
Medicines/pharmaceuticals of animal origin - This guideline provides
information for all clinical staff within Hospital and Health Services (HHS) on
best practice for avoidance of issues related to animal products.
Medicines/pharmaceuticals
of animal origin
V3.0 November 2020

Medicines/pharmaceuticals of animal origin - V3.0 November 2020 Page 2
Medicines/pharmaceuticals of animal origin - V3.0 November 2020
Published by the State of Queensland (Queensland Health), November 2020
This document is licensed under a Creative
Commons Attribution 3.0 Australia licence.
To view a copy of this licence, visit creativecommons.org/licenses/by/3.0/au
© State of Queensland (Queensland Health) 2020
You are free to copy, communicate and adapt the work, as long as you attribute the State of Queensland
(Queensland Health).
For more information contact:
Medication Services Queensland, Queensland Health, GPO Box 48, Brisbane QLD 4001,
email InfoMSQ@health.qld.gov.au
An electronic version of this document is available at
https://www.health.qld.gov.au/__data/assets/pdf_file/0024/147507/qh-gdl-954.pdf
Disclaimer:
The content presented in this publication is distributed by the Queensland Government as
an information source only. The State of Queensland makes no statements, representations
or warranties about the accuracy, completeness or reliability of any information contained
in this publication. The State of Queensland disclaims all responsibility and all liability
(including without limitation for liability in negligence) for all expenses, losses, damages
and costs you might incur as a result of the information being inaccurate or incomplete in
any way, and for any reason reliance was placed on such information.

Medicines/pharmaceuticals of animal origin - V3.0 November 2020 Page 3
Table of Contents
1 Purpose 4
2 Scope 4
3 Background 4
4 Religious restrictions 5
4.1 Organisations consulted: 7
5 Resources 7
6 Appendices 9
Appendix A - Porcine (Pig) products 9
Appendix B - Bovine (Cow) products 11
Bovine – Manufacture includes exposure to bovine materials “Bovine-Indirect 14
Appendix C Chinese Hamster Ovary (CHO) cells 17
Appendix D - Murine (mouse) 21
Appendix E - Equine (Horse) 25
Appendix F - Egg/Chicken 26
Appendix G – Other Animals 29
Appendix H - Excipients that may be of animal origin 31
7 References 32
8 Approval 33
9 Version Control 33

Medicines/pharmaceuticals of animal origin - V3.0 November 2020 Page 4
1 Purpose
This guideline provides information for clinicians to assist patients wishing to avoid animal
product, to make informed decisions about their treatment/care.
2 Scope
People with food allergies or intolerances, or who want to avoid animal products for
religious, cultural or secular reasons may want to know about the origin/source of drugs and
excipients contained within their medicines, to enable them to make a fully informed
decision about their treatment. This wish is supported by the Australian Charter of
Healthcare Rights which states that ‘patients have a right to have their culture, identity,
beliefs and choices recognised and respected’. This document provides information for
clinicians to assist patients in making this choice.
This guideline provides information for all clinicians involved in the medicines management
cycle within Hospital and Health Services (HHS). While the information contained in the
tables of products in the appendices is compiled from the best information available, it
should not be regarded as fully comprehensive. Information on enteral and infant feeds (and
many herbal and complimentary medicines) is not included in this document. Please refer to
a dietitian for advice on enteral/infant feed composition.
3 Background
Person-centred care is the gold standard approach to healthcare delivery and has been
shown to improve the safety and quality of health care, improve patient outcomes and
experience, and improve the performance of health service organisations
1
. For this reason,
healthcare professionals must take into consideration patients’ patients’ religious beliefs
and lifestyles when prescribing and administering medicines.
2
Many different medicines and vaccines, or specific formulations of a medicine such as
tablets, capsules, creams or mixtures contain animal products or are animal derived. For
example, gelatin is a partially hydrolysed collagen which is usually bovine (beef) or porcine
(pig) in origin. Gelatin is used in making capsule shells and is one of many types of
stabilisers added to pharmaceutical products such as vaccines.
3
Heparin, an injectable
anticoagulant, is prepared from a porcine source. Further examples of pharmaceutical
products known to be of animal origin are listed in the appendices
Several of the world’s most prominent religions have objected to the use of certain animal-
derived products, including Muslim, Hindu, Christian, Jewish and Buddhist faiths (see table
1). A growing number of individuals are also increasingly restricting their consumption and
use of animal-derived products for ethical reasons such as animal welfare and objections to
the intentional killing of animals, environmental concerns and perceived health benefits,
However, neither religious nor secular groups are homogeneous in their views on the use of

Medicines/pharmaceuticals of animal origin - V3.0 November 2020 Page 5
animal-derived products used in their care. Consequently, there is considerable diversity of
opinion, and membership of a particular group does not necessarily dictate an individual’s
convictions
4
. However, informing patients about the origins of their proposed medication (if
animal derived and no suitable synthetic alterative exists) will assist them in making
informed decisions regarding their treatment.
2
There may be provisions within various
religious groups to provide some form of dispensation, depending on the nature of the need
for treatment.
NB All medicines must undergo testing in non-human animals before they can proceed to testing in
humans in order to gain a product license
13
.
4 Religious restrictions
Table 1 Religious restrictions
Religion
Countries where widely
practiced (relevant to
Queensland)
Restrictions
Buddhism
Tibet, Bhutan, India, Nepal, Sri
Lanka, Burma, Thailand, Laos,
Cambodia, Malaysia, Vietnam, China,
Bangladesh, Korea, Japan,
Singapore, parts of Russia.
For some vegetarian Buddhists - all
animal products prohibited
however, no fixed rules.
Hinduism
India, Nepal, Bangladesh, Indonesia,
Pakistan, Sri Lanka, Philippines, Fiji,
UK, Mauritius, Bhutan, South Africa,
Burma, Singapore
For majority who are vegetarian –
all animal products including egg
prohibited
For those who are not vegetarian,
restrictions still include bovine*
and porcine products
Islam
Indonesia, India, Pakistan,
Bangladesh, Egypt, Turkey, Iran,
Nigeria, Ethiopia, Afghanistan,
Sudan, Iraq, Malaysia, Tanzania,
Somalia, Cote d’Ivoire, Congo,
Philippines, Sierra Leone, Thailand,
Eritrea, Lebanon
Porcine products prohibited
All animal products not killed in
the prescribed ritualistic way
(halal) prohibited
Products containing alcohol
prohibited
Please note gelatin is
contentious**

Medicines/pharmaceuticals of animal origin - V3.0 November 2020 Page 6
Religion
Countries where widely
practiced (relevant to
Queensland)
Restrictions
Jehovah’s
witnesses
A 24 hours a day
service for
clinicians treating
Jehovah’s
Witnesses is
available. The
number can be
found via the
‘Contacts’ Button
on their site
Australia, USA, Mexico, Brazil and
many other countries (240 in total)
Abstain from blood products e.g.
blood transfusions.
[The use of fractions derived from the
primary components of blood is not
absolutely prohibited see – religious and
ethical position medical therapy]
Queensland Blood Management
provide information on consent
and refusal see: consent-refusal
Many HHSs have procedures for
refusal of blood products.
Judaism
USA, Israel, France, Canada, UK,
Russia, Argentina, Ukraine, Brazil
and South Africa
All porcine and shellfish products
prohibited
Other rules about animal products
that can be ingested:
• land animals must be
mammals which chew their cud
and have cloven hooves
• birds of prey are prohibited
• fish must have fins and scales
• non-fish seafood is prohibited
e.g. shellfish
• meat and milk (any dairy)
cannot be mixed
Observers only consume kosher
products – complex set of rules
Seventh Day
Adventist
Australia, USA, South America, some
African countries
Some abstain from meat, but eggs
are permissible.
Sikh
India, Pakistan, Malaysia, Singapore,
Fiji, New Zealand, USA and UK
For some who are vegetarian – all
animal products including egg
prohibited
For those who are not vegetarian,
restrictions still include bovine and
porcine products
All animal products from halal
sources prohibited
Products containing alcohol
prohibited.
Adapted from Multicultural Clinical Support Resource Folder- Health and Religion available at
https://www.health.qld.gov.au/__data/assets/pdf_file/0025/158740/8mcrs_hlth_relgn.pdf
accessed 03/08/2020

Medicines/pharmaceuticals of animal origin - V3.0 November 2020 Page 7
4.1 Organisations consulted:
• Buddhist Council of Queensland (President)
• Hindu Society of Queensland (President)
• Brisbane Sikh Temple (President)
• Seventh-day Adventist Church South Queensland (President)
• Jehovah’s witnesses Hospital Information Services International Office
• The Islamic Council of Queensland (Secretary)
• Vegan Australia
• Queensland Jewish Board of Deputies Incorporated
5 Resources
A United Kingdom publication titled “Drugs of porcine origin and their clinical alternatives -
An introductory guide”
2
(written in 2004, accessed 26/08/2020) gives further information on
drugs of porcine origin and is available at:
http://archive.mcb.org.uk/wp-
content/uploads/2015/12/Drugs-Derived-From-Pigs-and-their-Clinical-
Alternatives_Booklet.pdf
A Canadian question and answer document produced by the Alberta Health Services –
Calgary provides healthcare professionals with an introduction to the religious and cultural
issues associated with drugs of animal origin and the need for informed choice in a
multicultural society.
5
This document, titled “Medications derived from animals and
culturally diverse patients” is available at:
https://www.albertahealthservices.ca/assets/programs/ps-1026227-health-care-religious-
beliefs.pdf
The Vegan society (England and Wales) has two blogs about medication:
Is my medication vegan? available at:
https://www.vegansociety.com/whats-new/blog/my-
medication-vegan and What vegans should know pre-operatively available at:
https://www.vegansociety.com/whats-new/blog/what-vegans-should-know-pre-operatively
The manufacturer’s Product Information (PI) gives details on the composition of the
medicine (i.e. listing the active and inactive constituents/ingredients) and provides some
form of description on how the medicine is produced (e.g. whether manufacture of the
product included exposure to animal derived materials). Also, Consumer Medicine
Information (CMI) leaflets are available for most prescription medicines which enable
patients to check the medicine’s ingredients. However, these leaflets are produced in English
only, so further assistance may be needed.
There appears to be no consistently practical way of identifying whether the gelatin in
products has come from beef or pork. This information is not always stated in
manufacturers’ PI or CMI leaflets but may be important for Jewish, Muslim and Hindu people
who may want to avoid even traces of these particular animal products. If patients are
concerned about whether the drug or excipients within their medicine are of animal origin,

Medicines/pharmaceuticals of animal origin - V3.0 November 2020 Page 8
they could seek the information from their pharmacist or doctor who can check the
medicine's PI or CMI. Alternatively, patients can call the National Prescribing Services (NPS)
Medicines Line (1300 888 763)
6
or contact the Medical Information Department of the
pharmaceutical company that makes the medicine
7
.
For further clarification, the patient could seek guidance from their religious organisation.

Medicines/pharmaceuticals of animal origin - V3.0 November 2020 Page 9
6 Appendices
Appendix A - Porcine (Pig) products
Product name Generic name Therapeutic class Comment
Clexane Enoxaparin
Anticoagulant,
Antithrombotics
derived from porcine
intestinal mucosa
Creon Pancrelipase
Digestive
supplements and
cholelitholytics
Pancreatic extract
(porcine)
Creon Micro Enteric
coated granules
Pancrelipase
Digestive
supplements and
cholelitholytics
Pancreatic extract
(porcine)
Curosurf Poractant alfa Respiratory agent
natural porcine lung
surfactant
Ethical Nutrients
Digestion plus
Herbal
gastrointestinal
preparations
pepsin (porcine)
Fragmin Dalteparin Anticoagulant pig mucosa
Gamunex
Normal
immunoglobulin
(Human)
Immunoglobulin
porcine parvovirus
(PPV) as a model for
human parvovirus
B19
Heparin sodium
injection
Heparin sodium Anticoagulant Porcine mucous
Heparinised saline Heparin sodium Anticoagulant Porcine mucous
Heparinised saline
injection
Heparin sodium Anticoagulant Porcine mucous
Imogam Rabies
Pasteurized
Rabies immune
globulin (human)
Rabies immune
globulin (human)
Porcine
pseudorabies virus
was selected to
model hepatitis B
virus and herpes
virus
M-M-R II Powder for
injection
Measles, mumps and
rubella virus vaccine
live
Vaccine Porcine gelatin
Obizur Susoctocog alfa Haemostatic agent Porcine sequence

Medicines/pharmaceuticals of animal origin - V3.0 November 2020 Page 10
Product name Generic name Therapeutic class Comment
Orgaran Danaparoid Haemostatic agent
From animal mucosa
(Porcine)
Panzytrat 25000
Amylase, Lipase,
Pancrelipase,
Protease
Digestive
supplement
Pancreatic extract
(porcine)
Prothrombinex-VF
Human prothrombin
complex
Haemostatic agent Heparin, porcine
ProQuad
Measles, Mumps,
Rubella and Varicella
Virus Vaccine Live
Vaccine
hydrolysed porcine
gelatin
Rotarix
Human rotavirus live
attenuated vaccine
Vaccine
Contains porcine
circovirus type 1
(PCV-1)
RotaTeq
Rotavirus vaccine
live oral pentavalent
Vaccine
porcine circoviruses
(PCV) 1 and 2
Varivax Refrigerated
Powder for injection
Live varicella vaccine. Vaccine
hydrolysed porcine
gelatin
Zostavax
Zoster virus vaccine
live
Vaccine
hydrolysed porcine
gelatin
Appendices A to G compiled from reference 9

Medicines/pharmaceuticals of animal origin - V3.0 November 2020 Page 11
Appendix B - Bovine (Cow) products
Product name Generic name Therapeutic class Comment
Artiss
Aprotinin (synthetic),
factor XIII,
fibrinogen, thrombin
and calcium chloride
dihydrate
Surgical antiseptics
and applications
Artiss contains
synthetic aprotinin.
As synthetic
aprotinin is
structurally identical
to bovine aprotinin,
the use of Artiss in
patients with
allergies to bovine
proteins should be
carefully evaluated
Benadryl Children's
Cough 2 years +
Herbal respiratory
preparation
lactoferrin - bovine
(from cow's milk)
BioHeme
Iron – vitamin and
mineral
bovine lactoferrin
(derived from cow's
milk)
Blackmores
Immunodefence
capsules
Immune supplement
Lactoferrin, bovine
(dairy)
Blackmores Joint
Formula, Joint
Formula Advanced &
Joint Formula
Advanced with MSM
Booster
Herbal analgesics
and anti-
inflammatories
Chondroitin sulfate
bovine
ChondroCare Excel
Herbal analgesics
and anti-
inflammatories
Chondroitin sulfate
bovine
ChondroPlex
Herbal analgesics
and anti-
inflammatories
Chondroitin sulfate
bovine
Duro-Tuss Children's
Cough Liquid
Herbal respiratory
preparation
lactoferrin bovine
Ethical Nutrients
Inner Health plus
capsules
Lactobacillus
acidophilus, Bovine
colostrum
Digestive
supplements
Ethical nutrients
inner health plus
powder
Lactobacillus
acidophilus, Bovine
colostrum
Digestive
supplements

Medicines/pharmaceuticals of animal origin - V3.0 November 2020 Page 12
Product name Generic name Therapeutic class Comment
Gelofusine Gelatin succinylated
Plasma volume
expander
Gelatin derived from
bovine material
Haemaccel Polygeline
Plasma volume
expander
Bovine Lactoferrin
Cow’s milk protein
Immune-5
Digestive
supplements and
cholelitholytics
Inner Health Plus
capsules and Inner
Health powder
Digestive
supplements and
cholelitholytics
Bovine colostrum
Jespect
Inactivated Japanese
encephalitis vaccine
Vaccine
Should not be
administered to
individuals who have
previously
experienced a
serious reaction to
bovine serum
albumin
Lactoferrin Enhanced
& Lactoferrin Plus SB
Herbal and other
complementary
medicines
Lactoferrin (bovine)
Methylpred,
Methylprednisolone
Alphapharm & Solu-
Medrol
Methylprednisolone
sodium succinate
Adrenal steroid
hormones
40 mg strength
includes lactose
from cow's milk
M-M-R II
Measles, mumps and
rubella virus vaccine
live
Vaccine
This product may
contain residual
foetal bovine serum
NovoSeven RT
Eptacog alfa
(activated)
Haemostatic agents
contains trace
amounts bovine IgG
protein and other
bovine proteins
ProQuad
Measles, Mumps,
Rubella and Varicella
(Oka/Merck) Virus
Vaccine Live
contains residual
components bovine
serum albumin

Medicines/pharmaceuticals of animal origin - V3.0 November 2020 Page 13
Product name Generic name Therapeutic class Comment
Quadracel
Pertussis vaccine
acellular, diphtheria
and tetanus toxoids
(adsorbed),
inactivated
poliovirus types 1, 2
and 3
bovine serum
albumin
Recombinate Octocog alfa
contains trace
amounts of mouse,
hamster and bovine
protein
Survanta Beractant
pulmonary
surfactant
bovine lung extract
Thyrogen Thyrotropin alfa Diagnostic Agents
Use with caution in
patients previously
treated with bovine
TSH
Tisseel VH S/D
Solution
Aprotinin - Factor XIII
- Fibrinogen, Calcium
chloride dihydrate -
Thrombin
Haemostatic agent
Use with caution in
patients with
allergies to bovine
proteins
Totally Natural
Products Exit Pain
Herbal analgesics
and anti-
inflammatories
chondroitin sulfate
(bovine)
Travelan Bovine colostrum Anti-diarrhoeal
VAQTA & VAQTA
Paediatric/adolescent
Hepatitis A virus bovine albumin
Varivax
Varicella zoster
vaccine, live
Vaccines
Inactive components
include hydrolysed
gelatin and traces of
bovine serum.
Vivaxim
Hepatitis A vaccine;
Salmonella typhi
vaccine
Vaccines
Bovine serum
albumin <10 ng
(Inactive component)
Vivotif Oral
Oral Typhoid Vaccine
Salmonella typhi
strain Ty21a
gelatin (bovine
derived)
Zostavax
Live varicella
vaccine.
bovine calf serum

Medicines/pharmaceuticals of animal origin - V3.0 November 2020 Page 14
Bovine – Manufacture includes exposure to bovine
materials “Bovine-Indirect
Product name Generic name Therapeutic class Comment
Adacel
Pertussis vaccine,
Diphtheria toxoid,
Tetanus toxoid,
Poliomyelitis vaccine
Vaccine
Adacel Polio
Acellular pertussis,
diphtheria, tetanus
and poliovirus type 1,
2 and 3
Vaccines
Avaxim Hepatitis A vaccine Vaccine
Boostrix
Diphtheria toxoid,
Tetanus toxoid,
Pertussis vaccine
Vaccine
Boostrix – IPV
suspension for
injection
Diphtheria toxoid,
Tetanus toxoid,
Pertussis vaccine,
Poliomyelitis vaccine
Vaccine
Engerix-B Adult and
Paediatric
Hepatitis B vaccine Vaccine
Havrix 1440 Hepatitis A vaccine Vaccine
Havrix Junior Hepatitis A vaccine Vaccine
Hexaxim
Diphtheria, tetanus,
pertussis, hepatitis B,
poliomyelitis and
Haemophilus
influenzae type b
conjugate vaccine
Vaccine
Hiberix
Haemophilus B
conjugate vaccine
Vaccine
Infanrix
Diphtheria, tetanus,
acellular pertussis
(DTPa) vaccine
Vaccine
Infanrix-IPV
Diphtheria, tetanus,
acellular pertussis
(DTPa) and
inactivated
poliovirus vaccine
Vaccine

Medicines/pharmaceuticals of animal origin - V3.0 November 2020 Page 15
Product name Generic name Therapeutic class Comment
Ipol
Poliovirus
(inactivated)
Vaccine
Merieux inactivated
rabies vaccine
Rabies vaccine Vaccine
M-M-R II
Measles, mumps and
rubella virus vaccine
live
Vaccine
This product may
contain residual
foetal bovine serum
NeisVac-C Vaccine
Meningococcal group
C polysaccharide
conjugate vaccine
Vaccines
Pneumovax 23
Pneumococcal
purified capsular
polysaccharides
Vaccine
Priorix
Measles, mumps &
rubella vaccine
Vaccines
Priorix-tetra
Varicella zoster
vaccine, Rubella
vaccine, Mumps
vaccine, Measles
vaccine
Vaccines
ProQuad
Measles, Mumps,
Rubella and Varicella
(Oka/Merck) Virus
Vaccine Live
Vaccines
contains residual
components of
bovine serum
albumin
Quadracel
Pertussis vaccine
acellular, diphtheria
and tetanus toxoids
(adsorbed),
inactivated
poliovirus types 1, 2
and 3
Vaccines
bovine serum
albumin
Rabipur Rabies vaccine Vaccines
contraindicated in
subjects with a
known severe
hypersensitivity to
chicken eggs, chicken
protein, bovine
gelatin
Rotarix Oral Liquid
Human Rotavirus
(live attenuated oral
vaccine)
Vaccines

Medicines/pharmaceuticals of animal origin - V3.0 November 2020 Page 16
Product name Generic name Therapeutic class Comment
RotaTeq Rotavirus vaccine Vaccines
Saizen & Saizen 3
Somatropin (rmc),
recombinant human
growth hormone
Pituitary hormones
prepared from
genetically
engineered
mammalian cells
(recombinant mouse
cells - C127)
transformed with a
bovine papilloma
virus vector
Stamaril Yellow Fever Vaccine Vaccines
Tripacel
Pertussis vaccine-
acellular, combined
with diphtheria and
tetanus toxoids
Vaccines
Twinrix & Twinrix
Junior
Combined hepatitis A
and hepatitis B
vaccine
Vaccines
Typhim Vi
Salmonella typhi Vi
polysaccharide
vaccine
Vaccines
Varilrix HSA-Free Live varicella vaccine Vaccines
Varivax
Varicella zoster
vaccine, live
Vaccines
Inactive components
include hydrolysed
gelatin and traces of
bovine serum.
Fluarix
Influenza virus
vaccine
Vaccine
ADT Booster Diphtheria toxoid Vaccine

Medicines/pharmaceuticals of animal origin - V3.0 November 2020 Page 17
Appendix C Chinese Hamster Ovary (CHO) cells
Product name Generic name Therapeutic class Comment
Actemra Tocilizumab Immunomodifiers
contraindicated in patients
with:
known hypersensitivity to
any CHO products
Adcetris
Brentuximab
vedotin
Antineoplastic
Advate Octocog alfa Haemostatic agent
Adynovate
Rurioctocog alfa
pegol
Haemostatic agent
derived from octocog alfa
(Advate)
Ajovy Fremanezumab
Antimigraine
preparations
fully humanised monoclonal
antibody produced in CHO
cells
Aldurazyme Laronidase
Enzyme
replacement
therapy
genetically engineered
Chinese hamster ovary cell
line
Aranesp Darbepoietin Haemopoietic agent produced in CHO cells
Avastin Bevacizumab Antineoplastic
produced by recombinant
DNA technology in a CHO
mammalian cell expression
system
Avonex Interferon beta-1a Immunomodifier
produced by mammalian
(CHO) cells
Bavencio Avelumab. Antineoplastic produced in CHO cells
Bemfola Follitropin alfa (rch) Pituitary hormone produced by a CHO cell
BeneFIX Nonacog alfa Haemostatic agent
contraindicated in patients
with previous allergy to
hamster protein
Besponsa
Inotuzumab
ozogamicin (rch)
Antineoplastic
Blincyto Blinatumomab (rch) Antineoplastic
Brenzys Etanercept.
Antirheumatoid
agents
Brineura Cerliponase alfa.
Endocrine and
metabolic agents

Medicines/pharmaceuticals of animal origin - V3.0 November 2020 Page 18
Product name Generic name Therapeutic class Comment
CellCept
Mycophenolate
mofetil.
Immunomodifiers
Based on Chinese hamster
inosine-5'-monophosphate
dehydrogenase
Cerezyme Imiglucerase
Enzyme
replacement
therapy
Cosentyx Secukinumab (rch) Immunomodifier
Darzalex Daratumumab Antineoplastic
Dupixent Dupilumab (rch) Immunomodifier
Elonva Corifollitropin alfa Pituitary hormones
Emgality Galcanezumab Antimigraine
Enbrel Etanercept Immunomodifier
Entyvio Vedolizumab Immunomodifier
Eprex Epoietin-alfa Haemopoietic agent
Eylea Aflibercept
Ophthalmic
medication
Fabrazyme Agalsidase beta
Enzyme
replacement
therapy
Fasenra Benralizumab Immunomodifier
Gonal-f Follitropin alfa Pituitary hormone
Granocyte Lenograstim Supportive therapy
expressed in a mammalian
host cell system, (CHO) cells
Hemlibra Emicizumab Haemostatic agent
Herceptin Trastuzumab Antineoplastic agent
contraindicated in patients
with known hypersensitivity
to CHO cell proteins
Hyqvia
Normal
immunoglobulin
(human) with
vorhyaluronidase
alfa
Immunoglobulin
The vorhyaluronidase alfa.is
produced from CHO cells

Medicines/pharmaceuticals of animal origin - V3.0 November 2020 Page 19
Product name Generic name Therapeutic class Comment
Ilumya Tildrakizumab (rch) Immunomodifier
Kadcyla
Trastuzumab
emtansine.
Antineoplastic agent
Kanjinti Trastuzumab Antineoplastic agent biosimilar to Herceptin.
Keytruda
Pembrolizumab
(rch)
Antineoplastic agent
Kogenate FS Octocog alfa Haemostatic agent
Trace amounts of mouse and
hamster protein present
Lemtrada Alemtuzumab (rch) Immunomodifier
Luveris 75 IU Lutropin alfa Pituitary hormone
Mabcampath Alemtuzumab Antineoplastic agent
Mabthera Rituximab Antineoplastic agent
Metalyse Tenecteplase Fibrinolytic agent
Mircera
Methoxy
polyethylene glycol-
epoetin beta
Haemopoietic agent
Recombinant DNA
technology in CHO cells
MycoCept Mycophenolate Immunomodifiers
Based on Chinese hamster
inosine-5'-monophosphate
dehydrogenase
Myozyme Alglucosidase alfa.
Endocrine and
metabolic agents
Naglazyme Galsulfase (rch)
Endocrine and
metabolic agent
NeoRecormon Epoietin beta Haemopoietic agent
Novicrit Epoetin lambda Haemopoietic agent Purified from a CHO cell
NovoSeven RT Eptacog alfa Haemostatic agent Baby hamster kidney cells
Nucala Mepolizumab Respiratory agent
Obizur Susoctocog alfa Haemostatic agent Baby hamster kidney cells

Medicines/pharmaceuticals of animal origin - V3.0 November 2020 Page 20
Product name Generic name Therapeutic class Comment
Ogivri Trastuzumab. Antineoplastic agent
contraindicated in patients
with hypersensitivity Chinese
hamster ovary cell proteins
Ontruzant Trastuzumab. Antineoplastic agent
biosimilar medicine to
Herceptin
Opdivo Nivolumab Antineoplastic agent
Orencia Abatacept Immunomodifier
Recombinant DNA
technology in CHO cells
Ovidrel
Choriogonadotropin
alfa
Pituitary hormone
Pergoveris
Follitropin alfa
(rch)/lutropin alfa
(rch)
Pituitary hormone
Perjeta Pertuzumab Antineoplastic agent
contraindicated in patients
with hypersensitivity to
Chinese hamster ovary cell
proteins
Praluent Alirocumab (rch)
Hypolipidaemic
agent
Prolia Denosumab
Affects calcium and
bone metabolism
Pulmozyme Dornase alfa Respiratory agent
contraindicated in patients
who have hypersensitivity to
Chinese hamster ovary cell
Puregon Follitropin beta Pituitary hormone
Rebif Interferon beta-1a Immunomodifier
Recombinate
Recombinate
antihaemophilic
factor
Haemostatic agent
Rekovelle Follitropin delta Pituitary hormone
Repatha Evolocumab
Hypolipidaemic
agent
Rixubis
Recombinant
Coagulation Factor
IX (rFIX), Nonacog
gamma (rch)
Haemostatic agent
contraindicated in patients
with known hypersensitivity
hamster protein
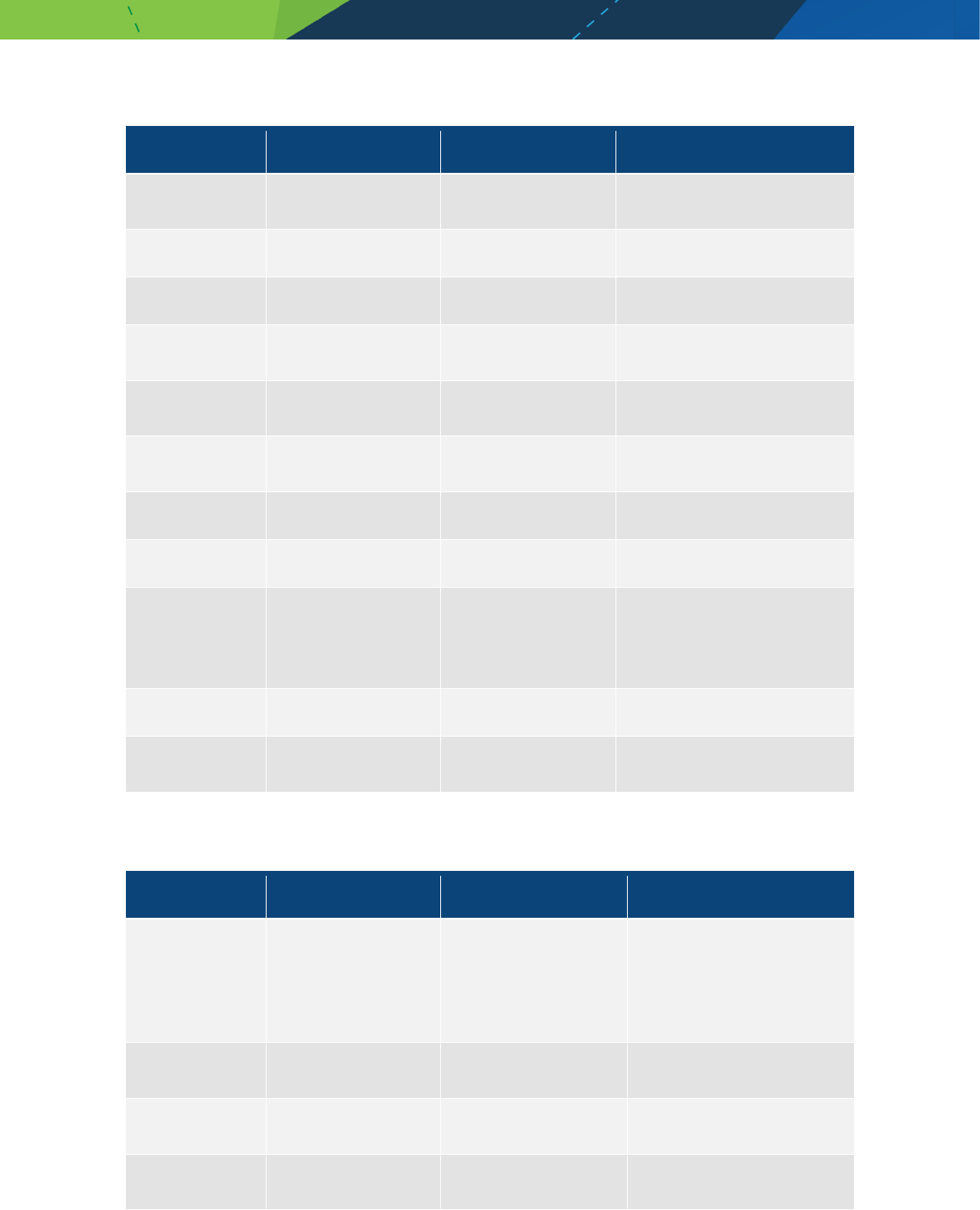
Medicines/pharmaceuticals of animal origin - V3.0 November 2020 Page 21
Product name Generic name Therapeutic class Comment
Strensiq Asfotase alfa rch
Endocrine and
metabolic agent
Sylvant siltuximab (rmc) Antineoplastic agent
Thyrogen Thyrotrophin alfa Diagnostic agent
Trazimera Trastuzumab
Antineoplastic
agents
biosimilar to Herceptin
Vectibix Panitumumab
Antineoplastic
agents
Recombinant DNA
technology in CHO cells
Vimizim Elosulfase alfa (rch)
Endocrine and
metabolic agent
Xgeva Denosumab Antineoplastic agent
Xolair Omalizumab Respiratory agent
Xyntha Moroctocog alfa Haemostatic agent
contraindicated in patients
with a known history of
hypersensitivity to hamster
proteins
Yervoy Ipilimumab Antineoplastic agent
Zinplava Bezlotoxumab
Antibiotics and anti-
infective
Appendix D - Murine (mouse)
Product name Generic name Therapeutic class Comment
Advate Octocog alfa Haemostatic agent
contains trace amounts of
mouse immunoglobulin G
Contraindicated in known
hypersensitivity to mouse
proteins
Adynovate
Rurioctocog alfa
pegol
Haemostatic agent
derived from octocog alfa
(Advate)
Arzerra Ofatumumab Antineoplastic agent
produced in a recombinant
murine cell line
Avastin Bevacizumab Antineoplastic agent
Humanised murine
antibody
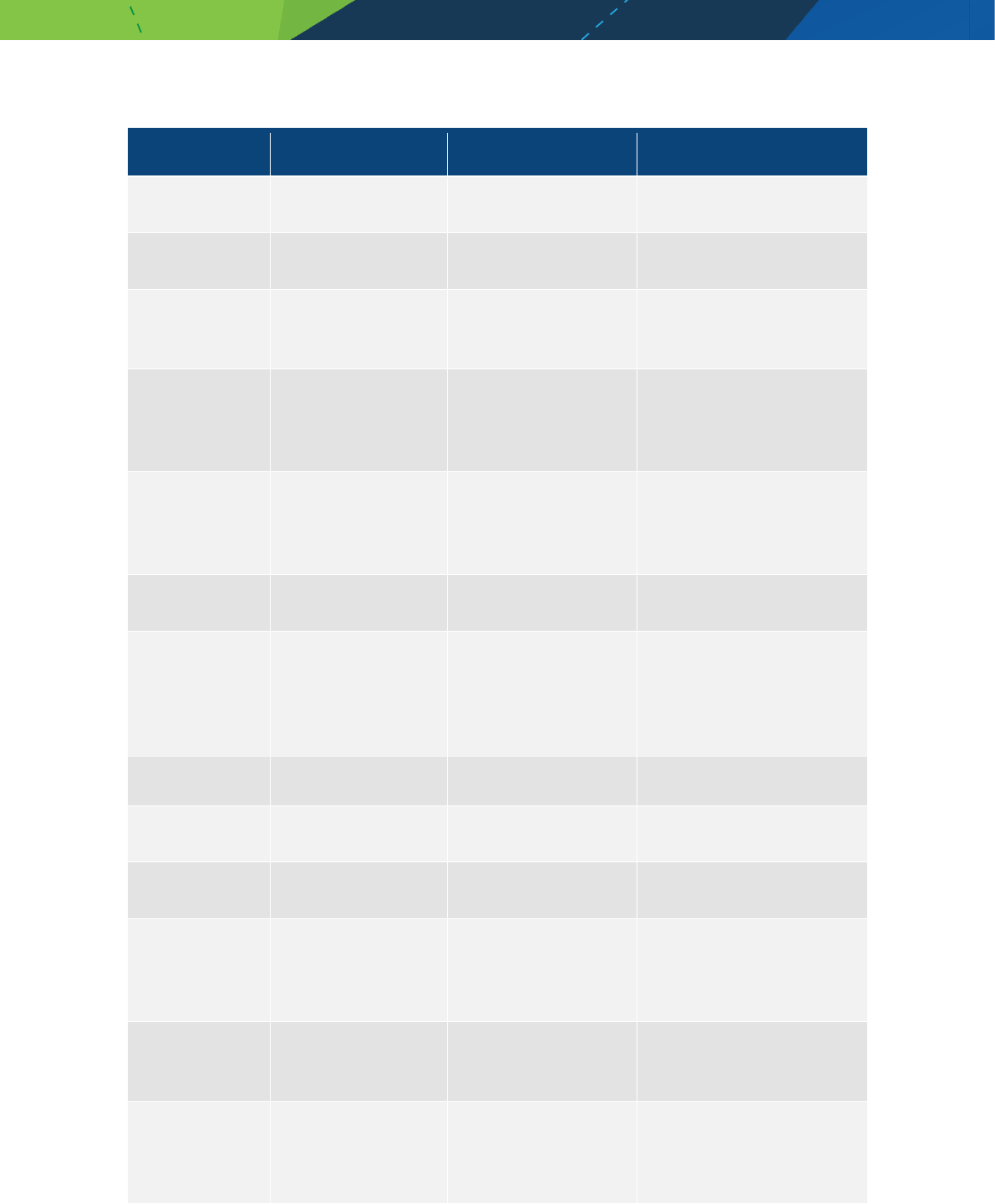
Medicines/pharmaceuticals of animal origin - V3.0 November 2020 Page 22
Product name Generic name Therapeutic class Comment
Ceprotin Protein C
Anticoagulant,
antithrombotic
may contain traces of
mouse protein
Cyramza Ramucirumab Antineoplastic agent
produced in murine (NS0)
cells
Empliciti Elotuzumab Antineoplastic agent
consists of regions of
mouse antibody grafted
onto human IgG1
Erbitux Cetuximab Antineoplastic agent
produced in mammalian
cell culture by mouse
myeloma cells (Sp2/0)
Gazyva Obinutuzumab Antineoplastic agent
contraindicated in patients
with known
hypersensitivity to murine
proteins
Herceptin Trastuzumab Antineoplastic agent
Murine anti-p185 HER2
antibody
Inflectra Infliximab Immunomodifier
biosimilar to Remicade
should not be given to
patients with a history of
hypersensitivity to murine
proteins
Kanjinti Trastuzumab Antineoplastic agent Biosimilar to Herceptin
Kogenate Octocog alfa Haemostatic agent
Trace amounts of mouse
protein are present
Kymriah Tisagenlecleucel Antineoplastic agent
contains a murine single
chain antibody fragment
Lemtrada Alemtuzumab Immunomodifier
humanised monoclonal
antibody with regions from
a murine monoclonal
antibody
Lucentis Ranibizumab
Ophthalmic
medication
Immunoglobulin G1, with
human-mouse monoclonal
rhuFab V2 κ-chain
MabCampath Alemtuzumab Immunomodifier
contraindicated in patients
with a known
hypersensitivity to murine
proteins
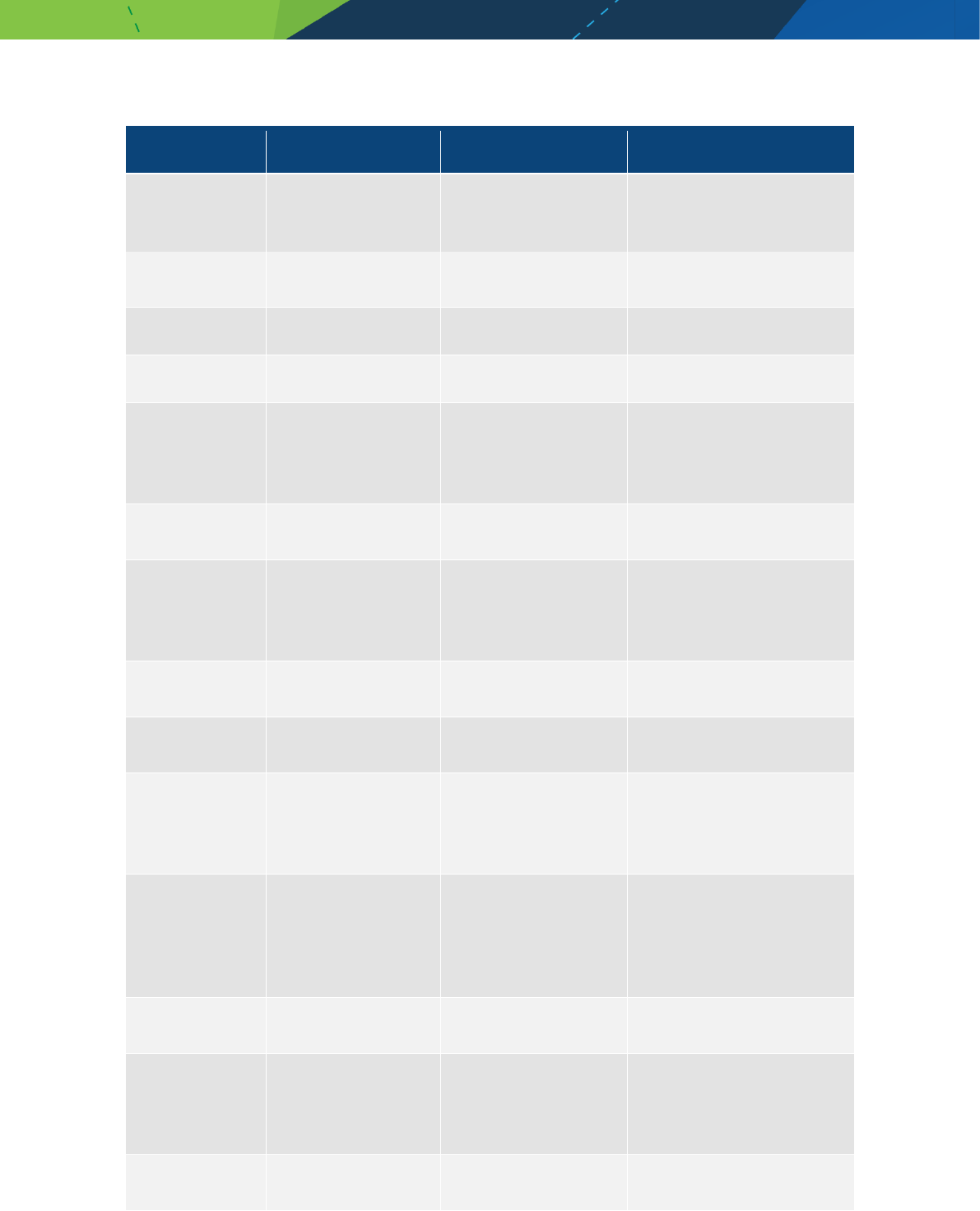
Medicines/pharmaceuticals of animal origin - V3.0 November 2020 Page 23
Product name Generic name Therapeutic class Comment
Mabthera Rituximab Antineoplastic agent
Genetically engineered
chimeric murine/human
monoclonal antibody
NovoSeven RT Eptacog alfa Haemostatic agent
contains trace amounts of
mouse IgG protein
Ogivri Trastuzumab Antineoplastic agent Biosimilar to Herceptin
Ontruzant Trastuzumab Antineoplastic agent Biosimilar to Herceptin
Praxbind Idarucizumab
Detoxifying agent,
antidote
humanised monoclonal
antibody derived from
murine IgG1 isotype
antibody molecule
Recombinate Octocog alfa Haemostatic agent
contains trace amounts of
mouse protein
Remicade Infliximab Immunomodifier
Monoclonal antibody
composed of human
constant and murine
variable regions
Renflexis Infliximab Immunomodifier
biosimilar medicine to
Remicade
Riximyo Rituximab Antineoplastic agent
biosimilar medicine to
Mabthera
Saizen Somatropin Pituitary hormone
prepared from genetically
engineered mammalian
cells (recombinant mouse
cells
Simponi
prefilled
syringe
solution
Golimumab
Antirheumatoid agent
Murine hybridoma cell line
with recombinant DNA
technology
Simulect Basiliximab Immunomodifier
Murine/human chimeric
monoclonal antibody
Soliris Eculizumab Immunomodifier
Human/murine hybrid
monoclonal antibody
produced by murine
myeloma cell culture
Sylvant siltuximab Antineoplastic agent
chimeric human murine
monoclonal antibody

Medicines/pharmaceuticals of animal origin - V3.0 November 2020 Page 24
Product name Generic name Therapeutic class Comment
Synagis Palivizumab Immunomodifier
5% murine amino acid
sequences
Trazimera Trastuzumab Antineoplastic agent Biosimilar to Herceptin
Tysabri Natalizumab Immunomodifier
Human/murine hybrid
monoclonal antibody
produced in murine
myeloma cells
Xolair Omalizumab Respiratory agent
recombinant monoclonal
antibody that contains
regions of a humanised
murine antibody
Yescarta
axicabtagene
ciloleucel
Antineoplastic agent
patient's own T cells are
harvested and genetically
modified ex vivo by
retroviral transduction to
express a chimeric antigen
receptor (CAR) comprising
a murine anti-CD19 single
chain variable fragment

Medicines/pharmaceuticals of animal origin - V3.0 November 2020 Page 25
Appendix E - Equine (Horse)
Product name Generic name
Therapeutic
class
Comment
ATGAM
Equine Antithymocyte
globulin
Immunomodifier
Black snake
antivenom
Black snake antivenom Antivenom
Brown snake
antivenom
Brown snake antivenom Antivenom
Death adder
antivenom
Death adder antivenom Antivenom
Duavive
Conjugated
estrogens/bazedoxifene.
Gonadal hormone
Polyvalent
snake
antivenom
Brown snake antivenom,
Death adder antivenom,
King brown snake
antivenom, Taipan
antivenom, Tiger snake
antivenom
Antivenom
Premarin
tablets
Oestrogens, Conjugated Gonadal hormone
Red back spider
antivenom
Red back spider
antivenom
Antivenom
Sea snake
antivenom
Sea snake antivenom Antivenom
Stonefish
antivenom
Stonefish antivenom Antivenom
TachoSil
Human fibrinogen and
human thrombin
Surgical
Preparation
equine collagen
Taipan
antivenom
Taipan antivenom Antivenom
Tiger snake
antivenom
Tiger snake antivenom Antivenom

Medicines/pharmaceuticals of animal origin - V3.0 November 2020 Page 26
Appendix F - Egg/Chicken
Product name Generic name Therapeutic use Comment
Afluria Quad
quadrivalent
influenza vaccine
Vaccine
manufactured in eggs.
trace amounts of
ovalbumin (< 1
microgram/0.5 mL dose),
may be present
Agrippal
Influenza virus
vaccine
Vaccine
vaccine may contain egg,
chicken
Cleviprex Clevidipine
Antihypertensive
agent
Contraindicated in
patients with known
allergies to eggs or egg
products as contains egg
lecithin
ClinOleic 20% Olive oil and soya oil
Parenteral vitamins,
minerals and
nutrition
Contraindicated in
patients with known
hypersensitivity to egg as
contains egg lecithin
Diprivan Propofol Anaesthetics Contains egg lecithin
Fluarix
Influenza virus
vaccine
Vaccine
prepared using whole
virus cultivated in
embryonated hen eggs
Fluad
Influenza virus
vaccine
Vaccine
grown in hens' eggs
May also contain chicken
proteins (such as
ovalbumin0
Fluad Quad
quadrivalent
influenza vaccine
Vaccine grown in hens' eggs
FluQuadri
Quadrivalent
Influenza Vaccine
Vaccine
propagated in chicken
eggs
Fluzone High-
Dose
trivalent influenza
vaccine
Vaccine
propagated in chicken
eggs
Fresofol 1%
Injection &
Fresofol 1%
MCT/LCT
Propofol Anaesthetics Contains egg lecithin
Influvac
Influenza virus
vaccine
Vaccine
prepared from virus
grown in the allantoic
cavity of embryonated
eggs

Medicines/pharmaceuticals of animal origin - V3.0 November 2020 Page 27
Product name Generic name Therapeutic use Comment
Intralipid Soya oil
Parenteral vitamins,
minerals and
nutrition
Contains egg lecithin
Kabiven G11% &
Kabiven G19%
Parenteral vitamins,
minerals and
nutrition
Contains egg lecithin
Kanuma Sebelipase alfa
endocrine and
metabolic agent
Contraindicated in
patients with
hypersensitivity to egg as
produced in egg white
M-M-R II
Measles, mumps and
rubella virus vaccine l
Vaccine
produced in chick embryo
cell culture
Olimel,
PeriOlimel
Parenteral vitamins,
minerals and
nutrition
contains egg lecithin
Priorix
Measles, mumps and
rubella virus vaccine
Vaccine
produced in chick embryo
cell culture, may contain
traces of egg protein
Priorix-Tetra &
ProQuad
Measles, mumps,
rubella and varicella
vaccine
Vaccine
produced in chick embryo
cell culture, may contain
traces of egg protein
Propofol Sandoz
Propofol-Lipuro
1%/2%
Provive 1% and
Provive MCT-LCT
1%
Propofol Anaesthetics Contains egg lecithin
Q-Vax & Q-Vax
Skin Test
Coxiella burnetii
vaccine
Vaccine
grown in the yolk sacs of
embryonated eggs. Trace
amounts of ovalbumin
may also be present
Rabipur Rabies vaccine Vaccine
Made from purified chick
embryo cell
Rixadone Risperidone Antipsychotic agent Contains Egg products
Stamaril Yellow Fever Vaccine Vaccine
propagated in chick
embryos
SmofKabiven
Electrolyte Free
& SmofKabiven
Parenteral vitamins,
minerals and
nutrition
Contains egg
phospholipids and Fish
oil

Medicines/pharmaceuticals of animal origin - V3.0 November 2020 Page 28
Product name Generic name Therapeutic use Comment
SMOFlipid
Parenteral vitamins,
minerals and
nutrition
Contains egg lecithin and
fish oil
Vaxigrip
Influenza virus
vaccine
Vaccine
prepared from virus
grown in the allantoic
cavity of embryonated
eggs
Visudyne Verteporfin
ophthalmic
medication
Contains egg
phosphatidylglycerol
sodium
Vitalipid N
Parenteral vitamins,
minerals and
nutrition
Contains egg lecithin

Medicines/pharmaceuticals of animal origin - V3.0 November 2020 Page 29
Appendix G – Other Animals
Product name Generic name Therapeutic uses Comment
Acarizax
house dust mite
extract
Antiallergy
preparation
Contains gelatin (fish)
Box Jellyfish
Antivenom
Box Jellyfish
Antivenom
antivenom
from the plasma of
sheep
Chondroitin Chondroitin
Complementary
osteoarthritis
From bovine or shark
cartilage
DigiFab
Digoxin binding
antibody
Digoxin-specific
antibody fragment
Antidote From sheep
Fluad
Inactivated
influenza vaccine
Vaccine
contains the oil
squalene which is
obtained from shark
liver
Funnel Web Spider
Antivenom
Funnel web spider
antivenom (rabbit)
antivenom
prepared from the
plasma of rabbits
Glucosamine Glucosamine
Complementary
osteoarthritis
From shellfish
Grazax Phleum pratense.
Antiallergy
preparation
contains gelatine (fish)
Human Insulin
(rys)
Protaphane
Mixtard 30/70.
Mixtard 50/50
Insulin Insulin preparation
Contain protamine
sulfate (a fish product)
Humira Adalimumab Immunomodifier
Mammalian cells (Not
specified)
Mono-plus
mononucleosis
test
Diagnostic Guinea pig, horse
Novoseven Eptacog alfa Haemostatic agent
Baby hamster kidney
(BHK) cells
Omacor
Eicosapentaenoic
acid (EPA) ethyl
ester,
docosahexaenoic
acid (DHA) ethyl
ester.
cardiovascular agent
obtained by the
transesterification of
the body oil of fat fish
species

Medicines/pharmaceuticals of animal origin - V3.0 November 2020 Page 30
Product name Generic name Therapeutic uses Comment
Protamine Sulfate
Injection
Haemostatic agent
Caution in patients
allergic to fish
SMOFlipid
Parenteral vitamins,
minerals and
nutrition
Contains egg lecithin
and fish oil
Thymoglobuline
Antithymocyte
globulin (rabbit)
Immunomodifier Rabbit
Compiled from reference 9

Medicines/pharmaceuticals of animal origin - V3.0 November 2020 Page 31
Appendix H - Excipients that may be of animal
origin
Product Source
Bee pollen
Gathered by bees and collected from legs of
bees
Chitin
From the exoskeletons of arthropods and
crustacean
Chymotrypsin Cochineal/Carmine/Carminic
acid
Ox pancreas
Cochineal/carmine/carmininc acid Red pigment from crushed cochineal insects
Disodium inosinate From meat extract
Gelatin
From pig skin, bovine hide and pork and cattle
bones. Used for many capsules
Glycerol May be derived from animal fats
Lactose
From cows’ milk (by-product of dairy
industry). Usually made synthetically
(Common filler in tablets)
Lanolin Fat extracted from sheep’s wool
Oleic oil and oleostearin From pressed tallow
Propolis Bee glue
Shellac Insect secretion
Stearic acid
Fat from cows, sheep, dogs or cats.
Can be obtained from vegetable sources
Trypsin Enzyme from pork pancreas
Compiled from references 11, 12

Medicines/pharmaceuticals of animal origin - V3.0 November 2020 Page 32
7 References
1. Reproduced with permission from Partnering with patients in their own care, developed by
the Australian Commission on Safety and Quality in Health Care (ACSQHC). ACSQHC: Sydney
2. United Kingdom. National Prescribing Centre. Task Force on Medicines Partnership. Drugs
of porcine origin and their clinical alternatives. An introductory guide. March 2004 [11
December 2007] Available from: http://archive.mcb.org.uk/wp-
content/uploads/2015/12/Drugs-Derived-From-Pigs-and-their-Clinical-
Alternatives_Booklet.pdf
3. Eldred BE, Dean AJ, McGuire TM, Nash AL. Vaccine components and constituents:
responding to consumer concerns. MJA 2006;184 (4):170-175.
4. Rodger, D., Blackshaw, B.P. Using animal-derived constituents in anaesthesia and surgery:
the case for disclosing to patients. BMC Med Ethics 20, 14 (2019. Available at
https://doi.org/10.1186/s12910-019-0351-4
5. Alberta Health Services – Calgary. Medications derived from animals and culturally diverse
patients. 2nd edition. Available at
https://www.albertahealthservices.ca/assets/programs/ps-1026227-health-care-religious-
beliefs.pdf
6. National Prescribing Services (NPS). Pharmaceutical excipients – where do we begin? 1
August 2011 DOI: 10.18773/austprescr.2011.060
7. What are excipients doing in medicinal products? Drug and Therapeutics Bulletin
2009;47:81-84. http://dx.doi.org/10.1136/dtb.2009.06.0026
8. United States. Immunization Action Coalition. World Health Organisation Regional Office
for the Eastern Mediterranean - The use of unlawful or juridically unclean substances in food
and medicine (correspondence dated 17 July 2001). [27 June 2007] Available from:
www.immunize.org/concerns/porcine.pdf
9. MIMS Online. Retrieved from http://www.mims.com.au
10. Australian Register of Therapeutic Goods (ARTG). Therapeutic Goods Administration.
Department of Health and Ageing. Australian Government available from
http://www.tga.gov.au/industry/artg.htm
11. Sriaandhal Sabalingam1, W. J. A. Banukie N. Jayasuriya. Pharmaceutical Excipients of
Marine and Animal origin: A Review Biological and Chemical Research (ISSN 2312-0088), Vol.6,
184-196
12. Alamgir A.N.M. (2018) Bioactive Compounds and Pharmaceutical Excipients Derived from
Animals, Marine Organisms, Microorganisms, Minerals, Synthesized Compounds, and
Pharmaceutical Drugs. In: Therapeutic Use of Medicinal Plants and their Extracts: Volume 2.
Progress in Drug Research, vol 74. Springer, Cham. https://doi.org/10.1007/978-3-319-92387-1_4
13 The Vegan society (England and Wales) Is my medication vegan? available at:
https://www.vegansociety.com/whats-new/blog/my-medication-vegan

Medicines/pharmaceuticals of animal origin - V3.0 November 2020 Page 33
8 Approval
Approving Officer:
Justin Lee
Director
Medication Services Queensland
2020
9 Version Control
Version Amendments Author/s Approved
2.0
Supersedes original version published in
2007
A Jagels November 2013
2.1
Fixed broken link to booklet on porcine
medicines. Updated organisational details
A Jagels April 2019
3.0 Review
J Quin
A Jagels
November 2020
