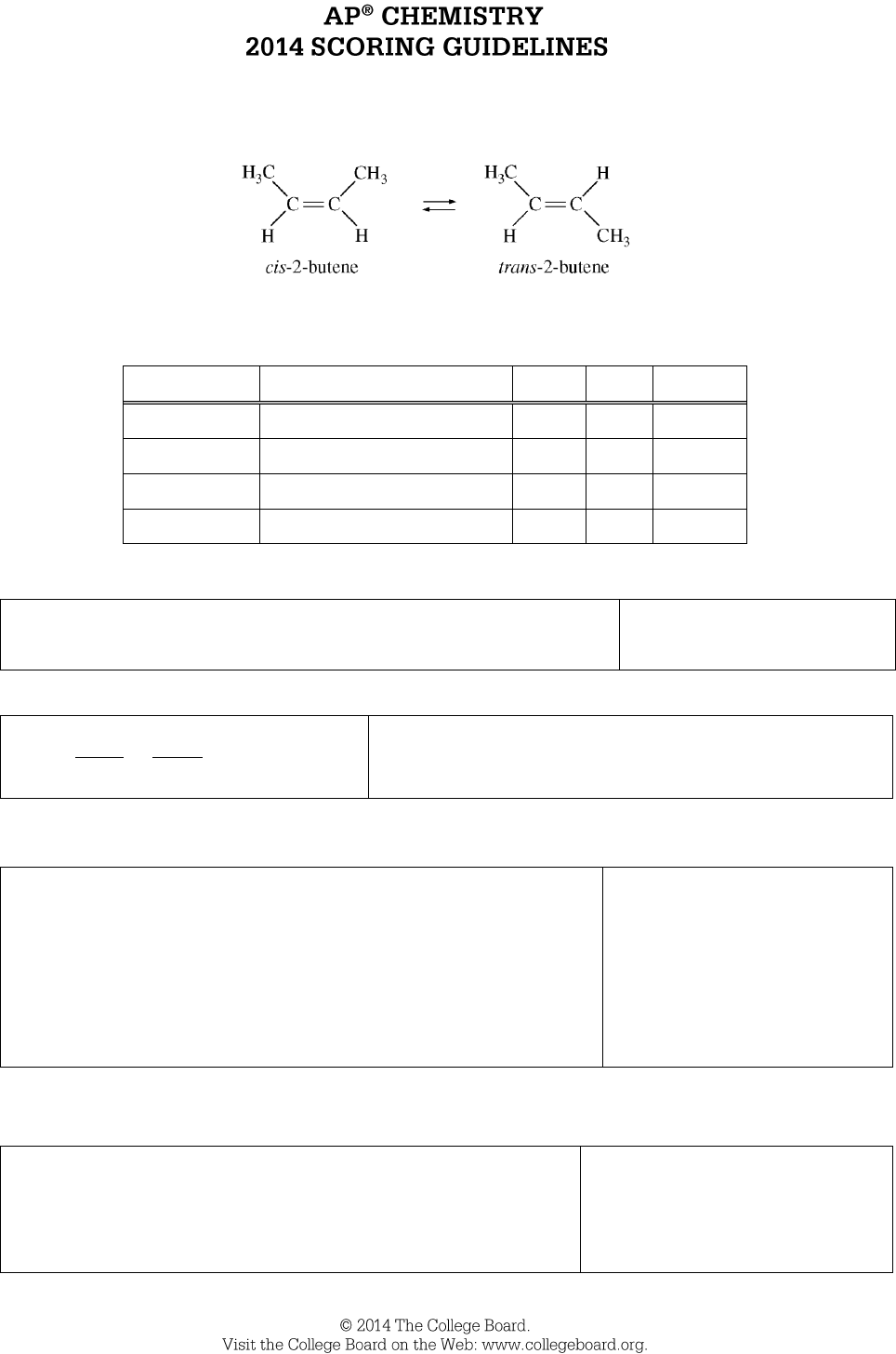
Question 7
(4 points)
The ha
l
f-life (t
1/2
) of the catalyzed isomerization of cis-2-butene gas to produce trans-2-butene gas,
represented above, was measured under various conditions, as shown in the table below.
Trial Number
Initial P
cis-2-butene
(torr)
V (L) T (K)
t
1/2
(s)
1
300.
2.00
350. 100.
2
600.
2.00
350. 100.
3
300.
4.00
350.
100.
4
300.
2.00
365 50.
(
a)
The reaction is first order. Explain how the data in the table are consistent with a first-order reaction.
For a first-order reaction, the half-life is independent of reactant
concentration (or pressure) at constant T, as shown in trials 1, 2, and 3.
1 point is earned for a
correct explanation.
(
b)
Calculate the rate constant, k, for the reaction at 350. K. Include appropriate units with your answer.
1/ 2
1
0.693 0.693
= = = 0.00693 s
100. s
k
t
-
1 poi
n
t is earned for correct numerical answer with units.
(
c)
Is the initial rate of the reaction in trial 1 greater than, less than, or equal to the initial rate in trial 2 ?
Justify your answer.
The initial rate in trial 1 is less than that in trial 2
because rate = k [cis-2-butene] or
rate = k
-2-butenecis
P
(
w
i
th reference
to values from both trials).
OR
because the initial concentration of cis-2-butene in trial 1 is less than
that in trial 2 and k is constant.
1 point is earned for the
correct answer with
justification.
(
d)
The half-life of the reaction in trial 4 is less than the half-life in trial 1. Explain why, in terms of activation
energy.
The temperature is higher in trial 4, meaning that the KE
avg
of the
molecules is greater. Consequently, in this trial a greater fraction
of collisions have sufficient energy to overcome the activation
energy barrier, thus the rate is greater.
1 poi
n
t is earned for a correct
answer with justification.

©2014 The College Board.
Visit the College Board on the Web: www.collegeboard.org.

©2014 The College Board.
Visit the College Board on the Web: www.collegeboard.org.

©2014 The College Board.
Visit the College Board on the Web: www.collegeboard.org.

©2014 The College Board.
Visit the College Board on the Web: www.collegeboard.org.

©2014 The College Board.
Visit the College Board on the Web: www.collegeboard.org.
AP
®
CHEMISTRY
2014 SCORING COMMENTARY
Question 7
Overview
T
he question required students to interpret kinetic data in the isomerization reaction of cis-2-butene to
trans-2-butene under various conditions in the gaseous state. Four trials were presented in a table that listed
the initial pressure of cis-2-butene, the volume of the reaction chamber, temperature, and half-life of the
reaction. Part (a) stated that the reaction is first order and asked for an explanation of how the data was
consistent with this fact. In part (b) students calculated the rate constant k for the reaction at 350 K. Part (c)
assessed students’ knowledge of the relationship between reaction rates, rate constant, and concentration
by asking for a prediction, with justification, about whether the initial rate of reaction in trial 1 would be
greater than, less than, or equal to that in trial 2. (Relative to trial 1, trial 2 had an identical volume,
temperature and half-life, but twice the initial partial pressure of reactant.) In part (d) students needed to
explain why, in terms of activation energy, the half-life of the reaction in trial 4 is less than the half-life of
trial 1. This part examined the ability to logically associate a higher temperature with greater average kinetic
energy of reactant molecules and thus a greater fraction of molecules able to collide with enough energy to
overcome the activation energy barrier.
Sample: 7
A
Score:
4
This response received all 4 possible points: 1 point in part (a), 1 point in part (b), 1 point in part (c), and 1
point in part (d).
Sample: 7
B
Score:
2
Part (a) did not earn a point because it omits any mention of half-life and makes no clear reference to
relevant data from the table. The answer to part (b) did not earn a point because, although it provides a
valid setup with the correct numerical value of k, it does not include units. The points were earned in parts
(c) and (d).
Sample: 7
C
Score: 1
In part (a) the point was not earned because the response makes only vague references to the data and
does
not mention half-life specifically. Part (b) did not earn the point because, although the setup and
numerical answer are correct, the answer has incorrect units. The point was not earned in part (c) because
the student said the initial rates are equal. The point was earned in part (d).
© 2014 The College Board.
Visit the College Board on the Web: www.collegeboard.org.
