
Suggested citation: European Centre for Disease Prevention and Control. Rapid Risk Assessment. Outbreak of yellow
fever in Angola, Democratic Republic of Congo and Uganda: First update, 27 May 2016. Stockholm: ECDC; 2016.
© European Centre for Disease Prevention and Control, Stockholm, 2016
Main conclusions and options for response
In the EU/EEA, the risk of yellow fever virus being introduced is limited to unvaccinated viraemic travellers
coming from epidemic areas. Given that outbreaks of yellow fever in urban settings have the potential for rapid
spread and that significant yellow fever epidemics are ongoing in Angola, DRC and Uganda, EU/EEA Member
States should consider a range of options for response.
Information for travellers to and EU citizens residing in areas with
active transmission
Travellers visiting countries where there is evidence of persistent or periodic yellow fever virus transmission and
EU citizens residing in these countries should:
• Be made aware of the risk of yellow fever;
• Check their vaccination status and get vaccinated. Vaccination against yellow fever is recommended for all
those ≥9 months old travelling to areas where there is evidence of persistent or periodic yellow fever virus
transmission. WHO publishes a list of countries, territories and areas with yellow fever vaccination
requirements and recommendations [1] which includes Angola, Democratic Republic of Congo and Uganda.
In Angola, the country requirement specifies that a yellow fever vaccination certificate is required for
travellers aged over nine months. To reduce the risk of serious adverse events, healthcare practitioners
should be aware of the contraindications and follow the manufacturers’ advice on precautions to take before
administering yellow fever vaccine [2].
• Take measures to prevent mosquito bites indoors and outdoors, especially between sunrise and sunset
when
Aedes
mosquito vectors are most active and biting. These measures include:
− The use of mosquito repellent in accordance with the instructions indicated on the product label.
− Wearing long-sleeved shirts and long trousers, especially during the hours when the type of mosquito
known to transmit the yellow fever virus (
Aedes
) is most active.
− Sleeping or resting in screened or air-conditioned rooms or using mosquito nets, at night and during the
day.
Options to prevent importation into EU/EEA countries
Implement the WHO International Health Regulations (IHR) Emergency Committee recommendation to only
allow travellers showing proof of a valid vaccination record for yellow fever to leave Angola. The procedure
should also be applied to land and sea borders. Entry screening in the EU, for proof of vaccination, would be of
RAPID RISK ASSESSMENT
Outbreaks of yellow fever in Angola,
Democratic Republic of Congo and Uganda
First update, 27 May 2016

RAPID RISK ASSESSMENT Outbreak of yellow fever in Angola, DRC and Uganda, first update – 27 May 2016
2
limited value because of the limited availability of direct flights and the high likelihood of indirect travel routes
into the EU.
• Alternatively, EU Member States, particularly those including areas with established populations of suitable
Aedes
mosquitoes, could prevent the arrival of viraemic travellers by requesting proof of valid vaccination
when issuing a visa.
Options to prevent transmission in EU/EEA countries
• Raise awareness of public health stakeholders, in particular clinicians and travel health clinics, concerning
the risk of yellow fever virus introduction into the EU through unvaccinated viraemic travellers coming from
epidemic areas.
• Clinicians should consider yellow fever among differential diagnoses for travellers returning from affected
areas.
• Ensure that clinicians and travel health clinics get updated information about areas with an ongoing yellow
fever outbreak to support their diagnosis in travellers returning from those areas.
• Apply strict personal prevention measures against
Aedes
mosquito bites for any suspected and confirmed
yellow fever cases through the use of a mosquito net in receptive areas for yellow fever transmission (i.e.
areas with active competent vectors and human populations susceptible to yellow fever infection).
• Implement focal vector control in the areas where unvaccinated viraemic travellers have stayed. This option
helps to reduce the risk of onward autochthonous transmission to the EU/EEA mainland and EU Overseas
Countries and Territories (OCT) and Outermost Regions (OMR), in areas where yellow fever vectors are
present. The vector competence of European
Aedes albopictus
mosquito populations needs to be assessed.
Source and date of request
ECDC internal decision, 17 May 2016
Public health issue
This document assesses the risk of yellow fever infections in EU/EEA countries related to the ongoing outbreak of
yellow fever in Angola, DRC and Uganda.
ECDC published a rapid risk assessment on 25 March 2016.
Consulted experts
ECDC experts (in alphabetical order): Kaja Kaasik Aaslav, Denis Coulombier, Tarik Derrough, Josep Jansa, Laurence
Marrama, Thomas Mollet, Ettore Severi, Bertrand Sudre, Wim Van Bortel.
Experts from the following institutions contributed to this risk assessment: World Health Organization Regional Office
for Europe, World Health Organization Country Office for Angola.
The following experts are acknowledged for their contribution to the mission and for providing comments on the
rapid risk assessment: Veerle Vanlerberghe (Institute of Tropical Medicine Antwerp, Belgium), Joana Haussig (Robert
Koch Institute, Postgraduiertenausbildung für angewandte Epidemiologie (PAE), Germany), Jonathan Baum (ECHO,
Mercator Fellowship on International Affairs, Germany), Amparo Laiseca (European Commission's Humanitarian Aid
and Civil Protection department, Kinshasa).
ECDC acknowledges the valuable contributions of all experts. Although experts from the World Health Organization
(WHO) reviewed the risk assessment, the views expressed in this document do not necessarily represent the views
of WHO. All experts have submitted declarations of interest and a review of these declarations did not reveal any
conflicts of interest.
Some of the data used in this rapid risk assessment were collected during a mission to Angola in May 2016.
Disease background information
Yellow fever is an acute viral haemorrhagic vector-borne disease affecting humans and non-human primates in 34
countries across sub-Saharan Africa and in tropical areas of 13 countries across South and Central America, from
Panama to the northern part of Argentina [1,2]. Autochthonous transmission of yellow fever has never been detected
in Asia, although the competent vector is present in south and south eastern areas of the continent [5].

RAPID RISK ASSESSMENT Outbreak of yellow fever in Angola, DRC and Uganda, first update – 27 May 2016
3
Yellow fever is caused by a virus of the
Flavivirus
genus of the
Flaviviridae
family and transmitted by infectious
mosquitoes of
Aedes
and
Haemagogus
genera. Monkeys and humans act as amplifying hosts. The virus originated
in Africa and was introduced to the Americas several hundred years ago.
In Africa, there are three cycles of yellow fever transmission:
1. The sylvatic cycle, in the forest, where the presence of the virus is maintained through transmission among
non-human primates by several species of sylvatic
Aedes
mosquitoes. Infected mosquitoes that bite humans
entering the forest cause sporadic cases of yellow fever.
2. Intermediate or rural cycle, at the margins of the forest and in the savannah, which are zones of emergence
of the virus, where the disease is transmitted by sylvatic
Aedes
mosquito and
Aedes aegypti
.
3. Urban cycle, when an infected human, returning to urban areas where the highly effective and anthropophilic
Aedes aegypti
vector is present, initiates human-to-human transmission cycles. Large epidemics, with tens of
thousands of deaths, have been recorded in Africa.
The competence of
Aedes albopictus
for the transmission of the yellow fever virus has been demonstrated in Brazil
using a Brazilian strain of yellow fever virus. In this study the dissemination and infection rates were lower than
those observed for
Aedes aegypti.
However, the ability of
Aedes albopictus
to transmit the yellow fever virus
cannot be ruled out in areas where infestation and biting indexes are high, even though its role in yellow fever
outbreaks has not been demonstrated [4-8].
The annual number of yellow fever cases reported to WHO is presented in Figure 1 [11]. Between 1987 and 1991,
recurrent yellow fever outbreaks were notified in Nigeria with more than 17 000 cases. In 2011, a large epidemic
took place in Burkina Faso, Nigeria and Sierra Leone. Two previous outbreaks of yellow fever have been
documented in Angola: one in 1971 (65 cases) and the other in 1988 (37 cases).
Figure 1.
Worldwide distribution of yellow fever cases reported to WHO, 1974–2014
Data source: 1974-2014 from WHO Global Health Observatory data repository
The number of reported cases is commonly underestimated. A study published in 2014 estimated that 130 000
(95% CI 51 000–380 000) severe cases and 78 000 (95% CI 19 000–180 000) deaths due to yellow fever had
occurred in Africa in 2013, accounting for about 90% of the global number of cases [10].
In the past two decades imported cases of yellow fever have been reported in Europe among unvaccinated
travellers returning from Ivory Coast (Germany, 1999), Gambia (Belgium, 2001) and Ghana (Spain, 2009) [11,12].
Up to 85% of infections in humans are either asymptomatic or only result in mild illness [15]. After an incubation
period of typically three to six days, infection occurs in one or two phases [4]. The initial symptoms include sudden
onset of fever, chills, severe headache, back pain, general body aches, nausea, vomiting, fatigue and weakness
[15]. Most people improve after the first phase but after a brief remission around 15 to 25% of cases develop the
severe form of the disease, characterised by high fever and jaundice, and in some cases bleeding, eventually
resulting in a shock and multiple organ failure. Up to 50% of severe cases may die. There is no specific treatment
for yellow fever [16]. Infection provides life-long immunity.
Yellow fever infection is challenging to diagnose, especially during the early stages. Differential diagnoses for
yellow fever include: malaria, haemorrhagic fevers (e.g. dengue haemorrhagic fever, Lassa fever, and Crimean-
Congo haemorrhagic fever), leptospirosis, viral hepatitis and liver failure (e.g. toxic hepatitis).

RAPID RISK ASSESSMENT Outbreak of yellow fever in Angola, DRC and Uganda, first update – 27 May 2016
4
The virus can be detected in blood specimens by RT-PCR, antigen-capture or viral isolation. For primary arbovirus
infections, a serological diagnosis can be made by detecting specific IgM antibodies one week after infection (for
secondary arbovirus infections, IgM and IgG need to be detected) [15].
Prevention and outbreak control
Yellow fever is effectively prevented through vaccination with the live attenuated vaccine [4].
Mass vaccination campaigns are the most effective public health strategy to control yellow fever outbreaks. A
global vaccine stockpile is managed by the International Coordinating Group (ICG) on Vaccine Provision, which
functions as a revolving fund for epidemic response [16]. In the long term, introducing preventive immunisation
through routine childhood vaccination in endemic countries can significantly reduce the burden of the disease.
Yellow fever vaccination is recommended for travellers ≥9 months old to areas where there is evidence of
persistent or periodic yellow fever virus transmission [1]. The vaccine is recommended to protect individual
travellers at risk of exposure to yellow fever and to prevent international spread of the disease from endemic
countries to countries with competent vectors [1]. Some adverse effects associated with the vaccine have been
reported and a case-by-case assessment of the risks and benefits of yellow fever vaccination should be considered
for some risk groups, such as older people or those with underlying health conditions [17]. According to WHO
‘when considering vaccination, any traveller must take into account the risk of being infected with yellow fever
virus, country entry requirements, as well as individual risk factors (e.g. age, immune status) for serious vaccine-
associated adverse events’ [1]. To reduce the risk of serious adverse events, healthcare providers should be aware
of the contra-indications and follow the manufacturer’s guidance on the precautions to be considered before
administering yellow fever vaccine [2]. Complementary preventive measures, especially for travellers, include using
insect repellent and wearing protective clothing.
The period of protection provided by yellow fever vaccination, and the term of validity of the certificate has been
changed from 10 years to life, in accordance with a Word Health Assembly resolution, and this should come into
effect from July 2016. On 19 May 2016, the Emergency Committee of the International Health Regulations (2005)
advised for immediate application of the policy of one lifetime dose of yellow fever vaccine in light of the limited
worldwide vaccine supply [20].
Mosquito control contributes to prevention of yellow fever outbreaks and is critical in situations where vaccination
coverage is low or the vaccine is not immediately available. Mosquito control includes killing adult mosquitoes and
larvae by using insecticides and larvicides, as well as eliminating mosquito breeding sites. Community involvement
through activities, such as cleaning household drains and covering water containers where mosquitoes can breed,
is a very important and effective way to control mosquitoes, but requires some time for preparation and
implementation [4,21].
Possible shortage of yellow fever vaccine has been a concern for several years. According to the latest update from
UNICEF dated May 2016,
during 2015, UNICEF increased total aggregate awards to suppliers to reach
approximately 98 million doses for 2016–2017. However, whereas supply can meet emergency stockpile and
routine requirements, it is insufficient to meet all preventive campaign demands, which increased the total demand
through UNICEF to 109 million doses
[22]. According to Lucey and Gostin, a vaccine shortage can be anticipated if
yellow fever spreads to other countries or regions, especially if large urban populations are to benefit from mass
vaccination campaigns [23].
Event background information
Situation in Angola
On 21 of January 2016, WHO was notified by the IHR focal point in Angola of an ongoing yellow fever outbreak.
The first cases reported were two males living in the municipality of Viana, a densely populated municipality on the
outskirts of Luanda. The first case presented with yellow fever symptoms to a private clinic on 5 December 2015
[24]. In the following months, suspected cases were reported in all 18 provinces of Angola and confirmed cases
were reported in 14 provinces.
Yellow fever infection was initially confirmed in three patients by PCR at the Zoonosis and Emerging Disease
Laboratory of the National Institute for Communicable Diseases in Johannesburg, South Africa in early January and
then at the Pasteur Institute in Dakar, Senegal on 20 January 2016. Following the confirmation of yellow fever
infection cases in Luanda province and other provinces of Angola, the national reporting system was enhanced to
collect epidemiological information on suspected cases and samples for laboratory confirmation.
From 21 January to 22 May 2016, the Angolan Ministry of Health notified 2 536 yellow fever cases, of which 747
were confirmed and 301 fatal (case fatality ratio: 11.9%), 88 of these being among confirmed cases (CFR: 11.8%).
The epidemic curve (Figure 2) shows that the highest number of suspected and confirmed cases was reported in
February and March 2016, with a peak of notification of more than 80 confirmed cases reported per week at the
end of February. Since April, the number of new cases has declined in Angola and in the two most affected
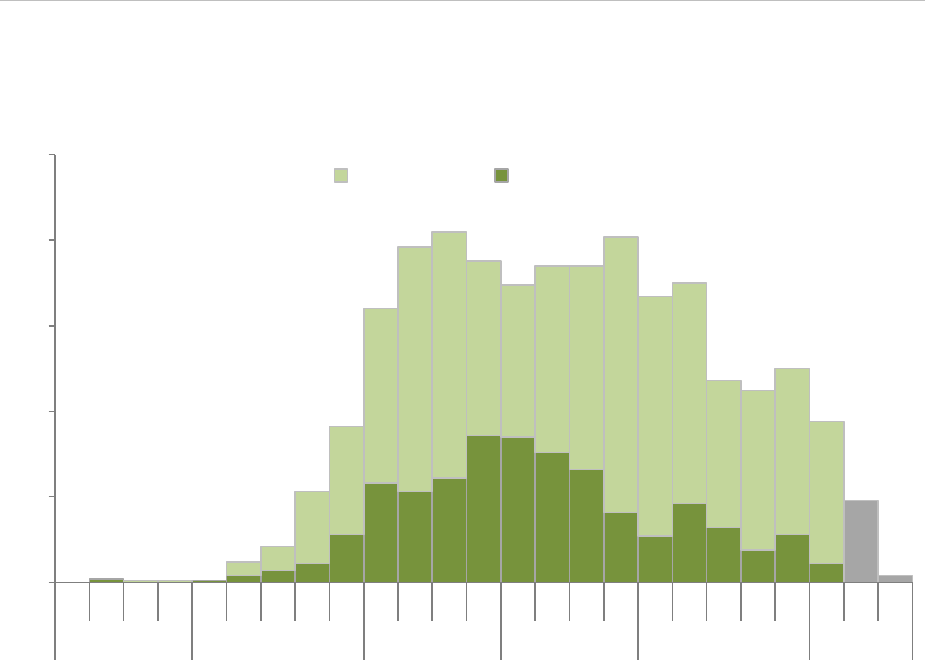
RAPID RISK ASSESSMENT Outbreak of yellow fever in Angola, DRC and Uganda, first update – 27 May 2016
5
provinces of Luanda and Huambo it has decreased to an average of 30 cases per week. However, transmission of
yellow fever has continued to spread to new areas and has increased in Benguela province.
Figure 2. Distribution of suspected and confirmed yellow fever cases per week of onset, Angola, 5
December 2015–22 May 2016
Data source: [25]
Note: Data from greyed columns is incomplete due to reporting delays
The epidemic curve should be interpreted with caution because it is likely that a significant proportion of cases has
not been diagnosed and reported, particularly during the early stages of the epidemic. Furthermore, the epicurve
presents cases by week of onset, therefore the number of cases presented for the most recent weeks will be an
underestimate because of the time lag between onset of symptoms, diagnosis and reporting. For these reasons,
figures after week 17 are incomplete. The recent decrease in the number of reported cases is occurring mainly in
Luanda and Huambo, the two largest urban areas of the country, following the implementation of vaccination
campaigns. However, Benguela, the third most populated urban area in the country, is currently reporting an
increasing number of cases, and new areas continue to experience transmission of yellow fever, indicating that the
epidemic is not yet under control.
At the time of the investigation, in addition to Luanda province, five provinces have confirmed local transmission
(Benguela, Cuanza Sul, Huambo, Huila and Uige). Overall, the three provinces with the highest number of reported
cases are Luanda, Huambo and Benguela, corresponding to the provinces where the three largest urban areas are
located (see Figure 3):
As of 23 May 2016, the province of Luanda had reported the highest number of confirmed cases (n=466). The
outbreak peaked between the last week of February and the second week of March 2016 with more than 50
confirmed cases reported per week. Since then, the number of cases has decreased, with five cases reported
for each of the last two weeks of April.
The province of Huambo, central Angola, reported the second highest number of confirmed cases after
Luanda province (n=121). The outbreak peaked between the last week of February and the third week of
March, with more than ten confirmed cases reported per week. The number of cases has decreased in recent
weeks, with four confirmed cases reported on week 17.
The province of Benguela is the third most affected after Luanda and Huambo with 66 confirmed cases. The
number of confirmed cases reported per week increased during April 2016, with the outbreak peaking in the
last week of April with 16 confirmed cases reported.
0
50
100
150
200
250
48 49 50 51 52 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20
Dec Jan Feb Mar Apr May
Suspected Confirmed

RAPID RISK ASSESSMENT Outbreak of yellow fever in Angola, DRC and Uganda, first update – 27 May 2016
6
Figure 3. Distribution of suspected and confirmed cases of yellow fever by province of reporting,
Angola, 5 December 2015–22 May 2016
Data source: Ministry of Health, Angola and WHO [25]
Cumulative attack rates during the epidemic period are highest in the two largest urban areas of the country
located in the province of Luanda, where the outbreak started in December 2015, and the province of Huambo,
affected in February 2016. Urban and peri-urban transmission cycles account for the highest number of cases, but
transmission has spread to more rural areas in recent weeks.
Most confirmed cases were in individuals between 15 and 30 years of age and overall 70% were in males. This
gender imbalance affects cases above 10 years of age. As is usual in yellow fever outbreaks, under-ascertainment
of asymptomatic and mild infections has been documented. In addition, a significant proportion of severe cases
may be unreported because private clinics are not consistently integrated into the surveillance system and a
significant proportion of the population in Angola regularly resort to traditional medicine.
The identification of suspected yellow fever cases by public health services is further affected by the concurrent
malaria epidemic that was particularly intense in the early months of 2016. A study found 47% of 232 confirmed
cases of yellow fever positive for malaria. Co-infection of yellow fever with dengue and chikungunya has been
identified, even though the laboratory assay used did not enable past and acute infections to be distinguished from
one other.
Imported cases from Angola have already been reported to DRC (41 confirmed cases), Kenya (two confirmed
cases) and the People’s Republic of China (11 confirmed cases).
In response to the epidemic, the first mass vaccination campaigns started on 2 February 2016. As of 15 of May,
the International Coordination Group for yellow fever vaccine (ICG) had released 11.7 million doses for Angola.
Vaccination campaigns took place throughout the province of Luanda in February and March, and in selected
municipalities of Huambo and Benguela provinces in April. Campaigns are currently in progress in selected
municipalities of the provinces of Benguela, Cuanza Sul, Huambo, Huíla and Uige. Due to limited vaccine supply,
the current yellow fever vaccination strategy focuses on mass vaccination campaigns of the population ≥6 months
of age in municipalities where local yellow fever transmission has been confirmed. While areas with local
transmission are a priority, the strategy does not allow intervention in as yet unaffected areas in order to prevent
establishment of transmission. Vaccine coverage analysis has shown that adult males are less likely to be
vaccinated than women and children, potentially explaining the over representation of young males among cases.
Yellow fever vaccine was integrated into routine immunisation in 1980. UNICEF estimated the yellow fever
childhood routine vaccination coverage in Angola to be 40–77 % between 2009 and 2014 [26]. Vaccine supplies
for routine childhood yellow fever vaccinations are currently insufficient.

RAPID RISK ASSESSMENT Outbreak of yellow fever in Angola, DRC and Uganda, first update – 27 May 2016
7
Vector control against
Aedes aegypti
is ongoing in urban areas, but is made difficult due to lack of information about
the distribution and abundance of the vector in the country.
Situation in the Democratic Republic of Congo (DRC)
As of 23 May, DRC has reported 590 cases of yellow fever, including 48 confirmed cases, of which 41 had a recent
travel history to Angola. Two were classified as resulting from sylvatic transmission in Tshuapa and le Bas Uélé
provinces in January 2016 and two were autochthonous cases – one in Kinshasa and one in Kongo Central province.
Three additional cases are under investigation. Confirmed cases have been reported in the provinces of Kongo central,
Kinshasa and Kwango where additional suspected cases are under investigation.
Figure 3. Distribution of yellow fever confirmed cases, by province of reporting, Democratic Republic
of Congo, 1 January –23 May 2016
Situation in Uganda
On 20 May 2016, WHO issued an update on the yellow fever outbreak in Uganda, which is unrelated to the outbreak
in Angola. Between 26 March and 19 May 2016, health authorities reported 60 yellow fever cases, including seven
deaths, in the districts of Masaka, Rukungiri, Ntungamo, Bukumansimbi, Kalungu, Lyantonde and Rakai. Seven cases
and two deaths were laboratory-confirmed. None of the cases had a recent travel history to Angola [27].
ECDC threat assessment for the EU
The large outbreak of yellow fever in Angola is of concern, given the risk that the virus will be introduced through
viraemic travellers to countries at risk of transmission, in particular neighbouring countries with receptive areas.
This extension of the epidemic would increase the number of travellers that could be exposed.
The outbreak needs to be controlled in the three countries, Angola, DRC and Uganda in order to prevent further
spread in the region and beyond. The number of new suspected and confirmed cases in Angola has been
decreasing and a mass vaccination campaign has already reached about half of the targeted population. However,
local transmission is still documented in many areas of the country and a potential vaccine shortage is predicted in
the coming months. The outbreak in Angola is not yet under control and is currently expanding to additional
provinces, posing a further challenge to the ongoing mass vaccination campaign. The possible shortage of the
yellow fever vaccine stock pile due to the ongoing outbreak in Angola and the start of vaccination in Uganda and
DRC poses a significant potential challenge for the current control strategy.
The rainy season in Angola ends in May, meaning that the conditions favouring vector abundance are likely to
decrease in the southern and central provinces, complementing the effect of the ongoing vaccination campaigns
and vector control measures in reducing the transmission rate. However, in the northern provinces environmental
conditions for mosquito transmission will probably remain suitable, allowing for new cases to occur in the coming
months, until the vaccination of the targeted population has reached an 80% coverage level, as recommended by
WHO [4].

RAPID RISK ASSESSMENT Outbreak of yellow fever in Angola, DRC and Uganda, first update – 27 May 2016
8
Currently, all regions in Angola should be considered as areas at high risk of yellow fever transmission. Large urban
areas, the province of Cabinda, where acquisition of infection was reported to have occurred for a case identified in
the Democratic Republic of Congo (DRC), and provinces in the Angolan northern provinces of Zaire, Uige, Malange
and Lunda Norte represent a significant risk for international spread. Imported confirmed cases from Angola have
been reported in DRC (41 cases), Kenya (two cases) and the People’s Republic of China (11 cases).
In DRC, the confirmation of the autochthonous circulation in the capital is a major concern as Kinshasa is densely
populated, posing the risk of extension to Brazzaville, the capital of Republic of the Congo, located across the Congo
River.
Risk for travellers and residents to affected areas
Unvaccinated travellers or residents in an epidemic area in Angola, DRC or Uganda are at risk of becoming
infected. Of particular concern are individuals who do not fulfil the vaccination criteria, such as new-born babies
and people with underlying health conditions, for whom strict individual vector control measures should be
enforced to prevent infection. Currently, all regions in Angola should be considered as areas at high risk of yellow
fever transmission. Despite an increase in vaccination coverage, areas with low coverage remain at high risk of
transmission.
Risk of international spread
The evolution of the situation in Angola, Uganda and DRC is of concern and is, according to WHO, a serious public
health event which warrants intensified national action and enhanced international support. The IHR Emergency
Committee decided on 19 May 2016 that, based on the information provided, the event does not constitute a
Public Health Emergency of International Concern (PHEIC) [20].
The outbreak in Angola is large and the number of yellow fever cases is, given the wide range of severity of its
clinical presentation, underestimated. In addition, people in the region frequently travel by road and plane to
neighbouring countries. Therefore the risk of exporting the virus to other countries is high. Viraemic patients
travelling to areas where suitable vectors and susceptible human populations are present risk causing local
transmission. Such areas exist in most of the inter-tropical zones of Africa, the Americas and Asia. Therefore, the
risk of international spread within Africa and beyond is currently high.
As yellow fever and dengue fever share the same mosquito vector (
Aedes aegypti
), any area where dengue
transmission has been documented could be suitable for local transmission of yellow fever if the virus is introduced
by a viraemic traveller. This could be the case in southern China, where dengue virus transmission occurs during
the warmer mosquito vector season, leading to local outbreaks in these areas. However, it has to be stated that
yellow fever has never been transmitted by the local
Aedes
species in South-East Asia [5].
Risk related to mass gatherings
The Rio de Janeiro 2016 Olympic Games (5–21 August 2016) and the Paralympic Games (7–18 September 2016)
are the two most prominent mass gathering events that will take place in the Americas in the coming months.
ECDC has published a specific risk assessment on these events, including an assessment for yellow fever virus
infection [28].
Risk of importation to the EU
As long as the WHO recommendation for travellers from areas experiencing epidemics to be vaccinated against
yellow fever is not enforced, the risk of yellow fever being imported into Europe exists, in relation to travellers
coming from affected countries who are not vaccinated against yellow fever. They may arrive in the EU/EEA and
become viraemic as they develop yellow fever. The risk in relation to returning EU/EEA travellers is limited since
they are likely to have been immunised.
The risk of the virus being imported into the local competent vector population in the EU through viraemic
travellers from Angola is considered to be moderate but possible, particularly in areas where
Aedes aegypti
is
present (Madeira). Potential local transmission of yellow fever in areas where
Aedes albopictus
is present in the
EU/EEA cannot be ruled out, following the introduction of the virus through a viraemic traveller. Some EU Overseas
Countries and Territories (OCT) and Outermost Regions (OMR) are located in the inter-tropical area with large
populations of competent
Aedes aegypti
mosquitoes. In these areas, the likelihood of importation is low because of
the limited travel patterns with Angola, but the risk of local transmission would be increased because of vector
availability.
There is sufficient capacity in the EU for the detection of yellow fever through several reference laboratories.
Risk of transmission in the EU
The risk of occurrence of yellow fever transmission in the EU/EEA is mainly related to areas where
Aedes aegypti
is
present. The mosquito is established in the Overseas Countries and Territories (OCT) and Outermost Regions
(OMR) of the EU, located in the yellow fever belt (inter-tropical area), as well as in the Black Sea region of

RAPID RISK ASSESSMENT Outbreak of yellow fever in Angola, DRC and Uganda, first update – 27 May 2016
9
Europe [29,30]. There are uncertainties about the capacity of
Aedes albopictus
to transmit yellow fever but
potential local transmission of yellow fever in areas where
Aedes albopictus
is present in the EU/EEA cannot be
ruled out, following the introduction of the virus through a viraemic traveller.
Conclusions and options for response
In the EU/EEA, the risk of yellow fever virus being introduced is limited to unvaccinated viraemic travellers coming
from epidemic areas. Given that outbreaks of yellow fever in urban settings have the potential for rapid spread and
that significant yellow fever epidemics are ongoing in Angola, DRC and Uganda, EU/EEA Member States should
consider a range of options for response.
Information for travellers to and EU citizens residing in areas with
active transmission
Travellers visiting countries where there is evidence of persistent or periodic yellow fever virus transmission and EU
citizens residing in these countries should:
• Be made aware of the risk of yellow fever;
• Check their vaccination status and get vaccinated. Vaccination against yellow fever is recommended for all those
≥ 9 months old travelling to areas where there is evidence of persistent or periodic yellow fever virus
transmission. WHO publishes a list of countries, territories and areas with yellow fever vaccination requirements
and recommendations [1] which includes Angola, Democratic Republic of Congo and Uganda. In Angola, the
country requirement specifies that a yellow fever vaccination certificate is required for travellers aged over nine
months. To reduce the risk of serious adverse events, healthcare practitioners should be aware of the
contraindications and follow the manufacturers’ advice on precautions to take before administering yellow fever
vaccine [2].
• Take measures to prevent mosquito bites indoors and outdoors, especially between sunrise and sunset when
Aedes
mosquito vectors are most active and biting. These measures include:
− The use of mosquito repellent in accordance with the instructions indicated on the product label.
− Wearing long-sleeved shirts and long trousers, especially during the hours when the type of mosquito
known to transmit the yellow fever virus (Aedes) is most active.
− Sleeping or resting in screened or air-conditioned rooms, or using mosquito nets, at night and during the
day.
Options to prevent importation into EU/EEA countries
• Implement the WHO International Health Regulations Emergency Committee recommendation to only allow
travellers showing proof of a valid vaccination record for yellow fever to leave Angola. The procedure should
also be applied to land and sea borders. Entry screening in the EU, for proof of vaccination, would be of limited
value because of the limited availability of direct flights and the high likelihood of indirect travel routes into the
EU
• Alternatively, EU Member States, particularly those including areas with established populations of suitable
Aedes mosquitoes, could prevent the arrival of viraemic travellers by requesting proof of valid vaccination when
issuing a visa.
Options to prevent transmission in EU/EEA countries
• Raise awareness of public health stakeholders, in particular clinicians and travel health clinics, concerning the
risk of yellow fever virus introduction into the EU through unvaccinated viraemic travellers coming from
epidemic areas.
• Clinicians should consider yellow fever among differential diagnoses for travellers returning from affected areas.
• Ensure that clinicians and travel health clinics get updated information about areas with an ongoing yellow
fever outbreak to support their diagnosis in travellers returning from those areas.
• Apply strict personal prevention measure against
Aedes
mosquito bites for any suspected and confirmed yellow
fever case through the use of a mosquito net in receptive areas for yellow fever transmission (i.e. areas with
active competent vectors and human population susceptible to yellow fever infection).
• Implement focal vector control in the areas where unvaccinated viraemic travellers have stayed. This option
helps to reduce the risk of onward autochthonous transmission in EU/EEA mainland and EU Overseas Countries
and Territories (OCT) and Outermost Regions (OMR), in areas with presence of yellow fever vectors. The vector
competence of European
Aedes albopictus
mosquito populations needs to be assessed.

RAPID RISK ASSESSMENT Outbreak of yellow fever in Angola, DRC and Uganda, first update – 27 May 2016
10
References
1. World Health Organization. List of countries, territories and areas. Yellow fever vaccination requirements
and recommendations; malaria situation; and other vaccination requirements. Geneva: WHO; 2015.
Available from: http://www.who.int/ith/2015-ith-county-list.pdf?ua=1.
2. Gershman M, Staples J. Yellow Fever: US Centers for Disease Control and Prevention. 2015. Available from:
http://wwwnc.cdc.gov/travel/yellowbook/2016/infectious-diseases-related-to-travel/yellow-fever.
3. Jentes ES. The revised global yellow fever risk map and recommendations for vaccination, 2010: consensus
of the Informal WHO Working Group on Geographic Risk for Yellow Fever (vol 11, pg 622, 2011). Lancet
Infect Dis. 2012 Feb;12(2):98-.
4. World Health Organization. Yellow fever (Fact Sheet) [Internet]. Geneva: World Health Organization; 2016
[cited 26 May 2016]. Available from: http://www.who.int/mediacentre/factsheets/fs100/en/.
5. Agampodi SB, Wickramage K. Is there a risk of yellow fever virus transmission in South Asian countries with
hyperendemic dengue? Biomed Res Int. 2013;2013:905043.
6. Mitchell C. Vector competence of North and South American strains of Aedes albopictus for certain
arboviruses: a review. J Am Mosq Control Assoc. 1991;7(3):446-51.
7. Miller BR, Ballinger ME.
Aedes albopictus
mosquitoes introduced into Brazil: vector competence for yellow
fever and dengue viruses. Trans R Soc Trop Med Hyg. 1988;82(3):476-7.
8. Mitchell CJ, Miller BR, Gubler DJ. Vector competence of
Aedes albopictus
from Houston, Texas, for dengue
serotypes 1 to 4, yellow fever and Ross River viruses. J Am Mosq Control Assoc. 1987 Sep;3(3):460-5.
9. Lourenço de Oliveira R, Vazeille M, Filippis A, Failloux A. Large genetic differentiation and low variation in
vector competence for dengue and yellow fever viruses of
Aedes albopictus
from Brazil, the United States,
and the Cayman Islands. 2003;69(1):105-14.
10. Johnson BW, Chambers TV, Crabtree MB, Filippis AM, Vilarinhos PT, Resende MC, et al. Vector competence
of Brazilian
Aedes aegypti
and
Ae. albopictus
for a Brazilian yellow fever virus isolate. Trans R Soc Trop Med
Hyg. 2002 Nov-Dec;96(6):611-3.
11. World Health Organization. Global Health Observatory data repository: World Health Organization; 2016
[cited 26 May 2016]. Available from: http://apps.who.int/gho/data/node.main.WHS3_50?lang=en.
12. Garske T, Van Kerkhove MD, Yactayo S, Ronveaux O, Lewis RF, Staples JE, et al. Yellow Fever in Africa:
estimating the burden of disease and impact of mass vaccination from outbreak and serological data. PLoS
Med. 2014 May;11(5):e1001638.
13. Bae HG, Drosten C, Emmerich P, Colebunders R, Hantson P, Pest S, et al. Analysis of two imported cases of
yellow fever infection from Ivory Coast and The Gambia to Germany and Belgium. J Clin Virol. 2005
Aug;33(4):274-80.
14. European Centre for Disease Prevention and Control. Annual epidemiological report 2014 – Emerging and
vector-borne diseases. Stockholm: ECDC; 2014. Available from:
http://ecdc.europa.eu/en/publications/Publications/emerging-vector-borne-diseases_annual-
epidemiological-report-2014.pdf.
15. Monath TP. Yellow fever: an update. Lancet Infect Dis. 2001 Aug;1(1):11-20.
16. Monath TP. Treatment of yellow fever. Antiviral Res. 2008 Apr;78(1):116-24.
17. Domingo C, Escadafal C, Rumer L, Mendez JA, Garcia P, Sall AA, et al. First international external quality
assessment study on molecular and serological methods for yellow fever diagnosis. PLoS One.
2012;7(5):e36291.
18. Yen C, Hyde TB, Costa AJ, Fernandez K, Tam JS, Hugonnet S, et al. The development of global vaccine
stockpiles. Lancet Infect Dis. 2015 Mar;15(3):340-7.
19. Lindsey NP, Schroeder BA, Miller ER, Braun MM, Hinckley AF, Marano N, et al. Adverse event reports
following yellow fever vaccination. Vaccine. 2008 Nov 11;26(48):6077-82.
20. World Health Organization. Meeting of the Emergency Committee under the International Health
Regulations (2005) concerning Yellow Fever Geneva2016 [cited 2016 May 19]. Available from:
http://www.who.int/mediacentre/news/statements/2016/ec-yellow-fever/en/.

RAPID RISK ASSESSMENT Outbreak of yellow fever in Angola, DRC and Uganda, first update – 27 May 2016
11
21. World Health Organization. Yellow fever Geneva: WHO; 2016 [cited 2016 March 22]. Available from:
http://www.who.int/csr/disease/yellowfev/en/.
22. United Nations Children's Fund. Yellow Fever Vaccine: Current Supply Outlook [internet]. 2016 [cited 30
May 2016]. Available from: http://www.unicef.org/supply/files/YF_number_3_Supply_Update.pdf.
23. Lucey D, Gostin LO. A Yellow Fever Epidemic: A New Global Health Emergency? JAMA. 9 May 2016.
24. World Health Organization. Yellow Fever – Angola. Disease Outbreak News 12 February. Geneva: WHO;
2016 [cited 22 March 2016]. Available from: http://who.int/csr/don/12-february-2016-yellow-fever-
angola/en/.
25. World Health Organization Regional Office for Africa. Situation Report: Yellow fever outbreak in Angola, 23
May 2016 Brazzaville: World Health Organization,; 2016 [cited 23 May 2016]. Available from:
http://www.afro.who.int/pt/yellow-fever/sitreps/item/8660-situation-report-yellow-fever-outbreak-in-angola-
23-may-2016.html.
26. United Nations Children's Fund. Yellow Fever Vaccine: Current Outlook. New York: UNICEF; 2015. Available
from: http://www.unicef.org/supply/files/Yellow_Fever_Vaccine_Current_Outlook_March_2015.pdf.
27. World Health Organization. Situation report. Yellow fever (24 April 2016) [Internet]. 2016 [cited 24 May
2016]. Available from: http://reliefweb.int/sites/reliefweb.int/files/resources/yf-outbreak-in-angola_sitrep-of-
25-april.pdf.
28. European Centre for Disease Prevention and Control. Potential risks to public health related to
communicable diseases at the Olympics and Paralympics Games in Rio de Janeiro, Brazil 2016 [Internet].
Stockholm ECDC; 2016. Available from:
http://ecdc.europa.eu/en/publications/_layouts/forms/Publication_DispForm.aspx?List=4f55ad51-4aed-
4d32-b960-af70113dbb90&ID=1486
29. European Centre for Disease Prevention and Control. VectorNet: A European network for sharing data on
the geographic distribution of arthropod vectors, transmitting human and animal disease agents [Internet].
Stockholm: ECDC; 2016. Available from:
http://ecdc.europa.eu/en/activities/diseaseprogrammes/emerging_and_vector_borne_diseases/Pages/VBOR
NET.aspx
30. Medlock JM, Hansford KM, Versteirt V, Cull B, Kampen H, Fontenille D, et al. An entomological review of
invasive mosquitoes in Europe. Bull Entomol Res. 2015 Dec;105(6):637-63.
