
January 2024
17 - 1
CDC/NHSN Surveillance Definitions for Specific Types of Infections
Introduction
This chapter contains the CDC/NHSN surveillance definitions and criteria for all specific types of infections.
This chapter also provides additional required criteria for the specific infection types that constitute organ
space surgical site infections (Refer to Chapter 9 Appendix for specific event types available for organ
space SSI attribution for each NHSN operative procedure category). Comments and reporting instructions
that follow the site-specific criteria provide further explanation and are integral to the correct
application of the criteria. Refer to Chapter 2 (Identifying HAIs in NHSN) for specific guidance for making
HAI determinations.
Infection criteria contained in this chapter may be necessary for determining whether a positive blood
specimen represents a primary bloodstream infection (BSI) or is secondary to a different type of infection
(see Appendix B Secondary Bloodstream Infection (BSI) Guide). A BSI that is identified as secondary to
another site of infection must meet one of the infection criteria detailed in this chapter or an eligible
infection criterion in the Patient Safety manual and meet other requirements. Secondary BSIs are not
reported as Laboratory Confirmed Bloodstream Infections in NHSN, nor can they be associated with the
use of a central line.
NOTES:
• See individual protocol chapters for infection criteria for urinary tract infections (UTI),
bloodstream infections (BSI), pneumonia (PNEU), ventilator-associated infections (VAE), and
surgical site infections (SSI).
• For NHSN reporting purposes, the term “organism(s)” in this chapter includes viruses.
The term “physician” for the purpose of application of the NHSN HAI criteria may be interpreted
to mean a surgeon, infectious disease physician, emergency physician, other physician on the
case, or physician’s designee (nurse practitioner or physician’s assistant).
• Organisms belonging to the following genera cannot be used to meet any NHSN definition:
Blastomyces, Histoplasma, Coccidioides, Paracoccidioides, Cryptococcus and Pneumocystis. These
organisms are typically causes of community-associated infections and are rarely known to cause
healthcare-associated infections, and therefore are excluded.
• Antibiograms of the blood and isolates from potential primary sites of infection do not have to
match for purposes of determining the source of BSIs (see “matching organisms” below).
• A matching organism is defined as one of the following:

January 2024 Surveillance Definitions
17 - 2
1. If genus and species are identified in both specimens, they must be the same.
a. Example: An intraabdominal specimen is used as an element to meet IAB definition and is
growing Enterobacter cloacae. A blood specimen with a collection date in the IAB
secondary BSI attribution period is reported to be growing Enterobacter cloacae. These
are considered matching organisms.
b. Example: An intraabdominal specimen is used as an element to meet IAB definition and is
growing Enterococcus faecium. A blood specimen with a collection date in the IAB
secondary BSI attribution period is reported to be growing Enterococcus faecalis. These
are NOT considered matching organisms as the species are different.
2. If the organism is less definitively identified in one specimen than the other, the lesser
identified organism must be identified to at least the genus level and at that level, the
organisms must be the same.
a. Example: A surgical wound growing Pseudomonas species is used to meet deep incisional
SSI criteria and a blood specimen growing Pseudomonas aeruginosa is collected in the SSI
secondary BSI attribution period. The organisms are considered matching at the genus
level and therefore the BSI is secondary to the SSI.
b. Example: PCR identifying Enterococcus faecalis in CSF meets the MEN definition. A
subsequent blood culture collected in the MEN secondary BSI attribution period is
identified as Enterococcus species. The organisms are considered to be matching and
therefore the BSI is secondary to MEN.
3. There are two exceptions to the definition:
a. Infections meeting LCBI 2 criteria with Staphylococcus or Streptococcus:
Example-(Staphylococcus): A patient has a fever and a previous chest tube site is
reddened, swollen and a culture is collected from the soft tissue. The chest tube site
culture is reported positive for Staphylococcus species. SST/ST definition is met. The next
day 2 blood culture sets are collected. The blood cultures are both positive for coagulase-
negative Staphylococcus. The organisms are NOT considered matching, because
Staphylococcus species could represent a coagulase-negative or a coagulase-positive
Staphylococcus. Therefore, the BSI would not be considered secondary to SST/ST.
Example-(Streptococcus): A patient has a fever and a previous chest tube is reddened
swollen and a culture is collected from the soft tissue. The chest tube site culture is
reported positive for Streptococcus species. SST/ST definition is met. The next day, 2
blood culture sets are collected. The blood cultures are both positive for Streptococcus,
viridans group. The organisms are NOT considered matching, because Streptococcus
species could represent a Streptococcus, viridans group or non- Streptococcus, viridans
group. Therefore, the BSI would not be considered secondary to SST/ST.
January 2024 Surveillance Definitions
17 - 3
b. In cases where an organism is identified only as “yeast” or “yeast not otherwise
specified”, the organism can be considered a match to other yeasts, when collected
during the required timeframe, whether more fully identified or not.
Example: A culture of tissue from the ulcer margin of a decubiti reported positive for
yeast is used as an element to meet DECU definition. A blood specimen collected in the
secondary BSI attribution period of the DECU is reported as Candida albicans. In this
example, the two organisms are considered matching organisms as the organisms are
complementary (specifically, Candida is a type of yeast) and because yeasts isolated from
non-sterile sites are commonly not identified to the genus or genus and species level.
NOTE: This exception is limited to yeast. It does not apply to identification of
organisms as Gram positive cocci, Gram negative rods, etc.
Example: A culture of tissue from ulcer margin of a decubiti reported positive for Gram
negative rod is used as an element to meet DECU definition. A blood specimen collected
in the secondary BSI attribution period of the DECU is reported as E. coli. In this example
the two organisms are NOT considered matching organisms.
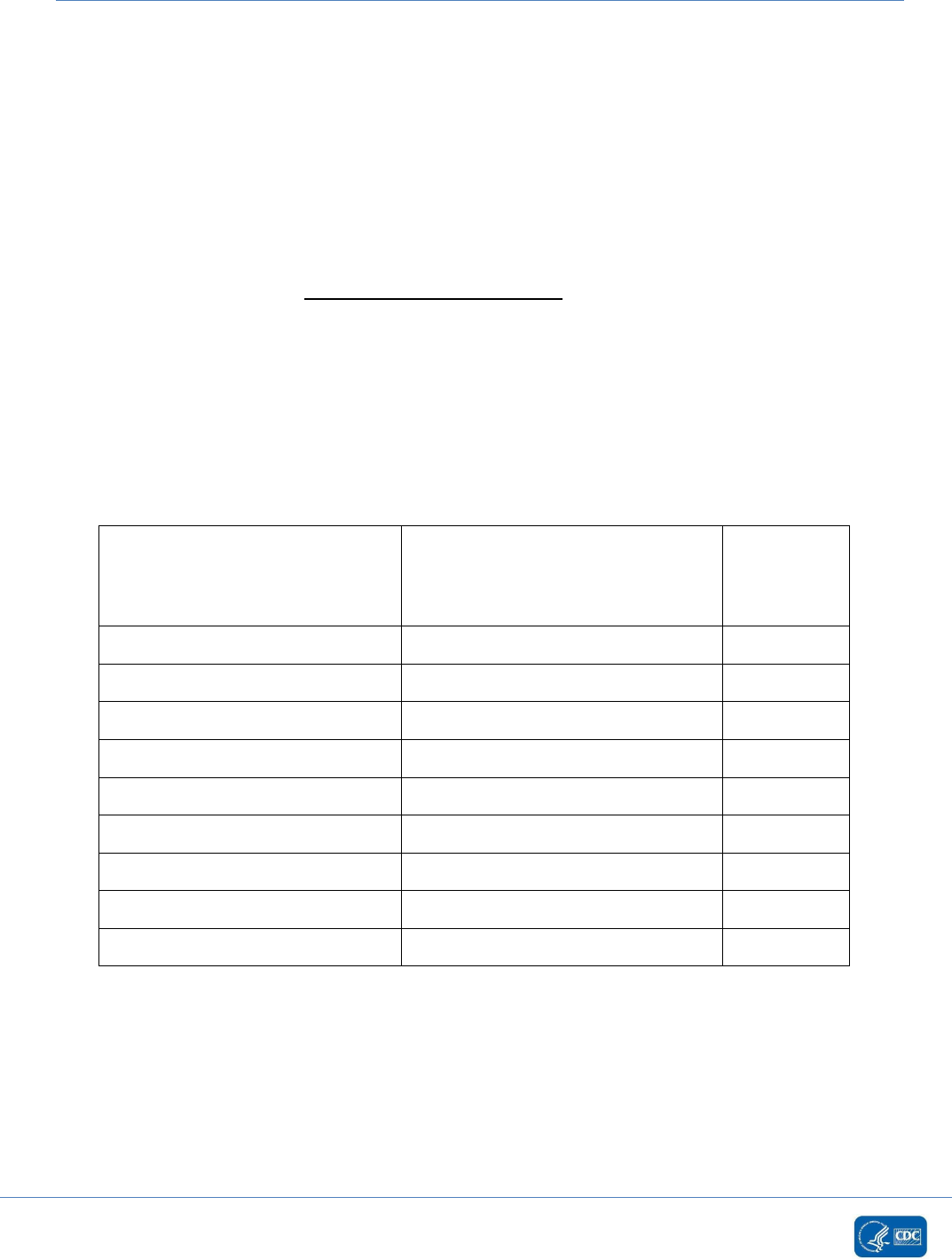
Examples for Determining Matching Organisms (correct selection for NHSN
reporting is bolded)
Identification # 1 Identification # 2
Matching
Organisms
Yes or No
Bacteroides vulgatus
Bacteroides fragilis
No
Enterococcus faecalis
Enterococcus
Yes
Enterococcus faecium
Enterococcus faecalis
No
Pseudomonas species
Pseudomonas aeruginosa
Yes
Coagulase-negative Staphylococcus
Staphylococcus aureus
No
Staphylococcus epidermidis
Coagulase-negative Staphylococcus
Yes
Staphylococcus species
Coagulase-positive Staphylococcus
No
Streptococcus species
Streptococcus Viridans Group
No
Yeast
Candida species
Yes
Infection criteria used for NHSN healthcare-associated infection surveillance have been grouped into 14
major types with some further categorized into specific infection types. For example, there are three
specific types of central nervous system infections (intracranial infection, meningitis or ventriculitis, and
spinal abscess/infection) that are grouped under the major type of CNS–Central Nervous System.
Infection criteria are listed in alphabetical order, according to their (abbreviated) major codes, and the
criteria for each of the specific types of infection follow it.

January 2024 Surveillance Definitions
17 - 4
Table of Contents
--
BJ – Bone and Joint Infection
6
BONE – Osteomyelitis
6
DISC – Disc space infection
6
JNT – Joint or bursa infection (not for use as Organ/Space SSI after HPRO or KPRO procedures)
7
PJI – Periprosthetic Joint Infection (for use as Organ/Space SSI following HPRO and KPRO only)
7
CNS – Central Nervous System
8
IC – Intracranial infection (brain abscess, subdural or epidural infection, encephalitis)
8
MEN – Meningitis or ventriculitis
9
SA – Spinal abscess/infection (spinal abscess, spinal subdural or epidural infection)
10
CVS – Cardiovascular System Infection
11
CARD – Myocarditis or pericarditis
11
ENDO – Endocarditis
12
MED – Mediastinitis
15
VASC – Arterial or venous infection excluding infections involving vascular access devices with
organisms identified in the blood
15
EENT – Eye, Ear, Nose, Throat, or Mouth Infection
16
CONJ – Conjunctivitis
16
EAR – Ear, mastoid infection
17
EYE – Eye infection, other than conjunctivitis
18
ORAL – Oral cavity infection (mouth, tongue, or gums)
18
SINU – Sinusitis
19
UR – Upper respiratory tract infection, pharyngitis, laryngitis, epiglottitis
19
GI – Gastrointestinal System Infection
20
CDI – Clostridioides difficile Infection
20
GE – Gastroenteritis (excluding C. difficile infections)
20
GIT – Gastrointestinal tract infection (esophagus, stomach, small and large bowel, and rectum)
excluding gastroenteritis, appendicitis, and C. difficile infection
21
IAB – Intraabdominal infection, not specified elsewhere, including gallbladder, bile ducts, liver
(excluding viral hepatitis), spleen, pancreas, peritoneum, retroperitoneal, subphrenic or
subdiaphragmatic space, or other intraabdominal tissue or area not specified elsewhere
22
NEC – Necrotizing enterocolitis
23
LRI – Lower Respiratory System Infection, Other Than Pneumonia
24
LUNG – Other infection of the lower respiratory tract and pleural cavity
24

January 2024 Surveillance Definitions
17 - 5
REPR – Reproductive Tract Infection
24
EMET – Endometritis
24
EPIS – Episiotomy infection
25
OREP –Deep pelvic tissue infection or other infection of the male or female reproductive tract
(for example, epididymis, testes, prostate, vagina, ovaries, uterus) including chorioamnionitis,
but excluding vaginitis, endometritis or vaginal cuff infections
25
VCUF – Vaginal cuff infection
25
SST-Skin and Soft Tissue Infection
26
BRST – Breast infection or mastitis
26
BURN – Burn infection
26
CIRC– Newborn circumcision infection
27
DECU – Decubitus ulcer infection (also known as pressure injury infection), including both
superficial and deep infections
27
SKIN – Skin infection (skin and /or subcutaneous) excluding decubitus ulcers, burns, and
infections at vascular access sites
27
ST – Soft tissue infection (muscle and/or fascia [for example, necrotizing fasciitis, infectious
gangrene, necrotizing cellulitis, infectious myositis, lymphadenitis, lymphangitis, or parotitis])
excluding decubitus ulcers, burns, and infections at vascular access sites
28
UMB – Omphalitis
29
USI – Urinary System Infection (kidney, ureter, bladder, urethra, or perinephric space
excluding UTI [see Chapter 7].)
29

January 2024 Surveillance Definitions
17 - 6
BJ-BONE AND JOINT INFECTION
BONE-Osteomyelitis
Osteomyelitis must meet at least one of the following criteria:
1. Patient has organism(s) identified from bone by culture or non-culture based microbiologic testing
method which is performed for purposes of clinical diagnosis and treatment, for example, not Active
Surveillance Culture/Testing (ASC/AST).
2. Patient has evidence of osteomyelitis on gross anatomic or histopathologic exam.
3. Patient has at least two of the following localized signs or symptoms: fever (>38.0°C), swelling*, pain
or tenderness*, heat*, or drainage*
And at least one of the following:
a. organism(s) identified from blood by culture or non-culture based microbiologic testing method
which is performed for purposes of clinical diagnosis and treatment, for example, not Active
Surveillance Culture/Testing (ASC/AST).
AND
imaging test evidence definitive for infection (for example, x-ray, CT scan, MRI, radiolabel scan
[gallium, technetium, etc.]), which if equivocal is supported by clinical correlation, specifically,
physician or physician designee documentation of antimicrobial treatment for osteomyelitis.
b. imaging test evidence definitive for infection (for example, x-ray, CT scan, MRI, radiolabel scan
[gallium, technetium, etc.]), which if equivocal is supported by clinical correlation, specifically,
physician or physician designee documentation of antimicrobial treatment for osteomyelitis.
* With no other recognized cause
Reporting Instructions
• Report mediastinitis following cardiac surgery that is accompanied by osteomyelitis as SSI-MED
rather than SSI-BONE.
• If a patient meets both organ space JNT and BONE report the SSI as BONE.
• After an HPRO or a KPRO if a patient meets both organ space PJI and BONE report the SSI as
BONE.
DISC-Disc space infection
Vertebral disc space infection must meet at least one of the following criteria:
1. Patient has organism(s) identified from vertebral disc space by culture or non-culture based
microbiologic testing method, which is performed for purposes of clinical diagnosis and treatment, for
example, not Active Surveillance Culture/Testing (ASC/AST).
2. Patient has evidence of vertebral disc space infection on gross anatomic or histopathologic exam.
3. Patient has at least one of the following: fever (>38.0°C) or pain* at the involved vertebral disc space
And at least one of the following:
a. organism(s) identified from blood by culture or non-culture based microbiologic testing method
which is performed for purposes of clinical diagnosis and treatment, for example, not Active
Surveillance Culture/Testing (ASC/AST)

January 2024 Surveillance Definitions
17 - 7
AND
imaging test evidence definitive for infection (for example, x-ray, CT scan, MRI, radiolabel scan
[gallium, technetium, etc.]), which if equivocal is supported by clinical correlation, specifically,
physician or physician designee documentation of antimicrobial treatment for vertebral disc
space infection.
b. imaging test evidence definitive for infection (for example, x-ray, CT scan, MRI, radiolabel scan
[gallium, technetium, etc.]), which if equivocal is supported by clinical correlation, specifically,
physician or physician designee documentation of antimicrobial treatment for vertebral disc
space infection.
* With no other recognized cause
JNT-Joint or bursa infection (not for use as Organ/Space SSI after HPRO or
KPRO procedures)
Joint or bursa infections must meet at least one of the following criteria:
1. Patient has organism(s) identified from joint fluid or synovial biopsy by culture or non-culture based
microbiologic testing method which is performed for purposes of clinical diagnosis and treatment, for
example, not Active Surveillance Culture/Testing (ASC/AST).
2. Patient has evidence of joint or bursa infection on gross anatomic or histopathologic exam.
3. Patient has a suspected joint infection and at least two of the following signs or symptoms: swelling*,
pain* or tenderness*, heat*, evidence of effusion*, or limitation of motion*.
And at least one of the following:
a. elevated joint fluid white blood cell count (per reporting laboratory’s reference range) OR
positive leukocyte esterase test strip of joint fluid.
b. organism(s) and white blood cells seen on Gram stain of joint fluid.
c. organism(s) identified from blood by culture or non-culture based microbiologic testing method
which is performed for purposes of clinical diagnosis and treatment, for example, not Active
Surveillance Culture/Testing (ASC/AST).
d. imaging test evidence definitive for infection (for example, x-ray, CT scan, MRI, radiolabel scan
[gallium, technetium, etc.]), which if equivocal is supported by clinical correlation, specifically,
physician or physician designee documentation of antimicrobial treatment for joint or bursa
infection.
* With no other recognized cause
Reporting Instruction
• If a patient meets both organ space JNT and BONE report the SSI as BONE.
PJI – Periprosthetic Joint Infection (for use as Organ/Space SSI following HPRO
and KPRO only)
Joint or bursa infections must meet at least one of the following criteria:

January 2024 Surveillance Definitions
17 - 8
1. Two positive periprosthetic specimens (tissue or fluid) with at least one matching organism, identified
by culture or non-culture based microbiologic testing method which is performed for purposes of
clinical diagnosis and treatment, for example, not Active Surveillance Culture/Testing (ASC/AST).
2. A sinus tract* communicating with the joint identified on gross anatomic exam.
3. Having three of the following minor criteria:
a. elevated serum C-reactive protein (CRP; >100 mg/L) and erythrocyte sedimentation rate (ESR;
>30 mm/hr.)
b. elevated synovial fluid white blood cell (WBC; >10,000 cells/μL) count OR “++” (or greater)
change on leukocyte esterase test strip of synovial fluid.
c. elevated synovial fluid polymorphonuclear neutrophil percentage (PMN% >90%)
d. positive histological analysis of periprosthetic tissue (>5 neutrophils (PMNs) per high power
field).
e. organism(s) identified from a single positive periprosthetic specimen (tissue or fluid) by culture
or non-culture based microbiologic testing method which is performed for purposes of clinical
diagnosis and treatment, for example, not Active Surveillance Culture/Testing (ASC/AST).
* A sinus tract is defined as a narrow opening or passageway that can extend in any direction through soft
tissue and results in dead space with potential for abscess formation.
Comments:
• A matching organism is defined on page 17-1. Organism(s) identified from hip or knee hardware
can be used to meet criterion 1.
• The NHSN definition of PJI is closely adapted from the Musculoskeletal Infection Society’s (MSIS’s)
definition of PJI (Proceedings of the International Consensus Meeting on Periprosthetic Joint
Infection, 2013).
• The standard laboratory cutoff values in criteria 3a - 3d are provided by NHSN for HPRO and KPRO
SSI surveillance purposes only. The NHSN laboratory cutoffs are not intended to guide clinicians in
the actual clinical diagnosis and management of acute or chronic PJI. Clinicians should refer to the
MSIS consensus definition for clinical use.
Reporting Instruction
• After an HPRO or a KPRO if a patient meets both organ space PJI and BONE report the SSI as
BONE.
CNS-CENTRAL NERVOUS SYSTEM INFECTION
IC-Intracranial infection (brain abscess, subdural or epidural infection,
encephalitis)
Intracranial infection must meet at least one of the following criteria:
1. Patient has organism(s) identified from brain tissue or dura by a culture or non-culture based
microbiologic testing method which is performed for purposes of clinical diagnosis or treatment, for
example, not Active Surveillance Culture/Testing (ASC/AST).
2. Patient has an abscess or evidence of intracranial infection on gross anatomic or histopathologic exam.

January 2024 Surveillance Definitions
17 - 9
3. Patient has at least two of the following signs or symptoms: headache*, dizziness*, fever (>38.0°C),
localizing neurologic signs*, changing level of consciousness*, or confusion*
And at least one of the following:
a. organism(s) seen on microscopic examination of brain or abscess tissue obtained by needle
aspiration or during an invasive procedure or autopsy.
b. imaging test evidence definitive for infection (for example, ultrasound, CT scan, MRI, radionuclide
brain scan, or arteriogram), which if equivocal is supported by clinical correlation, specifically,
physician or physician designee documentation of antimicrobial treatment for intracranial
infection.
c. diagnostic single antibody titer (IgM) or 4-fold increase in paired sera (IgG) for organism.
4. Patient ≤1 year of age has at least two of the following signs or symptoms: fever (>38.0°C),
hypothermia (<36.0°C), apnea*, bradycardia*, localizing neurologic signs*, or changing level of
consciousness*, for example, irritability, poor feeding, lethargy
And at least one of the following:
a. organism(s) seen on microscopic examination of brain or abscess tissue obtained by needle
aspiration or during an invasive procedure or autopsy.
b. imaging test evidence definitive for infection, (for example, ultrasound, CT scan, MRI,
radionuclide brain scan, or arteriogram), which if equivocal is supported by clinical correlation,
specifically, physician or physician designee documentation of antimicrobial treatment for
intracranial infection.
c. diagnostic single antibody titer (IgM) or 4-fold increase in paired sera (IgG) for organism.
* With no other recognized cause
Reporting Instructions
• Report as MEN if meningitis (MEN) and encephalitis (IC) are present together.
• Report as IC if meningitis (MEN) and a brain abscess (IC) are present together after operation.
• Report as SA if meningitis (MEN) and spinal abscess/infection (SA) are present together.
MEN-Meningitis or ventriculitis
Meningitis or ventriculitis must meet at least one of the following criteria:
1. Patient has organism(s) identified from cerebrospinal fluid (CSF) by a culture or non-culture based
microbiologic testing method which is performed for purposes of clinical diagnosis or treatment for
example, not Active Surveillance Culture/Testing (ASC/AST).
2. Patient has suspected meningitis or ventriculitis and at least two of the following:
i. fever (>38.0°C) or headache (Note: Elements of “i” alone may not be used to meet the two
required elements)
ii. meningeal sign(s)*
iii. cranial nerve sign(s)*
And at least one of the following:
a. increased white cells, elevated protein, and decreased glucose in CSF (per reporting laboratory’s
reference range).
b. organism(s) seen on Gram stain of CSF.

January 2024 Surveillance Definitions
17 - 10
c. organism(s) identified from blood by a culture or non-culture based microbiologic testing
method which is performed for purposes of clinical diagnosis or treatment, for example, not
Active Surveillance Culture/Testing (ASC/AST).
d. diagnostic single antibody titer (IgM) or 4-fold increase in paired sera (IgG) for organism.
3. Patient ≤1 year of age has suspected meningitis or ventriculitis and at least two of the following
elements:
i. fever (>38.0°C), hypothermia (<36.0°C), apnea*, bradycardia*, or irritability* (Note: Elements of
“i” alone may not be used to meet the required two elements).
ii. meningeal signs*
iii. cranial nerve signs*
And at least one of the following:
a. increased white cells, elevated protein, and decreased glucose in CSF (per reporting laboratory’s
reference range).
b. organism(s) seen on Gram stain of CSF.
c. organism(s) identified from blood by a culture or non-culture based microbiologic testing
method which is performed for purposes of clinical diagnosis or treatment, for example, not
Active Surveillance Culture/Testing (ASC/AST).
d. diagnostic single antibody titer (IgM) or 4-fold increase in paired sera (IgG) for organism.
* With no other recognized cause
Reporting Instructions
• Report CSF shunt infection as SSI-MEN if it occurs within 90 days of placement; if later or after
manipulation/access, it is considered CNS-MEN but is not reportable as an SSI.
• Report as MEN if meningitis (MEN) and encephalitis (IC) are present together.
• Report as IC if meningitis (MEN) and a brain abscess (IC) are present together after operation.
• Report as SA if meningitis (MEN) and spinal abscess/infection (SA) are present together.
SA-Spinal abscess/infection (spinal abscess, spinal subdural or epidural
infection)
Spinal abscess/infection must meet at least one of the following criteria:
1. Patient has organism(s) identified from abscess or from purulent material found in the spinal epidural
or subdural space by a culture or non-culture based microbiologic testing method which is performed
for purposes of clinical diagnosis or treatment, for example, not Active Surveillance Culture/Testing
(ASC/AST).
2. Patient has an abscess or other evidence of spinal infection on gross anatomic or histopathologic
exam.
3. Patient has at least one of the following localized signs or symptoms: fever (>38.0°C), back pain* or
tenderness*, radiculitis*, paraparesis*, or paraplegia*
And at least one of the following:
a. organism(s) identified from blood by a culture or non-culture based microbiologic testing
method which is performed for purposes of clinical diagnosis or treatment, for example, not
Active Surveillance Culture/Testing (ASC/AST)

January 2024 Surveillance Definitions
17 - 11
AND
imaging test evidence definitive for spinal abscess/infection, which if equivocal is supported by
clinical correlation, specifically, physician or physician designee documentation of antimicrobial
treatment for spinal abscess/infection.
b. imaging test evidence definitive for a spinal abscess/infection (for example, myelography,
ultrasound, CT scan, MRI, or other scans [gallium, technetium, etc.]) which if equivocal is
supported by clinical correlation, specifically, physician or physician designee documentation of
antimicrobial treatment for spinal abscess/infection.
* With no other recognized cause
Reporting Instruction
• Report as SA if meningitis (MEN) and spinal abscess/infection (SA) are present together after
operation.
CVS-CARDIOVASCULAR SYSTEM INFECTION
CARD-Myocarditis or pericarditis
Myocarditis or pericarditis must meet at least one of the following criteria:
1. Patient has organism(s) identified from pericardial tissue or fluid by a culture or non-culture based
microbiologic testing method which is performed for purposes of clinical diagnosis or treatment, for
example, not Active Surveillance Culture/Testing (ASC/AST).
2. Patient has at least two of the following signs or symptoms: fever (>38.0°C), chest pain*,
paradoxical pulse*, or increased heart size*
And at least one of the following:
a. abnormal EKG consistent with myocarditis or pericarditis.
b. evidence of myocarditis or pericarditis on histologic exam of heart tissue.
c. 4-fold rise in paired sera from IgG antibody titer.
d. pericardial effusion identified by echocardiogram, CT scan, MRI, or angiography.
3. Patient ≤1 year of age has at least two of the following signs or symptoms: fever (>38.0°C),
hypothermia (<36.0°C), apnea*, bradycardia*, paradoxical pulse*, or increased heart size*
And at least one of the following:
a. abnormal EKG consistent with myocarditis or pericarditis.
b. histologic examination of heart tissue shows evidence of myocarditis or pericarditis.
c. 4-fold rise in paired sera from IgG antibody titer.
d. pericardial effusion identified by echocardiogram, CT scan, MRI, or angiography.
* With no other recognized cause

January 2024 Surveillance Definitions
17 - 12
ENDO-Endocarditis
When meeting the Endocarditis (ENDO) definition:
• The ENDO Infection Window Period is defined as the 21 days during which all site-specific infection
criteria must be met. It includes the date the first positive diagnostic test that is used as an
element of the ENDO criterion was obtained, the 10 calendars days before and the 10 calendar
days after. The Infection Window Period is lengthened for this event to accommodate the
extended diagnostic timeframe that is frequently required to reach a clinical determination of
endocarditis.
• The RIT for Endocarditis (ENDO) is extended to include the remainder of the patient’s current
admission.
• When meeting the Endocarditis (ENDO) definition, the secondary BSI attribution period includes
the 21-day infection window period and all subsequent days of the patient’s current admission.
o As a result of this lengthy secondary BSI attribution period, secondary BSI pathogen
assignment for ENDO, is limited to organism(s) identified in blood specimen that match the
organism(s) used to meet the ENDO definition.
Example: If the ENDO definition was met using a site-specific specimen (for example, cardiac
vegetation) or using a blood specimen with S. aureus as the identified organism, if a blood
specimen collected during the ENDO secondary BSI attribution period is positive for S. aureus
and E. coli, while the S. aureus can be assigned to the ENDO event, it cannot be assumed the
E. coli can be assigned as a secondary BSI pathogen. The blood organism (E. coli) does not
match the organism (S. aureus) used to meet the ENDO definition. If the blood specimen can
be used to meet an ENDO definition criterion both organisms can be assigned. Otherwise, the
E. coli will need to be investigated as a separate BSI and identified as a secondary BSI to
another site-specific infection or determined to be a primary BSI.
Endocarditis of a natural or prosthetic heart valve must meet at least one of the following criteria:
1. Organism(s) identified from cardiac vegetation*
†
, embolized vegetation (for example, solid-organ
abscess) documented as originating from cardiac source, or intracardiac abscess by a culture or non-
culture based microbiologic testing method which is performed for purposes of clinical diagnosis or
treatment, for example, not Active Surveillance Culture/Testing (ASC/AST).
2. Organism(s) seen on histopathologic examination of cardiac vegetation*, embolized vegetation, for
example, solid organ abscess, documented as originating from cardiac source, or intracardiac abscess.
3. Endocarditis seen on histopathologic examination of cardiac vegetation* or intracardiac abscess.
4. At least one of the following echocardiographic evidence of endocarditis*
‡
:
i. vegetation on cardiac valve or supporting structures
ii. intracardiac abscess
iii. new partial dehiscence of prosthetic valve
And at least one of the following:
a. typical infectious endocarditis organism(s) (specifically, Viridans group streptococci,
Streptococcus bovis, Haemophilus spp., Actinobacillus actinomycetemcomitans,
Cardiobacterium hominis, Eikenella corrodens, Kingella spp., Staphylococcus aureus,

January 2024 Surveillance Definitions
17 - 13
Enterococcus spp.) identified from ≥2 matching blood collections drawn on separate occasions
with no more than 1 calendar day between specimens by a culture or non-culture based
microbiologic testing method which is performed for purposes of clinical diagnosis or
treatment, for example, not Active Surveillance Culture/Testing (ASC/AST).
b. Coxiella burnetii identified by anti-phase I IgG antibody titer >1:800 or identified from blood by
a culture or non-culture based microbiologic testing method which is performed for purposes of
clinical diagnosis or treatment, for example, not Active Surveillance Culture/Testing (ASC/AST).
5. At least three of the following (Note: Meaning one element from i, ii, iii, or iv and only one condition
within each element can be used.)
i. prior endocarditis, prosthetic valve, uncorrected congenital heart disease, history of rheumatic
heart disease, hypertrophic obstructive cardiomyopathy, or known IV drug use.
§
ii. fever (>38.0°C)
iii. vascular phenomena: major arterial emboli (specifically, embolic stroke, renal infarct, splenic
infarct or abscess, digital ischemic/gangrene from embolic source), septic pulmonary infarcts,
mycotic aneurysm (documented by imaging, seen in surgery, or described in gross pathological
specimen), intracranial hemorrhage, conjunctival hemorrhages, or Janeway’s lesions documented.
iv. immunologic phenomena: glomuleronephritis (documented in chart, or white cell or red blood cell
casts on urinalysis), Osler’s nodes, Roth’s spots, or positive rheumatoid factor.
And at least one of the following:
a. typical infectious endocarditis organism(s) (specifically, Viridans group streptococci,
Streptococcus bovis, Haemophilus spp., Actinobacillus actinomycetemcomitans,
Cardiobacterium hominis, Eikenella corrodens, Kingella spp., Staphylococcus aureus,
Enterococcus spp.) identified from ≥2 matching blood collections drawn on separate occasions
with no more than 1 calendar day between specimens by a culture or non-culture based
microbiologic testing method which is performed for purposes of clinical diagnosis or
treatment, for example, not Active Surveillance Culture/Testing (ASC/AST).
b. Coxiella burnetii identified by anti-phase I IgG antibody titer >1:800 or identified from blood by
a culture or non-culture based microbiologic testing method which is performed for purposes of
clinical diagnosis or treatment, for example, not Active Surveillance Culture/Testing (ASC/AST).
6. At least one of the following*‡:
i. vegetation on cardiac valve or supporting structures seen on echocardiogram
ii. intracardiac abscess seen on echocardiogram
iii. new partial dehiscence of prosthetic valve seen on echocardiogram
And at least one condition from three of the following elements:
a. prior endocarditis, prosthetic valve, uncorrected congenital heart disease, history of rheumatic
heart disease, hypertrophic obstructive cardiomyopathy, or known IV drug use.
§
b. fever (>38.0°C)
c. vascular phenomena: major arterial emboli (specifically, embolic stroke, renal infarct, splenic
infarct or abscess, digital ischemic/gangrene from embolic source), septic pulmonary infarcts,
mycotic aneurysm (documented by imaging, seen in surgery, or described in gross pathological
specimen), intracranial hemorrhage, conjunctival hemorrhages, or Janeway’s lesions
documented.
d. immunologic phenomena: glomuleronephritis (documented in chart, or white cell or red blood
cell casts on urinalysis), Osler’s nodes, Roth’s spots, or positive rheumatoid factor.
e. identification of organism(s) from the blood by at least one of the following methods:

January 2024 Surveillance Definitions
17 - 14
• recognized pathogen(s) identified from blood by a culture or non-culture based
microbiologic testing method which is performed for purposes of clinical diagnosis or
treatment, for example, not Active Surveillance Culture/Testing (ASC/AST).
• same common commensal organism(s) identified from ≥2 blood collections drawn on
separate occasions on the same or consecutive days by a culture or non-culture based
microbiologic testing method which is performed for purposes of clinical diagnosis or
treatment, for example, not Active Surveillance Culture/Testing (ASC/AST).
7. One condition from each of the following elements (a, b, c, d, and e):
a. prior endocarditis, prosthetic valve, uncorrected congenital heart disease, history of rheumatic
heart disease, hypertrophic obstructive cardiomyopathy, or known IV drug use.
§
b. fever (>38.0°C)
c. vascular phenomena: major arterial emboli (specifically, embolic stroke, renal infarct, splenic
infarct or abscess, digital ischemic/gangrene from embolic source), septic pulmonary infarcts,
mycotic aneurysm (documented by imaging, seen in surgery, or described in gross pathological
specimen), intracranial hemorrhage, conjunctival hemorrhages, or Janeway’s lesions
documented.
d. immunologic phenomena: glomuleronephritis (documented in chart, or white cell or red blood
cell casts on urinalysis), Osler’s nodes, Roth’s spots, or positive rheumatoid factor.
e. identification of organism(s) from the blood by at least one of the following methods:
• recognized pathogen(s) identified from blood by a culture or non-culture based
microbiologic testing method which is performed for purposes of clinical diagnosis or
treatment, for example, not Active Surveillance Culture/Testing (ASC/AST).
• same common commensal organism(s) identified from ≥2 blood collections drawn on
separate occasions on the same or consecutive days by a culture or non-culture based
microbiologic testing method which is performed for purposes of clinical diagnosis or
treatment, for example, not Active Surveillance Culture/Testing (ASC/AST).
Reporting Instructions
* Cardiac vegetation can be found on a cardiac valve, pacemaker/defibrillator lead or ventricular
assist device (VAD) components within the heart.
†
The following can also meet the definition of a “cardiac vegetation”:
• Positive culture from a cardiac valve, pacemaker/defibrillator lead or ventricular assist
device (VAD) components within the heart.
‡ Which if equivocal is supported by clinical correlation (specifically, physician or physician
designee documentation of antimicrobial treatment for endocarditis).
§ Elements of 5i, 6a and 7a documented during the current admission:
• May be documented outside of the ENDO infection window period or SSI surveillance
period.
• Should not be used to set the ENDO date of event.

January 2024 Surveillance Definitions
17 - 15
MED-Mediastinitis
Mediastinitis must meet at least one of the following criteria:
1. Patient has organism(s) identified from mediastinal tissue or fluid by a culture or non-culture based
microbiologic testing method which is performed for purposes of clinical diagnosis or treatment, for
example, not Active Surveillance Culture/Testing (ASC/AST).
2. Patient has evidence of mediastinitis on gross anatomic or histopathologic exam.
3. Patient has at least one of the following signs or symptoms: fever (>38.0°C), chest pain*, or sternal
instability. *
And at least one of the following:
a. purulent drainage from mediastinal area
b. mediastinal widening on imaging test
4. Patient ≤1 year of age has at least one of the following signs or symptoms: fever (>38.0°C),
hypothermia (<36.0°C), apnea*, bradycardia*, or sternal instability*
And at least one of the following:
a. purulent drainage from mediastinal area.
b. mediastinal widening on imaging test.
* With no other recognized cause
Comment:
• The mediastinal space is the area under the sternum and in front of the vertebral column,
containing the heart and its large vessels, trachea, esophagus, thymus, lymph nodes, and other
structures and tissues. It is divided into anterior, middle, posterior, and superior regions.
Reporting Instruction
• Report mediastinitis (MED) following cardiac surgery that is accompanied by osteomyelitis as SSI-
MED rather than SSI-BONE.
VASC-Arterial or venous infection excluding infections involving vascular
access devices with organisms identified in the blood
Note: If a patient meets the criteria for an LCBI in the presence of an arterial or vascular infection (VASC)
report as an LCBI not as a VASC. **
Arterial or venous infection must meet at least one of the following criteria:
1. Patient has organism(s) from extracted arteries or veins identified by a culture or non-culture based
microbiologic testing method which is performed for purposes of clinical diagnosis or treatment, for
example, not Active Surveillance Culture/Testing (ASC/AST).
2. Patient has evidence of arterial or venous infection on gross anatomic or histopathologic exam.
3. Patient has at least one of the following signs or symptoms: fever (>38.0°C), pain*, erythema*, or heat
at involved vascular site*
AND

January 2024 Surveillance Definitions
17 - 16
More than 15 colonies cultured from intravascular cannula tip using semi-quantitative culture method.
4. Patient has purulent drainage at involved vascular site.
5. Patient ≤1 year of age has at least one of the following signs or symptoms: fever (>38.0°C),
hypothermia (<36.0°C), apnea*, bradycardia*, lethargy*, pain*, erythema*, or heat at involved
vascular site*
AND
More than 15 colonies cultured from intravascular cannula tip using semi-quantitative culture
method.
* With no other recognized cause
Reporting Instructions
• Report infections of an arteriovenous graft, shunt, fistula or intravascular cannulation site without
organism(s) identified from blood as CVS-VASC.
• Report Organ Space VASC infections as an SSI and not an LCBI when you have an SSI with
secondary BSI.
• Report intravascular infections with organism(s) identified from the blood and meeting the LCBI
criteria, as BSI-LCBI.
**
Occasionally, a patient with both an eligible central line and another vascular access device will have
pus at the other access site. If there is pus at the site of one of the following vascular access devices and a
specimen collected from that site has at least one matching organism to an organism identified in the
blood during the BSI IWP, report such events marking the “pus at the vascular access site” field as “Yes.”
Vascular access devices included in this exception are limited to:
• Arterial catheters unless in the pulmonary artery, aorta or umbilical artery
• Arteriovenous fistulae
• Arteriovenous grafts
• Atrial catheters (also known as transthoracic intra-cardiac catheters, those catheters
inserted directly into the right or left atrium via the heart wall)
• Hemodialysis reliable outflow (HERO) dialysis catheters
• Intra-aortic balloon pump (IABP) devices
• Non-accessed CL (those neither inserted nor used during current admission)
• Peripheral IV or Midlines
EENT-EYE, ear, nose, throat, or mouth infection
CONJ-Conjunctivitis
1. Patient has at least one of the following signs or symptoms: pain, erythema, or swelling of
conjunctiva or around eye
And at least one of the following:
a. Patient has organism(s) identified from conjunctival scraping or purulent exudate obtained from
the conjunctiva or contiguous tissues, (for example, eyelid, cornea, meibomian glands, or
lacrimal glands) by a culture or non-culture based microbiologic testing method which is

January 2024 Surveillance Definitions
17 - 17
performed for purposes of clinical diagnosis or treatment, for example, not Active Surveillance
Culture/Testing (ASC/AST).
b. WBCs and organism(s) seen on Gram stain of exudate.
c. purulent exudate.
d. multinucleated giant cells seen on microscopic examination of conjunctival exudate or scrapings.
e. diagnostic single antibody titer (IgM) or 4-fold increase in paired sera (IgG) for organism.
Reporting Instructions
• Report other infections of the eye as EYE.
• Do not report chemical conjunctivitis, caused by silver nitrate (AgNO
3
), as a healthcare–associated
infection.
• Do not report a separate case of conjunctivitis (CONJ) that occurs as a part of another viral illness
(for example, UR).
EAR-Ear, mastoid infection
Ear and mastoid infections must meet at least one of the following criteria:
Otitis externa must meet at least one of the following criteria:
1. Patient has organism(s) identified from purulent drainage from ear canal by a culture or non-culture
based microbiologic testing method which is performed for purposes of clinical diagnosis or
treatment, for example, not Active Surveillance Culture/Testing (ASC/AST).
2. Patient has at least one of the following: fever (>38.0°C), pain*, or erythema*
AND
organism(s) seen on Gram stain of purulent drainage from ear canal.
Otitis media must meet at least one of the following criteria:
3. Patient has organism(s) identified from fluid from middle ear obtained during an invasive procedure
(for example, tympanocentesis) by a culture or non-culture based microbiologic testing method
which is performed for purposes of clinical diagnosis or treatment, for example, not Active
Surveillance Culture/Testing (ASC/AST).
4. Patient has at least two of the following: fever (>38.0°C), pain *, inflammation*, retraction* or
decreased mobility of eardrum*, or fluid behind eardrum*.
Otitis interna (labyrinthitis) must meet at least one of the following criteria:
5. Patient has organism(s) identified from fluid from inner ear obtained during an invasive procedure
by a culture or non-culture based microbiologic testing method which is performed for purposes of
clinical diagnosis or treatment, for example, not Active Surveillance Culture/Testing (ASC/AST).
6. Patient has a physician or physician designee diagnosis of inner ear infection.
Mastoiditis must meet at least one of the following criteria:
7. Patient has organism(s) identified from fluid or tissue from mastoid by a culture or non-culture
based microbiologic testing method which is performed for purposes of clinical diagnosis or
treatment, for example not Active Surveillance Culture/Testing (ASC/AST).
8. Patient has at least two of the following: fever (>38.0°C), pain or tenderness*, post auricular
swelling*, erythema*, headache*, or facial paralysis*.

January 2024 Surveillance Definitions
17 - 18
And at least one of the following:
a. organism(s) seen on Gram stain of fluid or tissue from mastoid.
b. imaging test evidence definitive for infection (for example, CT scan), which if equivocal is
supported by clinical correlation, specifically, physician or physician designee
documentation of antimicrobial treatment for mastoid infection.
* With no other recognized cause
EYE-Eye infection, other than conjunctivitis
An infection of the eye, other than conjunctivitis, must meet at least one of the following criteria:
1. Patient has organism(s) identified from anterior or posterior chamber or vitreous fluid by a culture or
non-culture based microbiologic testing method which is performed for purposes of clinical diagnosis
or treatment, for example, not Active Surveillance Culture/Testing (ASC/AST).
2. Patient has at least two of the following signs or symptoms with no other recognized cause: eye pain,
visual disturbance, or hypopyon
AND
Physician or physician designee initiates antimicrobial therapy within two days of onset or worsening
of symptoms.
ORAL-Oral cavity infection (mouth, tongue, or gums)
Oral cavity infections must meet at least one of the following criteria:
1. Patient has organism(s) identified from abscess or purulent material from tissues of oral cavity by a
culture or non-culture based microbiologic testing method which is performed for purposes of clinical
diagnosis or treatment, for example, not Active Surveillance Culture/Testing (ASC/AST).
2. Patient has an abscess or other evidence of oral cavity infection found on invasive procedure, gross
anatomic exam, or histopathologic exam.
3. Patient has at least one of the following signs or symptoms with no other recognized cause:
ulceration, raised white patches on inflamed mucosa, or plaques on oral mucosa.
And at least one of the following:
a. virus identified from mucosal scrapings or exudate by a culture or non-culture based
microbiologic testing method which is performed for purposes of clinical diagnosis or
treatment, for example, not Active Surveillance Culture/Testing (ASC/AST)
b. multinucleated giant cells seen on microscopic examination of mucosal scrapings or exudate
c. diagnostic single antibody titer (IgM) or 4-fold increase in paired sera (IgG) for organism.
d. fungal elements seen on microscopic exam of mucosal scrapings or exudate (for example, Gram
stain, KOH).
e. Physician or physician designee initiates antimicrobial therapy within 2 days of onset or
worsening of symptoms.
Reporting Instruction
• Report healthcare–associated primary herpes simplex infections of the oral cavity as ORAL;
recurrent herpes infections are not healthcare associated.

January 2024 Surveillance Definitions
17 - 19
SINU-Sinusitis
Sinusitis must meet at least one of the following criteria:
1. Patient has organism(s) identified from fluid or tissue from the sinus cavity obtained during an
invasive procedure by a culture or non-culture based microbiologic testing method which is
performed for purposes of clinical diagnosis or treatment, for example, not Active Surveillance
Culture/Testing (ASC/AST).
2. Patient has at least one of the following signs or symptoms: fever (>38.0°C), pain or tenderness over
the involved sinus*, headache*, purulent exudate*, or nasal obstruction*
AND
Imaging test evidence of sinusitis (for example, x-ray, CT scan).
* With no other recognized cause
UR-Upper respiratory tract infection, pharyngitis, laryngitis, epiglottitis
Upper respiratory tract infections must meet at least one of the following criteria:
1. Patient has at least two of the following signs or symptoms: fever (>38.0°C), erythema of pharynx*,
sore throat*, cough*, hoarseness*, tachypnea*, nasal discharge*, or purulent exudate in throat*
And at least one of the following:
a. organism(s) identified from upper respiratory site [specifically: larynx, nasopharynx, pharynx,
and epiglottis] by a culture or non-culture based microbiologic testing method which is
performed for purposes of clinical diagnosis or treatment, for example, not Active Surveillance
Culture/Testing (ASC/AST). Note: excludes sputum and tracheal aspirate because these are not
upper respiratory specimens.
b. diagnostic single antibody titer (IgM) or 4-fold increase in paired sera (IgG) for organism.
c. Physician or physician designee diagnosis of an upper respiratory infection.
2. Patient has an abscess on gross anatomical or histopathologic exam or imaging test.
3. Patient ≤1 year of age has at least two of the following signs or symptoms: fever (>38.0°C),
hypothermia (<36.0°C), apnea*, bradycardia*, nasal discharge*, or purulent exudate in throat*
And at least one of the following:
a. organism(s) identified from upper respiratory site [specifically larynx, nasopharynx, pharynx,
and epiglottis] by a culture or non-culture based microbiologic testing method which is
performed for purposes of clinical diagnosis or treatment, for example, not Active Surveillance
Culture/Testing (ASC/AST). Note: excludes sputum and tracheal aspirate because they are not
upper respiratory specimens.
b. diagnostic single antibody titer (IgM) or 4-fold increase in paired sera (IgG) for organism.
c. physician or physician designee diagnosis of an upper respiratory infection.
* With no other recognized cause

January 2024 Surveillance Definitions
17 - 20
GI-GASTROINTESTINAL SYSTEM INFECTION
CDI- Clostridioides difficile Infection
Clostridioides difficile infection must meet at least one of the following criteria:
1. Positive test for toxin-producing C. difficile on an unformed stool specimen (conforms to the shape of
the container).
2. Patient has evidence of pseudomembranous colitis on gross anatomic (includes endoscopic exams) or
histopathologic exam.
Note:
• When using a multi-testing methodology for CD identification, the result of the last test finding, which
is placed onto the patient medical record, will determine if GI-CDI criterion 1 is met.
Comments:
• The date of event for CDI criterion 1, will always be the specimen collection date of the unformed
stool, specifically, not the date of onset of unformed stool.
• A positive test for toxin-producing C. difficile and an unformed stool specimen is a single element,
and both are required to meet criterion.
Reporting Instructions
• Report the CDI and the GE or GIT if additional enteric organism(s) are identified and criteria are
met for GE or GIT.
• Report each new GI-CDI according to the Repeat Infection Timeframe (RIT) rule for HAIs (see
NHSN HAI definitions in Chapter 2 for further details and guidance).
• CDI laboratory-identified event (LabID Event) categorizations (for example, recurrent CDI assay,
incident CDI assay, healthcare facility-onset, community-onset, community-onset healthcare
facility-associated) do not apply to HAIs; including C. difficile associated gastrointestinal infections
(GI-CDI).
GE-Gastroenteritis (excluding C. difficile infections)
Gastroenteritis must meet at least one of the following criteria:
1. Patient has an acute onset of diarrhea (liquid stools for > 12 hours) and no likely noninfectious cause
(for example, diagnostic tests, therapeutic regimen other than antimicrobial agents, acute
exacerbation of a chronic condition, or psychological stress information).
2. Patient has at least two of the following signs or symptoms: nausea*, vomiting*, abdominal pain*,
fever (>38.0°C), or headache*
And at least one of the following:
a. an enteric pathogen is identified from stool or rectal swab by a culture or non-culture based
microbiologic testing method which is performed for purposes of clinical diagnosis or
treatment, for example, not Active Surveillance Culture/Testing (ASC/AST).
b. an enteric pathogen is detected by microscopy on stool
c. diagnostic single antibody titer (IgM) or 4-fold increase in paired sera (IgG) for organism.

January 2024 Surveillance Definitions
17 - 21
* With no other recognized cause
Comment:
• The reference to “enteric pathogens” describes pathogens that are not considered to be normal
flora of the intestinal tract. Enteric pathogens identified on culture or with the use of other
diagnostic laboratory tests include Salmonella, Shigella, Yersinia, Campylobacter, Listeria, Vibrio,
Enteropathogenic or Enterohemorrhagic E. coli or Giardia.
Reporting Instruction
• Report only GI-GIT using the event date as that of GI-GIT if the patient meets criteria for both GI-
GE and GI-GIT.
GIT-Gastrointestinal tract infection (esophagus, stomach, small and large
bowel, and rectum) excluding gastroenteritis, appendicitis, and C. difficile
infection
Gastrointestinal tract infections, excluding, gastroenteritis and appendicitis, must meet at least one of the
following criteria:
1. Patient has one of the following:
a. an abscess or other evidence of gastrointestinal tract infection on gross anatomic or
histopathologic exam.
b. abscess or other evidence of gastrointestinal tract infection on gross anatomic or
histopathologic exam (See Reporting Instructions)
AND
organism(s) identified from blood by a culture or non-culture based microbiologic testing
method, which is performed for purposes of clinical diagnosis or treatment, for example, not
Active Surveillance Culture/Testing (ASC/AST). The organism(s) identified in the blood
must contain at least one MBI organism on the NHSN Organism List that can be accessed via the
spreadsheet or the new NHSN Terminology Browser.
2. Patient has at least two of the following signs or symptoms compatible with infection of the organ or
tissue involved: fever (>38.0°C), nausea*, vomiting*, pain*or tenderness*, odynophagia*, or
dysphagia*
And at least one of the following:
a. organism(s) identified from drainage or tissue obtained during an invasive procedure
or from drainage from an aseptically-placed drain by a culture or non-culture based
microbiologic testing method which is performed for purposes of clinical diagnosis or
treatment, for example, not Active Surveillance Culture/Testing (ASC/AST).
b. organism(s) seen on Gram stain or fungal elements seen on KOH stain or multinucleated giant
cells seen on microscopic examination of drainage or tissue obtained during an invasive
procedure or from drainage from an aseptically-placed drain.
c. organism(s) identified from blood by a culture or non-culture based microbiologic testing
method which is performed for purposes of clinical diagnosis or treatment, for example, not
Active Surveillance Culture/Testing (ASC/AST). The organism(s) identified in the blood must

January 2024 Surveillance Definitions
17 - 22
contain at least one MBI organism on the NHSN Organism List that can be accessed via the
spreadsheet or the new NHSN Terminology Browser.
AND
imaging test evidence definitive for gastrointestinal infection (for example, endoscopic
exam, MRI, CT scan), which if equivocal is supported by clinical correlation, specifically,
physician or physician designee documentation of antimicrobial treatment for gastrointestinal
tract infection.
d. imaging test evidence definitive for gastrointestinal infection (for example, endoscopic exam,
MRI, CT scan), which if equivocal is supported by clinical correlation, specifically, physician or
physician designee documentation of antimicrobial treatment for gastrointestinal tract
infection.
* With no other recognized cause
Reporting Instructions
• Report only GI-GIT using the event date as that of GI-GIT if the patient meets criteria for both GI-
GE and GI-GIT.
• For GIT 1b: If an organism is identified on histopathologic exam, the blood specimen must contain
a matching organism.
• In patients > 1 year, pneumatosis intestinalis is considered an equivocal imaging finding for a
gastrointestinal tract infection (GIT). For patients ≤ 1 year, please review the NEC criteria.
IAB-Intraabdominal infection, not specified elsewhere, including gallbladder,
bile ducts, liver (excluding viral hepatitis), spleen, pancreas, peritoneum,
retroperitoneal, subphrenic or subdiaphragmatic space, or other
intraabdominal tissue or area not specified elsewhere
Intraabdominal infections must meet at least one of the following criteria:
1. Patient has organism(s) identified from an abscess or from purulent material from intraabdominal
space by a culture or non-culture based microbiologic testing method which is performed for
purposes of clinical diagnosis or treatment, for example, not Active Surveillance Culture/Testing
(ASC/AST).
2. Patient has at least one of the following:
a. abscess or other evidence of intraabdominal infection on gross anatomic or histopathologic
exam.
b. abscess or other evidence of intraabdominal infection on gross anatomic or histopathologic
exam
(See Reporting Instructions)
AND
organism(s) identified from blood by a culture or non-culture based microbiologic testing
method, which is performed for purposes of clinical diagnosis or treatment, for example, not
Active Surveillance Culture/Testing (ASC/AST). The organism(s) identified in the blood must
contain at least one MBI organism on the NHSN Organism List that can be accessed via the
spreadsheet or the new NHSN Terminology Browser.

January 2024 Surveillance Definitions
17 - 23
.
3. Patient has at least two of the following: fever (>38.0°C), hypotension, nausea*, vomiting*, abdominal
pain or tenderness*, elevated transaminase level(s)*, or jaundice*
And at least one of the following:
a. organism(s) seen on Gram stain and/or identified from intraabdominal fluid or tissue obtained
during invasive procedure or from an aseptically-placed drain in the intraabdominal space (for
example, closed suction drainage system, open drain, T-tube drain, CT guided drainage) by a
culture or non-culture based microbiologic testing method which is performed for purposes of
clinical diagnosis or treatment, for example, not Active Surveillance Culture/Testing (ASC/AST).
b. organism(s) identified from blood by a culture or non-culture based microbiologic testing
method which is performed for purposes of clinical diagnosis or treatment, for example, not
Active Surveillance Culture/Testing (ASC/AST). The organism(s) identified in the blood must
contain at least one MBI organism on the NHSN Organism List that can be accessed via the
spreadsheet or the new NHSN Terminology Browser.
AND
imaging test evidence definitive for infection (for example, ultrasound, CT scan, MRI, ERCP,
radiolabel scans [gallium, technetium, etc.] or on abdominal x-ray), which if equivocal is
supported by clinical correlation, specifically, physician or physician designee documentation of
antimicrobial treatment for intraabdominal infection.
†
* With no other recognized cause
Reporting Instructions
•
†
Biliary ductal dilatation is considered an equivocal finding for cholangitis.
• For IAB 2b: If an organism is identified on histopathologic exam, the blood specimen must contain
a matching organism.
• Do not report pancreatitis (an inflammatory syndrome characterized by abdominal pain, nausea,
and vomiting associated with high serum levels of pancreatic enzymes) unless it is determined to
be infectious in origin.
• Eligible laboratory results that represent transaminase levels include: serum glutamic oxaloacetic
transaminase (SGOT), serum glutamic pyruvic transaminase (SGPT), alanine transaminase (ALT) or
aspartate transaminase (AST). Consider the requirement for elevated transaminase level(s) met if
at least one is elevated as per the normal range provided by the laboratory.
NEC-Necrotizing enterocolitis (See Chapter 4)
Necrotizing enterocolitis (NEC) criteria include neither a site-specific specimen nor organism identified
from blood specimen. The pathophysiology of NEC is multifactorial. NEC definitions are provided to
facilitate the provision of an exception for assigning a BSI secondary to NEC and should not be used for HAI
surveillance as they are not designed, tested, or intended for this purpose.

January 2024 Surveillance Definitions
17 - 24
LRI- LOWER RESPIRATORY INFECTION, OTHER THAN PNEUMONIA
LUNG-Other infection of the lower respiratory tract and pleural cavity
Other infections of the lower respiratory tract must meet at least one of the following criteria:
1. Patient has organism(s) seen on Gram stain of lung tissue or pleural fluid or identified from lung tissue
or pleural fluid* (when pleural fluid was obtained during thoracentesis or within 24 hours of chest
tube placement) by a culture or non-culture based microbiologic testing method which is performed
for purposes of clinical diagnosis or treatment, for example, not Active Surveillance Culture/Testing
(ASC/AST).
2. Patient has a lung abscess or other evidence of infection (for example, empyema) on gross anatomic
or histopathologic exam.
3. Patient has imaging test evidence of abscess or infection (excludes imaging test evidence of
pneumonia) which if equivocal is supported by clinical correlation, specifically, physician or physician
designee documentation of antimicrobial treatment for lung infection).
Reporting Instruction
• If patient meets LUNG and PNEU report as PNEU only, unless the LUNG is a surgical site
organ/space infection, in which case, report both PNEU and SSI-LUNG.
*If a pleural fluid specimen is collected after a chest tube is repositioned OR after 24 hours of chest tube
placement, this pleural fluid specimen is not eligible for LUNG 1. Repositioning must be documented in
the patient record by a healthcare professional.
REPR-REPRODUCTIVE TRACT INFECTION
EMET-Endometritis
Endometritis must meet at least one of the following criteria:
1. Patient has organism(s) identified from endometrial fluid or tissue by a culture or non-culture based
microbiologic testing method which is performed for purposes of clinical diagnosis or treatment, for
example, not Active Surveillance Culture/Testing (ASC/AST).
2. Patient has suspected endometritis with at least two of the following signs or symptoms: fever
(>38.0°C), pain or tenderness (uterine or abdominal)*, or purulent drainage from uterus.
* With no other recognized cause
Reporting Instructions
• Do not report an HAI chorioamnionitis as EMET (see OREP).
• Do not report subsequent postpartum endometritis after a vaginal delivery as an HAI if a patient
is admitted with POA chorioamnionitis (OREP). (See next bullet for endometritis following a C-
section).
• Report as an organ space SSI-EMET if a C-section was performed on a patient with
chorioamnionitis, and the patient later develops endometritis.

January 2024 Surveillance Definitions
17 - 25
EPIS-Episiotomy infection
Episiotomy infections must meet at least one of the following criteria:
1. Postvaginal delivery patient has purulent drainage from the episiotomy.
2. Postvaginal delivery patient has an episiotomy abscess.
OREP- Deep pelvic tissue infection or other infection of the male or female
reproductive tract (for example, epididymis, testes, prostate, vagina, ovaries,
uterus) including chorioamnionitis, but excluding vaginitis, endometritis or
vaginal cuff infections
Other infections of the male or female reproductive tract must meet at least one of the following criteria:
1. Patient has organism(s) identified from tissue or fluid from affected site (excludes urine and vaginal
swabs) by a culture or non-culture based microbiologic testing method which is performed for
purposes of clinical diagnosis or treatment, for example, not Active Surveillance Culture/Testing
(ASC/AST).
2. Patient has an abscess or other evidence of infection of affected site on gross anatomic or
histopathologic exam.
3. Patient has suspected infection of one of the listed OREP sites and two of the following localized signs
or symptoms: fever (>38.0°C), nausea*, vomiting*, pain or tenderness*, or dysuria*
And at least one of the following:
a. organism(s) identified from blood by a culture or non-culture based microbiologic testing
method which is performed for purposes of clinical diagnosis or treatment, for example, not
Active Surveillance Culture/Testing (ASC/AST).
b. physician or physician designee initiates antimicrobial therapy within two days of onset or
worsening of symptoms.
* With no other recognized cause
Reporting Instructions
• Report endometritis as EMET.
• Report vaginal cuff infections as VCUF.
• If patient has epididymitis, prostatitis, or orchitis and meets OREP criteria, and they also meet UTI
criteria, report UTI only, unless the OREP is a surgical site organ/space infection, in which case, only
OREP should be reported.
VCUF-Vaginal cuff infection
Vaginal cuff infections must meet at least one of the following criteria:
1. Post hysterectomy patient has purulent drainage from the vaginal cuff on gross anatomic exam.
2. Post hysterectomy patient has an abscess or other evidence of infection at the vaginal cuff on gross
anatomic exam.

January 2024 Surveillance Definitions
17 - 26
3. Post hysterectomy patient has organism(s) identified from fluid or tissue obtained from the vaginal cuff
by a culture or non-culture based microbiologic testing method which is performed for purposes of
clinical diagnosis or treatment, for example, not Active Surveillance Culture/Testing (ASC/AST).
Reporting Instruction
• Report vaginal cuff infections as SSI-VCUF.
SST-SKIN AND SOFT TISSUE INFECTION
BRST-Breast infection or mastitis
A breast abscess or mastitis must meet at least one of the following criteria:
1. Patient has organism(s) identified from affected breast tissue or fluid obtained by invasive procedure
or from drainage from an aseptically-placed drain by a culture or non-culture based microbiologic
testing method which is performed for purposes of clinical diagnosis or treatment, for example, not
Active Surveillance Culture/Testing (ASC/AST).
2. Patient has a breast abscess or other evidence of infection on gross anatomic or histopathologic
exam.
3. Patient has fever (>38.0°C) and local inflammation of the breast,
AND
Physician or physician designee initiates antimicrobial therapy within 2 days of onset or worsening of
symptoms.
Reporting Instructions
• For SSI after a BRST procedure: if the infection is in the subcutaneous region report as a superficial
incisional SSI, and if the infection involves the muscle/fascial level report as a deep incisional SSI.
• BRST Criterion ‘3’ is not eligible as an Organ/Space SSI following a BRST procedure.
BURN-Burn infection
Burn infections must meet the following criteria:
1. Patient has a change in burn wound appearance or character*, such as rapid eschar separation, or dark
brown, black, or violaceous discoloration of the eschar,
AND
Organism(s) identified from blood by a culture or non-culture based microbiologic testing method
which is performed for purposes of clinical diagnosis or treatment, for example, not Active Surveillance
Culture/Testing (ASC/AST).
Reporting Instructions
• Report BURN in the setting of an infected burn covered with a temporary graft or dressing.
• In the setting of a permanent skin graft (autograft) over a burn wound, use the SKIN or ST criteria.

January 2024 Surveillance Definitions
17 - 27
CIRC-Newborn circumcision infection
Circumcision infection in a newborn (≤30 days old) must meet at least one of the following criteria:
1. Newborn has purulent drainage from circumcision site.
2. Newborn has at least one of the following signs or symptoms at circumcision site: erythema*,
swelling*, or tenderness*,
AND
Pathogen identified from circumcision site by a culture or non-culture based microbiologic testing
method which is performed for purposes of clinical diagnosis or treatment, for example, not Active
Surveillance Culture/Testing (ASC/AST).
3. Newborn has at least one of the following signs or symptoms at circumcision site: erythema*,
swelling*, or tenderness*,
AND
Common commensal is identified from circumcision site by a culture or non-culture based
microbiologic testing method which is performed for purposes of clinical diagnosis or treatment, for
example, not Active Surveillance Culture/Testing (ASC/AST),
AND
Physician or physician designee initiates antimicrobial therapy within two days on onset or worsening
of symptoms.
* With no other recognized cause
DECU-Decubitus ulcer infection (also known as pressure injury infection),
including both superficial and deep infections
Decubitus ulcer infections must meet the following criterion:
1. Patient has at least two of the following signs or symptoms: erythema*, tenderness*, or swelling of
decubitus wound edges*,
AND
Organism(s) identified from needle aspiration of fluid or biopsy of tissue from ulcer margin by a
culture or non-culture based microbiologic testing method which is performed for purposes of clinical
diagnosis or treatment, for example, not Active Surveillance Culture/Testing (ASC/AST).
* With no other recognized cause
SKIN-Skin infection (skin and/or subcutaneous) excluding decubitus ulcers,
burns, and infections at vascular access sites (See VASC).
Skin infections must meet at least one of the following criteria:
1. Patient has at least one of the following:

January 2024 Surveillance Definitions
17 - 28
• purulent drainage
• pustules
• vesicles
• boils (excluding acne)
2. Patient has at least two of the following localized signs or symptoms: pain* or tenderness*, swelling*,
erythema*, or heat*
And at least one of the following:
a. organism(s) identified from aspirate or drainage from affected site by a culture or non-culture
based testing method which is performed for purposes of clinical diagnosis and treatment for
example, not Active Surveillance Culture/Testing (ASC/AST). Identification of 2 or more
common commensal organisms without a recognized pathogen is not eligible for use. Common
Commensal organisms include, but not are not limited to, diphtheroids (Corynebacterium spp.
not C. diphtheria), Bacillus spp. (not B. anthracis), Propionibacterium spp., coagulase-negative
staphylococci (including S. epidermidis), viridans group streptococci, Aerococcus spp.,
Micrococcus spp., and Rhodococcus spp. Common Commensals on the NHSN Organism List can
be accessed via the spreadsheet or the new NHSN Terminology Browser.
b. multinucleated giant cells seen on microscopic examination of affected tissue.
c. diagnostic single antibody titer (IgM) or 4-fold increase in paired sera (IgG) for organism.
* With no other recognized cause
Reporting Instructions
• Do not report acne as a skin/soft tissue HAI.
• Report SKIN or ST criteria in the setting of a permanent skin graft (autograft) over a burn wound.
• Apply the site-specific definition (not SKIN) for the following:
o Report omphalitis in infants as UMB.
o Report infections of the circumcision site in newborns as CIRC.
o For decubitus ulcers, apply the DECU infection.
o Report infected burns as BURN.
o Report BURN in the setting of an infected burn covered with a temporary graft or
dressing.
o Report breast abscesses or mastitis as BRST.
o Report localized infection at a vascular access site as a VASC unless there is an organism
identified from blood, meeting LCBI criteria, which should instead be reported as an LCBI
(see VASC definition).
ST-Soft tissue infection (muscle and/or fascia [for example, necrotizing fasciitis,
infectious gangrene, necrotizing cellulitis, infectious myositis, lymphadenitis,
lymphangitis, or parotitis]) excluding decubitus ulcers, burns, and infections at
vascular access sites (See VASC).
Soft tissue infections must meet at least one of the following criteria:
1. Patient has organism(s) identified from tissue or drainage from affected site by a culture or non-
culture based microbiologic testing method which is performed for purposes of clinical diagnosis or
treatment, for example, not Active Surveillance Culture/Testing (ASC/AST)
2. Patient has purulent drainage at affected site.

January 2024 Surveillance Definitions
17 - 29
3. Patient has an abscess or other evidence of infection on gross anatomic or histopathologic exam
Reporting Instructions
• Report SKIN or ST criteria in the setting of a permanent skin graft (autograft) over a burn wound.
• Apply the site-specific definitions identified below (not ST) for the following:
o Report infected decubitus ulcers as DECU.
o Report infected burns as BURN.
o Report BURN in the setting of an infected burn covered with a temporary graft or dressing.
o Report infection of deep pelvic tissues as OREP.
o Report localized infection at a vascular access site as a VASC unless there is an organism
identified from blood, then it should be reported as an LCBI (see VASC definition).
UMB-Omphalitis
Omphalitis in a newborn (≤30 days old) must meet at least one of the following criteria:
1. Patient has erythema or drainage from umbilicus
And at least one of the following:
a. organism(s) identified from drainage or needle aspirate by a culture or non-culture based
microbiologic testing method which is performed for purposes of clinical diagnosis or
treatment, for example, not Active Surveillance Culture/Testing (ASC/AST)
b. organism(s) identified from blood by a culture or non-culture based microbiologic testing
method which is performed for purposes of clinical diagnosis or treatment, for example, not
Active Surveillance Culture/Testing (ASC/AST).
2. Patient has erythema and purulence at the umbilicus
Reporting instruction
• Report infection of the umbilical artery or vein related to umbilical catheterization as VASC if
there is no accompanying organism identified from blood specimen. However, if the patient
meets criteria for LCBI, report as a LCBI (see VASC).
• Catheterized umbilical venous catheter (UVC) or umbilical arterial catheter (UAC) sites are not
eligible for UMB criteria.
USI – URINARY SYSTEM INFECTION (kidney, ureter, bladder, urethra, or
perinephric space excluding UTI [see Chapter 7].)
Urinary system infections must meet at least one of the following criteria:
1. Patient has organism(s) identified from fluid (not urine) or tissue from affected site by a culture or
non-culture based microbiologic testing method which is performed for purposes of clinical diagnosis
or treatment, for example, not Active Surveillance Culture/Testing (ASC/AST)
2. Patient has an abscess or other evidence of infection on gross anatomical exam, during invasive
procedure, or on histopathologic exam.
3. Patient has one of the following signs or symptoms:
• fever (>38.0°C)

January 2024 Surveillance Definitions
17 - 30
• localized pain or tenderness*
And at least one of the following:
a. purulent drainage from affected site
b. organism(s) identified from blood by a culture or non-culture based microbiologic testing
method which is performed for purposes of clinical diagnosis or treatment, for example, not
Active Surveillance Culture/Testing (ASC/AST).
AND
imaging test evidence definitive for infection, for example, ultrasound, CT scan, magnetic
resonance imaging [MRI], or radiolabel scan [gallium, technetium]), which if equivocal is
supported by clinical correlation, specifically, physician or physician designee documentation of
antimicrobial treatment for urinary system infection.
4. Patient <1 year of age has at least one of the following signs or symptoms:
• fever (>38.0°C)
• hypothermia (<36.0°C)
• apnea*
• bradycardia*
• lethargy*
• vomiting*
And at least one of the following:
a. purulent drainage from affected site
b. organism(s) identified from blood by a culture or non-culture based microbiologic testing
method which is performed for purposes of clinical diagnosis or treatment, for example, not
Active Surveillance Culture/Testing (ASC/AST)
AND
imaging test evidence definitive for infection, for example, ultrasound, CT scans, magnetic
resonance imaging [MRI], or radiolabel scan [gallium, technetium]), which if equivocal is
supported by clinical correlation, specifically, physician or physician designee documentation of
antimicrobial treatment for urinary system infection.
* With no other recognized cause
Reporting Instructions
• Report infections following circumcision in newborns as SST-CIRC.
