TOXICOLOGICAL PROFILE FOR
NITRATE AND NITRITE
U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES
Public Health Service
Agency for Toxic Substances and Disease Registry
July 2017
ii NITRATE AND NITRITE
DISCLAIMER
Use of trade names is for identification only and does not imply endorsement by the Agency for Toxic
Substances and Disease Registry, the Public Health Service, or the U.S. Department of Health and Human
Services.
iii NITRATE AND NITRITE
UPDATE STATEMENT
A Toxicological Profile for Nitrate and Nitrite, Draft for Public Comment was released in September
2015. This edition supersedes any previously released draft or final profile.
Toxicological profiles are revised and republished as necessary. For information regarding the update
status of previously released profiles, contact ATSDR at:
Agency for Toxic Substances and Disease Registry
Division of Toxicology and Human Health Sciences
Environmental Toxicology Branch
1600 Clifton Road NE
Mailstop F-57
Atlanta, Georgia 30329-4027
iv NITRATE AND NITRITE
This page is intentionally blank.

v NITRATE AND NITRITE
FOREWORD
This toxicological profile is prepared in accordance with guidelines* developed by the Agency for Toxic
Substances and Disease Registry (ATSDR) and the Environmental Protection Agency (EPA). The
original guidelines were published in the Federal Register on April 17, 1987. Each profile will be revised
and republished as necessary.
The ATSDR toxicological profile succinctly characterizes the toxicologic and adverse health effects
information for these toxic substances described therein. Each peer-reviewed profile identifies and
reviews the key literature that describes a substance's toxicologic properties. Other pertinent literature is
also presented, but is described in less detail than the key studies. The profile is not intended to be an
exhaustive document; however, more comprehensive sources of specialty information are referenced.
The focus of the profiles is on health and toxicologic information; therefore, each toxicological profile
begins with a public health statement that describes, in nontechnical language, a substance's relevant
toxicological properties. Following the public health statement is information concerning levels of
significant human exposure and, where known, significant health effects. The adequacy of information to
determine a substance's health effects is described in a health effects summary. Data needs that are of
significance to the protection of public health are identified by ATSDR.
Each profile includes the following:
(A) The examination, summary, and interpretation of available toxicologic information and
epidemiologic evaluations on a toxic substance to ascertain the levels of significant human
exposure for the substance and the associated acute, subacute, and chronic health effects;
(B) A determination of whether adequate information on the health effects of each substance
is available or in the process of development to determine levels of exposure that present a
significant risk to human health of acute, subacute, and chronic health effects; and
(C) Where appropriate, identification of toxicologic testing needed to identify the types or
levels of exposure that may present significant risk of adverse health effects in humans.
The principal audiences for the toxicological profiles are health professionals at the Federal, State, and
local levels; interested private sector organizations and groups; and members of the public.
This profile reflects ATSDR’s assessment of all relevant toxicologic testing and information that has been
peer-reviewed. Staffs of the Centers for Disease Control and Prevention and other Federal scientists have
also reviewed the profile. In addition, this profile has been peer-reviewed by a nongovernmental panel
and was made available for public review. Final responsibility for the contents and views expressed in
this toxicological profile resides with ATSDR.
Patrick N. Breysse, Ph.D., CIH
Director, National Center for Environmental Health and
Agency for Toxic Substances and Disease Registry
Centers for Disease Control and Prevention

vi NITRATE AND NITRITE
*Legislative Background
The toxicological profiles are developed under the Comprehensive Environmental Response,
Compensation, and Liability Act of 1980, as amended (CERCLA or Superfund). CERCLA section
104(i)(1) directs the Administrator of ATSDR to “…effectuate and implement the health related
authorities” of the statute. This includes the preparation of toxicological profiles for hazardous
substances most commonly found at facilities on the CERCLA National Priorities List and that pose the
most significant potential threat to human health, as determined by ATSDR and the EPA. Section
104(i)(3) of CERCLA, as amended, directs the Administrator of ATSDR to prepare a toxicological profile
for each substance on the list. In addition, ATSDR has the authority to prepare toxicological profiles for
substances not found at sites on the National Priorities List, in an effort to “…establish and maintain
inventory of literature, research, and studies on the health effects of toxic substances” under CERCLA
Section 104(i)(1)(B), to respond to requests for consultation under section 104(i)(4), and as otherwise
necessary to support the site-specific response actions conducted by ATSDR.

vii NITRATE AND NITRITE
QUICK REFERENCE FOR HEALTH CARE PROVIDERS
Toxicological Profiles are a unique compilation of toxicological information on a given hazardous
substance. Each profile reflects a comprehensive and extensive evaluation, summary, and interpretation
of available toxicologic and epidemiologic information on a substance. Health care providers treating
patients potentially exposed to hazardous substances may find the following information helpful for fast
answers to often-asked questions.
Primary Chapters/Sections of Interest
Chapter 1: Public Health Statement: The Public Health Statement can be a useful tool for educating
patients about possible exposure to a hazardous substance. It explains a substance’s relevant
toxicologic properties in a nontechnical, question-and-answer format, and it includes a review of
the general health effects observed following exposure.
Chapter 2: Relevance to Public Health: The Relevance to Public Health Section evaluates, interprets,
and assesses the significance of toxicity data to human health.
Chapter 3: Health Effects: Specific health effects of a given hazardous compound are reported by type
of health effect (e.g.,death, systemic, immunologic, reproductive), by route of exposure, and by
length of exposure (acute, intermediate, and chronic). In addition, both human and animal studies
are reported in this section.
NOTE: Not all health effects reported in this section are necessarily observed in the clinical
setting. Please refer to the Public Health Statement to identify general health effects observed
following exposure.
Pediatrics: Four new sections have been added to each Toxicological Profile to address child health
issues:
Chapter 1 How Can (Chemical X) Affect Children?
Chapter 1 How Can Families Reduce the Risk of Exposure to (Chemical X)?
Section 3.7 Children’s Susceptibility
Section 6.6 Exposures of Children
Other Sections of Interest:
Section 3.8 Biomarkers of Exposure and Effect
Section 3.11 Methods for Reducing Toxic Effects
ATSDR Information Center
Phone: 1-800-CDC-INFO (800-232-4636) or 1-888-232-6348 (TTY)
Internet: http://www.atsdr.cdc.gov
The following additional materials are available online:
Case Studies in Environmental Medicine are self-instructional publications designed to increase primary
health care providers’ knowledge of a hazardous substance in the environment and to aid in the
evaluation of potentially exposed patients (see https://www.atsdr.cdc.gov/csem/csem.html).

viii NITRATE AND NITRITE
Managing Hazardous Materials Incidents is a three-volume set of recommendations for on-scene
(prehospital) and hospital medical management of patients exposed during a hazardous materials
incident (see https://www.atsdr.cdc.gov/MHMI/index.asp). Volumes I and II are planning guides
to assist first responders and hospital emergency department personnel in planning for incidents
that involve hazardous materials. Volume III—Medical Management Guidelines for Acute
Chemical Exposures—is a guide for health care professionals treating patients exposed to
hazardous materials.
Fact Sheets (ToxFAQs™) provide answers to frequently asked questions about toxic substances (see
https://www.atsdr.cdc.gov/toxfaqs/Index.asp).
Other Agencies and Organizations
The National Center for Environmental Health (NCEH) focuses on preventing or controlling disease,
injury, and disability related to the interactions between people and their environment outside the
workplace. Contact: NCEH, Mailstop F-29, 4770 Buford Highway, NE, Atlanta, GA
30341-3724 • Phone: 770-488-7000 • FAX: 770-488-7015 • Web Page:
https://www.cdc.gov/nceh/.
The National Institute for Occupational Safety and Health (NIOSH) conducts research on occupational
diseases and injuries, responds to requests for assistance by investigating problems of health and
safety in the workplace, recommends standards to the Occupational Safety and Health
Administration (OSHA) and the Mine Safety and Health Administration (MSHA), and trains
professionals in occupational safety and health. Contact: NIOSH, 395 E Street, S.W., Suite 9200,
Patriots Plaza Building, Washington, DC 20201 • Phone: 202-245-0625 or 1-800-CDC-INFO
(800-232-4636) • Web Page: https://www.cdc.gov/niosh/.
The National Institute of Environmental Health Sciences (NIEHS) is the principal federal agency for
biomedical research on the effects of chemical, physical, and biologic environmental agents on
human health and well-being. Contact: NIEHS, PO Box 12233, 104 T.W. Alexander Drive,
Research Triangle Park, NC 27709 • Phone: 919-541-3212 • Web Page:
https://www.niehs.nih.gov/.
Clinical Resources (Publicly Available Information)
The Association of Occupational and Environmental Clinics (AOEC) has developed a network of clinics
in the United States to provide expertise in occupational and environmental issues. Contact:
AOEC, 1010 Vermont Avenue, NW, #513, Washington, DC 20005 • Phone: 202-347-4976
• FAX: 202-347-4950 • e-mail: AOEC@AOEC.ORG • Web Page: http://www.aoec.org/.
The American College of Occupational and Environmental Medicine (ACOEM) is an association of
physicians and other health care providers specializing in the field of occupational and
environmental medicine. Contact: ACOEM, 25 Northwest Point Boulevard, Suite 700, Elk
Grove Village, IL 60007-1030 • Phone: 847-818-1800 • FAX: 847-818-9266 • Web Page:
http://www.acoem.org/.
The American College of Medical Toxicology (ACMT) is a nonprofit association of physicians with
recognized expertise in medical toxicology. Contact: ACMT, 10645 North Tatum Boulevard,
ix NITRATE AND NITRITE
Suite 200-111, Phoenix AZ 85028 • Phone: 844-226-8333 • FAX: 844-226-8333 • Web Page:
http://www.acmt.net.
The Pediatric Environmental Health Specialty Units (PEHSUs) is an interconnected system of specialists
who respond to questions from public health professionals, clinicians, policy makers, and the
public about the impact of environmental factors on the health of children and reproductive-aged
adults. Contact information for regional centers can be found at http://pehsu.net/findhelp.html.
The American Association of Poison Control Centers (AAPCC) provide support on the prevention and
treatment of poison exposures. Contact: AAPCC, 515 King Street, Suite 510, Alexandria VA
22314 • Phone: 701-894-1858 • Poison Help Line: 1-800-222-1222 • Web Page:
http://www.aapcc.org/.
x NITRATE AND NITRITE
This page is intentionally blank.
xi NITRATE AND NITRITE
CONTRIBUTORS
CHEMICAL MANAGER(S)/AUTHOR(S):
Carolyn Harper, Ph.D.
Sam Keith, M.S., C.H.P.
G. Daniel Todd, Ph.D.
Malcolm Williams, D.V.M., Ph.D.
ATSDR, Division of Toxicology and Human Health Sciences, Atlanta, GA
David W. Wohlers, Ph.D.
Gary L. Diamond, Ph.D.
Fernando Llados, Ph.D.
Christina Coley, B.S.
Mario Citra, Ph.D.
SRC, Inc., North Syracuse, NY
THE PROFILE HAS UNDERGONE THE FOLLOWING ATSDR INTERNAL REVIEWS:
1. Health Effects Review. The Health Effects Review Committee examines the health effects
chapter of each profile for consistency and accuracy in interpreting health effects and classifying
end points.
2. Minimal Risk Level Review. The Minimal Risk Level Workgroup considers issues relevant to
substance-specific Minimal Risk Levels (MRLs), reviews the health effects database of each
profile, and makes recommendations for derivation of MRLs.
3. Data Needs Review. The Environmental Toxicology Branch reviews data needs sections to
assure consistency across profiles and adherence to instructions in the Guidance.
4. Green Border Review. Green Border review assures the consistency with ATSDR policy.
xii NITRATE AND NITRITE
This page is intentionally blank.
xiii NITRATE AND NITRITE
PEER REVIEW
A peer review panel was assembled for nitrate and nitrite. The panel consisted of the following members:
1. Dr. John Fawell, Visiting Professor, School of Applied Sciences, Cranfield University, Cranfield,
Bedfordshire MK43 OAL, United Kingdom;
2. Dr. Richard B. Ferguson, Professor of Soil Science, Associate Head of the Department of
Agronomy & Horticulture, University of Nebraska-Lincoln, Lincoln, Nebraska; and
3. Dr. Stephen M. Roberts, Director, Center for Environmental & Human Toxicology; Professor,
College of Veterinary Medicine, College of Medicine, College of Public Health and Health
Professions, University of Florida, Gainesville, Florida.
These experts collectively have knowledge of nitrate’s and nitrite’s physical and chemical properties,
toxicokinetics, key health end points, mechanisms of action, human and animal exposure, and
quantification of risk to humans. All reviewers were selected in conformity with the conditions for peer
review specified in Section 104(I)(13) of the Comprehensive Environmental Response, Compensation,
and Liability Act, as amended.
Scientists from the Agency for Toxic Substances and Disease Registry (ATSDR) have reviewed the peer
reviewers' comments and determined which comments will be included in the profile. A listing of the
peer reviewers' comments not incorporated in the profile, with a brief explanation of the rationale for their
exclusion, exists as part of the administrative record for this compound.
The citation of the peer review panel should not be understood to imply its approval of the profile's final
content. The responsibility for the content of this profile lies with the ATSDR.
xiv NITRATE AND NITRITE
This page is intentionally blank.
CONTENTS
DISCLAIMER .............................................................................................................................................. ii
UPDATE STATEMENT ............................................................................................................................. iii
FOREWORD ................................................................................................................................................ v
QUICK REFERENCE FOR HEALTH CARE PROVIDERS .................................................................... vii
CONTRIBUTORS ....................................................................................................................................... xi
PEER REVIEW ......................................................................................................................................... xiii
CONTENTS ................................................................................................................................................ xv
LIST OF FIGURES ................................................................................................................................... xix
LIST OF TABLES ..................................................................................................................................... xxi
1. PUBLIC HEALTH STATEMENT FOR NITRATE AND NITRITE ..................................................... 1
2. RELEVANCE TO PUBLIC HEALTH ................................................................................................... 9
2.1 BACKGROUND AND ENVIRONMENTAL EXPOSURES TO NITRATE AND NITRITE
IN THE UNITED STATES ........................................................................................................... 9
2.2 SUMMARY OF HEALTH EFFECTS ........................................................................................... 9
2.3 MINIMAL RISK LEVELS (MRLs) ............................................................................................ 21
3. HEALTH EFFECTS .............................................................................................................................. 29
3.1 INTRODUCTION ........................................................................................................................ 29
3.2 DISCUSSION OF HEALTH EFFECTS BY ROUTE OF EXPOSURE ..................................... 29
3.2.1 Inhalation Exposure .............................................................................................................. 30
3.2.1.1 Death .............................................................................................................................. 30
3.2.1.2 Systemic Effects............................................................................................................. 31
3.2.1.3 Immunological and Lymphoreticular Effects ................................................................ 32
3.2.1.4 Neurological Effects ...................................................................................................... 32
3.2.1.5 Reproductive Effects ......................................................................................................
32
3.2.1.6 Developmental Effects ................................................................................................... 32
3.2.1.7 Cancer ............................................................................................................................ 32
3.2.2 Oral Exposure ........................................................................................................................ 34
3.2.2.1 Death .............................................................................................................................. 34
3.2.2.2 Systemic Effects............................................................................................................. 34
3.2.2.3 Immunological and Lymphoreticular Effects ................................................................ 71
3.2.2.4 Neurological Effects ...................................................................................................... 71
3.2.2.5 Reproductive Effects ...................................................................................................... 72
3.2.2.6 Developmental Effects ................................................................................................... 74
3.2.2.7 Cancer ............................................................................................................................ 80
3.2.3 Dermal Exposure ................................................................................................................. 105
3.2.3.1 Death ............................................................................................................................ 105
3.2.3.2 Systemic Effects........................................................................................................... 105
3.2.3.3 Immunological and Lymphoreticular Effects .............................................................. 105
3.2.3.4 Neurological Effects .................................................................................................... 105
3.2.3.5 Reproductive Effects .................................................................................................... 105
3.2.3.6 Developmental Effects ................................................................................................. 105
3.2.3.7 Cancer .......................................................................................................................... 105
3.3 GENOTOXICITY ...................................................................................................................... 105
3.4 TOXICOKINETICS ................................................................................................................... 109
3.4.1 Physiologically Based Pharmacokinetic (PBPK)/Pharmacodynamic (PD) Models ........... 115
xv NITRATE AND NITRITE
3.5 MECHANISMS OF ACTION ................................................................................................... 122
3.5.1 Pharmacokinetic Mechanisms ............................................................................................. 122
3.5.2 Mechanisms of Toxicity ...................................................................................................... 123
3.5.3 Animal-to-Human Extrapolations ....................................................................................... 123
3.6 TOXICITIES MEDIATED THROUGH THE NEUROENDOCRINE AXIS ........................... 124
3.7 CHILDREN’S SUSCEPTIBILITY ............................................................................................ 125
3.8 BIOMARKERS OF EXPOSURE AND EFFECT ..................................................................... 128
3.8.1 Biomarkers Used to Identify or Quantify Exposure to Nitrate and Nitrite ......................... 129
3.8.2 Biomarkers Used to Characterize Effects Caused by Nitrate and Nitrite ........................... 130
3.9 INTERACTIONS WITH OTHER CHEMICALS ..................................................................... 130
3.10 POPULATIONS THAT ARE UNUSUALLY SUSCEPTIBLE ................................................ 131
3.11 METHODS FOR REDUCING TOXIC EFFECTS .................................................................... 132
3.11.1 Reducing Peak Absorption Following Exposure ............................................................. 133
3.11.2 Reducing Body Burden ................................................................................................... 133
3.11.3 Interfering with the Mechanism of Action for Toxic Effects .......................................... 133
3.12 ADEQUACY OF THE DATABASE ........................................................................................ 134
3.12.1 Existing Information on Health Effects of Nitrate and Nitrite ........................................ 134
3.12.2 Identification of Data Needs ............................................................................................ 137
3.12.3 Ongoing Studies .............................................................................................................. 150
4. CHEMICAL AND PHYSICAL INFORMATION .............................................................................. 151
4.1 CHEMICAL IDENTITY ............................................................................................................ 151
4.2 PHYSICAL AND CHEMICAL PROPERTIES ......................................................................... 152
5. PRODUCTION, IMPORT/EXPORT, USE, AND DISPOSAL .......................................................... 161
5.1 PRODUCTION ..........................................................................................................................
161
5.2 IMPORT/EXPORT .................................................................................................................... 170
5.3 USE ............................................................................................................................................ 173
5.4 DISPOSAL ................................................................................................................................. 173
6. POTENTIAL FOR HUMAN EXPOSURE ......................................................................................... 177
6.1 OVERVIEW ............................................................................................................................... 177
6.2 RELEASES TO THE ENVIRONMENT ................................................................................... 181
6.2.1 Air ....................................................................................................................................... 181
6.2.2 Water ................................................................................................................................... 184
6.2.3 Soil ...................................................................................................................................... 190
6.3 ENVIRONMENTAL FATE ...................................................................................................... 191
6.3.1 Transport and Partitioning ................................................................................................... 192
6.3.2 Transformation and Degradation ........................................................................................ 193
6.3.2.1 Air ................................................................................................................................ 193
6.3.2.2 Water ............................................................................................................................ 194
6.3.2.3 Sediment and Soil ........................................................................................................ 194
6.4 LEVELS MONITORED OR ESTIMATED IN THE ENVIRONMENT .................................. 195
6.4.1 Air ....................................................................................................................................... 195
6.4.2 Water ................................................................................................................................... 196
6.4.3 Sediment and Soil ............................................................................................................... 199
6.4.4 Other Environmental Media ................................................................................................ 200
6.5 GENERAL POPULATION AND OCCUPATIONAL EXPOSURE ........................................ 204
6.6 EXPOSURES OF CHILDREN .................................................................................................. 211
6.7 POPULATIONS WITH POTENTIALLY HIGH EXPOSURES .............................................. 212
6.8 ADEQUACY OF THE DATABASE ........................................................................................
212
xvi NITRATE AND NITRITE
6.8.1 Identification of Data Needs ............................................................................................... 212
6.8.2 Ongoing Studies .................................................................................................................. 215
7. ANALYTICAL METHODS................................................................................................................ 217
7.1 BIOLOGICAL MATERIALS .................................................................................................... 217
7.2 ENVIRONMENTAL SAMPLES .............................................................................................. 220
7.3 ADEQUACY OF THE DATABASE ........................................................................................ 225
7.3.1 Identification of Data Needs ............................................................................................... 225
7.3.2 Ongoing Studies .................................................................................................................. 226
8. REGULATIONS, ADVISORIES, AND GUIDELINES ..................................................................... 227
9. REFERENCES .................................................................................................................................... 237
10. GLOSSARY ...................................................................................................................................... 273
APPENDICES
A. ATSDR MINIMAL RISK LEVELS AND WORKSHEETS ............................................................. A-1
B. USER’S GUIDE .................................................................................................................................. B-1
C. ACRONYMS, ABBREVIATIONS, AND SYMBOLS ...................................................................... C-1
xvii NITRATE AND NITRITE
xviii NITRATE AND NITRITE
This page is intentionally blank.
LIST OF FIGURES
3-1. Levels of Significant Exposure to Nitrate/Nitrite - Oral ..................................................................... 52
3-2. The Nitrate-Nitrite-Nitric Oxide Cycle in Humans .......................................................................... 113
3-3. Conceptual Representation of a Physiologically Based Pharmacokinetic (PBPK) Model for a
Hypothetical Chemical Substance .................................................................................................... 117
3-4. Structure of the Zeilmaker et al. (1996, 2010b) Model .................................................................... 119
3-5. Existing Information on Health Effects of Nitrate ............................................................................ 135
3-6. Existing Information on Health Effects of Nitrite ............................................................................ 136
5-1. Simplified Schematic of the Nitrogen Cycle .................................................................................... 168
6-1. Frequency of NPL Sites with Ammonium Nitrate Contamination ................................................... 178
6-2. Frequency of NPL Sites with Sodium Nitrate Contamination ......................................................... 179
6-3. Frequency of NPL Sites with Sodium Nitrite Contamination .......................................................... 180
xix NITRATE AND NITRITE
xx NITRATE AND NITRITE
This page is intentionally blank.
LIST OF TABLES
3-1. Levels of Significant Exposure to Nitrate/Nitrite - Oral ..................................................................... 36
3-2. Selected Cohort and Case-Control Studies Published Since 2006 Examining Possible
Associations Between Nitrate and Nitrite Intake and Cancer ............................................................ 81
3-3. Genotoxicity of Sodium Nitrite In Vivo ............................................................................................ 108
3-4. Genotoxicity of Sodium Nitrite In Vitro ........................................................................................... 110
3-5. Parameter Values for the Zeilmaker et al. (1996, 2010) PBPK Model of Nitrate and Nitrite in
Humans............................................................................................................................................. 120
4-1. Chemical Identity of Nitrate and Nitrite Ions ................................................................................... 153
4-2. Chemical Identity of Selected Inorganic Nitrate and Nitrite Compounds ........................................ 154
4-3. Chemical Identity of Ammonia and Urea ......................................................................................... 156
4-4. Physical and Chemical Properties of Selected Inorganic Nitrate and Nitrite Compounds ............... 157
4-5. Physical and Chemical Properties of Ammonia and Urea ................................................................ 158
5-1. Facilities that Produce, Process, or Use Nitrate Compounds ........................................................... 162
5-2. Facilities that Produce, Process, or Use Sodium Nitrite ................................................................... 164
5-3. Facilities that Produce, Process, or Use Ammonia ........................................................................... 166
5-4. Production of Ammonium Nitrate by the U.S. Chemical Industry................................................... 171
5-5. Production of Ammonia by the U.S. Chemical Industry .................................................................. 172
5-6. U.S. Imports and Exports (Metric Tons) of Selected Fertilizers 2000–2012 ................................... 174
5-7. U.S. Exports and Imports for Nitrate Fertilizers in 2012 (Short Tons) ............................................ 175
6-1. Releases to the Environment from Facilities that Produce, Process, or Use Nitrate Compounds .... 182
6-2. Releases to the Environment from Facilities that Produce, Process, or Use Sodium Nitrite............ 185
6-3. Releases to the Environment from Facilities that Produce, Process, or Use Ammonia ...................
187
6-4. Concentrations of Nitrate and Nitrite in Infant Food Products ......................................................... 202
6-5. Average Concentrations of Nitrate and Nitrite in Human Milk and Infant Formula ........................ 203
6-6. Geometric Mean and Selected Percentiles of Urine Concentrations of Urinary Nitrate (in mg/L) for
the U.S. P opulation from the National Health and Nutrition Examination Survey (NHANES) ...... 206
xxi NITRATE AND NITRITE
6-7. Geometric Mean and Selected Percentiles of Urine Concentrations of Urinary Nitrate (Creatinine
Corrected) (in mg/g of creatinine) for the U.S. Population f rom the National Health and Nutrition
Examination Survey (NHANES) ..................................................................................................... 208
7-1. Analytical Methods for Determining Nitrate and Nitrite in Biological Materials ............................ 218
7-2. Analytical Methods for Determining Nitrate and Nitrite in Environmental Samples ...................... 221
8-1. Regulations, Advisories, and Guidelines Applicable to Nitrate and Nitrite ..................................... 230
xxii NITRATE AND NITRITE
1 NITRATE AND NITRITE
1. PUBLIC HEALTH STATEMENT FOR NITRATE AND NITRITE
This Public Health Statement summarizes the Agency for Toxic Substances and Disease Registry’s
(ATSDR) findings on inorganic nitrate and nitrite, including chemical characteristics, exposure risks,
possible health effects from exposure, and ways to limit exposure. Nitrate and nitrite can be present in
organic or inorganic compounds, depending on their chemical structures. This profile pertains to
inorganic nitrate and nitrite, specifically the ionic forms of both nitrate and nitrite.
The U.S. Environmental Protection Agency (EPA) identifies the most serious hazardous waste sites in the
nation. These sites make up the National Priorities List (NPL) and are sites targeted for long-term federal
clean-up activities. Nitrate and nitrite are ubiquitous in the environment. Specific forms of nitrate and
nitrite have occasionally been identified in hazardous waste sites. Ammonium nitrate, sodium nitrate, and
sodium nitrite were identified in 7, 3, and 2 of the 1,832 hazardous waste sites, respectively, that have
been proposed for inclusion on the NPL. The total number of NPL sites evaluated for nitrate and nitrite is
not known. But the possibility remains that as more sites are evaluated, the number of sites at which
nitrate and/or nitrite are found may increase. This information is important because these future sites may
be sources of exposure, and overexposure to nitrate and/or nitrite may be harmful.
If you are exposed to nitrate and/or nitrite, many factors determine whether you’ll be harmed. These
include how much you are exposed to (dose), how long you are exposed (duration), how often you are
exposed (frequency), and how you are exposed (route of exposure). You must also consider the other
chemicals you are exposed to and your age, sex, diet, family traits, lifestyle, and state of health.
WHAT ARE NITRATE AND NITRITE?
Nitrate and nitrite are naturally occurring ionic species that are part of the earth’s nitrogen cycle. They
typically exist in the environment in highly water-soluble forms, in association with other ionic species
such as sodium and potassium. Nitrate and nitrite salts completely dissociate in aqueous environments.
Nitrite is readily oxidized (combines with oxygen) to form nitrate. Nitrate is generally stable in the
environment; however, it may be reduced to nitrite through biological processes involving plants,
microbes, etc.
In nature, plants utilize nitrate as an essential (key) nutrient. In commerce, the majority of nitrate is used
in inorganic fertilizers. Additional uses of commercial nitrate and nitrite include food preservation and
2 NITRATE AND NITRITE
1. PUBLIC HEALTH STATEMENT
the production of munitions and explosives. Sodium nitrite is also being used in medicines and
therapeutics; for example, as an antidote for cyanide poisoning and as a treatment for pulmonary arterial
hypertension.
WHAT HAPPENS TO NITRATE AND NITRITE WHEN THEY ENTER THE ENVIRONMENT?
Nitrate and nitrite ions naturally occur in the terrestrial (soil) and aquatic (water) environment as part of
the earth’s nitrogen cycle (see Figure 5-1) and can therefore be found in both soil and water. In nature,
nitrate and nitrite can also be found in igneous and volcanic rocks. Nitrate is formed naturally as an end
product of vegetable and animal decomposition, making this a principal source for nitrate ion in both
terrestrial (soil) and aquatic (water) environments. Nitrate and nitrite can also be released into the
atmospheric (air), terrestrial (soil), and aquatic (water) environments at places where human-made
materials such as fertilizers are produced or used. Human and animal wastes are important sources of
ammonia, a compound containing nitrogen, which undergoes chemical reaction to produce nitrite and
subsequently nitrate. In aerobic (containing oxygen) environments, ammonia is readily oxidized to nitrite
by ammonia-oxidizing bacteria; nitrite is oxidized to nitrate by nitrite-oxidizing bacteria. This two-stage
process is known as nitrification. Both human-made and natural sources of nitrogen may contribute to
nitrate aerosols in the atmosphere, as well as nitrate and nitrite ions in terrestrial (soil) and aquatic (water)
environments.
Nitrate and nitrite have been detected in surface waters, drinking water (including public and private
wells), and groundwater. Nitrate accounts for the majority of the total available nitrogen in surface
waters. Contamination of waters is a result of agricultural runoff (use of chemical fertilizer or animal
manure) and discharges from septic systems and municipal waste water treatment facilities. Nitrogen
exists naturally in soils, typically bound to organic matter or mineral soil material such as rocks.
Available forms of nitrogen, including nitrate and nitrite, are present in soils at a few kilograms
(kg)/hectare.
Nitrate and nitrite are a normal part of the human diet and can be found in vegetables, fruits, cured meats,
fish, dairy products, beers, cereals, and cereal products. Some salts, such as sodium nitrite, are
intentionally added to foods and beverages to preserve or cure them; inhibiting the formation of
microorganisms that may cause disease such as
botulism. Additionally, nitrites and nitrates may be
present in some medicines as they can be employed in medicinal and therapeutic uses.
3 NITRATE AND NITRITE
1. PUBLIC HEALTH STATEMENT
HOW MIGHT I BE EXPOSED TO NITRATE AND NITRITE?
The major source of overexposure of the general population to nitrate and nitrite is via ingestion of water,
foods, beverages, and/or medicines that contain nitrate and/or nitrite naturally or as an added preservative.
Nitrate and nitrite can be taken up by plants, especially leafy vegetables such as lettuce and spinach and
beet roots; vegetables account for about 80% of the nitrate in a typical human diet. Cured meats, meat
products, cheeses, and beverages may contain sodium nitrate and/or sodium nitrite as preservatives.
Relatively high nitrate concentrations are found in some privately owned wells with shallow depths and
permeable soils. Drinking of water from such sources, combined with nitrate intake from the diet, may
result in overexposure to nitrate in some individuals. Release of nitrate and/or nitrite to soil and water at
waste disposal sites could result in contamination of drinking water sources and increased uptake by
plants used for the human diet. Inhalation of nitrate or nitrite is not a likely exposure route of concern for
the general population, although inhalation of dust from fertilizer products containing nitrate salts is
possible. Dusts may also dissolve in sweat on skin, increasing the potential for dermal exposure.
HOW CAN NITRATE AND NITRITE ENTER AND LEAVE MY BODY?
Nitrate and nitrite could enter your body from the air you breathe; however, you are not likely to be
exposed to amounts of nitrate or nitrite in the air that might cause adverse health effects. Nitrate and
nitrite enter your body when you drink water or eat foods that contain these substances. Nitrate and
nitrite are also present in smokeless tobacco products. Certain bacteria and fungi in these products can
convert nitrate to nitrite, which can lead to the formation of carcinogenic nitrosamines. Neither nitrate
nor nitrite is likely to enter your body from soil. However, nitrate or nitrite in soil could enter the body of
young children if they put soil containing nitrate or nitrite in the mouth. Intake of some nitrate is a
normal part of the nitrogen cycle in humans. Both nitrate and nitrite can be produced inside the body as
well. Some of the nitrate in your body moves from blood to the salivary glands where some of it is
changed to nitrite. Nitrate and nitrite are widely distributed in the body. Nitrate and nitrite that enter your
body are no different chemically than nitrate and nitrite produced inside your body. Most nitrate in your
body leaves in the urine the same day it enters your body. Some nitrite in the stomach forms other
substances, some of which may be harmful. Nitrite in your blood can react with hemoglobin (which
carries oxygen to body tissues) and reduce the ability of hemoglobin to carry oxygen. Nitrite can also
form nitric oxide, which may be beneficial in some instances.
4 NITRATE AND NITRITE
1. PUBLIC HEALTH STATEMENT
HOW CAN NITRATE AND NITRITE AFFECT MY HEALTH?
Most people are not exposed to levels of nitrate and/or nitrite that would cause adverse health effects.
Young infants (<6 months of age) appeared to be particularly sensitive to the effects of nitrite on
hemoglobin after consuming formula prepared with drinking water that contained nitrate at levels higher
than recommended limits; some of these infants died. The cause of methemoglobinemia (a change to
hemoglobin that decreases the ability to transport oxygen to tissues) in many of these infants may have
been gastroenteritis from bacteria or viruses in the drinking water or from other sources not related to
nitrate. Some children and adults who ate food or drank fluids that contained unusually high levels of
nitrite experienced decreases in blood pressure, increased heart rate, reduced ability of the blood to carry
oxygen to tissues, headaches, abdominal cramps, vomiting, and even death.
There is limited evidence that nitrite may cause some cancers of the gastrointestinal tract in humans and
mice. Cancer could result from reactions between nitrite and certain other chemicals that may produce
cancer-causing substances. The International Agency for Research on Cancer (IARC) determined that
there is inadequate evidence for the carcinogenicity of nitrate in food or drinking water and limited
evidence for the carcinogenicity of nitrite in food (based on association with increased incidence of
stomach cancer). IARC determined that there is inadequate evidence for the carcinogenicity of nitrate,
limited evidence for the carcinogenicity of nitrite per se, and sufficient evidence for the carcinogenicity of
nitrite in combination with amines or amides. The overall conclusions of IARC were that “ingested
nitrate and nitrite under conditions that result in endogenous nitrosation is probably carcinogenic to
humans (Group 2A).” IARC noted that: (1) the endogenous nitrogen cycle in humans includes
interconversion of nitrate and nitrite; (2) nitrite-derived nitrosating agents produced in the acid stomach
environment can react with nitrosating compounds such as secondary amines and amides to generate
N-nitroso compounds; (3) nitrosating conditions are enhanced upon ingestion of additional nitrate, nitrite,
or nitrosatable compounds; and (4) some N-nitroso compounds are known carcinogens.
The U.S. EPA Integrated Risk Information System does not include a carcinogenicity evaluation for
nitrate or nitrite.
See Chapters 2 and 3 for more information on health effects of nitrate and nitrite.
5 NITRATE AND NITRITE
1. PUBLIC HEALTH STATEMENT
HOW CAN NITRATE AND NITRITE AFFECT CHILDREN?
This section discusses potential health effects of nitrate and nitrite exposure in humans from when they’re
first conceived to 18 years of age.
Children can experience the same effects as adults from overexposure to nitrate and/or nitrite. Young
infants (<6 months of age) who were fed formula prepared using nitrate-contaminated drinking water
sources appear to be particularly sensitive to the effects of nitrate on hemoglobin (i.e.,
methemoglobinemia), although bacterial infections may have been at least partially responsible for
increased sensitivity in these infants. It is not known whether nitrate or nitrite can cause birth defects.
Results of some studies suggest that ingestion of relatively high levels of nitrate or nitrite could cause
developmental effects, but other studies found no evidence for nitrate- or nitrite-related developmental
effects.
HOW CAN FAMILIES REDUCE THE RISK OF OVEREXPOSURE TO NITRATE AND
NITRITE?
If your doctor finds that you have been exposed to significant amounts of nitrate and/or nitrite, ask
whether your children might also be exposed. Your doctor might need to ask your state health department
to investigate. You may also contact the state or local health department with health concerns.
Much of the diet contains food with nitrate and possibly small amounts of nitrite. Some processed food
contains nitrate and/or nitrite as preservative. If you think that you are getting too much nitrate or nitrite
in your diet, consider eating less of those foods that contain high levels of nitrate or nitrite. This
consideration is particularly relevant to infants and small children. Don’t drink water containing levels of
nitrate or nitrite higher than guideline levels for drinking water.
ARE THERE MEDICAL TESTS TO DETERMINE WHETHER I HAVE BEEN OVEREXPOSED
TO NITRATE AND/OR NITRITE?
Methods are available to detect nitrate and nitrite in plasma and urine; however, these are usually not
available at a doctor’s office and are not clinically useful.
Routine blood tests are available to detect a condition known as methemoglobinemia, which is caused by
the presence of higher-than-normal levels of a form of hemoglobin. However, these tests cannot tell
6 NITRATE AND NITRITE
1. PUBLIC HEALTH STATEMENT
whether the high methemoglobin levels were caused by nitrate and nitrite or by some other substance or
disease.
For more information on the different substances formed by nitrate and nitrite breakdown and tests to
detect these substances in the body, see Chapters 3 and 7.
WHAT RECOMMENDATIONS HAS THE FEDERAL GOVERNMENT MADE TO PROTECT
HUMAN HEALTH?
The federal government develops regulations and recommendations to protect public health. Regulations
can be enforced by law. Federal agencies that develop regulations for toxic substances include the
Environmental Protection Agency (EPA), the Occupational Safety and Health Administration (OSHA),
and the Food and Drug Administration (FDA). Recommendations provide valuable guidelines to protect
public health but are not enforceable by law. Federal organizations that develop recommendations for
toxic substances include the Agency for Toxic Substances and Disease Registry (ATSDR) and the
National Institute for Occupational Safety and Health (NIOSH).
Regulations and recommendations can be expressed as “not-to-exceed” levels; that is, levels of a toxic
substance in air, water, soil, or food that do not exceed a critical value usually based on levels that affect
animals; levels are then adjusted to help protect humans. Sometimes these not-to-exceed levels differ
among federal organizations. Different organizations use different exposure times (e.g., an 8-hour
workday or a 24-hour day), different animal studies, or emphasize some factors over others, depending on
their mission.
Recommendations and regulations are also updated periodically as more information becomes available.
For the most current information, check with the federal agency or organization that issued the regulation
or recommendation.
The EPA lists maximum contaminant levels (MCL) and maximum contaminant level goals (MCLG) of
10 mg/L (or ppm) for nitrate (as nitrate-nitrogen; ~44 mg nitrate/L) and 1 mg/L (or ppm) for nitrite (as
nitrite-nitrogen; ~3.3 mg nitrite/L) in the 2012 Edition of the Drinking Water Standards and Health
Advisories. The FDA lists 10 mg/L nitrate (as nitrogen; ~44 mg nitrate/L), 1 mg/L nitrite (as nitrogen;
~3.3 mg nitrite/L), and 10 mg/L total nitrate and nitrite (as nitrogen) as allowable levels in bottled water.
OSHA has not set a legal limit for nitrate or nitrite in air. NIOSH has not set a recommended limit for
nitrate or nitrite in air.
7 NITRATE AND NITRITE
1. PUBLIC HEALTH STATEMENT
WHERE CAN I GET MORE INFORMATION?
If you have any questions or concerns, please contact your community or state health or environmental
quality department, or contact ATSDR at the address and phone number below. You may also contact
your doctor if experiencing adverse health effects or for medical concerns or questions. ATSDR can also
provide publicly available information regarding medical specialists with expertise and experience
recognizing, evaluating, treating, and managing patients exposed to hazardous substances.
• Call the toll-free information and technical assistance number at
1-800-CDCINFO (1-800-232-4636) or
• Write to:
Agency for Toxic Substances and Disease Registry
Division of Toxicology and Human Health Sciences
1600 Clifton Road NE
Mailstop F-57
Atlanta, GA 30329-4027
Toxicological profiles and other information are available on ATSDR’s web site:
http://www.atsdr.cdc.gov.
8 NITRATE AND NITRITE
1. PUBLIC HEALTH STATEMENT
This page is intentionally blank.
9 NITRATE AND NITRITE
2. RELEVANCE TO PUBLIC HEALTH
2.1 BACKGROUND AND ENVIRONMENTAL EXPOSURES TO NITRATE AND NITRITE IN
THE UNITED STATES
Nitrate and nitrite can be organic or inorganic chemicals depending on their chemical structures. This
profile pertains to inorganic nitrate and nitrite, specifically the nitrate anion and the nitrite anion. Nitrate
and nitrite occur naturally in the environment as part of the nitrogen cycle, and are produced both
endogenously and exogenously. Ammonia-oxidizing bacteria convert ammonia into nitrite; nitrite-
oxidizing bacteria convert nitrite into nitrate in aerobic environments. This two-stage process is known as
nitrification. Main sources of ammonia in the environment are decaying organic matter and human and
animal wastes. Nitrification, atmospheric fixation, and nitrogen fertilizers contribute to nitrite and nitrate
concentrations in the environment. In nature, salts of nitrate and nitrite completely dissociate and these
anions typically exist as ionic species. In the environment, nitrite is readily oxidized to nitrate. Nitrate is
generally stable in the environment; however, it may be reduced through biotic (living systems; plants,
microbes, etc.) processes to nitrite under anerobic conditions.
Nitrate and nitrite are ubiquitous in the environment and people are exposed to them primarily through the
ingestion of food and drinking water. Significant uptake of nitrate and nitrite occurs in all varieties of
plants; internal storage of nitrate (rather than metabolic conversion to ammonium and amino acids) can
occur in some plants, especially leafy vegetables such as lettuce and spinach. Vegetables account for
about 80% of the nitrate in a typical human diet. Nitrate and nitrite are also produced in the body as part
of the natural nitrate-nitrite-nitric oxide cycle.
2.2 SUMMARY OF HEALTH EFFECTS
Hematological Effects. In humans, ingested nitrate is nearly completely absorbed into the blood
from the small intestine and approximately 25% of the plasma nitrate enters the salivary glands where it is
secreted in saliva. As much as 20% of salivary nitrate (5% of ingested nitrate) is reduced to nitrite by
bacterial reductases in the mouth. This in vivo reduction of nitrate accounts for 80–85% of the body’s
nitrite and most of the rest comes from nitrite-containing food sources. Nitrite in the blood can react with
ferrous (Fe
2+
) hemoglobin (which transports oxygen) to form ferric (Fe
3+
) hemoglobin (methemoglobin, a
poor transporter of oxygen), and nitric oxide (which can also bind to deoxyhemoglobin) and nitrate.
10 NITRATE AND NITRITE
2. RELEVANCE TO PUBLIC HEALTH
Methemoglobinemia is a condition in which increased methemoglobin as a percentage of total
hemoglobin results in the expression of clinical signs that increase in severity with increasing percent
methemoglobin. In normal healthy individuals, methemoglobin levels are <1% of total hemoglobin.
Discoloration of the skin (cyanosis) is often observed at methemoglobin levels in the range of 3–15%;
most patients tolerate methemoglobin levels <10%. Tachycardia, weakness, and other signs of tissue
hypoxia may be observed at 10–20% methemoglobin levels. Symptoms involving the central nervous
system (e.g., headache, dizziness, fatigue) and dyspnea and nausea appear at >20% methemoglobin; the
severity of symptoms increases with increasing methemoglobin level. High risk of mortality occurs at
levels >70% methemoglobin. It should be noted that a patient with comorbidities that decrease oxygen
transport or delivery may develop moderate to severe symptoms at much lower methemoglobin levels
than a previously healthy patient. Furthermore, due to differences in the oxygen carrying capacity
between fetal hemoglobin and adult hemoglobin (which replaces fetal hemoglobin during the first year of
postnatal life), cyanosis in young infants with mostly fetal hemoglobin may not be detected at
methemoglobin levels eliciting clinical cyanosis in older infants with mostly adult hemoglobin.
As early as the mid-1900s, methemoglobinemia was reported in infants exposed to relatively large
amounts of nitrate from drinking water sources. Available data identify young bottle-fed infants (1–
3 months of age) as a subpopulation that is particularly susceptible to nitrate-induced
methemoglobinemia, especially those consuming formula prepared from drinking water sources
containing nitrate in excess of 10 mg nitrate-nitrogen/L (44 mg nitrate/L). Subsequent reports provide
additional evidence of associations between ingestion of nitrate from drinking water sources and elevated
methemoglobin levels in infants. Cyanosis and even death occurred in some of the reported cases.
Limited data are available regarding administration of controlled amounts of nitrate and methemoglobin
levels. A study reported methemoglobin levels as high as 5.3% of total hemoglobin in a group of four
infants (ages 11 days to 11 months) administered sodium nitrate in the formula for 2–18 days at a
concentration resulting in a dose of 50 mg nitrate/kg/day and as high as 7.5% in another group of four
infants (ages 2 days to 6 months) similarly treated at 100 mg nitrate/kg/day for 6–9 days. A study
reported methemoglobin levels as high as 6.9–15.9% among three infants (ages not specified) fed formula
prepared using water containing 108 mg nitrate/L.
Young children are somewhat less sensitive than infants to nitrate-induced methemoglobinemia. A study
evaluated methemoglobin levels in 102 children 1–8 years of age. Sixty-four of the children lived in
households where drinking water contained 22–111 mg nitrate-nitrogen/L (97–488 mg nitrate/L);
11 NITRATE AND NITRITE
2. RELEVANCE TO PUBLIC HEALTH
drinking water sources for the other 38 children (controls) contained <10 mg nitrate-nitrogen/L (<44 mg
nitrate/L). Methemoglobin measured 1.0–1.36% in those children 1–4 years of age and appeared to
increase with increasing nitrate intake, although there was no statistically significant change.
Methemoglobin levels in those children 5–8 years of age averaged 0.9–0.95%, independent of level of
exposure to nitrate.
Endocrine Effects. There is some evidence for nitrate-induced effects on thyroid function and
development. Nitrate is one of the substances that act as dose-dependent competitive inhibitors of the
sodium iodide symporter (NIS), which mediates the uptake of iodine by the thyroid. Sufficiently
decreased iodine uptake by the thyroid may result in decreased production of thyroid hormones
triiodothyronine (T3) and thyroxine (T4). Decreased thyroid hormone production causes increased
release of thyroid stimulating hormone (TSH) from the anterior pituitary gland leading to increased
uptake of iodine by the thyroid gland. Sufficiently inhibited uptake of iodine by the thyroid could result
in effects associated with thyroid dysfunction (e.g., hypothyroidism). Concern for nitrate-induced effects
on thyroid function has prompted scientists to perform studies designed to assess thyroid function relative
to drinking water and/or dietary nitrate levels. Some human studies provide suggestive evidence that
elevated levels of nitrate in drinking water and/or nitrate-rich diets may be associated with signs of
thyroid dysfunction. However, limitations of these studies include lack of individual dose-response data,
quantification of iodine intake, and control for other substances that may affect the thyroid; one study
relied on self-reported thyroid status and self-reported dietary nitrate intake. A study found no evidence
for nitrate-induced effects on thyroid function in adults ingesting sodium nitrate for 38 days at
15 mg/kg/day (which is 3 times the maximum acceptable daily intake of 5 mg sodium nitrate/kg/day set
by the Joint Expert Committee on Food Additives [JECFA] of the Food and Agriculture Organization of
the United Nations/World Health Organization and the European Commission's Scientific Committee on
Food).
Thyroid status has been assessed to some extent in animals consuming drinking water or food to which
nitrate salts had been added. There were no clinical signs of hypothyroidism or effects on serum T3 or T4
levels in adult Beagles or their puppies during exposure of the breeding dogs to sodium nitrate in the
drinking water for 1 year at concentrations in the range of 300–1,000 ppm (equivalent to 219–730 mg
nitrate/L). Decreased thyroidal
131
iodine uptake was noted in rats given food containing 0.5–2.5%
potassium nitrate (approximately 3,000–15,000 mg nitrate/kg food). Significantly increased uptake of
thyroidal
131
iodine; decreased serum T3, T4, and TSH levels; increased thyroid weight; and follicular
hyperplasia were noted in female Wistar rats administered sodium nitrate in the drinking water for
12 NITRATE AND NITRITE
2. RELEVANCE TO PUBLIC HEALTH
30 weeks at concentrations ≥250 mg/L (≥182 mg nitrate/L). In another study, significantly increased
serum T3 (34–44% lower than controls), increased thyroid weight (45–77% greater than controls), and
histopathologic thyroid lesions (glandular hypertrophy accompanied by vacuolization, increased colloidal
volume of the follicles, and flattened follicular epithelium) were observed in male Wistar rats receiving
drinking water for 5 months to which potassium nitrate had been added at concentrations ≥100 mg/L.
Significantly decreased serum T3 and T4 levels were observed in all groups of weanling male Wistar rats
with intakes in the range of 8.7–47.4 mg sodium nitrate/kg/day (equivalent to 6.4–34.6 mg
nitrate/kg/day). At doses ≥15.8 mg nitrate/kg/day, significantly increased serum TSH was also noted.
Groups of similarly-treated young adult male Wistar rats exhibited significantly decreased T3 and T4
levels and increased serum TSH at doses ≥15.8 mg nitrate/kg/day. Significantly increased thyroid gland
weight, increased TSH, decreased serum T3 and T4 levels, and decreased thyroid peroxidase activity
were reported in rats administered 3% potassium nitrate in the diet.
In a 13-week study of rats receiving drinking water to which potassium nitrite had been added, doses in
the range of 8.9–241.7 mg/kg/day (4.8–130.5 mg nitrite/kg/day), oral doses ≥13.3 mg nitrite/kg/day
(males) and ≥61.8 mg nitrite/kg/day (females) resulted in hypertrophy in the zona glomerulosa of the
adrenal gland. The effect on the adrenal gland was not observed in untreated controls or potassium
chloride controls. Similar results were obtained at estimated doses of 105.1 mg nitrite/kg/day (males) and
130.1 mg nitrite/kg/day (females) in a subsequent similarly-designed study. Results of a subsequent
study indicate that the effect on the adrenal gland of the rat is a physiological adaptation to repeated
episodes of hypotension caused by nitrite.
Metabolic Effects. Possible associations between nitrate and/or nitrite in drinking water and/or food
sources and risk of type 1 diabetes have been investigated in a number of case-control studies. Some
studies found no significant risk for childhood type 1 diabetes. In one case-control study that included
estimates of nitrate intake based on food frequency questionnaire results for children 0–14 years of age, a
significantly increased risk of type 1 diabetes was noted for children at the high end (≥75
th
percentile) of
estimated nitrate intake compared to those at the low end (<25
th
percentile). In an ecological study of
type 1 diabetes incidence rates by county in Colorado, children (<18 years of age) in counties with water
nitrate levels in the range of 0.77–8.2 mg/L had a significantly increased risk of type1 diabetes compared
to those in counties with water nitrate levels in the range of 0.0–0.084 mg/L. In another ecological study,
a significantly increased association between nitrate in drinking water (highest tertile versus lowest
tertile) and incidence of childhood type 1 diabetes was reported for children diagnosed between 1978 and
1994 in the Yorkshire Regional Health Authority in England. In a subsequent ecological study that
13 NITRATE AND NITRITE
2. RELEVANCE TO PUBLIC HEALTH
included portions of England and Scotland, the Drinking Water Inspectorate found no evidence for an
association between nitrate in the drinking water and incidence of childhood type 1 diabetes.
Cardiovascular Effects. Cardiovascular health is an end point of concern for nitrate and nitrite
because some nitrate is converted to nitrite in the body. Nitrite is a smooth muscle relaxant that can cause
hypotension and plasma nitrite is involved in the oxidation of hemoglobin to methemoglobin, which is
associated with hypotension, rapid pulse, and rapid breathing at high enough concentrations. Ingestion of
nitrite (from potassium nitrite or sodium nitrite sources) has been associated with severe
methemoglobinemia in adults and children; in some of these cases, symptoms included hypotension
and/or tachycardia. These cases were the result of consumption of food or drink that contained unusually
high levels of nitrite via contamination, inadvertent use of sodium nitrite instead of table salt, or ingestion
of a single sodium nitrite tablet (1 g; equivalent to 667 mg nitrite).
In a hospital-based study in Colorado that included 226 cases of hypertension among patients living in
areas where drinking water contained nitrate at concentrations ranging from 19 to 125 ppm (mean
52 ppm) and 261 cases from patients living in areas without nitrate in the drinking water, the mean annual
incidence rate for hypertension in the nitrate-exposed patients was only 5.9/1,000 compared to
7.9/1,000 for the control patients. However, the nitrate-exposed patients exhibited an earlier mean age at
hospitalization for hypertension (58.5 versus 65.2 years for controls); the toxicological significance of this
finding is uncertain because the incidence rate for hypertension was higher among control patients than
among patients exposed to nitrate in the drinking water.
In a study designed to evaluate the oral bioavailability of sodium nitrite in healthy volunteers (seven
females and two males; mean age 22.9 years), ingestion of 0.06 sodium nitrite per mmol hemoglobin
(~1.5–1.8 mg nitrite/kg) resulted in an average heart rate increase from 55 to 63 beats per minute (bpm)
and average mean arterial blood pressure decrease from 78 to 70 mmHg. At a higher intake (~2.9–3.6 mg
nitrite/kg), the average heart rate increased from 57 to 67 bpm and the average mean arterial blood
pressure decreased from 80 to 69 mmHg. The maximum effects on heart rate and blood pressure
occurred between 15 and 20 minutes following ingestion; heart rate and blood pressure returned to near-
baseline levels approximately 2 hours following ingestion at the low dose, but the effects had not returned
to baseline at 4 hours following ingestion at the high dose. The blood pressure-lowering effect of short-
term dietary supplementation of inorganic nitrate appears to be beneficial; however, there is some
uncertainty regarding potential health benefits of long-term nitrate supplementation to treat cardiovascular
diseases.
14 NITRATE AND NITRITE
2. RELEVANCE TO PUBLIC HEALTH
Gastrointestinal Effects. Ingestion of nitrite (from potassium nitrite or sodium nitrite sources) has
been associated with severe methemoglobinemia in adults and children; in many of these cases,
symptoms included abdominal cramps and vomiting. These cases were the result of consumption of food
or drink that contained unusually high levels of nitrite via contamination, inadvertent use of sodium nitrite
instead of table salt, or ingestion of a single sodium nitrite tablet (667 mg nitrite). In a study designed to
evaluate the oral bioavailability of sodium nitrite in healthy volunteers (seven females and two males;
mean age 22.9 years), one subject became nauseous and vomited within 20 minutes following ingestion
of 0.12 mmol sodium nitrite per mmol hemoglobin (~3.2 mg nitrite/kg); another subject reported nausea
within 30 minutes following ingestion of 0.12 mmol sodium nitrite per mmol hemoglobin (~2.9 mg
nitrite/kg).
Epithelial hyperplasia was noted in the forestomach of male and female B6C3F1 mice provided sodium
nitrite in the drinking water for 14 weeks at a concentrations resulting in estimated doses of 663.3 and
824.1 mg nitrite/kg/day, respectively); the no-observed-adverse-effect levels (NOAELs) for these lesions
in the males and females were 435.5 and 562.8 mg nitrite/kg/day, respectively. Similar results were noted
for male and female F344/N rats and male B6C3F1 mice treated for 104–105 weeks at estimated doses of
87.1, 100.5, and 147.4 mg nitrite/kg/day, respectively; the NOAELs for these lesions in the male and
female rats and male mice were 46.9, 53.6, and 80.4 mg nitrite/kg/day, respectively. Sodium nitrite
treatment did not result in increased incidences of forestomach lesions in other groups of male F344 rats
provided sodium nitrite in the drinking water at 2,000 mg/L (estimated dose of 208.4 mg nitrite/kg/day)
for 35 weeks or 51 weeks.
Neurological Effects. Neurological effects have been reported in humans and animals following
ingestion of nitrite; however, these effects may be secondary to nitrite-induced reductions in oxygen-
carrying capacity. Ingestion of nitrite (from potassium nitrite or sodium nitrite sources) has been
associated with severe methemoglobinemia in adults and children; in many of these cases, clinical signs
included dizziness, loss of consciousness, and/or convulsions. These cases were the result of
consumption of food or drink that contained unusually high levels of nitrite via contamination,
inadvertent use of sodium nitrite instead of table salt, or ingestion of a single sodium nitrite tablet
(667 mg nitrite).
Headache was induced in a male subject following consumption of a 10 mg sodium nitrite solution.
Headaches were induced in 8 out of 13 such tests. In a study designed to evaluate the oral bioavailability
15 NITRATE AND NITRITE
2. RELEVANCE TO PUBLIC HEALTH
of sodium nitrite in healthy volunteers (seven females and two males; mean age 22.9 years), headache
was reported by four out of the nine people after ingestion of 0.12 mmol sodium nitrite per mmol
hemoglobin (~2.9–3.6 mg nitrite/kg) and by four of nine subjects after ingestion of 0.06 mmol sodium
nitrite per mmol hemoglobin (~1.5–1.8 mg nitrite/kg).
Abnormalities in electroencephalograms (EEGs) were reported in male albino rats provided sodium nitrite
in the drinking water for 2 months at concentrations resulting in ≥9.38 mg nitrite/kg/day. The abnormal
readings persisted during up to 4.5 months following cessation of exposure to sodium nitrite. At the
highest dose (187.6 mg nitrite/kg/day), rats exhibited clinical signs of sedation and became motionless
during periods of electrical outbursts. Increased aggressive behavior was observed in male C57B1 mice
provided sodium nitrite in the drinking water at 1,000 mg/L for up to 13 weeks postweaning. The mice
had also been exposed via their parents during mating and their mothers during gestation and lactation.
Significantly reduced motor activity was reported in male mice provided sodium nitrite in the drinking
water. Sodium nitrite levels tested ranged from 100 to 2,000 mg/L; however, the study report did not
include specific information regarding the exposure levels that resulted in reduced motor activity.
Developmental Effects. A number of studies evaluated possible associations between
developmental end points and exposure to nitrate. The results provide some evidence of nitrate-related
developmental effects. The results are not adequate for quantitative risk assessment because estimations
of nitrate intakes were typically based on measurements of nitrate levels in drinking water sources at
selected time points and self-reported estimates of water consumption, possible confounding by other
potential toxicants was not evaluated, and most studies did not account for dietary nitrate or nitrite intake,
which is typically the major source of ingested nitrate and nitrite. Some studies reported significant
associations between selected developmental end points and nitrate in drinking water sources. One study
reported increased risk of intercalary limb defect associated with estimated total nitrite intake. Other
studies found no evidence of associations between nitrate and risk of developmental effects.
Cancer. Numerous case-control and cohort studies of carcinogenicity of ingested nitrate and nitrite in
humans have been reported. Many ecological studies have also been reported; however, interpretation of
outcomes of these studies is more uncertain because of various factors that contribute to ecologic bias
(group-based associations between exposure and cancer outcomes may not apply to individuals). In
general, outcomes of case-control and cohort studies have found no or weak associations between
exposure to nitrate and cancer in humans, with stronger associations with exposures to nitrite or intake of
high nitrite foods such as cured meat. Mechanistically, this outcome is consistent with nitrite being an
16 NITRATE AND NITRITE
2. RELEVANCE TO PUBLIC HEALTH
intermediate in the cancer mode of action of nitrate (see Section 3.5.2). This is further supported by
studies that have found interactions between cancer risk attributed to nitrite and exposure to antioxidants.
Uncertainties in estimates of cancer risk from exposure to nitrate or nitrite include those typical of
epidemiological studies in general: uncertainties in estimation of exposure (e.g., estimating long-term
dietary intakes from food frequency questionnaires or levels in public water supplies [PWS]), exposure
misclassification of individual outcomes (e.g., assigning group-level exposure estimates to individuals),
and adequacy of controlling for confounders (e.g., other factors contributing to the cancer). One
potentially important class of confounders is antioxidants that can influence the degree of nitrosation of
dietary amines and, thereby, the cancer risk from exposure to nitrate or nitrite.
The strongest and most consistent evidence of a carcinogenic role for nitrite is from studies of
gastrointestinal cancers and, in particular, gastric cancer. In general, these studies found significant
positive trends for cancer risk (risk increases with increasing intake), and three studies found elevated
cancer risk. Relative risks (RRs) were 1.71 (95% confidence interval [CI]: 1.24, 2.37) at a nitrite intake
of 1 mg/day and 2.5 (95% CI: 1.4, 4.3) at nitrite intakes ≥6 mg/day. Risk was modified by dietary
vitamin E and folate intake, with increased risk in association with higher nitrate/vitamin E or folate
ratios. Associations between exposure to nitrate or nitrite and colorectal cancer have been studied in
cohort and case-control studies and results are less consistent than for gastric cancer. Two studies found
elevated risk: 1.16 (95% CI: 1.04, 1.30) for colon cancer at nitrate-nitrogen levels >0.6 mg/L (>2.65 mg
nitrate/L drinking water; 1.5 (95% CI: 1.0, 2.1) for colon cancer at a dietary nitrite intake >1.26 mg/day,
and 1.7 (95% CI: 1.1, 2.5) at a dietary nitrite intake >1.26 mg/day. Risks were higher in populations
exposed to drinking water that had a calcium level >34.6 mg/L (odds ratio [OR] 1.37, 95% CI: 1.11; 1.69)
for nitrate <2.65 mg/L; or in populations exposed to nitrate in drinking water at levels >5 mg/L in
combination with a low vitamin C intake (OR 2.0, 95% CI: 1.2, 3.3).
Results have been mixed for other types of cancer. Some case-control or cohort studies found
associations between exposure to nitrite (or foods high in nitrite such as cured meat) and brain cancer in
children and adults, breast cancer, kidney cancer, testicular cancer, and non-Hodgkin’s lymphoma. Of
these studies, the highest risks were reported for brain cancers. Two case-control studies found elevated
relative risk of brain cancer in children in association with maternal exposure: 3.0 (95% CI: 1.2, 7.9) for
nitrite intakes >3.0 mg/day and 5.7 (95% CI: 1.2, 27.2) for astroglial tumors at drinking water exposures
≥5 mg/L. In general, case-control and cohort studies of cancers of larynx, liver, lung, mouth, pancreas,
and pharynx have found no consistent associations with exposures to nitrate or nitrite.
17 NITRATE AND NITRITE
2. RELEVANCE TO PUBLIC HEALTH
The potential carcinogenicity of nitrate has been investigated in several animal studies that employed the
oral exposure route. Studies in which negative results were reported include MCR-derived rats
(15/sex/group) provided 5,000 mg sodium nitrate/L (3,650 mg nitrate/L) in the drinking water for
84 weeks and sacrificed 20 weeks later, male white rats provided 4,000 mg sodium nitrate in the drinking
water for 273 days and sacrificed at 10 months, strain A male mice (n=40) provided 12,300 mg sodium
nitrate/L in the drinking water for 25 weeks and sacrificed 13 weeks later, female NMRI mice provided
1,000 mg calcium nitrate/L in the drinking water for 18 months, Fischer 344 rats (50/sex/group) fed diet
containing up to 5% sodium nitrate (1,517–1,730 mg nitrate/kg/day) for 2 years, and ICR mice
(10/sex/group) fed diets containing up to 5% sodium nitrate for 2 years. In one study, some groups of
male white rats were treated with drinking water containing 0.05% N-butyl-N-(4-hydroxybutyl)-
nitrosamine (BBNA, an inducer of urinary bladder cancer in laboratory animals) for 30 days, either alone
or followed by 4,000 mg sodium nitrate/L drinking water for 273 days. The group treated with BBNA
followed by sodium nitrate exhibited significantly increased incidence of urinary bladder carcinoma
(6/20 rats versus 1/18 rats treated with 0.05% BBNA only. These results indicate that sodium nitrate may
have promoted BBNA-induced bladder tumors.
The potential carcinogenicity of ingested nitrite has been investigated in numerous animal studies. Nitrite
treatment alone did not result in increased incidences of tumors in most studies. There was no evidence
of sodium nitrite-induced forestomach neoplasms among male and female F344/N rats provided sodium
nitrite in the drinking water for 2 years at concentrations of 750, 1,500, or 3,000 ppm (average doses in
the range of 35–150 mg sodium nitrite/kg/day or 23.3–100 mg nitrite/kg/day). Although the mid-dose
group of female rats exhibited a significantly increased incidence of mammary gland fibroadenoma, the
incidence in the high-dose group was not significantly different from that of controls; based on this
finding and the high historical background incidence of mammary gland fibroadenomas, the incidence in
the mid-dose group was not considered treatment related. Significantly decreased incidences of
mononuclear cell leukemia were observed in mid- and high-dose male and female rats. It was speculated
that increased methemoglobin concentrations may have played a role in the decreased incidences of
mononuclear cell leukemia. Significantly increased incidence of fibroma of the subcutis was noted in
mid-dose male rats; however, several factors (the incidence only slightly exceeded the historical range of
NTP controls, there was a lack of a dose-response characteristic, combined incidences of fibroma or
fibrosarcoma were within the historical range for NTP controls, and fibromas and fibrosarcomas are
common neoplasms in the skin of F344/N rats) suggested that the fibroma was not related to sodium
nitrite exposure. It was concluded that there was "no evidence of carcinogenic activity" of sodium nitrite
in the male or female F344/N rats under the conditions of the study.
18 NITRATE AND NITRITE
2. RELEVANCE TO PUBLIC HEALTH
In a similarly-designed study of B6C3F1 mice provided sodium nitrite in the drinking water (average
doses ranging from 45 to 220 mg sodium nitrite/kg/day or 30–146.7 mg nitrite/kg/day), female mice
exhibited a significantly positive trend for increased incidence of forestomach squamous cell papilloma or
carcinoma (combined) and the incidence in the high-dose female mice exceeded the historical range for
NTP controls; however, based on concurrent controls, incidences of squamous cell adenoma (1/50, 0/50,
1/50, and 3/50 for controls, 750, 1,500, and 3,000 ppm groups, respectively), squamous cell carcinoma
(0/50, 0/50, 0/50, and 2/50 for controls, 750, 1,500, and 3,000 ppm groups, respectively), and squamous
cell adenoma or carcinoma (1/50, 0/50, 1/50, and 5/50 for controls, 750, 1,500, and 3,000 ppm groups,
respectively) were not statistically significantly increased for any sodium nitrite exposure group. The
positive trend for incidences of forestomach squamous cell papilloma or carcinoma (combined) in the
female B6C3F1 mice was considered to provide "equivocal evidence of carcinogenic activity" of sodium
nitrite; there was "no evidence of carcinogenic activity" in the male B6C3F1 mice under the conditions of
the study. Incidences of alveolar/bronchiolar adenoma or carcinoma (combined) in sodium nitrite-
exposed groups of female mice were slightly greater than that of controls (incidences of 1/50, 6/50, 5/50,
and 6/50 for controls, 750, 1,500, and 3,000 ppm groups, respectively); however, incidences were within
that of historical NTP controls. Because the incidences did not exhibit exposure concentration-response
characteristics and were not accompanied by increased incidences of preneoplastic lesions, the study
authors did not consider them to be sodium nitrite exposure-related effects. Significantly increased
incidence of fibrosarcoma of the subcutis was noted in mid-dose female mice (incidences of 0/50, 5/50,
1/50, and 2/50 for 0, 750, 1,500, and 3,000 ppm groups, respectively) and exceeded the historical range
for controls; however, lack of exposure concentration-response characteristics and the fact that combined
incidence of fibroma or fibrosarcoma (0/50, 5/50, 1/50, and 3/50 for 0, 750, 1,500, and 3,000 ppm groups,
respectively) were within the historical range for controls suggest that these neoplasms were not related to
sodium nitrite exposure.
In two other studies of male and female F344 rats, addition of sodium nitrite to the drinking water at
concentrations as high as 2,000–3,000 ppm for up to 2 years did not result in significant increases in
tumor incidences at any site. Conversely, incidences of mononuclear cell leukemia were significantly
lower in the nitrite-treated groups relative to controls. In a 26-month study of male and female Sprague-
Dawley rats provided drinking water to which up to 2,000 ppm sodium nitrite was added, the study author
reported increased incidence of lymphomas, but not other types of tumors; however, two studies noted
that a working group sponsored by the U.S. FDA reevaluated the histology and did not confirm the results
of another study. A study reported that the working group considered the incidences of lymphomas to be
19 NITRATE AND NITRITE
2. RELEVANCE TO PUBLIC HEALTH
similar to those arising spontaneously in Sprague-Dawley rats. Increased incidences of total tumors and
lymphoreticular tumors were reported in rats fed diet to which sodium nitrite was added at 1,000 ppm
(total tumors: 58/96 versus 28/156 controls; lymphoreticular tumors 26/96 versus 9/156 controls); the
results were reported for F1 and F2 offspring that had been exposed via their mothers during gestation
and lactation and directly from the diet thereafter. In a 96-week study, a significantly increased incidence
of benign liver tumors among male CBA mice administered drinking water to which sodium nitrite was
added at a concentration resulting in author-estimated total dose of 1,600 mg sodium nitrite/mouse
compared to a group of untreated controls; however, there was no apparent sodium nitrite treatment-
related effect at a higher estimated dose (2,000 mg sodium nitrite/mouse).
Significantly increased incidences of forestomach squamous papillomas were reported for male and
female MRC Wistar rats provided drinking water to which sodium nitrite was added at 3,000 ppm on
5 days/week for life (5/22 males and 3/23 females versus 2/47 control males and 0/44 control females).
Dose-related decreases in time of onset and incidence of lymphomas, mononuclear cell leukemia, and
testicular interstitial-cell tumors were reported for male and female F344 rats administered reduced-
protein diet to which sodium nitrite was added for up to 115 weeks, compared to a group of controls
receiving reduced-protein only diet. There was no evidence of increased tumor incidences in male or
female ICR mice provided sodium nitrite in the drinking water for up to 109 weeks at concentrations as
high as 0.5% (5,000 ppm sodium nitrite), or in male or female Swiss mice or their offspring following a
single gavage administration of 10 mg/kg nitrite and subsequent exposure to 0.1% sodium nitrite
(1,000 ppm) in the drinking water during gestation days 15–21; terminal sacrifices occurred 10 months
following the initiation of treatment. There was no evidence of treatment-related effects on incidences of
nervous system tumors among male and female VM mice (susceptible to spontaneous development of
cerebral gliomas) provided drinking water to which sodium nitrite was added at 0.2% (2,000 ppm) from
weaning for a lifetime and others exposed via their mothers during gestation and lactation as well.
The potential carcinogenicity of combined exposure to sodium nitrite and selected nitrosatable substances
(oral exposures via combinations of drinking water, diet, and/or gavage dosing) has been well-studied in
laboratory animals. Many of the studies included sodium nitrite-only treatment groups for which there
was no evidence of sodium nitrite-induced carcinogenicity. However, one study reported significantly
increased incidence of hepatocellular neoplasms in female (but not male) F344 rats administered diet to
which sodium nitrite was added at 2,000 ppm for 2 years; significantly decreased incidence of
mononuclear-cell leukemia was observed as well.
20 NITRATE AND NITRITE
2. RELEVANCE TO PUBLIC HEALTH
Significantly increased incidences of selected tumor types were observed in some studies of laboratory
animals that employed coexposure to various amino compounds and sodium nitrite. These results were
typically attributed to in vivo nitrosation of amines by nitrite to produce carcinogenic N-nitrosoamines;
some of the studies did not include sodium nitrite-only treatment groups. Addition of sodium nitrite or
potassium nitrite to the food of rats in three other studies resulted in increased incidences of selected
tumors; analysis of the food revealed the presence of N-nitroso compounds (likely formed by nitrosation
in the presence of nitrite and selected amine compounds in the food), which were considered the probable
principal cause of the tumors. One study reported 30–70% incidences of malignant lymphomas, lung
adenomas, and hepatomas among maternal mice and their offspring following gavage treatment of the
dams with the fungicide, dodecylguanidine acetate, together with 0.05% sodium nitrite; the frequency of
spontaneous tumors in untreated controls was 6%. Dodecylguanidine acetate alone had no effect on
cancer incidence. One study found no significant increase in tumor incidences among male and female
MCR rats provided drinking water comprised of 0.5% nitrilotriacetic acid or iminodiacetic acid and 0.2 or
0.5% sodium nitrite on 5 days/week for a lifetime. There were no signs of treatment-related effects on
incidences of tumors at any site among groups of pregnant Syrian golden hamsters and their offspring fed
diets to which up to 1,000 ppm sodium nitrite and/or up to 1,000 ppm morpholine were added throughout
production of an F2 generation.
Based on available human data, one study determined that there is inadequate evidence for the
carcinogenicity of nitrate in food or drinking water and limited evidence for the carcinogenicity of nitrite
in food (based on association with increased incidence of stomach cancer). Evaluation of available
animal data resulted in the determination that there is inadequate evidence for the carcinogenicity of
nitrate, limited evidence for the carcinogenicity of nitrite per se, and sufficient evidence for the
carcinogenicity of nitrite in combination with amines or amides. The overall conclusions of a study were
that “ingested nitrate and nitrite under conditions that result in endogenous nitrosation is probably
carcinogenic to humans (Group 2A).” One study noted that: (1) the endogenous nitrogen cycle in
humans includes interconversion of nitrate and nitrite; (2) nitrite-derived nitrosating agents produced in
the acid stomach environment can react with nitrosating compounds such as secondary amines and
amides to generate N-nitroso compounds; (3) nitrosating conditions are enhanced upon ingestion of
additional nitrate, nitrite, or nitrosatable compounds; and (4) some N-nitroso compounds are known
carcinogens.
The U.S. EPA does not include a carcinogenicity evaluation for nitrate or nitrite.
21 NITRATE AND NITRITE
2. RELEVANCE TO PUBLIC HEALTH
2.3 MINIMAL RISK LEVELS (MRLs)
Estimates of exposure levels posing minimal risk to humans (MRLs) have been established for nitrate and
nitrite. An MRL is defined as an estimate of daily human exposure to a substance that is likely to be
without an appreciable risk of adverse effects (noncarcinogenic) over a specified duration of exposure.
MRLs are derived when reliable and sufficient data exist to identify the target organ(s) of effect or the
most sensitive health effect(s) for a specific duration within a given route of exposure. MRLs are based
on noncancerous health effects only and do not consider carcinogenic effects. MRLs can be derived for
acute, intermediate, and chronic duration exposures for inhalation and oral routes. Appropriate
methodology does not exist to develop MRLs for dermal exposure.
Although methods have been established to derive these levels (Barnes and Dourson 1988; EPA 1990),
uncertainties are associated with these techniques. Furthermore, ATSDR acknowledges additional
uncertainties inherent in the application of the procedures to derive less than lifetime MRLs. As an
example, acute inhalation MRLs may not be protective for health effects that are delayed in development
or are acquired following repeated acute insults, such as hypersensitivity reactions, asthma, or chronic
bronchitis. As these kinds of health effects data become available and methods to assess levels of
significant human exposure improve, these MRLs will be revised.
Inhalation MRLs
Inhalation MRLs were not derived for nitrate or nitrite due to lack of adequate human or animal data.
Limited human data are available. Al-Dabbagh et al. (1986) evaluated mortality rates among a cohort of
1,327 male workers involved in the manufacture of nitrate fertilizer for at least 1 year between 1946 and
1981 for a chemical company in northeast England and found no evidence of an association between
exposure to nitrate dusts and death from all respiratory diseases, ischemic heart disease, or other
circulatory diseases compared to mortality rates for the northern region of England. There was no
evidence of an association between exposure to nitrate dust and death from ischemic heart disease,
cerebrovascular disease, or all circulatory diseases in a census-based (England and Wales) mortality study
of workers involved in the production of nitrate fertilizers (Fraser et al. 1982, 1989). The study included
a cohort of 866 men from the 1961 census and 651 men from the 1971 census. These cohorts were
followed through 1985. Studies of workers in which outcomes are compared to the general population
(e.g., observed versus expected deaths) may be biased by a healthy worker effect, which may lower
estimated risks.
22 NITRATE AND NITRITE
2. RELEVANCE TO PUBLIC HEALTH
Available animal data are limited to a study in which dogs and sheep were exposed to aerosols of sodium
nitrate for short periods (Sackner et al. 1979). No signs of exposure-related pulmonary effects (e.g.,
respiratory resistance, static lung performance, functional residual capacity) were seen in anesthetized
dogs exposed at 10 mg sodium nitrate/m
3
(2.88 ppm) for 7.5 minutes or anesthetized dogs and conscious
sheep exposed for 4 hours at 5 mg sodium nitrate/m
3
(1.44 ppm). There was no evidence of exposure-
related cardiac effects (pulmonary and systemic arterial pressure, cardiac output, heart rate, arterial blood
gases) in anesthetized dogs or conscious sheep exposed at 5 mg sodium nitrate/m
3
(1.44 ppm) for 4 hours.
Oral MRLs
Nitrate
• An MRL of 4 mg/kg/day has been derived for acute-duration oral exposure (14 days or less) to
nitrate.
• An MRL of 4 mg/kg/day has been derived for intermediate-duration oral exposure (15–364 days)
to nitrate.
• An MRL of 4 mg/kg/day has been derived for chronic-duration oral exposure (365 days or more)
to nitrate.
Results from studies in laboratory animals are not an appropriate basis for oral MRL derivation due to
significant interspecies differences in kinetics of the nitrate-nitrite-nitric oxide pathway.
Most human exposure to nitrate and nitrite is through the diet. Vegetables are the major source of
exposure to nitrate; both nitrate and nitrite may be found in some meat, fish, and dairy products as well.
Estimates of daily dietary intake in the United States range from 103 mg nitrate/day from the normal diet
to as high as 367 mg nitrate/day for a vegetarian diet and from 1.2 mg nitrite/day for the normal and
vegetarian diets to 2.6 mg nitrite/day for a diet high in cured meat (Gangolli et al. 1994). Nitrate-
contaminated drinking water is another source of exposure to nitrate and nitrite; estimated oral intake
from drinking water sources may be as high as 319 mg nitrate/day and 1.2 mg nitrite/day (Gangolli et al.
1994).
Methemoglobinemia is the hallmark effect of overexposure to nitrate or nitrite. Although available
human data are limited by lack of information regarding bacterial contamination in drinking water and its
possible influence on methemoglobin levels, the weight-of-evidence indicates that bottle-fed infants (0–
23 NITRATE AND NITRITE
2. RELEVANCE TO PUBLIC HEALTH
<3 months of age) ingesting formula prepared using drinking water sources containing >44 mg nitrate/L
are at risk of methemoglobinemia (e.g., Bosch et al. 1950; Walton 1951). Proposed explanations for
increased susceptibility of infants to methemoglobinemia following ingestion of nitrate include:
(1) increased reduction of nitrate to nitrite in the newborn, (2) increased tendency for nitrite-induced
methemoglobin formation by fetal hemoglobin compared to adult hemoglobin, (3) lower levels of
NADH-dependent methemoglobin reductase (the major enzyme responsible for reduction of
methemoglobin to normal hemoglobin; also termed NADH-diaphorase, a soluble form of cytochrome-b5
reductase) in the newborn compared to older infants and adults, and (4) incompletely developed hepatic
microsomal enzyme system in the infant and consequent lower rate of hepatic reduction of circulating
nitrite compared to that of older children and adults. A portion of ingested nitrate is reduced to nitrite by
commensal bacteria in the mouth; however, the acid environment of the normal stomach does not support
the growth of such bacteria and most of the nitrate that reaches the stomach passes to the small intestine
from which it is nearly completely absorbed into the blood. Although Kanady et al. (2012) reported little
or no bacterial conversion of nitrate to nitrite in the saliva of a group of 10 infants during the first
2 postnatal months (considered mainly due to lower numbers of major nitrate-reducing oral bacteria than
adults), a higher pH in the stomach of the newborn may favor growth of nitrate-reducing bacteria,
resulting in increased reduction of nitrate to nitrite and increased plasma methemoglobin. Most
hemoglobin in the newborn is in the form of fetal hemoglobin, which appears to be more readily oxidized
to methemoglobin than adult hemoglobin; fetal hemoglobin is replaced by adult hemoglobin during early
postnatal life. Levels of NADH-dependent methemoglobin reductase (the major enzyme responsible for
reduction of methemoglobin to normal hemoglobin) in the newborn increase approximately 2-fold during
the first 4 months of postnatal life to reach adult levels. During the period of relatively lower
methemoglobin reductase levels, methemoglobin would not be expected to be as readily reduced,
resulting in increased susceptibility to methemoglobinemia. In apparent contrast, Ibrahim et al. (2012)
reported that blood nitrite levels in newborns approximately 1–2 days of age were 35–55% lower than
that of adults. However, one study that evaluated reduction rates of methemoglobin in human adult blood
and cord blood from term newborns estimated methemoglobin half-lives of 162 and 210 minutes,
respectively, indicating that methemoglobin reduction occurs more slowly in newborns than adults
(Power et al. 2007). Although specific mechanisms have not been elucidated, the increased susceptibility
to nitrite-induced methemoglobinemia in infants is well-documented.
Available human data provide some evidence of nitrate-induced developmental effects, limited human
data provide only suggestive evidence that elevated levels of nitrate in drinking water and/or nitrate-rich
diets may be associated with signs of thyroid dysfunction (Aschebrook-Kilfoy et al. 2012; Gatseva and
24 NITRATE AND NITRITE
2. RELEVANCE TO PUBLIC HEALTH
Argirova 2008; Rádiková et al. 2008; Tajtáková et al. 2006; Ward et al. 2010). Significant associations
between nitrate levels in drinking water and risk of childhood type 1 diabetes were reported by some
investigators (Kostraba et al. 1992; Parslow et al. 1997; Virtanen et al. 1994); others found no evidence
for such an association (Casu et al. 2000; Dahlquist et al. 1990; Moltchanova et al. 2004; van Maanen et
al. 2000; Zhao et al. 2001).
Although available data suggest that reports of methemoglobinemia among infants exposed to nitrate
from the drinking water may involve factors other than (or in addition to) nitrate exposure, the study of
Walton (1951) is selected as the principal study and methemoglobinemia is selected as the critical effect
for deriving acute-, intermediate-, and chronic-duration oral MRLs for nitrate to be protective of
particularly sensitive subpopulations (e.g., infants from birth to <3 months of age), including those with
gastrointestinal infections. Following ingestion of relatively large amounts of nitrate by healthy normal
individuals, blood methemoglobin levels increase rapidly, followed by a return to normal within several
hours following intake. Repeated ingestion for intermediate- or chronic-duration time periods would be
expected to result in changes in methemoglobin levels similar to those elicited from a single exposure.
Therefore, the acute-, intermediate- and chronic-duration oral MRL values are equivalent.
There is some evidence that methemoglobinemia in infants drinking formula prepared using drinking
water with relatively high levels of nitrate may be related to bacterial contamination of such water sources
and consequent gastrointestinal disorders, as well as overproduction of nitric oxide due to gastrointestinal
infection and inflammation (Avery 1999; Gupta et al. 1998; L’hirondel and L’hirondel 2002; Yano et al.
1982). On behalf of the World Health Organization (WHO), Fewtrell (2004) performed a literature-based
investigation of methemoglobinemia and drinking water concentrations >50 mg nitrate/L and concluded
that nitrate may be one of a number of cofactors in causing methemoglobinemia. Fewtrell (2004) noted a
paucity of information since the early 1990s linking methemoglobinemia to nitrate in drinking water,
although numerous reports describe water supplies worldwide that contain nitrate at levels >50 mg/L.
The acute-, intermediate-, and chronic-duration oral MRLs were calculated using estimated mean values
for drinking water ingestion rates (Kahn and Stralka 2009) and body weight (EPA 2008) and the
assumption that a drinking water level of 44 mg nitrate/L is a concentration not expected to cause
methemoglobinemia. A NOAEL of 4.33 mg nitrate/kg/day for infants <3 months of age was calculated
based on a drinking water NOAEL of 44 mg nitrate/L and estimations of water intake (0.525 L/day) and
body weight (5.33 kg) (i.e., [44 mg nitrate/L x 0.525 L/day] / 5.33 kg = 4.33 mg nitrate/kg/day). The
dose of 4.33 mg nitrate/kg/day for infants from birth to <3 months of age is selected as the point of
25 NITRATE AND NITRITE
2. RELEVANCE TO PUBLIC HEALTH
departure for deriving acute-, intermediate-, and chronic-duration oral MRLs for nitrate because infants
<3 months of age are particularly sensitive to nitrate-induced adverse effects. Application of a total
uncertainty factor of 1 is justified because the point of departure is a NOAEL for nitrate-induced effects
on methemoglobin in a sensitive human subpopulation (i.e., <3 month-old infants, which in many cases
may have been at increased risk of methemoglobinemia due to microbial contamination and associated
gastrointestinal infection, or which may have had gastroenteritis-associated methemoglobinemia unrelated
to nitrate intake). The resulting acute-, intermediate-, and chronic-duration oral MRLs for nitrate are
4 mg/kg/day and are considered to be highly conservative because they were derived using results from a
particularly sensitive population exhibiting nitrate-induced methemoglobinemia (infants <3 months of
age), and because increased risk of methemoglobinemia in the most sensitive population may have been
due in part to exposure to contaminants other than nitrate in the drinking water (refer to Appendix A for
additional details regarding derivation of oral MRLs for nitrate).
A physiologically based pharmacokinetic (PBPK) model approach to derivation of oral MRLs for nitrate
was initially considered, in which case a methemoglobin level of 10% of total hemoglobin would have
been considered a threshold for nitrate-induced methemoglobinemia in infants. However, although the
model of Zeilmaker et al. (1996, 2010b) simulates the kinetics of methemoglobin formation resulting
from gastrointestinal absorption of nitrate in adult humans, the model is not considered adequate for the
purpose of simulating the kinetics in infants.
Nitrite
• An MRL of 0.1 mg/kg/day has been derived for acute-duration oral exposure (14 days or less) to
nitrite.
• An MRL of 0.1 mg/kg/day has been derived for intermediate-duration oral exposure (15–
364 days) to nitrite.
• An MRL of 0.1 mg/kg/day has been derived for chronic-duration oral exposure (365 days or
more) to nitrite.
Ingestion of nitrite (from potassium nitrite or sodium nitrite sources) has been associated with severe
methemoglobinemia in adults and children (Aquanno et al. 1981; CDC 1997, 2002; Gautami et al. 1995;
Gowans 1990; Greenberg et al. 1945; Kaplan et al. 1990; Ringling et al. 2003; Sevier and Berbatis 1976;
Ten Brink et al. 1982; Walley and Flanagan 1987). In many of these cases, clinical signs included
dizziness, loss of consciousness, and/or convulsions (CDC 1997, 2002; Gautami et al. 1995; Greenberg et
26 NITRATE AND NITRITE
2. RELEVANCE TO PUBLIC HEALTH
al. 1945; Sevier and Berbatis 1976; Ten Brink et al. 1982). All cases were the result of consumption of
food or drink that contained unusually high levels of nitrite via contamination, inadvertent use of sodium
nitrite instead of table salt, or ingestion of a single sodium nitrite tablet (667 mg nitrite). Headache was
induced in a male subject following consumption of a 10 mg sodium nitrite solution (Henderson and
Raskin 1972). Headaches were induced in 8 out of 13 such tests. No information was located regarding
methemoglobin concentrations in infants following oral exposure to nitrite. The ingestion of nitrate
results in the formation of nitrite, which is the moiety responsible for methemoglobinemia. The study of
Walton (1951) is selected as the principal study and methemoglobinemia as the critical effect for deriving
acute-, intermediate-, and chronic-duration oral MRLs for nitrite to be protective of particularly sensitive
subpopulations (e.g., infants from birth to <3 months of age), including those with gastrointestinal
infections. On average, approximately 25% of an ingested dose of nitrate enters the saliva of an adult
where a portion (ca. 20% g/g) is reduced by commensal bacteria to nitrite (i.e., approximately 5% g/g of
ingested nitrate is reduced to nitrite in the saliva of an adult) (Spiegelhalder et al. 1976); most salivary
nitrite is absorbed into the blood in the small intestine. Therefore, the ingestion of nitrate at the oral MRL
of 4 mg/kg/day would be expected to result in the production of 0.2 mg nitrite/kg/day by an adult (i.e.,
0.2 mg nitrite/kg/day is 5% (g/g) of an oral dose of nitrate at the oral MRL of 4 mg/kg/day). Although
quantitative data are lacking regarding the effective blood nitrite level in a young infant from an ingested
dose of nitrate, young infants exhibit increased susceptibility to methemoglobinemia following nitrate
ingestion. Mechanisms responsible for increased susceptibility in infants may include greater reduction
of nitrate to nitrite (which may be higher in the stomach of an infant due to a higher pH), lower levels of
NADH-dependent methemoglobin reductase, slower rate of hepatic reduction of circulating nitrite, and/or
increased tendency for nitrite-induced methemoglobin formation by fetal hemoglobin compared to adult
hemoglobin. To account for increased susceptibility to methemoglobinemia following ingestion of nitrate
by infants, a modifying factor of 2 is applied to the point of departure (0.2 mg nitrite/kg/day ÷ 2 =
0.1 mg/kg/day). The modifying factor assumes that the effective methemoglobin level from a given
intake of nitrate by an infant is twice that of an adult (e.g., approximately 5% of an oral dose of nitrate is
converted to nitrite in the adult; the modifying factor of 2 accounts for up to 10% conversion in the
infant). The resulting acute-, intermediate-, and chronic-duration oral MRLs of 0.1 mg nitrite/kg/day are
considered protective of nitrite-induced methemoglobinemia for particularly sensitive subpopulations
(e.g., infants <3 months of age). The oral MRLs for nitrite are considered to be highly conservative
because they were derived using results from a particularly sensitive population exhibiting nitrate-induced
methemoglobinemia (infants <3 months of age), and because increased risk of methemoglobinemia in the
infants may have been due in part to exposure to contaminants other than nitrate in the drinking water
(refer to Appendix A for additional details regarding derivation of oral MRLs for nitrite).
27 NITRATE AND NITRITE
2. RELEVANCE TO PUBLIC HEALTH
Drinking water and dietary sources may contain both nitrate and nitrite; furthermore, as discussed in
Section 3.4, some nitrate is converted to nitrite in the body and nitrite can be converted to nitrate as well.
Overexposure to either nitrate or nitrite can result in elevated methemoglobin levels. At a worldwide
level, WHO (2011a, 2011b) provides guidance for combined exposure to nitrate and nitrite in drinking
water, which states that the sum of the ratios of the concentration of each to its guideline value should not
exceed 1.
28 NITRATE AND NITRITE
2. RELEVANCE TO PUBLIC HEALTH
This page is intentionally blank.
29 NITRATE AND NITRITE
3. HEALTH EFFECTS
3.1 INTRODUCTION
The primary purpose of this chapter is to provide public health officials, physicians, toxicologists, and
other interested individuals and groups with an overall perspective on the toxicology of nitrate and nitrite.
It contains descriptions and evaluations of toxicological studies and epidemiological investigations and
provides conclusions, where possible, on the relevance of toxicity and toxicokinetic data to public health.
A glossary and list of acronyms, abbreviations, and symbols can be found at the end of this profile.
3.2 DISCUSSION OF HEALTH EFFECTS BY ROUTE OF EXPOSURE
To help public health professionals and others address the needs of persons living or working near
hazardous waste sites, the information in this section is organized first by route of exposure (inhalation,
oral, and dermal) and then by health effect (e.g., death, systemic, immunological, neurological,
reproductive, developmental, and carcinogenic effects). These data are discussed in terms of three
exposure periods: acute (14 days or less), intermediate (15–364 days), and chronic (365 days or more).
Levels of significant exposure for each route and duration are presented in tables and illustrated in
figures. The points in the figures showing no-observed-adverse-effect levels (NOAELs) or lowest-
observed-adverse-effect levels (LOAELs) reflect the actual doses (levels of exposure) used in the studies.
LOAELs have been classified into "less serious" or "serious" effects. "Serious" effects are those that
evoke failure in a biological system and can lead to morbidity or mortality (e.g., acute respiratory distress
or death). "Less serious" effects are those that are not expected to cause significant dysfunction or death,
or those whose significance to the organism is not entirely clear. ATSDR acknowledges that a
considerable amount of judgment may be required in establishing whether an end point should be
classified as a NOAEL, "less serious" LOAEL, or "serious" LOAEL, and that in some cases, there will be
insufficient data to decide whether the effect is indicative of significant dysfunction. However, the
Agency has established guidelines and policies that are used to classify these end points. ATSDR
believes that there is sufficient merit in this approach to warrant an attempt at distinguishing between
"less serious" and "serious" effects. The distinction between "less serious" effects and "serious" effects is
considered to be important because it helps the users of the profiles to identify levels of exposure at which
major health effects start to appear. LOAELs or NOAELs should also help in determining whether or not
30 NITRATE AND NITRITE
3. HEALTH EFFECTS
the effects vary with dose and/or duration, and place into perspective the possible significance of these
effects to human health.
The significance of the exposure levels shown in the Levels of Significant Exposure (LSE) tables and
figures may differ depending on the user's perspective. Public health officials and others concerned with
appropriate actions to take at hazardous waste sites may want information on levels of exposure
associated with more subtle effects in humans or animals (LOAELs) or exposure levels below which no
adverse effects (NOAELs) have been observed. Estimates of levels posing minimal risk to humans
(Minimal Risk Levels or MRLs) may be of interest to health professionals and citizens alike.
A User's Guide has been provided at the end of this profile (see Appendix B). This guide should aid in
the interpretation of the tables and figures for Levels of Significant Exposure and the MRLs.
Nitrate (NO
3
-
) and nitrite (NO
2
-
) are naturally-occurring oxidation products of nitrogen. Nitrate may be
expressed in terms of ionic concentration (i.e., mg nitrate/L), or elemental concentration (i.e., mg nitrate-
nitrogen/L or mg nitrogen as nitrate/L). A concentration of nitrate expressed in elemental concentration
can be converted to its ionic concentration according to the following relationship: 1 mg nitrate-nitrogen
is equivalent to 4.4 mg nitrate. In aqueous environments, nitrate and nitrite salts such as sodium nitrate,
potassium nitrate, sodium nitrite, and potassium nitrite rapidly ionize. Sodium nitrate is approximately
27% sodium and 73% nitrate. To determine a nitrate dose from a sodium nitrate source, the quantity of
sodium nitrate is multiplied by the nitrate proportion (0.73). Thus a nitrate dose from a 5 mg sodium
nitrate source is 5x0.73=3.65 mg nitrate. The conversion factor for nitrate from a potassium nitrate
source is 0.61. Conversion factors for nitrite from sodium nitrite and potassium nitrite are 0.67 and 0.54,
respectively.
3.2.1 Inhalation Exposure
3.2.1.1 Death
No information was located regarding death in humans following inhalation exposure to nitrate or nitrite.
An inhalation LC
50
is an exposure level expected to result in 50% mortality. RTECS (2014) lists a rat
4-hour LC
50
of 5.5 mg/m
3
(1.95 ppm) for sodium nitrite and a rat 2-hour LC
50
of 85 mg/m
3
(24.42 ppm)
for potassium nitrite. No additional information was located regarding death in animals exposed to nitrate
or nitrite.
31 NITRATE AND NITRITE
3. HEALTH EFFECTS
3.2.1.2 Systemic Effects
No studies were located regarding gastrointestinal, hematological, musculoskeletal, hepatic, renal,
endocrine, dermal, ocular, or body weight effects in humans or animals after inhalation exposure to nitrate
or nitrite.
Respiratory Effects. Limited human data are available. Al-Dabbagh et al. (1986) evaluated the
mortality of a cohort of 1,327 male workers involved in the manufacture of nitrate fertilizer for at least
1 year between 1946 and 1981 for a chemical company in northeast England. There was no evidence of
an association between exposure to nitrate dusts and death from all respiratory diseases compared to
mortality rates for the northern region of England.
Available information in animals is limited to a study in which dogs and sheep were exposed to aerosols
of sodium nitrate for short periods (Sackner et al. 1979). There was no evidence of exposure-related
pulmonary effects (e.g., respiratory resistance, static lung performance, functional residual capacity) in
anesthetized dogs exposed at up to 10 mg sodium nitrate/m
3
(2.88 ppm) for 7.5 minutes or anesthetized
dogs or conscious sheep exposed at 5 mg sodium nitrate/m
3
(1.44 ppm) for 4 hours.
Cardiovascular Effects. Available information in humans is limited to results of mortality studies of
workers involved in the production of nitrate fertilizers. In general, studies of workers in which outcomes
are compared to the general population (e.g., observed versus expected deaths) may be biased by a
healthy worker effect, which may lower estimated risks. There was no evidence of an association
between exposure to nitrate dust and death from ischemic heart disease, cerebrovascular disease, or all
circulatory diseases in a census-based (England and Wales) mortality study of workers involved in the
production of nitrate fertilizers (Fraser et al. 1982, 1989). The study included a cohort of 866 men from
the 1961 census and 651 men from the 1971 census. These cohorts were followed through 1985. Al-
Dabbagh et al. (1986) evaluated the mortality of a cohort of 1,327 male workers involved in the
manufacture of nitrate fertilizer for at least 1 year between 1946 and 1981 for a chemical company in
northeast England. There was no evidence of an association between exposure to nitrate dusts and death
from ischemic heart disease or other circulatory diseases compared to mortality rates for the northern
region of England.
32 NITRATE AND NITRITE
3. HEALTH EFFECTS
Available information in animals is limited to a study in which dogs and sheep were exposed to aerosols
of sodium nitrate for short periods (Sackner et al. 1979). There was no evidence of exposure-related
cardiac effects (pulmonary and systemic arterial pressure, cardiac output, heart rate, arterial blood gases)
in anesthetized dogs or conscious sheep exposed at 5 mg sodium nitrate/m
3
(1.44 ppm) for 4 hours.
No information was located regarding the following effects in humans or animals exposed to nitrate or
nitrite via the inhalation route:
3.2.1.3 Immunological and Lymphoreticular Effects
3.2.1.4 Neurological Effects
3.2.1.5 Reproductive Effects
3.2.1.6 Developmental Effects
3.2.1.7 Cancer
Available information in humans is limited to results of mortality studies of workers involved in the
production of nitrate fertilizers. In general, studies of workers in which outcomes are compared to the
general population (e.g., observed versus expected deaths) may be biased by a healthy worker effect,
which may lower estimated risks. A census-based (England and Wales) mortality study of workers
involved in the production of nitrate fertilizers included 866 men from the 1961 census and 651 men from
the 1971 census; mortality rates among these workers were compared to mortality rates of men from
England and Wales (Fraser et al. 1982). At follow-up until 1978, slight excess of death from intestinal
cancer was noted among men from the 1961 census (6 observed versus 4.5 expected); excess of death
from all cancers, (19 versus 14.4 expected), esophageal cancer (1 versus 0.4 expected), gastric cancer
(2 versus 1.5 expected), intestinal cancer (1 versus 0.9 expected), rectal cancer (2 versus 0.6 expected),
and lung cancer (9 versus 6.4 expected) were observed in the 1971 census cohort. However, follow-up
through 1985 revealed no significant increased risk for cancer at any site (Fraser et al. 1989).
Al-Dabbagh et al. (1986) evaluated mortality rates within a cohort of 1,327 male workers involved in the
manufacture of nitrate fertilizer for at least 1 year between 1946 and 1981 for a chemical company in
northeast England; mortality rates were compared with those of the male population of the region.
Among 537 workers described as having been heavily exposed to nitrate dust (i.e., working in an
environment likely to have contained >10 mg nitrate/m
3
[>2.88 ppm]), slight excesses were noted for
deaths from lung cancer (25 observed versus 21.04 expected) and death from all malignant neoplasms
(59 observed versus 51.36 expected), but not for cancers of the esophagus, stomach, or bladder. After
categorizing the heavily-exposed workers by duration of exposure and time since first exposure, excess
33 NITRATE AND NITRITE
3. HEALTH EFFECTS
death from lung cancer was noted for those exposed for ≥10 years, with a lag time of ≥20 years since first
exposure (13 observed versus 8.11 expected). The study authors indicated that they were unable to adjust
for smoking.
Hagmar et al. (1991) evaluated mortality rates within a cohort of 2,131 male workers at a nitrate fertilizer
production facility in Sweden and compared them to mortality rates for men in the same county. Death
from prostate cancer (26 observed versus 16.1 expected) was in excess (standardized mortality ratio
[SMR] 161, 95% CI: 107, 239); however, risk of prostate cancer within this cohort was not enhanced
following application of a ≥10-year latency period. There was no significant increase in death from
tumors of the lips, oral cavity, pharynx, salivary glands, gastrointestinal tract, stomach, respiratory tract,
lung, urinary bladder, blood, or all sites combined.
Fandrem et al. (1993) evaluated incidences of selected cancers among 2,023 male workers who had been
employed for >1 year at a Norwegian nitrate fertilizer plant between 1945 and 1979. The average
historical concentration of nitrate in the workplace air was estimated to have been 10 mg/m
3
. The cohort
was followed from 1953 through 1988 and incidences of cancer among the workers were compared to
national rates. The study authors reported 30 incidences of lung cancer (27.5 expected: standardized
incidence ratio [SIR] 1.09; 95% CI 0.73, 1.53), 9 incidences of kidney cancer (7.6 expected: SIR 1.18;
95% CI 0.54, 2.25), and 9 incidences of pancreatic cancer (7.3 expected: SIR 1.23; 95% CI 0.56, 2.34).
There were fewer than expected cancers of the oesophagus, stomach, colon/rectum, pleura, bladder,
malignant melanoma, and all cancers combined. No association was found between gastric cancer and
cumulative exposure to nitrate, duration of employment, or time since first exposure.
Rafnsson and Gunnarsdóttir (1990) evaluated mortality rates among 603 male workers at a nitrate
fertilizer plant in Iceland who had been employed for >1 year between 1954 and 1985. Mortality data
were compared to national rates for men. The study authors reported nonstatistically significant excesses
of cancers of the large intestine (2 observed versus 1.25 expected: SMR 160; 95% CI 19, 578), rectum
(1 observed versus 0.61 expected: SMR 164; 95% CI 4, 913), pancreas (3 observed versus 1.31 expected:
SMR 229; 95% CI 47, 669), and respiratory tract (4 observed versus 2.88 expected: SMR 139; 95 CI 38,
356). There was no excess of death from stomach cancer (4 observed versus 4.32 expected: SMR 93;
95% CI 25, 237). This study is limited by low incidences of selected cancers and possible confounding
by the healthy worker effect.
34 NITRATE AND NITRITE
3. HEALTH EFFECTS
3.2.2 Oral Exposure
3.2.2.1 Death
As early as the mid-1900s, methemoglobinemia was reported in infants exposed to relatively large
amounts of nitrate from drinking water sources (e.g., Bosch et al. 1950; Bucklin and Myint 1960; Chapin
1947; Comly 1987; Donahoe 1949; Faucett and Miller 1946; Ferrant 1946; McLetchie and Robertson
1949; Medovy 1948; Robertson and Riddell 1949; Stafford 1947). Deaths occurred in some of these
cases. Ingestion of nitrite (from potassium nitrite or sodium nitrite sources) has been associated with
severe methemoglobinemia in adults and children (Aquanno et al. 1981; CDC 1997, 2002; Gautami et al.
1995; Gowans 1990; Greenberg et al. 1945; Kaplan et al. 1990; Ringling et al. 2003; Sevier and Berbatis
1976; Ten Brink et al. 1982; Walley and Flanagan 1987). Deaths occurred in some of these cases
following consumption of food or drink that contained unusually high levels of nitrite via contamination,
inadvertent use of sodium nitrite instead of table salt, or ingestion of a single sodium nitrite tablet
(667 mg nitrite).
An oral LD
50
is the dose expected to result in 50% mortality. Single oral doses of sodium nitrite at
multiple dose levels resulted in LD
50
values of 150 mg/kg (100 mg nitrite/kg) in rats (Imaizumi et al.
1980) and 265 mg/kg (178.2 mg nitrite/kg) in mice (Sheehy and Way 1974). RTECS (2014) lists oral
LD
50
values for sodium nitrate of 1,267, 3,500, and 2,680 mg/kg for the rat, mouse, and rabbit,
respectively; LD
50
values for sodium nitrite of 157.9, 175, and 186 mg/kg for the rat, mouse, and rabbit,
respectively; LD
50
values for potassium nitrate of 3,540 and 3,750 for the rat and 1,901 mg/kg for the
rabbit; and an LD
50
for potassium nitrite of 200 mg/kg. Among rats provided sodium nitrate in the
drinking water for 6 weeks, concentrations of sodium nitrate resulting in an estimated dose of 14,600 mg
nitrate/kg/day was lethal to 7/10 male rats; an estimated dose of 16,483.9 mg nitrate/kg/day was lethal to
10/10 female rats. Among male rats similarly treated with sodium nitrite, an estimated dose of
1,080.6 mg nitrite/kg/day was lethal to 4/10 rats. Inai et al. (1979) reported 100% mortality in male and
female mice (10/sex) provided sodium nitrite in the drinking water at concentrations resulting in
estimated doses of 330.8 and 354.1 mg nitrite/kg/day, respectively; the deaths occurred within the first
3 weeks of a 6-week study.
3.2.2.2 Systemic Effects
No studies were located regarding musculoskeletal or ocular effects in humans or animals after oral
exposure to nitrate or nitrite.
35 NITRATE AND NITRITE
3. HEALTH EFFECTS
The highest NOAEL values and all LOAEL values from each reliable study for systemic effects in each
species and duration category are recorded in Table 3-1 and plotted in Figure 3-1.
Respiratory Effects. No studies were located regarding respiratory effects in humans or animals
following oral exposure to nitrate or nitrite.
Cardiovascular Effects. Malberg et al. (1978) investigated possible associations between
hypertension and levels of nitrate in the drinking water in a hospital-based study in Colorado that included
226 cases of hypertension among patients living in areas where drinking water contained nitrate at
concentrations ranging from 19 to 125 ppm (mean 52 ppm) and 261 cases from patients living in areas
without nitrate in the drinking water. The mean annual incidence rate for the nitrate-exposed patients was
5.9/1,000 population versus 7.9/1,000 for the control patients. However, the nitrate-exposed patients
exhibited an earlier mean age at hospitalization for hypertension (58.5 years versus 65.2 years for
controls); the toxicological significance of this finding is uncertain because the incidence rate for
hypertension was higher among control patients than among patients exposed to nitrate in the drinking
water.
Cardiovascular health is an end point of concern for nitrate because some nitrate is converted to nitrite in
the body. Nitrite is a smooth muscle relaxant that can cause hypotension and plasma nitrite is involved in
the oxidation of hemoglobin to methemoglobin, which is associated with hypotension, rapid pulse, and
rapid breathing at high enough concentrations. Ingestion of nitrite (from potassium nitrite or sodium
nitrite sources) has been associated with severe methemoglobinemia in adults and children; in some of
these cases, symptoms included hypotension and/or tachycardia (Gowans 1990; Sevier and Berbatis 1976;
Ten Brink et al. 1982). These cases were the result of consumption of food or drink that contained
unusually high levels of nitrite via contamination, inadvertent use of sodium nitrite instead of table salt, or
ingestion of a single sodium nitrite tablet (667 mg nitrite).
In a study designed to evaluate the oral bioavailability of sodium nitrite in healthy volunteers (seven
females and two males; mean age 22.9 years), ingestion of 0.06 sodium nitrite per mmol hemoglobin
(~2.2–2.7 mg sodium nitrite/kg, or 1.5–1.8 mg nitrite/kg) resulted in an average heart rate increase from
55 to 63 bpm and average mean arterial blood pressure decrease from 78 to 70 mmHg (Kortboyer et al.
1997b). At a higher intake (0.12 mmol sodium nitrite per mmol hemoglobin; ~4.4–5.4 mg sodium
nitrite/kg, or 2.9–3.6 mg nitrite/kg), the average heart rate increased from 57 to 67 bpm and the average

83
100.5
92
178.2
104
4.33
106
0.2
84
6.7 16.75
88
104.2
NITRATE AND NITRITE
3. HEALTH EFFECTS
36
Table 3-1 Levels of Significant Exposure to Nitrate And Nitrite - Oral
a
Key to
Figure
Species
(Strain)
Exposure/
Duration/
Frequency
(Route)
ACUTE EXPOSURE
Death
1
Rat
(Sprague-
Dawley)
Once
(GW)
System
NOAEL
(mg/kg/day)
Less Serious
(mg/kg/day)
LOAEL
Serious
(mg/kg/day)
100.5 (LD50)
Reference
Chemical Form
Imaizumi et al. 1980
Sodium Nitrite
Comments
2
Mouse
(Swiss-
Webster)
Once
(GW)
178.2 M (LD50)
Sheehy and Way 1974
Sodium Nitrite
Systemic
3
Human NS
(F)
Hemato
b
4.33
Walton 1951
Nitrate
Dose based on a
drinking water level (44
mg nitrate/L) above
which nitrate could
cause
methemoglobinemia in
infants <3 months old.
4
Human NS
(F)
Hemato
c
0.2
Walton 1951
Nitrite
The NOAEL represents
the estimated nitrite
dose to an infant <3
months of age
consuming nitrate from
drinking water at up to
44 mg/L.
5
Rat
(Sprague-
Dawley)
Once
(GW)
Hemato 6.7
16.75 (8.6% methemoglobin)
Imaizumi et al. 1980
Sodium Nitrite
6
Rat
(Wistar)
1 or 3 d
1 x/d
(GW)
Hepatic 104.2 M
Lijinsky and Greenblatt 1972
Sodium Nitrite

78
150
150
86
53.6
80
13
93
113.2
12
1080.6
13
14600
16483.9
50
330.8
354.1
NITRATE AND NITRITE
3. HEALTH EFFECTS
37
Table 3-1 Levels of Significant Exposure to Nitrate And Nitrite - Oral (continued)
a
Key to
Figure
Species
(Strain)
System
Exposure/
Duration/
Frequency
(Route)
NOAEL
(mg/kg/day)
Less Serious
(mg/kg/day)
LOAEL
Serious
(mg/kg/day)
Reference
Chemical Form
Comments
7
Hepatic
Mouse
HaM/ICR
Once
(GW)
Bd Wt
Developmental
8
Rat
(NS)
Gd 15
Once
(GW)
150 M
150 M
53.6
A
sahina et al. 1971
Sodium Nitrite
Khera 1982
Sodium Nitrite
9
Mouse
(CD-1)
Gd 1-14, 16, or
18
1 x/d
(GW)
13
Globus and Samuel 1978
Sodium Nitrite
10
Mouse
(ICR)
Gd 7-18
(W)
INTERMEDIATE EXPOSURE
Death
11
Rat
(Fischer- 344)
6 wk
(W)
113.2
1080.6 F (4/10 died)
Shimada 1989
Sodium Nitrite
Maekawa et al. 1982
Sodium Nitrite
12
Rat
(Fischer- 344)
6 wk
(F)
14600 M (7/10 died)
16483.9 F (10/10 died)
Maekawa et al. 1982
Sodium Nitrate
13
Mouse
(ICR)
6 wk
(W)
330.8 M (death during first 3
treatment weeks)
Inai et al. 1979
Sodium Nitrite
354.1 F (death during first 3
treatment weeks)

101
4972
107
4.33
109
0.2
111
80
32
28.14 187.6
56
40.5
NITRATE AND NITRITE
3. HEALTH EFFECTS
38
Table 3-1 Levels of Significant Exposure to Nitrate And Nitrite - Oral (continued)
a
Key to
Figure
Species
(Strain)
Exposure/
Duration/
Frequency
(Route)
System
LOAEL
NOAEL
(mg/kg/day)
Less Serious
(mg/kg/day)
Serious
(mg/kg/day)
Reference
Chemical Form
Comments
14
Gn Pig
(NS)
143-204 d
(W)
Systemic
15
Human NS
(F)
Hemato
Sleight and Atallah 1968
potassium nitrate
4972 F (1/3 died)
Walton 1951
Nitrate
b
4.33
Dose based on a
drinking water level (44
mg nitrate/L) above
which nitrate could
cause
methemoglobinemia in
infants <3 months old.
16
Human NS
(F)
Hemato
Walton 1951
Nitrite
c
0.2
The NOAEL represents
the estimated nitrite
dose to an infant <3
months of age
consuming nitrate from
drinking water at up to
44 mg/L.
17
Rat
(Sprague-
Dawley)
12wk
1x/d
(G)
Metab
A
l-Gayyar et al. 2015
Sodium Nitrite
80 M (hyperglycemia, insulin
resistance)
18
Rat
(albino)
2 mo
(W)
Hemato
Behroozi et al. 1972
Sodium Nitrite
28.14 M
187.6 M (12.16% methemoglobin)
19
Rat
(Sprague-
Dawley)
16 wk
(W)
Hemato
Chow et al. 1980
Sodium Nitrate
40.5 M

58
18.6
98
6.4
15.8
6.4
6.4
113
34.8 34.8
34.8
20
60.16
158.77
85
167.5
NITRATE AND NITRITE
3. HEALTH EFFECTS
39
Table 3-1 Levels of Significant Exposure to Nitrate And Nitrite - Oral (continued)
a
Key to Species
Figure (Strain)
20
Rat
(Sprague-
Dawley)
21
Rat
(Wistar)
22
Rat
(Wistar)
23
Rat
(Wistar)
Exposure/
Duration/
Frequency
(Route)
System
NOAEL
(mg/kg/day)
Less Serious
(mg/kg/day)
LOAEL
Serious
(mg/kg/day)
Reference
Chemical Form
Comments
16 wk
(W)
Hemato 18.6 M
Chow et al. 1980
Sodium Nitrite
4 mo
(W)
Renal 6.4 M
15.8 M (increased urinary urea
and creatinine levels)
El-Wakf et al. 2008
Sodium Nitrate
Endocr 6.4 M (decreased serum T3
and T4; increased serum
TSH)
Bd Wt 6.4 M (11-12% depressed
mean body weight and
body weight gain)
4mo
Continuous
(W)
Bd Wt 34.8 M (9 and 30% depressed
mean body weight and
body weight gain,
respectively, among adult
rats)
34.8 M (24 and 39% depressed
mean body weight and
body weight gain,
respectively, among
young rats)
El-Wakf et al. 2015
Sodium Nitrate
Metab 34.8 M (hyperglycemia)
30 wk
(W)
Endocr 60.16 F
158.77 F (decreased serum T3,
T4, and TSH levels;
increased thyroid weight;
follicular hyperplasia)
Eskiocak et al. 2005
Sodium Nitrate
Rat 6 mo
Hemato 167.5 (peak methemoglobin of
Imaizumi et al. 1980
(Sprague-
(W)
33-88%)
Sodium Nitrite
Dawley)
24

74
208.4
71
183.1
183.1
10
3650
4121
7300
8241.9
7300
4121
14600
8241.9
NITRATE AND NITRITE
3. HEALTH EFFECTS
40
Table 3-1 Levels of Significant Exposure to Nitrate And Nitrite - Oral (continued)
a
Key to
Figure
Species
(Strain)
Exposure/
Duration/
Frequency
(Route)
System
NOAEL
(mg/kg/day)
Less Serious
(mg/kg/day)
LOAEL
Serious
(mg/kg/day)
Reference
Chemical Form
Comments
25
Rat
(Fischer- 344)
51 wk
(W)
Gastro 208.4 M
Kawabe et al. 1994
Sodium Nitrite
26
Rat
(Sprague-
Dawley)
10 mo
(F)
Hepatic 183.1
Lin and Ho 1992
Sodium Nitrite
Bd Wt 183.1
27
Rat
(Fischer- 344)
6 wk
(F)
Hemato 3650 M
4121 F
7300 M (discolored blood and
spleen indicative of
methemoglobinemia)
Maekawa et al. 1982
Sodium Nitrate
8241.9 F (discolored blood and
spleen indicative of
methemoglobinemia)
Bd Wt 7300 M
4121 F
14600 M (at least 10% depressed
body weight gain)
8241.9 F (at least 10% depressed
body weight gain)

9
186.1
270.2
372.2
540.3
372.2
540.3
744.4
1080.6
76
208.4
22
2416.6
2416.6
NITRATE AND NITRITE
3. HEALTH EFFECTS
41
Table 3-1 Levels of Significant Exposure to Nitrate And Nitrite - Oral (continued)
a
Key to
Figure
Species
(Strain)
Exposure/
Duration/
Frequency
(Route)
System
NOAEL
(mg/kg/day)
Less Serious
(mg/kg/day)
LOAEL
Serious
(mg/kg/day)
Reference
Chemical Form
Comments
28
Rat
(Fischer- 344)
6 wk
(W)
Hemato 186.1 M
270.2 F
372.2 M (discolored blood and
spleen indicative of
methemoglobinemia)
Maekawa et al. 1982
Sodium Nitrite
540.3 F (discoloration of blood
and spleen indicative of
methemoglobinemia)
Bd Wt 372.2 M
540.3 F
744.4 M (at least 10% depressed
body weight gain)
1080.6 F (at least 10% depressed
body weight gain)
29
Rat
(Fischer- 344)
35 wk
(W)
Gastro 208.4 M
Miyauchi et al. 2002
Sodium Nitrite
30
Rat
(Wistar)
4 wk
(F)
Endocr 2416.6 (increased thyroid
weight, decreased
thyroid peroxidase
activity, decreased serum
T3 and T4, increased
serum TSH)
Mukhopadhyay et al. 2005
potassium nitrate
Bd Wt 2416.6

1
77.1
53.6
134
87.1
17
41.9
61.8
107.6
130.5
4.8
16.8
13.3
61.8
NITRATE AND NITRITE
3. HEALTH EFFECTS
42
Table 3-1 Levels of Significant Exposure to Nitrate And Nitrite - Oral (continued)
a
Key to
Figure
Species
(Strain)
Exposure/
Duration/
Frequency
(Route)
System
NOAEL
(mg/kg/day)
Less Serious
(mg/kg/day)
LOAEL
Serious
(mg/kg/day)
Reference
Chemical Form
Comments
31
Rat
(Fischer- 344)
14 wk
(W)
Hemato 77.1 M
53.6 F
134 M (up to 10%
methemoglobin)
NTP 2001
Sodium Nitrite
87.1 F (up to 13%
methemoglobin)
32
Rat
(Wistar)
13 wk
(W)
Hemato 41.9 M
61.8 F
107.6 M (5.7% methemoglobin)
130.5 F (7.6% methemoglobin)
Til et al. 1988
potassium nitrite
Endocr 4.8 M
16.8 F
13.3 M (hypertrophy in zona
glomerulosa of adrenal
gland)
61.8 F (hypertrophy in zona
glomerulosa of adrenal
gland)

18
4.59
5.94
105.1
130.1
19
5.2
7.1
106.3
124.8
5.2
7.1
106.3
124.8
95
28.1
NITRATE AND NITRITE
3. HEALTH EFFECTS
43
Table 3-1 Levels of Significant Exposure to Nitrate And Nitrite - Oral (continued)
a
Key to Species
Figure (Strain)
33
Rat
(Wistar)
34
Rat
(Wistar)
35
Rat
(Sprague-
Dawley)
Exposure/
Duration/
Frequency
(Route)
System
NOAEL
(mg/kg/day)
Less Serious
(mg/kg/day)
LOAEL
Serious
(mg/kg/day)
Reference
Chemical Form
Comments
13 wk
(W)
Endocr 4.59 M
5.94 F
105.1 M (hypertrophy in zona
glomerulosa of adrenal
gland)
Til et al. 1997
potassium nitrite
130.1 F (hypertrophy in zona
glomerulosa of adrenal
gland)
13 wk
(W)
Hemato 5.2 M
7.1 F
106.3 M (increased
methemoglobin,
magnitude not specified)
Til et al. 1997
Sodium Nitrite
124.8 F (increased
methemoglobin,
magnitude not specified)
Endocr 5.2 M
7.1 F
106.3 M (hypertrophy in zona
glomerulosa of adrenal
gland)
124.8 F (hypertrophy in zona
glomerulosa of adrenal
gland)
F0 males:
15-28 d
F0 females:
58-71 d
F1 pups: 69 d
(F)
Bd Wt 28.1
Vorhees et al. 1984
Sodium Nitrite

21
9
13.5
9
13.5
66
82.5
35
118.1
36
1583
39
236.3
NITRATE AND NITRITE
3. HEALTH EFFECTS
44
Table 3-1 Levels of Significant Exposure to Nitrate And Nitrite - Oral (continued)
a
Key to Species
Figure (Strain)
36
Rat
(Wistar)
37
Mouse
Swiss
38
Mouse
Strain A
39
Mouse
Strain A
40
Mouse
Strain A
Exposure/
Duration/
Frequency
(Route)
System
NOAEL
(mg/kg/day)
Less Serious
(mg/kg/day)
LOAEL
Serious
(mg/kg/day)
Reference
Chemical Form
Comments
5 mo
(W)
Endocr 9 M
13.5 M (increases in serum T3
and thyroid weight;
nonneoplastic lesions in
thyroid gland)
Zaki et al. 2004
potassium nitrate
Bd Wt 9 M
13.5 M (16% lower mean body
weight than controls)
26 wk
(W)
Bd Wt 82.5
Greenblatt and Lijinsky 1974
Sodium Nitrite
25 wk
5 d/wk
(W)
Bd Wt 118.1 M
Greenblatt and Mirvish 1973
Sodium Nitrite
25 wk
5 d/wk
(W)
Bd Wt 1583 M
Greenblatt and Mirvish 1973
Sodium Nitrate
20 wk
5 d/wk
(W)
Bd Wt 236.3 M
Greenblatt and Mirvish 1973
Sodium Nitrite

2
435.5
562.8
663.3
824.1
231.2
160.8
435.5
298.1
435.5
824.1
663.3
31
9.38
82
165.4
NITRATE AND NITRITE
3. HEALTH EFFECTS
45
Table 3-1 Levels of Significant Exposure to Nitrate And Nitrite - Oral (continued)
a
Key to Species
Figure (Strain)
41
Mouse
(B6C3F1)
Neurological
42
Rat
(albino)
43
Rat
C57B1
Exposure/
Duration/
Frequency
(Route)
System
NOAEL
(mg/kg/day)
Less Serious
(mg/kg/day)
LOAEL
Serious
(mg/kg/day)
Reference
Chemical Form
Comments
14 wk
(W)
Gastro 435.5 M
562.8 F
663.3 M (focal hyperplasia in
forestomach)
NTP 2001
Sodium Nitrite
824.1 F (focal hyperplasia in
forestomach)
Hemato 231.2 M
160.8 F
435.5 M (extramedullary
hematopoiesis in spleen)
298.1 F (extramedullary
hematopoiesis in spleen)
Bd Wt 435.5 M
824.1 F
663.3 M (10% depressed final
mean body weight and
body weight gain)
2 mo
(W)
9.38 M (altered EEG)
Behroozi et al. 1972
Sodium Nitrite
F0: Mating,
gestation,
lactation
F1: 14 wk
postweaning
(W)
165.4 M (increased aggressive
behavior)
Gruener 1974
Sodium Nitrite

112
80
114
80
99
160
5
77.1
134
3
231.2
435.5
NITRATE AND NITRITE
3. HEALTH EFFECTS
46
Table 3-1 Levels of Significant Exposure to Nitrate And Nitrite - Oral (continued)
Exposure/
LOAEL
Duration/
a
Frequency
Key to Species
NOAEL Less Serious Serious
(Route)
Figure (Strain)
System
(mg/kg/day) (mg/kg/day) (mg/kg/day)
Reproductive
44
Rat
(Sprague-
Dawley)
12wk
1x/d
(G)
80 M
45
Rat
(Sprague-
Dawley)
12wk
1x/d
(G)
80 M
46
47
Rat
(Wistar)
2 generations
(F)
Rat
(Fischer- 344)
14 wk
(W)
160 F
77.1 M
134 M (7% decreased sperm
motility)
48
Mouse
(B6C3F1)
14 wk
(W)
231.2 M
435.5 M
(Increases in testicular
weight and serum FSH,
LH, and prolactin;
decreases in sperm
count and serum
testosterone)
(decreased serum
testosterone; increases
in testicular weight;
increased testicular
levels of
pro-inflammatory
cytokines, oxidative
stress markers, and
enzymes involved in
programmed cell death)
(degeneration in testis,
characterized by
increased size of residual
bodies within the lumen
of the seminiferous
tubules)
Reference
Chemical Form
Comments
A
lyoussef and Al-Gayya
r
2016a
Sodium Nitrite
A
lyoussef and Al-Gayya
r
2016b
Sodium Nitrite
Hugot et al. 1980
Sodium Nitrite
NTP 2001
Sodium Nitrite
NTP 2001
Sodium Nitrite

102
2230.8 4972
103
59.4
148.5
100
160
94
7.2
14.4
108
4.33
NITRATE AND NITRITE
3. HEALTH EFFECTS
47
Table 3-1 Levels of Significant Exposure to Nitrate And Nitrite - Oral (continued)
a
Key to
Figure
Species
(Strain)
Exposure/
Duration/
Frequency
(Route)
System
NOAEL
(mg/kg/day)
Less Serious
(mg/kg/day)
LOAEL
Serious
(mg/kg/day)
Reference
Chemical Form
Comments
49
Gn Pig
(NS)
143-204 d
(W)
2230.8 F
4972 F
Sleight and Atallah 1968
potassium nitrate
50
Gn Pig
(NS)
100-240 d
(W)
59.4 F
148.5 F (decreased number of
litters and live fetuses)
Sleight and Atallah 1968
potassium nitrite
Developmental
51
Rat
(Wistar)
2 generations
(F)
160
Hugot et al. 1980
Sodium Nitrite
52
Rat
(Sprague-
Dawley)
F0 males:
15-28 d
F0 females:
58-71 d
F1 pups: 69 d
(F)
CHRONIC EXPOSURE
Systemic
53
Human NS
(F)
Hemato
7.2
b
4.33
14.4 (increased pup mortality,
depressed preweaning
pup body weight, delayed
swimming development)
Vorhees et al. 1984
Sodium Nitrite
Walton 1951
Nitrate
Dose based on a
drinking water level (44
mg nitrate/L) above
which nitrate could
cause
methemoglobinemia in
infants <3 months old.

110
0.2
45
60.5
178.2
97
14.5
22.6
14
82.4
60.3
101
15
1517
832
1730
NITRATE AND NITRITE
3. HEALTH EFFECTS
48
a
Key to
Figure
54
55
56
57
58
Exposure/
Duration/
Frequency
Species
(Route)
(Strain)
Human NS
(F)
Table 3-1 Levels of Significant Exposure to Nitrate And Nitrite - Oral (continued)
LOAEL
System
Hemato
Bd Wt
Bd Wt
Bd Wt
Bd Wt
NOAEL
(mg/kg/day)
c
0.2
60.5 M
14.5 M
22.6 F
82.4 M
60.3 F
1517 M
832 F
Less Serious
(mg/kg/day)
178.2 M (approximately 15%
depressed mean body
weight)
101 F (more than 10% lower
mean body weight than
controls)
1730 F (up to 13% lower mean
body weight than
controls)
Serious
(mg/kg/day)
Reference
Chemical Form
Walton 1951
Nitrite
Grant and Butler 1989
Sodium Nitrite
Greenblatt et al. 1973
Sodium Nitrite
Maekawa et al. 1982
Sodium Nitrite
Maekawa et al. 1982
Sodium Nitrate
Comments
The NOAEL represents
the estimated nitrite
dose to an infant <3
months of age
consuming nitrate from
drinking water at up to
44 mg/L.
Study authors did not
specify whether
reported nitrite
consumption was nitrite
or sodium nitrite
Rat
(Fischer- 344)
Rat
(Wistar)
Rat
(Fischer- 344)
Rat
(Fischer- 344)
115 wk
(F)
67 wk
5 d/wk
(W)
104 wk
(W)
104 wk
(F)

4
46.9
53.6
87.1
100.5
25
172.8
86.4
69
176.8
204.5
176.8
204.5
176.8
204.5
204.5
176.8
NITRATE AND NITRITE
3. HEALTH EFFECTS
49
Table 3-1 Levels of Significant Exposure to Nitrate And Nitrite - Oral (continued)
Exposure/
Duration/
a
Key to Species
Frequency
Figure (Strain)
(Route)
59
Rat
(Fischer- 344)
105 wk
(W)
60
Rat
NS
24 mo
(W)
61
Rat
(Wistar)
29 mo
(F)
System
Gastro
Hemato
Hepatic
Gastro
Hemato
Hepatic
Bd Wt
LOAEL
NOAEL
(mg/kg/day)
Less Serious
(mg/kg/day)
Serious
(mg/kg/day)
Reference
Chemical Form
Comments
46.9 M
53.6 F
87.1 M (epithelial hyperplasia in
the forestomach)
NTP 2001
Sodium Nitrite
100.5 F (epithelial hyperplasia in
the forestomach)
86.4 M
172.8 M (12% methemoglobin)
Shuval and Gruener 1972
Sodium Nitrite
176.8 M
204.5 F
van Logten et al. 1972
Sodium Nitrite
176.8 M
204.5 F
176.8 M
204.5 F
204.5 F 176.8 M (10% lower mean body
weight)

6
80.4
147.4
147.4
110.6
23
38.5
39
24
38.5
39
43
108.4
87
110.4
53
298
NITRATE AND NITRITE
3. HEALTH EFFECTS
50
Table 3-1 Levels of Significant Exposure to Nitrate And Nitrite - Oral (continued)
a
Key to
Figure
Species
(Strain)
Exposure/
Duration/
Frequency
(Route)
System
NOAEL
(mg/kg/day)
Less Serious
(mg/kg/day)
LOAEL
Serious
(mg/kg/day)
Reference
Chemical Form
Comments
62
Mouse
(B6C3F1)
104-105 wk
(W)
Gastro 80.4 M
147.4 M (epithelial hyperplasia in
glandular stomach)
NTP 2001
Sodium Nitrite
Bd Wt 147.4 M
110.6 F
63
Dog
(Beagle)
1 yr
(W)
Endocr 38.5 M
39 F
Kelley et al. 1974
Sodium Nitrate
Reproductive
64
Dog
(Beagle)
1 yr
(W)
38.5 M
39 F
Kelley et al. 1974
Sodium Nitrate
Cancer
65
Rat
(Fischer- 344)
106 wk
(F)
108.4 F (CEL; hepatocellular
neoplasms)
Lijinsky 1984a; Lijinsky et al.
1983
Sodium Nitrite
66
Rat
(Fischer- 344)
104 wk
(F)
110.4 F (CEL: hepatocellular
neoplasms)
Lijinsky 1984b; Lijinsky et al.
1983
Sodium Nitrite
67
Rat
(Wistar)
Lifetime
(W)
298 (CEL; forestomach
tumors)
Mirvish et al. 1980
Sodium Nitrite
48
207.7
NITRATE AND NITRITE
3. HEALTH EFFECTS
51
Table 3-1 Levels of Significant Exposure to Nitrate And Nitrite - Oral (continued)
Exposure/
LOAEL
Duration/
a
Key to Species
Frequency
NOAEL Less Serious Serious
Reference
(Route)
Figure (Strain)
System
(mg/kg/day) (mg/kg/day) (mg/kg/day)
Chemical Form
Comments
68
Mouse Lifetime
207.7 M (CEL: lung carcinoma)
A
nderson et al. 1985
(Hybrid)
(W)
Sodium Nitrite
a The number corresponds to entries in Figure 3-1.
b NOAEL of of 4 mg/kg/day for nitrate was used to derive acute-, intermediate, and chronic-duration oral minimal risk levels (MRLs) of 4 mg/kg/day for nitrate, as described in detail in
Chapter 2 and Appendix A. The NOAEL was divided by an uncertainty factor of 1 for human variability because the NOAEL accounted for exposure of a particularly sensitive
subpopulation (infants <3 months of age).
c NOAEL of 0.2 mg/kg/day for nitrite was used to derive acute-, intermediate, and chronic-duration oral minimal risk levels (MRLs) of 0.1 mg/kg/day for nitrite, as described in detail in
Chapter 2 and Appendix A. The NOAEL represents the dose of nitrite that would be expected to enter the blood following ingestion of nitrate by an adult at the oral MRL value of 4
mg nitrate/kg/day assuming 5% reduction of an oral dose of nitrate to nitrite in the adult saliva complete absorption of nitrite from the digestive tract. The NOAEL of 0.2 mg/kg/day for
nitrite was divided by an uncertainty factor of 1 for human variability because the NOAEL was for exposure of a particularly sensitive subpopulation (infants <3 months of age). A
modifying factor of 2 was applied based on the assumption that the effective methemoglobin level from a given intake by an infant may be up to twice that of an adult.
Bd Wt = body weight; CEL = cancer effect level; d = day(s); EEG = electroencephalogram; Endocr = endocrine; (F) = feed; F = Female; Gastro = gastrointestinal; Gd = gestational
day; Gn pig = guinea pig; (GW) = gavage in water; Hemato = hematological; LD50 = lethal dose, 50% kill; LOAEL = lowest-observed-adverse-effect level; M = male; mo = month(s);
NOAEL = no-observed-adverse-effect level; NS = not specified; T3 = triiodothyronine ; T4 = thyroxine; TSH = thyroid stimulating hormone ; (W) = drinking water; wk = week(s); x =
time(s); yr = year(s)
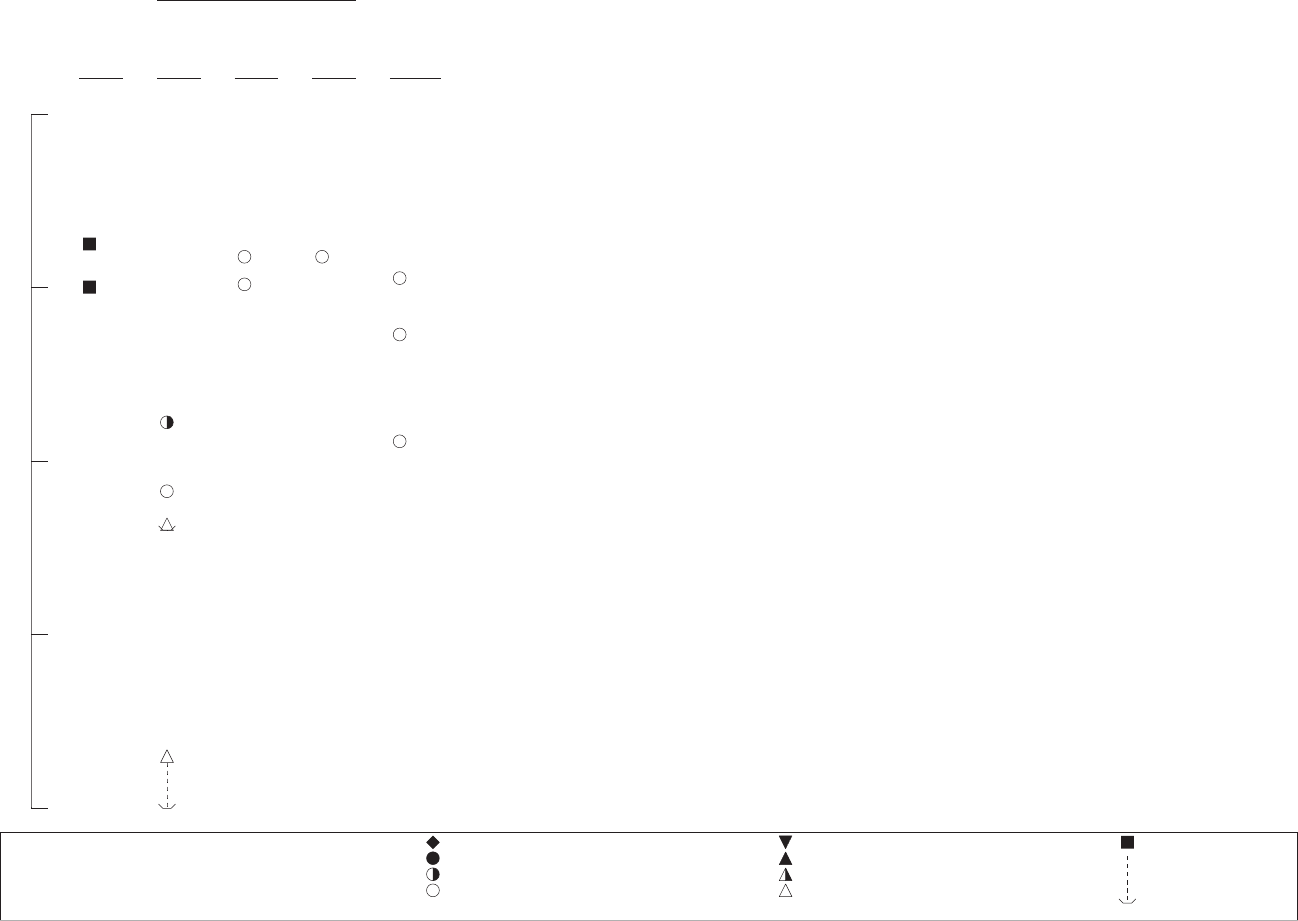
Death
1000
2m
7m 7m
10m
6r
1r
100
8r
5r
9m
10
3
5r
←MRL for Nitrate
1
4
←MRL for Nitrite
0.1
c-Cat
d-Dog
r-Rat
p-Pig
q-Cow
k-Monkey
m-Mouse
h-Rabbit
a-Sheep
f-Ferret
j-Pigeon
e-Gerbil
s-Hamster
g-Guinea Pig
n-Mink
o-Other
Cancer Effect Level-Animals
LOAEL, More Serious-Animals
LOAEL, Less Serious-Animals
NOAEL - Animals
Cancer Effect Level-Humans
LOAEL, More Serious-Humans
LOAEL, Less Serious-Humans
NOAEL - Humans
LD50/LC50
Minimal Risk Level
for effects
other than
Cancer
Hematological
Hepatic
Body Weight
Developmental
NITRATE AND NITRITE
3. HEALTH EFFECTS
52
Figure 3-1 Levels of Significant Exposure to Nitrate And Nitrite - Oral
Acute (≤14 days)
Systemic
mg/kg/day

Death
Gastroint
Hematol
Hepatic
Renal
Endocrin
mg/kg/day
100000
12r
12r
10000
27r
27r
14g
27r
27r
30r
11r
1000
41m
41m
41m
28r
41m 41m
15
←MRL for Nitrate
28r
13m
13m
41m
28r
41m
25r 29r
18r
28r
26r
24r
41m
23r
31r
32r
33r
34r 34r
32r
34r 34r
33r
100
31r
31r
32r 32r
23r
31r
32r
18r
20r
19r
32r
21r
36r
32r
10
36r
34r 34r
21r
21r
33r
34r 34r
32r
33r
1
16
←MRL for Nitrite
0.1
c-Cat
d-Dog
r-Rat
p-Pig
q-Cow
k-Monkey
m-Mouse
h-Rabbit
a-Sheep
f-Ferret
j-Pigeon
e-Gerbil
s-Hamster
g-Guinea Pig
n-Mink
o-Other
Cancer Effect Level-Animals
LOAEL, More Serious-Animals
LOAEL, Less Serious-Animals
NOAEL - Animals
Cancer Effect Level-Humans
LOAEL, More Serious-Humans
LOAEL, Less Serious-Humans
NOAEL - Humans
LD50/LC50
Minimal Risk Level
for effects
other than
Cancer
estinal
ogical
e
NITRATE AND NITRITE
3. HEALTH EFFECTS
53
Figure 3-1 Levels of Significant Exposure to Nitrate And Nitrite - Oral (Continued)
Intermediate (15-364 days)
Systemic

Body Weight
Metabolic
Neurological
Reproductive
Developmental
NITRATE AND NITRITE
3. HEALTH EFFECTS
54
mg/kg/day
100000
10000
1000
100
10
1
0.1
Figure 3-1 Levels of Significant Exposure to Nitrate And Nitrite - Oral (Continued)
Intermediate (15-364 days)
Systemic
27r
27r
27r
27r
49g
39m
30r
49g
28r
41m
41m
41m
28r
28r
28r
48m
38m
40m
26r
43r
50g
48m
46r
47r
51r
37m
17r
50g
44r 45r
47r
22r 22r
35r
22r
36r
52r
21r
36r
42r
52r
c-Cat
d-Dog
r-Rat
p-Pig
q-Cow
k-Monkey
m-Mouse
h-Rabbit
a-Sheep
f-Ferret
j-Pigeon
e-Gerbil
s-Hamster
g-Guinea Pig
n-Mink
o-Other
Cancer Effect Level-Animals
LOAEL, More Serious-Animals
LOAEL, Less Serious-Animals
NOAEL - Animals
Cancer Effect Level-Humans
LOAEL, More Serious-Humans
LOAEL, Less Serious-Humans
NOAEL - Humans
LD50/LC50
Minimal Risk Level
for effects
other than
Cancer

G
H
H
E
B
R
C
mg/kg/day
10000
58r
58r
1000
58r
67r
61r
68m
61r
61r 61r
55r
53
←MRL for Nitrate
61r
60r
61r
61r 61r
62m 62m
62m
66r
65r
57r
59r
100
59r
60r
57r
55r
62m
57r
59r
59r
63d 64d
63d
64d
56r
56r
10
1
54
*Doses represent the lowest dose tested per study that produced a tumorigenic
←MRL for Nitrite
response and do not imply the existence of a threshold for the cancer endpoint.
0.1
c-Cat
d-Dog
r-Rat
p-Pig
q-Cow
k-Monkey
m-Mouse
h-Rabbit
a-Sheep
f-Ferret
j-Pigeon
e-Gerbil
s-Hamster
g-Guinea Pig
n-Mink
o-Other
Cancer Effect Level-Animals
LOAEL, More Serious-Animals
LOAEL, Less Serious-Animals
NOAEL - Animals
Cancer Effect Level-Humans
LOAEL, More Serious-Humans
LOAEL, Less Serious-Humans
NOAEL - Humans
LD50/LC50
Minimal Risk Level
for effects
other than
Cancer
astrointestinal
ematological
epatic
ndocrine
ody Weight
eproductive
ancer*
NITRATE AND NITRITE
3. HEALTH EFFECTS
55
Figure 3-1 Levels of Significant Exposure to Nitrate And Nitrite - Oral (Continued)
Chronic (≥365 days)
Systemic
56 NITRATE AND NITRITE
3. HEALTH EFFECTS
mean arterial blood pressure decreased from 80 to 69 mmHg. The maximum effects on heart rate and
blood pressure occurred between 15 and 20 minutes following ingestion; heart rate and blood pressure
returned to near-baseline levels approximately 2 hours following ingestion at the low dose, but the effects
had not returned to baseline at 4 hours following ingestion at the high dose. The blood pressure-lowering
effect of short-term dietary supplementation of inorganic nitrate appears to be beneficial; however, there
is some uncertainty regarding potential health benefits of long-term nitrate supplementation to treat
cardiovascular diseases (Maccha and Schecter 2012; Siervo et al. 2013).
Gastrointestinal Effects. Ingestion of nitrite (from potassium nitrite or sodium nitrite sources) has
been associated with severe methemoglobinemia in adults and children; in many of these cases,
symptoms included abdominal cramps and vomiting (CDC 1997, 2002; Gautami et al. 1995; Gowans
1990; Greenberg et al. 1945; Sevier and Berbatis 1976; Ten Brink et al. 1982). These cases were the
result of consumption of food or drink that contained unusually high levels of nitrite via contamination,
inadvertent use of sodium nitrite instead of table salt, or ingestion of a single sodium nitrite tablet
(667 mg nitrite). In a study designed to evaluate the oral bioavailability of sodium nitrite in healthy
volunteers (seven females and two males; mean age 22.9 years), one subject became nauseous and
vomited within 20 minutes following ingestion of 0.12 mmol sodium nitrite per mmol hemoglobin
(~4.8 mg sodium nitrite/kg, or 3.2 mg nitrite/kg); another subject reported nausea within 30 minutes
following ingestion of 0.12 mmol sodium nitrite per mmol hemoglobin (~4.4 mg sodium nitrite/kg, or
2.9 mg nitrite/kg) (Kortboyer et al. 1997b).
In a population-based study, Nasseri-Moghaddam et al. (2011) evaluated the prevalence of acid
regurgitation and/or heartburn in regions of Tehran categorized by nitrate levels in drinking water
sources. The study authors reported a significantly increased prevalence of frequent (at least weekly) acid
regurgitation among residents living in areas with drinking water nitrate concentrations >100 mg/L
compared to those living in areas with drinking water nitrate concentrations <100 mg/L (OR 3.65; 95%
CI 1.32, 10.09).
NTP (2001) observed epithelial hyperplasia in the forestomach of male and female B6C3F1 mice
provided sodium nitrite in the drinking water for 14 weeks at a concentration (5,000 ppm) that resulted in
estimated sodium nitrite doses of 990 and 1,230 mg/kg/day, respectively (663.3 and 824.1 mg
nitrite/kg/day, respectively); NOAELs for these lesions in the males and females were 435.5 and
562.8 mg nitrite/kg/day, respectively. Similar results were noted for male and female F344/N rats and
male B6C3F1 mice treated for 104–105 weeks at estimated doses of 87.1, 100.5, and 147.4 mg
57 NITRATE AND NITRITE
3. HEALTH EFFECTS
nitrite/kg/day, respectively; NOAELs for these lesions in the male and female rats and male mice were
46.9, 53.6, and 80.4 mg nitrite/kg/day, respectively. Sodium nitrite treatment did not result in increased
incidences of forestomach lesions in other groups of male F344 rats provided sodium nitrite in the
drinking water at 2,000 mg/L (estimated dose of 208.4 mg nitrite/kg/day) for 35 weeks (Miyauchi et al.
2002) or 51 weeks (Kawabe et al. 1994).
Hematological Effects. As discussed in detail in Section 3.4 (Toxicokinetics) and Section 3.5
(Mechanisms of Action), some plasma nitrite, arising from reduction of ingested nitrate and via
endogenous production, is involved in the oxidation of hemoglobin-Fe
2+
(which transports oxygen) to
hemoglobin-Fe
3+
(methemoglobin, incapable of binding oxygen).
Methemoglobinemia is a condition in which increased methemoglobin as a percentage of total
hemoglobin results in the expression of clinical signs that increase in severity with increasing percent
methemoglobin (ATSDR 2013a; Bloom et al. 2013; Denshaw-Burke et al. 2013; Haymond et al. 2005).
In normal healthy individuals, methemoglobin levels are <1% of total hemoglobin. Discoloration (e.g.,
pale, gray blue) of the skin is often observed at methemoglobin levels in the range of 3–15%; most
patients tolerate methemoglobin levels <10%. Tachycardia, weakness, and other signs of tissue hypoxia
may be observed at 10–20% methemoglobin levels. Effects on the central nervous system (e.g.,
headache, dizziness, fatigue) and dyspnea and nausea appear at >20% methemoglobin; the severity of
symptoms increases with increasing methemoglobin level. High risk of mortality occurs at levels >70%
methemoglobin).
As early as the mid-1900s, methemoglobinemia was reported in infants exposed to relatively large
amounts of nitrate from drinking water sources (e.g., Bailey 1966; Bosch et al. 1950; Bucklin and Myint
1960; Chapin 1947; Comly 1987; Donahoe 1949; Faucett and Miller 1946; Ferrant 1946; McLetchie and
Robertson 1949; Medovy 1948; Robertson and Riddell 1949; Stafford 1947; Walton 1951). Available
data identify young bottle-fed infants (1–3 months of age) as a subpopulation that is particularly
susceptible to nitrate-induced methemoglobinemia, especially those consuming formula prepared from
drinking water sources containing nitrate in excess of 10 mg nitrate-nitrogen/L (44 mg nitrate/L)
(e.g., Bosch et al. 1950; Walton 1951); EPA established a maximum contaminant level (MCL) of
10 mg/L for nitrate-nitrogen in drinking water (EPA 2009c). Subsequent reports provide additional
evidence of associations between ingestion of nitrate from drinking water sources and elevated
methemoglobin levels in infants (e.g., Craun et al. 1981; Fan and Steinberg 1996; Fan et al. 1987;
Gruener and Toeplitz 1975; Gupta et al. 1999; Johnson et al. 1987; Jones et al. 1973; Miller 1971; Shuval
58 NITRATE AND NITRITE
3. HEALTH EFFECTS
and Gruener 1972; Simon et al. 1964; Super et al. 1981; Winton et al. 1971; Zeman et al. 2002).
Cyanosis and even death occurred in some of the reported cases. However, there is some evidence that
methemoglobinemia in infants who drank formula prepared using drinking water with relatively high
levels of nitrate may be related to bacterial contamination of such water sources and consequent
gastrointestinal disorders, as well as endogenous overproduction of nitric oxide due to gastrointestinal
infection and inflammation (Avery 1999; Gupta et al. 1998; Hegesh and Shiloah 1982; L’hirondel and
L’hirondel 2002; Yano et al. 1982).
Walton (1951) reviewed available literature and found 278 reported cases of infant methemoglobinemia.
Among those infants for whom data on nitrate levels in water sources used to prepare infant formula were
available (n=214), levels >50 mg nitrate-nitrogen/L (220 mg nitrate/L) were associated with 173 cases
(81%), levels of 21–50 mg/L (92–220 mg nitrate/L) were associated with 36 cases (17%), and levels of
11–20 mg nitrate-nitrogen (48–88 mg nitrate/L) were associated with 5 cases (2%). There were no cases
among those infants consuming water containing <10 mg nitrate-nitrogen/L (<44 mg nitrate/L).
Limitations include lack of information regarding the actual ages of the infants, total nitrate doses, and
other water source contaminants (e.g., bacterial levels).
Bosch et al. (1950) evaluated 139 reported cases of cyanosis among infants in Minnesota (90% of which
were <2 months of age; range 8 days to 5 months). Samples from 129 wells that served as water sources
to the cases revealed nitrate-nitrogen concentrations >100 mg/L (>440 mg nitrate/L) in 49 wells, 50–
100 mg/L (220–440 mg nitrate/L) in 53 wells, 21–50 mg/L (92–220 mg nitrate/L) in 25 wells, and 10–
20 mg/L (44–88 mg nitrate/L) in the other 2 wells. A major limitation of this study was the detection of
coliform organisms in 45 of 51 well water samples tested for bacterial contamination; bacteria in the
water source might have been a causal factor for gastrointestinal tract disturbances in some of the infants
and may have been at least partially responsible for increased susceptibility to nitrate-induced cyanosis
(e.g., gastrointestinal tract disturbances could have influenced conversion of ingested nitrate to nitrite or
absorption of nitrite).
Subsequent reports provide additional evidence of associations between ingestion of nitrate from drinking
water sources and elevated methemoglobin levels in infants (e.g., Craun et al. 1981; Fan and Steinberg
1996; Fan et al. 1987; Gruener and Toeplitz 1975; Gupta et al. 1999; Johnson et al. 1987; Jones et al.
1973; Miller 1971; Shuval and Gruener 1972; Simon et al. 1964; Super et al. 1981; Winton et al. 1971;
Zeman et al. 2002). Cyanosis and even death occurred in some of the reported cases.
59 NITRATE AND NITRITE
3. HEALTH EFFECTS
Simon et al. (1964) evaluated methemoglobin levels from 89 healthy infants with a nitrate-free water
source (group 1), 38 infants whose water source contained 50–100 mg nitrate/L (group 2), and 25 infants
whose water source contained >100 mg nitrate/L (group 3). Nitrite levels in the water sources measured
less than 0.3 mg/L (with the exception of a single measurement of 1 mg nitrite/L). For groups 1, 2, and 3,
methemoglobin levels averaged 1.0, 1.3, and 2.9%, respectively, in the first postnatal trimester (0–
3 months of age) and 0.8, 0.8, and 0.7 %, respectively, in the second trimester. Significantly increased
methemoglobin was observed only in the highest exposure group (>100 mg nitrate/L) and only during the
first trimester.
Super et al. (1981) evaluated associations between methemoglobin levels among infants 1–12 months of
age (relatively evenly distributed by month) and estimates of nitrate intake (based on measured drinking
water nitrate levels and considerations of liquid intake from other sources). When divided into two
groups according to estimated nitrate intake (310 infants ingesting ≤2.93 mg nitrate/kg/day and
102 infants ingesting >2.93 mg nitrate/kg/day), mean methemoglobin levels were 1.54 and 3.03%,
respectively. There were no striking age-related differences in frequency of infants with methemoglobin
levels >3%.
A nested case-control study included 26 cases of infants diagnosed with methemoglobinemia at
≤2 months of age and 45 age-matched controls (Zeman et al. 2002). Nitrate exposure levels were
categorized as low (<0.5 ppm), medium (1–10 ppm), or high (>10 ppm) according to estimated nitrate
levels reconstructed from parental responses to dietary questionnaires and environmental sampling
(1 ppm in the diet is equivalent to 1 mg/kg diet; 1 ppm in drinking water is equivalent to 1 mg/L).
Numbers of methemoglobinemia cases in the low-, medium-, and high-exposure categories were 0/26,
4/26, and 22/26, respectively, and estimated dietary nitrate intake ranged from 2.83 to 451.20 mg/kg/day
(mean 103.6 mg nitrate/kg/day); diarrheal disease was reported for 14/26 methemoglobinemia cases.
Numbers of controls in the low-, medium-, and high-exposure categories were 21/45, 11/45, and 13/45,
respectively, and estimated dietary nitrate intake ranged from 0 to 182 mg/kg/day (mean 11.2 mg
nitrate/kg/day) for the controls; diarrheal disease was reported for 13/45 controls. Univariate and
multifactorial analysis of risk factors for methemoglobinemia indicated that methemoglobinemia was
most strongly associated with dietary exposure to nitrate/nitrite (p=0.0318), but also significantly
associated with diarrheal disease (p=0.0376). Controls in the high-exposure category were less likely
than high-exposure methemoglobinemia cases to have experienced severe diarrhea and were more likely
to have been breastfed for >2 weeks. Major limitations to the study include the collection of information
60 NITRATE AND NITRITE
3. HEALTH EFFECTS
contributing to the exposure estimates several years following the occurrences of methemoglobinemia and
reliance on parental recollection of infant nutritional intake.
Sadeq et al. (2008) measured methemoglobin levels in children ranging in age from birth to 8 years of age
who either lived in a region where nitrate levels in 78 tested wells ranged from 15.39 to 246.9 mg/L or a
region supplied by municipal water with a mean nitrate level of 2.99 mg/L. The mean methemoglobin
level (0.205 g/dL) among 100 children in the region supplied by well water was slightly higher than that
of 37 children in the region supplied by municipal water (0.166 g/dL). The study authors stated that
0.24 g methemoglobin/dL is the equivalent of 2% methemoglobin, in which case mean methemoglobin
among the children in the region supplied by well water was approximately 1.7% of total hemoglobin
compared to a mean of 1.4% for the children in the region supplied by municipal water. The slight
increases in mean methemoglobin among the children in the region supplied by well water were
consistent within various age ranges (0–6, 7–11, 13–35, 36–71, and 72–95 months). The study authors
stated that methemoglobin ≤0.24 g/dL (2%) was considered to be within normal limits.
Craun et al. (1981) evaluated methemoglobin levels in 102 children 1–8 years of age. Sixty-four of the
children lived in households where drinking water contained 22–111 mg nitrate-nitrogen/L (97–488 mg
nitrate/L); drinking water sources for the other 38 children (controls) contained <10 mg nitrate-nitrogen/L
(<44 mg nitrate/L). Methemoglobin measured 1.0–1.36% in those children 1–4 years of age and
appeared to increase with increasing nitrate intake, although there was no statistically significant change.
Methemoglobin levels in those children 5–8 years of age averaged 0.9–0.95% independent of level of
exposure to nitrate.
In one longitudinal study of 357 pregnant women in south-central Minnesota, there was no apparent
association between estimated intake of nitrate from tap water and methemoglobin levels (Manassaram et
al. 2010). However, only four of the women used tap water with nitrate-nitrogen content above the EPA
(2009c) MCL of 10 mg/L.
Elevated methemoglobin levels and methemoglobinemia have been associated with consumption of foods
high in nitrate (e.g., borage, carrots, kohlrabi, spinach) by infants and small children (Greer and Shannon
2005; Keating et al. 1973; Martinez et al. 2013; Sanchez-Echaniz et al. 2001). In the study of Sanchez-
Echaniz et al. (2001), a homemade purée of mixed vegetables with high nitrate content was considered
the source of elevated methemoglobin levels (10–58% of total hemoglobin) among seven infants 7–
13 months of age.
61 NITRATE AND NITRITE
3. HEALTH EFFECTS
Limited data are available regarding administration of controlled amounts of nitrate and methemoglobin
levels. Cornblath and Hartmann (1948) administered sodium nitrate in the formula fed to four infants
(ages 11 days to 11 months) for 2–18 days at a concentration resulting in a dose of 50 mg nitrate/kg/day.
The highest observed level of methemoglobin was 5.3% of total hemoglobin; there was no evidence of
cyanosis. Among four other infants (ages 2 days to 6 months) similarly treated at 100 mg nitrate/kg/day
for 6–9 days, the only reported effect was that of 7.5% methemoglobin in a 10-day-old infant following
8 days of treatment in the absence of clinical cyanosis. Gruener and Toeplitz (1975) fed 104 infants
(1 week to 10 months of age) for 1 day with formula prepared using water containing 15 mg nitrate/L
(~0.8–1.5 mg nitrate/kg, based on age-specific values for water consumption [Kahn and Stralka 2009] and
body weight [EPA 2008]), increased to 108 mg nitrate/L for the next 3 days (~5.5–10.6 mg nitrate/kg/day,
based on age-specific values for water consumption [Kahn and Stralka 2009] and body weight [EPA
2008], and returned to 15 mg nitrate/L for one additional day. Mean methemoglobin levels were 0.89%
after the first day of feeding, 1.3, 0.91, and 0.93% after days 2, 3, and 4, and dropped to 0.8% on the fifth
day. Among three of these infants (ages not specified), methemoglobin levels reached 6.9, 13.9, and
15.9% during the high-dose days. Limitations of this study include the use of a wide range of ages and
the fact that only 57 of the 104 infants supplied blood samples on all 5 treatment days.
Ingestion of nitrite (from potassium nitrite or sodium nitrite sources) has been associated with severe
methemoglobinemia in adults and children (Aquanno et al. 1981; CDC 1997, 2002; Finan et al. 1998;
Gautami et al. 1995; Gowans 1990; Greenberg et al. 1945; Kaplan et al. 1990; Ringling et al. 2003;
Sevier and Berbatis 1976; Ten Brink et al. 1982; Walley and Flanagan 1987). These cases were the result
of consumption of food or drink that contained unusually high levels of nitrite via contamination,
inadvertent use of sodium nitrite instead of table salt, inadvertent use of sodium nitrite-contaminated
sugar, or ingestion of a single sodium nitrite tablet (667 mg nitrite).
In a study designed to evaluate the oral bioavailability of sodium nitrite in healthy volunteers (seven
females and two males; mean age 22.9 years), ingestion of 0.06 sodium nitrite per mmol hemoglobin
(~2.2–2.7 mg sodium nitrite/kg, or 1.5–1.8 mg nitrite/kg) resulted in a mean maximum methemoglobin
concentration of 0.309 mmol/L (range of 3.4–4.5% of total hemoglobin) at approximately 0.70 hours
following ingestion, and a mean half-life of approximately 1.07 hours for methemoglobin reduction
(Kortboyer et al. 1997b). At a higher intake (0.12 mmol sodium nitrite per mmol hemoglobin; ~4.4–
5.4 mg sodium nitrite/kg, or 2.9–3.6 mg nitrite/kg), the mean maximum methemoglobin concentration
62 NITRATE AND NITRITE
3. HEALTH EFFECTS
was 0.727 mmol/L (range of 7.7–10.9% of total hemoglobin) at approximately 1.14 hours following
ingestion, and a mean half-life of approximately 1.13 hours for methemoglobin reduction.
Increased methemoglobin levels have been reported in rats administered sodium nitrite orally. Imaizumi
et al. (1980) administered aqueous sodium nitrite to fasted Sprague-Dawley rats by gavage at 20, 25, 50,
100, or 150 mg/kg (6.7, 16.75, 33.5, 67, and 100.5 mg nitrite/kg, respectively) and observed
methemoglobin levels of 4.3, 8.6, 40.3, 64.7, and 45–80%, respectively, at 1 hour posttreatment. The
highest dose resulted in 50% mortality. Among surviving rats, methemoglobin levels returned to normal
after 24 hours. Imaizumi et al. (1980) administered sodium nitrite in the drinking water of other rats for
6 months at 0.5% (5,000 mg sodium nitrite/L or 3,333 mg nitrite/L). Methemoglobin levels as high as
88% were observed during evening hours of treatment day 18 when the rats were likely drinking water
and as low as 4% during morning and afternoon hours of the following day. The study authors did not
provide information regarding clinical signs or mortality, but stated that there was no effect on growth.
In a 14-week study of male and female Fischer-344 rats administered sodium nitrite in the drinking water,
clinical signs of cyanosis and brownish discoloration of mucous membranes and skin were noted at
concentrations ≥1,500 ppm (≥130 mg/kg or 87.1 mg nitrite/kg) in the females and ≥3,000 ppm
(≥225 mg/kg or 134 mg nitrite/kg) in the males (NTP 2001). The clinical signs were consistent with
increased methemoglobin, which measured as high as 13, 24, and 50% in the 1,500, 3,000, and 5,000 ppm
groups, respectively. Til et al. (1988) reported methemoglobin levels of 5.7 and 7.6% in male and female
Wistar rats, respectively, administered potassium nitrite in the drinking water for 13 weeks at
concentrations resulting in approximate doses of 107.6 and 130.5 mg nitrite/kg/day, respectively. Til et
al. (1997) reported similar effects on methemoglobin in rats similarly exposed to either potassium nitrite
or sodium nitrite; however, quantitative data were not included in the study report.
Behroozi et al. (1972) provided sodium nitrite in the drinking water of male albino rats for 2 months at
concentrations resulting in sodium nitrite doses of 0, 14, 42, and 280 mg/kg/day (0, 9.38, 28.14, and
187.6 mg nitrite/kg/day, respectively). Methemoglobin in all groups was approximately 0.5% prior to the
initiation of sodium nitrite treatment and remained at that level in the control group throughout the study.
Methemoglobin in the low-, mid- and high-dose groups averaged 1.1, 3.0, and 12.16%, respectively,
during the treatment period; following cessation of sodium nitrite exposure, methemoglobin levels in all
sodium nitrite-treated groups decreased to 0.3–0.7%.
63 NITRATE AND NITRITE
3. HEALTH EFFECTS
Chow et al. (1980) provided drinking water to male Sprague-Dawley rats for 16 weeks that contained 0 or
200 mg sodium nitrite/L (calculated dose of 18.6 mg nitrite/kg/day, based on EPA [1988] subchronic
reference values for body weight and food consumption). Methemoglobin averaged 0.5–3.0% in the
sodium nitrite-treated group and 0–1.2% in the controls.
Shuval and Gruener (1972) provided sodium nitrite in the drinking water to male rats for 24 months at 0,
100, 1,000, 2,000, or 3,000 mg/L (calculated doses of 0, 8.64, 86.4, 172.8, and 259.2 mg nitrite/kg/day,
based on EPA [1988] chronic default reference values for body weight and food consumption).
Methemoglobin levels in the three highest exposure groups averaged 5, 12, and 22% of total hemoglobin;
there were no treatment-related effects on hemoglobin levels.
Maekawa et al. (1982) added sodium nitrite to the food of male and female F-344 rats for 6 weeks at
concentrations ranging from 0.06 to 1% and sodium nitrate to the food of other rats at concentrations
ranging from 1.25 to 20%. Discoloration in blood and spleen were noted in rats from the two highest
exposure levels for sodium nitrite and sodium nitrate. These exposure levels were equivalent to doses
≥370 mg nitrite/kg/day and ≥7,300 mg nitrate/kg/day (based on EPA [1988] subchronic reference values
for body weight and food consumption in male and female F-344 rats). The study report did not include
information regarding methemoglobin levels.
Chow et al. (1980) provided drinking water to male Sprague-Dawley rats for 16 weeks that contained 0 or
400 mg sodium nitrate/L (calculated dose of 40.5 mg nitrate/kg/day, based on EPA [1988] subchronic
reference values for body weight and food consumption). There were no treatment-related effects on
mean methemoglobin levels.
Other hematological effects were noted in some animal studies that employed exposure to sodium nitrite
or potassium nitrite in the drinking water for periods of 13–115 weeks. Imaizumi et al. (1980) reported
decreased hemoglobin and irregularities in erythrocytes (irregular sizes and marked Heinz body
formation) in rats receiving 167.5 mg nitrite/kg/day. Til et al. (1988, 1997) noted slightly decreased
hemoglobin in male rats at ≥42 mg nitrite/kg/day, decreased packed cell volume and erythrocyte count at
approximately 108 mg nitrite/kg/day, and decreases in erythrocyte count, mean corpuscular volume and
mean corpuscular hemoglobin in female rats at 130 mg nitrite/kg/day. Initially decreased erythrocyte
counts were noted in male rats at ≥60 mg nitrite/kg/day (as much as 44% lower than controls at 8 weeks
of treatment, but returning to control levels by 52 weeks); significant decreases in mean corpuscular
volume, and hemoglobin in these rats were noted throughout the 115-week treatment period (Grant and
64 NITRATE AND NITRITE
3. HEALTH EFFECTS
Butler 1989). Significantly increased spleen weights were noted in male mice receiving sodium nitrite for
14 weeks at ≥ 435 mg nitrite/kg/day (39% greater than that of controls) and in male and female mice at
663 or 824 mg nitrite/kg/day (approximately 66% greater than their controls). The study authors
suggested that the increased spleen weights may have represented increased erythropoietic activity in
response to increased methemoglobin; however, methemoglobin data were not included in the study
report.
Hepatic Effects. No information was located regarding hepatic effects in humans following oral
exposure to nitrate or nitrite.
No indications of sodium nitrite-induced liver effects were observed in animal studies that included
assessment of liver function and/or histopathology (Asahina et al. 1971; Lijinsky and Greenblatt 1972;
Lin and Ho 1992; Shuval and Gruener 1972; van Logten et al. 1972).
Renal Effects. No information was located regarding renal effects in humans following oral exposure
to nitrate or nitrite.
El-Wakf et al. (2008) reported significantly increased urinary levels of urea and creatinine in male rats
provided sodium nitrate in the drinking water for 4 months at author-estimated doses of 21.7 and 47.4 mg
sodium nitrate/kg/day (15.8 and 34.6 mg nitrate/kg/day, respectively).
Endocrine Effects. Nitrate acts as a dose-dependent competitive inhibitor of the sodium iodide
symporter (NIS) that mediates the uptake of iodine by the thyroid. Sufficiently decreased iodine uptake
by the thyroid may result in decreased production of thyroid hormones T3 and T4. Decreased thyroid
hormone production causes increased release of TSH from the anterior pituitary gland and consequent
increased uptake of iodine by the thyroid gland. Sufficiently inhibited uptake of iodine by the thyroid
could result in effects associated with thyroid dysfunction (e.g., hypothyroidism). Concern for nitrate-
induced effects on thyroid function has prompted scientists to perform studies designed to assess thyroid
function relative to drinking water and/or dietary nitrate levels. Available human data provide suggestive
evidence that elevated levels of nitrate in drinking water and/or nitrate-rich diets may be associated with
signs of thyroid dysfunction. However, limitations of these studies include lack of individual dose-
response data, quantification of iodine intake, and control for other potential substances that may affect
the thyroid; one study relied on self-reported thyroid status and self-reported dietary nitrate intake.
65 NITRATE AND NITRITE
3. HEALTH EFFECTS
Tajtáková and coworkers evaluated thyroid function among schoolchildren (boys and girls 10 or 13 years
of age) from three areas in Slovakia; an agricultural area with drinking water sources containing nitrate at
51–274 mg/L (n=324), an area from a neighboring area where drinking water sources contained <2 mg
nitrate/L (n=168), and the city of Košice supplied with drinking water reported to be low in nitrate
(n=596) (Rádiková et al. 2008; Tajtáková et al. 2006). At the time of the study, measurements of urinary
iodine indicated that the children in the high- and low-nitrate areas were ingesting sufficient iodine.
Thyroid volume and density were estimated with the assistance of ultrasound equipment. Mean thyroid
volume was significantly higher in the 10- and 13-year-old children from the high-nitrate area
(5.10±0.14 mL for the 10-year-olds and 5.97±0.11 mL for the 13-year-olds) compared to that of the
children from the low-nitrate area (4.58±0.17 and 5.23±0.15 mL, respectively) and from the city of
Košice (4.77±0.10 and 4.87±0.10 mL, respectively). The frequency of hypoechogenicity (ultrasound
indicator of decreased thyroid density typically indicating destruction of normal thyroid tissue) was
significantly greater in children from the high-nitrate area compared to those from the low-nitrate areas
(13.7 versus 4.7% for the 10-year-olds and 10.6 versus 5.7% for the 13-year-olds). Blood samples
revealed TSH in the range of subclinical hypothyroidism in 13/324 children and positive anti-
thyroperoxidase antibodies (an indicator of subclinical thyroid disorder) in 8/324 of the children from the
high-nitrate area versus no cases in 109 children from the low-nitrate area. There were no significant
differences between children from the low- and high-nitrate areas regarding serum T3 or T4 levels.
Iodine status and goiter prevalence were evaluated in 156 schoolchildren (7–14 years of age) in an area of
rural Bulgaria where nitrate in the drinking water averaged 75 mg/L and 163 schoolchildren in a nearby
area drinking water nitrate averaged 8 mg/L at the time of the study (Gatseva and Argirova 2008). At the
time of the study, perchlorate was below the detection limit (1 µg/mL). Urinary iodine measurements
indicated that iodine intake was satisfactory for most children from each group. The goiter rate within the
high-nitrate areas was significantly higher than the goiter rate within the low-nitrate area (13.5 versus
5.9%). Familial thyroid disorders and chronic diseases were reported by families of 7.7% of the children
from the high-nitrate area and only 3.06% of the children from the low-nitrate area. In a similar study
that included two areas of Bulgaria, one with high nitrate in the drinking water (average of 93 mg/L) and
one with low nitrate in the drinking water (average 8 mg/L), pregnant women from the high- (n=26) and
low- (n=22) nitrate areas and children (3–6 years of age) from the high- (n=50) and low- (n=49) areas
were evaluated for iodine status and goiter frequency. Mean urinary iodine in the women from the high-
nitrate area was significantly lower than that of the women from the low-nitrate area (147.85±56.38
versus 230.55±61.56 µg/L). Iodine deficiency was indicated for 5/26 women and 11/50 of the children
from the high-nitrate area and 1/22 women and 5/49 children from the low-nitrate area. Goiter was
66 NITRATE AND NITRITE
3. HEALTH EFFECTS
reported for 9/26 women and 14/50 children from the high-nitrate area and 2/22 women and 7/49 children
from the low-nitrate area. Familial thyroid disorders and chronic diseases were reported for 9/26 women
and 3/50 children from the high-nitrate area and 2/22 women and 1/49 children from the low-nitrate area.
The differences in goiter rates may be the result of differences in iodine intake and reported familial
thyroid disorder and chronic disease prevalence.
Aschebrook-Kilfoy et al. (2012) reported an association between nitrate in private wells at estimated
levels >6.5 mg nitrate-nitrogen /L (>28.6 mg nitrate/L) and elevated serum TSH in women (but not men)
as an indicator of subclinical hypothyroidism (OR 1.60, 95% CI: 1.11, 2.32). The study included
2,543 Old Order Amish residing in several counties in Pennsylvania for whom TSH levels were available.
Nitrate levels in the wells were estimated by modeling data provided by the U.S. Geological Survey
(USGS) that monitored nitrate levels in 3,613 wells in the study area.
In one cohort of 21,977 older women in Iowa who had used the same water supply for >10 years, there
were no significant differences in prevalence of self-reported hypothyroidism or hyperthyroidism between
those using private wells as drinking water source (n=5,436) and those using public water sources
(n=16,541) (Ward et al. 2010). Sufficient data for public water sources were available from which to
evaluate prevalence of thyroid disorders by quartile of nitrate concentration in public water sources
defined as mean concentrations <0.36, 0.36–1.00, 1.01–2.46, and >2.46 mg nitrate-nitrogen/L (<1.58,
1.58–4.4, 4.41–10.82, and >10.82 mg nitrate/L, respectively). There was no apparent association between
nitrate in the drinking water and prevalence of self-reported hypothyroidism or hyperthyroidism when
comparing results by quartile. No nitrate measurement data were available for women using private
wells. Data for these women were compared to data for women in the lowest quartile of public water
sources, although it was estimated at the time of the study that 18% of the rural private wells in Iowa had
nitrate levels >10 mg nitrate-nitrogen/L (>44 mg nitrate/L). In the same study (Ward et al. 2010), dietary
nitrate intake was estimated using a food frequency questionnaire and published nitrate levels for various
food sources and the study subjects (3,018 cases of hypothyroidism and 937 cases of hyperthyroidism)
were divided into quartiles according to dietary nitrate intake (≤17.4, 17.5–27.7, 27.8–41.1, and >41.1 mg
nitrate-nitrogen/day; approximately equivalent to <77, 77–121.9, 122–181, and >181 mg nitrate mg/day,
respectively). Using the lowest quartile as a referent, associations were found for prevalence of
hypothyroidism (but not hyperthyroidism) for the second quartile (OR 1.13, 95% CI: 1.01, 1.27), third
quartile (OR 1.19, 95% CI: 1.06, 1.33), and fourth quartile (OR 1.24, 95% CI: 1.10, 1.40). A significant
trend was noted as well for increasing prevalence of hypothyroidism with increasing quartile of dietary
nitrate (p=0.001).
67 NITRATE AND NITRITE
3. HEALTH EFFECTS
In a randomized controlled study, 10 volunteers consumed sodium nitrate in aqueous solution at a dose of
15 mg/kg/day for 28 days; 10 other volunteers receiving distilled water served as controls. There were no
sodium nitrate treatment-related effects on thyroidal
131
iodine uptake or plasma thyroid hormone
concentrations (Hunault et al. 2007).
Thyroid status has been assessed to some extent in animals consuming drinking water or food to which
nitrate salts had been added. There were no clinical signs of hypothyroidism or effects on serum T3 or T4
levels in adult Beagles or their puppies during exposure of the breeding dogs to sodium nitrate in the
drinking water for 1 year at concentrations in the range of 300–1,000 ppm (equivalent to 219–730 mg
nitrate/L) (Kelley et al. 1974).
Decreased thyroidal
131
iodine uptake was noted in rats given food
containing 0.5–2.5% potassium nitrate (equivalent to 3,000–15,000 mg nitrate/kg food) (Bloomfield et al.
1961). Significantly increased uptake of thyroidal
131
iodine; decreased serum T3, T4, and TSH levels;
increased thyroid weight; and follicular hyperplasia were noted in female Wistar rats administered sodium
nitrate in the drinking water for 30 weeks at concentrations ≥250 mg/L (≥159 mg nitrate/kg/day, based on
reported average water intake and EPA [1988] subchronic reference body weight of 0.156 kg for the
female Wistar rat) (Eskiocak et al. 2005). In another study (Zaki et al. 2004), significantly decreased
serum T3 (34–44% lower than controls), increased thyroid weight (45–77% greater than controls), and
histopathologic thyroid lesions (glandular hypertrophy accompanied by vacuolization, increased colloidal
volume of the follicles, and flattened follicular epithelium) were observed in male Wistar rats receiving
drinking water for 5 months to which potassium nitrate had been added at concentrations resulting in
estimated doses ≥13.5 mg nitrate/kg/day (based on EPA [1988] subchronic reference values for body
weight and water consumption for the male Wistar rat).
El-Wakf et al. (2008) reported significantly decreased serum T3 and T4 levels (17–41% lower than
controls) in all groups of weanling male Wistar rats provided sodium nitrate in the drinking water for
4 months at concentrations resulting in author-estimated intakes in the range of 8.7–47.4 mg sodium
nitrate/kg/day (equivalent to 6.4–34.6 mg nitrate/kg/day). At estimated doses ≥15.8 mg nitrate/kg/day,
significantly increased serum TSH was also noted (26–30% higher than that of controls). Groups of
similarly-treated young adult male Wistar rats exhibited significantly decreased T3 and T4 levels (24–
47% lower than controls) and increased serum TSH (30–35% higher than controls) at estimated doses
≥15.8 mg nitrate/kg/day.
68 NITRATE AND NITRITE
3. HEALTH EFFECTS
In a 28-day study of rats receiving food to which potassium nitrate had been added to constitute 3% of the
diet, thyroid effects included significantly increased thyroid gland weight (45% greater than controls),
increased TSH (nearly 7-fold higher than that of controls), decreased serum T3 and T4 levels (61–63%
lower than controls), and decreased thyroid peroxidase activity (84% lower than controls)
(Mukhopadhyay et al. 2005). Based on reported body weight data and the EPA (1988) allometric
equation for calculating a food consumption rate for laboratory mammals (0.056 x body weight
0.6611
), an
estimated dose was 2,416 mg nitrate/kg/day.
Til et al. (1988) added potassium nitrite to the drinking water of male and female rats for 13 weeks at
concentrations resulting in estimated doses in the range of 8.9–199.2 mg/kg/day (4.8–108 mg
nitrite/kg/day) to the males and 10.9–241.7 mg/kg/day (5.9–130.5 mg nitrite/kg/day) to the females.
Doses ≥13.3 mg nitrite/kg/day (males) and ≥61.8 mg nitrite/kg/day (females) resulted in hypertrophy in
the zona glomerulosa of the adrenal gland. In this study, potassium was added to the drinking water of
each treatment group up to the level of potassium in the drinking water of the highest dose group.
Controls included groups with untreated drinking water and groups with potassium chloride-treated water.
The effect on the adrenal gland was not observed in the untreated controls or the potassium chloride
controls, indicating that the effect was the result of nitrite ion. Similar results were obtained at estimated
doses of 105.1 mg nitrite/kg/day (males) and 130.1 mg nitrite/kg/day (females) in a subsequent similarly-
designed study (Til et al. 1997) to evaluate effects at lower doses than those employed in the earlier study
(Til et al. 1988). Results of a subsequent study indicate that the effect on the adrenal gland of the rat is a
physiological adaptation to repeated episodes of hypotension caused by nitrite (RIVM 1996).
Dermal Effects. Available information regarding dermal effects following oral exposure to nitrate or
nitrite is limited to a case report in which ingestion of ammonium nitrate was considered a possible cause
of erythema dyschromicum perstans (ashy dermatosis) (Jablonska 1975).
Body Weight Effects. No information was located regarding body weight effects in humans
following ingestion of nitrate or nitrite.
No body weight effects were observed in some studies of laboratory animals provided sodium nitrate,
sodium nitrite, or potassium nitrite in the drinking water for intermediate exposure durations (4 weeks to
10 months) at concentrations resulting in estimated doses in the range of 1,583–7,300 mg nitrate/kg/day
(Maekawa et al. 1982; Mukhopadhyay et al. 2005) or 28–435.5 mg nitrite/kg/day (Greenblatt and
Lijinsky 1974; Greenblatt and Mirvish 1973; Greenblatt et al. 1971; Lin and Ho 1992; Maekawa et al.
69 NITRATE AND NITRITE
3. HEALTH EFFECTS
1982; NTP 2001; Vorhees et al. 1984). Depressed body weight and/or body weight gain (approximately
10% less that of controls) were observed in other studies at estimated doses of 8,241.9–14,600 mg
nitrate/kg/day (Maekawa et al. 1982) and 663.3–1,080.6 mg nitrite/kg/day (Maekawa et al. 1982; NTP
2001). In chronic-duration studies (≥365 days), doses in the range of 101–178.2 mg nitrite/kg/day and
1,730 mg nitrate/kg/day resulted in 10–15% depressed body weight in rats and mice (Grant and Butler
1989; Maekawa et al. 1982; van Logten et al. 1972).
Body weight data in the study report of Zaki et al. (2004) indicate as much as 16–25% depressed mean
body weight among male Wistar rats provided drinking water for 5 months that contained 150 or 500 mg
potassium nitrate/L (estimated doses of 13.5 and 45 mg nitrate/kg/day); however, data regarding food and
water consumption were not included in the study report. El-Wakf et al. (2008) provided drinking water
to weanling male Wistar rats for 4 months that contained 100, 250, or 500 mg sodium nitrate/L (estimated
doses of 6.4, 15.8, and 34.6 mg nitrate/kg/day) and reported mean final body weights that were 11, 29,
and 46%, respectively, less than that of control; however, data regarding food and water consumption
were not included in the study report. El-Wakf et al. (2015) provided young (3-week-old) and adult
(12-week-old) male Wistar rats with drinking water to which sodium nitrate was added at 550 mg/L
(estimated daily intake of 47.7 mg sodium nitrate/kg/day or 34.8 mg nitrate/kg/day) for 4 months;
controls received drinking water without added sodium nitrate. The sodium nitrate treatment resulted in
depressed body weight (24 and 9% less among the young and adult rats, respectively, compared to
controls) and depressed body weight gain (39 and 30% less among the young and adult rats, respectively,
compared to controls).
Metabolic Effects. Possible associations between nitrate and/or nitrite in drinking water and/or food
sources and risk of type 1 diabetes have been investigated in a number of epidemiological studies (Casu et
al. 2000; Dahlquist et al. 1990; Kostraba et al. 1992; Moltchanova et al. 2004; Parslow et al. 1997; van
Maanen et al. 2000; Zhao et al. 2001). Statistically significant associations between estimated nitrate
and/or nitrite intake were reported by some investigators, but were not observed by others. Limitations of
studies include the lack of quantitative dose-response data and the likelihood of confounding by other
potential toxicants. Therefore, there is considerable uncertainty regarding nitrate or nitrite intake and risk
of type 1 childhood diabetes.
A study in the Netherlands involved 1,064 cases of type 1 diabetes in a total of 2,829,020 children (0–
14 years of age) included in the analysis (van Maanen et al. 2000). Nitrate levels in drinking water were
determined by postal code. Two exposure categories were used. One category was based on equal
70 NITRATE AND NITRITE
3. HEALTH EFFECTS
numbers of children exposed to various levels of nitrate in the drinking water (0.25–2.08, 2.10–6.42, and
6.44–41.19 mg nitrate/L); the other category was based on cutoff values of 10 and 25 mg nitrate/L. The
study authors concluded that there was little evidence that nitrate in the drinking water was a risk factor
for childhood type 1 diabetes under the conditions of the study.
Zhao et al. (2001) found no significant association between nitrate in the drinking water and risk for
childhood type 1 diabetes in a study of 517 cases (0–15 years of age). The mean concentration of nitrate
in the drinking water was 6.62 mg/L (range 0.49–31.9 mg/L). Casu et al. (2000) found no significant
association between nitrate in tap water or bottled water and risk of type 1 diabetes among 1,975 cases
(0–29 years of age), 1,142 of which were <15 years of age. In this study, nitrate concentrations in tap and
bottled water were below the acceptable maximal concentration of 50 mg/L established by the European
Community and the recommended level of 25 mg/L. Moltchanova et al. (2004) found no significant
association between childhood type 1 diabetes and nitrate in the groundwater in Finland. The study
included 3,598 cases of childhood type 1 diabetes (ages 0–14 years) and 9,601,164 children at risk;
drinking water nitrate levels averaged 6.228 mg/L.
Dahlquist et al. (1990) evaluated a variety of nutrients and food additives (including nitrate) as possible
risk factors for type 1 diabetes among 339 children under 15 years of age and matched with 528 control
children in Sweden. Estimates of intake of the various nutrients and food additives were made based on
parental responses to food frequency questionnaires. Upon dividing the subjects into three groups
according to estimated nitrate intake (low=<25
th
percentile; medium=25–75
th
percentile; high=>75
th
percentile), a significant nonlinear trend for increased risk of type 1 diabetes with increasing nitrate intake
was noted. The high-nitrate intake group exhibited a significantly increased risk (crude OR 2.14, 95% CI:
1.64, 3.54) compared to the low-nitrate intake group; adjustment for age, sex, maternal age, maternal
education, and family history of type 1 diabetes did not significantly alter the results.
Kostraba et al. (1992) calculated incidence rates by county in Colorado (63 counties) for type 1 diabetes
in children (<18 years of age at diagnosis during the years 1978 and 1988; n=1,280) and compared the
rates to nitrate levels in potable water supplies. Children in counties with water nitrate levels in the range
of 0.77–8.2 mg/L had a significantly increased risk of type1 diabetes compared to those in counties with
water nitrate levels in the range of 0.0–0.084 mg/L.
Parslow et al. (1997) reported a significant increase association (SIR 115, 95% CI: 107,124) between
nitrate in drinking water (highest tertile versus lowest tertile) and incidence of childhood type 1 diabetes
71 NITRATE AND NITRITE
3. HEALTH EFFECTS
diagnosed between 1978 and 1994 in the Yorkshire Regional Health Authority in England. The study
subjects were 0–16 years of age, and nitrate levels in the drinking water were divided into tertiles (1.48–
<3.22, 3.22–<14.85, 14.85–40.01 mg/L). The study included 498 cases in a population of
225,708 children in the lowest tertile, 591 cases in a population of 232,373 children in the middle tertile,
and 708 cases in a population of 237,951 children in the highest tertile.
Virtanen et al. (1994) reported a significant association between estimated dietary nitrite intake by
children and mothers and risk for type 1 diabetes in all age groups of boys and girls (ages 0–4, 5–9, and
10–14 years). The study included 684 children with Type 1 diabetes, 595 control children, 548 case-
control pairs of fathers, and 620 case-control pairs of mothers in a nationwide Finnish study. Nitrate and
nitrite levels were estimated based on results from food frequency questionnaires and household water
data provided by the Finnish waterworks. Nitrate intake of the mother was associated with decreased risk
for childhood type 1 diabetes.
El-Wakf et al. (2015) provided young (3-week-old) and adult (12-week-old) male Wistar rats with
drinking water to which sodium nitrate was added at 550 mg/L (estimated daily intake of 47.7 mg sodium
nitrate/kg/day or 34.8 mg nitrate/kg/day) for 4 months; controls received drinking water without added
sodium nitrate. The sodium nitrate treatment induced hyperglycemia in both age groups. In a study of
Sprague-Dawley rats administered sodium nitrite by gavage at 80 mg/kg/day for 12 weeks, nitrite-induced
effects included inhibition of liver glycogenesis (generation of glycogen from glucose molecules) and
enhanced liver glycogenolysis (breakdown of glycogen) and gluconeogenesis (generation of glucose from
non-carbohydrate carbon substrates), accompanied by hyperglycemia and insulin resistance (Al-Gayyar et
al. 2015).
3.2.2.3 Immunological and Lymphoreticular Effects
No information was located regarding immunological or lymphoreticular effects in humans or animals
following oral exposure to nitrate or nitrite.
3.2.2.4 Neurological Effects
Ingestion of nitrite (from potassium nitrite or sodium nitrite sources) has been associated with severe
methemoglobinemia in adults and children; in many of these cases, clinical signs included dizziness, loss
of consciousness, and/or convulsions (CDC 1997, 2002; Gautami et al. 1995; Greenberg et al. 1945;
Sevier and Berbatis 1976; Ten Brink et al. 1982). These cases were the result of consumption of food or
72 NITRATE AND NITRITE
3. HEALTH EFFECTS
drink that contained unusually high levels of nitrite via contamination, inadvertent use of sodium nitrite
instead of table salt, or ingestion of a single sodium nitrite tablet (667 mg nitrite).
Headache was induced in a male subject following consumption of a 10 mg sodium nitrite solution
(Henderson and Raskin 1972). Headaches were induced in 8 out of 13 such tests. The tests were
performed to evaluate whether nitrite in frankfurters that the subject had previously ingested might be
cause for the headache he had developed shortly thereafter. In a study designed to evaluate the oral
bioavailability of sodium nitrite in healthy volunteers (seven females and two males; mean age
22.9 years), headache was reported by four of the nine people ingesting 0.12 mmol sodium nitrite per
mmol hemoglobin (~4.4–5.4 mg sodium nitrite/kg, or 2.9–3.6 mg nitrite/kg) and by four of the nine
subjects ingesting 0.06 mmol sodium nitrite per mmol hemoglobin (~2.2–2.7 mg sodium nitrite/kg, or
1.5–1.8 mg nitrite/kg) (Kortboyer et al. 1997b).
Abnormalities in electroencephalograms (EEGs) were reported in male albino rats provided sodium nitrite
in the drinking water for 2 months at concentrations resulting in author-reported doses ≥14 mg sodium
nitrite (≥9.38 mg nitrite/kg/day) (Behroozi et al. 1972). The abnormal readings persisted during up to
4.5 months following cessation of exposure to sodium nitrite. At the highest dose (187.6 mg
nitrite/kg/day), rats exhibited clinical signs of sedation and became motionless during periods of electrical
outbursts.
Gruener (1974) reported increased aggressive behavior in male C57B1 mice provided sodium nitrite in
the drinking water at 1,000 mg/L (estimated dose of 165.4 mg nitrite/kg/day) for up to 13 weeks
postweaning. The mice had also been exposed via their parents during mating and their mothers during
gestation and lactation. Shuval and Gruener (1972) reported significantly reduced motor activity in male
mice provided sodium nitrite in the drinking water. Sodium nitrite levels tested ranged from 100 to
2,000 mg/L; however, the study report did not include specific information regarding the exposure levels
that resulted in reduced motor activity.
3.2.2.5 Reproductive Effects
See Section 3.2.2.6 for information regarding results of case-control studies that evaluated reproductive/
developmental end points.
73 NITRATE AND NITRITE
3. HEALTH EFFECTS
Several animal studies included evaluation of selected reproductive end points. Among three female
guinea pigs provided potassium nitrate in the drinking water for up to 204 days of cohabitation at a
concentration resulting in estimated intake of 4,972 mg nitrate/kg/day, one female died and the other two
females produced a total of two litters (one live birth per litter) (Sleight and Atallah 1968). During
191 days of cohabitation, four control females produced eight litters and a total of 31 live births. There
was no gross or histopathologic evidence of treatment-related effects on reproductive organs. Sleight and
Atallah (1968) provided other guinea pigs with drinking water that contained potassium nitrite at
concentrations ranging from 300 to 10,000 ppm. Exposure levels ≥1,000 ppm potassium nitrite
(estimated doses ≥148.5 mg nitrite/kg/day) resulted in decreases in number of litters and live births;
histopathologic evaluations of reproductive organs revealed placental, uterine, and cervical lesions.
No treatment-related effects on implantations or resorptions were seen in female Wistar rats provided
sodium nitrite in the food throughout the production of two litters at concentrations resulting in estimated
doses as high as 160 mg nitrite/kg/day (Hugot et al. 1980). No treatment-related effects on fertility were
seen in breeding dogs provided sodium nitrate in the drinking water for 1 year at concentrations resulting
in doses as high as 39 mg nitrate/kg/day (Kelley et al. 1974).
Alavantić et al. (1988a) treated male mice with sodium nitrate or sodium nitrite by gavage for 3 days at
doses of 0, 600, or 1,200 mg/kg/day (sodium nitrate) or 0, 60, or 120 mg/kg/day (sodium nitrite); sperm-
head abnormalities were evaluated at 11 and 17 days following treatment. Frequencies of sperm-head
abnormalities in the low- and high-dose sodium nitrate-treated and the low-dose sodium nitrite-treated
groups were not significantly different from controls. However, the high-dose group of sodium nitrite-
treated mice exhibited significantly increased frequency of sperm-head abnormalities at 11 and 17 days
following treatment (approximately 1.5-fold greater than controls). Alavantić et al. (1988b) treated male
mice with sodium nitrate or sodium nitrite by gavage for 2 weeks at doses of 0, 600, or 1,200 mg/kg/day
(sodium nitrate) or 0, 60, or 120 mg/kg/day (sodium nitrite) and subsequently mated them to virgin
females. Evaluation of primary spermatocytes from parental males revealed significantly increased
frequency of sperm-head abnormalities in the high-dose sodium nitrate-treated group (1.4-fold greater
than controls) and the low- and high-dose sodium nitrite-treated groups (1.2- and 1.4-fold greater,
respectively, than controls). There was no treatment-related effect on frequency of sperm-head
abnormalities in F1 males. Fertility in the high-dose sodium nitrite-treated group was significantly
affected; only 31 of 49 females mated to the high-dose males became pregnant compared to 45 of
50 females mated to control males.
74 NITRATE AND NITRITE
3. HEALTH EFFECTS
Alyoussef and Al-Gayyar (2016a, 2016b) administered sodium nitrite to male Sprague-Dawley rats by
gavage at 0 or 80 mg/kg/day for 12 weeks. Sodium nitrite treatment resulted in increased testicular
weight (1.6–1.7-fold greater than controls); decreased serum testosterone levels (36–44% less than
controls); decreased epididymal sperm count (48% less than controls); decreased testicular anti-
inflammatory cytokine levels; increased serum luteinizing hormone (LH), follicle stimulating hormone
(FSH), and prolactin levels; and increased testicular levels of pro-inflammatory cytokines, oxidative stress
markers, and enzymes involved in programmed cell death.
NTP (2001) reported degeneration of the testis (characterized by increased size of residual bodies within
the lumen of the seminiferous tubules) in male mice provided sodium nitrite in the drinking water for
14 weeks at concentrations resulting in estimated doses ≥435.5 mg nitrite/kg/day; the biological
significance of this lesion was uncertain. In similarly-treated female mice, estrous cycles were
significantly increased (11 and 15%, respectively, longer than controls) at estimated doses of 298.1 and
824.1 mg nitrite/kg/day, but not at 562.8 mg nitrite/kg/day. Among similarly-treated male and female
rats, the males exhibited 7–18% decreased sperm motility at doses ≥134 mg nitrite/kg/day; there were no
treatment-related effects on vaginal cytology end points in the females at doses as high as 231 mg
nitrite/kg/day.
3.2.2.6 Developmental Effects
Several population-based, case-control studies evaluated possible associations between developmental
end points and exposure to nitrate from drinking water sources. The results are not adequate for
quantitative risk assessment because estimations of nitrate intakes were typically based on measurements
of nitrate levels in drinking water sources at selected time points and self-reported estimates of water
consumption, possible confounding by other potential toxicants was not evaluated, and most studies did
not account for dietary nitrate or nitrite intake which is typically the major source of ingested nitrate and
nitrite. Statistically significant associations between nitrate in the drinking water and selected
developmental end points (e.g., birth defects, spontaneous abortions) were reported by some investigators,
but were not observed by others.
Brender et al. (2013) evaluated possible relationships between prenatal exposure to nitrate in drinking
water and selected birth defects in a large population-based, case-control study that included 3,300 case
mothers and 1,121 control mothers who were participants in the National Birth Defects Prevention Study.
Nitrate levels were measured in public water supplies and in representative samples of bottled water sold
75 NITRATE AND NITRITE
3. HEALTH EFFECTS
in local stores; daily nitrate consumption was estimated from self-reported water consumption at home
and work. The lowest tertile of nitrate intake from water (<0.91 mg/day at conception or <1.0 mg/day
during preconception and the first trimester of pregnancy) represented the referent group. Within the
highest tertile (≥5.0 mg/day at conception; ≥5.42 mg/day during preconception and the first trimester of
pregnancy), significant associations were noted for risk of spina bifida (OR 2.02, 95% CI: 1.01, 2.04), any
limb deficiency (OR 1.79, 95% CI: 1.05, 3.08), any oral cleft defect (OR 1.45, 95% CI: 1.10, 1.92), cleft
lip without cleft palate (OR 1.82, 95% CI: 1.08, 3.07), cleft palate (OR 1.90, 95% CI: 1.17, 3.09), and any
neural tube defect (OR 1.43, 95% CI: 1.01, 2.04). Cases in the various tertiles ranged in number from
23 to 173. The study authors noted that higher estimated nitrate intakes from drinking water did not
increase associations between reported maternal intake of nitrosatable drugs and birth defects.
Dorsch et al. (1984) evaluated 218 cases of congenital malformations and matched controls between 1951
and 1979 in an area of South Australia. In an analysis of data by estimated level of nitrate in the drinking
water, the risk of malformations was significantly greater at nitrate levels of 5–15 mg/L (OR 2.6, 95% CI:
1.6, 4.1; 138 cases, 106 controls) and >15 mg/L (OR 4.1, 95% CI: 1.3, 13.1; 10 cases, 5 controls)
compared to those with nitrate levels <5 mg/L (70 cases, 107 controls).
Scragg et al. (1982) evaluated possible associations between maternal water source and frequency of
congenital malformations (mainly neural tube defects) in a locality in South Australia (258 cases and
matched controls). A referent group consisted of those women who used rainwater as the drinking water
source. Significantly increased risk of occurrence of a malformation was noted for those women who
drank water from a lake source (RR 2.8, 95% CL: 1.6, 5.1) and for women who used water from private
wells with nitrate levels typically >15 ppm (RR 4.1, 95% CL: 1.7, 10.0).
Cedergren et al. (2002) reported nonstatistically significant increased risk of cardiac defects among
infants of mothers exposed to nitrate in the drinking water at levels ≥2 mg/L (OR 1.18, 95% CI: 0.97,
1.44; 392 cases, 27,962 controls) compared to those with nitrate levels <2 mg/L; all measured nitrate
concentrations were below the Swedish maximum contaminant level. The study population included
75,832 infants born in a Swedish county between January 1982 and December 1996.
Croen et al. (2001) evaluated 538 cases of neural tube defects and 539 normal controls in an area of
California between June 1989 and May 1991. Exposure to nitrate in drinking water at concentrations
>45 mg/L was associated with statistically significantly increased risk of anencephaly (OR 4.0, 95% CI:
76 NITRATE AND NITRITE
3. HEALTH EFFECTS
1.0, 15.4), but no increased risk for spina bifida. Increased risk was also noted at nitrate levels <45 mg/L
among groundwater drinkers.
Arbuckle et al. (1988) evaluated mothers in the area of New Brunswick where private wells averaged
26 mg/L nitrate and public municipal sources averaged 0.1 mg/L nitrate. There was no statistically
significant increased risk for delivering a central nervous system-malformed infant by mothers using
private wells (OR 2.30, 95% CI: 0.73, 7.29). The study included 130 cases of central nervous system
birth defects for the years 1973–1983, each matched to 2 controls.
Aschengrau et al. (1993) found no statistically significant association between drinking water nitrate
levels (up to 4.5 mg/L) or nitrite levels (up to 0.15 mg/L) and frequency of congenital anomalies,
stillbirth, or neonatal death among 1,171 cases and 1,177 controls who delivered at a Massachusetts
hospital between August 1977 and March 1980.
Holtby et al. (2014) evaluated possible associations between nitrate in drinking water sources and
incidence of congenital anomalies in the agricultural region of Kings County, Nova Scotia, Canada
between 1988 and 2006. A mean level of 6.44 mg nitrate-nitrogen/L was calculated for rural wells
(equivalent to 28.34 mg nitrate/L), based on 1,113 water samples from 140 wells. A mean level of
2.03 mg nitrate-nitrogen/L was calculated for municipal water supplies (equivalent to 8.93 mg nitrate/L),
based on 53 water samples from 20 water sources (19 groundwater sources and 1 surface water source).
Nitrate-nitrogen concentration estimates were divided into tertiles (<1, 1–5.56, and >5.56 mg nitrate-
nitrogen/L; equivalent to <4.4, 4.4–24.46, and >24.46 mg nitrate/L). Overall, no significant association
was found between nitrate levels in drinking water sources and incidences of congenital malformations.
However, stratification of the data by conception before or after the onset of food fortification with folate
in Canada (instituted in 1998) resulted in an OR of 2.44 (95% CI 1.05, 5.66) for risk of congenital
anomalies with exposure of 1–5.56 mg nitrate-nitrogen/L (4.4–24.46 mg nitrate/L) for the time period
(1998–2006).
Ericson et al. (1988) found no association between frequency of neural tube defects and levels of nitrate
in the drinking water in a case-control study that included 1,458 cases of neural tube defects and
280 matched controls. The reported average nitrate levels in the water were 4.9 mg/L among the cases
and 5.1 mg/L among the controls.
77 NITRATE AND NITRITE
3. HEALTH EFFECTS
Super et al. (1981) evaluated the status of 486 infants in a geographical area of southwest Africa served
by 153 wells divided into regions of high nitrate (>20 mg/L) and low nitrate (≤20 mg/L). There was no
significant association between nitrate levels in drinking water sources and incidence of stillbirths,
prematurity, or birth size; however, an increased incidence of deaths during the first year of life was noted
for the high-nitrate region.
Winchester et al. (2009) investigated whether U.S. live births are at increased risk for birth defects when
conception occurs during months when surface water agrichemicals (including nitrate, atrazine, and other
pesticides) are at greatest concentrations (April–July). For the years 1996–2002, monthly agrichemical
concentrations were calculated using USGS’s National Water Quality Assessment data and live birth data
collected from the Centers for Disease Control and Prevention (CDC) natality data sets. Birth defects
were more likely to occur in live births conceived between April and July. However, this finding does not
necessarily implicate nitrate in the drinking water.
Brender et al. (2004) found no significant association between dietary nitrate or nitrite intake and risk of
offspring with neural tube defects at estimated total nitrite doses (dietary nitrite plus 5% dietary nitrate)
ranging from <7.5 to >10.53 mg/day. However, the risk of neural tube defect was significant among
those women with total nitrite doses >10.53 mg/day who also reported taking nitrosatable drugs (OR 7.5,
95% CI: 1.8, 45).
Huber et al. (2013) estimated daily nitrate and nitrite intakes among 6,544 mothers of infants with neural
tube defects, oral clefts, or limb deficiencies and 6,807 mothers of unaffected control infants, based on
results of food frequency questionnaires. The study included areas of 10 U.S. states, and the population
was divided into quartiles of estimated nitrate intake and nitrite intake. There was no statistically
significant increased risk of neural tube defect with any estimate of nitrate or nitrite intake. Similar
results were obtained for oral cleft and limb deficiency, with the exceptions of increased risk at the
highest quartile of cleft lip only (OR 1.32, 95% CI: 1.01, 1.72) and cleft lip with or without cleft palate
(OR 1.24, 95% CI: 1.05, 1.48) at the highest quartile of animal-based nitrite intake, and increased risk of
intercalary limb defect (OR 4.70, 95% CI: 1.23, 17.93) at the highest quartile of total nitrite intake.
Aschengrau et al. (1989) found no nitrate-related increased risk of spontaneous abortion in a study of
286 women who presented at a Massachusetts hospital between July 1976 and February 1978 with a
spontaneous loss through gestation week 27 and 1,391 controls.
78 NITRATE AND NITRITE
3. HEALTH EFFECTS
The CDC (1996) investigated a small cluster of spontaneous abortions (three women, six spontaneous
abortions) in close proximity to one another and to a hog farm in LaGrange County, Indiana during 1991–
1993. Well water on the hog farm contained >50 mg nitrate/L. Water samples from wells supplying the
women who aborted contained 19–26 mg nitrate/L. A mean concentration of 3.1 mg nitrate/L (1.6–
8.4 mg/L) was determined for well water supplies to residences of a comparison group of five women,
each having full-term birth within the same time period. During the investigation, another case was
identified in which a 35-year-old woman, living approximately 10 miles from the other three women, had
two spontaneous abortions after having five previous live births. Well water during the first four
pregnancies was found to contain 1.2 mg nitrate-nitrogen/L (5.3 mg nitrate/L); the spontaneous abortions
occurred after installation of a new well that was found to contain 28.7 mg nitrate-nitrogen/L (126 mg
nitrate/L). Although all four women delivered full-term, live-born infants after changing to nitrate-free
drinking water sources, the occurrences of spontaneous abortion may have been unrelated to nitrate-
containing drinking water.
George et al. (2001) evaluated the geographical and seasonal distribution of sudden infant death
syndrome (SIDS) in Sweden during the period 1990–1996 in relation to nitrate levels in drinking water
and changes in groundwater nitrate content. The local incidence of SIDS was correlated to maximally
recorded concentrations of nitrate in the drinking water. However, in addition to lack of dose-response
data for individuals, the SIDS incidence was declining during the study period, numbers of SIDS cases
were small in scarcely populated areas, and nitrate concentrations in groundwater sources may have
changed rapidly with weather changes and other factors.
Tabacova et al. (1997) evaluated maternal health among 61 pregnant women who lived near an
ammonium nitrate fertilizer plant and presented at a local prenatal care clinic. Tabacova et al. (1998)
evaluated the status of 51 mother-infant pairs in the same region. Nitrogen oxides in the air averaged
23.1 µg/m
3
with short-term peak levels as high as 238.5; nitrate concentrations in the public drinking
water supply measured 8–54 mg/L; nitrate levels in private wells measured as much as 13–400 mg/L. Of
the 61 pregnant mothers in the sample of Tabacova et al. (1997), only 10 had “normal” pregnancies.
Mothers diagnosed with anemia (41 cases), toxemia (20 cases), and/or threatened abortion/premature
labor (20 cases) exhibited ≥2-fold higher serum methemoglobin than those with “normal” pregnancies.
Of the 51 mothers in the sample of Tabacova et al. (1998), there were 38 full-term and normal-weight
infants, 7 full-term and low-weight infants, 6 premature deliveries, 1 Caesarean delivery, and 1 breech
delivery. Elevated methemoglobin was observed in serum from 28/51 of the mothers, and 24/51 cord
blood samples. Both maternal and cord blood methemoglobin levels were higher in cases of abnormal
79 NITRATE AND NITRITE
3. HEALTH EFFECTS
birth outcome. These results could not be directly linked to elevated nitrate intake from drinking water or
food sources.
Developmental end points have been assessed in some animal studies. No indications of treatment-
related developmental toxicity were seen in fetuses from pregnant mice administered sodium nitrite in the
drinking water during gestation days 7–18 at concentrations as high as 1,000 mg/L (approximate doses as
high as 113.2 mg nitrite/kg/day) (Shimada 1989). There were no signs of toxicity in offspring of pregnant
rats administered 80 mg sodium nitrite/kg (53.6 mg nitrite/kg) on gestation day 15; offspring were
observed for up to 140 days postpartum (Khera 1982). There were no signs of treatment-related
developmental effects during the production of two litters by female Wistar rats provided sodium nitrite
in the food at concentrations resulting in estimated doses as high as 160 mg nitrite/kg/day (Hugot et al.
1980). Among three female guinea pigs provided potassium nitrate in the drinking water for up to
204 days of cohabitation at a concentration resulting in estimated intake of 4,972 mg nitrate/kg/day, one
female died and the other two females produced a total of two litters (one live birth per litter) (Sleight and
Atallah 1968). During 191 days of cohabitation, four control females produced eight litters and a total of
31 live births. The only indication of a treatment-related effect on the offspring of pregnant mice
administered sodium nitrite by gavage at 0.5 mg/mouse/day (approximate dose of 13 mg nitrite/kg/day)
on gestation days 1–14, 16, or 18 was increased fetal hepatic erythropoiesis at gestation days 14 and 16,
which was thought to have been a response to nitrite-induced fetal methemoglobinemia (Globus and
Samuel 1978).
Significantly impaired auditory and visual discrimination learning behavior and retention of passive
avoidance responses (Nyakas et al. 1990), and delay in cholinergic and serotonergic fiber outgrowth in
cortical target areas of the brain (Nyakas et al. 1994), presumably due to nitrite-induced hypoxia, were
reported in offspring of Wistar rats provided sodium nitrite in the drinking water at 2,000 mg/L (1,334 mg
nitrite/L) during gestation day 13 until parturition. However, lack of information regarding body weight
and water consumption of the pregnant rats precludes estimation of nitrite doses to the pregnant dams.
Shuval and Gruener (1972) provided sodium nitrite in the drinking water of pregnant rats for 6 weeks
(that presumably included gestation and lactation) at concentrations of 2,000 or 3,000 mg/L (1,334 or
2,001 mg nitrite/L, respectively). There were no treatment-related effects on group litter sizes or pup
birth weights. However, during 3 weeks postpartum, 30 and 53% of the low- and high-dose pups died
(compared to 6% of control pups); surviving pups from the low- and high-dose groups exhibited 43 and
66% lower mean body weight than controls at 3 weeks postpartum. Lack of information regarding body
80 NITRATE AND NITRITE
3. HEALTH EFFECTS
weight and water consumption of the pregnant rats precludes estimation of nitrite doses to the pregnant
dams.
Increased pup mortality, depressed preweaning pup body weight, and delayed swimming development
were observed in offspring of male and female rats provided sodium nitrite in the diet at 0.025 or 0.05%
(estimated dose levels of 14.4 and 28.1 mg nitrite/kg/day, based on author-reported dose of 43 mg sodium
nitrite/kg/day for the high-dose group) (Vorhees et al. 1984). There were no treatment-related effects on
preweaning behavior that included surface righting, pivoting, negative geotaxis, or auditory startle and no
effects on postweaning survival, body weight, or most behavioral indices, with the exception of decreased
open-field behavior on days 40–45 in groups from dams exposed to 0.0125 or 0.05% (but not 0.025%)
sodium nitrite in the diet.
3.2.2.7 Cancer
Human Data. Numerous studies are available in which the carcinogenicity of ingested nitrate and nitrite
in humans was assessed. A comprehensive review of the cancer epidemiology studies of nitrate and
nitrite, published up to approximately 2007, is provided in IARC (2010). Up to that point, most studies
employed ecological designs and fewer case-control or cohort studies were available on cancers other
than gastrointestinal cancers. Since then, several cohort and case-control studies have been reported that
examine a variety of different cancer types (Aschebrook-Kilfoy et al. 2011, 2013a, 2013b; DellaValle et
al. 2013; Espejo-Herrera et al. 2015, 2016a, 2016b; Inoue-Choi et al. 2012, 2015; Kilfoy et al. 2010,
2011; Kim et al. 2007; Michaud et al. 2009; Ward et al. 2007, 2008; Wu et al. 2013; Yang et al. 2010;
Zeegers et al. 2006; Table 3-2). Ecological studies measure exposure and outcomes at the group level
rather than the individual level. Interpretation of outcomes of these studies is more uncertain because of
various factors that contribute to ecologic bias (group-based associations between exposure and cancer
outcomes may not apply to individuals). Ecological studies can be valuable for exploring causal
relationships when the exposures within exposure groups have low variability (homogenous), differences
in exposure are large between exposure groups, and when groups are assigned based on geography and
migration in and out of exposure areas is minimal (IARC 2010). A typical example of an ecological
design assigns group exposures based on residence within a public water supply (PWS) district, where the
average (or median) concentration of nitrate or nitrite in the PWS is the exposure metric and outcomes are
measured at the level of the PWS area (e.g., cancer incidences in two areas served by public water
supplies that have different nitrate or nitrite levels). The major limitation of this approach is that the
group-based exposure estimate may (and probably does not) apply to individuals and their cancer

81 NITRATE AND NITRITE
3. HEALTH EFFECTS
Table 3-2. Selected Cohort and Case-Control Studies Published Since 2006
Examining Possible Associations Between Nitrate and Nitrite Intake and
Cancer
Cancer
Nitrate and nitrite
Reference
type
Study design
intakes
Outcomes
a
Aschebrook-
Pancreatic
Cohort from NIH-
Quintile median
Kilfoy et al. AARP Diet and intake:
2011 Health Study, Nitrate from food:
1995–2006 34.8, 56.9, 75.0,
95.3, 150.3 mg/day;
303,156 cohort,
19.3, 29.9, 40.9,
1,728 cases
57.4,
94.8 mg/1,000 kcal
Nitrite from food: 0.8
,
1.0, 1.2, 1.2,
1.6 mg/day; 0.45,
0.57, 0.65, 0.74,
0.9 mg/1,000 kcal
Based on food
frequency
questionnaire,
24-hour recall, and
published food
nitrate and nitrite
levels
Aschebrook-
Thyroid
Cohort from
Quartile median
Kilfoy et al. Shanghai intake:
2013a Women’s Health Nitrate from food:
Study, 1996–2000 165.8, 257.8, 350.6,
506.8 mg/day;
73,317 cohort,
108.6, 164.2, 217.6,
164 cases
250.9 mg/1,000 kcal
Nitrite from food:
0.89, 1.27, 1.61,
2.14 mg/day; 0.62,
0.81, 0.95,
1.12 mg/1,000 kcal
Based on food
frequency
questionnaire,
24-hour dietary
recall, and published
food nitrate and
nitrite levels
Highest quintile vs lowest quintile:
Nitrate:
OR 1.01 (95% CI 0.85, 1.20)
Nitrite:
OR 0.92 (95% CI 0.78, 1.08)
No increased risk when accounting
for nitrite intake from plant sources,
animal sources, or processed meats
Adjustments: age, race, caloric
intake, smoking, family history of
cancer and diabet
es, BMI; intakes of
saturated fat, folate, vitamin C
Highest quartile vs lowest quartile:
Nitrate:
RR 0.93 (95% CI 0.42, 2.07)
Nitrite: RR 2.05 (95% CI 1.20, 3.51)
with total nitrite intake
RR 1.96 (95% CI 1.28, 2.99) for
nitrite intake from processed meat
Adjustments: age, caloric intake,
education, history of thyroid disease;
intakes of vitamin C, carotene, folate

82 NITRATE AND NITRITE
3. HEALTH EFFECTS
Table 3-2. Selected Cohort and Case-Control Studies Published Since 2006
Examining Possible Associations Between Nitrate and Nitrite Intake and
Cancer
Cancer
Nitrate and nitrite
Reference
type
Study design
intakes
Outcomes
a
Aschebrook-
Non-
Case-control with
Quartile median
Kilfoy et al. Hodgkin’s subjects from intake:
2013b lymphoma Nebraska
between 1999 and
Nitrate from food:
2002
22.0, 39.1, 57.5,
106.1 mg/day; 22.2,
348 cases,
38.2, 55.5, 88.3
470 controls
mg/1,000 kcal
Nitrite from food: 0.5,
0.6, 0.7, 0.9 mg/day;
0.49, 0.61, 0.71,
0.86 mg/1,000 kcal
Based on food
frequency
questionnaire and
published food
nitrate and nitrite
levels
Chiu et al.
Colon
Case-control from
Tertile median
2011 Taiwan Provincial Nitrate-nitrogen
Department of ranges in drinking
Health, 2003– water: <0.38, 0.39–
2007 0.57, ≥0.60 mg/L
(<1.67, 1.72–2.51,
3,707 cases,
≥2.64 mg nitrate/L)
3,707 controls
Based on PWS data
Highest quartile vs lowest quartile:
Nitrate:
OR 0.8 (95% CI 0.5, 1.3; p-trend
0.6)
Nitrite:
OR 1.3 (95% CI 0.8, 1.9; p-trend
0.4)
No significant associations for nitrate
or nitrite by lymphoma subtype
t(14;18)-positive or -negative)
Adjustments: sex, age, BMI, caloric
intake, education, family history of
cancer, vitamin C intake
Highest tertile vs lowest tertile:
OR 1.16 (95% CI 1.04, 1.30; p-trend
0.001)
OR 1.37 (95% CI 1.11, 1.69) with
drinking water calcium levels <34.6
mg/L
Adjustments: age, gender, marital
status, urbanization level of
residence

83 NITRATE AND NITRITE
3. HEALTH EFFECTS
Table 3-2. Selected Cohort and Case-Control Studies Published Since 2006
Examining Possible Associations Between Nitrate and Nitrite Intake and
Cancer
Cancer
Nitrate and nitrite
Reference
type
Study design
intakes
Outcomes
a
DellaValle et
al. 2013
Kidney
(RCC)
Cohort from NIH-
AARP Diet and
Health Study,
1995–2006
491,841cohort,
488 cases
Quintile median
intake:
Nitrate intake from
food: 19.3, 40.9, and
94.8 mg/1,000 kcal,
for quintiles 1, 3, and
5, respectively
Highest quintile vs lowest quintile:
Nitrate:
No increased risk (HR 0.98; 95% CI
0.84, 1.14 for total RCC)
Nitrite:
No increased risk for total nitrite (HR
1.02; 95% CI 0.87, 1.19 for total
Nitrite intake from
food: 0.5, 0.7, and
0.9 mg/1,000 kcal,
for quintiles 1, 3, and
5, respectively
Based on food
frequency
questionnaire and
published food
nitrate and nitrite
levels
RCC) or nitrite from plant sources
(HR 0.89; 95% CI 0.76, 1.04 for total
RCC)
Increased risk for nitrite from animal
sources (HR 1.28; 95% CI 1.10,
1.49; p-trend <0.01 for total RCC),
nitrite from processed meat sources
(HR 1.16; 95% CI 1.00, 1.35; p-
trend
0.04 for total RCC), nitrite from
animal sources other than
processed meat (HR 1.23 (95% CI
1.06, 1.43; p-trend 0.02 for total
RCC), nitrate and nitrite from
processed meat sources (HR 1.17;
95% CI 1.00, 1.37; p-trend 0.03 for
total RCC)
Risk of RCC mainly associated with
clear cell histological subtype (e.g.,
HR 1.68; 95% CI 1.25, 2.27; p-trend
<0.01 for nitrite from animal sources
and clear cell subtype)
Adjustments: age, sex, caloric
intake, race, smoking, family history
of cancer, BMI, alcohol intake,
education; history of hypertension,
diabetes

84
2015
NITRATE AND NITRITE
3. HEALTH EFFECTS
Table 3-2. Selected Cohort and Case-Control Studies Published Since 2006
Examining Possible Associations Between Nitrate and Nitrite Intake and
Cancer
Cancer
Nitrate and nitrite
Reference
type
Study design
intakes
Outcomes
a
Espejo-
Bladder
Case-control.
Herrera et al.
Spain, 1998–2001
556 controls,
531 cases
Espejo-
Breast
Case-control.
Herrera et al.
Spain, 2008–2013
2016b
1,520 controls,
1,245 cases
Average residential
No associations between risk of
ranges in drinking bladder cancer and average nitrate
water by tertiles: ≤5 level (OR 1.09; 95% CI 0.63, 1.87)
mg/L, >5–10 mg/L, for highest versus lowest level
>10 mg/L
For subjects with longest exposure
Based on historical duration (>20 years) to highest
records of nitrate levels (>9.5 mg/L), OR=1.42; 95%
levels in municipal CI 0.989 2.26
water sources
Stratification by intake of vitamin C,
vitamin E, meat, and gastric ulcer
did not modify the results
Adjustments: age, sex and area of
residence smoking status, NSAIDs
use, night-time urinary frequency,
time working in farm/agriculture
activities, tap water and vitamin C
daily intake, urinary infections (ever)
Average waterborne
No associations between dietary
ingested nitrate
nitrate intake or waterborne ingested
ranged from 2.9 ±1.9
nitrate and risk of breast cancer
mg/day (mean ± SD) overall, but increased risk (OR 1.64;
to 13.5 ±7.5 mg/day 95% CI 1.08, 2.49) among
postmenopausal women with both
Based on historical
high waterborne nitrate intake (>6
records of nitrate
mg/day) and high red meat intake
levels in municipal
(≥20 g/day)
water sources and
monitoring of other
Adjustments: study area, age,
sources
education, BMI, family history of
breast cancer, age at first birth, age
Average dietary
at menopause, oral contraceptives
nitrate intake ranged
use, energy intake
from 88.5±48.7
mg/day to 154±87.8
mg/day
Based on food
frequency
questionnaire and
published food
nitrate and nitrite
levels

85 NITRATE AND NITRITE
3. HEALTH EFFECTS
Table 3-2. Selected Cohort and Case-Control Studies Published Since 2006
Examining Possible Associations Between Nitrate and Nitrite Intake and
Cancer
Cancer
Nitrate and nitrite
Reference
type
Study design
intakes
Outcomes
a
Espejo-
Colorectal
Case-control.
Herrera et al.
Spain and Italy,
2016a 2008–2013
3,530 controls,
1,869 cases
(1,285 colon; 557
rectal)
Average waterborne
ingested nitrate
ranged from 3.4±3.3
mg/day to 19.7±22.6
mg/day
Tertiles: ≤5, <5–10,
>10 mg/day
Based on historical
records of nitrate
levels in municipal
water sources and
monitoring of other
sources
Mean dietary nitrate
intake was 118±72
mg/day overall
(102±70.5 mg/day
from vegetables and
6.2±3.3 mg/day from
animal sources)
Tertiles: <4.5, 4.5–
6.8, >6.8 mg/day
Based on food
frequency
questionnaire and
published food
nitrate and nitrite
levels
For highest versus lowest
waterborne nitrate intake:
OR 1.49 (95% CI 1.24, 1.78) for
colorectal cancer
OR 1.52 (95% CI 1.24, 1.86) for
colon cancer
OR 1.62 (95% CI 1.23, 2.14) for
rectal cancer
For risk of rectal cancer among
subjects with dietary intake of nitrate
from animal sources:
OR 1.59 (95% CI 1.22, 2.06) for mid
tertile
OR 1.55 (95% CI 1.17, 2.05) for
highest tertile
Greater risk among men than
women
Adjustments: sex, age, education,
physical activity, BMI, family history
of colorectal cancer, NSAIDs use,
energy intake, oral contraceptives
use

86 NITRATE AND NITRITE
3. HEALTH EFFECTS
Table 3-2. Selected Cohort and Case-Control Studies Published Since 2006
Examining Possible Associations Between Nitrate and Nitrite Intake and
Cancer
Cancer
Nitrate and nitrite
Reference
type
Study design
intakes
Outcomes
a
Inoue-Choi et
Breast
Cohort of post-
al. 2012 menopausal
women from Iowa
Women’s Health
Study, 1989–2008
34,388 cohort,
2,875 cases
Quintile median
nitrate intake from
drinking water: 1.6,
4.1, 9.4, 21.2, and
57.8 mg/2 L
Based on historical
database of Iowa
municipal water
supplies
Quintile median
nitrate intake from
food: 49.3, 78.7,
106.1, 140.2, 209.9
mg/day
Quintile median
nitrite intake from
food: 0.6, 0.9, 1.1,
1.4, 1.8 mg/day
Based on food
frequency
questionnaire and
published food
nitrate and nitrite
levels
Highest quintile vs lowest quintile:
Overall, no increased risk of breast
cancer with intake of nitrate and/or
nitrite from diet and/or drinking water
Significant trend for increasing HR
with increasing nitrite intake
HR 1.40 (95% CI 1.05, 1.87) for
nitrate and folate ≥400 µg/day
HR 1.0 95% CI
(0.79, 125) for nitrate
and folate <400 µg/day
Adjustments: age; caloric intake;
BMI; waist-hip ratio; education;
smoking; physical activity level;
alcohol intake; family history of
breast cancer; age at menopause;
age at first live birth; estrogen use;
intakes of alcohol, vitamin C, vitamin
E, flavonoids, cruciferae, red meat

87 NITRATE AND NITRITE
3. HEALTH EFFECTS
Table 3-2. Selected Cohort and Case-Control Studies Published Since 2006
Examining Possible Associations Between Nitrate and Nitrite Intake and
Cancer
Cancer
Nitrate and nitrite
Reference
type
Study design
intakes
Outcomes
a
Inoue-Choi et
Ovarian
Cohort of post-
Quartile median
al. 2015 menopausal nitrate levels in
women from Iowa drinking water: 0.31,
Women’s Health 0.75, 1.68, 3.81 mg
Study, 1986–2010 nitrate-nitrogen/L
(1.36, 3.3, 7.39,
28,555 cohort,
16.76 mg nitrate/L)
315 cases
Historical database
of Iowa municipal
water supplies
Quintile median
nitrate intake from
food: 49.5, 78.9,
106.2, 140.2, 209.2
mg/day
Quintile medium
nitrite dietary intake:
0.7, 0.9, 1.1, 1.4, 1.8
mg/day
Based on food
frequency
questionnaire and
published food
nitrate and nitrite
levels
Kilfoy et al.
Non-
Case-control of
Quartile ranges:
2010 Hodgkin’s women in
lymphoma Connecticut Nitrate from food:
between 1995 and
<63.9, 63.9 to <93.0,
2001 93.0 to <140.5,
≥140.5 mg/day
594 cases,
710 controls Nitrite from food:
<0.77, 0.77 to <0.99,
0.99 to <1.32, ≥1.32
mg/day
Based on food
frequency
questionnaire and
published food
nitrate and nitrite
levels
Highest quartile/quintile vs lowest
quartile/quintile:
HR 2.03 (95% CI 1.22, 3.38) for
highest quartile of nitrate in public
drinking water; association was
stronger when vitamin C intake was
≤190 mg/day and when red meat
servings exceeded 5 per week
Overall, no increased risk of ovarian
cancer with total intake of nitrate or
nitrite.
Adjustments: age, BMI, family
history of ovarian cancer, number of
live births, age at menarche, age at
menopause, age at first live birth,
oral contraceptive use, estrogen
use, history of unilateral
oophorectomy, and/or total energy
intake
Highest quartile vs lowest quartile:
Overall non-Hodgkin’s lymphoma:
OR 0.9 (95% CI 0.6, 1.2) for nitrate
OR 1.4 (95% CI 0.9, 2.2) for nitrite
Significant trend (p=0.04) for
follicular lymphoma with increasing
nitrate intake
OR 2.3 (95% CI 1.1, 4.9; p-trend
0.008) for follicular lymphoma with
nitrite intake
Adjustments: age; family history of
cancer; calories; intakes of vitamins
C, vitamin E, protein

88 NITRATE AND NITRITE
3. HEALTH EFFECTS
Table 3-2. Selected Cohort and Case-Control Studies Published Since 2006
Examining Possible Associations Between Nitrate and Nitrite Intake and
Cancer
Cancer
Nitrate and nitrite
Reference
type
Study design
intakes
Outcomes
a
Kilfoy et al.
2011
Thyroid
Cohort from NIH-
AARP Diet and
Health Study,
1995–1996 with
Quintile median
ranges:
Nitrate from food:
Highest quintile versus lowest
quintile:
Nitrate:
average of 7 years
of follow-up
490,194 cohort
(292,125 men;
198,069 women),
370 cases (170
men; 200 women)
29.6, 49.8, 70.2,
100.9, 166.8 mg/day
(19.4, 29.9, 40.9,
57.4,
94.8 mg/1,000 kcal)
Nitrite from food: 0.
6,
0.9, 1.1, 1.4, 1.9
mg/day (0.5, 0.6,
0.7, 0.7,
Men: RR 2.28 (95% CI 1.29, 4.04; p-
trend <0.01) for thyroid cancer
RR 2.10 (95% CI 1.09, 4.05; p-trend
0.05) for papillary thyroid cancer
RR 3.42 (95% CI 1.03, 11.4; p-
trend
<0.01) for follicular thyroid cancer
Women: RR 0.76 (95% CI 0.48,
1.10) for thyroid cancer
Nitrite:
0.9 mg/1,000 kcal)
Based on food
frequency
questionnaire and
published food
nitrate and nitrite
Men: RR 1.36 (95% CI 0.78, 2.37)
for thyroid cancer
RR 2.74 (95% CI 0.86, 8.77; p-trend
0.04) for follicular thyroid cancer
Women: RR 1.19 (95% CI 0.71,
1.98) for thyroid cancer
levels
Adjustments: sex; age; smoking
status; race; physical activity;
alcohol use; BMI; caloric intake;
education; family history of cancer;
intakes of vitamin C, beta-carotene,
folate
Kim et al.
2007
Stomach
Case-control with
subjects from two
Korean hospitals,
1997–1998
136 controls,
136 cases
Tertile median
values:
Nitrate from food:
240, 458, and 811
mg/day
Based on food
Highest tertile vs lowest tertile:
OR 1.13 (95% CI 0.42, 3.06)
OR 2.78 (95% CI 1.01, 7.67) for
nitrate/86.7 mg/mg vitamin E
OR 3.37 (95% CI 1.28, 8.87) for
nitrate/2.47 mg/µg folate
frequency
questionnaire and
published food
nitrate and nitrite
levels
Adjustments: age; sex; SES; family
history of gastric cancer; refrigerator
use; Helicobacter pylori infection;
intakes of charcoal grilled beef,
Korean cabbage kimichi, Dongchimi
,
spinach, garlic, mushroom, salty
foods

89 NITRATE AND NITRITE
3. HEALTH EFFECTS
Table 3-2. Selected Cohort and Case-Control Studies Published Since 2006
Examining Possible Associations Between Nitrate and Nitrite Intake and
Cancer
Cancer
Nitrate and nitrite
Reference
type
Study design
intakes
Outcomes
a
McElroy et
al. 2008
Colorectal
Wisconsin, United
States, 1990–
2001
Quintile cutoff range:
Nitrate in water:
Highest quintile versus lowest
quintile:
4,297 controls,
<0.5, 0.5–1.9, 2.0–
5.9, 6.0–9.9,
OR 1.52 (95% CI 0.95, 2.44) for all
colon cancer sites
1,476 cases,
all females
≥10 mg/L
Based on
OR 2.91 (95% CI 1.52, 5.56) for all
proximal colon sites
groundwater nitrate
data and spatial
interpolation to
individual residences
Adjustments: age
Michaud et
al. 2009
Brain
(glioma)
Combined
analysis of cohorts
from NHS I
(1980–2004),
NHS II (1991–
2005), and HPFS
(1986–2004)
230,655 cohort,
335 cases
Quintile cutoff
ranges, based on
baseline values:
Nitrate from food:
43–205 mg/day
Nitrite from food:
1.1–2.4 mg/day
NDMA from food:
0.02–0.09 mg/day
Highest tertile vs lowest tertile:
Nitrate: RR 1.02 (95% CI 0.66, 1.58)
Nitrite: RR 1.26 (95% CI 0.89, 1.79)
NDMA: RR 0.88 (95% CI: 0.57,
1.36)
Processed meat: RR 0.92 (95% CI
0.48, 1.77)
No effect of vitamin C, vitamin E, or
Based on food
frequency
questionnaire and
published food
nitrate and nitrite
ferric-reducing ability of plasma
Adjustments: age, caloric intake
levels

90 NITRATE AND NITRITE
3. HEALTH EFFECTS
Table 3-2. Selected Cohort and Case-Control Studies Published Since 2006
Examining Possible Associations Between Nitrate and Nitrite Intake and
Cancer
Cancer
Nitrate and nitrite
Reference
type
Study design
intakes
Outcomes
a
Ward et al.
Kidney
Case-control,
2007 (RCC) Iowa, United
States, 1986–
1989
2,434 controls,
406 cases
Quartile cutoff
ranges:
Nitrate from food:
<59.32, 59.32–
86.62, 86.63–
122.00,
≥122.01 mg/day
Nitrite from food:
<0.70, 0.70–0.93,
0.94–1.25,
≥1.26 mg/day
Nitrate in water:
<0.62, 0.62–<1.27,
1.27–≤2.78,
≥2.78 mg/L
Based on food
frequency
questionnaire and
published food
nitrate and nitrite
levels, and PWS
data
Highest quartile versus lowest
quartile:
Nitrate: OR 0.41 (
95% CI 0.28, 0.60)
for dietary nitrate
OR 0.89 (95% CI 0.57, 1.39) for
nitrate in water
Nitrite: OR 0.82 (95% CI 0.50, 1.33)
OR 1.00 (95% CI 0.63, 1.59) for
nitrite from animal sources
OR 1.91 (95% CI 1.04, 3.51) for red
meat intake ≥1.2 servings/day and
PWS nitrate >5 mg/L for >10 years
Adjustments: age, sex, BMI, caloric
intake, intakes of sodium and fat

91 NITRATE AND NITRITE
3. HEALTH EFFECTS
Table 3-2. Selected Cohort and Case-Control Studies Published Since 2006
Examining Possible Associations Between Nitrate and Nitrite Intake and
Cancer
Cancer
Nitrate and nitrite
Reference
type
Study design
intakes
Outcomes
a
Ward et al.
2008
Esophagus,
Stomach
Case-control,
Nebraska, United
States, 1988–
Quartile cutoff
ranges:
Highest quartile versus lowest
quartile:
1993
321 controls,
79 stomach
cases,
Nitrate-nitrogen in
water: <2.45, 2.45–
<2.58, 2.58–4.32,
>4.32 mg/L (<10.78,
10.78–<11.35,
Nitrate in water: OR 1.2 (95% CI 0.5,
2.7) for stomach cancer
OR 1.2 (95% CI 0.6, 2.7) for
esophageal cancer
84 esophagus
cases
11.35–19.01,
>19.01 mg nitrate/L)
Dietary nitrate from
plant sources: <16.9,
16.9–<26.2, 26.2–
Nitrate from plant sources: OR 1.6
(95% CI 0.7, 3.6) for stomach cancer
OR 0.8 (95% CI 0.3, 1.8) for
esophageal cancer
Nitrate and nitrite from animal
<38.8, >38.8 mg/day
nitrate-nitrogen
(<74.4, 74.4–
<115.3,
115.3–<170.7,
>170.7 mg
nitrate/day)
Nitrate and nitrite
sources: OR 1.6 (95% CI 0.7, 3.7)
for stomach cancer
OR 2.2 (95% CI 0.9, 5.7; p-trend
0.015) for esophageal cancer
Adjustments: year of birth; sex; BMI;
smoking; alcohol; caloric intake;
intakes of vitamin A, folate,
from animal sources:
<3.8, 3.8–<5.7, 5.7–
riboflavin, zinc, protein, carbohydrate
<8.3, ≥8.3 mg/day
Based on food
frequency
questionnaire and
published food
nitrate and nitrite
levels, and PWS
data
Wu et al.
2013
Prostate
Case-control with
subjects from
HPFS (1997–
2005)
630 controls,
630 cases
Quartile median
range:
Plasma nitrate
(cases): 29.39, and
51.47 µmol/L (1.82
and 3.19 mg/L)
Adjusted RR not significant; no
significant trend
RR 0.99 (95% CI 0.68, 1.48) for
highest plasma nitrate quintile
Adjustments: age, BMI, caloric
intake, time of blood draw, hours
since last meal before blood draw
year of blood draw, family history of
prostate cancer, smoking, history of
hypertension, history of diabetes,
physical activity

92 NITRATE AND NITRITE
3. HEALTH EFFECTS
Table 3-2. Selected Cohort and Case-Control Studies Published Since 2006
Examining Possible Associations Between Nitrate and Nitrite Intake and
Cancer
Cancer
Nitrate and nitrite
Reference
type
Study design
intakes
Outcomes
a
Yang et al.
2010
Breast
Case-control.
Seoul, South
Korea, 2004–2006
362 controls,
362 cases
Quintile median
nitrate intake from
food: 179.4, 299.7,
372.1, 492.5,
716.1 mg/day
Based on food
Adjusted OR not significant for
dietary nitrate, no significant trend.
Significant trend for increasing OR
with increasing nitrate/folate ratio;
OR significantly elevated in highest
nitrate/folate quintile.
frequency
questionnaire and
published food
nitrate levels
OR 1.54 (95% CI 0.88, 2.70) for
nitrate
OR 2.03 (95% CI 1.16, 3.54) for
nitrate/folate intake ratio (2.10)
Adjustments: age, education,
physical activity, family history of
breast cancer, parity, breast feeding,
menopause, oral contraceptive use;
intakes of soy protein, mushroom,
fat
Zeegers et
al. 2006
Bladder
Cohort from
Netherlands
Cohort Study,
1986–1996
Quintile median
nitrate intakes:
Nitrate from food:
Adjusted RR not significant for
nitrate in food or water; no significant
trend
cohort 120,852,
889 cases,
4,441 subcohort
57.4, 78.6, 97.8,
119.5, 158.9 mg/day
Nitrate from water:
Highest quintile vs lowest quintile:
RR 1.06 (95% CI 0.81, 1.37) for
nitrate from food
0.5, 1.4, 3.4, 5.6,
10.6 mg/day RR 1.06 (95% CI 0.82, 1.37) for
nitrate from water
Based on food
frequency
questionnaire and
published food
nitrate and nitrite
levels, and
RR 1.09 (95% CI
0.84, 1.42) for total
nitrate intake
Adjustments: age, sex, smoking
PWS data
a
Risk estimates (95% confidence limits)
AARP = American Association of Retired Persons; BMI = body mass index; HPFS = Health Professionals Follow-Up
Study; HR = multivariate hazard ratio; NDMA = nitrosodimethylamine; NIH = National Institutes of Health;
NHS = Nurses’ Health Study; NSAIDs = non-steroidal anti-inflammatories; OR = odds ratio; PWS = public water
supply; RCC = renal cell carcimoma; RR = relative risk; SD = standard deviation; SES = socioeconomic status
93 NITRATE AND NITRITE
3. HEALTH EFFECTS
outcomes. Exposure misclassification can occur for various reasons including dietary factors that
contribute to variability in dose of nitrosation precursors (e.g., nitrate or nitrite in fish, meat, and
vegetables) and nitrosatable compounds; consumption of antioxidants that can inhibit nitrosation (e.g.,
vitamin C, flavenoids, polyphenols); migration in and out of the PWS district; and ingestion of other
water sources (e.g., bottled water). Estimates of exposure from drinking water can be made at the
individual subject level. This can be accomplished with surveys of the individual’s residence history and
consumption patterns (e.g., percentage of drinking water consumed from the PWS and other sources, such
as bottled water), along with data on nitrate concentrations in the water supply (Inoue-Choi et al. 2012).
Dietary surveys (e.g., food frequency questionnaires, 24-hour recalls), coupled with data from residue
monitoring studies of market basket foods, can be used to estimate individual exposures to nitrosation
precursors in foods. However, in this approach, exposure misclassification can occur as a result of
ingestion of nitrosation precursors from non-market basket foods. Also, the diet survey is typically cross-
sectional, even in longitudinal studies, and results may not accurately reflect the average diets during the
entire follow-up period. Exposure misclassification can also occur in studies that examine associations at
the individual level. However, in these studies, exposure misclassification is likely to be non-differential
or independent of cancer status. As a result, exposure comparisons (exposed versus unexposed) would
tend to be biased towards the null if there truly is an effect of the exposure on cancer outcome, and if
more than two levels of exposure are being evaluated (e.g., high, low, versus no exposure), then the bias
can be in either direction for the middle levels of exposure and tend to be biased towards the null at the
highest level so that exposure-response relationships are distorted (e.g., the risk would be attenuated or
fall at the highest levels of exposure because of this bias). Most of the nitrate and nitrite ingested comes
from the diet (Zeegers et al. 2006); therefore, studies that quantify exposure only from drinking water are
weak designs for assessing cancer risk unless the water supply is extraordinarily contaminated (>20 mg
nitrate/L). Some studies have employed biomarkers (blood, plasma, saliva, or urine) as exposure metrics
(Armijo et al. 1981; Cuello et al. 1976; Forman et al. 1985; Joossens et al. 1996; Kamiyama et al. 1987;
Knight et al. 1990; Lin et al. 2003; Lu et al. 1986; Sierra et al. 1993; Tsugane et al. 1992; Wu et al. 1993,
2013). Biomarkers can provide more accurate estimates of the steady-state levels of nitrate (or nitrite) in
an individual; however, they may not reflect the cumulative absorbed dose or the dose of nitrosation
products that may contribute to cancers (e.g., N-nitrosodimethylamine) (Zeilmaker et al. 2010a). An
additional uncertainty that applies to all studies described in this summary is that cancer risk may be mis-
attributed to nitrite (or nitrosation precursors) as a result of other factors that contribute cancer risk that
co-vary with exposure to nitrite or nitrite precursors. These may include other carcinogens in drinking
water or diet. However, unless these risk factors have extremely strong associations with exposures to
nitrate or nitrite (or nitrosation precursors), confounding from these factors is unlikely to be a major
94 NITRATE AND NITRITE
3. HEALTH EFFECTS
source of uncertainty in interpretation of cancer risk estimates. One potentially important class of
confounders is anti-oxidants, which can interfere with nitrosation of dietary amines and, thereby, the
mode of carcinogenicity of nitrite, and may also interfere with other carcinogenic process that involve
reactive intermediates. In the discussions of individual studies, the terms “statistically significant” refer
to relative risks that are estimated to be ≥1 or trends that were reported by the investigators to be
statistically significant, typically p<0.05).
In general, outcomes of cohort and case-control studies have found no or weak associations between
nitrate intakes and cancer in humans, with stronger associations for exposures to nitrite or intake of high-
nitrite foods such as cured meat (Aschebrook et al. 2013; DellaValle et al. 2013; IARC 2010; Inoue-Choi
et al. 2012). Mechanistically, this outcome is consistent with nitrite being a reactive intermediate in the
cancer mode of action of nitrate (see Section 3.5.2).
Studies that form the basis for evidence of carcinogenicity of nitrate or nitrite are briefly described below.
Most of these studies are described in greater detail in IARC (2010). Studies published since IARC
(2010) are summarized in Table 3-2. Studies included in Table 3-2 estimated nitrate or nitrite intakes
from dietary survey instruments of individuals, in some cases, supplemented with estimates from drinking
water based on well water or PWS data and geographic location of the residence, or with biomarkers of
exposure. The table summarizes major features of the design of each study and the major outcomes.
Complete details of the outcomes for various design strata can be obtained from the cited references.
This summary of carcinogenicity of nitrate and nitrite in humans is intentionally biased for the sake of
brevity, in that it is restricted to case-control and cohort studies and emphasizes studies that have found
associations between nitrate or nitrite and cancer, while most studies that found no associations are not
described. Descriptions of important ecological studies and negative outcome studies can be found in
IARC (2010). In the summary below, reported risks are adjusted for co-variables, which differed across
studies. Most studies adjusted for age, sex, body mass index (BMI), caloric intake, family history of
cancer, smoking, and alcohol consumption. Some studies also adjusted for socioeconomic status,
education, and various dietary intakes (e.g., vitamin C, vitamin E, flavenoids, folate), as well as cancer
specific-adjustments (e.g., reproductive history in breast cancer studies). Estimates of risk for studies not
included in Table 3-2 were those reported in IARC (2010) where they were expressed as relative risk
(RR) without specification of the actual risk metric estimated in the study. Risk metrics reported in
Table 3-2 are ORs for case-control studies and RR or hazard ratio (HR) for cohort studies.
95 NITRATE AND NITRITE
3. HEALTH EFFECTS
Gastrointestinal Cancer. Associations between intake of nitrite and a variety of cancer types has been
studied; however, the strongest and most consistent evidence for carcinogenicity of nitrite derives from
studies of gastrointestinal cancers and, in particular, gastric cancer (Buiatti et al. 1990; Engel et al. 2003;
La Vecchia et al. 1994, 1997; Mayne et al. 2001; Palli et al. 2001; Risch et al. 1985; Rogers et al. 1995;
Ward et al. 2007, 2008). In general, these studies have found significant positive trends for cancer risk
(risk increases with increasing intake), and three studies found elevated cancer risk (Engel et al. 2003;
Kim et al. 2007; Risch et al. 1985). In the Risch et al. (1985) case-control study (246 cases,
246 controls), relative risk was 1.71 (95% CI: 1.24, 2.37) for a nitrite intake of 1 mg/day. In another case-
control study (369 cases, 695 controls) (Engel et al. 2003; Mayne et al. 2001), risk for stomach cancer
(non-cardia) was elevated at nitrite intakes ≥6 mg/day (OR 2.5, 95% CI: 1.4, 4.3). Risk increased with
decreasing vitamin C intake (RR 2.95, 95% CI: 1.90, 4.59). Additional support for antioxidants as effect
modifiers comes from a case-control study (136 cases, 136 controls) in which stomach cancer risk
increased in association with increasing ratio of nitrate to antioxidants in the diet (e.g., vitamin C, vitamin
E, folate) (Kim et al. 2007). Risk (OR) at the highest nitrate/vitamin E ratio (86.7 mg nitrate/mg vitamin
E) was 2.78 (95% CI: 1.01, 7.67). At the highest nitrate/folate ratio (2.47 mg nitrate/µg folate), an OR of
3.37 (95% CI: 1.28, 8.87) was determined.
Associations between exposure to nitrate or nitrite and colorectal cancer have been studied in cohort and
case-control studies (Chiu et al. 2011; De Roos et al. 2003; Knekt et al. 1999; Weyer et al. 2001). The
largest of the case-control studies (3,707 cases, 3,707 controls) (Chiu et al. 2011) found a significant
positive trend (chi-square for trend =13.26, p=0.001) for mortality from colon cancer with increasing
nitrate levels in drinking water (OR 1.16, 95% CI: 1.04, 1.30 at nitrate-nitrogen levels >0.6 mg/L;
>2.65 mg nitrate/L). Risks were higher in a stratum exposed to drinking water that had a calcium level
>34.6 mg/L (OR 1.37, 95% CI: 1.11, 1.69 for nitrate <2.64 mg/L). The De Roos et al. (2003) case-
control study (685 cases of colon cancer, 655 cases of rectal cancer, 2,434 controls) found elevated risk of
colon (RR 1.5, 95% CI: 1.0, 2.1) and rectal cancer (RR 1.7, 95% CI: 1.1, 2.5) at a dietary nitrite intake
>1.26 mg/day. Risk of colon cancer was higher in a stratum exposed to nitrate in drinking water at levels
>5 mg/L in combination with a low vitamin C intake (RR 2.0, 95% CI: 1.2, 3.3). Two meta-analyses
reported in IARC (2010) concluded that ingestion of cured meats was associated with increased risk of
colorectal cancer (Norat et al. 2002; Sandhu et al. 2001).
Central Nervous System Cancer. Cancer of the central nervous system has been studied extensively in
case-control studies (IARC 2010). Some studies found significant positive trends with nitrite and/or
cured meat intake; elevated risk was reported in a few studies (Blowers et al. 1997; Giles et al. 1994, Lee
96 NITRATE AND NITRITE
3. HEALTH EFFECTS
et al. 1997; Mueller et al. 2004; Pogoda and Preston-Martin 2001a, 2001b; Preston-Martin et al. 1996).
Risks increased with higher nitrite intake or cured meat/antioxidant ratios (Blowers et al. 1997; Preston-
Martin et al. 1996). The study of Preston-Martin and coworkers (Pogoda and Preston-Martin 2001a,
2001b; Preston-Martin et al. 1996) included 540 cases and 801 controls. Significantly increased risk (OR
3.0, 95% CI: 1.2, 7.9) was observed for central nervous system cancers (brain, cranial nerves, or cranial
meninges) in children of mothers reporting a nitrite intake >3.0 mg/day from cured meat during
pregnancy. The Mueller et al. (2004) case-control study (1,218 cases, 2,223 controls) found elevated risk
(RR 5.7, 95% CI: 1.2, 27.2) for astroglial tumors in children in association with maternal exposure to
drinking water to nitrite concentrations ≥5 mg/L during pregnancy. Risks for other types of brain tumors
were not elevated. A smaller case-control study (94 cases, 94 controls) found elevated risk of glioma in
women (with trend p=0.07) in association with intake of nitrite from cured meat (RR 2.1, 95% CI: 1.0,
4.6). Results of meta-analyses of brain cancer studies also support associations between intake of cured
meat during pregnancy and brain tumors in children and cured meat ingestion and brain tumors in adults
(Huncharek and Kupelnick 2004; Huncharek et al. 2003). A large cohort study (230,655 subjects,
335 cases) of associations between intakes of nitrate, nitrite, and nitrosodimethylamine (NDMA) and
glioma in adults did not find significant trends or elevated risk for glioma (Michaud et al. 2009,
Table 3-2).
Urinary Tract Cancer. Cancer of the urinary tract has been studied in several case-control and large
cohort studies (DellaValle et al. 2013; Espejo-Herrera et al. 2015; IARC 2010; Ward et al. 2007, Zeegers
et al. 2006). Positive trends for risk or elevated risk were found in some studies (DellaValle et al. 2013;
Ward et al. 2007; Wilkens et al. 1996). In the Wilkens et al. (1996) case-control study (272 cases,
522 controls), risk was elevated (trend p=0.05) in association with dietary nitrite intake (RR 2.0, 95% CI:
1.0, 4.0). In the Ward et al. (2007) case-control study (406 cases, 2,434 controls), risk of kidney cancer
was elevated in the strata who consumed >1.2 servings of red meat/day and who resided for >10 years in
a PWS district that had nitrate concentrations >5 mg/L (OR 1.91, 95% CI: 1.04, 3.51; see Table 3-2). A
large cohort study (491,841 subjects, 488 cases) found a significant positive trend and elevated risk for
renal cell carcinoma in association with nitrite intake from animal sources (HR 1.28, 95% CI: 1.10, 1.49
for renal cell carcinoma; HR 1.68, 95% CI: 1.25, 2.27 for clear cell carcinoma, both at 0.9 mg
nitrite/1,000 kcal) (DellaValle et al. 2013). The Zeegers et al. (2006) cohort study (120,852 subjects,
889 cases) found no association between bladder cancer and intake of nitrate from food or drinking water.
Wang et al. (2012) evaluated possible association between nitrate in drinking water and risk of bladder
cancer in a meta-analysis that included results from one ecological study (Morales et al. 1993), two cohort
studies (Weyer et al. 2001; Zeegers et al. 2006), and two case-control studies (Chiu et al.2007; Ward et al.
97 NITRATE AND NITRITE
3. HEALTH EFFECTS
2003) and found no evidence that nitrate in the drinking water was associated with risk of bladder cancer
(combined RR 1.27; 95% CI 0.75, 2.15) based on data for highest nitrate levels reported relative to
reference values from each study.
Reproductive Organ Cancer. A small number of case-control and cohort studies have examined
associations between exposure to nitrate or nitrite and cancers of breast, ovary, uterus, prostate, and testis
(Barbone et al. 1993; IARC 2010; Espejo-Herrera et al. 2016b; Inoue-Choi et al. 2012, 2015; Moller
1997;Wu et al. 2013; Yang et al. 2010). A cohort study of post-menopausal women (34,388 subjects,
2,875 cases) found a significant positive trend (p=0.04) and elevated risk (HR 1.40, 95% CI: 1.05, 1.87)
for breast cancer in association with consumption of public drinking water at ≥33.5 mg nitrate/2 L
(median 57.8 mg nitrate/2 L) among women who consumed folate at rates ≥400 µg/day; risk was not
elevated among those women who ingested folate at <400 µg/day (Inoue-Choi et al. 2012). Similarly
increased risk (HR 1.38, 95% CI: 1.05, 1.82) was noted for private well users who ingested folate at
>400 µg/day when compared to the lowest quintile of users of the public drinking water sources who
ingested folate at >400 µg/day. In contrast, Yang et al. (2010) reported elevated risk for breast cancer in
association with increasing dietary nitrate/folate ratio, with significantly elevated risk (OR 2.03, 95% CI:
1.16, 3.54) at nitrate/folate ratios in the range of 1.79–8.19. The contrasting effects of folate in these two
studies may reflect dose-dependent effect modification: an antioxidant effect at lower folate intakes and a
tumor promoting effect of folate at higher folate intakes (Inoue-Choi et al. 2012). Inoue-Choi et al.
(2015) reported increased risk of ovarian cancer (HR 2.03; 95% CI 1.22, 3.38) among subjects with
public water containing ≥2.98 mg nitrate-nitrogen/L (≥13.1 mg nitrate/L) in a cohort study of
28,555 post- menopausal women (315 ovarian cancer cases) in the Iowa Women’s Health Study.
Associations were stronger when vitamin C intake was ≤190 mg/day and when red meat servings
exceeded five per week. Espejo-Herrera et al. (2016b) reported increased risk (OR 1.64; 95% CI 1.08,
2.49) of breast cancer among postmenopausal women with both high waterborne nitrate intake
(>6 mg/day) and high red meat intake (≥20 g/day) in a case control study in Spain (1,245 cases,
1,520 controls). A case-control study of prostate cancer (630 cases, 630 controls) did not find significant
associations between prostate cancer risk and plasma nitrate concentrations (1.8–3.8 mg/L) (Wu et al.
2013). In the Moller (1997) case-control study (514 cases, 720 controls), elevated risk of testicular cancer
(OR 1.51, 95% CI: 1.03, 2.20) was found among men who had lived in areas during childhood with
drinking water containing >25 mg nitrate/L. Barbone et al. (1993) conducted a case-control study of
endometrial cancer (168 cases, 334 controls) and found a negative trend for risk (risk decreased with
increasing dietary nitrate intake).
98 NITRATE AND NITRITE
3. HEALTH EFFECTS
Reticuloendothelial Cancer. Associations between exposure to nitrate or nitrite and leukemia or non-
Hodgkin’s lymphoma have been studied in population-based case-control studies (Aschebrook-Kilfoy et
al. 2013; Chiu et al. 2008; Freedman et al. 2000; Kilfoy et al. 2010; Ward et al. 1996, 2006) and a
prospective cohort study (Weyer et al. 2001). One case-control study (181 cases, 142 controls) reported
elevated risk (OR 3.1, 95% CI: 1.7, 5.5) of non-Hodgkin’s lymphoma in association with dietary nitrite
(but not nitrate) at dietary nitrite intake >1.21 mg/day (Ward et al. 2006). Another case-control study
(156 cases, 527 controls) reported elevated risk (OR 2.0; 95% CI 1.1, 3.6) of non-Hodgkin’s lymphoma in
association with average nitrate levels ≥4 mg/L nitrate-nitrogen (17.6 mg nitrate/L) in the community
drinking water supply (Ward et al. 1996). Chiu et al. (2008) evaluated possible associations between diet
and non-Hodgkin’s lymphoma according to t(14;18) status (one of the most common chromosomal
abnormalities in non-Hodgkin’s lymphoma. Dietary factors in 60 t(14;18)-positive and 87 t(14; 18)-
negative cases were compared with 1,075 controls. The study authors reported increased risk (OR 2.8;
95% CI 1.3, 6.1) of t(14;18)-positive non-Hodgkin’s lymphoma for the highest tertile of dietary nitrite
(>1 mg/day) versus the lowest tertile (<1 mg/day). The Freedman et al. (2000) case-control study
(73 cases, 147 controls) found no association between non-Hodgkin’s lymphoma and nitrate levels in
public drinking water. Kilfoy et al. (2010) evaluated risk of non-Hodgkin’s lymphoma overall and by
histological type in relation to self-reported dietary nitrate and nitrite intake in a case-control study of
1,304 women. No significant association was found between risk of non-Hodgkin’s lymphoma overall
and dietary nitrate or nitrite. Significant positive trends were reported for follicular lymphoma and
increasing intakes of nitrate (p-trend =0.04) and nitrite (p-trend <0.01); a significant association (OR 2.3;
95% CI 1.1, 4.9) was noted for the highest nitrite intake quartile (≥1.32 mg/day). Aschebrook-Kilfoy et
al. (2013) estimated dietary intake of nitrate and nitrite intake via food frequency questionnaire among
348 non-Hodgkin’s lymphoma cases and 470 controls in Nebraska in 1999–2002 and reported
nonsignificant excess risk of non-Hodgkin’s lymphoma (OR 1.6; 95% CI 0.8, 2.9) among women in the
highest quartile of nitrite intake (median nitrite intake 0.86 mg/1,000 kcal) compared to the lowest
quartile (median nitrite intake 0.49 mg/kcal). An OR of 1.9 (95% CI 1.0, 3.4) was estimated for the
highest quartile based on nitrite intake from animal sources (median nitrite intake 0.41 mg/kcal versus
0.16 mg/kcal for the lowest quartile). There were no significant associations between estimated nitrate or
nitrite intake and risk of non-Hodgkin’s lymphoma subtypes. The Weyer et al. (2001) cohort study
(21,977 subjects, 105 cases of non-Hodgkin’s lymphoma, 94 cases of leukemia) did not find positive
associations or elevated risk of non-Hodgkin’s lymphoma or leukemia in association with dietary or
drinking water nitrate.
99 NITRATE AND NITRITE
3. HEALTH EFFECTS
Thyroid Cancer. Kilfoy et al. (2011) evaluated possible associations between dietary intake of nitrate and
nitrite and risk of thyroid cancer in a cohort of 292,125 men (170 thyroid cancer cases) and
198,069 women (200 thyroid cancer cases) from the NIH-AARP Diet and Health Study 1995–1996. The
study authors reported increased risk of thyroid cancer overall with nitrate intake among men (RR 2.28;
95% CI 1.29, 4.04; p-trend <0.01), but not women (RR 0.69; 95% CI 0.42, 1.15; p-trend 0.61). For
nitrate intake among the men, thyroid cancer risk was increased by subtype as well; RR 2.10; 95% CI
1.09, 4.05; p-trend 0.05 for papillary cancer and RR 2.74; 95% CI 0.86, 8.77; p-trend 0.04 for follicular
cancer. There were no significant associations between nitrite intake and risk of thyroid cancer among
men or women. Aschebrook-Kilfoy et al. (2013a) evaluated possible associations between dietary intake
of nitrate and nitrite and risk of thyroid cancer in a cohort of 73,317 women enrolled in the Shanghai
Women’s Health Study in 1996–2000 and followed-up for 11 years (164 thyroid cancer cases). The study
authors reported increased risk of thyroid cancer among the group with highest nitrite intake (RR 2.05;
95% CI 1.20, 3.51). The risk was strongest for nitrite intake from processed meats (RR 1.96; 95% CI
1.28, 2.99). Nitrate intake was not associated with increased risk (RR 0.93; 95% CI 0.42, 2.07). Meta-
analysis of the results from selected studies that evaluated risk of thyroid cancer with nitrate intake
(Aschebrook-Kilfoy et al. 2013a; Kilfoy et al. 2011; Ward et al. 2010) or nitrite intake (Aschebrook-
Kilfoy et al. 2013a; Kilfoy et al. 2011) indicated increased risk of thyroid cancer with nitrite intake (RR
1.48; 95% CI 1.09, 2.02), but not with nitrate intake (RR 1.36; 95% CI 0.67, 2.75) (Bahadoran et al.
2015).
Other Cancers. In general, case-control and cohort studies of cancers of larynx, liver, lung, mouth,
pancreas, or pharynx have found no consistent associations with exposure to nitrate or nitrite
(Aschebrook-Kilfoy et al. 2011; IARC 2010).
Studies of Laboratory Animals. The potential carcinogenicity of nitrate has been investigated in several
animal studies that employed the oral exposure route. Studies in which negative results were reported
include MCR-derived rats (15/sex/group) provided 5,000 mg sodium nitrate/L (3,650 mg nitrate/L) in the
drinking water for 84 weeks and sacrificed 20 weeks later (Lijinsky et al. 1973a), male white rats
provided 4,000 mg sodium nitrate in the drinking water for 273 days and sacrificed at 10 months (Pliss
and Frolov 1991), strain A male mice (n=40) provided 12,300 mg sodium nitrate/L in the drinking water
for 25 weeks and sacrificed 13 weeks later (Greenblatt and Mirvish 1973), female NMRI mice provided
1,000 mg calcium nitrate/L in the drinking water for 18 months (Mascher and Marth 1993), Fischer 344
rats (50/sex/group) fed diets containing up to 5% sodium nitrate (1,517–1,730 mg nitrate/kg/day) for
2 years (Maekawa et al. 1982), and ICR mice (10/sex/group) fed diets containing up to 5% sodium nitrate
100 NITRATE AND NITRITE
3. HEALTH EFFECTS
for 2 years (IARC 2010). In the study of Pliss and Frolov (1991) some groups of male white rats were
treated with drinking water containing 0.05% N-butyl-N-(4-hydroxybutyl)nitrosamine (BBNA, an
inducer of urinary bladder cancer in laboratory animals) for 30 days, either alone or followed by 4,000 mg
sodium nitrate/L drinking water for 273 days. The group treated with BBNA followed by sodium nitrate
exhibited a significantly increased incidence of urinary bladder carcinoma (6/20 rats versus 1/18 rats
treated with 0.05% BBNA only). These results indicate that sodium nitrate promoted BBNA-induced
bladder tumors.
The potential carcinogenicity of ingested nitrite has been investigated in numerous animal studies. Nitrite
treatment alone did not result in increased incidences of tumors in most studies. Nitrite doses (expressed
as nitrite/kg/day) reported in this Toxicological Profile for Nitrate and Nitrite were either provided by the
study authors or estimated using available body weight and oral intake data; otherwise, EPA (1988)
default reference values for body weight, food consumption, and water intake were used to calculate
doses.
NTP (2001) performed a cancer bioassay of male and female F344/N rats (50/sex/group) provided sodium
nitrite in the drinking water for 2 years at concentrations of 0, 750, 1,500, or 3,000 ppm. Author-reported
average doses were 35–130 mg sodium nitrite/kg/day (23.5–87.1 mg nitrite/kg/day) to the males and 40–
150 mg sodium nitrite/kg/day (26.8–100.5 mg nitrite/kg/day) to the females. There was no evidence of
sodium nitrite-induced forestomach neoplasms. Although the mid-dose group of female rats exhibited a
significantly increased incidence of mammary gland fibroadenoma, the incidence in the high-dose group
was not significantly different from that of controls; based on this finding and the high historical
background incidence of mammary gland fibroadenomas, the incidence in the mid-dose group was not
considered treatment related. Significantly decreased incidences of mononuclear cell leukemia were
observed in mid- and high-dose male and female rats. It was speculated that increased methemoglobin
concentrations may have played a role in the decreased incidences of mononuclear cell leukemia.
Significantly increased incidence of fibroma of the subcutis was noted in mid-dose male rats; however,
several factors (the incidence only slightly exceeded the historical range of NTP controls, lacked a dose-
response characteristic, combined incidences of fibroma or fibrosarcoma were within the historical range
for NTP controls, and fibromas and fibrosarcomas are common neoplasms in the skin of F344/N rats)
suggested that the fibroma was not related to sodium nitrite exposure. NTP (2001) concluded that there
was "no evidence of carcinogenic activity" of sodium nitrite in the male or female F344/N rats under the
conditions of the study.
101 NITRATE AND NITRITE
3. HEALTH EFFECTS
NTP (2001) also provided sodium nitrite in the drinking water of B6C3F1 mice (50/sex/group) for 2 years
at concentrations of 0, 750, 1,500, or 3,000 ppm. Author-reported average doses were 60–220 mg sodium
nitrite/kg/day (40.2–107.2 mg nitrite/kg/day) to the males and 45–160 mg sodium nitrite/kg/day (30.2–
107.2 mg nitrite/kg/day) to the females. Female mice exhibited a significant positive trend for increased
incidence of forestomach squamous cell papilloma or carcinoma (combined) and the incidence in the
high-dose female mice exceeded the historical range for NTP controls; however, based on concurrent
controls, incidences of squamous cell adenoma (1/50, 0/50, 1/50, and 3/50 for controls, 750, 1,500, and
3,000 ppm groups, respectively), squamous cell carcinoma (0/50, 0/50, 0/50, and 2/50 for controls, 750,
1,500, and 3,000 ppm groups, respectively), and squamous cell adenoma or carcinoma (1/50, 0/50, 1/50,
and 5/50 for controls, 750, 1,500, and 3,000 ppm groups, respectively) were not statistically significantly
increased for any sodium nitrite exposure group. NTP (2001) considered the positive trend for incidences
of forestomach squamous cell papilloma or carcinoma (combined) in the female B6C3F1 mice to provide
"equivocal evidence of carcinogenic activity" of sodium nitrite and noted that there was "no evidence of
carcinogenic activity" in the male B6C3F1 mice under the conditions of the study. Incidences of
alveolar/bronchiolar adenoma or carcinoma (combined) in sodium nitrite-exposed groups of female mice
were slightly greater than that of controls (incidences of 1/50, 6/50, 5/50, and 6/50 for controls, 750,
1,500, and 3,000 ppm groups, respectively); however, incidences were within that of historical NTP
controls. Because the incidences did not exhibit exposure concentration-response characteristics and
were not accompanied by increased incidences of preneoplastic lesions, the study authors did not consider
them to be sodium nitrite exposure-related effects. Significantly increased incidence of fibrosarcoma of
the subcutis was noted in mid-dose female mice (incidences of 0/50, 5/50, 1/50, and 2/50 for 0, 750,
1,500, and 3,000 ppm groups, respectively) and exceeded the historical range for NTP controls; however,
lack of exposure concentration-response characteristics and the fact that combined incidence of fibroma
or fibrosarcoma (0/50, 5/50, 1/50, and 3/50 for 0, 750, 1,500, and 3,000 ppm groups, respectively) were
within the historical range for NTP controls suggest that these neoplasms were not related to sodium
nitrite exposure.
In two other studies of male and female F344 rats, addition of sodium nitrite to the drinking water at
concentrations as high as 2,000–3,000 ppm for up to 2 years did not result in significant increases in
tumor incidences at any site (Lijinsky 1984a, 1984b; Lijinsky et al. 1983; Maekawa et al. 1982).
Conversely, incidences of mononuclear cell leukemia were significantly lower in the nitrite-treated
groups relative to controls. In a 26-month study of male and female Sprague-Dawley rats provided
drinking water to which up to 2,000 ppm sodium nitrite was added, the study author reported increased
incidence of lymphomas, but not other types of tumors (Newberne 1979); however, IARC (2010) and
102 NITRATE AND NITRITE
3. HEALTH EFFECTS
NTP (2001) noted that a working group sponsored by the U.S. FDA reevaluated the histology and did not
confirm the results of Newberne (1979). IARC (2010) reported that the working group considered the
incidences of lymphomas to be similar to those arising spontaneously in Sprague-Dawley rats. Shank and
Newberne (1976) reported increased incidences of total tumors and lymphoreticular tumors in rats fed
diet to which sodium nitrite was added at 1,000 ppm (total tumors: 58/96 versus 28/156 controls;
lymphoreticular tumors: 26/96 versus 9/156 controls); the results were reported for F1 and F2 offspring
that had been exposed via their mothers during gestation and lactation and directly from the diet
thereafter. In a 96-week study, Iurchenko et al. (1986) reported significantly increased incidences of
benign liver tumors among male CBA mice administered drinking water to which sodium nitrite was
added at a concentration resulting in author-estimated total dose of 1,600 mg sodium nitrite/mouse
compared to a group of untreated controls; however, there was no apparent sodium nitrite treatment-
related effect at a higher estimated dose (2,000 mg sodium nitrite/mouse).
Significantly increased incidences of forestomach squamous papillomas (by the life-table method) were
reported for male and female MRC Wistar rats provided drinking water to which sodium nitrite was
added at 3,000 ppm on 5 days/week for life (5/22 males and 3/23 females versus 2/47 control males and
0/44 control females) (Mirvish et al. 1980). The study authors stated that the sodium nitrite-treated rats
received a total dose of 63 g sodium nitrite/kg. Total numbers of rats and incidences of rats with
papillomas were small.
Grant and Butler (1989) added sodium nitrite to a reduced-protein diet and administered the diet to male
and female F344 rats for up to 115 weeks; a control group received reduced-protein diet alone. The study
authors reported dose-related decreases in time of onset and incidence of lymphomas, mononuclear cell
leukemia, and testicular interstitial-cell tumors in the nitrite-treated groups.
There was no evidence of increased tumor incidences in male or female ICR mice provided sodium nitrite
in the drinking water for up to 109 weeks at concentrations as high as 0.5% (5,000 ppm sodium nitrite)
(Inai et al. 1979), or in male or female Swiss mice or their offspring following a single gavage
administration of 10 mg/kg nitrite and subsequent exposure to 0.1% sodium nitrite (1,000 ppm) in the
drinking water during gestation days 15–21; terminal sacrifices occurred 10 months following the
initiation of treatment (Börzsönyi et al. 1978). Hawkes et al. (1992) found no evidence of treatment-
related effects on incidences of nervous system tumors among male and female VM mice (susceptible to
spontaneous development of cerebral gliomas) provided drinking water to which sodium nitrite was
103 NITRATE AND NITRITE
3. HEALTH EFFECTS
added at 0.2% (2,000 ppm) from weaning for a lifetime and others exposed via their mothers during
gestation and lactation as well.
The potential carcinogenicity of combined exposure to sodium nitrite and selected nitrosatable substances
(oral exposures via combinations of drinking water, diet, and/or gavage dosing) has been well-studied in
laboratory animals. Many of the studies included sodium nitrite-only treatment groups for which there
was no evidence of sodium-nitrite induced carcinogenicity (Anderson et al. 1985; Börzsönyi and Pintér
1977; Börzsönyi et al. 1976; Greenblatt and Lijinsky 1972, 1974; Greenblatt and Mirvish 1973;
Greenblatt et al. 1971, 1973; Hirose et al. 2002; Ivankovic 1979; Ivankovic and Preussman 1970; Kitano
et al. 1997; Murthy et al. 1979; Lijinsky 1984a, 1984b; Lijinsky and Reuber 1980; Mirvish et al. 1972;
Miyauchi et al. 2002; Rijhsinghani et al. 1982; Scheunig et al. 1979; Taylor and Lijinsky 1975a, 1975b;
van Logten et al. 1972; Yada et al. 2002; Yoshida et al. 1993, 1994). However, Lijinsky et al. (1983)
reported significantly increased incidences of hepatocellular neoplasms in female (but not male) F344 rats
administered diet to which sodium nitrite was added at 2,000 ppm for 2 years; significantly decreased
incidences of mononuclear-cell leukemia was observed as well.
Significantly increased incidences of selected tumor types were observed in some studies of laboratory
animals that employed coexposure to various amino compounds and sodium nitrite (Anderson et al. 1985;
Börzsönyi and Pintér 1977; Börzsönyi et al. 1976, 1978; Chan and Fong 1977; Greenblatt and Mirvish
1973; Greenblatt et al. 1971; Hirose et al. 1990; Iurchenko et al. 1986; Ivankovic 1979; Ivankovic and
Preussmann 1970; Kawabe et al. 1994; Murthy 1979; Lijinsky 1984a, 1984b; Lijinsky and Reuber 1980;
Lijinsky and Taylor 1977; Lijinsky et al. 1973b; Lin and Ho 1992; Maekawa et al. 1977; Mirvish et al.
1972, 1976, 1980; Miyauchi et al. 2002; Mokhtar et al. 1988; Newberne and Shank 1973; Nishiyama et
al. 1998; Nixon et al. 1979; Oka et al. 1974; Rijhsinghani et al. 1982; Rustia and Shubik 1974; Scheunig
et al. 1979; Shank and Newberne 1976; Tahira et al. 1988; Taylor and Lijinsky 1975a, 1975b; Weisburger
et al. 1980; Xiang et al. 1995; Yada et al. 2002; Yamamoto et al. 1989; Yoshida et al. 1993, 1994). These
results were typically attributed to in vivo nitrosation of amines by nitrite to produce carcinogenic
N-nitrosoamines; some of the studies did not include sodium nitrite-only treatment groups. Addition of
sodium nitrite or potassium nitrite to the food of rats in three other studies resulted in increased incidences
of selected tumors; analysis of the food revealed the presence of N-nitroso compounds (likely formed by
nitrosation in the presence of nitrite and selected amine compounds in the food), which were considered
the probable principal cause of the tumors (Aoyagi et al. 1980; Matsukura et al. 1977; Olsen et al. 1984).
Börzsönyi et al. (1978) reported 30–70% incidences of malignant lymphomas, lung adenomas, and
hepatomas among maternal mice and their offspring following gavage treatment of the dams with the
104 NITRATE AND NITRITE
3. HEALTH EFFECTS
fungicide, dodecylguanidine acetate, together with 0.05% sodium nitrite; the frequency of spontaneous
tumors in untreated controls was 6%. Dodecylguanidine acetate alone had no effect on cancer incidence.
Lijinsky et al. (1973a) found no significant increase in tumor incidences among male and female MCR
rats provided drinking water comprised of 0.5% nitrilotriacetic acid or iminodiacetic acid and 0.2 or 0.5%
sodium nitrite on 5 days/week for a lifetime.
There were no signs of treatment-related effects on incidences of tumors at any site among groups of
pregnant Syrian golden hamsters and their offspring fed diets to which sodium nitrite and/or morpholine
were added throughout production of an F2 generation (Shank and Newberne 1976). Fresh diet was
prepared every 2–7 days and 25% of the initial concentration of sodium nitrite was lost during 7 days
after preparation of the diet.
Based on available human data, IARC (2010) determined that there is inadequate evidence for the
carcinogenicity of nitrate in food or drinking water and limited evidence for the carcinogenicity of nitrite
in food (based on association with increased incidence of stomach cancer). Evaluation of available
animal data by IARC (2010) resulted in the determination that there is inadequate evidence for the
carcinogenicity of nitrate, limited evidence for the carcinogenicity of nitrite per se, and sufficient evidence
for the carcinogenicity of nitrite in combination with amines or amides. The overall conclusions of IARC
(2010) were that “ingested nitrate and nitrite under conditions that result in endogenous nitrosation is
probably carcinogenic to humans (Group 2A).” IARC (2010) noted that: (1) the endogenous nitrogen
cycle in humans includes interconversion of nitrate and nitrite; (2) nitrite-derived nitrosating agents
produced in the acid stomach environment can react with nitrosating compounds such as secondary
amines and amides to generate N-nitroso compounds; (3) nitrosating conditions are enhanced upon
ingestion of additional nitrate, nitrite, or nitrosatable compounds; and (4) some N-nitroso compounds are
known carcinogens.
The U.S. EPA IRIS (2002) does not include a carcinogenicity evaluation for nitrate or nitrite.
105 NITRATE AND NITRITE
3. HEALTH EFFECTS
3.2.3 Dermal Exposure
No relevant information was located regarding the following effects in humans or animals exposed to
nitrate or nitrite via the dermal route:
3.2.3.1 Death
3.2.3.2 Systemic Effects
3.2.3.3 Immunological and Lymphoreticular Effects
3.2.3.4 Neurological Effects
3.2.3.5 Reproductive Effects
3.2.3.6 Developmental Effects
3.2.3.7 Cancer
3.3 GENOTOXICITY
No studies were located regarding genotoxicity in human populations exposed to exogenous nitrite.
Limited information is available for nitrate. Kleinjans et al. (1991) examined the association between
nitrate levels in drinking water and frequency of sister chromatid exchanges (SCEs) in peripheral
lymphocytes from women from the Netherlands. Three groups were formed, low- (n=30), medium-
(n=30), and high- (n=18) exposure groups, based on the levels of nitrate in their drinking water. The
corresponding nitrate levels were 0.13, 32.0, and 133.5 mg/L. Regression analysis showed a good
correlation between levels on nitrate in water and nitrate body burden monitored by 24-hour urine levels
of nitrate. Examination of peripheral lymphocytes showed no significant association between 24-hour
urine excretion of nitrate and frequency of SCEs. Another study examined the frequency of
hypoxanthine-guanine phosphoribosyltransferase (HPRT) variants (an index of genetic risk) in peripheral
lymphocytes in groups of women from the Netherlands in relation to levels of nitrate in drinking water
(van Maanen et al. 1996a). A total of 50 subjects were exposed to concentrations of nitrate of 0.02 mg/L
(n=14), 17.5 mg/L (n=21), 25 mg/L (n=6), or 135 mg/L (n=9). The two lower concentrations were from
PWS, whereas the two highest originated from private wells. Analysis of 24-hour urine samples showed
a positive correlation between nitrate in drinking water and urinary nitrate. Also, salivary nitrate and
nitrite were similarly increased. Results of multiple regression analysis showed that the mean log
frequency of HPRT variants was significantly higher in the group exposed to 25 mg/L nitrate than in the
groups exposed to 0.02 and 17.5 mg/L nitrate. The analyses also showed a significant correlation
between frequency of HPRT variants and 24-hour urinary nitrate and salivary nitrite levels and between
24-hour urinary excretion of N-nitrosopyrrolidine and 24-hour urinary excretion of nitrate. The results
suggested that drinking water with nitrate poses a genetic risk due to the potential formation of
nitrosamines after endogenous reduction of nitrate to nitrite and reaction with amino compounds. A third
106 NITRATE AND NITRITE
3. HEALTH EFFECTS
study examined the frequency of SCEs and chromosomal aberrations in peripheral blood lymphocytes
from70 male and female Greek children (12–15 years of age) who were exposed to high nitrate in
drinking water (55.7–88.0 mg/L) (Tsezou et al. 1996). Controls consisted of 20 children from areas with
low nitrate content in the drinking water (0.70 mg/L). No measurements of nitrate or nitrite in biological
fluids were conducted in this study. Analyses of the results showed a significant increase in chromatid
and chromosome breaks in children exposed to nitrate levels ≥70.5 mg/L of drinking water. However,
levels of SCEs showed no significant increase with increasing nitrate levels. IARC (2010) noted that the
possibility that chemicals other than nitrate could have been responsible for the elevated chromosomal
aberrations could not be ruled out.
A limited number of studies have examined the in vivo genotoxicity of nitrate in laboratory animals.
Gavage administration of up to 500 mg/kg/day sodium nitrate to pregnant Syrian Golden hamsters on
gestation days 11 and 12 did not significantly affect the frequency of micronuclei, chromosomal
aberrations, morphological or malignant cell transformation, or drug-resistant mutations in embryonic
cells (Inui et al. 1979). In another in vivo study, oral administration of 150 mg/kg sodium nitrate (only
dose tested) to male Swiss mice did not inhibit testicular DNA synthesis measured 3.5 hours after dosing
(Friedman and Staub 1976). Gavage administration of up to 2,120 mg/kg/day sodium nitrate for 2 days to
male Wistar rats did not induce chromosomal aberrations in bone marrow cells examined 24 hours after
the last dose (Luca et al. 1985). A similar experiment with male Swiss mice showed induction of
chromosomal aberrations at 706.6 mg/kg/day sodium nitrate but not at 2,120 mg/kg/day (Luca et al.
1985). Daily administration of ≥78.5 mg/kg sodium nitrate for 2 weeks to rats resulted in a significant
dose-dependent increase in chromosomal aberrations in bone marrow cells 24 hours after the last dose
(Luca et al. 1985). Evaluation of micronuclei in mice treated daily for 2 weeks showed significant
increases (approximately 2-fold greater than controls) at the low concentrations tested, 78.5 and
235.5 mg/kg/day sodium nitrate, but not at 706.6 or 2,120 mg/kg/day, which the investigators attributed
to possible induction of cytotoxic effects (Luca et al. 1985). Alavantić et al. (1988a) treated male mice
with sodium nitrate by gavage for 3 days at doses of 0, 600, or 1,200 mg/kg/day; there was no sign of
treatment-related unscheduled DNA synthesis in spermatids analyzed 17 days following treatment.
Alavantić et al. (1988b) treated male mice with sodium nitrate by gavage for 2 weeks doses of 0, 600, or
1,200 mg/kg/day and subsequently mated them to virgin females; evaluation of primary spermatocytes
from F1 males revealed no sign of treatment-related heritable translocations.
In studies in vitro, neither potassium nitrate nor sodium nitrate in concentrations of up to 20 and
5 mg/plate, respectively, was mutagenic in various strains of Salmonella typhimurium (TA92, TA94,
107 NITRATE AND NITRITE
3. HEALTH EFFECTS
TA98, TA100, TA1535) (Ishidate et al. 1984), tested with and without metabolic activation. Lanthanum
nitrate hexahydrate also yielded negative results in S. typhimurium strains TA100 and TA1535 (Zeiger et
al. 1992). Tests for chromosomal aberrations in Chinese hamster fibroblast cells were positive for sodium
nitrate, but negative for potassium nitrate (Ishidate et al. 1984). IARC (2010) noted that since sodium
chlorite also yielded positive results in the same assay, the chromosomal aberrations induced by sodium
nitrate could have been due to the high osmotic pressure and sodium ion concentration. In another study,
incubation of Chinese hamster ovary cells with up to 10 mM ammonium nitrate for up to 24 hours in the
presence of metabolic activation or up to 48 hours without metabolic activation did not induce
chromosomal aberrations (Kim et al. 2011).
Several studies have examined the in vivo genotoxicity of nitrite using a variety of tests, a summary is
shown in Table 3-3. The results have been mixed, and at times inconsistent, between laboratories that
used the same tests. Administration of up to 7.3 mg sodium nitrite to pregnant mice (~290 mg/kg
assuming 0.025 kg body weight) on gestation days 7–18 via the drinking water did not induce
chromosomal aberrations in maternal bone marrow cells or in fetal liver cells (Shimada 1989). Negative
results for chromosomal aberrations were also reported in embryonic hamster cells after administration of
a single dose of up to 500 mg/kg sodium nitrite on gestation day 11 or 12 (Inui et al. 1979). However,
significantly increased incidences of chromosomal aberrations were reported in bone marrow cells from
male rats (ca. 2.1–2.4 times greater than controls), mice (ca. 4–5 times greater than controls), and rabbits
(ca. 2–3.6 times greater than controls) dosed with ≥1.7 mg/kg sodium nitrite (Luca et al. 1987). Rats and
mice were dosed twice by gavage, whereas rabbits received sodium nitrite via the drinking water for
3 months. No dose-response was apparent in the studies by Luca et al. (1987) over an approximately
27-fold dose range, suggesting that maximum response was already achieved with the lowest dose,
1.7 mg/kg. Sodium nitrite also induced micronuclei in polychromatic erythrocytes of mice dosed twice at
≥1.7 mg/kg (Luca et al. 1987) and in embryonic hamster cells after a single administration of 250 mg/kg
sodium nitrite to the pregnant dams (Inui et al. 1979). However, in another study (NTP 2001), sodium
nitrite did not induce micronuclei in male rat or mouse bone marrow cells after three intraperitoneal
injections at nonlethal doses up to 50 mg/kg/day (rats) and 125 mg/kg/day (mice). Evaluation of SCEs
also provided seemingly conflicting results. In a study by Giri et al. (1986), single doses of ≥5 mg/kg
sodium nitrite by gavage induced dose-related significant increases in SCEs in mouse bone marrow cells,
but Bambrilla et al. (1983) reported that a single gavage dose of 80 mg/kg sodium nitrite did not induce
SCEs in mouse bone marrow cells. Results from assays for DNA repair, DNA damage, or DNA
synthesis in mammalian cells from rats or mice generally yielded negative results (Bambrilla et al. 1983;
Friedman and Staub 1976; Hellmér and Bolcsfoldi 1992; Robbiano et al. 1990). Sodium nitrite induced

108 NITRATE AND NITRITE
3. HEALTH EFFECTS
Table 3-3. Genotoxicity of Sodium Nitrite In Vivo
Species (test system)
End point
Results
Reference
Mammalian cells:
Pregnant mouse bone marrow Chromosomal aberrations − Shimada 1989
cells
Mouse fetal liver cells
Chromosomal aberrations
–
Shimada 1989
Embryonic hamster cells
Chromosomal aberrations
−
Inui et al. 1979
Rat bone marrow cells
Chromosomal aberrations
+
Luca et al. 1987
Mouse bone marrow cells
Chromosomal aberrations
+
Luca et al. 1987
Rabbit bone marrow cells
Chromosomal aberrations
+
Luca et al. 1987
Mouse polychromatic
Micronuclei
+
Luca et al. 1987
erythrocytes
Rat bone marrow cells
Micronuclei
−
NTP 2001
Mouse bone marrow cells
Micronuclei
−
NTP 2001
Embryonic hamster cells
Micronuclei
+
Inui et al. 1979
Embryonic hamster cells
Malignant cell transformation
+
Inui et al. 1979
Embryonic hamster cells
Drug-resistant mutations
+
Inui et al. 1979
Mouse bone marrow cells
Sister chromatid exchange
+
Giri et al. 1986
Mouse bone marrow cells
Sister chromatid exchange
−
Brambrilla et al. 1983
Mouse host-mediated assay
Mutations in Salmonella
−
Couch and Friedman 1975
Mouse host-mediated assay
DNA repair in E. coli K-12
−
Hellmér and Bolcsfoldi 1992
uvrb/recA
Rat liver cells
DNA damage
−
Robbiano et al. 1990
Rat liver and gastric mucosa
DNA damage
−
Brambrilla et al. 1983
cells
Mouse testicular cells
DNA synthesis
−
Friedman and Staub 1976
Male mouse germ cells
Unscheduled DNA synthesis
−
Alavantić et al. 1988a
Male mouse germ cells
Heritable translocations
−
Alavantić et al. 1988b
Insect systems:
Drosophila melanogaster (wing
Somatic mutation + Graf et al. 1989
spot test)
+ = positive results; – = negative results
109 NITRATE AND NITRITE
3. HEALTH EFFECTS
malignant cell transformation and produced drug-resistant mutations in embryonic hamster cells
following treatment of the pregnant dams on gestation day 11 or 12 with a single dose of ≥125 mg/kg
(Inui et al. 1979). Alavantić et al. (1988a) treated male mice with sodium nitrite by gavage for 3 days at
doses of 0, 60, or 120 mg/kg/day; there was no sign of treatment-related unscheduled DNA synthesis in
spermatids analyzed 17 days following treatment. Alavantić et al. (1988b) treated male mice with sodium
nitrite by gavage for 2 weeks doses of 0, 60, or 120 mg/kg/day and subsequently mated them to virgin
females; evaluation of primary spermatocytes from F1 males revealed no sign of treatment-related
heritable translocations. In a host-mediated assay, mice were intraperitoneally inoculated with
S. typhimurium strain G46 and gavaged with sodium nitrite (Couch and Friedman 1975); the sodium
nitrite treatment did not induced increased frequency in S. typhimurium mutation rate in this host-
mediated assay. Finally, feeding sodium nitrite to larvae of Drosophila melanogaster induced somatic
mutations as assessed by the wing spot test (Graf et al. 1989).
Numerous studies have examined the genotoxicity of nitrite in in vitro assays. As shown in Table 3-4,
there seem to be more positive results than negative results in tests of gene mutations in prokaryotic
organisms, but it is difficult to draw a firm conclusion (Andrews et al. 1980, 1984; Balimandawa et al.
1994; Brams et al. 1987; De Flora 1981, De Flora et al. 1984; Ehrenberg et al. 1980; Ishidate et al. 1981,
1984; McCann et al. 1975; Törnqvist et al. 1983; Zeiger et al. 1992). However, it appears that the
addition of metabolic activation systems to the incubation mixtures did not make a difference in the
results. That is, tests that were positive without activation were also positive with activation; tests that
were negative without activation were also negative with activation. This would indicate that nitrite can
be a direct mutagenic chemical. In vitro tests that assessed chromosomal aberrations, SCEs, DNA repair,
and cell transformations in sodium nitrite-treated mammalian cells yielded positive results (Inoue et al.
1985; Ishidate et al. 1984; Luca et al. 1987; Lynch et al. 1983; Tsuda and Kato 1977; Tsuda et al. 1973,
1981). Nitrite enhanced neutrophil-induced DNA strand breakage in rat lung type II epithelial cells; the
enhancement was associated with an inhibition of neutrophil-derived myeloperoxidase (Knaapen et al.
2005).
3.4 TOXICOKINETICS
No information was located regarding the pharmacokinetics of nitrate or nitrite following inhalation or
dermal exposure. However, numerous reports are available regarding the pharmacokinetics of ingested
nitrate and nitrite. Comprehensive reviews of the available data (Bailey et al. 2012; Bryan and van
Grinsven 2013; IARC 2010; JECFA 2003a, 2003b; Lundberg and Weitzberg 2013; Lundberg and Govoni

110 NITRATE AND NITRITE
3. HEALTH EFFECTS
Table 3-4. Genotoxicity of Sodium Nitrite In Vitro
Results
With
Without
Species (test system)
End point
activation
activation
Reference
Prokaryotic organisms:
Salmonella typhimurium
Gene mutation
–
TA98
S. typhimurium TA100
Gene mutation
+
S. typhimurium TA98, TA100,
Gene mutation
+
TA1537
S. typhimurium TA100,
Gene mutation
+
TA1535
S. typhimurium TA98, TA100,
Gene mutation
−
TA1535, TA1537, TA1538
S. typhimurium TA100,
Gene mutation
+
TA1530, TA1535
S. typhimurium TA102,
Gene mutation
−
YG1024, DJ400, DJ460
S. typhimurium TA100
Gene mutation
+
S typhimurium TA97, TA98
Gene mutation
−
S. typhimurium TA1530
Gene mutation
NT
S typhimurium TA100,
Gene mutation
−
a
TA1535
S typhimurium TA98,
Gene mutation
−
TA1537, TA1538
S. typhimurium TA1535
Gene mutation
NT
Escherichia coli WP2, WP67,
DNA repair
+
CM871
Eukaryotic organisms:
Cultured human lymphocytes
Sister chromatid
NT
exchange
Chinese hamster ovary cells
Sister chromatid
NT
exchange
Chinese hamster ovary cells
Chromosomal
NT
aberrations
Monkey BS-C-1 fetal liver
Chromosomal
NT
cells
aberrations
HeLa cells
Chromosomal
NT
aberrations
Chinese hamster fibroblasts
Chromosomal
NT
aberrations
Syrian hamster embryo cells
Chromosomal
NT
aberrations
–
NTP 2001; Zeiger et al.
1992
+
NTP 2001; Zeiger et al.
1992
+
Ishidate et al. 1981
+
Ishidate et al. 1984
−
Andrews et al. 1980,
1984
+
Balimandawa et al. 1994
−
Balimandawa et al. 1994
NT
Brams et al. 1987
NT
Brams et al. 1987
+
Ehrenberg et al. 1980
+
De Flora 1981, 1984
−
De Flora 1981, 1984
(+)
McCann et al. 1975
+
De Flora et al. 1984
+
Inoue et al. 1985
+
Tsuda et al. 1981
+
Tsuda et al. 1981
+
Luca et al. 1987
+
Luca et al. 1987
+
Ishidate et al.1984
+
Tsuda and Kato 1977

111 NITRATE AND NITRITE
3. HEALTH EFFECTS
Table 3-4. Genotoxicity of Sodium Nitrite In Vitro
Results
With
Without
Species (test system)
End point
activation
activation
Reference
HeLa S3 carcinoma cells
DNA repair
NT
+
Lynch et al. 1983
Syrian hamster embryo cells
Cell transformation
NT
+
Tsuda et al. 1973
a
Reported as a decrease in mutagenicity in the presence of S9 mix; however, it was not specified whether the
decrease was relative to controls or sodium nitrite treatment in the absence of S9 mix.
+ = positive results; (+) = weakly positive; – = negative results; DNA = deoxyribonucleic acid; NT = not tested
112 NITRATE AND NITRITE
3. HEALTH EFFECTS
2004; Lundberg et al. 2008, 2009; Weitzberg and Lundberg 2013; Weitzberg et al. 2010; WHO 2011b)
serve as references for the major portion of toxicokinetic data presented in this section of the ATSDR
Toxicological Profile for Nitrate and Nitrite.
Ingestion is the major source of exposure to nitrate and nitrite. Vegetables are the main source of nitrate
in the diet (approximately 60–80% of total nitrate intake); nitrate in some drinking water sources may
contribute 15–20% of total nitrate intake. Small amounts of nitrate and nitrite are added to some animal-
based products to serve as preservatives and to enhance taste. Approximately 80–85% of nitrite in
humans is produced from in vivo reduction of nitrate.
The nitrate-nitrite-nitric oxide pathway in mammals includes a dietary component and an endogenous
component. Figure 3-2 depicts the metabolic pathways for ingested nitrate and nitrite, as well as the
endogenous production of nitric oxide via nitric oxide synthase (NOS). Numbers in brackets in the figure
coincide with those in the following description of the pathways. Ingested nitrate passes through the
stomach [1] to the small intestine [2] where it is nearly completely absorbed into the blood [3]. Following
a nitrate-containing meal, circulating nitrate concentrations are normally in the range of 20–40 µM,
depending on the type of diet and activity of nitric oxide synthases. Peak plasma nitrate levels are
reached 15–30 minutes following ingestion; the half-time of plasma nitrate is on the order of 5–6 hours.
Most nitrate that passes through the kidney [4] is reabsorbed into the blood [5]. However, some is
excreted in the urine [6]. In humans, approximately 25% of plasma nitrate is taken up by the salivary
glands and secreted in the saliva [7]; concentrations salivary nitrate can be as much as 10–20 times that of
plasma nitrate. Approximately 20% of the nitrate in saliva undergoes anaerobic, nitrate reductase-
catalyzed reduction to nitrite by commensal bacteria; thus, salivary secretion and reduction in saliva
results in conversion of approximately 5% of ingested nitrate to nitrite (Gangolli et al. 1994; Walker
1996). In vitro results using selected rat and mouse tissues and human liver tissues suggest a possible
metabolic pathway whereby some plasma nitrate could be reduced to nitrite by enzymes such as xanthine
oxidase (Jansson et al. 2008). Most salivary nitrate, however, passes to the small intestine and is
absorbed into the blood. A portion of nitrite (either produced from reduction of nitrate or ingested from
food sources) that enters the stomach is rapidly protonated to nitrous acid (HNO
2
), which decomposes
spontaneously to nitric oxide and other biologically active nitrogen oxides (e.g., nitrogen dioxide [NO
2
];
dinitrogen trioxide [N
2
O
3
]) in the acid environment of the stomach [8]; this process is enhanced in the
presence of reducing compounds such as ascorbic acid and polyphenols. Nitrite can also react with
proteins, amines, and amides in the stomach. Reaction of nitrite with some low-molecular-weight amines

NitrateNitrate
Nitrate
Nitrite
Nitrite
Nitrite
N
113 NITRATE AND NITRITE
3. HEALTH EFFECTS
Figure 3-2. The Nitrate-Nitrite-Nitric Oxide Cycle in Humans*
Kidney
Mouth
Nitrate
Nitrite
Small intestine
Nitrate Nitrite
Blood, tissues
Urine
[4]
[5]
[6]
Nitrate in
saliva
Nitric oxide
Physiological
functions
25% of blood nitrate taken up by salivary gland
HNO
2
NO
NO
2
N
2
O
3
N-nitroso
compounds
Nitrate
[1]
[2]
Nitrate Nitrite
[3]
L-arginine
NOS
[7]
[8]
[13]
[14]
[15]
Nitrate
deoxyHb
metHb
[10]
[11]
Nitrite
Stomach
[16]
oxyHb
metHb
[12]
oxyHb
metHb
Oral intake
Nitrate
Nitrite
*Numbers in brackets coincide with those in the descriptive text.
deoxyHb = deoxyhemoglobin; HNO
2
= nitrous acid; metHb = methemoglobin; NO = nitric oxide; NO
2
= nitrogen
dioxide; N
2
O
3
= dinitrogen trioxide; NOS = nitric oxide synthase; oxyHb = oxyhemoglobin
114 NITRATE AND NITRITE
3. HEALTH EFFECTS
(nitrosation) produces N-nitroso derivatives [9], including carcinogenic compounds, portions of which
can be absorbed and distributed via systemic circulation. However, most nitrite passes to the small
intestine where it is absorbed into the blood. Plasma levels of nitrite increase within 30 minutes
following ingestion of nitrate. Although the biological half-time of plasma nitrite is only 20–30 minutes,
plasma levels remain elevated for several hours due to the enterosalivary circulation of nitrate. Plasma
nitrite concentrations, which are normally 50–100 nM, may increase as much as 5 times after a nitrate-
rich meal. The production of N-nitroso derivatives from plasma nitrite occurs to some extent in selected
tissues.
Nitrite in the blood and tissues can be reduced to nitric oxide, which is involved in a variety of
physiological processes. In the presence of deoxyhemoglobin, reduction of nitrite to nitric oxide occurs
via oxidation of ferrous (Fe
2+
) hemoglobin (which transports oxygen) to ferric (Fe
3+
) hemoglobin
(methemoglobin, a poor transporter of oxygen) [10]. Methemoglobin is converted to deoxyhemoglobin
[11] in a reaction catalyzed by methemoglobin reductase. Nitrite can also react with oxyhemoglobin to
form nitrate and methemoglobin [12].
In addition to exogenous sources of nitrate and nitrite (e.g., diet), nitrate, nitrite, and nitric oxide are
produced endogenously. A major endogenous production mechanism is oxygen-dependent reduction of
L-arginine (a biologically-relevant amino acid) to nitric oxide [13], which occurs in most cells of the body
in the presence of nicotinamide adenine dinucleotide phosphate (NADPH) and cofactors flavin adenine
dinucleotide (FAD), tetrahydrobiopterin (BH
4
), heme, and calmodulin. Nitric oxide is involved in a
variety of physiological functions that include regulation of blood flow, platelet function, pulmonary
function, nerve function, host defense, and metabolic control. Nitric oxide may also be formed via an
oxygen-independent one-electron reduction of nitrite in acidic and hypoxic tissues [14]. Nitrite may serve
as an important source of nitric oxide under such acidic and hypoxic conditions because the half-time for
plasma nitrite (15–20 minutes) is much longer than that of nitric oxide (<6 seconds). Nitric oxide is
rapidly oxidized to nitrite in the presence of oxygen and ceruloplasmin [15]. Nitric oxide can also react
with oxyhemoglobin to form nitrate and methemoglobin [16]. Various physiological processes are
involved in maintaining a balance between systemic levels of nitrate, nitrite, and nitric oxide. The
endogenous nitrate-nitrite-nitric oxide pathway provides baseline levels of nitrate and nitrite in the body
which are supplemented by dietary intake. The total plasma nitrate and nitrite content consists of portions
entering the blood from oral intake and portions generated endogenously from nitric oxide in the body.
115 NITRATE AND NITRITE
3. HEALTH EFFECTS
As much as 60–75% of plasma nitrate is excreted unchanged in the urine within 24 hours following
ingestion. Under normal physiological conditions, nitrite is not detected in the urine and its presence in
urine is an indication of infection by nitrate-reducing organisms. Zhou et al. (2014) reported increased
urinary excretion of N-nitroso compounds following ingestion of nitrite from the drinking water of rats.
Minor urinary products of nitrate and nitrite metabolism include ammonia and urea. Nitrate and nitrite
are secreted to some extent in breast milk and perspiration. Fecal excretion of nitrate and nitrite is
negligible.
3.4.1 Physiologically Based Pharmacokinetic (PBPK)/Pharmacodynamic (PD) Models
Physiologically based pharmacokinetic (PBPK) models use mathematical descriptions of the uptake and
disposition of chemical substances to quantitatively describe the relationships among critical biological
processes (Krishnan et al. 1994). PBPK models are also called biologically based tissue dosimetry
models. PBPK models are increasingly used in risk assessments, primarily to predict the concentration of
potentially toxic moieties of a chemical that will be delivered to any given target tissue following various
combinations of route, dose level, and test species (Clewell and Andersen 1985). Physiologically based
pharmacodynamic (PBPD) models use mathematical descriptions of the dose-response function to
quantitatively describe the relationship between target tissue dose and toxic end points.
PBPK/PD models refine our understanding of complex quantitative dose behaviors by helping to
delineate and characterize the relationships between: (1) the external/exposure concentration and target
tissue dose of the toxic moiety, and (2) the target tissue dose and observed responses (Andersen and
Krishnan 1994; Andersen et al. 1987). These models are biologically and mechanistically based and can
be used to extrapolate the pharmacokinetic behavior of chemical substances from high to low dose, from
route to route, between species, and between subpopulations within a species. The biological basis of
PBPK models results in more meaningful extrapolations than those generated with the more conventional
use of uncertainty factors.
The PBPK model for a chemical substance is developed in four interconnected steps: (1) model
representation, (2) model parameterization, (3) model simulation, and (4) model validation (Krishnan and
Andersen 1994). In the early 1990s, validated PBPK models were developed for a number of
toxicologically important chemical substances, both volatile and nonvolatile (Krishnan and Andersen
1994; Leung 1993). PBPK models for a particular substance require estimates of the chemical substance-
specific physicochemical parameters, and species-specific physiological and biological parameters. The
116 NITRATE AND NITRITE
3. HEALTH EFFECTS
numerical estimates of these model parameters are incorporated within a set of differential and algebraic
equations that describe the pharmacokinetic processes. Solving these differential and algebraic equations
provides the predictions of tissue dose. Computers then provide process simulations based on these
solutions.
The structure and mathematical expressions used in PBPK models significantly simplify the true
complexities of biological systems. However, if the uptake and disposition of the chemical substance(s)
are adequately described, this simplification is desirable because data are often unavailable for many
biological processes. A simplified scheme reduces the magnitude of cumulative uncertainty. The
adequacy of the model is, therefore, of great importance, and model validation is essential to the use of
PBPK models in risk assessment.
PBPK models improve the pharmacokinetic extrapolations used in risk assessments that identify the
maximal (i.e., the safe) levels for human exposure to chemical substances (Andersen and Krishnan 1994).
PBPK models provide a scientifically sound means to predict the target tissue dose of chemicals in
humans who are exposed to environmental levels (for example, levels that might occur at hazardous waste
sites) based on the results of studies where doses were higher or were administered in different species.
Figure 3-3 shows a conceptualized representation of a PBPK model.
If PBPK models for nitrate and nitrite exist, the overall results and individual models are discussed in this
section in terms of their use in risk assessment, tissue dosimetry, and dose, route, and species
extrapolations.
Kinetics of absorption of nitrate from the gastrointestinal tract and elimination in urine can be described
mathematically with simple one-compartment first-order models (Schultz et al. 1985; Wagner et al.
1983). The complex kinetics of salivary secretion of nitrate, reduction and absorption in the
gastrointestinal tract, and binding to hemoglobin and formation of methemoglobin have been described
with a multicompartment model (Zeilmaker et al. 1996, 2010b).

117 NITRATE AND NITRITE
3. HEALTH EFFECTS
Figure 3-3. Conceptual Representation of a Physiologically Based
Pharmacokinetic (PBPK) Model for a
Hypothetical Chemical Substance
Inhaled chemical Exhaled chemical
Lungs
Liver
Fat
Slowly
perfused
tissues
Richly
perfused
tissues
Kidney
Skin
V
E
N
O
U
S
B
L
O
O
D
A
R
T
E
R
I
A
L
B
L
O
O
D
V
max
K
m
Ingestion
GI
Tract
Feces
Urine
Chemicals
contacting skin
Note: This is a conceptual representation of a physiologically based pharmacokinetic (PBPK) model for a
hypothetical chemical substance. The chemical substance is shown to be absorbed via the skin, by inhalation, or by
ingestion, metabolized in the liver, and excreted in the urine or by exhalation.
Source: adapted from Krishnan and Andersen 1994
118 NITRATE AND NITRITE
3. HEALTH EFFECTS
The Zeilmaker et al. (1996, 2010b) Model
Description of the Model. Zeilmaker et al. (1996, 2010b) developed a PBPK model for simulating
kinetics of methemoglobin formation resulting from absorption of nitrate in adult humans. The structure
of the model is depicted in Figure 3-4. Parameters and parameter values for the model are presented in
Table 3-5.
The model simulates absorption of nitrate from the gastrointestinal tract as a first-order transfer to a
central nitrate distribution compartment, which is assumed to be in equilibrium with blood plasma (k
a,NO
3
,
hour
-1
). The fraction of ingested nitrate that is absorbed is assumed to be 100% (F
a
=1). The model also
simulates delivery of endogenously produced nitrate to blood (zero-order K
end
= 162 mg NO
3
/24 hours).
Absorbed nitrate is eliminated from the central compartment by excretion into urine, metabolism (tissues
and gastrointestinal bacteria), and secretion into saliva. The metabolism and urinary pathways are
combined in the model into a single first-order pathway (k
el
, hour
-1
), a fraction of which goes to urine
(f
u
=0.56). Secretion of nitrate into saliva is simulated as a separate pathway. Secretion occurs by
capacity-limited transport mediated by a sodium/iodide (Na
+
/I
-
symporter, NIS) in the salivary gland
epithelium. Although the NIS has limited capacity for nitrate, relatively large nitrate doses and blood
nitrate concentrations are required to exceed linear blood-to-saliva kinetics in vivo, indicative of
saturation of the carrier. The model can simulate salivary secretion of nitrate as either a first-order
process (k
sec,NO
3
, hour
-1
), or a capacity-limited process (K
m
, mM; C
s,max,NO
3
, mg/L), depending on the dose
(<1,000 mg/70 kg; 14 mg/kg) or plasma nitrate concentration (<34 mg NO
3
/L). Nitrate is eliminated
from saliva by transfer to the gastrointestinal tract (flow-limited B, L/hour) or reduction to nitrite (first-
order k
conv
, hour
-1
). Nitrite in saliva undergoes transfer to the gastrointestinal tract (flow-limited B,
L/hour), from where it can be absorbed into blood (first-order k
a,NO
2
, hour
-1
) or be converted to other
metabolites and reaction products (first-order k
dec
, hour
-1
). Nitrite in blood is secreted into saliva (first-
order k
sec,NO
2
, hour
-1
) or reacts with hemoglobin to produce methemoglobin (first-order k
NO
2
, hour
-1
) and
nitrate. Methemoglobin is regenerated as a product of methemoglobin reductase (capacity-limited K
m,r
,
mM). Nitrate formed in the reaction of nitrite with hemoglobin is returned to blood (first-order z
⋅
k
NO
2
,
hour
-1
). Background production of methemoglobin from reactants other than nitrite is accounted for as a
background concentration of reactants (C
bg
, mM), which combines additively with the concentration of
nitrite (C
NO
2
, mM) to react with hemoglobin.

119 NITRATE AND NITRITE
3. HEALTH EFFECTS
Figure 3-4. Structure of the Zeilmaker et al. (1996, 2010b) Model*
*See Table 3-5 for explanation of symbols; solid lines = mass flows; dotted lines = functional relationships
Hb = hemoglobin; MetHb = methemoglobin
Source: Adapted from Zeilmaker et al. (2010b)

120 NITRATE AND NITRITE
3. HEALTH EFFECTS
Table 3-5. Parameter Values for the Zeilmaker et al. (1996, 2010) PBPK Model of
Nitrate and Nitrite in Humans
Parameter
Value (standard deviation or range)
Physiological parameters
Volume of saliva compartment (V
s
)
0.001 L
Salivary flow (B)
0.069 L/hour (0.042–0.120)
Nitrate parameters
Volume fraction (of body weight) of central nitrate distribution
0.30 (0.29–0.33)
compartment (V
NO3
)
Nitrate dose averaging time (
∆
t)
0.1 hours (drinking water); 0.8 hours
(vegetables)
Nitrate gastrointestinal absorption rate (k
a,NO3
)
>5 hour
-1
Nitrate gastrointestinal absorption fraction (F
a,NO3
)
1
Nitrate endogenous production (K
end
)
162 mg/24 hours
Nitrate elimination rate (k
el
)
0.14±0.01 hour
-1
Nitrate urinary elimination fraction (f
u
)
0.56±0.029
Nitrate blood-to-saliva secretion rate (k
sec,NO3
)
0.045±0.003 hour
-1
Nitrate blood-to-saliva half-maximum (K
M,s
)
104 mg/L
Nitrate blood-to-saliva maximum (C
max,s
)
2,258 mg/L
Nitrate-to-nitrite conversion rate in saliva (k
conv
)
19.95±1.75 hour
-1
Nitrite parameters
Volume fraction (of body weight) of central nitrite distribution
0.65±0.03
compartment (V
NO2
)
Nitrite gastrointestinal absorption rate (k
a,NO2
)
>5 hour
-1
Nitrite gastrointestinal absorption fraction (F
a,NO2
)
1
Nitrite blood-to-saliva secretion rate (k
sec,NO2
)
0.045±0.003 hour
-1
Nitrite gastrointestinal conversion rate to other products (k
dec
)
0.67 hour
-1
(at pH 1.5)
Hemoglobin/methemoglobin parameters
Nitrite reaction rate with hemoglobin (k
NO2
)
4.23±0.15 mM
-1
hour
-1
Methemoglobin reductase half maximum (K
M,r
)
0.012±0.0018 mM
Methemoglobin reductase maximum (V
max,r
)
4.23±0.15 mM/hour
Stoichiometric constant for regeneration of nitrate from
0.5±0.01
methemoglobin (z)
Hemoglobin concentration in blood (C
Hg
)
8 mM
Background methemoglobin concentration in blood (C
MetHb,bg
)
0.03 mM
Background concentration of hemoglobin oxidizing reactants in
0.0058 mM
blood(C
bg
)
a
Based on Zeilmaker et al. (2010b)
121 NITRATE AND NITRITE
3. HEALTH EFFECTS
Following ingestion of nitrate in a given medium (e.g., drinking water or vegetables), the ingested nitrate
dose is assumed to enter the absorption compartment at a rate (mg/hour) given by the oral dose (mg)
divided by a dose averaging time,
∆
t (hour), where the parameter, ∆t, is assigned a value specific for the
ingested medium.
Sources for model parameter estimates are presented in Table 3-5. Nine parameters were derived by
statistical optimization to experimental in vivo data (Kortboyer et al. 1997b, 1998b; Wagner et al. 1983).
Data from Wagner et al. (1983) were used to optimize parameters
∆
t
water
, k
a,NO
3
, k
sec,NO
3
, and k
conv
. Wagner
et al. (1983) measured plasma, saliva, and urine, and nitrite in plasma and saliva, in 12 healthy adults
following a single oral dose of
15
N-nitrate in drinking water. The parameter,
∆
t
veg
(for vegetables), was
optimized with data from a study in which plasma nitrate was measured in six adults before and following
a vegetable meal (Kortboyer et al. 1998b). The parameter, V
NO
2
, was optimized with data from a study in
which plasma nitrite concentrations were measured in nine adults before and following an intravenous
dose of sodium nitrite (Kortboyer et al. 1997b). Parameters describing reactions with hemoglobin and
methemoglobin (k
NO
2
, [K
m,r
, V
max,r
, z]) were derived by statistical optimization to experimental in vitro
studies in which reaction kinetics of nitrite with hemoglobin were measured in whole human blood
(Kosaka et al. 1979; Rodkey 1976). The remaining parameters were estimated from reported literature or
calculated from other parameters (Cortas and Wakid 1991; Kortboyer et al. 1995, 1997a, 1997b, 1998a,
1998b; Lambers et al. 2000
; McKnight et al. 1997; Mirvish et al. 1975; Schultz et al. 1985; Wagner et al.
1983).
Validation of the Model. The optimized model was evaluated by comparing predictions of plasma
nitrate and nitrite concentrations and blood methemoglobin concentrations in nine adults who consumed a
single oral dose of sodium nitrite (2.42 or 4.84 mg sodium nitrite/kg) (Kortboyer et al. 1997b). The
results of the evaluation are reported in Zeilmaker et al. (2010b) as overlay plots of observations and
predictions. Statistical evaluations of the agreement between predictions and observations were not
reported.
Risk Assessment. The model has been used to predict concentrations of methemoglobin that would
result from a vegetable meal and to evaluate whether the average daily intake of nitrate would result in
clinically significant methemoglobinemia (JECFA 2003a). JECFA (2003a) applied the model to make
predictions in adults and infants. In order to apply the model to infants, blood volume and volumes of the
central nitrate and nitrite compartments were scaled to infants (the exact scaling procedure or scaled
parameter values were not reported). JECFA (2003a) also applied the model to predict methemoglobin
122 NITRATE AND NITRITE
3. HEALTH EFFECTS
concentrations that might occur in patients who have inflammatory reactions to absorbed nitrite.
Absorbed doses of nitrate in patients were simulated in the model as an intravenous infusion of nitrite.
Target Tissues. The model was calibrated to predict concentrations of nitrate and nitrite in plasma
and blood methemoglobin concentrations in humans.
Species Extrapolation. The model simulates nitrate and nitrite kinetics in humans. Applications to
other species would require development of appropriate scaling methods, optimization, and validation.
Interroute Extrapolation. The model is currently configured to simulate kinetics associated with
intravenous and oral dosing. Simulation of other potential routes of exposure (e.g., inhalation, dermal)
would require development of models for the absorption of inhaled nitrate or nitrate deposited on the skin.
3.5 MECHANISMS OF ACTION
3.5.1 Pharmacokinetic Mechanisms
Ingestion is the major route of exposure to exogenous nitrate and nitrite. Nitrate is assumed to enter the
blood from the upper small intestinal tract via active transport (EPA 1990b), which may involve active
transport systems such as the sodium iodide symporter (NIS) because nitrate has been shown to be a
relatively weak competitive inhibitor of NIS (e.g., Eskandari et al. 1997) and the NIS-mediated uptake of
iodine from the intestine has been demonstrated (Nicola et al. 2009). Nitrite is readily absorbed via
diffusion across the gastric mucosa and wall of the small intestine (EPA 1990b). As described in detail in
Section 3.4, nitrate and nitrite are readily distributed throughout the body and a portion of plasma nitrate
is concentrated in the salivary gland at concentrations as much as 10 times that of plasma nitrate. Qin et
al. (2012) demonstrated that the scialic acid (SA)/H
+
cotransporter, sialin, is endogenously localized in the
plasma membrane of salivary gland cells and functions as an electrogenic 2NO
3
-
/H
+
cotransporter; this
active transport mechanism may be responsible for high concentrations of nitrate in the salivary gland.
Refer to Section 3.4 for information regarding metabolic pathways involved in the nitrate-nitrite-nitric
oxide cycle. No information was located regarding specific mechanisms involved in transfer of nitrate to
the urine.
123 NITRATE AND NITRITE
3. HEALTH EFFECTS
3.5.2 Mechanisms of Toxicity
The most sensitive and widely-recognized toxic effect of nitrate and nitrite is that of nitrite-induced
methemoglobinemia in which nitrite (ingested as nitrite, formed via bacterial reduction of ingested nitrate,
and/or produced as an endogenous product of the nitric oxide oxidation) reacts with ferrous (Fe
2+
)
hemoglobin (which transports oxygen) to form ferric (Fe
3+
) hemoglobin (methemoglobin, a poor
transporter of oxygen) (refer to Section 3.4 for additional information regarding the nitrate-nitrite-nitric
oxide pathway).
As stated in Section 3.2.2.2 (Endocrine Effects), nitrate is a dose-dependent competitive inhibitor of the
NIS, which mediates the uptake of iodine by the thyroid. Sufficiently decreased iodine uptake by the
thyroid might result in decreased production of thyroid hormones T3 and T4 and consequent adverse
effects associated with thyroid dysfunction (e.g., hypothyroidism), including effects on developing
fetuses.
Proposed mechanisms of carcinogenicity involve the production of N-nitrosamines via nitrosating
reactions that involve nitrite and amines or amides. Such reactions may occur within some food items
during storage or preparation or in the body (usually in the stomach) (Mirvish 1975). The National
Toxicology Program’s 12
th
Report on Carcinogens (NTP 2011) lists 17 N-nitroso compounds (mostly
nitrosamines) as reasonably anticipated to be a human carcinogen based on sufficient evidence of
carcinogenicity from studies in experimental animals and one nitrosourea compound as known to be a
human carcinogen and one nitrosourea compound (1-(2-chloroethyl)-3-(4-methylcyclohexyl)-
1-nitrosourea) as known to be a human carcinogen based on sufficient evidence of carcinogenicity from
studies in humans. The International Agency for Research on Cancer (IARC 2014) lists eight of these
compounds in Group 2A (probably carcinogenic to humans), another eight in Group 2B (possibly
carcinogenic to humans), and two compounds (N-nitrosopiperadine and 4-(N-nitrosomethylamino)-
1-(3-pyridyl)-1-butanone) in Group 1 (carcinogenic to humans). Interactions between nitrite and a variety
of drugs have been shown to result in the formation of carcinogenic N-nitroso compounds (Brambilla and
Martelli (2007).
3.5.3 Animal-to-Human Extrapolations
Interspecies differences in nitrate-nitrite-nitric acid pathways indicate that laboratory animals do not
represent reliable models of nitrate-nitrite-nitric oxide pathways for humans (EPA 1990b; Health Canada
2012; Kortboyer et al. 1997a, 1997b; Walker 1995; WHO 2011b). For example, Til et al. (1988) reported
124 NITRATE AND NITRITE
3. HEALTH EFFECTS
that the rate of conversion of nitrate to nitrite is much lower in rats than humans. Cohen and Myant
(1959) reported that the rat lacks the active transport mechanism (sodium iodide symporter) responsible
for secretion of plasma nitrate to the salivary gland in humans. Therefore, the rate of reduction of salivary
nitrate to nitrite in the rat is likely much less than the estimate of 25% reduction in human saliva.
3.6 TOXICITIES MEDIATED THROUGH THE NEUROENDOCRINE AXIS
Recently, attention has focused on the potential hazardous effects of certain chemicals on the endocrine
system because of the ability of these chemicals to mimic or block endogenous hormones. Chemicals
with this type of activity are most commonly referred to as endocrine disruptors. However, appropriate
terminology to describe such effects remains controversial. The terminology endocrine disruptors,
initially used by Thomas and Colborn (1992), was also used in 1996 when Congress mandated the EPA to
develop a screening program for “...certain substances [which] may have an effect produced by a
naturally occurring estrogen, or other such endocrine effect[s]...”. To meet this mandate, EPA convened a
panel called the Endocrine Disruptors Screening and Testing Advisory Committee (EDSTAC), and in
1998, the EDSTAC completed its deliberations and made recommendations to EPA concerning endocrine
disruptors. In 1999, the National Academy of Sciences released a report that referred to these same types
of chemicals as hormonally active agents. The terminology endocrine modulators has also been used to
convey the fact that effects caused by such chemicals may not necessarily be adverse. Many scientists
agree that chemicals with the ability to disrupt or modulate the endocrine system are a potential threat to
the health of humans, aquatic animals, and wildlife. However, others think that endocrine-active
chemicals do not pose a significant health risk, particularly in view of the fact that hormone mimics exist
in the natural environment. Examples of natural hormone mimics are the isoflavinoid phytoestrogens
(Adlercreutz 1995; Livingston 1978; Mayr et al. 1992). These chemicals are derived from plants and are
similar in structure and action to endogenous estrogen. Although the public health significance and
descriptive terminology of substances capable of affecting the endocrine system remains controversial,
scientists agree that these chemicals may affect the synthesis, secretion, transport, binding, action, or
elimination of natural hormones in the body responsible for maintaining homeostasis, reproduction,
development, and/or behavior (EPA 1997). Stated differently, such compounds may cause toxicities that
are mediated through the neuroendocrine axis. As a result, these chemicals may play a role in altering,
for example, metabolic, sexual, immune, and neurobehavioral function. Such chemicals are also thought
to be involved in inducing breast, testicular, and prostate cancers, as well as endometriosis (Berger 1994;
Giwercman et al. 1993; Hoel et al. 1992).
125 NITRATE AND NITRITE
3. HEALTH EFFECTS
As discussed in detail in Section 3.2.2.2 (Endocrine Effects), available human data provide some evidence
that elevated levels of nitrate in drinking water and/or nitrate-rich diets may be associated with signs of
thyroid dysfunction (Aschebrook-Kilfoy et al. 2012; Gatseva and Argirova 2008; Rádiková et al. 2008;
Tajtáková et al. 2006; Ward et al. 2010). In animals, orally-administered nitrate has been demonstrated to
cause decreased iodine uptake by the thyroid and changes in serum thyroid hormone levels (e.g.,
Bloomfield et al. 1961; El-Wakf et al. 2008; Eskiocak et al. 2005; Mukhopadhyay et al. 2005; Zaki et al.
2004).
No in vitro studies were located regarding endocrine disruption of nitrate or nitrite.
3.7 CHILDREN’S SUSCEPTIBILITY
This section discusses potential health effects from exposures during the period from conception to
maturity at 18 years of age in humans, when most biological systems will have fully developed. Potential
effects on offspring resulting from exposures of parental germ cells are considered, as well as any indirect
effects on the fetus and neonate resulting from maternal exposure during gestation and lactation.
Relevant animal and in vitro models are also discussed.
Children are not small adults. They differ from adults in their exposures and may differ in their
susceptibility to hazardous chemicals. Children’s unique physiology and behavior can influence the
extent of their exposure. Exposures of children are discussed in Section 6.6, Exposures of Children.
Children sometimes differ from adults in their susceptibility to adverse health effects from exposure to
hazardous chemicals, but whether there is a difference depends on the chemical(s) (Guzelian et al. 1992;
NRC 1993). Children may be more or less susceptible than adults to exposure-related health effects, and
the relationship may change with developmental age (Guzelian et al. 1992; NRC 1993). Vulnerability
often depends on developmental stage. There are critical periods of structural and functional
development during both prenatal and postnatal life that are most sensitive to disruption from exposure to
hazardous substances. Damage from exposure in one stage may not be evident until a later stage of
development. There are often differences in pharmacokinetics and metabolism between children and
adults. For example, absorption may be different in neonates because of the immaturity of their
gastrointestinal tract and their larger skin surface area in proportion to body weight (Morselli et al. 1980;
NRC 1993); the gastrointestinal absorption of lead is greatest in infants and young children (Ziegler et al.
1978). Distribution of xenobiotics may be different; for example, infants have a larger proportion of their
126 NITRATE AND NITRITE
3. HEALTH EFFECTS
bodies as extracellular water, and their brains and livers are proportionately larger (Altman and Dittmer
1974; Fomon 1966; Fomon et al. 1982; Owen and Brozek 1966; Widdowson and Dickerson 1964). Past
literature has often described the fetus/infant as having an immature (developing) blood-brain barrier that
is leaky and poorly intact (Costa et al. 2004). However, current evidence suggests that the blood-brain
barrier is anatomically and physically intact at this stage of development, and the restrictive intracellular
junctions that exist at the blood-CNS interface are fully formed, intact, and functionally effective
(Saunders et al. 2008, 2012).
However, during development of the brain, there are differences between fetuses/infants and adults that
are toxicologically important. These differences mainly involve variations in physiological transport
systems that form during development (Ek et al. 2012). These transport mechanisms (influx and efflux)
play an important role in the movement of amino acids and other vital substances across the blood-brain
barrier in the developing brain; these transport mechanisms are far more active in the developing brain
than in the adult. Because many drugs or potential toxins may be transported into the brain using these
same transport mechanisms—the developing brain may be rendered more vulnerable than the adult.
Thus, concern regarding possible involvement of the blood-brain barrier with enhanced susceptibility of
the developing brain to toxins is valid. It is important to note however, that this potential selective
vulnerability of the developing brain is associated with essential normal physiological mechanisms; and
not because of an absence or deficiency of anatomical/physical barrier mechanisms.
The presence of these unique transport systems in the developing brain of the fetus/infant is intriguing;
whether these mechanisms provide protection for the developing brain or render it more vulnerable to
toxic injury is an important toxicological question. Chemical exposure should be assessed on a case-by-
case basis. Research continues into the function and structure of the blood-brain barrier in early life
(Kearns et al. 2003; Saunders et al. 2012; Scheuplein et al. 2002).
Many xenobiotic metabolizing enzymes have distinctive developmental patterns. At various stages of
growth and development, levels of particular enzymes may be higher or lower than those of adults, and
sometimes unique enzymes may exist at particular developmental stages (Komori et al. 1990; Leeder and
Kearns 1997; NRC 1993; Vieira et al. 1996). Whether differences in xenobiotic metabolism make the
child more or less susceptible also depends on whether the relevant enzymes are involved in activation of
the parent compound to its toxic form or in detoxification. There may also be differences in excretion,
particularly in newborns given their low glomerular filtration rate and not having developed efficient
tubular secretion and resorption capacities (Altman and Dittmer 1974; NRC 1993; West et al. 1948).
127 NITRATE AND NITRITE
3. HEALTH EFFECTS
Children and adults may differ in their capacity to repair damage from chemical insults. Children also
have a longer remaining lifetime in which to express damage from chemicals; this potential is particularly
relevant to cancer.
Certain characteristics of the developing human may increase exposure or susceptibility, whereas others
may decrease susceptibility to the same chemical. For example, although infants breathe more air per
kilogram of body weight than adults breathe, this difference might be somewhat counterbalanced by their
alveoli being less developed, which results in a disproportionately smaller surface area for alveolar
absorption (NRC 1993).
As discussed in detail in Section 3.4 (Toxicokinetics), a portion of ingested nitrate is reduced to nitrite by
commensal bacteria in the mouth; however, the acid environment of the normal stomach does not support
the growth of such bacteria. Most nitrite (ingested or reduced from nitrate) is absorbed from the upper
gastrointestinal tract and enters the blood; plasma nitrite readily reacts with hemoglobin to form
methemoglobin. Sufficiently high levels of methemoglobin levels result in poor oxygen supply to tissues.
Clinical methemoglobinemia is generally indicated at methemoglobin levels >10% of total hemoglobin
and cyanosis is an early clinical sign. The first 6 months of postnatal life is a period of increased
susceptibility to methemoglobinemia (termed infantile methemoglobinemia or blue baby syndrome);
possible contributing factors to this increased susceptibility (pH of the infant stomach, proportion of fetal
hemoglobin to adult hemoglobin, and concentration of NADH-dependent methemoglobin reductase)
(Greer and Shannon 2005) are discussed below.
A portion of ingested nitrate is reduced to nitrite by commensal bacteria in the mouth; however, the acid
environment of the normal stomach does not support the growth of such bacteria and most of the nitrate
that reaches the stomach passes to the small intestine from which it is nearly completely absorbed into the
blood. However, a higher pH in the stomach of the newborn may favor growth of nitrate-reducing
bacteria and increased reduction of nitrate to nitrite and consequent increased plasma methemoglobin.
Most hemoglobin in the newborn is in a form termed fetal hemoglobin, which appears to be more readily
oxidized to methemoglobin than adult hemoglobin; fetal hemoglobin is replaced by adult hemoglobin
during early postnatal life. Levels of NADH-dependent methemoglobin reductase (the major enzyme
responsible for reduction of methemoglobin to normal hemoglobin) in the newborn increase
approximately 2-fold during the first 4 month of postnatal life to reach adult levels.
128 NITRATE AND NITRITE
3. HEALTH EFFECTS
There is some evidence that methemoglobinemia in infants drinking formula prepared using drinking
water with relatively high levels of nitrate may be related to bacterial contamination of such water sources
and consequent gastrointestinal disorders, as well as gastrointestinal infection and inflammation and the
ensuing overproduction of nitric oxide (Avery 1999). Kanady et al. (2012) reported little or no bacterial
conversion of nitrate to nitrite in the saliva of a group of 10 infants during the first 2 postnatal months that
was considered mainly due to lower numbers of major nitrate-reducing oral bacteria than adults. Ibrahim
et al. (2012) found that blood nitrite levels of newborns approximately 1–2 days of age were 35–55%
lower than that of adults.
Some investigators have reported significant associations between nitrate levels in drinking water (or
living in areas presumed to have elevated nitrate levels in drinking water sources) and risk of childhood
type 1 diabetes (Dahlquist et al. 1990; Kostraba et al. 1992; Parslow et al. 1997; Virtanen et al. 1994).
However, no such relationship was observed in two other studies (van Maanen et al. 2000; Zhao et al.
2001). Refer to Section 3.2.2.2 (Metabolic Effects) for summaries of these study reports.
Results of studies designed to assess possible associations between nitrate levels in drinking water
sources and developmental end points in humans provide equivocal evidence of nitrate-related effects on
the developing fetus and infant (see Section 3.2.2.6, Developmental Effects). There is limited evidence of
nitrate-induced thyroid dysfunction (see Section 3.2.2.2, Endocrine Effects), which could result in adverse
effects on the developing fetus of a pregnant mother.
3.8 BIOMARKERS OF EXPOSURE AND EFFECT
Biomarkers are broadly defined as indicators signaling events in biologic systems or samples. They have
been classified as markers of exposure, markers of effect, and markers of susceptibility (NAS/NRC
1989).
The National Report on Human Exposure to Environmental Chemicals provides an ongoing assessment
of a generalizable sample of the exposure of the U.S. population to environmental chemicals using
biomonitoring. This report is available at http://www.cdc.gov/exposurereport/. The biomonitoring data
for nitrate from this report is discussed in Section 6.5. A biomarker of exposure is a xenobiotic substance
or its metabolite(s) or the product of an interaction between a xenobiotic agent and some target
molecule(s) or cell(s) that is measured within a compartment of an organism (NAS/NRC 1989). The
preferred biomarkers of exposure are generally the substance itself, substance-specific metabolites in
129 NITRATE AND NITRITE
3. HEALTH EFFECTS
readily obtainable body fluid(s), or excreta. However, several factors can confound the use and
interpretation of biomarkers of exposure. The body burden of a substance may be the result of exposures
from more than one source. The substance being measured may be a metabolite of another xenobiotic
substance (e.g., high urinary levels of phenol can result from exposure to several different aromatic
compounds). Depending on the properties of the substance (e.g., biologic half-life) and environmental
conditions (e.g., duration and route of exposure), the substance and all of its metabolites may have left the
body by the time samples can be taken. It may be difficult to identify individuals exposed to hazardous
substances that are commonly found in body tissues and fluids (e.g., essential mineral nutrients such as
copper, zinc, and selenium). Biomarkers of exposure to nitrate and nitrite are discussed in Section 3.8.1.
Biomarkers of effect are defined as any measurable biochemical, physiologic, or other alteration within an
organism that, depending on magnitude, can be recognized as an established or potential health
impairment or disease (NAS/NRC 1989). This definition encompasses biochemical or cellular signals of
tissue dysfunction (e.g., increased liver enzyme activity or pathologic changes in female genital epithelial
cells), as well as physiologic signs of dysfunction such as increased blood pressure or decreased lung
capacity. Note that these markers are not often substance specific. They also may not be directly
adverse, but can indicate potential health impairment (e.g., DNA adducts). Biomarkers of effects caused
by nitrate and nitrite are discussed in Section 3.8.2.
A biomarker of susceptibility is an indicator of an inherent or acquired limitation of an organism's ability
to respond to the challenge of exposure to a specific xenobiotic substance. It can be an intrinsic genetic or
other characteristic or a preexisting disease that results in an increase in absorbed dose, a decrease in the
biologically effective dose, or a target tissue response. If biomarkers of susceptibility exist, they are
discussed in Section 3.10, Populations That Are Unusually Susceptible.
3.8.1 Biomarkers Used to Identify or Quantify Exposure to Nitrate and Nitrite
There are no biomarkers of exposure that are specific to nitrate or nitrite. Although nitrate and nitrite can
be detected in blood, saliva, and urine (mostly nitrate), nitrate and nitrite are also produced endogenously
via the nitrate-nitrite-nitric oxide pathway. Sources for nitrate and nitrite levels in the body may therefore
include not only ingested food and drinking water, but also oxidation of nitric oxide produced
endogenously. Similarly, N-nitroso compounds that may be detected in the blood or urine may indicate
exposure to nitrate or nitrite; however, these compounds may also be products of the endogenous nitrate-
nitrite-nitric oxide pathway.
130 NITRATE AND NITRITE
3. HEALTH EFFECTS
3.8.2 Biomarkers Used to Characterize Effects Caused by Nitrate and Nitrite
Biomarkers of effects from exposure to nitrate or nitrite are not specific to nitrate or nitrite. Blood
methemoglobin level has been used as a biomarker of nitrate and nitrite toxicity; however,
methemoglobinemia may be elicited by other substances such as selected drugs, pesticides, industrial and
commercial products, and medical conditions such as pediatric gastrointestinal infection, sepsis, and sickle
cell crisis (ATSDR 2013a). Methemoglobinemia may also be inherited (genetic conditions that result in
decreased activity of enzymes that reduce methemoglobin or the presence of hemoglobin M). Jansen et al.
(1995) reported a rapid 6-fold increase in urinary N-methylnicotinamide (a metabolite of tryptophan) in
four of eight volunteers following the ingestion of sodium nitrate at 10 mg/kg; however, little to no
increase in urinary N-methylnicotinamide was observed in the other four volunteers. Urinary levels of
various other N-nitroso compounds (e.g., nitrosoproline) have been measured as an index of nitrosation
(Ohshima and Bartsch 1988); however, N-nitroso compounds can form via endogenous nitrosation and do
not require the intake of nitrate or nitrite.
3.9 INTERACTIONS WITH OTHER CHEMICALS
Information regarding interactions between nitrate or nitrite and other substances is comprised mainly of
studies that assessed the tumorigenicity of oral exposure to sodium nitrite in the presence of selected
amino compounds or other substances suspected or known to cause cancer and studies that assessed
modulation of tumorigenicity by selected antioxidants. As discussed in Section 3.5 (Mechanisms of
Action), nitrosating reactions that involve nitrite and amines or amides may result in the production of
N-nitrosamines, some of which may be carcinogenic. Interactions between nitrite and a variety of drugs
may also result in the formation of carcinogenic N-nitroso compounds (Brambilla and Martelli (2007).
Adverse effects elicited in laboratory animals exposed to selected substances were enhanced or
diminished upon co-exposure to nitrite, although mechanisms for such nitrite-induced enhanced or
diminished responses have not been identified. For example, Kawabe et al. (1994) observed increased
severity of forestomach hyperplasia in groups of catechol- or 3-methoxycatechol-treated rats
coadministered sodium nitrite and increased thickness of forestomach mucosa (indication of cellular
proliferation) in rats treated with sodium nitrite in combination with phenolic compounds such as
t-butylhydroquinone, catechol, gallic acid, 1,2,4-benzenetriol, dl-3-(3,4-dihydroxyphenyl)-alanine, and
hydroquinone. Coadministration of sodium nitrite with catechol resulted in enhanced cellular
proliferation. Pregnant Syrian golden hamsters fed a diet containing nitrite and morpholine exhibited a
131 NITRATE AND NITRITE
3. HEALTH EFFECTS
higher incidence of liver-cell carcinoma (5/16 hamsters) compared to those fed diets containing
morpholine in the absence of nitrite (0/22) (Shank and Newberne 1976). Sodium nitrite treatment
resulted in increased incidences of forestomach papillomas and decreased incidences of glandular
stomach epithelial adenomas in rats provided drinking water to which sodium nitrite and either catechol
or 3-methoxycatechol were added either with or without coexposure to known carcinogens (Hirose et al.
1990, 1993). IARC (2010) summarized results from a Russian study (Ilnitsky and Kolpakova 1997) in
which sodium nitrite appeared to enhance the carcinogenic effect of leukemia viruses in mice. Hirose et
al. (2002) observed a reduction of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine-induced mammary
gland tumors in rats coexposed to sodium nitrite in the drinking water. Commoner et al. (1970) reported
an inhibition of the tumorigenic action of 2-acetylaminofluorene in rats co-treated with nitrite.
Nitrate, thiocyanate, and perchlorate are dose-dependent competitive inhibitors of the sodium-iodide
symporter (NIS), which mediates the uptake of iodine by the thyroid (De Groef et al. 2006).
Overexposure to any one of these competitive inhibitors could decrease iodine uptake and result in
thyroid dysfunction; this effect could be more severe during exposures to combinations of these
substances (and possibly other NIS competitive inhibitors).
3.10 POPULATIONS THAT ARE UNUSUALLY SUSCEPTIBLE
A susceptible population will exhibit a different or enhanced response to nitrate and nitrite than will most
persons exposed to the same level of nitrate and nitrite in the environment. Factors involved with
increased susceptibility may include genetic makeup, age, health and nutritional status, and exposure to
other toxic substances (e.g., cigarette smoke). These parameters result in reduced detoxification or
excretion of nitrate and nitrite, or compromised function of organs affected by nitrate and nitrite.
Populations who are at greater risk due to their unusually high exposure to nitrate and nitrite are discussed
in Section 6.7, Populations with Potentially High Exposures.
Infants 1–6 months of age appear to be particularly sensitive to nitrite-induced methemoglobinemia
following ingestion of formula prepared from drinking water containing elevated levels of nitrate (see
Section 3.7 for detailed discussion of biological factors that may be responsible for increased sensitivity
of infants). Infants with gastroenteritis may be at increased risk for nitrite-induced methemoglobinemia,
although nitrite and nitrate generation from oxidation of endogenous nitric oxide produced under
inflammatory conditions may be a major contributory factor (Avery 1999). Individuals with higher-than-
normal gastric pH (e.g., achlorhydria, a condition whereby gastric acid production is low or absent;
132 NITRATE AND NITRITE
3. HEALTH EFFECTS
individuals taking antacids) may be at increased risk of methemoglobinemia if the gastric environment
supports survival of nitrate-reducing bacteria.
Some epidemiological studies provide suggestive evidence of associations between exposure to nitrates in
drinking water and spontaneous abortions, intrauterine growth restriction, and selected birth defects (e.g.,
Brender et al. 2013; Bukowski et al. 2001; CDC 1996; Dorsch et al. 1984; Schmitz 1961; Tabacova et al.
1997, 1998). Results from these studies suggest that the pregnant mother and her developing fetus might
be particularly susceptible to nitrate/nitrite toxicity. However, estimates of nitrate intakes were typically
based on measurements of nitrate levels in drinking water sources at selected time points and self-
reported estimates of water consumption. Furthermore, possible confounding by other potential toxicants
was not evaluated and studies did not typically account for dietary nitrate or nitrite.
Other factors that may contribute to increased risk of methemoglobinemia include glucose-6-phosphate
dehydrogenase deficiency (which can result in decreased numbers of red blood cells); deficiency in
NADH-dependent methemoglobin reductase (the major enzyme responsible for the reduction of
methemoglobin to normal hemoglobin); diseases such as anemia, cardiovascular disease, lung disease,
and sepsis; and abnormal hemoglobin species including carboxyhemoglobin, sulfhemoglobin, and sickle
hemoglobin. Individuals consuming diets deficient in selected antioxidants (e.g., vitamin C, vitamin E)
might be at increased risk of cancer associated with the production of potentially carcinogenic N-nitroso
compounds (WHO 2011b).
3.11 METHODS FOR REDUCING TOXIC EFFECTS
This section will describe clinical practice and research concerning methods for reducing toxic effects of
exposure to nitrate and nitrite. Because some of the treatments discussed may be experimental and
unproven, this section should not be used as a guide for treatment of exposures to nitrate and nitrite.
When specific exposures have occurred, poison control centers, board certified medical toxicologists,
board-certified occupational medicine physicians and/or other medical specialists with expertise and
experience treating patients overexposed to nitrate and nitrite can be consulted for medical advice. The
following texts provide specific information about treatment following exposures to nitrate and nitrite:
Barclay PJ. 1998. Nitrates and nitrites. In: Viccellio P, ed. Emergency toxicology. 2
nd
ed.
Philadelphia, PA: Lippincott-Raven Publishers, 315-323.
Leikin JB, Paloucek FP, eds. 2008. Poisoning and toxicology handbook. 4th ed. Boca Raton, FL: CRC
Press, 830.
133 NITRATE AND NITRITE
3. HEALTH EFFECTS
Seifert SA. 2004. Nitrates and nitrites. In: Dart RC, ed. Medical toxicology. 3
rd
ed. Philadelphia, PA:
Lippincott Williams & Williams, 1174-1180.
Additional relevant information can be found in the front section of this profile under QUICK
REFERENCE FOR HEALTH CARE PROVIDERS.
3.11.1 Reducing Peak Absorption Following Exposure
Ingestion is the most likely route of overexposure to nitrate or nitrite. Nitrate and nitrite bind to activated
charcoal, which may be administered (1 g/kg without cathartic) within 1–2 hours following significant
ingestion (Seifert 2004). Use of mouthwash containing chlorhexidine (an active antibacterial) resulted in
a large decrease in the mean percent reduction of salivary nitrate to nitrite (van Maanen et al. 1996b).
3.11.2 Reducing Body Burden
No information was located regarding methods to reduce the body burden of nitrate or nitrite.
3.11.3 Interfering with the Mechanism of Action for Toxic Effects
Severe methemoglobinemia (methemoglobin levels generally >30% of total hemoglobin) can be reduced
by intravenous administration of methylene blue (1–2 mg/kg) (Barclay 1998; Leikin and Paloucek 2008;
Seifert 2004). Exchange transfusions may be considered for patients who do not respond to methylene
blue (particularly patients with glucose-6-phosphate dehydrogenase deficiency or hemoglobin M), and
patients where methylene blue is contraindicated (e.g., patients on serotonin uptake inhibitors) (ATSDR
2013a; Barclay 1998). In symptomatic patients, 100% oxygen and assisted ventilation should be
considered; seizures can be treated with oxygen and benzodiazepines, followed by phenobarbital (Seifert
2004). Hyperbaric oxygen therapy may be of some benefit, but has not been demonstrated in controlled
studies (Leikin and Paloucek 2008; Seifert 2004). Management of nitrite-induced hypotension involves
placement of the patient in Trendelenburg position, administration of intravenous isotonic fluids at 10–
20 mL/kg bolus and as required thereafter, and pressors such as dopamine or norepinephrine, as needed
(Seifert 2004).
In several rat studies, tumorigenicity associated with concurrent exposure to nitrite and various amino
compounds was modulated by coexposure to selected antioxidants such as ascorbic acid, catechol,
3-methoxycatechol, tert-butylhydroquinone, α-tocopherol, and propyl gallate (Chan and Fong 1977;
134 NITRATE AND NITRITE
3. HEALTH EFFECTS
Mirvish et al. 1976, 1983; Miyauchi et al. 2002; Mohktar et al. 1988; Yada et al. 2002; Yoshida et al.
1994); thioproline (which may serve as a nitrite scavenger when nitrosated to nitrosothioproline) (Tahira
et al. 1988); or soy bean (Mokhtar et al. 1988).
3.12 ADEQUACY OF THE DATABASE
Section 104(I)(5) of CERCLA, as amended, directs the Administrator of ATSDR (in consultation with the
Administrator of EPA and agencies and programs of the Public Health Service) to assess whether
adequate information on the health effects of nitrate and nitrite is available. Where adequate information
is not available, ATSDR, in conjunction with the National Toxicology Program (NTP), is required to
assure the initiation of a program of research designed to determine the adverse health effects (and
techniques for developing methods to determine such health effects) of nitrate and nitrite.
The following categories of possible data needs have been identified by a joint team of scientists from
ATSDR, NTP, and EPA. They are defined as substance-specific informational needs that if met would
reduce the uncertainties of human health risk assessment. This definition should not be interpreted to
mean that all data needs discussed in this section must be filled. In the future, the identified data needs
will be evaluated and prioritized, and a substance-specific research agenda will be proposed.
3.12.1 Existing Information on Health Effects of Nitrate and Nitrite
The existing data on health effects of inhalation, oral, and dermal exposure of humans and animals to
nitrate and nitrite are summarized in Figures 3-5 and 3-6. The purpose of this figure is to illustrate the
existing information concerning the health effects of nitrate and nitrite. Each dot in the figure indicates
that one or more studies provide information associated with that particular effect. The dot does not
necessarily imply anything about the quality of the study or studies, nor should missing information in
this figure be interpreted as a “data need”. A data need, as defined in ATSDR’s Decision Guide for
Identifying Substance-Specific Data Needs Related to Toxicological Profiles (Agency for Toxic
Substances and Disease Registry 1989), is substance-specific information necessary to conduct
comprehensive public health assessments. Generally, ATSDR defines a data gap more broadly as any
substance-specific information missing from the scientific literature.

135 NITRATE AND NITRITE
3. HEALTH EFFECTS
Figure 3-5. Existing Information on Health Effects of Nitrate
Death
Acute
Intermediate
Chronic
Immunologic/Lymphoretic
Neurologic
Reproductive
Developmental
Genotoxic
Cancer
Syste mic
Inhalation
Oral
Dermal
Human
Death
Acute
Intermediate
Chronic
Immunologic/Lymphoretic
Neurologic
Reproductive
Developmental
Genotoxic
Cancer
Syste mic
Inhalation
Oral
Dermal
Animal
Existing Studies

136 NITRATE AND NITRITE
3. HEALTH EFFECTS
Figure 3-6. Existing Information on Health Effects of Nitrite
Death
Acute
Intermediate
Chronic
Immunologic/Lymphoretic
Neurologic
Reproductive
Developmental
Genotoxic
Cancer
Syste mic
Inhalation
Oral
Dermal
Human
Death
Acute
Intermediate
Chronic
Immunologic/Lymphoretic
Neurologic
Reproductive
Developmental
Genotoxic
Cancer
Syste mic
Inhalation
Oral
Dermal
Animal
Existing Studies
137 NITRATE AND NITRITE
3. HEALTH EFFECTS
3.12.2 Identification of Data Needs
Acute-Duration Exposure. No information was located regarding the effects of acute-duration
inhalation exposure to nitrate or nitrite in humans. Available information in laboratory animals is limited.
RTECS (2014) lists a rat 4-hour LC
50
of 5.5 mg/m
3
(1.95 ppm) for sodium nitrite and a rat 2-hour LC
50
of
85 mg/m
3
(24.42 ppm) for potassium nitrite. There was no evidence of exposure-related pulmonary or
cardiac effects in anesthetized dogs exposed at up to 10 mg sodium nitrate/m
3
(2.88 ppm) for 7.5 minutes
or anesthetized dogs or conscious sheep exposed at 5 mg sodium nitrate/m
3
(1.44 ppm) for 4 hours.
Additional information regarding the effects of acute-duration inhalation exposure to nitrate or nitrite is
not considered necessary because the general population is not likely to be exposed to airborne nitrate or
nitrite concentrations at levels that might cause adverse health effects.
Refer to the section titled “Epidemiological and Human Dosimetry Studies” for a summary of available
information regarding noncancer effects in humans following oral exposure to nitrate or nitrite.
Among laboratory animals, acute oral LD
50
values range from 1,267 to 3,750 mg/kg for selected nitrate
salts (RTECS 2014) and from 150 to 200 mg/kg for selected nitrite salts (Imaizumi et al. 1980; RTECS
2014; Sheehy and Way 1974). Imaizumi et al. (1980) administered aqueous sodium nitrite to fasted
Sprague-Dawley rats by gavage and observed dose-related increased methemoglobin levels. Additional
studies regarding the effects of acute-duration oral exposure of laboratory animals to nitrate or nitrite are
not considered necessary, in part due to interspecies differences in kinetics of the nitrate-nitrite-nitric
oxide pathway.
No information was located regarding health effects in humans or animals following acute-duration
dermal exposure to nitrate or nitrite. Information regarding the effects of acute-duration dermal exposure
to nitrate or nitrite is not considered necessary because the general population is not likely to be dermally-
exposed to nitrate or nitrite concentrations at levels that might cause adverse health effects.
Intermediate-Duration Exposure. No information was located regarding the effects of
intermediate-duration inhalation exposure to nitrate or nitrite in humans or animals. The general
population is not likely to be exposed to airborne nitrate or nitrite concentrations at levels that might
cause adverse health effects.
138 NITRATE AND NITRITE
3. HEALTH EFFECTS
Refer to the section titled “Epidemiological and Human Dosimetry Studies” for a summary of available
information regarding noncancer effects in humans following oral exposure to nitrate or nitrite.
Epithelial hyperplasia was noted in the forestomach of mice provided sodium nitrite in the drinking water
for 14 weeks (NTP 2001). Another study found no evidence of treatment-related forestomach lesions in
male rats provided sodium nitrite in the drinking water for 35 weeks (Kawabe et al. 1994). Increased
methemoglobin levels and other evidence of hematological effects have been reported in laboratory
animals administered sodium nitrite or potassium nitrite orally for intermediate-duration time periods
(Behroozi et al. 1972; Chow et al. 1980; Grant and Butler 1989; Imaizumi et al. 1980; NTP 2001; Shuval
and Gruener 1972; Til et al. 1988, 1997). Several animal studies found no indications of sodium nitrite-
induced effects on liver function or histopathology (Asahina et al. 1971; Lijinsky and Greenblatt 1972;
Lin and Ho 1992; Shuval and Gruener 1972; van Logten et al. 1972). El-Wakf et al. (2008) reported
significantly increased urinary levels of urea and creatinine in male rats provided sodium nitrate in the
drinking water for 4 months. Sodium or potassium nitrate-induced effects on the endocrine system of
laboratory animals have been reported by several groups of investigators; effects include decreased serum
thyroidal iodine uptake, decreased serum thyroid hormone levels, increased thyroid weight, and follicular
hyperplasia (El-Wakf et al. 2008; Eskiocak et al. 2005; Mukhopadhyay et al. 2005; Zaki et al. 2004). Til
et al. (1988, 1997) observed adrenal gland hypertrophy in rats administered potassium nitrite in the
drinking water for 13 weeks; results of a subsequent study indicated that this effect was a physiological
adaptation to repeated episodes of hypotension caused by nitrite (RIVM 1996). Depressed body weight
and/or body weight gain were observed in some laboratory animals receiving nitrate or nitrite from the
drinking water for intermediate exposure durations (El-Wakf et al. 2008; Maekawa et al. 1982; Zaki et al.
2004). Intermediate-duration oral exposure to sodium nitrite in the drinking water of laboratory animals
has been associated with neurological effects such as abnormalities in EEGs (Behroozi et al. 1972),
increased aggressive behavior (Gruener 1974), and reduced motor activity (Shuval and Gruener 1972).
Available intermediate-duration oral studies in laboratory animals adequately characterize nitrate- and
nitrite-induced effects; additional animal studies do not appear necessary.
No information was located regarding health effects in humans or animals following intermediate-
duration dermal exposure to nitrate or nitrite. Information regarding the effects of intermediate-duration
dermal exposure to nitrate or nitrite is not considered necessary because the general population is not
likely to be dermally-exposed to nitrate or nitrite concentrations at levels that might cause adverse health
effects.
139 NITRATE AND NITRITE
3. HEALTH EFFECTS
Chronic-Duration Exposure and Cancer. Information regarding the effects of chronic-duration
inhalation exposure is limited. A cohort mortality study of male workers involved in the manufacture of
nitrate fertilizer for at least 1 year between 1946 and 1981 found no evidence of associations between
exposure to nitrate dusts and death from respiratory or circulatory diseases (Al-Dabbagh et al. 1986).
Among workers described as having been heavily exposed to nitrate dust, slight excesses were noted for
death from lung cancer and death from all malignant neoplasms, but not for cancers of the esophagus,
stomach, or bladder. After categorizing the heavily-exposed workers by duration of exposure and time
since first exposure, excess death from lung cancer was noted for those exposed for ≥10 years, with a lag
time of ≥20 years since first exposure. The study authors indicated that they were unable to adjust for
smoking. In a census-based mortality study of workers involved in production of nitrate fertilizer, there
was no evidence of associations between exposure to nitrate dust and death from circulatory diseases;
slight excesses were noted for deaths from lung cancer and death from all malignant neoplasms, but not
for cancers of the esophagus, stomach, or bladder (Fraser et al. 1982, 1989). No significant increased risk
for cancer at any site was observed at 7-year follow-up evaluation. In yet another cohort of workers at a
nitrate fertilizer production facility (Hagmar et al. 1991), death from prostate cancer was in excess;
however, risk of prostate cancer within this cohort was not enhanced following application of a ≥10-year
latency period, and there was no significant increase in death from tumors of the lips, oral cavity,
pharynx, salivary glands, gastrointestinal tract, stomach, respiratory tract, lung, urinary bladder, blood, or
all sites combined. The general population is not likely to be exposed to airborne nitrate or nitrite
concentrations at levels that might cause adverse health effects.
Refer to the section titled “Epidemiological and Human Dosimetry Studies” for a summary of available
information regarding noncancer effects in humans following oral exposure to nitrate or nitrite.
Numerous case-control and cohort studies regarding the carcinogenicity of ingested nitrate and nitrite in
humans have been reported (IARC 2010). Many ecological studies have also been reported; however,
interpretation of outcomes of these studies is more uncertain because of various factors that contribute to
ecologic bias (group-based associations between exposure and cancer outcomes may not apply to
individuals). In general, outcomes of case-control and cohort studies have found no or weak associations
between exposure to nitrate and cancer in humans, with stronger associations with exposures to nitrite or
intake of high nitrite foods such as cured meat (Aschebrook et al. 2013; DellaValle et al. 2013; IARC
2010; Inoue-Choi et al. 2012). Mechanistically, this outcome is consistent with nitrite and being a
reactive intermediate in the cancer mode of action of nitrate. This is further supported by studies that
found interactions between cancer risk attributed to nitrite and exposure to antioxidants (IARC 2010;
140 NITRATE AND NITRITE
3. HEALTH EFFECTS
Inoue-Choi et al. 2012; Kim et al. 2007; Yang et al. 2010). Uncertainties in estimates of cancer risk from
exposure to nitrate or nitrite include those typical of epidemiological studies in general: uncertainties in
estimation of exposure (e.g., estimating long-term dietary intakes from food frequency questionnaires or
levels in PWS), exposure misclassification of individual outcomes (e.g., assigning group-level exposure
estimates to individuals), and adequacy of controlling for confounders (e.g., other factors contributing to
the cancer). One potentially important class of confounders is antioxidants that can interfere with
nitrosation of dietary amines, and thereby the mode of carcinogenicity of nitrite, and may also interfere
with other carcinogenic processes that involve reactive intermediates.
The strongest and most consistent evidence for carcinogenicity of nitrite is from studies of gastrointestinal
cancers and, in particular, gastric cancer (Buiatti et al. 1990; Engel et al. 2003; La Vecchia et al. 1994,
1997; Mayne et al. 2001; Palli et al. 2001; Risch et al. 1985; Rogers et al. 1995; Ward et al. 2007, 2008).
Results have been mixed for other types of cancer. Some case-control or cohort studies found
associations between exposure to nitrite (or foods high in nitrite such as cured meat) and brain cancer in
children and adults (Blowers et al. 1997; Giles et al. 1994, Huncharek and Kupelnick 2004; Huncharek et
al. 2003; Lee et al. 1997; Pogoda and Preston-Martin 2001a, 2001b; Preston-Martin et al. 1996; Mueller
et al. 2004), breast cancer (Inoue-Choi et al. 2012; Yang et al. 2010), kidney cancer (DellaValle et al.
2013; Ward et al. 2007; Wilkens et al. 1996), testicular cancer (Moller 1997), and non-Hodgkin’s
lymphoma (Ward et al. 2006). Of these studies, the highest risks were reported for brain cancers. Two
case-control studies found elevated relative risk of brain cancer in children in association with maternal
nitrite intake (Mueller et al. 2004; Pogoda and Preston-Martin 2001a, 2001b; Preston-Martin et al. 1996).
In general, case-control and cohort studies of cancers of larynx, liver, lung, mouth, pancreas, and pharynx
have found no consistent associations with exposures to nitrate or nitrite (IARC 2010).
The potential carcinogenicity of nitrate has been investigated in several animal studies that employed the
oral exposure route. Studies in which negative results were reported include MCR-derived rats provided
sodium nitrate in the drinking water for 84 weeks (Lijinsky et al. 1973a), male white rats provided sodium
nitrate in the drinking water for 273 days (Pliss and Frolov 1991), strain A male mice provided sodium
nitrate in the drinking water for 25 weeks (Greenblatt and Mirvish 1973), female NMRI mice provided
calcium nitrate in the drinking water for 18 months (Mascher and Marth 1993), Fischer 344 rats fed diet
containing sodium nitrate for 2 years (Maekawa et al. 1982), and ICR mice fed diets containing sodium
nitrate for 2 years (IARC 2010). In the study of Pliss and Frolov (1991), some groups of male rats were
treated with drinking water containing BBNA (an inducer of urinary bladder cancer in laboratory animals)
for 30 days, either alone or followed by sodium nitrate in the drinking water for 273 days. The group
141 NITRATE AND NITRITE
3. HEALTH EFFECTS
treated with BBNA followed by sodium nitrate exhibited significantly increased incidence of urinary
bladder carcinoma. These results indicate that sodium nitrate promoted BBNA-induced bladder tumors.
The potential carcinogenicity of ingested nitrite has been investigated in numerous animal studies. Nitrite
treatment alone was not associated with tumor incidences in most studies (Börzsönyi et al. 1978; Hawkes
et al. 1992; Inai et al. 1979; Lijinsky 1984a, 1984b; Lijinsky et al. 1983; Maekawa et al. 1982; NTP
2001). Significantly increased incidences of forestomach squamous papillomas were reported for male
and female MRC Wistar rats provided drinking water to which sodium nitrite was added for life (Mirvish
et al. 1980). Dose-related decreases in time of onset and incidence of lymphomas, mononuclear cell
leukemia, and testicular interstitial-cell tumors were reported for male and female F344 rats administered
reduced-protein diet to which sodium nitrite was added for up to 115 weeks (Grant and Butler 1989). In a
96-week study, Iurchenko et al. (1986) reported a significantly increased incidence of benign liver tumors
among male CBA mice receiving sodium nitrite from the drinking water at an author-estimated total dose
of 1,600 mg sodium nitrite/mouse; however, there was no apparent sodium nitrite treatment-related effect
at a higher estimated dose (2,000 mg sodium nitrite/mouse). Increased incidences of total tumors and
lymphoreticular tumors were reported in rats fed diet to which sodium nitrite was added; the results were
reported for F1 and F2 offspring that had been exposed via their mothers during gestation and lactation
and directly from the diet thereafter (Shank and Newberne 1976). A positive trend for incidences of
forestomach squamous cell papilloma or carcinoma (combined) in female B6C3F1 mice administered
sodium nitrite in the drinking water for 2 years was considered to provide "equivocal evidence of
carcinogenic activity" of sodium nitrite (NTP 2001). In a 26-month study of male and female Sprague-
Dawley rats provided drinking water to which sodium nitrite was added, the study author reported
increased incidence of lymphomas, but not other types of tumors (Newberne 1979); however, IARC
(2010) and NTP (2001) noted that a working group sponsored by the U.S. FDA reevaluated the histology
and did not confirm the results of Newberne (1979). IARC (2010) reported that the working group
considered the incidences of lymphomas to be similar to those arising spontaneously in Sprague-Dawley
rats.
The potential carcinogenicity of combined exposure to sodium nitrite and selected nitrosatable substances
(oral exposures via combinations of drinking water, diet, and/or gavage dosing) has been well-studied in
laboratory animals. Many of the studies included sodium nitrite-only treatment groups for which there
was no evidence of sodium-nitrite induced carcinogenicity (Anderson et al. 1985; Börzsönyi and Pintér
1977; Börzsönyi et al. 1976; Greenblatt and Lijinsky 1972, 1974; Greenblatt and Mirvish 1973;
Greenblatt et al. 1971, 1973; Hirose et al. 2002; Ivankovic 1979; Ivankovic and Preussman 1970; Kitano
142 NITRATE AND NITRITE
3. HEALTH EFFECTS
et al. 1997; Murthy et al. 1979; Lijinsky 1984a, 1984b; Lijinsky and Reuber 1980; Mirvish et al. 1972;
Miyauchi et al. 2002; Rijhsinghani et al. 1982; Scheunig et al. 1979; Taylor and Lijinsky 1975a, 1975b;
van Logten et al. 1972; Yada et al. 2002; Yoshida et al. 1993, 1994). However, Lijinsky et al. (1983)
reported significantly increased incidences of hepatocellular neoplasms in female (but not male) F344 rats
administered diet to which sodium nitrite was added for 2 years.
Significantly increased incidences of selected tumor types were observed in some studies of laboratory
animals that employed coexposure to various amino compounds and sodium nitrite (Anderson et al. 1985;
Aoyagi et al. 1980; Börzsönyi and Pintér 1977; Börzsönyi et al. 1976, 1978; Chan and Fong 1977;
Greenblatt and Mirvish 1973; Greenblatt et al. 1971; Hirose et al. 1990; Iurchenko et al. 1986; Ivankovic
1979; Ivankovic and Preussmann 1970; Kawabe et al. 1994; Matsukura et al. 1977; Murthy 1979;
Lijinsky 1984a, 1984b; Lijinsky and Reuber 1980; Lijinsky and Taylor 1977; Lijinsky et al. 1973b; Lin
and Ho 1992; Maekawa et al. 1977; Mirvish et al. 1972, 1976, 1980; Miyauchi et al. 2002; Mokhtar et al.
1988; Newberne and Shank 1973; Nishiyama et al. 1998; Nixon et al. 1979; Oka et al. 1974; Olsen et al.
1984; Rijhsinghani et al. 1982; Rustia and Shubik 1974; Scheunig et al. 1979; Shank and Newberne 1976;
Tahira et al. 1988; Taylor and Lijinsky 1975a, 1975b; Weisburger et al. 1980; Xiang et al. 1995; Yada et
al. 2002; Yamamoto et al. 1989; Yoshida et al. 1993, 1994). These results were typically attributed to in
vivo nitrosation of amines by nitrite to produce carcinogenic N-nitrosoamines; some of the studies did not
include sodium nitrite-only treatment groups.
Based on available human data, IARC (2010) determined that there is inadequate evidence for the
carcinogenicity of nitrate in food or drinking water and limited evidence for the carcinogenicity of nitrite
in food (based on association with increased incidence of stomach cancer). Evaluation of available
animal data by IARC (2010) resulted in the determination that there is inadequate evidence for the
carcinogenicity of nitrate, limited evidence for the carcinogenicity of nitrite per se, and sufficient
evidence for the carcinogenicity of nitrite in combination with amines or amides. The overall conclusions
of IARC (2010) were that “ingested nitrate and nitrite under conditions that result in endogenous
nitrosation is probably carcinogenic to humans (Group 2A).” IARC (2010) noted that: (1) the
endogenous nitrogen cycle in humans includes interconversion of nitrate and nitrite; (2) nitrite-derived
nitrosating agents produced in the acid stomach environment can react with nitrosating compounds such
as secondary amines and amides to generate N-nitroso compounds; (3) nitrosating conditions are
enhanced upon ingestion of additional nitrate, nitrite, or nitrosatable compounds; and (4) some N-nitroso
compounds are known carcinogens. The U.S. EPA IRIS (2002) does not include a carcinogenicity
evaluation for nitrate or nitrite.
143 NITRATE AND NITRITE
3. HEALTH EFFECTS
No information was located regarding health effects in humans or animals following chronic-duration
dermal exposure to nitrate or nitrite. Information regarding the effects of chronic-duration dermal
exposure to nitrate or nitrite is not considered necessary because the general population is not likely to be
dermally-exposed to nitrate or nitrite concentrations at levels that might cause adverse health effects.
Genotoxicity. Limited information is available regarding the potential genotoxicity of nitrate in
human studies. One study found no significant association between urinary excretion of nitrate and
frequency of SCEs in peripheral lymphocytes (Kleinjans et al. 1991). In another study, frequency of
HPRT variants in peripheral lymphocytes was associated with nitrate levels in drinking water, urinary and
salivary nitrite levels, and urinary excretion of nitrate and N-nitrosopyrrolidine (van Maanen et al. 1996a).
The results suggest that drinking water with nitrate poses a genetic risk due to the potential formation of
nitrosamines after endogenous reduction of nitrate to nitrite and reaction with amino compounds. Tsezou
et al. (1996) reported a significant increase in chromatid and chromosome breaks in children exposed to
nitrate in drinking water.
A limited number of studies have examined the in vivo genotoxicity of nitrate in laboratory animals;
results were negative for frequency of micronuclei, chromosomal aberrations, morphological or malignant
cell transformation, or drug-resistant mutations in embryonic cells in one study (Inui et al. 1979),
inhibition of testicular DNA synthesis in another study (Friedman and Staub 1976), and chromosomal
aberrations in bone marrow cells in a 2-day study (Luca et al. 1985). However, daily administration of
sodium nitrate for 2 weeks resulted in significant dose-dependent increase in chromosomal aberrations in
bone marrow cells (Luca et al. 1985). Gavage administration of 706.6 mg/kg/day sodium nitrate for
2 days to male Swiss mice showed induction of chromosomal aberrations; however, this effect was not
observed at a much higher dose (Luca et al. 1985). Evaluation of micronuclei in mice treated daily for
2 weeks showed significant increases at the low concentrations tested (78.5 and 235.5 mg/kg/day sodium
nitrate), but not at 706.6 or 2,120 mg/kg/day; the investigators attributed the result to possible induction
of cytotoxic effects (Luca et al. 1985).
Neither potassium nitrate, sodium nitrate, nor lanthanum nitrate hexahydrate were mutagenic to multiple
strains of S. typhimurium either with or without metabolic activation (Ishidate et al. 1984; Zeiger et al.
1992). Tests for chromosomal aberrations in Chinese hamster fibroblast cells were positive for sodium
nitrate, but negative for potassium nitrate (Ishidate et al. 1984). IARC (2010) noted that since sodium
chlorite also yielded positive results in the same assay, the chromosomal aberrations induced by sodium
144 NITRATE AND NITRITE
3. HEALTH EFFECTS
nitrate could have been due to the high osmotic pressure and sodium ion concentration. Ammonium
nitrate did not induce chromosomal aberrations in Chinese hamster ovary cells with or without metabolic
activation (Kim et al. 2011).
In vivo tests for nitrite conducted in mammalian cells yielded negative results for chromosomal
aberrations, SCEs, DNA repair, and cell transformations (Inoue et al. 1985; Ishidate et al. 1984; Lynch et
al. 1983; Tsuda and Kato 1977; Tsuda et al. 1973, 1981). Numerous studies have examined the in vitro
genotoxicity of nitrite; more positive results than negative results were found in tests of gene mutations in
prokaryotic organisms, but it is difficult to draw a firm conclusion (Andrews et al. 1980, 1984;
Balimandawa et al. 1994; Brams et al. 1987; De Flora 1981, De Flora et al. 1984; Ehrenberg et al. 1980;
Ishidate et al. 1984; Törnqvist et al. 1983; Zeiger et al. 1992). However, it appears that the addition of
metabolic activation systems to the incubation mixtures did not make a difference in the results. This
would indicate that nitrite could be a direct mutagenic chemical.
Additional in vivo and in vitro studies could be designed to further assess the genotoxicity of nitrate and
nitrite.
Reproductive Toxicity. Refer to the section titled “Developmental Toxicity” for information
regarding results of case-control studies that evaluated reproductive/developmental end points.
Several animal studies included evaluation of selected reproductive end points. Sleight and Atallah
(1968) reported death and reduced litter production among female guinea pigs provided potassium nitrate
in the drinking water for up to 204 days of cohabitation at a concentration resulting in estimated intake of
4,972 mg nitrate/kg/day. Reduced litter production was the likely result of maternal toxicity rather than
reproductive toxicity per se. Sleight and Atallah (1968) also reported decreases in number of litters and
live births and histopathologic lesions in reproductive organs (placenta, uterus, and cervix) of guinea pigs
administered sodium nitrite in the drinking water. No treatment-related reproductive effects were seen in
female Wistar rats provided sodium nitrite in the food throughout the production of two litters (Hugot et
al. 1980) or in breeding dogs provided sodium nitrate in the drinking water for 1 year (Kelley et al. 1974).
NTP (2001) reported degeneration of the testis in male mice provided sodium nitrite in the drinking water
for 14 weeks, and significantly increased estrous cycles in similarly-treated female mice. Among
similarly-treated male and female rats, the males exhibited decreased sperm motility.
145 NITRATE AND NITRITE
3. HEALTH EFFECTS
A multi-generation reproductive toxicity study in laboratory animals could be designed to more
comprehensively assess the reproductive toxicity potential of ingested nitrate and nitrite.
Developmental Toxicity. A number of studies evaluated possible associations between
developmental end points and exposure to nitrate in humans. The results provide some evidence of
nitrate-related developmental effects. The results are not adequate for quantitative risk assessment
because (1) estimations of nitrate intakes were typically based on measurements of nitrate levels in
drinking water sources at selected time points and self-reported estimates of water consumption;
(2) possible confounding by other potential toxicants was not evaluated; and (3) most studies did not
account for dietary nitrate or nitrite intake, which is typically the major source of ingested nitrate and
nitrite. Some studies reported significant associations between selected developmental end points and
nitrate in drinking water sources (Brender et al. 2013; Croen et al. 2001; Dorsch et al. 1984; Scragg et al.
1982). One study reported increased risk of intercalary limb defect associated with estimated total nitrite
intake (Huber et al. 2013). Other studies found no evidence of associations between nitrate and risk of
developmental effects (Arbuckle et al. 1988; Aschengrau et al. 1989, 1993; Brender et al. 2004;
Cedergren et al. 2002; Ericson et al. 1988; Huber et al. 2013; Super et al. 1981). Tabacova et al. (1997,
1998) evaluated maternal health among pregnant women and their infants who lived near an ammonium
nitrate fertilizer plant. Nitrogen oxides in the air averaged 23.1 µg/m
3
with short-term peak levels as high
as 238.5; nitrate concentrations in the public drinking water supply measured 8–54 mg/L and nitrate
levels in private wells measured as much as 13–400 mg/L. Results indicated that both maternal and cord
blood methemoglobin levels were higher in cases of abnormal birth outcome.
Developmental end points have been assessed in some animal studies. Some studies found no indication
of nitrite treatment-related developmental toxicity (Hugot et al. 1980; Khera 1982; Shimada 1989). One
study reported increased fetal hepatic erythropoiesis, which was thought to have been a response to
nitrite-induced fetal methemoglobinemia (Globus and Samuel 1978). Significantly impaired auditory and
visual discrimination learning behavior and retention of passive avoidance responses (Nyakas et al. 1990),
and delay in cholinergic and serotonergic fiber outgrowth in cortical target areas of the brain (Nyakas et
al. 1994), presumably due to nitrite-induced hypoxia, were reported in offspring of Wistar rats provided
sodium nitrite in the drinking water. Shuval and Gruener (1972) reported decreases in postpartum
survival and pup body weight during 3 weeks postpartum following addition of sodium nitrite to the
drinking water of pregnant rats for 6 weeks; no treatment-related effects were observed regarding group
litter sizes or pup birth weights. Increased pup mortality, depressed preweaning pup body weight, and
delayed swimming development were observed in offspring of male and female rats provided sodium
146 NITRATE AND NITRITE
3. HEALTH EFFECTS
nitrite in the diet (Vorhees et al. 1984). There were no treatment-related effects on preweaning behavior
(surface righting, pivoting, negative geotaxis, or auditory startle) and no effects on postweaning survival,
body weight, or most behavioral indices among pups from dams exposed to sodium nitrite in the diet.
Additional human data are needed to comprehensively assess the developmental toxicity potential of
ingested nitrate and nitrite.
Immunotoxicity. No information was located regarding immunological or lymphoreticular effects in
humans or animals following exposure to nitrate or nitrite by any route. An animal study could be
designed to assess the potential immunotoxicity of ingested nitrate and nitrite.
Neurotoxicity. No information was located regarding the neurotoxicity of nitrate in humans or
animals. Ingestion of nitrite has been associated with severe methemoglobinemia in adults and children;
in many of these cases, clinical signs included dizziness, loss of consciousness, and/or convulsions (CDC
1997, 2002; Gautami et al. 1995; Greenberg et al. 1945; Sevier and Berbatis 1976; Ten Brink et al. 1982).
These cases were the result of consumption of food or drink that contained unusually high levels of nitrite
via contamination, inadvertent use of sodium nitrite instead of table salt, or ingestion of a single sodium
nitrite tablet (667 mg nitrite). Headache was induced in a male subject following consumption of a 10 mg
sodium nitrite solution (Henderson and Raskin 1972). In a study designed to evaluate the oral
bioavailability of sodium nitrite in healthy volunteers, headache was reported after ingestion of nitrite at
doses as low as approximately 1.5–1.8 mg nitrite/kg (Kortboyer et al. 1997b).
Abnormalities in EEGs were reported in male albino rats provided sodium nitrite in the drinking water for
2 months at concentrations resulting in ingestion of ≥9.38 mg nitrite/kg/day (Behroozi et al. 1972). At the
highest dose (187.6 mg nitrite/kg/day), rats exhibited clinical signs of sedation and became motionless
during periods of electrical outbursts. Increased aggressive behavior was observed in male C57B1 mice
provided sodium nitrite in the drinking water at 1,000 mg/L for up to 13 weeks postweaning (Gruener
1974). The mice had also been exposed via their parents during mating and via their mothers during
gestation and lactation. Significantly reduced motor activity was reported in male mice provided sodium
nitrite in the drinking water (Shuval and Gruener 1972).
The nervous system is not expected to be a particularly sensitive target of nitrate toxicity; available data
for nitrite appear adequate for the purpose of hazard identification. Additional neurotoxicity studies do
not appear necessary.
147 NITRATE AND NITRITE
3. HEALTH EFFECTS
Epidemiological and Human Dosimetry Studies. Oral exposure to nitrate and nitrite is
ubiquitous because nitrate and nitrite are part of the normal diet. Elevated methemoglobin levels are
commonly associated with levels of nitrate in drinking water sources or ingestion of nitrate; clinical signs
of methemoglobinemia may be observed at sufficiently high nitrate levels, particularly among newborn
infants (e.g., Bosch et al. 1950; Chapin 1947; Comly 1987; Craun et al. 1981; Donahoe 1949; Fan and
Steinberg 1996; Fan et al. 1987; Faucett and Miller 1946; Ferrant 1946; Gruener and Toeplitz 1975;
Gupta et al. 1999; Johnson et al. 1987; Jones et al. 1973; Medovy 1948; Miller 1971; Robertson and
Riddell 1949; Sadeq et al. 2008; Shuval and Gruener 1972; Simon et al. 1964; Stafford 1947; Super et al.
1981; Walton 1951; Winton et al. 1971; Zeman et al. 2002). Although oral exposure to nitrate has been
associated with methemoglobinemia in bottle-fed infants receiving drinking water containing measurable
levels of nitrate, available studies are limited by lack of accounting for substances in the drinking water
(e.g., bacteria) that may have contributed to the methemoglobinemia and the fact that many of the infants
exhibited gastroenteritis, which in itself can trigger increased methemoglobin levels. Therefore,
additional information regarding the effects of oral exposure of infants to nitrate would serve to reduce
uncertainty as to the role of nitrate in the observed methemoglobinemia cases reported in the literature.
Available human data provide suggestive evidence that elevated levels of nitrate in drinking water and/or
nitrate-rich diets may be associated with signs of thyroid dysfunction (Aschebrook-Kilfoy et al. 2012;
Gatseva and Argirova 2008; Rádiková et al. 2008; Tajtáková et al. 2006; Ward et al. 2010). However,
limitations of these studies include lack of individual dose-response data, quantification of iodine intake,
and control for other potential substances that may affect the thyroid; one study relied on self-reported
thyroid status and self-reported dietary nitrate intake. Additional studies should focus on possible
associations between nitrate and/or nitrite and thyroid status.
Possible associations between nitrate and/or nitrite in drinking water and/or food sources and risk of type
1 diabetes have been investigated in a number of epidemiological studies. Significant associations were
reported in some studies (Dahlquist et al. 1990; Kostraba et al. 1992; Parslow et al. 1997; Virtanen et al.
1994), but not in other studies (Casu et al. 2000; Moltchanova et al. 2004; van Maanen et al. 2000; Zhao
et al. 2001). Limitations of studies include the lack of quantitative dose-response data and the likelihood
of confounding by other potential toxicants. Additional studies should focus on possible associations
between nitrate and/or nitrite and risk of type 1 diabetes.
148 NITRATE AND NITRITE
3. HEALTH EFFECTS
Ingestion of nitrite has been associated with severe methemoglobinemia in adults and children (Aquanno
et al. 1981; CDC 1997, 2002; Gautami et al. 1995; Gowans 1990; Greenberg et al. 1945; Kaplan et al.
1990; Ringling et al. 2003; Sevier and Berbatis 1976; Ten Brink et al. 1982; Walley and Flanagan 1987),
typically following consumption of food or drink that contained unusually high levels of nitrite via
contamination, inadvertent use of sodium nitrite instead of table salt, or ingestion of a single sodium
nitrite tablet (667 mg nitrite). Other effects noted in some of these cases include hypotension and/or
tachycardia, abdominal cramps, vomiting, dizziness, loss of consciousness, convulsions, and even death.
In a study designed to evaluate the oral bioavailability of sodium nitrite in healthy volunteers, ingestion of
approximately 1.5–1.8 mg nitrite/kg resulted in increased percent methemoglobin and average heart rate,
and decreased mean arterial blood pressure (Kortboyer et al. 1997b). Higher ingested doses resulted in
more pronounced effects and included nausea and vomiting. Additional information regarding effects of
oral exposure to nitrite at lower dose levels would be useful in determining minimal risk levels for nitrite
toxicity if populations with such exposure characteristics are identified.
Data needs relating to both prenatal and childhood exposures, and developmental effects expressed either
prenatally or during childhood, are discussed in detail in the Developmental Toxicity subsection above.
Biomarkers of Exposure and Effect
Exposure. There are no biomarkers of exposure that are specific to nitrate or nitrite. Although nitrate
and nitrite can be detected in blood, saliva, and urine (mostly nitrate), nitrate and nitrite are also produced
endogenously via the nitrate-nitrite-nitric oxide pathway. Sources for nitrate and nitrite levels in the body
may therefore include not only ingested food and drinking water, but also oxidation of nitric oxide
produced endogenously. Similarly, N-nitroso compounds that may be detected in the blood or urine may
indicate exposure to nitrate or nitrite; however, these compounds may also be products of the endogenous
nitrate-nitrite-nitric oxide pathway.
Effect. Biomarkers of effects from exposure to nitrate or nitrite are not specific to nitrate or nitrite.
Blood methemoglobin level has been used as a biomarker of nitrate and nitrite toxicity; however,
methemoglobinemia may be elicited by other substances such as selected drugs, pesticides, industrial and
commercial products, and medical conditions such as pediatric gastrointestinal infection, sepsis, and
sickle cell crisis (ATSDR 2013a). Methemoglobinemia may also be inherited (genetic conditions that
result in decreased activity of enzymes that reduce methemoglobin or the presence of hemoglobin M).
Urinary levels of various N-nitroso compounds have been measured as an index of nitrosation; however,
149 NITRATE AND NITRITE
3. HEALTH EFFECTS
N-nitroso compounds can form via endogenous nitrosation and do not require the intake of nitrate or
nitrite.
Absorption, Distribution, Metabolism, and Excretion. No information was located regarding
the pharmacokinetics of nitrate or nitrite following inhalation or dermal exposure. However, numerous
reviews are available regarding the pharmacokinetics of ingested nitrate and nitrite (Bailey et al. 2012;
Bryan and van Grinsven 2013; IARC 2010; JECFA 2003a, 2003b; Lundberg and Govoni 2004; Lundberg
and Weitzberg 2013; Lundberg et al. 2008, 2009; Weitzberg and Lundberg 2013; Weitzberg et al. 2010;
WHO 2011b). Ingestion is the major source of exposure to nitrate and nitrite. The data adequately
describe the pharmacokinetics of nitrate and nitrite; additional studies do not appear necessary.
A PBPK model (Zeilmaker et al. 1996, 2010b) simulates the kinetics of methemoglobin formation
resulting from gastrointestinal absorption of nitrate in adult humans. The model is adequate for this
purpose; however, the model is not considered adequate for the purpose of simulating the kinetics in
infants. Additional information is needed to adapt the model to infants for the purpose of quantitative risk
assessment.
Comparative Toxicokinetics. Significant differences exist regarding the kinetics of the nitrate-
nitrite-nitric oxide pathway in humans and laboratory animals, thus precluding the usefulness of results
from laboratory animals to evaluate the toxicokinetics of nitrate or nitrite in humans.
Methods for Reducing Toxic Effects. Ingestion is the most likely route of overexposure to nitrate
or nitrite. Methods for reducing peak absorption include oral administration of activated charcoal within
a short period following significant ingestion (Seifert 2004) and use of mouthwash containing
chlorhexidine (an active antibacterial), which may decrease the reduction of salivary nitrate to nitrite (van
Maanen et al. 1996b). No information was located regarding methods to reduce the body burden of
nitrate or nitrite. Adequate data are available regarding methods for reducing nitrate- or nitrite-induced
methemoglobinemia (e.g., Barclay 1998; Leikin and Paloucek 2008; Seifert 2004). In several rat studies,
tumorigenicity associated with concurrent exposure to nitrite and various amino compounds was
modulated by coexposure to selected antioxidants such as ascorbic acid, catechol, 3-methoxycatechol,
tert-butylhydroquinone, α-tocopherol, and propyl gallate (Chan and Fong 1977; Mirvish et al. 1976, 1983;
Miyauchi et al. 2002; Mohktar et al. 1988; Yada et al. 2002; Yoshida et al. 1994); thioproline (which may
serve as a nitrite scavenger when nitrosated to nitrosothioproline) (Tahira et al. 1988); or soy bean
(Mokhtar et al. 1988).
150 NITRATE AND NITRITE
3. HEALTH EFFECTS
Children’s Susceptibility. Data needs relating to both prenatal and childhood exposures, and
developmental effects expressed either prenatally or during childhood, are discussed in detail in the
Developmental Toxicity subsection above.
Ingestion of relatively large amounts of nitrate or nitrite can result in methemoglobinemia. The first
6 months of postnatal life is a period of increased susceptibility to methemoglobinemia; possible
contributing factors to this increased susceptibility include a higher pH in the infant stomach, greater
proportion of fetal hemoglobin (which appears to be more readily oxidized to methemoglobin than adult
hemoglobin), and higher concentration of NADH-dependent methemoglobin reductase (an enzyme
involved in the reduction of methemoglobin to hemoglobin). Some investigators have reported
significant associations between nitrate levels in drinking water (or living in areas presumed to have
elevated nitrate levels in drinking water sources) and risk of childhood type 1 diabetes (Dahlquist et al.
1990; Kostraba et al. 1992; Parslow et al. 1997; Virtanen et al. 1994). However, no such relationship was
observed in two other studies (van Maanen et al. 2000; Zhao et al. 2001). Refer to Section 3.2.2.2
(Metabolic Effects) for summaries of these study reports.
Child health data needs relating to exposure are discussed in Section 6.8.1, Identification of Data Needs:
Exposures of Children.
3.12.3 Ongoing Studies
The following ongoing study pertaining to nitrate was identified in National Institutes of Health (NIH)
Research Portfolio Online Reporting Tools (RePORTER 2014): Dr. Paul A Romitti, College of Public
Health, University of Iowa, is evaluating risk of birth defects associated with nitrate in drinking water.
151 NITRATE AND NITRITE
4. CHEMICAL AND PHYSICAL INFORMATION
4.1 CHEMICAL IDENTITY
Information regarding the chemical identity of nitrate and nitrite is provided in Table 4-1 and information
regarding the chemical identity of selected inorganic nitrate and nitrite compounds is provided in
Table 4-2. Information regarding ammonia and urea is provided in Table 4-3.
Inorganic nitrate and nitrite are naturally occurring ionic species that are part of the earth’s nitrogen cycle
(see Figure 5-1). These anions are the products formed via the fixation of nitrogen and oxygen.
Chemical processes, biological processes, and microbial processes in the environment convert nitrogen
compounds to nitrite and nitrate via nitrogen fixation and nitrification. Compounds such as urea are
converted via hydrolysis to ammonia, protonation of ammonia to ammonium (cation), followed by
oxidation of ammonium to form nitrite, and then oxidation to form nitrate. Nitrate and nitrite are not
neutral compounds, but rather the ionic (anionic; negatively charged) portions of compounds, commonly
found in commerce as organic and inorganic salts. As used in this profile, the word “ion” is implied and
not used, unless added for clarity.
Nitrate and nitrite typically exist in the environment as highly water-soluble inorganic salts, often bound
when not solubilized to metal cations such as sodium or potassium. The nitrate ion is the more stable
form as it is chemically unreactive in aqueous solution; however, it may be reduced through biotic
processes with nitrate reductase to the nitrite ion. The nitrite ion is readily oxidized back to the nitrate ion
via Nitrobacter (a genus of proteobacteria), or conversely, the nitrite ion may be reduced to various
compounds (IARC 2010; WHO 2011b).
Under certain conditions, nitrite may be converted to a class of compounds called N-nitrosamines. In
foods, endogenous production of N-nitrosamines occurs when nitrite reacts with secondary amines or
amides. Several factors, including the presence of antioxidants, such as vitamin C, affect the rate of
formation. N-nitrosamines are a class of chemical compounds that have a nitroso (N=O) group bonded to
an amine (-N(R)R’) with a general chemical structure of RN(R’)-N=O (IARC 94).
There is a wide range of both organic and inorganic nitrate and nitrite compounds. Common nitrate and
nitrite salts include potassium nitrate, potassium nitrite, sodium nitrate, sodium nitrite, and ammonium
nitrate; these salts are highly soluble in water, dissociate under environmental conditions, and exist as
152 NITRATE AND NITRITE
4. CHEMICAL AND PHYSICAL INFORMATION
ions (WHO 1978, 2011b). Common inorganic fertilizers that contribute to environmental concentrations
of nitrate and nitrite include ammonia and urea.
4.2 PHYSICAL AND CHEMICAL PROPERTIES
Information regarding the physical and chemical properties of selected inorganic nitrate and nitrite
compounds is provided in Table 4-4 and information regarding the physical and chemical properties of
ammonia and urea is provided in Table 4-5.

153 NITRATE AND NITRITE
4. CHEMICAL AND PHYSICAL INFORMATION
Table 4-1. Chemical Identity of Nitrate and Nitrite Ions
a
Characteristic
Nitrate ion
Nitrite ion
Synonym(s)
Nitrate ion; nitrate(1-); nitrate ion
(NO
3
-
); nitrate ion(1-); nitrato; nitric
acid, ion (1-)
Nitrite ion; nitrite (1-); nitrite anion;
nitrite ion (NO
2
-
); nitrite ion (1-);
nitrogen dioxide(1-); nitrogen
peroxide ion (1-); nitrous acid,
ion (1-)
Registered trade name(s)
No data
No data
Chemical formula
NO
3
-
NO
2
-
Chemical structure
b
O
O
N
+
O
O
N
O
Ionic weight
62.005
45.995
Identification numbers:
CAS registry
14797-55-8
14797-65-0
NIOSH RTECS
No data
No data
EPA hazardous waste
No data
No data
DOT/UN/NA/IMDG shipping
c
UN3218; UN1447
No data
HSDB
Not applicable
Not applicable
NCI
No data
No data
EPA Pesticide Chemical
Code
No data
No data
a
All information obtained from IARC (2010), except where noted.
b
HSDB 2007.
c
ChemIDplus 2014.
CAS = Chemical Abstracts Service; DOT/UN/NA/IMDG = Department of Transportation/United Nations/North
America/International Maritime Dangerous Goods Code; EPA = Environmental Protection Agency;
HSDB = Hazardous Substances Data Bank; NCI = National Cancer Institute; NIOSH = National Institute for
Occupational Safety and Health; RTECS = Registry of Toxic Effects of Chemical Substances

154 NITRATE AND NITRITE
4. CHEMICAL AND PHYSICAL INFORMATION
Table 4-2. Chemical Identity of Selected Inorganic Nitrate and Nitrite
Compounds
a
Ammonium
Sodium
Sodium
Potassium
Potassium
Characteristic
nitrate
nitrate
nitrite
nitrate
nitrite
Synonym(s)
Nitric acid,
ammonium salt;
ammonium nitrate
(NH
4
NO
3
); Emulite;
EXP 200; German
saltpeter; Norge
saltpeter; Norway
saltpeter;
Norwegian
saltpeter; Plenco
12203; Varioform I;
ZhVK
Nitric acid,
sodium salt;
Chile saltpeter;
niter; nitric acid
sodium
salt(1:1);
saltpeter; soda
niter; nitrate of
soda
;
cubic
niter; nitratine
Nitrous acid,
sodium salt;
nitrous acid
soda; nitrous
acid sodium
salt (1:1)
Nitric acid,
potassium
salt; niter;
nitre; nitric
acid
potassium salt
(1:1);
saltpeter;
saltpetre;
nitrate of
potash
Nitrous acid,
potassium
salt; Chile
saltpeter;
niter; nitric
acid sodium
salt (1:1);
salpeter; soda
niter
Registered trade
name(s)
No data
No data
No data
No data
No data
Chemical formula
NH
4
NO
3
NaNO
3
NaNO
2
KNO
3
KNO
2
Chemical structure
Trigonal NH
4
+
NO
3
-
Na
+
NO
3
-
Trigonal
Na
+
NO
2
-
Orthorhombic
K
+
NO
3
-
K
+
NO
2
-
N
H
H
H
H
+
N
+
O
O
O
Na
+
N
+
O
O
O
N
O
O
Na
+
K
+
N
+
O
O
N
O
O
K
+
O
Identification
numbers:
CAS registry
6484-52-2
7631-99-4
7632-00-0
7757-79-1
7758-09-0
NIOSH RTECS
BR9050000
WC5600000
RA1225000
TT3700000
TT3750000
EPA hazardous
No data
No data
No data
No data
No data
waste
DOT/UN/NA/IMDG
UN 2426; UN 0223;
UN 1498;
UN 1500;
UN 1486;
UN 1488;
shipping UN 1942; UN 2067; IMO 5.1 IMO 5.1 IMO 5.1 IMO 5.1
UN 2068; UN 2069;
UN 2070; UN 2071;
UN 2072; UN 0222
IMO 5.1; NA 1942;
IMO 1.1; IMO 9.0
HSDB
475
726
757
1227
1216
NCI
No data
No data
No data
No data
No data
NFPA instability
hazard
b
3
No data
No data
No data
No data

155 NITRATE AND NITRITE
4. CHEMICAL AND PHYSICAL INFORMATION
Table 4-2. Chemical Identity of Selected Inorganic Nitrate and Nitrite
Compounds
a
Ammonium
Sodium
Sodium
Potassium
Potassium
Characteristic
nitrate
nitrate
nitrite
nitrate
nitrite
EPA Pesticide
076101
c
076104
c
076204
c
076103
c
076203
c
Chemical Code
a
All information obtained from IARC 2010 and HSDB 2007, except where noted.
b
NFPA 2002; instability hazard 3 = materials that in themselves are capable of detonation or explosive
decomposition or explosive reaction, but that require a strong initiating source or that must be heated under
confinement before initiation.
c
EPA 2014f.
CAS = Chemical Abstracts Service; DOT/UN/NA/IMDG = Department of Transportation/United Nations/North
America/International Maritime Dangerous Goods Code; EPA = Environmental Protection Agency;
HSDB = Hazardous Substances Data Bank; NCI = National Cancer Institute; NFPA = National Fire Protection
Association; NIOSH = National Institute for Occupational Safety and Health; RTECS = Registry of Toxic Effects of
Chemical Substances

156 NITRATE AND NITRITE
4. CHEMICAL AND PHYSICAL INFORMATION
Table 4-3. Chemical Identity of Ammonia
a
and Urea
b
Characteristic
Ammonia
Urea
Synonym(s)
Anhydrous ammonia, ammonia gas;
Alphahydrate; carbamide; carbonyl
aqua ammonia; liquid ammonia
diamide; carbonyldiamine; isourea
Registered trade name(s)
BCMW; BUSAN 1215
UAL-37; N-Dure; UF-Concentrate-
85; Ureacin-20
Chemical formula
NH
3
CH
4
N
2
O
Chemical structure
H
O
H
2
N
N
H
H
NH
2
Ionic weight
17.03
60.06
Identification numbers:
CAS registry
7664-41-7
57-13-6
NIOSH RTECS
No data
No data
EPA hazardous waste
No data
No data
DOT/UN/NA/IMDG shipping
UN 1005; UN 3318; UN 2672; UN
No data
2073
HSDB
162
163
NCI
No data
No data
EPA Pesticide Chemical
005302
085702
Code
a
HSDB 2012.
b
HSDB 2003.
CAS = Chemical Abstracts Service; DOT/UN/NA/IMDG = Department of Transportation/United Nations/North
America/International Maritime Dangerous Goods Code; EPA = Environmental Protection Agency;
HSDB = Hazardous Substances Data Bank; NCI = National Cancer Institute; NIOSH = National Institute for
Occupational Safety and Health; RTECS = Registry of Toxic Effects of Chemical Substances

157 NITRATE AND NITRITE
4. CHEMICAL AND PHYSICAL INFORMATION
Table 4-4. Physical and Chemical Properties of Selected Inorganic Nitrate and
Nitrite Compounds
a
Ammonium
Potassium
Potassium
Characteristic
nitrate
Sodium nitrate
Sodium nitrite
nitrate
nitrite
CAS
6484-52-2
7631-99-4
7632-00-0
7757-79-1
7758-09-0
Molecular weight
80.043
84.995
68.985
101.103
85.093
Color
White; colorless
White; colorless
White to pale
Colorless
Pale yellow
(pure); gray or yellow
brown (fertilizer
grade)
Physical state
Solid
Solid
Solid
Solid
Solid
Melting point
169.7°C
306°C; 308°C
271°C
334°C; 337°C
440°C
Boiling point
Decomposes at
380°C;
320°C;
400°C;
537°C;
~210°C (200– decomposes decomposes decomposes explodes
260°C)
Density:
1.725 g/cm
3
2.26 g/cm
3
2.17 g/cm
3
2.11 g/cm
3
1.915 g/cm
3
at 20°C/4°C
Odor
Odorless
Odorless
b
No data
Odorless
No data
Odor threshold:
No data
No data
No data
No data
No data
Taste threshold
No data
No data
No data
No data
No data
Solubility:
Water at 25°C
213 g/100 g
91.2
c
g/100 g
84.8 g/100 g
38.3 g/100 g
312 g/100 g
Organic solvent(s)
Acetone,
Ammonia,
Ammonia,
Ammonia;
Ammonia;
ammonia, hydrazine, ethanol, glycerol; sl sol alcohol
ethanol, ethanol, methanol ethanol
isopropanol, methanol,
methanol
acetone, glycerol
Partition coefficients
Not available
Not available
Not available
Not available
Not available
Vapor pressure
Not available
Not available
Not available
Not available
Not available
Henry's law
Not available
Not available
Not available
Not available
Not available
constant
Flashpoint
Not available
Flames up when
498ºC; May
Not flammable
Not flammable
heated to 540°C explode above
530°C
Flammability limits
Not available
Not available
Not available
Not available
Not available
Explosive limits
Not available
Not available
>1,000°C
Not available
Not available
a
All information obtained from HSDB 2007, unless otherwise noted.
b
Lewis 2002.
c
Lide 2013.
CAS = Chemical Abstracts Service; HSDB = Hazardous Substances Data Bank

158 NITRATE AND NITRITE
4. CHEMICAL AND PHYSICAL INFORMATION
Table 4-5. Physical and Chemical Properties of Ammonia
a
and Urea
b
Characteristic
Ammonia
Urea
CAS
7664-41-7
57-13-6
Molecular weight
17.03
60.06
Color
Colorless
White
Physical state
Gas
Crystal/powder
Melting point
-77.7°C
132.70°C
Boiling point
-33.35 °C at 760 mm Hg
Decomposes
Density:
at 20°C/4°C
0.696 g/L (liquid)
1.3230
Odor
Sharp, pungent, irritating
Slight odor of ammonia; odorless
Odor threshold:
Water: 1.5 mg/L; Air: 5.2 µL/L
No data
Taste threshold
No data
No data
Solubility:
Water
4.82x10
5
mg/L at 24°C
5.45x10
5
mg/L at 25°C
Organic solvent(s)
Alcohol, chloroform, ether
Alcohol; acetic acid; pyrimidine
Partition coefficients
No data
-2.11
Vapor pressure at 25°C
7.51x10
3
mm Hg
1.2x10
-5
mm Hg
Henry's law constant
1.61x10
-5
atm m
3
/mole at 25°C
No data
Flashpoint
No data
No data
Flammability limits
No data
No data
Explosive limits
No data
No data
a
HSDB 2012
b
HSDB 2003
CAS = Chemical Abstracts Service; HSDB = Hazardous Substances Data Bank
159 NITRATE AND NITRITE
4. CHEMICAL AND PHYSICAL INFORMATION
Nitrate is the most oxidized form of nitrogen present in the environment (oxidation state of nitrogen +5).
It accounts for the majority of the total available nitrogen in surface waters (Environment Canada 2012),
perhaps due to its formation by converting the ammonium ion (e.g., from fertilizer and manure) through a
2-step oxidation process, first to nitrite and then to nitrate. In compounds, nitrate and nitrite typically
exist in an oxidation state of 1
-
. Nitrate is the conjugate base of nitric acid (HNO
3
), a strong acid with
pKa of -1.38 at 25°C (Dean 1985). Nitric acid and salts of nitric acid completely dissociate in aqueous
solutions, except for nitrates of mercury and bismuth (Environment Canada 2012; WHO 1978). Nitrite is
the conjugate base of nitrous acid (HNO
2
), a weak acid with a pKa of 3.14 at 25°C (Dean 1985); nitrite
readily decomposes to yield water and dinitrogen trioxide (N
2
O
3
), or nitric acid, nitric oxide (NO), and
water (H
2
O) (WHO 1978, 2011b).
160 NITRATE AND NITRITE
4. CHEMICAL AND PHYSICAL INFORMATION
This page is intentionally blank.
161 NITRATE AND NITRITE
5. PRODUCTION, IMPORT/EXPORT, USE, AND DISPOSAL
5.1 PRODUCTION
Table 5-1 lists the production year, number of facilities, the state where each facility is located, and the
range (in pounds) for each domestic manufacturer that reported the production or formulation of nitrate
compounds in 2012 (TRI12 2014). Table 5-2 lists Toxics Release Inventory (TRI) data for sodium
nitrite, a common nitrite salt. Table 5-3 lists the TRI data for ammonia. Manufacturers are required to
report Toxics Release Inventory (TRI) data to satisfy EPA requirements. The TRI data should be used
with caution since only certain types of facilities are required to report (EPA 2005). Facilities that must
report to the TRI include industries in a specific business sector such as manufacturing, mining, or electric
generation, employ ≥10 full-time employees, and manufacture or process 25,000 pounds of a TRI-listed
chemical or use >10,000 pounds of a TRI listed chemical per calendar year. Therefore, there are some
facilities that may be processing or using nitrate and/or nitrite, but are not required to report to TRI
because they do not meet the regulatory criteria. The amounts reported in Tables 5-1, 5-2, and 5-3
represent those reported by all facilities in each state that are required to report to the TRI and represent
the range of minimum to maximum amounts of each chemical present on-site at these facilities during the
year. This is not an exhaustive list.
Nitrate and nitrite are not stable compounds, but rather the ionic portions of compounds such as inorganic
salts. As used in this profile, the word “ion” is implied and not used, unless added for clarity. Nitrate and
nitrite occur naturally in the environment as a part of the nitrogen cycle. Nitrogen fixation is part of the
natural process by which free nitrogen gas (N
2
) is converted to nitrite, then to nitrate, used by plants, and
returned as free N
2
to the atmosphere. This is called the nitrogen cycle, and is shown in Figure 5-1. This
cycle occurs through the global environment (Newton 2005). Nitrogen exists naturally in soils. Topsoils
contain nitrogen, at content levels as high as 2 to 4 tons/hectare (roughly 1.2–2.4 kg/m
3
in the upper
15 cm of soil; topsoil depths can range between 0 and 30 cm [Hill Laboratories 2014]), typically bound to
organic matter and mineral soil material; available forms of nitrogen, including nitrate, are present in soils
at a few kg/hectare (Taylor 2004). Nitrate is also formed naturally as an end product of oxidation of
vegetable debris and animal and human waste, mainly urine disposed of in waste water. This process is
known as nitrification, which is a microbial process that converts ammonia to nitrate and is the principal
source for nitrate in the terrestrial and aquatic environment (Environment Canada 2012). Under aerobic
conditions, the ammonium ion (e.g., from fertilizer or manure, or discharge from municipal and onsite
waste water treatment systems) is converted to nitrite ion via ammonia-oxidizing bacteria (Nolan 1999).

162 NITRATE AND NITRITE
5. PRODUCTION, IMPORT/EXPORT, USE, AND DISPOSAL
Table 5-1. Facilities that Produce, Process, or Use Nitrate Compounds
Minimum
Maximum
Number of
amount on site amount on site
State
a
facilities
in pounds
b
in pounds
b
Activities and uses
c
AK
2
1,000,000
9,999,999
1, 5, 12, 14
AL
56
0
49,999,999
1, 2, 3, 4, 5, 6, 7, 8, 10, 11, 12, 13, 14
AR
26
0
49,999,999
1, 3, 4, 5, 7, 9, 11, 12, 13
AZ
32
0
99,999,999
1, 4, 5, 6, 7, 11, 12
CA
139
0
499,999,999
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
CO
39
0
10,000,000,000
1, 5, 7, 11, 12, 13, 14
CT
26
0
99,999
1, 3, 5, 7, 8, 10, 12
DC
4
1,000
9,999
7, 8
DE
6
0
9,999,999
1, 5, 7, 13, 14
FL
36
0
9,999,999
1, 3, 4, 5, 6, 7, 9, 11, 12, 13, 14
GA
57
0
99,999,999
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
GU
1
100,000
999,999
1, 5
HI
7
0
99,999
1, 5, 9
IA
46
0
999,999,999
1, 3, 4, 5, 7, 8, 10, 11, 12, 13
ID
25
0
9,999,999
1, 2, 3, 5, 6, 7, 8, 10, 12, 13, 14
IL
108
0
499,999,999
1, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
IN
62
0
9,999,999
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
KS
27
0
999,999,999
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13
KY
44
0
999,999
1, 2, 3, 5, 6, 7, 8, 10, 11, 12, 13
LA
48
0
999,999,999
1, 2, 3, 4, 5, 6, 7, 9, 10, 11, 12, 13, 14
MA
42
0
9,999,999
1, 3, 5, 6, 7, 11, 12, 13
MD
21
0
999,999
1, 3, 4, 5, 6, 7, 8, 10, 13, 14
ME
12
0
99,999
1, 5, 11, 12
MI
105
0
9,999,999
1, 5, 6, 7, 8, 9, 10, 11, 12, 14
MN
53
0
999,999,999
1, 2, 3, 4, 5, 6, 7, 9, 10, 11, 12, 14
MO
37
0
49,999,999
1, 2, 3, 4, 5, 6, 7, 9, 10, 11, 12, 13, 14
MS
28
100
49,999,999
1, 2, 3, 4, 5, 6, 7, 8, 10, 11, 12
MT
10
100
999,999
1, 3, 5, 7, 11, 12, 13
NC
41
0
9,999,999
1, 3, 4, 5, 6, 7, 9, 10, 11, 12, 13
ND
10
100
999,999
1, 5, 7, 8, 13
NE
25
100
99,999,999
1, 3, 4, 5, 6, 7, 10, 12, 13
NH
7
0
99,999
1, 5, 7, 10, 11, 12
NJ
40
100
999,999
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 12, 13, 14
NM
14
0
499,999,999
1, 5, 6, 10, 11, 12
NV
27
0
499,999,999
1, 2, 3, 5, 6, 7, 10, 11, 12, 13, 14
NY
73
0
999,999
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
OH
111
0
499,999,999
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
OK
37
100
99,999,999
1, 3, 4, 5, 6, 7, 10, 11, 12, 14
OR
40
0
49,999,999
1, 2, 3, 4, 5, 6, 7, 8, 10, 11, 12

163 NITRATE AND NITRITE
5. PRODUCTION, IMPORT/EXPORT, USE, AND DISPOSAL
Table 5-1. Facilities that Produce, Process, or Use Nitrate Compounds
Minimum
Maximum
Number of
amount on site amount on site
State
a
facilities
in pounds
b
in pounds
b
Activities and uses
c
PA
67
0
499,999,999
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13
PR
7
100
999,999
1, 3, 5, 7, 8, 14
RI
6
100
99,999
1, 2, 3, 5, 6, 12
SC
43
0
999,999
1, 2, 3, 5, 6, 7, 10, 11, 12, 13, 14
SD
8
1,000
9,999,999
1, 5
TN
45
0
49,999,999
1, 3, 4, 5, 6, 7, 8, 10, 11, 12, 13, 14
TX
132
0
49,999,999
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
UT
38
0
49,999,999
1, 2, 3, 4, 5, 6, 7, 9, 10, 12, 13
VA
42
0
9,999,999
1, 3, 5, 7, 8, 9, 10, 11, 12, 13
VT
6
100
9,999,999
1, 5, 6, 10, 12
WA
46
0
99,999,999
1, 3, 4, 5, 6, 7, 9, 12, 13, 14
WI
127
0
499,999,999
1, 4, 5, 7, 9, 10, 11, 12, 13, 14
WV
18
0
9,999,999
1, 3, 4, 5, 6, 7, 10, 12, 13, 14
WY
5
10,000
99,999,999
1, 3, 4, 6, 7, 11
a
Post office state abbreviations used.
b
Amounts on site reported by facilities in each state.
c
Activities/Uses:
1. Produce
6. Reactant
11. Manufacturing Aid
2. Import 7. Formulation Component 12. Ancillary/Other Uses
3. Onsite use/processing 8. Article Component 13. Manufacturing Impurity
4. Sale/Distribution 9. Repackaging 14. Process Impurity
5. Byproduct
10. Chemical Processing Aid
Source: TRI13 2014 (Data are from 2013)

164 NITRATE AND NITRITE
5. PRODUCTION, IMPORT/EXPORT, USE, AND DISPOSAL
Table 5-2. Facilities that Produce, Process, or Use Sodium Nitrite
Minimum
Maximum
Number of amount on site amount on site
State
a
facilities
in pounds
b
in pounds
b
Activities and uses
c
AL
7
1,000
999,999
1, 5, 6, 7, 10, 11, 12
AR
8
0
99,999
2, 3, 5, 6, 8, 9, 12
AZ
3
1,000
9,999
12
CA
11
1,000
499,999,999
1, 3, 6, 7, 9, 11
CO
2
1,000
99,999
7, 9, 11
FL
1
10,000
99,999
12
GA
11
1,000
99,999
1, 5, 6, 7, 9, 11
IA
3
100
99,999
6, 10
ID
1
10,000
99,999
11
IL
32
0
999,999
1, 3, 4, 5, 6, 7, 9, 10, 11, 12
IN
20
100
9,999,999
1, 2, 3, 5, 6, 7, 9, 10, 11, 12, 13
KS
2
1,000
9,999
10, 12
KY
10
100
999,999
6, 7, 10, 11, 12
LA
9
1,000
9,999,999
1, 5, 6, 7, 10, 11, 12
MA
5
1,000
99,999
6, 12
MD
1
10,000
99,999
2, 3, 11
MI
43
0
9,999,999
2, 3, 6, 7, 8, 9, 10, 11, 12
MN
6
10,000
999,999
10, 12
MO
13
0
99,999,999
2, 3, 6, 7, 10, 11, 12
MS
6
1,000
99,999
7, 10, 11, 12
NC
4
1,000
99,999
1, 5, 7, 12
NE
4
1,000
99,999
7, 8, 9
NJ
9
1,000
999,999
7, 9, 11, 12
NM
1
1,000
9,999
12
NV
1
10,000
99,999
2, 3, 12
NY
8
0
9,999,999
1, 4, 5, 7, 10, 11, 12
OH
35
100
999,999
1, 2, 3, 5, 6, 7, 10, 11, 12
OK
3
100
999,999
1, 5, 7, 11
OR
2
10,000
99,999
11
PA
15
0
9,999,999
1, 5, 6, 7, 10, 11, 12
RI
1
100
999
1, 5, 12
SC
19
100
9,999,999
1, 2, 3, 5, 6, 7, 8, 10, 11, 12
SD
3
1,000
99,999
1, 5, 7
TN
5
1,000
999,999
2, 3, 4, 7, 8, 9, 10, 11, 12
TX
38
0
9,999,999
1, 5, 6, 7, 8, 9, 10, 11, 12, 13
UT
1
0
0
0
VA
4
1,000
999,999
7, 8, 11, 12
WA
1
0
0
0

165 NITRATE AND NITRITE
5. PRODUCTION, IMPORT/EXPORT, USE, AND DISPOSAL
Table 5-2. Facilities that Produce, Process, or Use Sodium Nitrite
Minimum
Maximum
Number of
amount on site amount on site
State
a
facilities
in pounds
b
in pounds
b
Activities and uses
c
WI
13
100
999,999
7, 9, 11, 12
WV
4
1,000
9,999,999
1, 5, 7, 11, 13
a
Post office state abbreviations used.
b
Amounts on site reported by facilities in each state.
c
Activities/Uses:
1. Produce
6. Reactant
11. Manufacturing Aid
2. Import 7. Formulation Component 12. Ancillary/Other Uses
3. Onsite use/processing 8. Article Component 13. Manufacturing Impurity
4. Sale/Distribution 9. Repackaging 14. Process Impurity
5. Byproduct
10. Chemical Processing Aid
Source: TRI13 2014 (Data are from 2013)

166 NITRATE AND NITRITE
5. PRODUCTION, IMPORT/EXPORT, USE, AND DISPOSAL
Table 5-3. Facilities that Produce, Process, or Use Ammonia
Minimum
Maximum
Number of amount on site amount on site
State
a
facilities
in pounds
b
in pounds
b
Activities and uses
c
AK
6
0
999,999
1, 2, 3, 5, 11, 12
AL
70
0
49,999,999
1, 2, 3, 4, 5, 6, 7, 9, 10, 11, 12, 13, 14
AR
49
0
49,999,999
1, 2, 3, 5, 6, 7, 9, 10, 11, 12, 13
AS
1
1,000
9,999
12
AZ
20
0
49,999,999
1, 5, 6, 7, 9, 10, 11, 12
CA
120
0
99,999,999
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
CO
19
0
9,999,999
1, 2, 3, 4, 5, 7, 9, 10, 11, 12, 13, 14
CT
15
0
999,999
1, 2, 3, 5, 6, 7, 8, 10, 11, 12
DC
2
10,000
99,999
12
DE
7
1,000
9,999,999
1, 3, 5, 6, 7, 11, 12
FL
64
0
499,999,999
1, 2, 3, 5, 6, 7, 9, 10, 11, 12, 13
GA
81
0
99,999,999
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13
HI
9
0
999,999
1, 3, 5, 6, 7, 9, 10, 11, 12, 13, 14
IA
79
100
999,999,999
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
ID
19
100
49,999,999
1, 3, 4, 5, 6, 7, 9, 10, 11, 12, 13
IL
112
100
99,999,999
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 14
IN
65
0
9,999,999
1, 2, 3, 5, 6, 7, 9, 10, 11, 12, 13, 14
KS
37
0
99,999,999
1, 2, 3, 4, 5, 6, 7, 9, 10, 11, 12, 13, 14
KY
47
0
49,999,999
1, 2, 3, 5, 6, 7, 9, 10, 11, 12, 13, 14
LA
72
0
499,999,999
1, 2, 3, 4, 5, 6, 7, 9, 10, 11, 12, 13, 14
ME
10
0
999,999
1, 2, 3, 5, 6, 7, 8, 10, 11, 12, 13
MI
70
0
9,999,999
1, 2, 3, 5, 6, 7, 8, 10, 11, 12, 13, 14
MN
61
0
49,999,999
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13
MO
46
0
9,999,999
1, 2, 3, 5, 6, 7, 8, 9, 10, 11, 12, 13
MS
34
0
99,999,999
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13
MT
10
0
9,999,999
1, 2, 3, 5, 6, 9, 10, 12, 13
NC
86
0
49,999,999
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
ND
13
0
99,999,999
1, 3, 4, 5, 6, 9, 10, 11, 12
NE
45
100
499,999,999
1, 2, 3, 4, 5, 6, 7, 8, 10, 11, 12, 13, 14
NH
9
0
9,999,999
1, 3, 5, 6, 10, 11, 12
NJ
43
0
999,999
1, 2, 3, 4, 5, 6, 7, 9, 10, 11, 12, 13
NM
6
0
99,999
1, 3, 5, 6, 11, 12, 13, 14
NV
12
0
9,999,999
1, 2, 3, 5, 6, 7, 9, 12, 13, 14
NY
50
0
999,999
1, 2, 3, 5, 6, 7, 8, 9, 10, 11, 12, 14
OH
115
0
10,000,000,000
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
OK
24
0
99,999,999
1, 2, 3, 4, 5, 6, 7, 9, 10, 11, 12, 13, 14
OR
31
0
9,999,999
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13
PA
95
0
49,999,999
1, 2, 3, 4, 5, 6, 7, 9, 10, 11, 12, 13, 14

167 NITRATE AND NITRITE
5. PRODUCTION, IMPORT/EXPORT, USE, AND DISPOSAL
Table 5-3. Facilities that Produce, Process, or Use Ammonia
Minimum
Maximum
Number of
amount on site amount on site
State
a
facilities
in pounds
b
in pounds
b
Activities and uses
c
PR
10
0
999,999
1, 2, 3, 4, 5, 6, 7, 10, 12
RI
10
1,000
9,999,999
1, 2, 3, 4, 5, 6, 9, 10, 11
SC
54
0
499,999,999
1, 3, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
SD
10
1,000
999,999
1, 2, 5, 7, 10, 11, 13
TN
70
0
999,999
1, 2, 3, 5, 6, 7, 8, 9, 10, 11, 12, 13
TX
211
0
99,999,999
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
UT
27
0
9,999,999
1, 2, 3, 4, 5, 6, 7, 9, 10, 11, 12
WA
29
0
9,999,999
1, 2, 3, 5, 6, 7, 9, 10, 11, 12, 13
WI
81
0
49,999,999
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
WV
34
0
49,999,999
1, 2, 3, 5, 6, 7, 8, 10, 11, 12, 13, 14
WY
14
0
99,999,999
1, 2, 3, 4, 5, 6, 7, 10, 12, 13
a
Post office state abbreviations used.
b
Amounts on site reported by facilities in each state.
c
Activities/Uses:
1. Produce
6. Reactant
11. Manufacturing Aid
2. Import 7. Formulation Component 12. Ancillary/Other Uses
3. Onsite use/processing 8. Article Component 13. Manufacturing Impurity
4. Sale/Distribution 9. Repackaging 14. Process Impurity
5. Byproduct
10. Chemical Processing Aid
Source: TRI13 2014 (Data are from 2013)

168 NITRATE AND NITRITE
5. PRODUCTION, IMPORT/EXPORT, USE, AND DISPOSAL
Figure 5-1. Simplified Schematic of the Nitrogen Cycle
Adapted from EEA 2010; EPA 2012a; Vitousek et al. 1997
169 NITRATE AND NITRITE
5. PRODUCTION, IMPORT/EXPORT, USE, AND DISPOSAL
This oxidation process is an intermediate step in the nitrogen cycle, followed by further oxidation of
nitrite to nitrate ion via nitrite-oxidizing bacteria. These two reactions are mediated by aerobic
chemolithotrophs, Nitrosomonas and Nitrobactor, respectively (WHO 1978). Microbial conversion of
nitrate to nitrite (reduction) may also occur, especially after prolonged storage of vegetables that make the
environment anaerobic.
In nature, nitrate can also be found in igneous and volcanic rocks; however, the high solubility of nitrogen
salts makes minerals containing nitrate rare. Major minerals known are saltpeter (KNO
3
) found in India,
and Chile saltpeter (NaNO
3
) found in deserts of northern Chile (Environment Canada 2012; Hammerl and
Klapotke 2006).
Plants and mammals naturally contain nitrate and nitrite (WHO 2011b). Assimilation of nitrite from soils
occurs via reduction of nitrate to nitrite, which is facilitated by various bacteria and catalyzed by nitrate
reductase (WHO 1978). Mammals endogenously produce nitrate and excrete it in their waste products
(WHO 1978, 2011b).
Various industrial process produce nitrate in their waste streams. Specifically, potassium nitrate, calcium
nitrate, silver nitrate, and sodium nitrate used in several industrial applications have waste waters with
high-nitrate concentrations (Environment Canada 2012).
A major source of anthropogenic nitrate and nitrite is artificial fertilizers (WHO 1978). The majority of
nitrate in the environment derived from fertilizers does not solely originate from nitrate-containing
fertilizers; it also comes from ammonium and urea fertilizers. Nitrate from ammonium and urea
fertilizers is produced through biological processes involving hydrolysis of urea to ammonium and
ammonium nitrification (Kissel et al. 2008). Approximately 11.5 million tons of nitrogen are applied
yearly (as of 1994) in the United States as fertilizer in agricultural areas (Nolan et al. 1997). The
Association of American Plant Food Control Officials and The Fertilizer Institute reported that the United
States used 13.5 thousand tons of nitrogen fertilizer in 2012 (TFI 2014). Ammonium, calcium,
potassium, and sodium salts are all used in commercial fertilizers compounds (IARC 2010; WHO 2011b).
The most common nitrite salt, sodium nitrite, is produced commercially via the reaction of nitrogen
oxides with sodium carbonate or sodium hydroxide solution, typically at a pH higher than 8 (Hammerl
and Klapotke 2006). In 2004, global production of sodium nitrate was about 63 kilotons (IARC 2010).
Ammonium nitrate is manufactured through the reaction of nitric acid and ammonium (HSDB 2007).
Global production of ammonium nitrate in 2002 was reported at 13,608 kilotons (IARC 2010). Between
170 NITRATE AND NITRITE
5. PRODUCTION, IMPORT/EXPORT, USE, AND DISPOSAL
1998 and 1999, 90 kilotons of Canadian fertilizers were nitrate compounds: 82% as ammonium nitrate
and the remaining 18% from calcium nitrate, calcium ammonium nitrate, and potassium nitrate
(Environment Canada 2012).
According to the 2011 SRI Directory of Chemical Producers, there are 15 domestic producers of
ammonium nitrate in the United States, with an annual capacity of 2,290 metric tons (SRI 2011). There
were six producers of sodium nitrate, two producers of sodium nitrite, and one producer of potassium
nitrite; however, no production volumes or capacities were reported for any of these substances (SRI
2011). Production of ammonium nitrate in 2004 by the United States chemical industry was reported as
6,558 thousands of metric tons and preliminary production data reported 6,353 thousands of metric tons
for the year 2005 (HSDB 2007). Production of ammonium nitrate by the U.S. chemical industry in 1994
through 2003 is listed in Table 5-4. U.S. production of sodium nitrate in 1982 was estimated as
4.75x10
7
kg and at least 5.0x10
7
kg in 1977; U.S. production of sodium nitrite in 1977 was reported as at
least 5.0x10
6
kg; U.S. production of potassium nitrate in 1972 and 1975 were reported as 4.23x10
7
and
9.89x10
7
kg, respectively (HSDB 2007).
Production of ammonia by the U.S. chemical industry in 1995 through 2002 is listed in Table 5-5.
According to 2012 Chemical Data Reporting (CDR) data, the total reported production volumes for
ammonia and urea were 1.75 x10
10
kg/year and 1.17 x10
10
, respectively (EPA 2014g) Consumption
patterns indicate that the major use for these chemicals is in the fertilizer industry (HSDB 2003, 2012).
5.2 IMPORT/EXPORT
In 1984, United States imports of ammonium nitrate were 1.14x10
11
g (109,247 metric tons) and exports
in 1975 were reported as 3.18x10
10
(31,298 metric tons) (HSDB 2007). In 1986, U.S. imports of
potassium nitrate were 3.62x10
6
g (3.56 metric tons) and exports in 1975 were reported as negligible
(HSDB 2007). In 1985, U.S. imports of sodium nitrate were 6.44x10
7
g (63.4 metric tons) and exports in
1985 were reported as 4.81x10
6
(4.73 metric tons) (HSDB 2007). In 1984, U.S. imports of sodium nitrite
were 8.14x10
9
g (8,011 metric tons) and exports in 1984 were reported as 4.03x10
11
(396,635 metric tons)
(exports related to general sodium compounds) (HSDB 2007).

171 NITRATE AND NITRITE
5. PRODUCTION, IMPORT/EXPORT, USE, AND DISPOSAL
Table 5-4. Production of Ammonium Nitrate by the U.S. Chemical Industry
Year
Thousands of metric tons
1994
7,771
1995
7,700
1996
7,708
1997
7,804
1998
8,235
1999
6,920
2000
7,237
2001
5,833
2002
6,436
2003
5,733
Source: HSDB 2007

172 NITRATE AND NITRITE
5. PRODUCTION, IMPORT/EXPORT, USE, AND DISPOSAL
Table 5-5. Production of Ammonia by the U.S. Chemical Industry
Year
Millions of metric tons
1994
64,510
1995
35,600
1999
16.6
2000
15.7
2001
9.5
2002
10.8
Source: HSDB 2012
173 NITRATE AND NITRITE
5. PRODUCTION, IMPORT/EXPORT, USE, AND DISPOSAL
The U.S. Department of Agriculture (USDA 2013) has compiled annual import/export data on nitrate
fertilizers (ammonium nitrate, potassium nitrate, and sodium nitrate) for years 2000–2012. The volumes
for ammonium nitrate are provided in Table 5-6, followed by a 2012 comparison for all three fertilizers
(Table 5-7).
5.3 USE
The majority of nitrate in commerce is used in common inorganic fertilizers. Ammonia, urea, ammonium
nitrate, sodium nitrate, potassium nitrate, and calcium nitrate are used as commercial fertilizers;
ammonium nitrate and sodium nitrate are also used in munitions and explosives. These chemicals have
uses defined in several other industrial and consumer categories. Nitrate and nitrite salts are used as
preservatives in beverages. Additional uses include oxidizing agents, in instant cold packs and for the
production of nitrous oxide (ammonium nitrate), and for glass making (potassium nitrate) (EPA 2009a;
IARC 2010; Taylor 2004; WHO 2011b). Potassium and ammonium nitrate may also be used in
pyrotechnics, herbicides, and insecticides (HSDB 2007). Sodium nitrite is mainly used in the food
industry as a preservative, in cured meats for preventing botulism (e.g., it inhibits microbial activity of
certain Clostridium species in cheeses), and in the chemical, pharmaceutical, and agricultural industries
(Hammerl and Klapotke 2006; HSDB 2007; WHO 2011b). Sodium nitrite also has therapeutic uses such
as an antidote for cyanide poisoning and as an antifungal topical agent, for example against MRSA strains
(HSDB 2007; Ormerod et al. 2011; Pokorny and Maturana 2006). Due to the bioactivity of NO, an
endogenous metabolite of nitrite produced under hypoxic conditions, sodium nitrite is being used in
medicinal applications, such as for the treatment of pulmonary arterial hypertension (Blood and Power
2015; Lundberg et al. 2008; Rix et al. 2015). Potassium nitrate has been added to some toothpastes for
cavity prevention and to reduce sensitivity, as well as being used as a curing agent and color fixative in
meats (HSDB 2007). In nature, plants utilize nitrate as an essential nutrient (WHO 2011b).
5.4 DISPOSAL
Disposal methods for anthropogenic sources of nitrate and nitrite are general; unused portions of the
material should be recycled for the approved use or returned to the manufacturer or supplier, while leaks
or spills should be resolved wearing appropriate protective equipment and taking care not to create a
flammable or explosive environment. Response to a small liquid spill involves stopping the leak, soaking
up the liquid with vermiculite or sand, and placing it in a non-combustible container. Response to a large
liquid spill on land involves diking, product recovery, treating residue with soda ash and neutralizing it
with HCl, and flushing residue from the area with water. Response to a solid spill involves picking up the

174 NITRATE AND NITRITE
5. PRODUCTION, IMPORT/EXPORT, USE, AND DISPOSAL
Table 5-6. U.S. Imports and Exports (Metric Tons) of Selected Fertilizers 2000–
2012
Ammonium
Ammonium
Calcium
Calcium
Potassium
Potassium
Sodium
Sodium
nitrate nitrate nitrate nitrate nitrate nitrate nitrate nitrate
Year
exports
imports
exports
imports
exports
imports
exports
imports
2012
335,080
851,196
Not reported
38,550
15,746
159,135
3,348
148,898
2011
314,764
633,974
Not reported
33,998
16,449
114,861
3,286
90,470
2010
317,737
548,976
Not reported
34,490
9,991
76,849
2,429
70,156
2009
195,455
450,664
Not reported
123,168
8,449
73,871
2,536
79,741
2008
188,818
706,955
Not reported
204,552
4,322
132,571
5,783
149,467
2007
194,038
1,107,220
Not reported
187,640
Not reported
135,912
3,139
72,892
2006
127,244
1,150,523
Not reported
156,997
Not reported
149,633
2,827
68,416
2005
82,237
907,618
Not reported
119,448
Not reported
86,961
2,289
66,655
2004
109,972
1,055,949
Not reported
126,498
Not reported
66,381
2,838
62,812
2003
51,856
1,203,985
Not reported
90,989
Not reported
78,754
2,465
85,565
2002
98,218
989,507
Not reported
99,200
Not reported
100,712
2,810
72,568
2001
19,277
925,534
Not reported
127,586
Not reported
50,791
2,199
89,422
2000
21,611
838,035
Not reported
108,269
Not reported
40,941
2,264
96,067
Source: USDA 2013

175 NITRATE AND NITRITE
5. PRODUCTION, IMPORT/EXPORT, USE, AND DISPOSAL
Table 5-7. U.S. Exports and Imports for Nitrate Fertilizers in 2012 (Short Tons)
Fertilizer
Exports
Imports
Ammonium nitrate
369,362
938,283
Potassium nitrate
17,357
175,416
Sodium nitrate
3,691
164,132
Urea
370,694
7,654,464
Anhydrous ammonia
41,504
6,938,744
Aqua ammonia
6,549
96,517
All fertilizers
a
10,783,383
35,552,395
a
Includes nitrogen, potassium and phosphate fertilizers
Source: USDA 2013
176 NITRATE AND NITRITE
5. PRODUCTION, IMPORT/EXPORT, USE, AND DISPOSAL
material with implements (e.g., shovels, broom, and pan), placing in a non-combustible container, and
loosely capping the container. Spills to water can be treated with activated charcoal. Ultimate disposal of
the chemicals should take into account several factors (the material's impact on air quality; migration
characteristics; effects on animal, aquatic, and plant life) and must take into account compliance with
environmental and public health regulations. Generally, this involves treatment with sodium carbonate,
neutralization with HCl, and disposal of the resulting sludge in a secure landfill. If incineration is used,
processes to remove nitrogen dioxide and nitrogen oxide should be included (HSDB 2007).
177 NITRATE AND NITRITE
6. POTENTIAL FOR HUMAN EXPOSURE
6.1 OVERVIEW
Nitrate and nitrite are ubiquitous in the environment. Specific salts have occasionally been identified in
hazardous waste sites. Ammonium nitrate, sodium nitrate, and sodium nitrite were identified in 7, 3, and
2, of the 1,832 hazardous waste sites, respectively that have been proposed for inclusion on the EPA
National Priorities List (NPL) (ATSDR 2015). However, the number of sites evaluated for these
substances is not known. The frequency of these sites can be seen in Figures 6-1, 6-2, and 6-3.
Nitrate may enter the environment via natural and anthropogenic sources. Nitrate and nitrite occur
naturally in the environments as a part of the earth’s nitrogen cycle. A major source of anthropogenic
nitrate and nitrite is artificial fertilizers, and various industrial processes also produce nitrate in their waste
streams (Environment Canada 2012; WHO 1978). Inorganic fertilizer and nitrification of animal waste
are the principal sources of nitrate in the environment (Environment Canada 2012; Nolan et al. 1997).
However, contributions from human waste must be taken into account as well. Point and non-point
anthropogenic sources that contribute include industrial waste water, mining (explosives) waste water,
agricultural and urban runoff, feedlot discharges, septic system and landfill leachate, lawn fertilizers,
storm sewer overflow, and nitric oxide and nitrogen dioxide from vehicle exhaust (Environment Canada
2012). Additionally, organic forms of nitrogen in the environment from various sources may undergo
ammonification to form inorganic ammonia and ammonium, and nitrification to form nitrate, and have the
potential to be released into surface waters (Environment Canada 2012). Inorganic nitrate and nitrite in
soil and water can be taken up by plants used for human consumption (ATSDR 2013a).
Exposure from drinking water of private wells is a source of concern as elevated concentrations have been
reported in some wells, yet these water sources are not routinely tested, monitored, or regulated since they
are not covered by the Safe Drinking Water Act (SDWA). About 15% of Americans use private wells as
a source of drinking water and an important percentage of them may have a septic system serving their
homes. Additionally, nitrate and nitrite exposure can occur from the ingestion of foods containing high
levels of these chemicals (ATSDR 2013a).

178 NITRATE AND NITRITE
6. POTENTIAL FOR HUMAN EXPOSURE
Figure 6-1. Frequency of NPL Sites with Ammonium Nitrate Contamination

179 NITRATE AND NITRITE
6. POTENTIAL FOR HUMAN EXPOSURE
Figure 6-2. Frequency of NPL Sites with Sodium Nitrate Contamination

180 NITRATE AND NITRITE
6. POTENTIAL FOR HUMAN EXPOSURE
Figure 6-3. Frequency of NPL Sites with Sodium Nitrite Contamination
181 NITRATE AND NITRITE
6. POTENTIAL FOR HUMAN EXPOSURE
6.2 RELEASES TO THE ENVIRONMENT
The Toxics Release Inventory (TRI) data should be used with caution because only certain types of
facilities are required to report (EPA 2005). This is not an exhaustive list. Manufacturing and processing
facilities are required to report information to the TRI only if they employ 10 or more full-time
employees; if their facility is included in Standard Industrial Classification (SIC) Codes 10 (except 1011,
1081, and 1094), 12 (except 1241), 20–39, 4911 (limited to facilities that combust coal and/or oil for the
purpose of generating electricity for distribution in commerce), 4931 (limited to facilities that combust
coal and/or oil for the purpose of generating electricity for distribution in commerce), 4939 (limited to
facilities that combust coal and/or oil for the purpose of generating electricity for distribution in
commerce), 4953 (limited to facilities regulated under RCRA Subtitle C, 42 U.S.C. section 6921 et seq.),
5169, 5171, and 7389 (limited S.C. section 6921 et seq.), 5169, 5171, and 7389 (limited to facilities
primarily engaged in solvents recovery services on a contract or fee basis); and if their facility produces,
imports, or processes ≥25,000 pounds of any TRI chemical or otherwise uses >10,000 pounds of a TRI
chemical in a calendar year (EPA 2005).
Nitrate is released into the environment through both natural and anthropogenic sources. Naturally
occurring nitrate and nitrite are part of the earth’s nitrogen cycle. Anthropogenic sources, including
animal and human organic wastes as well as nitrogen-containing fertilizers, increase the concentrations of
nitrate in the environment. Nitrate and nitrite are present in the environment, in soils and water, and to a
lesser extent, in air, plant materials, and meat products. Concentrations of nitrite in plants and water are
low relative to nitrate concentration due to the fact that nitrite is easily oxidized to nitrate (WHO 1978).
Nitrate is the ion detected in the majority of groundwater and surface water samples because the nitrite
ion is easily oxidized to nitrate in the environment; the nitrate ion is stable and is chemically unreactive
under most environmental conditions (IARC 2010; WHO 2011b).
6.2.1 Air
Estimated releases of 301,654 pounds (~137 metric tons) of nitrate compounds to the atmosphere from
2,110 domestic manufacturing and processing facilities in 2013, accounted for about 0.1% of the
estimated total environmental releases from facilities required to report to the TRI (TRI13 2014). These
releases are summarized in Table 6-1. Estimated releases of 65,201 (~30 metric tons) pounds of sodium
nitrite were released to the atmosphere from 363 domestic manufacturing and

182 NITRATE AND NITRITE
6. POTENTIAL FOR HUMAN EXPOSURE
Table 6-1. Releases to the Environment from Facilities that Produce, Process, or
Use Nitrate Compounds
a
Reported amounts released in pounds per year
b
Total release
On- and off-
State
c
RF
d
Air
e
Water
f
UI
g
Land
h
Other
i
On-site
j
Off-site
k
site
AK
2
0
310,000
0
1,730,003
0
2,040,003
No data
2,040,003
AL
56
5
10,970,520
5,000
589,240
1,003
11,012,324
553,444
11,565,768
AR
26
0
3,776,021
0
14,399
4,217
3,790,167
4,470
3,794,637
AZ
32
3,295
0
0
39,894
78
43,189
78
43,267
CA
139
7,172
1,967,150
20,684
956,477
65,682
2,831,868
185,297
3,017,165
CO
39
1
1,850,266
0
54,944
50
1,904,854
407
1,905,261
CT
26
58,098
202,953
0
698
54,000
261,051
54,698
315,749
DC
4
0
0
0
0
0
0
No data
0
DE
6
0
2,850,359
0
0
0
2,850,359
No data
2,850,359
FL
36
479
850,888
23,373,722
264,705
0
24,261,115
228,680
24,489,794
GA
57
511
12,284,962
0
352,756
196,086
12,573,225
261,090
12,834,315
GU
1
0
181,244
0
196
0
181,440
No data
181,440
HI
7
0
439,915
0
0
0
439,915
No data
439,915
IA
46
23,005
6,737,465
0
133,387
28
6,886,605
7,280
6,893,885
ID
25
53
2,462,856
0
1,590,643
0
4,021,989
31,564
4,053,553
IL
108
28,202
6,428,670
14,007
454,232
2,346
6,875,892
51,565
6,927,456
IN
62
439
19,965,218
0
3,287,114
12,527
19,965,662
3,299,636
23,265,298
KS
27
38,262
108,068
340,905
98,569
21
585,186
639
585,825
KY
44
990
5,031,265
0
313,935
532
5,305,617
41,104
5,346,721
LA
48
4,502
10,169,890
1,576,528
55,015
0
11,753,676
52,259
11,805,934
MA
42
10
115
25,928
27,091
217,377
145
270,376
270,520
MD
21
0
739,290
0
84,199
35
739,687
83,837
823,524
ME
12
1,209
2,854,965
0
63
0
2,856,209
28
2,856,237
MI
103
10,021
1,714,827
0
228,050
33,121
1,732,091
253,928
1,986,019
MN
53
1,076
1,471,856
0
78,276
250
1,536,094
15,364
1,551,458
MO
37
2,852
1,752,877
0
241,834
5,100
1,977,139
25,524
2,002,663
MS
28
372
6,495,644
0
329
0
6,496,345
No data
6,496,345
MT
10
0
234,169
0
43,891
0
272,860
5,200
278,060
NC
41
1
6,563,023
0
236,361
236,692
6,793,505
242,572
7,036,077
ND
10
0
113,400
0
15,640
0
129,040
No data
129,040
NE
25
187
11,785,649
0
243,650
40
12,022,108
7,418
12,029,526
NH
7
125
0
0
0
39
125
39
164
NJ
40
0
5,313,118
0
41,966
376
5,354,858
602
5,355,460
NM
14
55,000
42,240
0
662,620
0
406,331
353,529
759,860
NV
27
6
2,800
0
3,947,279
2
3,729,262
220,825
3,950,087
NY
73
5,337
5,797,905
0
437,314
28,473
5,804,333
464,696
6,269,029
OH
111
1,987
6,081,057
134,614
182,994
64,665
6,219,720
245,598
6,465,318
OK
37
13,010
4,246,811
534,620
180,969
0
4,970,061
5,349
4,975,410
OR
40
1,000
542,093
0
6,833
0
545,071
4,855
549,926

183 NITRATE AND NITRITE
6. POTENTIAL FOR HUMAN EXPOSURE
Table 6-1. Releases to the Environment from Facilities that Produce, Process, or
Use Nitrate Compounds
a
Reported amounts released in pounds per year
b
Total release
On- and off-
State
c
RF
d
Air
e
Water
f
UI
g
Land
h
Other
i
On-site
j
Off-site
k
site
PA
67
9,139
7,212,765
0
66,541
2,428
7,224,885
65,988
7,290,874
PR
7
0
0
1,465
120
34
1,465
154
1,619
RI
6
0
121
0
0
20,098
121
20,098
20,219
SC
43
3,001
2,346,088
0
178,745
458
2,376,696
151,596
2,528,293
SD
8
0
2,995,074
2,000
338,782
6
3,280,324
55,538
3,335,862
TN
45
270
2,748,175
470,956
125,258
1,678
2,772,445
573,892
3,346,337
TX
131
788
14,234,564
7,762,004
876,395
7,165
21,822,716
1,058,200
22,880,916
UT
37
329
97,000
0
1,282,016
29
714,559
664,815
1,379,374
VA
42
3,558
10,978,189
0
4,587
1
10,981,823
4,511
10,986,334
VT
6
0
124,890
0
57,395
0
124,890
57,395
182,285
WA
46
8,770
1,190,505
0
981,730
0
1,631,774
549,231
2,181,004
WI
127
591
2,162,376
0
2,339,390
31,174
3,734,315
799,216
4,533,531
WV
18
18,000
2,140,714
0
2,138
0
2,160,673
179
2,160,852
WY
5
0
633
6,569,900
249
0
6,570,782
No data
6,570,782
Total
2,110
301,654
188,570,641
40,832,332
22,848,913
985,811
242,566,590
10,972,761
253,539,351
a
The TRI data should be used with caution since only certain types of facilities are required to report. This is not an exhaustive list.
Data are rounded to nearest whole number.
b
Data in TRI are maximum amounts released by each facility.
c
Post office state abbreviations are used.
d
Number of reporting facilities.
e
The sum of fugitive and point source releases are included in releases to air by a given facility.
f
Surface water discharges, waste water treatment-(metals only), and publicly owned treatment works (POTWs) (metal and metal
compounds).
g
Class I wells, Class II-V wells, and underground injection.
h
Resource Conservation and Recovery Act (RCRA) subtitle C landfills; other onsite landfills, land treatment, surface impoundments,
other land disposal, other landfills.
i
Storage only, solidification/stabilization (metals only), other off-site management, transfers to waste broker for disposal, unknown
j
The sum of all releases of the chemical to air, land, water, and underground injection wells.
k
Total amount of chemical transferred off-site, including to POTWs.
RF = reporting facilities; UI = underground inject
ion
Source: TRI13 2014 (Data are from 2013)
184 NITRATE AND NITRITE
6. POTENTIAL FOR HUMAN EXPOSURE
processing facilities in 2013, accounted for about 0.8% of the estimated total environmental releases from
facilities required to report to the TRI (TRI13 2014). These releases are summarized in Table 6-2.
Estimated releases of 125,680,001 pounds (~57,007 metric tons) of ammonia were released to the
atmosphere from 2,292 domestic manufacturing and processing facilities in 2013, accounted for about
77% of the estimated total environmental releases from facilities required to report to the TRI (TRI13
2014). These releases are summarized in Table 6-3.
6.2.2 Water
Estimated releases of 188,570,641 pounds (~85,534 metric tons) of nitrate compounds to surface water
from 2,110 domestic manufacturing and processing facilities in 2013, accounted for about 74% of the
estimated total environmental releases from facilities required to report to the TRI (TRI13 2014). These
releases are summarized in Table 6-1. Estimated releases of 2,472,668 pounds (~1,122 metric tons) of
sodium nitrite compounds to surface water from 363 domestic manufacturing and processing facilities in
2013, accounted for about 30% of the estimated total environmental releases from facilities required to
report to the TRI (TRI13 2014). These releases are summarized in Table 6-2. Estimated releases of
4,221,440 pounds (~1,914 metric tons) of ammonia to surface water from 2,292 domestic manufacturing
and processing facilities in 2013, accounted for about 2.6% of the estimated total environmental releases
from facilities required to report to the TRI (TRI13 2014). These releases are summarized in Table 6-3.
EPA (2009d) reported that the Mississippi River drains >40% of the area of the contiguous 48 states and
carries roughly 15 times more nitrate than any other river in the country. EPA (2009d) noted that the
nitrate load in the Mississippi rose from 200,000 to 500,000 tons per year in the 1950s and 1960s to an
average of approximately 1,000,000 tons per year during the 1980s and 1990s; the data indicate that the
nitrate load decreased slightly in the early 2000s.
Nitrate is commonly detected in various surface waters and groundwaters such as shallow, rural domestic
wells. Contamination of water systems is a consequence of inorganic fertilizer use, animal manures,
septic systems, and waste water treatment (ATSDR 2013a; Nolan 1999; WHO 2011b). Ammonium ions
in sludge from waste water treatment plants, as well as effluents from those plants and septic systems, are
rapidly converted to nitrate (WHO 1978). Various industrial process produce nitrate in their waste
streams. For example, potassium nitrate, calcium nitrate,

185 NITRATE AND NITRITE
6. POTENTIAL FOR HUMAN EXPOSURE
Table 6-2. Releases to the Environment from Facilities that Produce, Process, or
Use Sodium Nitrite
a
Reported amounts released in pounds per year
b
Total release
On- and
State
c
RF
d
Air
e
Water
f
UI
g
Land
h
Other
i
On-site
j
Off-site
k
off-site
AL
7
0
0
0
360
0
0
360
360
AR
7
0
254
0
0
0
254
No data
254
AZ
3
0
0
0
9,117
0
9,117
No data
9,117
CA
11
7
0
0
5
0
7
5
12
CO
2
255
0
0
0
0
255
No data
255
FL
1
0
0
0
0
0
0
No data
0
GA
11
98
648,256
0
101,148
68
749,502
68
749,570
IA
3
0
2,517
0
0
0
2,517
No data
2,517
ID
1
0
0
0
0
0
0
No data
0
IL
32
1,637
18,600
0
90,065
15
20,237
90,080
110,317
IN
20
1
1,130,853
0
3,312,017
4,896
1,130,854
3,316,913
4,447,767
KS
2
0
0
0
0
0
0
No data
0
KY
10
1,016
0
0
41,435
36,320
27,291
51,480
78,771
LA
9
0
48,000
1,500,000
107
0
1,548,000
107
1,548,107
MA
5
0
0
0
0
0
0
No data
0
MD
1
16
0
0
0
103
16
103
119
MI
42
12,992
4
0
181,207
2,490
18,745
177,948
196,693
MN
6
0
194,173
0
0
0
194,173
No data
194,173
MO
13
2,871
0
0
412
3,685
2,871
4,097
6,968
MS
6
8,489
7,895
0
26,296
3
16,384
26,299
42,683
NC
4
0
4,455
0
0
0
4,455
No data
4,455
NE
4
0
21,200
0
1,182
1,637
21,467
2,552
24,019
NJ
9
200
68,032
0
2,898
0
68,290
2,840
71,130
NM
1
0
0
0
0
0
0
No data
0
NV
1
2
0
0
33,641
0
33,642
No data
33,642
NY
8
11,807
4,925
0
2,800
220
16,732
3,020
19,752
OH
35
13,067
731
0
77,303
1,134
13,798
78,437
92,235
OK
3
289
9,010
0
17,405
0
9,299
17,405
26,704
OR
2
0
0
0
430
0
0
430
430
PA
15
250
0
0
7
0
250
7
257
RI
1
0
0
0
0
0
0
No data
0
SC
19
1,530
69,403
0
787
190
70,933
977
71,910
SD
3
6
121
25
5,500
0
5,652
0
5,652
TN
5
239
0
0
153
0
239
153
392
TX
38
34
199,659
198,969
54,487
0
449,818
3,331
453,149
UT
1
No data
No data
No data
No data
No data
No data
No data
No data
VA
4
0
0
0
40
0
0
40
40
WA
1
No data
No data
No data
No data
No data
No data
No data
No data

186 NITRATE AND NITRITE
6. POTENTIAL FOR HUMAN EXPOSURE
Table 6-2. Releases to the Environment from Facilities that Produce, Process, or
Use Sodium Nitrite
a
Reported amounts released in pounds per year
b
Total release
On- and
State
c
RF
d
Air
e
Water
f
UI
g
Land
h
Other
i
On-site
j
Off-site
k
off-site
WI
13
10,231
28
0
69,018
0
10,259
69,018
79,277
WV
4
165
44,552
0
3
0
44,717
3
44,720
Total
363
65,201
2,472,668
1,698,994
4,027,823
50,760
4,469,774
3,845,673
8,315,446
a
The TRI data should be used with caution since only certain types of facilities are required to report. This is not an
exhaustive
list. Data are rounded to nearest whole number.
b
Data in TRI are maximum amounts released by each facility.
c
Post office state abbreviations are used.
d
Number of reporting facilities.
e
The sum of fugitive and point source releases are included in releases to air by a given facility.
f
Surface water discharges, waste water treatment-(metals only), and publicly owned treatment works (
POTWs) (metal
and metal compounds).
g
Class I wells, Class II-V wells, and underground injection.
h
Resource Conservation and Recovery Act (RCRA) subtitle C landfills; other onsite landfills, land treatment, surface
impoundments, other land disposal, other landfills.
i
Storage only, solidification/stabilization (metals only), other off-site management, transfers to waste broker for
disposal, unknown
j
The sum of all releases of the chemical to air, land, water, and underground injection wells.
k
Total amount of chemical transferred off-site, including to POTWs.
RF = reporting facilities; UI = underground injection
Source: TRI13 2014 (Data are from 2013)

187 NITRATE AND NITRITE
6. POTENTIAL FOR HUMAN EXPOSURE
Table 6-3. Releases to the Environment from Facilities that Produce, Process, or
Use Ammonia
a
Reported amounts released in pounds per year
b
Total release
On- and off-
State
c
RF
d
Air
e
Water
f
UI
g
Land
h
Other
i
On-site
j
Off-site
k
site
AK
6
23,099
7,012
136
24,075
0
54,323
No data
54,323
AL
70
4,060,001
209,278
9,343
43,646
294
4,287,915
34,647
4,322,563
AR
47
1,837,750
114,277
0
5,499
511
1,956,076
1,961
1,958,037
AS
1
20
0
0
0
0
20
No data
20
AZ
20
336,273
0
0
722
0
336,989
6
336,995
CA
118
2,801,456
26,830
2,870
165,409
369
2,978,123
18,811
2,996,934
CO
19
269,063
17,833
0
101,218
2,430
385,942
4,602
390,544
CT
15
74,775
155
0
0
0
74,930
No data
74,930
DC
2
165
0
0
0
0
165
No data
165
DE
7
58,659
6,071
0
23
0
64,730
23
64,753
FL
63
5,607,959
244,014
464,183
960,078
0
6,343,579
932,655
7,276,234
GA
81
12,615,696
268,976
0
166,494
153
12,967,436
83,883
13,051,319
HI
9
100,496
1,000
1,200
0
0
102,696
No data
102,696
IA
79
7,333,058
108,999
0
210,562
6,621
7,545,358
113,882
7,659,240
ID
19
2,844,444
27,824
0
167,548
0
3,023,068
16,749
3,039,817
IL
112
3,081,028
110,035
0
69,293
4,620
3,246,667
18,309
3,264,976
IN
64
1,600,854
45,833
707,485
77,195
0
2,423,117
8,250
2,431,367
KS
37
3,056,601
6,490
38,214
49,185
15,483
3,130,592
35,381
3,165,972
KY
47
971,022
55,064
0
48,663
1,845
1,036,520
40,073
1,076,593
LA
72
13,461,749
585,745
4,582,747
326,520
0
18,630,317
326,444
18,956,761
MA
30
178,727
41
0
1,622
0
178,768
1,622
180,390
MD
16
523,890
48,675
0
2
0
572,565
2
572,567
ME
10
753,203
89,718
0
0
0
842,921
No data
842,921
MI
70
1,681,959
36,582
9,790
7,181
7,875
1,731,377
12,010
1,743,387
MN
61
1,870,843
49,112
0
35,092
2,270
1,938,690
18,627
1,957,317
MO
46
422,032
228,193
0
44,490
251
654,800
40,166
694,966
MS
34
4,700,259
188,194
0
2,181
0
4,889,803
830
4,890,633
MT
10
449,711
5,420
0
264,118
0
719,205
44
719,249
NC
86
2,661,108
85,427
0
73,410
110
2,781,752
38,302
2,820,054
ND
13
16,199,585
4,476
11,500
474,130
0
16,689,625
66
16,689,691
NE
44
974,508
26,655
0
162,935
4,643
1,021,771
146,970
1,168,741
NH
9
127,211
447
0
0
2
127,658
2
127,660
NJ
43
527,774
9,790
0
24,984
74
537,605
25,017
562,622
NM
6
98,819
0
2,300
11,561
0
112,680
No data
112,680
NV
12
132,670
560
0
228,759
1
361,989
1
361,990
NY
50
687,039
50,197
0
974
274
737,570
915
738,485
OH
115
6,430,630
100,258
1,715,361
76,575
2,560
8,244,156
81,229
8,325,384
OK
24
5,433,575
18,832
696,880
137,282
0
6,282,692
3,877
6,286,569
OR
31
1,156,034
35,599
0
9,980
0
1,192,997
8,616
1,201,613

188 NITRATE AND NITRITE
6. POTENTIAL FOR HUMAN EXPOSURE
Table 6-3. Releases to the Environment from Facilities that Produce, Process, or
Use Ammonia
a
Reported amounts released in pounds per year
b
Total release
On- and off-
State
c
RF
d
Air
e
Water
f
UI
g
Land
h
Other
i
On-site
j
Off-site
k
site
PA
95
1,739,875
77,649
59
710,403
4,905
1,829,424
703,467
2,532,891
PR
10
366,211
0
0
1,499
0
366,211
1,499
367,710
RI
10
7,130
0
0
0
2,500
7,130
2,500
9,630
SC
54
3,581,123
163,436
0
15,085
60,935
3,747,126
73,453
3,820,579
SD
10
102,302
1,809
1
21,448
0
104,819
20,741
125,560
TN
70
2,512,358
418,374
0
90,697
0
2,932,376
89,053
3,021,430
TX
209
4,853,142
297,133
17,187,730
397,858
468
21,789,218
947,114
22,736,332
UT
27
520,482
1,484
8
1,162,922
120
1,684,865
151
1,685,016
VA
49
4,358,663
105,431
0
30,198
32,318
4,471,823
54,787
4,526,610
VT
2
4,543
5,357
0
76
0
9,900
76
9,976
WA
29
878,866
55,744
0
100,110
3,880
940,195
98,405
1,038,600
WI
81
593,142
45,129
15,241
14,091
0
640,527
27,076
667,603
WV
34
332,238
227,614
137,238
47,192
13
602,602
141,693
744,294
WY
14
686,181
8,666
297,557
2,791
0
995,195
No data
995,195
Total
2,292
125,680,001
4,221,440
25,879,844
6,565,774
155,524
158,328,597
4,173,986
162,502,583
a
The TRI data should be used with caution since only certain types of facilities are required to report. This is not an
exhaustive list. Data are rounded to nearest whole number.
b
Data in TRI are maximum amounts released by each facility.
c
Post office state abbreviations are used.
d
Number of reporting facilities.
e
The sum of fugitive and point source releases are included in releases to air by a given facility.
f
Surface water discharges, waste water treatment-(metals only), and publicly owned treatment works (POTWs) (metal and
metal compounds).
g
Class I wells, Class II-V wells, and underground injection.
h
Resource Conservation and Recovery Act (RCRA) subtitle C landfills; other onsite landfills, land treatment, surface
impoundments, other land disposal, other landfills.
i
Storage only, solidification/stabilization (metals only), other off-site management, transfers to waste broker for disposal,
unknown
j
The sum of all releases of the chemical to air, land, water, and underground injection wells.
k
Total amount of chemical transferred off-site, including to POTWs.
R
F = reporting facilities; UI = underground injection
Source: TRI13 2014 (Data are from 2013)
189 NITRATE AND NITRITE
6. POTENTIAL FOR HUMAN EXPOSURE
silver nitrate, and sodium nitrate used in several industrial applications have waste waters with high-
nitrate concentrations (Environment Canada 2012). Discharges of these waste streams increase the
concentrations of nitrate and nitrite in surface waters. Treatment of these waste streams may only remove
a portion of nitrogen. Factors such as nitrogen loading, population density, soil drainage characteristics,
and woodland to cropland ratios, affect the transport of nitrogen from land to water (Nolan et al. 1997;
Zhang et al. 1998). Increased levels of nitrite in drinking water may also be a consequence of
contamination by boiler fluid additives (ATSDR 2013a). High risk waters for nitrate contamination
include areas having soils with high permeability, high-nitrogen input, and low woodland to cropland
ratios (Nolan et al. 1997; Zhang et al. 1998).
Natural sources of nitrate and nitrite include wet and dry deposition of atmospheric nitric acid and nitrate
ion. These are formed in the atmosphere as a result of nitrogen cycling. Atmospheric deposition is a
-
factor for nitrate concentrations in water systems (Momen et al. 1999). Atmospheric nitrogen (NO
3
,
NO
2
-
, and NH
4
+
), mainly from natural sources but a result of anthropogenic sources as well, have been
estimated to contribute 182 kilotons of inorganic nitrogen per year to Canadian surface waters via wet and
dry deposition (Environment Canada 2012). In the United States, deposition contributes an estimated
3.2 million tons (3,200 kilotons) of nitrogen per year to watersheds (Momen et al. 1999; Nolan 1999;
Nolan et al. 1997). Owens et al. (1994) reported that nitrogen input to a grass pasture from precipitation
was equivalent to 10% of the nitrogen fertilizer applied during a 5-year period. The concentration of
nitrate-nitrogen in the precipitation during 1975–1980 was reported as 1.1 mg/L (ppm), which correlated
to an input of 12.0 kg nitrate-nitrogen/hectare. A 10-year average was also evaluated for the years 1980–
1990, which resulted in an input of 8.9 kg nitrate-nitrogen/hectare (Owens et al. 1994).
Stagnation of nitrate-containing and oxygen-poor drinking water in galvanized steel pipes and
chlorination disinfectant residues can lead to conditions where nitrite is formed via chemical reactions in
the distribution pipes by Nitrosomonas bacteria (WHO 2011b).
A U.S. Geological Survey (USGS) study across the United States showed that 7% of 2,388 domestic
wells and about 3% of 384 public-supply wells were contaminated with nitrate levels above the EPA
drinking water standard of 10 mg/L (10 ppm) (ATSDR 2013a). Between 1994 and 1996, 24 lakes in the
Adirondack Park, United States, were studied to assess the contribution of in-lake processes, atmospheric
deposition, and watershed cover on the lakes’ nitrate concentrations (Momen et al. 1999). Weighted
means for nitrate concentrations as a result of precipitation near the lakes were reported for all seasons
during the study period and ranged from 13.86 to 35.52 µeq/L. Nitrogen concentrations throughout the
190 NITRATE AND NITRITE
6. POTENTIAL FOR HUMAN EXPOSURE
study period ranged from 2.1 to 22 µmol/L. Both atmospheric deposition and average lake depth were
considered strong factors in concentrations of nitrate in lakes. It was concluded that the average lake
depth was the most important factor; greater average depths correlated to higher nitrate concentrations.
This was attributed to decreased contact time with lake sediment, decreasing the potential for removal
processes (Momen et al. 1999).
Concentrations of nitrate in freshwater downstream from an open-pit coal mining operation have been
reported to exceed 44 mg nitrate/L (Nordin and Pommen 1986). This is attributed to high nitrate levels in
waste streams due to explosive residues. Monitoring studies conducted by the USGS indicate that nitrate
and nitrite levels are several times greater in streams and groundwater in areas classified as agricultural
use rather than as urban use, mixed use, or undeveloped land (USGS 2010a, 2010b).
Policies implemented by the European Union (EU) to reduce nitrogen emissions from agricultural point
sources were reviewed by Velthof et al. (2014). The Nitrates Directive (ND) was implemented to protect
water quality across Europe by inhibiting nitrates released by agricultural sources from leaching into
groundwater and surface waters through the use of good farming practices. Although regional differences
in emissions were large throughout the entire EU, nitrate leaching into groundwater and surface waters
was estimated to decrease by 16% in nitrate leaching vulnerable zones over the period of 2000–2008,
primarily as a result of lower nitrogen emissions from fertilizers and manures (Velthof et al. 2014).
Seawater nitrate concentrations that occur naturally due to nitrification processes can be as high as 2.4 mg
nitrate/L (Environment Canada 2012). Assimilation into biological systems can deplete nitrate
concentrations in marine environments, causing seasonal variations in nitrate concentrations. Winter
concentrations off the Canadian Atlantic coast were reported to be 0.54 mg nitrate/L, a magnitude higher
than summer concentrations of <0.03 mg nitrate/L (Environment Canada 2012).
6.2.3 Soil
Estimated releases of 22,848,913 pounds (~10,364 metric tons) of nitrate compounds to soils from
2,110 domestic manufacturing and processing facilities in 2013, accounted for about 9% of the estimated
total environmental releases from facilities required to report to the TRI (TRI13 2014). An additional
40,832,332 pounds (~18,521 metric tons), constituting about 16% of the total environmental emissions,
were released via underground injection (TRI13 2014). These releases are summarized in Table 6-1. An
estimated release of 4,027,823 pounds (~1,826 metric tons) of sodium nitrite were emitted to soils from
191 NITRATE AND NITRITE
6. POTENTIAL FOR HUMAN EXPOSURE
363 domestic manufacturing and processing facilities in 2013, accounted for about 48% of the estimated
total environmental releases from facilities required to report to the TRI (TRI13 2014). An additional
1,698,994 pounds (~771 metric tons), constituting about 20% of the total environmental emissions, were
released via underground injection (TRI13 2014). These releases are summarized in Table 6-2. An
estimated release of 6,565,774 pounds (~2,978 metric tons) of ammonia were emitted to soils from
2,292 domestic manufacturing and processing facilities in 2013, accounted for about 4% of the estimated
total environmental releases from facilities required to report to the TRI (TRI13 2014). An additional
25,879,844 pounds (~11,739 metric tons), constituting about 16% of the total environmental emissions,
were released via underground injection (TRI13 2014). These releases are summarized in Table 6-3.
In 2012, 13.5 million tons of nitrogen was added to soils as fertilizer (TFI 2014). Therefore, it should be
noted that the totals provided here, for nitrate compounds, ammonia, and ammonium nitrite alone, may be
insignificant, yet contribute to and are representative of, nitrogen releases to the environment.
6.3 ENVIRONMENTAL FATE
Nitrate and nitrite occur naturally in water and soils as part of the nitrogen cycle. Plants and mammals
naturally contain nitrate and nitrite (WHO 2011b). Nitrate is the primary source of nitrogen for plants
(EPA 2009a). Assimilation of nitrite from soils occurs via reduction of nitrate to nitrite, which is
facilitated by various bacteria and catalyzed via nitrate reductase (WHO 1978). The most common forms
of nitrogen that plants assimilate include ammonium (NH
4
+
), nitrate (NO
3
-
), and urea ((NH
2
)2CO)
(Cornell University 2009). Transport, partitioning, and transformation are controlled by various
physicochemical properties, degradation, and other loss processes. Mammals endogenously produce
nitrate and excrete them in their waste products (WHO 1978). Anthropogenic and natural sources of
ammonia in the environment, such as fertilizers or animal waste products, are converted to nitrite via
Nitrosomonas bacteria and then to nitrate via Nitrobactor bacteria. These products may be assimilated
into plants and subsequently the atmosphere, or may leach into groundwater when they are present in
excessive amounts (WHO 2011b).
Nitrate is the most oxidized form of nitrogen present in the environment (oxidation state of +5) and
accounts for the majority of the total available nitrogen in surface waters (Environment Canada 2012).
Nitrate is the conjugate base of nitric acid, HNO
3
, a strong acid with pKa of -1.37 (WHO 1978). Nitric
acid and salts of nitric acid completely dissociate in aqueous solutions (Environment Canada 2012; WHO
1978). Nitrite is the conjugate base of nitrous acid, HNO
2
, a weak acid with a pKa of 3.37; nitrite readily
192 NITRATE AND NITRITE
6. POTENTIAL FOR HUMAN EXPOSURE
decomposes to yield water and dinitrogen trioxide or nitric acid, nitric oxide, and water (WHO 1978;
WHO 2011b).
6.3.1 Transport and Partitioning
Nitrate and nitrite are inorganic water-soluble salts with the potential for rapid migration through soils to
surface water and groundwater (Nolan 1999; Taylor 2004; EPA 2009a). Sorption of anions such as
nitrate is insignificant in most soils; therefore, leaching of excess soil nitrate into oceans, lakes, streams,
and groundwater is an important consideration (Taylor 2004). Drainage characteristics of soils are
strongly related to nitrate levels in shallow wells near agricultural areas (Nolan et al. 1997; Zhang et al.
1998). Other factors affecting leaching potential include the texture of the soil, pH, precipitation rates,
tillage, and the types of crops or vegetation that may be planted in the soils.
The mobility of nitrate in a mid-European semi-natural grassland ecosystem as a function of plant
diversity was investigated (Scherer-Lorenzen et al. 2003). The greatest leaching was observed in bare
ground plots as well as plots planted only with legumes. Experiments with plots containing a wider
variety of plant species indicated that total nitrate plant uptake increased and leaching losses decreased
with increasing plant diversity due to greater root biomass within the soils. The leaching of nitrate
decreased in the following order: bare plots > pure legumes > legumes + grasses > legumes + grasses +
herbs (Scherer-Lorenzen et al. 2003). Annual nitrate leaching in an apple orchard was 4.4–5.6 times
greater in plots treated with conventional farming practices (calcium nitrate fertilizer) as compared to
plots treated by organic farming practices, in which nitrogen application was accomplished by loadings of
chicken manure and alfalfa meal (Kramer et al. 2006). Reduced leaching was accompanied by increased
denitrification in the organic treatment areas. Kitchen et al. (2015) investigated groundwater nitrate as a
result of leaching due to agriculture cropping systems over time (1994–2004) and found the greatest
decreases in groundwater nitrate concentration occurred as groundwater moved through an in-field tree
line or through a riparian zone.
Nitrate leaching from croplands with high fertilizer use is a major source of groundwater nitrate
concentrations. In Nebraska, groundwater concentrations of nitrate have been correlated with nitrogen-
containing fertilizer application rates and residual nitrogen in surface soils (Schepers et al. 1991). The
reduction of nitrate concentrations in groundwater through agricultural management practices was
assessed in Nebraska’s central Platte River valley (Exner et al. 2010). Groundwater nitrate concentration
reports were studied from 1986 to 2003. Peak levels, during 1988, in the primary aquifers were
193 NITRATE AND NITRITE
6. POTENTIAL FOR HUMAN EXPOSURE
26.8 mg nitrate-nitrogen/L. A gradual decline was observed with the implementation of fertilizer
management regulations. In 2003, nitrate-nitrogen levels in the aquifer averaged 22.0 mg/L.
Nitrate in soils and surface water are susceptible to denitrification resulting in gaseous losses to the
atmosphere (Taylor 2004). Nitrate in the atmosphere, emitted by denitrification, industrial processes, and
vehicle exhaust, is deposited on land and water in precipitation, gases, and dry particles (Nolan 1999;
Taylor 2004). Atmospheric deposition is a factor for nitrate concentrations in water systems (Momen et
al. 1999; Nolan 1999; Nolan et al. 1997).
6.3.2 Transformation and Degradation
Nitrate and nitrite has the potential to move into various environmental compartments and are subject to
abiotic and biotic degradation processes. Transformation and degradation processes include
denitrification to atmospheric nitrogen and plant uptake (Newton 2005; Nolan 1999). Conversion is
achieved via biotic process carried out by auto- and heterotrophic bacteria (Hammerl and Klapotke 2006).
Under aerobic conditions in aquatic systems, ammonia and nitrite are converted to nitrate via nitrification.
Conversion is achieved through a biotic process carried out by autotrophic nitrifying bacteria. Under
anaerobic conditions in aquatic systems, bacteria convert nitrate to nitrite, which is further reduced to the
gaseous compounds nitric oxide (NO), nitrous oxide (N
2
O), and N
2
(nitrogen). These compounds are
subsequently released to the atmosphere. Results from a study of denitrification in riverbed sediments
found that potential rates for denitrification are limited by environmental conditions such as available
organic carbon and temperature, rather than concentration of nitrate itself (Pfenning and McMahon1997).
Higher rates were demonstrated in experiments with added carbon sources. Additionally, higher rates of
denitrification were measured at 22°C compared to those at 4°C (Pfenning and McMahon1997).
6.3.2.1 Air
Nitrogen compounds are formed in the air by natural phenomena such as lightning (Hord et al. 2011), or
may be discharged into air from industrial processes, motor vehicles, agricultural practices, or emitted by
denitrification processes. Nitrate is present in air primarily as nitric acid and inorganic aerosols, as well
as nitrate radicals and organic gases or aerosols (WHO 2011b). Nitrate in the atmosphere is subject to
wet and dry deposition and are deposited on land via precipitation, gases, and dry particles (Nolan 1999;
Taylor 2004).
194 NITRATE AND NITRITE
6. POTENTIAL FOR HUMAN EXPOSURE
6.3.2.2 Water
In surface waters, assimilation by plants and algae accounts for the majority of nitrate loss. Reducing
conditions of water system including dissolved oxygen (DO) and dissolved organic carbon (DOC), as
well as temperature and pH, influence the extent of bacterially mediated nitrate loss processes in water
systems (Nolan 1999; WHO 2011b). Biologically mediated reduction processes of nitrate and nitrite
were found to be positively related to DO and inversely related to iron, manganese, ammonium, and DOC
concentrations (Nolan 1999).
Rates of denitrification were examined by measuring N
2
O production using riverbed sediment and
groundwater or surface water collected from the South Platte alluvial aquifer in Colorado, an area with
high nitrate levels due to anthropogenic activity such as fertilizer use, farming practices, and septic
drainage and runoff (Pfenning and McMahon 1996). The greatest N
2
O production was observed in
microcosms containing high levels of organic carbon. The type of organic carbon source was also shown
to be correlated with the denitrification rate. Higher N
2
O production rates were observed with acetate-
amended sediments as compared to sediments amended with fulvic acids. Surface water-derived fulvic
acids resulted in higher denitrification rates as compared to groundwater-derived fulvic acids. Reduction
in microbial activity due to temperature gradients was also investigated. Denitrification rates decreased
by nearly 80% in laboratory experiments when the temperature was lowered from 22 to 4°C.
6.3.2.3 Sediment and Soil
In soils, nitrite is oxidized to nitrate and the majority of nitrate is assimilated by plants and algae (WHO
2011b). Under aerobic conditions, residual or excess nitrate is expected to leach into groundwater and is
not expected to undergo considerable degradation and/or denitrification. Under anaerobic conditions
degradation of nitrate into atmospheric nitrogen is an important removal process (WHO 2011b). Poorly
drained soils, which lack oxygen, promote nitrate conversion to gaseous nitrogen (Nolan et al. 1997).
Denitrification rates were examined using soil samples obtained from different areas of an apple orchard
and measuring the rates of gaseous nitrogen and N
2
O production as well as nitrate leaching (Kramer et al.
2006). Specifically denitrification rates were measured at different locations of the orchard treated with
organic farming practices, conventional farming practices for orchards in the state of Washington, and
integrated treatments from horticultural and pest management practices of conventional and organic
farming practices. Nitrogen application in the conventional plots used calcium nitrate based fertilizer,
while the organic plots used chicken manure and alfalfa meal. The integrated plots used equal parts of
195 NITRATE AND NITRITE
6. POTENTIAL FOR HUMAN EXPOSURE
calcium nitrate fertilizer and chicken manure. Nitrogen emissions were higher in the organically treated
plots, as compared to the conventional and integrated plots. Nitrate leaching was much greater (4.4–
5.6 times higher) in the conventional plots as compared to the organically treated plots.
6.4 LEVELS MONITORED OR ESTIMATED IN THE ENVIRONMENT
Reliable evaluation of the potential for human exposure to nitrate and nitrite depends in part on the
reliability of supporting analytical data from environmental samples and biological specimens.
Concentrations of nitrate and nitrite in unpolluted atmospheres and in pristine surface waters are often so
low as to be near the limits of current analytical methods. In reviewing data on nitrate and nitrite levels
monitored or estimated in the environment, it should also be noted that the amount of chemical identified
analytically is not necessarily equivalent to the amount that is bioavailable. The analytical methods
available for monitoring nitrate and nitrite in a variety of environmental media are detailed in Chapter 7.
Nitrate occurs naturally in the environments as a part of the earth’s nitrogen cycle. Elevated levels may
be present due to anthropogenic sources such as fertilizers, and human or animal wastes. High levels of
nitrate in drinking water pose a health risk to infants, children, and pregnant or nursing women (EPA
2009a).
6.4.1 Air
Anthropogenic emissions of nitrogen oxides (NO
x
) are now of the same order of magnitude as natural
emissions (Hammerl and Klapotke 2006). Air pollution is considered a minor source of exposure to
nitrate (WHO 2011b). Nitrate in the atmosphere is generally a result of nitrogen oxides released into the
atmosphere that are oxidized to nitric acid, in turn forming nitrate particles (Matsumoto and Tanaka
1996). Atmospheric levels of particulate nitrate are highly dependent on temperature and the chemical
composition of aerosol and gases in the atmosphere, especially particulate ammonium nitrate and gaseous
nitric acid (Matsumoto and Tanaka 1996). Reported atmospheric nitrate concentrations range from low
concentrations of 0.1–0.4 μg/m
3
up to higher-level concentrations ranging from 1 to 40 μg/m
3
(WHO
1978, 2011b). Concentrations in Netherland air samples have been reported to range from 1 to 14 μg/m
3
.
Indoor nitrate aerosol concentrations of 1.1–5.6 μg/m
3
appear to be related to outdoor concentrations
(WHO 2011b). Zhuang et al. (1999) evaluated the concentrations of fine and coarse particle nitrate in the
atmosphere over Hong Kong. The average daily concentrations for fine and coarse particle nitrate were
found to be 0.583 and 1.663 µg/m
3
, respectively.
196 NITRATE AND NITRITE
6. POTENTIAL FOR HUMAN EXPOSURE
6.4.2 Water
Nitrate and nitrite concentrations in water are typically expressed as either mg nitrate/L (ppm nitrate) and
mg nitrite/L (ppm nitrite), or mg nitrate as nitrogen (nitrate-nitrogen/L) and mg nitrite as nitrogen (nitrite-
nitrogen/L) (IARC 2010). The federal drinking-water standard maximum contaminant level (MCL) for
nitrate is 10 mg nitrate-nitrogen/L and the MCL for nitrite is 1 mg nitrite-nitrogen/L (EPA 2009c; USGS
2010a; WHO 2011b). Inorganic nitrate and nitrite are very soluble in water and occur naturally in
groundwater and surface water as a result of the earth’s nitrogen cycle. Naturally occurring background
levels of nitrate (concentrations expected if there were no effects of human development and
anthropogenic sources) have been estimated as 1.0 and 0.24 mg nitrate-nitrogen/L for groundwater and
streams, respectively, in the United States (USGS 2010a).
A comprehensive report analyzed nutrient levels in 5,101 wells from 51 different study areas (Burow et
al. 2010; USGS 2010a). Monitoring data from 1993 to 2003 indicated that nitrate levels in groundwater
varied widely across the nation, with some of the highest levels observed in the Northeast (particularly
southern Pennsylvania), the Midwest, the state of California, and select regions of the Northwest
(Washington state and Idaho). The report concluded that nitrate levels in deep aquifers were likely to
continue to increase as shallow groundwater with high levels of nitrate gravitate downward (USGS
2010a). The highest levels of nitrate were observed in oxic groundwater (water containing >0.5 ppm DO)
as opposed to anoxic groundwaters and shallow wells in agricultural areas, which tended to have greater
levels than in urban areas (USGS 2010a). Burow et al. (2010) analyzed these data and reported that
nitrate concentrations exceeded the MCL (10 mg nitrate-nitrogen/L) in 437 wells (8%). Levels exceeded
the MCL in 20% of wells classified as agricultural land-use setting, and 3% were above the MCL in wells
classified as urban use. In monitoring data from bank and in-stream wells in the San Joaquin River in
California, collected between 2006 and 2008, the concentration of nitrate exceeded the detection limit
(0.01 mg/L) in 5% of the groundwater samples and the concentrations in surface waters ranged from 1 to
3 mg/L. It was reported that 17 of the 26 nested monitoring wells, along the river bed and river bank, had
no detectable concentrations of nitrate during the monitoring period (USGS 2013a).
Monitoring data obtained from 1991 to 1995 in shallow groundwater of coastal plains in the Albemarle-
Pamlico Drainage Unit, in North Carolina and Virginia, have indicated the presence of increased nitrate
concentrations as a result of agriculture and anthropogenic sources. Shallow groundwater concentrations
are higher at inner coastal sites with well-drained soils compared with outer coastal sites. Areas with
anthropogenic nitrogen sources, such as fertilizer and manure, had aquifer concentrations >3 mg nitrate-
197 NITRATE AND NITRITE
6. POTENTIAL FOR HUMAN EXPOSURE
nitrogen/L. Levels <2 mg nitrate-nitrogen/L were reported in aquifer waters with greater DOC
concentrations. Groundwaters from areas having well-drained soils had a median concentration of
approximately 0.4 mg nitrate-nitrogen/L. Two of the 20 inner coastal wells had levels >10 mg nitrate-
nitrogen/L. Groundwater concentration of nitrate was nearly undetectable in waters underneath poorly
drained soils in the outer coast (median 0.05 mg nitrate-nitrogen/L). The North Carolina Division of
Water Quality (NCDWQ) selected groundwater samples susceptible to contamination; 40% of the
15 wells in the inner coastal plain of the Albemarle-Pamlico Drainage had levels >10 mg nitrate-
nitrogen/L (USGS 2012).
Nitrate and nitrite were the two most detected inorganic chemicals reported in public water systems
(PWSs) in an analysis supporting the U.S. EPA’s second Six-Year Review of National Primary Drinking
Water Regulations. Occurrence data for nitrate from a Six-Year Review-ICR Dataset include
1,052,487 analytical results from 119,537 public water systems (groundwater 114,764; surface water
4,773) across 44 states during the time period from 1998 to 2005 (EPA 2009a). These water systems are
reported to serve a combined population of 229,508,036. Nitrate was detected in approximately 70% of
the water systems (groundwater 69.4%; surface water 81.3%) at a median concentration of 1.8 mg nitrate-
nitrogen/L (groundwater systems 1.6 mg nitrate-nitrogen/L; surface water systems 2.71 mg nitrate-
nitrogen/L). Maximum concentrations detected in groundwater and surface water systems were 99 and
48.5 mg nitrate-nitrogen/L, respectively. Seven states in the review reported at least one detection of
nitrate greater than the MCL of 10 mg nitrate-nitrogen/L in >5% of their systems. Overall, the
2,973 systems with detections exceeding the MCL serve a combined population of 16,777,093 (EPA
2009a). Occurrence data for nitrite from the Six-Year Review-ICR Dataset includes 397,175 analytical
results from 86,313 public water systems (groundwater 82,738; surface water 3,575) across 44 states
during the time period from 1998 to 2005 (EPA 2009a). These water systems are reported to serve a
combined population of 207,984,813. Nitrite was detected in approximately 22% of the water systems
(groundwater 22%; surface water 23%) at a median concentration of 0.02 mg nitrite-nitrogen/L.
Maximum concentrations detected in groundwater and surface water systems were 13 and 8.68 mg nitrite-
nitrogen/L, respectively. Four states in the review reported at least one nitrite detection greater than the
MCL (1 mg nitrite-nitrogen/L) in more than 1% of their systems. Overall, the 635 systems with
detections exceeding the MCL serve a combined population of 10,067,031 (EPA 2009a).
In 1991, 12% of 631 private wells located on farmlands across 18 states in the United States reported
concentrations >10.2 mg nitrate-nitrogen/L (Bruning-Fann et al. 1994; IARC 2010). Additionally, in
1994, levels of nitrate found in drinking waters across nine mid-western U.S. states ranged from 0.01 to
198 NITRATE AND NITRITE
6. POTENTIAL FOR HUMAN EXPOSURE
266 mg nitrate-nitrogen/L, with a mean value of 8.4 mg nitrate-nitrogen/L and 10% of the water supplies
had concentrations >10 mg nitrate-nitrogen/L (CDC 1998). California groundwater is relied on for
drinking water in 70% of its cities. A study in 1987 indicated that ~10% of the sampled California wells
and >7% of the public water systems in Tulare County, California had levels >45 mg nitrate/L (>10 mg
nitrate-nitrogen/L) (Zhang et al. 1998).
Studies regarding nitrate levels in drinking water outside the United States were summarized (IARC
2010). Zhang et al. (1996) reported that several cities in China (with populations between 10,000 and
100,000) had water supplies with concentrations >11.3 mg nitrate-nitrogen/L. Twenty-eight percent of
public wells monitored in India and 13% in Saudi Arabia had reported levels >11.2 mg nitrate-nitrogen/L
as well. In 1990, average concentrations in Canadian municipal drinking waters were reported to range
from 0.1 to 3.3 mg nitrate/L (0.02–0.75 mg nitrate-nitrogen/L) (Environment Canada 2012). It has been
estimated that 2% of the European population receive their drinking water from private wells and an
estimated 2.4 million people are exposed to water supplies containing nitrate concentrations above
guideline levels (Gangolli et al. 1994). Nitrate concentrations in many European countries have been
reported to be gradually increasing over the last few decades. An average annual increase of 0.7 mg
nitrate/L (0.2 mg nitrate-nitrogen/L) has been observed in some rivers of the United Kingdom (WHO
2011b). Nitrate levels in drinking water in Denmark have increased about 400% over the period from
1940 to 1983 (Moller et al. 1989).
Nitrate was reported to be the most frequently detected nutrient in U.S. streams that exceeded its MCL. It
exceeded the MCL in 2% of all samples obtained (566 out of 27,555) and in at least 1 sample of 50 of the
499 streams surveyed from 1992 to 2001 (USGS 2010a). Many of the streams with levels above the
MCL were located in the upper Midwestern Corn Belt where application rates of fertilizer and manure are
high. Nitrite samples from five streams exceeded the nitrite MCL (USGS 2010a). Flow-adjusted nitrate
concentrations decreased in 25% of 166 streams and rivers sampled by the USGS over the period 1993–
2003; however, concentrations increased over this time period in 20 of the 166 sites (12%) surveyed
(USGS 2009). Decreases in levels were attributed to changes in nitrogen use patterns and implementation
of pollution control strategies. Multiple factors such as land use, nitrogen loading from fertilizer, manure
use, and atmospheric deposition affected the trends observed.
Annual trends of nitrate levels at eight sites along the Mississippi River Basin were studied by the USGS
from 1980 to 2010 (USGS 2013b). Flow-normalized nitrate concentrations were generally reported to be
level or increasing at all of the monitoring sites from 1980 to 2000; however, select locations showed
199 NITRATE AND NITRITE
6. POTENTIAL FOR HUMAN EXPOSURE
greater increases or actual decreases in levels since 2000. The greatest increases over the 30-year period
were observed in the upper Mississippi River (Clinton, Iowa) and Missouri River (Herman, Missouri).
Decreasing flow-normalized nitrate levels over the 30-year period were observed at the Iowa River near
Wapello, Iowa and Illinois River at Valley City, Illinois, suggesting that recent land management and
farming practices may be reducing the nitrate fluxes in these areas (USGS 2013b).
Many surface water bodies in the United States have a large percentage of total nitrate load contributed by
base flow (groundwater discharge, release from other watershed storages, and long-term interflow) in
addition to surface runoff. Mean annual base-flow nitrate levels for 148 surface water and shallow
groundwater sites in the United States from 1990 to 2006 were typically reported to be <1 mg nitrate-
nitrogen/L; however, values as high as 8.48, 11.44, 8.29, and 8.25 mg nitrate-nitrogen/L were reported for
Tulpehocken Creek, Pennsylvania; Indian Creek, Illinois; Salt Creek, Illinois; and Clifty Creek, Indiana,
respectively (USGS 2010b). The highest levels typically were reported for agricultural land use sites that
had frequent fertilizer and manure applications and highly permeable underlying bedrock.
Due to assimilation of nitrate by algae and other plant-life, concentrations of nitrate in surface water are
typically lower than that detected in groundwaters (IARC 2010). Tables 1.4 and 1.5 in the IARC report
summarize concentrations of nitrate from various regions around the globe. The global concentrations in
groundwater were reported to range from 0.02 to 110 mg nitrate-nitrogen/L; mean values ranged from
2.2 to 42.9 mg nitrate-nitrogen/L. Global concentrations of nitrate in surface water were reported to range
from 0 to 22 mg nitrate-nitrogen/L; mean values range from 0.1 to 8.3 mg nitrate-nitrogen/L). Nitrate
levels in rainwater as high as 5 mg nitrate/L (1 mg nitrate-nitrogen/L) have been observed in various
industrial areas (WHO 2011b).
6.4.3 Sediment and Soil
Levels of nitrate and nitrite in soil vary considerably as a function of soil properties, temperature,
precipitation rates, nitrogen loadings, farming practices (tillage, crops planted), and seasonal changes. In
well-drained aerobic soils, the conversion of ammonia into nitrate (nitrification) increases the soil-nitrate
content and in anaerobic soils with high organic matter (such as waterlogged soils or wetlands),
denitrification decreases the levels of nitrate and nitrite in soils. Acidic soils tend to have lower levels of
nitrate since the nitrification process ceases at pH levels below 4.5 (USDA 2014). Typical nitrate levels
in humid temperate soils fluctuate from about 20–65 kg-nitrogen/hectare in cropped soils and 25–150 kg-
200 NITRATE AND NITRITE
6. POTENTIAL FOR HUMAN EXPOSURE
nitrogen/hectare in bare soils (USDA 2014). Certain locations near the South Platte River in northeastern
Colorado had nitrate levels exceeding 500 kg-nitrogen/hectare (Shaffer et al. 1995).
6.4.4 Other Environmental Media
Nitrate and nitrite are common food preservatives. Levels of nitrite and nitrate were evaluated through
several processing steps and storage conditions. Nitrite is oxidized to nitrate during storage. Nitrate
remained relatively constant over a 2-week storage period, while nitrite levels declined over the same
time period. The study also observed minimal influence of the nitrate levels formed from the amount of
nitrite added. Additionally, using nitrate alone resulted in the formation of nitrite after thermal
processing. Cooking resulted in losses of both nitrite and nitrate; 50% nitrite and 10–15% nitrate
remained in the prepared sausage analyzed (Perez-Rodriguez et al. 1996).
Nitrate and nitrite are present in vegetables, fruits, cured meats, fish, dairy products, beers, cereals, and
cereal products (Gangolli et al. 1994). Nitrate content of foodstuffs is typically higher than nitrite content
(ATSDR 2013a; WHO 2011b). Cured meats have concentrations of <2.7–945 mg of nitrate/kg and <0.2–
1.7 mg nitrite/kg (IARC 2010; WHO 2011b). Concentrations of nitrate in vegetables and fruit is strongly
affected by processing of the food, fertilizer use, and growing conditions and range from 30 to 6,000
mg/kg (ppm) (IARC 2010; WHO 2011b). Celery, lettuce, red beetroot, and spinach have high levels of
nitrate (200–>2,500 mg/kg [ppm]). Parsley, leek, endive, Chinese cabbage, and fennel have high levels
of nitrate ranging from 100 to 250 mg/kg (ppm). Cabbage, dill, and turnips have medium levels of nitrate
ranging from 50 to 100 mg/g. Vegetables with low levels of nitrate (20–50 mg/g) include broccoli,
carrots, cauliflower, cucumber, and pumpkin. Very low levels of nitrate (<20 mg/g) are found in
artichokes, asparagus, eggplant, garlic, onions, green beans, mushrooms, peas, peppers, potatoes, summer
squash, sweet potatoes, tomatoes, and watermelons (ATSDR 2013a). Nitrite concentrations are typically
<10 mg/kg (ppm) and rarely reach 100 mg/kg (ppm); however, exceptions include damaged, outdated,
pickled, and fermented foods in which levels may be as high as 400 mg/kg (ppm) (WHO 2011b). Data
for nitrate and nitrite in foodstuffs are not lacking and several papers have been published that summarize
numerous studies, including IARC (94 2010) and Gangolli et al. (1994). Gangolli et al. (1994) cited a
paper reporting the average daily intakes of nitrate and nitrite in the United States to be 106 and
4.1 mg/day, respectively.
Marshall and Trenerry (1996) analyzed several food types purchased from local supermarkets in Australia
for both nitrate and nitrite. Nitrite was not detected in fruit juices in this study. Nitrate and nitrite was
201 NITRATE AND NITRITE
6. POTENTIAL FOR HUMAN EXPOSURE
detected in various cheeses at levels <10 mg/kg (ppm). Canned meat products had small amounts of
nitrite (<10 mg/kg [ppm]), while nitrate levels were higher (10–25 mg/kg [ppm]). Several samples of ice
were also analyzed for the presence of nitrite and nitrate, and all samples were below Australian food
standards (10 mg/L nitrate; 1 mg/L nitrite).
Nitrite and nitrate levels were determined in several whey-containing food products (Oliveira et al. 1995).
In total, 231 samples from powdered modified milk, powdered non-fat milk, a dairy beverage, and
strawberry- and chocolate-flavored instant mixes were evaluated. Mean nitrate levels ranged between
7.3 and 532 mg/kg (ppm). Mean nitrite levels ranged between 1.1 and 2.5 mg/kg (ppm). One serving of
the product with the highest reported nitrate levels (a chocolate-flavored instant mix) was calculated to be
51.9 mg/serving. One serving of the product with the highest reported nitrite levels (powdered non-fat
milk) was calculated to be 0.1 mg/serving.
From 1993 to 1997, nitrate and nitrite levels were monitored in Danish lettuce, leek, potato, beetroot,
Chinese cabbage, and white cabbage, and spinach (Peterson and Stoltze 1999). Seasonal variation was
observed in nitrate levels. Lettuce exhibited higher concentrations in winter as opposed to summer.
Overall nitrite concentrations were low. Average nitrate concentrations were 2,760, 1,783, 198, and
158 mg/kg fresh weight for lettuce, fresh spinach, leeks, and potatoes, respectively. Average nitrite
concentrations were 11, 0.91, 0.80, 0.15, and 0.14 mg/kg fresh weight for fresh spinach, beetroot,
potatoes, leeks, and lettuce, respectively. The average daily intakes, estimated from consumption surveys
of the vegetables in the study, were 40 mg/day nitrate and 0.09 mg/day nitrite.
Table 6-4 contains data on infant foods examined for nitrate and nitrite (Cortesi et al. 2015). Food
samples of animal origin were composed of a variety of sources such as poultry, beef, rabbit, lamb, and
turkey. Foods samples of plant origin were composed of a variety of sources such peas, legumes,
vegetable broths, cream of pumpkin and carrots, and mixed vegetables. Mixed-origin samples were
composed of both plant and animal sources. The highest average concentration of nitrate was found in
foods of plant origin (45.5 mg/kg), while the highest average concentration of nitrite was found in foods
of animal origin (14.82 mg/kg).
Jones et al. (2014) reported nitrate and nitrite concentrations in fresh breast milk, freeze-thawed breast
milk, freeze thawed colostrum, and several commercially available infant formulas. Data are tabulated in
Table 6-5. Fresh breast milk was collected from 11 mothers of term infants and 13 mothers of preterm
infants. Samples of colostrum (milk expressed days 1–3), transition milk (expressed days 4–7), and

202 NITRATE AND NITRITE
6. POTENTIAL FOR HUMAN EXPOSURE
Table 6-4. Concentrations of Nitrate and Nitrite in Infant Food Products
Food type
Nitrate (mg/kg)
Nitrite (mg/kg)
Homogenized samples of animal
0.35–83.2
6.6–48.87
origin
Freeze dried samples of animal
2.01–80.26
1.3–74.74
origin
Homogenized samples of plant
4.82–131.68
2.26–20.71
origin
Freeze dried samples of plant
19.41–85.03
1.34–6.62
origin
Homogenized samples of mixed
3.77–67.31
1.98–80.22
origin
Source: Cortesi et al. 2015

203 NITRATE AND NITRITE
6. POTENTIAL FOR HUMAN EXPOSURE
Table 6-5. Average Concentrations of Nitrate and Nitrite in Human Milk and Infant
Formula
Milk type
Nitrate (µmol/L)
Nitrite (µmol/L)
Fresh breast milk
16
0.1
Freeze-thawed breast milk
20
0.04
Preterm fresh
12
0.07
Preterm freeze-thawed
22
0.03
Term fresh
11.5
0.13
Term freeze-thawed
12.5
0.04
Freeze-thawed colostrum
41
0.16
Infant formula
43
0.29
Freeze-thawed colostrum
44
0.15
Transition milk
Not reported
0.05
Mature milk
Not reported
0.025
Source: Jones et al. 2014
204 NITRATE AND NITRITE
6. POTENTIAL FOR HUMAN EXPOSURE
mature milk (expressed days >7) were analyzed. Concentrations of nitrate and nitrite in the 10 formulas
evaluated were 9–61 and not detected–1.4 µmol/L, respectively.
6.5 GENERAL POPULATION AND OCCUPATIONAL EXPOSURE
The general public is typically exposed to nitrate and nitrite via ingestion of water and foods that contain
these chemicals. Inhalation and dermal exposure may be possible; however, these routes are not as
prominent. Oral exposure to nitrate and nitrite from contaminated drinking water and food is the
prominent route. Nitrate and nitrite overexposure may occur through ingestion of foods containing high
levels of nitrate and nitrite (ATSDR 2013a). Inorganic nitrate and nitrite can be taken up by plants,
especially leafy vegetables such as lettuce and spinach as well as beet root; vegetables account for about
80% of the nitrate in a typical human diet (ATSDR 2013a; Hord 2011; Lundberg et al. 2009; Peterson and
Stoltze 1999). Contaminated foodstuffs from improper storage of commercial and prepared baby foods
have caused overexposure in children (Dusdieker et al. 1994; Greer and Shannon 2005; Sanchez-Echaniz
et al. 2001).
Iammarino et al. (2014) analysed 75 samples of spinach and 75 samples of lettuce, collected from June
2010 to December 2011, for nitrate and nitrite. Spinach had a greater number of detections compared
with the lettuce samples. Mean nitrate concentrations ranged from 155.5 to 2,149.6 mg/kg; mean nitrite
concentrations ranged from 16.3 to 101.6 mg/kg. Four spinach samples and five lettuce samples had
concentrations of nitrate >2,000 mg/kg. Quantifiable concentrations of nitrite were detected in
15 samples of spinach (28.5–197.5 mg/kg) and one sample of lettuce (66.5 mg/kg).
The remainder of the nitrate in a typical diet comes from drinking water (about 21%) and from meat and
meat products (about 6%) in which sodium nitrate is used as a preservative and color-enhancing agent
(ATSDR 2013a; Lundberg et al. 2008; Saito et al. 2000). For bottle-fed infants, the major source of
nitrate exposure is from contaminated drinking water used to dilute formula, especially when the water is
boiled prior to use (ATSDR 2013a; Fewtrell 2004). A review by Jones et al. (2015) reported daily nitrate
ingestion concentrations for adults. An intake of approximately 3 mg/kg/day for adults was based on a
typical adult diet.
The Fourth National Report on Human Exposures to Environmental Chemicals, published and updated by
the Centers for Disease Control and Prevention (CDC 2013), reported the following data from the
National Health and Nutrition Examination Survey (NHANES) 1999–2008. Nitrate levels in the urine
205 NITRATE AND NITRITE
6. POTENTIAL FOR HUMAN EXPOSURE
(see Table 6-6), and urine (creatinine corrected) (see Table 6-7) were evaluated for various ages and
ethnicities. Mean values of nitrate in the urine were 42.7 and 46.3 mg/L for 7,697 members of the general
U.S. population sampled during 2005–2006 and 7,629 members of the general U.S. population sampled
during 2007–2008, respectively. The highest geometric mean (creatinine corrected) during 2001–2002 of
72.0 mg/L was determined from 374 samples from 6–11 year olds; the highest geometric mean (creatinine
corrected) during 2005–2006 of 60.8 mg/L was determined from 1,054 samples from 6–11 year olds; and
the highest geometric mean during 2007–2008 of 70.2 mg/L was determined from 1,143 samples from 6–
11 year olds. Throughout all survey years, females had a higher geometric mean than males. In the
survey years 2007–2008, 3,789 female samples yielded a mean of 51.0 mg/g, while 5,351 male samples
yielded a mean of 44.6 mg/g (CDC 2013).
No information was located regarding absorption of inhaled inorganic nitrate or nitrite in humans or
laboratory animals. Inhalation of inorganic nitrate or nitrite is not a likely exposure route of concern for
the general population, although inhalation of dust from fertilizer products containing nitrate salts is
possible.
Occupational exposure is primarily via inhalation and dermal routes. Industrial workers and farmers may
be exposed via inhalation of dusts. Dusts may also dissolve in sweat on skin, increasing the potential for
dermal exposure.
Vegetable consumption is a considerable source of nitrate, and drinking water with high levels of nitrate
is also a major contributing factor. Several studies have been conducted assessing exposure to drinking
waters with high levels of nitrate. In 1986 until 1987, Moller et al. (1989) studied 294 Danish adults
between the ages of 20 and 64 years who were exposed to various levels of nitrate in their drinking water
and diet. Twenty-one drinking water supplies contained low (0–5 mg nitrate/L [ppm nitrate])
intermediate (35–59 mg nitrate/L [ppm nitrate]) and high (≥60 mg nitrate/L [ppm nitrate]) nitrate levels,
with mean concentrations of 0.3, 46.5, and 84.4 mg nitrate/L (ppm nitrate), respectively. The median
exposures of total dietary nitrate for the low, intermediate, and high water categories were 37, 89, and
123 mg nitrate/day, respectively. Mean nitrate levels detected in the participant’s 24-hour urine samples
for the low, intermediate, and high water concentration categories were reported as 36, 55, and 73 mg
nitrate, respectively. Overall, the dietary contribution was calculated to be 17% from water and 83%
from food for the low group, and increased to approximately 60% from water and 40% from food for the
intermediate and high groups. Fifty-nine Canadian adults between the ages of 20–74 years used tap water
with low (<3 mg nitrate-nitrogen/L) and high (>3 mg nitrate-nitrogen/L) concentrations of nitrate. The

206 NITRATE AND NITRITE
6. POTENTIAL FOR HUMAN EXPOSURE
Table 6-6. Geometric Mean and Selected Percentiles of Urine Concentrations of
Urinary Nitrate (in mg/L) for the U.S. Population from the National Health and
Nutrition Examination Survey (NHANES)
Geometric
Selected percentiles (95% CI)
Survey
years
mean
(95% CI)
50
th
75
th
90
th
95
th
Sample
size
Total
2001–2002
2005–2006
2007–2008
48.2 (46.2–
50.3)
42.7 (39.6–
46.1)
46.3 (44.6–
48.1)
49.0 (46.0–
52.0)
47.8 (44.4–
51.2)
50.3 (48.4–
52.0)
78.0 (73.0–
83.0)
74.6 (69.8–
79.4)
76.0 (72.7–
79.1)
100 (100–
130)
108 (101–
114)
110 (104–
116)
140 (130–
150)
133 (125–
144)
138 (132–
146)
1,617
7,697
7,629
Age group
6–11 years
2001–2002
2005–2006
2007–2008
62.2 (53.8–
71.8)
51.2 (47.4–
55.4)
55.2 (51.7–
58.9)
68.0 (58.0–
79.0)
54.7 (51.9–
58.2)
60.2 (56.1–
64.3)
94.0 (84.0–
100)
79.2 (72.8–
87.2)
84.5 (80.1–
92.5)
130 (100–
160)
113 (101–
128)
117 (107–
135)
150 (120–
380)
141 (120–
158)
149 (132–
189)
374
1,054
1,143
12–19 years
2001–2002
2005–2006
2007–2008
57.4 (53.5–
61.6)
52.5 (48.5–
56.8)
55.5 (51.5–
59.7)
66.0 (60.0–
69.0)
57.5 (52.3–
62.6)
56.8 (51.9–
61.5)
91.0 (86.0–
95.0)
84.2 (79.9–
88.4)
84.1 (76.2–
94.5)
120 (100–
130)
119 (111–
124)
119 (107–
133)
150 (130–
160)
144 (129–
153)
144 (133–
162)
827
2,106
1,135
≥20 years
2001–2002
2005–2006
2007–2008
45.4 (43.3–
47.5)
40.5 (37.4–
43.9)
44.2 (42.5–
45.9)
49.0 (46.0–
52.0)
45.0 (41.3–
48.3)
48.1 (46.0–
49.7)
78.0 (73.0–
83.0)
71.7 (67.1–
77.4)
73.2 (70.1–
76.5)
100 (100–
130)
105 (98.0–
113)
107 (101–
113)
140 (130–
150)
129 (122–
142)
135 (128–
146)
1,617
4,537
5,351
Gender
Males
2001–2002
2005–2006
2007–2008
57.5 (54.6–
60.6)
48.4 (44.6–
52.6)
51.9 (49.9–
54.1)
63.0 (59.0–
67.0)
52.7 (48.3–
57.8)
56.1 (53.8–
58.0)
89.0 (83.0–
94.0)
79.4 (72.8–
86.5)
79.5 (75.7–
83.5)
130 (100–
140)
110 (103–
121)
112 (105–
119)
150 (140–
170)
136 (123–
152)
137 (131–
149)
1,335
3,765
3,839
Females
2001–2002
2005–2006
2007–2008
40.7 (38.4–
43.2)
37.9 (35.1–
40.8)
41.4 (39.3–
43.7)
43.0 (41.0–
48.0)
42.0 (38.2–
46.0)
43.9 (41.5–
46.3)
72.0 (68.0–
76.0)
69.2 (65.5–
73.4)
71.3 (67.5–
74.8)
100 (98.0–
120)
104 (96.6–
110)
108 (99.8–
115)
130 (120–
150)
130 (124–
140)
138 (132–
149)
1,483
3,932
3,790

207 NITRATE AND NITRITE
6. POTENTIAL FOR HUMAN EXPOSURE
Table 6-6. Geometric Mean and Selected Percentiles of Urine Concentrations of
Urinary Nitrate (in mg/L) for the U.S. Population from the National Health and
Nutrition Examination Survey (NHANES)
Geometric
Selected percentiles (95% CI)
Survey
years
mean
(95% CI)
50
th
75
th
90
th
95
th
Sample
size
Race/ethnicity
Mexican
Americans
2001–2002
2005–2006
2007–2008
53.2 (48.7–
58.2)
47.8 (44.7–
51.2)
48.7 (45.0–
52.6)
59.0 (52.0–
66.0)
52.4 (49.9–
56.2)
51.9 (47.4–
56.7)
84.0 (79.0–
91.0)
77.9 (75.1–
83.1)
75.6 (69.7–
81.3)
120 (100–
150)
113 (104–
120)
111 (100–
122)
160 (130–
180)
148 (133–
156)
148 (127–
164)
707
1,972
1,505
Non-Hispanic
blacks
2001–2002
2005–2006
2007–2008
53.8 (47.8–
60.5)
45.9 (42.1–
50.0)
47.5 (45.0–
50.3)
58.0 (51.0–
64.0)
50.4 (46.6–
54.9)
50.3 (48.6–
52.5)
84.0 (77.0–
93.0)
75.0 (68.8–
80.8)
74.7 (71.9–
77.3)
120 (100–
130)
101 (95.3–
110)
105 (97.1–
116)
140 (130–
170)
127 (114–
148)
134 (125–
150)
680
2,078
1,707
Non-
Hispanic
whites
2001–2002
2005–2006
2007–2008
46.3 (44.1–
48.6)
41.2 (37.6–
45.2)
45.0 (42.7–
47.5)
51.0 (47.0–
53.0)
46.2 (41.3–
50.6)
49.2 (46.1–
52.6)
81.0 (78.0–
85.0)
73.3 (67.3–
80.1)
75.5 (70.5–
80.0)
120 (100–
130)
107 (98.2–
115)
108 (101–
116)
140 (130–
150)
129 (122–
142)
134 (128–
140)
1,228
3,056
3,190
CI = confidence interval
Source: CDC 2013

208 NITRATE AND NITRITE
6. POTENTIAL FOR HUMAN EXPOSURE
Table 6-7. Geometric Mean and Selected Percentiles of Urine Concentrations of
Urinary Nitrate (Creatinine Corrected) (in mg/g of creatinine) for the
U.S. Population from the National Health and Nutrition
Examination Survey (NHANES)
Geometric
Selected percentiles (95% CI)
Survey
years
mean
(95% CI)
50
th
75
th
90
th
95
th
Sample
size
Total
2001–2002
2005–2006
2007–2008
49.8 (47.7–
51.9)
42.6 (40.2–
45.1)
47.7 (45.9–
49.7)
46.9 (44.2–
49.6)
42.4 (40.1–
44.7)
46.0 (44.0–
48.3)
63.8 (61.2–
67.7)
59.7 (55.8–
64.1)
66.5 (62.4–
70.5)
90.9 (84.3–
98.8)
85.5 (81.3–
91.3)
98.0 (92.3–
102)
120 (111–
128)
113 (106–
118)
127 (119–
135)
1,616
7,697
7,628
Age group
6–11 years
2001–2002
2005–2006
2007–2008
72.0 (66.1–
78.4)
60.8 (57.4–
64.5)
70.2 (65.7–
74.9)
66.0 (62.6–
70.4)
57.3 (53.6–
60.6)
65.9 (62.4–
69.5)
87.0 (80.2–
97.7)
76.5 (70.9–
82.1)
89.0 (83.2–
96.5)
129 (96.5–
144)
109 (95.0–
123)
128 (112–
152)
144 (130–
235)
134 (121–
164)
173 (140–
216)
374
1,054
1,143
12–19 years
2001–2002
2005–2006
2007–2008
44.8 (43.4–
46.2)
39.8 (37.8–
41.9)
43.4 (41.3–
45.5)
43.8 (42.6–
45.0)
38.1 (36.3–
40.3)
40.5 (38.6–
43.5)
56.2 (52.2–
59.7)
51.8 (47.7–
56.0)
56.0 (52.7–
59.2)
73.2 (65.2–
85.1)
70.6 (63.1–
78.8)
76.6 (69.0–
86.2)
93.4 (79.0–
104)
88.9 (79.4–
103)
98.0 (85.4–
121)
826
2,106
1,134
≥20 years
2001–2002
2005–2006
2007–2008
48.3 (45.9–
50.9)
41.4 (38.9–
43.9)
46.5 (44.6–
48.4)
46.9 (44.2–
49.6)
41.0 (38.8–
43.7)
44.7 (42.5–
47.0)
63.8 (61.2–
67.7)
58.6 (54.8–
63.1)
65.0 (60.7–
69.2)
90.9 (84.3–
98.8)
85.3 (80.2–
91.0)
96.1 (90.0–
102)
120 (111–
128)
111 (105–
116)
125 (117–
132)
1,616
4,537
5,351
Gender
Males
2001–2002
2005–2006
2007–2008
47.6 (44.7–
50.7)
40.1 (37.5–
42.9)
44.6 (42.8–
46.6)
46.1 (43.4–
48.7)
39.5 (36.7–
42.8)
42.8 (40.7–
45.1)
61.3 (58.0–
64.5)
55.3 (51.6–
59.5)
60.6 (58.1–
64.4)
86.7 (77.1–
97.3)
77.2 (70.7–
83.0)
85.6 (81.1–
91.3)
114 (97.3–
125)
95.9 (89.6–
102)
111 (101–
121)
1,335
3,765
3,839
Females
2001–2002
2005–2006
2007–2008
51.9 (49.9–
54.1)
45.1 (42.8–
47.6)
51.0 (48.9–
53.2)
51.2 (48.4–
53.0)
45.0 (42.4–
47.4)
50.0 (47.5–
52.7)
69.1 (66.7–
71.2)
64.4 (60.0–
69.6)
72.3 (67.8–
76.8)
100 (91.7–
111)
96.8 (87.8–
105)
107 (101–
116)
129 (118–
140)
128 (117–
134)
146 (129–
163)
1,481
3,932
3,789

209 NITRATE AND NITRITE
6. POTENTIAL FOR HUMAN EXPOSURE
Table 6-7. Geometric Mean and Selected Percentiles of Urine Concentrations of
Urinary Nitrate (Creatinine Corrected) (in mg/g of creatinine) for the
U.S. Population from the National Health and Nutrition
Examination Survey (NHANES)
Geometric
Selected percentiles (95% CI)
Survey
years
mean
(95% CI)
50
th
75
th
90
th
95
th
Sample
size
Race/ethnicity
Mexican
Americans
2001–2002
2005–2006
2007–2008
50.9 (45.7–
56.8)
44.6 (42.6–
46.7)
48.7 (45.1–
52.7)
48.1 (44.9–
51.4)
44.0 (42.2–
45.3)
47.0 (43.5–
50.5)
67.4 (60.3–
77.6)
60.4 (58.1–
62.9)
64.8 (59.7–
69.9)
97.3 (85.7–
117)
89.9 (82.0–
95.4)
93.5 (88.9–
101)
135 (100–
161)
120 (111–
128)
128 (108–
156)
707
1,972
1,505
Non-Hispanic
blacks
2001–2002
2005–2006
2007–2008
38.7 (36.3–
41.3)
32.9 (30.9–
35.0)
35.9 (34.2–
37.7)
38.0 (34.6–
41.3)
31.9 (29.8–
34.1)
34.8 (33.3–
36.6)
53.5 (50.3–
57.4)
45.4 (41.5–
49.6)
49.0 (44.8–
53.6)
70.3 (64.9–
79.3)
64.0 (60.1–
68.0)
69.1 (63.5–
77.4)
91.7 (78.9–
100)
81.2 (75.3–
89.6)
87.8 (78.1–
97.4)
679
2,078
1,706
Non-
Hispanic
whites
2001–2002
2005–2006
2007–2008
51.4 (49.4–
53.4)
43.7 (40.8–
46.8)
49.0 (46.7–
51.4)
49.1 (46.9–
51.5)
43.9 (40.8–
46.8)
47.4 (44.9–
50.5)
66.9 (64.2–
69.4)
61.4 (56.5–
66.2)
68.2 (63.3–
73.1)
95.2 (87.7–
100)
85.5 (80.6–
91.9)
98.3 (91.2–
105)
124 (115–
132)
110 (102–
116)
126 (118–
135)
1,227
3,056
3,190
CI = confidence interval
Source: CDC 2013
210 NITRATE AND NITRITE
6. POTENTIAL FOR HUMAN EXPOSURE
mean urinary nitrate excretion of the participants who consumed drinking water with low-nitrate levels
was 15.0 mg nitrate-nitrogen/day, while the mean value for the participants who consumed drinking water
with higher nitrate levels was 22 mg nitrate-nitrogen/day (Levellois et al. 2000). Higher correlations for
total nitrate intake and urinary excretion were found with dietary nitrate intake as opposed to water nitrate
intake.
Various scenarios for nitrate and nitrite intake have been considered and it has been found that dietary
intake contributes the majority of exposure occurrences. Approximately 89–99% of an adult’s daily
intake of both nitrate and nitrite is from their food when an average to high vegetable diet is consumed
with average water intake. The daily intake contribution from food decreases to 33–56% for nitrate and
7.7–14% for nitrite when average to high consumption of water with nitrate levels (50 mg nitrate/L [ppm
nitrate]) is considered (IARC94_2010). Gangolli et al. (1994) also reported that 88–96% of the average
dietary intake of nitrate comes from food sources (85% of which is attributed to vegetables), while 4–12%
comes from drinking waters. Exposure to dietary nitrate may increase exposure to nitrite due to
endogenous production. Ingested nitrate is readily absorbed from the upper gastrointestinal tract into the
blood and is mainly excreted in the urine (Gangolli et al. 1994). Portions of blood nitrate are transported
to human saliva where it is mostly metabolized to nitrite; approximately 5% of dietary nitrate is
metabolized to nitrite (Gangolli et al. 1994). Gangolli et al. (1994) estimated that the human adult intakes
of nitrate are 2.4 mg nitrate/kg (ppm nitrate) body weight/day from food and 0.33–4.1 mg nitrate/kg body
weight/day from water; the human adult intakes of nitrite are 0.04–0.07 mg nitrite/kg body weight/day
from food and <0.002–0.07 mg nitrite/kg body weight/day from water. These estimates do not account
for the endogenous production of nitrite. Worldwide dietary exposures were estimated from data
collected in the 1997 Total Diet Study in the United Kingdom and additional dietary studies.
Representative exposure estimates for nitrate and nitrite were reported as 58–218 and 0.7–1.6 mg
nitrite/day, respectively (IARC 2010).
Estimated daily intake values of nitrate and nitrite have been calculated based on data from the United
Kingdom and the United States. An individual with an average water intake (1.4 L/day) and average food
consumption or a high vegetable diet is estimated to consume levels 52–80 or 140–220 mg nitrate/day,
respectively. An individual with an average water intake (1.4 L/day) and average food consumption or a
high vegetable diet is estimated to consume levels 0.74 or 2.2 mg nitrite/day, respectively (IARC 2010).
211 NITRATE AND NITRITE
6. POTENTIAL FOR HUMAN EXPOSURE
Dermal exposure to inorganic nitrate or nitrite is not a likely route of concern for the general population,
although absorption following dermal exposure to dust from fertilizer products containing nitrate salts is
possible.
6.6 EXPOSURES OF CHILDREN
This section focuses on exposures from conception to maturity at 18 years in humans. Differences from
adults in susceptibility to hazardous substances are discussed in Section 3.7, Children’s Susceptibility.
Children are not small adults. A child’s exposure may differ from an adult’s exposure in many ways.
Children drink more fluids, eat more food, breathe more air per kilogram of body weight, and have a
larger skin surface in proportion to their body volume than adults. A child’s diet often differs from that of
adults. The developing human’s source of nutrition changes with age: from placental nourishment to
breast milk or formula to the diet of older children who eat more of certain types of foods than adults. A
child’s behavior and lifestyle also influence exposure. Children crawl on the floor, put things in their
mouths, sometimes eat inappropriate things (such as dirt or paint chips), and may spend more time
outdoors. Children also are generally closer to the ground and have not yet developed the adult capacity
to judge and take actions to avoid hazards (NRC 1993).
Children will be exposed to nitrate and nitrite through ingestion of food and drinking water. Nitrate is
commonly detected in various surface waters and groundwaters. High nitrate concentrations in drinking
water are commonly found in privately owned wells, with shallow depths and permeable soils. It has
been estimated that about 15 million families in the United States use private well drinking water. Based
on monitoring data and birthrates from 2000, it was estimated that 40,000 infants <6 months old would be
living in households using drinking water with nitrate levels that exceed the federal standard (10 mg
nitrate-nitrogen/L) (Fewtrell 2004). Additionally, boiling water from private wells may concentrate
nitrate in the water, which may lead to higher exposure of children whose infant foods are prepared using
water that is boiled first (Fewtrell 2004). Gangolli et al. (1994) estimated that the infant intake of nitrate
and nitrite from food is negligible, while the infant intakes of nitrate and nitrite from water are 1.7–8.3 mg
nitrate/kg body weight/day and <0.02 mg nitrite/kg body weight/day, respectively. A review by Jones et
al. (2015) reported dietary nitrate and nitrite concentrations for newborn infants. An intake of
approximately 0.15 mg/kg/day for infants was based on a mean of reported concentrations in breast milk
and formula. Ingestion based on breast milk intake of approximately 150 mL/kg/day, was reported as
0.12 mL/kg/day for nitrate and 0.0007 mL/kg/day for nitrite.
212 NITRATE AND NITRITE
6. POTENTIAL FOR HUMAN EXPOSURE
Infants may have higher exposures as compared to adults if the water source used for formula has high
levels of nitrates and nitrites. Human breast milk has been shown to contain, although not concentrate,
nitrate and is not considered a significant source of infant exposure (IARC 2010).
6.7 POPULATIONS WITH POTENTIALLY HIGH EXPOSURES
Populations using well water in agricultural areas may be exposed to greater levels of nitrate and nitrite as
compared to populations living in urban areas since groundwater in agricultural communities typically
has greater levels of nitrate and nitrite than urban water (Burow et al. 2010). Furthermore, workers who
are employed in occupations where fertilizer use is common (e.g., farming, greenhouse operations) may
be exposed to nitrate and nitrite through dermal routes and inhalation of dust particles.
6.8 ADEQUACY OF THE DATABASE
Section 104(i)(5) of CERCLA, as amended, directs the Administrator of ATSDR (in consultation with the
Administrator of EPA and agencies and programs of the Public Health Service) to assess whether
adequate information on the health effects of nitrate and nitrite is available. Where adequate information
is not available, ATSDR, in conjunction with NTP, is required to assure the initiation of a program of
research designed to determine the health effects (and techniques for developing methods to determine
such health effects) of nitrate and nitrite.
The following categories of possible data needs have been identified by a joint team of scientists from
ATSDR, NTP, and EPA. They are defined as substance-specific informational needs that if met would
reduce the uncertainties of human health assessment. This definition should not be interpreted to mean
that all data needs discussed in this section must be filled. In the future, the identified data needs will be
evaluated and prioritized, and a substance-specific research agenda will be proposed.
6.8.1 Identification of Data Needs
Physical and Chemical Properties. The physical and chemical properties of nitrate salts are
discussed in Chapter 4. These salts are highly soluble in water and dissociate under environmental
conditions and exist as ions (WHO 1978, 2011b). No data needs are identified.
213 NITRATE AND NITRITE
6. POTENTIAL FOR HUMAN EXPOSURE
Production, Import/Export, Use, Release, and Disposal. According to the Emergency
Planning and Community Right-to-Know Act of 1986, 42 U.S.C. Section 11023, industries are required
to submit substance release and off-site transfer information to the EPA. The TRI, which contains this
information for 2012, became available in November of 2013. This database is updated yearly and should
provide a list of industrial production facilities and emissions. Import/export data are available for
ammonium nitrate (USDA 2013). Data for the other compounds assessed in this profile would be useful.
Environmental Fate. The transport and fate of nitrate and nitrite compounds have been studied
(Kramer et al. 2006; Pfenning and McMahon 1996; WHO 2011b). These substances are highly mobile in
soils. Transformation and degradation processes include denitrification to atmospheric nitrogen and plant
uptake (Newton 2005; Nolan 1999). Conversion is achieved via biotic process carried out by auto- and
heterotrophic bacteria (Hammerl and Klapotke 2006). Under aerobic conditions in aquatic systems,
ammonia and nitrite are converted to nitrate via nitrification. Conversion is achieved through a biotic
process carried out by autotrophic nitrifying bacteria. Under anaerobic conditions in aquatic systems,
bacteria convert nitrate to nitrite, which is further reduced to the gaseous compounds nitric oxide (NO),
nitrous oxide (N
2
O), and N
2
(nitrogen). No data needs are identified.
Bioavailability from Environmental Media. Nitrate and nitrite are readily absorbed following
ingestion from water or food sources.
Data assessing absorption from intake of food sources and water containing nitrate and nitrite has been
studied (Gangolli et al. 1994; Kortboyer et al. 1997b). Several reports have indicated the correlation of
methemoglobinemia in adults and children and elevated nitrite levels in the blood (CDC 1997, 2002;
Gautami et al. 1995; Gowans 1990; Greenberg et al. 1945; Sevier and Berbatis 1976; Ten Brink et al.
1982). Adequate data for intake of nitrate and nitrite from drinking water and food are available (ATSDR
2013a; Gangolli et al. 1994; Hord 2011; JECFA 2003c; Lundberg et al. 2009; Peterson and Stoltze 1999).
Data are lacking for absorption from the lungs and skin. Further data may be useful to establish whether
uptake via inhalation or dermal contact of dust is a notable source of exposure, since this may occur
during application of fertilizers containing these chemicals.
Food Chain Bioaccumulation. Nitrate ion and nitrite ion are both a natural part of the earth’s
nitrogen cycle. Plants and mammals naturally contain nitrate and nitrite (WHO 2011b). Assimilation of
nitrite from soils occurs via reduction of nitrate to nitrite, which is facilitated by various bacteria and
catalyzed by nitrate reductase (WHO 1978). Data are available to indicate that nitrate and nitrite may be
214 NITRATE AND NITRITE
6. POTENTIAL FOR HUMAN EXPOSURE
concentrated in several plants and waters intended for human consumption (JECFA 2003c; Peterson and
Stoltze 1999; Zhang et al. 1996, 2003). No data needs are identified.
Exposure Levels in Environmental Media. Reliable monitoring data for the levels of nitrate and
nitrite in contaminated media at hazardous waste sites are needed so that the information obtained on
levels of nitrate and nitrite in the environment can be used in combination with the known body burden of
nitrate and nitrite to assess the potential risk of adverse health effects in populations living in the vicinity
of hazardous waste sites.
Exposure Levels in Humans. Humans are exposed to nitrate and nitrite primarily through the
ingestion of drinking water and consumption of food. Estimated intakes are available (Gangolli et al.
1994; IARC 2010). Biomonitoring data for nitrate levels in urinary samples have been reported (CDC
2013). Continued monitoring of nitrate and nitrite levels in humans is needed.
This information is necessary for assessing the need to conduct health studies on these populations.
Exposures of Children. Children are exposed to nitrate and nitrite by the same exposure routes as
adults (e.g., ingestion of food and water). Data from the NHANES survey discussed in Section 6.5
indicated that higher urinary nitrate levels were typically observed in children as compared to adults.
Continued monitoring of nitrate and nitrite levels in children is needed.
Child health data needs relating to susceptibility are discussed in Section 3.12.2, Identification of Data
Needs: Children’s Susceptibility.
Exposure Registries. No exposure registries for nitrate or nitrite were located. This substance is not
currently one of the compounds for which a sub-registry has been established in the National Exposure
Registry. The substance will be considered in the future when chemical selection is made for sub-
registries to be established. The information that is amassed in the National Exposure Registry facilitates
the epidemiological research needed to assess adverse health outcomes that may be related to exposure to
this substance.
215 NITRATE AND NITRITE
6. POTENTIAL FOR HUMAN EXPOSURE
6.8.2 Ongoing Studies
No ongoing environmental fate studies for nitrate or nitrite were identified using NIH RePORTER or the
Defense Technical Information Center (DTIC) online database. Nitrate and nitrite levels and trends are
monitored in major watersheds and drinking water by organizations such as the USGS and USDA. These
reports are typically available from their websites.
216 NITRATE AND NITRITE
6. POTENTIAL FOR HUMAN EXPOSURE
This page is intentionally blank.
217 NITRATE AND NITRITE
7. ANALYTICAL METHODS
The purpose of this chapter is to describe the analytical methods that are available for detecting,
measuring, and/or monitoring nitrate and nitrite, and other biomarkers of exposure and effect to nitrate
and nitrite. The intent is not to provide an exhaustive list of analytical methods. Rather, the intention is
to identify well-established methods that are used as the standard methods of analysis. Many of the
analytical methods used for environmental samples are the methods approved by federal agencies and
organizations such as EPA and the National Institute for Occupational Safety and Health (NIOSH). Other
methods presented in this chapter are those that are approved by groups such as the Association of
Official Analytical Chemists (AOAC) and the American Public Health Association (APHA).
Additionally, analytical methods are included that modify previously used methods to obtain lower
detection limits and/or to improve accuracy and precision.
7.1 BIOLOGICAL MATERIALS
Several methods are available for the analysis of nitrate and nitrite in biological media; details of selected
methods are provided in Table 7-1.
Following the ingestion of nitrate and nitrite, they are readily absorbed from the upper gastrointestinal
tract into the blood and readily excreted in human urine as nitrate. This process is essentially complete at
18 hours following ingestion; minor urinary products of nitrate and nitrite metabolism include ammonia
and urea (Gangolli et al. 1994). Portions of blood nitrate are transported to human saliva where it is
mostly metabolized to nitrite. In human blood and tissues, nitrite is typically oxidized to nitrate.
Concentrations of nitrate in urine and saliva fluctuate; therefore, in order to evaluate exposure more
precisely, a 24-hour collection of urine is recommended. Analysis is achieved via hydrazine reduction
(IARC 2010; Levallois et al. 2000).
Levels of nitrate and nitrite in plasma, urine, and saliva can be measured by gas chromatography/mass
spectrometry (GC/MS) (Bondonno et al. 2012; Tsikas 2005). Frozen samples are treated with
tetraoctylammonium bromide and derivatizing reagent pentafluorobenzyl bromide in acetone solutions at
elevated temperature. Acetone is removed by evaporation under a nitrogen atmosphere and the remaining
aqueous phase is extracted with an isooctane/toluene solution and analyzed by GC/MS (m/z = 62 for
nitrate and 46 for nitrite). Sample procedures must involve precautionary steps to minimize the

218 NITRATE AND NITRITE
7. ANALYTICAL METHODS
Table 7-1. Analytical Methods for Determining Nitrate and Nitrite in Biological
Materials
Sample
Analytical
Sample
Percent
matrix
Preparation method
method
detection limit
recovery
Reference
Urine
Hydrazine reduction
GC-MS
5 ng/L
Not reported
Levallois et al.
2000
Plasma,
Sample derivitization
GC-MS
Not reported
Not reported
Bondonna et al.
urine, or with tetraoctyl- 2012
saliva ammonium bromide
and pentafluorobenzyl
bromide
Plasma
Deproteinization;
HPLC-UV
0.3–20 µM
Not reported
Hibbs et al.
(serum) Griess reaction spectrometry; (nitrite) 1992; Sun et al.
GC-MS
4–
81 µM (nitrate)
2003; Tsikas
2005
Urine
Griess
GC-MS
690 µmol/
Not reported
Hibbs et al. 1992
24 hours
Urine
International standard
IC-MS/MS
500 µg/L (nitrate)
95
Valentín-Blasini
dilution
et al. 2007
Urine
24-Hour specimen
Colorimetric
Not reported
Not reported
Tietz 1970
(metabolite- collected, preserve (Berthelot
ammonia) with HCl and
reaction)
refrigerate
Urine
24-Hour specimen
Indophenol
Not reported
Not reported
Huizenga et
(metabolite-
analyzed immediately, reaction
al. 1994)
ammonia)
or stored up to 8
weeks at -20 °C
Whole blood
Deprotonized using
ace
tonitrile followed by
purification
HPLC/direct
conductivity
detection
0.4 µmol/L
(nitrite)
Yan et al. 2016
GC-MS = gas chromatography-mass spectrometry; HPLC = high-performance liquid chromatography;
IC-MS/MS = ion chromatography-mass spectrometry/mass spectrometry; UV = ultraviolet
219 NITRATE AND NITRITE
7. ANALYTICAL METHODS
endogenous contribution of analytes in the laboratory chemicals and materials (glassware, filtration
equipment, etc.) used for sample collection and work up (Tietz 1970; Tsikas 2005). Chemical
interferences in samples resulting in reduction of nitrate to nitrite, or conversely, the oxidation of nitrite to
nitrate during analysis should be evaluated and accounted for. Microbial conversion via hydrolysis may
cause an increase in values (Tietz 1970). Preparation of blood samples must involve procedures that limit
the oxidation of nitrite by oxyhaemoglobin and loss due to methods requiring acidification or
derivatization. Possible interferences in ammonia quanitification and to the Griess assay, such as
anticoagulants, must also be factored (Huizenga 1994; Tsikas 2005).
The Griess assay is one of the first methods used to measure levels of nitrate and nitrite in biological and
environmental samples. The method involves reduction of nitrate to nitrite followed by a diazotization
reaction and then measuring the absorbance of the diazo chromophore in the visible spectrum. To
determine the levels of nitrate and nitrite separately, the procedure is first carried out without the
preliminary reduction step in order to quantify the level of nitrite solely. This assay was originally
performed using sulfanilic acid, which forms a diazonium cation with nitrite under acidic conditions
followed by coupling with α-naphthylamine to form a diazo compound, which contains a strong
absorption band at about 540 nm (Tsikas 2005). Other methods include diazotizing with sulfanilamide
and coupling with N-(1-naphthyl)-ethylenediamine dihydrochloride to form the diazo compound (EPA
1993). GC/MS methods were shown to provide superior quantification of nitrate and nitrite in human
plasma and urine samples when compared to the Griess assays (Tsikas 2005)
Reverse-phase high performance liquid chromatography (HPLC) by means of ion pairing in the mobile
phase without derivitization followed by ultraviolet (UV) detection around 210 nm has also been used to
detect nitrate in urine samples (Tsikas 2005). Urinary nitrate levels can be measure using ion
chromatography-tandem mass spectrometry (IC-MS/MS) by means of internal standard dilution
(Valentín-Blasini et al. 2007).
The level of methemoglobin in the blood is often the biomarker for assessing nitrate exposure
(Manassaram et al. 2010). Methemoglobin can be measured in blood collected via finger stick samples.
Samples are analyzed with portable AVOXimeter 4000 whole-blood oximeter devices. The device
measures total hemoglobin, and further characterizes percentages of oxyhemoglobin, carboxyhemoglobin,
and methemoglobin. The accuracy and precision of the method were reported as ±0.5 and ±0.7%,
respectively. Refer to ATSDR (Agency for Toxic Substance and Disease Registry 2013b) for discussion
of other nitrate and nitrite laboratory tests.
220 NITRATE AND NITRITE
7. ANALYTICAL METHODS
Yan et al. (2016) developed a simple method for the quantitative determination of nitrite in whole blood
samples employing ion chromatography and electrochemical detection. The blood sample is prepared by
adding acetonitrile followed by purification using mini-cartridges to remove interfering compounds. The
detection limit for the method is reported as 0.4 µmol/L.
7.2 ENVIRONMENTAL SAMPLES
Methods are available for determining the level of nitrate and nitrite in a variety of environmental
matrices. A summary of representative methods is shown in Table 7-2.
Ion chromatography and spectrometry methods are the most common analytical techniques employed for
the detection and quantification of nitrate and nitrite in environmental samples; detection limits range
from 0.01 to 1 mg/L (ppm) (IARC 2010; WHO 2011b). Samples must be analyzed as soon as is
reasonably possible in order to minimize any changes in the sample due to microbial transformations.
Sample preservation using chemicals and or deep freezing methods have been reported; however,
interference with the analysis can occur in certain methods (Mulvaney 1996).
Methods based on the Griess assay are available for the determination of nitrate and nitrite in potable
water, raw water and wastewater (EPA 1993; WHO 2011b). The limit of detection for the International
Organization for Standardization ISO method 6777/1 lies within the range of 0.005–0.01 mg/L (ppm)
(WHO 2011b). A continuous-flow spectrometric method (ISO method 7890-1) for the determination of
nitrite, nitrate or the sum of both in various types of water is suitable at concentrations ranging from
0.05 to 5 mg/L (ppm) for nitrite and from 1 to 100 mg/L (ppm) for nitrite and nitrate, both in the
undiluted sample (WHO 2011b).
NIOSH method 7903 employs ion chromatography for the determination of nitric acid in air (NIOSH
1994a). Method 7903 is an analytical technique for determining inorganic acids by measuring the total
concentration of airborne anions. Particulate nitrate has been successfully detected and quantified in
atmospheric samples via ion chromatographic techniques and NO
x
chemiluminescent analyzers (Small et
al. 1975; Yoshizumi et al. 1985)

221 NITRATE AND NITRITE
7. ANALYTICAL METHODS
Table 7-2. Analytical Methods for Determining Nitrate and Nitrite in
Environmental Samples
Sample
Analytical
Sample
Percent
matrix
a
Preparation method
method
detection limit
recovery
Reference
Air/water
Nitrite prepared using
(nitrate, Griess-Ilosvay reaction;
nitrite) nitrate prepared using
hydrazine reduction;
aqueous extracts from
aerosol filters are
analyzed without
pretreatment
Air (nitrate)
Personal air sampled at
0.2–0.5 L/minute for
total sample size of 3–
100 L using silica gel
sample tube; boil
sorbent from sample
tube in bicarbonate/
carbonate buffer for
10 minutes
Air (nitrite)
Ambient air is sampled
at 0.025 L/minute for 3-
L
air sample using glass
sorbent tubes with glass
wool retainers; add
adsorbing solution; add
solution of hydrogen
peroxide, sulfanilamide
and NEDA to extracted
sample, set for
10 minutes
Air (nitrite)
Ambient air is sampled
at 0.025 L/minute for 3-
L
air sample using
diffusive sampler tubes
with three
triethanolamine screens;
add adsorbing solution;
add solution of hydrogen
peroxide, sulfanilamide
and NEDA to extracted
sample, set for
10 minutes
Water
Drinking water or river
(nitrate) water samples are
prepared with
lanthanum (III) chloride
and placed into the cell
UV spectrometry
0.07 ppm
Not
(nitrite); reported
0.2 ppm
(nitrate)
Ion
0.7 μg/sample
Not
chromatography/ reported
conductivity
detector;
NIOSH 7903
Visible
1 μg/sample
Not
absorption reported
spectro-
photometry;
NIOSH 6014
Visible
0.01 μg/sample
Not
absorption reported
spectro-
photometry;
NIOSH 6700
Voltammetry/
20 µg/L
Not
static mercury reported
drop electrode
Oms et al. 1995
NIOSH 1994a
NIOSH 1994b
NIOSH 1998
Markusova et al.
1996

222 NITRATE AND NITRITE
7. ANALYTICAL METHODS
Table 7-2. Analytical Methods for Determining Nitrate and Nitrite in
Environmental Samples
Sample
Analytical
Sample
Percent
matrix
a
Preparation method
method
detection limit
recovery
Reference
Drinking
Clean samples can be
IC/CD
Not reported
93–114
IARC 2010
water used directly (nitrite-N)
83–113
(nitrate-N)
Soil (nitrate)
Soil samples are added
Voltammetry/
20 µg/L
Not
Markusova et al.
to Gohler solutions static mercury reported 1996
followed by drop electrode
neutralization before
analysis
Soil (nitrite,
Extraction of field
UV-Vis
Not reported
Not
Mulvaney 1996
nitrate) samples with 2 M KCL; (λ=540 nm) reported
nitrate reduction;
diazoitization and
coupling with resulting
dye formation
Foods and
Blended/pureed food
Capillary ion
Not reported
>73
Marshall and
juices samples are mixed with electrophoresis (nitrate); Trenerry 1996
(nitrate, water and filtered >88
nitrite)
(nitrite)
Food (nitrate,
Direct injection
HePI-MS
Not reported
Not
Pavlov and
nitrite)
reported
Attygalle 2013
Fish and
Fish muscle
FIA spectrometry
0.01 µg/mL
97.8–
Monser et al. 2002
water homogenized, digested (nitrite); 102.1
(nitrate, with perchloric acid and 0.025 µg/mL (nitrite);
nitrite) centrifuged; nitrate (nitrate) 98.5–
reduction to nitrite using 101.6
copperised cadmium (nitrate)
redactor; reaction with
phosphomolybdenum
blue complex and
ammonium chloride
Water and
Food: homogenization
Potentiometry
0.0037 µ/mL
98.9–
Wardak and
vegetables with deionized water with solid contact
(nitrate)
105.9 Grabarczky 2016
(nitrate)
and heated at 80°C;
ISE (nitrate)
cooled and diluted with
deionized water
Water: direct analysis
Cured meat
Reaction with
Colorimetry;
Not reported
Not
IARC 2010
(nitrite) sulfanilamide followed absorbance reported
by reaction with NEDA
540 nm
Milk and milk
Suspension in buffer
FIA
0.5 mg/kg
Not
IARC 2010
products solution; centrifuge and spectrometry; (nitrate) reported
(nitrate, reduce with cadmium; absorbance 1.0 mg/kg
nitrite) react with sulfanilamide 540 nm (nitrite)
followed by react
ion with
NEDA

223 NITRATE AND NITRITE
7. ANALYTICAL METHODS
Table 7-2. Analytical Methods for Determining Nitrate and Nitrite in
Environmental Samples
Sample
Analytical
Sample
Percent
matrix
a
Preparation method
method
detection limit
recovery
Reference
Dairy
Extract cheese slurry
Spectro-
≥1 µg/g nitrate
Not
IARC 2010
products and using ZnSO
4
and NaOH;
photometry; reported
cheese reduce in Jones absorbance at
(nitrate, reductor using zinc and 522 nm
nitrite)
CdSO
4
Fried bacon
Grind frozen sample;
GC
Not reported
Not
IARC 2010
(N-nitros- vacuum distill with reported
amines) NaOH and mineral oil;
extract and dry with
DCM and anhydrous
NaSO
4
; concentrate
DCM = dichloromethane; FIA = flow injection analysis; GC = gas chromatography; HePI-MS = helium-plasma
ionization-mass spectrometry; IC/CD = ion chromatography/conductivity detector; ISE = ion-selective electrodes;
NEDA = N-1-naphthylethylenediamine dihydrochloride; NIOSH = National Institute for Occupational Safety and
Health; UV = ultraviolet absorbance detection
224 NITRATE AND NITRITE
7. ANALYTICAL METHODS
A sequential injection method coupled with spectrophotometry has been developed for the detection of
nitrate and nitrite in environmental samples such as atmospheric aerosol filter extracts and waste water
samples (Oms et al. 1995). The method is advantageous due to the small volumes of sample and reagents
required for analysis. The detection limits were reported as 0.07 ppm for nitrite and 0.2 ppm for nitrate.
Nitrite is analyzed using the Griess-Ilosvay reaction; nitrate is reduced to nitrite using hydrazine sulphate.
Markusova et al. (1996) developed a sensitive voltammetric method that can determine nitrate levels in
drinking water, river water, or soil extracts three orders of magnitude lower than the allowed levels of
nitrate in drinking water. The method employs a multi-purpose electrochemical analyzer and a
voltammetric cell. Water samples are prepared with lanthanum (III) chloride and placed into the cell; soil
samples are added to Gohler solutions followed by neutralization before analysis. The reported limit of
detection is 20 µg nitrate/L (20 ppb) (5 µg NO
3
—
N/L).
The most commonly used method for soil and soil extract analysis of nitrite is a modified Griess-Ilosvay
colorimetric method using a continuous flow analyzer. Nitrites react with primary aromatic amines to
form a diazonium salt which is then coupled with an aromatic compound; the resulting complex has a
characteristic absorbance band in the UV-Vis spectrum. The concentration of nitrite is proportional to the
color intensity of the resulting azo compound measured using a spectrophotometer or colorimeter. This
technique is also used for sensitive analysis of nitrate following reduction to nitrite. Cadmium reduction
to nitrite is achieved in a column of copperized cadmium with an ammonium chloride (NH4Cl) matrix at
pH between 5 and 10. Other various reducing agents have been reported. Analysis for nitrate must
account for initial concentrations of nitrite in the sample prior to reduction. Maximum accuracy is seen
when absorbance is measured at wavelengths of 540 nm; however, wavelengths between 510 and 550 nm
are acceptable (Mulvaney 1996).
Pavlov and Attygalle (2013) developed an analytical method with minimal sample preparation employing
helium-plasma ionization-mass spectrometry. Nitrate was successfully identified and quantified using
this solvent-less ambient pressure mass spectrometry technique in various foodstuffs. Samples of fruit
juice and meat pieces (i.e., tomato and celery juice, hot dog and beef) can be placed onto glass slides and
analyzed directly without any modification. Quantification of nitrate in such complex matrices is
suggested to be determined with accuracy by spiking with known quantities of radiolabeled nitrate. The
method detection limit for determining the nitrate concentration is in the range of 20 ng/sample and
depends on the specific sample matrix.
225 NITRATE AND NITRITE
7. ANALYTICAL METHODS
Capillary ion electrophoresis has been successfully employed for the determination of nitrite and nitrate in
foods and juices (Marshall and Trenerry 1996). The authors tested the procedure using cheese, cabbage,
fruit juices, and meats. Percent recovery for three processed meat samples ranged from 88 to 118% for
nitrite and from 73 to 106% for nitrate.
7.3 ADEQUACY OF THE DATABASE
Section 104(i)(5) of CERCLA, as amended, directs the Administrator of ATSDR (in consultation with the
Administrator of EPA and agencies and programs of the Public Health Service) to assess whether
adequate information on the health effects of nitrate and nitrite is available. Where adequate information
is not available, ATSDR, in conjunction with NTP, is required to assure the initiation of a program of
research designed to determine the health effects (and techniques for developing methods to determine
such health effects) of nitrate and nitrite.
The following categories of possible data needs have been identified by a joint team of scientists from
ATSDR, NTP, and EPA. They are defined as substance-specific informational needs that if met would
reduce the uncertainties of human health assessment. This definition should not be interpreted to mean
that all data needs discussed in this section must be filled. In the future, the identified data needs will be
evaluated and prioritized, and a substance-specific research agenda will be proposed.
7.3.1 Identification of Data Needs
Methods for Determining Biomarkers of Exposure and Effect.
Exposure. Nitrate and nitrite may be converted to many other compounds in the body, such as N-nitroso
compounds, including nitrosamines. Approximately 25% of absorbed nitrate is secreted to saliva and
about 20% of this is reduced to nitrite. Nitrite is converted to nitric oxide by the acidic environment on
the stomach. Methods exist for the measurement of nitroso compounds and nitrite in plasma and salivary
nitrite (Bondonno et al. 2012). Nitrate in the diet may contribute to nitric oxide levels in the body, and
increases in these levels can be a biomarker of exposure. Ammonia is a minor urinary product of nitrite
and nitrate in which analytical methods are available (Huizenga et al. 1994; Tietz 1970). N-Methyl-
nicotinamide has also been shown to be a potential biomarker of exposure to nitrate and nitrite and there
are methods to measure this (Jansen et al. 1995). No data needs were identified.
226 NITRATE AND NITRITE
7. ANALYTICAL METHODS
Effect. Methemoglobinemia caused by the presence of higher-than-normal levels of methemoglobin is a
biomarker of effect for exposure to high levels of nitrate; however, this effect is not unique for nitrate and
nitrite since other substances may also cause this condition (Bruning-Fann and Kaneene 1993). Methods
are available to measure methemoglobin in the blood (Manassaram et al. 2010).
Methods for Determining Parent Compounds and Degradation Products in Environmental
Media. Methods are available for determining nitrate and nitrite levels in environmental samples such
as air (NIOSH 1994a; Small et al. 1975; Yoshizumi et al. 1985) and water (EPA 1993; Markusova et al.
1996; WHO 2011b).
7.3.2 Ongoing Studies
No ongoing analytical methodologies for nitrate or nitrite were identified using the NIH RePORTER
version 6.1.0 or the DTIC online database.
227 NITRATE AND NITRITE
8. REGULATIONS, ADVISORIES, AND GUIDELINES
MRLs are substance-specific estimates that are intended to serve as screening levels. They are used by
ATSDR health assessors and other responders to identify contaminants and potential health effects that
may be of concern at hazardous waste sites.
MRLs of 4 mg nitrate/kg/day have been derived for acute-, intermediate, and chronic-duration oral
exposure (≤14 days) to nitrate. The MRLs are based on a no-adverse-effect concentration (NOAEC) of
10 mg nitrate-nitrogen/L (44 mg nitrate/L) in drinking water used to prepare formula for infants
<6 months of age (Walton 1951). A NOAEL of 4.33 mg nitrate/kg/day at the NOAEC of 44 mg nitrate/L
was calculated based on estimates of 0.525 L/day for water intake (Kahn and Stralka 2009) and 5.33 kg
for body weight (EPA 2008) of an infant from birth to <3 months of age. A total uncertainty factor of 1
was applied because the point of departure is a NOAEL for nitrate-induced effects on methemoglobin in a
sensitive human subpopulation (i.e., <3-month-old infants, which in many cases may have been at
increased risk of methemoglobinemia due to microbial contamination and associated gastrointestinal
infection). Following ingestion of relatively large amounts of nitrate by healthy normal individuals,
blood methemoglobin levels increase rapidly, followed by a return to normal within several hours
following intake. Repeated ingestion for intermediate- or chronic-duration time periods would be
expected to result in changes in methemoglobin levels similar to those elicited from a single exposure.
Therefore, the acute-, intermediate- and chronic-duration oral MRL values are equivalent. Refer to
Appendix A for additional information regarding derivation of oral MRLs for nitrate.
MRLs of 0.1 mg nitrite/kg/day have been derived for acute-, intermediate, and chronic-duration oral
exposure (≤14 days) to nitrite. The ingestion of nitrate results in the formation of nitrite, which is the
moiety responsible for methemoglobinemia. In adults, approximately 5% of an oral dose of nitrate is
reduced to nitrite in the saliva, most of which is absorbed into the blood in the small intestine. Based on
the assumption of 100% absorption of ingested nitrite, an oral dose of 0.2 mg nitrite/kg/day by an adult
would be expected to result in a nitrite blood level similar to that achieved following ingestion of nitrate
at the oral MRL dose of 4 mg nitrate/kg/day (i.e., 0.2 mg nitrite/kg/day is 5% of an oral dose of nitrate at
the MRL of 4 mg nitrate/kg/day). A modifying factor of 2 was applied to the point of departure (0.2 mg
nitrite/kg/day ÷ 2 = 0.1 mg nitrite/kg/day) because young infants exhibit increased susceptibility to
methemoglobinemia following nitrate ingestion; the modifying factor assumes that the effective
methemoglobin level from a given intake of nitrate by an infant is up to twice that of an adult. Following
ingestion of relatively large amounts of nitrate by healthy normal individuals, blood methemoglobin
228 NITRATE AND NITRITE
8. REGULATIONS, ADVISORIES, AND GUIDELINES
levels increase rapidly, followed by a return to normal within several hours following intake. Repeated
ingestion for intermediate- or chronic-duration time periods would be expected to result in changes in
methemoglobin levels similar to those elicited from a single exposure. Therefore, the acute-,
intermediate-, and chronic-duration oral MRL values are equivalent. Refer to Appendix A for additional
information regarding derivation of oral MRLs for nitrite.
EPA (IRIS 2002) derived an oral reference dose (RfD) of 1.6 mg nitrate-nitrogen/kg/day (i.e., 1.6 mg
nitrogen from nitrate; ~7 mg nitrate/kg/day) based on a NOAEL of 1.6 mg nitrate-nitrogen/kg/day and a
LOAEL of 1.8–3.2 mg nitrate-nitrogen/kg/day (7.92–14.08 mg nitrate/kg/day) for early clinical signs of
methemoglobinemia in excess of 10% among formula-fed infants 0–3 months of age (Bosch et al. 1950;
Walton 1951). An uncertainty factor of 1 was employed because available data defined the NOAEL for
the critical effect in the most sensitive human subpopulation.
EPA (IRIS 2002) derived an RfD of 0.1 mg nitrite-nitrogen/kg/day (~0.33 mg nitrite/kg/day) based on a
NOAEL of 10 mg nitrate-nitrogen/L and a LOAEL of 11–20 mg nitrate-nitrogen/L for early clinical signs
of methemoglobinemia in excess of 10% (Walton 1951). The NOAEL of 10 mg nitrate-nitrogen/L was
converted to an estimated dose of 1 mg nitrate-nitrogen/kg/day based assumptions that a 10-kg child
would ingest 1 L of water/day. EPA applied a modifying factor of 10 to the NOAEL of 1 mg nitrate-
nitrogen/kg/day from the Walton (1951) study to account for the direct toxicity of nitrite, resulting in an
RfD of 0.1 mg nitrite-nitrogen/kg/day. As described in a Drinking Water Criteria Document for
Nitrate/Nitrite (EPA 1990a), the modifying factor of 10 was used to account for an estimated rate of 10%
conversion of ingested nitrate to nitrite in infants compared to an estimated rate of 5% conversion in
adults.
Based on available human data, IARC (2010) determined that there is inadequate evidence for the
carcinogenicity of nitrate in food or drinking water and limited evidence for the carcinogenicity of nitrite
in food (based on association with increased incidence of stomach cancer). Evaluation of available
animal data by IARC (2010) resulted in the determination that there is inadequate evidence for the
carcinogenicity of nitrate, limited evidence for the carcinogenicity of nitrite per se, and sufficient evidence
for the carcinogenicity of nitrite in combination with amines or amides. The overall conclusions of IARC
(2010) were that “ingested nitrate and nitrite under conditions that result in endogenous nitrosation is
probably carcinogenic to humans (Group 2A).” IARC (2010) noted that: (1) the endogenous nitrogen
cycle in humans includes interconversion of nitrate and nitrite; (2) nitrite-derived nitrosating agents
produced in the acid stomach environment can react with nitrosating compounds such as secondary
229 NITRATE AND NITRITE
8. REGULATIONS, ADVISORIES, AND GUIDELINES
amines and amides to generate N-nitroso compounds; (3) nitrosating conditions are enhanced upon
ingestion of additional nitrate, nitrite, or nitrosatable compounds; and (4) some N-nitroso compounds are
known carcinogens.
Neither nitrate nor nitrite have been classified as to their carcinogenicity by the U.S. EPA Integrated Risk
Information System (IRIS 2002), the National Toxicology Program (NTP, 2011), or the American
Conference of Governmental Industrial Hygienists (ACGIH 2013).
The EPA lists maximum contaminant levels (MCL) and maximum contaminant level goals (MCLG) of
10 mg/L for nitrate (as nitrate-nitrogen; ~44 mg nitrate/L) and 1 mg/L for nitrite (as nitrite nitrogen;
~3.3 mg nitrite/L) in the 2012 Edition of the Drinking Water Standards and Health Advisories (EPA
2012b).
The international and national regulations, advisories, and guidelines regarding nitrate and nitrite in air,
water, and other media are summarized in Table 8-1.

230 NITRATE AND NITRITE
8. REGULATIONS, ADVISORIES, AND GUIDELINES
Table 8-1. Regulations, Advisories, and Guidelines Applicable to Nitrate and
Nitrite
Agency
Description
Information
Reference
INTERNATIONAL
Guidelines:
IARC
Carcinogenicity classification
IARC 2014
Nitrate or nitrite (ingested)
under conditions that result in
endogenous nitrosation
Group 2A
a
WHO
Air quality guidelines
No data
WHO 2010
Drinking water quality guidelines
Nitrate (as NO
3
-
)
50 mg/L
b
WHO 2011a
Nitrite (as NO
2
-
)
3 mg/L
c
Combined nitrate plus nitrite
The sum of the ratios of the
concentrations as reported
or detected in the sample of
each to its guideline value
should not exceed 1
NATIONAL
Regulations and
guidelines:
a. Air
ACGIH
TLV-TWA
No data
ACGIH 2013
AIHA
ERPGs
No data
AIHA 2013
DOE
Nitrate(s)
PAC-1
d
30 mg/m
3
DOE 2012
PAC-2
PAC-3
330 mg/m
3
2,000 mg/m
3
Ammonium nitrate
PAC-1
d
PAC-2
PAC-3
6.7 mg/m
3
73 mg/m
3
440 mg/m
3
Potassium nitrate
PAC-1
d
PAC-2
PAC-3
0.074 mg/m
3
0.82 mg/m
3
600 mg/m
3
Sodium nitrate
PAC-1
d
PAC-2
PAC-3
12 mg/m
3
130 mg/m
3
250 mg/m
3

231 NITRATE AND NITRITE
8. REGULATIONS, ADVISORIES, AND GUIDELINES
Table 8-1. Regulations, Advisories, and Guidelines Applicable to Nitrate and
Nitrite
Agency
Description
Information
Reference
NATIONAL (cont.)
EPA
Sodium nitrite
PAC-1
d
2.3 mg/m
3
PAC-2
26 mg/m
3
PAC-3
280 mg/m
3
EPA
AEGLs
No data
EPA 2013a
Hazardous air pollutant
No data
EPA 2014a
42 USC 7412
NAAQS
No data
EPA 2014d
NIOSH
REL
No data
NIOSH 2014
STEL
No data
IDLH
No data
OSHA
PEL (8-hour TWA) for general
industry
No data
OSHA 2013b
29 CFR 1910.1000,
Table Z-2
Highly hazardous chemicals
No data
OSHA 2013a
29 CFR 1910.119,
Appendix A
b. Water
EPA
Designated as hazardous
substances in accordance with
Section 311(b)(2)(A) of the Clean
Water Act
No data
EPA 2013b
40 CFR 116.4
Drinking water contaminant
candidate list
No data
EPA 2009b
74 FR 51850
Drinking water standards and
health advisories
EPA 2012b
Nitrate
MCL
10 mg nitrogen/L
(~44 mg nitrate/L)
e
MCLG
10 mg nitrogen/L
(~44 mg nitrate/L)
f
Health advisory for 1 day for
10-kg child
100 mg nitrogen/L
(~440 mg nitrate/L)
e
Health advisory for 10 days
for 10-kg child
100 mg nitrogen/L
(~440 mg nitrate/L)
e
DWEL
No data

232 NITRATE AND NITRITE
8. REGULATIONS, ADVISORIES, AND GUIDELINES
Table 8-1. Regulations, Advisories, and Guidelines Applicable to Nitrate and
Nitrite
Agency
Description
Information
Reference
NATIONAL (cont.)
EPA
Nitrite
MCL
MCLG
Health advisory for 1 day for
10-kg child
Health advisory for 10 days
for 10-kg child
DWEL
Nitrate + nitrite (both as
nitrogen)
MCL
MCLG
National primary drinking water
standards
Nitrate
MCL
Potential health effects from
long-term exposure above
the MCL
Common sources of
contaminant in drinking water
Public Health Goal
Nitrite
MCL
Potential health effects from
long-term exposure above
the MCL
Common sources of
contaminant in drinking water
Public Health Goal
1 mg nitrogen/L
(~3.3 mg nitrite/L)
f
1 mg nitrogen/L
(~3.3 mg nitrite/L)
f
10 mg nitrogen/L
(~33 mg nitrite/L)
f
10 mg nitrogen/L
(~33 mg nitrite/L)
f
No data
10 mg/L
10 mg/L
10 mg nitrogen/L
(~44 mg nitrate/L)
e
Serious illness; symptoms
include shortness of breath
and blue-baby syndrome
g
Runoff from fertilizer use;
leaching from septic tanks,
sewage; erosion of natural
deposits
10 mg nitrogen/L
(~44 mg nitrate/L)
e
1 mg nitrogen/L
(~3.3 mg nitrite/L)
f
Serious illness; symptoms
include shortness of breath
and blue-baby syndrome
g
Runoff from fertilizer use;
leaching from septic tanks,
sewage; erosion of natural
deposits
1 mg nitrogen/L
(~3.3 mg nitrite/L)
f
EPA 2009c

233 NITRATE AND NITRITE
8. REGULATIONS, ADVISORIES, AND GUIDELINES
Table 8-1. Regulations, Advisories, and Guidelines Applicable to Nitrate and
Nitrite
Agency
Description
Information
Reference
NATIONAL (cont.)
EPA
National recommended water
quality criteria: human health for
the consumption of (at 10
-4
risk)
EPA 2014e
Nitrates
Water + organism
10,000 μg nitrogen/L
Organism only
No data
Reportable quantities of
hazardous substances designated
pursuant to Section 311 of the
Clean Water Act
EPA 2013d
40 CFR 117.3
Sodium nitrite
100 pounds
c. Food
FDA
Bottled water (allowable limits)
Nitrate
10 mg nitrogen/L
(~44 mg nitrate/L)
e
FDA 2013
21 CFR 165.110
Nitrite
1 mg nitrogen/L
(~3.3 mg nitrite/L)
f
Total nitrate and nitrite (as
nitrogen)
10 mg/L
EAFUS
h
FDA 2014
Potassium nitrate, sodium
nitrate, potassium nitrite, and
sodium nitrite
Yes
d. Other
ACGIH
Carcinogenicity classification
No data
ACGIH 2013
EPA
Nitrate
Carcinogenicity classification
No data
EPA 1990a; IRIS
2002
RfC
No data
RfD
1.6 mg nitrogen/kg/day
(~7 mg nitrate/kg/day)
e
Nitrite
Carcinogenicity classification
No data
RfC
No data
RfD
0.1 mg nitrogen/kg/day
(~0.33 mg nitrite/kg/day)
f
Identification and listing of
hazardous waste
No data
EPA 2013c
40 CFR 261,
Appendix VIII

234 NITRATE AND NITRITE
8. REGULATIONS, ADVISORIES, AND GUIDELINES
Table 8-1. Regulations, Advisories, and Guidelines Applicable to Nitrate and
Nitrite
Agency
Description
Information
Reference
NATIONAL (cont.)
EPA
Inert pesticide ingredients in
pesticide products approved for
nonfood use only
Ammonium nitrate, potassium
nitrate, sodium nitrate,
potassium nitrite, and sodium
nitrite
Master Testing List
RCRA waste minimization PBT
priority chemical list
Standards for owners and
operators of hazardous waste TSD
facilities; groundwater monitoring
list
Superfund, emergency planning,
and community right-to-know
Designated CERCLA
hazardous substance and
reportable quantity pursuant to
Section 311(b)(2) of the
Clean Water Act
Sodium nitrite
Nitrate compounds (water
dissociable; reportable only
when in aqueous solution);
sodium nitrite
Superfund, emergency planning,
and community right-to-know
Extremely hazardous
substances and its threshold
planning quantity
TSCA chemical lists and reporting
periods
TSCA health and safety data
reporting
Yes
No data
No data
No data
100 pounds
Effective date of toxic
chemical release reporting;
01/01/1995
No data
No data
No data
EPA 2014b
EPA 2014c
EPA 1998
63 FR 60332
EPA 2013e
40 CFR 264,
Appendix IX
EPA 2013f
40 CFR 302.4
EPA 2013h
40 CFR 372.65
EPA 2013g
40 CFR 355,
Appendix A
EPA 2013i
40 CFR 712.30
EPA 2013j
40 CFR 716.120

235 NITRATE AND NITRITE
8. REGULATIONS, ADVISORIES, AND GUIDELINES
Table 8-1. Regulations, Advisories, and Guidelines Applicable to Nitrate and
Nitrite
Agency
Description
Information
Reference
NATIONAL (cont.)
NTP
Carcinogenicity classification
No data
NTP 2011
a
Group 2A: probably carcinogenic to humans.
b
As nitrate ion (or 11 mg/L as nitrate-nitrogen) to protect against methemoglobinemia in bottle-fed infants (short-term
exposure).
c
As nitrite ion (or 0.9 mg/L as nitrite-nitrogen) to protect against methemoglobinemia in bottle-fed infants (short-term
exposure).
d
PAC-1: mild, transient health effects; PAC-2: irreversible or other serious health effects that could impair the ability
to take protective action; PAC-3: life-threatening health effects (DOE 2012).
e
1 mg nitrate-nitrogen/L (i.e., nitrogen from nitrate) ~4.4 mg nitrate/L
f
1 mg nitrite-nitrogen/L (i.e., nitrogen from nitrite) ~3.3 mg nitrite/L
g
Infants below the age of 6 months who drink water containing nitrate and/or nitrite in excess of the MCL could
become seriously ill and, if untreated, may die (EPA 2009b).
h
The EAFUS list of substances that contains ingredients added directly to food that FDA has either approved as food
additives or listed or affirmed as GRAS.
ACGIH = American Conference of Governmental Industrial Hygienists; AEGL = acute exposure guideline level;
AIHA = American Industrial Hygiene Association; CERCLA = Comprehensive Environmental Response,
Compensation, and Liability Act; CFR = Code of Federal Regulations; DOE = Department of Energy;
DWEL = drinking water equivalent level; EAFUS = Everything Added to Food in the United States;
EPA = Environmental Protection Agency; ERPG = emergency response planning guidelines; FDA = Food and Drug
Administration; FR = Federal Register; GRAS = generally recognized as safe; IARC = International Agency for
Research on Cancer; IDLH = immediately dangerous to life or health; IRIS = Integrated Risk Information System;
MCL = maximum contaminant level; MCLG = maximum contaminant level goal; NAAQS = National Ambient Air
Quality Standards; NIOSH = National Institute for Occupational Safety and Health; NTP = National Toxicology
Program; OSHA = Occupational Safety and Health Administration; PAC = protective action criteria; PBT = persistent,
bioaccumulative, and toxic; PEL = permissible exposure limit; RCRA = Resource Conservation and Recovery Act;
REL = recommended exposure limit; RfC = inhalation reference concentration; RfD = oral reference dose;
STEL = short-term exposure limit; TLV = threshold limit value; TSCA = Toxic Substances Control Act;
TSD = treatment, storage, and disposal; TWA = time-weighted average; USC = United States Code; WHO = World
Health Organization
236 NITRATE AND NITRITE
8. REGULATIONS, ADVISORIES, AND GUIDELINES
This page is intentionally blank.
_______________________
237 NITRATE AND NITRITE
9. REFERENCES
ACGIH. 2013. Threshold limit values for chemical substances and physical agents and biological
exposure indices. Cincinnati, OH: American Conference of Governmental Industrial Hygienists.
http://www.acgih.org/home.htm. January 08, 2014.
Adlercreutz H. 1995. Phytoestrogens: Epidemiology and a possible role in cancer protection.
Environmental Health Perspectives Supplement 103(7):103-112.
AIHA. 2013. Emergency response planning guidelines (ERPG). Fairfax, VA: American Industrial
Hygiene Association. https://www.aiha.org/get-
involved/AIHAGuidelineFoundation/EmergencyResponsePlanningGuidelines/Pages/default.aspx.
January 08, 2014.
Alavantic D, Sunjevaric I, Cerovic G, et al. 1988a. In-vivo genotoxicity of nitrates and nitrites in germ
cells of male mice. 2. Unscheduled DNA synthesis and sperm abnormality after treatment of spermatids.
Mutat Res 204(4):697-702.
Alavantic D, Sunjevaric I, Pecevski J, et al. 1988b. In vivo genotoxicity of nitrates and nitrites in germ
cells of male mice. 1. Evidence for gonadal exposure and lack of heritable effects. Mutat Res
204(4):689-695.
Al-Dabbagh S, Forman D, Bryson D, et al. 1986. Mortality of nitrate fertilizer workers. Br J Ind Med
43(8):507-515.
Al-Gayyar MM, Alyoussef A, Hamdan AM, et al. 2015. Cod liver oil ameliorates sodium nitrite-induced
insulin resistance and degradation of rat hepatic glycogen through inhibition of cAMP/PKA pathway.
Life Sci 120:13-21. 10.1016/j.lfs.2014.11.002.
Altman PL, Dittmer DS. 1974. Biological handbooks: Biology data book. Vol. III. 2nd ed. Bethesda,
MD: Federation of American Societies of Experimental Biology, 1987-2008, 2041.
Alyoussef A, Al-Gayyar MM. 2016a. Thymoquinone ameliorated elevated inflammatory cytokines in
testicular tissue and sex hormones imbalance induced by oral chronic toxicity with sodium nitrite.
Cytokine 83:64-74. 10.1016/j.cyto.2016.03.018.
Alyoussef A, Al-Gayyar MM. 2016b. Thymoquinone ameliorates testicular tissue inflammation induced
by chronic administration of oral sodium nitrite. Andrologia 48(5):501-508. 10.1111/and.12469.
Andersen ME, Krishnan K. 1994. Relating in vitro to in vivo exposures with physiologically based tissue
dosimetry and tissue response models. In: Salem H, ed. Animal test alternatives: Refinement,
reduction, and replacement. New York, NY: Marcel Dekker, Inc., 9-25.
Andersen ME, Clewell HJ, Gargas ML, et al. 1987. Physiologically based pharmacokinetics and the risk
assessment process for methylene chloride. Toxicol Appl Pharmacol 87(2):185-205.
* Not cited in text
238 NITRATE AND NITRITE
9. REFERENCES
Anderson LM, Giner-Sorolla A, Haller IM, et al. 1985. Effects of cimetidine, nitrite, cimetidine plus
nitrite, and nitrosocimetidine on tumors in mice following transplacental plus chronic lifetime exposure.
Cancer Res 45(8):3561-3566.
Andrews AW, Fornwald JA, Lijinsky W. 1980. Nitrosation and mutagenicity of some amine drugs.
Toxicol Appl Pharmacol 52:237-244.
Andrews AW, Lijinsky W, Snyder SW. 1984. Mutagenicity of amine drugs and their products of
nitrosation. Mutat Res 135:105-108.
Aoyagi M, Matsukura N, Uchida E, et al. 1980. Induction of liver tumors in Wistar rats by sodium nitrite
given in pellet diet. J Natl Cancer Inst 65(2):411-414.
Aquanno JJ, Chan KM, Dietzler DN. 1981. Accidental poisoning of two laboratory technologists with
sodium nitrite. Clin Chem 27(6):1145-1146.
Arbuckle TE, Sherman GJ, Corey PN, et al. 1988. Water nitrates and CNS birth defects: A population-
based case-control study. Arch Environ Health 43(2):162-167.
Armijo R, Gonzalez A, Orellana M, et al. 1981. Epidemiology of gastric cancer in Chile: 2. Nitrate
exposures and stomach cancer frequency. Int J Epidemiol 10(1):57-62.
Asahina S, Friedman MA, Arnold E, et al. 1971. Acute synergistic toxicity and hepatic necrosis
following oral administration of sodium nitrite and secondary amines to mice. Cancer Res 31(9):1201-
1205.
Aschebrook-Kilfoy B, Cross AJ, Stolzenberg-Solomon RZ, et al. 2011. Pancreatic cancer and exposure
to dietary nitrate and nitrite in the NIH-AARP Diet and Health Study. Am J Epidemiol 174(3):305-315.
Aschebrook-Kilfoy B, Heltshe SL, Nuckols JR, et al. 2012. Modeled nitrate levels in well water supplies
and prevalence of abnormal thyroid conditions among the Old Order Amish in Pennsylvania. Environ
Health (Lond) 11:6-16.
Aschebrook-Kilfoy B, Shu X, Gao Y, et al. 2013a. Thyroid cancer risk and dietary nitrate and nitrite
intake in the Shanghai Women's Health Study. Int J Cancer 132:897-904.
Aschebrook-Kilfoy B, Ward MH, Dave BJ, et al. 2013b. Dietary nitrate and nitrite intake and risk of
non-Hodgkin lymphoma. Leuk Lymphoma 54(5):945-950. 10.3109/10428194.2012.734613.
Aschengrau A, Zierler S, Cohen A. 1989. Quality of community drinking water and the occurrence of
spontaneous abortion. Arch Environ Health 44(5):283-290.
Aschengrau A, Zierler S, Cohen A. 1993. Quality of community drinking water and the occurrence of
late adverse pregnancy outcomes. Arch Environ Health 48(2):105-113.
ATSDR. 1989. Decision guide for identifying substance-specific data needs related to toxicological
profiles; Notice. Agency for Toxic Substances and Disease Registry. Fed Regist 54(174):37618-37634.
ATSDR. 2013a. ATSDR case studies in environmental medicine. Nitrate/nitrite toxicity. Atlanta, GA:
Agency for Toxic Substances and Disease Registry. U.S. Department of Health and Human Services.
http://www.atsdr.cdc.gov/csem/nitrate_2013/docs/nitrite.pdf. May 20, 2014.
239 NITRATE AND NITRITE
9. REFERENCES
ATSDR. 2013b. Nitrate/nitrite toxicity. Clinical assessment-laboratory tests [CE original date:
December 5, 2013, CE renewal date: December 5, 2015, CE expiration date: December 5, 2017.
http://www.atsdr.cdc.gov/csem/nitrate_2013/docs/nitrite.pdf. September 15, 2016.
ATSDR. 2015. Ammonium nitrate, sodium nitrate, and sodium nitrite . Full SPL data. Substance
priority list (SPL) resource page. Agency for Toxic Substances and Disease Registry, Centers for Disease
Control and Prevention. http://www.atsdr.cdc.gov/SPL/resources. August 3, 2016.
Avery AA. 1999. Infantile methemoglobinemia: Reexamining the role of drinking water nitrates.
Environ Health Perspect 107(7):583-586.
Bahadoran Z, Mirmiran P, Ghasemi A, et al. 2015. Is dietary nitrate/nitrite exposure a risk factor for
development of thyroid abnormality? A systematic review and meta-analysis. Nitric Oxide 47:65-76.
10.1016/j.niox.2015.04.002.
Bailey SJ, Vanhatalo A, Winyard PG, et al. 2012. The nitrate-nitrite-nitric oxide pathway: Its role in
human exercise physiology. Eur J Sport Sci 12(4):309-320.
http://dx.doi.org/10.1080/17461391.2011.635705. January 1, 2013.
Bailey WP. 1966. Methemoglobinemia--acute nitrate poisoning in infants: Second report. J Am
Osteopath Assoc 66(4):431-434.
Balimandawa M, de Meester C, Leonard A. 1994. The mutagenicity of nitrite in the Salmonella/
microsome test system. Mutat Res 321(1-2):7-11.
Barbone F, Austin H, Partridge EE. 1993. Diet and endometrial cancer: A case-control study. Am J
Epidemiol 137(4):393-403.
Barclay PJ. 1998. Nitrates and nitrites. In: Viccellio P, ed. Emergency toxicology. 2nd ed.
Philadelphia, PA: Lippincott-Raven Publishers, 315-323.
Barnes DG, Dourson M. 1988. Reference dose (RfD): Description and use in health risk assessments.
Regul Toxicol Pharmacol 8(4):471-486.
Bauer SE, Koch D, Unger N, et al. 2007. Nitrate aerosols today and in 2030: A global simulation
including aerosols and tropospheric ozone. Atmos Chem Phys 7(19):5043-5059.
Behroozi K, Robinson S, Gruener N, et al. 1972. The effect of chronic exposure to sodium nitrite on the
electroencephalogram of rats. Environ Res 5(4):409-417.
Berger GS, ed. 1994. Epidemiology of endometriosis. In: Endometriosis: Modern surgical
management of endometriosis. New York, NY: Springer-Verlag, 3-7.
Blood AB, Power GG. 2015. Nitrite: On the journey from toxin to therapy. Clin Pharmacokinet
54(3):221-223. 10.1007/s40262-014-0231-5.
Bloom JC, Schade AE, Brandt JT. 2013. Toxic responses of the blood. In: Klaassen CD, ed. Casarett
and Doull's toxicology: The basic science of poisons. 8th ed. New York: McGraw Hill Education, 532-
533.
240 NITRATE AND NITRITE
9. REFERENCES
Bloomfield RA, Welsch CW, Garner GB, et al. 1961. Effect of dietary nitrate on thyroid function.
Science 134(3491):1690.
Blowers L, Preston-Martin S, Mack WJ. 1997. Dietary and other lifestyle factors of women with brain
gliomas in Los Angeles County (California, USA). Cancer Causes Control 8(1):5-12.
Bondonno CP, Croft KD, Puddey IB, et al. 2012. Nitrate causes a dose-dependent augmentation of nitric
oxide status in healthy women. Food Funct 3(5):522-527.
Börzsönyi M, Pintér A, Surján A, et al. 1976. Transplacental induction of lymphomas in Swiss mice by
carbendazim and sodium nitrite. Int J Cancer 17:742-747.
Börzsönyi M, Pintér A. 1977. The carcinogenicity of N-nitroso compounds formed endogenously in
mice from benzimidazole carbamate pesticides. Neoplasma 21(1):119-122.
Börzsönyi M, Pintér A, Surján A, et al. 1978. Carcinogenic effect of a quanidine pesticide administered
with sodium nitrite on adult mice and on the offspring after prenatal exposure. Cancer Lett 5(2):107-113.
Bosch HM, Rosenfield AB, Huston R, et al. 1950. Methemoglobinemia and Minnesota well supplies. J
Am Water Works Assoc 42(2):161-170.
Brambilla G, Martelli A. 2007. Genotoxic and carcinogenic risk to humans of drug-nitrite interaction
products. Mutat Res 635(1):17-52.
Brambilla G, Cavanna M, Faggin P, et al. 1983. Genotoxic effects in rodents given high oral doses of
ranitidine and sodium nitrite. Carcinogenesis 4(10):1281-1285.
Brams A, Buchet JP, Crutzen-Fayt MC, et al. 1987. A comparative study, with 40 chemicals, of the
efficiency of the Salmonella assay and the SOS chromotest (kit procedure). Toxicol Lett 38:1-2.
Brender JD, Olive JM, Felkner M, et al. 2004. Dietary nitrites and nitrates, nitrosatable drugs, and neural
tube defects. Epidemiology 15(3):330-336.
Brender JD, Weyer PJ, Romitti PA, et al. 2013. Prenatal nitrate intake from drinking water and selected
birth defects in offspring of participants in the National Birth Defects Prevention Study. Environ Health
Perspect. 121(9):1083-1089.
Bronson KF. 2008. Forms of inorganic nitrogen in soil. In: Schepers JS, Raun WR, eds. Nitrogen in
agricultural systems. Madison, WI: American Society of Agronomy, Inc., Crop Science Society of
America, Inc., Soil Science Society of America, Inc., 31-55.
Bruning-Fann CS, Kaneene JB. 1993. The effects of nitrate nitrite and n-nitroso compounds on human
health: A review. Vet Hum Toxicol 35(6):521-538.
Bruning-Fann C, Kaneene JB, Miller RA, et al. 1994. The use of epidemiological concepts and
techniques to discern factors associated with the nitrate concentration of well water on swine farms in the
USA. Sci Total Environ 153(1-2):85-96.
Bryan NS, van Grinsven H. 2013. The role of nitrate in human health. In: Sparks D, ed. Advances in
agronomy. Vol. 119. Delaware: Elsevier, Inc., 153-181.
241 NITRATE AND NITRITE
9. REFERENCES
Bucklin R, Myint MK. 1960. Fatal methemoglobinemia due to well water nitrates. Ann Intern Med
52:703-705.
Buiatti E, Palli D, Decarli A, et al. 1990. A case-control study of gastric cancer and diet in Italy: II.
Association with nutrients. Int J Cancer 45(5):896-901.
Bukowski J, Somers G, Bryanton J. 2001. Agricultural contamination of groundwater as a possible risk
factor for growth restriction or prematurity. J Occup Environ Med 43(4):377-383.
Burow KR, Nolan BT, Rupert MG, et al. 2010. Nitrate in groundwater of the United States, 1991−2003.
Environ Sci Technol 44(13):4988-4997.
Casu A, Carlini M, Contu A, et al. 2000. Type 1 diabetes in Sardinia is not linked to nitrate levels in
drinking water. Diabetes Care 23(7):1043-1044.
CDC. 1996. Spontaneous abortions possibly related to ingestion of nitrate-contaminated well water-
LaGrange County, Indiana, 1991-1994. MMWR Morb Mortal Wkly Rep 45(26):569-572.
CDC. 1997. Methemoglobinemia attributable to nitrite contamination of potable water through boiler
fluid additives-New Jersey, 1992 and 1996. MMWR Morb Mortal Wkly Rep 46(9):202-204.
CDC. 1998. A survey of the quality of water drawn from domestic wells in nine midwest states. Centers
for Disease Control and Prevention, National Center for Environmental Health.
http://www.cdc.gov/nceh/hsb/disaster/pdfs/A%20Survey%20of%20the%20Quality%20ofWater%20Draw
n%20from%20Domestic%20Wells%20in%20Nine%20Midwest%20States.pdf. May 21, 2014.
CDC. 2002. Methemoglobinemia following unintentional ingestion of sodium nitrite - New York, 2002.
Centers for Disease Control and Prevention, U.S. Department of Health and Human Services. Morbidity
and Mortality Weekly Report 51(29):639-642.
CDC. 2013. Fourth national report on human exposure to environmental chemicals, updated tables,
September 2013. Atlanta, GA: Centers for Disease Control and Prevention, Department of Health and
Human Services. http://www.cdc.gov/exposurereport/pdf/FourthReport_UpdatedTables_Sep2013.pdf.
May 20, 2014.
Cedergren MI, Selbing AJ, Lofman O, et al. 2002. Chlorination byproducts and nitrate in drinking water
and risk for congenital cardiac defects. Environ Res 89(2):124-130.
Chan WC, Fong YY. 1977. Ascorbic acid prevents liver tumour production by aminopyrine and nitrite
in the rat. Int J Cancer 20(2):268-270.
Chapin FJ. 1947. Methemoglobinemia from nitrates in well water. J Mich State Med Soc 46(8):938.
ChemID Plus. 2014. Nitrate ion and nitrite ion. ChemIDplus: A Toxnet database. Bethesda, MD: U.S.
National Library of Medicine. http://chem.sis.nlm.nih.gov/chemidplus/. May 22, 2014.
Chiu BC, Dave BJ, Ward MH, et al. 2008. Dietary factors and risk of t(14;18)-defined subgroups of
non-Hodgkin lymphoma. Cancer Causes Control 19(8):859-867. 10.1007/s10552-008-9148-3.
Chiu HF, Tsai SS, Chen PS, et al. 2011. Does calcium in drinking water modify the association between
nitrate in drinking water and risk of death from colon cancer? J Water Health 9(3):498-506.
242 NITRATE AND NITRITE
9. REFERENCES
Chiu HF, Tsai SS, Yang CY. 2007. Nitrate in drinking water and risk of death from bladder cancer: An
ecological case-control study in Taiwan. J Toxicol Environ Health A 70(12):1000-1004.
10.1080/15287390601171801.
Chow CK, Chen CJ, Gairola C. 1980. Effect of nitrate and nitrite in drinking water on rats. Toxicol Lett
6(3):199-206.
Clewell HJ, Andersen ME. 1985. Risk assessment extrapolations and physiological modeling. Toxicol
Ind Health 1(4):111-131.
Cohen B, Myant NB. 1959. Concentration of salivary iodide: A comparative study. J Physiol
145(3):595-610.
Comly HH. 1987. Landmark article Sept 8, 1945: Cyanosis in infants caused by nitrates in well water.
JAMA 257(20):2788-2792.
Commoner B, Woolum JC, Senturia BH, et al. 1970. The effects of 2-acetylaminofluorene and nitrite on
free radicals and carcinogenesis in rat liver. Cancer Res 30(8):2091-2097.
Cornblath M, Hartmann AF. 1948. Methemoglobinemia in young infants. J Pediatr 33:421-425.
Cornell University. 2009. Nitrogen: All forms are not equal. Cornell University, Cooperative
Extension. www.greenhouse.cornell.edu/crops/factsheets/nitrogen_form.pdf. November 17, 2014.
Cortas NK, Wakid NW. 1991. Pharmacokinetic aspects of inorganic nitrate ingestion in man.
Pharmacol Toxicol 68(3):192-195.
Cortesi ML, Vollano L, Francesca M, et al. 2015. Determination of nitrate and nitrite levels in infant
foods marketed in Southern Italy. CyTA - Journal of Food 13(4):629-634.
10.1080/19476337.2015.1035337.
Costa LG, Aschner M, Vitalone A, et al. 2004. Developmental neuropathology of environmental agents.
Annu Rev Pharmacol Toxicol 44:87-110.
Couch DB, Friedman MA. 1975. Interactive mutagenicity of sodium nitrite, dimethylamine, methylurea,
and ethylurea. Mutat Res 31:109-114.
Craun GF, Greathouse DG, Gunderson DH. 1981. Methemoglobin levels in young children consuming
high nitrate well water in the USA. Int J Epidemiol 10(4):309-317.
Croen LA, Todoroff K, Shaw GM. 2001. Maternal exposure to nitrate from drinking water and diet and
risk for neural tube defects. Am J Epidemiol 153(4):325-331.
Cuello C, Correa P, Haenszel W, et al. 1976. Gastric cancer in Colombia: I. Cancer risk and suspect
environmental agents. J Natl Cancer Inst 57(5):1015-1020.
Dahlquist GG, Blom LG, Persson LA, et al. 1990. Dietary factors and the risk of developing insulin
dependent diabetes in childhood. Br Med J 300(6735):1302-1306.
243 NITRATE AND NITRITE
9. REFERENCES
Dean JA. 1985. Table 5-7. Proton-transfer reactions of inorganic materials in water at 25 °C. In:
Lange's handbook of chemistry. Thirteenth ed. New York, NY: McGraw Hill Book Company, 5-16.
De Flora S. 1981. Study of 106 organic and inorganic compounds in the Salmonella/microsome test.
Carcinogenesis 2:283-298.
De Flora S, Zanacchi P, Camoirano A, et al. 1984. Genotoxic activity and potency of 135 compounds in
the Ames reversion test and in a bacterial DNA-repair test. Mutat Res 133:161-198.
De Groef B, Decallonne BR, Van der Geyten S, et al. 2006. Perchlorate versus other environmental
sodium/iodide symporter inhibitors: Potential thyroid-related health effects. Eur J Endocrinol 155(1):17-
25.
DellaValle CT, Daniel CR, Aschebrook-Kilfoy B, et al. 2013. Dietary intake of nitrate and nitrite and
risk of renal cell carcinoma in the NIH-AARP Diet and Health Study. Br J Cancer 108:205-212.
Denshaw-Burke M, DelGiacco E, Curran AL, et al. 2013. Methemoglobinemia. In: Medscape.
http://emedicine.medscape.com/article/204178-overview. May 13, 2014.
De Roos AJ, Ward MH, Lynch CF, et al. 2003. Nitrate in public water supplies and the risk of colon and
rectum cancers. Epidemiology 14(6):640-649.
DOE. 2012. Protective action criteria (PAC). Oak Ridge, TN: U.S. Department of Energy and
Subcommittee on Consequence Assessment and Protective Actions (SCAPA).
http://orise.orau.gov/emi/scapa/chem-pacs-teels/default.htm. January 08, 2014.
Donahoe WE. 1949. Cyanosis in infants with nitrates in drinking water as cause. Pediatrics 3(3):308-
311.
Dorsch MM, Scragg RK, McMichael AJ, et al. 1984. Congenital malformations and maternal drinking
water supply in rural South Australia: A case-control study. Am J Epidemiol 119(4):473-486.
*Druckrey H, Steinhoff D, Beuthner H, et al. 1963. Screening of nitrite for chronic toxicity in rats.
Arzneim Forsch 13:320-323.
Dusdieker LB, Getchell JP, Liarakos TM, et al. 1994. Nitrate in baby foods: Adding to the nitrate
mosaic. Arch Pediatr Adolesc Med 148(5):490-494.
DWI. 1999. Nitrate in drinking water and childhood-onset insulin-dependent diabetes mellitus in
Scotland and central England. Drinking Water Inspectorate. DWI0801.
http://dwi.defra.gov.uk/research/completed-research/reports/dwi0801.pdf. November 17, 2014.
EEA. 2010. Data and maps. Maps and graphs. The nitrogen cycle. European Environment Agency.
http://www.eea.europa.eu/data-and-maps/figures/the-nitrogen-cycle. July 8, 2014.
Ehrenberg L, Hussain S, Noor Saleh M, et al. 1980. Nitrous esters: A genetical hazard from nitrogen
oxides (NO
x
)? Hereditas 92:127-130.
Ek CJ, Dziegielewska KM, Habgood MD, et al. 2012. Barriers in the developing brain and
neurotoxicology. Neurotoxicology 33(3):586-604.
244 NITRATE AND NITRITE
9. REFERENCES
El-Wakf AM, Hassan HA, El-said FG, et al. 2008. Hypothyroidism in male rats of different ages
exposed to nitrate polluted drinking water. Mansoura J Forensic Med Clin Toxicol 15(2):77-89.
El-Wakf AM, Hassan HA, Mahmoud AZ, et al. 2015. Fenugreek potent activity against nitrate-induced
diabetes in young and adult male rats. Cytotechnology 67(3):437-447. 10.1007/s10616-014-9702-7.
Engel LS, Chow WH, Vaughan TL, et al. 2003. Population attributable risks of esophageal and gastric
cancers. J Natl Cancer Inst 95(18):1404-1413.
Environment Canada. 2012. Nitrate ion. Canadian environmental quality guidelines. http://ceqg-
rcqe.ccme.ca/download/en/197/. May 20, 2014.
EPA. 1990a. The drinking water criteria document on nitrate/nitrite. Washington, DC: U.S.
Environmental Protection Agency. PB91142836.
EPA. 1990b. Interim methods for development of inhalation reference concentrations. Washington, DC:
U.S. Environmental Protection Agency, Office of Health and Environmental Assessment, Office of
Research and Development. EPA600890066A. PB90238890.
EPA. 1993. Method 353.2. Determination of nitrate-nitrite nitrogen by automated colorimetry.
Cincinnati, OH: U.S. Environmental Protection Agency. http://water.epa.gov/scitech/methods/cwa/
bioindicators/upload/2007_07_10_methods_method_353_2.pdf. May 21, 2014.
EPA. 1997. Special report on environmental endocrine disruption: An effects assessment and analysis.
Washington, DC: U.S. Environmental Protection Agency, Office of Pollution Prevention and Toxics.
EPA630R96012.
EPA. 1998. RCRA waste minimization PBT priority chemical list. U.S. Environmental Protection
Agency. Fed Regist 63 FR 60332. http://www.gpo.gov/fdsys. January 08, 2014.
EPA. 2008. Child-specific exposure facts handbook. Washington, DC: U.S. Environmental Protection
Agency, National Center for Environmental Assessment, Office of Research and Development.
EPA600R06096F.
EPA. 2009a. Contaminant occurrence support document for category 2 contaminants for the second six-
year review of national primary drinking water regulations. U.S. Environmental Protection Agency.
EPA815B09011.
http://water.epa.gov/lawsregs/rulesregs/regulatingcontaminants/sixyearreview/second_review/upload/6Ye
arCategory2Report_final.pdf. May 21, 2014.
EPA. 2009b. Drinking water contaminant candidate list. U.S. Environmental Protection Agency. Fed
Regist 74 FR 51850:51850 -51862. http://www.gpo.gov/fdsys. January 08, 2014.
EPA. 2009c. National primary drinking water regulations. Washington, DC: U.S. Environmental
Protection Agency, Office of Ground Water and Drinking Water. EPA816F090004.
http://water.epa.gov/drink/contaminants/. January 08, 2014.
EPA. 2009d. Nitrogen and phosphorus loads in large rivers. Report on the environment. U.S.
Environmental Protection Agency.
http://cfpub.epa.gov/eroe/index.cfm?fuseaction=detail.viewInd&lv=list.listbyalpha&r=216594&subtop=2
00. June 17, 2015.
245 NITRATE AND NITRITE
9. REFERENCES
EPA. 2012a. CADDIS volume 2: Sources. stressors & responses. Ammonia: Simple conceptual
diagram. Nitrogen cycle. U.S. Environmental Protection Agency.
http://www.epa.gov/caddis/ssr_amm_nitrogen_cycle_popup.html. July 7, 2014.
EPA. 2012b. Drinking water standards and health advisories. Washington, DC: U.S. Environmental
Protection Agency, Office of Water. EPA822S12001.
http://water.epa.gov/drink/standards/hascience.cfm. January 08, 2014.
EPA. 2013a. Acute exposure guideline levels (AEGLs). Washington, DC: U.S. Environmental
Protection Agency, Office of Pollution Prevention and Toxics. http://www.epa.gov/oppt/aegl/. January
08, 2014.
EPA. 2013b. Designated as hazardous substances in accordance with section 311(b)(2)(a) of the Clean
Water Act. U.S. Environmental Protection Agency. Code of Federal Regulations 40 CFR 116.4.
http://www.gpo.gov/fdsys. January 08, 2014.
EPA. 2013c. Identification and listing of hazardous waste. Hazardous constituents. U.S. Environmental
Protection Agency. Code of Federal Regulations 40 CFR 261, Appendix VIII.
http://www.gpo.gov/fdsys. January 08, 2014.
EPA. 2013d. Reportable quantities of hazardous substances designated pursuant to section 311 of the
Clean Water Act. U.S. Environmental Protection Agency. Code of Federal Regulations 40 CFR 117.3.
http://www.gpo.gov/fdsys. January 08, 2014.
EPA. 2013e. Standards for owners and operators of hazardous waste TSD facilities. Groundwater
monitoring list. U.S. Environmental Protection Agency. Code of Federal Regulations 40 CFR 264,
Appendix IX. http://www.gpo.gov/fdsys. January 08, 2014.
EPA. 2013f. Superfund, emergency planning, and community right-to-know programs. Designation,
reportable quantities, and notifications. U.S. Environmental Protection Agency. Code of Federal
Regulations 40 CFR 302.4. http://www.gpo.gov/fdsys. January 08, 2014.
EPA. 2013g. Superfund, emergency planning, and community right-to-know programs. Extremely
hazardous substances and their threshold planning quantities. U.S. Environmental Protection Agency.
Code of Federal Regulations 40 CFR 355, Appendix A. http://www.gpo.gov/fdsys. January 08, 2014.
EPA. 2013h. Superfund, emergency planning, and community right-to-know programs. Toxic chemical
release reporting. U.S. Environmental Protection Agency. Code of Federal Regulations 40 CFR 372.65.
http://www.gpo.gov/fdsys. January 08, 2014.
EPA. 2013i. Toxic Substances Control Act. Chemical lists and reporting periods. U.S. Environmental
Protection Agency. Code of Federal Regulations 40 CFR 712.30. http://www.gpo.gov/fdsys. January
08, 2014.
EPA. 2013j. Toxic Substances Control Act. Health and safety data reporting. U.S. Environmental
Protection Agency. Code of Federal Regulations 40 CFR 716.120. http://www.gpo.gov/fdsys. January
08, 2014.
EPA. 2014a. Hazardous air pollutants. Clean Air Act. U.S. Environmental Protection Agency. United
States Code 42 USC 7412. http://www.epa.gov/ttn/atw/orig189.html. January 08, 2014.
246 NITRATE AND NITRITE
9. REFERENCES
EPA. 2014b. Inert ingredients permitted for use in nonfood pesticide products. Washington, DC: U.S.
Environmental Protection Agency. http://iaspub.epa.gov/apex/pesticides/f?p=124:1. January 08, 2014.
EPA. 2014c. Master testing list. Washington, DC: U.S. Environmental Protection Agency, Office of
Pollution Prevention and Toxics. http://www.epa.gov/opptintr/chemtest/pubs/mtl.html. January 08,
2014.
EPA. 2014d. National ambient air quality standards (NAAQS). Washington, DC: U.S. Environmental
Protection Agency, Office of Air and Radiation. http://www.epa.gov/air/criteria.html. January 08, 2014.
EPA. 2014e. National recommended water quality criteria. Washington, DC: U.S. Environmental
Protection Agency, Office of Water, Office of Science and Technology.
http://water.epa.gov/scitech/swguidance/standards/criteria/current/index.cfm. January 08, 2014.
EPA. 2014f. Ammonium nitrate, CAS registry 6484-52-2; sodium nitrate, CAS registry 7631-99-4;
sodium nitrite, CAS registry 7632-00-0; potassium nitrate, CAS registry 7757-79-1; potassium nitrite,
CAS registry 7758-09-0. Pesticide chemical search. U.S. Environmental Protection
Agency. http://iaspub.epa.gov/apex/pesticides/f?p=CHEMICALSEARCH:1:0. May 23, 2014.
EPA. 2014g. 2012. Chemical data reporting (CDR) information on the production and use of chemicals
manufactured or imported into the United States. U.S. Environmental Protection Agency.
http://java.epa.gov/oppt_chemical_search/. November 17, 2014.
Ericson A, Kallen B, Lofkvist E. 1988. Environmental factors in the etiology of neural tube defects: A
negative study. Environ Res 45(1):38-47.
Eskandari S, Loo DDF, Dai G, et al. 1997. Thyroid Na+/I- symporter. J Biol Chem. 272(43):27230-
27238.
Eskiocak S, Dundar C, Basoglu T, et al. 2005. The effects of taking chronic nitrate by drinking water on
thyroid functions and morphology. Clin Exp Med 5(2):66-71.
Espejo-Herrera N, Cantor KP, Malats N, et al. 2015. Nitrate in drinking water and bladder cancer risk in
Spain. Environ Res 137:299-307. 10.1016/j.envres.2014.10.034.
Espejo-Herrera N, Gracia-Lavedan E, Boldo E, et al. 2016a. Colorectal cancer risk and nitrate exposure
through drinking water and diet. Int J Cancer 139(2):334-346. 10.1002/ijc.30083.
Espejo-Herrera N, Gracia-Lavedan E, Pollan M, et al. 2016b. Ingested nitrate and breast cancer in the
Spanish multicase-control study on cancer (MCC-Spain). Environ Health Perspect 124(7):1042-1029.
10.1289/ehp.1510334.
Exner ME, Perea-Estrada H, Spalding RF. 2010. Long-term response of groundwater nitrate
concentrations to management regulations in Nebraska's central Platte valley. Sci World J 10:286-297.
Fan AM, Steinberg VE. 1996. Health implications of nitrate and nitrite in drinking water: An update on
methemoglobinemia occurrence and reproductive and developmental toxicity. Regul Toxicol Pharmacol
23(1 Part 1):35-43.
247 NITRATE AND NITRITE
9. REFERENCES
Fan AM, Willhite CC, Book SA. 1987. Evaluation of the nitrate drinking water standard with reference
to infant methemoglobinemia and potential reproductive toxicity. Regul Toxicol Pharmacol 7(2):135-
148.
Fandrem SI, Kjuus H, Andersen A, et al. 1993. Incidence of cancer among workers in a Norwegian
nitrate fertiliser plant. Br J Ind Med 50(7):647-652.
Faucett RL, Miller HC. 1946. Methemoglobinemia occurring in infants fed milk diluted with well water
of high nitrate content. J Pediatr 29(5):593-596.
FDA. 2013. Requirements for specific standardized beverages. U.S. Food and Drug Administration.
Code of Federal Regulations 21 CFR 165.110. http://www.gpo.gov/fdsys. January 08, 2014.
FDA. 2014. Everything added to food in the United States (EAFUS). Washington, DC: U.S. Food and
Drug Administration. http://www.accessdata.fda.gov/scripts/fcn/fcnnavigation.cfm?rpt=eafuslisting.
January 08, 2014.
Ferrant M. 1946. Methemoglobinemia; two cases in newborn infants caused by nitrates in well water. J
Pediatr 29(5):585-592.
Fewtrell L. 2004. Drinking-water nitrate, methemoglobinemia, and global burden of disease: A
discussion. Environ Health Perspect 112(14):1371-1374.
Finan A, Keenan P, Donovan FO, et al. 1998. Methaemoglobinaemia associated with sodium nitrite in
three siblings. BMJ 317(7166):1138-1139.
Fomon SJ. 1966. Body composition of the infant: Part 1: The male reference infant. In: Faulkner F,
ed. Human development. Philadelphia, PA: WB Saunders, 239-246.
Fomon SJ, Haschke F, Ziegler EE, et al. 1982. Body composition of reference children from birth to age
10 years. Am J Clin Nutr 35(Suppl 5):1169-1175.
Forman D, Al-Dabbagh S, Doll R. 1985. Nitrates, nitrites and gastric cancer in Great Britain. Nature
313(6004):620-625.
Fraser P, Chilvers C, Day M, et al. 1989. Further results from a census based mortality study of fertilizer
manufacturers. Br J Ind Med 46(1):38-42.
Fraser P, Chilvers C, Goldblatt P. 1982. Census-based mortality study of fertilizer manufacturers. Br J
Ind Med 39(4):323-329.
Freedman DM, Cantor KP, Ward MH, et al. 2000. A case-control study of nitrate in drinking water and
non-Hodgkin's lymphoma in Minnesota. Arch Environ Health 55(5):326-329.
Friedman MA, Staub J. 1976. Inhibition of mouse testicular DNA synthesis by mutagens and
carcinogens as a potential simple mammalian assay for mutagenesis. Mutat Res 37(1):67-76.
Gangolli SD, Van Den Brandt PA, Feron VJ, et al. 1994. Nitrate, nitrite and N-nitroso compounds. Eur
J Pharmacol 292:1-38.
248 NITRATE AND NITRITE
9. REFERENCES
Gatseva PD, Argirova MD. 2008. Iodine status and goitre prevalence in nitrate-exposed schoolchildren
living in rural Bulgaria. Public Health 122(5):458-461.
Gautami S, Rao RN, Raghuram TC, et al. 1995. Accidental acute fatal sodium nitrite poisoning. J
Toxicol Clin Toxicol 33(2):131-133.
George M, Wiklund L, Aastrup M, et al. 2001. Incidence and geographical distribution of sudden infant
death syndrome in relation to content of nitrate in drinking water and groundwater levels. Eur J Clin
Invest 31(12):1083-1094.
Giles GG, McNeil JJ, Donnan G, et al. 1994. Dietary factors and the risk of glioma in adults: Results of
a case-control study in Melbourne, Australia. Int J Cancer 59(3):357-362.
Giri AK, Talukder G, Sharma A. 1986. Sister chromatid exchange induced by metanil yellow and nitrite
singly and in combination in vivo on mice. Cancer Lett 31(3):299-303.
Giwercman A, Carlsen E, Keiding N, et al. 1993. Evidence for increasing incidence of abnormalities of
the human testis: A review. Environ Health Perspect 101(Supp 2):65-71.
Globus M, Samuel D. 1978. Effect of maternally administered sodium nitrite on hepatic erythropoiesis
in fetal CD-1 mice. Teratology 18(3):367-378.
Gowans WJ. 1990. Fatal methaemoglobinaemia in a dental nurse. A case of sodium nitrite poisoning.
Br J Gen Pract 40(340):470-471.
Graf U, Frei H, Kaegi A, et al. 1989. Thirty compounds tested in the Drosophila wing spot test. Mutat
Res 222(4):359-374.
Grant D, Butler WH. 1989. Chronic toxicity of sodium nitrite in the male F344 rat. Food Chem Toxicol
27(9):565-571.
Greenberg M, Birnkrant WB, Schiftner JJ. 1945. Outbreak of sodium nitrite poisoning. Am J Public
Health Nations Health 35(11):1217-1220.
Greenblatt M, Lijinsky W. 1972. Failure to induce tumors in Swiss mice after concurrent administration
of amino acids and sodium nitrite. J Natl Cancer Inst 48(5):1389-1392.
Greenblatt M, Lijinsky W. 1974. Carcinogenesis and chronic toxicity of nitrillotriacetic acid in Swiss
mice. J Natl Cancer Inst 52(4):1123-1126.
Greenblatt M, Mirvish SS. 1973. Dose-response studies with concurrent administration of piperazine
and sodium nitrite to strain A mice. J Natl Cancer Inst. 50(1):119-124.
Greenblatt M, Kommineni VR, Lijinsky W. 1973. Null effect of concurrent feeding of sodium nitrite
and amino acids to MRC rats. J Natl Cancer Inst 50(3):799-802.
Greenblatt M, Mirvish S, So BT. 1971. Nitrosamine studies: Induction of lung adenomas by concurrent
administration of sodium nitrite and secondary amines in Swiss mice. J Natl Cancer Inst 46(5):1029-
1034.
249 NITRATE AND NITRITE
9. REFERENCES
Greer FR, Shannon M. 2005. Infant methemoglobinemia: The role of dietary nitrate in food and water.
Pediatrics 116(3):784-786.
Gruener N. 1974. The effect of nitrites on isolation-induced aggression in mice. Pharmacol Biochem
Behav 2(2):267-269.
Gruener N, Toeplitz R. 1975. The effect of changes in nitrate concentration in drinking water on
methemoglobin levels in infants. Int J Environ Stud 7(3):161-163.
Gupta SK, Fitzgerald JF, Chong SK, et al. 1998. Expression of inducible nitric oxide synthase (iNOS)
mRNA in inflamed esophageal and colonic mucosa in a pediatric population. Am J Gastroenterol
93(5):795-798.
Gupta SK, Gupta RC, Seth AK, et al. 1999. Adaptation of cytochrome-b5 reductase activity and
methaemoglobinaemia in areas with a high nitrate concentration in drinking-water. Bull World Health
Organ 77(9):749-753.
Guzelian PS, Henry CJ, Olin SS. 1992. Similarities and differences between children and adults:
Implications for risk assessment. Washington, DC: International Life Sciences and Press Institute Press.
Hagmar L, Bellander T, Andersson C, et al. 1991. Cancer morbidity in nitrate fertilizer workers. Int
Arch Occup Environ Health 63(1):63-68.
Hammerl A, Klapotke TM. 2006. Nitrogen: Inorganic chemistry. In: Encyclopedia of inorganic
chemistry. Wiley Online Library.
http://onlinelibrary.wiley.com/doi/10.1002/0470862106.ia157/abstract. May 20, 2014.
Hawkes CH, Cavanagh JB, Darling JL, et al. 1992. Chronic low-dose exposure of sodium nitrite in VM-
strain mice: Central nervous system changes. Hum Exp Toxicol 11(4):279-281.
Haymond S, Cariappa R, Eby CS, et al. 2005. Laboratory assessment of oxygenation in
methemoglobinemia. Clin Chem 51(2):434-444.
Health Canada. 2012. Nitrate and nitrite in drinking water. Health Canada. Federal-Provincial-
Territorial Committee on Drinking Water. http://www.hc-sc.gc.ca/ewh-
semt/alt_formats/pdf/consult/_2012/nitrite-nitrite/nitrite-nitrite-eng.pdf. May 21, 2014.
Hegesh E, Shiloah J. 1982. Blood nitrates and infantile methemoglobinemia. Clin Chim Acta
125(2):107-115.
Hellmer L, Bolcsfoldi G. 1992. An evaluation of the Escherichia coli K-12 uvrB/recA DNA repair host-
mediated assay: II. In vivo results for 36 compounds tested in the mouse. Mutat Res 272(2):161-173.
Henderson WR, Raskin NH. 1972. "Hot dog'' headache: Individual susceptibility to nitrite. Lancet
2(Dec):1162-1163.
Hibbs JB, Westenfelder C, Taintor R, et al. 1992. Evidence for cytokine-inducible nitric oxide synthesis
from L-arginine in patients receiving interleukin-2 therapy. J Clin Invest 89(3):867-877.
Hirose M, Fukushima S, Hasegawa R, et al. 1990. Effects of sodium nitrite and catechol or 3-
methoxycatechol in combination on rat stomach epithelium. Jpn J Cancer Res 81(9):857-861.
250 NITRATE AND NITRITE
9. REFERENCES
Hirose M, Nishikawa A, Shibutani M, et al. 2002. Chemoprevention of heterocyclic amine-induced
mammary carcinogenesis in rats. Environ Mol Mutagen 39(2-3):271-278.
Hirose M, Tanaka H, Takahashi S, et al. 1993. Effects of sodium nitrite and catechol, 3-
methoxycatechol, or butylated hydroxyanisole in combination in a rat multiorgan carcinogenesis model.
Cancer Res 53(1):32-37.
Hoel DG, Davis DL, Miller AB, et al. 1992. Trends in cancer mortality in 15 industrialized countries,
1969-1986. J Natl Cancer Inst 84(5):313-320.
Holtby CE, Guernsey JR, Allen AC, et al. 2014. A population-based case-control study of drinking-
water nitrate and congenital anomalies using geographic information systems (GIS) to develop individual-
level exposure estimates. Int J Environ Res Public Health 11(2):1803-1823. 10.3390/ijerph110201803.
Hord NG. 2011. Dietary nitrates, nitrites, and cardiovascular disease. Curr Atheroscler Rep 13(6):484-
492.
HSDB. 2003. Urea. Hazardous Substances Data Bank. National Library of Medicine.
http://toxnet.nlm.nih.gov. November 17, 2014.
HSDB. 2007. Ammonium nitrate, sodium nitrate, sodium nitrite, potassium nitrate, and potassium
nitrite. Hazardous Substances Data Bank. National Library of Medicine. http://toxnet.nlm.nih.gov. May
21, 2014.
HSDB. 2012. Ammonia. Hazardous Substances Data Bank. National Library of Medicine.
http://toxnet.nlm.nih.gov. November 17, 2014.
Huber JC, Jr., Brender JD, Zheng Q, et al. 2013. Maternal dietary intake of nitrates, nitrites and
nitrosamines and selected birth defects in offspring: A case-control study. Nutrition journal 12:34.
Hugot D, Causeret J, Richir C. 1980. The influence of large amounts of sodium nitrite on reproductive
performances in female rats. Ann Nutr Aliment 34:1115-1124.
Huizenga JR, Tangerman A, Gips CH. 1994. Determination of ammonia in biological fluids. Ann Clin
Biochem 31(Pt 6):529-543.
Hunault CC, Lambers AC, Mensinga TT, et al. 2007. Effects of sub-chronic nitrate exposure on the
thyroidal function in humans. Toxicol Lett 175(1-3):64-70.
Huncharek M, Kupelnick B. 2004. A meta-analysis of maternal cured meat consumption during
pregnancy and the risk of childhood brain tumors. Neuroepidemiology 23(1-2):78-84.
Huncharek M, Kupelnick B, Wheeler L. 2003. Dietary cured meat and the risk of adult glioma: A meta-
analysis of nine observational studies. J Environ Pathol Toxicol Oncol 22(2):129-137.
Iammarino M, Di Taranto A, Cristino M. 2014. Monitoring of nitrites and nitrates levels in leafy
vegetables (spinach and lettuce): A contribution to risk assessment. J Sci Food Agric 94(4):773-778.
10.1002/jsfa.6439.
251 NITRATE AND NITRITE
9. REFERENCES
IARC. 2010. Ingested nitrate and nitrite. In: Ingested nitrate and nitrite, and cyanobacterial peptide
toxins. IARC Monographs on the evaluation of the carcinogenic risk of chemicals to humans. Vol. 94.
Lyon, France: International Agency for Research on Cancer, World Health Organization, 45-325.
IARC. 2014. Agents classified by the IARC monographs. Volumes 1-109. Lyon, France: International
Agency for Research on Cancer. http://monographs.iarc.fr/ENG/Classification/index.php. May 15, 2014.
Ibrahim YI, Ninnis JR, Hopper AO, et al. 2012. Inhaled NO therapy increases blood nitrite, nitrate and
S-nitrosohemoglobin concentrations in infants with pulmonary hypertension. J Pediatr 160(20):245-251.
Ilnitsky AP, Kolpakova AS. 1997. The enhancing effect of sodium nitrite on virus-induced leukemia in
mice. Cancer Detect Prev 21(4):312-318.
Imaizumi K, Tyuma I, Imai K, et al. 1980. In vivo studies on methemoglobin formation by sodium
nitrite. Int Arch Occup Environ Health 45(2):97-104.
Inai K, Aoki Y, Tokuoka S. 1979. Chronic toxicity of sodium nitrite in mice, with reference to its
tumorigenicity. Gann 70(2):203-208.
Inoue K, Shibata T, Kosaka H, et al. 1985. Induction of sister-chromatid exchanges by n-
nitrosocimetidine in cultured human lymphocytes and its inhibition by chemical compounds. Mutat Res
156:117-121.
Inoue-Choi M, Jones RR, Anderson KE, et al. 2015. Nitrate and nitrite ingestion and risk of ovarian
cancer among postmenopausal women in Iowa. Int J Cancer 137(1):173-182. 10.1002/ijc.29365.
Inoue-Choi M, Ward MH, Cerhan JR, et al. 2012. Interaction of nitrate and folate on the risk of breast
cancer among postmenopausal women. Nutr Cancer 64(5):685-694.
Inui N, Nishi Y, Mori M, et al. 1979. Detection of 8-azaguanine resistant mutants of embryonic cells
induced by products formed in the stomach on oral administration of sodium nitrite plus aminopyrine to
pregnant golden hamsters. Proceedings of the Japan Academy, Series B 55(6):286-289.
IRIS. 2002. Nitrite (CASRN 14797-65-0), nitrate (CASRN 14797-55-8). Integrated Risk Information
System. Washington, DC: U.S. Environmental Protection Agency. http://www.epa.gov/iris/. January
08, 2014.
Ishidate MJ, Sofuni T, Yoshikawa K. 1981. Chromosomal aberration tests in vitro as a primary
screening tool for environmental mutagens and/or carcinogens. Gann Monogr Cancer Res 27:95-108.
Ishidate MJ, Sofuni T, Yoshikawa K, et al. 1984. Primary mutagenicity screening of food additives
currently used in Japan. Food Chem Toxicol 22(8):623-636.
Iurchenko VA, Linnik AB, Il'nitskii AP. 1986. [Carcinogenic hazard of small doses of nitrite in
connection with the endogenous synthesis of nitroso compounds]. Eksp Onkol 8(1):41-44.
Ivankovic S. 1979. Teratogenic and carcinogenic effects of some chemicals during prenatal life in rats,
Syrian golden hamsters, and minipigs. Natl Cancer Inst Monogr 51:103-115.
Ivankovic S, Preussmann R. 1970. Transplacental production of malignant tumors after oral dose of
ethylurea and nitrite to rats. Naturwissenschaften 57:460.
252 NITRATE AND NITRITE
9. REFERENCES
Jablonska S. 1975. Ingestion of ammonium nitrate as a possible cause of erythema dyschromicum
perstans (ashy dermatosis). Dermatologica 150(5):287-291.
Jansen EHJ, Van Den Berg RH, Boink ABT, et al. 1995. A new physiological biomarker for nitrate
exposure in humans. Toxicol Lett 77(1-3):265-269.
Jansson EA, Huang L, Malkey R, et al. 2008. A mammalian functional nitrate reductase that regulates
nitrite and nitric oxide homeostasis. Nat Chem Biol 4(7):411-417.
JECFA. 2003a. Nitrate (and potential endogenous formation of N-nitroso compounds). WHO Food
Additives Series 50. Joint Expert Committee on Food Additives. International Programme on Chemical
Safety. http://www.inchem.org/documents/jecfa/jecmono/v50je06.htm. May 22, 2014.
JECFA. 2003b. Nitrite (and potential endogenous formation of N-nitroso compounds). WHO Food
Additives Series 50. Joint Expert Committee on Food Additives. International Programme on Chemical
Safety. 1057. http://www.inchem.org/documents/jecfa/jecmono/v50je05.htm. December 18, 2013.
JECFA. 2003c. Nitrate and nitrite: Intake assessment. WHO Food Additives Series 50. Joint Expert
Committee on Food Additives. 1059. http://www.inchem.org/documents/jecfa/jecmono/v50je07.htm.
December 18, 2013.
Johnson CJ, Bonrud PA, Dosch TL, et al. 1987. Fatal outcome of methemoglobinemia in an infant. J
Am Med Assoc 257(20):2796.
Jones HJ, Sethney HT, Schoenhals GW, et al. 1973. Grandmother's poisoned well: Report of a case of
methemoglobinemia in an infant in Oklahoma. J Okla State Med Assoc 66(2):60-66.
Jones JA, Hopper AO, Power GG, et al. 2015. Dietary intake and bio-activation of nitrite and nitrate in
newborn infants. Pediatr Res 77(1-2):173-181. 10.1038/pr.2014.168.
Jones JA, Ninnis JR, Hopper AO, et al. 2014. Nitrite and nitrate concentrations and metabolism in breast
milk, infant formula, and parenteral nutrition. JPEN J Parenter Enteral Nutr 38(7):856-866.
10.1177/0148607113496118.
Joossens JV, Hill MJ, Elliott P, et al. 1996. Dietary salt, nitrate and stomach cancer mortality in 24
countries. European Cancer Prevention (ECP) and the INTERSALT Cooperative Research Group. Int J
Epidemiol 25(3):494-504.
Kahn HD, Stralka K. 2009. Estimated daily average per capita water ingestion by child and adult age
categories based on USDA's 1994-1996 and 1998 continuing survey of food intakes by individuals. J
Expo Sci Environ Epidemiol 19(4):396-404.
Kamiyama S, Ohshima H, Shimada A, et al. 1987. Urinary excretion of N-nitrosamino acids and nitrate
by inhabitants in high and low-risk areas for stomach cancer in northern Japan. IARC Sci Publ 84:497-
502.
Kanady JA, Aruni AW, Ninnis JR, et al. 2012. Nitrate reductase activity of bacteria in saliva of term and
preterm infants. Nitric Oxide 27(4):193-200.
253 NITRATE AND NITRITE
9. REFERENCES
Kaplan A, Smith C, Promnitz DA, et al. 1990. Methaemoglobinaemia due to accidental sodium nitrite
poisoning. Report of 10 cases. S Afr Med J 77(6):300-301.
Kawabe M, Takaba K, Yoshida Y, et al. 1994. Effects of combined treatment with phenolic compounds
and sodium nitrite on two-stage carcinogenesis and cell proliferation in the rat stomach. Jpn J Cancer Res
85(1):17-25.
Kearns GL, Abdel-Rahman SM, Alander SW, et al. 2003. Developmental pharmacology-drug
disposition, action, and therapy in infants and children. N Engl J Med 349(12):1157-1167.
Keating JP, Lell ME, Strauss AW, et al. 1973. Infantile methemoglobinemia caused by carrot juice. N
Engl J Med 288(16):824-826.
Kelley ST, Oehme FW, Hoffman SB. 1974. Effect of chronic dietary nitrates on canine thyroid function.
Toxicol Appl Pharmacol 27(1):200-203.
Khera KS. 1982. Reduction of teratogenic effects of ethylenethiourea in rats by interaction with sodium
nitrite in vivo. Food Chem Toxicol 20:273-278.
Kilfoy BA, Ward MH, Zheng T, et al. 2010. Risk of non-Hodgkin lymphoma and nitrate and nitrite from
the diet in Connecticut women. Cancer Causes Control 21(6):889-896. 10.1007/s10552-010-9517-6.
Kilfoy BA, Zhang Y, Park Y, et al. 2011. Dietary nitrate and nitrite and the risk of thyroid cancer in the
NIH-AARP Diet and Health Study. Int J Cancer 129(1):160-172. 10.1002/ijc.25650.
Kim HJ, Lee SS, Choi BY, et al. 2007. Nitrate intake relative to antioxidant vitamin intake affects
gastric cancer risk: A case-control study in Korea. Nutr Cancer 59(2):185-191.
Kim SJ, Rim KT, Kim JK, et al. 2011. Evaluation of the genetic toxicity of cyclopentane and
ammonium nitrate in vitro mammalian chromosomal aberration assay in Chinese hamster ovary cells. Saf
Health Work 2(1):17-25.
Kissel DE, Cabrera ML, Paramasivam S. 2008. Ammonium, ammonia, and urea reactions in soils. In:
Schepers JS, Raun WR, eds. Nitrogen in agricultural systems. Madison, WI: American Society of
Agronomy, Inc., Crop Science Society of America, Inc., Soil Science Society of America, Inc., 101-155.
Kitano M, Takada N, Chen T, et al. 1997. Carcinogenicity of methylurea or morpholine in combination
with sodium nitrite in rat multi-organ carcinogenesis bioassay. Jpn J Cancer Res 88(9):797-806.
Kitchen NR, Blanchard PE, Lerch RN. 2015. Long-term agroecosystem research in the central
Mississippi river basin: Hydrogeologic controls and crop management influence on nitrates in loess and
fractured glacial till. J Environ Qual 44(1):58-70. 10.2134/jeq2014.09.0405.
Kleinjans JC, Albering HJ, Marx A, et al. 1991. Nitrate contamination of drinking water evaluation of
genotoxic risk in human populations. Environ Health Perspect 94(0):189-194.
Knaapen AM, Schins RP, Borm PJ, et al. 2005. Nitrite enhances neutrophil-induced DNA strand
breakage in pulmonary epithelial cells by inhibition of myeloperoxidase. Carcinogenesis 26(9):1642-
1648. 10.1093/carcin/bgi116.
254 NITRATE AND NITRITE
9. REFERENCES
Knekt P, Jarvinen R, Dich J, et al. 1999. Risk of colorectal and other gastro-intestinal cancers after
exposure to nitrate, nitrite and N-nitroso compounds: A follow-up study. Int J Cancer 80(6):852-856.
Knight TM, Forman D, Pirastu R, et al. 1990. Nitrate and nitrite exposure in Italian populations with
different gastric cancer rates. Int J Epidemiol 19(3):510-515.
Komori M, Nishio K, Kitada M, et al. 1990. Fetus-specific expression of a form of cytochrome P-450 in
human livers. Biochemistry 29(18):4430-4433.
Kortboyer JM, Boink ABTJ, Schothorst RC, et al. 1998b. Healthy volunteer study investigating the
feasibility of an oral bioavailability study of nitrate from vegetables. The Netherlands: National Institute
of Public Health and the Environment. RIVM Report 235802012.
Kortboyer JM, Boink ABTJ, Zeilmaker MJ, et al. 1997a. Methemoglobin formation due to nitrite:
Dose-effect relationship in vitro. The Netherlands: National Institute of Public Health and the
Environment. RIVM Report 235802006.
Kortboyer JM, Colbers EHP, Vaessen HAMG, et al. 1995. A pilot-study to investigate nitrate and nitrite
kinetics in healthy volunteers with normal and artificially increased gastric pH after sodium nitrate
ingestion. In: Health aspects of nitrate and its metabolites (particularly nitrite). Strasbourg: Publishing
and Documentation Service, Council of Europe Press, 269-286.
Kortboyer JM, Olling M, Zeilmaker MJ, et al. 1997b. The oral bioavailability of sodium nitrite
investigated in healthy adult volunteers Bilthoven, Netherlands: National Institute for Public Health and
the Environment. RIVM Report No. 235802007.
Kortboyer JM, Schothorst RC, Zeilmaker MJ, et al. 1998a. Intravenous administration of sodium nitrite
in healthy volunteers: A single ascending dose study. The Netherlands: National Institute of Public
Health and the Environment. RIVM Report 235802011.
Kosaka H, Imaizumi K, Imai K, et al. 1979. Stoichiometry of the reaction of oxyhemoglobin with nitrite.
Biochim Biophys Acta 581(1):184-188.
Kostraba JN, Gay EC, Rewers M, et al. 1992. Nitrate levels in community drinking waters and risk of
IDDM: An ecological analysis. Diabetes Care 15(11):1505-1508.
Kramer SB, Reganold JP, Glover JD, et al. 2006. Reduced nitrate leaching and enhanced denitrifier
activity and efficiency in organically fertilized soils. Proc Natl Acad Sci USA 103(12):4522-4527.
Krishnan K, Andersen ME. 1994. Physiologically based pharmacokinetic modeling in toxicology. In:
Hayes AW, ed. Principles and methods of toxicology. 3rd ed. New York, NY: Raven Press, Ltd., 149-
188.
Krishnan K, Anderson ME, Clewell HJ, et al. 1994. Physiologically based pharmacokinetic modeling of
chemical mixtures. In: Yang RSH, ed. Toxicology of chemical mixtures. Case studies, mechanisms,
and novel approaches. San Diego, CA: Academic Press, 399-437.
La Vecchia C, Ferraroni M, D'Avanzo B, et al. 1994. Selected micronutrient intake and the risk of
gastric cancer. Cancer Epidemiol Biomarkers Prev 3(5):393-398.
255 NITRATE AND NITRITE
9. REFERENCES
La Vecchia C, Negri E, Franceschi S, et al. 1997. Case-control study on influence of methionine, nitrite,
and salt on gastric carcinogenesis in northern Italy. Nutr Cancer 27(1):65-68.
Lambers AC, Kortboyer JM, Schothorst RC, et al. 2000. The oral bioavailability of nitrate from
vegetables investigated in health volunteers. The Netherlands: National Institute of Public Health and the
Environment. RIVM Report 235802014.
Lee M, Wrensch M, Miike R. 1997. Dietary and tobacco risk factors for adult onset glioma in the San
Francisco Bay Area (California, USA). Cancer Causes Control 8(1):13-24.
Leeder JS, Kearns GL. 1997. Pharmacogenetics in pediatrics: Implications for practice. Pediatr Clin
North Am 44(1):55-77.
Leikin JB, Paloucek FP. 2008. Poisoning and toxicology handbook. 4th ed. Boca Raton, FL: CRC
Press, 830.
Leung H. 1993. Physiologically-based pharmacokinetic modelling. In: Ballantyne B, Marrs T, Turner
P, eds. General and applied toxicology. Vol. 1. New York, NY: Stockton Press, 153-164.
Levallois P, Ayotte P, Van Maanen JMS, et al. 2000. Excretion of volatile nitrosamines in a rural
population in relation to food and drinking water consumption. Food Chem Toxicol 38(11):1013-1019.
Lewis RJ. 2002. Sodium nitrate. In: Sax's dangerous properties of industrial materials. Hoboken, NJ:
John Wiley & Sons, Inc, 3265.
L'hirondel J, L'hirondel JL. 2002. The case against nitrate: A critical examination. In: Nitrate and man.
Toxic, harmless or beneficial? Wallingford, UK: CABI Publishing, 35-69.
Lide DR. 2013. Sodium nitrate. In: CRC handbook of chemistry and physics. 94th ed. Boca Raton,
FL: Taylor & Francis Group, LLC, 4-90.
Lijinsky W. 1984a. Induction of tumors of the nasal cavity in rats by concurrent feeding of thiram and
sodium nitrite. J Toxicol Environ Health 13(4-6):609-614.
Lijinsky W. 1984b. Induction of tumours in rats by feeding nitrosatable amines together with sodium
nitrite. Food Chem Toxicol 22(9):715-720.
Lijinsky W, Greenblatt M. 1972. Carcinogen dimethylnitrosamine produced in vivo from nitrite and
aminopyrine. Nat New Biol 236(67):177-178.
Lijinsky W, Reuber MD. 1980. Tumors induced in Fischer 344 rats by the feeding of disulfiram together
with sodium nitrite. Food Cosmet Toxicol 18(1):85-87.
Lijinsky W, Taylor HW. 1977. Feeding tests in rats on mixtures of nitrite with secondary and tertiary
amines of environmental importance. Food Cosmet Toxicol 15:269-274.
Lijinsky W, Greenblatt M, Kommineni C. 1973a. Brief communication: Feeding studies of
nitrilotriacetic acid and derivatives in rats. J Natl Cancer Inst 50(4):1061-1063.
Lijinsky W, Kovatch R, Riggs CW. 1983. Altered incidences of hepatic and hemopoietic neoplasms in
F344 rats fed sodium nitrite. Carcinogenesis 4(9):1189-1191.
256 NITRATE AND NITRITE
9. REFERENCES
Lijinsky W, Taylor HW, Snyder C, et al. 1973b. Malignant tumours of liver and lung in rats fed
aminopyrine or heptamethyleneimine together with nitrite. Nature 244(5412):176-178.
Lin JK, Ho YS. 1992. Hepatotoxicity and hepatocarcinogenicity in rats fed squid with or without
exogenous nitrite. Food Chem Toxicol 30(8):695-702.
Lin K, Shen W, Shen Z, et al. 2003. Estimation of the potential for nitrosation and its inhibition in
subjects from high- and low-risk areas for esophageal cancer in southern China. Int J Cancer 107(6):891-
895.
Livingston AL. 1978. Forage plant estrogens. J Toxicol Environ Health 4(2-3):301-324.
Lu SH, Ohshima H, Fu HM, et al. 1986. Urinary excretion of n nitrosamino-acids and nitrate by
inhabitants of high-risk and low-risk areas for esophageal cancer in northern China: Endogenous
formation of nitrosoproline and its inhibition by vitamin C. Cancer Res 46(3):1485-1491.
Luca D, Luca V, Cotor F, et al. 1987. In vivo and in vitro cytogenetic damage induced by sodium nitrite.
Mutat Res 189(3):333-340.
Luca D, Raileanu L, Luca V, et al. 1985. Chromosomal aberrations and micronuclei induced in rat and
mouse bone marrow cells by sodium nitrate. Mutat Res 155(3):121-126.
Lundberg JO, Govoni M. 2004. Inorganic nitrate is a possible source for systemic generation of nitric
oxide. Free Radic Biol Med 37(3):395-400.
Lundberg JO, Weitzberg E. 2013. Biology of nitrogen oxides in the gastrointestinal tract. Gut
62(4):616-629.
Lundberg JO, Gladwin MT, Ahluwalia A, et al. 2009. Nitrate and nitrite in biology, nutrition and
therapeutics. Nat Chem Biol 5(12):865-869.
Lundberg JO, Weitzberg E, Gladwin MT. 2008. The nitrate-nitrite-nitric oxide pathway in physiology
and therapeutics. Nat Rev Drug Discov 7(2):156-167.
Lynch SC, Gruenwedel DW, Russell GF. 1983. Mutagenic activity of a nitrosated early Maillard
product: DNA synthesis (DNA repair) induced in HeLa S3 carcinoma cells by nitrosated 1-(N-L-
tryptophan)-1-deoxy-D-fructose. Food Chem Toxicol 21(5):551-556.
Machha A, Schechter AN. 2012. Inorganic nitrate: A major player in the cardiovascular health benefits
of vegetables? Nutr Rev 70(6):367-372.
Maekawa A, Ishiawata H, Odashima S. 1977. Transplacental carcinogenesis and chemical determination
of 1-butyl-1-nitrosourea in stomach content after simultaneous oral administration of 1-butylurea and
sodium nitrite to ACI rats. Gann. 68(1):81-88.
Maekawa A, Ogiu T, Onodera H, et al. 1982. Carcinogenicity studies of sodium nitrite and sodium
nitrate in F-344 rats. Food Chem Toxicol 20(1):25-33.
Malberg JW, Savage EP, Osteryoung J. 1978. Nitrates in drinking water and the early onset of
hypertension. Environ Pollut 15:155-160.
257 NITRATE AND NITRITE
9. REFERENCES
Manassaram DM, Backer LC, Messing R, et al. 2010. Nitrates in drinking water and methemoglobin
levels in pregnancy: A longitudinal study. Environ Health 9:60.
Markusova K, Kohutova L, Dzurov J. 1996. Programmed-potential voltammetric determination of
nitrate as a static mercury drop electrode in natural waters and soil extracts. Electroanalysis 8(6):582-
584.
Marshall PA, Trenerry VC. 1996. The determination of nitrite and nitrate in foods by capillary ion
electrophoresis. Food Chem 57(2):339-345.
Martinez A, Sanchez-Valverde F, Gil F, et al. 2013. Methemoglobinemia induced by vegetable intake in
infants in northern Spain. J Pediatr Gastroenterol Nutr 56(5):573-577.
10.1097/MPG.0b013e3182849d2b.
Mascher F, Marth E. 1993. Metabolism and effect of nitrates. Cent Eur J Public Health 1(1):49-52.
Matsukura N, Kawachi T, Sasajima K, et al. 1977. Induction of liver tumors in rats by sodium nitrite and
methylguanidine. Z Krebsforsch 90(1):87-94.
Matsumoto K, Tanaka H. 1996. Formation and dissociation of atmospheric particulate nitrate and
chloride: An approach based on phase equilibrium. Atmos Environ 30(4):639-648.
Mayne ST, Risch HA, Dubrow R, et al. 2001. Nutrient intake and risk of subtypes of esophageal and
gastric cancer. Cancer Epidemiol Biomarkers Prev 10(10):1055-1062.
Mayr U, Butsch A, Schneider S. 1992. Validation of two in vitro test systems for estrogenic activities
with zearalenone, phytoestrogens and cereal extracts. Toxicology 74(2-3):135-149.
McCann J, Choi E, Yamasaki E, et al. 1975. Detection of carcinogens as mutagens in the
salmonella/microsome test: Assay of 300 chemicals. Proc Natl Acad Sci USA 72:5135-5139.
McElroy JA, Trentham-Dietz A, Gangnon RE, et al. 2008. Nitrogen-nitrate exposure from drinking
water and colorectal cancer risk for rural women in Wisconsin, USA. J Water Health 6(3):399-409.
McKnight GM, Smith LM, Drummond RS, et al. 1997. Chemical synthesis of nitric oxide in the
stomach from dietary nitrate in humans. Gut 40(2):211-214.
McLetchie NGB, Robertson HE. 1994. Nitrate poisoning from well-water. Can Med Assoc J 60:230-
233.
Medovy H. 1948. Well-water methemoglobinemia in infants, its occurrence in rural Manitoba and
Ontario. J Lancet 68(5):194-196.
Michaud DS, Holick CN, Batchelor TT, et al. 2009. Prospective study of meat intake and dietary
nitrates, nitrites, and nitrosamines and risk of adult glioma. Am J Clin Nutr 90(3):570-577.
Miller LW. 1971. Methemoglobinemia associated with well water. J Am Med Assoc 216(10):1642-
1643.
258 NITRATE AND NITRITE
9. REFERENCES
Mirvish SS, Bulay O, Runge RG, et al. 1980. Study of the carcinogenicity of large doses of
dimethylnitramine, n-nitroso-l-proline, and sodium nitrite administered in drinking water to rats. J Natl
Cancer Inst 64(6):1435-1442.
Mirvish SS, Greenblatt M, Kommineni VR. 1972. Nitrosamide formation in vivo: Induction of lung
adenomas in Swiss mice by concurrent feeding of nitrite and methylurea or ethylurea. J Natl Cancer Inst
48(5):1311-1315.
Mirvish SS, Patil K, Ghadirian P, et al. 1975. Disappearance of nitrite from the rat stomach:
Contribution of emptying and other factors. J Natl Cancer Inst 54(4):869-875.
Mirvish SS, Pelfrene AF, Garcia H, et al. 1976. Effect of sodium ascorbate on tumor induction in rats
treated with morpholine and sodium nitrite, and with nitrosomorpholine. Cancer Lett 2(2):101-108.
Mirvish SS, Salmasi S, Cohen SM, et al. 1983. Liver and forestomach tumors and other forestomach
lesions in rats treated with morpholine and sodium nitrite, with and without sodium ascorbate. J Natl
Cancer Inst 71(1):81-85.
Miyauchi M, Nakamura H, Furukawa F, et al. 2002. Promoting effects of combined antioxidant and
sodium nitrite treatment on forestomach carcinogenesis in rats after initiation with N-methyl-N'-nitro-N-
nitrosoguanidine. Cancer Lett 178(1):19-24.
Mokhtar NM, el-Aaser AA, el-Bolkainy MN, et al. 1988. Effect of soybean feeding on experimental
carcinogenesis-III. Carcinogenicity of nitrite and dibutylamine in mice: A histopathological study. Eur J
Cancer Clin Oncol 24(3):403-411.
Moller H. 1997. Work in agriculture, childhood residence, nitrate exposure, and testicular cancer risk: A
case-control study in Denmark. Cancer Epidemiol Biomarkers Prev 6(2):141-144.
Moller H, Landt J, Jensen P, et al. 1989. Nitrate exposure from drinking water and diet in a Danish rural
population. Int J Epidemiol 18(1):206-212.
Moltchanova E, Rytkonen M, Kousa A, et al. 2004. Zinc and nitrate in the ground water and the
incidence of Type 1 diabetes in Finland. Diabet Med 21(3):256-261.
Momen B, Zehr JP, Boylen CW, et al. 1999. Determinants of summer nitrate concentration in a set of
Adirondack lakes, New York. Water Air Soil Pollut 111(1-4):19-28.
Monser L, Sadok S, Greenway GM, et al. 2002. A simple simultaneous flow injection method based on
phosphomolybdenum chemistry for nitrate and nitrite determinations in water and fish samples. Talanta
57:511-518.
Morales Suarez-Varela M, Llopis Gonzalez A, Tejerizo Perez ML, et al. 1993. Concentration of nitrates
in drinking water and its relationship with bladder cancer. J Environ Pathol Toxicol Oncol 12(4):229-
236.
Morales Suarez-Varela M, Llopis Gonzalez A, Tejerizo Perez ML. 1995. Impact of nitrates in drinking
water on cancer mortality in Valencia, Spain. Eur J Epidemiol 11(1):15-21.
Morselli PL, Franco-Morselli R, Bossi L. 1980. Clinical pharmacokinetics in newborns and infants:
Age-related differences and therapeutic implications. Clin Pharmacokinet 5(6):485-527.
259 NITRATE AND NITRITE
9. REFERENCES
Mueller BA, Searles Nielsen S, Preston-Martin S, et al. 2004. Household water source and the risk of
childhood brain tumours: Results of the SEARCH International Brain Tumor Study. Int J Epidemiol
33(6):1209-1216.
Mulvaney RL. 1996. Nitrogen-inorganic forms. In: Sparks DL, ed. Methods of soil analysis. Part 3.
Chemical Methods. Madison, WI: Soil Science Society of America, Inc. and American Society of
Agronomy Inc., 1123-1184.
Mukhopadhyay S, Ghosh D, Chatterjee A, et al. 2005. Evaluation of possible goitrogenic and anti-
thyroidal effect of nitrate, a potential environmental pollutant. Indian J Physiol Pharmacol 49(3):284-288.
Murthy AS, Baker JR, Smith ER, et al. 1979. Neoplasms in rats and mice fed butylurea and sodium
nitrite separately and in combination. Int J Cancer 23(2):253-259.
NAS/NRC. 1989. Report of the oversight committee. Biologic markers in reproductive toxicology.
Washington, DC: National Academy of Sciences, National Research Council, National Academy Press,
15-35.
Nasseri-Moghaddam S, Mofid A, Razjouyan H. 2011. Dietary nitrate may have a role in development of
gastro-esophageal reflux disease. Arch Iran Med 14(5):312-314.
Newberne PM. 1979. Nitrite promotes lymphoma incidence in rats. Science 204(4397):1079-1081.
Newberne PM, Shank RC. 1973. Induction of liver and lung tumours in rats by the simultaneous
administration of sodium nitrite and morpholine. Food Cosmet Toxicol 11(5):819-825.
Newton WE. 2005. Nitrogen fixation. In: Kirk-Othmer encyclopedia of chemical toxicology.
http://onlinelibrary.wiley.com/doi/10.1002/0471238961.1409201814052320.a01.pub2/abstract. May 21,
2014.
NFPA. 2002. In: Spencer AB, Colonna GR, eds. Fire protection guide to hazardous materials. Quincy,
MA: National Fire Protection Association, 49-19, 704-710.
Nicola JP, Basquin C, Portulano C, et al. 2009. The Na+/I- symporter mediates active iodide uptake in
the intestine. Am J Physiol Cell Physiol. 296(4):C654-662.
NIOSH. 1994a. Acids, inorganic. Method 7903, Issue 2. NIOSH Manual of Analytical Methods.
National Institute for Occupational Safety and Health. http://www.cdc.gov/niosh/docs/2003-
154/pdfs/7903.pdf. May 20, 2014.
NIOSH. 1994b. Nitric oxide and nitrogen dioxide. Method 6014, Issue 1. NIOSH Manual of Analytical
Methods. National Institute for Occupational Safety and Health. http://www.cdc.gov/niosh/docs/2003-
154/pdfs/6014-1.pdf. May 20, 2014.
NIOSH. 1998. Nitric dioxide (diffusive sampler). Method 6700, Issue 2. NIOSH Manual of Analytical
Methods. National Institute for Occupational Safety and Health. http://www.cdc.gov/niosh/docs/2003-
154/pdfs/6700.pdf. May 20, 2014.
260 NITRATE AND NITRITE
9. REFERENCES
NIOSH. 2014. NIOSH pocket guide to chemical hazards. Atlanta, GA: National Institute for
Occupational Safety and Health, Centers for Disease Control and Prevention.
http://www.cdc.gov/niosh/npg/. January 08, 2014.
Nishiyama K, Ando-Lu J, Ishimura S, et al. 1998. Initiating and promoting effects of concurrent oral
administration of ethylenethiourea and sodium nitrite on uterine endometrial adenocarcinoma
development in Donryu rats. In Vivo 12(4):363-368.
Nixon JE, Koller LD, Exon JH. 1979. Effect of methylmercury chloride on transplacental tumors
induced by sodium nitrite and ethylurea in rats. J Natl Cancer Inst 63:1057-1963.
Nolan BT. 1999. Nitrate behavior in ground waters of the Southeastern USA. J Environ Qual
28(5):1518-1527.
Nolan BT, Ruddy BC, Hitt KJ, et al. 1997. Risk of nitrate in groundwaters of the United States: A
national perspective. Environ Sci Technol 31(8):2229-2236.
Norat T, Lukanova A, Ferrari P, et al. 2002. Meat consumption and colorectal cancer risk: Dose-
response meta-analysis of epidemiological studies. Int J Cancer 98(2):241-256.
Nordin RN, Pommen LW. 1986. Water quality criteria for nitrogen (nitrate, nitrite, and ammonia).
Victoria, BC: Ministry of Environment and Parks Province of British Columbia.
NRC. 1993. Pesticides in the diets of infants and children. Washington, DC: National Research
Council. National Academy Press. PB93216091.
NTP. 2001. Toxicology and carcinogenesis studies of sodium nitrite in F 344/N rats and B6C3F1 mice
(drinking water studies). National Toxicology Program Technical Report Series 495.
NTP. 2011. Report on carcinogens. Research Triangle Park, NC: U.S. Department of Health and
Human Services, Public Health Service, National Toxicology Program. http://ntp-
server.niehs.nih.gov/ntp/roc/twelfth/roc12.pdf. January 08, 2014.
Nyakas C, Buwalda B, Kramers RJ, et al. 1994. Postnatal development of hippocampal and neocortical
cholinergic and serotonergic innervation in rat: Effects of nitrite-induced prenatal hypoxia and
nimodipine treatment. Neuroscience 59(3):541-559.
Nyakas C, Markel E, Bohus B, et al. 1990. Protective effect of the calcium antagonist nimodipine on
discrimination learning deficits and impaired retention behavior caused by prenatal nitrite exposure in
rats. Behav Brain Res 38(1):69-76.
Ohshima H, Bartsch H. 1988. Urinary N-nitrosamino acids as an index of exposure to N-nitroso
compounds. In: Bartsch H, Hemminki K, O'Neil IK, eds. Methods for detecting DNA damaging agents
in humans: Applications in cancer epidemiology and prevention. Vol. 89. International Agency for
Research on Cancer Institute of Occupational Health, Finland Commission of the European Communities,
83-91.
Oka K, Betto K, Nishimori I. 1974. Development of sarcomata in the livers of albino rats given sodium
nitrite and dimethylamine. (Report 1). Acta Med Nagasaki 18:13-25.
261 NITRATE AND NITRITE
9. REFERENCES
Oliveira CP, Gloria MB, Barbour JF, et al. 1995. Nitrate, nitrite, and volatile nitrosamines in whey-
containing food products. J Agric Food Chem 43(4):967-969.
Olsen P, Gry J, Knudsen I, et al. 1984. Animal feeding study with nitrite-treated meat. International
Agency for Research on Cancer. IARC Sci Publ 57:667-676.
Oms MT, Cerda A, Cerda V. 1995. Sequential injection analysis of nitrites and nitrates. Anal Chim
Acta 315(3):321-330.
Ormerod AD, Shah AAJ, Li H, et al. 2011. An observational prospective study of topical acidified nitrite
for killing methicillin-resistant Staphylococcus aureus (MRSA) in contaminated wounds. BioMed
Central. BMC Res Notes 4(1):1-7. http://dx.doi.org/10.1186/1756-0500-4-458. July 9, 2014.
OSHA. 2013a. List of highly hazardous chemicals, toxics, and reactives. Occupational safety and health
standards. Occupational Safety and Health Administration. Code of Federal Regulations 29 CFR
1910.119, Appendix A. http://www.osha.gov/law-regs.html. January 08, 2014.
OSHA. 2013b. Toxic and hazardous substances. Occupational safety and health standards.
Occupational Safety and Health Administration. Code of Federal Regulations 29 CFR 1910.1000, Table
Z-1. http://www.osha.gov/law-regs.html. January 08, 2014.
Owen GM, Brozek J. 1966. Influence of age, sex and nutrition on body composition during childhood
and adolescence. In: Falkner F, ed. Human development. Philadelphia, PA: WB Saunders, 222-238.
Owens LB, Edwards WM, Van Keuren RW. 1994. Groundwater nitrate levels under fertilized grass and
grass-legume pastures. J Environ Qual 23(4):752-758.
Palli D, Russo A, Decarli A. 2001. Dietary patterns, nutrient intake and gastric cancer in a high-risk area
of Italy. Cancer Causes Control 12(2):163-172.
Parslow RC, McKinney PA, Law GR, et al. 1997. Incidence of childhood diabetes mellitus in Yorkshire,
northern England, is associated with nitrate in drinking water: An ecological analysis. Diabetologia
40(5):550-556.
Pavlov J, Attygalle AB. 2013. Direct detection of inorganic nitrate salts by ambient pressure helium-
plasma ionization mass spectrometry. Anal Chem 85(1):278-282.
Perez-Rodriguez ML, Bosch-Bosch N, Garcia-Mata M. 1996. Monitoring nitrite and nitrate residues in
frankfurters during processing and storage. Meat Sci 44(1-2):65-73.
Petersen A, Stoltze S. 1999. Nitrate and nitrite in vegetables on the Danish market: Content and intake.
Food Addit Contam 16(7):291-299.
Pfenning KS, McMahon PB. 1997. Effect of nitrate, organic carbon, and temperature on potential
denitrification rates in nitrate-rich riverbed sediments. J Hydrol 187(3-4):283-295.
Pliss GB, Frolov AG. 1991. Sodium nitrite as possible promotor of carcinogenesis in the bladder of rats.
Vopr Onkol 37(2):203-206.
262 NITRATE AND NITRITE
9. REFERENCES
Pogoda JM, Preston-Martin S. 2001a. Maternal cured meat consumption during pregnancy and risk of
paediatric brain tumour in offspring: Potentially harmful levels of intake. Public Health Nutr 4(2):183-
189.
Pogoda JM, Preston-Martin S. 2001b. Letter to the editor: Maternal cured meat consumption during
pregnancy and risk of paediatric brain tumour in offspring: Potentially harmful levels of intake, authors'
response. Public Health Nutr 4:1304-1305.
Pokomy L, Maturana I, Bortle WH. 2006. Sodium nitrate and nitrite. In: Kirk-Othmer encyclopedia of
chemical technology. John Wiley & Sons, Inc. 10.1002/0471238961.1915040916151115.a01.pub2.
Power GG, Bragg SL, Oshiro BT, et al. 2007. A novel method of measuring reduction of nitrite-induced
methemoglobin applied to fetal and adult blood of humans and sheep. J Appl Physiol 103:1359-1365.
Preston-Martin S, Pogoda JM, Mueller BA, et al. 1996. Maternal consumption of cured meats and
vitamins in relation to pediatric brain tumors. Cancer Epidemiol Biomarkers Prev 5(8):599-605.
Qin L, Liu X, Sun Q, et al. 2012. Sialin (SLC17A5) functions as a nitrate transporter in the plasma
membrane. Proc Natl Acad Sci USA. 109(33):13434-13439.
Rádiková Z, Tajtakova M, Kocan A, et al. 2008. Possible effects of environmental nitrates and toxic
organochlorines on human thyroid in highly polluted areas in Slovakia. Thyroid 18(3):353-362.
Rafnsson V, Gunnarsdottir H. 1990. Mortality study of fertiliser manufacturers in Iceland. Br J Ind Med
47(11):721-725.
RePORTER. 2014. Ammonium nitrate, nitrates, potassium nitrate, sodium nitrate, sodium nitrite.
Research Portfolio Online Reporting Tools (RePORT). National Institutes of Health, U.S. Department of
Health and Human Services. http://projectreporter.nih.gov/reporter.cfm. April 7, 2014.
Rijhsinghani KS, Abrahams C, Krakower C, et al. 1982. Tumor induction in C57BL X C3HF1 mice
following single oral administration of diethylamine hydrochloride (DEA . HCl) and sodium nitrite
(NaNO2). Cancer Detect Prev 5(3):283-290.
Ringling S, Boo T, Bottei E. 2003. Methemoglobinemia from nitrite-contaminated punch. J Toxicol
Clin Toxicol 41(5):730-731.
Risch HA, Jain M, Choi NW, et al. 1985. Dietary factors and the incidence of cancer of the stomach.
Am J Epidemiol 122(6):947-959.
RIVM. 1996. On the etiology of nitrite-induced hypertrophy of the zona glomerulosa of rats: II. The
possible role of feed. National Institute for Public Health and the Environment. RIVM Report
235802004.
http://www.rivm.nl/en/Documents_and_publications/Scientific/Reports/1996/juli/On_the_etiology_of_nit
rite_induced_hypertrophy_of_the_zona_glomerulosa_of_rats_II_The_possible_role_of_feed?sp=cml2bX
E9ZmFsc2U7c2VhcmNoYmFzZT0yMjkwMDtyaXZtcT1mYWxzZTs=&pagenr=2291. July 8, 2015.
Rix PJ, Vick A, Attkins NJ, et al. 2015. Pharmacokinetics, pharmacodynamics, safety, and tolerability of
nebulized sodium nitrite (AIR001) following repeat-dose inhalation in healthy subjects. Clin
Pharmacokinet 54(3):261-272. 10.1007/s40262-014-0201-y.
263 NITRATE AND NITRITE
9. REFERENCES
Robbiano L, Carlo P, Finollo R, et al. 1990. DNA damage induced in rats by oral administration of
chlordiazepoxide plus sodium nitrite or of N-nitrosochlordiazepoxide. Toxicol Appl Pharmacol
102(1):186-190.
Robertson HE, Riddell WA. 1949. Cyanosis of infants produced by high nitrate concentration in rural
waters of Saskatchewan. Can J Public Health 40(2):72-77.
Rodkey FL. 1976. A mechanism for the conversion of oxyhemoglobin to methemoglobin by nitrite.
Clin Chem 22(12):1986-1990.
Rogers MA, Vaughan TL, Davis S, et al. 1995. Consumption of nitrate, nitrite, and
nitrosodimethylamine and the risk of upper aerodigestive tract cancer. Cancer Epidemiol Biomarkers
Prev 4(1):29-36.
RTECS. 2014. Ammonium nitrate, sodium nitrate, potassium nitrite, potassium nitrate and nitrous acid,
sodium salt. Registry of Toxic Effects on Chemical Substances. National Institute of Occupational
Safety and Health. MDL Information Systems, Inc. July 8, 2014.
Rustia M, Shubik P. 1974. Prenatal induction of neurogenic tumors in hamsters by precursors ethylurea
and sodium nitrite. J Natl Cancer Inst 52(2):605-608.
Sackner MA, Dougherty RD, Chapman GA, et al. 1979. Effects of sodium nitrate aerosol on
cardiopulmonary function of dogs, sheep and man. Environ Res 18(2):421-436.
Sadeq M, Moe CL, Attarassi B, et al. 2008. Drinking water nitrate and prevalence of
methemoglobinemia among infants and children aged 1-7 years in Moroccan areas. Int J Hyg Environ
Health 211(5-6):546-554.
Saito T, Takeichi S, Osawa M, et al. 2000. A case of fatal methemoglobinemia of unknown origin but
presumably due to ingestion of nitrate. Int J Legal Med 113(3):164-167.
http://dx.doi.org/10.1007/s004140050290. May 23, 2014.
Sanchez-Echaniz J, Benito-Fernandez J, Mintegui-Raso S. 2001. Methemoglobinemia and consumption
of vegetables in infants. Pediatrics 107(5):1024-1028.
Sandhu MS, White IR, McPherson K. 2001. Systematic review of the prospective cohort studies on meat
consumption and colorectal cancer risk: A meta-analytical approach. Cancer Epidemiol Biomarkers Prev
10(5):439-446.
Saunders NR, Ek CJ, Habgood MD, et al. 2008. Barriers in the brain: A renaissance? Trends Neurosci
31(6):279-286.
Saunders NR, Liddelow SA, Dziegielewska KM. 2012. Barrier mechanisms in the developing brain.
Front Pharmacol 3(10.3389/fphar.2012.00046):Article 46.
Schepers JS, Moravek MG, Alberts EE, et al. 1991. Maize production impacts on groundwater quality.
J Environ Qual 20(1):12-16.
Scherer-Lorenzen M, Palmborg C, Prinz A, et al. 2003. The role of plant diversity and composition for
nitrate in leaching in grasslands. Ecology 84(6):1539-1552.
264 NITRATE AND NITRITE
9. REFERENCES
Scheunig G, Horn KH, Mehnert WH. 1979. [Induction of tumors in Wistar-rats after oral application of
aminopyrine and nitrite (author's translation)]. Arch Geschwulstforsch 49(3):220-228.
Scheuplein R, Charnley G, Dourson M. 2002. Differential sensitivity of children and adults to chemical
toxicity. I. Biological basis. Regul Toxicol Pharmacol 35(3):429-447.
Schmitz JT. 1961. Methemoglobinemia-a cause of abortions? Preliminary report. Obstet Gynecol
17:413-415.
Schultz DS, Deen WM, Karel SF, et al. 1985. Pharmacokinetics of nitrate in humans: Role of
gastrointestinal absorption and metabolism. Carcinogenesis 6(6):847-852.
Scragg RK, Dorsch MM, McMichael AJ, et al. 1982. Birth defects and household water supply.
Epidemiological studies in the Mount Gambier region of South Australia. Med J Aust 2(12):577-579.
Seifert SA. 2004. Nitrates and nitrites. In: Dart RC, ed. Medical toxicology. 3rd ed. Philadelphia, PA:
Lippincott Williams & Williams, 1174-1180.
Sevier JN, Berbatis CG. 1976. Accidental sodium nitrite ingestion. Med J Aust 1(May):847.
Shaffer MJ, Wylie BK, Hall MD. 1995. Identification and mitigation of nitrite leaching hot spots using
NLEAP-GIS technology. J Contam Hydrol 20:253-263.
Shank RC, Newberne PM. 1976. Dose-response study of the carcinogenicity of dietary sodium nitrite
and morpholine in rats and hamsters. Food Cosmet Toxicol 14(1):1-8.
*Sharma MK, Sharma H, Bapna N. 2011. A study of toxicological effects of high nitrate ingestion in
rabbits. Journal of Biomedical Sciences and Research 3(3):439-443.
*Sharma MK, Sharma H, Bapna N. 2013. Histopathological changes in the liver of rabbits exposed to
high nitrate ingestion in drinking water. J Clin Diagn Res 7(8):1552-1554.
Sheehy MH, Way JL. 1974. Nitrite intoxication: Protection with methylene blue and oxygen. Toxicol
Appl Pharmacol 30(Nov):221-226.
Shimada T. 1989. Lack of teratogenic and mutagenic effects of nitrite on mouse fetuses. Arch Environ
Health 44(1):59-63.
Shuval HI, Gruener N. 1972. Epidemiological and toxicological aspects of nitrates and nitrites in the
environment. Am J Public Health 62(8):1045-1052.
Sierra R, Chinnock A, Ohshima H, et al. 1993. In vivo nitrosoproline formation and other risk factors in
Costa Rican children from high- and low-risk areas for gastric cancer. Cancer Epidemiol Biomarkers
Prev 2(6):563-568.
Siervo M, Lara J, Ogbonmwan I, et al. 2013. Inorganic nitrate and beetroot juice supplementation
reduces blood pressure in adults: A systematic review and meta-analysis. J Nutr 143(6):818-826.
Simon C, Manzke H, Kay H, et al. 1964. Occurrence, pathogenesis, and possible prophylaxis of nitrite
induced methemoglobinemia. Z Kinderheilkd 91:124-138.
265 NITRATE AND NITRITE
9. REFERENCES
Sleight SD, Atallah OA. 1968. Reproduction in the guinea pig as affected by chronic administration of
potassium nitrate and potassium nitrite. Toxicol Appl Pharmacol 12:179-185.
Small H, Stevens TS, Bauman WC. 1975. Novel ion exchange chromatographic method using
conductimetric detection. Anal Chem 47:1801-1809.
*Smyth HF, Jr, Carpenter CP, Weil CS, et al. 1969. Range-finding toxicity data: List VII. Am Ind Hyg
Assoc J. 30(5):470-476.
SRI. 2011. Sodium nitrate, sodium nitrite and potassium nitrite. In: 2011 Directory of chemical
producers. Menlo Park, CA: SRI Consulting, 816, 849.
Stafford GE. 1947. Methemoglobinemia in infants from water containing high concentrations of nitrates.
Nebr State Med J 32(10):392-394.
Steinhorn RH. 2008. Evaluation and management of the cyanotic neonate. Clin Pediatr Emerg Med
9(3):169-175.
Sun J, Zhang X, Broderick M, et al. 2003. Measurement of nitric oxide production in biological systems
by using Griess Reaction assay. Sensors 3(8):276-284. http://www.mdpi.com/1424-8220/3/8/276. May
24, 2014.
Super M, Heese HdV, MacKenzie D, et al. 1981. An epidemiological study of well-water nitrates in a
group of south west African/Namibian infants. Water Res 15:1265-1270.
Tabacova S, Baird DD, Balabaeva L. 1998. Exposure to oxidized nitrogen: Lipid peroxidation and
neonatal health risk. Arch Environ Health 53(3):214-221.
Tabacova S, Balabaeva L, Little RE. 1997. Maternal exposure to exogenous nitrogen compounds and
complications of pregnancy. Arch Environ Health 52(5):341-347.
Tahira T, Ohgaki H, Wakabayashi K, et al. 1988. The inhibitory effect of thioproline on carcinogenesis
induced by n-benzylmethylamine and nitrite. Food Chem Toxicol 26(6):511-516.
Tajtáková M, Semanova Z, Tomkova Z, et al. 2006. Increased thyroid volume and frequency of thyroid
disorders signs in schoolchildren from nitrate polluted area. Chemosphere 62(4):559-564.
Taylor AW. 2004. Fertilizers. In: Kirk-Othmer encyclopedia of chemical toxicology.
http://onlinelibrary.wiley.com/doi/10.1002/0471238961.0605182008150606.a01.pub2/abstract. May 21,
2014.
Taylor HW, Lijinsky W. 1975a. Tumor induction in rats by feeding aminopyrine or oxytetracycline with
nitrite. Int J Cancer 16(2):211-215.
Taylor HW, Lijinsky W. 1975b. Tumor induction in rats by feeding heptamethyleneimine and nitrite in
water. Cancer Res 35(3):812-815.
Ten Brink WA, Wiezer JH, Luijpen AF, et al. 1982. Nitrite poisoning caused by food contaminated with
cooling fluid. J Toxicol Clin Toxicol 19(2):139-147.
266 NITRATE AND NITRITE
9. REFERENCES
TFI. 2014. History of U.S. fertilizer use. The Fertilizer Institute:
http://httpwww.tfi.org/sites/default/files/images/history_of_us_fert_use.pdf. November 17, 2014.
Thomas K, Colborn T. 1992. Organochlorine endocrine disruptors in human tissue. In: Colborn T,
Clement C, eds. Chemically induced alterations in sexual and functional development: The
wildlife/human connection. Princeton, NJ: Princeton Scientific Publishing, 365-394.
Tietz NW, ed. 1970. Fundamentals of clinical chemistry. Philadelphia, PA: W.B. Saunders Company,
665, 666, 729-731.
Til HP, Falke HE, Kuper CF, et al. 1988. Evaluation of the oral toxicity of potassium nitrite in a 13-
week drinking-water study in rats. Food Chem Toxicol 26(10):851-859.
Til HP, Kuper CF, Falke HE. 1997. Nitrite-induced adrenal effects in rats and the consequences for the
no-observed-effect level. Food Chem Toxicol 35(3-4):349-355.
Toernqvist M, Rannug U, Jonsson A, et al. 1983. Mutagenicity of methyl nitrite in Salmonella
typhimurium. Mutat Res 117:47-54.
TRI13. 2014. TRI explorer: Providing access to EPA’s toxics release inventory data. Washington, DC:
Office of Information Analysis and Access. Office of Environmental Information. U.S. Environmental
Protection Agency. Toxics Release Inventory. http://www.epa.gov/triexplorer/. November 17, 2014.
Tsezou A, Kitsiou-Tzeli S, Galla A, et al. 1996. High nitrate content in drinking water: Cytogenetic
effects in exposed children. Arch Environ Health 51(6):458-461.
Tsikas D. 2005. Methods of quantitative analysis of the nitric oxide metabolites nitrite and nitrate in
human biological fluids. Free Radic Res 39(8):797-815.
Tsuda H, Kato K. 1977. High rate of endoreduplications and chromosomal aberrations in hamster cells
treated with sodium nitrite in vitro. Mutat Res 56(1):69-74.
Tsuda H, Inui N, Takayama S. 1973. In vitro transformation of newborn hamster cells by sodium nitrite.
Biochem Biophys Res Commun 55:1117-1124.
Tsuda H, Kushi A, Yoshida D, et al. 1981. Chromosomal aberrations and sister-chromatid exchanges
induced by gaseous nitrogen dioxide in cultured Chinese hamster cells. Mutat Res 89(4):303-310.
Tsugane S, Tsuda M, Gey F, et al. 1992. Cross-sectional study with multiple measurements of biological
markers for assessing stomach cancer risks at the population level. Environ Health Perspect 98:207-210.
USDA. 2013. U.S. imports (1995-2012) and exports (1990-2012) of selected fertilizers, by country.
U.S. Department of Agriculture. http://www.ers.usda.gov/data-products/fertilizer-
importsexports/standard-tables.aspx. April 1, 2014.
USDA. 2014. Soil quality indicators. Soil nitrate. U.S. Department of Agriculture, Natural Resources
Conservation Service.
http://www.nrcs.usda.gov/wps/PA_NRCSConsumption/download?cid=stelprdb1243373&ext=pdf. July
10, 2014.
267 NITRATE AND NITRITE
9. REFERENCES
USGS. 2009. Nutrient trends in streams and rivers of the United States, 1993-2003. National Water-
Quality Assessment Program. Reston, VA: U.S. Geological Survey. U.S. Department of the Interior.
Scientific Investigations Report 2008-5202.
USGS. 2010a. The quality of our nation's water-nutrients in the nation's streams and groundwater, 1992-
2004. National Water-Quality Assessment Program. Reston, VA: U.S. Geological Survey. U.S.
Department of the Interior. Circular 1350.
USGS. 2010b. Nitrate loads and concentrations in surface-water base flow and shallow groundwater for
selected basins in the United States, water years 1990-2006. National Water-Quality Assessment
Program. Reston, VA: U.S. Geological Survey. U.S. Department of the Interior. Scientific
Investigations Report 2010-5098.
USGS. 2012. Nitrate-nitrogen concentrations in shallow ground water of the coastal plain of the
Albemarle-Pamlico drainage study units, North Carolina and Virginia. U.S. Geological Survey. U.S.
Department of the Interior. Fact Sheet 241-96. http://nc.water.usgs.gov/reports/fs24196/. May 20, 2014.
USGS. 2013a. Groundwater contributions of flow, nitrate, and dissolved organic carbon to the lower San
Joaquin River, California, 2006-08. Scientific Investigations Report 2013–5151.
http://pubs.usgs.gov/sir/2013/5151/pdf/sir2013-5151.pdf. September 5, 2016.
USGS. 2013b. Nitrate in the Mississippi River and its tributaries, 1980-2010: An update. National
Water-Quality Assessment Program. Reston, VA: U.S. Geological Survey. U.S. Department of the
Interior. Scientific Investigations Report 2013-5169.
Valentín-Blasini L, Blount BC, Delinsky A. 2007. Quantification of iodide and sodium-iodide symporter
inhibitors in human urine using ion chromatography tandem mass spectrometry. J Chromatogr A
1155(1):40-46.
van Logten MJ, den Tonkelaar EM, Kroes R, et al. 1972. Long-term experiment with canned meat
treated with sodium nitrite and glucono-delta-lactone in rats. Food Cosmet Toxicol 10(4):475-488.
van Maanen JM, Albering HJ, de Kok TM, et al. 2000. Does the risk of childhood diabetes mellitus
require revision of the guideline values for nitrate in drinking water? Environ Health Perspect
108(5):457-461.
van Maanen JM, van Geel AA, Kleinjans JC. 1996b. Modulation of nitrate-nitrite conversion in the oral
cavity. Cancer Detect Prev. 20(6):590-596.
van Maanen JMS, Welle IJ, Hageman G, et al. 1996a. Nitrate contamination of drinking water:
Relationship with HPRT variant frequency in lymphocyte DNA and urinary excretion of N-nitrosamines.
Environ Health Perspect 104(5):522-528.
Velthof GL, Lesschen JP, Webb J, et al. 2014. The impact of the Nitrates Directive on nitrogen
emissions from agriculture in the EU-27 during 2000-2008. Sci Total Environ 468-469:1225-1233.
Vieira I, Sonnier M, Cresteil T. 1996. Developmental expression of CYP2E1 in the human liver:
Hypermethylation control of gene expression during the neonatal period. Eur J Biochem 238(2):476-483.
Virtanen SM, Jaakkola L, Rasanen L, et al. 1994. Nitrate and nitrite intake and the risk for type 1
diabetes in Finnish children. Childhood Diabetes in Finland Study Group. Diabet Med 11(7):656-662.
268 NITRATE AND NITRITE
9. REFERENCES
Vitousek PM, Aber JD, Howarth RW, et al. 1997. Human alteration of the global nitrogen cycle:
Sources and consequences. Ecological Society of America. Ecol Appl 7(3):737-750.
http://www.esa.org/esa/?post_type=document&p=2770. 2014/07/08.
Vorhees CV, Butcher RE, Brunner RL, et al. 1984. Developmental toxicity and psychotoxicity of
sodium nitrite in rats. Food Chem Toxicol 22(1):1-6.
Wagner DA, Schultz DS, Deen WM, et al. 1983. Metabolic fate of an oral dose of
15
N-labeled nitrate in
humans: Effect of diet supplementation with ascorbic acid. Cancer Res 43(4):1921-1925.
Walker R. 1995. The conversion of nitrate into nitrite in several animal species and man. In: Health
aspects of nitrate and its metabolites (particularly nitrite). Proceedings of an international workshop,
Bilthoven (Netherlands) 8-10 November 1994. Strasbourg: Council of Europee Press, 115-123.
Walker R. 1996. The metabolism of dietary nitrites and nitrates. 658th Meeting of the Biochemical
Society, Liverpool, England, UK, April 16-19, 1996. Biochemical Society Transactions 24(3):780-785.
Walley T, Flanagan M. 1987. Nitrite-induced methaemoglobinaemia. Postgrad Med J 63(742):643-644.
Walton G. 1951. Survey of literature relating infant methemoglobinemia due to nitrate-contaminated
water. Am J Public Health 41:986-996.
Wang W, Fan Y, Xiong G, et al. 2012. Nitrate in drinking water and bladder cancer: A meta-analysis. J
Huazhong Univ Sci Technolog 32(6):912-918. 10.1007/s11596-012-1057-8.
Ward MH, Cantor KP, Riley D, et al. 2003. Nitrate in public water supplies and risk of bladder cancer.
Epidemiology 14(2):183-190. 10.1097/01.ede.0000050664.28048.df.
Ward MH, Cerhan JR, Colt JS, et al. 2006. Risk of non-Hodgkin lymphoma and nitrate and nitrite from
drinking water and diet. Epidemiology 17(4):375-382.
Ward MH, Heineman EF, Markin RS, et al. 2008. Adenocarcinoma of the stomach and esophagus and
drinking water and dietary sources of nitrate and nitrite. Int J Occup Environ Health 14(3):193-197.
Ward MH, Kilfoy BA, Weyer PJ, et al. 2010. Nitrate intake and the risk of thyroid cancer and thyroid
disease. Epidemiology 21(3):389-395.
Ward MH, Mark SD, Cantor KP, et al. 1996. Drinking water nitrate and the risk of non-Hodgkin's
lymphoma. Epidemiology 7(5):465-471.
Ward MH, Rusiecki JA, Lynch CF, et al. 2007. Nitrate in public water supplies and the risk of renal cell
carcinoma. Cancer Causes Control 18(10):1141-1151.
Wardak C, Grabarczyk M. 2016. Analytical application of solid contact ion-selective electrodes for
determination of copper and nitrate in various food products and drinking water. J Environ Sci Health B
51(8):519-524. 10.1080/03601234.2016.1170545.
Weisburger JH, Marquardt H, Hirota N, et al. 1980. Induction of cancer of the glandular stomach in rats
by an extract of nitrite-treated fish. J Natl Cancer Inst 64(1):163-167.
269 NITRATE AND NITRITE
9. REFERENCES
Weitzberg E, Lundberg JO. 2013. Novel aspects of dietary nitrate and human health. Annu Rev Nutr
33:129-159.
Weitzberg E, Hezel M, Lundberg JO. 2010. Nitrate-nitrite-nitric oxide pathway: Implications for
anesthesiology and intensive care. Anesthesiology 113(6):1460-1475.
West JR, Smith HW, Chasis H. 1948. Glomerular filtration rate, effective renal blood flow, and maximal
tubular excretory capacity in infancy. J Pediatr 32:10-18.
Weyer PJ, Cerhan JR, Kross BC, et al. 2001. Municipal drinking water nitrate level and cancer risk in
older women: The Iowa Women's Health Study. Epidemiology 12(3):327-338.
WHO. 1978. Nitrates, nitrites and N-nitroso compounds. Environmental Health Criteria 5. World
Health Organization. http://www.inchem.org/documents/ehc/ehc/ehc005.htm. July 8, 2014.
WHO. 2010. WHO guidelines for indoor air quality: Selected pollutants. Geneva, Switzerland: World
Health Organization. http://www.euro.who.int/__data/assets/pdf_file/0009/128169/e94535.pdf. January
08, 2014.
WHO. 2011a. Guidelines for drinking-water quality. Geneva, Switzerland: World Health Organization.
http://www.who.int/water_sanitation_health/publications/2011/dwq_guidelines/en/index.html. January
08, 2014.
WHO. 2011b. Nitrate and nitrite in drinking-water. Background document for development of WHO
Guidelines for Drinking-water Quality. Geneva, Switzerland: World Health Organization.
WHO/SDE/WSH/07.01/16/Rev/1.
http://www.who.int/entity/water_sanitation_health/dwq/chemicals/nitratenitrite_background.pdf.
December 18, 2013.
Widdowson EM, Dickerson JWT. 1964. Chemical composition of the body. In: Comar CL, Bronner F,
eds. Mineral metabolism: An advance treatise. Volume II: The elements Part A. New York, NY:
Academic Press, 1-247.
Wilkens LR, Kadir MM, Kolonel LN, et al. 1996. Risk factors for lower urinary tract cancer: The role
of total fluid consumption, nitrites and nitrosamines, and selected foods. Cancer Epidemiol Biomarkers
Prev 5(3):161-166.
Winchester PD, Huskins J, Ying J. 2009. Agrichemicals in surface water and birth defects in the United
States. Acta Paediatr 98:664-669.
Winton EF, Tardiff RG, McCabe LJ. 1971. Nitrate in drinking water. J Am Water Works Assoc
63(2):95-98.
Wu T, Wang Y, Ho S, et al. 2013. Plasma levels of nitrate and risk of prostate cancer: A prospective
study. Cancer Epidemiol Biomarkers Prev 22(7):1210-1218.
Wu Y, Chen J, Ohshima H, et al. 1993. Geographic association between urinary excretion of N-nitroso
compounds and oesophageal cancer mortality in China. Int J Cancer 54(5):713-719.
Xiang YY, Wang DY, Tanaka M, et al. 1995. Efficient and specific induction of esophageal tumors in
rats by precursors of N-nitrososarcosine ethyl ester. Pathol Int 45(6):415-421.
270 NITRATE AND NITRITE
9. REFERENCES
Yada H, Hirose M, Tamano S, et al. 2002. Effects of antioxidant 1-O-hexyl-2,3,5-
trimethylhydroquinone or ascorbic acid on carcinogenesis induced by administration of aminopyrine and
sodium nitrite in a rat multi-organ carcinogenesis model. Jpn J Cancer Res 93(12):1299-1307.
Yamamoto K, Nakajima A, Eimoto H, et al. 1989. Carcinogenic activity of endogenously synthesized n
nitrosobis-2-hydroxypropylamine in rats administered bis-2-hydroxypropylamine and sodium nitrite.
Carcinogenesis 10(9):1607-1612.
Yan H, Zhuo X, Shen B, et al. 2016. Determination of nitrite in whole blood by high-performance liquid
chromatography with electrochemical detection and a case of nitrite poisoning. J Forensic Sci 61(1):254-
258. 10.1111/1556-4029.12918.
Yang YJ, Hwang SH, Kim HJ. 2010. Dietary intake of nitrate relative to antioxidant vitamin in relation
to breast cancer risk: A case-control study. Nutr Cancer 62(5):555-566.
Yano SS, Danish EH, Hsia YE. 1982. Transient methemoglobinemia with acidosis in infants. J Pediatr
100(3):415-418.
Yoshida A, Harada T, Hayashi S, et al. 1994. Endometrial carcinogenesis induced by concurrent oral
administration of ethylenethiourea and sodium nitrite in mice. Carcinogenesis 15(10):2311-2318.
Yoshida A, Harada T, Maita K. 1993. Tumor induction by concurrent oral administration of
ethylenethiourea and sodium nitrite in mice. Toxicol Pathol 21(3):303-310.
Yoshizumi K, Aoki K, Matsuoka T, et al. 1985. Determination of nitrate by a flow system with a
chemiluminescent nitrogen oxides analyzer. Anal Chem 57(3):737-740.
Zaki A, Ait Chaoui A, Talibi A, et al. 2004. Impact of nitrate intake in drinking water on the thyroid
gland activity in male rat. Toxicol Lett 147(1):27-33.
Zeegers MP, Selen RF, Kleinjans JC, et al. 2006. Nitrate intake does not influence bladder cancer risk:
The Netherlands cohort study. Environ Health Perspect 114(10):1527-1531.
Zeiger E, Anderson B, Haworth S, et al. 1992. Salmonella mutagenicity tests: V. Results from the
testing of 311 chemicals. Environ Mol Mutagen 21:2-141.
Zeilmaker MJ, Bakker MI, Schothorst R, et al. 2010a. Risk assessment of N-nitrosodimethylamine
formed endogenously after fish-with-vegetable meals. Toxicol Sci 116(1):323-335.
http://toxsci.oxfordjournals.org/content/116/1/323.abstract. May 20, 2014.
Zeilmaker MJ, Meulenbelt J, Kortboyer JM, et al. 1996. Safety evaluation of nitrate: Mathematical
modeling of nitrite formation in man and its application in the risk assessment of nitrate. Bilthoven, The
Netherlands: National Institute of Public Health and the Environment. Report number 235802.002.
Zeilmaker MJ, Meulenbelt J, Slob W. 2010b. Supplementary Information A2-1. Mathematical model of
human nitrate/nitrite kinetics, including the formation of methemoglobin in the blood (Supplement to:
Toxicol Sci 116(1):323-335). Toxicol Sci.
http://toxsci.oxfordjournals.org/content/suppl/2010/03/23/kfq093.DC1/toxsci-09-0763-File002.doc. April
1, 2014.
271 NITRATE AND NITRITE
9. REFERENCES
Zeman CL, Kross B, Vlad M. 2002. A nested case-control study of methemoglobinemia risk factors in
children of Transylvania, Romania. Environ Health Perspect 110(8):817-822.
Zhang M, Geng S, Smallwood KS. 1998. Assessing groundwater nitrate contamination for resource and
landscape management. Ambio 27(3):170-174.
Zhang WL, Tian ZX, Zhang N, et al. 1996. Nitrate pollution of groundwater in northern China. Agric
Ecosyst Environ 59(3):223-231.
Zhang XL, Bing Z, Xing Z, et al. 2003. Research and control of well water pollution in high esophageal
cancer areas. World J Gastroenterol 9(6):1187-1190.
Zhao HX, Mold MD, Stenhouse EA, et al. 2001. Drinking water composition and childhood-onset type
1 diabetes mellitus in Devon and Cornwall, England. Diabet Med 18(9):709-717.
Zhou L, Anwar MM, Zahid M, et al. 2014. Urinary excretion of N-nitroso compounds in rats fed sodium
nitrite and/or hot dogs. Chem Res Toxicol 27(10):1669-1674. 10.1021/tx5000188.
Zhuang H, Chan CK, Fang M, et al. 1999. Size distributions of particulate sulfate, nitrate, and
ammonium at a coastal site in Hong Kong. Atmos Environ 33(6):843-853.
Ziegler EE, Edwards BB, Jensen RL, et al. 1978. Absorption and retention of lead by infants. Pediatr
Res 12(1):29-34.
272 NITRATE AND NITRITE
9. REFERENCES
This page is intentionally blank.
273 NITRATE AND NITRITE
10. GLOSSARY
Absorption—The taking up of liquids by solids, or of gases by solids or liquids.
Acute Exposure—Exposure to a chemical for a duration of 14 days or less, as specified in the
Toxicological Profiles.
Adsorption—The adhesion in an extremely thin layer of molecules (as of gases, solutes, or liquids) to the
surfaces of solid bodies or liquids with which they are in contact.
Adsorption Coefficient (K
oc
)—The ratio of the amount of a chemical adsorbed per unit weight of
organic carbon in the soil or sediment to the concentration of the chemical in solution at equilibrium.
Adsorption Ratio (Kd)—The amount of a chemical adsorbed by sediment or soil (i.e., the solid phase)
divided by the amount of chemical in the solution phase, which is in equilibrium with the solid phase, at a
fixed solid/solution ratio. It is generally expressed in micrograms of chemical sorbed per gram of soil or
sediment.
Benchmark Dose (BMD)—Usually defined as the lower confidence limit on the dose that produces a
specified magnitude of changes in a specified adverse response. For example, a BMD
10
would be the
dose at the 95% lower confidence limit on a 10% response, and the benchmark response (BMR) would be
10%. The BMD is determined by modeling the dose response curve in the region of the dose response
relationship where biologically observable data are feasible.
Benchmark Dose Model—A statistical dose-response model applied to either experimental toxicological
or epidemiological data to calculate a BMD.
Bioconcentration Factor (BCF)—The quotient of the concentration of a chemical in aquatic organisms
at a specific time or during a discrete time period of exposure divided by the concentration in the
surrounding water at the same time or during the same period.
Biomarkers—Broadly defined as indicators signaling events in biologic systems or samples. They have
been classified as markers of exposure, markers of effect, and markers of susceptibility.
Cancer Effect Level (CEL)—The lowest dose of chemical in a study, or group of studies, that produces
significant increases in the incidence of cancer (or tumors) between the exposed population and its
appropriate control.
Carcinogen—A chemical capable of inducing cancer.
Case-Control Study— A type of epidemiological study that examines the relationship between a
particular outcome (disease or condition) and a variety of potential causative agents (such as toxic
chemicals). In a case-control study, a group of people with a specified and well-defined outcome is
identified and compared to a similar group of people without the outcome.
Case Report—Describes a single individual with a particular disease or exposure. These may suggest
some potential topics for scientific research, but are not actual research studies.
Case Series—Describes the experience of a small number of individuals with the same disease or
exposure. These may suggest potential topics for scientific research, but are not actual research studies.
274 NITRATE AND NITRITE
10. GLOSSARY
Ceiling Value—A concentration that must not be exceeded.
Chronic Exposure—Exposure to a chemical for 365 days or more, as specified in the Toxicological
Profiles.
Cohort Study—A type of epidemiological study of a specific group or groups of people who have had a
common insult (e.g., exposure to an agent suspected of causing disease or a common disease) and are
followed forward from exposure to outcome. At least one exposed group is compared to one unexposed
group.
Cross-sectional Study—A type of epidemiological study of a group or groups of people that examines
the relationship between exposure and outcome to a chemical or to chemicals at one point in time.
Data Needs—Substance-specific informational needs that, if met, would reduce the uncertainties of
human health risk assessment.
Developmental Toxicity—The occurrence of adverse effects on the developing organism that may result
from exposure to a chemical prior to conception (either parent), during prenatal development, or
postnatally to the time of sexual maturation. Adverse developmental effects may be detected at any point
in the life span of the organism.
Dose-Response Relationship—The quantitative relationship between the amount of exposure to a
toxicant and the incidence of the adverse effects.
Embryotoxicity and Fetotoxicity—Any toxic effect on the conceptus as a result of prenatal exposure to
a chemical; the distinguishing feature between the two terms is the stage of development during which the
insult occurs. The terms, as used here, include malformations and variations, altered growth, and in utero
death.
Environmental Protection Agency (EPA) Health Advisory—An estimate of acceptable drinking water
levels for a chemical substance based on health effects information. A health advisory is not a legally
enforceable federal standard, but serves as technical guidance to assist federal, state, and local officials.
Epidemiology—Refers to the investigation of factors that determine the frequency and distribution of
disease or other health-related conditions within a defined human population during a specified period.
Genotoxicity—A specific adverse effect on the genome of living cells that, upon the duplication of
affected cells, can be expressed as a mutagenic, clastogenic, or carcinogenic event because of specific
alteration of the molecular structure of the genome.
Half-life—A measure of rate for the time required to eliminate one half of a quantity of a chemical from
the body or environmental media.
Immediately Dangerous to Life or Health (IDLH)—A condition that poses a threat of life or health, or
conditions that pose an immediate threat of severe exposure to contaminants that are likely to have
adverse cumulative or delayed effects on health.
Immunologic Toxicity—The occurrence of adverse effects on the immune system that may result from
exposure to environmental agents such as chemicals.
275 NITRATE AND NITRITE
10. GLOSSARY
Immunological Effects—Functional changes in the immune response.
Incidence—The ratio of new cases of individuals in a population who develop a specified condition to
the total number of individuals in that population who could have developed that condition in a specified
time period.
Intermediate Exposure—Exposure to a chemical for a duration of 15–364 days, as specified in the
Toxicological Profiles.
In Vitro—Isolated from the living organism and artificially maintained, as in a test tube.
In Vivo—Occurring within the living organism.
Lethal Concentration
(LO)
(LC
LO
)—The lowest concentration of a chemical in air that has been reported
to have caused death in humans or animals.
Lethal Concentration
(50)
(LC
50
)—A calculated concentration of a chemical in air to which exposure for
a specific length of time is expected to cause death in 50% of a defined experimental animal population.
Lethal Dose
(LO)
(LD
Lo
)—The lowest dose of a chemical introduced by a route other than inhalation that
has been reported to have caused death in humans or animals.
Lethal Dose
(50)
(LD
50
)—The dose of a chemical that has been calculated to cause death in 50% of a
defined experimental animal population.
Lethal Time
(50)
(LT
50
)—A calculated period of time within which a specific concentration of a chemical
is expected to cause death in 50% of a defined experimental animal population.
Lowest-Observed-Adverse-Effect Level (LOAEL)—The lowest exposure level of chemical in a study,
or group of studies, that produces statistically or biologically significant increases in frequency or severity
of adverse effects between the exposed population and its appropriate control.
Lymphoreticular Effects—Represent morphological effects involving lymphatic tissues such as the
lymph nodes, spleen, and thymus.
Malformations—Permanent structural changes that may adversely affect survival, development, or
function.
Minimal Risk Level (MRL)—An estimate of daily human exposure to a hazardous substance that is
likely to be without an appreciable risk of adverse noncancer health effects over a specified route and
duration of exposure.
Modifying Factor (MF)—A value (greater than zero) that is applied to the derivation of a Minimal Risk
Level (MRL) to reflect additional concerns about the database that are not covered by the uncertainty
factors. The default value for a MF is 1.
Morbidity—State of being diseased; morbidity rate is the incidence or prevalence of disease in a specific
population.
Mortality—Death; mortality rate is a measure of the number of deaths in a population during a specified
interval of time.
276 NITRATE AND NITRITE
10. GLOSSARY
Mutagen—A substance that causes mutations. A mutation is a change in the DNA sequence of a cell’s
DNA. Mutations can lead to birth defects, miscarriages, or cancer.
Necropsy—The gross examination of the organs and tissues of a dead body to determine the cause of
death or pathological conditions.
Neurotoxicity—The occurrence of adverse effects on the nervous system following exposure to a
hazardous substance.
No-Observed-Adverse-Effect Level (NOAEL)—The dose of a chemical at which there were no
statistically or biologically significant increases in frequency or severity of adverse effects seen between
the exposed population and its appropriate control. Effects may be produced at this dose, but they are not
considered to be adverse.
Octanol-Water Partition Coefficient (K
ow
)—The equilibrium ratio of the concentrations of a chemical
in n-octanol and water, in dilute solution.
Odds Ratio (OR)—A means of measuring the association between an exposure (such as toxic substances
and a disease or condition) that represents the best estimate of relative risk (risk as a ratio of the incidence
among subjects exposed to a particular risk factor divided by the incidence among subjects who were not
exposed to the risk factor). An OR of greater than 1 is considered to indicate greater risk of disease in the
exposed group compared to the unexposed group.
Organophosphate or Organophosphorus Compound—A phosphorus-containing organic compound
and especially a pesticide that acts by inhibiting cholinesterase.
Permissible Exposure Limit (PEL)—An Occupational Safety and Health Administration (OSHA)
regulatory limit on the amount or concentration of a substance not to be exceeded in workplace air
averaged over any 8-hour work shift of a 40-hour workweek.
Pesticide—General classification of chemicals specifically developed and produced for use in the control
of agricultural and public health pests (insects or other organisms harmful to cultivated plants or animals).
Pharmacokinetics—The dynamic behavior of a material in the body, used to predict the fate
(disposition) of an exogenous substance in an organism. Utilizing computational techniques, it provides
the means of studying the absorption, distribution, metabolism, and excretion of chemicals by the body.
Pharmacokinetic Model—A set of equations that can be used to describe the time course of a parent
chemical or metabolite in an animal system. There are two types of pharmacokinetic models: data-based
and physiologically-based. A data-based model divides the animal system into a series of compartments,
which, in general, do not represent real, identifiable anatomic regions of the body, whereas the
physiologically-based model compartments represent real anatomic regions of the body.
Physiologically Based Pharmacodynamic (PBPD) Model—A type of physiologically based dose-
response model that quantitatively describes the relationship between target tissue dose and toxic end
points. These models advance the importance of physiologically based models in that they clearly
describe the biological effect (response) produced by the system following exposure to an exogenous
substance.
277 NITRATE AND NITRITE
10. GLOSSARY
Physiologically Based Pharmacokinetic (PBPK) Model—Comprised of a series of compartments
representing organs or tissue groups with realistic weights and blood flows. These models require a
variety of physiological information: tissue volumes, blood flow rates to tissues, cardiac output, alveolar
ventilation rates, and possibly membrane permeabilities. The models also utilize biochemical
information, such as blood:air partition coefficients, and metabolic parameters. PBPK models are also
called biologically based tissue dosimetry models.
Prevalence—The number of cases of a disease or condition in a population at one point in time.
Prospective Study—A type of cohort study in which the pertinent observations are made on events
occurring after the start of the study. A group is followed over time.
q
1
*—The upper-bound estimate of the low-dose slope of the dose-response curve as determined by the
multistage procedure. The q
1
* can be used to calculate an estimate of carcinogenic potency, the
incremental excess cancer risk per unit of exposure (usually μg/L for water, mg/kg/day for food, and
μg/m
3
for air).
Recommended Exposure Limit (REL)—A National Institute for Occupational Safety and Health
(NIOSH) time-weighted average (TWA) concentration for up to a 10-hour workday during a 40-hour
workweek.
Reference Concentration (RfC)—An estimate (with uncertainty spanning perhaps an order of
magnitude) of a continuous inhalation exposure to the human population (including sensitive subgroups)
that is likely to be without an appreciable risk of deleterious noncancer health effects during a lifetime.
The inhalation reference concentration is for continuous inhalation exposures and is appropriately
expressed in units of mg/m
3
or ppm.
Reference Dose (RfD)—An estimate (with uncertainty spanning perhaps an order of magnitude) of the
daily exposure of the human population to a potential hazard that is likely to be without risk of deleterious
effects during a lifetime. The RfD is operationally derived from the no-observed-adverse-effect level
(NOAEL, from animal and human studies) by a consistent application of uncertainty factors that reflect
various types of data used to estimate RfDs and an additional modifying factor, which is based on a
professional judgment of the entire database on the chemical. The RfDs are not applicable to
nonthreshold effects such as cancer.
Reportable Quantity (RQ)—The quantity of a hazardous substance that is considered reportable under
the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA). Reportable
quantities are (1) 1 pound or greater or (2) for selected substances, an amount established by regulation
either under CERCLA or under Section 311 of the Clean Water Act. Quantities are measured over a
24-hour period.
Reproductive Toxicity—The occurrence of adverse effects on the reproductive system that may result
from exposure to a hazardous substance. The toxicity may be directed to the reproductive organs and/or
the related endocrine system. The manifestation of such toxicity may be noted as alterations in sexual
behavior, fertility, pregnancy outcomes, or modifications in other functions that are dependent on the
integrity of this system.
Retrospective Study—A type of cohort study based on a group of persons known to have been exposed
at some time in the past. Data are collected from routinely recorded events, up to the time the study is
undertaken. Retrospective studies are limited to causal factors that can be ascertained from existing
records and/or examining survivors of the cohort.
278 NITRATE AND NITRITE
10. GLOSSARY
Risk—The possibility or chance that some adverse effect will result from a given exposure to a hazardous
substance.
Risk Factor—An aspect of personal behavior or lifestyle, an environmental exposure, existing health
condition, or an inborn or inherited characteristic that is associated with an increased occurrence of
disease or other health-related event or condition.
Risk Ratio—The ratio of the risk among persons with specific risk factors compared to the risk among
persons without risk factors. A risk ratio greater than 1 indicates greater risk of disease in the exposed
group compared to the unexposed group.
Short-Term Exposure Limit (STEL)—A STEL is a 15-minute TWA exposure that should not be
exceeded at any time during a workday.
Standardized Mortality Ratio (SMR)—A ratio of the observed number of deaths and the expected
number of deaths in a specific standard population.
Target Organ Toxicity—This term covers a broad range of adverse effects on target organs or
physiological systems (e.g., renal, cardiovascular) extending from those arising through a single limited
exposure to those assumed over a lifetime of exposure to a chemical.
Teratogen—A chemical that causes structural defects that affect the development of an organism.
Threshold Limit Value (TLV)—An American Conference of Governmental Industrial Hygienists
(ACGIH) concentration of a substance to which it is believed that nearly all workers may be repeatedly
exposed, day after day, for a working lifetime without adverse effect. The TLV may be expressed as a
Time Weighted Average (TLV-TWA), as a Short-Term Exposure Limit (TLV-STEL), or as a ceiling
limit (TLV-C).
Time-Weighted Average (TWA)—An average exposure within a given time period.
Toxic Dose
(50)
(TD
50
)—A calculated dose of a chemical, introduced by a route other than inhalation,
which is expected to cause a specific toxic effect in 50% of a defined experimental animal population.
Toxicokinetic—The absorption, distribution, and elimination of toxic compounds in the living organism.
Uncertainty Factor (UF)—A factor used in operationally deriving the Minimal Risk Level (MRL) or
Reference Dose (RfD) or Reference Concentration (RfC) from experimental data. UFs are intended to
account for (1) the variation in sensitivity among the members of the human population, (2) the
uncertainty in extrapolating animal data to the case of human, (3) the uncertainty in extrapolating from
data obtained in a study that is of less than lifetime exposure, and (4) the uncertainty in using lowest-
observed-adverse-effect level (LOAEL) data rather than no-observed-adverse-effect level (NOAEL) data.
A default for each individual UF is 10; if complete certainty in data exists, a value of 1 can be used;
however, a reduced UF of 3 may be used on a case-by-case basis, 3 being the approximate logarithmic
average of 10 and 1.
Xenobiotic—Any substance that is foreign to the biological system.
A-1 NITRATE AND NITRITE
APPENDIX A. ATSDR MINIMAL RISK LEVELS AND WORKSHEETS
The Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA) [42 U.S.C.
9601 et seq.], as amended by the Superfund Amendments and Reauthorization Act (SARA) [Pub. L. 99–
499], requires that the Agency for Toxic Substances and Disease Registry (ATSDR) develop jointly with
the U.S. Environmental Protection Agency (EPA), in order of priority, a list of hazardous substances most
commonly found at facilities on the CERCLA National Priorities List (NPL); prepare toxicological
profiles for each substance included on the priority list of hazardous substances; and assure the initiation
of a research program to fill identified data needs associated with the substances.
The toxicological profiles include an examination, summary, and interpretation of available toxicological
information and epidemiologic evaluations of a hazardous substance. During the development of
toxicological profiles, Minimal Risk Levels (MRLs) are derived when reliable and sufficient data exist to
identify the target organ(s) of effect or the most sensitive health effect(s) for a specific duration for a
given route of exposure. An MRL is an estimate of the daily human exposure to a hazardous substance
that is likely to be without appreciable risk of adverse noncancer health effects over a specified route and
duration of exposure. MRLs are based on noncancer health effects only and are not based on a
consideration of cancer effects. These substance-specific estimates, which are intended to serve as
screening levels, are used by ATSDR health assessors to identify contaminants and potential health
effects that may be of concern at hazardous waste sites. It is important to note that MRLs are not
intended to define clean-up or action levels.
MRLs are derived for hazardous substances using the no-observed-adverse-effect level/uncertainty factor
approach. They are below levels that might cause adverse health effects in the people most sensitive to
such chemical-induced effects. MRLs are derived for acute (1–14 days), intermediate (15–364 days), and
chronic (365 days and longer) durations and for the oral and inhalation routes of exposure. Currently,
MRLs for the dermal route of exposure are not derived because ATSDR has not yet identified a method
suitable for this route of exposure. MRLs are generally based on the most sensitive substance-induced
endpoint considered to be of relevance to humans. Serious health effects (such as irreparable damage to
the liver or kidneys, or birth defects) are not used as a basis for establishing MRLs. Exposure to a level
above the MRL does not mean that adverse health effects will occur.
MRLs are intended only to serve as a screening tool to help public health professionals decide where to
look more closely. They may also be viewed as a mechanism to identify those hazardous waste sites that
A-2 NITRATE AND NITRITE
APPENDIX A
are not expected to cause adverse health effects. Most MRLs contain a degree of uncertainty because of
the lack of precise toxicological information on the people who might be most sensitive (e.g., infants,
elderly, nutritionally or immunologically compromised) to the effects of hazardous substances. ATSDR
uses a conservative (i.e., protective) approach to address this uncertainty consistent with the public health
principle of prevention. Although human data are preferred, MRLs often must be based on animal studies
because relevant human studies are lacking. In the absence of evidence to the contrary, ATSDR assumes
that humans are more sensitive to the effects of hazardous substance than animals and that certain persons
may be particularly sensitive. Thus, the resulting MRL may be as much as 100-fold below levels that
have been shown to be nontoxic in laboratory animals.
Proposed MRLs undergo a rigorous review process: Health Effects/MRL Workgroup reviews within the
Division of Toxicology and Human Health Sciences, expert panel peer reviews, and agency-wide MRL
Workgroup reviews, with participation from other federal agencies and comments from the public. They
are subject to change as new information becomes available concomitant with updating the toxicological
profiles. Thus, MRLs in the most recent toxicological profiles supersede previously published levels.
For additional information regarding MRLs, please contact the Division of Toxicology and Human
Health Sciences, Agency for Toxic Substances and Disease Registry, 1600 Clifton Road NE, Mailstop
F-57, Atlanta, Georgia 30329-4027.

A-3 NITRATE AND NITRITE
APPENDIX A
MINIMAL RISK LEVEL (MRL) WORKSHEET
Chemical Name: Nitrate
CAS Numbers: 14797-55-8
Date: July 2017
Profile Status: Final
Route: [ ] Inhalation [x] Oral
Duration: [x] Acute [x] Intermediate [x] Chronic
Graph Key: 3 (Acute), 15 (Intermediate), 53 (Chronic)
Species: Human
Minimal Risk Level: 4 [x] mg/kg/day [ ] ppm
Reference: Walton G. 1951. Survey of literature relating to infant methemoglobinemia due to nitrate-
contaminated water. Am J Public Health 41:986-996.
Experimental design: Walton (1951) reviewed available literature and found 278 reported cases of infant
methemoglobinemia in a total of 14 U.S. states from which information was available. Cases were
grouped by state according to ranges of nitrate levels in drinking water sources.
Effect noted in study and corresponding doses: Among methemoglobinemia cases for which nitrate
levels in water sources used to prepare infant formula were available, 173 cases were associated with
>50 mg nitrate-nitrogen/L (220 mg nitrate/L), 36 cases with 21–50 mg nitrate-nitrogen/L (92–220 mg
nitrate/L), and 5 cases with 11–20 mg nitrate-nitrogen (48–88 mg nitrate/L). None of the
methemoglobinemia cases were associated with drinking water sources measuring <10 mg nitrate-
nitrogen/L (<44 mg nitrate/L). Limitations of the contributing studies include lack of information
regarding the actual ages of the infants, total nitrate doses, and other water source contaminants (e.g.,
bacterial levels).
Following ingestion of relatively large amounts of nitrate by healthy normal individuals, blood
methemoglobin levels increase rapidly, followed by a return to normal within several hours following
intake. Repeated ingestion for intermediate- or chronic-duration time periods would be expected to result
in changes in methemoglobin levels similar to those elicited from a single exposure. Therefore, the
acute-, intermediate-, and chronic-duration oral MRL values are equivalent.
Dose and end point used for MRL derivation: 4.33 mg nitrate/kg/day
[x] NOAEL [ ] LOAEL
Uncertainty Factors used in MRL derivation:
[ ] 10 for use of a LOAEL
[ ] 10 for extrapolation from animals to humans
[x] 1 for human variability
A total uncertainty factor of 1 is justified because the point of departure is a NOAEL for nitrate-induced
effects on methemoglobin in a particularly sensitive human subpopulation (i.e., <3-month-old infants,
which in many cases may have been at increased risk of methemoglobinemia due to microbial
contamination and associated gastrointestinal infection).

A-4 NITRATE AND NITRITE
APPENDIX A
Was a conversion factor used from ppm in food or water to a mg/body weight dose? Nitrate may be
expressed in terms of ionic concentration (i.e., mg nitrate/L), or elemental concentration (i.e., mg nitrate-
nitrogen/L or mg nitrogen as nitrate/L). A concentration of nitrate expressed in elemental concentration
(mg nitrogen per liter from nitrate source) can be converted to its ionic concentration (mg NO
3
-
)
according to the following relationship: 1 mg nitrate-nitrogen = 4.4 mg nitrate (i.e., the proportion of N
-
in NO
3
is 14 [atomic mass of N] ÷ 62 [molecular mass of NO
3
-
] = 0.226).
Table A-1 presents estimated nitrate doses to infants (birth–<3 months of age) calculated using estimated
mean values for drinking water ingestion rates (Kahn and Stralka 2009) and body weight (EPA 2008) and
assuming a drinking water level of 44 mg nitrate/L as a concentration not expected to cause
methemoglobinemia; the calculated doses of 4.31–4.34 mg nitrate/kg/day represent NOAELs for the age
ranges. The TWA-based calculated dose of 4.33 mg nitrate/kg/day for the age range of birth–<3 months
is selected as the point of departure for deriving acute-, intermediate-, and chronic-duration oral MRLs for
nitrate.
Table A-1. Estimated Nitrate Dose to Infants of Selected Age Ranges Assuming a
Drinking Water Level of 44 mg Nitrate/L
a
Age range
Water intake (L/day)
b
Body weight (kg)
c
Nitrate dose (mg/kg/day)
d
Birth–<1 month
0.470
4.8
4.31
1–<3 months
0.552
5.6
4.34
Birth–<3 months
0.525
e
5.33
e
4.33
a
Considered a no-adverse-effect concentration for nitrate intake by infants up to 6 months of age, based on weight-
of-evidence analysis of available human data.
b
Estimated mean water intake (combined direct intake [ingested largely as a beverage] and indirect intake [added in
preparation of food or beverages]) from community water; data from Table 3-14 of EPA (2008) and Table 2 of Kahn
and Stralka (2009).
c
Estimated mean body weight; data from Table 8-1 of EPA (2008).
d
Nitrate dose = 44 mg nitrate/L (NOAEL) x water intake (L/day) / body weight (kg).
e
Calculated TWA for birth–<1 month and 1–<3 months (e.g., TWA water intake for birth–<3 months = (0.470 L/day x
1 month) + (0.552 L/day x 2 months)/3 months = 0.525 L/day.
EPA = Environmental Protection Agency; NOAEL = no-observed-adverse-effect level; TWA = time-weighted average
If an inhalation study in animals, list conversion factors used in determining human equivalent dose: Not
applicable.
Was a conversion used from intermittent to continuous exposure? Not applicable.
Other additional studies or pertinent information that lend support to this MRL: Methemoglobinemia is a
condition in which increased methemoglobin as a percentage of total hemoglobin results in the expression
of clinical signs that increase in severity with increasing percent methemoglobin (ATSDR 2013a; Bloom
et al. 2013; Denshaw-Burke et al. 2013; Haymond et al. 2005). In normal healthy individuals,
methemoglobin levels are <1% of total hemoglobin. Discoloration (e.g., pale, gray blue) of the skin is
often observed at methemoglobin levels in the range of 3–15%; most patients tolerate methemoglobin
levels <10%. Tachycardia, weakness, and other signs of tissue hypoxia may be observed at 10–20%
methemoglobin levels. Effects on the central nervous system (e.g., headache, dizziness, fatigue) and
dyspnea and nausea appear at >20% methemoglobin; the severity of symptoms increases with increasing
methemoglobin level. High risk of mortality occurs at levels >70% methemoglobin.
Proposed explanations for increased susceptibility of infants to methemoglobinemia following ingestion
of nitrate include: (1) increased reduction of nitrate to nitrite in the newborn, (2) increased tendency for
A-5 NITRATE AND NITRITE
APPENDIX A
nitrite-induced methemoglobin formation by fetal hemoglobin compared to adult hemoglobin, (3) lower
levels of NADH-dependent methemoglobin reductase (the major enzyme responsible for reduction of
methemoglobin to normal hemoglobin; also termed NADH-diaphorase, a soluble form of cytochrome-b5
reductase) in the newborn compared to older infants and adults, and (4) incompletely developed hepatic
microsomal enzyme system in the infant and consequent lower rate of hepatic reduction of circulating
nitrite compared to that of older children and adults. A portion of ingested nitrate is reduced to nitrite by
commensal bacteria in the mouth; however, the acid environment of the normal stomach does not support
the growth of such bacteria and most of the nitrate that reaches the stomach passes to the small intestine
from which it is nearly completely absorbed into the blood. Although Kanady et al. (2012) reported little
or no bacterial conversion of nitrate to nitrite in the saliva of a group of 10 infants during the first
2 postnatal months (considered mainly due to lower numbers of major nitrate-reducing oral bacteria than
adults), a higher pH in the stomach of the newborn may favor growth of nitrate-reducing bacteria,
resulting in increased reduction of nitrate to nitrite and increased plasma methemoglobin. Most
hemoglobin in the newborn is in the form of fetal hemoglobin, which appears to be more readily oxidized
to methemoglobin than adult hemoglobin; fetal hemoglobin is replaced by adult hemoglobin during early
postnatal life. Levels of NADH-dependent methemoglobin reductase in the newborn increase
approximately 2-fold during the first 4 months of postnatal life to reach adult levels. During the period of
relatively lower methemoglobin reductase levels, methemoglobin would not be expected to be as readily
reduced, resulting in increased susceptibility to methemoglobinemia. In apparent contrast, Ibrahim et al.
(2012) reported that blood nitrite levels in newborns approximately 1–2 days of age were 35–55% lower
than that of adults. However, one study that evaluated reduction rates of methemoglobin in human adult
blood and cord blood from term newborns estimated methemoglobin half-lives of 162 and 210 minutes,
respectively, indicating that methemoglobin reduction occurs more slowly in newborns than adults
(Power et al. 2007). Although specific mechanisms have not been elucidated, the increased susceptibility
to nitrite-induced methemoglobinemia in infants is well-documented.
Bosch et al. (1950) evaluated 139 reported cases of cyanosis among infants in Minnesota (90% were
<2 months of age; range 8 days to 5 months). Samples from 129 wells that served as water sources to the
cases revealed nitrate-nitrogen concentrations >100 mg/L (>440 mg nitrate/L) in 49 wells, 50–100 mg/L
(220–440 mg nitrate/L) in 53 wells, 21–50 mg/L (92–220 mg nitrate/L) in 25 wells, and 10–20 mg/L (44–
88 mg nitrate/L) in the other 2 wells. A major limitation of this study was the detection of coliform
organisms in 45 of 51 well water samples tested for bacterial contamination.
A nested case-control study included 26 cases of infants diagnosed with methemoglobinemia at
≤2 months of age and 45 age-matched controls (Zeman et al. 2002). Nitrate exposure levels were
categorized as low (<0.5 ppm), medium (1–10 ppm), or high (>10 ppm) according to estimated nitrate
levels reconstructed from parental responses to dietary questionnaires and environmental sampling.
Numbers of methemoglobinemia cases in the low, medium, and high exposure categories were 0/26, 4/26,
and 22/26, respectively, and estimated dietary nitrate intake ranged from 2.83 to 451.20 mg/kg/day (mean
103.6 mg nitrate/kg/day). Diarrheal disease was reported for 14/26 methemoglobinemia cases. Numbers
of controls in the low, medium, and high exposure categories were 21/45, 11/45, and 13/45, respectively,
and estimated dietary nitrate intake ranged from 0 to 182 mg/kg/day (mean 11.2 mg nitrate/kg/day) for
the controls; diarrheal disease was reported for 13/45 controls. Univariate and multifactorial analysis of
risk factors for methemoglobinemia indicated that methemoglobinemia was most strongly associated with
dietary exposure to nitrate/nitrite (p=0.0318), but also significantly associated with diarrheal disease
(p=0.0376). Controls in the high exposure category were less likely than high exposure
methemoglobinemia cases to have experienced severe diarrhea and were more likely to have been
breastfed for >2 weeks. Major limitations to the study include the collection of information contributing
to the exposure estimates several years following the occurrences of methemoglobinemia and reliance on
parental recollection of infant nutritional intake.

A-6 NITRATE AND NITRITE
APPENDIX A
Results from other studies suggest an association between nitrate in drinking water sources and elevated
methemoglobin among infants. Average methemoglobin levels of 1.0, 1.3, and 2.9% during the first
postnatal trimester (0–3 months of age) were reported among groups healthy infants with water sources
that were nitrate-free or contained 50–100 mg nitrate/L or >100 mg nitrate/L, respectively (Simon et al.
1964). At the end of the second trimester (6 months), methemoglobin averaged 0.7–0.8% for each group.
Super et al. (1981) reported mean methemoglobin levels of 1.54% among infants ingesting ≤2.93 mg
nitrate/kg/day and 3.03% among infants ingesting >2.93 mg nitrate/kg/day.
Limited data are available regarding administration of controlled amounts of nitrate and methemoglobin
levels. Cornblath and Hartmann (1948) administered sodium nitrate in the formula fed to four infants
(ages 11 days to 11 months) for 2–18 days at a concentration resulting in a dose of 50 mg nitrate/kg/day.
The highest observed level of methemoglobin was 5.3% of total hemoglobin; there was no evidence of
cyanosis. Among four other infants (ages 2 days to 6 months) similarly treated at 100 mg nitrate/kg/day
for 6–9 days, the only reported effect was that of 7.5% methemoglobin in a 10-day-old infant following
8 days of treatment in the absence of clinical cyanosis. Gruener and Toeplitz (1975) fed 104 infants
(1 week to 10 months of age) for 1 day with formula prepared using water containing 15 mg nitrate/L
(~0.8–1.5 mg nitrate/kg, based on age-specific values for water consumption [Kahn and Stralka 2009] and
body weight [EPA 2008]), increased to 108 mg nitrate/L for the next 3 days (~5.5–10.6 mg nitrate/kg/day,
based on age-specific values for water consumption [Kahn and Stralka 2009] and body weight [EPA
2008], and returned to 15 mg nitrate/L for 1 additional day. Mean methemoglobin levels were 0.89%
after the first day of feeding, 1.3, 0.91, and 0.93% after days 2, 3, and 4, and dropped to 0.8% on the fifth
day. Among three of these infants (ages not specified), methemoglobin levels reached 6.9, 13.9, and
15.9% during the high-dose days. Limitations of this study include the use of a wide range of ages and
the fact that only 57 of the 104 infants supplied blood samples on all 5 treatment days.
Agency Contacts (Chemical Managers): Carolyn Harper, Ph.D.

A-7 NITRATE AND NITRITE
APPENDIX A
MINIMAL RISK LEVEL (MRL) WORKSHEET
Chemical Name: Nitrite
CAS Numbers: 14797-65-0
Date: July 2017
Profile Status: Final
Route: [ ] Inhalation [x] Oral
Duration: [x] Acute [x] Intermediate [x] Chronic
Graph Key: 4 (Acute), 16 (Intermediate), 54 (Chronic)
Species: Human
Minimal Risk Level: 0.1 [x] mg/kg/day [ ] ppm
Reference: Walton G. 1951. Survey of literature relating to infant methemoglobinemia due to nitrate-
contaminated water. Am J Public Health 41:986-996.
Experimental design: Walton (1951) reviewed available literature and found 278 reported cases of infant
methemoglobinemia in a total of 14 U.S. states from which information was available. Cases were
grouped by state according to ranges of nitrate levels in drinking water sources.
Effect noted in study and corresponding doses: Among methemoglobinemia cases for which nitrate
levels in water sources used to prepare infant formula were available, 173 cases were associated with
>50 mg nitrate-nitrogen/L (220 mg nitrate/L), 36 cases with 21–50 mg nitrate-nitrogen/L (92–220 mg
nitrate/L), and 5 cases with 11–20 mg nitrate-nitrogen (48–88 mg nitrate/L). None of the
methemoglobinemia cases were associated with drinking water sources measuring <10 mg nitrate-
nitrogen/L (<44 mg nitrate/L). Limitations of the contributing studies include lack of information
regarding the actual ages of the infants, total nitrate doses, and other water source contaminants (e.g.,
bacterial levels).
Following ingestion of relatively large amounts of nitrate by healthy normal individuals, blood
methemoglobin levels increase rapidly, followed by a return to normal within several hours following
intake. Repeated ingestion of nitrate or nitrite for intermediate- or chronic-duration time periods would
be expected to result in changes in methemoglobin levels similar to those elicited from a single exposure.
Therefore, the acute-, intermediate-, and chronic-duration oral MRL values are equivalent.
Dose and end point used for MRL derivation: 0.2 mg nitrite/kg/day. The ingestion of nitrate results in
the formation of nitrite, which is the moiety responsible for methemoglobinemia. On average,
approximately 25% of an ingested dose of nitrate enters the saliva of an adult where a portion (ca. 20%
g/g) is reduced by commensal bacteria to nitrite (i.e., approximately 5% g/g of ingested nitrate is reduced
to nitrite in the saliva of an adult (Spiegelhalder et al. 1976); most salivary nitrite is absorbed into the
blood in the small intestine. Therefore, the ingestion of 0.2 mg nitrite/kg/day by an adult would be
expected to result in a nitrite blood level similar to that achieved following ingestion of 4 mg
nitrate/kg/day, based on essentially 100% absorption of the ingested dose of nitrite (i.e., 0.2 mg
nitrite/kg/day is 5% of an oral dose of nitrate at the oral MRL of 4 mg nitrate/kg/day).
[x] NOAEL [ ] LOAEL
Uncertainty Factors used in MRL derivation:
[ ] 10 for use of a LOAEL
[ ] 10 for extrapolation from animals to humans

A-8 NITRATE AND NITRITE
APPENDIX A
[x] 1 for human variability
A total uncertainty factor of 1 is justified because the point of departure is a NOAEL for nitrate-induced
effects on methemoglobin in a particularly sensitive human subpopulation (i.e., <3-month-old infants,
which in many cases may have been at increased risk of methemoglobinemia due to microbial
contamination and associated gastrointestinal infection).
Modifying factor used in MRL derivation:
[x] 2 because young infants exhibit increased susceptibility to methemoglobinemia following
nitrate ingestion; the modifying factor assumes that the effective methemoglobin level from a
given intake of nitrate by an infant is up to twice that of an adult; however, quantitative data
regarding conversion of nitrate to nitrite in the infant are lacking.
Was a conversion factor used from ppm in food or water to a mg/body weight dose? Nitrate may be
expressed in terms of ionic concentration (i.e., mg nitrate/L), or elemental concentration (i.e., mg nitrate-
nitrogen/L or mg nitrogen as nitrate/L). A concentration of nitrate expressed in elemental concentration
(mg nitrogen per liter from nitrate source) can be converted to its ionic concentration (mg NO
3
-
)
according to the following relationship: 1 mg nitrate-nitrogen = 4.4 mg nitrate (i.e., the proportion of N
-
in NO
3
is 14 [atomic mass of N] ÷ 62 [molecular mass of NO
3
-
] = 0.226).
A concentration of 44 mg nitrate/L (10 mg nitrate-nitrogen/L) in drinking water used to prepare infant
formula represents a NOAEC for infants <3 months of age. Table A-1 presents estimated nitrate doses to
infants (birth–<3 months of age) calculated using estimated mean values for drinking water ingestion
rates (Kahn and Stralka 2009) and body weight (EPA 2008) and assuming a drinking water level of
44 mg nitrate/L as a concentration not expected to cause methemoglobinemia; the calculated doses of
4.31–4.34 mg nitrate/kg/day represent NOAELs for the age ranges. The TWA-based calculated dose of
4.33 mg nitrate/kg/day for the age range of birth–<3 months is selected as the point of departure for
deriving acute-, intermediate-, and chronic-duration oral MRLs for nitrite.
If an inhalation study in animals, list conversion factors used in determining human equivalent dose: Not
applicable.
Was a conversion used from intermittent to continuous exposure? Not applicable.
Other additional studies or pertinent information that lend support to this MRL: Methemoglobinemia is a
condition in which increased methemoglobin as a percentage of total hemoglobin results in the expression
of clinical signs that increase in severity with increasing percent methemoglobin (ATSDR 2013a; Bloom
et al. 2013; Denshaw-Burke et al. 2013; Haymond et al. 2005). In normal healthy individuals,
methemoglobin levels are <1% of total hemoglobin. Discoloration (e.g., pale, gray blue) of the skin is
often observed at methemoglobin levels in the range of 3–15%; most patients tolerate methemoglobin
levels <10%. Tachycardia, weakness, and other signs of tissue hypoxia may be observed at 10–20%
methemoglobin levels. Effects on the central nervous system (e.g., headache, dizziness, fatigue) and
dyspnea and nausea appear at >20% methemoglobin; the severity of symptoms increases with increasing
methemoglobin level. High risk of mortality occurs at levels >70% methemoglobin.
Proposed explanations for increased susceptibility of infants to methemoglobinemia following ingestion
of nitrate include: (1) increased reduction of nitrate to nitrite in the newborn, (2) increased tendency for
nitrite-induced methemoglobin formation by fetal hemoglobin compared to adult hemoglobin, (3) lower
levels of NADH-dependent methemoglobin reductase (the major enzyme responsible for reduction of
methemoglobin to normal hemoglobin; also termed NADH-diaphorase, a soluble form of cytochrome-b5
A-9 NITRATE AND NITRITE
APPENDIX A
reductase) in the newborn compared to older infants and adults, and (4) incompletely developed hepatic
microsomal enzyme system in the infant and consequent lower rate of hepatic reduction of circulating
nitrite compared to that of older children and adults. A portion of ingested nitrate is reduced to nitrite by
commensal bacteria in the mouth; however, the acid environment of the normal stomach does not support
the growth of such bacteria and most of the nitrate that reaches the stomach passes to the small intestine
from which it is nearly completely absorbed into the blood. Although Kanady et al. (2012) reported little
or no bacterial conversion of nitrate to nitrite in the saliva of a group of 10 infants during the first
2 postnatal months (considered mainly due to lower numbers of major nitrate-reducing oral bacteria than
adults), a higher pH in the stomach of the newborn may favor growth of nitrate-reducing bacteria,
resulting in increased reduction of nitrate to nitrite and increased plasma methemoglobin. Most
hemoglobin in the newborn is in the form of fetal hemoglobin, which appears to be more readily oxidized
to methemoglobin than adult hemoglobin; fetal hemoglobin is replaced by adult hemoglobin during early
postnatal life. Levels of NADH-dependent methemoglobin reductase in the newborn increase
approximately 2-fold during the first 4 months of postnatal life to reach adult levels. During the period of
relatively lower methemoglobin reductase levels, methemoglobin would not be expected to be as readily
reduced, resulting in increased susceptibility to methemoglobinemia. In apparent contrast, Ibrahim et al.
(2012) reported that blood nitrite levels in newborns approximately 1–2 days of age were 35–55% lower
than that of adults. However, one study that evaluated reduction rates of methemoglobin in human adult
blood and cord blood from term newborns estimated methemoglobin half-lives of 162 and 210 minutes,
respectively, indicating that methemoglobin reduction occurs more slowly in newborns than adults
(Power et al. 2007). Although specific mechanisms have not been elucidated, the increased susceptibility
to nitrite-induced methemoglobinemia in infants is well-documented.
Bosch et al. (1950) evaluated 139 reported cases of cyanosis among infants in Minnesota (90% were
<2 months of age; range 8 days to 5 months). Samples from 129 wells that served as water sources to the
cases revealed nitrate-nitrogen concentrations >100 mg/L (>440 mg nitrate/L) in 49 wells, 50–100 mg/L
(220–440 mg nitrate/L) in 53 wells, 21–50 mg/L (92–220 mg nitrate/L) in 25 wells, and 10–20 mg/L (44–
88 mg nitrate/L) in the other 2 wells. A major limitation of this study was the detection of coliform
organisms in 45 of 51 well water samples tested for bacterial contamination.
A nested case-control study included 26 cases of infants diagnosed with methemoglobinemia at
≤2 months of age and 45 age-matched controls (Zeman et al. 2002). Nitrate exposure levels were
categorized as low (<0.5 ppm), medium (1–10 ppm), or high (>10 ppm) according to estimated nitrate
levels reconstructed from parental responses to dietary questionnaires and environmental sampling.
Numbers of methemoglobinemia cases in the low, medium, and high exposure categories were 0/26, 4/26,
and 22/26, respectively, and estimated dietary nitrate intake ranged from 2.83 to 451.20 mg/kg/day (mean
103.6 mg nitrate/kg/day). Diarrheal disease was reported for 14/26 methemoglobinemia cases. Numbers
of controls in the low, medium, and high exposure categories were 21/45, 11/45, and 13/45, respectively,
and estimated dietary nitrate intake ranged from 0 to 182 mg/kg/day (mean 11.2 mg nitrate/kg/day) for
the controls; diarrheal disease was reported for 13/45 controls. Univariate and multifactorial analysis of
risk factors for methemoglobinemia indicated that methemoglobinemia was most strongly associated with
dietary exposure to nitrate/nitrite (p=0.0318), but also significantly associated with diarrheal disease
(p=0.0376). Controls in the high exposure category were less likely than high exposure
methemoglobinemia cases to have experienced severe diarrhea and were more likely to have been
breastfed for >2 weeks. Major limitations to the study include the collection of information contributing
to the exposure estimates several years following the occurrences of methemoglobinemia and reliance on
parental recollection of infant nutritional intake.
Results from other studies suggest an association between nitrate in drinking water sources and elevated
methemoglobin among infants. Average methemoglobin levels of 1.0, 1.3, and 2.9% during the first
postnatal trimester (0–3 months of age) were reported among groups healthy infants with water sources

A-10 NITRATE AND NITRITE
APPENDIX A
that were nitrate-free or contained 50–100 mg nitrate/L or >100 mg nitrate/L, respectively (Simon et al.
1964). At the end of the second trimester (6 months), methemoglobin averaged 0.7–0.8% for each group.
Super et al. (1981) reported mean methemoglobin levels of 1.54% among infants ingesting ≤2.93mg
nitrate/kg/day and 3.03% among infants ingesting >2.93 mg nitrate/kg/day.
Limited data are available regarding administration of controlled amounts of nitrate and methemoglobin
levels. Cornblath and Hartmann (1948) administered sodium nitrate in the formula fed to four infants
(ages 11 days to 11 months) for 2–18 days at a concentration resulting in a dose of 50 mg nitrate/kg/day.
The highest observed level of methemoglobin was 5.3% of total hemoglobin; there was no evidence of
cyanosis. Among four other infants (ages 2 days to 6 months) similarly treated at 100 mg nitrate/kg/day
for 6–9 days, the only reported effect was that of 7.5% methemoglobin in a 10-day-old infant following
8 days of treatment in the absence of clinical cyanosis. Gruener and Toeplitz (1975) fed 104 infants
(1 week to 10 months of age) for 1 day with formula prepared using water containing 15 mg nitrate/L
(~0.8–1.5 mg nitrate/kg, based on age-specific values for water consumption [Kahn and Stralka 2009] and
body weight [EPA 2008]), increased to 108 mg nitrate/L for the next 3 days (~5.5–10.6 mg nitrate/kg/day,
based on age-specific values for water consumption [Kahn and Stralka 2009] and body weight [EPA
2008], and returned to 15 mg nitrate/L for 1 additional day. Mean methemoglobin levels were 0.89%
after the first day of feeding, 1.3, 0.91, and 0.93% after days 2, 3, and 4, and dropped to 0.8% on the fifth
day. Among three of these infants (ages not specified), methemoglobin levels reached 6.9, 13.9, and
15.9% during the high-dose days. Limitations of this study include the use of a wide range of ages and
the fact that only 57 of the 104 infants supplied blood samples on all 5 treatment days.
In a study designed to evaluate the oral bioavailability of sodium nitrite in healthy volunteers (seven
females and two males; mean age 22.9 years), ingestion of ~2.2–2.7 mg sodium nitrite/kg (1.5–1.8 mg
nitrite/kg) resulted in maximum methemoglobin concentrations ranging from 3.4 to 4.5% of total
hemoglobin at approximately 0.70 hours following ingestion (Kortboyer et al. 1997b). At a higher intake
(~4.4–5.4 mg sodium nitrite/kg, or 2.9–3.6 mg nitrite/kg), the maximum methemoglobin concentrations
ranged from 7.7 to 10.9% of total hemoglobin at approximately 1.14 hours following ingestion.
Agency Contacts (Chemical Managers): Carolyn Harper, Ph.D.
B-1 NITRATE AND NITRITE
APPENDIX B. USER'S GUIDE
Chapter 1
Public Health Statement
This chapter of the profile is a health effects summary written in non-technical language. Its intended
audience is the general public, especially people living in the vicinity of a hazardous waste site or
chemical release. If the Public Health Statement were removed from the rest of the document, it would
still communicate to the lay public essential information about the chemical.
The major headings in the Public Health Statement are useful to find specific topics of concern. The
topics are written in a question and answer format. The answer to each question includes a sentence that
will direct the reader to chapters in the profile that will provide more information on the given topic.
Chapter 2
Relevance to Public Health
This chapter provides a health effects summary based on evaluations of existing toxicologic,
epidemiologic, and toxicokinetic information. This summary is designed to present interpretive, weight-
of-evidence discussions for human health end points by addressing the following questions:
1. What effects are known to occur in humans?
2. What effects observed in animals are likely to be of concern to humans?
3. What exposure conditions are likely to be of concern to humans, especially around hazardous
waste sites?
The chapter covers end points in the same order that they appear within the Discussion of Health Effects
by Route of Exposure section, by route (inhalation, oral, and dermal) and within route by effect. Human
data are presented first, then animal data. Both are organized by duration (acute, intermediate, chronic).
In vitro data and data from parenteral routes (intramuscular, intravenous, subcutaneous, etc.) are also
considered in this chapter.
The carcinogenic potential of the profiled substance is qualitatively evaluated, when appropriate, using
existing toxicokinetic, genotoxic, and carcinogenic data. ATSDR does not currently assess cancer
potency or perform cancer risk assessments. Minimal Risk Levels (MRLs) for noncancer end points (if
derived) and the end points from which they were derived are indicated and discussed.
Limitations to existing scientific literature that prevent a satisfactory evaluation of the relevance to public
health are identified in the Chapter 3 Data Needs section.
Interpretation of Minimal Risk Levels
Where sufficient toxicologic information is available, ATSDR has derived MRLs for inhalation and oral
routes of entry at each duration of exposure (acute, intermediate, and chronic). These MRLs are not
meant to support regulatory action, but to acquaint health professionals with exposure levels at which
adverse health effects are not expected to occur in humans.
B-2 NITRATE AND NITRITE
APPENDIX B
MRLs should help physicians and public health officials determine the safety of a community living near
a hazardous substance emission, given the concentration of a contaminant in air or the estimated daily
dose in water. MRLs are based largely on toxicological studies in animals and on reports of human
occupational exposure.
MRL users should be familiar with the toxicologic information on which the number is based. Chapter 2,
"Relevance to Public Health," contains basic information known about the substance. Other sections such
as Chapter 3 Section 3.9, "Interactions with Other Substances,” and Section 3.10, "Populations that are
Unusually Susceptible" provide important supplemental information.
MRL users should also understand the MRL derivation methodology. MRLs are derived using a
modified version of the risk assessment methodology that the Environmental Protection Agency (EPA)
provides (Barnes and Dourson 1988) to determine reference doses (RfDs) for lifetime exposure.
To derive an MRL, ATSDR generally selects the most sensitive end point which, in its best judgement,
represents the most sensitive human health effect for a given exposure route and duration. ATSDR
cannot make this judgement or derive an MRL unless information (quantitative or qualitative) is available
for all potential systemic, neurological, and developmental effects. If this information and reliable
quantitative data on the chosen end point are available, ATSDR derives an MRL using the most sensitive
species (when information from multiple species is available) with the highest no-observed-adverse-effect
level (NOAEL) that does not exceed any adverse effect levels. When a NOAEL is not available, a
lowest-observed-adverse-effect level (LOAEL) can be used to derive an MRL, and an uncertainty factor
(UF) of 10 must be employed. Additional uncertainty factors of 10 must be used both for human
variability to protect sensitive subpopulations (people who are most susceptible to the health effects
caused by the substance) and for interspecies variability (extrapolation from animals to humans). In
deriving an MRL, these individual uncertainty factors are multiplied together. The product is then
divided into the inhalation concentration or oral dosage selected from the study. Uncertainty factors used
in developing a substance-specific MRL are provided in the footnotes of the levels of significant exposure
(LSE) tables.
Chapter 3
Health Effects
Tables and Figures for Levels of Significant Exposure (LSE)
Tables and figures are used to summarize health effects and illustrate graphically levels of exposure
associated with those effects. These levels cover health effects observed at increasing dose
concentrations and durations, differences in response by species, MRLs to humans for noncancer end
points, and EPA's estimated range associated with an upper- bound individual lifetime cancer risk of 1 in
10,000 to 1 in 10,000,000. Use the LSE tables and figures for a quick review of the health effects and to
locate data for a specific exposure scenario. The LSE tables and figures should always be used in
conjunction with the text. All entries in these tables and figures represent studies that provide reliable,
quantitative estimates of NOAELs, LOAELs, or Cancer Effect Levels (CELs).
The legends presented below demonstrate the application of these tables and figures. Representative
examples of LSE Table 3-1 and Figure 3-1 are shown. The numbers in the left column of the legends
correspond to the numbers in the example table and figure.

B-3 NITRATE AND NITRITE
APPENDIX B
LEGEND
See Sample LSE Table 3-1 (page B-6)
(1) Route of Exposure. One of the first considerations when reviewing the toxicity of a substance
using these tables and figures should be the relevant and appropriate route of exposure. Typically
when sufficient data exist, three LSE tables and two LSE figures are presented in the document.
The three LSE tables present data on the three principal routes of exposure, i.e., inhalation, oral,
and dermal (LSE Tables 3-1, 3-2, and 3-3, respectively). LSE figures are limited to the inhalation
(LSE Figure 3-1) and oral (LSE Figure 3-2) routes. Not all substances will have data on each
route of exposure and will not, therefore, have all five of the tables and figures.
(2) Exposure Period. Three exposure periods—acute (less than 15 days), intermediate (15–
364 days), and chronic (365 days or more)—are presented within each relevant route of exposure.
In this example, an inhalation study of intermediate exposure duration is reported. For quick
reference to health effects occurring from a known length of exposure, locate the applicable
exposure period within the LSE table and figure.
(3) Health Effect. The major categories of health effects included in LSE tables and figures include
death, systemic, immunological, neurological, developmental, reproductive, and cancer.
NOAELs and LOAELs can be reported in the tables and figures for all effects but cancer.
Systemic effects are further defined in the "System" column of the LSE table (see key number
18).
(4) Key to Figure. Each key number in the LSE table links study information to one or more data
points using the same key number in the corresponding LSE figure. In this example, the study
represented by key number 18 has been used to derive a NOAEL and a Less Serious LOAEL
(also see the two "18r" data points in sample Figure 3-1).
(5) Species. The test species, whether animal or human, are identified in this column. Chapter 2,
"Relevance to Public Health," covers the relevance of animal data to human toxicity and
Section 3.4, "Toxicokinetics," contains any available information on comparative toxicokinetics.
Although NOAELs and LOAELs are species specific, the levels are extrapolated to equivalent
human doses to derive an MRL.
(6) Exposure Frequency/Duration. The duration of the study and the weekly and daily exposure
regimens are provided in this column. This permits comparison of NOAELs and LOAELs from
different studies. In this case (key number 18), rats were exposed to “Chemical x” via inhalation
for 6 hours/day, 5 days/week, for 13 weeks. For a more complete review of the dosing regimen,
refer to the appropriate sections of the text or the original reference paper (i.e., Nitschke et al.
1981).
(7) System. This column further defines the systemic effects. These systems include respiratory,
cardiovascular, gastrointestinal, hematological, musculoskeletal, hepatic, renal, and
dermal/ocular. "Other" refers to any systemic effect (e.g., a decrease in body weight) not covered
in these systems. In the example of key number 18, one systemic effect (respiratory) was
investigated.
(8) NOAEL. A NOAEL is the highest exposure level at which no adverse effects were seen in the
organ system studied. Key number 18 reports a NOAEL of 3 ppm for the respiratory system,
which was used to derive an intermediate exposure, inhalation MRL of 0.005 ppm (see
footnote "b").

B-4 NITRATE AND NITRITE
APPENDIX B
(9) LOAEL. A LOAEL is the lowest dose used in the study that caused an adverse health effect.
LOAELs have been classified into "Less Serious" and "Serious" effects. These distinctions help
readers identify the levels of exposure at which adverse health effects first appear and the
gradation of effects with increasing dose. A brief description of the specific end point used to
quantify the adverse effect accompanies the LOAEL. The respiratory effect reported in key
number 18 (hyperplasia) is a Less Serious LOAEL of 10 ppm. MRLs are not derived from
Serious LOAELs.
(10) Reference. The complete reference citation is given in Chapter 9 of the profile.
(11) CEL. A CEL is the lowest exposure level associated with the onset of carcinogenesis in
experimental or epidemiologic studies. CELs are always considered serious effects. The LSE
tables and figures do not contain NOAELs for cancer, but the text may report doses not causing
measurable cancer increases.
(12) Footnotes. Explanations of abbreviations or reference notes for data in the LSE tables are found
in the footnotes. Footnote "b" indicates that the NOAEL of 3 ppm in key number 18 was used to
derive an MRL of 0.005 ppm.
LEGEND
See Sample Figure 3-1 (page B-7)
LSE figures graphically illustrate the data presented in the corresponding LSE tables. Figures help the
reader quickly compare health effects according to exposure concentrations for particular exposure
periods.
(13) Exposure Period. The same exposure periods appear as in the LSE table. In this example, health
effects observed within the acute and intermediate exposure periods are illustrated.
(14) Health Effect. These are the categories of health effects for which reliable quantitative data
exists. The same health effects appear in the LSE table.
(15) Levels of Exposure. Concentrations or doses for each health effect in the LSE tables are
graphically displayed in the LSE figures. Exposure concentration or dose is measured on the log
scale "y" axis. Inhalation exposure is reported in mg/m
3
or ppm and oral exposure is reported in
mg/kg/day.
(16) NOAEL. In this example, the open circle designated 18r identifies a NOAEL critical end point in
the rat upon which an intermediate inhalation exposure MRL is based. The key number 18
corresponds to the entry in the LSE table. The dashed descending arrow indicates the
extrapolation from the exposure level of 3 ppm (see entry 18 in the table) to the MRL of
0.005 ppm (see footnote "b" in the LSE table).
(17) CEL. Key number 38m is one of three studies for which CELs were derived. The diamond
symbol refers to a CEL for the test species-mouse. The number 38 corresponds to the entry in the
LSE table.

B-5 NITRATE AND NITRITE
APPENDIX B
(18) Estimated Upper-Bound Human Cancer Risk Levels. This is the range associated with the upper-
bound for lifetime cancer risk of 1 in 10,000 to 1 in 10,000,000. These risk levels are derived
from the EPA's Human Health Assessment Group's upper-bound estimates of the slope of the
cancer dose response curve at low dose levels (q
1
*).
(19) Key to LSE Figure. The Key explains the abbreviations and symbols used in the figure.

B-6
SAMPLE
NITRATE AND NITRITE
APPENDIX B
1
→
Table 3-
1. Levels of Significant Exposure to [Chemical x]
– Inhalation
LOAEL (effect)
Exposure
Less serious
Serious (ppm)
Key to
frequency/
NOAEL
(ppm)
figure
a
Species
duration
System
(ppm)
Reference
2
3
4
→
INTERMEDIATE EXPOSURE
5
→
Systemic
↓
18
Rat
→
CHRONIC EXPOSURE
Cancer
38
Rat
39
Rat
40
Mouse
6
↓
13 wk
5 d/wk
6 hr/d
18 mo
5 d/wk
7 hr/d
89
–
104 wk
5 d/wk
6 hr/d
79
–
103 wk
5 d/wk
6 hr/d
7
8
9
↓
↓
↓
Resp
3
b
10 (hyperplasia)
11
↓
20
(CEL, multiple
organs)
10
(CEL, lung tumors
,
nasal tumors)
10
(CEL, lung tumors,
hemangiosarcomas)
10
↓
Nitschke et al. 1981
Wong et al. 1982
NTP 1982
NTP 1982
12
→
a
The number corresponds to entries in Figure 3
-1.
b
Used to derive an intermediate inhalation Minimal Risk Level (MRL) of 5x10
-3
ppm; dose adjusted for intermittent exposure and divided
by an uncertainty factor of 100 (10 for extrapolation from animal to humans, 10 for human variability).

B-7 NITRATE AND NITRITE
APPENDIX B
Chronic (≥ 365 days) Intermediate (15-364 days)
B-8 NITRATE AND NITRITE
APPENDIX B
This page is intentionally blank.
C-1 NITRATE AND NITRITE
APPENDIX C. ACRONYMS, ABBREVIATIONS, AND SYMBOLS
ACGIH American Conference of Governmental Industrial Hygienists
ACOEM American College of Occupational and Environmental Medicine
ADI acceptable daily intake
ADME absorption, distribution, metabolism, and excretion
AED atomic emission detection
AFID alkali flame ionization detector
AFOSH Air Force Office of Safety and Health
ALT alanine aminotransferase
AML acute myeloid leukemia
AOAC Association of Official Analytical Chemists
AOEC Association of Occupational and Environmental Clinics
AP alkaline phosphatase
APHA American Public Health Association
AST aspartate aminotransferase
atm atmosphere
ATSDR Agency for Toxic Substances and Disease Registry
AWQC Ambient Water Quality Criteria
BAT best available technology
BCF bioconcentration factor
BEI Biological Exposure Index
BMD/C benchmark dose or benchmark concentration
BMD
X
dose that produces a X% change in response rate of an adverse effect
BMDL
X
95% lower confidence limit on the BMD
X
BMDS Benchmark Dose Software
BMR benchmark response
BSC Board of Scientific Counselors
C centigrade
CAA Clean Air Act
CAG Cancer Assessment Group of the U.S. Environmental Protection Agency
CAS Chemical Abstract Services
CDC Centers for Disease Control and Prevention
CEL cancer effect level
CELDS Computer-Environmental Legislative Data System
CERCLA Comprehensive Environmental Response, Compensation, and Liability Act
CFR Code of Federal Regulations
Ci curie
CI confidence interval
CLP Contract Laboratory Program
cm centimeter
CML chronic myeloid leukemia
CPSC Consumer Products Safety Commission
CWA Clean Water Act
DHEW Department of Health, Education, and Welfare
DHHS Department of Health and Human Services
DNA deoxyribonucleic acid
DOD Department of Defense
DOE Department of Energy
DOL Department of Labor
DOT Department of Transportation
C-2 NITRATE AND NITRITE
APPENDIX C
DOT/UN/ Department of Transportation/United Nations/
NA/IMDG North America/Intergovernmental Maritime Dangerous Goods Code
DWEL drinking water exposure level
ECD electron capture detection
ECG/EKG electrocardiogram
EEG electroencephalogram
EEGL Emergency Exposure Guidance Level
EPA Environmental Protection Agency
F Fahrenheit
F
1
first-filial generation
FAO Food and Agricultural Organization of the United Nations
FDA Food and Drug Administration
FEMA Federal Emergency Management Agency
FIFRA Federal Insecticide, Fungicide, and Rodenticide Act
FPD flame photometric detection
fpm feet per minute
FR Federal Register
FSH follicle stimulating hormone
g gram
GC gas chromatography
gd gestational day
GLC gas liquid chromatography
GPC gel permeation chromatography
HPLC high-performance liquid chromatography
HRGC high resolution gas chromatography
HSDB Hazardous Substance Data Bank
IARC International Agency for Research on Cancer
IDLH immediately dangerous to life and health
ILO International Labor Organization
IRIS Integrated Risk Information System
Kd adsorption ratio
kg kilogram
kkg kilokilogram; 1 kilokilogram is equivalent to 1,000 kilograms and 1 metric ton
K
oc
organic carbon partition coefficient
K
ow
octanol-water partition coefficient
L liter
LC liquid chromatography
LC
50
lethal concentration, 50% kill
LC
Lo
lethal concentration, low
LD
50
lethal dose, 50% kill
LD
Lo
lethal dose, low
LDH lactic dehydrogenase
LH luteinizing hormone
LOAEL lowest-observed-adverse-effect level
LSE Levels of Significant Exposure
LT
50
lethal time, 50% kill
m meter
MA trans,trans-muconic acid
MAL maximum allowable level
mCi millicurie
MCL maximum contaminant level
C-3 NITRATE AND NITRITE
APPENDIX C
MCLG maximum contaminant level goal
MF modifying factor
MFO mixed function oxidase
mg milligram
mL milliliter
mm millimeter
mmHg millimeters of mercury
mmol millimole
mppcf millions of particles per cubic foot
MRL Minimal Risk Level
MS mass spectrometry
mt metric ton
NAAQS National Ambient Air Quality Standard
NAS National Academy of Science
NATICH National Air Toxics Information Clearinghouse
NATO North Atlantic Treaty Organization
NCE normochromatic erythrocytes
NCEH National Center for Environmental Health
NCI National Cancer Institute
ND not detected
NFPA National Fire Protection Association
ng nanogram
NHANES National Health and Nutrition Examination Survey
NIEHS National Institute of Environmental Health Sciences
NIOSH National Institute for Occupational Safety and Health
NIOSHTIC NIOSH's Computerized Information Retrieval System
NLM National Library of Medicine
nm nanometer
nmol nanomole
NOAEL no-observed-adverse-effect level
NOES National Occupational Exposure Survey
NOHS National Occupational Hazard Survey
NPD nitrogen phosphorus detection
NPDES National Pollutant Discharge Elimination System
NPL National Priorities List
NR not reported
NRC National Research Council
NS not specified
NSPS New Source Performance Standards
NTIS National Technical Information Service
NTP National Toxicology Program
ODW Office of Drinking Water, EPA
OERR Office of Emergency and Remedial Response, EPA
OHM/TADS Oil and Hazardous Materials/Technical Assistance Data System
OPP Office of Pesticide Programs, EPA
OPPT Office of Pollution Prevention and Toxics, EPA
OPPTS Office of Prevention, Pesticides and Toxic Substances, EPA
OR odds ratio
OSHA Occupational Safety and Health Administration
OSW Office of Solid Waste, EPA
OTS Office of Toxic Substances
C-4 NITRATE AND NITRITE
APPENDIX C
OW Office of Water
OWRS Office of Water Regulations and Standards, EPA
PAH polycyclic aromatic hydrocarbon
PBPD physiologically based pharmacodynamic
PBPK physiologically based pharmacokinetic
PCE polychromatic erythrocytes
PEL permissible exposure limit
PEL-C permissible exposure limit-ceiling value
pg picogram
PHS Public Health Service
PID photo ionization detector
pmol picomole
PMR proportionate mortality ratio
ppb parts per billion
ppm parts per million
ppt parts per trillion
PSNS pretreatment standards for new sources
RBC red blood cell
REL recommended exposure level/limit
REL-C recommended exposure level-ceiling value
RfC reference concentration (inhalation)
RfD reference dose (oral)
RNA ribonucleic acid
RQ reportable quantity
RTECS Registry of Toxic Effects of Chemical Substances
SARA Superfund Amendments and Reauthorization Act
SCE sister chromatid exchange
SGOT serum glutamic oxaloacetic transaminase (same as aspartate aminotransferase or AST)
SGPT serum glutamic pyruvic transaminase (same as alanine aminotransferase or ALT)
SIC standard industrial classification
SIM selected ion monitoring
SMCL secondary maximum contaminant level
SMR standardized mortality ratio
SNARL suggested no adverse response level
SPEGL Short-Term Public Emergency Guidance Level
STEL short term exposure limit
STORET Storage and Retrieval
TD
50
toxic dose, 50% specific toxic effect
TLV threshold limit value
TLV-C threshold limit value-ceiling value
TOC total organic carbon
TPQ threshold planning quantity
TRI Toxics Release Inventory
TSCA Toxic Substances Control Act
TWA time-weighted average
UF uncertainty factor
U.S. United States
USDA United States Department of Agriculture
USGS United States Geological Survey
VOC volatile organic compound
WBC white blood cell
C-5 NITRATE AND NITRITE
APPENDIX C
WHO World Health Organization
> greater than
≥ greater than or equal to
= equal to
< less than
≤ less than or equal to
% percent
α alpha
β beta
γ gamma
δ delta
μm micrometer
μg microgram
q
1
*
cancer slope factor
– negative
+ positive
(+) weakly positive result
(–) weakly negative result
