
FilmArray
®
Meningitis/Encephalitis Panel
(Injection Vials)
x3
To avoid contamination always wear gloves and work behind a protective shield.
Step 2: Hydrate Pouch
q Unscrew Hydration Injection Vial, leaving cap in Pouch Loading
Station, and insert into pouch hydration port.
q Forcefully push down to puncture seal and wait as Hydration Solution
is drawn into pouch.
x3
BioFire Diagnostics, LLC
390 Wakara Way
Salt Lake City, Utah 84108
USA
FLM1-SUB-0065
BioFire Diagnostics, LLC
390 Wakara Way
Salt Lake City, Utah 84108
USA
FLM1-SUB-0065
BioFire Diagnostics, LLC
390 Wakara Way
Salt Lake City, Utah 84108
USA
FLM1-SUB-0065
BioFire Diagnostics, LLC
390 Wakara Way
Salt Lake City, Utah 84108
USA
FLM1-SUB-0065
BioFire Diagnostics, LLC
390 Wakara Way
Salt Lake City, Utah 84108
USA
FLM1-SUB-0065
BioFire Diagnostics, LLC
390 Wakara Way
Salt Lake City, Utah 84108
USA
FLM1-SUB-0065
BioFire Diagnostics, LLC
390 Wakara Way
Salt Lake City, Utah 84108
USA
FLM1-SUB-0065
Step 3: Prepare Sample Mix
q Add Sample Buffer to Sample Injection Vial:
• Invert Sample Buffer Ampoule so that tip is facing up.
Note: Do not touch the tip of the ampoule.
• Firmly pinch textured plastic tab on side of ampoule until seal snaps.
• With the tip facing down, dispense Sample Buffer into Sample
Injection Vial using a slow, forceful squeeze, followed by a 2
nd
squeeze. Avoid generating excessive bubbles.
q Using transfer pipette, draw cerebrospinal fluid (CSF) specimen to 2
nd
line.
q Add to Sample Injection Vial.
q Tightly close lid of Sample Injection Vial.
q Mix sample by gently inverting Sample Injection Vial 3 times.
q Return Sample Injection Vial to red well of Pouch Loading Station.
Warning: The Sample Buffer is harmful if swallowed, can cause
serious eye damage and/or skin irritation.
Step 4: Load Sample Mix
q Unscrew Sample Injection Vial from cap.
q Pause for 3-5 seconds, then remove Sample Injection Vial, leaving
cap in Pouch Loading Station.
q Insert Sample Injection Vial into pouch sample port.
q Forcefully push down to puncture seal.
q Wait as Sample Mix is drawn into pouch.
x3
BioFire Diagnostics, LLC
390 Wakara Way
Salt Lake City, Utah 84108
USA
FLM1-SUB-0065
BioFire Diagnostics, LLC
390 Wakara Way
Salt Lake City, Utah 84108
USA
FLM1-SUB-0065
BioFire Diagnostics, LLC
390 Wakara Way
Salt Lake City, Utah 84108
USA
FLM1-SUB-0065
BioFire Diagnostics, LLC
390 Wakara Way
Salt Lake City, Utah 84108
USA
FLM1-SUB-0065
BioFire Diagnostics, LLC
390 Wakara Way
Salt Lake City, Utah 84108
USA
FLM1-SUB-0065
BioFire Diagnostics, LLC
390 Wakara Way
Salt Lake City, Utah 84108
USA
FLM1-SUB-0065
BioFire Diagnostics, LLC
390 Wakara Way
Salt Lake City, Utah 84108
USA
FLM1-SUB-0065
0:05
Step 5: Run Pouch
q Follow instructions on computer for initiating a test.
q The pouch will click into place when properly seated.
Note: If the pouch does not insert easily, ensure that the lid is opened completely.
FilmArray
™
Panel
Step 1: Prepare Pouch
q Insert pouch into Pouch Loading Station.
q Place Sample Injection Vial into red well.
q Place Hydration Injection Vial into blue well.
x3
BioFire Diagnostics, LLC
390 Wakara Way
Salt Lake City, Utah 84108
USA
FLM1-SUB-0065
BioFire Diagnostics, LLC
390 Wakara Way
Salt Lake City, Utah 84108
USA
FLM1-SUB-0065
BioFire Diagnostics, LLC
390 Wakara Way
Salt Lake City, Utah 84108
USA
FLM1-SUB-0065
BioFire Diagnostics, LLC
390 Wakara Way
Salt Lake City, Utah 84108
USA
FLM1-SUB-0065
BioFire Diagnostics, LLC
390 Wakara Way
Salt Lake City, Utah 84108
USA
FLM1-SUB-0065
BioFire Diagnostics, LLC
390 Wakara Way
Salt Lake City, Utah 84108
USA
FLM1-SUB-0065
BioFire Diagnostics, LLC
390 Wakara Way
Salt Lake City, Utah 84108
USA
FLM1-SUB-0065
x3
BioFire Diagnostics, LLC
390 Wakara Way
Salt Lake City, Utah 84108
USA
FLM1-SUB-0065
BioFire Diagnostics, LLC
390 Wakara Way
Salt Lake City, Utah 84108
USA
FLM1-SUB-0065
BioFire Diagnostics, LLC
390 Wakara Way
Salt Lake City, Utah 84108
USA
FLM1-SUB-0065
BioFire Diagnostics, LLC
390 Wakara Way
Salt Lake City, Utah 84108
USA
FLM1-SUB-0065
BioFire Diagnostics, LLC
390 Wakara Way
Salt Lake City, Utah 84108
USA
FLM1-SUB-0065
BioFire Diagnostics, LLC
390 Wakara Way
Salt Lake City, Utah 84108
USA
FLM1-SUB-0065
x3
BioFire Diagnostics, LLC
390 Wakara Way
Salt Lake City, Utah 84108
USA
FLM1-SUB-0065
BioFire Diagnostics, LLC
390 Wakara Way
Salt Lake City, Utah 84108
USA
FLM1-SUB-0065
BioFire Diagnostics, LLC
390 Wakara Way
Salt Lake City, Utah 84108
USA
FLM1-SUB-0065
BioFire Diagnostics, LLC
390 Wakara Way
Salt Lake City, Utah 84108
USA
FLM1-SUB-0065
BioFire Diagnostics, LLC
390 Wakara Way
Salt Lake City, Utah 84108
USA
FLM1-SUB-0065
BioFire Diagnostics, LLC
390 Wakara Way
Salt Lake City, Utah 84108
USA
FLM1-SUB-0065
x3
BioFire Diagnostics, LLC
390 Wakara Way
Salt Lake City, Utah 84108
USA
FLM1-SUB-0065
BioFire Diagnostics, LLC
390 Wakara Way
Salt Lake City, Utah 84108
USA
FLM1-SUB-0065
BioFire Diagnostics, LLC
390 Wakara Way
Salt Lake City, Utah 84108
USA
FLM1-SUB-0065
BioFire Diagnostics, LLC
390 Wakara Way
Salt Lake City, Utah 84108
USA
FLM1-SUB-0065
BioFire Diagnostics, LLC
390 Wakara Way
Salt Lake City, Utah 84108
USA
FLM1-SUB-0065
BioFire Diagnostics, LLC
390 Wakara Way
Salt Lake City, Utah 84108
USA
FLM1-SUB-0065
x3
BioFire Diagnostics, LLC
390 Wakara Way
Salt Lake City, Utah 84108
USA
FLM1-SUB-0065
BioFire Diagnostics, LLC
390 Wakara Way
Salt Lake City, Utah 84108
USA
FLM1-SUB-0065
BioFire Diagnostics, LLC
390 Wakara Way
Salt Lake City, Utah 84108
USA
FLM1-SUB-0065
BioFire Diagnostics, LLC
390 Wakara Way
Salt Lake City, Utah 84108
USA
FLM1-SUB-0065
BioFire Diagnostics, LLC
390 Wakara Way
Salt Lake City, Utah 84108
USA
FLM1-SUB-0065
BioFire Diagnostics, LLC
390 Wakara Way
Salt Lake City, Utah 84108
USA
FLM1-SUB-0065
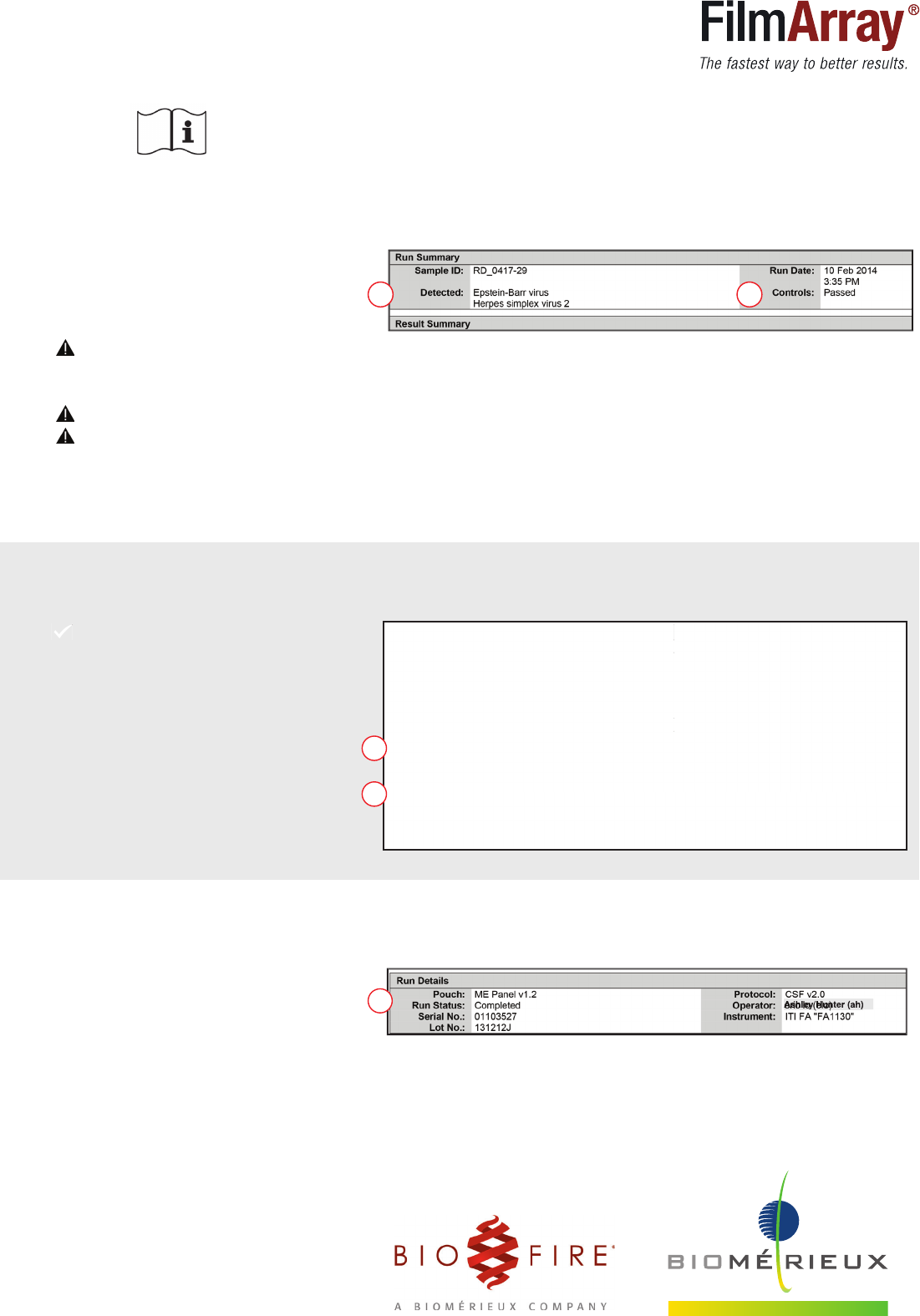
1. 2.
Ashley Hunter (ah)
5.
3. “ Detected”, pathogen was
detected
4. “Not Detected”, pathogen was not
detected
The Run Summary Section displays information about the sample and a summary of the
control and test results.
1. Detected:
• Names of any detected pathogens
• ‘None’, no pathogens were detected
• ‘
Invalid’, RETEST SAMPLE
2. Controls:
• ‘Passed’, results are valid
• ‘ Failed’, RETEST SAMPLE
• ‘
Invalid’, RETEST SAMPLE
The Results Summary Section lists the test results for each pathogen targeted
by the Respiratory Panel.
5. ‘Completed’, run is complete
‘Incomplete’, ‘Aborted’, or any other
error message, RETEST SAMPLE
Note: If repeated “Error” messages are obtained contact BioFire Diagnostics, the local bioMérieux sales representative, or an
authorized distributor.
The Run Details Section displays information about the pouch, instrument,
run status and operator.
Understanding the FilmArray Run Report
Mar. 2015 / RFIT-PRT-0489-01 / FR / A / bioMérieux S.A. RCS Lyon 673 620 399 / Imprinted in France.
Manufactured by:
BioFire Diagnostics, LLC
390 Wakara Way, Salt Lake City,
UT 84108, USA
BioFire is a subsidiary 100% owned by bioMérieux.
www.biofiredx.com
bioMérieux S.A.
69280 Marcy I’Etoile
France
Tel.: +33 (0) 4 78 87 20 00
Fax: +33 (0) 4 78 87 20 90
www.biomerieux.com
The purchase of this product includes a limited, non-transferable license under speci c claims of one or more US patents listed on the BioFire Diagnostics website (http://www.bio redx.com/LegalNotices/) owned by BioFire and/or the Research Foundation of The University of Utah. The blue logo, BioFire
and FilmArray logos, and BIOMÉRIEUX, BIOFIRE, and FILMARRAY are used, pending, and/or registered trademarks belonging to bioMérieux or BioFire or one of their subsidiaries or companies. Document and/or picture not legally binding. Modi cations can be made without prior notice. No part of this
document may be reproduced or transmitted in any form or by any means, electronic or mechanical, for any purpose, without the express written permission of bioMérieux / BioFire Diagnostics.
4.
3.
