinical Social Workers and Psychologists;
• Health plan benefit administrators; and
• Members of the Green Mountain Care Board (whether or not they are licensed HCPs).
How the Law Impacts Pfizer Colleague Activities
All colleagues (regardless of division, business unit or role) who engage in activities that involve Vermont
HCPs, regardless of where the HCP practices or where the interaction occurs, should be aware that
Vermont prohibits Pfizer from providing meals and certain other items of value to Vermont HCPs. In
addition, certain expenditures have to be reported, so all colleagues must ensure that their records on these
expenditures are accurate and complete.
You must make a good faith effort to determine whether an HCP is licensed in Vermont. To help you
determine whether an HCP holds a VT license, you can check the HCP Lookup Tool. Sales Colleagues
can also access this information by looking up the HCP in their Veeva CRM tablet or iPad. Note that Veeva
CRM flags most (but not all) VT HCPs.
Meals
All meals to Vermont HCPs are prohibited. This prohibition includes the provision of coffee and doughnuts,
or other food items of nominal value, even if these items are for non-prescribing staff in a physician’s office.
There is a limited exception for meals provided as compensation to Vermont HCPs who are providing
services pursuant to a bona fide contract with Pfizer and those provides in connection with a job interview.
In addition, refreshments such as coffee and snacks provided by Pfizer at a booth at a convention/congress
are also permissible.

White Guide – Chapter 15: State Laws: HCP and State Employee Restrictions
Rev. 12/20
Page 278 of 405
Gift Ban
In addition to the prohibition on meals, colleagues cannot provide Vermont HCPs with any item of value
unless the item is explicitly allowed under the law.
The following items are allowed under Vermont law:
• Starters;
• Peer-reviewed academic, scientific, or clinical articles or journals that have been RC approved;
• Articles, journals, and other educational items;
• Certain conference sponsorships;
• Rebates and discounts;
• Authorized expenditures related to clinical trials; and
• Compensation at fair market value for bona fide consulting services, including research and product
development meetings.
Marketing Research
The Prescribed Products Gift Ban and Disclosure Law prohibits Pfizer from providing payments to Vermont-
licensed HCPs in connection with marketing research surveys (including blinded surveys).
Paid market research surveys involving Vermont-licensed HCPs are banned. The restriction applies
whether the survey is conducted directly by Pfizer or through an independent third party survey research
organization.
Helpful Points
Vermont prohibits all meals with VT HCPs (regardless of where the meal takes place) except as
noted below.
Snacks of nominal value (e.g., coffee, drinks, cookies, etc.) are also prohibited, except when
provided at a booth at a convention/congress.
You must not invite VT HCPs to Pfizer speaker programs at which food is provided even if the
program is conducted outside of Vermont.
There is an exception for meals provided as compensation for services performed under a bona
fide consulting contract or in connection with a job interview.
You are required to make a good faith effort to determine whether an HCP is licensed in VT before
inviting an HCP to a speaker program. You can consult the HCP Lookup Tool for a list of VT HCPs
or look up the HCP in the Veeva CRM as noted above.
The meal and gift restrictions apply even when a VT HCP is located in another state.

White Guide – Chapter 15: State Laws: HCP and State Employee Restrictions
Rev. 12/20
Page 279 of 405
Disclosure of Expenditures to Vermont HCPs
Most allowable expenditures to Vermont HCPs, or other institutions covered by the law (e.g., Vermont
academic institutions, Vermont nonprofit hospital foundations, and professional, educational, and patient
organizations representing or serving health care providers or consumers in Vermont), must be disclosed,
regardless of the amount.
This includes tracking and disclosing the distribution of samples, coupons, and vouchers. Vermont’s law
defines “sample” as a unit of a prescription drug, biological product, or medical device that is not intended
to be sold and is intended to promote the sale of the drug, product, or device, including starter packs and
coupons or vouchers that allow any individual to receive a prescribed product for free or at a discounted
price.
Items exempt from disclosure are:
• Refreshments and other snacks provided at a booth at a convention/congress;
• Rebates and discounts;
• Royalties and licensing fees for patent rights;
• Labels on prescribed products;
• Reasonable expenses related to an interview by a manufacturer in connection with a bona fide
employment opportunity; and
• Prescribed products distributed free of charge or at a discounted price pursuant to a Pfizer Patient
Assistance Program.
The Law: Vermont Price Disclosure Law
The Vermont Price Disclosure Law requires that, when marketing directly to Vermont HCPs, Pfizer disclose
the Average Wholesale Price (AWP) “per pill” of each drug marketed, as well as the prices of other drugs
in the same therapeutic class. Two types of disclosure are required:
• Long Form Disclosure: Disclosure of price-related information posted on Pfizer’s website; and
• Short Form Disclosure: Written disclosure of price information which must be provided to the
prescriber at the point of specific detailing or promotional activity (whether in person, by mail, by
telephone, or electronically).
Both the long and short Vermont price disclosure forms may be accessed at
http://www.pfizer.com/vtprescribers/.
The following table identifies which forms are required in connection with typical promotional activities.

White Guide – Chapter 15: State Laws: HCP and State Employee Restrictions
Rev. 12/20
Page 280 of 405
Promotional Activity
Action Required
Face-to-face meeting with HCPs (detailing, exhibit
booths, professional conferences) in Vermont.
Provide short form to each HCP for each product
promoted or detailed.
Mailing to HCPs.
Include short form with mailing for each product
promoted.
Telephone calls.
Inform Vermont HCP that short form will be mailed;
mail short form for each product promoted to
business address within 24 hours.
E-mails or electronic communications.
Include short form for each product promoted as
an attachment or as conspicuous and separate
section of the e-mail.
Marketing activities which do not require price disclosure in Vermont include placement of advertisements
and marketing to state or private payers as well as hospitals.
Vermont can impose significant penalties on Pfizer for failure to comply with this law. If you have any
questions, please contact the team attorney with responsibility for Vermont.
• Refer any questions to the team attorney with responsibility for the relevant state.
For More Information

Rev. 12/20
Page 281 of 405
CHAPTER #16 – FEDERAL
EMPLOYEE INTERACTIONS AND
LOBBYING

White Guide – Chapter 16: Federal Employee Interactions and Lobbying
Rev. 12/20
Page 282 of 405
Chapter
#16
FEDERAL EMPLOYEE INTERACTIONS
AND LOBBYING
CONTENTS
Introduction................................................................................................................................................ 284
Federal Employee Interactions ................................................................................................................. 284
Promotional Activities ................................................................................................................................ 285
Impact of Formulary Status on Ability to Promote .............................................................................. 285
Site Visits/Providing Promotional Materials/Educational Materials .................................................... 288
Starters ............................................................................................................................................... 288
Gifts to Federal Employees ....................................................................................................................... 289
General Rules ..................................................................................................................................... 289
Educational Items ............................................................................................................................... 290
Refreshments ..................................................................................................................................... 290
Inviting Government Employees to Speak or Present at Events ....................................................... 291
Inviting Government Employees to Attend Events (Non-Speakers/Presenters) ................................ 292
Supporting Independent Medical Education ....................................................................................... 294
Summary of Guidelines Regarding Federal Employee Interaction ........................................................... 294
General ............................................................................................................................................... 294
For DoD or IHS facilities, if provision of meals is permitted, the following conditions must also
be met: ................................................................................................................................................ 295
Key Points to Ensure Compliance ...................................................................................................... 295
Lobbying .................................................................................................................................................... 296
Federal Lobbying ................................................................................................................................ 296
Who Is a “Lobbyist?” ................................................................................................................................. 297
What Is Lobbying? .................................................................................................................................... 298
Reporting Lobbying Time and Expense .................................................................................................... 300
State-Specific Laws ................................................................................................................................... 301
States’ General Lobbying Disclosure Laws ........................................................................................ 301
States’ Lobbying Laws Impacting Marketing ...................................................................................... 302
Federal Employee Interactions and Lobbying

White Guide – Chapter 16: Federal Employee Interactions and Lobbying
Rev. 12/20
Page 283 of 405
State Restrictions on Gifts to Legislators ........................................................................................... 302
State Formularies ............................................................................................................................... 302
Campaign Contributions ............................................................................................................................ 303
The Pfizer Political Action Committee ....................................................................................................... 303
General ...................................................................................................................................................... 304
For More Information ................................................................................................................................. 304

White Guide – Chapter 16: Federal Employee Interactions and Lobbying
Rev. 12/20
Page 284 of 405
Chapter #16 Federal Employee Interactions and Lobbying
This Chapter summarizes: (a) the important rules you must understand and follow when engaging in
promotional and non-promotional activities with U.S. federal government agencies, including the
Department of Veterans Affairs (VA), the Department of Defense (DoD), the Department of Health
and Human Services (DHHS), and federal government employees; and (b) certain key Pfizer policies
regarding lobbying registration and disclosure. This Chapter is relevant to any colleague who: (1) interacts
with federal government employees, including healthcare professionals (HCPs) and formulary decision-
makers; or (2) engages in lobbying activities with any elected or appointed state or federal government
official or public employee (including state Medicaid agency employees and public hospital and government
HCPs).
Each colleague is responsible for adhering to Pfizer's policies governing interactions with federal
employees and lobbying activities involving federal or state government officials and public
employees. Non-compliance with these policies puts the Company at risk and can subject
colleagues to internal disciplinary action, up to and including termination, and external civil and
criminal sanctions.
As Pfizer’s sales to the federal government continue to increase, interactions with government officials (e.g.,
Director of Medicaid) and government employees (e.g., a physician at a federal institution or at a federal
prison) are becoming more commonplace. Pfizer’s customers include federal government agencies and
institutions, including the VA and its hospitals, the DoD and its medical facilities, and the DHHS, including
the Indian Health Service (IHS) and the Centers for Disease Control and Prevention (CDC). Pfizer
sales colleagues may interact with HCPs and other employees who work for these government agencies
and institutions on a full- or part-time basis or otherwise qualify as federal government employees. Account
managers may also interact with federal government employees who make decisions on formularies and
purchasing.
Introduction
Federal Employee Interactions

White Guide – Chapter 16: Federal Employee Interactions and Lobbying
Rev. 12/20
Page 285 of 405
Department of Veterans Affairs (VA)
Federal agency that provides patient care, services, and benefits to U.S. veterans.
Department of Defense (DoD)
Federal agency that oversees the four branches of the U.S. military (Army, Navy, Marine Corps,
and Air Force).
Centers for Disease Control and Prevention (CDC)
Federal agency that is responsible for the of promotion health and quality of life by preventing and
controlling disease, injury, and disability.
Indian Health Service (IHS)
Federal agency that is responsible for providing federal health services to American Indians and
Alaska Natives.
Promotional activities that are permissible when conducted with HCPs who are not federal government
employees may be prohibited when these same activities are conducted with HCPs who are federal
government employees. Interactions with federal employees are governed by the Standards of Ethical
Conduct established by the Office of Government Ethics (OGE), other government-wide OGE
regulations, agency-specific regulations and policies, and institution and site-specific policies and
procedures. Interactions with VA employees are further restricted by the more specific rules contained in
Veterans Health Administration (VHA) handbook 1004.07 (“Financial Relationships Between VHA
Healthcare Professionals and Industry”), VHA Directive 1108.10 “Promotion of Drugs and Drug-related
Supplies by Pharmaceutical Company Representatives”, and 38 C.F.R. 1.220, On-site Activities by
Pharmaceutical Company Representatives at VA Medical Facilities.
Impact of Formulary Status on Ability to Promote
Sales colleagues must comply with federal agency, institution, and local site policies regarding drug
promotion, including those that regulate promotion based on formulary status. In some cases, local
regulations will prohibit any discussion of products that are either not on the institution’s formulary or are
on the formulary with restrictions. In all cases, you must accurately and clearly represent the formulary
status of the product being discussed.
At VA facilities and other VA points of care, promotion of formulary and non-formulary drugs, including those
with established Criteria-For-Use (CFU) is permitted only under limited circumstances. CFU means clinical
Promotional Activities

White Guide – Chapter 16: Federal Employee Interactions and Lobbying
Rev. 12/20
Page 286 of 405
criteria developed by the Department of Veterans Affairs (VA) at a National level that describe how certain
drugs may be used. VA's CFU are available to the public at www.pbm.va.gov. Exceptions may be applied
at the local level for operational reasons. In all cases, the Veterans Integrated Service Network (VISN)
Director, the facility Chief of Pharmacy, or his or her designee must provide approval of the promotional
activity. Key VA-specific rules are set forth below.
VA-Specific Promotional Rules: VA National Formulary (VANF) and Non-VANF drugs and drug-related
supplies may be promoted in VA medical centers (including Community-Based Outpatient Clinics (CBOCs)
and other VA medical facilities) provided that all of the following conditions are met:
(1) The promotion has been approved by the VA medical facility’s Chief of Pharmacy Services, or
designee;
(2) The promotion is consistent with the existing Pharmacy Benefits Management (PBM) Criteria-for-Use
guidance;
• NOTE: Sales representatives may access information regarding VA Criteria-for-Use from the PBM
Web site at: www.pbm.va.gov.
(3) The drugs or drug-related supplies are discussed, displayed, and represented accurately;
(4) The promotion has significant educational value and does not inappropriately divert VA staff from other
activities they would otherwise perform during duty hours, including patient care and other educational
activities; and
(5) The drug or drug-related supply has not been classified by VA as non-promotable.
38 C.F.R. 1.220 On-site Activities by Pharmaceutical Company Representatives at VA Medical Facilities.
Non-VANF drugs and drug-related supplies where PBM Criteria-for-Use have not been developed, may be
promoted in VA medical centers (including CBOCs and other VA medical facilities) provided that all of the
following conditions are met:
(1) The promotion is specifically permitted by the VA medical facility’s Chief of Pharmacy Services, or
designee;
(2) Drugs or drug-related supplies are discussed, displayed, and represented accurately;
(3) The promotion has significant educational value and does not inappropriately divert VA staff from other
activities they would otherwise perform during duty hours, including patient care and other educational
activities; and

White Guide – Chapter 16: Federal Employee Interactions and Lobbying
Rev. 12/20
Page 287 of 405
(4) The drug or drug-related supply has not been classified by VA as non-promotable. NOTE: The PBM
maintains a National listing of formulary medications that are not to be promoted or detailed by sales
representatives on https://www.pbm.va.gov/PBM/NationalFormulary.asp Web site.
38 C.F.R. 1.220 On-site Activities by Pharmaceutical Company Representatives at VA Medical Facilities.
Products with Criteria-for-Use: It is possible that product-specific information and recommendations in
the CFU may be inconsistent with product labeling. Therefore, sales colleagues can discuss CFU product-
specific recommendations and clinical recommendations only if approved by the relevant brand RC. It is
important to highlight, when having such discussions, that the CFU was independently developed by the
VA and that Pfizer does not necessarily endorse them. In the event that the CFU is inconsistent with
product labeling, for example, when they recommend use of a Pfizer product over a competitor when there
is no head to head data, or when the use is recommended in a patient population that is different from that
in the label, the brand RC may consider allowing sales colleagues to refer HCPs to the VA website for
review of the CFU or leaving a copy behind, without discussing them. If copies of CFU are approved by
the brand RC as a leave-behind, they should be distributed separately from any promotional materials and
include prominent disclaimers that the CFU was independently developed by the VA, that Pfizer does not
endorse the CFU or recommend using the product as described in the CFU and attach a copy of the
approved product labeling.
In all cases where there is any question as to whether promotional materials are consistent with Pfizer
policies, your team attorney must be consulted before providing those promotional materials to the
customer.
Products with Criteria-for-Use
The VA provider mentioned to me he tried prescribing Product X but was told he must
first try Product Y. What should I do?
Representatives may acknowledge in general the existence of a CFU and refer the
provider to their internal website or pharmacy for more information. The specific VA
product CFU cannot be discussed by representatives unless permitted by the brand RC.
VA establishes CFUs that are similar to prior-authorizations per its VANF process.
These may require trial through VANF drugs, generics, or certain circumstances to
exist.

White Guide – Chapter 16: Federal Employee Interactions and Lobbying
Rev. 12/20
Page 288 of 405
Site Visits/Providing Promotional Materials/Educational Materials
You must make an appointment prior to visiting VA Facilities for the purpose of promotional activity. Use
and reference to promotional materials can be driven by facility-specific policy. VA policy, for example,
requires that promotional materials referenced on a VA site must be approved by the VA medical facility’s
Chief of Pharmacy Services or his or her designee. Also, it does not permit company representatives to
leave promotional materials in patient areas. At DoD facilities you should follow the process of each facility
regarding appointments and promotional activity.
In addition, be aware of rules pertaining to how you are expected to conduct yourself when leaving
promotional materials for HCPs at federal institutions. For example, VA facilities do not permit marketing
to students (including residents), and do not permit waiting in patient-care areas or paging employees via
a public address/paging system.
• Any promotional programs or educational materials that sales colleagues wish to use or circulate at VA
facilities must be RC-approved and submitted to the Facility Chief of Pharmacy Services at least 60
days prior to your educational program or meeting for review and approval. No materials may be used
without obtaining such approval. Additionally, without permission from the VA Pharmacy Benefits
Management Service, patient education materials may not contain the name or logo of the
manufacturer or promote a specific medication.
VA Appointment Requirement
Do sales colleagues have to make an appointment before calling on HCPs who work
at VA facilities?
Yes. Additionally, once on-site you may only detail HCPs with whom you have made
an appointment.
Starters
Many federal government institutions, such as VA clinics and hospitals, may prohibit pharmaceutical
companies from leaving starters, samples, or free goods. You must always learn the sample policies and
procedures of any institution that you call on and follow those rules. If there is any question as to whether
these policies and procedures might conflict with Pfizer policy or the Prescription Drug Marketing Act
(PDMA), you must consult your team attorney before leaving starters with that customer.
Federal rules and policies regarding sampling/starters/donations must be followed in all cases where
samples are provided to or in federal facilities. Accordingly, starters cannot be left for federal employees
at the federal institution at which the employee works even in cases where the starters are intended for use
outside of the government (e.g., in private practice settings).

White Guide – Chapter 16: Federal Employee Interactions and Lobbying
Rev. 12/20
Page 289 of 405
The VA has documented procedures, policies, and regulations regarding the donation of drug samples by
pharmaceutical representatives at VA medical facilities. All drug donations (including samples) must be
submitted in advance for approval by the VA Medical Facility Director. Additionally, all usage information
about the product must be forwarded to the VA Pharmacy Executive or VISN Formulary Committee. After
approval, all samples must be delivered to the Office of the Chief of Pharmacy Services for proper storage,
documentation, and dispensing. Pharmaceutical company representatives are strictly prohibited from
providing drug samples to VA providers outside of the Pharmacy Service for any reason, including VA staff
member’s personal use.
Additionally, if donated products (including samples) are intended to be used solely to allow VA clinicians
to gain familiarity with the products, such use must be pre-approved by the VISN Pharmacist Executive
and/or VISN Formulary Committee. Information about the trial use of the product must be forwarded to the
VA Pharmacy Benefits Management Office and/or the VISN Formulary Committee.
In general, donated drugs should not labeled as “sample” or similar wording. However, on rare occasion,
the VA will make exceptions to this labeling rule if it is in the best interest of the patient (e.g., product
shortage). 38 C.F.R. 1.220 On-site Activities by Pharmaceutical Company Representatives at VA Medical
Facilities.
Providing Starters to the VA
I’ve been told by an HCP at a VA facility that pharmaceutical companies cannot leave
starters with the VA. Is this correct?
VA policy permits pharmaceutical companies to provide samples/starters to VA
medical facilities upon approval by the VA Medical Facility Director. Samples/starters
must be delivered to the Office of the Chief of Pharmacy Services for proper storage,
documentation, and dispensing. The distribution of samples/starters directly to VA
HCPs is inconsistent with the VA’s policy. Moreover, if the products are intended to
be used solely to allow VA clinicians to gain familiarity with the product, such use must
be pre-approved by the VISN Pharmacist Executive and/or VISN Formulary
Committee
General Rules
Like the PhRMA Code’s guidelines on gifts to HCPs, the federal government places restrictions on the
acceptance of gifts by its employees, including HCPs. Under the overarching federal gift rules, a federal
government employee may not accept any single gift (which can include anything of value, such as meals,
Gifts to Federal Employees

White Guide – Chapter 16: Federal Employee Interactions and Lobbying
Rev. 12/20
Page 290 of 405
travel, lodging, entertainment) that has a retail or market value of more than $20, nor can a federal
government employee accept gifts with an aggregate value of more than $50 annually from a single
“source,” e.g., a single company, like Pfizer. As discussed in this section, there are additional rules that
further limit these general restrictions.
To help ensure that Pfizer maintains compliance with the federal rules, the only “gifts” that
colleagues can provide to federal government employees (including HCPs) are Pfizer-approved
educational items and modest refreshments (without alcoholic beverages) under the circumstances
outlined in this Chapter. As a reminder meals at VA facilities are prohibited.
Further, any gifts, including refreshments, provided to federal government employees will be subject to
Pfizer’s HCP Payment Disclosure Policy. All HCPs, including those employed by the VA and DoD, may
“opt-out” of receiving these items by notifying their Pfizer sales colleague or by contacting [email protected].
For additional information on Pfizer’s HCP Payment Disclosure Policy, see Chapter 18: Meals, Educational
Items, Greenstone Giveaways, and HCP Payment Disclosure.
Educational Items
There is an exception to the general gift restrictions that allows a federal government employee to accept
unsolicited gifts of informational materials with a value of $100 or less from a single source in a calendar
year. To qualify, the materials must be: (i) educational or instructive in nature; (ii) not primarily created for
entertainment, display, or decoration; and (iii) contain information that relates in whole or in part to the
following categories: (A) the employee's official duties or position, profession, or field of study; (B) a general
subject matter area, industry, or economic sector affected by or involved in the programs or operations of
the agency; or (C) another topic of interest to the agency or its mission. A federal government employee
may only exceed the $100 limit with prior written authorization from his or her Designated Agency Ethics
Official (DAEO). Before providing informational materials to a federal government employee, you must
contact Team Attorney for prior approval.
Refreshments
As outlined above, federal law and Pfizer policy place limits and/or restrictions on the offering of food, meals,
and refreshments to federal employees. These must be followed by all Pfizer colleagues.
Modest Refreshments: Modest refreshments (such as coffee and donuts, not including alcoholic
beverages) in some cases can be offered to federal government employees when incidental to a scheduled
meeting or legitimate educational interchange not otherwise prohibited by the facility or local rules. In these
cases, modest refreshments are not considered “gifts” and do not count toward the $50 annual cap for each
federal government employee. Also, offering even modest refreshments on a regular, repeated, or routine
basis is not allowed, and alcohol is always prohibited.

White Guide – Chapter 16: Federal Employee Interactions and Lobbying
Rev. 12/20
Page 291 of 405
Site-Specific Rules/VA On-Site Prohibition: The VA prohibits pharmaceutical representatives from
providing meals or refreshments of any type or value to VA staff (including volunteers) or non-VA
staff while on-site at a VA facility (hospital, office, other agency offices). Other federal government
agencies, including DoD and IHS, have their own rules concerning interactions on-site at their facilities.
Pfizer sales colleagues must review the local site rules of all federal healthcare facilities that they visit to
determine whether in-office or in-hospital meals and/or refreshment are permissible.
When meals are permitted, you also must comply strictly with the following limitations:
• You must obtain confirmation from the federal employee that he or she is permitted to accept the in-
office or in hospital meal under all applicable laws and rules, including any local site rules.
• You may not offer meals on a regular, repeated, or routine basis to any federal government employees,
including any HCP or group of HCPs;
• Each meal must have a total value of $20 or less;
• You must confirm that offering the meal will not cause Pfizer to exceed the $50 ceiling on gifts to any
federal employee (this ceiling applies to Pfizer as a whole and not to specific Pfizer colleagues); and
• The meal must take place at the HCP’s office or hospital when hosted by a Pfizer colleague.
Inviting Government Employees to Speak or Present at Events
Pfizer colleagues must contact Team Attorney for more information before scheduling an event or meeting
at which a full- or part-time federal employee will speak or extending an invitation to any federal employee
to attend an event.
Speaker/Free Attendance: Federal government employees, including HCPs, may accept an offer of free
attendance to speak at a Pfizer-sponsored event and may accept meals provided at the event that are
provided to all participating speakers on the same day. Pfizer policy requires obtaining approval in writing
by the DAEO of any such engagement. However, federal government employees are generally prohibited
from accepting compensation for speaking engagements that relate to their official duties. This includes
receiving compensation to speak to other government employees on Pfizer’s behalf.
In limited circumstances, federal government employees may be compensated to speak on matters that
are not related to their official duties. The conflict-of-interest regulations require that any such engagement
be pre-approved in writing by the federal government employee’s DAEO. (Approval from other federal
government employees who are not the DAEO is not sufficient.) In assessing such an engagement, the
DAEO will consider whether the federal government employee:
• Is speaking in his or her individual capacity and not as part of his or her official duties;
• Is speaking because he or she is a subject matter expert on a topic and not because of his or her official
position;

White Guide – Chapter 16: Federal Employee Interactions and Lobbying
Rev. 12/20
Page 292 of 405
• Is not speaking on a matter pending before his or her government agency or institution;
• Is speaking on his or her personal time rather than government working time; and
• Is not conveying information that draws on ideas or official data that is nonpublic information.
Pfizer policy requires receipt of DAEO approval in writing prior to such speaking engagement or
confirmation from the Government Employee, in writing, that they received approval from the DAEO.
Inviting Government Employees to Attend Events (Non-Speakers/Presenters)
On occasion, Pfizer may wish to invite federal government employees to events, including off-site
educational speaker programs, as non-speakers. Under those circumstances, free attendance is
considered a gift. Free attendance and meals provided to all attendees in a group setting may be allowed
under an exception to the gift restriction that applies for “widely attended gatherings.” Importantly, to qualify
for this exception, the federal government employee must receive prior written approval from his or her
DAEO before accepting the invitation to attend. (Approval from other Federal Government Employees who
are not the DAEO is not sufficient.) Pfizer policy thus requires receipt of DAEO approval or confirmation
from the Federal Government Employee, in writing, that they received approval from the DAEO prior to
Federal Government Employee attendance at this type of event and all invitations must be contingent upon
receiving this approval.
Pfizer policy further requires that any meal being provided is in connection with a legitimate educational
speaker program that:
• Satisfies Pfizer’s standards for a speaker program as set forth in Orange Guide Chapter 9: Speaker
Programs for HCPs; and
• Is not offered on a regular or repeated basis to a federal government-employed HCP.
Lunch and Learn
A sales colleague would like to call on a HCP employed by the VA who has a busy
schedule. Due to her crowded schedule, the HCP has offered to meet with the
representative during her lunch hour every other Tuesday. May the representative
have a “lunch and learn” with the HCP in her office on alternating Tuesdays and bring
a modest lunch for the HCP, such as a sandwich and soda?
No. VA Rules prohibit providing a meal to VA employees at VA facilities. Additionally,
because the HCP is a federal government employee, even if the on-site meal
prohibition did not apply, Pfizer prohibits colleagues from providing meals to federal
government employees. Under the policy, colleagues may only provide modest
refreshments without alcoholic beverages.

White Guide – Chapter 16: Federal Employee Interactions and Lobbying
Rev. 12/20
Page 293 of 405
Speaker Program Meals
A sales colleague has invited a DoD HCP to a speaker program that qualifies as a
“widely attended gathering.” If the DoD HCP attends the speaker program after
confirming in writing with her employer that attendance is permitted, is it permissible
for the DoD HCP to receive the same meal as the other attendees in the group setting
if it’s more than $20 in value? Or, is Pfizer required to provide a meal of $20 or less
in value?
The Pfizer Colleague needs to confirm that the federal government employee has
received DAEO approval in writing (approval by others within the agency will not be
sufficient). If DAEO written approval has been obtained, the exception will be met and
the meal provided at the event will not be considered a gift. The HCP thus can have
the same meal as the other event attendees.
Part-Time VA Employees
One of my customers works three days a week at his private practice and two days a
week at a VA hospital. When I provide him meals at his private office, am I required
to follow the VA/DoD limitations set forth in the Orange Guide?
Yes. HCPs who work part-time for the VA are still required to follow the policies of the
VA as if they are full-time employees.
Compliance Responsibility
If a HCP at a VA facility asks me to provide him with something that would be
considered a gift, isn’t it the HCP’s responsibility to make sure that he or she is in
compliance with applicable gift rules? How can Pfizer get in trouble?
It is your responsibility to make sure that you do not take action that causes the HCP
to violate the gift rules. While the ethics rules place compliance requirements on the
federal employee, under criminal law, private companies can be held accountable for
their actions, including any that result in federal employee violations of ethics rules.
Additionally, if Pfizer provides a gift to a federal HCP, it can trigger certain reporting
obligations for the company. In addition, providing the gift may violate the local
institution’s policies and result in Pfizer being excluded from the facility.
Accordingly, at no time should you ever provide a federal government employee with
any gift or meal, except as described in this Policy, even if the item has been approved
for distribution to non-government HCPs or the item is requested by the federal
government employee. If you are ever in doubt, treat the HCP as if he or she was a
government employee and follow the applicable rules herein and at the HCP’s local
facility.
White Guide – Chapter 16: Federal Employee Interactions and Lobbying
Rev. 12/20
Page 294 of 405
Engaging Part-Time Government Employees as Speakers
May I engage an HCP who works part-time at a federal government institution to be a
paid speaker at a Pfizer conference?
You may only engage the HCP once he or she can provide a written approval from
his/her DAEO authorizing the engagement. Additionally, all Pfizer policies related to
engaging HCPs as speakers and properly conducting speaker programs must be
followed. Please see chapter 9 of the Orange Guide.
Supporting Independent Medical Education
Federal government agencies and institutions often ask Pfizer to support their independent medical
education programs. Pfizer may be permitted to support these activities through independent educational
grants. Grant requestors must submit all requests for funding through www.pfizer.com/independentgrants.
Requests will be reviewed according to Pfizer’s standards for supporting independent medical education.
For more information on Pfizer’s educational grant process, refer to GNT01-GSOP “Independent Medical
Grants” for further details.
General
• Do not provide anything of value to a federal government employee (including HCPs) other than Pfizer-
approved educational items and modest refreshments (not including alcoholic beverages); additionally,
on-site meals and modest refreshments at VA facilities are strictly prohibited.
• Only provide federal government employees with educational materials that are pre-approved in
accordance with this Chapter.
• Never provide free aloholic beverages to federal government employees.
• On site at facilities of any federal government agencies, understand and comply with the applicable
rules. For instance, if you are visting a DoD or IHS facility, you are responsible for identifying any
unique rules that apply to that facility and complying with them.
• Do not engage a federal government employee, including a HCP, to speak on Pfizer’s behalf without
evidence that the employee’s DAEO has approved the engagement in writing or unless the Federal
Government Employee has confirmed in writing that the engagement has been approved by the
employee’s DAEO.
• Do not provide a federal government employee, including a HCP, free attendance to an event without
evidence that the employee’s DAEO has approved in writing the employee’s attendance.
Summary of Guidelines Regarding Federal Employee Interaction

White Guide – Chapter 16: Federal Employee Interactions and Lobbying
Rev. 12/20
Page 295 of 405
• Even if an item may be provided under the federal ethics rules and agency/facility-specific
requirements, any such item, including refreshments, provided to a U.S.-licensed physician may be
reportable under the relevant state laws and/or Sunshine Act.
• Additionally, always check the State Laws: HCP and State Employee Restrictions chapter for additional
guidance.
• You must understand and comply with the sample policies of any institution that you call on, and to the
extent that there is any question as to whether they might conflict with Pfizer policy or the PDMA, consult
your team attorney.
• You must submit RC-approved educational materials to the Chief of Pharmacy Services at least 60
days prior to your educational program or meeting. Additionally, without permission from the VA
Pharmacy Benefits Management Service, patient education materials may not contain the name or logo
of the manufacturer or promote a specific medication.
For DoD or IHS facilities, if provision of meals is permitted, the following conditions must
also be met:
• Meals may not be offered on a regular, repeated, or routine basis to an HCP or group of HCPs;
• Meals must comply with the $20 per occasion and $50 per year limits discussed above; and
• The federal government employee must confirm in advance that he or she is permitted to accept an in-
office or in-hospital meal under the Standards of Ethical Conduct and the local site rules.
• Only RC-approved (and, in the case of the VA, approval by the VA medical facility's Chief
of Pharmacy Services or designee) nominally-priced educational materials may be provided
to a government HCP.
•
Government officials may be given RC-approved educational materials only-gifts of any
value, including meals, are prohibited.
•
Public employees may be given approved educational materials subject to each institution's
policies and applicable law.
•
Every communication with a state government official or his or her staff must be coordinated
through the relevant GRD. Communications with federal government officials or staff must
be coordinated through the Washington, D.C. Pfizer office.
Key Points to Ensure Compliance

White Guide – Chapter 16: Federal Employee Interactions and Lobbying
Rev. 12/20
Page 296 of 405
Federal and state lobbying laws regulate interactions with government officials and public employees that
are intended to influence legislation, regulations, or government policies. Pfizer is required by federal law
and many state laws to disclose publicly its lobbying expenditures on a regular basis.
Federal Lobbying
The Federal Lobbying Disclosure Act (LDA), as amended by the Honest Leadership and Open Government
Act (HLOGA), requires Pfizer to report expenses incurred for all its federal lobbying activities. This includes
not only time and expenses spent by those Pfizer colleagues who are registered as federal “lobbyists,” but
also time and expenses of those Pfizer colleagues who support Pfizer’s federal lobbying effort.
Pfizer’s grassroots advocacy programs present additional opportunities for colleagues to interact with
government officials and public employees about healthcare policy. To help ensure that Pfizer complies
with all registration and reporting requirements, all of your interactions with government officials must be
• Sales Colleagues should spend no more than one hour per week or four hours per month,
if at all, on political activities related to Pfizer business.
•
Do not suggest, offer or provide campaign contributions in exchange for a promise to
perform any official act.
•
Pfizer must report certain expenditures made towards lobbying efforts to the federal
government as well as many state governments.
•
Even if you are not a “lobbyist,” your time spent supporting the lobbying efforts of others
within the Company is reportable under federal law.
•
Each state’s reporting requirements are different – be sure to check with your GRD or team
attorney if you are unsure whether you need to register as a lobbyist and/or which activities
must be reported.
•
For more information on state specific restrictions on interactions with state-employed
HCPs, see the State Laws: HCP and State Employee Restrictions Chapter.
Key Points to Ensure Compliance
Lobbying

White Guide – Chapter 16: Federal Employee Interactions and Lobbying
Rev. 12/20
Page 297 of 405
coordinated either through the Pfizer Grassroots program, the Washington, D.C. office, or a Pfizer State
Government Relations Director (GRD), depending on the nature of the interaction.
Like the rules that govern your interactions with healthcare professionals, lobbying, ethics, gift, and
campaign finance laws regulate interactions with government officials and sometimes public employees as
well. In addition to becoming familiar with the information in this Chapter, you should check with your GRD,
or team attorney about the relevant laws in your region, since the specific state or local laws applicable to
you may vary depending upon the state in which you work.
Under federal law, a “lobbyist” is any individual who is employed by Pfizer and has: (1) made more than
one “lobbying contact” within a three-month period; and (2) spends at least 20% of his or her time engaged
in lobbying for Pfizer in that three-month period.
This pertains only to Pfizer colleagues and not to independent contractors retained by Pfizer. A “lobbying
contact” is any oral or written communication, including e-mail, with certain executive and legislative branch
employees made with regard to federal legislation, a rule, regulation, or any other program, policy or
position of the U.S. Government. Affected executive and legislative branch employees include Members
of Congress and their staff, the White House, Secretary and Deputy Secretary positions within the federal
agencies, and some members of the military.
Most Pfizer colleagues do not qualify to be registered as lobbyists because they do not spend 20% of their
time “lobbying” during the reporting period (three-month intervals); however, it is important to remember
that even if you are not a “lobbyist,” federal law requires Pfizer to report your time spent supporting the
lobbying efforts of others within the Company.
Calculating Lobbying Contacts
I am a Public Affairs colleague. I called Congressman A’s office and spoke with a
member of his staff to request the congressman call me back. Two days later, the
congressman returned my call, and I explained I was calling about access to
medication for the elderly, and we set up a time to meet. Does this count as two
“lobbying contacts” for purposes of determining whether I am a lobbyist under federal
law? I thought requesting meetings did not count as lobbying?
This would likely count as one lobbying contact. The purpose of your first call was to
contact the congressman, which you were unable to do. On the second call, however,
you did speak with the congressman, and you explained the purpose of your call,
which was to discuss some aspect of federal law or policy. While you did call to set
up a face-to-face meeting, you also discussed policy issues during the telephone call.
Who Is a “Lobbyist?”

White Guide – Chapter 16: Federal Employee Interactions and Lobbying
Rev. 12/20
Page 298 of 405
The two telephone calls would be considered one lobbying contact and the in-person
meeting would count as a second lobbying contact.
Determining Time Engaged in Lobbying Activities
I am a Public Affairs colleague. From time to time, I call congressional staff members
and ask a series of prepared questions to gauge perceptions of healthcare issues or
policy perspectives. Does the amount of time I spend on those calls factor into the
20% threshold for registering as a lobbyist?
It depends. If the questions pertain to the status of legislation affecting Pfizer’s
interests, the calls may have been made in an effort to influence the congressional
members for whom the staff members work, and the calls therefore would be
considered lobbying contacts. If the questions constitute routine information-gathering
and there is not an attempt to influence a covered official, then the communications
will not amount to lobbying contacts. If you are unsure if your call would count towards
the 20% threshold, please consult your GRD or team attorney. Remember, even if
you do not qualify as a “lobbyist,” you still may need to keep track of your time spent
on some of these types of activities for the Company’s federal lobbying disclosure
report.
The LDA defines “lobbying activities” as lobbying contacts, as defined above, and any efforts in support of
these contacts, including preparation and planning activities, research, and other background work
intended for use in lobbying contacts. Reportable expenses include time spent by Pfizer colleagues in
meetings with federal officials for the purpose of influencing federal laws, regulations or policies, and
expenses incurred in connection with lobbying, such as expenses for travel, lodging or food. Pfizer is
required to file quarterly reports that provide a list of the specific issues that were addressed by “lobbying
activities” and an estimate of the total expenses incurred in connection with these lobbying activities.
Although most Pfizer colleagues do not qualify as “lobbyists,” the time Pfizer colleagues spend in supporting
the lobbying efforts of others within the Company is reportable, including:
• Developing “talking points” or “white papers” if they are used for lobbying purposes;
• Attending internal meetings or discussions regarding lobbying strategy (e.g., identifying federal officials
who should be targeted or developing and testing messages);
• Fees paid to outside consultants for analyses, studies, or reports, if they are used for lobbying;
• Negotiating contracts with government agencies;
• Providing educational information or materials to influence government formulary decisions; and
What Is Lobbying?

White Guide – Chapter 16: Federal Employee Interactions and Lobbying
Rev. 12/20
Page 299 of 405
• Promotional interactions with certain state hospital administrators or HCPs.
The federal definition of lobbying does not include:
• Drafting and developing comments to proposed regulations in a formal agency rulemaking proceeding;
• Representing Pfizer in an agency adjudicatory matter or criminal proceeding;
• Drafting legislation, regulations, or legal analyses (applicable to attorney work-product only);
• Preparing for and providing “on the record” testimony in a congressional or agency hearing;
• Communicating with government officials as part of Pfizer’s Grassroots advocacy program;
• Requesting a meeting with a congressional or agency official or his or her staff, if the request does not
include an attempt to influence the official; and
• Responding to a request by an official for reports, information, statistics, subpoenas, or similar
documents.
Pfizer’s Grassroots advocacy program works to inform and educate colleagues on public policy issues and
provide colleagues the opportunity to engage in policy debates by making their voices heard in Washington,
D.C. and state capitols across the country. There may be other activities developed by a State Action Team
(formerly called State Resource Team) or the Regional Council that involve interaction with government
officials or public employees and would be subject to the Pfizer policies in this Chapter.
To help ensure that Pfizer complies with all registration and reporting requirements, all of your interactions
with state government officials must be coordinated through a GRD. Interactions with federal government
officials must be coordinated through the Washington, D.C. Pfizer office. If calling on HCPs who work for
a state or federal facility or institution, check with your team attorney to find out whether your promotional
activities are considered “lobbying” in your state.
Lobbying Do's and Don'ts
Do
Don't
Provide only RC-approved educational materials
to government officials.
Discuss Pfizer products or specific Pfizer
activities.
Coordinate all your activities with government
officials through your GRD.
Spend more than one hour per week or four hours
per month, if at all, on lobbying activities related to
Pfizer business.
Report your lobbying activities as required.
Experiment or try something new without
checking with your GRD or team attorney.

White Guide – Chapter 16: Federal Employee Interactions and Lobbying
Rev. 12/20
Page 300 of 405
As discussed in this Chapter, the laws in the state in which you work will determine whether you are
engaged in “lobbying” activities which require Pfizer to register the time and expenses related to them.
If you have been engaged in federal “lobbying activities,” you must track and report the following on the
form available at http://ecf.pfizer.com/sites/LobbyingDisclosureReporting.
• A reasonable estimate of the time spent on lobbying activities, rounded to the nearest hour;
• A description of the specific activity;
• The policy topic(s) worked on; and
• Any expenses associated with these efforts.
You should fill out the form only when you have engaged in federal lobbying activity. Do not fill it out when
you have engaged in state lobbying activity (see the section on state-specific Laws below). The information
from the online form is collected for the Company’s quarterly federal LDA reports which are filed on April
20th, July 20th, October 20th, and January 20th of each year with both the U.S. House of Representatives
and the U.S. Senate. If you have engaged in federal lobbying activity during a reporting period,
please make sure you complete an online form no later than one week after the close of the reporting
period, or by April 7th, July 7th, October 7th, and January 7
th
,of that year.
Determining Time Engaged in Lobbying Activities
When I fill out Pfizer’s lobbying form, I have to include the issue that pertained to the
lobbying efforts I supported. If the work I did was about a particular Senate bill, can I
just write the bill number?
No, while the bill number must be reported under the law, the number alone is not a
sufficient description of the issue for purposes of disclosing Pfizer’s lobbying activity
and filing the federal report. You should try and be as specific as possible, and include,
in addition to the bill number, the bill’s name, the bill title and/or section heading if one
exists, and the specific provisions that were the subject of your work.
If ever in doubt, consult with a GRD, the Washington, D.C. Pfizer office, or your team attorney to
verify whether your activities subject you to registration or reporting requirements.
Reporting Lobbying Time and Expense

White Guide – Chapter 16: Federal Employee Interactions and Lobbying
Rev. 12/20
Page 301 of 405
There are two types of lobbying disclosure laws enacted by states that may require you to record and report
certain information. The first category is similar to the federal LDA and requires Pfizer to report on a regular
basis the lobbying activities undertaken in or directed towards a particular state. The second category
affects colleagues who meet with certain state officials or state employees.
States’ General Lobbying Disclosure Laws
Pfizer has a State Government Relations program which is active in almost all 50 states. As part of this
effort, certain Pfizer colleagues have registered as lobbyists and have reporting requirements similar to
those on the federal level. The laws differ in each state. Depending on the particular state law, if you
participate in Pfizer’s Grassroots advocacy programs and other interactions with state government officials
or public employees, Pfizer may be required to register you as a lobbyist or make certain disclosures about
your activities. If you have questions regarding whether your participation in state lobbying activities triggers
disclosure requirements, you should consult with the GRD responsible for the state. If the GRD determines
that you are required to disclose your activities, you will receive a compliance form or timesheet to complete.
Reportable lobbying activities and expenses may include:
• Meetings with government officials or staff;
• Time spent reviewing policy issues in preparation for a meeting with government officials;
• Time spent communicating, including by letter or e-mail, with government officials about policy issues;
and
• Any food, travel, lodging, or other expenses you may incur while engaged in lobbying activities.
State procurement or contract lobbying laws may also apply to you if you are involved with the sale of Pfizer
products to state institutions (such as public hospitals and state prisons) or their reimbursement through
state agencies (such as Medicaid). These laws seek to prevent inappropriate influence over state
employees responsible for purchasing products with taxpayer money.
While procurement and contract lobbying laws vary from state to state, most involve registering individuals
who interact with state officials regarding state purchase contracts and disclosing lobbyist compensation
and lobbying expenses incurred, such as meals (food and beverage), travel, and lodging. To ensure
appropriate tracking and disclosure, check with a GRD or your team attorney before engaging in these or
related activities.
State-Specific Laws

White Guide – Chapter 16: Federal Employee Interactions and Lobbying
Rev. 12/20
Page 302 of 405
States’ Lobbying Laws Impacting Marketing
Several states have enacted laws that require pharmaceutical representatives who interact with state
officials or state employees to register with the state and report their “lobbying” expenditures. In particular,
numerous states have laws under which marketing activities involving Medicaid Pharmaceutical
and Therapeutics Committee members may be considered lobbying. For example, when certain
threshold limits are met, Louisiana requires pharmaceutical representatives to register with the Board of
Ethics and file semi-annual reports detailing expenditures as they relate to marketing activities directed
towards members of the Medicaid Pharmaceutical and Therapeutics Committee.
In Colorado, an amendment to the Colorado Constitution prohibits individuals considered lobbyists from
giving anything of value, including gifts and meals, to government employees. Various other states, and
even counties, also have lobbying registration and disclosure requirements (e.g., New York and Miami-
Dade County, Florida). To ensure that expenses and interactions are properly tracked, please consult with
the relevant team attorney before engaging in any marketing interactions with state or local government
employees.
State Restrictions on Gifts to Legislators
Many states place restrictions on gifts from the general public and lobbyists to legislators. These range
from a general prohibition to specific dollar limits. The link below outlines some of these restrictions at
http://www.ncsl.org/research/ethics/50-state-table-gift-laws.aspx. There are differences in what a lobbyist
can provide to a legislator and what a legislator can receive from the public, a lobbyist or an outside interest.
Consult your team attorney for specific restrictions.
State Formularies
Attempts to influence state formulary decisions are currently considered lobbying in many states. As a
result, registration and/or reporting may be required. If you are interacting with members of a state
committee or agency that make decisions with respect to their state’s formulary you should check with the
GRD with responsibility for that state prior to those interactions to determine whether any of your activity
could be considered lobbying.
Every Pfizer colleague is responsible for adhering to Pfizer’s policies regarding lobbying
registration and disclosure. Non-compliance with these policies puts the Company at risk and can
subject Pfizer colleagues to disciplinary action up to and including termination.

White Guide – Chapter 16: Federal Employee Interactions and Lobbying
Rev. 12/20
Page 303 of 405
It is important to understand the difference between lobbying and grassroots advocacy efforts and
campaign contributions. Lobbying and grassroots advocacy efforts are intended to influence government
policy. Campaign contributions are intended to influence campaigns and elections.
While corporations like Pfizer are permitted to lobby government officials, federal and various state laws
prohibit corporations from making financial contributions to support a candidate’s election. This prohibition
applies to both monetary and “in kind” donations, such as employee time and the use of corporate resources
on behalf of a campaign committee.
In addition, federal and state anti-bribery laws impose criminal penalties for offering gifts or campaign
contributions to government officials in exchange for a change in policy, entering into a federal or state
contract, or agreeing to engage in any other official act.
For this reason, you are prohibited from discussing past, present, or future campaign contributions
with a government official or public employee.
Corporations are not allowed to make direct contributions to any candidates running for federal office, and
similar restrictions may apply in certain states as well. However, corporations can sponsor political action
committees (PACs), which are supported by voluntary contributions from eligible employees. These
corporate-sponsored PACs can then contribute directly to candidates running for federal office and for state
office where applicable. A PAC is subject to federal laws and regulations, reporting requirements, and
monetary limits on campaign contributions.
Pfizer sponsors a PAC. The Pfizer PAC is a non-partisan committee that supports candidates who value
biopharmaceutical innovation and are open to real dialogue on issues that affect patient access to
medicines. For more information on the Pfizer PAC, please visit
http://sharepoint.pfizer.com/sites/USGovernmentRelations/SitePages/Home_New.aspx.
Before interacting with any federal or state government official or public employee in a way not described
here, seek guidance from a GRD, the Washington, D.C. Pfizer office, or your team attorney.
Campaign Contributions
The Pfizer Political Action Committee

White Guide – Chapter 16: Federal Employee Interactions and Lobbying
Rev. 12/20
Page 304 of 405
Leaving Educational Items with Public Employees
If I leave RC-approved, nominally priced educational (PhRMA Code compliant) items
with an HCP at a federal prison, do I have to track it? What about a state prison
system?
Yes. Under Pfizer’s HCP Payment Disclosure Policy, educational items valued $10 or
more must be disclosed and items valued less than $10 may also be subject to
disclosure so all items must be tracked for reporting purposes. Also, a reporting
obligation may be triggered under applicable state law. Because state laws differ by
state, it is imperative that you check with your team attorney before leaving any item
with an HCP at a state prison.
HCPs Who Sit on State Formulary Committees
One of the physicians I call on also happens to sit on a state formulary review
committee. If I am calling on this physician to discuss his private practice only, and
not his role on the state formulary review committee, must I treat him differently than
any other physician who does not sit on a formulary committee?
Maybe. The extent to which HCPs who sit on state formulary committees can interact
with pharmaceutical representatives varies widely, depending on the specific laws in
your state. Check with the relevant team attorney to ensure your interactions are
compliant with applicable state law.
• Lobbying questions may be referred to the relevant GRD, the Washington, D.C. Pfizer office, or team
attorney.
• For more information on state specific laws, see Chapter 17: State Laws: HCP and State Employee
Restrictions.
• For more information on Pfizer’s HCP Payment Disclosure Policy, see Chapter 18: Meals, Educational
Items, Greenstone Giveaways, and HCP Payment Disclosure.
• For more information on Pfizer’s educational grant process refer to GNT01-GSOP 1.0 “Independent
Medical Grants”.
For More Information
General

White Guide – Chapter 16: Federal Employee Interactions and Lobbying
Rev. 12/20
Page 305 of 405
• For more information about the Pfizer PAC, visit
http://sharepoint.pfizer.com/sites/USGovernmentRelations/SitePages/Home_New.aspx.
• Take the online training module (training module for the online form) on how to complete the federal
Lobbying Disclosure form.
• Federal Employee Interaction questions may be referred to your lead BU National Account Manager or
team attorney.

Rev. 12/20
Page 306 of 405
CHAPTER #17 – PUBLICATIONS

White Guide – Chapter 17: Publications
Rev. 12/20
Page 307 of 405
Chapter
#17
PUBLICATIONS
CONTENTS
Introduction................................................................................................................................................ 308
Key Points to Ensure Compliance ...................................................................................................... 309
Publication Planning .................................................................................................................................. 310
Authorship and Disclosure ........................................................................................................................ 311
Compendia ................................................................................................................................................ 312
Payments to Authors and Contracting with Authors ................................................................................. 312
Supplements ............................................................................................................................................. 313
Publication of ISR Study Results .............................................................................................................. 314
For More Information ................................................................................................................................. 314
Publications

White Guide – Chapter 17: Publications
Rev. 12/20
Page 308 of 405
Chapter #17 Publications
Pfizer supports the timely submission of manuscripts associated with Pfizer-sponsored clinical studies
whether the results are positive, negative or neutral. Pfizer also supports other types of publications, such
as abstracts, congress posters and presentations, review articles, manuscripts, including those coming
from health economics/outcomes research studies, and abstract and manuscript Plain Language
Summaries (PLS).
This Chapter summarizes the policies and procedures for managing Pfizer-supported publications,
including author selection, informing external authors of Pfizer’s publication policies, payments to authors,
contracts with authors, publication development and disclosure of Pfizer support.
Publications subject to the requirements of this Chapter include:
• Submissions to peer-reviewed medical and scientific journals, such as primary and secondary
manuscripts from Pfizer-sponsored clinical studies, review articles, and letters to the editor;
• Submissions to scientific congresses such as abstracts, posters, and presentations;
• Book chapters; and
• Publications (e.g., publications based on epidemiology analyses, surveillance studies, health
economics and outcomes research) that mention a Pfizer product or are in support of a Pfizer
product disease state/therapeutic area reviews.
Note that non-clinical publications are separately addressed by the Non-Clinical Disclosure Guideline
available on the R&D Compliance CNTR here.
Pfizer colleagues, external authors, and vendors (e.g., publications agencies and medical writers)
who are involved with Pfizer-supported publications must understand and follow Pfizer’s
publications policies. Non-compliance with these policies puts the Company at risk and can subject
Pfizer colleagues to disciplinary action up to and including termination of employment.
Introduction

White Guide – Chapter 17: Publications
Rev. 12/20
Page 309 of 405
Pfizer publications are developed to support the safe and appropriate use of a Pfizer product and are not
intended to encourage the use of a Pfizer product in order to impact prescribing, purchase or
recommendation. Pfizer publications fulfill the Company’s commitment to the truthful, accurate, and
objective disclosure of data from Pfizer-sponsored or collaborative clinical studies in a timely manner,
•
Publications are not marketing tools
. While they may eventually be used in a promotional
context, the planning and development of publications must be based on medical and
scientific needs and must be independent of commercial and brand strategies.
• All members of a Publications Sub-Committee (PSC)
must understand their roles and
responsibilities and the applicable Pfizer policies.
•
Commercial, marketing and sales colleagues must not be involved in the funding, preparation,
planning, review or content development of publications, and must not influence or attempt to
influence the publication planning process or content of publications.
• The selection of authors must be consistent with the
International Committee of Medical
Journal Editors (ICMJE) authorship criteria (http://www.icmje.org) and
the Pharmaceutical
Research and Manufacturers of America (PhRMA)
Principles on Conduct of Clinical Trials
and Communication of Clinical Trial Results.
•
Pfizer colleagues must be listed as authors if they satisfy the four ICMJE criteria for
authorship. General supervision of a research group that is conducting or supervising a
project, or solely performing data analysis, is not sufficient for authorship.
•
Pfizer does not compensate authors who are investigators in a Pfizer-sponsored study for
work associated with the preparation of the primary abstract, congress presentation, or
manuscript regarding that study.
• In those rare circumstances where Healthcare Providers (HCPs) or
Healthcare Institutions
(HCIs)
are paid to author or produce publications, Pfizer must ensure that payments are
consistent with Fair Market Value (FMV)
determination and other applicable requirements of
Pfizer policy, including
CP #207: Global Policy on Interactions with Healthcare Professionals
(GPIHP) and My Anti-Corruption Policies and Procedures (MAPP)
. In addition, Pfizer must
contract directly with HCPs for work on publications; a third-party may not contract with an
HCP on Pfizer’s behalf.
Key Points to Ensure Compliance

White Guide – Chapter 17: Publications
Rev. 12/20
Page 310 of 405
regardless of whether or not the results are favorable to Pfizer. Specific timelines apply to submission of
primary manuscripts disclosing the primary end point(s) results of Pfizer-sponsored interventional clinical
studies in patients and preventive interventional studies in healthy subjects (e.g., prophylactic vaccine
studies) to a peer-reviewed journal.
Pfizer colleagues must ensure that any engagement of HCPs or HCIs to author or develop publications
does not give rise to inappropriate financial relationships with, or influence over, those HCPs or HCIs.
Importantly, the process by which authors are selected and compensated, if not structured appropriately,
may violate federal or various states’ anti-kickback statutes. For example, if an HCP is being paid to author
a publication, but in reality, is not actually contributing or performing any author responsibilities, the
government might question whether the HCP was chosen and/or paid as an inducement for his or her
continued or increased prescribing of a Pfizer product. Even if an HCP has contributed substantially to the
development of a publication, it could still raise questions whether any compensation received was based
on FMV or could be viewed as a potential kickback. Conversely, omitting an individual’s name as an author
on a scientific article, when the individual’s contribution satisfied the ICMJE criteria, may be viewed as a
form of research misconduct.
Publications supported by a Pfizer product team are managed by the product’s multidisciplinary PSC which
is responsible for developing and implementing the publication plan. The PSC’s purpose is to ensure that
clinical study results are published in a timely manner, identify gaps in medical knowledge about the product
and determine whether existing science can address those gaps through a Pfizer-supported publication,
and ensure publication integrity and compliance with Pfizer publication policies and procedures.
The PSC is chaired by the Clinical/Medical Lead responsible for overseeing the publication program for a
product and may include other Medical and Clinical colleagues (who can also be ad hoc members), a
Biostatistician, and a Publications Specialist. Commercial, Marketing and Sales Colleagues are not
permitted to be members of the PSC.
In addition, Commercial, Marketing and Sales Colleagues are not permitted to:
Attend PSC meetings and receive PSC meeting minutes;
Influence the decision-making process as it relates to publication planning;
Make decisions regarding prioritization of publications;
Select or suggest authors or scientific congresses and journals;
Make decisions regarding the order of authors’ names on the by-line;
Publication Planning

White Guide – Chapter 17: Publications
Rev. 12/20
Page 311 of 405
Author a medical or scientific publication;
Review or comment on draft publications;
Contract with a vendor for publications or pay a vendor for publications; and
Liaise with authors or vendors to discuss publications.
Pfizer has adopted the authorship criteria established by the ICMJE as well as the Pharmaceutical
Research and Manufacturers of America (PhRMA) Principles on Conduct of Clinical Trials and
Communication of Clinical Trial Results, link. In accordance with the ICMJE guidelines, authors must meet
all four of the following conditions:
• Substantial contributions to the conception or design of the study, acquisition of data, or analysis
and interpretation of data; and
• Drafting the publication or revising it critically with respect to important intellectual content; and
• Final approval of the version to be published; and
• Agreement to be accountable for all aspects of the work in ensuring that questions related to the
accuracy or integrity of any part of the work are appropriately investigated and resolved.
Only individuals who meet all 4 of the ICMJE criteria should be named as authors on Pfizer publications
and all those who fulfill these criteria should be named in the byline. Any Pfizer employee who meets the
criteria for authorship should be listed as an author. Those who do not meet the criteria for authorship, but
have contributed in some way to the publication may be acknowledged elsewhere, as appropriate.
Pursuant to the ICMJE criteria, general supervision of the research group that is conducting or supervising
a project is not sufficient for authorship. Participation solely in collection or analysis of data also does not
justify authorship; ̶ substantial involvement in drafting or revising the publication is required. All individuals
providing editorial and medical writing support must work under the direction of the authors.
A former or current member of a study’s Data Monitoring Committee (DMC) cannot author a publication,
even if the study has been completed and the DMC disbanded. This is because DMC members have
privileged access to unblinded interim data and other safety and efficacy-related information. Therefore, to
avoid actual or perceived bias in publications related to the study, DMC members may not serve as authors.
In addition to the ICMJE criteria, authors of a Pfizer-supported publication must ensure that development
of a publication is consistent with journal or congress guidelines, including applicable disclosure obligations.
Further, the authors should obtain and adhere to the publisher’s requirements for acknowledging financial
and material support and any other actual or perceived conflicts of interest. Authors must acknowledge in
Authorship and Disclosure

White Guide – Chapter 17: Publications
Rev. 12/20
Page 312 of 405
the publication all those who provided editorial support, the funding source, and the author’s relationship
with Pfizer. Authors must also determine the content and type of publication, the order of names on the
byline, and where the publication will be submitted. All authors should be given a reasonable amount of
time to review and approve a proposed publication.
Clinical and Medical Controlled Document CMCD CT37-GSOP: Development of Pfizer Publications
includes specific recommended wording for disclosure/acknowledgement statements in a variety of
situations. For example, where a publication reports the results of a Pfizer-sponsored study, the statement
should read, “This study was sponsored by Pfizer Inc.”
Development of publications supported by Pfizer Country offices (i.e., local publications) that do not come
from a Pfizer-sponsored interventional clinical study or non-interventional post-authorization safety study
(NI-PASS) should follow the process detailed in CT37-SOP-PCO01: Development of Pfizer Regional
Offices and Country Offices Publications.
Either prior to or during development of a publication, the Pfizer Publication Owner (PPO), or designee,
is responsible for ensuring that an External Author Letter is sent to each potential external author that
describes, and requests written acknowledgment of, Pfizer’s policies on authorship and disclosure. In
addition, prior to submission of a manuscript or abstract reporting the results of a Pfizer-sponsored
interventional study or NI-PASS, Pfizer requires the completion of a Data Checklist to help ensure the
quality of the underlying data. The PPO, study statistician and Publications Specialist must also perform a
Final Check to confirm that the manuscript is compliant with Pfizer’s policy and that the data are accurate
and support the statistical interpretation.
Generally, Pfizer does not actively engage with compendia regarding Pfizer products. Colleagues in U.S.
Medical Information who receive an information request from an External Drug Compendium to review a
product monograph may review the document for accuracy and completeness. All other Pfizer colleagues
who receive an information request from an External Drug Compendium should consult with the relevant
Product Counsel prior to responding.
In addition, colleagues in U.S. Medical Information may be notified of, or independently identify, inaccurate
or incomplete product information (e.g., errors in dosages, omission of safety information) in External Drug
Compendia product monographs. In these cases colleagues may proactively inform the External Drug
Compendia of any such errors pursuant to the Guidance Document: Contacting External Drug Compendia.
Any other Pfizer colleague that identifies or is made aware of any errors should notify the U.S. Medical
Information colleague responsible for the relevant product.
Payments to Authors and Contracting with Authors
Compendia

White Guide – Chapter 17: Publications
Rev. 12/20
Page 313 of 405
Pfizer does not pay investigators of a Pfizer-sponsored interventional or non-interventional study for work
associated with the preparation of the primary abstract, scientific congress poster/presentation, manuscript
or abstract or manuscript PLS for the study. However, Pfizer may pay authors, including investigators, for
the development of publications such as secondary publications, post-hoc analyses, meta-analyses and
review articles.
Pfizer publication activities involving payment to an author must have a documented business rationale
prior to engaging with an HCP for work on the publication. The business rationale must include specific
details about the publication activities to be performed (e.g., a description of the proposed work to be done,
the type of work product to be generated, and the purpose of the work). Publication activities related to an
FDA-approved product require legal approval of the business rationale. Agreements for publication
activities must include the applicable contract language. Engagement Owners should consult with their
contracting lead for the required language.
External authors who will be compensated for their work on a Pfizer publication must enter into a written
agreement describing the scope of work to be performed, the fees to be paid in connection with the
publication, and the compliance obligations of the authors, including representations that they will adhere
to the authorship criteria and disclosure obligations described above, before work on the publication begins.
For payments related to the development of publications, Pfizer must contract with and make payments to
HCPs and HCIs directly. A vendor may not contract with, and may not make payments to, an HCP or
HCI on Pfizer’s behalf.
All payments to authors must be in accordance with a centrally managed, pre-set rate structure that is
determined based on FMV analysis conducted by Pfizer, and all payments to HCPs or HCIs must be
recorded and disclosed pursuant to governmental and other transparency requirements.
Note that Pfizer does not compensate authors for their time presenting a poster or an oral presentation at
a congress or similar meeting. However, Pfizer may provide authors of Pfizer publications with funding for
registration and reasonable travel expenses associated with such presentations. Congress presenter travel
is managed by Pfizer’s North America Customer Engagement & Events (NA CE&E) group. The relevant
Publications Specialist should be made aware of such activities.
Journal supplements are collections of papers that deal with related issues or topics. They may be
published as part of a regular issue of a journal or as a separate issue, and generally are funded by sources
other than the journal’s publisher. Pfizer-funded supplements are permitted under Pfizer policy. However,
because supplements are a paid communication mechanism, they are governed by promotional standards
and must comply with REG08-POL Requirements for the Content and Approval of Promotional Activities
Supplements

White Guide – Chapter 17: Publications
Rev. 12/20
Page 314 of 405
and/or Materials. As a result, supplements must be consistent with the product label and cannot contain
information about products or indications that are not yet approved. To ensure compliance with this
restriction, an overview or synopsis of the supplement must be reviewed and approved by the Product
Counsel prior to contracting. The business rationale process must also be completed if an HCP will be
engaged to develop the supplement. All contracts must ensure that Pfizer has the final decision on the
supplement’s content. In addition, unlike other types of publications, supplements must be reviewed by
the relevant product Review Committee (RC) prior to journal submission. It is the PPO’s responsibility
to ensure the PSC-reviewed supplement is not submitted to the journal without RC approval. In the RC
meeting, Marketing also has an opportunity to review the supplement.
As with publications related to the results of Pfizer-sponsored studies, Pfizer supports the exercise of
academic freedom and encourages investigators to publish the results of an Investigator-Sponsored-
Research study (ISR) or Clinical Research Collaboration (CRC), whether the results are favorable for a
Pfizer product. In our contracts, Pfizer requests an opportunity to review proposed publications or other
public disclosures of such studies prior to publication. Pfizer also expects the investigator or institution to
comply with recognized ethical standards concerning publications and authorship, including the disclosure
of Pfizer support of the study in any publication of study results.
Support for and management of ISRs, and CSCs and any subsequent publications (as well as publications
related to Pfizer-sponsored clinical studies) is further described in White Guide Chapter 9: Pfizer-Sponsored
and Non-Sponsored Clinical Research Including Investigator-Sponsored Research Studies (ISRs).
• CP #402: Scientific and Technical Publications and Presentations
• CP #207: Global Policy on Interactions with Healthcare Professionals (GPIHP)
• My Anti-Corruption Policies and Procedures (MAPP)
• CMCD CT20-POL: Public Disclosure of Pfizer Clinical Study Data and Authorship
• CMCD CT37-GSOP: Development of Pfizer Publications
• CT37-SOP-PCO01: Development of Pfizer Regional Offices and Country Offices Publications
• ICMJE Guidelines on Authorship and Contributorship
• PhRMA Principles on Conduct of Clinical Trials and Communication of Clinical Trial Results
• Guidance Document: Contacting External Drug Compendia
Publication of ISR Study Results
For More Information

White Guide – Chapter 17: Publications
Rev. 12/20
Page 315 of 405
• White Guide Chapter 9: Pfizer-Sponsored and Non-Sponsored Clinical Research Including
Investigator-Sponsored Research Studies (ISRs)
• Refer any other questions or concerns to a member of Pfizer’s Publications Management Team.
Click here for the Publications Management Team contact list.

Rev. 12/20
Page 316 of 405
CHAPTER #18 – MEALS,
EDUCATIONAL ITEMS,
GREENSTONE GIVEAWAYS, AND
HCP PAYMENT DISCLOSURE

White Guide – Chapter 18: Meals, Educational Items,
Greenstone Giveaways, and HCP Payment Disclosure
Rev. 12/20
Page 317 of 405
Chapter
#18
MEALS, EDUCATIONAL ITEMS,
GREENSTONE GIVEAWAYS, AND
HCP PAYMENT DISCLOSURE
CONTENTS
Introduction................................................................................................................................................ 318
Key Points to Ensure Compliance ...................................................................................................... 319
Meals to HCPs .......................................................................................................................................... 320
General Rules and Restrictions .......................................................................................................... 320
Meals Provided by Field Sales Colleagues and Their Immediate Managers ..................................... 322
Meals Provided by Senior Sales Colleagues, Headquarters Colleagues, and Account
Management Colleagues Permitted to Host Out-Of-Office Meals ..................................................... 326
Legitimate Business Reason .............................................................................................................. 326
Meal Planning & Execution ................................................................................................................. 327
Attendance by Other Colleagues at Meals Hosted by Senior Sales, HQ, and Account
Management Colleagues .................................................................................................................... 327
Business Meals Provided by Greenstone and Sterile Injectables Colleagues ......................................... 329
Educational Items to HCPs ....................................................................................................................... 331
Greenstone Giveaway Items ..................................................................................................................... 332
HCP Payment Disclosure Policy ............................................................................................................... 332
Overview ............................................................................................................................................. 332
Items Included in Reporting ................................................................................................................ 333
Disclosure of the Value of Meals ................................................................................................. 333
Disclosure of the Value of Educational Items and Non-Disclosure of Patient Materials ............. 334
Recording Disclosable Payments and Items ...................................................................................... 334
Opting Out of Receiving Disclosable Items ........................................................................................ 335
Access and Use of Open Payments and other Transparency Data for Analytics ....................... 336
For More Information ................................................................................................................................. 337
Meals, Educational Items, Greenstone Giveaways,
and HCP Payment Disclosure

White Guide – Chapter 18: Meals, Educational Items,
Greenstone Giveaways, and HCP Payment Disclosure
Rev. 12/20
Page 318 of 405
Chapter #18 Meals, Educational Items, Greenstone
Giveaways, and HCP Payment Disclosure
The Pharmaceutical Research and Manufacturers of America (PhRMA) Code on Interactions with
Healthcare Professionals (PhRMA Code) provides that occasional meals may be offered to U.S.
Healthcare Professionals (HCPs). In addition, it allows colleagues to give occasional approved
educational items to U.S. HCPs if the items are valued at $100 or less.
As of August 1, 2013, pharmaceutical manufacturers operating in the United States are required to report
to the government payments and other transfers of value made to U.S.-licensed physicians and teaching
hospitals in accordance with the “Sunshine Act” or “Open Payments” provisions. Starting January 1, 2021,
reporting expands under the SUPPORT Act to also include payments and transfers of value provided to
the following HCPs:
• Physician Assistant
• Nurse Practitioner
• Clinical Nurse Specialist
• Certified Registered Nurse Anesthetist
• Certified Nurse Midwife
Certain state laws and federal institutions create additional restrictions and disclosure obligations (broader
than the Sunshine Act) regarding payments and other items provided to U.S. HCPs, as described in the
State Laws: HCP and State Employee Restrictions Chapter 17 and the Federal Employee Interactions and
Lobbying Chapter 4 in this Guide. HCP payment disclosure is just one of the many ways Pfizer is fulfilling
its commitment to increased transparency and public candor.
This Chapter addresses Pfizer policies regarding the provision and disclosure of meals, educational
items, or other items of value to U.S. HCPs or certain institutions. Non-compliance with these
policies puts the Company at risk and can subject Pfizer colleagues to disciplinary action up to and
including termination of employment.
Introduction

White Guide – Chapter 18: Meals, Educational Items,
Greenstone Giveaways, and HCP Payment Disclosure
Rev. 12/20
Page 319 of 405
•
Pfizer’s Field Force T&E Expense Procedure can be found on MyPfieldNet.
•
Except where restricted by law or Pfizer policy, a Pfizer colleague may provide food and
beverage to HCPs if the value is modest by local standards. For out-of-office meals, the
total cost cannot exceed $135 per attendee, including tax, tip, and delivery charges. For
in-office or in-hospital meals, the total cost, including tax, tip, and delivery charges, may not
exceed $40.
•
When an educational or promotional presentation includes a modest meal, the meal must
never be the primary focus of the interaction – it should be incidental to the dissemination
of approved information and must comply with the PhRMA Code.
•
The PhRMA Code prohibits non-educational items from being offered to U.S. HCPs or
members of their staff. Accordingly, only Pfizer Review Committee-approved (RC-
approved) educational items may be provided to HCPs and their staff.
•
Pfizer’s payment disclosure policy applies to payments, meals, snacks, reimbursable travel
expenses, approved educational items, and other transfers of value provided to HCPs.
Pfizer also discloses payments to certain institutions, as well as payments related to clinical
research, which are attributed to the principal investigators.
•
Certain state laws and federal institutions (e.g., VA/DoD) also limit and/or require the
disclosure of payments and items of value provided to HCPs. These laws and restrictions
are described in the State Laws: HCP and State Employee Restrictions Chapter and the
Federal Employee Interactions and Lobbying Chapter in this Guide. Additional information
is also available on Global Policy Xchange on GCO On Demand
under the State Healthcare
Law Compliance tab and on MyPfieldNet under the Compliance tab.
•
Licensed prescribers in Minnesota or licensed HCPs in Vermont, or employees of a
Vermont HCP, may not be invited to any speaker program (in-office or out-of-office) if food
will be provided.
•
Other reportable HCPs, incuding physicians, have the option to “opt out” of eating a meal
at a speaker program where a meal is provided, in which case the value of the meal will not
be reported for them.
Key Points to Ensure Compliance
White Guide – Chapter 18: Meals, Educational Items,
Greenstone Giveaways, and HCP Payment Disclosure
Rev. 12/20
Page 320 of 405
General Rules and Restrictions
Pfizer policy and the PhRMA Code permit colleagues to provide meals to U.S. HCPs on occasion in
appropriate circumstances – such as meals in connection with informational presentations or discussions
providing scientific or educational value – so long as (1) the meal is modest as judged by local standards,
(2) the meal is never the primary focus of the interaction, and (3) the presentation occurs in a venue and
manner conducive to informational communication. Recreational and entertainment venues are prohibited.
In addition, under Pfizer policy, out-of-office meals to U.S. HCPs cannot exceed $135 per attendee
(including the cost of food, beverage, tax, tip, and delivery charges) and meals in an in-office or in-
hospital setting cannot exceed $40 (including food, beverage, tax, tip, and delivery charges). Any
pre-dinner food or beverages must be included in the $135 cap and reported for purposes of the Sunshine
Act. In addition, solely providing alcoholic beverages or excessive amounts thereof is prohibited and not
considered conducive to a business discussion. No other expenses (e.g., room fees) may be paid to the
office or hospital in connection with meals conducted in an in-office or in-hospital setting. Meal costs for
• HCPs may permanently “opt out” of being offered meals, snacks, or educational items by
contacting PTI@Pfizer.com
. If an HCP has permanently “opted out” but nonetheless
accepts payments, meals, or other disclosable items of value from Pfizer, they will be
subject to disclosure. Disclosures pursuant to the Sunshine Act are posted on the Open
Payments website maintained by CMS at http://www.cms.gov/OpenPayments/index.html.
•
Colleagues who interact with HCPs are responsible for verifying their “opt out” status. Sales
Colleagues should consult the HCP profiles on Veeva CRM to view an HCP’s “opt out”
status. A permanent “opt out” list, accessible to all colleagues, is also available on
Global
Policy Xchange on GCO On Demand.
•
Colleagues must correctly record in the applicable finance and payment system(s)
information necessary to identify institutions and HCPs and the payments or items of value
provided to them.
•
In-scope payments or other transfers of value provided to U.S.-licensed HCPs and certain
U.S. institutions through external parties, such as
Contract Research Organizations
(CROs) and Contract Sales Organizations (CSOs), are also subject to disclosure.
Key Points to Ensure Compliance
Meals to HCPs

White Guide – Chapter 18: Meals, Educational Items,
Greenstone Giveaways, and HCP Payment Disclosure
Rev. 12/20
Page 321 of 405
meals with HCP attendees may not be split or divided between internal colleagues or with individuals who
are employed by co-promote partners.
The PhRMA Code restrictions on out-of-office meals apply only to sales representatives and their
immediate managers. If and when Pfizer colleagues are permitted to provide meals to HCPs varies based
on each colleague’s role, but always requires a legitimate business reason. The table below provides a
high-level summary:
Host
restaurant
meals?
Host
in-office
meals?
Host
in-hospital
meals?
Host
speaker
program
meals?
Host meals at
conventions?
Sales Representative
No
Yes
Yes
Yes
No
District Manager
No
Yes
Yes
Yes
No
Regional Business
Director, Regional
President, National
Sales Lead
Yes
Yes
Yes
Yes
Yes
Greenstone and
Sterile Injectables
Please see Business Meals Provided by Greenstone
and Sterile Injectables Colleagues Section
Account Manager,
including AD, DE,
KAM, VAM, ADM (only
if such colleague
does not directly
supervise Sales
representatives)
Only for non-
HCPs or
HCPs who
do not
regularly
treat patients
or fill
prescriptions
Yes
Yes
Yes
Only for non-
HCPs or
HCPs who do
not regularly
treat patients
or fill
prescriptions
HQ Marketing/Medical
Yes
Yes
Yes
Yes
Yes
Several states and the U.S. Department of Veterans Affairs (VA)/Department of Defense (DoD) also
impose meal limitations and reporting requirements that are stricter than the PhRMA Code and/or our Pfizer
policy. See White Guide Chapter 15 and Chapter 4 of the Orange Guide for more information. You cannot
provide any food or other support in connection with an accredited continuing medical education activity
(ACCME, ACPE, or ANCC). Note that “medical education” is not limited to medical education for physicians
but also includes education for other HCPs, including pharmacists. Any type of financial support for
accredited continuing education, including payment for event expenses or meals, must be funded through
White Guide – Chapter 18: Meals, Educational Items,
Greenstone Giveaways, and HCP Payment Disclosure
Rev. 12/20
Page 322 of 405
an independent professional education grant. Requests for these grants should be sent by the requestor
through Pfizer’s Global Medical Grants.
Before providing any meals or other items of value to HCPs, colleagues should refer to the State Laws:
HCP and State Employee Restrictions Chapter and the Federal Employee Interactions and Lobbying
Chapter in this Guide. To determine whether an HCP is licensed in Massachusetts, Minnesota, New Jersey
or Vermont, Sales Colleagues should consult the physician profiles on Veeva CRM, and other colleagues
should search the HCP Lookup Tool. Additional information on state law restrictions and other tools are
available under the State Healthcare Law Compliance tab on GCO Policy Xchange on GCO on Demand.
Account Manager Out-of-Office Meals with HCPs
Can a KAM host an out-of-office meal with an HCP who serves as the medical director
of a hospital system?
It depends. Account Managers such as KAMs can provide out-of-office meals to an
HCP who is not regularly treating patients. For pharmacists, to be eligible for an out-
of-office meal with a colleague who is permitted to host, they must be not regularly
filling patient prescriptions. Typically, an HCP or pharmacist who treats patients or fills
prescriptions one day per week (i.e., approximately 20%) or less is not “regularly
treating patients.” As always, there must be a legitimate business reason (related to
the HCP’s responsibilities outside of treating patients) for meeting over a meal, and
the interaction must be conducted in accordance with the provisions of this Chapter,
including any other state law or restriction.
Meals Provided by Field Sales Colleagues and Their Immediate Managers
Meals provided to U.S. HCPs by Sales representatives and their immediate managers in connection with
informational presentations must be limited to in-office and in-hospital settings. The only times a Sales
representative or their immediate manager may provide out-of-office meals to HCPs are during in-person
Pfizer speaker programs where trained speakers (typically contracted external HCPs) present RC-
approved information about Pfizer products, disease states, or other healthcare topics, using content
controlled by Pfizer. For more information about speaker programs, see Orange Guide Chapter 9: Speaker
Programs for HCPs and White Guide Chapter 4: Marketing Programs. In accordance with the Statement
on Application of PhRMA Code Section 2 During Emergency Periods, it may be permissible for colleagues
to provide in-office meals to appropriate attendees as an ancillary offering to virtual informational
presentations or virtual speaker programs conducted during traditional mealtimes. Colleagues should follow
the appropriate guidance in the ‘Working Virtually’ section of MyPfieldNet.

White Guide – Chapter 18: Meals, Educational Items,
Greenstone Giveaways, and HCP Payment Disclosure
Rev. 12/20
Page 323 of 405
It is inappropriate for a Pfizer colleague to include an HCP’s spouse or other guest in any Pfizer-provided
meal, unless the spouse or guest is otherwise an appropriate attendee under Pfizer policies.
It is never appropriate for a Pfizer colleague to offer “take-out” meals or meals to be eaten without the Pfizer
colleague present or virtually present under certain circumstances. Meals must be incidental to the
provision of informational presentations and discussions. Therefore, only individual HCPs and office staff
members who have a role in patient care and engage in an educational discussion with the Pfizer colleague
can partake in the meal. For this reason, and to ensure proper reporting for disclosure purposes, Pfizer
colleagues should instruct HCPs and their staff not to unwrap or consume meals provided by Pfizer prior
to the arrival of a Pfizer colleague.
Meals where you anticipate a large number of attendees (e.g. >15 attendees), including virtual
presentations, may require additional pre-planning and discussion with your manager about logistics to
ensure there is a meaningful opportunity to engage in educational discussions with all HCPs and office
staff members who partake in the meal, and to ensure accurate disclosure. Every office operates in a
different way, so you should identify the precise circumstances you will encounter and how to best manage
the meal. You should consider inviting another Pfizer colleague to assist or conduct a single presentation
to provide education to all attendees at the same time, while ensuring that they can see and hear a fair and
balanced presentation. You are not permitted to conduct a meal if you cannot ensure there will be a
meaningful opportunity to provide information or education to all attendees who partake in the meal.
“Meals” Defined
Does taking an HCP out for a cup of coffee constitute a meal?
No. Under Pfizer policy, food or beverage items of nominal value ($10 per attendee
or less) – such as coffee, other non-alcoholic beverages, or pastries, are considered
a snack and not considered a meal. Pfizer policy permits a Sales representative or
their immediate manager to make an occasional educational presentation to an HCP
out of the HCP’s office or hospital (such as in a coffee shop near the HCP’s office),
along with offering a snack (not a meal), in circumstances where meals are not
permitted in an in-office or in-hospital setting, unless further restricted by state law or
other laws or policies. Offering a snack (as defined above) out of an HCP’s office or
hospital should be limited to only one or two HCPs at a time.
In all cases, the value of any food or beverages provided to a U.S.-licensed physician,
regardless of amount, is potentially subject to the requirements of the State Laws
Chapter. In addition, these activities may also require public disclosure by Pfizer.
Thus, the Pfizer colleague providing the item of value must properly record the
expense as described later in this Chapter.

White Guide – Chapter 18: Meals, Educational Items,
Greenstone Giveaways, and HCP Payment Disclosure
Rev. 12/20
Page 324 of 405
Providing a Meal to Office Staff
If a Pfizer colleague is bringing lunch to a medical office for HCPs to eat during a
product discussion, can the colleague also provide lunch to non-HCPs (e.g., office
staff) in attendance?
Yes, the PhRMA Code provides that when conducting in-office (“lunch and learn”)
programs for HCPs it is permissible to provide the meal to members of an HCP’s staff
who also attend the presentation or otherwise receive educational information unless
further restricted by state law or other laws or policies. Nevertheless, colleagues
should be thoughtful about feeding only those office members who have a legitimate
need to be provided the education.
Can a Pfizer colleague provide lunch to HCPs or medical office staff who do not attend
the informational presentation or receive educational information?
Any individual who consumes a meal must receive educational information incidental
to their meal. If an HCP or office staff member unexpectedly steps away or excuses
themselves without receiving an educational presentation, the hosting colleague
should schedule a near-term follow-up to ensure the information is conveyed.
Can a Pfizer colleague set up a monthly appointment, in an HCP’s office, that includes
a meal or snack?
Maybe. If there is a business rationale to provide educational information, it is
appropriate to provide a meal or snack approximately once a month to the same
attendees. Under the PhRMA Code, meals may only be provided to HCPs on an
occasional basis. Providing a meal or snack more than once a month may be
appropriate if there is new information to share or different attendees.
Providing “In-Office” Meals to Remotely-Based Customers
How is “in-office” meal defined for customers who are based remotely? Can a Sales
Colleague or their immediate manager host a non-restaurant meal in temporary
meeting space rented by customers who do not have a corporate office?
Sales Colleagues and their immediate managers are limited to providing an “in-office”
meal under the PhRMA code to ensure the meal is incidental to a substantive
interaction and in the setting where the HCP typically conducts professional
conversations. Some HCP customers are field-based without a formal corporate

White Guide – Chapter 18: Meals, Educational Items,
Greenstone Giveaways, and HCP Payment Disclosure
Rev. 12/20
Page 325 of 405
Providing “In-Office” Meals to Remotely-Based Customers
office, e.g., retail pharmacy managers (licensed pharmacists who manage a territory
of chain pharmacies for large retailers). These customers occasionally rent hotel or
other meeting space to conduct business. In such instances, the customer-rented
space, excluding all restaurants and restaurant meeting rooms, may be considered
“in-office” for purposes of this Chapter, as that is where the customer conducts
professional conversations.
If the customer-rented space is at a restaurant or restaurant meeting room, it is not
considered “in-office,” and you may not provide a meal at such a location. Sales
representatives and their immediate managers may only expense a meal at the
customer-rented location incidental to a promotional presentation and in accordance
with all requirements of this Chapter; no other expenses such as the meeting space
rental may be incurred. As with other “in-office” promotional opportunities, Pfizer
colleagues must follow all Pfizer policies for detailing and should leave the customers’
meeting space after the promotional discussion and incidental meal are concluded, in
no way involving themselves in the customers’ other business dealings. If colleagues
have questions or concerns about promotional opportunities with remotely-based
customers, including the provision of meals, they should consult with their team
attorney.
Providing in-Hospital Meals
What qualifies as an appropriate “in-hospital” meal? Can a Sales representative or
their immediate manager host a meal at a hospital food court or a cafeteria within the
hospital complex?
An in-hospital meal takes place in offices, conference rooms, or hospital locations that
are considered part of the hospital complex. Sales representatives or their immediate
managers may provide a meal at a hospital food court or cafeteria on hospital grounds
in conjunction with an informational presentation, if it is considered part of the hospital
complex. No other expenses (e.g., room fees) may be paid to the office or hospital in
connection with meals conducted in an in-hospital setting.

White Guide – Chapter 18: Meals, Educational Items,
Greenstone Giveaways, and HCP Payment Disclosure
Rev. 12/20
Page 326 of 405
Providing Meals to Pharmacists
May you provide a meal to a pharmacists or pharmacy technicians?
Yes, however you may not provide a meal to a pharmacist or pharmacy technician in
Vermont. For Massachusetts, Nevada and D.C., pharmacists and pharmacy
technicians any food and beverage expenses must be reported and disclosed
individually.
Meals Provided by Senior Sales Colleagues, Headquarters Colleagues, and Account
Management Colleagues Permitted to Host Out-Of-Office Meals
All colleagues are subject to the general rules and restrictions set forth at the beginning of this section.
However, the PhRMA Code restriction on out-of-office meals is not applicable to senior Sales Colleagues
above District Manager level, Headquarters (HQ) (e.g. Marketing, HQ Medical, senior business leadership)
colleagues, or Account Management colleagues for meals with non-HCPs or HCPs who do not regularly
treat patients. These colleagues may provide occasional modest food or beverage items to HCPs in
restaurants or other appropriate venues (such as Pfizer’s offices), as long as there is a legitimate business
reason for hosting the meal. All out-of-office meals hosted by senior Sales Colleagues, HQ colleagues,
and Account Management colleagues, must follow the requirements of this Chapter.
Legitimate Business Reason
To determine whether the legitimate business reason requirement is satisfied, appropriate colleagues
hosting out-of-office meals should determine whether the proposed interaction is consistent with their role
and responsibilities, and whether an interaction over a meal is an appropriate way to achieve their goals
and objectives. Some examples of legitimate business purposes might include a discussion regarding local
market payer challenges, account dynamics, or understanding how HCPs manage a particular disease
state. It would not be a legitimate business purpose to host a meal solely to build a relationship with an
HCP or to facilitate the introduction of one HCP to another.
The central focus must be the business interaction, with the meal being incidental to that primary purpose.
At all times, colleagues must exercise sound judgment and discretion when providing meals in conjunction
with a business interaction.
Further, for all Sales Colleagues, it is presumed that discussions regarding unapproved indications for
Pfizer products, pipeline products, or disease states or therapeutic areas for which Pfizer has no product,
are impermissible and thus cannot constitute a legitimate business reason for hosting or attending a meal
with an HCP.

White Guide – Chapter 18: Meals, Educational Items,
Greenstone Giveaways, and HCP Payment Disclosure
Rev. 12/20
Page 327 of 405
Meal Planning & Execution
All out-of-office meals must follow the requirements below:
1. In general, attendance should be limited to no more than 3 HCP attendees at an out-of-office meal to
ensure that there is a meaningful opportunity for the hosting colleague to engage with all attendees to
meet their objectives. If the hosting colleague believes there is a legitimate justification for including
more than 3 HCPs, they should discuss with their manager and align on how the host will ensure there
is a meaningful opportunity for them to engage with all attendees.
2. The host must have a legitimate business objective for the interaction and consider having a list of
topics and questions or other presentation to facilitate the legitimate business discussion with the
attendees for each meal. The host should assess whether the information to be gathered is needed
and ensure it is not duplicative of information already available. The materials should be discussed,
prior to the meal, with the host’s manager, and reviewed as needed, by the team attorney, GPC and/or
brand medical depending on their content, and consistent with RC-approved content. The host’s
legitimate business objectives should be made available upon request to the host’s manager in
connection with their review of the colleague’s expenses.
3. Any materials and questions to be utilized to facilitate the discussion must be on-label and consistent
with overall brand strategy, unless you are a colleague who is permitted by Pfizer policy to engage with
HCPs regarding an unapproved product or indication, or disease states or therapeutic areas for which
Pfizer has no product. Colleagues should consult their team attorney for any questions regarding
whether the topics to be discussed at a proposed meal with an HCP are appropriate.
4. To the extent you are aware that multiple Pfizer colleagues (e.g., RBDs from different geographies or
colleagues from both Marketing and Sales) wish to discuss the same topic or use the same materials
with different HCPs, the colleagues must all coordinate to ensure that the overall number of events and
HCP attendees is appropriate to achieve the business need.
5. Following the meal, consistent with guidance on information sharing between functional roles, the host
must share the information gathered with the Brand Team or other Pfizer colleagues, as appropriate,
to determine how the information will be utilized to further Pfizer’s business. Potential hosts should use
these deliverables and insights to assess the need for future meals for the same geography, disease
state or product.
Attendance by Other Colleagues at Meals Hosted by Senior Sales, HQ, and Account
Management Colleagues
When determining who may be in attendance for an out-of-office meal hosted by an appropriate colleague,
you must always ensure that the topics of discussion are appropriate for all colleagues in attendance and
the ratio of Pfizer colleagues to HCPs is conducive to the business discussion. For example, Senior Sales
or HQ colleagues should not discuss a proposed speaker agreement with an HCP in the presence of a

White Guide – Chapter 18: Meals, Educational Items,
Greenstone Giveaways, and HCP Payment Disclosure
Rev. 12/20
Page 328 of 405
sales representative or District Manager. The number of colleagues in attendance for meals hosted by a
Senior Sales, HQ, or Account Management colleague must be limited to the minimum necessary to facilitate
an appropriate business discussion with all external attendees.
Because sales representatives and District Managers are not permitted to host out-of-office meals under
the PhRMA Code, their attendance at out-of-office meals hosted by Senior Sales or HQ colleagues must
be carefully considered. The decision to include a sales representative and/or District Manager should be
based on their specific expertise relating to the customer, account, or local dynamics and only permitted if
necessary, to assist the Senior Sales or HQ colleague in meeting their objectives in an introductory meeting
with an HCP. Once an introduction has been made, future attendance by sales representatives and/or a
District Manager at a meal with that same HCP would generally be unnecessary. The Senior Sales
colleague or HQ colleague must provide a clear justification to their immediate manager for any additional
meals with the same HCP and sales representatives and/or District Managers. Sales representatives and
District Managers may not attend out-of-office meals for the purpose of conducting promotional activities or
discussions that they cannot host on their own (e.g. detailing at a restaurant) or to meet their own objectives
of building a relationship with an HCP. The legitimate business reason for the meal must be to meet the
objectives of the hosting Senior Sales or HQ colleague, not the objectives of the sales representative or
District Manager in attendance.
Any attendance by medical colleagues (HQ or field-based) should be consistent with guidance on joint
commercial-medical activities in this guide and the Green Guide. Medical participation is subject to review
and approval by the team attorney as well as the GPC (if needed). Sales representatives and medical
colleagues may not attend the same out of-office meal without prior consultation with the team attorney or
GPC.
Sales Colleagues Attending Non-Speaker Program Restaurant Meals
An Account Manager plans to provide a restaurant meal to an HCP C-Suite executive
who does not regularly treat patients for an appropriate business discussion focused
on the delivery of patient care or similar topics related to the HCP’s primary role as an
administrator or executive. Would it be acceptable for a Sales representative to
accompany the Account Manager?
The circumstances under which this would be appropriate are extremely rare. A Sales
representative may only join an Account Manager who is permitted to provide out-of-
office business meals if the topics of discussion are appropriate for all colleagues in
attendance and in accordance with Pfizer policies on joint interactions. Those policies
generally permit Sales representatives to participate in joint meetings with an Account
Manager and the customer on an infrequent basis when there is a legitimate business

White Guide – Chapter 18: Meals, Educational Items,
Greenstone Giveaways, and HCP Payment Disclosure
Rev. 12/20
Page 329 of 405
Sales Colleagues Attending Non-Speaker Program Restaurant Meals
need to do so and the programs or materials to be discussed are RC-approved for joint
sales and account management customer interactions. Colleagues should consult
their team attorney for any questions regarding appropriate attendees at an out-of-
office meal based on the topics for discussion.
May a Sales representative or District Manager attend a restaurant meal with an HCP
if there is no appropriate colleague present, but the parties each agree to pay their
own way?
No, this would not be in the spirit of the PhRMA Code or Pfizer policy.
Legitimate Business Reason
Pfizer is hosting a promotional booth staffed by Marketing colleagues at a medical
conference. Can a Marketing colleague take a group of physicians out to a restaurant
meal to discuss new Pfizer RC-approved data on a Pfizer product?
Yes. This would be considered a legitimate business purpose since it is permissible
for Marketing colleagues to discuss RC-approved content with HCPs so long as they
adhere to the Four Core Compliance Principles. Marketing colleagues may provide a
modest meal incidental to the discussion, unless restricted by state law. For more
information, see the State Laws: HCP and State Employee Restrictions Chapter in this
Guide.
Greenstone Colleagues and Sterile Injectables Colleagues (Sterile Injectables SHRs, DBMs, and Sales
Directors) who do not provide clinical detailing of products may host off-site business meals or snacks for
non-HCP customers and HCPs who hold administrative positions and dedicate very little time, if any, to
seeing patients or filling prescriptions, generally following the sections on “Meals to HCPs” and “Meals
Provided by Senior Sales Colleagues, Headquarters Colleagues, and Account Management Colleagues
Permitted to Host Out-Of-Office Meals.” If there is doubt as to whether a particular customer’s role is
administrative, please consult with your manager or Legal/Compliance contact. In addition, any local, state,
or hospital policies or restrictions must be considered to ensure compliance. Inclusion of a customer’s
Business Meals Provided by Greenstone
and Sterile Injectables Colleagues

White Guide – Chapter 18: Meals, Educational Items,
Greenstone Giveaways, and HCP Payment Disclosure
Rev. 12/20
Page 330 of 405
spouse or other guest in the meal is not appropriate unless the spouse or guest has a legitimate
independent business reason to attend.
Before providing any meals or other items of value to customers, colleagues should refer to the State Laws:
HCP and State Employee Restrictions Chapter and the Federal Employee Interactions and Lobbying
Chapter in this Guide.
ACT
Host
Restaurant
Meals?
Host
In-office
Meals?
Host
In-hospital
Meals?
Host
Speaker
Programs?
Host Meals at
Conventions?
Greenstone and
Sterile Injectables
Colleagues with
Administrative
Customers
Only for non-
HCPs or
HCPs who do
not regularly
see patients
or fill
prescriptions.
See guidance
found in
“Meals to
HCPs” and
“Meals
Provided by
Senior Sales
Colleagues,
Headquarters
Colleagues,
and Account
Management
Colleagues
Permitted to
Host Out-Of-
Office Meals”
sections
above.
Yes. See
guidance
found in
“Meals to
HCPs”
section
above.
Yes. See
guidance
found in
“Meals to
HCPs”
section
above.
Yes
Only for non-
HCPs or HCPs
who do not
regularly see
patients or fill
prescriptions.
See guidance
found in
“Meals to
HCPs” and
“Meals
Provided by
Senior Sales
Colleagues,
Headquarters
Colleagues,
and Account
Management
Colleagues
Permitted to
Host Out-Of-
Office Meals”
sections
above.
In-Office/In-Hospital Meals for Greenstone and Sterile Injectables Customers
I am meeting with an HCP with a purely administrative role. We are meeting in his
office. What can I spend?
All in-office or in-hospital meals must be modest by local standards and may not
exceed a total cost of $40, including tax, tip, and delivery charges. Keep in mind that
additional local, state and hospital restrictions may apply.
White Guide – Chapter 18: Meals, Educational Items,
Greenstone Giveaways, and HCP Payment Disclosure
Rev. 12/20
Page 331 of 405
In accordance with the PhRMA Code and Pfizer policy, RC-approved educational items valued at $100 or
less may be provided on occasion to HCPs or members of their staff. Non-educational items are prohibited
from being offered, even if the items are practice-related and of minimal value (such as pens, pads, mugs,
etc.). Educational items that do not directly benefit a patient or are not intended to be used by or with a
patient, such as textbooks and reprints, are reportable under the Sunshine Act. If you have a question
about whether a specific educational item is approved to be provided to HCPs, consult the relevant product
Legal or Regulatory colleague, or submit your question to StateHealthcareLawCompl[email protected]m.
Further, as with meals, several states and the VA/DoD also impose limitations which are stricter than the
PhRMA Code or Pfizer policy on educational items (and other items of value) that may be provided to
HCPs. For instance, to ensure compliance with Minnesota state law, Pfizer policy prohibits colleagues from
providing certain educational items to prescribers licensed to practice in that state. Before providing
educational items to HCPs, colleagues should refer to the State Laws: HCP and State Employee
Restrictions Chapter and the Federal Employee Interactions and Lobbying Chapter in this Guide. For
further information, and to determine where an HCP is licensed to practice, consult the HCP Lookup Tool
and the other references available on Global Policy Xchange on GCO On Demand under the “State
Healthcare Law Compliance” tab. Sales Colleagues should also consult the State Law Restriction field in
Veeva CRM.
Out-of-Pocket Gifts for HCPs
Can I pay for a gift for an HCP out of my own pocket if I do not expense it?
No. It is not appropriate to purchase personal gifts, or any other items of value for
HCPs in the course of doing business, even if you pay out-of-pocket and do not seek
reimbursement from Pfizer. The gesture could appear to be an attempt to illegally
influence prescribing in violation of anti-kickback laws. This principle applies to any
item of value expensed personally, including meals. Remember that The Summary of
Pfizer Policies on Business Conduct (the “Blue Book”) and Corporate Policy (CP)
#203: Conflicts of Interest require you to avoid even the appearance of a conflict of
interest.
Educational Items to HCPs

White Guide – Chapter 18: Meals, Educational Items,
Greenstone Giveaways, and HCP Payment Disclosure
Rev. 12/20
Page 332 of 405
Items of nominal value, such as pens, may be distributed by Greenstone Colleagues at booths at trade
shows and conferences, provided the criteria listed below are met.
• The majority of attendees at the trade show must be non-HCPs or non-practicing HCPs (e.g.,
GPO meetings, wholesaler trade shows, pharmacy buyer conventions);
• No other Pfizer brands with clinical detailing messages are represented at the event.
Giveaway items must not, under any circumstances, be distributed in the field (e.g., at hospitals or to
practicing pharmacists), nor may these items be made available through the PROMOS online catalog or
other sources accessible by all colleagues.
Giveaway Items for Greenstone Colleagues
Can Greenstone Colleagues offer pens at their booth during a trade show?
Yes. Greenstone Colleague may offer giveaway items of nominal value at a booth or
table at meetings and conventions. However, Colleagues must ensure that there are
no other Pfizer brands being promoted with clinical detailing messages at the same
event (no “detailed” products are displayed), and the majority of the attendees at the
meeting or convention are non-HCPs and/or non-practicing HCPs. Prior to arranging
to distribute giveaway items at an event, please contact the convention or meeting
organizers to confirm that no other Pfizer teams will be attending and exhibiting
detailed products.
Overview
Pfizer committed to publicly disclose payments and the value of meals, reimbursable travel expenses, and
educational items that it provides to HCPs and to U.S. institutions in connection with clinical research, along
with the names of the associated principal investigators. Pfizer disclosed on its public website payments
and the value of meals, reimbursable travel expenses, and educational items that are provided.
Pfizer’s disclosure policy is broader than the requirements of the Sunshine Act and defines “HCP”
more broadly than the definition found in the Act. This is so because certain states have different
reporting standards, and individuals other than those described in the Sunshine Act can influence
or cause the administration, prescription, purchase, or recommendation of prescription medicines.
Greenstone Giveaway Items
HCP Payment Disclosure Policy

White Guide – Chapter 18: Meals, Educational Items,
Greenstone Giveaways, and HCP Payment Disclosure
Rev. 12/20
Page 333 of 405
Items Included in Reporting
Pfizer’s disclosures may include the following types of payments and non-cash items provided directly or
indirectly to a broad range of U.S. healthcare professionals and institutions:
• Meals (including snacks/refreshments);
• Business travel expenses;
• Educational Items (e.g., textbooks and reprints);
• Research support (all payments or transfers of value related to R&D, such as clinical site
payments, study drug, and equipment that is leased, loaned, or given):
o Investigator-Sponsored Research (ISR);
o Non-interventional/Observational Studies;
o Pre-clinical Research;
o Phase I-IV Pfizer-Sponsored Clinical Studies;
o Clinical Research Collaborations (CRCs); and
o Outcomes Research Studies.
• Consulting Fees and Honoraria;
• Promotional Speaking Fees;
• Publication support (e.g., editorial support provided by an agency);
• Charitable Contributions;
• Grants; and
• Royalty and License Payments.
Disclosure of the Value of Meals
As described in this Chapter, colleagues are permitted to provide occasional modest meals to U.S. HCPs
in appropriate circumstances. Currently, subject to state laws that may also impose meal limitations and
reporting requirements that are stricter than the PhRMA Code or Pfizer policy, Pfizer’s disclosures include
all meals provided to U.S.-licensed HCPs, regardless of value. Although not treated as “meals,” snacks
and refreshments of nominal value ($10 or less per attendee) must be appropriately recorded in expense
reports, as directed in this Chapter.
When meals are provided in connection with an informational presentation to a group, the disclosable value
is calculated by taking into account both actual and expected attendees. Therefore, to ensure appropriate
accounting for the per-person value, all attendees who partake in the meal (HCPs and non-HCP office
staff), as well as all expected attendees and those who do not partake in the meal but did attend, should
be tracked. (See Pfizer’s Field Force T&E Expense Procedure is available on MyPfieldNet.)

White Guide – Chapter 18: Meals, Educational Items,
Greenstone Giveaways, and HCP Payment Disclosure
Rev. 12/20
Page 334 of 405
Disclosure of Snacks and Refreshments Provided at Exhibit Booths
We are planning to have an exhibit booth at a state physicians’ annual convention, at
which we intend to make coffee and pastries of nominal value ($10 per attendee or
less) available. Do I need to track and report the refreshments provided to U.S.-
licensed HCPs visiting the Pfizer booth?
No. As a general rule, snacks and refreshments of nominal value do not need to be
tracked at an exhibit booth when conducted in a large-scale convention or conference
setting (greater than 5o attendees).
Disclosure of the Value of Educational Items and Non-Disclosure of Patient Materials
As discussed in this Chapter, under Pfizer’s policies and PhRMA Code guidelines, RC-approved
educational items valued at $100 or less may be provided on occasion to U.S.-licensed HCPs. The value
of these educational items (such as textbooks) is included in Pfizer’s public disclosures. Note that reprints
and other educational materials that enhance an HCP’s skills are considered reportable transfers of value
under the Sunshine Act.
Generally, Pfizer-created branded and unbranded promotional materials, literature and other leave-behind
written materials are NOT subject to disclosure under the Sunshine Act. Likewise, items that are to be used
by or with patients, such as an anatomical model or patient education materials, are NOT disclosable under
the Sunshine Act. However, some of these items are subject to disclosure under state laws (e.g., Vermont).
Accordingly, all of these items must be tracked for business purposes. Such items include:
• Copay cards;
• Savings cards;
• Pill dispensers;
• Brochures;
• Vouchers;
• Prescription stamps; and
• Pamphlets.
Recording Disclosable Payments and Items
Colleagues must properly record all payments, meals (including the number and classification of
attendees), and other items that may be disclosable, regardless of value, as part of the regular expense
reporting process. Colleagues are expected to:

White Guide – Chapter 18: Meals, Educational Items,
Greenstone Giveaways, and HCP Payment Disclosure
Rev. 12/20
Page 335 of 405
• Obtain full and complete names, titles, addresses, and state license numbers for all U.S.-
licensed HCPs receiving payment for, or otherwise participating in, activities involving disclosable
items, including attendees at meetings, presentations, and speaker programs where meals are
provided;
• Ensure that information about payments and non-cash items given to U.S.-licensed HCPs is
accurately recorded in the appropriate system (e.g., Ariba ePay and Purchase Orders; PT&E’s
“My HCP”, “HCP”, “Other HCP” categories; Centris’s “Attendee” section; CVENT Attendee
registry; Veeva CRM);
• Classify budgets and expenses using the appropriate codes and ensure invoices can be
attributed to the HCP through the Pfizer Physician ID Number; and
• Never approve expense reports or invoices that lack full names and appropriate expense
allocation.
Identifying HCP Meal Attendees in Sales Colleague Expense Reports
A Sales Colleague has provided an in-office meal to a mixed group including both
physicians who are on and not on her TCL, as well as office staff. Which individuals
must the Sales Colleague identify by name in her meal expense report?
All individuals who are licensed to prescribe medicines in the United States must
be identified by name in the meal expense report, regardless of whether they appear
on the colleague’s TCL. These include doctors of medicine or osteopathy, medical
residents, dentists, podiatrists, optometrists, chiropractors, and advanced practice
nurses, such as nurse practitioners and physician assistants, who are legally
authorized to prescribe by the state in which they practice. Non-prescribers, including
registered nurses, pharmacists and office staff, do not need to be identified by name,
unless the meal is with a NV HCP for any dollar amount, or a DC HCP that is $25 or
more or a MA HCP that is $50 or more. In these circumstances all individuals must be
listed individually in the expense report. For further information regarding appropriate
use of the travel & expense system, Sales Colleagues should consult the Pfizer Travel
& Expense guidelines available on MyPfieldNet. Please also see the State Laws: HCP
and State Employee Restrictions Chapter in this Guide, for further details on who
qualifies as an HCP in Nevada, Massachusetts and D.C.
Opting Out of Receiving Disclosable Items
If a U.S.-licensed HCP expresses a desire to opt out of receiving food, beverages, or other disclosable
items, the notified colleague must: (1) immediately make Pfizer aware of the opt out by e-mailing all relevant
information to PTI@Pfizer.com; and (2) inform other colleagues who may interact with that HCP, so that

White Guide – Chapter 18: Meals, Educational Items,
Greenstone Giveaways, and HCP Payment Disclosure
Rev. 12/20
Page 336 of 405
the HCP’s request can be honored. The HCP may also submit questions or an opt out request directly to
It is critical for Pfizer colleagues to make sure that the U.S.-licensed HCPs with whom they interact are
aware of Pfizer’s disclosure policy and the meaning of an “opt out.” An HCP who does not want to have
items reported should not be offered – and must not accept – any payments, food, or other disclosable
items from Pfizer. Pfizer maintains a record of HCPs who have “opted out” of receiving disclosable items
from Pfizer on MyPfieldNet and Global Policy Xchange on GCO On Demand.
If a U.S.-licensed HCP accepts a disclosable payment or item of value, that information will be subject
to disclosure regardless of any prior opt out request.
If an HCP who has opted out subsequently chooses to opt back in, the notified colleague or the HCP should
contact [email protected]m.
Access and Use of Open Payments and other Transparency Data for Analytics
The Transparency team has created resources, which include CMS Open Payments competitor and Pfizer
internal payment datasets, that enable certain analyses and business insights. For specific data requests
or information regarding access to these datasets and dashboards for analytics, please visit the Payment
Transparency Portal or contact the Transparency team directly at
GlobalTransparencyAnalytics@pfizer.com. If you have questions about the appropriate use of data please
consult your BU, Divisional or Functional Compliance Lead.
Understanding the Opt Out Process
Can a Sales representative provide a meal to an office with multiple HCPs, if some
HCPs have opted out and others have chosen not to opt out?
Generally, yes. However, any HCPs in the office who have opted out may not
consume the meal.
What happens if an HCP who has previously opted out eats a meal that was provided
for other HCPs in the office or at a joint meeting or event?
The HCP must be informed that any meals consumed will be reported, and the HCP’s
name must be included in the list of attendees in the relevant expense system (e.g.,
PT&E), so that an appropriate portion of the meal expense can be allocated to that
HCP.

White Guide – Chapter 18: Meals, Educational Items,
Greenstone Giveaways, and HCP Payment Disclosure
Rev. 12/20
Page 337 of 405
Understanding the Opt Out Process
An HCP is willing to provide consulting services for zero compensation, including no
travel expense reimbursements. Will this arrangement be subject to disclosure?
Probably not. The HCP should still sign a “zero fee” consulting agreement to
memorialize the terms. Please contact ENG[email protected]om or your team attorney
with any questions.
The Disclosure Process
Will U.S-licensed HCPs have the opportunity to review their Sunshine Act data before
it is posted on the CMS Open Payments website?
Yes. After Pfizer submits data to CMS, and prior to the information becoming public,
HCPs have a 45-day period to review their data and raise inquiries with Pfizer. Pfizer
then has an additional 15 days to investigate and respond.
How should I handle complaints by HCPs about Pfizer’s disclosure policy? What if an
HCP believes that the information in Pfizer’s disclosures is incorrect?
Pfizer has a dedicated staff to address transparency questions and concerns.
Colleagues should e-mail questions to PTI@pfizer.com. If the HCP has a concern
about a particular transaction disclosed on Open Payments, please direct the HCP to
raise a dispute in the Open Payments portal directly or send an e-mail to
• For more information about the PhRMA Code, refer to the PhRMA website at
http://www.phrma.org/codes-and-guidelines/phrma-code-on-interactions-with-healthcare-
professionals.
• For more information on Pfizer’s meal and educational item guidelines based on the PhRMA Code,
including an FAQ on the PhRMA Code, refer to the PhRMA Guidelines tab on Global Policy Xchange
on GCO On Demand, or e-mail StateHealthcareLawCompliance@pfizer.com.
• For more information regarding processes for capturing and recording promotional meals in PT&E,
refer to the guidance available here.
• To determine whether an HCP is licensed in Massachusetts, Minnesota, New Jersey or Vermont, Sales
representatives should consult the physician profile within Veeva CRM, and other colleagues should
For More Information

White Guide – Chapter 18: Meals, Educational Items,
Greenstone Giveaways, and HCP Payment Disclosure
Rev. 12/20
Page 338 of 405
consult the HCP Lookup Tool. Additional information on state law restrictions and other tools is
available under the State Healthcare Law Compliance tab on Global Policy Xchange on GCO On
Demand.
• For more information on Pfizer’s HCP transparency practices, refer to the Payment Transparency Portal
or HCP Payment Disclosure tab on Global Policy Xchange on GCO On Demand or e-mail
• For more information on the National Physician Payment Transparency Program (Open Payments)
under the Affordable Care Act of 2010, commonly known as the Sunshine Act, and its implementing
regulations, refer to the guidance available on the CMS website.
• For more information on Open Payments, please see https://www.cms.gov/OpenPayments/About/Law-
and-Policy.html.
• More information on Pfizer’s Field Force T&E Expense Procedure is available on MyPfieldNet.

Rev. 12/20
Page 339 of 405
CHAPTER #19 – SAVINGS AND FREE
TRIAL PROGRAMS

White Guide – Chapter 19: Savings and Free Trial Programs
Rev. 12/20
Page 340 of 405
Chapter
#19
SAVINGS AND FREE TRIAL PROGRAMS
CONTENTS
Introduction................................................................................................................................................ 341
Key Points to Ensure Compliance ...................................................................................................... 341
Applicability ............................................................................................................................................... 343
Pfizer Savings and Free Trial Programs ................................................................................................... 344
Overview ............................................................................................................................................. 344
Savings Programs .............................................................................................................................. 344
Discount and Direct Purchase Programs ..................................................................................... 345
Free Trial Programs ..................................................................................................................... 345
Legal Framework for Savings and Free Trial Programs ........................................................................... 346
Anti-Kickback Statute ......................................................................................................................... 346
Beneficiary Inducement Statute .......................................................................................................... 347
State Laws .......................................................................................................................................... 347
Savings and Free Trial Programs Development and Implementation ...................................................... 347
Materials Describing Savings and Free Trial Programs ........................................................................... 348
State Law Requirements ........................................................................................................................... 349
For More Information ................................................................................................................................. 350
Savings and Free Trial Programs

White Guide – Chapter 19: Savings and Free Trial Programs
Rev. 12/20
Page 341 of 405
Chapter #19 Savings and Free Trial Programs
Pfizer believes that all patients should have access to the medicines prescribed by their Healthcare
Providers (HCPs). To that end, Pfizer develops and offers various patient savings programs that reduce
a patient’s out-of-pocket costs or offer medicines at a discounted price (such as copay coupons/cards,
discount programs, and direct purchase programs) and certain other programs that provide a limited supply
of Product at no cost to the patient for the purpose of determining efficacy and tolerability (such as voucher
programs and free trial programs) (collectively, “Savings and Free Trial Programs”).
The Office of Inspector General (OIG) of the U.S. Department of Health and Human Service has
cautioned that programs that provide free or reduced-price product or savings on out-of-pocket costs to
patients implicate the federal Anti-Kickback Statute (AKS) and raise substantial risks of fraud and abuse.
This Chapter provides a framework for the structure, operation, and implementation of Pfizer Savings and
Free Trial Programs to help patients access or try their prescribed Pfizer medications and to facilitate
compliance with applicable federal and state laws and guidance from the OIG.
Compliance with this Chapter is critical to ensure that the Savings and Free Trial Programs comport
with evolving government laws and guidance. Non-compliance with these policies puts the
Company at risk and can subject Pfizer colleagues to disciplinary actions up to and including
termination of employment.
• Savings and Free Trial Programs must be structured, reviewed and approved by Pricing &
Access Legal, and implemented consistent with this Chapter and through a centralized
process managed by North America Commercial Operations (NA CO)
as described in
this Chapter and the Savings and Free Trial Programs
Standard Operating Procedure
(SOP).
•
Each Pfizer Savings and Free Trial Program must conform with all applicable laws and
regulations, and be structured and implemented consistent with administrative guidance,
including the 2014 OIG Special Advisory Bulletin on Pharmaceutical Manufacturer
Copayment Coupons and other OIG guidance.
Key Points to Ensure Compliance
Introduction

White Guide – Chapter 19: Savings and Free Trial Programs
Rev. 12/20
Page 342 of 405
• Program owners wishing to initiate a new program or make changes to an existing program
must complete the Savings and Free Trial Program Approval Form and submit it to NA CO.
• Every Savings or Free Trial Program must be reviewed and approved by
Pricing & Access
Legal prior to implementation through the process outlined in the SOP.
•
Program owners must not engage a new Savings or Free Trial Program vendor or enter into
new or amended Statements of Work (SOW)
without coordinating with NA CO, who must
lead any such engagement. All vendors that implement Pfizer Savings or Free Trial
Programs must comply with applicable business rules and standard operating procedures,
Pfizer policies, and each such vendor's applicable policies.
•
Pfizer Savings and Free Trial Program vendors administering programs that are designed to
exclude Federal Health Care Program beneficiaries and/or patients in certain states must
apply robust government exclusion controls.
•
Each Pfizer Savings and Free Trial Program must be designed to meet a sound business
need that takes into account the specific characteristics of the Pfizer medication, patient
population, competitive landscape, and other patient support offerings available or proposed
for the product; such business need must focus on appropriate patient access to the
medication after an independent prescribing decision has been made, and must not be
intended to influence a prescribing decision.
•
Pfizer Savings and Free Trial Programs are intended for the exclusive use and benefit of
eligible program patients. Pfizer Savings and Free Trial Programs are not intended to, and
may not be designed to, benefit HCPs, pharmacies, or other third parties that are not
patients.
•
Pfizer must provide Savings and Free Trial Programs to patients only where prescribers have
made an independent clinical decision that the Pfizer product is medically appropriate for the
individual patient.
•
Program owners may use HCP prescribing volumes as a proxy for measuring patient need
when determining appropriate allocation of Savings and Free Trial Program offers; however,
such allocations to HCPs must not be intended to reward the HCPs' prescribing of Pfizer
products.
Key Points to Ensure Compliance

White Guide – Chapter 19: Savings and Free Trial Programs
Rev. 12/20
Page 343 of 405
This Chapter applies to all U.S. Pfizer colleagues and contractors that perform activities related to Savings
and Free Trial Programs. This includes, but is not limited to, Pfizer colleagues from the following functional
areas: Brand teams, Compliance, Global Health & Patient Access, Legal, and North America Commercial
Operations (NA CO). Any programs, tools or resources offered directly by Pfizer that offer free product or
financial assistance with a patient’s out-of-pocket costs are subject to this Chapter and the Savings and
Free Trial Programs SOP. This Chapter does not apply to the Pfizer Patient Assistance Program (Pfizer
PAP) and the Institutional Patient Assistance Program (which provide free product to eligible patients who
demonstrate financial need), Starters, and the Interim Care Program (or similar programs intended to
address an insurance delay). Information on these programs can be found in White Guide Chapter 10: The
Pfizer PAP and Donations to ICPAPS and White Guide Chapter 14: Starters.
A program owner is the colleague with primary responsibility for design and execution of Pfizer Patient
Savings Programs, which offer patient savings (typically without regard to financial need) on product
prescriptions, in the form of either a reduction in cost-sharing (e.g., copay or co-insurance) or reduced cash
price. Program owners must consult with NA CO and Pricing & Access Legal prior to implementation of a
proposed program if they have questions about the applicability of this Chapter and the SOP.
Savings or discount programs that offer financial assistance with a patient’s out-of-pocket costs that are
sponsored and operated by third parties (e.g., third-party payers, pharmacy benefit managers, or retail
pharmacies) are not subject to this Chapter; however, Pfizer colleagues interested in such third party
programs not operated by Pfizer must consult with Pricing & Access Legal and BU Compliance prior to
entering into any such programs.
• Brand Teams should work with the Global Products Counsels (GPCs) and Business Unit
(BU)
Compliance to ensure that Savings and Free Trial Program distribution channels and
allocation strategies are appropriate and consistent with this Chapter, and applicable state
and federal law, taking into account scrub lists, opt-out lists, and state law restrictions. The
GPC may consult with Pricing & Access Legal, as necessary.
•
Pfizer must ensure that all Savings and Free Trial Programs maintain adequate controls to
limit program use to eligible patients, including appropriate terms and conditions and patient
enrollment/activation requirements, as applicable.
Key Points to Ensure Compliance
Applicability

White Guide – Chapter 19: Savings and Free Trial Programs
Rev. 12/20
Page 344 of 405
Overview
Pfizer Savings and Free Trial Programs include both commercial programs offered by Brand teams, which
are centrally operated by NA CO, and certain programs offered by Global Health & Patient Access. Free
trial programs are not based on financial need and offer a limited free supply of medicine to patients newly
prescribed a Pfizer medication so they can determine efficacy and tolerability.
Savings Programs
Copay Programs1
9
Copay Programs help to reduce patients’ out-of-pocket costs for a Pfizer drug. Copay Programs may be
offered in many forms, including paper copay coupons (distributed by Pfizer or downloadable from a product
website), physical or virtual copay cards, electronic coupons (typically offered through the pharmacy
adjudication system or prescriber’s electronic medical records or e-prescribing system), debit cards, or
customer rebates.
Cost Sharing Programs
• Copay Coupon/Card – a program that reduces a patient’s out-of-pocket costs immediately at the
point of sale.
• Electronic Copay Savings Program – sends an offer electronically directly to a pharmacy or
dispensing physician that reduces an eligible patient’s out-of-pocket cost for a product immediately
at the point of sale without the patient presenting a physical coupon or card.
• Debit Card – a preloaded card that may be used at the point of sale to pay the pharmacy/dispensing
HCP for the patient’s out-of-pocket costs.
• Customer Rebates – an offer of post-purchase reimbursement from Pfizer directly to an eligible
patient for his/her out-of-pocket cost (e.g., copay or co-insurance amount) for a prescription after the
patient pays the cost at the point of sale and submits the receipt and other required documentation
as proof of purchase to the Pfizer program vendor.
• Copay programs do not include: Direct Purchase/Discounts (e.g., Viagra Direct), PBM Rebates, etc.
All proposals for Copay Programs must be reviewed by Pricing & Access Legal and may require additional
controls to ensure compliance with applicable laws and OIG Guidance. In general, Copay Programs are
available for commercially-insured patients only and require Pricing & Access Legal review and approval.
Pfizer may consider offering Copay Programs to uninsured or cash paying patients.
Copay Programs must not be used by Federal Healthcare Program beneficiaries for product that is
reimbursed by a Federal Health Care Program, such as Medicare, Medicaid, or TRICARE.
9
The May 23, 2018 Corporate Integrity Agreement refers to these types of programs as “cost-sharing” programs.
Pfizer Savings and Free Trial Programs

White Guide – Chapter 19: Savings and Free Trial Programs
Rev. 12/20
Page 345 of 405
Copay Programs and Federal Beneficiaries
May Copay Program offers be used by patients using Medicare, Medicaid or other
federal health care program?
No. Copay Program offers (e.g., copay cards or copay coupons) must never be used
by a federal healthcare beneficiary patient whose product is reimbursed by a Federal
Health Care Program, such as Medicare, Medicaid, or TRICARE, or any other federal
or state healthcare program.
Note: While Brand teams may consider offering Copay Programs to uninsured or cash
paying patients, which may include Federal Health Care Program beneficiaries (who
will purchase the product outside of their government program benefit), Pricing &
Access Legal review and approval is required.
Discount and Direct Purchase Programs
Discount and Direct Purchase Programs allow uninsured and/or underinsured patients to either:
(1) Use a program card to purchase certain Pfizer medications at a reduced cash price, outside of any
insurance benefit, through participating pharmacies; or
(2) Purchase certain Pfizer medications directly from Pfizer at a fixed cash price outside of the patient’s
insurance benefit.
These programs are offered to eligible patients without regard to the purchase of any other product or
service and typically without regard to the patient’s income. Participating patients with insurance must not
seek reimbursement from their insurance provider or count the cost of product purchased through these
programs toward their plan’s deductible or any out-of-pocket cost calculations/limitations. This means, for
example, that if a patient has Medicare Part D and is permitted to participate in this type of program, then
if they seek to purchase a prescription outside of their Medicare Part D benefits they must not seek
reimbursement from their Part D plan or count the cost of product purchased through these programs
towards their True Out of Pocket (TrOOP) costs.
Free Trial Programs
Vouchers and other free trial programs provide patients with a limited supply of free product to allow patients
and prescribers to evaluate the safety and efficacy of the product for a patient newly prescribed the product.
Vouchers and free trial programs must not be provided for the purpose of financial assistance. These
programs are analogous to a Starter; however, the free product is provided directly to the patient by a
program vendor or a participating pharmacy, rather than from a physician. Vouchers and free trial programs

White Guide – Chapter 19: Savings and Free Trial Programs
Rev. 12/20
Page 346 of 405
must not be positioned to HCPs or provided for the purpose of addressing long term issues, such as patient
access, insurance coverage or financial assistance. Brand teams must provide implementation guidance
to sales representatives about the appropriate characterization of these resources, including messaging
that directs patients to RxPathways or the product specific HUB to understand other resources that may be
available to address patient access to the medication and financial need. Sales representatives may
distribute both starters and vouchers to the same HCP office in accordance with the following principles.
Sales representatives should clearly indicate the appropriate use of these resources to HCPs, including
that: (1) vouchers are not intended to address financial hardship and insurance delays; and (2) an individual
patient should receive either a voucher or starter, but not both. The intent is to prevent a patient from
receiving both Starters and vouchers
Federal Health Care Program patients may be eligible to participate in properly structured voucher and free
trial programs. However, the free product must be provided to patients without regard to the purchase of
any other product or service, and the free product must not be eligible for reimbursement by any insurer.
Anti-Kickback Statute
The OIG broadly defines ‘‘copayment coupons’’ as any form of direct support offered by manufacturers to
insured patients, including print coupons, electronic coupons, debit cards, and direct reimbursements. If
offered to Federal Health Care Program patients (where the federal program covers and reimburses the
product), such programs constitute remuneration offered to consumers to induce the purchase of specific
prescription drugs and thus implicate the AKS.
Although manufacturers typically expressly prohibit the use of copayment coupon programs for products
that are reimbursable by Federal Health Care Programs due to the risk of violating the law, in recent years
the OIG has observed that steps taken to exclude such use are generally inadequate. In 2014, the OIG
issued a report and a Special Advisory Bulletin questioning the effectiveness of safeguards manufacturers
have in place to ensure that patients do not use copayment coupons to obtain prescription drugs paid for
by Medicare. The OIG concluded that manufacturers are responsible for operating their copayment coupon
programs in compliance with federal law and must take appropriate steps to prevent their programs from
inducing the purchase of drugs paid for by the Federal Health Care Programs. The OIG stated that failure
to take such additional steps may be considered evidence of intent to induce the purchase of drugs paid
for by these programs, in violation of the AKS.
In addition, while there is no safe harbor for vouchers or other free trial programs, the OIG has said that
sample programs (e.g., Pfizer’s Starter Programs) that comply with the Prescription Drug Marketing Act
(PDMA) generally will not raise AKS risks because physicians are prohibited from reselling or potentially
billing third-party payors (including Federal Health Care Programs) for samples they receive for free from
manufacturers. Further, the OIG has applied its guidance on samples to a program that provided a free
Legal Framework for Savings and Free Trial Programs

White Guide – Chapter 19: Savings and Free Trial Programs
Rev. 12/20
Page 347 of 405
one-time trial supply that allowed physicians and patients to test the product’s safety and tolerability and
incorporated various safeguards to prevent Federal Health Care Programs from bearing any cost.
Beneficiary Inducement Statute
The beneficiary inducement statute prohibits any person from offering anything of value to any Medicare or
Medicaid beneficiary that such person knows or should know is likely to influence the beneficiary to order
or receive a federally reimbursed product from a particular provider, practitioner, or supplier.
While pharmaceutical manufacturers like Pfizer are not considered “providers, practitioners, or suppliers”
for purposes of the statute, the statute applies to manufacturer programs that could influence a Medicare
or Medicaid beneficiary to choose a particular pharmacy or physician. Thus, if a Savings and Free Trial
Program would encourage a beneficiary to use a particular healthcare provider or pharmacy, the program
may implicate the beneficiary inducement statute.
State Laws
Certain states have enacted laws that limit the use of copay coupons, copay cards, vouchers, and other
Savings and Free Trial Programs in those states. Some state laws require Pfizer to report certain data
relating to Savings and Free Trial Programs. Many states also have laws that seek to protect consumers
from inappropriate marketing and sales practices. Virtually all states have broad laws prohibiting “unfair”
or “deceptive” trade practices, which have been interpreted to require that manufacturers provide clear and
conspicuous information regarding terms and conditions of the program offer on the offer materials (e.g.,
card or voucher) and any related advertising.
Pfizer colleagues should consult with NA CO and Pricing & Access Legal if they have questions regarding
the applicability of state laws to Savings and Free Trial Programs. More information on State Laws can be
found in White Guide Chapter15.
Either Commercial colleagues or colleagues in Global Health & Patient Access may be program owners of
Savings and Free Trial Programs. Accordingly, they are responsible for identifying the types of Savings
and Free Trial Programs to offer to patients for a particular Pfizer medication, and they manage the budget
for their respective Savings and Free Trial Programs.
Savings and Free Trial Programs must be structured, approved, and implemented consistent with this
Chapter and through a centralized process managed by NA CO, as described in the Savings and Free Trial
Programs Standard Operating Procedure. In order to initiate new programs or make changes to existing
programs, a program owner must complete the Savings and Free Trial Program Design Form and submit
it to NA CO.
Savings and Free Trial Programs Development and Implementation

White Guide – Chapter 19: Savings and Free Trial Programs
Rev. 12/20
Page 348 of 405
NA CO is responsible for overseeing the development, approval, and implementation of all Pfizer Savings
and Free Trial Programs, in coordination with Pricing & Access Legal and the relevant Savings and Free
Trial Program owner. This includes, among other things, owning and managing all Savings and Free Trial
Program vendor relationships, in coordination with Pricing & Access Legal, Global Procurement, and the
relevant program owner. Program owners may not engage a new Savings and Free Trial Program vendor
or enter into new or amended Statements of Work (SOW) without coordinating with NA CO, who will lead
any such engagement.
Pricing & Access Legal and GPC provide legal guidance to Brand Teams, Global Health & Patient Access,
and NA CO with respect to Savings and Free Trial Programs. Every Savings or Free Trial Program must
be reviewed and approved by Pricing & Access Legal prior to initial implementation or
implementation of changes to the programs through the process outlined in the SOP.
The relevant Savings and Free Trial Program owner, in coordination with NA CO and in consultation with
the GPC, is responsible for facilitating the allocation and distribution of physical and digital Savings and
Free Trial Program offers, consistent with this Chapter, the Savings and Free Trial Programs SOP, and
applicable state and federal law.
The chart below contains tips on what Pfizer colleagues must do and must not do when developing a
Savings or Free Trial Program:
DO
DO NOT
Consult with NA CO on the development,
approval, and implementation of all Pfizer Savings
and Free Trial Programs.
Engage a new Savings or Free Trial Program
vendor or enter into new or amended SOW without
coordinating with NA CO.
Complete the Savings and Free Trial Program
Design Form and submit it to NA CO for every
new and changing Savings or Free Trial Program
prior to implementation.
Design a voucher program to address access issues
or financial need.
Prior to initiating a new Savings or Free Trial
Program or making changes to existing programs,
you must submit the Savings or Free Trial
Program Design Form to NA CO and receive
approval from Pricing & Access Legal.
Contract with a new or different vendor without
Legal and NA CO approval of the Savings or Free
Trial Program Design Form.
All materials describing Savings and Free Trial Programs, including the terms and conditions, and any
limitations or patient eligibility requirements, regardless of the intended audience, must be reviewed and
approved by the applicable Review Committee and must be:
• Accurate and not misleading;
• Clear and transparent regarding the offering, patient eligibility, and program terms and conditions; and
Materials Describing Savings and Free Trial Programs

White Guide – Chapter 19: Savings and Free Trial Programs
Rev. 12/20
Page 349 of 405
• Compliant with the requirements outlined in this Chapter and the Savings and Free Trial Program SOP,
REG-08 and its associated work instructions, and all other relevant Pfizer policies, SOPs, and guidance.
Below is a summary of relevant state law requirements. It is not intended to be a complete list. Brand
teams should consult with NA CO and Pricing & Access Legal to ensure compliance with these
requirements, and any new laws.
Controlled Substances. A number of states have adopted broad restrictions on various manufacturer
practices relating to controlled substances, including coupons and other forms of copay relief.
Massachusetts, for example, prohibits discounts, rebates, vouchers, or other reductions in an individual’s
out of pocket expenses, including copayments and deductibles for any Schedule II opioid prescription drug.
Manufacturers that have products that are regulated as controlled substances can provide copay support
programs only on a limited basis. Any proposed Pfizer copay programs for a product that is scheduled as
a controlled substance are subject to review and approval by the applicable GPC, with consultation with
Pricing & Access Legal as appropriate.
California. California prohibits manufacturers from offering discounts, repayments, product vouchers or
other reductions to an individual’s out-of-pocket health plan expenses for a drug if a lower cost generic drug
is covered by the patient’s health plan on a lower cost-sharing tier and that is the therapeutically equivalent
to the drug, or if the active ingredients of the drug are available without a prescription at a lower cost.
This prohibition does not apply to, among other things, the following:
• Branded drugs until the first “Therapeutically Equivalent” generic product approved by the FDA has
been nationally available for at least three months (note: this does not apply to biologics/biosimilars);
• The individual has completed any applicable step therapy or prior authorization requirements for the
branded prescription drug as mandated by the individual’s health plan; or
• A discount, repayment, product voucher, or other reduction in an individual’s out-of-pocket expenses
is not associated with his or her health insurance, health care service plan, or other health coverage.
Program owners should consult with NA CO for more information about the applicability of California law to
a particular program.
Massachusetts. Massachusetts prohibits discounts, rebates, product vouchers or other reductions in an
individual’s out-of-pocket expenses (including copayments and deductibles) for both any prescription drugs
that have an AB-rated generic equivalent and for any Schedule II opioid prescription drug. In addition, for
prescription drugs that are not Schedule II opioids or that do not have an AB-rated generic equivalent,
Massachusetts prohibits manufacturers from offering discounts, rebates, product vouchers, or copay
State Law Requirements

White Guide – Chapter 19: Savings and Free Trial Programs
Rev. 12/20
Page 350 of 405
support programs if the manufacturer excludes or favors any pharmacy in the redemption of such discount,
rebate, product voucher or copay program voucher (e.g., if patients can use a copay card at a limited
number of pharmacies in the state).
Colorado and Louisiana. Copay offerings are not permitted to be offered directly to pharmacists in
Louisiana and Colorado.
Tennessee. Contact NA CO for assistance information regarding requirements to register programs with
the TN Division of Commerce & Insurance.
• Savings and Free Trial Programs Standard Operating Procedure.
• Global Commercial Content: Review and Approval Procedure – U.S. Addendum.
• Chapter 2 (Advertising and Promotional Labeling).
• Chapter 10 (The Pfizer PAP and Donations to ICPAPS).
• Chapter 11 (Privacy: Protecting Personal Information).
• Chapter 14 (Starters).
• White Guide Chapter 15 (State Laws).
For More Information

Rev. 12/20
Page 351 of 405
CHAPTER 20 – NON-DISCOUNT
ARRANGEMENTS WITH SPECIALTY
PHARMACIES AND OTHER
ACCOUNTS

White Guide – Chapter 20: Non-discount Arrangements
with Specialty Pharmacies and Other Accounts
Rev. 12/20
Page 352 of 405
Chapter
#20
NON-DISCOUNT ARRANGEMENTS
WITH SPECIALTY PHARMACIES
AND OTHER ACCOUNTS
CONTENTS
Introduction................................................................................................................................................ 353
Anti-Kickback Analysis .............................................................................................................................. 353
Overview of Interactions with Specialty Pharmacies ................................................................................ 355
Types of Contractual Relationships Between Pfizer and SPPs ................................................................ 356
Non-Discount Arrangements .............................................................................................................. 356
Discount Arrangements with SPPs..................................................................................................... 357
Materials Provided to SPPs ...................................................................................................................... 357
Privacy Requirements & SPP Data ........................................................................................................... 357
Field Commercial Colleagues and Interactions with SPPs ....................................................................... 358
SAS NAD Interactions with SPPs ....................................................................................................... 358
Other Field Commercial Colleague Interactions with SPPs ............................................................... 358
Sales Interactions with Specialty Pharmacy HCPs and SPP Office Staff .......................................... 358
Interactions with Specialty Pharmacy Sales Representatives. .......................................................... 359
Approval Process for Sales Representative Interaction ........................................................................... 360
Field Commercial Colleague SPP Interaction Approval Process ....................................................... 360
For More Information ................................................................................................................................. 361
Non-discount Arrangements with Specialty
Pharmacies and Other Accounts

White Guide – Chapter 20: Non-discount Arrangements
with Specialty Pharmacies and Other Accounts
Rev. 12/20
Page 353 of 405
Chapter #20 Non-Discount Arrangements with Specialty
Pharmacies and Other Accounts
Pfizer enters into a variety of contractual arrangements with its customers, including non-discount
arrangements with customers to procure goods or services on behalf of Pfizer. Specialty pharmacies
sometimes referred to as Specialty Pharmacy Providers (SPPs) are amongst these customers. This
Chapter discusses non-discount arrangements between Pfizer and SPPs or other accounts. Chapter 20
of the Orange Guide contains additional discussion about non-discount arrangements with SPPs when
Pfizer enters into such arrangements with specialty pharmacies to participate in Defined Networks or to
provide Supplemental Services to patients that have been prescribed a Pfizer product; Chapter 15 and
Chapter 20 of the Orange Guide contains a discussion of non-discount arrangements with SPPs and
organized customers, including managed care organizations, retail pharmacies, group purchasing
organizations, and integrated delivery networks.
The Anti-Kickback Statute prohibits payments intended to induce the purchase, prescription, endorsement,
or recommendation a product that is reimbursed under federal or state healthcare programs. Under Pfizer
policy, all customers are treated as if they are subject to the Anti-Kickback Statute, even though they may
not participate in a federal healthcare program. Recognizing that the federal Anti-Kickback Statute, if read
literally, could restrict many otherwise legitimate marketing activities and even some non-promotional
activities, the U.S. Department of Health and Human Services (HHS), Office of Inspector General (OIG)
has defined certain “safe harbors.” Activities that fall entirely within a safe harbor, such as legitimate
service arrangements, do not violate the Anti-Kickback Statute. The Anti-Kickback Statute and safe harbors
are critical to consider when entering into non-discount arrangements with any customer, including a
specialty pharmacy. This will help ensure that the proposed non-discount arrangement has a legitimate
business purpose and that Pfizer is procuring a needed good or service at Fair Market Value (FMV). Any
discussions regarding arrangements to purchase, prescribe or recommend a Pfizer product, or to improve
the price or discount at which a customer can purchase Pfizer’s products, must be separate and apart from
any discussions regarding a proposed service agreement with an account. Discussing these separate and
otherwise legitimate arrangements together could lead to conflation between the discounts (i.e. including
best price reporting) and services safe harbors thereby removing safe harbor protection and subjecting
Pfizer to potential liability under the Anti-Kickback Statute.
In order to ensure compliance, when entering into non-discount arrangements with SPPs or accounts
remember the following principles:
Introduction
Anti-Kickback Analysis

White Guide – Chapter 20: Non-discount Arrangements
with Specialty Pharmacies and Other Accounts
Rev. 12/20
Page 354 of 405
• Ensure the non-discount arrangement serves a legitimate business purpose. Only purchase those
goods or services for which Pfizer has a bona fide need. Paying for unneeded goods, services or data
can increase the risk that the arrangement is viewed as an illegal kickback.
• Do not attempt to procure a service from an account that the account is already legally obligated to
provide whether by statute, regulation or contract.
• Do not interfere with carefully defined contractual arrangements between Pfizer and an account,
including by providing any items of value to SPP or account personnel other than as expressly stated
in Pfizer’s contract with the SPP or account.
• To avoid pricing concerns, do not combine discount arrangements with other types of transactions. Do
not discuss discount arrangements under which an SPP or account may be eligible for a discount on
Pfizer products in conjunction with non-discount arrangements under which Pfizer seeks to procure an
item of value or service from the SPP or account – this applies to actual conversations with Accounts
as well as written documents or presentations.
• Do not imply unstated performance requirements (including therapeutic conversion) in discount or non-
discount (e.g. distribution or services agreements). In the event a SPP or account makes a
statement(s) regarding switching patients in exchange for discounts or service fees, immediately refer
such statement(s) to either the applicable global product counsel or Pricing & Access Legal for
appropriate follow up.
• Do not leverage Pfizer’s ability to purchase goods or services from a Customer in order to induce or
influence the purchase, prescribing or recommendation of Pfizer products from that Customer. For
example, do not condition the purchase of data from an account on formulary access for a Pfizer
product.
• Do not interfere with the independent clinical judgement of HCPs (including prescribers and SPP
clinicians) or offer any payments in exchange for patient referrals. Do not encourage SPP or account
personnel to suggest to patients or a prescribing HCP that the patient should switch to a Pfizer product
from their existing therapy.
• Do not interfere with the relationship between the patient and his/her HCP (including both prescribers
and SPP clinicians).
• Do not steer or implement SPP or account arrangements to steer HCPs or patients to one particular
SPP to the exclusion of other SPPs within a Defined SPP Network.
• Do not use SPP personnel (including SPP clinicians or sales representatives) as an extension of the
Pfizer sales force, or as a mechanism for delivering Pfizer promotional messaging to either HCPs or
patients.
• Always ensure you are paying FMV for the good or service based upon Pfizer’s FMV analysis. Paying
too much for a good, service, or data increases the risk that the arrangement may be viewed as a
kickback.

White Guide – Chapter 20: Non-discount Arrangements
with Specialty Pharmacies and Other Accounts
Rev. 12/20
Page 355 of 405
• Consult your team attorney or Pricing & Access Legal for all arrangements covered under this Chapter
20. Pricing & Access Legal will ensure all arrangements are appropriately memorialized in a written
contract and help mitigate compliance risks by structuring the non-discount arrangement so that it
meets the applicable anti-kickback safe harbor (i.e. Personal Services safe harbor).
Personal Services Safe Harbor
This safe harbor protects legitimate service arrangements recorded in a written agreement, of at
least one year in duration, where the compensation is determined in advance and on a fair market
value basis. Pfizer is required to ensure all service agreements meet the Personal Services safe-
harbor.
Fair Market Value
Price at which an asset or service passes from a willing seller to a willing buyer based on market
demand and supply. Pfizer is required to pay any person or entity in a position to purchase,
prescribe, endorse, or recommend our products fair market value for the good or service Pfizer
receives in return.
Recently, there has been an increase in the development of “specialty” medications. Specialty medications
often have specialized administration, storage, or distribution requirements commonly have a higher cost
of therapy than traditional medications and may be subject to additional regulatory requirements (i.e. FDA
mandated Risk Evaluation Mitigation Strategies (REMS) requirements). Specialty drugs are generally
dispensed by “Specialty Pharmacy Providers” (SPPs), which are distinct from traditional retail pharmacies
in their more comprehensive coordination of many aspects of patient care, disease management,
distribution, and patient access to therapy. SPPs have developed expertise in overcoming payer access
challenges and have been shown to help improve clinical and economic outcomes for patients with
complex, chronic or rare conditions. SPPs employ health care professionals, including both pharmacists
and nurses, provide patient education, help ensure appropriate medication use, promote clinically
appropriate adherence by identifying drug-drug interactions, or by referring patients back to their prescribing
physician when a therapy is not working as expected. SPPs can help patients gain access to therapy by:
performing prescription intake and dispensing, conducting benefits investigation, providing clinical support
for inbound patient calls, providing eligible patients with information about third party funding sources (e.g.,
out-of-pocket assistance with co-pay resources and contact information for third party co-pay foundations),
Overview of Interactions with Specialty Pharmacies

White Guide – Chapter 20: Non-discount Arrangements
with Specialty Pharmacies and Other Accounts
Rev. 12/20
Page 356 of 405
providing prior authorization and appeals support, and a number of other services as part of their normal
business operations or “core services.”
As discussed in Orange Guide Chapter 15 and Orange Guide Chapter 12, Pfizer enters into a variety of
discount arrangements. These arrangements are separate from the non-discount arrangements Pfizer
enters into to procure services.
Pfizer enters into non-discount arrangements with specialty pharmacies to participate in Defined Specialty
Pharmacy Provider (SPP) Networks and/or to provide Supplemental Services to patients that have been
prescribed a Pfizer product dispensed by such specialty pharmacies. All of these arrangements raise
specific legal risks if not handled by Pfizer colleagues in an appropriate manner.
Non-Discount Arrangements
Defined Distribution Networks: Through distribution agreements, Pfizer contracts with certain SPPs that
meet pre-defined and consistently applied objective inclusion criteria to dispense a particular Pfizer brand
as part of a ‘Defined SPP Network’. Defined SPP Networks are designed to ensure consistent and high-
quality patient care and facilitate patient access across payers and geographic regions. Any Defined SPP
Network must comply with all laws, regulations and administrative guidance, including 340B must offer
requirements, the personal services and management contracts safe harbor to the Anti-Kickback Statute,
as well as Pfizer internal policies and procedures. Defined SPP Network inclusion criteria are determined
by the Specialty Access Solutions (SAS) COE and Pricing & Access Legal. All pharmacies seeking
access to a Defined SPP Network must be reviewed by the SAS COE against such pre-defined objective
inclusion criteria.
Supplemental Services Contracts: Through service agreements, Pfizer contracts with SPPs to provide
specific services to help support patients prescribed a Pfizer product. These services – known as
‘Supplemental Services’ – are in addition to a SPP’s ‘Core Services’ (i.e., services that the SPP provides
to its patients as part of its normal business practice). Supplemental Services may include, but are not
limited to, the SPP’s dissemination of Pfizer patient educational materials, product adherence counseling,
or provision of data or information to Pfizer about product usage. Such agreements are only entered into
where there is a bona fide business and/or patient need for the services, for which payment is consistent
with Fair Market Value (FMV), and the services contracted are not part of a SPP’s Core Services.
Supplemental Services must be: (i) non-promotional in nature; (ii) limited to patients with a prescription for
a Pfizer Product consistent with an FDA-approved indication and dosing regimen; and (iii) transparent as
to Pfizer funding.
Types of Contractual Relationships Between Pfizer and SPPs

White Guide – Chapter 20: Non-discount Arrangements
with Specialty Pharmacies and Other Accounts
Rev. 12/20
Page 357 of 405
Pfizer’s SAS COE team within Payer and Channel Access manages Pfizer’s SPP strategy and operations
and must be engaged for all non-discount types of arrangements listed above.
Discount Arrangements with SPPs
Separate from distribution or services agreements, Pfizer may engage in discount arrangements with SPPs,
such as channel discounts. Such discount arrangements are negotiated by SAS National Account
Directors (NADs). Discount arrangements must be negotiated and contracted separately from distribution
agreements or service agreements. Discount arrangements must not be contingent upon the performance
of Supplemental Services. Certain SAS COE team members (i.e., Directors, Strategy and Innovation;
Directors, Operations and Execution; and Directors, Analytics of the SAS COE) are specifically firewalled
from discussions concerning SPP product discounts and should not participate in these discussions.
Pfizer may provide certain Pfizer-approved materials to SPPs for distribution to patients. Development of
SPP-related materials is managed by the SAS COE and Pricing & Access Legal is responsible for the
review of patient educational materials intended to be provided to/by the SPP. SPP materials must be
educational in nature. Other than SPP welcome kits/ brochures, SPP materials must not contain Pfizer
product brand names.
Privacy laws also limit the scope of permissible activities that Pfizer may engage in when SPPs are
interacting with patients on Pfizer’s behalf. For example, the Health Insurance Portability and
Accountability Act (HIPAA) and some state privacy laws restrict certain activities in which SPPs, and
accounts are paid or otherwise provided remuneration, directly or indirectly, by Pfizer in exchange for
communicating with targeted patients or clinicians. Under these laws, certain communication programs
require prior written patient authorization. In limited circumstances, SPPs and Accounts may implement
such programs without securing patient authorizations; however, in such cases Pfizer and the applicable
SPPs and Accounts must ensure that the arrangements comply with the terms of the limited exceptions to
the patient authorization requirement under the HIPAA marketing rules. State privacy laws may also be
implicated by certain marketing arrangements. For more information about HIPAA and state privacy laws,
see White Guide Chapter 11: Privacy: Protecting Personal Information. Please consult a Pricing & Access
attorney or your team attorney if you have questions on the permitted scope of SPP or Account interactions
and communications involving patients or clinicians.
Any patient data procured through a SPP Supplemental Services agreement must be in a de-identified
format only, consistent with applicable privacy laws and regulations. Pfizer-sponsored SPP
communications transmitted over telephone systems including any automatic dialing systems, artificial, or
Materials Provided to SPPs
Privacy Requirements & SPP Data

White Guide – Chapter 20: Non-discount Arrangements
with Specialty Pharmacies and Other Accounts
Rev. 12/20
Page 358 of 405
prerecorded voice messages, SMS text messages, and fax communications, must comply with the
Telephone Consumer Protection Act (TCPA). These programs may not be implemented at an SPP until
an executed contract is in place, and contracted activities may not commence until appropriate training and
other on-boarding activities have been completed. Pfizer colleagues may not share any de-identified
prescription data or other proprietary information such as Fingertip Formulary with Specialty Pharmacy
personnel or prescribers.
Prohibitions on ROI Analysis. Pfizer prohibits personnel from conducting any analysis of SPP data or
reports (e.g., return on investment (ROI) analysis that would violate applicable laws, regulations and/or
company policies and procedures). To mitigate the risk of any impermissible analysis, no Pfizer personnel
other than the SAS COE colleagues may calculate any measures of program impact on product utilization
or financials or ROI.
SAS NAD Interactions with SPPs
SPPs are sometimes owned or affiliated with other healthcare organizations. For SPPs aligned to payers,
pharmacy benefits managers and similar organizations (collectively, “Organized SPP Customers”), the
Specialty Access Solutions National Account Directors (SAS NADs) serve as the primary Pfizer
contact for Organized SPP Customers and work to support the engagement of SPPs through contracting
activities, contract implementation, and SPP program onboarding, among other things. A listing of such
Organized Customers can be found at the SPP Engagement Matrix at this link. SAS NADs also manage
relationships through aggregators that contract with the non-Organized SPP Customers for Supplemental
Services.
Other Field Commercial Colleague Interactions with SPPs
In addition to Organized SPP Customers, certain SPPs may operate independently or may be affiliated with
Integrated Delivery Systems (IDNs) or academic medical centers that are approved to dispense Pfizer
brands. These institutions may be supported by KAMs or similar Account Management roles. In order to
ensure effective understanding and implementation of the Key Compliance Principles listed above, Field
Commercial Colleagues other than SAS NADs (e.g. Sales, KAMs, and other Account Management roles)
must receive prior approval from their BU management, PCA, the global product counsel and Pricing &
Access Legal before engaging with SPP personnel. The process for obtaining such approval is set forth
below.
Sales Interactions with Specialty Pharmacy HCPs and SPP Office Staff
As a general rule, subject to the approval identified above, Pfizer Sales Representatives may engage with
HCPs and office staff at SPPs (including SPPs at IDNs/COEs) consistent with the requirements of Chapter
Field Commercial Colleagues and Interactions with SPPs

White Guide – Chapter 20: Non-discount Arrangements
with Specialty Pharmacies and Other Accounts
Rev. 12/20
Page 359 of 405
2 of this Guide. Any such interactions must arise from and be narrowly tailored to: 1) educate an SPP HCP
about the clinical profile of a Pfizer product; 2) educate SPP office staff about patient support programs
available to patients; or 3) further an otherwise approved purpose. Such interactions should be for
educational purposes only and must not include express or implied requests for the SPP personnel to
recommend Pfizer products either to patients or prescribing HCPs. Sales Representatives must not attempt
to influence the SPP personnel’s decision-making and may only use materials and messaging specifically
approved for use with SPPs during these interactions. In addition, Sales Representatives must not make
any express or implied requests for the SPP personnel to recommend Pfizer products either to patients or
HCPs.
Pfizer Sales Representatives are not permitted to discuss product discounts, rebates, reimbursement
details to SPPs, participation in Pfizer’s SPP Defined Networks, or Purchase or Service Agreements with
any personnel at an SPP. In the event that a Pfizer Sales Representative learns that the SPP wishes to
discuss such a contractual arrangement, the matter must be referred to the SAS NAD covering the SPP.
Finally, Sales Representatives must not share complaints about SPP performance issues that are relayed
to them by HCPs or others (e.g., slow dispense times, inadequate inventory, etc.) to any SPP personnel
directly or indirectly. Rather, the Sales Representatives must elevate potential SPP performance issues
that they learn about to their sales manager, who can share the information with the appropriate SAS NAD.
Interactions with Specialty Pharmacy Sales Representatives.
SPPs employ their own sales representatives to call on HCPs and promote the services of the SPP to
encourage referrals.
Subject to the approval process referenced below, Pfizer Sales Representatives may have limited
interactions with SPP sales representatives on a periodic, infrequent basis, where there is a legitimate
business purpose to share appropriate information. Pfizer sales representatives must not use these
contacts for relationship building with the SPP, to gather general payer information regarding Pfizer
products, or to seek information about market shares of competitive products within the SPP, top
prescribers, product volumes and utilization information on specific products, as this information may be
subject to restrictions on further use by Pfizer or third parties. Moreover, Pfizer Sales Representatives must
not share call or HCP target lists with SPP sales representatives and must not jointly meet with the SPP
representative and a prescriber or HCP office.
In addition, Pfizer Sales Representatives must not provide Pfizer materials to the SPP sales representative.
In the event that a SPP sales representative reaches out to a Pfizer Sales Representative with a product
question or request for product information, the Pfizer Sales Representative should refer the SPP sales
representative to the SPP’s own clinical personnel to answer the product-related question. SPP sales

White Guide – Chapter 20: Non-discount Arrangements
with Specialty Pharmacies and Other Accounts
Rev. 12/20
Page 360 of 405
representatives must not attend educational presentations provided by Pfizer Sales Representatives or by
Pfizer medical colleagues for SPP HCPs.
In interactions with SPP sales representatives, Pfizer Sales Representatives must never share or discuss
patient-specific information (even if de-identified), including information regarding the status of fulfillment of
prescriptions at the SPP or with a payer. Pfizer Sales Representatives are also prohibited from sharing
information about an HCP’s prescribing patterns or coordinating targeting of or visits to HCPs with the SPP
business representative; such actions may be perceived as inappropriately “steering” business to a
particular SPP.
Field Commercial Colleague SPP Interaction Approval Process
To ensure that commercial colleagues’ interactions with SPPs are consistent with these principles, a team
seeking approval for such interactions must obtain approval from their global product counsel, Pricing &
Access Legal and for organized SPPs the Payor Channel Access team. Among the factors to be considered
are:
• The duration and frequency of these interactions;
• The training requirements associated with the SPP engagements; and
• The risk mitigation strategies and tactics associated with these interactions.
Requests for approvals must be reviewed periodically. Periodic renewal of the required approval will help
to ensure that the factors on which the initial approval was based still exist, and that sufficient guidance,
training, and monitoring are in place. As with other field-based roles, colleagues partaking in such activities
may be subject to ongoing monitoring, field rides, and audits.
Non-compliance with these policies puts the Company at risk and can subject colleagues to
disciplinary action up to and including termination of employment.
Approval Process for Sales Representative Interaction

White Guide – Chapter 20: Non-discount Arrangements
with Specialty Pharmacies and Other Accounts
Rev. 12/20
Page 361 of 405
• For more information about HIPAA, see White Guide Chapter 11: Privacy: Protecting Personal
Information.
• General Questions regarding this chapter should be referred to your manager and/or the Pricing &
Access Legal Team.
• Orange Guide – Chapter 12: Discount Arrangements and Contracting.
• Orange Guide – Chapter 15: Service Agreements and Other Non-Discount Arrangements with
Accounts.
• Orange Guide – Chapter 20: Special Types of Services Agreements – Interactions with Specialty
Pharmacies.
For More Information

Rev. 12/20
Page 362 of 405
CHAPTER #21 – PATIENT SUPPORT
ROLES

White Guide – Chapter 21: Patient Support Roles
Rev. 12/20
Page 363 of 405
Chapter
#21
PATIENT SUPPORT ROLES
CONTENTS
Introduction................................................................................................................................................ 364
Patient Support Role Descriptions ............................................................................................................ 364
Field Reimbursement Managers (FRMs) ........................................................................................... 364
Clinical Educators (CEs) ..................................................................................................................... 365
Patient Affairs Liaisons (PALs) ........................................................................................................... 365
Key Points to Ensure Compliance for Patient Support Roles ............................................................ 365
Guidance Principles for Patient Support Roles ......................................................................................... 366
Provide only Limited Support .............................................................................................................. 366
Non-Promotional Nature of Patient Support Roles ............................................................................. 366
No Promotion of Pfizer Products ........................................................................................................ 367
Independence from Colleagues with Marketing or Sales Responsibilities ......................................... 367
Educating HCPs and Patients about Patient Support Roles .............................................................. 368
Appropriate Selection of Patient Support Role Customers/Audiences .............................................. 368
Patient Support Role Creation Process and Approval ....................................................................... 369
Contract Patient Support Organization ............................................................................................... 370
For More Information ................................................................................................................................. 370
Patient Support Roles

White Guide – Chapter 21: Patient Support Roles
Rev. 12/20
Page 364 of 405
Chapter #21 Patient Support Roles
Pfizer is committed to supporting patient access to the medicines prescribed by Healthcare Providers
(HCPs) in a manner consistent with all applicable laws and regulations. As part of this commitment, some
Pfizer brands may offer brand-specific reimbursement and patient support activities that are carried out by
field-based colleagues (hereinafter “Patient Support Roles”). Patient Support Roles are field-based,
external roles that seek to expand access to, reimbursement of, and education about Pfizer products in a
non-promotional manner. As of this Chapter’s publication, Patient Support roles include Field
Reimbursement Managers (FRMs), Clinical Educators (CEs) and Patient Affairs Liaisons (PALs).
Although Patient Support Roles are external facing roles, they are not intended to promote Pfizer products.
This Chapter provides guidance for teams who interact with, and colleagues who directly and indirectly
manage, Patient Support Roles, and supplements applicable policies and procedures including the Blue
Book and the Orange Guide Chapter 21.
To ensure compliance with applicable laws, the following principles govern Pfizer Patient Support activities:
• Patient Support Roles must not provide substantial, independent value to HCPs, HCP practices,
patients or their caregivers;
• Patient Support Role activities must be made available in a non-discriminatory fashion to all appropriate
HCPs and eligible patients; and
• Patient Support Role offerings must be unrelated to the volume or value of business generated by any
HCP or healthcare facility or to any decision by a patient to use a Pfizer product.
As of the writing of this Chapter, the Patient Support Roles include the following:
Field Reimbursement Managers (FRMs)
FRMs are subject-matter experts on reimbursement, access, and coverage issues affecting Pfizer products.
They educate HCPs and their staff on matters relating to reimbursement, access, and coverage to facilitate
appropriate patient access to prescribed Pfizer products. FRMs may also respond to patient-specific
access and reimbursement questions from HCPs and office staff. FRMs may engage commercial and
government payers, including Medicare Administrative Contractors and Medicare Carrier Advisory
Committees, to discuss systemic obstacles to and support policy-level decisions about patient access to
Pfizer products. FRMs may attend relevant state and regional societies and associations meetings to keep
apprised of reimbursement and coverage developments that may affect Pfizer products. FRMs can also
Introduction
Patient Support Role Descriptions

White Guide – Chapter 21: Patient Support Roles
Rev. 12/20
Page 365 of 405
coordinate with Pfizer Hubs concerning individual patient cases. In addition to this chapter, FRMs should
consult role- and/or BU specific guidance on their activities and interactions.
Clinical Educators (CEs)
HCP-Facing CEs educate HCPs and relevant office staff on topics such as relevant disease states, proper
administration of Pfizer products, safety and tolerability matters (including monitoring and management),
contraindications, warnings, and other relevant Product characteristics. Patient-Facing CE’s are
responsible for educating patients and their caregivers on disease awareness and management and
providing basic information on proper use and administration of Pfizer products and related devices.
Patient Affairs Liaisons (PALs)
PALs are field based, non-promotional, community-facing colleagues who serve as educational resources
for both local advocacy groups and individual patients and caregivers. The PALs primary function is to
engage in proactive outreach to local advocacy and patient groups to understand their goals, objectives
and needs, and to develop strong working partnerships to help advance the needs of the patient community.
PALs may staff exhibits and displays at patient meetings and conferences, as well as educate patients and
their caregivers on disease awareness and management.
• Patient Support Roles may provide only Limited Support
•
Support may not replace services HCPs would otherwise conduct or pay office staff or a third
party to perform.
•
Activities must be connected to a Pfizer product, and may not constitute routine business
support or address practice-level issues.
• Patient Support Roles are non-promotional:
o They may not engage in promotional activities;
o
Patient Support Roles must remain independent from and may not be directed by HQ
and Field Commercial Colleagues with marketing or sales responsibilities; and
o
Patient Support Roles may not be used to promote or differentiate Pfizer or its products.
• Patient Support Roles activities and materials must be on-label.
• Ensure Patient Support Roles, materials and tactics protect patient privacy.
Key Points to Ensure Compliance for Patient Support Roles

White Guide – Chapter 21: Patient Support Roles
Rev. 12/20
Page 366 of 405
Provide only Limited Support
As discussed in Chapter 1, federal and state anti-kickback laws prohibit payments -- or any other exchange
of in-kind value -- intended to induce the purchase, prescription, endorsement, or recommendation of a
product that is reimbursed under federal or state healthcare programs. Patient Support Roles may implicate
anti-kickback laws if used to induce patients to request or physicians to prescribe Pfizer products. To
mitigate this risk, Patient Support Roles must never offer patients or HCPs services that have “independent
value,” which is defined as “more than limited support in connection with the purchase or prescription of a
Pfizer product.” Any activities that defray costs or expenses that the HCP, patient or caregiver would
otherwise incur may be seen as providing independent value and are therefore prohibited. See the below
chart for examples of prohibited activities that provide “independent value.”
Non-Promotional Nature of Patient Support Roles
Patient Support Roles, while externally facing roles, are not intended to promote Pfizer products. To remain
non-promotional, Patient Support Roles must ensure their activities and interactions, with both external
parties and internal colleagues, are not, and do not appear, promotional.
Prohibited “Independent Value” Activities
• Providing any consultant-type services or back-office support that eliminate an expense that the HCP
would otherwise incur.
• Accessing a patient record or working with staff to fill out clinical parts of statements of medical
necessity, prior authorization forms, or appeals forms.
• Performing profitability or other financial analyses for an HCP practice, or otherwise advising a
practice on how it could operate more profitably.
• Providing any routine practice operating services (e.g., filling out forms, submitting claims, coding
records, processing bills, compiling documentation for PA or appeals and/or submitting
PAs/appeals).
• Providing general advice on billing or coding issues not related to Pfizer products or devices that are
necessary for the use of the product
• Providing medical advice, a patient should otherwise receive from their HCP.
• Performing services for a patient or caregiver they would otherwise have to do for themselves.
Guidance Principles for Patient Support Roles

White Guide – Chapter 21: Patient Support Roles
Rev. 12/20
Page 367 of 405
No Promotion of Pfizer Products
Patient Support Roles, while externally facing roles, are prohibited from promoting Pfizer products. FRMs
may not promote or detail any Pfizer product, and may not discuss clinical product information with HCPs
or office staff. Similarly, while PALs, HCP- and Patient-Facing CEs are responsible for delivering some
product-related information, it must be delivered in a manner that is non-promotional, and without claims
as to a product’s efficacy or safety. Therefore, Patient Support Roles’ RC approved materials may not
contain promotional claims about Pfizer products. Rather, Patient Support Role materials should be
narrowly tailored to supporting patient access to and education about Pfizer products. All Patient Support
Role activities and materials should be on-label.
In addition, Patient Support Role activities should not have promotional tactics, goals or objectives. Patient
Support Role operating plans, tactical plans and goals should be consistent with their non-promotional
nature and independent from commercial goals. Lastly, Patient Support Roles performance metrics and
accountabilities must be independent of commercial objectives and goals. Patient Support Roles may not
be measured by prescription, sales or revenue generation. And in no instance should a “return on
investment (ROI)” calculation be done, or the “value” of Patient Support Roles be determined by comparing
pre- and post- Patient Support Role interaction prescribing patterns.
All Patient Support materials for use with HCPs, patients and caregivers must be reviewed and
approved by the applicable Review Committee. While these materials are not intended to promote
Pfizer products, inclusion of certain information or formatting (i.e., brand name, brand colors) may
trigger the need for product safety information and filing with the FDA. The Review Committee will
make the necessary determinations and filings.
Independence from Colleagues with Marketing or Sales Responsibilities
To remain non-promotional, Patient Support Role activities must be independent from promotional activities
and influence. Patient Support Role activities may not be directed by Pfizer colleagues with sales or brand
marketing responsibilities. Consistent with this, Patient Support Role managers (the “Patient Support Role
Team Lead”) and managers of Patient Support Role Team Leads, should not have any direct sales or brand
marketing responsibilities. Teams seeking to create Patient Support Roles should work with Legal Division
to ensure appropriate reporting lines.

White Guide – Chapter 21: Patient Support Roles
Rev. 12/20
Page 368 of 405
Patient Support Roles must limit their interactions with colleagues who have sales and marketing
responsibilities. See the Orange Guide Chapter 21 for more information.
Educating HCPs and Patients about Patient Support Roles
Communication about the availability of Patient Support Roles can raise significant legal concerns if such
communication seeks to induce the prescription, purchase or referral of Pfizer products. Therefore, Pfizer
may not promote the availability of Patient Support Roles as a reason to prescribe Pfizer products.
Furthermore, Pfizer should not use Patient Support Roles to differentiate Pfizer products from competitor
products or suggest that Patient Support Role activities provide substantial independent value to any HCP,
patient or caregiver. Lastly, Patient Support Roles may not be used as levers to gain access for Sales
Colleagues or Account Managers, and the availability of Patient Support Role offerings may not be made
contingent on providing access to other Pfizer colleagues. Teams may create materials for the purpose of
educating HCPs and patients about Patient Support Roles, their availability and how they can facilitate
patient access to and education about Pfizer products. However, such materials should not “promote,”
make claims about, or position Patient Support Roles as providing “value” to or conducting “services” for
the HCP or staff. And all such materials should be reviewed and approved by the applicable RC.
Appropriate Selection of Patient Support Role Customers/Audiences
Patient Support Roles should avoid off label discussions and select their audiences accordingly. Patient
Support Roles should not seek to engage HCPs or patients and caregivers of HCPs practicing in specialties
excluded from Sales Colleague or Account Manager call lists as those specialties generally prescribe the
relevant product for off-label purposes.
Patient Support Role activities must be made available in a non-discriminatory fashion to all appropriate
HCPs and eligible patients, unrelated to the volume or value of business generated by any HCP and any
decision by a patient to use a Pfizer medicine. Rather, the decision to engage should be based on the
customer’s or patient’s need for the information the Patient Support Role provides. Consistent with this,
Sales Colleagues and Account Managers may not direct or influence Patient Support Role Colleagues to
engage with certain audiences. In addition, Sales Colleagues and Account Managers must refer requests
for inclusion on Patient Support Role call and contact lists to the applicable Patient Support Role Team
Lead. Such requests may not be sent directly to the Patient Support Role colleagues. Sales Colleagues
and Account Managers may also provide the relevant Patient Support Role colleague’s contact information
to HCPs and offices who request it. Patient Support Role Leads and Brand teams, in consultation with
Legal, may identify additional methods for Sales Colleagues and Account Managers to pass along HCP
referrals (i.e., iPad apps, e-mail templates, etc.).

White Guide – Chapter 21: Patient Support Roles
Rev. 12/20
Page 369 of 405
While Sales Colleagues and Account Managers may not influence the selection of HCPs with whom
Patient Support Roles engage, Sales Colleagues and Account Managers can notify Patient Support
Role Colleagues if they learn an issue about an ongoing Patient Support Role engagement in an
unsolicited manner. For example, if an HCP asks a Sales Colleague about the status of an ongoing
benefit investigation by an FRM, the Sales Colleague may contact the FRM directly and alert the
FRM to the issue about which the HCP is seeking follow up. In such instances, the Sales Colleague
should not collect or transmit any personal health information. And Sales Colleagues and Account
Managers should not solicit such queries from Patient Support Role audiences.
A Patient Support Role team lead, in consultation with Legal and Compliance, may use HCP or office
prescribing and diagnosis data – among other factors – to inform their decision on whether to include an
HCP or office on the Patient Support Role contact list. This data may be used to determine whether Pfizer
products are being prescribed, whether they are being prescribed for on or off label uses, and the eligible
patient population seen by the HCP office with the goal of ascertaining the HCP or office’s need for the
approved information that the Patient Support Role is permitted to share. However, such data must not be
used to reward high prescribing HCPs with Patient Support Role engagement, or to target high prescribers
of a competitor product for conversion of patients to a Pfizer product.
Patient Support Role Creation Process and Approval
To ensure that Pfizer’s Patient Support Role activities are consistent with these principles, a team seeking
to create a Patient Support Role or make a material change to an existing Patient Support Role must obtain
approval from the Pfizer Legal Division. Legal will evaluate the risks and benefits of implementing the role
or its proposed activities. Among the factors to be considered are:
• How, consistent with its non-promotional nature, the Patient Support Role will support access to and
education about the Pfizer product;
• The relative efficacy, safety, and cost of the Pfizer product;
• The clinical appropriateness of any increased utilization that may result from the proposed Patient
Support Role activities;
• Whether, when viewed independently or in connection with other relevant Pfizer offerings, the proposed
Patient Support Role activities could constitute independent value to an HCP, patient or caregiver; and
• The need for and feasibility of any risk mitigation strategies and tactics.
Patient Support Role approvals must be renewed periodically. Periodic renewal of the required approval
will help to ensure that the factors on which the initial approval was based still exist, and that sufficient
guidance, training and monitoring are in place. The frequency with which approvals must be renewed will
be set forth in more detailed guidance governing the process to approve Patient Support Roles. As with

White Guide – Chapter 21: Patient Support Roles
Rev. 12/20
Page 370 of 405
other field-based roles, colleagues in Patient Support Role activities will be subject to ongoing monitoring,
field rides and audits.
The Legal Division will create a process to facilitate the review and approval of team requests to
create or materially change an existing Patient Support Role and renew approvals as necessary.
Contact your team or attorney or Pricing & Access Legal for more information on the approval and
renewal process.
Contract Patient Support Organization
Pfizer may contract with vendors to carry out Patient Support Role activities. In addition to business factors,
Vendors should be selected based on their reputation for compliance and ability to facilitate robust training
and self-monitor activities. Patient Support Role vendors will be expected to: (i) comply with all applicable
Pfizer policies and state and federal laws and report any deviations thereof; (ii) participate in any trainings
deemed necessary by Legal and Compliance; and (iii) complete any training or compliance certifications
deemed necessary by Legal or Compliance. All vendor personnel involved in the execution and
management of Patient Support Roles must be trained on the legal principles and relevant Pfizer policies
governing their role prior to participating in such activities.
Vendors must execute a contract with Pfizer prior to carrying out any activities on Pfizer’s behalf. The
contract must adequately set forth vendor’s business and compliance obligations. Any compensation to
which the vendor may be entitled must be fair market value for bona fide services. Vendor compensation
may not consider the number of prescription or referrals the vendor’s activity may generate. Teams seeking
to hire vendors to carry out Patient Support Role activities must work with Procurement and the Legal
Division on, and submit to any process supporting, vendor selection and hiring.
Teams seeking to create a Patient Support Role should work with their Global Product Counsel and Pricing
& Access Legal to help ensure compliance.
• For more information, see the Orange Guide: Chapter 21 - Patient Support Roles.
• Refer any questions to your manager, Legal or Compliance.
For More Information

Rev. 12/20
Page 371 of 405
CHAPTER #22 – INDEPENDENT
MEDICAL GRANTS

White Guide – Chapter 22: Independent Medical Grants
Rev. 12/20
Page 372 of 405
Chapter
#22
INDEPENDENT MEDICAL GRANTS
CONTENTS
Introduction................................................................................................................................................ 373
Key Points to Ensure Compliance ...................................................................................................... 373
Investigator Sponsored Research ...................................................................................................... 375
Scientific Validity ................................................................................................................................. 375
Nature and Basis of Pfizer Support .................................................................................................... 377
Independence and Investigator Responsibilities ................................................................................ 377
Regulatory and Ethical Framework .................................................................................................... 378
IND Requirements ........................................................................................................................ 378
Institutional Review Board (IRB)/Independent Ethics Committee (IEC) Approvals ..................... 379
Publication of ISR Study Results ........................................................................................................ 379
General Research Grants ......................................................................................................................... 379
General Research vs. ISR Grants ...................................................................................................... 379
Independent Quality Improvement and Medical Education Grants .......................................................... 380
Overview ............................................................................................................................................. 380
Application Submission ...................................................................................................................... 381
Application Review, Notification, and Payment .................................................................................. 381
Pfizer May Not Influence Grant Funded Activities .............................................................................. 382
Promotional Opportunities at Medical Education Conferences .......................................................... 383
Complimentary Exhibit or Display Space ........................................................................................... 384
For More Information ................................................................................................................................. 384
Independent Medical Grants

White Guide – Chapter 22: Independent Medical Grants
Rev. 12/20
Page 373 of 405
Chapter #22 Independent Medical Grants
Independent Medical Grants (IMGs) must only be used to support bona fide independent initiatives (e.g.,
research, quality improvement, or education) that are scientifically and ethically sound. Independent
Medical Grant types include:
• Investigator Sponsored Research (ISR)
• General Research
• Quality Improvement
• Medical Education
Key Points to Ensure Compliance
Pfizer’s IMG processes must be conducted in accordance with all applicable local laws and regulations,
applicable Pfizer policies and SOPs, as well as applicable country or region-specific industry and
professional standards and aligned with Pfizer’s medical and/or scientific areas of program scope.
Interactions between Pfizer and requesting organizations must be conducted in a manner that ensures
independence of the research, project or activity that is being supported. Pfizer colleagues must not control
or influence the development or content of an IMG request or the resulting research, project, initiative, or
medical education activity.
Pfizer’s evaluation of an IMG request must not be influenced by a grant requester’s or grantee’s, or their
organization's generation of past or potential future business for Pfizer but is determined solely by the
required evaluation of the components of the proposal (e.g., scientific merit of the grant, potential grantee’s
qualifications to conduct the proposed activities described in the grant request). Pfizer does not provide
IMGs based upon past, present, or future prescribing, purchasing, or recommending of Pfizer product(s) by
HCPs or healthcare organizations.
A grant must never be offered or provided as an inducement to approve, reimburse, prescribe, purchase,
or recommend a Pfizer product or to influence the outcome of a clinical trial, or to improperly benefit Pfizer’s
business activities.
The amount of financial support must be relative to the nature of the research or program and not dependent
on Pfizer’s relationship with the grant requester or their organization.
Introduction

White Guide – Chapter 22: Independent Medical Grants
Rev. 12/20
Page 374 of 405
Pfizer does not accept tangible benefits (e.g., goods or services) in return for supporting a grant. Refer to
USFR-SOP-02-01 and R&D SOP 201 for information on requirements and process for external funding
requests involving receipt of tangible benefits.
Pfizer does not own the results (e.g., data) or end product (e.g., medical device) developed as a result of
the grant.
There must be no involvement of commercial colleagues (including sales, marketing, account management,
commercial development, and access colleagues) in any aspect of the review and approval of the project,
project design, set-up, and execution, including providing any funding directly from a commercial budget.
IMG requests are managed by the Global Medical Grants group in Pfizer’s Worldwide Medical & Safety
organization. With the exception of Pfizer’s Competitive Grant Program, requests for independent medical
grant support must be initiated by an external organization, not solicited by Pfizer. Refer to GNT01-GSOP
– Independent Medical Grants for more information.
Each request is evaluated based on objective criteria including the institution’s ability to properly oversee
and conduct the study/project/education in compliance with applicable regulations and guidelines, design,
budget, and scientific rationale. Whether Pfizer will benefit from the outcomes of the
study/project/education in any way should not be a basis for approval. Thus, all IMGs are reviewed by
Medical Affairs, Research Unit colleagues and/or Global Medical Grants colleagues, and sales and
marketing personnel may not influence any decision-making.
In addition to increased scrutiny of clinical trial conduct and payments to HCPs in recent years, the HHS
Office of Inspector General (OIG) has issued compliance guidance addressing independent medical
grants. This guidance and government investigations reinforce that funding must not be used to induce or
reward the purchase of a manufacturer's products. Further, ISR or General Research studies must not be
used as “seeding” studies for unapproved indications. Buttressing fraud and abuse laws, trade
organizations including the Advanced Medical Technology Association (AdvaMed) and the
Pharmaceutical Research and Manufacturers of America (PhRMA) have issued compliance guidelines
regarding ISR studies. All relevant local, anti-corruption, and anti-kickback laws also apply.
Why support ISR studies? ISR studies expand therapeutic area and product knowledge, including
safety information. Researchers may identify new ways of using existing treatments or
investigational compounds, or they may focus on under-studied patient populations.
As a result of this regulatory framework, and because they are independent, Pfizer colleagues should not
be involved in the design, conduct, supervision, management, or monitoring of any study/project/education
that is supported by an IMG. Such actions would be out of compliance with the SOP governing IMGs,

White Guide – Chapter 22: Independent Medical Grants
Rev. 12/20
Page 375 of 405
GNT01-GSOP – Independent Medical Grants. In addition to the above risks, involvement of Pfizer
colleagues in these initiatives could result in Pfizer becoming subject to liability for the study generally
and/or for ensuring regulatory compliance of the study.
Investigator Sponsored Research
ISR grants, by definition, are those grants that support research that involves a Pfizer asset. Either the
grant includes investigational product (e.g. drug, vaccine, medical device) and/or the intent of the research
is to study a Pfizer therapy. Pfizer accepts proposals for ISR grants submitted by interested investigators
and institutions through its online web portal. Investigators may propose clinical studies of approved and
investigational uses of marketed products, or investigational Pfizer compounds and devices; in vitro or
animal studies; observational studies; or other types of independent research on disease states.
Pfizer cannot support requests that involve ongoing or pre-existing research projects. To ensure Pfizer
receives all necessary information, Pfizer requires the investigator to submit requests through the Global
Medical Grants System (GMGS) at https://www.pfizer.com/purpose/independent-grants.
Pfizer Medical (non-Commercial) and Research teams may also choose to implement a Competitive Grant
Program for research relating to a particular product, disease, or area of scientific inquiry. These programs
typically have a defined set of research criteria and are limited to a certain timeframe. They are publicized
broadly to a specific audience via professional journals or websites and typically have an external
independent advisory committee review and approve the program’s competitive grant recipients.
• The recipients of ISR study support must be chosen on the basis of the merits of their research
proposals and their scientific qualifications.
• An asset’s or product’s ISR strategy is determined by the Medical Asset or Medical Affairs
Leadership. Commercial colleagues may not attend strategy development meetings or otherwise
influence or participate in the decision to fund an ISR study. Refer to Refer to GNT01-GSOP –
Independent Medical Grants for additional guidance on ISR strategy determination.
Scientific Validity
Grant Reviewers, Pfizer colleagues representing various aspects of expertise, review ISR proposals for
medical and scientific merit and study feasibility. Grant Reviewers consider the investigator’s qualifications,
including his or her experience, training, and capability to perform all sponsor responsibilities such as filing
for any necessary regulatory approvals. The ISR investigator must agree, in accordance with standard
industry grant agreement terms, to provide Pfizer with a copy of final study results and any resulting
publications for Pfizer’s review. All information related to an approved ISR grant, including the scientific
rationale for support, is documented in the Global Medical Grants System (GMGS). Reviewers and
Approvers must record their assessment and decision to approve or reject a request directly in GMGS.
There is no option to conditionally approve a grant request.

White Guide – Chapter 22: Independent Medical Grants
Rev. 12/20
Page 376 of 405
ISR Support
Can a Pfizer colleague provide information on how to request an ISR grant?
Yes, colleagues can provide information on how to request an ISR grant, but they
cannot assist an HCP with the development of the protocol or the grant application.
Pfizer posts Areas of Interest on the Global Medical Grants website, so colleagues
should direct HCPs to the website if they have questions about grant support. In
addition, Pfizer also publishes Requests for Proposals through our Competitive Grants
Program, and HCPs can respond if they choose.
Can Pfizer support an ISR grant request involving an off-label use of a Pfizer product?
Yes. Research involving investigational use for an unapproved indication is not
considered “off-label” use of a Pfizer product and would be eligible for ISR support
only if the proposed research is intended to provide valuable scientific or clinical
information, improve clinical care, lead to new or improved treatments, or otherwise
benefit patients. Any decision to support such a proposal must be based on the
scientific merits of the proposal and must not be an attempt to influence the HCP’s
prescribing behavior. Pfizer may only support ISR requests and associated
investigators based on their credentials and research capabilities. Colleagues may
respond to an HCP’s unsolicited questions about the process for ISR requests but
should not proactively encourage or seek out ISR proposals for any reason.
ISR Support
Could our clinical personnel help an ISR applicant with a proposed protocol?
No, Pfizer must not be involved in any aspect of study protocol or project development,
nor the conduct or monitoring of the research/quality improvement program. For ISR
grants that include a Pfizer asset, or the research is assessing a Pfizer asset, medical
colleagues may provide feedback on an ISR request solely for the purposes of: (1)
safety assessments and safety reporting, including study drug dosing and handling, or
(2) describing why a specific ISR request was denied. Medical colleagues can also
discuss research and quality improvement areas of interest, track study progress and
require, minimally, a study status report twice per year.

White Guide – Chapter 22: Independent Medical Grants
Rev. 12/20
Page 377 of 405
Nature and Basis of Pfizer Support
An ISR grant may include Pfizer product (including marketed or investigational products, finished goods,
and/or pure substance), funding, or both product and funding. ISR grants are only provided to support
specified, prospectively approved research projects. ISR grants may not be provided to support general
research, educational or training programs, or studies being conducted on behalf of Pfizer nor where
services are being provided for Pfizer’s benefit, such as development of software, technology, or
methodologies to which Pfizer would be granted ownership. In accordance with customary industry
intellectual property practices, Pfizer might require certain non-exclusive license rights to Pfizer product-
related inventions arising from Pfizer grant funding. ISR grants may not support studies that would involve
or are intended to assist Pfizer with new product registration, a change in Pfizer product labeling, or other
regulatory approval efforts.
See Refer to GNT01-GSOP – Independent Medical Grants and CMCD CT44–GSOP: Clinical and Research
Collaborations, for additional details.
Further, when considering ISRs for a given asset program, it should be understood that Pfizer does not
own the data and therefore cannot use study results for promotion. Brand teams, however, may seek RC
approval for promotional use of a published ISR study reprint, if it meets the guidelines outlined in White
Guide Chapter 2: Advertising and Promotional Materials.
ISR grants may not be provided to reward or influence the prescribing practices of the investigator or
institution. ISR grants must not be based in any way on any preexisting or future business relationships
with the investigator or institution or on any decisions the investigator or institution has made or may make
in the future related to Pfizer or Pfizer products.
Funding for an ISR study must represent fair market value for the activities being funded, including
applicable institutional overhead. As part of Pfizer’s review of an ISR study proposal, the Grant Approver
must assess the reasonableness of the study budget.
Pfizer support can only be provided once an ISR agreement has been fully executed and Pfizer has
received all of the required documents outlined in the agreement.
Independence and Investigator Responsibilities
All ISRs must follow GNT01-GSOP – Independent Medical Grants requirements. Since ISR grants support
independent research, all ISR protocols must be developed by the external investigator and/or institution
and, as the sponsor of the independent research, the grantee must assume all legal and regulatory
responsibilities. Pfizer may not be involved in any aspect of study protocol development, nor may Pfizer
be involved in the conduct or monitoring of the research. Pfizer may provide feedback on an ISR proposal
solely for the purposes of: (1) safety assessments and safety reporting, including study drug dosing and

White Guide – Chapter 22: Independent Medical Grants
Rev. 12/20
Page 378 of 405
handling; or (2) describing why a specific ISR proposal was denied. Pfizer can also discuss research areas
of interest, track study progress and require, minimally, a study status report twice a year. For any studies
undertaken by a third-party researcher where Pfizer is collaborating on clinical study design, conduct, or
data analysis, or where Pfizer intends to use and rely on the data, CMCD CT44-GSOP Clinical Research
Collaborations applies, not GNT01-GSOP – Independent Medical Grants.
Safety Information & Adverse Event Monitoring – ISRs
Are Pfizer study teams obligated to report safety information from an independent ISR
study? Can the ISR sponsor choose what type of information to report to regulatory
authorities?
Study sponsors cannot choose what safety information they report to regulatory
authorities. The ISR sponsor is required to record and evaluate all safety information
received from any source and provide expedited reports to regulatory authorities and
Pfizer of adverse events that are both serious and unexpected. However if a Pfizer
colleague becomes aware of an adverse event, Pfizer must also report it in accordance
with CMCD AEM01-POL: Adverse Event Monitoring (AEM) System. For ISRs, all
investigators, IRBs and IECs, as well as the relevant regulatory authorities, should be
immediately informed of significant unanticipated problems such as new safety
information. If significant safety information is discovered after study participants have
agreed to be involved in the study, the study participants must be provided this new
information, regardless of whether it may affect their willingness to continue to be
involved in the study.
Regulatory and Ethical Framework
IND Requirements
As with Pfizer-sponsored studies, investigator-sponsored clinical studies of drugs and biological products
in the United States must be conducted under an Investigational New Drug (IND) application, unless an
exemption applies. An IND is required for clinical studies involving an unapproved drug or vaccine and,
generally, for those studies that involve an FDA-approved drug or vaccine if the study will be used to support
a new indication, advertising claim, or significant change in product labeling, or if the study involves an
increased level of risk associated with the use of the drug (21 CFR 312.2). For clinical trials in the United
States utilizing a Pfizer product, Pfizer requires documentation of IND submission or exemption from the
ISR investigator-sponsor.

White Guide – Chapter 22: Independent Medical Grants
Rev. 12/20
Page 379 of 405
Institutional Review Board (IRB)/Independent Ethics Committee (IEC) Approvals
Unless an exemption applies, all applicable investigator-sponsored clinical studies must be reviewed and
approved by a qualified Institutional Review Board (IRB) or Independent Ethics Committee (IEC) to
help ensure the protection of the rights and welfare of study participants.
Publication of ISR Study Results
Pfizer supports the exercise of academic freedom and encourages investigators to publish the results of
ISR studies, whether or not the results are favorable to a Pfizer product. As with sponsored studies, Pfizer
may request an opportunity to review proposed publications or other public disclosures of the results in
advance to prevent inadvertent disclosure of Pfizer proprietary information. Pfizer may request a delay in
publication, if necessary, to protect intellectual property rights. Pfizer also expects the investigator or
institution to comply with recognized ethical standards concerning publications and authorship, including
the disclosure of Pfizer support of the study in any publication of study results. Note that it is not permissible
for Pfizer to author, contribute to the content or edit a manuscript that is based on an independent
investigator-sponsored clinical study supported with an ISR grant.
An ISR grant may include funding for publication costs, including manuscript preparation. This will be
specifically documented in the ISR agreement and associated project budget. Pfizer must decide before
the grant is awarded whether to include publication support in the ISR grant. Pfizer cannot make this
decision after completion of the study, as it could create the appearance that Pfizer’s decision was based
on whether the results of the project are favorable to a Pfizer product.
General Research vs. ISR Grants
General Research grants support the development or refinement of specific and defined medical knowledge
based upon medical and scientific merit. This grant type is used to support research that would otherwise
not be defined as an ISR, including support for an institution’s general research fund, Health Services
Research, Registry Development and/or Queries, Outcomes Research, and Research Fellowships*. A
General Research grant cannot include a Pfizer asset, nor can it support research that involves the study
of a Pfizer asset.
*NOTE: Fellowships supported by GMG use RFPs under the GMG Competitive Grants Program with final
decisions rendered by External Review Panels. To support unsolicited fellowships (i.e., those funding
requests to support fellowships initiated by organizations, and not initiated by Pfizer), colleagues should
refer to their divisional external funding SOP.
General Research Grants

White Guide – Chapter 22: Independent Medical Grants
Rev. 12/20
Page 380 of 405
These grants support interventional, non-interventional, outcomes, registry or other types of research that
does not involve a Pfizer asset (drug, compound). And like ISRs, General Research grants must follow
GNT01-GSOP – Independent Medical Grants requirements. All General Research protocols must be
developed by the external investigator and/or institution and, as the sponsor of the independent research,
the grantee must assume all legal and regulatory responsibilities. Pfizer may not be involved in any aspect
of study protocol development, nor may Pfizer be involved in the conduct or monitoring of the research.
General Research grant requests must be submitted by the external investigator and/or institution through
the GMGS. Pfizer colleagues may not submit a request on an investigator’s behalf. Since General
Research grants do not involve a Pfizer asset, grantees are not obligated to report Adverse Events to Pfizer,
but they are required to record and evaluate all safety information received from any source and provide
expedited reports to regulatory authorities of adverse events that are both serious and unexpected.
However if a Pfizer colleague becomes aware of an adverse event, Pfizer must also report it in accordance
with CMCD AEM01-POL: Adverse Event Monitoring (AEM) System.
Overview
Pfizer provides non-promotional funding to third-party organizations in the form of Independent Medical
Education (IME) and Quality Improvement (QI) grants. An IME grant refers to funding given to a third-
party entity to support an activity or initiative which serves to maintain, develop, or increase the knowledge,
skills, and/or professional performance of Healthcare Professionals (HCPs). A QI grant refers to funding
given to a third-party entity for QI which consists of systematic and continuous actions that lead to
measurable improvement in healthcare services and the health status of targeted patient groups. These
grant types are covered by SOP GNT01 – Independent Medical Grants.
Legitimate professional and educational initiatives that can be supported with IME grants include, but are
not limited to, activities like accredited Continuing Medical Education (CME)/Continuing Education (CE)
for HCPs. IME grants are permissible only if they are “independent,” which means that colleagues may not
influence the development or content of the supported activity or how it is conducted. For example,
colleagues cannot choose nor have any input on the topic, or the speakers who participate in the activity.
Additionally, if Pfizer colleagues are solicited by external organizations to serve as faculty, colleagues are
required to ascertain whether funding has been provided by Pfizer for the specific medical education
activity. Any independent CME/CE activity supported by Pfizer precludes Pfizer colleagues from serving
as faculty for that CME activity.
The review and approval of requests for IME and QI grants in the U.S. (and Puerto Rico) is managed by
the office of Global Medical Grants (GMG). GMG, a part of WMS, works with therapeutic area
representatives from BU Medical and Legal to develop IME and QI strategies for clinical areas of interest.
Independent Quality Improvement and Medical Education Grants

White Guide – Chapter 22: Independent Medical Grants
Rev. 12/20
Page 381 of 405
To be considered for funding, a grant request should align with these strategies and must meet all of the
criteria of an appropriate educational activity or QI initiative, including that it is independent, and information
provided is balanced, accurate, and not misleading, delivered to a broad audience, and reasonable in cost.
Additional criteria must be met when responding to a Request For Proposal (RFP) prepared by GMG in
collaboration with External Review Panels and/or in partnership with other third-party organizations
Under no circumstances does Pfizer condition grant funding upon past, present, or future
prescribing, purchasing, or recommending of Pfizer products, nor will Pfizer accept any benefits in
return for providing an IME or QI grant. By requiring the review and approval of these requests by GMG
(or when applicable, by External Review Panels), Pfizer seeks to minimize the risk that an IME or QI grant
could be approved, or perceived to have been approved, for an improper purpose.
Industry support of IME grants has been under increasing scrutiny by Congress and the U.S. Department
of Health and Human Services Office of Inspector General (OIG). In an effort to be more transparent,
Pfizer publicly reports grants and charitable contributions provided to medical, scientific, and patient
organizations in the United States, on the Pfizer website.
Application Submission
All requests for U.S. IME or QI grants must be submitted by the external organization directly to GMG via
Pfizer’s online Grant Management System (GMS) at www.pfizer.com/independentgrants. All
submissions, required documentation, and decisions are recorded and archived in GMS.
Types of organizations eligible to apply for grants include hospitals, academic medical centers, schools of
nursing or pharmacy, professional societies and associations, and other institutions specializing in specific
healthcare-related disciplines (e.g., public health, quality improvement). Eligible organizations may submit
a request for support of QI/health services initiatives and independent accredited or non-accredited
professional educational programs and activities. Requests for accredited IME must be submitted by
accredited organizations. Examples of qualified accreditations include ACCME, AAFP, and AOA, ACPE,
ANCC, AANP, AAPA, and NCOA. Providers must be in compliance with Pfizer standards as well as the
guidelines of the OIG, ACCME, and other relevant bodies, as applicable. Pfizer does not support requests
from individual physicians, private practice groups, or institutions that appear to have significant conflicts of
interest. For example, organizations where practicing HCPs have a proprietary or ownership interest in the
organization will not be eligible to apply for IME or QI grants from Pfizer. Additionally, funding from GMG
may not be used to support food and beverage for learners or audience participants.
Application Review, Notification, and Payment
GMG will review application submissions for completeness, alignment with IME or QI goals, compliance
with Pfizer policies, and other requirements. For those requests submitted in response to an RFP, final
decisions will be rendered by External Review Panels. Due to limited funding, not all grant requests will be
White Guide – Chapter 22: Independent Medical Grants
Rev. 12/20
Page 382 of 405
approved. Requestors will receive an e-mail notification when a grant is approved or denied. Funds are
sent directly to the requesting organization.
Colleague Roles in Grant Process
May a Field Commercial Colleague communicate with grant requestors regarding the
status of grant requests?
No. These colleagues must not be part of the submission, review, or approval process.
Requestors must communicate only with members of the GMG team regarding grant
requests, funding, or denials. Colleagues must direct requestors to the GMG website
at www.pfizer.com/independentgrants, or the dedicated e-mail address
Pfizer May Not Influence Grant Funded Activities
Colleagues may not offer suggestions regarding topics, content, or speakers to a CME/CE provider,
program sponsor, or speaker at a CME/CE medical education activity. Even if colleagues are asked to
provide input on topics or speakers, colleagues must decline. If a provider or speaker were to implement
Pfizer suggestions, the independence of that medical education program could be compromised.
Additionally, colleagues must not provide logistical support at an IME or QI activity.
On occasion, Pfizer may be offered promotional opportunities in connection with an IME or QI activity, such
as exhibit space or time to conduct a speaker program. Such opportunities may be accepted only under
strictly limited conditions. For information on promotional opportunities at CME/CE activities, see the
section below.
Colleagues’ Role in Preserving Independence
May a colleague provide input on the content of a CME/CE activity in order to inform
the accredited provider that the information is inaccurate or unreasonably favors Pfizer
products?
No. To preserve independence, colleagues, including those in GMG, must not provide
input or in any way influence the content of a CME/CE activity.

White Guide – Chapter 22: Independent Medical Grants
Rev. 12/20
Page 383 of 405
Colleagues’ Role in Preserving Independence
May a colleague provide input on the content of a non-CME/CE activity funded through
GMG? Similarly, can a colleague provide logistical assistance for a non-CE event
funded through GMG?
No. Pfizer considers all grant-funded activities, even non-CME/CE activities, to be
independent. Colleagues may not influence any grant-funded activity in any way.
Promotional Opportunities at Medical Education Conferences
Colleagues may not under any circumstances fund or provide a meal or any other type of expense
associated with a third party’s medical education conference or activity where CME/CE credit is being
offered.
If Pfizer is offered the opportunity to conduct a speaker program in connection with an accredited medical
education activity (ACCME, AAFP, or AOA), this may be done only under the following conditions:
• The Pfizer program must be conducted in a room physically separated from the space where
CME/CE content is being provided.
• At the start of the program, the speaker must clearly communicate to attendees that it is a separate
Pfizer promotional presentation not certified for CME/CE credit.
• Pfizer cannot provide meals or beverages in connection with the Pfizer program. Any meals
provided by a CME/CE provider must be made available to all CME/CE event attendees, including
those not attending the Pfizer presentation.
• No advice or guidance may be provided regarding the content of the medical education activity.
• No financial or other support, including payment for event expenses or meals, setting up logistics,
or handling non-Pfizer speaker arrangements, may be provided in connection with the Pfizer
program (subject to vary narrow exceptions for logistical expenses discussed in Orange Guide
Chapter 9: Speaker Programs for HCPs). As discussed above, financial support may only be
funded by an independent medical education grant approved by GMG.
If colleagues are offered an opportunity to conduct a speaker program at an event where CME/CE is not
being provided, the above restrictions do not apply; however, Sales Colleagues must still follow all
applicable Pfizer policies for promotional speaker programs (including the policies outlined in Orange Guide
Chapter 9: Speaker Programs for HCPs).

White Guide – Chapter 22: Independent Medical Grants
Rev. 12/20
Page 384 of 405
Complimentary Exhibit or Display Space
If exhibit opportunities are available at an event – whether or not CME/CE credit is being offered – Pfizer
may pay for placement of an exhibit or display at fair market value (FMV). From time to time the event
organizers may offer Pfizer complimentary exhibit and display space. If such complimentary offerings are
tied to a GMG-approved grant, then Pfizer may only accept complimentary exhibit space when it is equally
offered to all potential exhibitors.
• More information on Independent Medical Grants can be found on the GMG website,
www.pfizer.com/purpose/independent-grants. More information about requests for pure drug
substance alone can be found at the Compound Transfer Program website www.pfizer.com/ctp.
• For more information on SOPs please refer to the eSOP portal.
• Questions may be referred to a Medical colleague, your manager, or Pfizer Legal counsel.
For More Information

Rev. 12/20
Page 385 of 405
APPENDIX – ACRONYM LIST

White Guide – Appendix – Acronym List
Rev. 12/20
Page 386 of 405
Acronym
Definition
3PL
Third Party Logistics Provider
A
ABM
Area Business Manager
ACA
Affordable Care Act
ACCME
Accreditation Council for Continuing Medical Education
ACO
Accountable Care Organization
ACPE
Accreditation Council for Pharmacy Education
ADE
Adverse Drug Event
ADR
ADR – Adverse Drug Reaction
AE
Adverse Event
AER
Adverse Event Report
AfME
Africa/Middle East
AGC
Assistant General Counsel
AHP
Animal Healthcare Providers
AI
Active Ingredient
AKS
Anti-Kickback Statute
AMA
American Medical Association
AMCP
Academy of Managed Care Pharmacy
AMP
Average Manufacturer Price
ANCC
American Nurses Credentialing Center
AP
Accounts Payable
A&P
Advertising & Promotion
APC
Ambulatory Payment Classification
API
Active Pharmaceutical Ingredient
APN
Administrative Professionals Network
ARIBA
Pfizer Accounts System

White Guide – Appendix – Acronym List
Rev. 12/20
Page 387 of 405
Acronym
Definition
ASC
Administrative Services Contract
ASP
Average Sales Price
A/V
Audio Visual
AWP
Average Wholesale Price
B
BA
Business Analytics
BAA
Business Associate Agreement
BAMs
Biosimilars Account Managers
BB
Blue Book
BI
Benefits Investigation
Big 4
Department of Veterans Affairs, Department of Defense, Public Health Service & Coast
Guard
BLA
Biological License Application
BP
Best Price
BRE
Business Rules Engine
BRF
Business Rationale Form
BT
Business Technology
BU
Business Unit
BV
Benefits Verification
C
CAP
Consumer Assistance Program
CBO
Chief Business Office or Congressional Budget Office
CBOCs
Community Based Outpatient Clinics
CCQRO
Chief Compliance, Quality, and Risk Officer
CDA
Confidentiality Disclosure Agreement

White Guide – Appendix – Acronym List
Rev. 12/20
Page 388 of 405
Acronym
Definition
CDC
Center for Disease Control and Prevention
CDE
Customer Data Evaluation
CDHC
Consumer-Direct Health Care
CEs
Clinical Educators
CE
Continuing Education
CE&E
Customer Events & Engagement
CEO
Chief Executive Officer
CEP
Customer Engagement Program
CERF
Contract Execution Request Form
CFC
Customer Facing Colleagues
CFO
Chief Financial Officer
CFR
Code of Federal Regulations
CFU
Criteria-For-Use
CH
Consumer Health
CHC
Community Health Centers
CHIP
Children’s Health Insurance Program
CI
Clinical Investigator or Competitive Intelligence
CIA
Corporate Integrity Agreement
CISRs
Contracted Inside Sales Representatives
CLM
Contract Lifecycle Management or Closed Loop Marketing
CMCDs
Clinical and Medical Controlled Documents
CMD
Chief Medical Director
CME
Continuing Medical Education

White Guide – Appendix – Acronym List
Rev. 12/20
Page 389 of 405
Acronym
Definition
CMP
Comprehensive Medical Plan or Civil Monetary Penalty
CMS
Centers for Medicare & Medicaid Services
CO
Commercial Operations or Contracting Officer
COB
Coordination of Benefits
COBRA
Consolidated Omnibus Budget Reconciliation Act
CoE
Center of Excellence
COT
Class of Trade
CP
Corporate Policy
CPA
Capital Project Appropriations
CPI-U
Consumer Price Index – Urban
CPT
Current Procedural Terminology
CRCs
Clinical Research Collaborations
CRGs
Colleague Resource Groups
CROs
Contract Research Organizations
CSA
Cross-Site Access or Clinical Supply Agreement
CSO
Contract Sales Organization
CV
Cardiovascular
D
DAEO
Designated Agency Ethics Official
DataXfer
Designated Agency Ethics Official
DBM
District Business Manager
DDQ
Due Diligence Questionnaire
D&E
Development & Equity

White Guide – Appendix – Acronym List
Rev. 12/20
Page 390 of 405
Acronym
Definition
DEs
Director of National Employer Accounts
DFG
Digital for Growth
DHHS
Department of Health and Human Services
D&I
Diversity & Inclusion
DL
Distribution List
DLA
Defense Logistics Agency
DMC
Data Monitoring Committee
DME
Durable Medical Equipment
DoD
Department of Defense
DOJ
Department of Justice
DP
Direct Price
DPA
Data Purchase Agreement
DRA
Deficit Reduction Act of 2005
DRG
Diagnosis-Related Group
DSA
Distribution Services Agreement
DSCSA
Drug Supply Chain Security Act
DSH
Disproportionate-Share Hospital
DSU
Drug Safety Unit
DTC
Direct-to-Consumer
DTCA
Direct-to-Consumer Advertising
E
EA
Expanded Access
EAC
Estimated Acquisition Cost

White Guide – Appendix – Acronym List
Rev. 12/20
Page 391 of 405
Acronym
Definition
ECP
Essential Community Providers
E&D
Exhibit & Display
EF
Enabling Functions
EFR
Electronic File Room
EGWP
Employer Group Waiver Plans
EHB
Essential Health Benefits
EHR
Electronic Health Record
EH&S
Environmental Health & Safety
ELT
Executive Leadership Team
EMB
Pfizer Established Medicines (EMBU)
EMR
Electronic Medical Record
EOB
Explanation of Benefits
EPO
Exclusive Provider Organization
eRIM
Enterprise Records and Information Management
ERISA
Employee Retirement and Income Security Act of 1974
ERP
Emerging Risk Program or Expert Review Panels
ESI
Employer-Sponsored Insurance
ETMS
Electronic Territory Management System
EU
European Union
F
FAQs
Frequently Asked Questions
FCP
Federal Ceiling Price
FCPA
Foreign Corrupt Practices Act

White Guide – Appendix – Acronym List
Rev. 12/20
Page 392 of 405
Acronym
Definition
FDA
U.S. Food and Drug Administration
FDB
First Data Bank
FF
Field Force
FFS
Fee-For-Service
FMAP
Federal Medical Assistance Percentage
FMV
Fair Market Value
FPL
Federal Poverty Level
FQHC
Federally Qualified Health Center
FRF
Funding Request Form
FRMs
Field Reimbursement Managers
FSS
Federal Supply Schedule
FTC
Federal Trade Commission
FTO
Freedom To Operate
FUL
Federal Upper Limit
G
GC
General Counsel
GCMA
Global Content Management and Approval Program
GCN
Generic Code Number (6-character, First Data Bank)
GCO
Global Commercial Operations
GCP
Good Clinical Practice
GEP
Global Established Pharma
GG
Green Guide
GH&V
Global Health and Value

White Guide – Appendix – Acronym List
Rev. 12/20
Page 393 of 405
Acronym
Definition
GIP
Global Innovative Pharma
GMG
Global Medical Grants
GMI
Global Medical Information
GMS
Grant Management System
GO
Government Official
GPC
Global Product Counsel
GPD
Global Product Development
GPI
Generic Product Identifier (14-character, Medi-Span)
GPM
Global Performance Management
GPO
Global Purchasing Organization
GPP
Global Performance Plan
GRD
Government Relations Director
GSOC
Global Security Operations Center
GTC
Global Trade Controls
H
HAS
Hematology Account Specialist
HBU
Hospital Business Unit
HCEI
Healthcare Economic Information
HCP
Healthcare Professional
HCPCS
Healthcare Common Procedure Coding System
HCPMID
Healthcare Professional Medical Identification
HDAS
Health Data Analytics Specialist
HDHP/SO
High Deductible Health Plan with Savings Option

White Guide – Appendix – Acronym List
Rev. 12/20
Page 394 of 405
Acronym
Definition
HDL
High-Density lipoprotein
HHS
U.S. Department of Health and Human Services
HIE or HIX
Health Insurance Exchange
HIPAA
Health Insurance Portability and Accountability Act
HIT
Health Information Technology
HITECH
Health Information Technology for Economic & Clinical Health
HLOGA
Honest Leadership and Open Government Act
HMO
Health Maintenance Organization
HOPD
Hospital Outpatient Department
HR
Human Resources
HRoD
HR on Demand
HRSA
Health Resources and Services Administration
HSA
Health Savings Account
I
ICD
Informed Consent Document
ICMJE
International Committee of Medical Journal Editors
ICPAP
Independent Charity Patient Assistance Program
I&I
Inflammation & Immunology
IDE
Investigational Device Exemption
IDN
Integrated Delivery Network
IDP
Individual Development Plan
IEC
Independent Ethics Committee
IGLC
Independent Grants for Learning and Change

White Guide – Appendix – Acronym List
Rev. 12/20
Page 395 of 405
Acronym
Definition
IHS
Indian Health Service
IM
Internal Medicine
IMB
Pfizer Innovative Medicines (IMBU)
IME
Independent Medical Education
IND
Investigational New Drug
INDA
Investigational New Drug Application
IP
Intellectual Property
IPA
Independent Practice Association
IPAP
Institutional Patient Assistance Program
IPGS
Intellectual Property Global Services
IPSM
Intellectual Property Strategy Management
IRB
Institutional Review Board
ISP
Internet Service Provider
ISRs
Investigator-Sponsored Research Organizations or Inside Sales Representatives
K
KAMs
Key Account Managers
KFF/HRET
Kaiser Family Foundation/Health Research and Educational Trust
KITs
Key Intelligence Topics
KIQs
Key Intelligence Questions
KOL
Key Opinion Leader
KPI
Key Performance Indicator

White Guide – Appendix – Acronym List
Rev. 12/20
Page 396 of 405
Acronym
Definition
L
LCA
Least Costly Alternative
LDA
Lobbying Disclosure Act
LDET
Legal Division Executive Team
LIS
Low Income Subsidy
LMR
Life Management Resources
LOD
Legal on Demand
LOE
Loss of Exclusivity
LPAT
Legal Planning Advisory Team
LTC
Long-Term Care
LTD
Long-Term Disability
LVP
Local Vet Policy
M
MA
Medicare Advantage
M&A
Merger & Acquisition
MAC
Maximum Allowable Cost or Medication Advisory Committee
MA-PD
Medicare Advantage-Prescription Drug Plan
MAPP
My Anti-Corruption Policy and Procedures
MCC
Managed Care Customer
MCM
Multichannel Marketing
MCO
Managed Care Organization
M&E
Meetings & Events or Mergers & Acquisitions
MedPAC
Medicare Payment Advisory Commission

White Guide – Appendix – Acronym List
Rev. 12/20
Page 397 of 405
Acronym
Definition
MMA
Medicare Prescription Drug, Improvement, and Modernization Act of 2003
MOS
Medical Outcome Specialist or Manufacturing Operations Solutions
MRC
Medical Review Committee
MSA
Master Services Agreement
MYRA
My Reporting App
N
NAD
National Account Director
NAMs
National Account Managers
NCD
New Channel Director
NCPAS
Non-Clinical Publication Approval System
NDA
New Drug Application or Non-Disclosure Agreement
NDC
National Drug Code (11-character)
Non-FAMP
Non-Federal Average Manufacturer Price
NRx
New Prescriptions
NSAID
Nonsteroidal Anti-Inflammatory Drug
NYO
New York Office
O
OAM
Oncology Account Manager
OAS
Oncology Account Specialist
OBRA 90
Omnibus Budget Reconciliation Act of 1990
OCP
Organized Customer and Payer
OFG
Organizing For Growth
OG
Orange Guide (Field Guide)

White Guide – Appendix – Acronym List
Rev. 12/20
Page 398 of 405
Acronym
Definition
OGE
Office of Government Ethics
OIG
Office of Inspector General (of the Department of Health & Human Services)
OM
Organization Manager
ONC
Oncology
OOO
Office of Ombudsman or Out of Office
OOP
Out of Pocket
OPA
Office of Pharmacy Affairs (part of HRSA)
OPD
Outpatient Prescription Drug
OPE
Out of Pocket Expense
OPH
Office of Public Health
OOO
Office of the Ombudsman
OPPS
Outpatient Prospective Payment System
OTC
Over-the-Counter
P
P2L
Power2Learn
P4P
Pay for Performance
PA
Prior Authorization
PAC
Political Action Committee or Program Activity Coordinator
PAL
Pfizer Authorization Limit
PALs
Patient Affairs Liaisons
PAP
Patient Assistance Program
PBG
Physician Buying Group or Pfizer Biopharmaceutical Group
PBM
Pharmacy Benefit Manager

White Guide – Appendix – Acronym List
Rev. 12/20
Page 399 of 405
Acronym
Definition
PCA
Payer and Channel Access
PCCs
Pfizer Colleague Councils
PCEC
Pfizer Compliance Education Center
PCMH
Patient Centered Medical Home
PCP
Primary Care Physician
PCRs
Pharmaceutical Company Representatives
PDL
Preferred Drug List
PDMA
Prescription Drug Marketing Act
PDP
Prescription Drug Plan
PEH
Pfizer Essential Health
PEM
Pfizer Established Medicines
PERS
Public Employees’ Retirement System
PFE
Pfizer
Pfizer
CAReS
Pfizer Compassionate Access Request System
PGRP
Pfizer Global Recovery Program
PGS
Pfizer Global Supply
PHI
Patient Health and Impact or Protected Health Information
PHR
Professional Healthcare Representative
PhRMA
Pharmaceutical Research and Manufacturers of America
PHS
Public Health Service
PI
Package Insert or Prescribing Information
PIE
Preapproval Investigational Exchange
PIGO
Potentially Influencing Government Official

White Guide – Appendix – Acronym List
Rev. 12/20
Page 400 of 405
Acronym
Definition
PIH
Pfizer Innovative Health
PII
Personal Identifying Information
PLA
Pfizer Legal Alliance
PLI
Performance and Leadership Insights
PM
Pfizer Medical
PMA
Premarket Approval
PMPM
Per Member Per Month
PO
Purchase Order
POC
Point of Contact or Proof of Concept
POI
Position of Influence
POS
Point of Sale or Point of Service
PPACA
Patient Protection and Affordable Care Act
PPAF
Pfizer Patient Assistance Foundation
PPARx
Partnership for Prescription Assistance
PPO
Preferred Provider Organization
PPS
Prospective Payment System
PQCG
Product Quality Complaint Group
PRO
Peer Review Organization
PROMOS
Marketing Materials
PRxP
Pfizer RxPathways
PSAO
Pharmacy Services Administrative Organization
PSO
Provider-Sponsored Organization
P&T
Pharmacy and Therapeutics

White Guide – Appendix – Acronym List
Rev. 12/20
Page 401 of 405
Acronym
Definition
PT&E
Pfizer Travel and Expense
PTE
Patent Term Extension
Q
QEs
Quality Events
QGO
Qualified Government Official
QHP
Qualified Health Plan
QI
Quality Improvement
QIG
Quality Improvement Grant
QMS
Quality Management System
R
RAMP
Risk Assessment Mitigation Plan
RBC
Responsible Business Communication
RBD
Regional Business Director
RC
Review Committee or Regional Coordinator
RCD
Regional Channel Director
RCI
Referable Compliance Issue or Reportable Compliance Issue
RCL
Risk Counselor Lead
RCP
Retail Community Pharmacies
R&D
Research and Development
RD
Rare Diseases
RDS
Retiree Drug Subsidy
REG
Regulatory, Environmental and Global Supply
REMS
Risk Evaluation and Mitigation Strategy

White Guide – Appendix – Acronym List
Rev. 12/20
Page 402 of 405
Acronym
Definition
RFP
Request for Proposal
RM
Regional Manager
ROI
Return on Investment
RP
Reference Price
RPA
Robinson-Patman Act
RPS
Restricted Party Screening
RRS
Resource Reporting System
RxPathway
s
Pfizer’s Patient Assistance Program
S
SAF
Starter Activity Form
SAP
Statistical Analysis Plan
SARs
Starter Activity Reports
SAS
Specialty Access Solutions
SBL
Skill Based-Learning
SCHIP
State Children’s Health Insurance Program
SCOD
Specified Covered Outpatient Drug
SD
State Director or Sales Director or Senior Director
SE
Step-Edit
SFA
Sales Force Automation
SFG
Simplicity For Growth
SMART
Specific, Measurable, Actionable, Realistic, Time-Bound
SMD
Software Medical Devices
SNF
Skilled Nursing Facility

White Guide – Appendix – Acronym List
Rev. 12/20
Page 403 of 405
Acronym
Definition
SOC
Standard of Care
SOM
State Office Manager
SOP
Standard Operating Procedure
SOW
Statement of Work
SP
SharePoint
SPA
Single-Point of Accountability
SPAP
State Pharmaceutical Assistance Program
SPI
Sensitive Personal Information
SPNA
Speaker Program Needs Assessment
SPP
Specialty Pharmacy Provider
SSA
Social Security Administration
SSI
Supplemental Security Income
StratCO
Strategy and Commercial Operations
STD
Short-Term Disability
SVP
Senior Vice President
T
TBO
Tactical Business Objectives
TCL
Territory Credit List or Territory Call List
TIPPS
Transparency in Drug Purchasing Solutions
TMAC
Therapeutic maximum allowable cost
TPA
Third-party (claims) Administrator or Third Party Agreement
TPAA
Third Party Access Agreement
TPRM
Third Party Risk Management
TrOOP
True Out-Of-Pocket

White Guide – Appendix – Acronym List
Rev. 12/20
Page 404 of 405
Acronym
Definition
U
U&C
Usual and Customary (price)
UCR
Usual, Customary, and Reasonable
UM
Utilization Management
UMR
Unsolicited Medical Requests
URA
Unit Rebate Amount
USFDA
United States Food and Drug Administration
URO
Urology
USMI
US Medical Information
USP
United States Pharmacopeia
V
VA
Department of Veterans Affairs (Veterans Administration)
VAC
Vaccine
VADs
Vaccine Account Directors
VAMs
Vaccine Account Managers
VANF
VA National Formulary
VP
Vice President
W
WAC
Wholesale Acquisition Cost
WAMP
Widely Available Market Price
WBB
Worldwide Biopharmaceutical Business
WG
White Guide
WHO
World Health Organization
WL
Warning Letter

White Guide – Appendix – Acronym List
Rev. 12/20
Page 405 of 405
Acronym
Definition
WMS
Worldwide Medical and Safety
WRDM
Worldwide Research & Development and Medical
Y
YRR
Your Reporting Responsibilities
