
Paci)c University
CommonKnowledge
'05<<9<3%5F@606.;@@6@A.;A'AB162@ <99242<32.9A5%?<32@@6<;@
'B::2?
Ca&eine Intake During Pregnancy and Weight of
O&spring in Childhood: A Systematic Review
Gina M. McCoy
Pacic University
Evelyn L. Evans
Pacic University
<99<DA56@.;1.116A6<;.9D<?8@.A 5R=@0<::<;@=.06O0B21B=.
%.?A<3A52 "21606;2.;12.9A5'062;02@<::<;@
N6@.=@A<;2%?<720A6@/?<B45AA<F<B3<?3?22.;1<=2;.002@@/FA52<99242<32.9A5%?<32@@6<;@.A<::<; ;<D92142A5.@/22;.002=A213<?
6;09B@6<;6;'05<<9<3%5F@606.;@@6@A.;A'AB162@/F.;.BA5<?6G21.1:6;6@A?.A<?<3<::<; ;<D92142<?:<?26;3<?:.A6<;=92.@20<;A.0A
<::<; ;<D92142=.06O0B21B
&20<::2;1216A.A6<;
"0<F6;.".;1C.;@C29F;!.L26;2;A.82B?6;4%?24;.;0F.;1*2645A<3$L@=?6;46;56915<<1'F@A2:.A60
&2C62D School of Physician Assistant Studies
5R=@0<::<;@=.06O0B21B=.

Ca&eine Intake During Pregnancy and Weight of O&spring in Childhood:
A Systematic Review
Abstract
Background56915<<1</2@6AF0B??2;A9F.L20A@<;2A56?1<3A52);6A21'A.A2@IF<BA5"22A6;40?6A2?6.3<?
<C2?D2645A.;1</2@6AF6;056915<<1;<A<;9F6;0?2.@2@A52?6@8<3/26;4<C2?D2645A<?</2@26;.1B9A5<<1
/BA.9@<6;0?2.@2@A52?6@8<30<:<?/616A62@<3</2@6AF6;09B16;4AF=216./2A2@:2A./<960@F;1?<:2;<;
.90<5<960@A2.A<52=.A6A6@5F=2?A2;@6<;</@A?B0A6C2@922=.=;2..;1@96==210.=6A.932:<?.92=6=5F@6@'2C2?.9
3.0A<?@5.C2/22;6:=960.A21A<5.C2.0.[email protected]?29.A6<;@56=<?.@@<06.A6<;D6A52E02@@6C2D2645A4.6;5<D2C2?
;2D@AB162@.?2@B442@A6;4A5.A.@64;6O0.;A6;PB2;02<;A52D2645A<3A52056916@=?2@2;A in-uteroN20B??2;A
?2C62D2E=9<?2@A52?29.A6<;@56=/2AD22;0.L26;20<;@B:=A6<;1B?6;4=?24;.;0F.;1A52D2645A<3<L@=?6;4
6;056915<<1N2=B?=<@2<3A56@?2C62D6@A<<?4.;6G2.;10?6A60.99F.==?.6@2A520B??2;A1.A.6;<?12?A<
?2.964;<B?@A.;1.?1<30.?2D6A5A52:<@A?202;A6;3<?:.A6<;%?2C2;A6;4056915<<1</2@6AF6@<B?/2@A96;2<3
1232;@23<?<B?05691?2;.;1A5252.9A5<3<B?;.A6<;
Method0<:=?252;@6C2@2.?05<3"!#%B/"21*2/<3'062;02#!'$.;1
"!#$C61D.@0<;1B0A21B@6;4A523<99<D6;482FD<?1@:.A2?;.9=?24;.;AD<:2;0.L26;2.;1
056915<<1</2@6AFN2@2.?05=?<1B021OC2.?A6092@<3D56053<B?D2?2?292C.;AA<A52A<=60.;1:2A.99
29646/696AF0?6A2?6.N23<B?.==960./92.?A6092@D2?2A52;?2C62D21B@6;4M0?6A2?6.
ResultsN?22<3A5229646/92@AB162@@B442@AA5.AA52?26@.;.@@<06.A6<;/2AD22;56452?92C29@<30.L26;2
6;A.826;=?24;.;0F.;16;0?2.@21D2645A6;056915<<10<:=.?21A<9<D2?92C29@<3:.A2?;.90.L26;26;A.82
$;2@AB1F2C2;@B442@A@0<:=92A2.C<61.;02<30.L26;2:.F/2.1C6@./92.Q2?O;16;4@?2C2.96;0?2.@21"
3?<:6;3.;0FA<056915<<1D2?2.@@<06.A21D6A5.;F.:<B;A<30.L26;26;A.821B?6;4=?24;.;0F;0<;A?.@A.
@AB1F/FA52&2@2.?05;@A6ABA2.A#.A6<;D6125691?2;I@<@=6A.9161;<A@B==<?AA52A52<?FA5.A6;0?2.@6;4
:.A2?;.90.L26;20<;@B:=A6<;1B?6;4=?24;.;0F6;0?2.@2@A52?6@8<3056915<<1</2@6AF
ConclusionN2B@2<30.L26;21B?6;4=?24;.;0F:.F/296;821A<056915<<1</2@6AF%?<C612?@@5<B91
0<;@612?46C6;4@A?60A2??20<::2;1.A6<;@A5.;A520B??2;A$4B61296;2@<3:41.F.;1:.F2C2;
21B0.A2=?24;.;A:<A52?@A<296:6;.A20.L26;2.9A<42A52?
Keywords".A2?;.9=?24;.;AD<:2;0.L26;2056915<<1</2@6AF
Degree Type
.=@A<;2%?<720A
Degree Name
".@A2?<3'062;026;%5F@606.;@@6@A.;A'AB162@
Keywords
".A2?;.9=?24;.;AD<:2;0.L26;2056915<<1</2@6AF
Subject Categories
"21606;2.;12.9A5'062;02@
N6@0.=@A<;2=?<720A6@.C.69./92.A<::<; ;<D92142 5R=@0<::<;@=.06O0B21B=.

Copyright and terms of use
3F<B5.C21<D;9<.121A56@1<0B:2;A16?20A9F3?<:A52D2/<?3?<:<::<; ;<D92142@22A52
J&645A@K@20A6<;<;A52=?2C6<B@=.423<?A52A2?:@<3B@2
If you have received this document through an interlibrary loan/document delivery service, the
following terms of use apply:
<=F?645A6;A56@D<?86@5291/FA52.BA5<?@+<B:.F1<D;9<.1<?=?6;A.;F=<?A6<;<3A56@1<0B:2;A
3<?=2?@<;.9B@2<;9F<?3<?.;FB@2A5.A6@.99<D21/F3.6?B@2(6A92H)'E02=A3<?=2?@<;.9
<?3.6?B@2F<B<?F<B?/<??<D6;496/?.?F:.F;<A?2=?<1B02?2:6E?2=B/96@5=<@AA?.;@:6A<?
16@A?6/BA2A56@1<0B:2;A<?.;F=<?A6<;A52?2<3D6A5<BAA52=2?:6@@6<;<3A520<=F?645A<D;2?,#<A2
3A56@1<0B:2;A6@9602;@21B;12?.?2.A6C2<::<;@9602;@2@22J&645A@K<;A52=?2C6<B@=.42
D5605.99<D@/?<.12?[email protected]2?645A@F<B?B@26@4<C2?;21/FA52A2?:@<3A5.A9602;@2-
;>B6?62@?24.?16;43B?A52?B@2<3A52@2:.A2?6.9@@5<B91/2.11?2@@21A<<::<; ;<D92142&645A@
%.06O0);6C2?@6AF!6/?.?F<99242*.F<?2@A?<C2$&:.696;>B6?62@
:.F/216?20A21A<0<=F?645A=.06O0B21B
N6@0.=@A<;2=?<720A6@.C.69./92.A<::<; ;<D92142 5R=@0<::<;@=.06O0B21B=.

1
Caffeine Intake During Pregnancy and Weight of Offspring in Childhood:
A Systematic Review
Evelyn Evans, PA-S and Gina McCoy, PA-S
A Clinical Graduate Project Submitted to the Faculty of
the School of Physician Assistant Studies
Pacific University
Hillsboro, OR
For the Masters of Science Degree, August 10, 2019
Faculty Advisor: Kim Lovato, MS, PA-C and Alison McLellan, MMS, PA-C
Clinical Graduate Project Coordinator: Annjanette Sommers, PA-C, MS
2
|| Biography ||
Evelyn Evans hails from San Luis Obispo, California. She attended the University of California,
Davis earning a Bachelor of Arts in Studio Art with a minor in Human Physiology in 2014.
Evelyn’s medical background includes work as an emergency department scribe, physical
therapy aide, and volunteer medical assistant in free clinics in both Sacramento and San
Francisco. She is excited to assume the role of provider in a variety of fields throughout her
career as a PA.
Gina McCoy is from Overland Park, Kansas. She received a Bachelor of Arts in Psychology
from Central College of Pella, Iowa in 2014. Part of Gina’s clinical background involves
working with the pediatric population at Rocky Mountain Hospital for Children in Denver, CO.
She is interested in pursuing a career in either pediatrics or obstetrics & gynecology.
3
|| Abstract ||
Background: Childhood obesity currently affects one third of the United States’ youth. Meeting criteria for
overweight and obesity in childhood not only increases the risk of being overweight or obese in adulthood, but also
increases the risk of comorbidities of obesity including: type 2 diabetes, metabolic syndrome, non-alcoholic
steatohepatitis, hypertension, obstructive sleep apnea, and slipped capital femoral epiphysis. Several factors have
been implicated to have a causal relationship or association with excessive weight gain; however, new studies are
suggesting that a significant influence on the weight of the child is present in-utero. The current review explores the
relationship between caffeine consumption during pregnancy and the weight of offspring in childhood. The purpose
of this review is to organize and critically appraise the current data in order to realign our standard of care with the
most recent information. Preventing childhood obesity is our best line of defense for our children and the health of
our nation.
Method: A comprehensive search of MEDLINE-PubMed, Web of Science, CINAHL-EBSCO, and MEDLINE-
Ovid was conducted using the following key words: maternal, pregnant women, caffeine, and childhood obesity.
The search produced five articles of which four were relevant to the topic and met all eligibility criteria. The four
applicable articles were then reviewed using GRADE criteria.
Results: Three of the 4 eligible studies suggest that there is an association between higher levels of caffeine intake
in pregnancy and increased weight in childhood compared to lower levels of maternal caffeine intake. One study
even suggests complete avoidance of caffeine may be advisable after findings reveal increased BMI from infancy to
childhood were associated with any amount of caffeine intake during pregnancy. In contrast, a study by the Research
Institute at Nationwide Children’s Hospital did not support the theory that increasing maternal caffeine consumption
during pregnancy increases the risk of childhood obesity.
Conclusion: The use of caffeine during pregnancy may be linked to childhood obesity. Providers should consider
giving stricter recommendations than the current ACOG guidelines of <200mg/day and may even educate pregnant
mothers to eliminate caffeine altogether.
Keywords: Maternal, pregnant women, caffeine, childhood obesity
4
|| Table of Contents ||
Biography.…..….…………….………………………………………………………………...….2
Abstract.….……..…………..…………………………………………………………..................3
Table of Contents …...……………..………………………………………………………….......4
List of Tables …...…….…………….…………………………………………………….............5
List of Abbreviations.……....…………….…………………………………………….................5
Background……………………………….………………………………………………….........6
Methods……………..………………………..…………………………………………...........….7
Results.….………………..……………………………..………………………………...…….…8
Discussion………………………..……....…………………………………………………..…..15
Conclusion…………………………………………………………………………………..…...18
References...……………...……………………………………………………………...............20
Table 1……....…….……………………………………………………………………..…........22
Table 2……....…….……………………………………………………………………..…........23
Figure 1..…....…….……………………………………………………………………..…........24

5
List of Tables and Figures
Table 1: Characteristics of Reviewed Studies and GRADE profile.
Table 2: Maternal caffeine intake in pregnancy and risk of overweight/obesity at age 3 years, 5
years and 8 years.
Figure 1: Mean difference in BMI z-score at ages 4 and 7 years by maternal serum paraxanthine
(micrograms/liter) at <20 and 26+ weeks, adjusted for maternal age, prepregnant weight,
smoking, education, gestation at blood draw, and diabetes.
List of Abbreviations
ACOG American Congress of Obstetricians and Gynecologists
CDC Centers for Disease Control and Prevention
GRADE Grading of Recommendations, Assessment, Development, and Evaluations
SGA Small-for-Gestational Age
6
BACKGROUND
It is no secret that childhood obesity has swiftly reached epidemic proportions as nearly a
third of our nation’s youth now qualifies as being overweight or obese.
1
The issue of increased
weight in childhood is especially problematic given that those who meet criteria for overweight
and obesity in childhood are much more likely to be overweight and obese in adulthood and to
suffer the systemic consequences of obesity including increased morbidity and mortality.
2
Therefore, the etiology and prevention of obesity among our pediatric population ought to be of
critical importance to health care providers in order to promote health and wellness throughout
the lifespan.
Initial strategies for remedying the issue of obesity in the pediatric population have
focused on modifying environmental factors such as the child’s diet, exercise, and access to
healthy food. However, these strategies may have failed to rectify the issue at its source; new
research suggests that the earliest environmental factors impacting weight in childhood emerge
from inside the womb.
3
The theory of fetal programming argues that threats to a fetus in utero
may cause permanent physiological changes that predispose the individual to diseases in
adulthood.
3
Caffeine is widely recognized as a threat to fetal development due to its connection
with increased risk of spontaneous abortion, preterm delivery and small-for-gestational age
(SGA) newborns.
4
However, current studies are now suggesting that the consumption of
caffeine during pregnancy may have implications well beyond the postnatal period. The current
review compiles evidence from four studies that explore an association between maternal
caffeine intake and childhood obesity.
7
METHODS
The initial literature search was conducted via MEDLINE-PubMed and Web of Science
online databases using the keywords: pregnant women, caffeine and childhood obesity. Both
search engines produced the same three articles
5,5,6
, all three of which matched the eligibility
criteria. Eligibility required human studies published in English no earlier than 2008 with
abstracts that contained 1 or more of the key search terms identified (See Abstract). A
subsequent search of CINAHL-EBSCO using the aforementioned keyword search produced zero
articles. A follow-up search was conducted using the keywords: maternal, caffeine and childhood
obesity using MEDLINE-PubMed, Web of Science and MEDLINE-Ovid. Of the five articles
produced by this search, three were the same articles produced by the initial literature search, one
article was not relevant to the current review of literature and one additional article met
eligibility criteria.
RESULTS
The online literature searches produced a total of 6 unique articles, 4 of which were
relevant and met eligibility criteria. These articles included 3 prospective cohort studies
6,7,8
and 1
case control study.
5
Klebanoff et al
This case-control study
5
which was part of the Collaborative Perinatal Project, assessed
the relationship between maternal serum caffeine metabolite levels and body mass index of
offspring at ages 4 and 7 years. Pregnant women were recruited from 1 site in the U.S between
1959 and 1965 and tracked from pregnancy to delivery. Paraxanthine, caffeine’s primary
metabolite, was measured in women’s serum to objectively quantify maternal caffeine intake.
8
Serum levels were measured at 2 points during pregnancy, <20 weeks’ and ≥26 weeks’ gestation.
The blood samples were frozen at -20 degrees Celsius and analyzed between 1997 and 1999.
5
Height and weight measurements were taken at study visits when children were ages 4
and 7 years. The Centers for Disease Control 2000 growth charts were utilized to calculate sex
and age specific BMIs. Children with an average z-score, percent BMI ≥85
th
percentile, and
percent BMI ≥95
th
percentile for age and sex, were considered important outcomes. Furthermore
persistent obesity was characterized as a BMI ≥95
th
percentile at ages 4 and 7 years.
5
Adjustments were made for a variety of possible confounders including maternal age,
race, education, weight prior to pregnancy, smoking at enrollment, gestation at blood draw, and
diabetes during pregnancy. Upon analysis, it was recognized that paraxanthine levels tended to
be higher in women who were older, had smoked heavily at registration, were less educated, and
were Caucasian. Children at both ages were observed to have higher BMIs with increasing
maternal age, amount of maternal smoking, and increased maternal prepregnant weight.
Ultimately a significant relationship was not demonstrated between maternal serum paraxanthine
and increased risk of childhood obesity. A connection between serum paraxanthine was observed
with increased risk of elevated BMI in offspring but only up to about 1000 µg/liter. The
association flattened or fell at increased paraxanthine concentrations. Adjusting for confounders
greatly decreased any associations that were found (Figure 1).
5
Limitations of the study include not gathering information on the diet or exercise of
mothers and their children, nor the incidence of breastfeeding after birth. Another flaw of the
study is the difference in caffeine consumption, prevalence of maternal smoking, incidence of
prepregnant obesity, and nutritional supplementation for mothers and babies between the
population in the 1960s and present day. Lastly, paraxanthine was only measured at 2 points
9
during pregnancy, and using it as a proxy for caffeine consumption may have skewed the
results.
5
Li et al
This prospective cohort study
6
assessed in-utero caffeine exposure and the risk of
childhood obesity of offspring over a 15-year follow up period. One thousand sixty-three women
with positive pregnancy tests in the San Francisco area were incorporated into a study of the risk
factors for miscarriage on patients from Kaiser Permanente Northern California (KPNC) from
1996 to 1998. Of the 1063 women who were early in their pregnancies, 829 of them birthed live
children who were included in the study, and 28 children were excluded due to failure to receive
pediatric care in the KPNC system. Ultimately 615 mother-child pairs were included in the
study. To determine caffeine consumption for the first or early second trimesters of their
pregnancies, women reported all beverages consumed since their last menstrual period during in-
person interviews. They were questioned on type, timing, frequency, amount, and patterns of
beverage consumption since becoming pregnant. Caffeine consumption was reported on a daily
or weekly basis including caffeine from coffee, tea, 17 kinds of soda, and hot chocolate. Total
daily caffeine intake was calculated using conversion factors. The caffeine in decaffeinated tea
proved difficult to calculate, leading the authors to exclude 46 participants who drank
decaffeinated teas exclusively. Women were grouped into the following categories of caffeine
consumption during pregnancy for data analysis: 0mg, <150mg per day, and ≥150mg per day.
6
Weight and height of offspring from birth onward were obtained using paper and
electronic medical records. For those children that stayed in the KPNC system, data was
collected for over 15 years until the end of the study on May 31
st
, 2013. BMI was calculated if
both height and weight had been recorded on the same day. Almost 100% of mother and baby
10
pairs had multiple BMI measurements with the average number of child measurements being 17.
Children were considered obese if they had a BMI at or above the 95
th
percentile after age 2, per
the 2000 Centers for Disease Control definition of obesity. Furthermore, children were
considered to have persistent obesity rather than temporary/transitory obesity if more than half of
their BMIs during follow up met criteria for obesity, or if after age 11 years their final
measurement was over the 95
th
percentile.
6
When set against women who did not consume caffeine during pregnancy, it was found
that women who consumed greater than 150mg of caffeine per day were more likely to be older,
Caucasian, smokers, multiparous, and tended not to breastfeed their offspring. Multiple factors
were controlled for including gender and age of children at weight measurements, prepregnancy
BMI and race/ethnicity, and maternal age and smoking. The study observed an 87% increased
risk of obesity in children of women consuming any amount of caffeine during pregnancy
compared to the children of women whose caffeine consumption was 0. Daily maternal caffeine
intake ≥150mg showed a more than doubled risk of offspring obesity and daily maternal caffeine
consumption < 150mg was associated with a 77% increase in offspring obesity risk. After
analyzing children with persistent obesity separately from children with transitory obesity, a
statistically significant linear dose-response relationship between amount of daily maternal
caffeine intake and obesity in offspring was found in female children with persistent obesity.
Caffeine source as well as factors such as preexisting maternal conditions and lifestyle patterns
of offspring did not appear to alter the results.
6
Limitations of this study include not ruling out all possible confounders such as added
sugar in beverages. Caffeine intake was only reported in the first two trimesters, thus caffeine
consumption could have changed in the third trimester leading to inaccurate estimations.
6
11
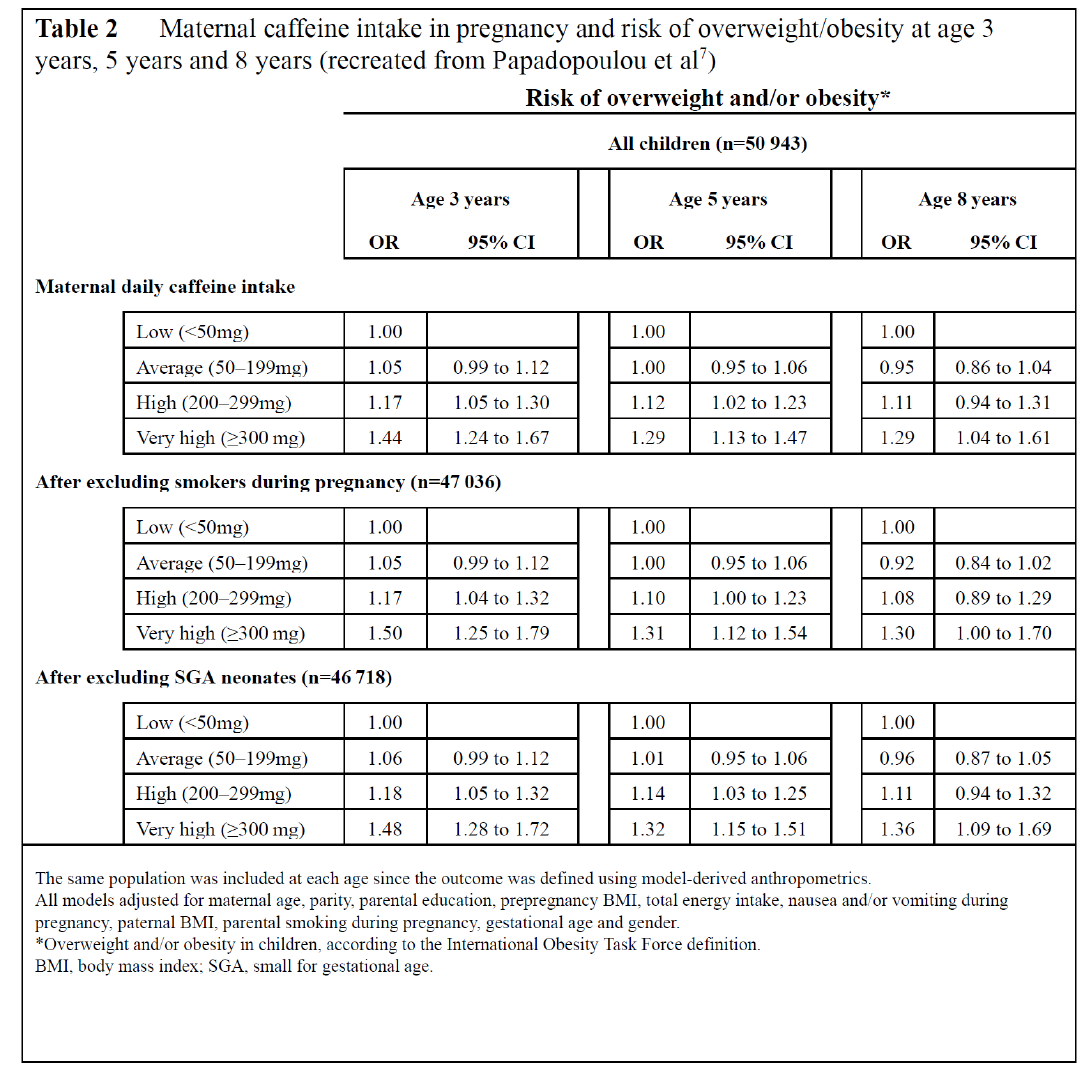
Papadopoulou et al
This was a prospective cohort study,
7
embedded in the Norwegian Mother and Child
Cohort study, that looked for connections between caffeine intake in pregnant women and weight
in offspring until age 8 years. Taking place across Norway, the study recruited mothers from
2002-2008. Exclusion criteria consisted of multiple gestations, stillbirths, fetal malformations
and chromosomal abnormalities. Caffeine intake was self-reported during mid-pregnancy using
the Food Frequency Questionnaire (FFQ) which was created for the study to assess diet during
the first 4-5 months of pregnancy. Only mother-child pairs who had a completed FFQ and data
on small for gestational age as well as offspring height and weight were included in the study,
leaving 50 943 pairs as the study cohort. Using the FFQ, mean daily caffeine consumption was
calculated from a variety of sources including coffee, black tea, soda, energy drinks, chocolate
beverages, sandwich spread, desserts, and cocoa containing foods. Caffeine intake was grouped
into the following categories: low (0-49mg/day), average (50-199mg/day), high (200-
299mg/day), and very high (≥300mg/day).
7
Body growth information including weight and length/height of singleton offspring were
collected at the same 11 age points from 6 weeks to 8 years old. The study used a WHO weight
gain z-score of >0.67 to define excess infant growth and criteria from the International Obesity
Task Force to define children as overweight.
7
Statistical adjustments were made for maternal age, education, BMI, smoking, caloric
intake and nausea and vomiting, as well as for paternal smoking. Gestational age and gender of
offspring was accounted for. A negative control analysis was also performed to assess if paternal
caffeine intake could be a possible confounder.
12
A comparison of pregnant women revealed that the more caffeine a woman consumed the
more likely she was to be above 30 years, multiparous, have a higher energy intake, smoke, and
not experience nausea or vomiting during pregnancy. She also tended to have lower education,
be obese prior to pregnancy and have a partner who was obese and smoked. The study
demonstrated that average, high, and very high caffeine intake were associated with higher risk
of excess infant growth. Any amount of caffeine exposure proved to increase the risk of
becoming overweight in offspring at ages 3 and 5, with a continued elevated risk at 8 years for
children exposed to very high levels of caffeine in-utero (Table 2). Even exposure to small
quantities of caffeine was related to higher BMIs from age 1 month to age 8 and very high
exposure demonstrated an increased velocity of weight gain from infancy to age 8. The negative
paternal control yielded very little change in the connection between maternal caffeine
consumption and risk of excess infant growth and overweight at 3 years old.
7
A limitation of this study is that not all participants could contribute anthropomorphic
data at 8 years leading to possible outcome misclassification. Varying percentages of the
population had either not reached 8 years old by the end of the study, could not contribute
information, or were lost to follow up. Self-reporting of diet is another limitation.
7
Voerman et al
This population-based prospective cohort study,
8
performed in Rotterdam, the
Netherlands, looked at the relationship between maternal caffeine consumption in pregnancy
with growth patterns, body fat composition and insulin levels of offspring from birth to age 6
years. Nestled within the Generation R study which focused on fetal life through childhood, the
study included pregnant women enrolled between 2001 and 2005. Originally 8879 women were
13
enrolled during pregnancy and ultimately 5562 were involved in the follow-up 6 years later,
contributing information on the BMI, body composition, and insulin levels of their children.
8
Maternal caffeine ingestion was assessed by mail in questionnaire during all 3 trimesters
of pregnancy with response rates over 75% in each trimester. Women provided information on
the average number of daily cups of coffee and tea consumed, and the caffeine content was then
calculated using standard caffeine amounts for coffee and tea in the Netherlands. Caffeine intake
was divided into 4 categories: <2 units, 2-3.9 units, 4-5.9 units, ≥6 units, with each unit equal to
90mg of caffeine (a standard cup of coffee).
8
Children’s height and weight were collected using medical records from birth,
subsequently measured at 6 months and then in 12-month intervals until age 3 years. Body mass
index (BMI) was calculated beginning at 6 months of age and then sex and age appropriate
standard deviation scores (SDS) were made for study participants utilizing North-European and
Dutch growth charts. At age 3 children were described as overweight or obese according to The
Obesity Task Force definition of obesity for girls and boys specifically. Dual-Energy X-ray
absorptiometry (DXA) was used to calculate total body fat mass and android/gynoid fat mass
ratios in children at age 6 years. Additionally, abdominal ultrasound was utilized to ascertain pre-
peritoneal fat mass in place of visceral fat. Insulin and C-peptide levels were measured using
fasting venipuncture blood draws in children at 6 years old.
8
Questionnaires aided in gathering information such as maternal demographics, smoking
and alcohol consumption during pregnancy, sources of nutrition in offspring during infancy, and
the amount of TV watched by 6-year-old children. Hospital and midwife registries provided data
on pregnancy disorders like gestational diabetes and hypertension.
8
14
Women with caffeine intakes ≥6 units per day tended to have higher education levels,
have never given birth before, and be European. Mothers were excluded from analysis due to
failure to report all caffeine consumption during pregnancy or from loss to follow up, though the
difference in their caffeine intake compared to women included in the analysis was minimal.
When analyzing the results of the study, standard 95% confidence intervals were utilized and P
values of <0.05 were considered significant. Compared with the offspring of pregnant women
who consumed <2 units of caffeine per day, children of women consuming ≥6 units of daily
caffeine had increased weight gain from birth to 3 years old as well as increased BMI from 6
months to 3 years old. Additionally, the children of mothers consuming 4-5.9 and ≥6 units of
daily caffeine had increased childhood BMI and total body fat mass. Elevated android/gynoid fat
mass ratio was solely observed in offspring of women with ≥6 units of daily caffeine intake. The
completely adjusted model revealed a greater risk of being overweight in the children of women
who consumed ≥6 units of caffeine during pregnancy set against the children of women who
consumed <2 units (odds ratio:1.25 (95% CI: 0.68, 2.30)). No association was found with
caffeine consumption in pregnancy and childhood insulin or c-peptide levels.
8
Though a large population of pregnant women and their children were studied, there was
a 30% loss to follow up at 6 years. Other limitations include the study’s inability to fully adjust
for specific diets of pregnant women and their children, and the exclusion of dietary sources of
caffeine beyond coffee and tea. Because questionnaires were used to establish caffeine intake,
results could be subject to underreporting. Lastly it was assumed that a cup of coffee was
125mL, potentially causing a mis-categorization of maternal caffeine consumption if volumes
strayed from the expected value.
8
15
DISCUSSION
Caffeine is the most commonly used psychoactive drug in the world. It can be found in a
wide variety of foods and beverages including coffee, tea, soda, chocolate, weight loss pills, and
certain types of pain medication.
9
Caffeine’s ability to cross the placenta and blood-brain barrier
allows entry of the drug into the fetal blood supply. Exposure of the fetus to caffeine is further
augmented during pregnancy as a result of prolonged elimination of the drug.
10
Animal studies
yield several biological postulates that may explain how fetal caffeine exposure can lead to
weight gain by altering: (1) adenosine regulation,
11
which plays an important role in
development, (2) leptin levels
12
, which regulates feelings of hunger, and (3) the hypothalamus-
pituitary-axis
13
, which increases metabolic syndrome susceptibility.
In light of the potential teratogenic effect of caffeine on fetal development, several
recommendations have been put forth to limit in utero caffeine exposure. The American
Congress of Obstetricians and Gynecologists (ACOG) suggests that pregnant women limit
caffeine to no more than 200mg/day.
14
Despite this recommendation, Weng et al found that 75%
of U.S. pregnant women continue to consume caffeine during pregnancy and 15% of U.S
pregnant women report consuming 200mg of caffeine or more per day while pregnant.
Considering the prevalence of caffeine consumption in pregnancy and its possible association
with increased weight in childhood, there exists a window of opportunity for the prevention of
childhood obesity as it relates to environmental exposures in the womb.
Despite the conflicting results presented in the aforementioned articles, 3 of the 4
studies
6,7,8
reviewed found an association between maternal caffeine intake and increased weight
in childhood. Of the 3 articles
6,7,8
that showed a statistically significant correlation between in-
utero caffeine exposure and increased weight in childhood, 2 studies
6,7
found that any caffeine
16
intake led to adverse effects on childhood weight. Bearing in mind that ACOG recommends
limiting caffeine consumption to less than or equal to 200mg/day during pregnancy,
14
consideration should be taken to create stricter guidelines and possibly to recommend complete
elimination of caffeine intake during pregnancy.
Each study measured caffeine intake differently. Voerman et al
8
limited their study of
7857 mothers by collecting information on maternal caffeine intake solely from caffeinated and
decaffeinated coffee and tea. Voerman et al
8
reasoned that from 2002-2006, when their data was
collected, coffee and tea accounted for 96% of overall caffeine consumption.
8
However, the
Papadopoulou et al
7
study was conducted between 2002-2008 with a larger sample size of 50943
pregnant mothers and the results showed “adverse effects on child’s growth even at low caffeine
intakes, in the range of the recommendations, that are mostly due to consumption of foods and
drinks other than coffee (chocolate, black tea, caffeinated sodas)”. Li et al
13
measured caffeine
intake from coffee, tea, soda, energy drinks and hot chocolate and found similar effects of
caffeine exposure as Papadopoulou et al.
14
These results are not surprising as many foods and
beverages contain caffeine that consumers may not be aware of and these sources of caffeine
may play a larger role on in-utero exposure to caffeine than previously suspected.
All 3 of the prospective cohort studies
6,7,8
in this review used self-report to measure
maternal caffeine intake. There are several factors that can reduce the accuracy of these
measurements. The studies that used self-report were conducted in different countries: the United
States,
6
Norway,
7
and the Netherlands.
8
A coffee serving in the Netherlands is typically 125mL
and contains about 90mg of caffeine, assuming the coffee is caffeinated. However, a standard
U.S coffee mug holds 350mL of fluid, which equates to 142mg of caffeine. A U.S. coffee mug is
often referred to as a “cup” of coffee, while the US customary unit, cup, is a unit of volume equal
17
to 8oz. Furthermore, espresso-containing drinks which are commonly referred to as coffee drinks
are now a prominent source of caffeine in the United States. In fact, “gourmet” espresso drinks
now account for 59% of coffee consumed daily in the United States.
15
Compared to coffee
which averages 12-16mg of caffeine per ounce, one ounce of espresso contains 63mg of
caffeine.
16
However, the amount of caffeine in coffee or espresso varies by brand, type of bean,
roasting method and preparation technique.
16
Depending on how the researchers educated
mothers on how to self-report their intake of caffeine-containing beverages, the self-reported
measurements may not accurately represent maternal caffeine consumption.
Klebanoff et al
5
attempted to quantify maternal caffeine intake more objectively by
measuring serum paraxanthine, the primary metabolite of caffeine. Using a biomarker for
caffeine may have limited reporting errors that influenced the results of the other studies in this
review. However, Klebanoff et al drew maternal serum from 1959-1966, nearly 5 decades before
the remaining studies in this review collected their data.
5
This difference is not to be taken
lightly. During World War II, the United States began rationing coffee available to the general
public. In 1946, just as WWII ended, the supplies of coffee increased, and the annual per capita
coffee consumption peaked to an all-time high. Klebanoff et al consider increased maternal
intake to be a strength of their study
5
; however, this unique time in U.S. history leads to a
multitude of confounding factors that may limit this study’s validity including: lack of prenatal
vitamin recommendations, higher prevalence of smoking, wide usage of synthetic chemical
pesticides, etc. Furthermore, several factors determine how fast an individual can metabolize
food, namely pregnancy, smoking, regulation of TSH, exercise, etc. Anything that can change an
individual’s metabolism will also affect the amount of paraxanthine in the serum. For these
18
reasons, the quality of evidence was downgraded and provided very low quality evidence based
on GRADE criteria.
Despite several limitations, the highest quality evidence available suggests a link between
maternal caffeine intake and increased weight in childhood. The World Health Organization
considers obesity a top public health challenge of the 21
st
century, yet, over 75% of U.S.
pregnant women consume caffeine on a daily basis.
6
This is an area where providers can use
patient education and motivational interviewing in order to prevent serious implications
throughout a lifespan. Providers should consider more stringent caffeine intake recommendations
or complete abstinence from maternal caffeine intake to all pregnant women and those
attempting to become pregnant in order to prevent childhood obesity and its comorbidities.
CONCLUSION
The articles in this review were contradictory regarding the association between maternal
caffeine intake and childhood obesity. Two articles found that any amount of caffeine intake
during pregnancy had an adverse effect on weight of offspring in childhood. Voerman et al found
that higher levels of caffeine are linked to increased weight gain from birth to 6 years and a
higher BMI from 6 months to 6 years. Only one article found no association with maternal
caffeine intake and weight in childhood; however this study had very low quality evidence and
studied women who were pregnant in the 1950s and 60s.
Despite the contradictory nature of these articles and the poor quality of evidence
provided, there is sufficient evidence to suggest a link between maternal caffeine intake and
increased weight in childhood. As childhood obesity rates are quickly rising and the majority of
pregnant mothers throughout the U.S. are still consuming caffeine, this should be an area of
19
concern for providers. This review seeks to encourage providers to recommend pregnant patients
limit caffeine intake below ACOG guidelines and, perhaps, eliminate the use of caffeine entirely.

20
References
1. Ogden, C. L., Carroll, M. D., Kit, B. K. & Flegal, K. M. Prevalence of Childhood and Adult
Obesity in the United States, 2011-2012. JAMA 311, 806–814 (2014).
2. Guo, S. S. & Chumlea, W. C. Tracking of body mass index in children in relation to
overweight in adulthood. Am. J. Clin. Nutr. 70, 145S-148S (1999).
3. Kwon, E. J. & Kim, Y. J. What is fetal programming?: a lifetime health is under the control
of in utero health. Obstet. Gynecol. Sci. 60, 506–519 (2017).
4. Greenwood, D. C. et al. Caffeine intake during pregnancy and adverse birth outcomes: a
systematic review and dose-response meta-analysis. Eur. J. Epidemiol. Dordr. 29, 725–34
(2014).
5. Klebanoff, M. A. & Keim, S. A. Maternal Serum Paraxanthine During Pregnancy and
Offspring Body Mass Index at Ages 4 and 7 Years: Epidemiology 26, 185–191 (2015).
6. Li, D.-K., Ferber, J. R. & Odouli, R. Maternal caffeine intake during pregnancy and risk of
obesity in offspring: a prospective cohort study. Int. J. Obes. 2005 39, 658–664 (2015).
7. Papadopoulou, E. et al. Maternal caffeine intake during pregnancy and childhood growth and
overweight: results from a large Norwegian prospective observational cohort study. BMJ
Open 8, e018895 (2018).
8. Voerman, E. et al. Maternal caffeine intake during pregnancy, early growth and body fat
distribution at school-age. The Generation R Study. Obes. Silver Spring Md 24, 1170–1177
(2016).
9. Scientific Opinion on the safety of caffeine. EFSA J. 13, 4102
10. Brazier, J. L., Ritter, J., Berland, M., Khenfer, D. & Faucon, G. Pharmacokinetics of caffeine
during and after pregnancy. Dev. Pharmacol. Ther. 6, 315–322 (1983).
21
11. Buscariollo, D. L. et al. Embryonic Caffeine Exposure Acts via A1 Adenosine Receptors to
Alter Adult Cardiac Function and DNA Methylation in Mice. PLOS ONE 9, e87547 (2014).
12. Wu, Y. et al. Prenatal caffeine exposure induced a lower level of fetal blood leptin mainly
via placental mechanism. Toxicol. Appl. Pharmacol. 289, 109–116 (2015).
13. Caffeine-Induced Activated Glucocorticoid Metabolism in the Hippocampus Causes
Hypothalamic-Pituitary-Adrenal Axis Inhibition in Fetal Rats. Available at:
http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0044497. (Accessed: 16th
July 2018)
14. Moderate Caffeine Consumption During Pregnancy - ACOG. Available at:
https://www.acog.org/Clinical-Guidance-and-Publications/Committee-Opinions/Committee-
on-Obstetric-Practice/Moderate-Caffeine-Consumption-During-Pregnancy. (Accessed: 16th
July 2018)
15. Sethi, S. A Surprising New Trend In Coffee. Forbes Available at:
https://www.forbes.com/sites/simransethi/2017/12/01/a-surprising-new-trend-in-coffee/.
(Accessed: 27th July 2018)
16. Roberts, C. Is There More Caffeine in Espresso Than in Coffee? Consumer Reports
Available at: https://www.consumerreports.org/coffee/is-there-more-caffeine-in-espresso-
than-in-coffee/. (Accessed: 27th July 2018)
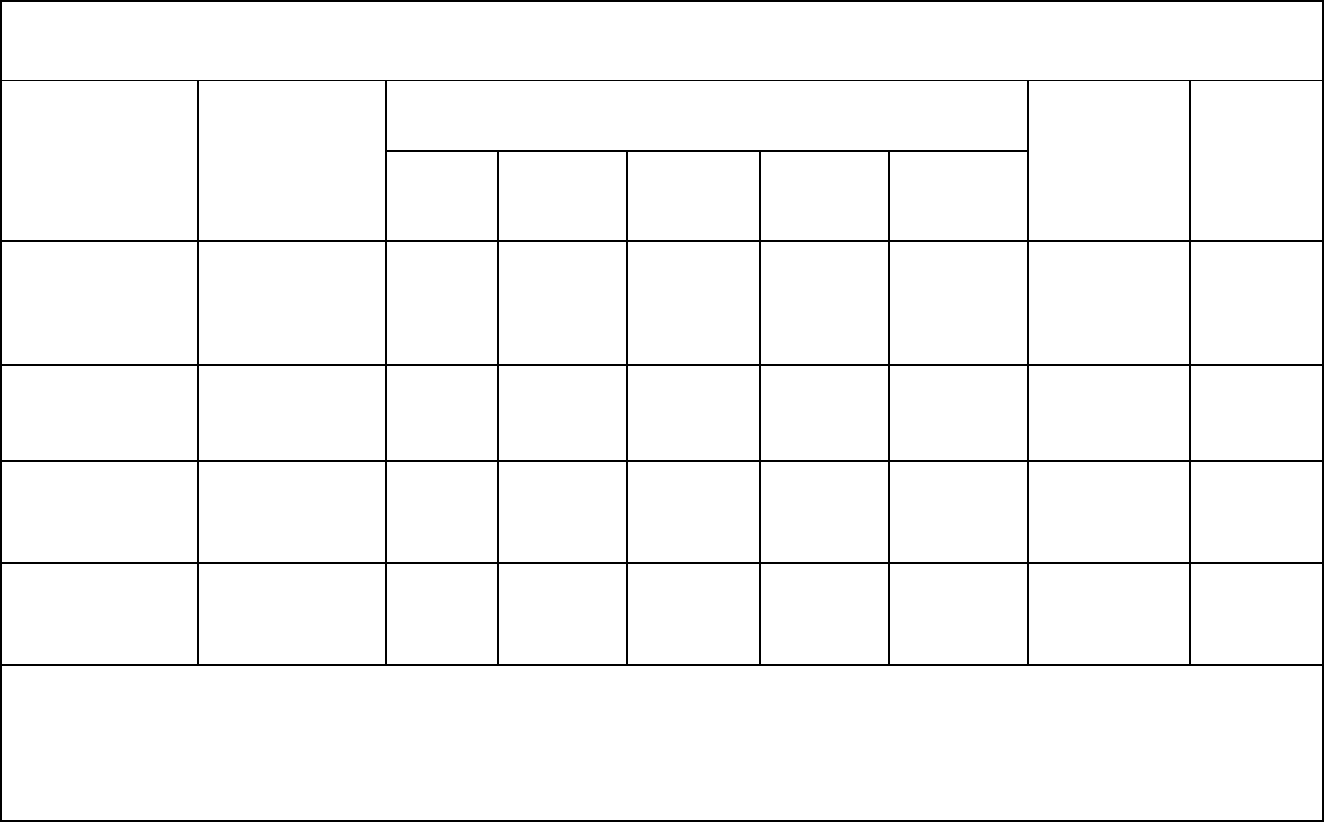
22
Table 1: Quality Assessment of Reviewed Articles
Study
Design
Downgrade Criteria
Upgrade
Criteria
Quality
Limitations
Indirectness
Inconsistency
Imprecision
Publication
bias
Klebanoff et al
5
Case Control
Study
Serious
a
Serious
Not
Serious
Not
Serious
Likely
None
Very low
Li et al
6
Prospective
Cohort
Serious
b
Not
Serious
Not
Serious
Not
Serious
Unlikely
Dose
Response
Very low
Papadopoulou et
al
7
Prospective
Cohort
Serious
b,c
Not
Serious
Not
Serious
Serious
Unlikely
None
Very low
Voerman et al
8
Prospective
Cohort
Serious
b,c
Not
Serious
Not
Serious
Not
Serious
Unlikely
None
Very low
a
Study published more than 50 years ago, did not collect diet or exercise information, and used paraxanthine to measure caffeine
consumption
b
Strong use of self-reported data
c
High rate of lost to follow up or ineligible data
23

24
© 2018 Klebanoff et al.
5
, CC-BY-NC 4.0.

